Abstract
In the present study, we used an unsupervised classification algorithm to reveal both consistency and degeneracy in neural network connectivity during anger and anxiety. Degeneracy refers to the ability of different biological pathways to produce the same outcomes. Previous research is suggestive of degeneracy in emotion, but little research has explicitly examined whether degenerate functional connectivity patterns exist for emotion categories such as anger and anxiety. Twenty-four subjects underwent functional magnetic resonance imaging (fMRI) while listening to unpleasant music and self-generating experiences of anger and anxiety. A data-driven model building algorithm with unsupervised classification (subgrouping Group Iterative Multiple Model Estimation) identified patterns of connectivity among 11 intrinsic networks that were associated with anger vs anxiety. As predicted, degenerate functional connectivity patterns existed within these overarching consistent patterns. Degenerate patterns were not attributable to differences in emotional experience or other individual-level factors. These findings are consistent with the constructionist account that emotions emerge from flexible functional neuronal assemblies and that emotion categories such as anger and anxiety each describe populations of highly variable instances.
Keywords: intrinsic networks, emotion, degeneracy, constructionism
Affective neuroscience has long assumed that there are dedicated neural circuits associated with specific emotion categories (e.g. fear vs anger). Yet, growing research suggests that instances of an emotion category such as fear do not derive from a singular anatomical network in either the human (Touroutoglou et al., 2015; Wager et al., 2015; Huang et al., 2018) or non-human (Barrett and Finlay, 2018; LeDoux and Daw, 2018) brain. Instead, it is likely that multiple neural pathways can produce the same emotional experience or behavior (Barrett, 2017). When multiple mechanisms can achieve the same function, this is called ‘degeneracy’. The present report examines for the first time whether there is degeneracy in neural network patterns associated with emotions.
Degeneracy is a well-documented principle that makes complex systems adaptive and robust to insult; it is frequently observed in biological systems (Tononi et al., 1999; Sporns et al., 2000; Whitacre and Bender, 2010; see also Edelman and Gally, 2001 for multiple examples). For instance, there is degeneracy in the genetic code, where 64 codon triplets code for only 20 different amino acids (Shu, 2017). There is also degeneracy in the immune system, where many different antigens produce the same immune response by binding to the same T-cell (Edelman and Gally, 2001; Eisen, 2001). Finally, degeneracy has been documented in functional brain activity. In rodents, different assemblies of neurons in the medial prefrontal cortex support the same social behavior across time (Liang et al., 2018) and degenerate assemblies of brain regions produce the same defensive behavior across different contexts (Barrett and Finlay, 2018). In humans, two distinct neural pathways are associated with reading familiar words aloud (Seghier et al., 2008).
In emotion, degeneracy may occur because categories such as ‘anger’ or ‘anxiety’ name variable populations of instances that differ in their physiological, behavioral and cognitive features depending on the situation they occur in (Barrett, 2017). For instance, one instance of anxiety might involve increased heart rate and freezing when looking down a deep ravine, whereas another might involve increased heart rate and running quickly through a dark alley (see Satpute and Lindquist, 2019). Each of these instances would involve different sensations, behaviors and cognitions and hence different neural circuitry, despite involving similar subjective feelings of anxiety. This idea is consistent with the theory of constructed emotion, which proposes that emotions are variable populations of instances that arise from functional connectivity between intrinsic networks that serve domain-general psychological functions (Barrett and Satpute, 2013; Touroutoglou et al., 2015; Wager et al., 2015; Clark-Polner et al., 2017).
To our knowledge, little work has explicitly examined degenerate neural network patterns associated with emotion categories in humans. However, growing evidence is suggestive of the hypothesis that degenerate neural network patterns are associated with the same emotion category. First, neuroimaging evidence suggests that the neural activation associated with an emotion category such as anger varies significantly by context (Wilson-Mendenhall et al., 2011) and across individuals (Azari et al., 2020; Koide-Majima et al., 2020). Second, lesion evidence in twins is consistent with the notion that the amygdala, long thought to be part of an evolved fear network (Panksepp, 1998; Adolphs, 2017), supports fearful behaviors for some individuals, but not others. Monozygotic twins share genetics and patterns of neural functional connectivity (Adhikari et al., 2018). Yet, following bilateral amygdala lesions due to Urbach–Wiethe disease, one monozygotic twin shows impaired fear perception and startle responses (Adolphs et al., 1994, 1995; Siebert et al., 2003; Bach et al., 2015), and the other does not (Becker et al., 2012). The maintenance of fearful behaviors in one twin, but not in the other, suggests that degenerate neural pathways may exist for instances of the emotion category fear. Collectively, these findings lead to the hypothesis that multiple functional neural network patterns can result in instances of the same emotion category in healthy humans.
Consistency and degeneracy in functional connectivity patterns
Affective neuroscience increasingly relies on a network-based understanding of emotion (Lindquist and Barrett, 2012; Engen et al., 2017; Pessoa, 2017). To date, this work has almost exclusively sought a single average functional connectivity pattern for each emotion category. It is often at least implicitly assumed that this average pattern consistently represents all instances of that emotion category (e.g. Lee and Hsieh, 2014; Saarimäki et al., 2022). Individual differences, if they exist, may influence the strength of connections within an average pattern, but it is not generally assumed that there are degenerate patterns for the same emotion category.
Our goal in the present study was to first reveal consistent patterns in functional connectivity patterns associated with anger and anxiety. Within these patterns, we also sought evidence for degeneracy, if it exists. We used the well-validated continuous music technique (CMT; Eich and Metcalfe, 1989; Eich et al., 1994) to evoke anger and anxiety in the MRI scanner, following evidence that music-induced emotions are associated with broad-scale changes in neural networks (Wilkins et al., 2014; Liu et al., in press). Across blocks, participants listened to unpleasant and highly activating classical music while using imagery to self-generate experiences of anger and anxiety. After each induction, participants rated the intensity of anger, anxiety, arousal and unpleasantness they felt using a visual analogue scale (VAS).
We focused specifically on functional connectivity among11 functional intrinsic networks during anger and anxiety. These networks are present at rest but also describe functional connectivity patterns during a range of cognitive tasks (Smith et al., 2009; Shirer et al., 2012), including emotions (Smith et al., 2009; Lindquist and Barrett, 2012; Engen et al., 2017; Saviola et al., 2020; Sorella et al., 2022). Indeed, prior studies (Saarimäki et al., 2022) and meta-analytic work have observed that connectivity patterns among regions within some of these networks can differentiate emotions such as anger and fear (Wager et al., 2015). Network-based approaches to emotion also predict that connectivity between networks may underlie emotion (Lindquist and Barrett, 2012; Barrett and Satpute, 2013; Pessoa, 2017).
We used regions of interest (ROIs) from Shirer et al.’s (2012) parcellation of functional connectivity networks to quantify 11 a priori networks. See Table 1 for a priori networks, the corresponding names in the Shirer et al. parcellation and examples of associated psychological functions. We specifically focused on the canonical default mode network (Raichle et al., 2001; Greicius et al., 2003; Raichle, 2015), salience network (Seeley et al., 2007), frontoparietal control network (Dosenbach et al., 2007; Fair et al., 2007) and dorsal attention network (DAN) (Corbetta and Shulman, 2002; Fox et al., 2006; Vossel et al., 2014), given their links to cognition and emotion in the literature (e.g. Smith et al., 2009; Wager et al., 2015; see Barrett and Satpute, 2013; Lindquist and Barrett, 2012). We additionally included three networks from Shirer et al. (2012) based on their involvement in emotional experiences. First, we included the sensorimotor network because emotions are often characterized by motor action or motor action planning (Hajcak et al., 2007). Next, we involved the basal ganglia network because emotions involve the computation of motivational salience and enactment of motivated behavior (Arsalidou et al., 2013). Finally, we included the language network because regions associated with semantic knowledge and retrieval are frequently activated during experiences of emotion (Brooks et al., 2017). We were limited in how many networks we could select due to Group Iterative Multiple Model Estimation (GIMME)’s processing limitations; we thus did not include networks associated with primary sensory processing such as the auditory, primary visual and higher visual networks, although it would be interesting to examine these in future research.
Table 1.
Networks of interest and examples of their associated functions
| A priori network | Associated Shirer et al. (2012) network(s) | Associated functions |
|---|---|---|
| DMN | vDMN; dDMN; PCUN | Self-referential processing (Raichle et al., 2001); abstract, heteromodal, representations of emotion concept knowledge (Satpute and Lindquist, 2019) |
| FPC | Left executive control network (lECN)a; right executive control network (rECN)a | Cognitive control (Dosenbach et al., 2007) |
| SAL | aSAL; pSAL | Visceromotor control and representation of the viscera (Craig, 2002; Kleckner et al., 2017) |
| DAN | Visuospatial | Voluntary direction of visual attention (Corbetta and Shulman, 2002; Fox et al., 2006; Vossel et al., 2014) |
| BG | Basal Ganglia | Motivational salience (Arsalidou et al., 2013) |
| SMN | Sensorimotor | Motor engagement and planning (Hajcak et al., 2007) |
| Language (Lang) | Language | Access to semantic emotion concepts (Brooks et al., 2017) |
Note that we refer to lECN and rECN as lFPC and rFPC from here out.
We reduced the ROIs to a single network variable using principal component analysis (PCA) and submitted those variables to a directed functional connectivity approach called the GIMME (Gates and Molenaar, 2012). GIMME outperforms 38 alternative functional connectivity approaches (Gates and Molenaar, 2012), recovers true connections with very few false positives (Nestler and Humberg, 2021) and is considered one of the best-performing directed connectivity methods (Mumford and Ramsey, 2014). To test hypotheses about consistency and degeneracy, we used the data-driven model selection component of GIMME [subgrouping GIMME (s-GIMME); Gates et al., 2017a]. This unsupervised clustering approach allowed us to identify subgroups of data with distinct functional connectivity patterns.
We first used s-GIMME on all anger and anxiety inductions to examine whether it would reveal between-network connectivity patterns corresponding to each emotion category. We did not have a priori predictions about directed functional connectivity between networks since no studies to our knowledge have examined this question. Nonetheless, we predicted that we might replicate the involvement of specific networks in specific emotion inductions based on prior literature. For instance, meta-analytic evidence finds greater functional co-activation among regions within the DAN and default mode network during anger vs fear (Wager et al., 2015). In contrast, the meta-analytic evidence shows greater functional co-activation within the basal ganglia network and sensorimotor network during fear vs anger (Wager et al., 2015). Still, other studies find that state anxiety during a resting state scan correlates with connectivity within the salience and default mode networks (Saviola et al., 2020). Another study found that social anxiety disorder is associated with connectivity between nuclei of the basal ganglia and regions of the frontoparietal control and salience networks (Anteraper et al., 2014).
Next, we used s-GIMME to examine whether there were degenerate patterns of functional connectivity within each emotion category induction. Unsupervised approaches are uniquely poised to identify degeneracy, if it exists, because they rely on data-driven clustering and do not rely on researcher-defined categories (Azari et al., 2020). Our major prediction was that our unsupervised approach would reveal multiple degenerate patterns for each anger and anxiety. However, we did not have a priori hypotheses about specific functional connectivity patterns that would constitute degenerate pathways for each emotion category because no prior work has examined this question.
Method
Participants
Twenty-four (13 female) community members were recruited to take part in a neuroimaging experiment on ‘music and the brain’. We used this cover story on the basis of prior research showing that participants who had familiarity with, or who especially liked music selections, showed functional connectivity changes in networks associated with emotion while listening to music (Wilkins et al., 2014). Participants were right-handed, healthy and had no history of psychiatric illness. The mean age was 22.92 (s.d. = 4.95), and participants had on average 4.04 (s.d. = 6.01) years of formal or self-taught music training.
Procedure
Participants completed six 5 min runs in a single fMRI session (see Supplementary Material for scanning protocol and imaging parameters). Relevant to this report, participants completed three runs in which they underwent emotion inductions using the CMT. See Supplementary Material for CMT instructions and music selections. The CMT induces reliable and valid changes in participants’ emotional experiences by asking them to listen to instrumental music selections normed to induce a desired affective state (e.g. neutral, low arousal; unpleasant, high arousal). Throughout, participants are asked to use the music to self-generate instances of a specific mood or emotion. Participants first engaged in a neutral music induction. Next, participants completed either the anger induction or the anxiety induction; the induction order and music selection were both counterbalanced across participants. After each induction, participants rated the extent to which they felt anger, anxiety, unpleasantness and arousal on a scale from ‘not at all’ (1) to ‘very’ (10) using a VAS.
fMRI analysis
Preprocessing
Data were preprocessed using the CONN functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), which implements preprocessing steps from Statistical Parametric Mapping version 12. Functional data were realigned and unwarped, slice-timing corrected, examined for excessive motion using the Artifact Detection Tools toolbox (https://www.nitrc.org/projects/artifact_detect/), co-registered to structural images, normalized to 2 mm isotropic voxels in Montreal Neurological Institute space and spatially smoothed using an 8 mm full width at half maximum Gaussian kernel. We used spatial smoothing following other recent network approaches in the literature (Hwang et al., 2019; Lima Portugal et al., 2020; Finn and Bandettini, 2021), including those that have employed s-GIMME (Gates et al., 2017a; McCormick et al., 2019b). Rather than using global signal regression, which may potentially induce spurious negative correlations among intrinsic networks (Murphy et al., 2009), functional data were denoised using the CompCor toolbox (Behzadi et al., 2007). CompCor is a component-based correction method which regresses signals from five principal components of white matter and cerebrospinal fluid, rather than the average signal from all voxels in the brain.
Time-series extraction
We next used the CONN toolbox to extract time series from ROIs within 11 intrinsic functional networks (Table 1). This produced a data file containing 150 denoised time points per condition for each participant for each ROI in the Shirer et al., (2012) parcellation.
Network analyses
Network estimation
We used the PCA function from the ‘FactoMineR’ R package (Lê et al., 2008) to reduce ROI time series across participants into sets of uncorrelated principal components for each network, per each condition. We used the first principal component to represent functional connectivity within each network of interest. Data reduction techniques such as PCA (as well as independent component analysis and partial least squares analysis, e.g. Addis et al., 2004; Smith et al., 2009) are frequently used to produce a single estimate of a network among correlated ROIs. Doing so was necessary as the block-Toeplitz design employed by GIMME increases computation time non-linearly with the introduction of each additional variable. Choice of data reduction does not appear to bias GIMME results: GIMME performs similarly well in true path recovery, regardless of whether PCA, scaling indicators, sum scores, pseudo-Maximum Likelihood estimation or model-implied instrumental variables with two-stage least squares are used to reduce data (Gates et al., 2020).
Data reduction was advantageous because it allowed us to test our hypotheses about between-network connectivity rather than connectivity between ROIs within a single network (e.g. Gates et al., 2014; McCormick and Telzer, 2018) or single ROIs spanning networks (e.g. Yang et al., 2015; Zelle et al., 2017; McCormick et al., 2019a). PCA also ensured that only those ROIs common variance during our emotion tasks contributed to network estimates. This issue is particularly relevant since the Shirer et al. (2012) parcellation chose ROIs based on their functional connectivity during rest, autobiographical recall, mental math and recalling song lyrics (Shirer et al. 2012) but did not include any explicitly emotional tasks. We did not assess functional connectivity patterns among subsequent principal components in our analyses because we were not interested in examining connectivity patterns among what could be considered ‘subnetworks’ of our a priori networks. See Table 2 for the percentage of variance explained by each first principal component and Supplementary Tables S1–11 for individual ROI loadings. Visualizations of the first and second component loadings are available on our OSF site: https://osf.io/z3f7t/.
Table 2.
Variance explained in descending order by the first principal component for a priori networks in the anger and anxiety inductions
| Network | Number of ROIs | Variance explained |
|---|---|---|
| Anger induction | ||
| rFPC | 6 | 53.13 |
| PCUN | 4 | 52.71 |
| aSAL | 7 | 46.55 |
| lFPC | 6 | 44.54 |
| vDMN | 10 | 40.35 |
| Lang | 7 | 38.15 |
| SM | 6 | 36.49 |
| BG | 5 | 35.94 |
| dDMN | 9 | 35.50 |
| pSAL | 12 | 26.20 |
| DAN | 11 | 25.12 |
| Mean | 7.5 | 39.51 |
| Anxiety induction | ||
| rFPC | 6 | 49.20 |
| PCUN | 4 | 48.96 |
| aSAL | 7 | 47.90 |
| vDMN | 10 | 42.58 |
| lFPC | 6 | 40.51 |
| Lang | 7 | 39.12 |
| dDMN | 9 | 37.24 |
| BG | 5 | 36.98 |
| SM | 6 | 36.22 |
| DAN | 11 | 27.09 |
| pSAL | 12 | 25.53 |
| Mean | 7.5 | 39.21 |
Directed functional connectivity
We used the s-GIMME algorithm within the ‘GIMME’ R package (Lane et al., 2021) to estimate directed functional connectivity among the 11 networks of interest. See Figure 1 for a schematic of the GIMME and s-GIMME algorithms.
Fig. 1.
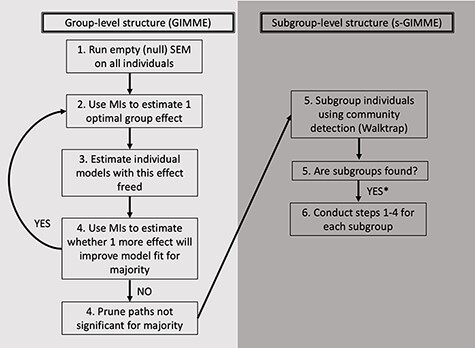
Schematic of GIMME and s-GIMME algorithms. Adapted from Gates et al. (2017a). The s-GIMME algorithm builds on the GIMME algorithm, which uses individual-level networks to iteratively identify optimal group-level paths. MIs refer to modification indices. S-GIMME then uses community detection to identify subgroups of individuals with similar paths. *Note that s-GIMME also identifies individual-level paths, which we do not represent here.
The GIMME algorithm (Gates and Molenaar, 2012) arrives at robust individual-specific and group-level models of directed brain connectivity using unified structural equation models (uSEMs; Kim et al., 2007) that represent connectivity paths between variables that are both contemporaneous (represented as solid arrows in Figures 3–5) and lagged (lag-1; represented as dashed arrows in Figures 3–5; Beltz and Gates, 2017). We grant equal interpretative weight to both contemporaneous and lagged paths in the present report since we do not have a priori hypotheses about lagged relationships. Moreover, the temporal resolution of fMRI (seconds) is slower than the biological process it aims to capture (milliseconds), so it is possible that some relationships estimated to be contemporaneous are truly lagged (Lane et al., 2019).
Fig. 3.
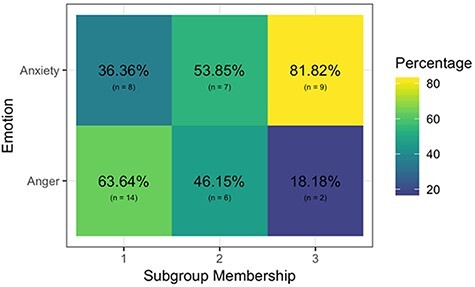
Composition of the unsupervised subgroup solution for all scans from the anger and anxiety conditions. S-GIMME revealed consistent brain patterns for anger (Subgroup 1) and anxiety (Subgroup 3), as well as a mixed subgroup (Subgroup 2). Percentages reflect the number of scans per emotion condition in each subgroup out of the total scans in that subgroup.
Building from GIMME results, s-GIMME (Lane et al., 2021) uses the unsupervised Walktrap community detection algorithm (Pons and Latapy, 2005) to identify N subgroups of individuals who share paths (represented as colored arrows in Figures 3–5; see Gates et al., 2017a for the validation of s-GIMME). S-GIMME also identifies paths that are unique for individuals, but we do not visualize these herein for interpretive ease. Subgroups are defined by (i) having similar connectivity paths within block-Toeplitz (lag-1 and lag-0) correlation matrices, as determined via the GIMME uSEM algorithm and (ii) having paths with the same sign. We operationalized an interpretable subgroup as one consisting of  4 individuals, since smaller subgroups might reflect noise or ungeneralizable idiosyncrasies.
4 individuals, since smaller subgroups might reflect noise or ungeneralizable idiosyncrasies.
Consistency analyses
We first performed s-GIMME analyses on all runs across the anger and anxiety inductions, with the goal of revealing data-driven evidence for consistency in neural network connectivity patterns for the categories of anger and anxiety, respectively. We used Student’s t-tests to examine whether participants’ self-reported VAS ratings of anger and anxiety characterized these subgroups. Note that because each participant contributed two scans (anger and anxiety) to the combined analysis, comparisons between subgroups are not fully independent. However, separate VAS scores were collected following both emotion inductions, and we examined whether VAS scores within each group differed.
Degeneracy analyses
We next performed s-GIMME analyses on each anger and anxiety induction, with the goal of revealing data-driven evidence for degeneracy in neural network connectivity patterns for anger and anxiety, if it exists.
We compared all subgroups based on participants’ VAS ratings of experience (anger, anxiety, unpleasantness arousal). We used non-parametric Mann–Whitney U-tests (Mann and Whitney, 1947) due to the small sample sizes of subgroups in this analysis. Effect sizes for the U-test are reported using r, the rank biserial correlation (Cureton, 1956). We used the Bonferroni correction to correct for multiple comparisons and report effect sizes and bootstrapped 95% confidence intervals (CIs) where applicable.
Ruling out alternative explanations
There could be alternate explanations for our unsupervised groups. One possibility is that the unsupervised groups we revealed in both the consistency and degeneracy analyses merely reflected individual connectomes—stable patterns in functional connectivity that are unique to individuals (Horien et al., 2019). To rule this explanation out, we first computed the number of unique participants that contributed to each subgroup in the consistency analysis. Next, we computed whether the same individuals tended to group together in the degeneracy analyses. We visualized the latter information using an alluvial plot (produced by the ggalluvial R package; Brunson and Read, 2020). Third, we performed an internal validation on the neutral condition to examine whether the same individuals tended to group together in neutral and emotion conditions. Fourth, it was possible that factors such as age, self-identified sex, music training or experimental factors such as counterbalance order impacted subgroup formation. We report the latter two analyses in the Supplementary Material.
Results
Consistency analyses
We assessed whether the unsupervised s-GIMME procedure revealed network connectivity patterns that differentiated the anger induction runs from the anxiety induction runs. S-GIMME identified three major subgroups (Figure 2). We do not report on two additional subgroups that contained one anger run each since they did not meet our a priori criterion for subgroup size. As predicted, one subgroup contained predominantly anger inductions (Subgroup 1; n = 22; 63.64% anger inductions). The other contained predominantly anxiety inductions (Subgroup 3; n = 11; 81.82% anxiety inductions). A third subgroup consisted of both anger and anxiety inductions (Subgroup 2, n = 13; 46.15% anger inductions and 53.85% anxiety inductions; see Figure 3).
Fig. 2.
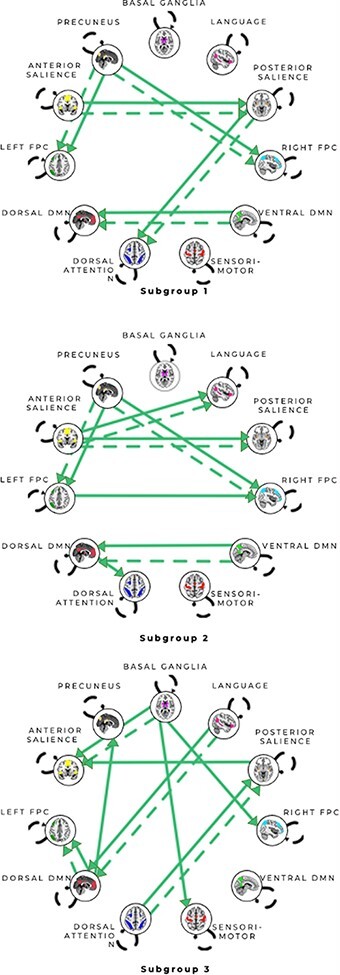
Connectivity patterns for unsupervised subgroup solution for all scans from the anger and anxiety conditions. All arrows represent subgroup-level paths there are no shared paths across these subgroups. Solid arrows represent contemporaneous relationships, and dashed arrows represent lagged (X at time minus 1 (T-1) predicts Y at time (T)) relationships. Autoregressive paths (X at T-1 predicts X at T) appear as dashed loops. Subgroup 1 consisted predominantly of anger runs (63.6% anger runs); Subgroup 2 was a mixed group (46.2% anger runs vs 53.9% anxiety runs) and Subgroup 3 consisted predominantly of anxiety runs (81.8% anxiety runs). See Figures S5–7 for matrices reflecting the counts of paths visualized here.
To assess whether s-GIMME was subgrouping individuals based on their felt emotions, we next compared self-reported emotion ratings of anger and anxiety within each subgroup. In Subgroup 1, anger inductions were associated with more intense reports of anger (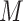 = 4.90) than anxiety inductions (
= 4.90) than anxiety inductions (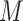 = 1.79), t(19.64) = 4.50, P < 0.001, d = 1.77. In Subgroup 2, anxiety inductions were associated with more intense reports of anxiety (
= 1.79), t(19.64) = 4.50, P < 0.001, d = 1.77. In Subgroup 2, anxiety inductions were associated with more intense reports of anxiety (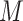 = 4.44) than anger inductions (
= 4.44) than anger inductions (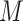 = 2.30), t(5.80) = −3.15, P = 0.02, d = 1.36. Anger and anxiety inductions in Subgroup 2 did not differ in the intensity of either anger or anxiety (P > 0.1).
= 2.30), t(5.80) = −3.15, P = 0.02, d = 1.36. Anger and anxiety inductions in Subgroup 2 did not differ in the intensity of either anger or anxiety (P > 0.1).
Degeneracy analyses
Anger
We assessed whether the unsupervised s-GIMME procedure revealed degenerate patterns within the anger induction. s-GIMME revealed two major subgroups of individuals (N = 10, N = 12; see Figure 4). A third subgroup consisted of two participants and did not meet our a priori criterion for subgroup size. Consistent with the degeneracy hypothesis, participants did not differ in the self-reported intensity of anger, anxiety or arousal (Table 3). Subgroup 1 reported instances of anger that were significantly more unpleasant (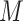 = 4.91) than Subgroup 2 (
= 4.91) than Subgroup 2 (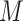 = 2.81), but these findings did not survive the Bonferroni correction (Table 3).
= 2.81), but these findings did not survive the Bonferroni correction (Table 3).
Fig. 4.
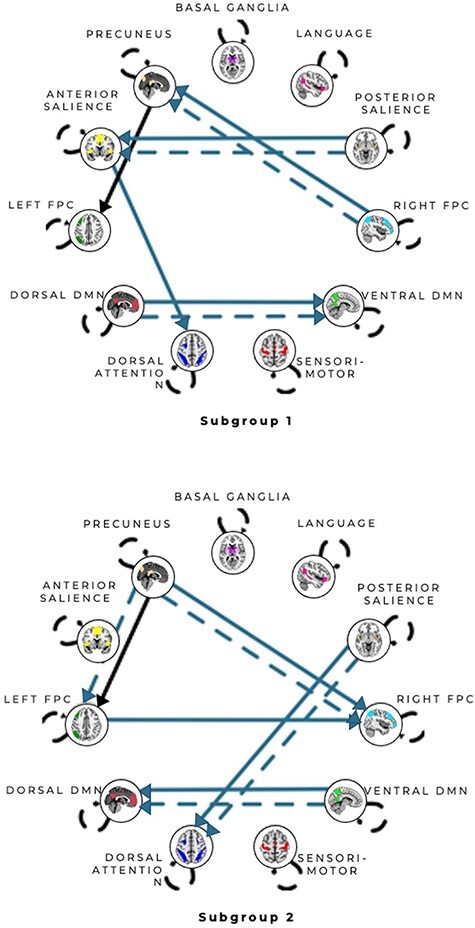
Connectivity patterns for unsupervised subgroup solution for the anger condition. All arrows represent subgroup-level paths with the exception of the solid arrow from the precuneus network to left FPC network path which was shared by both subgroups. Solid arrows represent contemporaneous relationships and dashed arrows represent lagged (X at T-1 predicts Y at T) relationships. Autoregressive paths (X at T-1 predicts X at T) appear as dashed loops. See Supplementary Figures S8–9 for matrices reflecting the counts of paths visualized here.
Table 3.
Comparisons of Anger Subgroups 1 and 2 using Mann–Whitney U-tests
| Measure | Scale/subscale | Results | Effect size |
|---|---|---|---|
| VAS | Anger | U = 81.5, P = 0.166 | r = 0.30, 95% CI [0.02, 0.66] |
| Anxiety | U = 74.5, P = 0.356 | r = 0.20, 95% CI [0.01, 0.60] | |
| Arousal | U = 48.5, P = 0.468 | r = 0.16, 95% CI [0.01, 0.57] | |
| Unpleasantness | U = 96.5, P = 0.018a | r = 0.51, 95% CI [0.18, 0.77] |
The P-value does not survive a Bonferroni correction of α = 0.0125.
Anxiety
We assessed whether the unsupervised s-GIMME procedure revealed degenerate patterns within the anxiety induction. s-GIMME revealed two major subgroups of individuals (N = 12, N = 12; see Figure 5). Subgroup 2 showed marginally greater self-reported anxiety (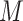 = 5.66) than Subgroup 1 (
= 5.66) than Subgroup 1 (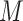 = 3.99), U = 42.5, P = 0.094, r = 0.34, 95% CI [0.02, 0.69], but sensitivity analysis suggested that this effect was driven by an outlier. With that outlier removed, there were no significant differences in anxiety between groups’ self-reported anxiety, anger, arousal or unpleasantness (Table 4).
= 3.99), U = 42.5, P = 0.094, r = 0.34, 95% CI [0.02, 0.69], but sensitivity analysis suggested that this effect was driven by an outlier. With that outlier removed, there were no significant differences in anxiety between groups’ self-reported anxiety, anger, arousal or unpleasantness (Table 4).
Fig. 5.
Connectivity patterns for unsupervised subgroup solution for the anxiety condition. All arrows represent subgroup-level paths with the exception of a Language network to the dDMN path, which was shared by both subgroups. Solid arrows represent contemporaneous relationships, and dashed arrows represent lagged (X at T-1 predicts Y at T) relationships. Autoregressive paths (X at T-1 predicts X at T) appear as dashed loops. See Supplementary Figures S10–11 for matrices reflecting the counts of paths visualized here.
Table 4.
Comparisons of Anxiety Subgroups 1 and 2 using Mann–Whitney U-tests
| Measure | Scale/subscale | Results | Effect size |
|---|---|---|---|
| VAS | Anger | U = 74.5, P = 0.908 | r = 0.02, 95% CI [0.00, 0.48] |
| Anxiety | U = 42.5, P = 0.157 | r = 0.30, 95% CI [0.02, 0.66] | |
| Arousal | U = 61.5, P = 0.564 | r = 0.12, 95% CI [0.01, 0.55] | |
| Unpleasantness | U = 62.5, P = 0.603 | r = 0.11, 95% CI [0.01, 0.51] |
Ruling out alternative explanations
We first assessed whether unsupervised patterns in the consistency analyses were attributable to the stable connectivity patterns of individuals. The first subgroup in the consistency analysis consisted predominantly of anger inductions; of the 22 total runs included, there were 18 unique participants. Both anger and anxiety runs were included for 5 participants (27.8%), but 13 participants (72%) contributed only an anger run. In contrast, the third subgroup in the consistency analysis consisted predominantly of anxiety inductions; of the 11 total runs included, there were 10 unique participants. Both anxiety and anger runs were included for three participants (30%), but seven participants (70%) contributed only an anxiety run. Finally, the second subgroup consisted of a mix of both anger and anxiety runs. Of the 13 runs included, there were 9 unique participants. Four participants (44%) had both anger and anxiety runs included, and five participants (56%) contributed only an anxiety run.
We next assessed whether unsupervised patterns in the degeneracy analyses were attributable to the stable connectivity patterns of individuals. See Figure 6 for a visualization of participants’ contributions to different subgroups within anger and anxiety inductions. Fifteen of the 24 participants (63%) appeared in distinct subgroups across inductions. Specifically, six of the participants in Anger Subgroup 1 were in Anxiety Subgroup 1, whereas the other four were in Anxiety Subgroup 2. Similarly, six of the participants in Anger Subgroup 2 were in Anxiety Subgroup 1, and the other six were in Anxiety Subgroup 2. A within-study validation on the neutral condition furthermore suggests that s-GIMME is not sorting based on individual connectivity patterns (see Supplementary Material). Degenerate subgroups furthermore did not differ (P > 0.1) based on age, self-identified sex, time spent in formal music training or counterbalanced order of the experiment (see Supplementary Materials).
Fig. 6.
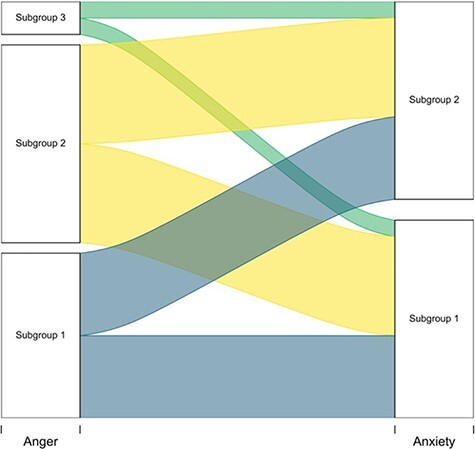
Participant assignment to degenerate patterns for anger and anxiety. The height of the ribbons represents the magnitude of participants flowing from one subgroup to the next.
Discussion
We used a directed functional connectivity approach alongside a data-driven subgrouping procedure to reveal between-network connectivity during anger and anxiety inductions. The GIMME approach is among the best directed functional connectivity approaches (Mumford and Ramsey, 2014), and its data-driven subgrouping procedure (s-GIMME) was particularly advantageous for addressing our hypotheses that both consistent and degenerate pathways exist for anger and anxiety.
Consistency in between-network patterns for emotions
To assess whether s-GIMME revealed consistent patterns associated with anger and anxiety, we first performed unsupervised analysis on all runs from the anger and anxiety inductions. The unsupervised approach suggested that there are functional connectivity patterns that consistently describe instances of anger vs anxiety. Subgroup 1 consisted of 63.64% anger inductions, and participants in those inductions reported more intense anger experiences than participants in anxiety inductions within that subgroup. Subgroup 1 was characterized by connectivity between subnetworks of salience (SAL) [from anterior SAL (aSAL) to posterior SAL (pSAL)] and subnetworks of the default mode (DMN) [from ventral DMN (vDMN) to dorsal DMN (dDMN)] as well as connectivity from pSAL to dorsal attention (DAN) and from precuneus (PCUN) to both left and right frontoparietal control (FPC). These findings are consistent with meta-analytic evidence that anger experiences routinely produce increased activation in regions of lateral prefrontal cortex contributing to the FPC and insula contributing to the aSAL (Lindquist et al., 2012; Sorella et al., 2021). These findings are also consistent with meta-analytic connectivity approaches showing that the DAN and DMN show greater within-network connectivity in anger inductions than fear inductions (Wager et al., 2015).
Subgroup 3 consisted of 81.82% anxiety inductions, and participants in those inductions reported more intense anxiety experiences than participants in anger scans within that subgroup. Subgroup 3 was characterized by connectivity from dDMN to PCUN, from Lang to dDMN and from basal ganglia (BG) to several other networks, including sensorimotor (SMN), aSAL and right FPC. These findings are consistent with meta-analytic evidence that both BG and SMN show greater within network connectivity in fear vs anger inductions (Wager et al., 2015). They are also consistent with evidence that social anxiety disorder is associated with increased connectivity between nuclei of the basal ganglia and regions within aSAL and FPC (Anteraper et al., 2014). Subgroup 3’s greater connectivity between dDMN and PCUN is consistent with evidence that state anxiety correlates with greater functional integration of precuneus with other DMN nodes at rest (Saviola et al., 2020).
Finally, Subgroup 2 was a mix of anger and anxiety inductions (46.15% vs 53.85%). One interpretation is that this subgroup reflects inductions in which individuals were experiencing unpleasantness, but not a clear feeling of anger or anxiety. Consistent with this interpretation, inductions in this subgroup were associated with equivalent levels of self-reported anger and anxiety, regardless of which emotion induction participants were assigned to. Subgroup 2 was similar to Subgroup 1 in terms of connectivity between aspects of the SAL (from aSAL to pSAL), between aspects of DMN (from vDMN to dDMN) and from the precuneus to left and right FPC. It was relatively dissimilar to Subgroup 3, which had BG to aSAL and SM connectivity and connectivity from pSAL to aSAL. It is possible that in the absence of clear functional connectivity patterns related to anger or anxiety, the unsupervised procedure was picking up on stable individual connectivity patterns. Indeed, although most scans belonged to the anxiety condition (53.85%), 44% of participants contributed both an anger and anxiety induction to this subgroup.
Degeneracy in between-network patterns for emotions
To assess whether s-GIMME revealed degenerate patterns associated with anger and anxiety, we next performed an unsupervised analysis on all runs from within the anger induction and from within the anxiety induction. The unsupervised approach revealed evidence for neural degeneracy: we observed distinct functional connectivity patterns within each emotion category. For instance, two subgroups of functional connectivity patterns characterized the anger induction. Participants in both subgroups experienced the same degree of anger, despite hints that Anger Subgroup 1 may have experienced instances of anger as more unpleasant (Table 3). On the one hand, this finding may seem at odds with the notion of degeneracy, but there is evidence that instances of the same emotion category (e.g. anger) vary in features such as valence or arousal (Kuppens et al., 2013, 2017; Wilson-Mendenhall et al., 2014). Since within-salience connectivity correlates with the intensity of negative effect (Seeley et al., 2007; Touroutoglou et al., 2012), these valence-based differences may explain the connectivity between aSAL and pSAL in Anger Subgroup 1 and lack thereof in Anger Subgroup 2. Anger Subgroups 1 and 2 were furthermore differentiated by connectivity between aspects of the salience network and DAN. Anger Subgroup 1 had directed connectivity between aSAL and DAN, whereas Anger Subgroup 2 had directed connectivity between pSAL and DAN. Insofar as pSAL represents visceral sensations (Craig, 2002, 2009), these findings may suggest a route by which visceral sensations can drive attentional in anger.
Two subgroups of functional connectivity patterns also characterized the anxiety induction, with participants experiencing similar degrees of anxiety across both. Anxiety subgroups differed primarily in their involvement of aSAL and PCUN vs pSAL and Lang. Of interest, Anxiety Subgroup 2 showed no connectivity between sub-networks of the DMN (vDMN, dDMN and PCUN), a pattern that also characterizes high trait anxiety (Modi et al., 2015; Imperatori et al., 2019).
Ruling out alternate explanations
We conducted multiple analyses to rule out alternate explanations of our findings. One alternate explanation may be that we have revealed not degeneracy, but stable individual-level connectivity patterns. Yet, individuals were not consistently sorted into the same subgroups across analyses, and individual-level factors (e.g. age, self-identified sex and music training) did not predict subgroup composition. Moreover, experimental factors such as experiment counterbalance order did not describe our subgroups.
Of course, we did not rule out all alternate explanations for our findings. For instance, we cannot rule out that our findings reveal stable ‘subtypes’ of anger and anxiety that will be replicated again and again across instances and people (Scarantino, 2009; Silva et al., 2013; Adolphs, 2017). This possibility would be at odds with the notion of degeneracy, which would predict different patterns for the same emotion category, even within people over time.
Limitations
There are several limitations to the present research. First, we were limited by a relatively small sample size. However, our analytic approach relies on the uSEM framework, in which time points—rather than participants—serve as sampling units. In the present study, each participant has 150 time points per run and s-GIMME recovers reliable subgroups with as few as 60 timepoints (Lane et al., 2019) and in samples of comparable size (Gates et al., 2017b). Ideally, these preliminary findings would be replicated and extended in large neuroimaging data sets in the future. One possibility would be to employ existing, large, open-access data sets such as the Human Connectome Project (Glasser et al., 2016). Yet, many such data sets tend to focus on facial expression-viewing, which likely taps different processes than emotional experience (see Lindquist et al., 2012).
The highly idiographic music and imagery-based induction emotion induction we used had advantages for robustly inducing emotion but also had limitations. Our induction required participants to self-generate actual or prospective scenarios that they find most personally relevant. This may have produced more heterogeneity in the data than more standardized inductions (e.g. viewing pictures or movies). It is also possible that it produced brain activation that was ancillary to the emotion itself. Indeed, we cannot rule out that some of the patterns we observed, especially those implicating networks involved in attention such as the DAN and FPC, are related to the task and not emotional experiences per se. Nonetheless, both DAN and FPC are frequently implicated in emotion across tasks in the literature (Lindquist et al., 2012; Wager et al., 2015), suggesting that they may in fact be integral to emotional experiences. Moreover, participants were not routinely grouped into the same subgroup, suggesting that individual differences in task strategy were unlikely to alone drive effects.
Future directions and conclusion
Recent methodological advances in neuroscience have enabled researchers to study emotions as dynamic, contextualized experiences. This approach is more consistent with the emerging scientific picture of the nature of emotion. This work contributes to this movement by showing that there is some consistency in the patterns of neural connectivity associated with specific emotional experiences. Nonetheless, meaningful degeneracy also underlies these average patterns. Future research must not only replicate evidence for degeneracy but must also speak to its function. Degeneracy is thought to be adaptive in biological systems, and so a next step would be to examine how degeneracy is related to emotional function and dysfunction. Variation in functional connectivity produces adaptive network function (Ghosh et al., 2008; Deco et al., 2009; McDonnell and Ward, 2011) and is related to cognitive flexibility (Cohen, 2018). An interesting future direction would thus be to investigate degeneracy within individuals across instances of an emotion to examine whether within-person degeneracy is associated with wellness vs psychopathology. We look forward to future research examining the variable pathways to emotional experiences.
Supplementary Material
Acknowledgements
C.M.D., PhD, and S.T.L., PhD, completed this research while at the University of North Carolina at Chapel Hill and are now Quantitative UX Researcher at Meta Platforms, Inc. and Data Science Manager at Netflix, Inc., respectively. The authors would like to thank Taylor L. Killian for her help with editing an earlier version of this manuscript and Robert Kraft for assistance with magnetic resonance imaging (MRI) acquisition.
Contributor Information
Cameron M Doyle, Department of Psychology and Neuroscience, University of North Carolina, Chapel Hill, NC 27599, USA.
Stephanie T Lane, Department of Psychology and Neuroscience, University of North Carolina, Chapel Hill, NC 27599, USA.
Jeffrey A Brooks, Department of Psychology, University of California, Berkeley, CA 84720, USA; Hume AI, New York, NY 10010, USA.
Robin W Wilkins, Gateway University of North Carolina Greensboro MRI Center, Greensboro, NC 27412, USA.
Kathleen M Gates, Department of Psychology and Neuroscience, University of North Carolina, Chapel Hill, NC 27599, USA.
Kristen A Lindquist, Department of Psychology and Neuroscience, University of North Carolina, Chapel Hill, NC 27599, USA; Biomedical Research Imaging Center, School of Medicine, University of North Carolina, Chapel Hill, NC 27599, USA.
Funding
This research was supported by the University of North Carolina at Chapel Hill, the University of North Carolina, Greensboro, and the Gateway University of North Carolina Greensboro MRI Center. C.M.D. was supported by a National Science Foundation Graduate Research Fellowship.
Conflict of interest
The authors declare that they had no conflict of interest with respect to their authorship or the publication of this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary data
Supplementary data are available at SCAN online.
References
- Addis D.R., McIntosh A.R., Moscovitch M., Crawley A.P., McAndrews M.P. (2004). Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. NeuroImage, 23(4), 1460–71. [DOI] [PubMed] [Google Scholar]
- Adhikari B.M., Jahanshad N., Shukla D., et al. (2018). Comparison of heritability estimates on resting state fMRI connectivity phenotypes using the ENIGMA analysis pipeline. Human Brain Mapping, 39(12), 4893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio H., Damasio A. (1994). Impaired recognition of emotion in facial expressions following bilateral dam age to the human amygdala. Nature, 372(6507), 669–72. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio H., Damasio A.R. (1995). Fear and the human amygdala. Journal of Neuoscience, 15(9), 5879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. (2017). How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Social Cognitive and Affective Neuroscience, 12, 24–31.doi: 10.1093/scan/nsw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anteraper S.A., Triantafyllou C., Sawyer A.T., Hofmann S.G., Gabrieli J.D., Whitfield-Gabrieli S. (2014). Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connectivity, 4(2), 81–90. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Duerden E.G., Taylor M.J. (2013). The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Human brain mapping, 34(11), 3031–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari B., Westlin C., Satpute A.B., et al. (2020). Comparing supervised and unsupervised approaches to emotion categorization in the human brain, body, and subjective experience. Scientific Reports, 10(1), 20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D.R., Hurlemann R., Dolan R.J. (2015). Impaired threat prioritisation after selective bilateral amygdala lesions. Cortex, 63, 206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F. (2017). The theory of constructed emotion: an active inference account of interoception and categorization. Social cognitive and affective neuroscience, 12(1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Finlay B.L. (2018). Concepts, goals and the control of survival-related behaviors. Current Opinion in Behavioral Sciences, 24, 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Satpute A.B. (2013). Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23(3), 361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B., Mihov Y., Scheele D., et al. (2012). Fear processing and social networking in the absence of a functional amygdala. Biological Psychiatry, 72(1), 70–7. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz A.M., Gates K.M. (2017). Network Mapping with GIMME. Multivariate Behavioral Research, 52(6), 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J.A., Shablack H., Gendron M., Satpute A.B., Parrish M.H., Lindquist K.A. (2017). The role of language in the experience and perception of emotion: a neuroimaging meta-analysis. Social Cognitive and Affective Neuroscience, 12(2), 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson J.C., Read Q.D. (2020). ggalluvial: alluvial plots in “ggplot2”. R Package Version 0.12.3. Available: http://corybrunson.github.io/ggalluvial/. Retrieved October 10, 2019.
- Clark-Polner E., Johnson T.D., Barrett L.F. (2017). Multivoxel pattern analysis does not provide evidence to support the existence of basic emotions. Cerebral Cortex, 27(3), 1944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R. (2018). The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage, 180, 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–15. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Craig A.D.B. (2009). How do you feel—now? The anterior insula and human awareness. Nature Review Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Cureton E.E. (1956). Rank-biserial correlation. Psychometrika, 21(3), 287–90. [Google Scholar]
- Deco G., Jirsa V., Mcintosh A.R., Sporns O., Kötter R., Raichle M.E. (2009). Key role of coupling, delay, and noise in resting brain fluctuations. Proceedings of the National Academy of Sciences of the United States of America, 106(25), 10302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G.M., Gally J.A. (2001). Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences, 98(24), 13763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich E., Macaulay D., Ryan L. (1994). Mood dependent memory for events of the personal past. Journal of Experimental Psychology General, 123(2), 201–15. [DOI] [PubMed] [Google Scholar]
- Eich E., Metcalfe J. (1989). Mood dependent memory for internal versus external events. Journal of Experimental Psychology Learning, Memory, and Cognition, 15(3), 443–55. [Google Scholar]
- Eisen H.N. (2001). Specificity and degeneracy in antigen recognition: yin and yang in the immune system. Annual Review of Immunology, 19(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Engen H.G., Kanske P., Singer T. (2017). The neural component-process architecture of endogenously generated emotion. Social Cognitive and Affective Neuroscience, 12(2), 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., et al. (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Bandettini P.A. (2021). Movie-watching outperforms rest for functional connectivity-based prediction of behavior. NeuroImage, 235, 117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Molenaar P.C.M., Iyer S.P., Nigg J.T., Fair D.A. (2014). Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLoS One, 9, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Lane S.T., Varangis E., Giovanello K., Guiskewicz K. (2017a). Unsupervised classification during time-series model building. Multivariate Behavioral Research, 52(2), 129–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Lane S.T., Varangis E., Giovanello K., Guskiewicz K. (2017b). Unsupervised classification during time-series model building. Multivariate Behavioral Research, 52(2), 129–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Fisher Z.F., Bollen K.A. (2020). Latent variable GIMME using model implied instrumental variables (MIIVs). Psychological Methods, 25(2), 227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Molenaar P.C.M. (2012). Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. NeuroImage, 63, 310–9. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Rho Y., McIntosh A.R., Kötter R., Jirsa V.K. (2008). Noise during rest enables the exploration of the brain’s dynamic repertoire. PLoS Computational Biology, 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Smith S.M., Marcus D.S., et al. (2016). The Human Connectome Project’s neuroimaging approach. Nature Neuroscience, 19(9), 1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Molnar C., George M.S., Bolger K., Koola J., Nahas Z. (2007). Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology, 44(1), 91–7. [DOI] [PubMed] [Google Scholar]
- Horien C., Shen X., Scheinost D., Constable R.T. (2019). The individual functional connectome is unique and stable over months to years. NeuroImage, 189, 676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-A., Jastorff J., Van den Stock J., Van de Vliet L., Dupont P., Vandenbulcke M. (2018). Studying emotion theories through connectivity analysis: evidence from generalized psychophysiological interactions and graph theory. NeuroImage, 172, 250–62. [DOI] [PubMed] [Google Scholar]
- Hwang K., Shine J.M., D’Esposito M. (2019). Frontoparietal activity interacts with task-evoked changes in functional connectivity. Cerebral Cortex (New York, N.Y.: 1991), 29(2), 802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatori C., Farina B., Adenzato M., et al. (2019). Default mode network alterations in individuals with high-trait-anxiety: an EEG functional connectivity study. Journal of Affective Disorders, 246(March), 611–8. [DOI] [PubMed] [Google Scholar]
- Kim J., Zhu W., Chang L., Bentler P.M., Ernst T. (2007). Unified structural equation modeling approach for the analysis of multisubject, multivariate functional MRI data. Human Brain Mapping, 28(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner I.R., Zhang J., Touroutoglou A., et al. (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1(5), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide-Majima N., Nakai T., Nishimoto S. (2020). Distinct dimensions of emotion in the human brain and their representation on the cortical surface. NeuroImage, 222, 117258. [DOI] [PubMed] [Google Scholar]
- Kuppens P., Tuerlinckx F., Russell J.A., Barrett L.F. (2013). The relation between valence and arousal in subjective experience. Psychological Bulletin, 139(4), 917–40. [DOI] [PubMed] [Google Scholar]
- Kuppens P., Tuerlinckx F., Yik M., et al. (2017). The relation between valence and arousal in subjective experience varies with personality and culture. Journal of Personality, 85(4), 530–42. [DOI] [PubMed] [Google Scholar]
- Lane S.T., Gates K.M., Fisher Z. et al. (2021). gimme: Group Iterative Multiple Model Estimation Available at https://github.com/GatesLab/gimme/. Retrieved August 1, 2019.
- Lane S.T., Gates K.M., Pike H.K., Beltz A.M., Wright A.G.C. (2019). Uncovering general, shared, and unique temporal patterns in ambulatory assessment data. Psychological Methods, 24(1), 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S., Josse J., Husson F. (2008). FactoMineR: a package for multivariate analysis. Journal of Statistical Software, 25(1), 1–18. [Google Scholar]
- LeDoux J., Daw N.D. (2018). Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nature Reviews Neuroscience, 19(5), 269. [DOI] [PubMed] [Google Scholar]
- Lee Y.-Y., Hsieh S. (2014). Classifying different emotional states by means of EEG-based functional connectivity patterns. PLoS One, 9(4), e95415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Zhang L., Barbera G., et al. (2018). Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron, 100, 700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Portugal L.C., Alves R.D.C.S., Junior O.F., et al. (2020). Interactions between emotion and action in the brain. NeuroImage, 214, 116728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences, 35(3), 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Barrett L.F. (2012). A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences, 16(11), 533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhao X., Tang Q., Li W., Liu G. (in press). Dynamic functional network connectivity associated with musical emotions evoked by different tempi. Brain Connectivity. 10.1089/brain.2021.0069. [DOI] [PubMed] [Google Scholar]
- Mann H.B., Whitney D.R. (1947). On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics, 18(1), 50–60. [Google Scholar]
- McCormick E.M., Gates K.M., Telzer E.H. (2019a). NeuroImage model-based network discovery of developmental and performance-related differences during risky decision-making. NeuroImage, 188, 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick E.M., Gates K.M., Telzer E.H. (2019b). Model-based network discovery of developmental and performance-related differences during risky decision-making. NeuroImage, 188, 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick E.M., Telzer E.H. (2018). Contributions of default mode network stability and deactivation to adolescent task engagement. Scientific Reports, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell M.D., Ward L.M. (2011). The benefits of noise in neural systems: bridging theory and experiment. Nature Reviews Neuroscience, 12(7), 415–26. [DOI] [PubMed] [Google Scholar]
- Modi S., Kumar M., Kumar P., Khushu S. (2015). Aberrant functional connectivity of resting state networks associated with trait anxiety. Psychiatry Research: NeuroImaging, 234(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Ramsey J.D. (2014). Bayesian networks for fMRI: a primer. NeuroImage, 86, 573–82. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage, 44(3), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler S., Humberg S. (2021). GIMME’s ability to recover group-level path coefficients and individual-level path coefficients. Methodology, 17, 58–91. [Google Scholar]
- Panksepp J. (1998). Affective Neuroscience. Vol. 349, New York: Oxford University Press. [Google Scholar]
- Pessoa L. (2017). A network model of the emotional brain. Trends in Cognitive Sciences, 21(5), 357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons P., Latapy M. (2005). Computing communities in large networks using random walks. Computer and Information Sciences (ISCIS), 10, 284–93. [Google Scholar]
- Raichle M.E., Macleod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–47. [DOI] [PubMed] [Google Scholar]
- Saarimäki H., Glerean E., Smirnov D., et al. (2022). Classification of emotion categories based on functional connectivity patterns of the human brain. NeuroImage, 247, 118800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute A.B., Lindquist K.A. (2019). The default mode network’s role in discrete emotion. Trends in Cognitive Sciences, 23(10), 851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviola F., Pappaianni E., Monti A., Grecucci A., Jovicich J., De Pisapia N. (2020). Trait and state anxiety are mapped differently in the human brain. Scientific Reports, 10(1), 11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarantino A. (2009). Core affect and natural affective kinds. Philosophy of Science, 76(5), 940–57. [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Lee H.L., Schofield T., Ellis C.L., Price C.J. (2008). Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage, 42(3), 1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J.-J. (2017). A new integrated symmetrical table for genetic codes. BioSystems, 151, 21–6. [DOI] [PubMed] [Google Scholar]
- Siebert M., Markowitsch H.J., Bartel P. (2003). Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain, 126(12), 2627–37. [DOI] [PubMed] [Google Scholar]
- Silva B.A., Mattucci C., Krzywkowski P., et al. (2013). Independent hypothalamic circuits for social and predator fear. Nature Neuroscience, 16(12), 1731–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorella S., Grecucci A., Piretti L., Job R. (2021). Do anger perception and the experience of anger share common neural mechanisms? Coordinate-based meta-analytic evidence of similar and different mechanisms from functional neuroimaging studies. NeuroImage, 230, 117777. [DOI] [PubMed] [Google Scholar]
- Sorella S., Vellani V., Siugzdaite R., Feraco P., Grecucci A. (2022). Structural and functional brain networks of individual differences in trait anger and anger control: an unsupervised machine learning study. European Journal of Neuroscience, 55(2), 510–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Edelman G.M. (2000). Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Networks, 13(8–9), 909–22. [DOI] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G.M. (1999). Measures of degeneracy and redundancy in biological networks. Proceedings of the National Academy of Sciences of the United States of America, 96(6), 3257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Barrett L.F. (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage, 60(4), 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Lindquist K.A., Dickerson B.C., Barrett L.F. (2015). Intrinsic connectivity in the human brain does not reveal networks for “basic” emotions. Social Cognitive and Affective Neuroscience, 10(9), 1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist, 20(2), 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Kang J., Johnson T.D., Nichols T.E., Satpute A.B., Barrett L.F. (2015). A Bayesian model of category-specific emotional brain responses. PLoS Computational Biology, 11(4), 1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre J., Bender A. (2010). Degeneracy: a design principle for achieving robustness and evolvability. Journal of Theoretical Biology, 263(1), 143–53. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–41. [DOI] [PubMed] [Google Scholar]
- Wilkins R.W., Hodges D.A., Laurienti P.J., Steen M., Burdette J.H. (2014). Network science and the effects of music preference on functional brain connectivity: from Beethoven to Eminem. Scientific Reports, 4(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Mendenhall C.D., Barrett L.F., Simmons W.K., Barsalou L.W. (2011). Grounding emotion in situated conceptualization. Neuropsychologia, 49(5), 1105–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Mendenhall C.D., Barrett L.F., Barsalou L.W. (2014). Variety in emotional life: within-category typicality of emotional experiences is associated with neural activity in large-scale brain networks. Social Cognitive and Affective Neuroscience, 10, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Gates K.M., Molenaar P., Li P. (2015). Neural changes underlying successful second language word learning: an fMRI study. Journal of Neurolinguistics, 33, 29–49. [Google Scholar]
- Zelle S.L., Gates K.M., Fiez J.A., Sayette M.A., Wilson S.J. (2017). The first day is always the hardest: functional connectivity during cue exposure and the ability to resist smoking in the initial hours of a quit attempt. NeuroImage, 151(March 2016), 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


