Abstract
Traditionally, lust and pride have been considered pleasurable, yet sinful in the West. Conversely, guilt is often considered aversive, yet valuable. These emotions illustrate how evaluations about specific emotions and beliefs about their hedonic properties may often diverge. Evaluations about specific emotions may shape important aspects of emotional life (e.g. in emotion regulation, emotion experience and acquisition of emotion concepts). Yet these evaluations are often understudied in affective neuroscience. Prior work in emotion regulation, affective experience, evaluation/attitudes and decision-making point to anterior prefrontal areas as candidates for supporting evaluative emotion knowledge. Thus, we examined the brain areas associated with evaluative and hedonic emotion knowledge, with a focus on the anterior prefrontal cortex. Participants (N = 25) made evaluative and hedonic ratings about emotion knowledge during functional magnetic resonance imaging (fMRI). We found that greater activity in the medial prefrontal cortex (mPFC), ventromedial PFC (vmPFC) and precuneus was associated with an evaluative (vs hedonic) focus on emotion knowledge. Our results suggest that the mPFC and vmPFC, in particular, may play a role in evaluating discrete emotions.
Keywords: emotion concepts, emotion words, fMRI, emotion evaluation
Pride and lust are ranked among the seven cardinal sins of Western canon (Aquinas, 2006). The belief in the sinfulness of pride and lust stands out because they are emotions that are often considered hedonically pleasant while being evaluated as bad in Western religious tradition. Even today, many people recognize that pride and lust are often evaluated as ‘bad’ emotions despite being believed to be experientially pleasant emotions. Thus, pride and lust offer an intuitive example of how evaluations of emotions and beliefs about their hedonic properties can diverge. Indeed, this divergence exists for a range of different emotions (for discussions see Parrott, 1993; Larsen, 2000; Dweck, 2017; Ford and Gross, 2019).
Evidence of divergence between hedonic and evaluative aspects of emotion suggests that people may place values on different emotions that are distinct from hedonics. These values include attitudes toward specific emotions, as well as the appropriateness and desirability of these emotions (Eid and Diener, 2001; Wood et al., 2009; Harmon-Jones et al., 2011; Yoon et al., 2018). For instance, some people vary in the extent to which they fear unpleasant emotions such as anger, anxiety and depression. Greater fear of unpleasant emotions is tied to more intense depressive symptoms (Yoon et al., 2018). Moreover, cross-cultural studies have shown that social norms contribute to the desirability of positively and negatively valenced emotions (Tsai et al., 2006) or even fear of a positive emotion (happiness; Joshanloo et al., 2014). Additional work has indirectly demonstrated that people hold beliefs about the usefulness or social acceptability of certain negative emotions, such as anger or shame. For example, in one study, participants tend to upregulate anger when expecting to negotiate with another person, but not when expecting to cooperate with that person (Tamir and Ford, 2012). Further, whether participants reported more frequent encounters with situations that elicited anger vs shame depended on which emotion was socially sanctioned and which was socially condemned in their culture (Boiger et al., 2013). Consistent with norms in their respective countries, US participants tended to report that they encountered situations that elicited anger more than situations that elicited shame. In contrast, Japanese participants tended to report the opposite.
These studies suggest that there is a divergence between evaluative and hedonic aspects of ‘emotion knowledge’. Emotion knowledge refers to a person’s store of beliefs and experiences about an emotion category (for similar treatments see Salovey and Mayer, 1990; Barrett, 2006, 2009; Niedenthal, 2008; Russell, 2009; Lindquist et al., 2015). It includes knowledge about normative views on the hedonic or evaluative properties of that emotion. Consider that, although pride, happiness and lust are normatively assumed to be hedonically pleasurable in Western contexts, studies have found that they may be evaluated negatively in certain cultural contexts (Eid and Diener, 2001; Stenstrom and Curtis, 2012; Joshanloo et al., 2014; see also Aquinas, 2006). Conversely, although guilt, shame and anger are often assumed to be hedonically unpleasant, studies have found they may be evaluated as positive in specific cultural contexts (Eid and Diener, 2001; Tamir and Ford, 2012; Boiger et al., 2013).
For neuroscience, in particular, research on evaluative vs hedonic emotion knowledge raises questions about their neural bases and the extent to which they overlap. Studies have implicated prefrontal cortical areas including the medial prefrontal cortex (mPFC), ventromedial prefrontal cortex (vmPFC) and right dorsolateral prefrontal cortex (dlPFC) in judging the valence of affective stimuli (Anderson et al., 2003; Small et al., 2003; Grimm et al., 2006). Further, activity in the mPFC and dlPFC are correlated with judgments of valence but not judgments of arousal associated with emotion-related words (e.g. distressed, excited, content, bored, idle, etc.; Posner et al., 2009). Several multivariate studies have also found evidence that areas in the anterior mPFC, among others, are involved in representing stimulus valence across sensory modalities (Baucom et al., 2012; Chikazoe et al., 2014; Skerry and Saxe, 2014; Bush et al., 2018; Kim et al., 2020; Shinkareva et al., 2020). Studies on attitudes and evaluations similarly suggest that the mPFC and vmPFC are associated with evaluative judgments. These include making good–bad judgments about concepts like murder, happiness or gun control (Cunningham et al., 2004), agreement or disagreement with evaluative statements (Zysset et al., 2002; Schroeter et al., 2010) and judging whether two evaluations are compatible (e.g. ‘Chris likes parties’ and ‘parties are good’; Wood et al., 2005). Other studies have shown mPFC activation during moral (Greene et al., 2001, 2004; Moll et al., 2001) and aesthetic judgments (Jacobsen et al., 2006; Kirk et al., 2009; Zhang et al., 2016, 2017). Finally, damage to the vmPFC, in particular, produces disruptions in valuation during decision-making, such as deficits in valuing future (vs immediate) rewards, insensitivity to punishment, impaired reversal learning and erratic preference judgments (Bechara et al., 1994, 1997; Fellows and Farah, 2003, 2007). Collectively, these studies demonstrate that anterior prefrontal areas support judgments concerning valence across different domains.
We were particularly interested in the vmPFC’s role in focusing on evaluative (vs hedonic) dimensions of emotion knowledge. Studies have shown that vmPFC regulates subcortical structures involved in affective experience such as the amygdala and striatum (Roy et al., 2012; Rae et al., 2015). Other work has further suggested that the vmPFC, along with and dlPFC are involved in inhibitory control and affective regulation (Cunningham and Zelazo, 2007; Goldin et al., 2008; Kober et al., 2010). Meanwhile, studies in value-based decision-making have implicated the vmPFC in evaluating the healthiness and taste of foods when choosing between healthy and unhealthy foods (Hare et al., 2009; Nook and Zaki, 2015; Schmidt et al., 2018). Notably, for self-reported dieters, both the ratings of healthiness and taste moderated the relationship between vmPFC and food choice during successful self-control, that is, when choosing less tasty but healthy foods over tasty but unhealthy foods (Hare et al., 2009). In contrast, only taste ratings moderated the relationship between the vmPFC and food choice during unsuccessful self-control (choosing tasty but less healthy foods over healthy foods).
These findings (Hare et al., 2009) suggest that the vmPFC is involved in more than the representation of hedonic value. Instead, it is consistent with the hypothesis that the vmPFC plays a role in evaluation more broadly and that it integrates hedonic and other pertinent information (e.g. healthiness of a food) during decision-making. Further consistent with this hypothesis, vmPFC activity to healthy and unhealthy foods is also affected by the foods’ popularity (Nook and Zaki, 2015). When participants were blind to how their peers evaluated different foods, vmPFC activation was greater when viewing unhealthy vs healthy foods. However, once participants were presented with alleged peer ratings (like vs dislike) about the popularity of different foods, vmPFC activity tracked with popularity rather than healthiness or unhealthiness. Together, these studies suggest that the vmPFC, alongside the dlPFC, plays an important role in evaluative focus in certain domains. However, little is known about neural responses when the evaluative focus is on emotion knowledge.
To determine which brain regions were engaged when participants focused on evaluative vs hedonic dimensions of emotion knowledge, participants made judgments about the evaluative (bad vs good) and hedonic (unpleasant vs pleasant) properties of different emotion categories. These categories were represented by emotional words. Using emotion words as stimuli enabled us to emphasize the processes involved in representing emotion knowledge over the processes that generate affective experiences. Indeed, there are now hundreds of studies that have examined the neural correlates of affective and emotional experiences which likely engage a wide variety of processes including those related to the generation of affect, retrieval of emotion knowledge and more (Vytal and Hamann, 2010; Satpute et al., 2013; Lindquist et al., 2016). However, few studies have specifically examined emotion knowledge separately from affect generation, let alone neural activity associated with the evaluation of emotions in particular.
Method
Participants
A total of 25 right-handed, native-English speaking participants (11 females and 14 males), aged 21–40 years (M = 29.84, s.d. = 5.46), took part in the study. Participants received $40 per hour for taking part in the scanning session. Participants were included in the study if they were between the ages of 18 and 55, were native English speakers, were right-handed, had no metal in their bodies and had no history of psychiatric illness. Participants provided written informed consent prior to taking part in the study. All procedures were approved by the institutional review board at the California Institute of Technology.
Experimental task
To investigate neural activity associated with representing emotion words on evaluative vs hedonic dimensions, we designed a task with a 2 (Task: Evaluative vs Hedonic) by 10 (Emotion Category: Anger, Disgust, Fear, Guilt, Sad, Calm, Excited, Happy, Lust and Pride) repeated measures factorial design. The ten emotion words were chosen to have a balance of positively and negatively valenced words presumed to show variability in the agreement of their evaluative vs hedonic traits based on prior behavioral work. For example, whereas anger, guilt, lust and pride are commonly thought to diverge in their hedonic and evaluative aspects (Eid and Diener, 2001; Stenstrom and Curtis, 2012; Tamir and Ford, 2012; Boiger et al., 2013), the other emotions might be expected to show less divergence. In a separate set of behavioral studies, we found that the valuation of emotion depended on the valuation dimension (evaluative vs hedonic) and emotion category in question (Lee et al., under review). Certain emotions such as lust, pride, guilt, anger among others showed divergence between their evaluative and hedonic valence, whereas others were less so (e.g. misery, joy and awe).
In the experimental trials, participants were first presented with the rating scale before one of ten emotion words appeared on screen for 4 s. During this 4 s window, participants were instructed to make a rating. In one block, participants rated the words on the extent to which they thought the emotion was evaluatively good or bad in general. In the other block, participants rated the words on whether the emotion described was hedonically pleasant or unpleasant in general. Participants provided a rating along a continuous rating scale (a line with a cursor) with ‘good’ (or ‘pleasant’) (0) and ‘bad’ (or ‘unpleasant’) (1) being the other anchor. For example, a rating at the midpoint of the scale would be recorded as 0.50, which is functionally a response of ‘neither.’ For ease of interpretability, participants’ evaluative and hedonic ratings were reverse scored such that bad/unpleasant = 0 and good/pleasant = 1. This was done by subtracting the ratings from 1.
Participants also completed a standard odd–even task for use as a baseline task (Stark and Squire, 2001). In the baseline task, participants were presented with a number (1, 3, 4, 5, 6 or 8) and made a response by making a button press on the trackball if the number was ‘5.’ In total, participants completed 8 experimental blocks, repeating the evaluative and hedonic blocks twice within a run across 2 runs for a total of 40 emotion word trials for each type of judgment (evaluative vs hedonic). Additionally, prior to entering the scanner, participants completed a total of four practice blocks, with evaluative and hedonic ratings being repeated twice within a run. Block order was randomized.
Apparatus
Participants were scanned using a Siemens TIM Trio (Erlangen, Germany) 3 T scanner equipped with a 32-channel head coil. Functional image acquisition in was done using a T2*-weighted echo planar imaging (EPI) pulse sequence (TR = 1 s, TE = 3 s, flip angle = 60°, 2.5 mm isotropic resolution, interleaved transverse acquisition). Anatomical image acquisition was done using a T1-weighted sequence (TR = 2.4 s, TE = 2.6 s, flip angle = 8°, 1 mm isotropic resolution). We presented the task using a projector onto a screen that participants could see via a mirror attached to the head coil.
Data Analysis
We used fmriprep (https://fmriprep.readthedocs.io/en/stable/index.html) to preprocess the functional images. We coregistered the functional images with each participant’s T1-weighted structural image, applied motion correction and slice-time correction before and normalized the functional images using the MNI-ICBM152 template before applying spatial smoothing (6 mm full width half max). First and second level analyses were conducted in NeuroElf v1.1 rc2 (http://neuroelf.net/) using MATLAB version R2018b. To examine neural correlates of the evaluative vs hedonic qualities of emotion knowledge, we first conducted analyses that modeled the two functional runs as a general linear model (GLM) with a separate regressor for the evaluative and hedonic rating conditions and a single nuisance regressor accounting for subject head movement. The GLM convolved each regressor with the canonical hemodynamic response function. We then computed subtraction contrasts from the first-level GLMs (Evaluative > Hedonic) for second-level group maps. We used AlphaSim Monte Carlo simulation (smoothness was estimated from the data) to correct for family-wise error (FWE) at the voxel level across the whole brain using a voxel-wide threshold at P < 0.005, t > 2.80, FWE P < 0.05.
Results
Behavioral Results
To examine whether participants distinguished between evaluative and hedonic aspects of emotion knowledge, we first examined their ratings. Based on previous behavioral studies (Lee et al., under review), we expected a two-way interaction between the type of rating and the emotion category. Thus, we conducted a 2 (Judgment type: Evaluative vs hedonic) × 10 (Emotion category: anger, disgust, fear, guilt, sad, calm, excited, happy, lust, pride) repeated measures ANOVA. Consistent with our hypothesis, we found a significant interaction between judgment type and emotion, F (9, 207) = 7.51, P < 0.001, η2 = 0.25. We also found a significant main effect of emotion, F (9, 207) = 145.09, P < 0.001, η2 = 0.86. We did not find a main effect of judgment type, F (1, 23) < 0.01, P = 0.96, η2 < 0.01. The main effect of emotion category will be interpreted in the context of the significant interaction.
Post-hoc comparisons revealed significant pairwise differences between evaluative and hedonic ratings for guilt, excited, lust and pride. Participants rated guilt to be more ‘good’ (M = 0.18, s.d. = 0.19) than ‘pleasant’ (M = 0.11, s.d. = 0.12), t (24) = 2.27, P = 0.03. Conversely, participants rated excited to be more ‘pleasant’ (M = 0.89, s.d. = 0.11) than ‘good’ (M = 0.85, s.d. = 0.12), t (24) = 2.58, P = 0.02. Similarly, participants rated lust as being more ‘pleasant’ (M = 0.55, s.d. = 0.26) than ‘good’ (M = 0.42, s.d. = 0.27), t (24) = 3.29, P < 0.01. Finally, participants rated pride to be more ‘pleasant’ (M = 0.70, s.d. = 0.20) than ‘good’ (M = 0.62, s.d. = 0.25), t (24) = 2.32, P = 0.03. Notably, differences were evident mostly in emotions (guilt, lust and pride) that are commonly viewed as having divergent evaluative and hedonic valence in Western culture. Additionally, we examined the response times for participants’ ratings to examine whether task difficulty might account for any differences between evaluative vs hedonic ratings. Participants’ response times did not significantly differ between evaluative and hedonic ratings, F (1, 23) = 0.19, P = 0.67, η2 = 0.01. Thus, task difficulty is unlikely to account for our results.
In sum, the behavioral findings suggest that participants adopted an evaluative focus when making good/bad judgments and a hedonic focus when making pleasant/unpleasant judgments of emotion categories. To be sure, we did not expect mean differences in ratings to occur for each and every emotion category. Indeed, it stands to reason that even if evaluative and hedonic ratings are similar for certain emotion categories, participants may nonetheless be focusing on and retrieving different informational dimensions. For example, that happiness was rated similarly on both evaluative and hedonic rating dimensions does not mean that participants were processing happiness in the same way. Thus, these findings suggest more simply that participants followed task instructions: they adopted an evaluative focus when judging emotion concepts along the ‘good or bad’ dimension, and a hedonic focus when judging emotion concepts along the ‘pleasant or unpleasant’ dimension, across emotion concepts.
Neuroimaging results
Our goal was to examine the extent to which neural activity in frontal areas would be associated with accessing evaluative (vs hedonic) emotion knowledge. First, we compared neural activity during the task to baseline to see whether the task would engage salience areas typically associated with affect. Consistent with our expectations, we did not find that making ratings about emotion words engaged the amygdala, anterior cingulate cortex, the insula, the ventral striatum or other areas commonly associated with generating affective experiences. Rather, we found activation in a number of regions including the left inferior frontal gyrus, in an area associated with a variety of processes including language, alongside general task-positive engagement (Figure 1). We also found activation in a number of visual and motor areas that included primary visual cortex and extrastriate visual areas in the cuneus, as well as the pre-central and post-central gyri (Figure 1). This finding is potentially attributable to different sources including the increased visual (processing semantically meaningful words vs single digit numbers) and motor demands of the task compared to baseline (moving the trackball when making ratings in the experimental vs making a button press during the baseline task). Together, the results are consistent with the notion that the emotion judgment task drew more on areas involved in linguistic processing rather than areas associated with affective experience.
Fig. 1.
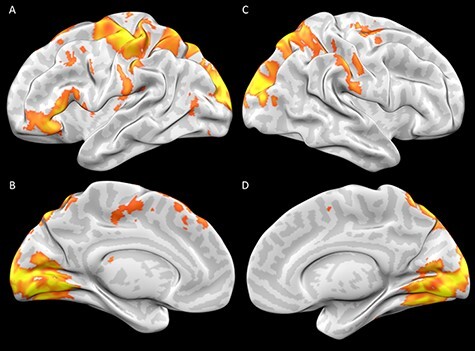
Activation during evaluative and hedonic judgments of emotion concepts relative to baseline. Figure 1A shows a lateral view and Figure 1B shows a medial view of activation in the left hemisphere associated both evaluative and hedonic judgments. Figure 1C shows a lateral view and Figure 1D shows a medial view of activation in the right hemisphere during both evaluative and hedonic judgments. All results are FWE corrected at P < 0.05 using a nominal voxel-wise threshold of P = 0.005 and cluster-extent threshold of k = 134.
To examine the brain areas associated with evaluative (vs hedonic) emotion knowledge, we contrasted conditions for evaluative vs hedonic rating blocks. When participants made evaluative (vs hedonic) judgments, we found a significant cluster of activation in the anteriormPFC, k = 347; peak voxel at [21, 66, 0]; t (24) = 3.28, P < 0.005 (Figure 2A) that included the vmPFC, k = 22; peak voxel at [6, 60, −15]; t (24) = 3.07, P < 0.005 (Figure 2B). During evaluative (vs hedonic) judgments, we also found greater activity in the midline precuneus, k = 108; peak voxel at [6, −57, 42]; t (24) = 3.23, P < 0.005 (Figure 2B). Access to evaluative emotion knowledge was associated with increased activity in these areas (Figure 2C). Conversely, when participants made hedonic (vs evaluative) judgments, we found increased activation in the left post-central gyrus, k = 107; peak voxel at [–39, −21, 60]; t (24) = 3.29, P < 0.005. Given that we did not find a significant difference in participants’ response times in the evaluative and hedonic blocks, task difficulty is unlikely to account for these findings. Overall, our results point to the mPFC and precuneus as being important in evaluative vs hedonic emotion knowledge in general.
Fig. 2.
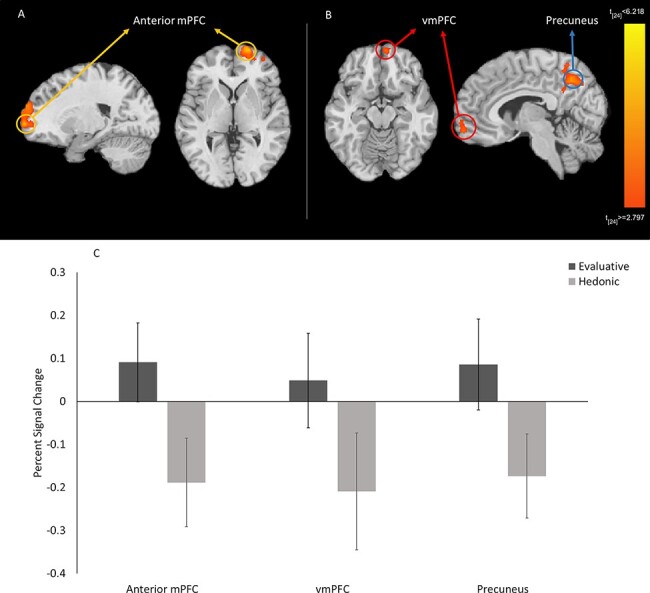
Anterior prefrontal and precuneus activation during evaluative > hedonic judgments. Figure 2A shows the cluster of activation in the anterior medial prefrontal cortex (mPFC) in the right hemisphere, while Figure 2B shows clusters of activation in the ventromedial prefrontal cortex and midline precuneus. Peak voxels are circled. All results are FWE corrected, P < 0.05, voxel-wise P = 0.005, k-threshold = 105. Figure 2C shows neural activity in the mPFC, the ventromedial PFC (vmPFC) and precuneus during evaluative vs hedonic judgments. Note that the cluster of activation in the vmPFC is not statistically independent from the anterior mPFC, as it was part of larger cluster of activation with the peak being in the anterior mPFC. Error bars reflect 95% confidence intervals. Effect sizes should be interpreted with caution.
Discussion
Emotion knowledge is multifaceted (Barrett, 2006; Tsai et al., 2006; Niedenthal, 2007; Tsai, 2007; Lindquist et al., 2015; Hoemann et al., 2020) and believed to play an important role in shaping affective experiences, whether through regulation (Izard et al., 2011; Torre and Lieberman, 2018) or emotion construction (Barrett, 2006, 2009; Lindquist, 2013). Indeed, multiple processes, including emotion knowledge, are thought to come online when experiencing discrete emotions (Lazarus, 1991; Tsai, 2007; Barrett, 2009; Tamir, 2009; Roseman, 2011; Lindquist, 2013). Emotion knowledge likely comes online alongside other processes involved in directing attention, driving autonomic changes, preparing for or initiating motor responses, etc. (Barrett et al., 2007; Satpute et al., 2013). Yet, there is a dearth of affective neuroscience studies that identify brain regions that are engaged when focusing on different dimensions of emotion knowledge vs other components involved in emotion experiences. For example, many studies investigate the brain basis of positive and negative valence by examining brain activity following various affective stimuli (e.g. videos, images, faces; Phan et al., 2002; Lindquist et al., 2016). However, this approach does not differentiate processes related to emotion knowledge vs affect generation and conflates evaluative (good and bad) and hedonic (pleasant and unpleasant) aspects of emotion into valence.
Our current functional magnetic resonance imaging (fMRI) study addresses this gap by identifying functional activation patterns associated with focusing on evaluative and hedonic emotion. Our results showed that focusing on evaluative (vs hedonic) aspects of emotion knowledge was associated with greater activity in the anterior mPFC, vmPFC and midline precuneus. Importantly, these areas were not engaged when participants completed the rating task in general. Instead, the task engaged areas consistent with the visual, motor, and linguistic demands of making ratings about words. Moreover, we did not find activity in areas often associated with affective processing such as the amygdala, anterior cingulate cortex, the insula and the ventral striatum. These findings suggest these areas may be more involved in generation of affect vs emotion knowledge (Satpute et al., 2013). Overall, our results suggest that the anterior mPFC and the precuneus may play a role in accessing evaluative emotion knowledge. This interpretation lines up with prior work showing that regions in the anterior mPFC are engaged during evaluation more generally (Zysset et al., 2002; Cunningham et al., 2004; Wood et al., 2005; Hampton et al., 2006; Schroeter et al., 2010).
Of course, one limitation of our study is that, although our instructions were intended to be as general as possible so as not to bias participants’ ratings, emotion knowledge is both rich and highly contextualized (Barrett, 2006; Tsai et al., 2006; Niedenthal, 2007; Tsai, 2007; Lindquist et al., 2015; Hoemann et al., 2020). Even within evaluative and hedonic emotion knowledge, there are likely different aspects that are embedded within specific contexts—just like other forms of concept knowledge (Barsalou, 2009; Yee and Thompson-Schill, 2016; Yee, 2019). For example, evaluative emotion knowledge about whether an emotion is good or bad will likely change depending on the context (e.g. happiness might be good during a celebration but bad during a funeral). The good-bad dimension might include additional subdivisions into appropriateness, usefulness or morality. Given our instructions, we cannot be certain whether participants made their emotion judgments with reference to specific situations or in a situation-general way. For these reasons, we suggest the more limited interpretation that the areas of the anterior medial prefrontal cortex and precuneus may be relevant when the focus is on evaluating emotion, but future work is needed to address exactly how the contents of different dimensions of emotion knowledge are represented.
While our study asked participants to provide evaluative ratings of emotion in general, we did not examine the source of their evaluations. Indeed, participants may have based their judgments on beliefs they thought that other people might hold (i.e. normative beliefs), beliefs that they personally hold or a combination of both. Moreover, it seems plausible that emotion evaluations will likely be driven by different sources for different people depending, for example, on the extent to which one’s personal beliefs are shared with the cultural milieu that underlies their perceptions of normative beliefs or are influenced from other sources (e.g. in cases when one might reject certain cultural or religious institutions in favor of others, and then re-evaluate emotions accordingly). Going forward, a richer behavioral examination of the sources of emotion evaluations and how they vary across individuals would be an important step toward developing a more thorough examination of how the brain represents emotion evaluations and emotion knowledge more generally.
Implications
Our findings point to several questions for future work examining how people develop emotion knowledge and evaluations about emotion categories. For example, when do children develop evaluative emotion knowledge? Children acquire knowledge about emotion categories (e.g. anger, fear, disgust, etc.) over the course of development (Bridges, 1932; Camras, 1992; Russell and Widen, 2002; Widen et al., 2015; Nook et al., 2017; Hoemann et al., 2019, 2020; Shablack and Lindquist, 2019). For instance, it is not until two to three years of age that children begin to reliably categorize faces based on prototypical emotion categories (Widen, 2016; Hoemann et al., 2019). Notably, two- to three-year old children’s ability to consistently categorize prototypical emotion faces above and beyond valence depends on the presence of emotion labels (Russell and Widen, 2002). In contrast, young infants at three to four months of age are only able to categorize emotional faces based on valence (Walker-Andrews, 1997). Even then, these infants are only able to reliably do so when there is accompanying information such as vocal expressions.
Relevant for questions about the acquisition of emotion evaluative knowledge, Atzil and Gendron (2017) have noted that the ability of children and adolescents to regulate their emotions coincides with the development of the default mode network. The default mode has been proposed to support emotion knowledge (Satpute and Lindquist, 2019). The ability to successfully regulate emotion increases linearly with age (McRae et al., 2010). This coincides with linear increases in the strength of functional connectivity between the mPFC and posterior cingulate cortex (PCC) over the course of adolescence (Fair et al., 2008). Intriguingly, the mPFC and PCC are earliest nodes in a nascent default mode network to show functional connectivity with other areas at early stages of development (Gao et al., 2009). By one year of age, these two areas are already highly connected to other areas. Given that our findings implicate the mPFC and the precuneus in evaluative emotion knowledge, researchers might begin to investigate how the ability to evaluate emotions changes with developmental changes in connectivity between the mPFC and the PCC/precuneus and other default mode areas.
Our results may also help clarify the role of prefrontal areas in emotion regulation. Many studies of emotion regulation have focused on activity in the mPFC—the vmPFC in particular—during emotion regulation (Harenski and Hamann, 2006; Urry et al., 2006; Koenigsberg et al., 2009; McRae et al., 2010, 2012; Dolcos et al., 2014). However, a meta-analysis of studies on the neural correlates of emotion regulation did not find that the vmPFC was consistently activated during reappraisal (Buhle et al., 2014). Instead, prefrontal areas associated with reappraisal included the lateral mPFC and the dorsomedial PFC, which have been implicated in cognitive control. Yet, an important gap in the literature on emotion regulation in general is that studies generally tell participants when and how to regulate their emotions. In turn, these studies rarely examine decisions during the emotion regulation process.
In contrast, in everyday life emotion regulation involves a series of decisions. For example, a person decides whether to regulate or experience an instance of emotion. If deciding to regulate, they might then choose between regulation strategies (e.g. distraction vs reappraisal; see Sheppes et al., 2011, 2014). Our results raise the possibility that one role of the vmPFC in emotion regulation is supporting the decision-making process. Specifically, the vmPFC might be involved in evaluation of emotions during decisions to regulate. While speculative, this would be consistent with the vmPFC’s established role in decision-making (Bechara et al., 1994, 1997; Fellows and Farah, 2003; Grabenhorst and Rolls, 2011) and in evaluation (Zysset et al., 2002; Cunningham et al., 2004; Hare et al., 2009; Nook and Zaki, 2015). Indeed, studies on reward learning and decision-making that have implicated anterior prefrontal areas with valuation (O’Doherty et al., 2001a, 2001b; Dom et al., 2005; Lin et al., 2012; Gross et al., 2014). Similarly, lesions to anterior prefrontal areas produce deficits in evaluation that result in disruptions in reward-learning (Hornak et al., 2004; Tsuchida et al., 2010; Vaidya and Fellows, 2016), decision-making (Bechara et al., 1997; Clark et al., 2008; Moretti et al., 2009; Koenigs et al., 2010; but see Sanfey et al., 2003) and judging the appropriateness of behaviors (Beer et al., 2003; Berlin et al., 2004; Leopold et al., 2012; Cameron et al., 2018).
Thus, future work might examine whether the vmPFC’s role in emotion regulation involves supporting decision-making during voluntary regulation. If so, then one might expect greater activity in the vmPFC when participants are faced with a decision to regulate or not. In contrast, if the vmPFC’s involvement is more tied to processes involved in regulation itself, vmPFC activity might be associated with a specific regulation strategy or regulation success. Emotion regulation likely involves a number of processes, such as evaluating one’s affective state and current situation, categorizing an instance of emotion, reappraisal, etc. Investigating and distinguishing between these processes and their neural bases during voluntary emotion regulation may offer novel insights into the mechanisms of emotion regulation.
Conclusion
People hold evaluations about specific emotions, just as they hold evaluations about external events or other internal mental states. And they distinguish evaluations about specific emotions from beliefs about the hedonic experience of those same emotions. We find evidence that prefrontal areas often implicated in valence (Anderson et al., 2003; Small et al., 2003; Anders et al., 2004; Dolcos et al., 2004; Grimm et al., 2006; Posner et al., 2009) may help support these evaluations. Anterior prefrontal areas were particularly sensitive to evaluative (vs hedonic) emotion knowledge in our study. These prefrontal areas and the precuneus show increased activation when participants evaluate the goodness or badness of emotions compared to when they judge the pleasantness or unpleasantness of these emotions.
Supplementary Material
Acknowledgements
We would like to thank Alessia Iancarelli, Kieran McVeigh, Stephanie Fielder and Yiyu Wang for their helpful feedback and comments during the writing process.
Contributor Information
Kent M Lee, Department of Psychology, Northeastern University, Boston, MA 02115, USA.
SuhJin Lee, Department of Neurobiology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Ajay B Satpute, Department of Psychology, Northeastern University, Boston, MA 02115, USA.
Funding
This research was supported by the Division of Behavioral and Cognitive Sciences of the National Science Foundation (1947972) and the National Cancer Institute (U01CA193632) and the National Institute of Mental Health (F32MH122062-01A1) of the National Institutes of Health.
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
Supplementary data
Supplementary data is available at SCAN online.
References
- Anders S., Lotze M., Erb M., Grodd W., Birbaumer N. (2004). Brain activity underlying emotional valence and arousal: a response‐related fMRI study. Human Brain Mapping, 23(4), 200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Stappen I., et al. (2003). Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience, 6(2), 196–202. [DOI] [PubMed] [Google Scholar]
- Aquinas T. (2006). Question 84: of the cause of sin, in respect to one sin being the cause of another. In: Perry, S.K., editor. Summa Theologica, Vol. 1, Fourth Article (I–II, Q. 84, Art. 4)), Urbana, Illinois: Project Gutenberg. Available: https://www.gutenberg.org/cache/epub/17897/pg17897.html [April 1, 2021]. [Google Scholar]
- Atzil S., Gendron M. (2017). Bio-behavioral synchrony promotes the development of conceptualized emotions. Current Opinion in Psychology, 17, 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F. (2006). Solving the emotion paradox: categorization and the experience of emotion. Personality and Social Psychology Review, 10, 20–46. [DOI] [PubMed] [Google Scholar]
- Barrett L.F., Mesquita B., Ochsner K.N., Gross J.J. (2007). The experience of emotion. Annual Review of Psychology, 58, 373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F. (2009). Variety is the spice of life: a psychological construction approach to understanding variability in emotion. Cognition and Emotion, 23, 1284–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou L.W. (2009). Simulation, situated conceptualization, and prediction. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 364(1521), 1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucom L.B., Wedell D.H., Wang J., Blitzer D.N., Shinkareva S.V. (2012). Decoding the neural representation of affective states. NeuroImage, 59(1), 718–27. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1-3), 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Tranel D., Damasio A.R. (1997). Deciding advantageously before knowing the advantageous strategy. Science, 275, 1293–5. [DOI] [PubMed] [Google Scholar]
- Beer J.S., Heerey E.A., Keltner D., Scabini D., Knight R.T. (2003). The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology, 85(4), 594. [DOI] [PubMed] [Google Scholar]
- Berlin H.A., Rolls E.T., Kischka U. (2004). Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain, 127(5), 1108–26. [DOI] [PubMed] [Google Scholar]
- Boiger M., Mesquita B., Uchida Y., Feldman Barrett L. (2013). Condoned or condemned: the situational affordance of anger and shame in the United States and Japan. Personality and Social Psychology Bulletin, 39(4), 540–53. [DOI] [PubMed] [Google Scholar]
- Bridges K.M.B. (1932). Emotional development in early infancy. Child Development, 3, 324–41. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K.A., Gardner J., Privratsky A.A., Chung M.-H., James G.A., Kilts C.D. (2018). Brain states that encode perceived emotion are reproducible but their classification accuracy is stimulus-dependent. Frontiers in Human Neuroscience, 12, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C.D., Reber J., Spring V.L., Tranel D. (2018). Damage to the ventromedial prefrontal cortex is associated with impairments in both spontaneous and deliberative moral judgments. Neuropsychologia, 111, 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camras L.A. (1992). Expressive development and basic emotions. Cognition & Emotion, 6(3–4), 269–83. [Google Scholar]
- Chikazoe J., Lee D.H., Kriegeskorte N., Anderson A.K. (2014). Population coding of affect across stimuli, modalities and individuals. Nature Neuroscience, 17(8), 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L., Bechara A., Damasio H., Aitken M.R.F., Sahakian B.J., Robbins T.W. (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain, 131(5), 1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Raye C.L., Johnson M.K. (2004). Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience, 16(10), 1717–29. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Zelazo P.D. (2007). Attitudes and evaluations: a social cognitive neuroscience perspective. Trends in Cognitive Sciences, 11(3), 97–104. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage, 23(1), 64–74. [DOI] [PubMed] [Google Scholar]
- Dolcos S., Katsumi Y., Dixon R.A. (2014). The role of arousal in the spontaneous regulation of emotions in healthy aging: a fMRI investigation. Frontiers in Psychology, 5, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G., Sabbe B., Hulstijn W., Van Den Brink W. (2005). Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. The British Journal of Psychiatry, 187(3), 209–20. [DOI] [PubMed] [Google Scholar]
- Dweck C.S. (2017). From needs to goals and representations: foundations for a unified theory of motivation, personality, and development. Psychological Review, 124(6), 689. [DOI] [PubMed] [Google Scholar]
- Eid M., Diener E. (2001). Norms for experiencing emotions in different cultures: Inter- and intranational differences.. Journal of Personality and Social Psychology, 81(5), 869–85. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., et al. (2008). The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 4028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. (2003). Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain, 126(8), 1830–7. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. (2007). The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se?. Cerebral Cortex, 17(11), 2669–74. [DOI] [PubMed] [Google Scholar]
- Ford B.Q., Gross J.J. (2019). Why beliefs about emotion matter: an emotion-regulation perspective. Current Directions in Psychological Science, 28(1), 74–81. [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., et al. (2009). Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences, 15(2), 56–67. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Sommerville R.B., Nystrom L.E., Darley J.M., Cohen J.D. (2001). An fMRI investigation of emotional engagement in moral judgment. Science, 293(5537), 2105–8. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Nystrom L.E., Engell A.D., Darley J.M., Cohen J.D. (2004). The neural bases of cognitive conflict and control in moral judgment. Neuron, 44(2), 389–400. [DOI] [PubMed] [Google Scholar]
- Grimm S., Schmidt C.F., Bermpohl F., et al. (2006). Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—an fMRI study. NeuroImage, 30(1), 325–40. [DOI] [PubMed] [Google Scholar]
- Gross J., Woelbert E., Zimmermann J., Okamoto-Barth S., Riedl A., Goebel R. (2014). Value signals in the prefrontal cortex predict individual preferences across reward categories. Journal of Neuroscience, 34(22), 7580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton A.N., Bossaerts P., O’doherty J.P. (2006). The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. Journal of Neuroscience, 26(32), 8360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–8. [DOI] [PubMed] [Google Scholar]
- Harenski C.L., Hamann S.B. (2006). Neural correlates of regulating negative emotions related to moral violations. NeuroImage, 30(1), 313–24. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Amodio D.M., Gable P.A. (2011). Attitudes toward emotions. Journal of Personality and Social Psychology, 101(6), 1332–50. [DOI] [PubMed] [Google Scholar]
- Hoemann K., Xu F., Barrett L.F. (2019). Emotion words, emotion concepts, and emotional development in children: a constructionist hypothesis. Developmental Psychology, 55(9), 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann K., Wu R., LoBue V., Oakes L.M., Xu F., Barrett L.F. (2020). Developing an understanding of emotion categories: lessons from objects. Trends in Cognitive Sciences, 24, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J., O’Doherty J.P., Bramham J., et al. (2004). Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience, 16(3), 463–78. [DOI] [PubMed] [Google Scholar]
- Izard C.E., Woodburn E.M., Finlon K.J., Krauthamer-Ewing E.S., Grossman S.R., Seidenfeld A. (2011). Emotion knowledge, emotion utilization, and emotion regulation. Emotion Review, 3(1), 44–52. [Google Scholar]
- Jacobsen T., Schubotz R.I., Höfel L., Cramon D.Y. (2006). Brain correlates of aesthetic judgment of beauty. NeuroImage, 29(1), 276–85. [DOI] [PubMed] [Google Scholar]
- Joshanloo M., Lepshokova Z.K., Panyusheva T., et al. (2014). Cross-cultural validation of fear of happiness scale across 14 national groups. Journal of Cross-Cultural Psychology, 45(2), 246–64. [Google Scholar]
- Kim J., Weber C.E., Gao C., Schulteis S., Wedell D.H., Shinkareva S.V. (2020). A study in affect: predicting valence from fMRI data. Neuropsychologia, 143, 107473. [DOI] [PubMed] [Google Scholar]
- Kirk U., Skov M., Hulme O., Christensen M.S., Zeki S. (2009). Modulation of aesthetic value by semantic context: an fMRI study. NeuroImage, 44(3), 1125–32. [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Kruepke M., Newman J.P. (2010). Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia, 48(7), 2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., et al. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry, 66(9), 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R.J. (2000). Toward a science of mood regulation. Psychological Inquiry, 11(3), 129–41. [Google Scholar]
- Lazarus R.S. (1991). Progress on a cognitive-motivational-relational theory of emotion. American Psychologist, 46(8), 819. [DOI] [PubMed] [Google Scholar]
- Lee S., McVeigh K., Garcia M., Carrillo V., Kim J., Satpute A.B. (under review). Disentangling Valence in Emotion Knowledge: The Good, the Pleasant, and the Desirable. [Google Scholar]
- Leopold A., Krueger F., Dal Monte O., et al. (2012). Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Social Cognitive and Affective Neuroscience, 7(8), 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Adolphs R., Rangel A. (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7(3), 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A. (2013). Emotions emerge from more basic psychological ingredients: a modern psychological constructionist model. Emotion Review, 5, 356–68. [Google Scholar]
- Lindquist K.A., MacCormack J.K., Shablack H. (2015). The role of language in emotion: predictions from psychological constructionism. Frontiers in Psychology, 6, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D.E., Gross J.J., Ochsner K.N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22(2), 248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., et al. (2012). The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Eslinger P.J., de Oliveira-souza R. (2001). Frontopolar and anterior temporal cortex activation in a moral judgment task: preliminary functional MRI results in normal subjects. Arquivos de Neuro-Psiquiatria, 59(3B), 657–64. [DOI] [PubMed] [Google Scholar]
- Moretti L., Dragone D., Di Pellegrino G. (2009). Reward and social valuation deficits following ventromedial prefrontal damage. Journal of Cognitive Neuroscience, 21(1), 128–40. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M. (2007). Embodying emotion. Science, 316(5827), 1002–5. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M. (2008). Emotion concepts. In: Lewis, M., Haviland-Jones, J.M., Barrett, L.F., editors. Handbook of Emotions, 3rd edn, pp. 587–600, New York, NY, US: Guilford Publications. [Google Scholar]
- Nook E.C., Sasse S.F., Lambert H.K., McLaughlin K.A., Somerville L.H. (2017). Increasing verbal knowledge mediates development of multidimensional emotion representations. Nature Human Behaviour, 1(12), 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nook E.C., Zaki J. (2015). Social norms shift behavioral and neural responses to foods. Journal of Cognitive Neuroscience, 27(7), 1412–26. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Kringelbach M.L., Rolls E.T., Hornak J., Andrews C. (2001a). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(1), 95–102. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Rolls E.T., Francis S., Bowtell R., McGlone F. (2001b). Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology, 85(3), 1315–21. [DOI] [PubMed] [Google Scholar]
- Parrott W.G. (1993). Beyond hedonism: motives for inhibiting good moods and for maintaining bad moods. In: Wegner, D.M., Pennebaker, J.W., editors. Handbook of Mental Control, 278–305, Englewood Cliffs, NJ, US: Prentice-Hall. [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331–48. [DOI] [PubMed] [Google Scholar]
- Posner J., Russell J.A., Gerber A., et al. (2009). The neurophysiological bases of emotion: an fMRI study of the affective circumplex using emotion-denoting words. Human Brain Mapping, 30(3), 883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.L., Hughes L.E., Anderson M.C., Rowe J.B. (2015). The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. Journal of Neuroscience, 35(2), 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman I.J. (2011). Emotional behaviors, emotivational goals, emotion strategies: multiple levels of organization integrate variable and consistent responses. Emotion Review, 3(4), 434–43. [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A. (2009). Emotion, core affect, and psychological construction. Cognition and Emotion, 23, 1259–83. [Google Scholar]
- Russell J.A., Widen S.C. (2002). A label superiority effect in children’s categorization of facial expressions. Social Development, 11(1), 30–52. [Google Scholar]
- Salovey P., Mayer J.D. (1990). Emotional intelligence. Imagination, Cognition and Personality, 9(3), 185–211. [Google Scholar]
- Sanfey A.G., Hastie R., Colvin M.K., Grafman J. (2003). Phineas gauged: decision-making and the human prefrontal cortex. Neuropsychologia, 41(9), 1218–29. [DOI] [PubMed] [Google Scholar]
- Satpute A.B., Shu J., Weber J., Roy M., Ochsner K.N. (2013). The functional neural architecture of self-reports of affective experience. Biological Psychiatry, 73, 631–8. [DOI] [PubMed] [Google Scholar]
- Satpute A.B., Lindquist K.A. (2019). The default mode network’s role in discrete emotion. Trends in Cognitive Sciences, 23(10), 851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L., Tusche A., Manoharan N., Hutcherson C., Hare T., Plassmann H. (2018). Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. Journal of Neuroscience, 38(25), 5799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Ettrich B., Menz M., Zysset S. (2010). Traumatic brain injury affects the frontomedian cortex—an event-related fMRI study on evaluative judgments. Neuropsychologia, 48(1), 185–93. [DOI] [PubMed] [Google Scholar]
- Shablack H., Lindquist K.A. (2019). The role of language in the development of emotion. In: LoBue, V., Perez-Edgar, K., Buss, K., editors. The Handbook of Emotional Development, 451–78, New York, NY, US: Springer. [Google Scholar]
- Sheppes G., Scheibe S., Suri G., Gross J.J. (2011). Emotion-regulation choice. Psychological Science, 22(11), 1391–6. [DOI] [PubMed] [Google Scholar]
- Sheppes G., Scheibe S., Suri G., Radu P., Blechert J., Gross J.J. (2014). Emotion regulation choice: a conceptual framework and supporting evidence. Journal of Experimental Psychology: General, 143(1), 163. [DOI] [PubMed] [Google Scholar]
- Shinkareva S.V., Gao C., Wedell D. (2020). Audiovisual representations of valence: a cross-study perspective. Affective Science, 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry A.E., Saxe R. (2014). A common neural code for perceived and inferred emotion. The Journal of Neuroscience, 34(48), 15997–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.M., Gregory M.D., Mak Y.E., Gitelman D., Mesulam M.M., Parrish T. (2003). Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron, 39(4), 701–11. [DOI] [PubMed] [Google Scholar]
- Stark C.E.L., Squire L.R. (2001). When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America, 98(22), 12760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstrom D.M., Curtis M. (2012). Pride, sloth/lust/gluttony, envy, greed/wrath: rating the seven deadly sins. Interdisciplinary Journal of Research on Religion, 8. [Google Scholar]
- Tamir M. (2009). What do people want to feel and why? Pleasure and utility in emotion regulation. Current Directions in Psychological Science, 18(2), 101–5. [Google Scholar]
- Tamir M., Ford B.Q. (2012). When feeling bad is expected to be good: emotion regulation and outcome expectancies in social conflicts. Emotion, 12(4), 807. [DOI] [PubMed] [Google Scholar]
- Torre J.B., Lieberman M.D. (2018). Putting feelings into words: affect labeling as implicit emotion regulation. Emotion Review, 10, 116–24. [Google Scholar]
- Tsai J.L., Knutson B., Fung H.H. (2006). Cultural variation in affect valuation. Journal of Personality and Social Psychology, 90(2), 288. [DOI] [PubMed] [Google Scholar]
- Tsai J.L. (2007). Ideal affect: cultural causes and behavioral consequences. Perspectives on Psychological Science, 2(3), 242–59. [DOI] [PubMed] [Google Scholar]
- Tsuchida A., Doll B.B., Fellows L.K. (2010). Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. Journal of Neuroscience, 30(50), 16868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., Van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26(16), 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A.R., Fellows L.K. (2016). Necessary contributions of human frontal lobe subregions to reward learning in a dynamic, multidimensional environment. Journal of Neuroscience, 36(38), 9843–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K., Hamann S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. Journal of Cognitive Neuroscience, 22(12), 2864–85. [DOI] [PubMed] [Google Scholar]
- Walker-Andrews A.S. (1997). Infants’ perception of expressive behaviors: differentiation of multimodal information. Psychological Bulletin, 121(3), 437. [DOI] [PubMed] [Google Scholar]
- Widen S.C., Pochedly J.T., Russell J.A. (2015). The development of emotion concepts: a story superiority effect in older children and adolescents. Journal of Experimental Child Psychology, 131, 186–92. [DOI] [PubMed] [Google Scholar]
- Widen S.C. (2016). The development of children’s concepts of emotion. Handbook of Emotions, 4, 307–18. [Google Scholar]
- Wood J.N., Romero S.G., Knutson K.M., Grafman J. (2005). Representation of attitudinal knowledge: role of prefrontal cortex, amygdala and parahippocampal gyrus. Neuropsychologia, 43(2), 249–59. [DOI] [PubMed] [Google Scholar]
- Wood J.V., Heimpel S.A., Manwell L.A., Whittington E.J. (2009). This mood is familiar and I don’t deserve to feel better anyway: mechanisms underlying self-esteem differences in motivation to repair sad moods. Journal of Personality and Social Psychology, 96(2), 363–80. [DOI] [PubMed] [Google Scholar]
- Yee E. (2019). Abstraction and Concepts: When, How, Where, What and Why?. Taylor & Francis. [Google Scholar]
- Yee E., Thompson-Schill S.L. (2016). Putting concepts into context. Psychonomic Bulletin & Review, 23(4), 1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Dang V., Mertz J., Rottenberg J. (2018). Are attitudes towards emotions associated with depression? A conceptual and meta-analytic review. Journal of Affective Disorders, 232, 329–40. [DOI] [PubMed] [Google Scholar]
- Zhang W., Lai S., He X., Zhao X., Lai S. (2016). Neural correlates for aesthetic appraisal of pictograph and its referent: an fMRI study. Behavioural Brain Research, 305, 229–38. [DOI] [PubMed] [Google Scholar]
- Zhang W., He X., Lai S., et al. (2017). Neural substrates of embodied natural beauty and social endowed beauty: an fMRI study. Scientific Reports, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S., Huber O., Ferstl E., von Cramon D.Y. (2002). The anterior frontomedian cortex and evaluative judgment: an fMRI study. NeuroImage, 15(4), 983–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


