Abstract
Given the complexity of antimicrobial resistance and the dire implications of misusing antimicrobials, it is imperative to identify accurate and meaningful ways to understand and communicate the realities, challenges, and opportunities associated with antimicrobial utilization and measurement in all sectors, including in animal agriculture. The objectives of this article are to (i) describe how antimicrobials are regulated and used in US animal agriculture and (ii) highlight realities, challenges, and opportunities to foster multidisciplinary understanding of the common goal of responsible antimicrobial use. Recognition of the realities of medicine, practice, and policy in the agricultural setting is critical to identify realistic opportunities for improvement and collaboration.
Keywords: antimicrobials, animal agriculture, animal husbandry, antimicrobial stewardship, multidisciplinary collaboration, One Health
This article describes how antimicrobials are regulated and used in US animal agriculture and highlights realities, challenges, and opportunities to foster multidisciplinary understanding of the common goal of responsible antimicrobial use.
Antimicrobials are used in humans and other animals. Professional and public attention paid to antimicrobial use (AU) in human healthcare is nearly always framed in the context of individual patient health and safety as well as public health. In contrast, appraisals of AU in animals rarely include the context of animal health and welfare or security of the human food supply. Given the complexity of antimicrobial resistance and the dire implications of misusing antimicrobials, it is imperative to identify accurate and meaningful ways to understand and communicate the realities, challenges, and opportunities associated with antimicrobial utilization and measurement in all sectors, including in animal agriculture. The objectives of this article are (i) to describe how antimicrobials are regulated and used in animal agriculture in the United States (US) and (ii) to highlight realities, challenges, and opportunities to foster multidisciplinary understanding of the common goal of responsible AU. We describe approval of antimicrobials for use in food-producing animals, federal policy influencing AU in nonhuman animals, animal husbandry approaches to prevent infection (including biosecurity), challenges to reduce AU, and opportunities for improving antimicrobial stewardship (AS). Recognition of the realities of medicine, practice, and policy in the agricultural setting is critical to identify realistic opportunities for improvement and collaboration.
ANTIMICROBIAL USE IN FOOD-PRODUCING ANIMALS
Food-producing animals are defined under the US Food, Drug, and Cosmetic Act as either major species (cattle, swine, chickens, turkeys) or minor species, which include all other animals from which food (eg, meat, milk, eggs) is derived [1].
Indications: How Antimicrobials Are Used in Food-Producing Animals
Antimicrobials may be administered to individual animals or groups of animals. Antimicrobials are administered to food-producing animals for the purposes of disease treatment, disease control, and disease prevention [2] (Table 1). Antimicrobials have been used for production (eg, growth promotion) purposes since the 1940s, under the premise that AU reduces gastrointestinal flora competing for nutrients and microbial metabolites that suppress growth, allowing the animal to better harness nutrition it consumes [3]. Use of medically important antimicrobials (MIAs) for growth promotion has not been legal in the US since January of 2017, although several had been historically used for that purpose [4]. MIAs are antimicrobials identified as essential to human medicine by the US Food and Drug Administration (FDA). They are used in human healthcare, animal agriculture, and clinic-based veterinary medicine [5]. All drugs on the FDA MIA list are equally subject to regulations in US animal agriculture, regardless of their relative importance to healthcare or inclusion in the World Health Organization critically important antimicrobials list [4, 6]. Some non-MIAs that have retained drug-label uses for production purposes remain available for growth promotion.
Table 1.
American Veterinary Medical Association Definitions of Antimicrobial Use
| Antibiotic Purpose | AVMA Definition for Individual Animals | AVMA Definition for Groups of Animals |
|---|---|---|
| Disease treatment | Administration of an antimicrobial as a remedy for an individual animal with evidence of infectious disease | Administration of an antimicrobial to those animals within the group with evidence of infectious disease |
| Disease control | Administration of an antimicrobial to an individual animal with a subclinical infection to reduce the risk of the infection becoming clinically apparent, spreading to other tissues or organs, or being transmitted to other individuals | Administration of an antimicrobial to reduce the incidence of infectious disease in a group of animals that already has some individuals with evidence of infection |
| Disease prevention | Administration of an antimicrobial to an individual animal to mitigate the risk for acquiring a disease or infection that is anticipated based on history, clinical judgment, or epidemiological knowledge. | Administration of an antimicrobial to a group of animals, none of which have evidence of disease or infection, when transmission of existing undiagnosed infections, or the introduction of pathogens, is anticipated based on history, clinical judgment, or epidemiological knowledge. |
Source: [2].
Abbreviation: AVMA, American Veterinary Medical Association.
AU regulations are framed around the uses of treatment, control, prevention, and production [5, 7, 8, 9]. The American Veterinary Medical Association (AVMA) Committee on Antimicrobials underscores the importance of concise definitions for the broad-purpose categories and clarity around indication for AU to the practice of clinical care and AS (Table 1) [2, 10]. Disease treatment refers to the administration of antimicrobials to individuals with clinical or diagnostic evidence of infection [10]. Selection of an antimicrobial in human healthcare is largely based upon individual patient factors, with some decision making informed by the potential for disease transmission. The latter consideration is central in animal agriculture settings, with veterinarians accounting for disease status (eg, incubating, subclinical but infectious) of others in the population. The objective of AU for disease control (sometimes referred to as metaphylaxis) is to reduce the frequency of a disease that already exists within a population. Some animals might demonstrate clinical illness whereas others are infected but not (yet) showing clinical signs [2, 10]. This is analogous to the practice of administering antiviral chemoprophylaxis to all nursing home residents or hospitalized patients residing in an affected unit during an influenza outbreak [11]. Because food animals raised in groups are often exposed to production-related stressors (eg, weaning, transport, insect vectors), antimicrobials are used to prevent morbidity related to opportunistic pathogens [12]. When used for disease prevention, antimicrobials are administered to an animal or to a group of animals not yet demonstrating clinical signs of disease in situations where disease is likely to occur in the absence of AU. For example, bacitracin methylenedisalicylate is labeled for use in broiler chickens for prevention of necrotic enteritis caused or complicated by Clostridium spp or other organisms susceptible to bacitracin [13]. Administration of this non-MIA drug to an entire flock through feed can reduce need for MIA (eg, water-soluble penicillin or lincomycin) use for disease treatment [14].
Routes of Antimicrobial Administration for Food-Producing Animals: Guided by Clinical Indication, Animals Affected, and Drug Label
Route of antimicrobial administration depends on animal species, production class (eg, class: cattle, subclass: beef cattle), indication, duration, number of animals to be treated, and antimicrobial formulation. Antimicrobials may be administered by injection (subcutaneous, intramuscular or in ovo), oral bolus, topical application, or mixed into feed or water. Administration of antimicrobials in feed and water facilitates timely therapy of multiple animals while also preventing handling-related stress or injury to animals and caretakers. The lay public, nonveterinary, and nonagriculture professional sectors have a general misunderstanding that antimicrobial administration through feed equates to growth promotion use. However, feeding of MIAs for growth promotion has not been legal in the US since January 2017 [4]. MIAs may be delivered in feed, as labeled and under veterinarian oversight, only for the purposes of disease treatment, control, and prevention. Administering medications by feed or water makes medicating larger groups of animals more feasible than individual administration (eg, 15 000 turkeys receiving antimicrobials for disease control). When an individual animal needs treatment, other routes of administration (eg, injection, oral) are often used.
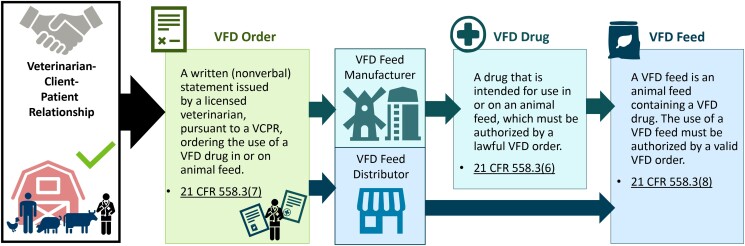
Dosing of antimicrobials administered in feed is based on expected average daily feed intake. Antimicrobials to be administered in finished feed (referred to by FDA as type C feed) usually come in a premixture (referred to by FDA as a type A medicated article) with stated grams of drug per pound of premixture. After receipt of a lawful veterinary feed directive (VFD, described further below), the feed manufacturer may add the premixture to feed to generate a finished feed product with drug concentrations (generally grams per ton) approved by FDA (Figure 1). These drug concentrations for labeled species, production class, clinical indications, withdrawal times, and other conditions of use are established by drug manufacturers during the FDA application process. Animal feed manufacturing is regulated under current Good Manufacturing Practices by FDA and analogous regulations at the state level [16]. Both the veterinarian and the feed manufacturer provide oversight to ensure medicated feed use aligns with FDA-approved drug labels (eg, species, production class, indication) for both the feed and the drug premixture. Extralabel (ie, off-label) use of MIAs in feed for cattle, swine, chickens, and turkeys is prohibited.
Figure 1.
Infographic showing lawful veterinary feed directive process when in-feed antimicrobial drugs are deemed necessary by a veterinarian. Image adapted from the California Department of Food and Agriculture [15]. Abbreviations: CFR, Code of Federal Regulations; VCPR, veterinarian-client-patient relationship; VFD, veterinary feed directive.
Antimicrobials may also be administered to groups of animals via drinking water. Because sick animals might continue drinking when not eating, this route can be preferable (eg, in poultry production). Use of medicated drinking water does not require the feed distributor to create a new feed ration for the affected group. Water administration dosing is based on expected water intake, as determined by on-farm measurements and standard reference tables. A soluble powder formulation of the antimicrobial is mixed into a stock solution according to the drug label, and the stock solution is added to the drinking water at an established rate to deliver a precise amount (milligrams) of drug per gallon of drinking water. Many livestock and poultry operations have meters to accurately record water consumption by a herd or flock [17].
Feed and water are essential routes of pharmaceutical administration in US food animal production. Because veterinarians and producers have detailed feed and water intake data for groups of animals, and animals housed in a group are usually at the same age or life stage, and of similar genetic background, the dose received by each animal in the group is relatively uniform. Part of the FDA approval process requires demonstration of this uniformity [18–20]. Post–FDA approval, samples of medicated feed are routinely tested and assayed at feed mills to assure drug product uniformity [21]. Dose determination for nearly all species and pharmaceuticals in veterinary medicine is established on a milligrams per kilogram (not per-head) basis [22].
Drug Residue Prevention: A Multilayered Approach, From Labeled Withdrawal Times to Premarket Testing
Antimicrobial residues are antimicrobial parent compounds or their metabolites that remain in foods of animal origin (eg, meat, eggs, milk) from treated animals. The US Department of Agriculture (USDA) Food Safety and Inspection Service administers the National Residue Program, which aims to protect consumers from adulterated or misbranded food products [23]. Through this program, the Food Safety and Inspection Service collects and tests samples of domestic and imported meat, poultry, and egg products for chemical residues, including antimicrobials, other veterinary drugs, and contaminants. Regulation and drug labeling requirements enacted in 1976 have helped ensure the safety of the US food supply. Drugs for use in food animals are labeled with a “withdrawal time,” or the mandatory minimum time between cessation of drug administration and entry of that animal's products (eg, meat, milk, eggs) into the food chain. Withdrawal times vary by drug, animal, and animal product and range from days to weeks [24]. If a drug can be and is used in an extralabel manner, the veterinarian must determine a sufficient withdrawal time. The Food Animal Residue Avoidance Databank assists veterinarians in establishing this expanded withdrawal time [25]. The consequences of detectible antimicrobial residues are considerable for producers. For example, the penalty for selling milk contaminated with veterinary drugs can be thousands of dollars per violation, including, for example, being responsible for the cost of the disposal of the contaminated load(s), in addition to payment for all milk in a contaminated multifarm tanker and loss of sales until the farm's milk has been shown to be negative for all residues [26]. Farms with 1 or more violations may lose their permit to sell milk. Statutes may differ between states, but in Minnesota, for example, every tanker load of milk is tested for β-lactam drug residues prior to delivering that load to a creamery for further processing. Samples taken directly from individual dairy farmers are tested monthly for 4 months out of every 6-month period [26]. Accurate record keeping and attention to appropriate AU and withdrawal time are essential for US dairies.
USDA works with states to provide education and resources to producers on the topic of drug-residue prevention. It is a reality of veterinary practice that antimicrobial selection (eg, antimicrobials with shorter withdrawal times) is at times influenced by labeled withdrawal times, to ensure therapy fits into the timeframe dictated by the production and marketing situation of a particular farm or individual animal. Residue violations in meat are rare in the US. In fiscal year 2019, 21 of 7767 (0.27%) tissue samples analyzed under USDA-scheduled sampling showed chemical residues, including 2 (ceftiofur and tetracycline) with antibiotic residues [23]. USDA also tests when an inspector suspects that a slaughter animal might have a chemical residue. In 2019, 523 of 174 308 (0.3%) inspector-generated samples were positive for violative residue. Ceftiofur, penicillin, and sulfadimethoxine were most common, accounting for 62.7% of total violative residues. When a meat residue is detected, the producer receives a warning letter from FDA and an onsite visit to review records and assess the cause of the residue [27]. Violators with 2 or more violations in a 12-month period are named on a USDA repeat violators list. The list is referenced by meat processors and livestock markets. Some processors may put violating producers on probation, refusing to purchase animals without documentation that they are free of medications. In some cases, FDA prohibits producers from selling animals for meat if they have been found neglectful or failed to prevent residues [27].
POLICIES GUIDING ANTIBIOTIC USE
FDA Guidance for Industry: Progress Toward Improved Antimicrobial Oversight Through Pharmaceutical Labeling
Since 2003, FDA has released several guidance for industry (GFI) documents related to AU in animal agriculture (Table 2). GFI #213 in 2013 directed removal of growth promotion indications from MIA labels. Because neither the FDA Animal Medicinal Drug Use Clarification Act (AMDUCA) nor the FDA VFD final rule allows extralabel use of animal drugs in the feed of food-producing animals, removal of these label claims eliminated the use of MIAs for production uses in the US and made it illegal to use them in this fashion [28, 31, 32]. The US GFI #213 was fully implemented in January 2017. Although the use of antimicrobials for production purposes is not explicitly banned in the US, the removal of label claims for growth promotion, feed efficiency, and/or improved weight gain has effectively eliminated usage of MIA for production purposes [28, 31, 32]. Data collected by FDA demonstrate an overall reduction in antimicrobial sales subsequent to GFI #213 implementation, although those data cannot be used to assess changes in actual AU [33].
Table 2.
Food and Drug Administration Guidance for Industry Related to Antimicrobial Use in Agriculture
| GFI# | Publication Year | Title | Description |
|---|---|---|---|
| 152 | 2003 | Evaluating the Safety of Antimicrobial New Animal Drugs With Regard to Their Microbiological Effects on Bacteria of Human Health Concern |
|
| 209 | 2012 | The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals |
|
| 213 | 2013 | New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209 |
|
| 263 | 2021 | Recommendations for Sponsors of Medically Important Antimicrobial Drugs Approved for Use in Animals to Voluntarily Bring Under Veterinary Oversight All Products That Continue to be Available Over-the-Counter |
|
AMDUCA and the VFD final rule constraints on extralabel use of feed-administered antimicrobials are pertinent not only to the discussion of AU for production purposes but also to AU for treatment, control, and prevention indications. Animal pharmaceuticals are reviewed for safety and efficacy and are approved for specific labeled use(s) by the FDA prior to sale by drug manufacturers [1]. Unlike in human healthcare, where off-label use is allowed at the prescriber's discretion and is common, pharmaceutical labeling dictates how drugs can be used in food-producing animals. Veterinarians (and feed manufacturers) who do not comply with regulations are subject to regulatory action by FDA, state agriculture agencies, and disciplinary action by state veterinary licensing boards [34, 35]. Withdrawal of production uses from MIA labels and removal of over-the-counter labeling for MIAs administered through feed or water presented a major shift in how MIAs can be legally used [5, 31, 36]. Label-related constraints also determine what options are available to optimize AU through AS interventions.
Veterinary Feed Directive Rule and Veterinarian-Client-Patient Relationship: Ensuring Veterinary Oversight for MIA Administered Through Feed
The FDA VFD final rule outlines the process that veterinarians take to authorize use of drugs, including MIAs, intended for administration through animal feed. An VFD order is a written statement authorizing use of a drug (alone or in combination with another drug) [31] in animal feed. This order authorizes the animal owner or owner's representative to obtain and use animal feed that contains the drug for use in accordance with conditions for use permitted by the FDA. More practically, when a veterinarian writes an VFD order, a copy is provided to the animal owner and to the feed distributor (Figure 1). The owner can then purchase feed that contains that drug from that feed distributor. Veterinarians utilize VFD orders only for in-feed medications. Prescriptions are utilized for antimicrobials administered through water or other routes.
For an VFD order to be lawful, the issuing veterinarian must be licensed to practice veterinary medicine in the state in which the animals will ingest the feed; be operating in the course of the veterinarian's professional practice; and be in compliance with all applicable veterinary licensing and practice requirements, including issuing the VFD order in the context of a veterinarian-client-patient relationship as defined by the State or Federal government [24, 37]. Under a valid veterinarian-client-patient relationship, the veterinarian has assumed responsibility for making medical judgments regarding health of the animal(s) and need for medical treatment, and the owner/caretaker of the animal(s) has agreed to follow the instructions of the veterinarian; the veterinarian has sufficient knowledge of the animal(s) to initiate at least a general or preliminary diagnosis; and the veterinarian is readily available for follow-up in case of adverse reactions or treatment failure. The veterinarian must have recently seen and be personally acquainted with the keeping and care of the animal(s) through examination and/or by visits to the premises where the animal(s) are kept [24, 37].
FDA conducts inspections to verify that antimicrobials used in or on animal feed are authorized appropriately according to the VFD rule [38]. When the final rule was first implemented, inspections focused on education of veterinarians, producers, and feed distributors. In 2018, FDA began documenting VFD violations, which have been rare through fiscal year 2021 [38].
Outstanding Policy Issues That Influence Antimicrobial Stewardship
FDA and other partners in improving AU for food-producing animals have identified and continue efforts on other regulatory issues that influence AS [39]. Two of these issues are outlined here. FDA has acknowledged that use of the “MIA” term has become increasingly broad and that the MIA list and the risk-based framework for describing the human health impact of antimicrobials should be updated to reflect this evolution. Beyond the challenge of defining MIAs in the US, identifying ways to better harmonize the content and context of lists created by other countries and nongovernmental organizations is essential [40].
As part of FDA's 5-year plan for supporting AS in veterinary settings, the agency outlined an objective to “ensure that all MIA drugs used in or on the feed or in drinking water of food-producing animals have an appropriately defined duration of use” [39]. As of plan publication, approximately 40% of MIAs approved for use included at least 1 indication lacking a defined use duration [39]. All approved uses of MIAs in non-feed dosage forms have appropriately defined durations of use [41]. Since 2019, FDA has provided research funding opportunities with the objective of generating data to support establishment of targeted durations of use for certain MIAs approved for feed administration to food-producing animals. When bundled with other tenets of judicious AU and AS, use of antimicrobials for the shortest effective duration is critical to reduce overall antimicrobial pressure on microorganisms. Insufficient duration may lead to treatment failure and may contribute to risk of resistance development. Because MIAs cannot be used extralabel in feed, challenges to AS can arise even for drugs with a labeled duration, since that duration cannot be adjusted in response to clinical realities (eg, early discontinuation in the case of clinical resolution).
An FDA concept paper has stimulated discussion and public comment on establishment of targeted durations of use for MIA [41]. The objective is to “outline for animal drug sponsors and other stakeholders a potential framework for how to voluntarily revise the product use conditions (eg, dosage regimen, instructions for use), as necessary, to better target when and for how long a drug may be used to effectively treat, control, or prevent the disease(s) for which the product is indicated.” Ensuring there is a labeled duration for all antimicrobial drugs used for food-producing animals is a topic to which nonregulatory entities have also directed attention [42]. As in human medicine, further study into optimal durations is necessary and has become a funding priority for FDA [38]. Since MIA cannot be used in animals for shorter durations than dictated on the label, label changes are also paramount to driving practice change.
PREVENTION OF INFECTIONS
As in human healthcare, prevention of infections is critical to reducing the need for AU in animal agriculture settings. Approaches to disease prevention include biosecurity, vaccination, attention to best practices in management and husbandry, and diagnostic testing. Quality assurance certification programs, like those for pork and beef production, provide structure for production practices that improve food safety, animal health and welfare, worker safety, and environmental stewardship [43, 44]. Research is also ongoing to explore the role of probiotics in microbiota modulation, with some success seen for prevention of Lawsonia intracellularis infection of pigs and reduction of Clostridium perfringens and Salmonella colonization in chickens [45, 46]. Selection of specific genetic traits has long been a part of animal husbandry, and genomic advancements have facilitated acquisition of traits conferring reduced disease susceptibility alongside those associated with increased yield and feed conversion efficiency [47]. Targeted genetic editing techniques can increase host resistance to major animal pathogens, including porcine reproductive and respiratory syndrome (PRRS) virus, which is a major cause of morbidity and mortality in swine populations. PRRS virus, a highly mutable RNA virus, is challenging to control with vaccination alone. PRRS is often associated with secondary bacterial infection, for which AU is common [48]. Despite technological advances, gene editing approaches that might address major challenges like PRRS lack broad acceptance by regulators and consumers in the US [49] and some US trading partners. Incentive programs such as that from the Presidential Advisory Council on Combating Antibiotic Resistance aim to incentivize innovation and provide return on investment for prevention initiatives for animal species, including development of vaccines and diagnostics and exploration of antibiotic alternatives [50, 51].
CHALLENGES AND OPPORTUNITIES FOR FOOD-ANIMAL AU
Continued improvement is needed to meet the AS objectives of effective antimicrobial therapy, optimized AU, and prevention of the emergence and persistence of antimicrobial resistance . The AVMA has outlined 5 core principles of AS in veterinary medicine [52]. These core principles include commitment to stewardship, advocating for a system of care to prevent common diseases, selecting and using antimicrobial drugs judiciously, evaluating antimicrobial drug use practices, and educating and building expertise. Veterinarians and food-animal producers face numerous challenges to AS practice, but opportunities exist to improve AU while advancing animal health.
For every food-producing species, efficacy of AU for specific indications must be better quantified, alternative therapeutic options identified, or both. Given differences in geographic location, disease pressures, livestock genetics, and management, the generalizability of field research outcomes can be limited. Establishment of effective AS actions must be a site-specific exercise, guided by available best practices and outcomes measurement, and the impact of these practices on overall AU must be tracked nationally.
Measurement of Antimicrobial Use
In human healthcare, measures such as the Centers for Disease Control and Prevention's National Healthcare Safety Network standardized antimicrobial administration ratio have allowed facilities to compare AU of hospitals of similar size and acuity [53, 54]. A similar system does not exist for veterinary medicine in the US. Comparisons of AU among food-animal species often make measurement and benchmarking more challenging and are, in general, not useful. Species differences in disease pressures, metabolic processes, lifespans, and body size do not lend to simplistic comparisons, such as comparisons of AU tonnage [55, 56]. Antimicrobial medications commonly used in food-producing animals are not used in human healthcare (eg, lincomycin, florfenicol, and tiamulin in turkey production [57]), confounding the comparison between human and animal use. While antimicrobial days of therapy per 1000 patient-days have all but become the standard comparator for human AU, most metrics in animal husbandry are in milligram per kilogram of liveweight, resulting in an inherent inability to compare AU across species. Differences in drug potency and incidence of diseases within species also hampers comparison. For example, the total grams of antibiotic used for gangrenous dermatitis in turkeys, which are affected at older age and heavier weight, is larger than for the same condition in poultry affected at a younger age [57]. Some comparisons are made using adjustments, such as “defined daily dose animal” metrics and/or population correction unit for calculating biomass, but these calculations do not account for variabilities in disease incidence, availability of approved drugs, management conditions, or lifespan, and there is international variability in how metrics like population correction unit are calculated [58]. Underlying all discussions of AU measurement is the challenge of available data, some of which are maintained only in paper-based records. While AU measurement itself is not the goal of antimicrobial stewardship, collection of accurate data is imperative to improve practice.
Since 2009, FDA has collected and reported sales and distribution data on antimicrobials approved for use in US food-producing animals. While these data are relatively easy to collect and useful to track changes in overall antibiotic sales over time, they do not equate to on-farm AU and, until 2016, they did not include differentiation of sales by animal species [59–61]. The FDA publishes annual summaries of these data [62]. The most recent annual summary reports an overall reduction of 38% from a 2015 peak of sales and distribution in the US [63].
In 2016, FDA's Center for Veterinary Medicine funded two 5-year cooperative agreements [64] to collect species-specific AU data for the major food-producing animals [14, 57, 65–67]. These data were intended to address the limitations of sales data and fill gaps related to antimicrobial indication. One change in AU patterns identified through the studies in broiler chickens and turkeys was a decrease in in-feed MIAs (eg, tetracyclines) and a concomitant prioritization of non-MIAs for targeted diseases [14]. Although the data collected through these cooperative agreements provide insight into AU practices, they do not represent a long-term solution to AU tracking in the US.
Animal Health and Welfare
AU is an essential component of veterinary medicine and the ability to maintain animal health and welfare. Initiatives to optimize, and overall reduce, AU do not eliminate the need for antimicrobials, including MIAs, to address clinical bacterial infections. The balance between AS, animal health and welfare, and food security is insufficiently recognized outside of agriculture and veterinary medicine. The common perception that the welfare of animals raised in US organic production systems is superior to that of animals in conventional systems often overlooks the reality that antimicrobials cannot be used for animals marketed as organically produced, even for infection treatment (US 7 Code of Federal Regulations Part 205 [68]). Organic animal production in the European Union allows for use of antimicrobials in organically certified livestock when treatment is needed to ensure animal welfare is maintained (Commission Regulation No 889/2008 [69]). The organic rule in the US states, “Producers may not withhold treatment from sick or injured animals. However, animals treated with a prohibited substance may not be sold as organic.” The cost of raising animals in an organic setting is greater than raising animals conventionally, in large part because of added costs of animal feed, which must be comprised of organic ingredients. Marketing animal products under a conventional label after raising the animal under organic conditions presents significant financial loss. Surveyed US veterinarians and producers of major animal commodities acknowledge that the main reasons for raising animals without antimicrobials are market-driven, and many indicate that there are times when a “raised without antibiotics” label takes priority over animal health and welfare [70].
INTERDISCIPLINARY COLLABORATION
Sharing Realities and Challenges Across Disciplines: Opportunities for Multidisciplinary Collaboration
The authors of this paper are infectious diseases pharmacists, veterinarians, and public health professionals. This multisector collaboration has produced a contribution to the scientific literature that recognizes the prevailing view of AU in animal agriculture and addresses it by providing essential information about the regulatory environment and clinical realities that are often overlooked in publications and commentary from professionals outside of veterinary medicine. We are also clear about opportunities for improvement and collaboration.
Opportunities for healthcare professionals to collaborate with veterinary professionals to support AS include the following:
Collaborate at state and national levels to identify meaningful AU measures and reduction goals. Given the incomparable nature of food-producing species, management approaches, disease pressures, and lack of data on rates of inappropriate use, establishment of broad reduction targets (eg, reduction of MIA use in animal agriculture by 50%) might not reflect progress made through impactful AS interventions for specific clinical situations and drugs and may have negative consequences on food security. Neglecting the need for setting-specific measures and practice improvement goals can further segregate professionals in healthcare and animal agriculture.
Contact state or local health departments to get more engaged. Public health organizations increasingly support One Health [71] approaches to AS through education and engagement opportunities. The value of pharmacists and healthcare providers to inform public health stewardship initiatives cannot be overstated.
Engage with local and state partners to support AS in companion animal veterinary settings. Veterinarians face fewer regulatory limitations when prescribing for companion animals, like dogs and cats. MIAs are commonly used for these patients. Microbiota sharing between humans and household pets makes antimicrobial resistance surveillance and AS initiatives essential [72, 73]. Companion animals can harbor pathogens of public health significance, including carbapenemase-producing Enterobacterales (CPE) [74–76]. In a recent large CPE outbreak occurring in an academic small animal hospital, 5 of 6 patients colonized or infected with CPE had received at least 4 antibiotic drugs in the last 30 days, including enrofloxacin [77]. AS in companion animal settings is a priority for the AVMA and FDA, and evidence-based approaches from healthcare are increasingly explored in this field [78]. Clinic-based veterinary medicine and healthcare have similarities in structure, function, pharmacy services, clinical considerations, and prescriber behavior. As such, evidence-based practices from healthcare settings (eg, preauthorization, prospective audit with feedback, formulary restriction, retrospective review, AU tracking, and benchmarking) could benefit clinic-based veterinary AS programs. Multidisciplinary collaboration in this space is welcome.
Engage on the essential action of incorporating both setting-specific and One Health AS content into all health professions curricula, including preprofessional and postgraduate training.
CONCLUSIONS
All sectors that use antimicrobials, including but not limited to veterinary medicine (in animal agriculture, aquaculture, clinics, and other settings), horticulture, and human healthcare, have potential to contribute to the problem of antimicrobial resistance and are therefore an important part of AS-driven solutions. The One Health concept challenges us to improve the health of all sectors, knowing that humans cannot experience optimal health without a healthy animal population and environment. Here we sought to objectively describe how antimicrobials are regulated and used in US animal agriculture for a nonveterinary audience, including the realities, challenges, progress, and opportunities for improvement. Together we can ensure that this shared resource is preserved to maintain the health of both humans and animals.
Contributor Information
Krista D Gens, Department of Pharmacy, Abbott Northwestern Hospital, Minneapolis, Minnesota, USA.
Randall S Singer, University of Minnesota College of Veterinary Medicine, Saint Paul, Minnesota, USA.
Thomas J Dilworth, Department of Pharmacy Services, Advocate Aurora Health, Milwaukee, Wisconsin, USA.
Emily L Heil, University of Maryland School of Pharmacy, Baltimore, Maryland, USA.
Amanda L Beaudoin, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Notes
Acknowledgments. The authors acknowledge Chris Evans, PharmD, BCPS, for review and comments that greatly improved the manuscript.
Financial support. R. S. S. was partially supported by grant U01FD005878 from the U.S. Food and Drug Administration.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. US Food and Drug Administration . Federal Food, Drug, and Cosmetic Act. 2018. Available at: https://www.fda.gov/regulatory-information/laws-enforced-fda/federal-food-drug-and-cosmetic-act-fdc-act. Accessed 7 September 2021.
- 2. American Veterinary Medical Association . AVMA definitions of antimicrobial use for treatment, control, and prevention. 2019. Available at: https://www.avma.org/resources-tools/avma-policies/avma-definitions-antimicrobial-use-treatment-control-and-prevention. Accessed 7 September 2021.
- 3. Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 2005; 84:634–43. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . List of medically important antimicrobial drugs affected by GFI #213. 2021. Available at: https://www.fda.gov/animal-veterinary/judicious-use-antimicrobials/list-medically-important-antimicrobial-drugs-affected-gfi-213. Accessed 10 September 2021.
- 5. US Food and Drug Administration . CVM GFI #209: the judicious use of medically important antimicrobial drugs in food-producing animals. 2019. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-209-judicious-use-medically-important-antimicrobial-drugs-food-producing-animals. Accessed 7 September 2021.
- 6. World Health Organization . Critically important antimicrobials for human medicine. 2007. Available at: https://www.who.int/publications-detail-redirect/9789241595742. Accessed 7 September 2021.
- 7. State of California . Bill text—SB-27 livestock: use of antimicrobial drugs. 2015. Available at: https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201520160SB27. Accessed 7 September 2021.
- 8. World Health Organization . WHO guidelines on use of medically important antimicrobials in food-producing animals. 2018. Available at: https://apps.who.int/iris/handle/10665/330086. Accessed 7 September 2021. [DOI] [PMC free article] [PubMed]
- 9. Maryland General Assembly . Keep antibiotics effective act of 2017 legislation—SB0422. 2017. Available at: https://mgaleg.maryland.gov/mgawebsite/legislation/details/sb0422?ys=2017rs. Accessed 7 September 2021.
- 10. Smith DR, Gaunt PS, Plummer PJ, et al. The AVMA's definitions of antimicrobial uses for prevention, control, and treatment of disease. J Am Vet Med Assoc 2019; 254:792–7. [DOI] [PubMed] [Google Scholar]
- 11. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abell KM, Theurer ME, Larson RL, White BJ, Apley M. A mixed treatment comparison meta-analysis of metaphylaxis treatments for bovine respiratory disease in beef cattle. J Anim Sci 2017; 95:626–35. [DOI] [PubMed] [Google Scholar]
- 13. Proctor A, Phillips GJ. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front Vet Sci 2019; 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer RS, Porter LJ, Schrag NFD, Davies PR, Apley MD, Bjork K. Estimates of on-farm antimicrobial usage in broiler chicken production in the United States, 2013–2017. Zoonoses Public Health 2020; 67(Suppl 1):22–35. [DOI] [PubMed] [Google Scholar]
- 15. California Department of Food and Agriculture . Annual summary report veterinary feed directives. 2019. Available at: https://www.cdfa.ca.gov/is/ffldrs/pdfs/AUS_VFD_Summary_Report_2019.pdf. Accessed 23 September 2022.
- 16. US Food and Drug Administration . Current good manufacture practices guidances. 2022. Available at: https://www.fda.gov/animal-veterinary/guidance-industry/current-good-manufacture-practices-guidances. Accessed 3 June 2022.
- 17. D Swayne, Ed. Diseases of poultry, 14th ed. Hoboken, New Jersey: Wiley-Blackwell; 2020. [Google Scholar]
- 18. US Food and Drug Administration . CVM GFI #13: evaluation of effectiveness of new animal drugs for use in free-choice feeds-medicated block. 1985. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-13-evaluation-effectiveness-new-animal-drugs-use-free-choice-feeds-medicated-block. Accessed 18 July 2022.
- 19. US Food and Drug Administration . CVM GFI #23: medicated free choice feeds–manufacturing control. 2021. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-23-medicated-free-choice-feeds-manufacturing-control. Accessed 18 July 2022.
- 20. US Food and Drug Administration . CVM GFI #264: standardized medicated feed assay limits. 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-264-standardized-medicated-feed-assay-limits. Accessed 18 July 2022.
- 21. US Code of Federal Regulations . 21 CFR 558.4: requirement of a medicated feed mill license. 2022. Available at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-E/part-558/subpart-A/section-558.4. Accessed 18 July 2022.
- 22. Educational Concepts, LLC . Plumb's. 2021. Available at: https://plumbs.com/. Accessed 29 November 2021.
- 23. US Department of Agriculture, Food Safety and Inspection Service . Residue sampling plan: fiscal year 2020 blue book. 2019. Available at: http://www.fsis.usda.gov/node/1982. Accessed 30 September 2021.
- 24. US Food and Drug Administration . US Code of Federal Regulations title 21. 2015. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=558.6. Accessed 30 September 2021.
- 25. Food Animal Residue Avoidance and Depletion Program . Food Animal Residue Avoidance Databank. Available at: http://www.farad.org/. Accessed 27 May 2022.
- 26. State of Minnesota . Sec. 32D.19 MN statutes. 2017. Available at: https://www.revisor.mn.gov/statutes/2017/cite/32D.19. Accessed 9 February 2022.
- 27. Minnesota Department of Agriculture . Avoiding drug residues in meat. 2022. Available at: https://www.mda.state.mn.us/sites/default/files/inline-files/Avoiding-Drug-Residues-Meat-2.21.pdf. Accessed 23 September 2022.
- 28. US Food and Drug Administration . CVM GFI #263: recommendations for sponsors of medically important antimicrobial drugs approved for use in animals to voluntarily bring under veterinary oversight all products that continue to be available over-the-counter. 2021. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-263-recommendations-sponsors-medically-important-antimicrobial-drugs-approved-use-animals. Accessed 7 September 2021.
- 29. US Food and Drug Administration . CVM GFI #152: evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. 2003. Available at: https://www.fda.gov/files/animal%20&%20veterinary/published/CVM-GFI–152-Evaluating-the-Safety-of-Antimicrobial-New-Animal-Drugs-with-Regard-to-Their-Microbiological-Effects-on-Bacteria-of-Human-Health-Concern.pdf. Accessed 30 September 2021.
- 30. US Food and Drug Administration . Concept paper: potential approach for ranking of antimicrobial drugs according to their importance in human medicine: a risk management tool for antimicrobial new animal drugs. 2020. Available at: https://www.fda.gov/media/142846/download. Accessed 9 February 2022.
- 31. US Federal Register . Veterinary feed directive. 2015. Available at: https://www.federalregister.gov/documents/2015/06/03/2015-13393/veterinary-feed-directive. Accessed 7 September 2021.
- 32. US Food and Drug Administration . Animal Medicinal Drug Use Clarification Act of 1994 (AMDUCA). 1994. Available at: https://www.fda.gov/animal-veterinary/guidance-regulations/animal-medicinal-drug-use-clarification-act-1994-amduca. Accessed 24 October 2022.
- 33. US Food and Drug Administration . 2020 summary report on antimicrobials sold or distributed for use in food-producing animals. 2021. Available at: https://www.fda.gov/media/154820/download. Accessed 23 September 2022.
- 34. US Food and Drug Administration . FDA regulation of medicated feed. 2021. Available at: https://www.fda.gov/animal-veterinary/resources-you/fda-regulation-medicated-feed. Accessed July 18, 2022.
- 35. US Food and Drug Administration . Types of FDA enforcement actions. 2018. Available at: https://www.fda.gov/animal-veterinary/resources-you/types-fda-enforcement-actions. Accessed 24 April 2022.
- 36. US Food and Drug Administration . FDA's strategy on antimicrobial resistance—questions and answers. 2019. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fdas-strategy-antimicrobial-resistance-questions-and-answers. Accessed 9 February 2022.
- 37. Office of the Federal Register . US Code of Federal Regulations. 21 CFR 530.3—definitions. 2017. Available at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-E/part-530/subpart-A/section-530.3. Accessed 29 November 2021.
- 38. US Food and Drug Administration . FDA-TRACK: progress on FDA’s support of antimicrobial stewardship in veterinary settings. 2022. Available at: https://www.fda.gov/about-fda/fda-track-agency-wide-program-performance/fda-track-progress-fdas-support-antimicrobial-stewardship-veterinary-settings. Accessed 23 September 2022.
- 39. US Food and Drug Administration, Center for Veterinary Medicine . Supporting antimicrobial stewardship in veterinary settings: goals for fiscal years 2019–2023. 2018. Available at: https://www.fda.gov/files/animal%20&%20veterinary/published/Supporting-Antimicrobial-Stewardship-in-Veterinary-Settings--Goals-for-Fiscal-Years-2019-2023.pdf. Accessed 9 February 2022.
- 40. Scott HM, Acuff G, Bergeron G, et al. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann N Y Acad Sci 2019; 1441:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. US Food and Drug Administration Center for Veterinary Medicine . Potential approach for defining durations of use for medically important antimicrobial drugs intended for use in or on feed: a concept paper. 2021. Available at: https://www.fda.gov/media/144927/download. Accessed 9 February 2022.
- 42. Pew Charitable Trusts . FDA must establish limits for all animal antibiotics. 2021. Available at: https://pew.org/3aCM9ek. Accessed 29 December 2021.
- 43. Beef Quality Assurance . Beef quality assurance national manual. 2019. Available at: https://www.bqa.org/Media/BQA/Docs/bqa_manual_final.pdf. Accessed 9 February 2022.
- 44. America’s Pork Producers . Pork quality assurance plus education handbook version 4. 2018. Available at: http://www.porkcdn.com/sites/all/files/documents/PQAPlus/V4.0/Forms/PQAv4e_Handbook.pdf. Accessed 29 December 2021.
- 45. Knap I, Kehlet AB, Bennedsen M, et al. Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poult Sci 2011; 90:1690–4. [DOI] [PubMed] [Google Scholar]
- 46. Opriessnig T, Karuppannan AK, Beckler D, Ait-Ali T, Cubas-Atienzar A, Halbur PG. Bacillus pumilus probiotic feed supplementation mitigates Lawsonia intracellularis shedding and lesions. Vet Res 2019; 50:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pollock J, Low AS, McHugh RE, et al. Alternatives to antibiotics in a One Health context and the role genomics can play in reducing antimicrobial use. Clin Microbiol Infect 2020; 26:1617–21. [DOI] [PubMed] [Google Scholar]
- 48. Burkard C, Opriessnig T, Mileham AJ, et al. Pigs lacking the scavenger receptor cysteine-rich domain 5 of CD163 are resistant to porcine reproductive and respiratory syndrome virus 1 infection. J Virol 2018; 92:e00415-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Eenennaam AL, De Figueiredo Silva F, Trott JF, Zilberman D. Genetic engineering of livestock: the opportunity cost of regulatory delay. Annu Rev Anim Biosci 2021; 9:453–78. [DOI] [PubMed] [Google Scholar]
- 50. US Department of Health and Human Services . Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria. Recommendations for incentivizing the development of vaccines, diagnostics, and therapeutics to combat antibiotic-resistance. 2017. Available at: https://www.hhs.gov/sites/default/files/paccarb-final-incentives-report-sept-2017.pdf. Accessed 30 September 2021.
- 51. Pew Charitable Trusts . Alternatives to antibiotics in animal agriculture. 2017. Available at: http://pew.org/2uvar2Y. Accessed 30 September 2021.
- 52. American Veterinary Medical Association . Antimicrobial stewardship definition and core principles. Available at: https://www.avma.org/resources-tools/avma-policies/antimicrobial-stewardship-definition-and-core-principles. Accessed 24 October 2022.
- 53. US Centers for Disease Control and Prevention, National Healthcare Safety Network . Keys to success with the SAAR analysis resources. 2022. Available at: https://www.cdc.gov/nhsn/ps-analysis-resources/keys-to-success-saar.html. Accessed 6 March 2022.
- 54. Shealy S, Kohn J, Yongue E, et al. Application of standardized antimicrobial administration ratio as a motivational tool within a multi-hospital healthcare system. Pharm J Pharm Educ Pract 2021; 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aidan H, Ziana A. Preserving antibiotics, rationally. N Engl J Med 2013; 369:2474–6. [DOI] [PubMed] [Google Scholar]
- 56. Van Boeckel TP, Glennon EE, Chen D, et al. Reducing antimicrobial use in food animals. Science 2017; 357:1350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singer RS, Porter LJ, Schrag NFD, Davies PR, Apley MD, Bjork K. Estimates of on-farm antimicrobial usage in turkey production in the United States, 2013–2017. Zoonoses Public Health 2020; 67(Suppl 1):36–50. [DOI] [PubMed] [Google Scholar]
- 58. Radke BR. Towards an improved estimate of antimicrobial use in animals: adjusting the “population correction unit” calculation. Can J Vet Res 2017; 81:235–40. [PMC free article] [PubMed] [Google Scholar]
- 59. Bondt N, Jensen VF, Puister-Jansen LF, van Geijlswijk IM. Comparing antimicrobial exposure based on sales data. Prev Vet Med 2013; 108:10–20. [DOI] [PubMed] [Google Scholar]
- 60. Merle R, Meyer-Kühling B. Sales data as a measure of antibiotics usage: concepts, examples and discussion of influencing factors. Vet Med Sci 2019; 6:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Office of the Federal Register . Antimicrobial animal drug sales and distribution reporting. 2016. Available at: https://www.federalregister.gov/documents/2016/05/11/2016-11082/antimicrobial-animal-drug-sales-and-distribution-reporting. Accessed 30 September 2021.
- 62. US Food and Drug Administration . ADUFA reports. 2021. Available at: https://www.fda.gov/industry/animal-drug-user-fee-act-adufa/adufa-reports. Accessed 30 September 2021.
- 63. US Food and Drug Administration . FDA releases annual summary report on antimicrobials sold or distributed in 2020 for use in food-producing animals. 2021. Available at: https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2020-use-food-producing. Accessed 27 May 2022.
- 64. National Institutes of Health . Antimicrobial use and resistance data collection (U01). 2016. Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-FD-16-046.html. Accessed 3 June 2022.
- 65. Davies PR, Singer RS. Antimicrobial use in wean to market pigs in the United States assessed via voluntary sharing of proprietary data. 2020. Zoonoses Public Health 2020; 67(Suppl 1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schrag NFD, Godden SM, Apley MD, Singer RS, Lubbers BV. Antimicrobial use quantification in adult dairy cows. Part 3—use measured by standardized regimens and grams on 29 dairies in the United States. Zoonoses Public Health 2020; 67(Suppl 1):82–93. [DOI] [PubMed] [Google Scholar]
- 67. Hope KJ, Apley MD, Schrag NFD, Lubbers BV, Singer RS. Antimicrobial use in 22 U.S. beef feedyards: 2016–2017. Zoonoses Public Health 2020; 67(Suppl 1):94–110. [DOI] [PubMed] [Google Scholar]
- 68. Office of the Federal Register . US Code of Federal Regulations. 7 CFR Part 205—National Organic Program. 2022. Available at: https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205?toc=1. Accessed 8 February 2022.
- 69. European Union . Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. 2008. Available at: https://www.legislation.gov.uk/eur/2008/889/contents. Accessed 8 February 2022.
- 70. Singer RS, Porter LJ, Thomson DU, Gage M, Beaudoin A, Wishnie JK. Raising animals without antibiotics: U.S. producer and veterinarian experiences and opinions. Front Vet Sci 2019; 6:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. US Centers for Disease Control and Prevention . One Health. 2022. Available at: https://www.cdc.gov/onehealth/index.html. Accessed 27 May 2022.
- 72. Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013; 2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tun HM, Konya T, Takaro TK, et al. Exposure to household furry pets influences the gut microbiota of infants at 3–4 months following various birth scenarios. Microbiome 2017; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Montgomery MP. Multidrug-Resistant Campylobacter jejuni outbreak linked to puppy exposure—United States, 2016–2018. MMWR Morb Mortal Wkly Rep 2018; 67:1032–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stolle I, Prenger-Berninghoff E, Stamm I, et al. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J Antimicrob Chemother 2013; 68:2802–8. [DOI] [PubMed] [Google Scholar]
- 76. Grönthal T, Österblad M, Eklund M, et al. Sharing more than friendship—transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Euro Surveill 2018; 23:1700497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cole SD, Peak L, Tyson GH, Reimschuessel R, Ceric O, Rankin SC. New Delhi metallo-β-lactamase-5–producing Escherichia coli in companion animals, United States. Emerg Infect Dis 2020; 26:381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. University of Minnesota . Handbook of antibiotic stewardship in companion animal veterinary settings—antimicrobial resistance and stewardship initiative. 2020. Available at: https://arsi.umn.edu/handbook-antibiotic-stewardship-companion-animal-veterinary-settings. Accessed 30 September 2021.