Abstract
Migraine headaches are widespread, debilitating and considered a main cause of disability worldwide. Symptoms of migraines include unilateral, pulsating pain that can last for hours to days, frequently associated with photophobia and phonophobia, nausea, or vomiting, and often aggravated by physical activity. The Canadian Headache Society and American Headache Society guidelines suggest strong evidence of the efficacy of triptans, acetaminophen, aspirin, diclofenac sodium, naproxen and ibuprofen for the acute treatment of migraines. The use of calcitonin gene-related peptide (CGRP) antagonists for the treatment and prevention of migraines has been gaining utilization since the approval of the first agent in this class in 2018. There are increasing available options for the acute treatment of migraines. The purpose of this article is to provide a narrative review of the pharmacological and clinical characteristics of ubrogepant and rimegepant and to discuss their implications for use.
Keywords: calcitonin gene-related peptide receptor antagonist, gepants, migraine, rimegepant, ubrogepant
Introduction
Migraines are a type of headache disorder that are widespread, debilitating and considered a main cause of disability worldwide.1 Approximately 75% of adults aged between 18 and 65 years experience headaches, and more than 30% of those adults report suffering from migraines. Migraines are more prevalent in adults between the ages of 35 and 45 years and, due to hormonal differences, women are twice as likely to suffer from migraines compared to men.2–4 Ineffective migraine treatment can increase emergency department visits and are estimated to result in a significant economic burden in the United States of approximately US$36 billion due to direct and indirect costs.5,6 Symptoms of migraines include unilateral, pulsating pain that may last hours to days associated with photophobia and phonophobia, nausea, or vomiting, and are often aggravated by physical activity.7,8 Other symptoms may include autonomic dysfunction and sensory manifestations, including visual disturbances referred to as auras (e.g. flashing lights, spots or lines). Various triggers, including stress, diet, irregular sleep and hormonal changes, can contribute to migraine attacks.6,7
The exact underlying pathophysiology of migraines remains unclear; however, it is believed to be due to the dilation of intracranial blood vessels, which is what initially led to the discovery of triptans (5-HT1B/1D agonists). Triptans, being non-selective vasoconstrictors, provide relief during acute migraine attacks but remain contraindicated in patients with cardiovascular diseases.9 Triptans have been the cornerstone of migraine abortive treatment options until the introduction of calcitonin gene-related peptide (CGRP) receptor antagonists, also known as ‘gepants’, to the market. Although triptans continue to be used with great efficacy and tolerability, CGRP inhibitors offer a new targeted treatment alternative for migraine headaches.
The Canadian Headache Society10 and the American Headache Society (AHS)11 published guidelines on the acute management of migraines in 2013 and 2015, respectively. These guidelines suggest strong evidence of the efficacy of triptans, acetaminophen, aspirin, diclofenac potassium, naproxen and ibuprofen for the acute treatment of migraines. Different approaches exist to treatment selection based on the severity of the migraine and varying opinions on the use of other acute migraine treatments such as dihydroergotamine, codeine-containing compounds, and tramadol, amongst others.10–12 The AHS published a position paper in 2019 to address numerous new treatment options available on the market for migraine treatment. The consensus statement focused on evidence-based treatments, improved outcomes with early treatment of migraine attacks, avoidance of the overuse of acute medications, and self-administered medications.12 Unfortunately, the AHS statement was published before the FDA approval of rimegepant and ubrogepant, both of which are CGRP receptor antagonists.
The first CGRP inhibitor, erenumab (Aimovig®, Amgen Inc.), was approved by the FDA in May 2018 as a subcutaneous injection indicated for migraine prevention.13 Other injectable monoclonal CGRP inhibitors for migraine prevention include fremanezumab (Ajovy®, Teva Pharmaceuticals USA Inc.), galcanezumab (Emgality®, Eli Lily and Company) and eptinezumab (Vyepti®, Lundbeck Seattle BioPharmaceuticals Inc.).14–16 Atogepant (Qulipta®, Forest Laboratories Ireland Ltd) is an oral CGRP antagonist approved for the prevention of migraines in adults.17
For the acute management of migraines in adults, the FDA-approved ubrogepant (Ubrelvy®, Allergan USA Inc.) in December 2019 and rimegepant (Nurtec® ODT, Biohaven Pharmaceuticals) in February 2020.18,19 In addition to acute treatment, rimegepant received further FDA approval for the prevention of migraines in adults in May 2021.19 Table 1 includes all FDA-approved CGRP inhibitors. Biohaven Pharmaceuticals is currently investigating a new CGRP antagonist, zavegepant, for both acute and preventative treatment of migraines with the potential to be available through various routes of administration, including nasal, and oral.20
Table 1.
| Drug | Formulation | Indication | FDA approval date |
|---|---|---|---|
| Atogepant (Qulipta®) | PO | Prevention | September 2021 |
| Eptinezumab (Vyepti®) | IV | Prevention | February 2020 |
| Erenumab (Aimovig®) | SQ | Prevention | May 2018 |
| Fremanezumab (Ajovy®) | SQ | Prevention | September 2018 |
| Galcanezumab (Emgality®) | SQ | Prevention | September 2018 |
| Rimegepant (Nurtec® ODT) | PO | Acute treatment | February 2020 |
| Prevention | May 2021 | ||
| Ubrogepant (Ubrelvy®) | PO | Acute treatment | December 2019 |
IV, intravenous; ODT, orally disintegrating tablet; PO, by mouth; SQ, subcutaneous.
With increasing available options for the acute treatment of migraines, the purpose of this article is to provide a narrative review of the pharmacological and clinical characteristics of ubrogepant and rimegepant and to discuss their implications for use.
Methods
PubMed and Medline searches were conducted to identify the relevant and up-to-date literature on acute migraine management and CGRP receptor antagonist use (i.e. rimegepant and ubrogepant). Primary literature relating to the efficacy and safety of rimegepant and ubrogepant were included as well as other relevant literature. Poster presentations with data pertaining to the clinical trials provided by the drug manufacturer were reviewed and included if considered applicable to this narrative review.
Review
Chemical composition
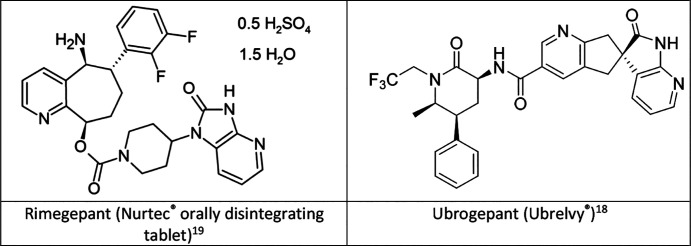
Rimegepant is chemically identified as (5S,6S,9R)-5- amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridine-9-y1 4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridine-1-y1)-1-piperidinecarboxylate hemisulfate sesquihydrate.19 The chemical name for ubrogepant is (3′S)-N-((3S,5S,6R)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl)-2′-oxo-1′,2′,5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3′-pyrrolo[2,3-b]pyridine]-3-carboxamide.18 The chemical structures are shown in Figure 1.
Figure 1.
Chemical structure of rimegepant and ubrogepant.
Clinical pharmacology
CGRP has been found to be elevated in the blood serum during migraine attacks and is suggested to be a key neurotransmitter in the pathogenesis of migraines.7,21 The discovery of CGRP as a neuropeptide acting on the central and peripheral nervous system with a relation to migraine pathophysiology dates back to the mid-1980s.22 CGRP release results in vasodilation of the cranial blood vessels leading to mast cell activation, neurogenic inflammation, and further amplification and progression of migraine pain transmission.7,23,24 Rimegepant and ubrogepant exert their effect by inhibiting CGRP receptors and migraine attacks are believed to be aborted or reduced in frequency by blockade of CGRP activity.18,19
Pharmacokinetics
The absolute bioavailability of rimegepant following oral administration of the orally disintegrating tablet (ODT) formulation is approximately 64% with the maximum concentration (Cmax) at 90 minutes. The time to reach the maximum concentration (Tmax) was delayed by approximately 60–90 minutes when rimegepant was administered to individuals under fed conditions. The elimination half-life of rimegepant in healthy individuals is about 11 hours. In clinical efficacy and safety trials, rimegepant was administered without regard to meals; therefore, the impact of food on the efficacy of the medication remains unknown. Rimegepant is readily distributed with a volume of distribution of 120 L. Binding to human plasma proteins is approximately 96%. The drug is primarily metabolized by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C9, and it is eliminated primarily in its unchanged form at approximately 77%. The route of elimination was studied by the oral administration of radiolabelled rimegepant in healthy men, with 78% being recovered in the faeces and 24% in the urine. The exposure of rimegepant was approximately 40% greater in patients with moderate renal impairment (creatinine clearance [CrCl] 30–59 mL/min) compared to patients with normal renal function following a 75-mg dose. However, when comparing patients with severe renal impairment (CrCl 15—29 mL/min) to patients with normal renal function, there was no clinically significant difference in overall drug exposure. When evaluating the impact of hepatic impairment on rimegepant, there was approximately a two-fold increase in drug exposure in individuals with severe hepatic impairment (Child–Pugh class C).19
Ubrogepant’s Cmax was observed within 90 minutes following its oral administration. A high-fat meal demonstrated a 2-hour delay to Tmax and a 22% reduction in Cmax with no change in the area under the curve (AUC). The clinical studies evaluating the efficacy of ubrogepant were conducted without regard to food. Ubrogepant is readily distributed with a volume of distribution of 350 L. Binding to human plasma proteins is 87%. The drug is primarily metabolized by CYP3A4 with the main pharmacological activity resulting from the parent compound. Ubrogepant is mainly excreted via the biliary/faecal route, with the renal route being responsible for only minor drug excretion. Mild and moderate renal impairment (CrCl 30–89 mL/min) had no impact on the pharmacokinetics of ubrogepant compared to patients with normal renal clearance. There are limited data to determine the effect of severe renal impairment (CrCl 15–29 mL/min) and end-stage renal disease (ESRD) on ubrogepant. Ubrogepant exposure was increased by 7%, 50% and 115% in patients with mild (Child–Pugh Class A), moderate (Child–Pugh Class B) and severe hepatic impairment (Child–Pugh Class C), respectively. The significant impact of severe hepatic impairment on the overall exposure of ubrogepant resulted in recommended dose adjustments for patients with Child–Pugh Class C.18
Clinical studies
Historically, the efficacy of acute migraine treatments was based on four co-primary endpoints: pain relief and the reduction of photophobia, phonophobia and nausea at 2 hours. In 2018, the acute treatment trial guidelines were updated by the FDA to evaluate treatment efficacy based on pain freedom and freedom from the most bothersome symptom (MBS) determined by each patient (i.e. photophobia, phonophobia or nausea) at 2 hours.25 The efficacy and safety of rimegepant and ubrogepant for the acute treatment of migraines with and without aura in adults were evaluated in pivotal phase III studies of similar design. Of note, the efficacy and safety in paediatric patients have not been established.
The two multicentred, randomized, double-blind and placebo-controlled phase III trials evaluating rimegepant 75 mg in adults (18 years of age and older) in the United States with at least a 1-year history of moderate-to-severe migraines and a frequency of two to eight times per month were conducted. The two studies differed in that rimegepant was administered either as an ODT (study 1)27 or oral tablet (study 2)26 formulation. The ODT formulation was developed by Biohaven Pharmaceuticals to enhance rimegepant absorption and allow favourable administration without any liquids in patients experiencing nausea and vomiting. Patients were allowed to use their own acute treatment as a rescue, excluding the use of triptans. Rimegepant demonstrated 2-hour pain freedom rates of 20% and 21% compared to placebo at 11% to 12% (p<0.001). The most common MBS were photophobia (52–59%), phonophobia (15–20%) and nausea (22–32%). The 2-hour freedom rates from the MBS were 35% for rimegepant compared to 27% for placebo (p<0.001) in the first trial and 38% for rimegepant and 25% for the placebo arm (p<0.001) in the second trial. Pain freedom was sustained up to 24 hours in 16% of participants in the rimegepant group compared to 6% in the placebo group (p<0.05) in study 1. A similar sustained response of pain freedom up to 24 hours was observed in study 2 with 12% for rimegepant and 7% for placebo (p value not reported).26,27 The efficacy endpoints for rimegepant are summarized in Table 2. A long-term 52-week safety study revealed no safety concerns or new adverse effects in patients receiving an average of eight or more doses over 4 weeks.28
Table 2.
Results of the primary efficacy and key secondary outcome measures in rimegepant studies.
| Outcome measures | Study 127 | Study 226 | ||
|---|---|---|---|---|
| Rimegepant 75 mg ODT |
Placebo | Rimegepant 75 mg |
Placebo | |
| Pain free at 2 h | n=669 | n=682 | n=537 | n=535 |
| Number (%) | 142 (21.2) | 74 (10.9) | 105 (19.6) | 64 (12.0) |
| p value | – | <0.001 | – | <0.001 |
| MBS free at 2 h | n=669 | n=682 | n=537 | n=535 |
| Number (%) | 235 (35.1) | 183 (26.8) | 202 (37.6) | 135 (25.2) |
| p value | – | 0.0009 | – | <0.001 |
| Sustained pain freedom 2–24 h | n=669 | n=682 | n=537 | n=535 |
| Number (%) | 105 (15.7) | 38 (5.6) | 66 (12.3) | 38 (7.1) |
| p value | – | <0.05 | – | NR |
MBS, most bothersome symptom (i.e. photophobia, phonophobia and nausea); NR, not reported; ODT, orally disintegrating tablet.
The efficacy of ubrogepant was evaluated in two phase III, randomized, double-blind, multicentred, placebo-controlled trials (ACHIEVE I and ACHIEVE II) conducted in the United States. The studies included participants aged 18–75 years with at least a 1-year history of migraines with or without aura. ACHIEVE I randomly assigned participants to receive ubrogepant 50 mg, ubrogepant 100 mg or placebo. The 2-hour pain freedom rates were 19% for ubrogepant 50 mg (p=0.002) and 21% for ubrogepant 100 mg (p<0.001) compared to 12% for the placebo arm. The 2-hour freedom from MBS was 39% and 38% for the ubrogepant 50 mg (p=0.002) and 100 mg (p=0.002) groups, respectively, compared to 28% in the placebo group. Pain freedom was sustained for up to 24 hours in 13% of patients receiving ubrogepant 50 mg (p value not reported) and 15% for ubrogepant 100 mg (p=0.004) compared to placebo at 9%.29 ACHIEVE II evaluated the efficacy of ubrogepant in three randomized groups receiving either ubrogepant 25 mg, ubrogepant 50 mg or placebo. The 2-hour pain freedom was 21%, 22% and 14% in the ubrogepant 25 mg (p=0.03), ubrogepant 50 mg (p=0.01) and placebo groups, respectively. The absence of MBS was 34% for ubrogepant 25 mg (p=0.07) and 39% for ubrogepant 50 mg (p=0.01) compared to 27% in the placebo group. Pain freedom was sustained for 24 hours in 13% of the 25 mg group (p value not reported) and 14% of the 50 mg (p=0.01) compared to 8% in the placebo group.30,31 The results of ACHIEVE I and II are summarized in Table 3. Participants were allowed to use a rescue treatment (i.e. NSAIDs, triptans) or a second dose of ubrogepant if the pain was still moderate at 2 hours post initial dose. Patients in the placebo group received only a placebo throughout the study, whereas the ubrogepant group was rerandomized to ubrogepant or placebo as an optional second dose. The second dose of ubrogepant 50 mg showed efficacy in up to 55% of patients who experienced pain relief following the initial dose compared to 33% of patients receiving placebo as their second dose (p=0.0091). Taking a second dose of ubrogepant increased the likelihood of achieving pain freedom compared to a single dose alone.32 A 52-week long-term safety study revealed no new safety concerns or side-effects when ubrogepant was used for up to eight doses per month.33
Table 3.
Results of the primary and key secondary efficacy outcome measures in ubrogepant studies.
| Outcome measures | ACHIEVE I29 | ACHIEVE II30 | ||||
|---|---|---|---|---|---|---|
| Ubrogepant 50 mg |
Ubrogepant 100 mg |
Placebo | Ubrogepant 25 mg |
Ubrogepant 50 mg |
Placebo | |
| Pain free at 2 h | n=422 | n=448 | n=456 | n=435 | n=464 | n=456 |
| Number (%) | 81 (19.2) | 95 (21.2) | 54 (11.8) | 90 (20.7%) | 101 (21.8) | 65 (14.3) |
| p value | 0.002 | <0.001 | – | 0.03 | 0.01 | – |
| MBS free at 2 h | n=420 | n=448 | n=454 | n=434 | n=463 | n=456 |
| Number (%) | 162 (38.6) | 169 (37.7) | 126 (27.8) | 148 (34.1) | 180 (38.9) | 125 (27.4) |
| p value | 0.002 | 0.002 | – | 0.07 | 0.01 | – |
| Sustained pain freedom 2–24 h | n=418 | n=441 | n=452 | n=432 | n=457 | n=451 |
| Number (%) | 53 (12.7) | 68 (15.4) | 39 (8.6) | 55 (12.7) | 66 (14.4) | 37 (8.2) |
| p value | NR | 0.004 | – | NR | 0.01 | – |
MBS, most bothersome symptom (i.e. photophobia, phonophobia and nausea); NR, not reported; ODT, orally disintegrating tablet.
Safety profile
Whilst long-term studies and post-marketing reports will ultimately shed more light on the overall safety use of rimegepant and ubrogepant, the data from current studies suggest that both medications are generally well tolerated.18,19 CGRP inhibitors as a class are reported to have fewer adverse drug reactions and warnings/precautions compared to other medications approved for migraine treatment (i.e. triptans).7 Vasoconstriction is not observed with the use of CGRP antagonists; therefore, they should be safe to use in patients with stable cardiovascular disease. However, there are insufficient data in patients with vascular events (e.g. stroke, myocardial infarction) within 6 months.34–36
The most common adverse effects reported for both rimegepant and ubrogepant were nausea, somnolence and dry mouth in 1–4% of patients compared with placebo. In the clinical study evaluating rimegepant for the use of migraine prevention with an increased frequency of administration, abdominal pain and dyspepsia were reported in approximately 2% more patients when compared to placebo.18,19
The labelling of rimegepant cites no major contraindications to its use other than avoidance of the agent in patients with a history of hypersensitivity to rimegepant or any excipients found in the formulation and concomitant use with moderate/strong CYP3A4 inhibitors and inducers. Ubrogepant, on the other hand, is contraindicated in patients concomitantly using a strong CYP3A4 inhibitor (e.g. ketoconazole, clarithromycin).18,19
Studies regarding the use of rimegepant and ubrogepant in pregnant women have not been conducted; however, data from animal reproduction studies demonstrated potential for fetal harm. As a post-marketing requirement, a pregnancy exposure registry was created to monitor pregnancy outcomes related to rimegepant. In the clinical studies, the number of participants 65 years or older was low for an adequate comparison to younger participants; however, no differences were noted in the efficacy and safety of rimegepant and ubrogepant in this group of patients compared to adults younger than 65 years of age. As is typical with newly approved agents, the use of rimegepant and ubrogepant in patients younger than 18 years of age has not been evaluated.18,19
Drug interactions
As in vitro data revealed that rimegepant is metabolized at a higher rate by CYP3A4, to a lesser extent by CYP2C9 and is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), studies were conducted to assess the impact of other drugs on rimegepant. A single 75-mg dose of rimegepant with itraconazole, a strong CYP3A4 inhibitor, resulted in an increase in the AUC and Cmax of approximately 4-fold and 1.5-fold, respectively. In a similar study evaluating the impact of rifampin, a strong CYP3A4 inducer, the overall exposure of rimegepant decreased (AUC by 80% and Cmax by 64%) and was believed to have an impact on the efficacy of the drug. CYP2C9 inhibition is not expected to significantly affect rimegepant exposure. The interaction of rimegepant with quinidine, a potent P-gp inhibitor, led to an increase in the AUC and Cmax by 1.6-fold and 1.7-fold, respectively. Similarly, cyclosporine, a potent P-gp and BCRP inhibitor, was studied concomitantly with rimegepant and demonstrated an increase in rimegepant exposure (AUC by 1.6-fold and Cmax by 1.4-fold). BCRP inhibitors are not expected to have a significant impact on rimegepant exposure given these findings.19
Ubrogepant in vitro studies demonstrated that CYP3A4 is primarily responsible for its metabolism and is a substrate of P-gp and BCRP. The concomitant use of ubrogepant and ketoconazole, a strong CYP3A4 inhibitor, resulted in an increase in the AUC by 9.7-fold and 5.3-fold in the Cmax. Verapamil, a moderate CYP3A4 inhibitor, led to a 3.5-fold and 2.8-fold increase in the AUC and Cmax of ubrogepant, respectively. The use of ubrogepant with strong CYP3A4 inhibitors is contraindicated based on its labelling. An 80% reduction in ubrogepant was observed when administered in tandem with rifampin, a strong CYP3A4 inducer. The use of ubrogepant with strong CYP3A4 inducers is not recommended, and a potential dose adjustment is noted in the labelling when administered with weak or moderate CYP3A4 inducers. The use of P-gp and/or BCRP inhibitors is expected to result in an increase in ubrogepant exposure, and dose adjustments are recommended for ubrogepant when clinically relevant.18
Dosing and administration
The recommended dose of rimegepant is 75 mg taken by mouth as needed, with a maximum dose of 75 mg over a 24-hour period. It is suggested to not exceed 18 doses in a 30-day period given the lack of safety data available with regard to more frequent rimegepant administration. Rimegepant is supplied as an ODT in a blister package and can be placed on or below the tongue. In patients with severe hepatic impairment (Child–Pugh Class C), the use of rimegepant is not recommended due to an increase in the plasma concentration of the medication. The use of rimegepant in patients with ESRD (CrCl of less than 15 mL/min) or on dialysis has not been studied; therefore, its use is not recommended in this group of patients.19
Ubrogepant is dosed at 50 mg or 100 mg orally and, if needed, a second dose can be repeated at least 2 hours apart following the initial dose. The maximum amount of ubrogepant in a 24-hour period is 200 mg, and it is recommended to not treat more than eight migraines in a 30-day period given the need for additional safety data. Patients with severe hepatic impairment (Child–Pugh Class C) and renal impairment (CrCl 15–29 mL/min) should only receive a maximum of 50 mg per dose. The use of ubrogepant in patients with ESRD (CrCl <15 mL/min) should be avoided altogether.18
Cost
The average wholesale price for a 75-mg ODT of rimegepant (Nurtec® ODT) is US$137.89 and US$112.45 for a 50- or 100-mg oral tablet of ubrogepant (Ubrelvy®).37 This is significantly greater than traditional oral agents indicated for the acute treatment of migraines, the majority of which have generic availability but are comparable to injectable and intranasal formulations. A cost comparison of agents commonly used to treat acute migraines in adults is provided in Table 4.37,38
Table 4.
| Drug | Usual adult treatment dose | Cost for one full dosea (US$) |
|---|---|---|
| Triptans (serotonin 5-HT1B, 1D receptor agonists) | ||
| Almotriptan | 12.5 mg PO as a single dose | 42 |
| Eletriptan (Relpax®) | 40 mg PO as a single dose | 61 |
| Frovatriptan (Frova®) | 2.5 mg PO as a single dose | 72 |
| Naratriptan (Amerge®) | 2.5 mg PO as a single dose | 25–29 |
| Sumatriptan (Imitrex®, Onzetra® Xsail®, Sumavel DosePro®, Tosymra®, Zembrace® SymTouch®) | 50–100 mg PO as a single dose | 25 |
| 6 mg SQ as a single dose | 15–85 | |
| One 20 mg spray as a single dose in one nostril | 49–81 | |
| 11 mg (powder, breath-activated) insufflated in each nostril | 141 | |
| Rizatripan (Maxalt®) | 5–10 mg PO as a single dose | 6–36 |
| Zolmitriptan (Zomig®) | 2.5 mg PO as a single dose | 1–55 |
| One (2.5–5 mg) spray as a single dose in one nostril | 106–111 | |
| Ergot derivative | ||
| Dihydroergotamine (DHE 45®, Migranal®, Trudhesa®) | 1 mg IV as a single dose | 150–199 |
| 1 mg IM as a single dose | ||
| 1 mg SQ as a single dose | ||
| One (0.5 mg or 0.75 mg) spray into each nostril repeat in 15 minutes (4 sprays/dose) | 66–514 | |
| Ditan (serotonin 5-HT1F receptor agonist) | ||
| Lasmiditan (Reyvow®) | 50–100 mg PO as a single dose | 106 |
| Gepants (calcitonin gene-related peptide (CGRP) receptor antagonists) | ||
| Rimegepant (Nurtec®) | 75 mg PO as a single dose | 138 |
| Ubrogepant (Ubrelvy®) | 50–100 mg PO as a single dose | 112 |
Cost is based on the average wholesale price (AWP) at the usual adult treatment dose rounded to the nearest dollar. The lowest AWP noted in Red Book Online is provided for generic products.
IM, intramuscular; IV, intravenous; PO, by mouth; SQ, subcutaneous.
Conclusion
Migraine is a common neurological condition that can lead to significant debilitation. Rimegepant and ubrogepant are oral CGRP inhibitors used as abortive agents for the treatment of migraines in adults, with known efficacy in resolving acute attacks and an improved safety profile compared to traditional acute migraine therapies. Gepants offer an effective and safe alternative to triptans as a first-line treatment for migraine cessation; however, the familiarity and cost of triptans continue to drive their use amongst patients suffering from migraines. Patients who are not experiencing adequate relief, those unable to tolerate other migraine abortive agents (e.g. triptans) or those with comorbid cardiovascular diseases would greatly benefit from a CGRP receptor antagonist.
Acknowledgements
None.
Footnotes
Contributions: ERO conducted the literature search, reviewed the literature, and developed and wrote the manuscript. MK contributed to the methods and to the writing of this manuscript. EQ reviewed the manuscript and provided additions and/or corrections. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/10/dic.2022-3-5-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Ocheretyaner ER, Kofman M, Quattrocchi E. https://doi.org/10.7573/dic.2022-3-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.CDC. Acute migraine. [Accessed January 12, 2022]. https://www.cdc.gov/acute-pain/migraine/index.html .
- 2.WHO. Headache disorders. [Accessed January 12, 2022]. https://www.who.int/news-room/fact-sheets/detail/headache-disorders .
- 3.Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21–34. doi: 10.1111/head.12482. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonafede M, Sapra S, Shah N, et al. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700–714. doi: 10.1111/head.13275. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty D, Lippman S. CGRP inhibitors for migraine. Innov Clin Neurosci. 2020;17(4–6):39–40. [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. New Drug class employs novel mechanism for migraine treatment and prevention. [Accessed January 12, 2022]. https://www.fda.gov/drugs/news-events-human-drugs/new-drug-class-employs-novel-mechanism-migraine-treatment-and-prevention .
- 8.International Headache Society. The International Classification of Headache Disorders. 3rd edition. 1. Vol. 18. Cephalagia: 2018. pp. 1–211. [DOI] [PubMed] [Google Scholar]
- 9.Mercer SE, Chaturvedula PV, Conway CM, et al. Azepino-indazoles as calcitonin gene-related peptide (CGRP) receptor antagonists. Bioorg Med Chem Lett. 2021;31:127624. doi: 10.1016/j.bmcl.2020.127624. [DOI] [PubMed] [Google Scholar]
- 10.Worthington WI, Pringsheim T, Gawel M, et al. Canadian Headache Society Guideline: acute drug therapy for migraine headache. Can J Neurol Sci. 2013;40(Suppl 5):S1–S80. [PubMed] [Google Scholar]
- 11.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20. doi: 10.1111/head.12499. [DOI] [PubMed] [Google Scholar]
- 12.American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18. doi: 10.1111/head.13456. [DOI] [PubMed] [Google Scholar]
- 13.Aimovig (erenumab-aooe) prescribing information. Thousand Oaks, CA: Amgen Inc; Nov, 2021. [Accessed September 20, 2022]. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Aimovig/aimovig_pi_hcp_english.pdf . [Google Scholar]
- 14.Ajovy (fremanezumab-vfrm) prescribing information. North Wales, PA: Teva Pharmaceuticals USA Inc; Sep, 2021. [Accessed September 20, 2022]. https://www.ajovy.com/globalassets/ajovy/ajovy-pi.pdf . [Google Scholar]
- 15.Emgality (galcanezumab-gnlm) prescribing information. Indianapolis, IN: Eli Lily and Company; Mar, 2021. [Accessed September 20, 2022]. https://pi.lilly.com/us/emgality-uspi.pdf . [Google Scholar]
- 16.Vyepti (eptinezumab-jjmr) prescribing information. Bothell, WA: Lundbeck Seattle BioPharmaceuticals Inc; Sep, 2021. [Accessed September 20, 2022]. https://www.lundbeck.com/content/dam/lundbeck-com/americas/united-states/products/neurology/vyepti_pi_us_en.pdf . [Google Scholar]
- 17.Qulipta (atogepant) prescribing information. Dublin, Ireland: Forest Laboratories Ireland Ltd; Oct, 2021. [Accessed September 20, 2022]. https://www.rxabbvie.com/pdf/qulipta_pi.pdf . [Google Scholar]
- 18.Ubrelvy (ubrogepant) prescribing information. Madison, NJ: Allergan USA Inc; Mar, 2021. [Accessed September 20, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf . [Google Scholar]
- 19.ODT. Nurtec (rimegepant) prescribing information. New Haven, CT: Biohaven Pharmaceuticals Inc; Dec, 2021. [Accessed September 20, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212728s000lbl.pdf . [Google Scholar]
- 20.Pfizer Inc. Pfizer completes acquisition of Biohaven Pharmaceuticals. [Accessed October 20, 2022]. www.pfizer.com/news/press-release/press-release-detail/pfizer-completes-acquisition-biohaven-pharmaceuticals .
- 21.Tepper SJ. History and review of anti-Calcitonin Gene-Related Peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58(Suppl 3):238–275. doi: 10.1111/head.13379. [DOI] [PubMed] [Google Scholar]
- 22.McCulloch J, Uddman R, Kingman TA, et al. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci USA. 1986;83(15):5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wattiez AS, Sowers LP, Russo AF. Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting. Expert Opin Ther Targets. 2020;24(2):91–100. doi: 10.1080/14728222.2020.1724285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matteis ED, Guglielmetti M, Ornello R, et al. Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother. 2020;20(6):627–641. doi: 10.1080/14737175.2020.1772758. [DOI] [PubMed] [Google Scholar]
- 25.Cooper W, Doty EG, Hochstetler H, et al. The current state of acute treatment for migraine in adults in the United States. Postgrad Med. 2020;132(7):581–589. doi: 10.1080/00325481.2020.1767402. [DOI] [PubMed] [Google Scholar]
- 26.Lipton R, Croop R, Stock E, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist for migraine. N Engl J Med. 2019;381(2):142–149. doi: 10.1056/NEJMoa1811090. [DOI] [PubMed] [Google Scholar]
- 27.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomized, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–748. doi: 10.1016/S0140-6736(19)31606-X. [DOI] [PubMed] [Google Scholar]
- 28.Croop R, Berman G, Kudrow D, et al. Long-term safety of rimegepant 75 mg for the acute treatment of migraine. Neurology. 2020;94(Suppl 15):4829. [Google Scholar]
- 29.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230–2241. doi: 10.1056/NEJMoa1813049. [DOI] [PubMed] [Google Scholar]
- 30.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II Randomized Clinical Trial. JAMA. 2019;322(19):1887–1898. doi: 10.1001/jama.2019.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60(4):686–700. doi: 10.1111/head.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ailani J, Blumenfeld A, Klein B, et al. An optional second dose of ubrogepant is effective in achieving 2-hour pain freedom in the acute treatment of migraine. Neurology. 2020;94(Suppl 15):166. [Google Scholar]
- 33.Ailani J, Lipton RB, Hutchinson S, et al. Long-term safety evaluation of ubrogepant for the acute treatment of migraine: phase 3, randomized, 52-week extension trial. Headache. 2020;60(1):141–152. doi: 10.1111/head.13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conway C, Croop R, Dubowski G, et al. Cardiovascular safety of rimegepant 75 mg in 3 randomized clinical trials and system evaluations from in vitro, ex vivo, and in vivo nonclinical assays (2141) Neurology. 2020;94(Suppl 15):2141. [Google Scholar]
- 35.Rubio-Beltran E, Chan KY, Danser AJ. Characteristics of the calcitonin gene-related peptide receptor antagonists ubrogepant and atogepant in human isolated coronary, cerebral and middle meningeal arteries. Cephalalgia. 2020;40(4):357–366. doi: 10.1177/0333102419884943. [DOI] [PubMed] [Google Scholar]
- 36.Severt L, Silberstein SD, Blumendfeld AM, et al. Safety and efficacy of ubrogepant in participants with moderate to high cardiovascular risk. Neurology. 2020;64(Suppl 15):107. [Google Scholar]
- 37.Lexicomp. [Accessed January 23, 2022]. https://online.lexi.com/lco/action/home .
- 38.Micromedex Solutions Website. [Accessed January 23, 2022]. https://www.micromedexsolutions.com/home/dispatch .