Abstract
Background
The efficacy of a single administration of darvadstrocel (expanded allogeneic adipose-derived mesenchymal stem cells) for treating complex perianal fistulas in patients with Crohn’s disease was demonstrated in a randomized, double-blind trial (ADMIRE-CD [Adipose Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn\'s Disease] trial). The current chart review study (INSPECT [A retrospectIve chart review study evaluatINg the longer-term effectiveneSs of darvadstrocel in PatiEnts who CompleTed ADMIRE-CD]) evaluated the longer-term effectiveness and safety of darvadstrocel.
Methods
Eligible patients had completed at least 52 weeks in the ADMIRE-CD trial. Data on clinical remission and fistula relapse outcomes were collected retrospectively at 104 and 156 weeks after treatment. Adverse events of special interest (tumorigenicity and ectopic tissue formation) were collected up to 208 weeks after treatment.
Results
Eighty-nine patients were included (43 darvadstrocel patients, 46 control subjects). At 52, 104, and 156 weeks posttreatment, clinical remission was observed in 29 (67.4%) of 43, 23 (53.5%) of 43, and 23 (53.5%) of 43 darvadstrocel-treated patients, compared with 24 (52.2%) of 46, 20 (43.5%) of 46, and 21 (45.7%) of 46 control subjects, respectively. In patients with clinical remission at week 52, this remission was sustained at 104 and 156 weeks after treatment in 19 (65.5%) of 29 and 16 (55.2%) of 29 darvadstrocel-treated patients and in 17 (70.8%) of 24 and 13 (54.2%) of 24 control subjects, respectively. Time to fistula relapse and incidence of fistula relapse or new fistula occurrence were not significantly different between groups. Tumorigenicity was reported for 1 (2.2%) patient in the control group (malignant epidermoid carcinoma). No ectopic tissue formation was reported.
Conclusions
Real-world follow-up of patients from the ADMIRE-CD trial indicates that clinical remission of complex perianal fistulas can be sustained in the long term irrespective of whether it is achieved through darvadstrocel administration or maintenance treatment regimens and confirms a favorable long-term safety profile of darvadstrocel.
Keywords: perianal fistulizing Crohn’s disease, stem cell therapy, fistula remission
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract, which can lead to complications such as the formation of complex perianal fistulas.1 Complex perianal fistulas may have branches with multiple external openings, may be associated with a perianal abscess or an anorectal stricture, or may have a rectovaginal tract, and can lead to a substantial impairment in quality of life.2-5 Complex perianal fistulas are estimated to occur in up to 28% of patients with CD in the first 20 years after diagnosis, although it has been speculated that, owing to underreporting of symptoms, the actual frequency of perianal fistulas may be higher than documented in the literature.5-8
Although a range of medical and surgical treatment options are available for complex perianal fistulas, their management remains difficult, and many patients experience a poor response to treatment or encounter frequent relapses or recurrences of fistulas.9 Furthermore, proctitis and rectal ulceration in patients with complex perianal fistulas can lead to anorectal stricture, which further reduces therapeutic options. Antibiotics, anti-tumor necrosis factor (anti-TNF) therapies, and immunomodulators, in combination with surgical fistula curettage and seton placement for drainage, have shown some efficacy in the treatment of complex perianal fistulas in patients with CD4; however, most treatments are unable to provide long-term healing of fistulas.10 Anti-TNF therapies have demonstrated efficacy in several clinical trials, and infliximab, a monoclonal antibody for the treatment of immune-mediated diseases, was recommended in the 2019 European Crohn’s and Colitis Organisation guidelines as a first-line medical therapy following adequate drainage of complex perianal fistulas.4,11-14 However, systematic reviews and meta-analyses of trials assessing the efficacy of various therapies for the treatment of perianal fistulas in CD have reported that approximately only one-third of patients treated with anti-TNF therapies achieved fistula remission, and high rates of fistula recurrences has been reported 1 year after discontinuation of infliximab treatment.15,16 A combination of anti-TNF therapy and thiopurine immunomodulators has also been used, but the evidence for improvement in outcomes is weak.13 Data with other biological therapies for complex perianal fistula are scarce. Common surgical approaches to close fistulas include ligation of the intersphincteric fistula tract, mucosal advancement flap surgery, or fistula laser closure.10 Although surgical procedures can achieve fistula closure, it is only possible in cases in which there is a single internal opening without proctitis; furthermore, these procedures can be associated with an increased risk of fecal incontinence.10,17 For a number of patients, a diverting ostomy or proctectomy may be required. Consequently, alternative treatment options are needed to achieve long-term fistula healing, to prevent recurrence, to maintain fecal continence, and to avoid a permanent diverting ostomy, and hence to improve and to maintain the quality of life of patients with CD.
Stem cell–based therapies have become an attractive approach for some immune-mediated refractory diseases. Mesenchymal stem cells have potent anti-inflammatory and immunomodulatory properties, and the delivery of stem cells directly to fistula tracts can increase cell numbers locally to aid fistula healing.18 Mesenchymal stem cells have shown efficacy in the treatment of complex perianal fistulas in a number of studies.19,20 Adipose-derived mesenchymal stem cells (ASCs) are easy to harvest and can differentiate into various cell types. Promising outcomes from several studies that investigated the use of ASCs to treat complex perianal fistulas in patients with CD are summarized in the 2019 European Crohn’s and Colitis Organisation guidelines on therapeutics in CD.19-21 These guidelines not only report that “allogeneic ASC therapy could be an effective and safe treatment for complex perianal fistulas in patients with CD,” but also note the lack of long-term follow-up data.21
Darvadstrocel (DVS) (Cx601, Alofisel; TiGenix S.A.U., Madrid, Spain) is an expanded allogeneic ASC therapy for the treatment of complex perianal fistulas in patients with CD.22 The safety and efficacy of DVS were demonstrated in the ADMIRE-CD (Adipose Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn\'s Disease) phase 3, double-blind, randomized trial (NCT01541579), during which DVS or placebo (control group), in combination with standard of care, was administered locally after fistula curettage and closure of the internal opening. The primary endpoint of combined remission at 24 weeks after treatment (defined as a clinical assessment of closure (no longer draining despite gentle finger compression) of all treated external openings draining at baseline, and the absence of collections larger than 2 cm (in 2 diameters) assessed by magnetic resonance imaging) was met in 51.5% (n = 53 of 103) and 35.6% (n = 36 of 101) of patients in the DVS and control groups, respectively (P = .021).23 Remission rates achieved in the ADMIRE-CD trial were irrespective of any ongoing anti-TNF or immunomodulator therapy.
A follow-up study found that, at 52 weeks after treatment in the ADMIRE-CD trial, 56.3% (n = 58 of 103) and 38.6% (n = 39 of 101) of patients were in combined remission in the DVS and control groups, respectively (P = .010).24 Among the patients included in the ADMIRE-CD trial extended follow-up study (n = 40), clinical remission at 104 weeks was observed in 56% (n = 14 of 25) and 40% (n = 6 of 15) of patients in the DVS and control groups, respectively (95% confidence interval [CI], −15.5 to 47.5).25 Combined remission was not evaluated in this extended follow-up study.
The primary aim of the INSPECT (A retrospectIve chart review study evaluatINg the longer-term effectiveneSs of darvadstrocel in PatiEnts who CompleTed ADMIRE-CD) study was to evaluate whether the responses to DVS observed during the ADMIRE-CD trial were maintained beyond 104 weeks after treatment, based on a retrospective chart review assessment.
Methods
Study Design
A retrospective chart review study (INSPECT) was conducted over a period of 104 weeks for patients with CD with complex perianal fistulas who had previously been enrolled in the ADMIRE-CD trial. Full details of the ADMIRE-CD trial design and patient eligibility criteria have been published previously.23 The INSPECT study was conducted in 7 countries (Austria, Belgium, France, Germany, Italy, the Netherlands, and Spain) across 35 sites (14 of the 49 sites that participated in the ADMIRE-CD trial were not willing or able to participate in the INSPECT study). Patient data were collected from October 2013 to November 2018.
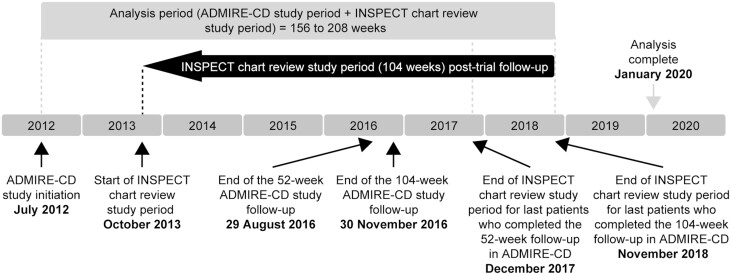
The total analysis period varied by patient depending on how long they had been followed up within the ADMIRE-CD trial (Figure 1): for example, a patient with a last ADMIRE-CD trial visit at 52 weeks could contribute a total of 156 weeks of data to the INSPECT study (52 weeks of ADMIRE-CD trial period and 104 weeks of chart review period), whereas a patient with a last ADMIRE-CD trial visit at 104 weeks could contribute a total of 208 weeks of data to the INSPECT study (104 weeks of ADMIRE-CD trial period and 104 weeks of chart review period). However, to present remission results for all patients at the same time after treatment, clinical remission data are only reported up to week 156 here (the number of patients with data collected at 208 weeks after treatment was limited by the number who completed the 104-week follow-up period in ADMIRE-CD, and hence, there were too few to permit robust analyses).
FIGURE 1.
INSPECT study design. ADMIRE-CD, Adipose Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn\'s Disease; INSPECT, A retrospectIve chart review study evaluatINg the longer-term effectiveneSs of darvadstrocel in PatiEnts who CompleTed ADMIRE-CD.
Inclusion and Exclusion Criteria
Patients included in the INSPECT study had previously been enrolled in the ADMIRE-CD trial, completed at least 52 weeks of trial follow-up, received treatment with DVS or placebo as part of the trial, and provided informed consent (if required by local regulations). Patients were excluded if no information relating to patient care within the 104-week INSPECT chart review period was available.
Study Treatment (in the ADMIRE-CD Trial)
After vigorous curettage of all fistula tracts, removal of setons (if in place), and closing of internal openings with sutures, patients received a single administration of DVS (suspension of 5 × 106 cells/mL; 120 × 106 cells in total, in sterile buffered solution) or placebo (24 mL saline solution; control group) at a patient group ratio of 1:1, injected around the internal fistula openings and along the fistula tract.23
Objectives
The objective of the INSPECT study was to compare the proportion of patients in clinical remission and in sustained clinical remission in the DVS group with that in the control group at 104 and 156 weeks after treatment. These analyses pertain only to fistulas treated in the ADMIRE-CD trial. Clinical remission was defined as closure of all external fistula openings that were draining at baseline in the ADMIRE-CD trial, based on clinical assessment (closed fistulas were those no longer draining despite gentle finger compression, or fistulas that were no longer spontaneously draining, or in absence of this level of detail, fistulas that were no longer draining). In the absence of documented assessments of fistula draining status, medical record documentation stating that the patient was in remission or had a complete response was used to define clinical remission.
Additional objectives included the incidence of fistula relapse and time to fistula relapse (in patients in clinical remission 52 weeks after treatment) and the proportion of patients with new fistula occurrence or fistula relapse during the INSPECT chart review period in the DVS and control groups. In addition, the incidence of adverse events of special interest (ectopic tissue formation and tumorigenicity) that occurred during the 104-week INSPECT chart review period was compared between groups (these data were collected up to 208 weeks after treatment for patients who completed 104 weeks of follow-up in the ADMIRE-CD trial).
Data Analysis
The proportion of patients in clinical remission was compared at 52, 104, and 156 weeks after treatment. Of those patients in clinical remission at 52 weeks, the proportion with sustained remission at 104 and 156 weeks was evaluated. Descriptive statistics were used to compare outcomes for patients between treatment groups. Continuous data were described by mean ± SD and median (interquartile range), and categorical variables by frequency and percentage. Significant differences were determined using t tests or Mann-Whitney U tests. The time to fistula relapse was analyzed using survival analysis techniques, and Cox regression analyses were used to account for the ADMIRE-CD trial baseline characteristics, using age, sex, perianal fistulizing disease duration, and baseline Crohn’s Disease Activity Index score as covariates in the adjusted hazard ratio. Kaplan-Meier curves were used to illustrate survival probability estimates.
Results
Patient Disposition, Demographics, and Disease Characteristics
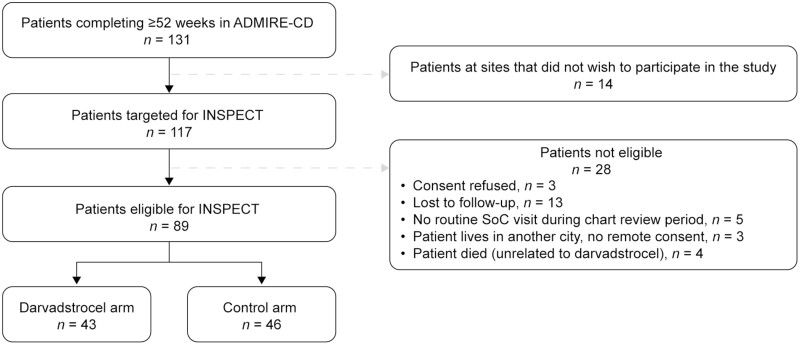
Of the 131 patients who completed at least 52 weeks of follow-up in the ADMIRE-CD trial, 89 were eligible for inclusion in the INSPECT study (Figure 2). Of these patients, 43 had been treated with DVS (28 patients completed 52 weeks of follow-up and 15 patients completed 104 weeks of follow-up in the ADMIRE-CD trial) and 46 patients were in the control group (34 patients completed 52 weeks of follow-up and 12 completed 104 weeks of follow-up in the ADMIRE-CD trial). Demographics and disease characteristics of patients in the INSPECT study were broadly similar to the intention-to-treat population in the ADMIRE-CD trial (n = 212) (Table 1 and Supplementary Data Content, Table 1).23
FIGURE 2.
CONSORT flow diagram for the INSPECT study. ADMIRE-CD, Adipose Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn\'s Disease; INSPECT, A retrospectIve chart review study evaluatINg the longer-term effectiveneSs of darvadstrocel in PatiEnts who CompleTed ADMIRE-CD; SoC, standard of care.
TABLE 1.
Demographics and disease characteristics for patients enrolled in the INSPECT study
| Patient Demographic/Disease Characteristic | DVS Group (n = 43) | Control Group (n = 46) |
|---|---|---|
| Age, y | 42.1 ± 13.7 | 37.7 ± 13.5 |
| Male | 24 (55.8) | 25 (54.3) |
| Race | ||
| White | 40 (93.0) | 42 (91.3) |
| Black or African American | 3 (7.0) | 1(2.2) |
| Missing | 0 | 3 (6.5) |
| Weight at INSPECT baseline, kga | 73.5 ± 15.0 | 75.4 ± 17.7 |
| Smoking status | ||
| Smoker | 8 (18.6) | 4 (8.7) |
| Nonsmoker | 7 (16.3) | 6 (13.0) |
| Unknown | 28 (65.1) | 36 (78.3) |
| Concomitant medications at INSPECT baselinea | ||
| Immunosuppressants only | 3 (7.0) | 7 (15.2) |
| Anti-TNF only | 18 (41.9) | 14 (30.4) |
| Anti-TNF and immunosuppressants | 15 (34.9) | 17 (37.0) |
| Neither | 7 (16.3) | 8 (17.4) |
| Duration of CD at INSPECT baseline, ya | 12.7 ± 11.1 | 11.0 ± 8.4 |
| Duration of perianal fistulizing disease at INSPECT baseline, ya,b | 4.7 ± 5.9 | 3.0 ± 3.6 |
| Fistula typec | ||
| High intersphincteric | 3 (7.0) | 3 (6.5) |
| High transsphincteric | 13 (30.2) | 19 (41.3) |
| Extrasphincteric | 2 (4.7) | 3 (6.5) |
| Suprasphincteric | 1 (2.3) | 1 (2.2) |
| Low intersphincteric | 5 (11.6) | 4 (8.7) |
| Low transsphincteric | 5 (11.6) | 4 (8.7) |
| Unknown | 20 (46.5) | 16 (34.8) |
| Number of internal openings | ||
| 0 | 0 | 1 (2.2) |
| 1 | 35 (81.4) | 39 (84.8) |
| 2 | 8 (18.6) | 6 (13.0) |
| Number of external openings | ||
| 1 | 22 (51.2) | 30 (65.2) |
| 2 | 15 (34.9) | 13 (28.3) |
| 3 | 6 (14.0) | 3 (6.5) |
| Anatomic complexity of fistula | ||
| Single-tract fistula | 20 (46.5) | 29 (63.0) |
| Multiple-tract fistula | 23 (53.5) | 17 (37.0) |
Values are mean ± SD or n (%).
Abbreviations: ADMIRE-CD, Adipose Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn\'s Disease; CD, Crohn’s disease; DVS, darvadstrocel; INSPECT, A retrospectIve chart review study evaluatINg the longer-term effectiveneSs of darvadstrocel in PatiEnts who CompleTed ADMIRE-CD; TNF, tumor necrosis factor.
The INSPECT baseline was 1 day after completing the ADMIRE-CD trial.
Duration of perianal fistulizing disease was collected from medical charts as part of INSPECT. All other variables were taken from the ADMIRE-CD trial.
The sum may exceed the number of patients because patients may have had multiple fistulas in different locations.
Clinical Remission
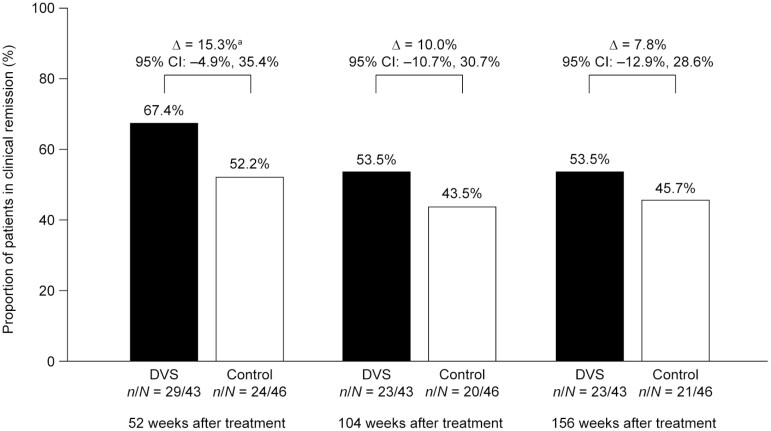
At 52, 104, and 156 weeks after treatment, 67.4% (n = 29 of 43), 53.5% (n = 23 of 43), and 53.5% (n = 23 of 43) of patients in the DVS group were in clinical remission, compared with 52.2% (n = 24 of 46), 43.5% (n = 20 of 46), and 45.7 % (n = 21 of 46) of patients in the control group, respectively. At each time point, the proportion of patients in clinical remission was numerically higher in the DVS group than in the control group: the difference between the DVS group and the control group was 15.3% (95% CI, −4.9% to 35.4%) at 52 weeks, 10.0% (95% CI, −10.7% to 30.7%) at 104 weeks, and 7.8% (95% CI, −12.9% to 28.6%) at 156 weeks (Figure 3).
FIGURE 3.
Clinical remission in patients with Crohn’s disease–related complex perianal fistulas, at 52, 104, and 156 weeks after treatment with darvadstrocel (DVS) vs control. CI, confidence interval. aDiscrepancies in percentage difference between DVS and control group are due to rounding.
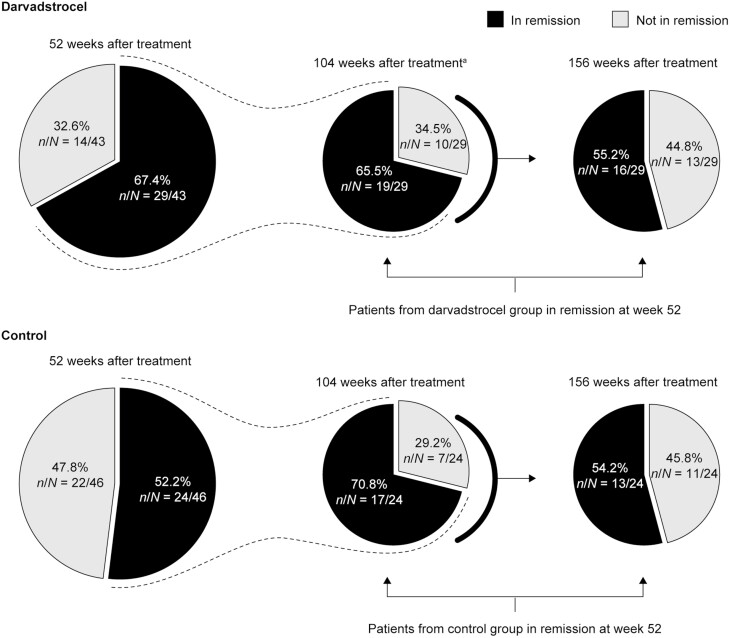
In the subgroup of patients in clinical remission at 52 weeks after treatment (n = 29 in the DVS group and n = 24 in the control group), remission was sustained in 65.5% (n = 19 of 29) and 55.2% (n = 16 of 29) of patients at 104 and 156 weeks after treatment, respectively, in the DVS group and in 70.8% (n = 17 of 24) and 54.2% (n = 13 of 24) of patients, respectively, in the control group. The difference between the DVS and control groups was −5.3% (95% CI, −30.4% to 19.8%) at 104 weeks and 1.0% (95% CI, −25.9% to 27.9%) at 156 weeks (Figure 4).
FIGURE 4.
Sustained clinical remission in patients with Crohn’s disease-related complex perianal fistulas treated with darvadstrocel vs control. aDVS group: of the 23 patients in clinical remission 104 weeks after treatment (Figure 3), 4 had not achieved remission by 52 weeks and are therefore not included in those reported to be in sustained remission at 104 weeks (n = 19). Control group: of the 20 patients in clinical remission 104 weeks after treatment (Figure 3), 3 had not achieved remission by 52 weeks and are therefore not included in those reported to be in sustained remission at 104 weeks (n = 17).
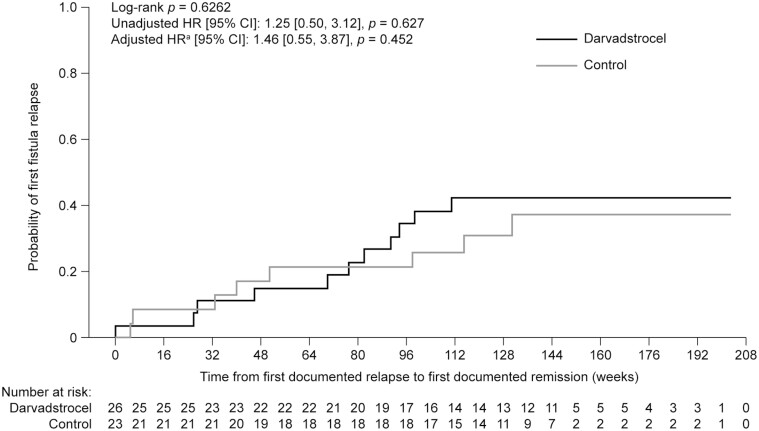
Incidence of Fistula Relapse and Time to Fistula Relapse
In patients reported to be in clinical remission at 52 weeks after treatment and who had data available (n = 26 in the DVS group and n = 23 in the control group), the incidence of fistula relapse at 156 weeks after treatment was not significantly different between groups: 38.5% (n = 10 of 26) in the DVS group and 34.8% (n = 8 of 23) in the control group, respectively (Supplementary Data Content, Table 2). The rate of first fistula relapse over time was not significantly different between groups: the unadjusted hazard ratio was 1.25 (95% CI, 0.50 to 3.12; P = .627) and the adjusted hazard ratio was 1.46 (95% CI, 0.55 to 3.87; P = .452) (Figure 5). These data do not account for the influence of concomitant medication or changes in treatment regimens. A summary of concomitant medical treatments, which may have influenced patient outcomes, is shown in Supplementary Data Content, Table 3.
FIGURE 5.
Probability of first fistula relapse over time for patients who were in remission 52 weeks after treatment with darvadstrocel vs control. CI, confidence interval; HR, hazard ratio. aBaseline characteristics accounted in the adjusted HR include age, sex, perianal fistulizing disease duration, and baseline Crohn\'s Disease Activity Index score. Censoring rules are that patients are counted as an event at first documentation of the outcome or as a right-censored value at the end of follow-up (i.e. earliest of 104 weeks after ADMIRE-CD, death, or last contact with site). Patient numbers represent the analysis population subject to availability of data.
New Fistula Occurrence or Fistula Relapse
For all patients in the INSPECT study (N = 89), regardless of remission status at 52 weeks, the proportion of patients with a new fistula occurrence or fistula relapse at 156 weeks was 25.6% (n = 11 of 43) and 21.7% (n = 10 of 46) in the DVS and control groups, respectively (difference, 3.83%; 95% CI, −0.138 to 0.215).
Further categorical outcomes at 156 weeks, including the proportions of patients who developed perianal abscesses or required rescue medication or surgical intervention, were similar in the DVS and control groups and are shown in Supplementary Data Content, Table 2.
Safety
During the 104-week INSPECT chart review period, tumorigenicity was reported for 1 (2.2%) of 46 patients in the control group (a malignant epidermoid carcinoma from which the patient had not recovered by the end of the study period). One (2.3%) of 43 patients in the DVS group had a benign fibroadenoma (uterine leiomyoma) that was considered not related to DVS, and the patient recovered from this nonmalignant event with sequelae. Both events were reported between 156 and 208 weeks after treatment. There were no reports of events related to ectopic tissue formation.
Discussion
The INSPECT study is a retrospective study to evaluate the long-term effectiveness and key safety endpoints of DVS treatment of complex perianal fistulas in patients with CD. This is the first study to report long-term, real-world data for 156 weeks after treatment with DVS. Findings from this study show that DVS can lead to sustained fistula healing in patients with CD and is therefore an attractive option among the current treatment and management strategies for patients with complex perianal fistulas.
Of those patients treated with DVS and in clinical remission at 52 weeks after treatment, more than half sustained clinical remission for up to 156 weeks. A similar proportion of patients showed sustained remission in the control group (likely owing to the surgical procedures and continuing medical treatments received by the control group, including 2 fistula curettages and closure of the internal opening). These findings indicate that clinical remission can be sustained long-term irrespective of whether it is achieved through DVS administration or maintenance treatment regimens. However, at 52 weeks, fewer patients had achieved clinical remission after control treatments than in the DVS group and this study shows that the benefit of DVS treatment can be sustained over the long term. In contrast, it has been reported that maintenance dosing of biological therapies is necessary to preserve the initial response to treatment of complex perianal fistulas.26 Similarly, surgical repairs of complex perianal fistulas often do not achieve a permanent cure, leading to multiple procedures and an increased risk of complications such as fecal incontinence.9,27
In the ADMIRE-CD trial, safety data were collected for 52 weeks after treatment with DVS. Comprehensive adverse event data were not collected during the INSPECT chart review period, as this was not considered to be relevant for this long-term retrospective study 52 to 208 weeks after treatment; however, tumorigenicity and ectopic tissue formation data were collected, as these were adverse events that might occur over long-term treatment with a stem cell therapy.28,29 A report from the Cell Products Working Party and the Committee for Advanced Therapeutics recommended that, despite a lack of evidence of tumorigenicity of mesenchymal stem cells in clinical and nonclinical studies, the risk of tumor formation should be explored in clinical settings with long-term follow-up.28 During the INSPECT chart review period, no adverse events of special interest (including tumorigenicity) related to treatment were reported, confirming observations from the ADMIRE-CD trial.23,24 These findings are in accordance with previous studies in which local injections of mesenchymal stem cells (autologous or allogeneic) were reported to have a favorable safety profile with no treatment-related tumor development in patients with perianal fistulas.30 In contrast, anti-TNF therapies, used for the treatment of complex perianal fistulas and other conditions, are associated with an increased risk of opportunistic infections, and there are reported cases of lymphoma and other malignancies in patients treated with anti-TNF therapies.28,31
This study had a number of limitations, including small patient numbers. Potential bias may arise from the fact that the patient population in this chart review included only those who completed at least 52 weeks of follow-up in the ADMIRE-CD trial; however, the demographic profile and clinical characteristics of patients included in the INSPECT chart review were similar to that of the whole intention-to-treat population in the ADMIRE-CD trial.23 Furthermore, because some patients in this study completed only 52 weeks, whereas others completed up to 104 weeks in the ADMIRE-CD trial, the proportion of time spent enrolled in the phase 3 ADMIRE-CD trial vs the time enrolled in the INSPECT chart review varied. Consequently, certain outcomes may have been influenced by time spent in the ADMIRE-CD trial. This limitation was partly mitigated by the fact that treatment in the ADMIRE-CD trial was a single administration of DVS or placebo at the start of the study and that, after treatment, all patients continued with concomitant therapies and management of complex perianal fistulas (real-world management of complex perianal fistulas was maintained throughout the INSPECT study). A comparison of the sustained remission outcomes between groups at 156 weeks could also be biased because fewer patients in the control group achieved clinical remission than in the DVS group, hence unidentified confounders in these patient groups may influence the long-term remission outcomes. Analyses of subgroups to evaluate clinical outcomes by baseline patient and disease characteristics or concomitant medication could minimize the risk of bias; however, small sample sizes prohibited such comparisons. Finally, an inherent limitation of chart reviews is that datasets can be incomplete, preventing robust comparisons across all outcomes. In the INSPECT study, data were collected at the original ADMIRE-CD trial sites, but some patients were followed up at a different site to where they were enrolled, which could have contributed to the missing data.
Conclusions
Clinical remission of complex perianal fistulas, previously reported at 52 weeks after DVS treatment in patients with CD, can be sustained for up to 156 weeks in more than half of patients. While clinical remission after control treatment can also be sustained for the same period of time, a numerically smaller proportion of patients in the control group achieved clinical remission than in the DVS-treated group. In accordance with the ADMIRE-CD trial, no new treatment-related safety concerns of tumorigenicity or ectopic tissue formation were identified. DVS may represent an effective minimally invasive option to achieve long-term remission of complex perianal fistulas in patients with CD.
Supplementary Material
Acknowledgments
The authors acknowledge the investigators and coordinators of the INSPECT study for their contributions and to thank the patients for consenting and participating in this chart review. The authors acknowledge Dr Barbara Zhang for her contributions to the collection and interpretation of safety data.
Contributor Information
Julian Panés, Inflammatory Bowel Disease Unit, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Gerd Bouma, Amsterdam Gastroenterology Endocrinology and Metabolism, Department of Gastroenterology and Hepatology, Amsterdam UMC, Amsterdam, the Netherlands.
Marc Ferrante, Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium.
Torsten Kucharzik, Department of Internal Medicine and Gastroenterology, University Teaching Hospital Lüneburg, University of Hamburg, Lüneburg, Germany.
Maria Nachury, U1286 - INFINITE - Institute for Translational Research in Inflammation, Centre Hospitalier Universitaire de Lille, University of Lille, Lille, France.
Fernando de la Portilla de Juan, Departamento de Cirugia, Hospital Universitario Virgen del Rocío, University of Seville, Seville, Spain.
Walter Reinisch, Department for Gastroenterology and Hepatology, Medical University of Vienna, Vienna, Austria; Division of Gastroenterology, McMaster University, Hamilton, ON, Canada.
Francesco Selvaggi, Unit of Colorectal Surgery, Department of Medical, Surgical, Neurological, Metabolic and Ageing Sciences, University of Campania Luigi Vanvitelli, Naples, Italy.
Jörg Tschmelitsch, Department of Surgery, Hospital Barmherzige Brüder, Sankt Veit an der Glan, Austria.
Neil R Brett, Evidera, Montréal, QC, Canada.
Martin Ladouceur, Evidera, Montréal, QC, Canada.
Matthias Binek, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Gary Hantsbarger, Takeda Pharmaceutical Company Limited, Cambridge, MA, USA.
Sarah Campbell-Hill, Takeda UK Ltd, London, United Kingdom.
Chitra Karki, Takeda Pharmaceutical Company Limited, Cambridge, MA, USA.
Christianne Buskens, Amsterdam Gastroenterology Endocrinology and Metabolism, Amsterdam UMC, Location AMC, University of Amsterdam, Amsterdam, the Netherlands.
Funding
The INSPECT study and the ADMIRE-CD trial were sponsored by Takeda Pharmaceuticals International Co. Medical writing support was provided by Sally McTaggart, PhD, of Oxford PharmaGenesis (Oxford, United Kingdom) and was funded by Takeda Pharmaceuticals International Co.
Conflicts of Interest
J.P. has received research grants from AbbVie and Pfizer; received speaker fees from AbbVie, Ferring, Janssen Pharmaceuticals, Merck, Pfizer, Shire (a Takeda company), Takeda Pharmaceuticals Int. Co., and Theravance Biopharma; and served as a consultant for AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Genentech, GlaxoSmithKline, GoodGut, Janssen Pharmaceuticals, Merck, Nestlé, Origo, Pandion Therapeutics, Pfizer, Progenity, Robarts Clinical Trials, Roche, Takeda Pharmaceuticals Int. Co., Theravance Biopharma, and Wassermann. G.B. has received consulting fees from Roche and Takeda Pharmaceuticals Int. Co. (not related to this study or study topic). M.F. has received grants from Amgen, Biogen, Janssen Pharmaceuticals, Pfizer, and Takeda Pharmaceuticals Int. Co.; personal fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celltrion, Dr. Falk Pharma GmbH, Ferring Pharmaceuticals, Janssen Pharmaceuticals, Lamepro, Lilly, Medtronic, MSD, Mylan, Pfizer, Sandoz, Takeda Pharmaceuticals International Co., Thermo Fisher Scientific, and Truvion Healthcare; and nonfinancial support from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celltrion, Falk, Ferring Pharmaceuticals, Janssen Pharmaceuticals, Lamepro, Lilly, Medtronic, MSD, Mylan, Pfizer, Sandoz, Takeda Pharmaceuticals Int. Co., Thermo Fisher Scientific, and Truvion Healthcare. T.K. has no conflicts of interest to disclose. M.N. has received consulting and teaching fees from AbbVie, Adacyte Therapeutics, Amgen, Arena Pharmaceuticals, Biogen, CTMA, Celltrion, Ferring Pharmaceuticals, Fresenius Kabi, Janssen Pharmaceuticals, Mayoli Spindler, MSD, Pfizer, and Takeda Pharmaceuticals International Co. F.d.l.P.J. has received lecture fees from Ethicon (a Johnson & Johnson company), Medtronic, and Takeda Pharmaceuticals Int. Co.; and consultancy fees from Ethicon (a Johnson & Johnson company), LOGSA Group, Medtronic, and Takeda Pharmaceuticals Int. Co. W.R. has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; served as a consultant for Abbott Laboratories, AbbVie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, AstraZeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Dr. Falk Pharma GmbH, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Landos Biopharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestlé, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Teva Pharma, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; served on the advisory board for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC; and received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, Janssen, MSD, Sandoz, and Takeda. F.S. has received consulting fees from Takeda Pharmaceuticals Int. Co. J.T. has no conflicts of interest to disclose. N.R.B. and M.L. are employees of Evidera, which received funding from Takeda Pharmaceuticals Company Ltd for this study. M.B. is an employee of Takeda Pharmaceuticals Int. AG and receives stock/stock options. G.H. is an employee of Takeda Pharmaceuticals Company Ltd and receives stock/stock options. S.C.-H. is an employee of Takeda Pharmaceuticals UK Ltd and receives stock/stock options. C.K. is an employee of Takeda Pharmaceuticals Company Ltd and receives stock/stock options. C.B. has received unrestricted grant support from Boehringer Ingelheim and Roche; received consultancy fees and/or speaker fees from Galapagos, MST, Takeda Pharmaceuticals Int. Co., and Tillotts Pharma; and served on the advisory board for Johnson & Johnson Energy Devices.
Data Availability
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The dataset will be provided after its deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
- 1. Baumgart DC, Sandborn WJ.. Crohn’s disease. Lancet. 2012;380:1590-1605. [DOI] [PubMed] [Google Scholar]
- 2. Kasparek MS, Glatzle J, Temeltcheva T, et al. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007;50:2067-2074. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. . AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508-1530. [DOI] [PubMed] [Google Scholar]
- 4. Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, et al. Management of complex perianal Crohn’s disease. Ann Gastroenterol. 2017;30:33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adegbola SO, Dibley L, Sahnan K, et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: a qualitative exploration. Health Qual Life Outcomes. 2020;18:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz DA, Tagarro I, Carmen Díez M, Sandborn WJ.. Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Inflamm Bowel Dis. 2019;25:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eglinton TW, Barclay ML, Gearry RB, Frizelle FA.. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012;55:773-777. [DOI] [PubMed] [Google Scholar]
- 9. Panes J, Reinisch W, Rupniewska E, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: systematic review. World J Gastroenterol. 2018;24:4821-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lightner AL, Ashburn JH, Brar MS, et al. Fistulizing Crohn’s disease. Curr Probl Surg. 2020;57:100808. [DOI] [PubMed] [Google Scholar]
- 11. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398-1405. [DOI] [PubMed] [Google Scholar]
- 12. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885. [DOI] [PubMed] [Google Scholar]
- 13. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4-22. [DOI] [PubMed] [Google Scholar]
- 14. Gionchetti P, Dignass A, Danese S, et al. ECCO . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135-149. [DOI] [PubMed] [Google Scholar]
- 15. Ardizzone S, Maconi G, Colombo E, et al. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10:91-96. [DOI] [PubMed] [Google Scholar]
- 16. Lee MJ, Parker CE, Taylor SR, et al. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1879-1892. [DOI] [PubMed] [Google Scholar]
- 17. Norton C, Dibley LB, Bassett P.. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J Crohns Colitis. 2013;7:e302-e311. [DOI] [PubMed] [Google Scholar]
- 18. DelaRosa O, Dalemans W, Lombardo E.. Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol. 2012;23:978-983. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Olmo D, Schwartz DA.. Cumulative evidence that mesenchymal stem cells promote healing of perianal fistulas of patients with Crohn’s disease-going from bench to bedside. Gastroenterology. 2015;149:853-857. [DOI] [PubMed] [Google Scholar]
- 20. Choi S, Jeon BG, Chae G, Lee SJ.. The clinical efficacy of stem cell therapy for complex perianal fistulas: a meta-analysis. Tech Coloproctol. 2019;23:411-427. [DOI] [PubMed] [Google Scholar]
- 21. Adamina M, Bonovas S, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2020;14:155-168. [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency. Alofisel European public assessment report summary. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel. Accessed January 11, 2022. [Google Scholar]
- 23. Panés J, García-Olmo D, Van Assche G, et al. ADMIRE CD Study Group Collaborators . Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [DOI] [PubMed] [Google Scholar]
- 24. Panés J, García-Olmo D, Van Assche G, et al. ADMIRE CD Study Group Collaborators . Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154:1334-1342.e4. [DOI] [PubMed] [Google Scholar]
- 25. Spinelli A, García-Olmo D, Van Assche G, et al. Stem cell therapy (darvadstrocel) for treatment-refractory complex perianal fistulas in patients with Crohn’s disease: 2-year efficacy and safety data. Abstracts presented at the 13th European Colorectal Congress (#eccstgallen), 1-5.12.2019, St Gallen, Switzerland. Techn Coloproctol. 2020;24:629-662. [Google Scholar]
- 26. Lopez N, Ramamoorthy S, Sandborn WJ.. Recent advances in the management of perianal fistulizing Crohn’s disease: lessons for the clinic. Expert Rev Gastroenterol Hepatol. 2019;13:563-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bell SJ, Williams AB, Wiesel P, et al. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145-1151. [DOI] [PubMed] [Google Scholar]
- 28. Barkholt L, Flory E, Jekerle V, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies-bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753-759. [DOI] [PubMed] [Google Scholar]
- 29. Bernardo ME, Fibbe WE.. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107-117. [DOI] [PubMed] [Google Scholar]
- 30. Ciccocioppo R, Klersy C, Leffler DA, et al. Systematic review with meta-analysis: Safety and efficacy of local injections of mesenchymal stem cells in perianal fistulas. JGH Open. 2019;3:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Food and Drug Administration. Remicade. Accessed January 11, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103772s5389s5391s5394lbl.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The dataset will be provided after its deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.