Abstract
Magnetic resonance imaging-guided laser interstitial thermal therapy (LITT) is an ablative procedure using heat from a laser to provide cytoreduction in tissue. It is a minimally invasive procedure that has been used in intracranial pathologies such as high-grade gliomas, metastatic lesions, epilepsy, and other lesions. While LITT may offer a more acceptable complication profile compared to open surgery, the role of laser therapy for intracranial lesions in current treatment paradigms continues to evolve. This review will focus on the background and application of LITT, the current evidence for its use, and future directions for the technology.
Keywords: glioma surgery, laser interstitial thermal therapy, laser surgery, LITT
Laser interstitial thermal therapy (LITT) is a minimally invasive procedure utilizing the nonionizing radiation emitted from laser light to produce a thermal effect on tissue, resulting in cell death.1 LITT is a novel and minimally invasive procedure that has transformed the treatment of various intracranial pathologies. Previously, there were concerns regarding the ability of surgeons to control and monitor thermal damage. However, recent advances in technology have paired LITT with magnetic resonance imaging (MRI) and thermometry, providing surgeons with optimal thermal control.
Currently, LITT systems allow surgeons to selectively ablate tumors and lesions in the brain that may have previously been considered difficult to resect or tumors that have recurred after multiple therapies. LITT is particularly useful in cases where tumors are in difficult-to-access locations or where patients may be considered risky surgical candidates. Recently, LITT has shown that it is not only effective in ablating lesions directly but may also be utilized to augment the tumor microenvironment through mechanisms such as increasing the permeability of the blood-brain barrier (BBB),2 making it more accessible for directed drug therapies and immunologic responses. In the past decade, interest in LITT has been reignited as technological advances in intraoperative MRI have significantly enhanced the efficacy and safety of the procedure. In this review, we describe the history, mechanism, and clinical applications of the LITT procedure.
Historical Perspective on Lasers in Neurosurgery
Lasers have been used in the field of neurosurgery for over 50 years. Ruby lasers were the first to be used to treat malignant tumors in mice and guinea pigs.3 While their use led to almost early death in the animals, the experiments showed that lasers could be used to effectively destruct tumor tissue.4 The CO2 laser was used shortly thereafter by surgeons to attempt to treat brain tumors.5 However, initial uses of lasers resulted in incomplete tumor ablation which was attributed to poor targeting of tumor tissue and a lack of control by the surgeon over the thermal effect.6 While these early studies were promising in the potential to use lasers to ablate tumors, the procedure needed further refinement before its widespread adoption.
A neodymium: yttrium-aluminum-garnet (Nd:YAG) laser was later discovered in 1980 and the LITT procedure was created.7 The Nd:YAG laser utilizes a flexible fiberoptic cable allowing it to penetrate deeply into neuronal tissue.8 Brown first used the Nd:YAG laser and was able to successfully produce tissue coagulation in response.1 His discovery led to numerous animal studies followed by clinical trials and the establishment of LITT as a feasible neurosurgical therapy.5,7,9 Neurosurgeons were able to combine stereotactic guidance with LITT. The addition of real-time MRI thermometry enhanced the LITT procedure and permitted better targeting of tissues with hyperthermia. Recent advances in imaging, surgical navigation, and thermal monitoring have reintroduced LITT as a promising treatment for intracranial lesions. LITT offers a minimally invasive alternative approach for tumor management.
LITT Technology and Operative Approach
Lasers have long been used to treat brain tumors based on the principle of hyperthermia. The hypoxic tumor microenvironment may permit the tumor cells to be more susceptible to heat damage.9 The laser interacts with the tissue to create a thermal reaction, generating heat that is deadly to the tumor cells.10 The laser’s optical fiber emits photons that are absorbed by tumor chromophores causing excitation and the subsequent release of thermal energy.8 If the tissue is maintained at an elevated temperature for an extended period of time, protein denaturation followed by cellular necrosis and tissue coagulation will result.11 Intrinsic properties of the tissue as well as the characteristics of the laser will influence the speed and pattern of tissue heating, impacting the overall result of the ablation. Prolonged tissue hyperthermia leads to apoptosis of target tissue (cancer cells), and maintaining tissue temperature between 43°C and 45°C for more than 10 min can sensitize tumor cells to chemotherapy and radiation likely due to BBB disruption and the DNA damage response.9,11 When temperatures reach above 50°C, protein denaturation and tumor necrosis will occur.9 The extent of tissue damage and cell death that occurs is dependent on the temperature reached in the treated tissue and the length of time that that temperature was maintained for. The Arrhenius thermal dose model can be used to estimate the amount of tissue damage that will occur.12 Using this algorithm, the MRI software can generate thermal maps that actively monitor thermal change and tumor necrosis in real time.
In the United States, there are currently two FDA-approved LITT systems in use: the NeuroBlate System (Monteris Medical, Inc.) and the Visualase Thermal Therapy System (Medtronic, Inc.). These systems have varying wavelengths and cooling methods, but their underlying biological mechanisms remain similar. The Visualase system uses a 15-Watt 980 nm diode laser that is cooled with saline. The NeuroBlate system uses a 12-Watt 1064 nm Nd:YAG continuous wave laser that is cooled with CO2. The Nd:YAG laser is better suited for soft tissue that contains a high volume of white matter as the laser has demonstrated a high penetration depth in the 1000–1100 nm wavelength range.9 This wavelength is near the infrared window and therefore the laser’s scattering is greater than its absorption, further supporting deeper penetration.9 The diode laser is beneficial in that its wavelength is ideal for water absorption. Given that the brain is a water-heavy environment, these properties allow for the creation of faster lesions with sharp thermal gradients.9 Shorter procedures are preferable as they minimize unwanted collateral damage.
LITT involves the implementation of stereotactic techniques in an intraoperative or interventional MRI suite.13 Prior to the operation, a post-gadolinium axial volumetric MRI scan is obtained to delineate tumor tissue from surrounding brain parenchyma for surgical planning. Routinely, a stereotactic needle biopsy is performed prior to LITT treatment for a tissue diagnosis. Once the patient is positioned for surgery after anesthesia induction, neuronavigation registration, and a stereotactic plan is generated to target the lesion. A trajectory is chosen that avoids sulci, the ependyma, and eloquent pathways.14 Confirming the surgical trajectory is imperative to avoiding scar tissue and key structures like the ventricles or blood vessel entry points. It is easiest to localize lesions that are well-circumscribed, spherical, and deep-seated.
After burr hole placement and a stereotactic biopsy are completed, a stereotactic bolt is placed. Multiple bolts can be placed into the bone to accommodate multiple LITT treatments, depending on the size of the lesion.15 The LITT fiber is placed through the stereotactic bolt to the tumor target. MRI is then used to confirm proper positioning of the LITT catheter fiber and proximity to the tumor target.16 Pretreatment images serve as reference for depicting important surrounding anatomical structures and are overlaid with thermal measurements for the actual LITT procedure. Thermal imaging by real-time MR thermometry occurs for the duration of the procedure and is designed for crucial feedback and monitoring during the LITT procedure.16
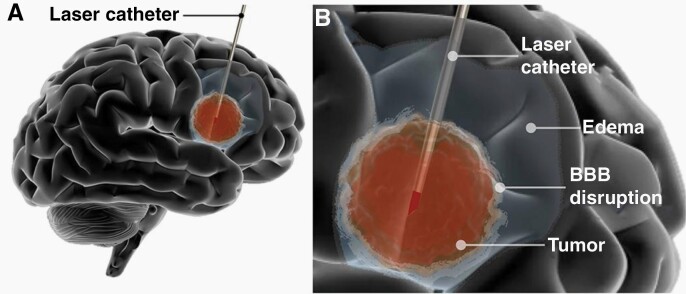
Tumor ablation begins when the laser fiber interstitially delivers heat to the tumor tissue (see Figure 1) and reaches 43°C for approximately 10 min.14 This temperature and timeframe induce cell death and thermal destruction of targeted lesions. MRI thermometry provides visual representations of temperature over a period of time and is used to track the temperature within the lesion. Maps of live temperature changes and estimated irreversible damage from LITT are generated during the procedure. The irreversible damage map reflects complete ablation of the lesion at the end of the session. Additional MRI sequences are obtained after LITT to confirm proper targeting of the lesion and no hemorrhage is present.
Fig. 1.
Illustration showing Laser Interstitial Thermal Therapy (LITT). (A) Representation of the intratumoral placement of the laser catheter and tumor ablation. (B) Representation of ablation with post-LITT contrast enhancement related to BBB disruption and peri-lesional edema (Permission from Skandalakis et al.25).
The LITT system is comprised of 3 components including the actual laser catheter, computer planning workstation, and use of MRI for proper targeting of tissues and real-time thermometry. The laser itself has a light source, fibers, an applicator, a sheath, and a diffusion tip. The laser light is generated by the source and transmitted via the optical fibers. Sapphire fibers are preferable as they are heat resistant and able to transmit the laser with minimal energy absorption.14 A cooled tip is essential for LITT as it allows the ablation to be carried out for longer periods of time without charring the tumor and also preventing off-target heating.17 Charring can interfere with the laser by decreasing absorption and heat transmission. An external workstation is used during the LITT procedure to provide real-time thermal maps and imaging of the target being treated. MRI images are sent to the work station in real-time, permitting the software to estimate the level of tissue necrosis that has occurred. Both the Visualase and NeuroBlate systems have safety mechanisms built in that trigger deactivation if a certain temperature threshold is breached. MRI is utilized during LITT to accurately identify the lesion and then plan the trajectory of the laser accordingly. Throughout the procedure, it is used to visualize and measure heat exposure within the tumor and outside the tumor borders using magnetic resonance thermometry. Intraoperative MRI is necessary for successful thermal ablation for temperature maintenance and control and was the essential component that allowed LITT to become a widely used treatment.
Post-LITT Lesion Transformation
After the LITT procedure is completed, MRI and histologic changes can be found at the LITT target site. These changes have a unique pattern comprising 5 concentric zones, 3 of which form within the first 3 months and 2 form up to 6 months after LITT. The zones are described as follows18–20:
Primary Stages (0–3 Months Post-procedure)
Light guide track zone: region where the laser fiber is placed. There is minimal structural damage18–20
Central zone: region forms intraoperatively around the tip of the laser fiber. It can be characterized by coagulative necrosis and tumor cell death which is positive for apoptotic markers (ie, cleaved caspase-3 and cleaved PARP-1). 18–20 The size of this zone can plateau or enlarge during and after the LITT procedure. MRI findings consist of hyperintensity seen on T1-weighted images and hypointensity seen on T2-weighted images. Histologically, there is damage to the mitochondria, intracellular membranes, blood vessels, and extracellular matrix.18–20
Peripheral zone: region undergoes delayed liquefactive necrosis. It can be characterized by intracellular edema, thrombosed vessels, and cell death. The peripheral zone is similar to the central zone in terms of its size and MRI features. The size of the zone increases with time but eventually stabilizes or decreases. MRIs reflect hyperintense T1-weighted images and hypointense T2-weighted imaging signals. Histological staining reveals a layer of astrocytes distinguishing the necrotic lesion from normal brain parenchyma.18–20 Additionally, there is infiltration of cells involved in phagocytosis, including granulocytes, lymphocytes, and macrophages.
Secondary Stages (2 Weeks to 6 months Post-procedure)
4. Outer thin enhancing zone: region is seen at the margin of the peripheral zone with distinctive vascular anatomy and reflects formation of granulation tissue and damage of the BBB. The damage causes this zone to have a unique “eggshell”-like appearance. Residual long-term enhancement is indicative of inflammation and additional formation of granulation tissue.18–20
5. Marginal zone: outermost region of the lesion with features of reversible perilesional edema. The edema seen in this zone occurs 1 to 3 days after ablation and reflects mild to severe progression that can be evaluated on T2-weighted MRIs.18
Evidence for Use
In 1990, Sugiyama et al. performed some of the first studies on the efficacy of LITT as a treatment for deep-seated brain lesions.21 Traditionally, resection was the primary form of therapy used to treat these lesions, but the invasive nature of removing hard-to-reach tumors prompted the search for an equally, if not more, effective approach. Sugiyama et al. showed, through a histological study on cats, that necrosis occurred in the central zone of lesions treated with laser hyperthermia.21 Edema was also present, but it was confined to the heated zone surrounding the lesion. In the clinical study, three of the five patients showed no evidence of recurrence up to 31 months following LITT. Of the two patients who did not survive, one patient showed evidence of recurrence but not in the irradiated site. These groundbreaking studies prompted further research into LITT as a potential therapy, in addition to its applications beyond just primary tumors.
High-Grade Gliomas
LITT is an emerging treatment for primary brain tumors and outcomes have been described in several studies, most commonly for progressive and recurrent glioblastomas (GBMs). Many clinical studies have been performed on both new and recurrent high-grade gliomas (HGGs), of which a non-exhaustive list is provided in Table 1. One of the first GBM studies where LITT was implemented involved investigating the outcomes of 16 patients with recurrent GBM.22,23 These patients were divided into two groups (Group 1: 10 patients; Group 2: 6 patients). Minimal complications were seen from LITT, but, interestingly, the two groups had vastly different overall survival (OS) times (Group 1: 5.2 months; Group 2: 11.2 months). Investigators attributed the differences in OS to their mastery of the technique after treating the first patient group and the lengthened time between the LITT procedure and diagnosis of recurrence for the first patient group (Group 1: 2 months vs Group 2: 0.3 months).22
Table 1.
Clinical Trials for New and Recurrent High-grade Glioma
| Study | System | Patients (n) | Pretreatment Tumor Volume | Treatment Time | Tumor Volume Ablated (%) | Imaging Modality | Complications |
|---|---|---|---|---|---|---|---|
| Sugiyama et al.21 | Nd:YAG | 5 | 1.2–3 cm | 30-40 min | NR | CCTS | NR |
| Bettag et al.67 | Nd:YAG | 5 | 1–3.5 cm | NR | NR | CT, MRI | NR |
| Sakai et al.68 | Nd:YAG | 5 | NR | NR | 100% in 4/5 patients | CCTS | None |
| Kahn et al.20 | Nd:YAG | 8 | 1.8–3.4 cm | 10 min | 15–87% | CT | None |
| Schwabe et al.19 | Nd:YAG | 18 | 2–3.5 cm | 2–23 min | NR | MRI | NR |
| Reimer et al.69 | Nd:YAG | 4 | 1–3.5 cm | 3–13 min | NR | MRI | Hemorrhage |
| Leonardi et al.70 | Nd:YAG | 24 | 2.1–3.3 cm | NR | NR | MRI | Neurological deficit |
| Leonardi and Lumenta71 | Nd:YAG | 24 | 2.1–3.3 cm | NR | NR | MRI | Neurological deficit |
| Schwarzmaier et al.22 | Nd:YAG | 16 | 21.6 ± 18.6 cm3 | NR | NR | MRI | None |
| Jethwa et al.24 | Viusalase | 20 | 0.37–68.9 cm3 | 1.83–35.9 min | NR | MRI | Hemicraniectomy from edema |
| Hawasli et al.72 | NeuroBlate | 17 | 14.1 ± 10.7 cm3 | 3.11–10.32 h | 8.5–67.7 cm3 (45), 6.2–47.3 (52) | MRI | Transient neurological deficit, DVT, meningitis, hyponatremia |
| Sloan et al.62 | NeuroBlate | 10 | 2.6–19 cm3 | 7.5–50.2 min | 2.6–19.01 cm3 | MRI | Vascular injury, neurological deficits |
| Mohammadi et al.61 | NeuroBlate | 34 | 0.7–49.9 cm3 | NR | 91%–98% | MRI | Neurological deficit, DVT, hyponatremia, wound infection |
| Leuthardt et al.58 | NeuroBlate | 19 | <3 cm diameter | NR | NR | MRI | None |
| Rennert et al.73 | NeuroBlate | 38 | NR | NR | NR | MRI | NR |
| de Groot et al.30 | NeuroBlate | 89 | 7.7–8.5 | NR | 91%–99% | MRI | Neurological deficit, DVT |
Abbreviations: CCTS, computer-controlled temperature system; CT, computed tomography; MRI, magnetic resonance imaging; Nd:YAG; neodymium-doped yttrium aluminum garnet; NR, not recorded; DVT, deep vein thrombosis.
The technical rigor and feasibility of LITT were explored by Jethwa et al., where post-LITT complications were assessed in 20 patients with various brain tumor types, including GBM, anaplastic astrocytoma, ependymoma, meningioma, hemangioblastoma, primitive neuroectodermal tumors, chordoma, and cerebral metastases.24 The accuracy of laser insertion was recorded for all 20 procedures with 83.9% accuracy achieved. Postoperative complications included refractory brain edema and arterial and pituitary abrasions.24,25 Neuro-oncology studies documenting LITT efficacy and safety have been synthesized and compared to standard treatment modalities in a systematic literature review.26 Repeated surgical resection resulted in the longest OS (24.4 months) followed by LITT (20.9 months), brachytherapy (18.9 months), chemotherapy (16 months), and one-time surgical resection (14.8 months). Postoperative complications, including surgical site infections, hemorrhage, and permanent neurologic deficits, occurred in 16.7% LITT cases compared to 11% of the surgical resection cases.26
More recent studies focus on comparing the outcomes of LITT-treated new versus recurrent GBM. One study involved evaluating 54 patients with GBM treated with 58 LITT procedures,15,27 Poorer progression-free survival (PFS) and OS were seen in patients with primarily treated tumors compared to recurrent malignancies (PFS: 3.6 [primary] vs 7.3 [recurrent]; OS: 9.1 [primary] vs 11.8 [recurrent]). The tumors’ genomic alterations did not impact patient outcomes. A case study evaluating patients with GBM receiving LITT found that 5 of 13 recurrent patients demonstrated tumor shrinkage, however, none of the 8 progressive patients demonstrated a reduction in tumor size. The study importantly noted that the patients newly diagnosed tumors were in older patients and had a higher percentage of isocitrate dehydrogenase (IDH)-wildtype tumors.17 The impact of the extent of ablation on LITT-treated GBM outcomes has been queried by many investigators. A systematic review that evaluated 6 case series with a total of 63 patients diagnosed with recurrent HGGs found that complete ablation of the tumor resulted in increased OS (9.7 months) compared to the cases where HGGs were partially ablated (4.6 months).28 Conversely, a 25-patient meta-analysis of 4 LITT studies for progressive malignant gliomas found that there was no association between extent of ablation and patient outcome.29 In a recent prospective multicenter study of both new and recurrent GBM undergoing LITT as opposed to surgical resection, de Groot et al. found that newly diagnosed patients receiving adjuvant chemoradiotherapy had a median OS of 16 months, comparable to patients undergoing traditional surgical resection followed by chemotherapy and radiation therapy.30
LITT has also been used to gauge the efficacy of MRIs in identifying residual tumor tissue. Mahammadi et al., found that in post-LITT MRIs for GMs, areas where diffusion-weighted imaging signal is decreased and the diffusion coefficient along the tumor border is increased may be indicative of residual tumor. These findings suggest that improving MRI and LITT technologies are crucial to improving outcomes for GBM patients treated with LITT.31
LITT has also been used as a neoadjuvant for GBM standard-of-care treatments. Recent studies have shown that LITT causes a disruption in the BBB, allowing for improved penetration of autologous immune cells, as well as improve the efficacy of immunotherapeutics, such as ipilimumab and checkpoint inhibitors.2, 32, 33 A retrospective multicenter study comparing GBMs that were treated with LITT and radiochemotherapy to GBMs treated with radiochemotherapy alone identified several predictors of OS and PFS. Higher OS rates strongly correlated with younger age and smaller tumor size.34 Multivariate analysis revealed that extent of ablation was associated with improved PFS; however, there was no significant difference in PFS between the group that received LITT and the group that did not receive LITT.34 Investigators have also proposed combining LITT with cycles of the immunotherapeutic agent bevacizumab. A case report has been published where patients received LITT prior to their bevacizumab treatment scheme. While the 3 patients did not have complications with the combination treatment, all of them experienced local disease progression.35
While there are variable outcomes for patients who have received LITT to treat primary brain tumors, improvements to LITT technologies can greatly enhance the efficacy of this treatment for neuro-oncology patients. Additional work is required to comprehensively evaluate the potential benefits of LITT.
Cerebral Metastases
While surgical resection, chemotherapy, stereotactic radiosurgery (SRS), and whole-brain radiation have been standard regimens to treat cerebral metastases, LITT is a promising option for patients who are resistant to these treatments and cannot withstand surgery (Table 2). Broadly, LITT does not adversely impact patient outcomes and is associated with minor postoperative complications.
Table 2.
Clinical Trials for Cerebral Metastasis and Radiation Necrosis
| Study | System | Patients (n) | Lesion | Pretreatment Lesion Volume | Treatment Time | Lesion Volume Ablated (%) | Imaging Modality | Complications |
|---|---|---|---|---|---|---|---|---|
| Sujijantarat et al.74 | NeuroBlate | 25 | Radiation necrosis | 0.3–12.6 cc | NR | 65% | MRI | Seizure, hemiparesis, DVT |
| Ahluwalia et al.63 | NeuroBlate | 42 | Metastasis, Radiation necrosis | 0.4–38.6 cm3 | NR | NR | MRI | Seizures, hemiparesis, cerebral edema |
| Carpentier et al.75 | Visualase | 6 | Metastasis | 9–25 mm diameter | 30–225 sec | NR | MRI | None |
| Carpentier et al.76 | Visualase | 7 | Metastasis | NR | 30–180 sec | 43%–50% contrast-enhancing tumor | MRI | Hemorrhage, cerebellar syndrome |
| Rennert et al.73 | Neuroblate | 34 | Metastasis | NR | NR | NR | MRI | NR |
Abbreviations: MRI, magnetic resonance imaging; NR, not recorded.
Forty-two patients participating in a prospective multicenter open-label phase II study reported a median OS of 86.5% at 12 weeks and 72.2% at 26 weeks, with 48% of patients experiencing a complete response.35 However, this study must be carefully considered since one patient opted for chemotherapy between assessments and only 16 patients were followed-up. A systematic review of 13 LITT-treated metastases articles reported a 1-year survival rate ranging between 0% and 65%.36
Clinical studies have shown comparable clinical outcomes between LITT and surgical resection. Compared to surgical resection, there was no significant difference in OS, PFS, or steroid use 1 month-post treatment. A 75-patient clinical study where one group of patients were surgically treated for their metastases and another group underwent LITT with similar tumors (surgery: 41, LITT: 34) reported a negligible difference in clearance of preoperative symptoms for the patients who received surgery (89.7%) compared to those who were treated with LITT (87.0%).37,38 Additionally, there was no difference between OS (surgery: 49.5% vs LITT: 56.6%), 2-year PFS (surgery: 61.1% vs LITT: 60.0%), or steroid use 1 month-post treatment (surgery: 47.4% vs LITT: 34.8%). Moreover, when accounting for tumor size, investigators found no significant difference between OS and PFS for tumors smaller than 3 cm3.37
There is a paucity of studies evaluating LITT outcomes for posterior fossa metastases, however, outcomes from 2 small studies where posterior fossa lesions were treated seem promising.39,40 In one study where 4 patients with cerebellar metastases received LITT, patients reported clearance of preoperative symptoms without the use of steroids.39 Tumor volume increased to, on average, 486.9% of the original tumor size 1-day post-LITT. Depending on the location, an increase in tumor volume can be characteristic for posterior fossa tumors after LITT. The extrapolated average time for the tumor size to reduce below original size was 294.5 days. Edema volume from the preoperative MRI decreased from 17.8 cm3 to 3.4 cm3 on the most recent postoperative MRI during follow-up.39 Another case series involved using LITT to treat 8 patients with posterior fossa tumors.40 Tumor types included 3 cerebral metastases and 3 primary brain tumors (2 pilocytic astrocytomas and 1 GBM), as well as 2 radiation necrosis (RN) lesions. Two patients (cerebral metastasis and GBM) were reported to have local disease progression. One metastasis patient required surgical resection of the tumor 7.7 months post-LITT; however, the remaining 7 patients experienced tumor shrinkage or stability at a median follow-up of 14.8 months.
Studies have consistently shown that the cerebral metastases outcomes are associated with the extent of ablation. Specifically, the volume of ablation closely correlates with longer PFS rates. Ahluwalia et al., noted a local disease progression rate of 25% and 62.5% for patients with completely ablated metastases and partially ablated metastases, respectively.35 In Alattar et al., where LITT was performed after patients underwent radiation therapy, tumors that were completely ablated had improved local control, defined as larger preoperative contrast enhancing Volume (cEV) compared to smaller postoperative cEV, and PFS.36 Six months post LITT, local control rates were 60% for patients with partially ablated metastases and 85% for patients with completely ablated metastases. Furthermore, tumor progression was not seen in patients with complete tumor ablation. Another retrospective study of 25 patients where 24 were treated with LITT prior to undergoing surgical resection and/or radiosurgery for their metastases reported a significantly longer PFS when more than 97% of the tumor was ablated.5,41 When accounting for tumor size, there was significantly shorter PFS for tumors larger than the median volume of lesions (5.62 cm3), proving that PFS correlates with tumor size. Despite findings describing strong associations between tumor size and PFS, investigators must continue to explore the relationship between these two variables.5
Radiation Necrosis
LITT for RN has been a large area of study over the past decade. RN is a common, late complication of SRS, and may lead to complications in up to 32% of patients.42 Rahmathulla et al. introduces LITT as an attractive potential treatment for medically refractory RN, due to its minimally invasive nature and low adverse risk profile compared to other RN treatments.43 In the multicenter prospective trial assessing the efficacy of NeuroBlate for post-SRS LITT, Ahluwalia et al. found that in 19 patients with biopsy-proven RN, there was a 91% PFS rate upon last follow up.35 Smith et al. specifically examined the cases of 25 patients with confirmed post-radiation treatment effect or RN, and found a statistically significant increase in postoperative mental health and vitality scores at 12 months after treatment.44 A potential benefit of LITT in the treatment paradigm for post-SRS RN is the ability to minimize prolonged steroid use. In a recent multicenter study on 72 post-SRS RN patients, Sankey et al. found that patients who underwent LITT (vs medical management) stopped steroids a median 208 days earlier, and were three times more likely to be weaned off steroids by the end of the study.45
Spinal Metastases
Over the past decade, LITT has been trialed in patients with metastatic spinal tumors, as an alternative treatment option to separation surgery. Ahrar and Stafford first reported LITT in the spine, and found that LITT was safe and reliable, however, patients did not have epidural disease burden.46 Tatsui et al. performed spinal laser interstitial thermotherapy (SLITT) in conjunction with spinal stereotactic radiosurgery in 11 patients with spinal metastases to determine if SLITT could function as a suitable alternative to separation surgery.47 The degree of epidural spinal cord compression (ESCC) was scored, as well as the tumor thickness. The median preoperative ESCC score was significantly higher than the postoperative score.47 The 2-month follow-up also revealed a significant decrease in mean tumor thickness from 8.82 mm to 6.36 mm. There was no evidence of a change in thickness during patient follow-up ranging from 3.4 to 6.6 months following treatment. Additionally, there was a significant decrease in the mean Visual Analog Scale (VAS) score for pain from 6.18 preoperatively, to 4.27 at the 1-month follow-up and 2.18 at the 2-month follow-up. The VAS score for quality of life increased by 10% following treatment.47
Low-grade Gliomas
Gliomas with IDH 1 and 2 mutations are genetically distinct subtype of glioma, associated with younger age of onset and prolonged survival compared to IDH wildtype tumors.48 LITT has been shown in several cases to be an effective treatment alternative to open surgery in cases of tumors that are difficult to access and may lead to increased morbidity with surgical resection, or in achieving seizure freedom.49,50 In a single-center, retrospective study, Johnson et al. found that in 22 patients with IDH 1/2 mutant gliomas receiving LITT, 22.7% experienced progression with a median follow-up of 1.8 years.51 Further studies are warranted to better understand the effect of laser therapy on LGG tumors and patient outcomes.
Meningiomas
In addition to intra-axial lesions, LITT has also been trialed in dural-based lesions, such as meningiomas. Several small cases series have found that LITT may be a useful alternative in the case of tumor progression and poor surgical candidacy.52–54 In a series by Ivan et al., grade I meningiomas had a 52% reduction in size after 3 months following treatment.53 After 7–10 months posttreatment, no patients demonstrated radiographic recurrence.54 LITT will likely have a limited role in treatment of meningiomas due to favorable surgical outcomes, however, there may be a role in these select cases.
LITT and Radiotherapy
In addition to conventional external beam radiation therapy, interstitial radiation treatments, known as brachytherapy, have been employed to deliver local radiation to the tumor bed. This may be particularly useful in the case of HGGs, where the majority of recurrence is in close proximity to the site of initial tumor core.55 Gammatile therapy (GTT), an FDA-approved therapy for recurrent tumors where surgical resection is able to be performed, delivers a large dose of local radiation, while sparing surrounding tissue.56 Compared to external beam radiotherapy (EBRT), GTT reduces the risk of radiation-induced necrosis, and has been shown to be more cost-effective compared to EBRT.57 However, use of GTT eliminates the possibility of adjuvant EBRT, which may be problematic in the case of local recurrence beyond the tumor-treated field. Both LITT and brachytherapy have been shown to disrupt the BBB, with peak permeability 1–2 weeks post-procedure, allowing for potentially increased efficacy of adjuvant chemotherapy.58 In a review of 5 studies of patients with recurrent gliomas, Banerjee et al., found similar survival rates with LITT compared to high-dose brachytherapy.59 Additionally, cerebral metastatic lesions previously treated with LITT with local recurrence that received salvage brachytherapy have shown potential benefit for local control.60 Despite this preliminary evidence, there are no prospective published trials to date examining the role for brachytherapy for LITT-refractory lesions, or the role for LITT in multifocal GBM or cerebral metastases where surgical resection and brachytherapy have been used for other lesions. Further investigation is warranted to better establish the relationship between salvage therapies.
Future Directions
Many prior studies have referenced small sample sizes as a major limitation that impacted validity.44,47,61–63 In addition, the lack of baseline for both pre- and postoperative therapies that patients received caused difficulty in establishing a direct association between LITT and positive outcomes.37 Another commonly mentioned limitation was biases associated with patient selection based on a number of varying factors.22,61,62,64 Future clinical trials that include a larger cohort of patients and that are designed to control for confounding variables associated with differences in systemic treatment and selection biases would be greatly beneficial. In addition, future studies should include more prolonged follow-up times to truly determine the efficacy of LITT.11,44,47,64 The treatment’s value would also be better elucidated if subsequent trials directly compared treatment groups to one another, such as the study performed by Hong et al.37 and to a control group.7,44,47,63 Currently, there are active studies of LITT for both newly diagnosed as well as recurrent GBM (NCT02970448, NCT04181684, NCT04699773). Future areas of study include expanding upon initial studies in low-grade gliomas49,50 pediatric brain tumors,65 and recurrent extra-axial lesions.53 There is an expanding role for use of laser therapy for a wide variety of brain tumors, and future trials are needed to better assess patient outcomes as an alternative to open surgery.
From the first clinical trials examining LITT, the treatment has been known to both stimulate the immune system, as well as increase the permeability of the BBB. Fever-range hyperthermia (defined as above 38°C) resulting from laser hyperthermia elicits both an innate (via natural killer cell activity) and adaptive immune response (via antigen-presenting cells and T cells), as well as increases the production of heat shock proteins, which upregulate the anti-tumoral immune response.66 Leuthardt et al. observed a series of patients with recurrent GBM who underwent LITT ablation and found that BBB disruption was present immediately following LITT, with a peak three weeks post-procedure, and BBB closure after 6 weeks.58 Additionally, it was determined that BBB disruption was not only present at the tumor margin but in a peritumoral zone approximately 1–2 cm beyond the radiographic margin. This spatial and temporal window to the tumor microenvironment allows the opportunity for drug delivery, including chemotherapy and immunotherapy drug agents. Despite current ongoing trials, there are no current randomized controlled trials assessing the efficacy of LITT with adjuvant immunotherapy in the treatment of brain tumors.
Conclusion
For the past 3 decades, LITT has been used for the treatment of brain tumors. As it is not currently feasible to have randomized controlled trials assessing the utility of LITT for the treatment of brain tumors, prospective studies and case series have shown that LITT is safe and effective in providing cytoreduction and local disease control for primary and metastatic brain lesions. The applications for laser therapy have broadened beyond neurosurgical oncology to other areas of neurosurgery such as epilepsy surgery, and as the technology improves, further applications may be on the horizon.
Contributor Information
Alexander J Schupper, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, New York, USA.
Tori Chanenchuk, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, New York, USA.
Anna Racanelli, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, New York, USA.
Gabrielle Price, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, New York, USA.
Constantinos G Hadjipanayis, Department of Neurosurgery, Icahn School of Medicine, Mount Sinai Downtown Union Square, Mount Sinai Health System, New York, New York, USA.
Supplement sponsorship. This supplement is sponsored by GT Medical Technologies, Inc., the makers of GammaTile® Therapy for brain tumors. GammaTile is FDA-cleared to deliver radiation therapy in patients with newly diagnosed malignant intracranial neoplasms and recurrent intracranial neoplasms. For full product and safety information, refer to the instructions for use.
Conflict of Interest. Constantinos Hadjipanayis is a consultant for NXDC (NX Development Corporation), Synaptive Medical Inc, Stryker Corp, and Hemerion. He receives royalties from NXDC.
Copyrighted Material/Consent Forms. This manuscript did not use previously copyrighted materials or signed patient consent forms.
References
- 1. Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700–709. [DOI] [PubMed] [Google Scholar]
- 2. Melnick K, Shin D, Dastmalchi F, et al. Role of laser interstitial thermal therapy in the management of primary and metastatic brain tumors. Curr Treat Options Oncol. 2021;22(12):108. [DOI] [PubMed] [Google Scholar]
- 3. Fine S, Klein E, Nowak W, et al. Interaction of laser radiation with biologic systems. I. Studies on interaction with tissues. Fed Proc. 1965;24(Suppl 14):35–47. [PubMed] [Google Scholar]
- 4. Stellar S. A Study of the Effects of Laser Light on Nervous Tissue. New York: New York University; 1967. [Google Scholar]
- 5. Salehi A, Kamath AA, Leuthardt EC, Kim AH. Management of intracranial metastatic disease with laser interstitial thermal therapy. Front Oncol. 2018;8:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosomoff HL, Carroll F. Reaction of neoplasm and brain to laser. Arch Neurol. 1966;14(2):143–148. [DOI] [PubMed] [Google Scholar]
- 7. Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37:E1. [DOI] [PubMed] [Google Scholar]
- 8. Barker FG II, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709–723. [DOI] [PubMed] [Google Scholar]
- 9. Rahmathulla G, Recinos PF, Kamian K, et al. MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. 2014;87(2):67–82. [DOI] [PubMed] [Google Scholar]
- 10. Bhuyan BK. Kinetics of cell kill by hyperthermia. Cancer Res. 1979;39(6 Pt 2):2277–2284. [PubMed] [Google Scholar]
- 11. Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors—the neuroblate system. Expert Rev Med Devices. 2014;11(2):109–119. [DOI] [PubMed] [Google Scholar]
- 12. Ascher PW:. Newest ultrastructural findings after the use of a CO2-laser on CNS tissue. Acta Neurochir Suppl (Wien) 1979;28(2):572–581. [PubMed] [Google Scholar]
- 13. Menovsky T, Beek JF, van Gemert MJ, Roux FX, Bown SG. Interstitial laser thermotherapy in neurosurgery: a review. Acta Neurochir (Wien). 1996;138(9):1019–1026. [DOI] [PubMed] [Google Scholar]
- 14. Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery 2019;84(4):836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holste KG, Orringer DA. Laser interstitial thermal therapy. Neurooncol Adv. 2019;2(1):vdz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016;41(4):E12. [DOI] [PubMed] [Google Scholar]
- 18. Schober R, Bettag M, Sabel M, Ulrich F, Hessel S. Fine structure of zonal changes in experimental Nd:YAG laser-induced interstitial hyperthermia. Lasers Surg Med. 1993;13(2):234–441. [DOI] [PubMed] [Google Scholar]
- 19. Schwabe B, Kahn T, Harth T, Ulrich F, Schwarzmaier HJ. Laser-induced thermal lesions in the human brain: short- and long-term appearance on MRI. J Comput Assist Tomogr. 1997;21(5):818–825. [DOI] [PubMed] [Google Scholar]
- 20. Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr. 1994;18(4):519–532. [DOI] [PubMed] [Google Scholar]
- 21. Sugiyama K, Sakai T, Fujishima I, et al. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg. 1990;54–55:501–505. [DOI] [PubMed] [Google Scholar]
- 22. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208–215. [DOI] [PubMed] [Google Scholar]
- 23. Montemurro N, Anania Y, Cagnazzo F, Perrini P. Survival outcomes in patients with recurrent glioblastoma treated with laser interstitial thermal therapy (LITT): a systematic review. Clin Neurol Neurosurg. 2020;195:105942. [DOI] [PubMed] [Google Scholar]
- 24. Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 Suppl Operative):133–144; 144– 145. [DOI] [PubMed] [Google Scholar]
- 25. Skandalakis GP, Rivera DR, Rizea CD, et al. Hyperthermia treatment advances for brain tumors. Int J Hyperthermia. 2020;37(2):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Archavlis E, Tselis N, Birn G, et al. Survival analysis of HDR brachytherapy versus reoperation versus temozolomide alone: a retrospective cohort analysis of recurrent glioblastoma multiforme. BMJ Open. 2013;3(3):e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu EK, Sulman EP, Wen PY, Kurz SC. Novel Therapies for glioblastoma. Curr Neurol Neurosci Rep. 2020;20(7):19. [DOI] [PubMed] [Google Scholar]
- 28. Lee I, Kalkanis S, Hadjipanayis CG. Stereotactic laser interstitial thermal therapy for recurrent high-grade gliomas. Neurosurgery. 2016;79(Suppl 1):S24–S34. [DOI] [PubMed] [Google Scholar]
- 29. Ivan ME, Mohammadi AM, De Deugd N, et al. Laser ablation of newly diagnosed malignant gliomas: a meta-analysis. Neurosurgery. 2016;79(Suppl 1):S17–S23. [DOI] [PubMed] [Google Scholar]
- 30. de Groot JF, Kim AH, Prabhu S, et al. Efficacy of laser interstitial thermal therapy (LITT) for newly diagnosed and recurrent IDH wild-type glioblastoma. Neurooncol Adv. 2022;4(1):vdac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahammedi A, Bachir S, Escott EJ, Barnett GH, Mohammadi AM, Larvie M. Prediction of recurrent glioblastoma after laser interstitial thermal therapy: the role of diffusion imaging. Neurooncol Adv. 2019;1(1):vdz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naylor MF, Zhou F, Geister BV, et al. Treatment of advanced melanoma with laser immunotherapy and ipilimumab. J Biophotonics. 2017;10(5): 618–622. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Chongsathidkiet P, Crawford BM, et al. Plasmonic gold nanostarmediated photothermal immunotherapy for brain tumor ablation and immunologic memory. Immunotherapy. 2019;11(15):1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammadi AM, Sharma M, Beaumont TL, et al. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: a multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery 2019;85(6):762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–811. [DOI] [PubMed] [Google Scholar]
- 36. Alattar AA, Bartek J, Chiang VL, Mohammadi AM, et al. Stereotactic laser ablation as treatment for brain metastases recurring after stereotactic radiosurgery: a systematic literature review. World Neurosurg 2019;128:134–142. [DOI] [PubMed] [Google Scholar]
- 37. Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142(2):309–317. [DOI] [PubMed] [Google Scholar]
- 38. Moravan MJ, Fecci PE, Anders CK, et al. Current multidisciplinary management of brain metastases. Cancer 2020;126(7):1390–1406. [DOI] [PubMed] [Google Scholar]
- 39. Eichberg DG, VanDenBerg R, Komotar RJ, Ivan ME. Quantitative volumetric analysis following magnetic resonance-guided laser interstitial thermal ablation of cerebellar metastases. World Neurosurg. 2018;110:e755–e765. [DOI] [PubMed] [Google Scholar]
- 40. Borghei-Razavi H, Koech H, Sharma M, et al. Laser interstitial thermal therapy for posterior fossa lesions: an initial experience. World Neurosurg. 2018;117:e146–e153. [DOI] [PubMed] [Google Scholar]
- 41. Bastos DCA, Rao G, Oliva ICG, et al. Predictors of local control of brain metastasis treated with laser interstitial thermal therapy. Neurosurgery 2020;87(1):112–122. [DOI] [PubMed] [Google Scholar]
- 42. Williams BJ, Suki D, Fox BD, et al. Stereotactic radiosurgery for metastatic brain tumors: a comprehensive review of complications. J Neurosurg. 2009;111:439–448. [DOI] [PubMed] [Google Scholar]
- 43. Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200. [DOI] [PubMed] [Google Scholar]
- 44. Smith CJ, Myers CS, Chapple KM, Smith KA. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(Suppl 1):S59–S72. [DOI] [PubMed] [Google Scholar]
- 45. Sankey EW, Grabowski MM, Srinivasan ES, Griffin AS, Howell EP, Otvos B, Tsvankin V, Barnett GH, Mohammadi AM, Fecci PE. Time to steroid independence after laser interstitial thermal therapy vs medical management for treatment of biopsy-proven radiation necrosis secondary to stereotactic radiosurgery for brain metastasis. Neurosurgery. 2022;90(6):684–690. [DOI] [PubMed] [Google Scholar]
- 46. Ahrar K, Stafford RJ. Magnetic resonance imaging-guided laser ablation of bone tumors. Techn Vasc Interv Radiol. 2011;14(3):177–182. [DOI] [PubMed] [Google Scholar]
- 47. Tatsui CE, Stafford RJ, Li J, et al. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J Neurosurg Spine. 2015;23(4):400–411. [DOI] [PubMed] [Google Scholar]
- 48. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hafez DM, Liekweg C, Leuthardt EC. Staged laser interstitial thermal therapy (LITT) treatments to left insular low-grade glioma. Neurosurgery. 2020;86(3):E337–E342. [DOI] [PubMed] [Google Scholar]
- 50. Easwaran TP, Lion A, Vortmeyer AO, et al. Seizure freedom from recurrent insular low-grade glioma following laser interstitial thermal therapy. Childs Nerv Syst. 2020;36(5):1055–1059. [DOI] [PubMed] [Google Scholar]
- 51. Johnson GW, Han RH, Smyth MD, Leuthardt EC, Kim AH. Laser interstitial thermal therapy in grade 2/3 IDH1/2 mutant gliomas: a preliminary report and literature review. Curr Oncol. 2022;29(4):2550–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rammo R, Scarpace L, Nagaraja T, Lee I. MR-guided laser interstitial thermal therapy in the treatment of recurrent intracranial meningiomas. Lasers Surg Med. 2018;51:245–250. 10.1002/lsm.23045. [DOI] [PubMed] [Google Scholar]
- 53. Ivan ME, Diaz RJ, Berger MH, et al. Magnetic resonance-guided laser ablation for the treatment of recurrent dural-based lesions: a series of five cases. World Neurosurg. 2017;98:162–170. 10.1016/j.wneu.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 54. Ruiz A, Diaz RJ, Buttrick S, et al. Preliminary experience on laser interstitial thermal ablation therapy in the treatment of extra-axial masses indications, imaging characterization and outcomes. Cureus. 2018;10(6):e2894. 10.7759/cureus.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Archavlis E, Tselis N, Birn G, Ulrich P, Zamboglou N. Salvage therapy for recurrent glioblastoma multiforme: a multimodal approach combining fluorescence-guided resurgery, interstitial irradiation, and chemotherapy. Neurol Res. 2014;36(12):1047–1055. [DOI] [PubMed] [Google Scholar]
- 56. Mahase SS, Navrazhina K, Schwartz TH, Parashar B, Wernicke AG. Intraoperative brachytherapy for resected brain metastases. Brachytherapy 2019;18(3):258–270. [DOI] [PubMed] [Google Scholar]
- 57. Parikh BB, Neil EC. Evolving strategies to potentially further optimize surgical interventions in brain cancer. Curr Oncol Rep. 2020;22(4):32. [DOI] [PubMed] [Google Scholar]
- 58. Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM, Shimony JS, Tran DD. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Banerjee C, Snelling B, Berger MH, et al. The role of magnetic resonance-guided laser ablation in neurooncology. Br J Neurosurg. 2015;29(2):192–196. [DOI] [PubMed] [Google Scholar]
- 60. Yu KKH, Imber BS, Moss NS. Multimodality durable salvage of recurrent brain metastases refractory to LITT, SRS and immunotherapy with resection and cesium-131 brachytherapy: case report and literature review. BMJ Case Rep. 2021;14(12):e245369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202. [DOI] [PubMed] [Google Scholar]
- 63. Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2019;130:804–811. [DOI] [PubMed] [Google Scholar]
- 64. Le S, Ho AL, Fisher RS, et al. Laser interstitial thermal therapy (LITT): Seizure outcomes for refractory mesial temporal lobe epilepsy. Epilepsy Behav. 2018;89:37–41. [DOI] [PubMed] [Google Scholar]
- 65. Tovar-Spinoza Z, Choi H. Magnetic resonance-guided laser interstitial thermal therapy: report of a series of pediatric brain tumors. J Neurosurg. 2016;17:723–733. [DOI] [PubMed] [Google Scholar]
- 66. Hatzfeld-Charbonnier AS, Lasek A, Castera L, et al. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol. 2007 May;81(5):1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl (Wien). 1991;52:81–83. doi: 10.1007/978-3-7091-9160-6_23. PMID: 1792975. [DOI] [PubMed] [Google Scholar]
- 68. Sakai T, Fujishima I, Sugiyama K, Ryu H, Uemura K. Interstitial laserthermia in neurosurgery. J Clin Laser Med Surg. 1992;10(1):37–40. doi: 10.1089/clm.1992.10.37. PMID: 10149909. [DOI] [PubMed] [Google Scholar]
- 69. Reimer P, Bremer C, Horch C, Morgenroth C, Allkemper T, Schuierer G. MR-monitored LITT as a palliative concept in patients with high grade gliomas: preliminary clinical experience. J Magn Reson Imaging. 1998;8(1):240–244. doi: 10.1002/jmri.1880080140. PMID: 9500287. [DOI] [PubMed] [Google Scholar]
- 70. Leonardi MA, Lumenta CB, Gumprecht HK, von Einsiedel GH, Wilhelm T. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR-unit. Minim Invasive Neurosurg. 2001;44(1):37–42. doi: 10.1055/s-2001-13581. PMID: 11409310. [DOI] [PubMed] [Google Scholar]
- 71. Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical expierence. Minim Invasive Neurosurg. 2002;45(4):201–207. doi: 10.1055/s-2002-36203. PMID: 12494354. [DOI] [PubMed] [Google Scholar]
- 72. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017. doi: 10.1227/NEU.0000000000000144. PMID: 24056317; PMCID: PMC3871404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rennert RC, Khan U, Tatter SB, et al. Patterns of clinical use of stereotactic laser ablation: analysis of a multicenter prospective registry. World Neurosurg. 2018;116:e566–e570. doi: 10.1016/j.wneu.2018.05.039. Epub 2018 May 14. PMID: 29772367. [DOI] [PubMed] [Google Scholar]
- 74. Sujijantarat N, Hong CS, Owusu KA, et al. Laser interstitial thermal therapy (LITT) vs. bevacizumab for radiation necrosis in previously irradiated brain metastases. J Neurooncol. 2020;148(3):641–649. doi: 10.1007/s11060-020-03570-0. Epub 2020 Jun 29. PMID: 32602021. [DOI] [PubMed] [Google Scholar]
- 75. Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21-8; discussion ONS28-9. doi: 10.1227/01.neu.0000335007.07381.df. PMID: 18728600. [DOI] [PubMed] [Google Scholar]
- 76. Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950. doi: 10.1002/lsm.21138. Epub 2011 Nov 22. PMID: 22109661. [DOI] [PubMed] [Google Scholar]