Abstract
BACKGROUND
The key oncogene B-cell lymphoma 6 (BCL6) drives malignant progression by promoting proliferation, overriding DNA damage checkpoints and blocking cell terminal differentiation. However, its functions in the placenta and the endometrium remain to be defined.
OBJECTIVE AND RATIONALE
Recent studies provide evidence that BCL6 may play various roles in the human placenta and the endometrium. Deregulated BCL6 might be related to the pathogenesis of pre-eclampsia (PE) as well as endometriosis. In this narrative review, we aimed to summarize the current knowledge regarding the pathophysiological role of BCL6 in these two reproductive organs, discuss related molecular mechanisms, and underline associated research perspectives.
SEARCH METHODS
We conducted a comprehensive literature search using PubMed for human, animal and cellular studies published until October 2021 in the following areas: BCL6 in the placenta, in PE and in endometriosis, in combination with its functions in proliferation, fusion, migration, invasion, differentiation, stem/progenitor cell maintenance and lineage commitment.
OUTCOMES
The data demonstrate that BCL6 is important in cell proliferation, survival, differentiation, migration and invasion of trophoblastic cells. BCL6 may have critical roles in stem/progenitor cell survival and differentiation in the placenta and the endometrium. BCL6 is aberrantly upregulated in pre-eclamptic placentas and endometriotic lesions through various mechanisms, including changes in gene transcription and mRNA translation as well as post-transcriptional/translational modifications. Importantly, increased endometrial BCL6 is considered to be a non-invasive diagnostic marker for endometriosis and a predictor for poor outcomes of IVF. These data highlight that BCL6 is crucial for placental development and endometrium homeostasis, and its upregulation is associated with the pathogenesis of PE, endometriosis and infertility.
WIDER IMPLICATIONS
The lesson learned from studies of the key oncogene BCL6 reinforces the notion that numerous signaling pathways and regulators are shared by tumors and reproductive organs. Their alteration may promote the progression of malignancies as well as the development of gestational and reproductive disorders.
Keywords: B-cell lymphoma 6, placenta, pre-eclampsia, endometriosis, infertility, proliferation, migration, fusion and differentiation, inflammation, stem and progenitor cells
Introduction
B-cell lymphoma 6 (BCL6), a key oncogene, is a master regulator of humoral immunity and lymphoma survival (Bunting and Melnick, 2013; Cardenas et al., 2017). Interestingly, recent studies show that it has important functions in trophoblastic cells (Louwen et al., 2014; Muschol-Steinmetz et al., 2016; Jasmer et al., 2017; Ritter et al., 2020) and is upregulated in pre-eclamptic placenta (Enquobahrie et al., 2008; Sitras et al., 2009; Winn et al., 2009; Nishizawa et al., 2011; Xiang et al., 2013; Louwen et al., 2014; Trifonova et al., 2014; Sober et al., 2015; Than et al., 2018; Guo et al., 2021a; Ren et al., 2021) as well as in endometriotic lesions (Evans-Hoeker et al., 2016; Almquist et al., 2017; Gong et al., 2017; Yoo et al., 2017; Likes et al., 2019; Nezhat et al., 2020; Sansone et al., 2021; Shen et al., 2021). These interesting observations provide evidence that this key oncogene may play physiological and pathological roles in the placenta and the endometrium.
BCL6 was identified as a locus affected by chromosomal translocations in diffuse large B-cell lymphomas (Ye et al., 1993). It plays a major role in many lymphomas independent of genetic lesions, where it drives the malignant phenotype by promoting proliferation, deactivating DNA damage checkpoints, and inhibiting cell terminal differentiation (Cardenas et al., 2017). BCL6 is a master regulator of B cell differentiation in germinal centers (GCs), being critical for the initiation and maintenance of GC reactions, and a key oncogene in B-cell lymphomagenesis (Basso and Dalla-Favera, 2010; Hatzi and Melnick, 2014). Beside B cells in GCs, BCL6 is also selectively expressed in follicular helper T cells (TFH) (Nurieva et al., 2009). There is compelling evidence demonstrating that BCL6 is a lineage-defining transcription factor essential for TFH differentiation (Nurieva et al., 2009) and maintenance (Alterauge et al., 2020).
BCL6 binds to canonical DNA sequences in the regulatory region of diverse target genes and recruits corepressor complexes that repress gene transcription. It consists of an N-terminal BTB/POZ domain mediating transcriptional repression, an unstructured middle region containing a second repression domain (RD2), and a series of six C2H2 zinc fingers at the C-terminus that bind to DNA sequences and other proteins (Hatzi and Melnick, 2014). To mediate repression, its N-terminal BTB domain recruits the corepressor proteins SMRT (silencing mediator of retinoic acid and thyroid hormone receptor), NCOR (nuclear receptor corepressor) and BCOR (BCL6 interacting corepressor) to an extended groove motif along the BTB dimer interface (Ahmad et al., 2003; Ghetu et al., 2008), whereas the central RD2 region interacts with CtBP (C-terminal binding protein), NuRD (nucleosome remodeling domain), MTA2 (metastasis-associated protein 2) and HDAC2 (histone deacetylase 2) to assist the repression of genes (Huang et al., 2014). These interactions enable BCL6 to repress the transcription of diverse genes (Basso et al., 2010; Basso and Dalla-Favera, 2012; Hatzi and Melnick, 2014), including proliferation inhibitors such as CDKN1A (cyclin-dependent kinase inhibitor 1A), CDKN1B, CDKN2A and CDKN2B, DNA damage response regulators like ATR (ataxia telangiectasia and rad3-related protein), CHEK1 (checkpoint kinase 1) and TP53 (tumor protein 53) and cell differentiation suppressors including IRF4 (interferon regulatory factor 4) and PRDM1 (positive regulatory domain containing 1) (Hatzi and Melnick, 2014).
As BCL6 is also found in various tissues including skeletal muscle, breast and prostate (Bajalica-Lagercrantz et al., 1998; Logarajah et al., 2003), it is not surprising that numerous studies demonstrate its involvement in solid tumors. Increased BCL6 expression has been reported in breast cancer (Bos et al., 2003; Logarajah et al., 2003; Walker et al., 2015), gastric cancer (Hirata et al., 2009), ovarian cancer (Wang et al., 2015), non-small-cell lung cancer (Sun et al., 2016) and glioblastoma (Ruggieri et al., 2014; Song et al., 2018), which is associated with a range of malignant characteristics including tumor cell proliferation, survival, migration, invasion and therapy resistance (Bos et al., 2003; Hurtz et al., 2011; Walker et al., 2015; Cardenas et al., 2017; Guo et al., 2021b). These observations highlight that BCL6 is also an important oncoprotein in solid tumors, although the precise molecular mechanisms remain to be elucidated.
Intriguingly, BCL6 is richly expressed in the human placenta, and its increased expression is associated with the common disease of pregnancy, pre-eclampsia (PE) (Enquobahrie et al., 2008; Winn et al., 2009; Sitras et al., 2009; Nishizawa et al., 2011; Xiang et al., 2013; Louwen et al., 2014; Trifonova et al., 2014; Sober et al., 2015; Than et al., 2018; Guo et al., 2021a; Ren et al., 2021). Recent studies also demonstrate that BCL6 is a diagnostic marker for endometriosis and its associated infertility (Evans-Hoeker et al., 2016; Almquist et al., 2017; Gong et al., 2017; Yoo et al., 2017; Likes et al., 2019; Nezhat et al., 2020; Sansone et al., 2021; Shen et al., 2021). These data suggest that BCL6 is an important player in normal development as well as in disease of the placenta and the endometrium. In this narrative review, we have summarized the available data and discussed the pathophysiological roles of BCL6 in these two reproductive organs.
Methods
A comprehensive search of the PubMed database was conducted for this narrative review to identify peer-reviewed publications in English until October 2021, focused on the expression and roles of BCL6 in the human placenta, and the pathogenesis of PE and endometriosis. The search included the keywords BCL6, proliferation, migration, invasion, differentiation, fusion, inflammation and infertility, either alone or in combination with ‘placenta’, ‘pre-eclampsia’, ‘endometrium’, ‘endometriosis’ or ‘in vitro fertilization’.
BCL6 in the placenta and pre-eclampsia
The human placenta
The placenta is a transient organ essential for fetal growth and development (Burton and Fowden, 2015). Being a multifunctional organ, the placenta fulfills a plethora of tasks such as providing nutrients and oxygen to the growing fetus, producing/releasing hormones into both the fetal and maternal circulation, and removing waste products (Gude et al., 2004). The placenta also serves as a defense front against pathogens and provides a protective environment through multiple mechanisms including triggering interferon type III signaling, microRNA (miRNA)-mediated autophagy and the nuclear factor-κB pathway (Roland et al., 2016; Kreis et al., 2020). The key events of placental development depend on the differentiation of progenitor cells, called villous cytotrophoblasts (vCTBs), into the syncytiotrophoblast (STB) as well as extravillous trophoblasts (EVTs) (Turco and Moffett, 2019). The STB, formed and maintained by fusion of proliferative progenitor vCTBs, builds the key interface between maternal and fetal blood (Arora et al., 2017). Moreover, vCTBs of the anchoring villi undergo a degree of epithelial-mesenchymal transitions and differentiate into invasive interstitial EVTs (iEVTs), which invade the maternal decidua and are able to remodel the uterine spiral arteries to ensure adequate placental perfusion (O’Tierney-Ginn and Lash, 2014). In addition, these iEVTs also develop and invade into other lumen structures of the uterine wall such as veins, glands, and lymph vessels (Huppertz, 2019). During the early stages of placental development, trophoblast cell lineage specification and differentiation are tightly regulated by a variety of molecular signaling pathways, including Wnt and Notch, which control trophoblast stemness/differentiation and formation of invasive trophoblast progenitors, respectively (Knofler et al., 2019).
Pre-eclampsia
An imbalance between trophoblast proliferation and differentiation causes severe placental pathologies and pregnancy complications such as PE (Huppertz et al., 2002). PE is a gestational hypertensive disorder with a global prevalence of up to 8% (Steegers et al., 2010). It is not only a leading cause of maternal and perinatal mortality and morbidity worldwide, but also a risk factor of cardiovascular and metabolic complications for both mother and offspring later in life (MacKay et al., 2001; Rana et al., 2019). PE is defined by concurrent hypertension (blood pressure ≥ 140/90 mmHg) and proteinuria or any other end-organ damage, including liver or brain, occurring after 20 weeks of gestation (Phipps et al., 2019). Clinically, PE can be classified into distinct subgroups: early-onset PE with delivery at <34 + 0 weeks of gestation; preterm PE with delivery at <37 + 0 weeks of gestation; late-onset PE with delivery at ≥34 + 0 weeks of gestation; and term PE with delivery at ≥37 + 0 weeks of gestation (Poon et al., 2019). Pathologically, PE is generally regarded to progress in two stages: placental dysfunction caused by genetic, maternal and immunological factors, and the release of placental antiangiogenic factors and inflammatory mediators inducing a generalized maternal endothelial activation leading to maternal symptoms such as hypertension and proteinuria (Roberts and Hubel, 2009; Phipps et al., 2019). This may further lead to multiorgan dysfunction (Chappell et al., 2021) and maternal decompensation including eclampsia and the HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome (Flint et al., 2019). Mechanistically, PE is a multifactorial disease that encompasses several subclasses, including the subclass featured with maternal vascular malperfusion and the subclass with signs of allograft rejection (Leavey et al., 2015, 2016, 2019).
The etiology of PE is not completely understood, and defective placentation is considered to be causative for the development of PE. Diverse pathological processes have been proposed for abnormal placentation and placental ischemia, including abnormal spiral artery remodeling and immune intolerance at the maternal–fetal interface leading to the release of soluble toxic factors into the maternal circulation (Phipps et al., 2019; Rana et al., 2019). These soluble factors include high levels of circulating antiangiogenic factors, such as sFLT1 (soluble fms-like tyrosine kinase 1) and soluble endoglin and low levels of circulating proangiogenic factors, like VEGF (vascular endothelial growth factor) and PlGF (placental growth factor), causing an imbalanced angiogenic state, and contribute to maternal manifestations of PE including systemic endothelial dysfunction, inflammation, and organ failures (Romero and Chaiworapongsa, 2013; Rana et al., 2019). The pathological cellular and molecular events in the pre-eclamptic placenta are varied, such as failed transformation of proliferative vCTBs into the invasive endovascular subtype (Zhou et al. 1997), deficient aggrephagy, aberrant pyroptosis, compromised unfolded protein response, abnormal release of placental extracellular vesicles containing miRNAs and long non-coding RNAs (lncRNAs), defective fusion, increased syncytial knots and excessive STB stress (Rana et al., 2019; Redman et al., 2020; Bai et al., 2021; Nakashima et al., 2021).
BCL6 in the placenta and its involvement in pre-eclampsia
Transcriptomic profiling analyses of pre-eclamptic placentas have revealed diverse sets with hundreds of genes specific for placental dysfunction (Sober et al., 2015; Leavey et al., 2016; Brew et al., 2016; Than et al., 2018). Interestingly, BCL6 is one of the prominent genes frequently altered in pre-eclamptic placentas shown by systematic meta- and expression network analysis (Kleinrouweler et al., 2013; Tejera et al., 2013; Vaiman et al., 2013; Brew et al., 2016). In fact, BCL6 has been repeatedly reported to be upregulated in placentas of patients with early- as well as late-onset PE (Table I) (Enquobahrie et al., 2008; Winn et al., 2009; Sitras et al., 2009; Nishizawa et al., 2011; Xiang et al., 2013; Louwen et al., 2014; Trifonova et al., 2014; Sober et al., 2015; Than et al., 2018; Guo et al., 2021a; Ren et al., 2021). Of importance, BCL6 was highly expressed in preterm pre-eclamptic placentas and its overexpression activated the expression of ARNT2 (aryl hydrocarbon receptor nuclear translocator 2) (Than et al., 2018), an interaction partner of hypoxia-inducible factor 1α (HIF-1α) (Maltepe et al., 2000) involved in trophoblast invasion and the pathogenesis of PE (Rosario et al., 2008; Chakraborty et al., 2016). Upregulation of these two transcription regulatory genes, BCL6 and ARNT2, led to dysregulation of most predominant PE-related genes such as FLT1 under hypoxia (Than et al., 2018).
Table I.
BCL6 expression in human pre-eclamptic placentas.
| Reference | Sample number/patient information | Method/dataset/GEO | Main results |
|---|---|---|---|
| Enquobahrie et al. (2008) |
|
Microarray (Operon’s Human Genome Array Ready Oligo Set™ version 2.1) and qPCR. | 58 genes (56 up- and 2 downregulated) were differentially expressed in PE placentas, including upregulated BCL6, with a fold change of 1.78 (P = 0.0154). |
|
| |||
| Winn et al. (2009) |
|
Microarray platform high-density HG-U133A and HGU133B GeneChips (Affymetrix, Santa Clara, CA); GEO accession number: GSE14722. | 55 genes were differentially expressed in PE placentas, including upregulated BCL6, with a fold change of 1.69. |
|
| |||
| Sitras et al. (2009) |
|
30 K Human Genome Survey Microarray v.2.0 (Applied Biosystems); GEO accession number: GSE10588. | 213 genes were significantly upregulated in severe PE placentas, including BCL6, with a fold change of 2.2 (P = 0.01). 82 genes were downregulated. 168 genes were differentially expressed between EO- versus LO-PE placentas, suggesting differences in pathophysiology. |
|
| |||
| Nishizawa et al. (2011) |
|
Microarray experiments with Affymetrix GeneChip system (Affymetrix, Santa Clara, CA, USA). GEO accession number: GSE24129. | 62 genes were differentially expressed in both PE and FGR. TP53-downstream apoptosis-related genes, such as BCL6 and BAX, were found to be more highly upregulated in PE- than in FGR placentas. BCL6 was upregulated in PE placentas with a fold change of 2.02 (P = 0.0024). |
|
| |||
| Xiang et al. (2013) |
|
Microarray experiments with Roche Nimblegen Gene Expression 126135 K Arrays. GEO accession number: GSE43942. | 1312 genes were differentially expressed in PE placentas, including upregulated BCL6 (fold change 2.51, P = 0.0026; Supplementary Table S2). |
|
| |||
| Trifonova et al. (2014) |
|
HT-12 BeadChip microarrays (Illumina, USA). | 63 genes were differentially expressed in PE placentas (50 up- and 13 downregulated). BCL6 was upregulated with a fold change of 2.36 (P = 0.0006). |
|
| |||
| Louwen et al. (2014) |
|
Self-designed TaqMan gene array, Applied Biosystems (Darmstadt, Germany); qPCR verification. | BCL6 gene was upregulated in LO-PE with a fold change of 1.47 (P = 0.087). Increased BCL6 may be involved in deregulated cell cycle regulation, survival, and differentiation processes of villous trophoblasts in pre-eclamptic placentas. |
|
| |||
| Sober et al. (2015) |
|
Library preparation with Nextera™ Technology (Illumina) total RNA sequencing was performed on Illumina HiSeq2000. | 199 and 98 genes were differentially expressed in PE without IUGR and PE with IUGR, respectively. BCL6 was in the top-gene list and upregulated in LO-PE with IUGR (log2 fold change 0.77, P = 0.00081, Supplementary Data S3). |
|
| |||
| Than et al. (2018) |
|
Whole-Human Genome Oligo Microarray G4112A (Agilent Technologies, Santa Clara, CA); GEO accession numbers GSE65866, GSE65940, and GSE66273. | BCL6 upregulation sensitized trophoblasts to ischemia and repressed differentiation (log fold change 1.86, absolute fold change 3.64, P = 0.00065; Supplementary Data S1). BCL6 was highly expressed in preterm but not in term PE placentas, suggesting its potential role only in the pathology of preterm cases. |
| Guo et al. (2021) |
|
The study performed a deeply re-analyzing of publicly available datasets (Agilent-039494 SurePrint G3 Human GE v2 8x60K Microarray 039381 (Feature Number version)). | 1016 genes were differentially expressed in severe EO-PE. Severe LO-PE showed a deregulation of 133 genes. The comparison between EO-PE versus LO-PE identified 226 differentially expressed genes. BCL6 was upregulated only in EO-PE with a log fold change of 1.603 (P = 0.00026). |
|
| |||
| Ren et al. (2021) |
|
TruSeq RNA Kit (Illumina) followed by paired-end sequencing using Illumina Hiseq 2500. | 2977 genes, which are enriched with metabolism-related pathways and transporter functions, were differentially expressed in severe EO-PE, whereas 375 genes, which are enriched with immune-related pathways, were differentially expressed in severe LO-PE. BCL6 was upregulated in both EO- and LO-PE (log2 fold change 0.85, P = 1.51E−06; Supplementary Table S3). |
BCL6, B-cell lymphoma 6; CS, cesarean section; eCS, emergency cesarean section after the onset of labor; EO-PE, early-onset pre-eclampsia; FGR, fetal growth restriction; GEO, Gene Expression Omnibus; HELLP, Hemolysis, Elevated Liver enzymes and Low Platelets; IUGR, intrauterine growth restriction; LO-PE, late-onset pre-eclampsia; opVD, operative vaginal delivery; PE, pre-eclampsia; VD, vaginal delivery; wk, week.
To investigate the significance of BCL6 in normal placental development and the pathogenesis of PE, we have analyzed gene expression of pre-eclamptic placentas compared with control samples using an array containing genes involved in several important signaling pathways (Louwen et al., 2014). The data showed that genes associated with migration and angiogenesis were mostly altered in pre-eclamptic placentas. Notably, the BCL6 gene, among several crucial genes, was significantly increased and correlated with enhanced FLT1 and LEPTIN in pre-eclamptic placenta samples (Louwen et al., 2014), hallmark genes of PE. In support of this observation, BCL6 has been shown to be a key regulator of target genes of three critical pathways in the placenta, namely FOXO (forkhead box O), ribosome and HIF1 signaling (Ren et al., 2021). Collectively, these studies underscore the notion that BCL6 is an important regulator that is deregulated in malignant cells as well as pre-eclamptic trophoblasts (Louwen et al., 2012).
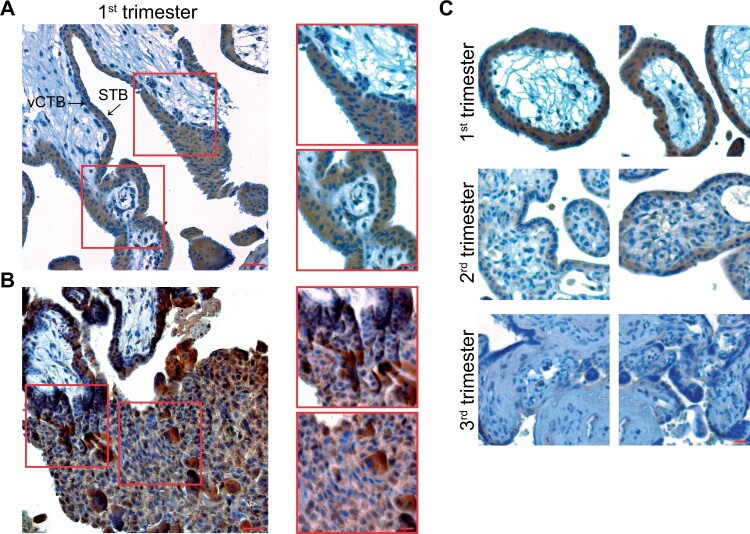
While the BCL6 protein was mainly localized in the nuclei of vCTBs in term placentas (Louwen et al., 2014), it is abundantly present in nuclei as well as in the cytoplasm of vCTBs, the STB and in syncytial sprouts in first trimester placentas (Fig. 1A). BCL6 is also expressed in cell column trophoblasts (CCTs) of anchoring villi in first trimester placentas (Ritter et al., 2020). The high presence of BCL6 in the CCTs, the source for all subtypes of invasive EVTs (Huppertz, 2019), suggests its roles in both proliferation and differentiation in those cells. Moreover, BCL6 is also richly present in iEVTs of the maternal decidua (Fig. 1B). Of note, the expression of BCL6 in vCTBs and the STB decreases during gestation (Fig. 1C). In addition, HTR-8/SVneo cells, a widely used first trimester trophoblast cell line (Graham et al., 1993), express much more BCL6 than other trophoblastic cell lines (Muschol-Steinmetz et al., 2016). These observations suggest that BCL6 may be crucial in early placental development and could be associated with the pathogenesis of PE.
Figure 1.
BCL6 is expressed in various trophoblast types of first trimester placentas and its expression declines during gestation. (A) First trimester placental tissue sections were immunohistochemically stained for BCL6 and DNA. Left: a representative image is shown. Scale: 50 µm. Right: enlarged villi. Scale: 20 µm. STB, syncytiotrophoblast; vCTB, villous cytotrophoblast. (B) Representative EVTs positively stained with BCL6 antibody in the decidua of first trimester placenta (left, scale: 50 µm) and enlarged regions with positive EVTs (right, scale: 20 µm). EVT, extravillous trophoblast. (C) Representative images are shown for first (upper panel), second (middle panel) and third trimester placental tissues (lower panel) stained for BCL6 and DNA. Scale: 50 µm.
Potential molecular mechanisms by which BCL6 is upregulated in pre-eclampsia
The aberrant expression of BCL6 in hematopoietic malignancy is attributed to a variety of mechanisms (Cardenas et al., 2017). These include altered gene transcription (Pasqualucci et al., 2003; Saito et al., 2007; Yang and Green, 2019), deregulated epigenetics (Lai et al., 2010), activated heat shock protein 90 (HSP90) (Cerchietti et al., 2009) and changed post-translational modifications (Yang and Green, 2019) such as phosphorylation (Niu et al., 1998; Phan et al., 2007), acetylation (Bereshchenko et al., 2002) and methylation (Lu et al., 2018). In cancer cells, the heat shock factor 1 (HSF1) was shown to bind to the BCL6 promoter and activate its gene expression upon stress (Fernando et al., 2019). In addition, deregulated miRNAs and lncRNAs were reported to induce aberrant expression of BCL6 in various cancer cells (Wei et al., 2015; Sun et al., 2016; Yan et al., 2016; Li et al., 2018; Wang et al., 2020). These data highlight that BCL6 is upregulated and stabilized in malignant cells through diverse mechanisms including transcriptional and translational regulation and post-translational modifications.
BCL6 may be pivotal in controlling a variety of challenging situations associated with PE, which are characterized by a myriad of diverse stressors such as oxidation, chronic hypoxia and inflammation. To respond to these stresses, the placenta must activate its response machinery, which could give rise to the highly expressed and stabilized BCL6, a central component of the stress response (Phan et al., 2007; Fernando et al., 2019).
Similar to malignant cells, the mechanisms by which BCL6 is upregulated in pre-eclamptic placentas might also be multilateral (Fig. 2). First, HSP90 is a major regulator stabilizing and activating BCL6 (Cardenas et al., 2017), and studies have indicated a link between PE and deregulated HSP90 (Padmini et al., 2012; Ren et al., 2021), which could affect the stability and activity of BCL6 in pre-eclamptic placentas. It will also be of interest to study if PE-related proinflammatory cytokines, such as tumor necrosis factor-alpha (TNFα) and IL8, which increase and activate the HSP members (Jee et al., 2021), could be responsible for the stabilized BCL6 in pre-eclamptic placentas. Second, maternal circulating IL6 was shown to be increased in PE (Lau et al., 2013), which could contribute to the high expression of BCL6, as reported in TFH cells (Nurieva et al., 2009). Third, BCL6 may also be stabilized by post-translational modifications, such as phosphorylation by the mitogen-activated protein kinase (MAPK) and acetylation by the cyclic AMP response element binding protein, which have been reported to be deregulated in pre-eclamptic placentas (Qi et al., 2021; Sadeghi et al., 2021). Fourth, three CpG islands on Chromosome 3 were reported to be differentially methylated in trophoblasts from pre-eclamptic placentas (Than et al., 2018), suggestive of a potential methylation alteration in the BCL6 promoter. Fifth, HSF1 activated BCL6 gene expression in GC B cells as well as in cancer cells under stress (Fernando et al., 2019), and it will be of importance to examine whether HSF1 is increased in stressed trophoblasts and involved in BCL6 gene activation in pre-eclamptic placentas. Sixth, the expression of aberrant miRNAs and lncRNAs has been observed in pregnancy complications including PE (Yang and Meng, 2019; Ali et al., 2020), and deregulated miRNAs may result in enhanced expression of BCL6 in pre-eclamptic placentas. The detailed mechanisms by which BCL6 is highly expressed and stabilized in pre-eclamptic placentas remain to be elucidated.
Figure 2.
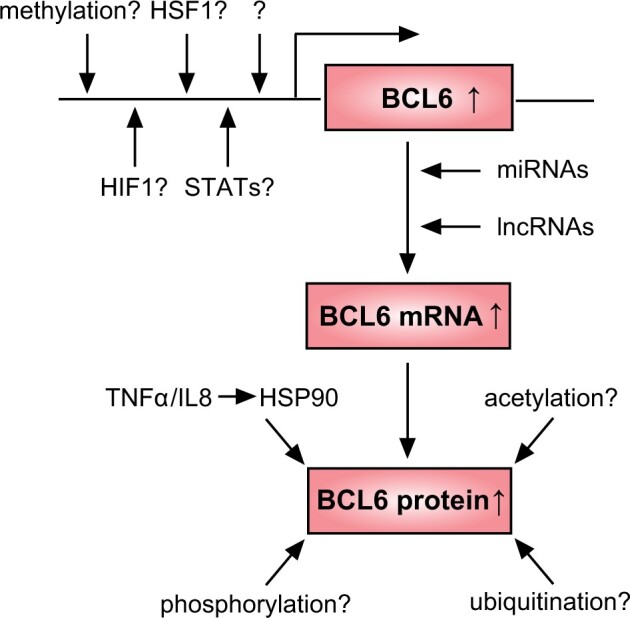
Proposed working model by which BCL6 is upregulated in pre-eclamptic placentas. Upregulation and activation of BCL6 in pre-eclamptic placentas may be caused at transcriptional, translational and post-translation level. HIF1, hypoxia-inducible factor 1; HSF1, heat shock factor 1; HSP90, heat shock protein 90; IL8, interleukin 8; lncRNA, long non-coding RNA; miRNA, microRNA; STAT, signal transducer and activator of transcription; TNFα, tumor necrosis factor alpha.
Functions of BCL6 in trophoblasts of the placenta
BCL6 promotes proliferation and survival of trophoblasts
Rapidly and dynamically regulated proliferation of placental cells, including trophoblasts, mesenchymal stem/stromal cells and endothelial cells, is mandatory for proper placental development. Beginning at implantation, trophoblast lineages derived from extraembryonic tissue expand rapidly to form placental villi during the first weeks of gestation (Hemberger et al., 2020; Aplin and Jones, 2021). During the 40 weeks of gestation, these villi undergo dynamic morphological changes and develop into highly vascularized structures (Burton et al., 2009). By term, extensive branching morphogenesis of the villi results in a total epithelial surface area of ∼12–14 m2 to meet the increasing demands of the growing fetus (Knofler et al., 2019). Dynamic and rapid development of the placenta is highly co-ordinated by spatiotemporal regulation of trophoblast proliferation through a great number of signaling pathways and various regulators.
BCL6 is highly expressed in vCTBs (Fig. 1) and CCTs in the first trimester (Ritter et al., 2020), and its expression is significantly lower in second trimester placentas (Prater et al., 2021), suggestive of its importance in trophoblast expansion and differentiation in early placental development. Indeed, inhibition of BCL6 significantly reduced proliferation and resulted in apoptosis in trophoblastic cell lines, in particular under stress situation (Muschol-Steinmetz et al., 2016). The genes CDKN1A and TP53, coding for the important cell cycle regulators p21 and p53, respectively, are controlled by BCL6 in normal GC B cells (Basso et al., 2010). However, the expression of CDKN1A was hardly changed, whereas TP53 was significantly decreased in trophoblastic cells depleted of BCL6 (Muschol-Steinmetz et al., 2016), indicating that BCL6 may differentially affect gene expression depending on the cellular context. Interestingly, BCL6 upregulation was associated with overexpression of cyclin D1, p53 and HIF-1α in breast cancer tissue (Bos et al., 2003), which might be responsible for enhanced proliferation, invasion and stress response. By targeting distinct genes and pathways, BCL6 functions as a proliferation promoter and survival guardian in various tissues including the placenta. Enhancing BCL6 might be one of the molecular mechanisms that allow cytotrophoblasts to proliferate under low oxygen conditions in early pregnancy, which are normally stressful for cells in other tissues.
BCL6 blocks differentiation and fusion processes of vCTBs
The multinucleated STB has a limited lifespan and must be regularly replenished with fresh cytoplasm and nuclei by controlled differentiation and fusion of its underlying vCTBs (Aplin and Jones, 2021; Jaremek et al., 2021). Importantly, vCTBs are a heterogeneous cell population showing different cellular features with different functions (Aplin and Jones, 2021). Based on single-cell RNA sequencing of human placental samples from the first and second trimester, at least three subpopulations of vCTBs were reported: one population expressed cell cycle genes belonging to the cycling fraction, the second showed high differentiation genes related to cellular fusion such as syncytin 1 and 2, and the third population was proposed to be a G0 ‘reserve’ cell population, possibly progenitor/stem cells (Liu et al., 2018). These observations suggest that vCTB development and progression are highly orchestrated, ensuring a functional STB throughout gestation. Integration of multiple signaling pathways and transcription factors has been implicated in the process of vCTB differentiation, including repression of Wnt and activin/transforming growth factor-beta (TGFβ) signaling as well as activation of cAMP/protein kinase A (PKA) and MAPK pathways (Jaremek et al., 2021). vCTB differentiation also involves the activity of numerous transcription factors and epigenetic regulators, including PPARγ (peroxisome proliferator-activated receptor γ), DLX3 (distal-less homeobox 3), GCM1 (glial cell missing-1), TFAP2A (transcription factor AP-2), OVOL1 (OVO homolog-like 1), p21 (Lu et al., 2017; Kreis et al., 2021) and many others (Knofler et al., 2019). BCL6 might be one of these many factors involved in vCTB differentiation and fusion, based on its functions in other cell types and tissues (Reljic et al., 2000; Logarajah et al., 2003). In fact, BCL6 has been found to be one of the key transcription factors for regulating vCTB differentiation toward the STB (Liu et al., 2018).
Interestingly, suppression of BCL6 using siRNA promoted the fusion rate of BeWo cells, a widely used fusogenic trophoblastic cell line (Zachariades et al., 2011), as well as primary vCTBs by enhancing the levels of fusion-related genes ERVW-1 (endogenous retrovirus group W member 1, syncytin 1), HERV-FRD (endogenous retrovirus group FRD member 1, syncytin 2) and CGB3 (chorionic gonadotrophin beta 3, β-hCG). The amount of secreted β-hCG from cells depleted of BCL6 was increased about 1.5-fold relative to control cells. In contrast, BeWo cells stably overexpressing BCL6, mimicking the situation observed in PE, displayed decreased fusion capacity. Importantly, reduction of BCL6 significantly facilitated the fusion capability of BeWo cells under hypoxia (Jasmer et al., 2017). As knockdown of BCL6 enhanced the expression of the fusion-related genes, such as ERVW-1, HERV-FRD and CGB3, in trophoblastic cell lines as well as in primary vCTBs (Jasmer et al., 2017), it is tempting to suggest that BCL6 could directly target these genes. These data indicate BCL6 as a negative regulator for differentiation and fusion of vCTBs into the STB, and increased BCL6 in pre-eclamptic placenta may impair proper differentiation and successful fusion, contributing to the pathogenesis of PE.
Interestingly, connective tissue growth factor was reported to regulate the fusion of osteoclast precursors by inhibiting BCL6 (Choi et al., 2020). BCL6 was also shown to block differentiation and fusion of osteoclasts in mouse (Miyamoto 2011). Moreover, enhanced BCL6 inhibited lymphocyte terminal differentiation in lymphomagenesis (Reljic et al., 2000). In addition, increased BCL6 in breast tissues impeded differentiation of epithelial cells and supported the progressive development of cancer (Logarajah et al., 2003). These data strongly suggest that BCL6 plays an important role in controlling differentiation in various tissues by targeting the respective genes.
BCL6 facilitates migration and invasion of EVTs
The finely tuned regulation by a multitude of regulators and pathways is fundamental for proper migration and invasion of EVTs. Multiple pathways, such as FAK/Rho (focal adhesion kinase/Rho), PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase B), TGFβ and Wnt signaling, have been reported to regulate migration and invasion of EVTs (Pollheimer and Knofler, 2005; Knofler and Pollheimer, 2012; Knofler et al., 2019). In particular, Wnt signaling is critical for regulating EVT function (Sonderegger et al., 2010). Alterations in the Wnt pathway were reported to contribute to the pathogenesis of PE by affecting migration and invasion of trophoblasts (Wang et al., 2018; Chen et al., 2021). Intriguingly, Wnt signaling is targeted by BCL6 in GC B cells (Basso et al., 2010). BCL6 was also reported to be associated with migration and invasion of breast cancer cells (Yu et al., 2015), glioblastoma cells (Song et al., 2018) and ovarian cancer cells (Wang et al., 2015). It is thus reasonable to hypothesize that BCL6 might play similar roles in trophoblast migration and invasion.
Indeed, as in malignant cells (Bos et al., 2003; Cardenas et al., 2017), BCL6 is crucial in the migration and invasion of trophoblastic cells (Ritter et al., 2020). Knockdown of BCL6 reduced the motility of trophoblastic cells by affecting numerous genes of regulatory proteins in the actin cytoskeleton, including PAK1 (p21 activated kinase 1) and ACTG1 (gamma actin 1), in ECM (extracellular matrix) remodeling such as MMP2 (matrix metalloproteinase 2) and CAV1 (caveolin 1), as well as in the MAPK and PI3K pathway like RPS6KA (ribosomal protein S6 kinase alpha-1) and DUSP16 (dual-specificity protein phosphatase 16) (Ritter et al., 2020). Suppression of BCL6 also decreased the cellular signals of p-FAK, p-Akt and p-Erk1/2, which are important for cell migration and invasion (Ritter et al., 2020). These data imply that BCL6, like in cancer cells, acts as a key player in the migration and invasion of trophoblasts through the regulation of diverse cellular activities, which could be of vital importance in early placental development.
Potential impact of BCL6 on trophoblast stem/progenitor cells
Key events of placental development include the spatially and temporally balanced regulation of stem cell/progenitor maintenance and self-renewal, and their timely differentiation into other cell types (Knofler et al., 2019). The molecular mechanisms that control trophoblastic stem cell/progenitor quiescence, self-renewal, lineage commitment and differentiation into all trophoblast types during early placental development remain to be fully elucidated.
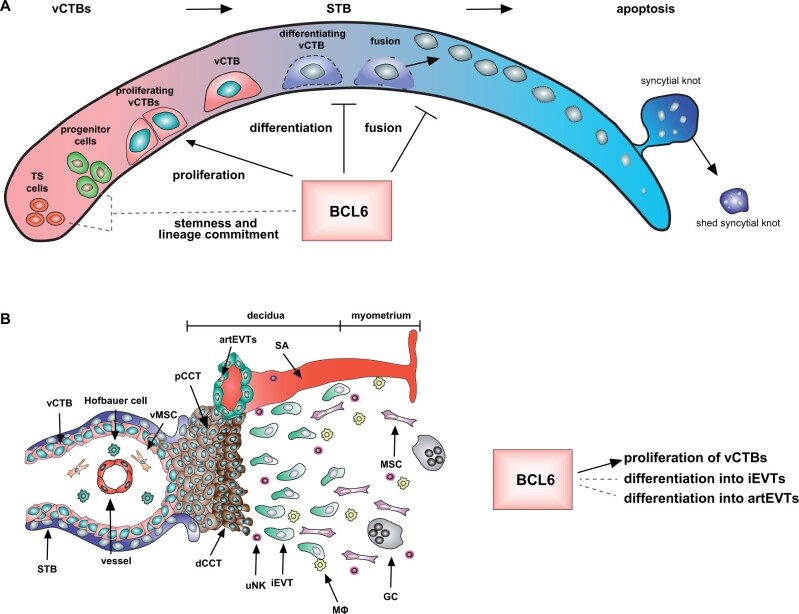
BCL6 has been demonstrated to be involved in the maintenance and differentiation of stem/progenitor cells in various tissues and cell types (Hurtz et al., 2011; Pietras et al., 2014; Tiberi et al., 2014; Bonnefont et al., 2019; Fabre et al., 2020; Solanki et al., 2020; Kawabata et al., 2021; Li et al., 2021). Recently, it has been revealed that the motifs of the two transcription factors, BCL6 and CBX3 (chromobox protein homolog 3), are significantly overrepresented in early placental villous tissues (Prater et al., 2021). Notably, CBX3 is a BCL6 target gene (Polo et al., 2007), indicating a regulatory role of BCL6 over CBX3, strengthening BCL6’s significance in the early placental development. In support of this notion, BCL6 is richly present in vCTBs in first trimester placenta (Ritter et al., 2020) (Fig. 1), and some of vCTBs have been proposed as G0 progenitors/stem cells (Liu et al., 2018; Aplin and Jones, 2021). BCL6 is also highly expressed in CCTs of anchoring villi (Ritter et al., 2020). The proximal CCTs undergo a multistep differentiation process and further differentiate into non-proliferating distal CCTs, ready to invade the maternal decidua (Silini et al., 2020). The high presence of BCL6 in vCTBs and CCTs of first trimester placenta suggests its potential roles in stem/progenitor cell maintenance/survival and differentiation (Fig. 3), which require further investigations.
Figure 3.
BCL6 may affect stemness and differentiation of trophoblast stem/progenitor cells. (A) Potential functions of BCL6 in vCTBs. BCL6 may promote vCTB proliferation, inhibit differentiation and fusion of vCTBs into the STB, and possibly affect stemness and lineage commitment of trophoblast stem/progenitor cells. (B) Potential functions of BCL6 in trophoblasts within the cell column. BCL6 may facilitate proliferation of vCTBs, and affect differentiation of vCTBs into iEVTs and artEVTs. artEVT, endoarterial EVT; dCCT, distant cell column trophoblast; EVT, extravillous trophoblast; GC, giant cell; iEVT, interstitial EVT; MФ, macrophage; pCCT, proximal cell column trophoblast; SA, spiral artery; STB, syncytiotrophoblast; vCTB, villous cytotrophoblast; vMSC, villous mesenchymal stromal cell; uNK, uterine natural killer.
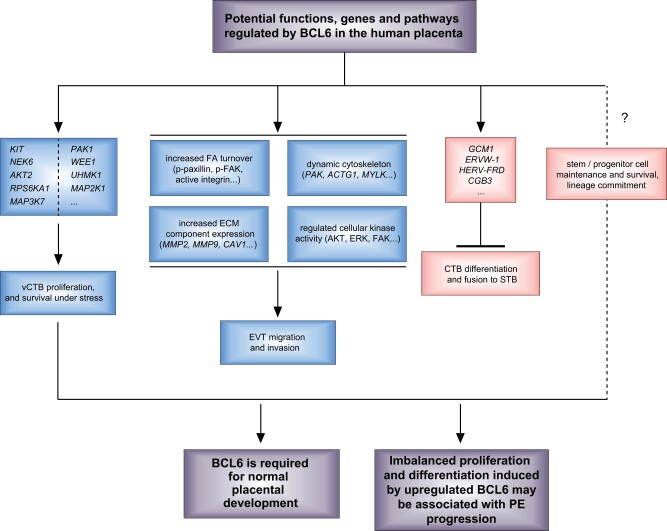
Taken together, BCL6 may play multiple cellular roles in trophoblasts including proliferation, survival, migration, invasion, differentiation and fusion. While increased BCL6 in pre-eclamptic placenta may promote proliferation and survival of vCTBs (Muschol-Steinmetz et al., 2016), and facilitate migration and invasion of EVTs (Ritter et al., 2020), it could impair differentiation and fusion of vCTBs into the STB (Jasmer et al., 2017) and compromise EVT differentiation into further subtypes, such as endoarterial EVTs. This imbalanced proliferation and differentiation might be associated with the progression of PE (Fig. 4).
Figure 4.
Potential functions of BCL6 in the human placenta. BCL6 promotes proliferation and survival of vCTBs, facilitates migration and invasion of EVTs, and inhibits differentiation and fusion of vCTBs into the STB. BCL6 may also affect trophoblast stem/progenitor cell survival and differentiation. Upregulated BCL6 may cause imbalanced proliferation and differentiation, contributing to the pathogenesis of PE. ACTG, actin gamma; AKT2, AKT serine/threonine kinase 2; CAV1, caveolin 1; CGB3, chorionic gonadotropin subunit beta 3; ECM, extracellular matrix; ERVW-1, endogenous retrovirus group W member 1, syncytin1; FAK, focal adhesion kinase; GCM1, glial cells missing transcription factor 1; HERV-FRD, human endogenous retrovirus group FRD, syncytin 2; KIT, receptor tyrosine kinase; MAP2K1, mitogen-activated protein kinase kinase 1; MAP3K7, mitogen-activated protein kinase kinase kinase 7; MAPK, mitogen-activated protein kinase 1; MMP, matrix metallopeptidase; MYLK, myosin light chain kinase; NEK6, NIMA-related kinase 6; PAK1, p21-activated kinase 1; RPS6KA1, ribosomal protein S6 kinase A1; UHMK1, U2 small nuclear RNA auxiliary factor 2 homology motif kinase 1; WEE1, G2 checkpoint kinase.
BCL6 in endometriosis and its related infertility
The uterine endometrium
Deregulated BCL6 is not only linked to PE but also to endometriosis, a common disorder of the uterine endometrium. The uterine endometrium, the inner uterine cell layer, is composed of two layers, the functionalis and the basalis, the latter being responsible for regeneration of the former during the proliferative phase (Cooke et al., 2013). Depending on the phases of the menstrual cycle, the endometrium undergoes cyclical regeneration, differentiation and shedding in response to the steroid hormones estrogen and progesterone under the control of the hypothalamic-pituitary-ovarian axis (Jabbour et al., 2006; Hawkins and Matzuk, 2008; Garry et al., 2009). While estrogen induces mitosis of endometrial cells including luminal and glandular epithelial cells, stromal fibroblasts and vascular components throughout the proliferative phase, progesterone causes secretory transformation of epithelial cells and differentiation of stromal fibroblasts after ovulation. In addition to the regulation by the steroid hormones, these dynamic processes of the endometrium are supported by cytokines/chemokines, such as IL6, IL10 and monocyte chemotactic protein 1, produced by endometrial stromal cells (Large and DeMayo, 2012; Vallve-Juanico et al., 2019).
The extraordinarily high regenerative capacity of the endometrium indicates the presence of stem cell-like endometrial cell populations capable of self-renewal and differentiation. Indeed, numerous studies have demonstrated the presence of a stem cell population in the basalis of the endometrium, which is responsible for the formation of endometrial stromal fibroblasts (Schwab and Gargett, 2007; Masuda et al., 2012; Yin et al., 2019). Additionally, several putative endometrial stem cell-like populations have been identified, including side population cells, endometrial mesenchymal stem cells (eMSCs), and bone marrow mesenchymal stem cells (BM-MSCs) (Yilmaz and Bulun 2019). The side population may represent endometrial stem cells coffering migration and angiogenesis abilities (Masuda et al., 2010), whereas eMSCs are self-renewing, multipotent and clonogenic mesenchymal stem cells giving rise to mesodermal lineages in vitro (Gargett et al., 2016). Additionally, BM-MSCs may improve endometrial regeneration through secreting paracrine factors to stimulate resident endometrial stem cells (Yilmaz and Bulun, 2019). The co-ordinated regulation of these self-renewal and differentiation populations is essential for dynamic endometrial tissue regeneration and homeostasis.
In recent years, enormous progress has been made in the generation and application of self-organizing 3D organoids. 3D organoids of normal and decidualized human endometrium have been established and these organoids were able to differentiate upon treatment with reproductive hormones (Turco et al., 2017). Functionally, human endometrial organoids were successfully used to study ciliogenesis of multi-ciliated endometrial cells (Haider et al., 2019). Recently, a 3D model of the human decidua, consisting of hormone-responsive endometrial stromal fibroblasts and endometrial epithelial cells, was reported (Cheung et al., 2021). These technical developments are of importance in elucidating the pathogenesis of endometrium-related diseases such as endometriosis.
Endometriosis and its related infertility
Endometriosis is an estrogen-dependent inflammatory disorder of the uterine endometrium (Bulun et al., 2019). About 5–10% of women of reproductive age are affected and up to 50% of these women are infertile (Bulun, 2009; de Ziegler et al., 2010; Taylor et al., 2021). The etiology of endometriosis remains to be defined. Among several hypotheses, ‘retrograde menstruation’ (Sampson, 1927a,b) is believed to be the primary mechanism of lesion formation (Smolarz et al., 2021). Important features of endometriosis are the high production of estrogen and deregulated expression of its receptors in endometriotic regions (Chantalat et al., 2020), and progesterone resistance (Patel et al., 2017). Endometriosis is characterized by endometrial-like tissue outside its normal location lining the uterus (Giudice, 2010). Depending on its location, endometriosis can be classified as peritoneal endometriotic implants, rectovaginal nodules and ovarian endometriomas (Bulun et al., 2019; Kuan et al., 2021). The symptoms of endometriosis are dysmenorrhea, dyspareunia, non-cyclic chronic pelvic pain and infertility (Bulun, 2009; Bulun et al., 2019). Its treatment includes pharmacological and surgical therapy (Brichant et al., 2021; Taylor et al., 2021).
Endometriosis is associated with infertility owing to numerous factors such as distorted anatomy, local inflammatory effects on oocyte quality and an inhospitable endometrial environment for embryo implantation (Vallve-Juanico et al., 2019). It is likely that ≤50% of infertile women (Meuleman et al., 2009) and ≤70% of women with endometriosis will not have a live birth (Bulletti et al., 2010). Moreover, a larger study, based on fertilized sibling oocytes transferred into women with and without endometriosis, demonstrated reduced implantation, clinical pregnancy, ongoing pregnancy and live birth rates in women with endometriosis (Prapas et al., 2012). These findings highlight that endometriosis is associated with infertility and failure of IVF.
Despite the high incidence of endometriosis, its cellular and molecular mechanisms are poorly understood. The growth of endometriotic tissue outside the uterine cavity requires degradation of the ECM, peritoneal invasion and the growth of ectopic endometrial stromal and glandular cells (Santanam et al., 2002; Van Langendonckt et al., 2002). The biological alterations in endometriotic lesions include increased proliferation, enhanced inflammation, decreased apoptosis, a deregulated immune response and progesterone resistance (Young and Lessey, 2010). It is generally regarded that genetic, epigenetic, environmental, autoimmune and allergic factors are among the etiological factors (Smolarz et al., 2021). Specifically, the endometrium of patients with endometriosis exhibited aberrant global DNA-methylation profiles and altered gene expression (Houshdaran et al., 2016). The deregulated immune response and inflammatory regulation, systemically and locally, were reported to play central roles in its etiology and pathophysiology (Vallve-Juanico et al., 2019; Giacomini et al., 2021). In fact, the endometriotic stromal cells, including stromal fibroblasts and immune cells, expressed and secreted large amounts of immune molecules such as IL1β, IL6 and TNFα (Tseng et al., 1996; Hornung et al., 2001). High estrogen production is of paramount importance in the pathogenesis of endometriosis, as it promotes endometriotic cell survival, inflammation and lesion progression (Bulun, 2009; Yilmaz and Bulun, 2019). Moreover, the underlying pathologic mechanisms include defectively programmed endometrial mesenchymal progenitor/stem cells (Bulun et al., 2019). Studies also highlight the involvement of the MAPK and the WNT/β-catenin signaling pathway in the pathogenesis of endometriosis through multiple mechanisms, including proliferation, apoptosis, migration and angiogenesis (Giacomini et al., 2021).
Furthermore, many factors have been investigated as potential biomarkers for the diagnosis of endometriosis, such as IL6, IL8, hepatocyte growth factor, fibroblast growth factor, epidermal growth factor, VEGF- and platelet-derived growth factor (Smolarz et al., 2021). These growth factors and cytokines promote proliferation, invasion and angiogenesis. Notably, among these factors, IL6 is the most characteristic of endometriosis, with a sensitivity of 63% and a specificity of 69% (Nisenblat et al., 2016). IL6 impacts various vital cellular activities, including proliferation and migration by mediating the phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3) (Yuan et al., 1994; Heinrich et al., 2003). Indeed, increased phosphorylated STAT3 was observed in endometriotic lesions (Kim et al., 2015). Interestingly, BCL6 is upregulated by STAT3 (Arguni et al., 2006; Walker et al., 2013), suggesting a likelihood that BCL6 could be highly expressed in endometriotic lesions.
Increased BCL6 in endometriosis and its related infertility
BCL6 is upregulated at both mRNA and protein levels in the endometrium of women with endometriosis and is considered to be a potential diagnostic marker for endometriosis (Table II) (Evans-Hoeker et al., 2016; Almquist et al., 2017; Gong et al., 2017; Yoo et al., 2017; Likes et al., 2019; Nezhat et al., 2020; Sansone et al., 2021; Shen et al., 2021). The first work came from Evans-Hoeker and colleagues reporting that BCL6 was expressed in endometrial cells during the secretory phase of the menstrual cycle and was overexpressed in eutopic endometrium from women with endometriosis (Evans-Hoeker et al., 2016). The authors reported that aberrant BCL6 expression had a high sensitivity and specificity for the diagnosis of all stages of endometriosis, indicative of BCL6 as a biomarker for endometriosis. In support of this finding, another large cohort study showed a high positive predictive value of BCL6 expression for the diagnosis of endometriosis (Nezhat et al., 2020). In combination with Sirtuin 1 (SIRT1), a histone deacetylase and gene silencer, BCL6 was shown to be increased in endometrial cells of women with endometriosis (Yoo et al., 2017). In addition, BCL6 was upregulated together with ERK1/ERK2 in endometriotic tissues from rats with experimentally induced endometriosis (Nahari and Razi, 2018).
Table II.
BCL6 expression in human endometriosis.
| Reference | Sample number/patient information | Methods | Main results |
|---|---|---|---|
| Evans-Hoeker et al. (2016) |
|
|
|
|
| |||
| Almquist et al. (2017) |
|
Prospective cohort study, IHC and HSCORE with BCL6 antibody clone LN22 (Leica Biosystems). |
|
|
| |||
| Gong et al. (2017) |
|
qPCR and IHC with rabbit BCL6 antibody (1:100; cat no. bs2734R, BIOSS, Beijing, China). | The mRNA and protein level of BCL6 in the endometrium during the mid-luteal phase was significantly increased in the RIF group. |
|
| |||
| Yoo et al. (2017) |
|
Results were based on the WB band intensity (BCL6 antibody 561520; BD Pharmingen, San Jose, CA) and correlation coefficient = 0.5659, P < 0.0001, between BCL6 and SIRT1 expression. |
|
|
| |||
| Likes et al. (2019) |
|
|
|
|
| |||
| Nezhat et al. (2020) |
|
|
|
| Sansone et al. (2021) |
|
BCL6 and SIRT1 levels were measured in serum, plasma, urine and cervical mucus using ELISA. |
|
|
| |||
| Shen et al. (2021) |
|
Flow cytometry for endometrial B-cell subsets analysis. | 1–5% of endometrial immune cells were B cells. FAS+IgD-BCL6+ GC B cells were present in the endometrium, indicating their roles in the human endometrial environment. TFH-like cells did not have a higher expression of BCL6. |
BCL6, B-cell lymphoma 6; GC, germinal center; GLI1, glioma-associated oncogene homolog 1; HSCORE, a method for quantifying the staining intensity and the percentage of stained cells in IHC; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma viral oncogene homolog; qPCR, quantitative PCR; RIF, repeated implantation failure; SIRT1, Sirtuin 1; TFH, T follicular helper cells; UI, unexplained infertility; WB, western blot analysis.
Endometriosis is associated with infertility and IVF failure (Prapas et al., 2012). Intriguingly, the prevalence of elevated endometrial BCL6 in women with unexplained infertility (UI) has been reported to be 75.3% (Almquist et al., 2017) and 80% (Evans-Hoeker et al., 2016). A prospective study in patients with UI following IVF showed a live birth rate of 11.5% versus 58% in patients with and without elevated BCL6, respectively (Almquist et al., 2017). It was also reported that the mRNA and protein levels of endometrial BCL6, along with IL21 and CXCR5 (chemokine receptor type 5), were significantly increased in women with repeated implantation failure (Gong et al., 2017). In support of these observations, Likes et al. (2019) reported that women with suspected endometriosis and aberrant endometrial BCL6 expression had worse reproductive outcomes following embryo transfer, including a high miscarriage rate, poor implantation rate, and low live birth rate and clinical pregnancy rate. Increased endometrial BCL6 is thus considered to be a negative predictor for patients with endometriosis undergoing IVF (Nezhat et al., 2020). Although more investigations are required, these findings demonstrate an association of upregulated BCL6 with endometriosis, its related infertility and IVF failure.
At the molecular level, BCL6 and SIRT1 have been shown to co-localize in the nuclei of endometriotic cells, interact with each other and bind to and repress the promoter of GLI1 (Yoo et al., 2017), a critical mediator of progesterone action in the Indian Hedgehog pathway (Wei et al., 2010). Similarly, the BCL6/BCOR/SIRT1 complex was observed to affect the growth of human medulloblastoma cells by suppressing GLI1 and GLI2 of the Sonic Hedgehog pathway (Tiberi et al., 2014). These data indicate that BCL6 is an important regulator of the Hedgehog pathway, which renders upregulated BCL6 as a prime candidate driving the progesterone resistance that is crucial in the pathophysiology of endometriosis (Yoo et al., 2017). In addition, among the immune cells, TFH and GC B cells, controlled by BCL6 (Hatzi and Melnick, 2014; Alterauge et al., 2020), were present in the endometrium (Shen et al., 2021), suggesting the importance of these cells through GC reactions. Among these observations, the association of increased endometrial BCL6 with progesterone resistance (Yoo et al., 2017) is of particular importance. Progesterone is the key hormone for adequate decidualization, proper implantation and successful pregnancy, as it is crucial for preparing the endometrium for implantation by blocking the proliferative effect of estrogen and by inducing genes permitting embryo attachment, as well as for regulating trophoblast invasion and migration by controlling MMP activity (Halasz and Szekeres-Bartho, 2013; Nagy et al., 2021). Abnormal endometrial BCL6-associated progesterone resistance and its other cellular activities, including deregulated proliferation, invasion and immune modulation, may be responsible for a defective endometrium, deficient endometrial receptivity, poor reproductive outcomes, and failed IVF. These data also suggest a potential involvement of BCL6 in endometrial-blastocyst cross talk during implantation.
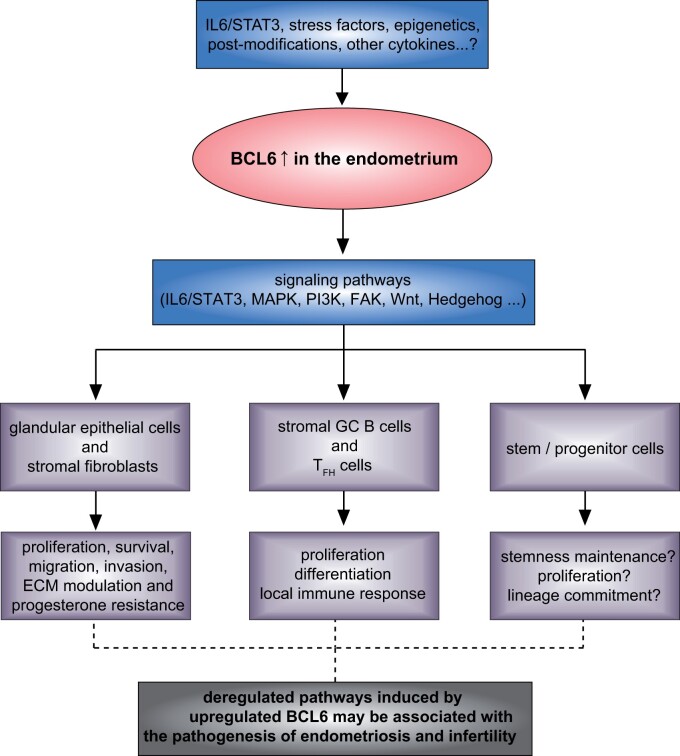
As in trophoblasts (Fig. 2), BCL6 may be overexpressed and stabilized in endometriotic tissues by diverse causes, including altered epigenetic regulation, increased IL6/STAT3, other cytokines, stress factors, miRNAs, lncRNAs and miscellaneous protein post-translational modifications. Increased BCL6 may regulate multiple signaling pathways including FAK, MAPK/ERK, PI3K/AKT, WNT and Hedgehog pathways in endometriotic cells, as occurs in lymphoma cells (Basso et al., 2010). This might affect vital cellular activities such as cell proliferation, survival, differentiation, migration, invasion, immune response and inflammation regulation (Basso et al., 2010; Louwen et al., 2014; Muschol-Steinmetz et al., 2016; Jasmer et al., 2017; Ritter et al., 2020), impact various functionalities of endometriotic cells, including glandular epithelial cells, stromal fibroblasts, stromal GC B and TFH cells and stem/progenitor cells, and contribute to the pathophysiology of endometriosis and its related infertility (Fig. 5).
Figure 5.
Increased BCL6 in endometriosis. Schematic illustration shows that various factors may upregulate and stabilize BCL6, which may impact vital cellular activities in diverse cell types of endometrial tissues through multiple signaling pathways, affecting the pathogenesis of endometriosis and its related infertility. ECM, extracellular matrix; FAK, focal adhesion kinase; GC, germinal center; IL6, interleukin 6; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; STAT3, signal transducer and activator of transcription 3; TFH cells, T follicular helper cells.
Conclusion and perspectives
We have summarized the current data from bench to clinic showing BCL6’s potential function in the placenta and its critical involvement in PE and endometriosis. BCL6 is significantly increased in pre-eclamptic placentas as well as in endometriotic lesions. Upregulated BCL6 regulates a variety of cellular activities, including proliferation, migration and invasion, and may also influence stem/progenitor cell differentiation in pre-eclamptic placentas as well as in endometriotic lesions. Increased endometrial BCL6 is considered to be a diagnostic marker for endometriosis and a predictor for poor IVF outcomes. Proper regulation of BCL6 may be crucial to orchestrate endometrial decidualization, implantation and placentation for a successful pregnancy.
Although some work has been done, the detailed molecular mechanisms by which BCL6 exerts multiple functions in the placenta as well as in the endometrium have not been completely elucidated. It is paramount to explore whether increased BCL6 is one of the causes for the initiation of PE and endometriosis owing to epigenetic modifications, or a pathological consequence of the disease-related high-stress conditions such as chronic hypoxia, inflammation and oxidation. Further investigations are warranted to examine whether BCL6 is able to co-determine the fate of various stem/progenitor cells in the placenta and the endometrium.
Recent progress in the establishment of human trophoblast stem cells (Okae et al., 2018), placental organoids (Haider et al., 2018; Turco et al., 2018) and endometrial organoids (Turco et al., 2017; Cheung et al., 2021; Song and Fazleabas, 2021) has provided novel tools to study the molecular regulatory network of placentation, the endometrial cycle and the pathophysiology of gestational and reproductive diseases like PE and endometriosis. These new models, in combination with specific small molecule inhibitors targeting BCL6 or gene editing, will allow us to explore the individual roles of BCL6 in stemness and differentiation of trophoblastic and endometrial stem/progenitor cells. New omics technologies (Cao et al., 2017; Habib et al., 2017) will be of great help to delineate the gene landscapes controlled by BCL6 in diverse trophoblastic and endometrial cell types. Being equipped with these novel tools and techniques, we are just at the beginning of a journey to unveil the multifaceted roles of BCL6 in placental development, endometrial homeostasis, and the pathogenesis of PE and endometriosis.
Contributor Information
Frank Louwen, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Nina-Naomi Kreis, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Andreas Ritter, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Alexandra Friemel, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Christine Solbach, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Juping Yuan, Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Hospital Frankfurt, J. W. Goethe-University, Frankfurt, Germany.
Data Availability
The data underlying this article are available in the article.
Authors’ roles
FL and JY conceived the review concept. JY performed the literature search. FL, CS and JY analyzed and discussed the data. JY wrote the manuscript. NNK greatly modified the manuscript, summarized the expression data of BCL6 in pre-eclampsia (Table I) and in endometriosis (Table II), and contributed to Fig. 1. AR drew Figs 2–5 and performed a critical reading of the manuscript. AF contributed to Fig. 1. NNK, AR and AF were intensively involved in BCL6 studies included in this article. All authors improved the manuscript and approved the submission.
Funding
This work was funded by the Division of Obstetrics and Prenatal Medicine, Department of Gynecology and Obstetrics, University Frankfurt Hospital, Germany.
Conflict of interest
The authors declare no conflicts of interest.
References
- Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, Mayer S, Takahashi S, Licht JD, Prive GG.. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell 2003;12:1551–1564. [DOI] [PubMed] [Google Scholar]
- Ali A, Bouma GJ, Anthony RV, Winger QA.. The role of LIN28-let-7-ARID3B pathway in placental development. Int J Mol Sci 2020;21:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almquist LD, Likes CE, Stone B, Brown KR, Savaris R, Forstein DA, Miller PB, Lessey BA.. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: a cohort study. Fertil Steril 2017;108:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterauge D, Bagnoli JW, Dahlstrom F, Bradford BM, Mabbott NA, Buch T, Enard W, Baumjohann D.. Continued Bcl6 expression prevents the transdifferentiation of established Tfh cells into Th1 cells during acute viral infection. Cell Rep 2020;33:108232. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Jones CJP.. Cell dynamics in human villous trophoblast. Hum Reprod Update 2021;27:904–922. [DOI] [PubMed] [Google Scholar]
- Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M, Tokuhisa T.. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol 2006;18:1079–1089. [DOI] [PubMed] [Google Scholar]
- Arora N, Sadovsky Y, Dermody TS, Coyne CB.. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017;21:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K, Li X, Zhong J, Ng EHY, Yeung WSB, Lee CL, Chiu PCN.. Placenta-derived exosomes as a modulator in maternal immune tolerance during pregnancy. Front Immunol 2021;12:671093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajalica-Lagercrantz S, Piehl F, Farnebo F, Larsson C, Lagercrantz J.. Expression of the BCL6 gene in the pre- and postnatal mouse. Biochem Biophys Res Commun 1998;247:357–360. [DOI] [PubMed] [Google Scholar]
- Basso K, Dalla-Favera R.. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol 2010;105:193–210. [DOI] [PubMed] [Google Scholar]
- Basso K, Dalla-Favera R.. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev 2012;247:172–183. [DOI] [PubMed] [Google Scholar]
- Basso K, Saito M, Sumazin P, Margolin AA, Wang K, Lim WK, Kitagawa Y, Schneider C, Alvarez MJ, Califano A. et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood 2010;115:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko OR, Gu W, Dalla-Favera R.. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet 2002;32:606–613. [DOI] [PubMed] [Google Scholar]
- Bonnefont J, Tiberi L, van den Ameele J, Potier D, Gaber ZB, Lin X, Bilheu A, Herpoel A, Velez Bravo FD, Guillemot F. et al. Cortical neurogenesis requires Bcl6-mediated transcriptional repression of multiple self-renewal-promoting extrinsic pathways. Neuron 2019;103:1096–1108.e1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, van Diest PJ, van der Groep P, Greijer AE, Hermsen MA, Heijnen I, Meijer GA, Baak JP, Pinedo HM, van der Wall E. et al. Protein expression of B-cell lymphoma gene 6 (BCL-6) in invasive breast cancer is associated with cyclin D1 and hypoxia-inducible factor-1alpha (HIF-1alpha). Oncogene 2003;22:8948–8951. [DOI] [PubMed] [Google Scholar]
- Brew O, Sullivan MH, Woodman A.. Comparison of normal and pre-eclamptic placental gene expression: a systematic review with meta-analysis. PLoS One 2016;11:e0161504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichant G, Laraki I, Henry L, Munaut C, Nisolle M.. New therapeutics in endometriosis: a review of hormonal, non-hormonal, and non-coding RNA treatments. Int J Mol Sci 2021;22:10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletti C, Coccia ME, Battistoni S, Borini A.. Endometriosis and infertility. J Assist Reprod Genet 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J.. Endometriosis. Endocr Rev 2019;40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting KL, Melnick AM.. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr Opin Immunol 2013;25:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Charnock-Jones DS, Jauniaux E.. Regulation of vascular growth and function in the human placenta. Reproduction 2009;138:895–902. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL.. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci 2015;370:20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ. et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 2017;357:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas MG, Oswald E, Yu W, Xue F, MacKerell AD Jr, Melnick AM.. The expanding role of the BCL6 oncoprotein as a cancer therapeutic target. Clin Cancer Res 2017;23:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerchietti LC, Lopes EC, Yang SN, Hatzi K, Bunting KL, Tsikitas LA, Mallik A, Robles AI, Walling J, Varticovski L. et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med 2009;15:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Cui W, Rosario GX, Scott RL, Dhakal P, Renaud SJ, Tachibana M, Rumi MA, Mason CW, Krieg AJ. et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A 2016;113:E7212–E7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, Vergriete K, Buscail E, Lluel P, Fontaine C. et al. Estrogen receptors and endometriosis. Int J Mol Sci 2020;21:2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell LC, Cluver CA, Kingdom J, Tong S.. Pre-eclampsia. Lancet 2021;398:341–354. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang J, Fan X, Zhang Y, Zhoua M, Li X, Wang L.. LASP2 inhibits trophoblast cell migration and invasion in preeclampsia through inactivation of the Wnt/beta-catenin signaling pathway. J Recept Signal Transduct Res 2021;41:67–73. [DOI] [PubMed] [Google Scholar]
- Cheung VC, Peng CY, Marinic M, Sakabe NJ, Aneas I, Lynch VJ, Ober C, Nobrega MA, Kessler JA.. Pluripotent stem cell-derived endometrial stromal fibroblasts in a cyclic, hormone-responsive, coculture model of human decidua. Cell Rep 2021;35:109138. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoo JH, Lee JH, Lee Y, Bae MK, Kim YD, Kim HJ.. Connective tissue growth factor (CTGF) regulates the fusion of osteoclast precursors by inhibiting Bcl6 in periodontitis. Int J Med Sci 2020;17:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Spencer TE, Bartol FF, Hayashi K.. Uterine glands: development, function and experimental model systems. Mol Hum Reprod 2013;19:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ziegler D, Borghese B, Chapron C.. Endometriosis and infertility: pathophysiology and management. Lancet 2010;376:730–738. [DOI] [PubMed] [Google Scholar]
- Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA.. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol 2008;199:566 e561–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, Schammel DP, Young SL.. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci 2016;23:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre MS, Stanton NM, Slatter TL, Lee S, Senanayake D, Gordon RMA, Castro ML, Rowe MR, Taha A, Royds JA. et al. The oncogene BCL6 is up-regulated in glioblastoma in response to DNA damage, and drives survival after therapy. PLoS One 2020;15:e0231470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando TM, Marullo R, Pera Gresely B, Phillip JM, Yang SN, Lundell-Smith G, Torregroza I, Ahn H, Evans T, Gyorffy B. et al. BCL6 evolved to enable stress tolerance in vertebrates and is broadly required by cancer cells to adapt to stress. Cancer Discov 2019;9:662–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint EJ, Cerdeira AS, Redman CW, Vatish M.. The role of angiogenic factors in the management of preeclampsia. Acta Obstet Gynecol Scand 2019;98:700–707. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Deane JA.. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 2016;22:137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R, Hart R, Karthigasu KA, Burke C.. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod 2009;24:1393–1401. [DOI] [PubMed] [Google Scholar]
- Ghetu AF, Corcoran CM, Cerchietti L, Bardwell VJ, Melnick A, Prive GG.. Structure of a BCOR corepressor peptide in complex with the BCL6 BTB domain dimer. Mol Cell 2008;29:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini E, Minetto S, Li Piani L, Pagliardini L, Somigliana E, Vigano P.. Genetics and inflammation in endometriosis: improving knowledge for development of new pharmacological strategies. Int J Mol Sci 2021;22:9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Zhu Y, Pang N, Ai H, Gong X, La X, Ding J.. Increased levels of CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells, and associated factors Bcl-6, CXCR5, IL-21 and IL-6 contribute to repeated implantation failure. Exp Ther Med 2017;14:5931–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK.. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993;206:204–211. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG.. Growth and function of the normal human placenta. Thromb Res 2004;114:397–407. [DOI] [PubMed] [Google Scholar]
- Guo F, Zhang B, Yang H, Fu Y, Wang Y, Huang J, Cheng M, Li X, Shen Z, Li L. et al. Systemic transcriptome comparison between early- and late-onset pre-eclampsia shows distinct pathology and novel biomarkers. Cell Prolif 2021a;54:e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liu Y, Lv J, Zou B, Chen Z, Li K, Feng J, Cai Z, Wei L, Liu M. et al. BCL6 confers KRAS-mutant non-small-cell lung cancer resistance to BET inhibitors. J Clin Invest 2021b;131:e133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K. et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods 2017;14:955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Gamperl M, Burkard TR, Kunihs V, Kaindl U, Junttila S, Fiala C, Schmidt K, Mendjan S, Knofler M. et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology 2019;160:2282–2297. [DOI] [PubMed] [Google Scholar]
- Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep 2018;11:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz M, Szekeres-Bartho J.. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol 2013;97:43–50. [DOI] [PubMed] [Google Scholar]
- Hatzi K, Melnick A.. Breaking bad in the germinal center: how deregulation of BCL6 contributes to lymphomagenesis. Trends Mol Med 2014;20:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SM, Matzuk MM.. The menstrual cycle: basic biology. Ann N Y Acad Sci 2008;1135:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F.. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemberger M, Hanna CW, Dean W.. Mechanisms of early placental development in mouse and humans. Nat Rev Genet 2020;21:27–43. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Ogasawara N, Sasaki M, Mizushima T, Shimura T, Mizoshita T, Mori Y, Kubota E, Wada T, Tanida S. et al. BCL6 degradation caused by the interaction with the C-terminus of pro-HB-EGF induces cyclin D2 expression in gastric cancers. Br J Cancer 2009;100:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN.. Regulated on activation, normal T-cell-expressed and -secreted mRNA expression in normal endometrium and endometriotic implants: assessment of autocrine/paracrine regulation by in situ hybridization. Am J Pathol 2001;158:1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC.. Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol Reprod 2016;95:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Gonzalez DG, Cote CM, Jiang Y, Hatzi K, Teater M, Dai K, Hla T, Haberman AM, Melnick A.. The BCL6 RD2 domain governs commitment of activated B cells to form germinal centers. Cell Rep 2014;8:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. Int J Mol Sci 2019;21:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Kaufmann P, Kingdom J.. Trophoblast turnover in health and disease. Fet Mat Med Rev 2002;13:103–118. [Google Scholar]
- Hurtz C, Hatzi K, Cerchietti L, Braig M, Park E, Kim YM, Herzog S, Ramezani-Rad P, Jumaa H, Muller MC. et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med 2011;208:2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO.. Endocrine regulation of menstruation. Endocr Rev 2006;27:17–46. [DOI] [PubMed] [Google Scholar]
- Jaremek A, Jeyarajah MJ, Jaju Bhattad G, Renaud SJ.. Omics approaches to study formation and function of human placental syncytiotrophoblast. Front Cell Dev Biol 2021;9:674162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer B, Muschol-Steinmetz C, Kreis NN, Friemel A, Kielland-Kaisen U, Bruggmann D, Jennewein L, Allert R, Solbach C, Yuan J. et al. Involvement of the oncogene B-cell lymphoma 6 in the fusion and differentiation process of trophoblastic cells of the placenta. Oncotarget 2017;8:108643–108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee B, Dhar R, Singh S, Karmakar S.. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front Cell Dev Biol 2021;9:648463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata KC, Zong H, Meydan C, Wyman S, Wouters BJ, Sugita M, Goswami S, Albert M, Yip W, Roboz GJ. et al. BCL6 maintains survival and self-renewal of primary human acute myeloid leukemia cells. Blood 2021;137:812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW.. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod 2015;30:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinrouweler CE, van Uitert M, Moerland PD, Ris-Stalpers C, van der Post JA, Afink GB.. Differentially expressed genes in the pre-eclamptic placenta: a systematic review and meta-analysis. PLoS One 2013;8:e68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knofler M, Haider S, Saleh L, Pollheimer J, Gamage T, James J.. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol Life Sci 2019;76:3479–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knofler M, Pollheimer J.. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta 2012;33(Suppl):S55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis NN, Friemel A, Jennewein L, Hoock SC, Hentrich AE, Nowak T, Louwen F, Yuan J.. Functional analysis of p21(Cip1/CDKN1A) and its family members in trophoblastic cells of the placenta and its roles in preeclampsia. Cells 2021;10:2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis NN, Ritter A, Louwen F, Yuan J.. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells 2020;9:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan KKW, Gibson DA, Whitaker LHR, Horne AW.. Menstruation dysregulation and endometriosis development. Front Reprod Health 2021;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Fatemi M, Dhasarathy A, Malone C, Sobol SE, Geigerman C, Jaye DL, Mav D, Shah R, Li L. et al. DNA methylation prevents CTCF-mediated silencing of the oncogene BCL6 in B cell lymphomas. J Exp Med 2010;207: 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MJ, DeMayo FJ.. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol 2012;358:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW.. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 2013;70:412–427. [DOI] [PubMed] [Google Scholar]
- Leavey K, Bainbridge SA, Cox BJ.. Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. PLoS One 2015;10:e0116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavey K, Benton SJ, Grynspan D, Kingdom JC, Bainbridge SA, Cox BJ.. Unsupervised placental gene expression profiling identifies clinically relevant subclasses of human preeclampsia. Hypertension 2016;68:137–147. [DOI] [PubMed] [Google Scholar]
- Leavey K, Grynspan D, Cox BJ.. Both “canonical” and “immunological” preeclampsia subtypes demonstrate changes in placental immune cell composition. Placenta 2019;83:53–56. [DOI] [PubMed] [Google Scholar]
- Li H, Hu B, Hu S, Luo W, Sun D, Yang M, Liao Z, Wei H, Zhao C, Li D. et al. High expression of BCL6 inhibits the differentiation and development of hematopoietic stem cells and affects the growth and development of chickens. J Anim Sci Biotechnol 2021;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Liu H, Li Y, Wang Y, Zhao L, Wang H.. miR-339-5p inhibits lung adenocarcinoma invasion and migration by directly targeting BCL6. Oncol Lett 2018;16:5785–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likes CE, Cooper LJ, Efird J, Forstein DA, Miller PB, Savaris R, Lessey BA.. Medical or surgical treatment before embryo transfer improves outcomes in women with abnormal endometrial BCL6 expression. J Assist Reprod Genet 2019;36:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fan X, Wang R, Lu X, Dang YL, Wang H, Lin HY, Zhu C, Ge H, Cross JC. et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res 2018;28:819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logarajah S, Hunter P, Kraman M, Steele D, Lakhani S, Bobrow L, Venkitaraman A, Wagner S.. BCL-6 is expressed in breast cancer and prevents mammary epithelial differentiation. Oncogene 2003;22:5572–5578. [DOI] [PubMed] [Google Scholar]
- Louwen F, Muschol-Steinmetz C, Friemel A, Kampf AK, Tottel E, Reinhard J, Yuan J.. Targeted gene analysis: increased B-cell lymphoma 6 in preeclamptic placentas. Hum Pathol 2014;45:1234–1242. [DOI] [PubMed] [Google Scholar]
- Louwen F, Muschol-Steinmetz C, Reinhard J, Reitter A, Yuan J.. A lesson for cancer research: placental microarray gene analysis in preeclampsia. Oncotarget 2012;3:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Fernando TM, Lossos C, Yusufova N, Liu F, Fontan L, Durant M, Geng H, Melnick J, Luo Y. et al. PRMT5 interacts with the BCL6 oncoprotein and is required for germinal center formation and lymphoma cell survival. Blood 2018;132:2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Wang R, Zhu C, Wang H, Lin HY, Gu Y, Cross JC, Wang H.. Fine-tuned and cell-cycle-restricted expression of fusogenic protein syncytin-2 maintains functional placental syncytia. Cell Rep 2017;21:1150–1159. [DOI] [PubMed] [Google Scholar]
- MacKay AP, Berg CJ, Atrash HK.. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol 2001;97:533–538. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Keith B, Arsham AM, Brorson JR, Simon MC.. The role of ARNT2 in tumor angiogenesis and the neural response to hypoxia. Biochem Biophys Res Commun 2000;273:231–238. [DOI] [PubMed] [Google Scholar]
- Masuda H, Anwar SS, Buhring HJ, Rao JR, Gargett CE.. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant 2012;21:2201–2214. [DOI] [PubMed] [Google Scholar]
- Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H. et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One 2010;5:e10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T.. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril 2009;92:68–74. [DOI] [PubMed] [Google Scholar]
- Miyamoto T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med 2011;60:101–105. [DOI] [PubMed] [Google Scholar]
- Muschol-Steinmetz C, Jasmer B, Kreis NN, Steinhauser K, Ritter A, Rolle U, Yuan J, Louwen F.. B-cell lymphoma 6 promotes proliferation and survival of trophoblastic cells. Cell Cycle 2016;15:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B, Szekeres-Bartho J, Kovacs GL, Sulyok E, Farkas B, Varnagy A, Vertes V, Kovacs K, Bodis J.. Key to life: physiological role and clinical implications of progesterone. Int J Mol Sci 2021;22:11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahari E, Razi M.. Silymarin amplifies apoptosis in ectopic endometrial tissue in rats with endometriosis; implication on growth factor GDNF, ERK1/2 and Bcl-6b expression. Acta Histochem 2018;120:757–767. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Shima T, Tsuda S, Aoki A, Kawaguchi M, Furuta A, Yasuda I, Yoneda S, Yamaki-Ushijima A, Cheng SB. et al. Aggrephagy deficiency in the placenta: a new pathogenesis of preeclampsia. Int J Mol Sci 2021;22:2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhat C, Rambhatla A, Miranda-Silva C, Asiaii A, Nguyen K, Eyvazzadeh A, Tazuke S, Agarwal S, Jun S, Nezhat A. et al. BCL-6 overexpression as a predictor for endometriosis in patients undergoing in vitro fertilization. JSLS 2020;24:e2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N.. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;7:CD012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa H, Ota S, Suzuki M, Kato T, Sekiya T, Kurahashi H, Udagawa Y.. Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Ye BH, Dalla-Favera R.. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev 1998;12:1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C.. Bcl6 mediates the development of T follicular helper cells. Science 2009;325:1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tierney-Ginn PF, Lash GE.. Beyond pregnancy: modulation of trophoblast invasion and its consequences for fetal growth and long-term children’s health. J Reprod Immunol 2014;104-105:37–42. [DOI] [PubMed] [Google Scholar]
- Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T.. Derivation of human trophoblast stem cells. Cell Stem Cell 2018;22:50–63.e56. [DOI] [PubMed] [Google Scholar]
- Padmini E, Uthra V, Lavanya S.. Effect of HSP70 and 90 in modulation of JNK, ERK expression in preeclamptic placental endothelial cell. Cell Biochem Biophys 2012;64:187–195. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R.. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood 2003;101: 2914–2923. [DOI] [PubMed] [Google Scholar]
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN.. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017;96:623–632. [DOI] [PubMed] [Google Scholar]
- Phan RT, Saito M, Kitagawa Y, Means AR, Dalla-Favera R.. Genotoxic stress regulates expression of the proto-oncogene Bcl6 in germinal center B cells. Nat Immunol 2007;8:1132–1139. [DOI] [PubMed] [Google Scholar]
- Phipps EA, Thadhani R, Benzing T, Karumanchi SA.. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 2019;15:275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras A, Katz AM, Ekstrom EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC.. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 2014;14:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollheimer J, Knofler M.. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta 2005;26(Suppl A):S21–30. [DOI] [PubMed] [Google Scholar]
- Polo JM, Juszczynski P, Monti S, Cerchietti L, Ye K, Greally JM, Shipp M, Melnick A.. Transcriptional signature with differential expression of BCL6 target genes accurately identifies BCL6-dependent diffuse large B cell lymphomas. Proc Natl Acad Sci U S A 2007;104:3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD. et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet 2019;145:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapas Y, Goudakou M, Matalliotakis I, Kalogeraki A, Matalliotaki C, Panagiotidis Y, Ravanos K, Prapas N.. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod Biomed Online 2012;25:543–548. [DOI] [PubMed] [Google Scholar]
- Prater M, Hamilton RS, Wa Yung H, Sharkey AM, Robson PAbd, Hamid NE, Jauniaux E, Charnock-Jones DS, Burton GJ, Cindrova-Davies T.. RNA-Seq reveals changes in human placental metabolism, transport and endocrinology across the first-second trimester transition. Biol Open 2021;10:bio058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Yao C, Xing J, Qin Y.. Hypoxia-induced GPR4 suppresses trophoblast cell migration and proliferation through the MAPK signaling pathway. Reprod Toxicol 2021;99:1–8. [DOI] [PubMed] [Google Scholar]
- Rana S, Lemoine E, Granger JP, Karumanchi SA.. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res 2019;124:1094–1112. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Staff AC, Roberts JM.. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol 2020;226:S907–S927. [DOI] [PubMed] [Google Scholar]
- Reljic R, Wagner SD, Peakman LJ, Fearon DT.. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 2000;192:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Gao Y, Gao Y, Liang G, Chen Q, Jiang S, Yang X, Fan C, Wang H, Wang J. et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 2021;11:5028–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A, Safdar BK, Jasmer B, Kreis NN, Friemel A, Roth S, Solbach C, Louwen F, Yuan JP.. The function of oncogene B-cell lymphoma 6 in the regulation of the migration and invasion of trophoblastic cells. Int J Mol Sci 2020;21:8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Hubel CA.. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30(Suppl A):S32–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland CS, Hu J, Ren CE, Chen H, Li J, Varvoutis MS, Leaphart LW, Byck DB, Zhu X, Jiang SW.. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci 2016;73:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T.. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest 2013;123:2775–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario GX, Konno T, Soares MJ.. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 2008;314: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri S, Tamma R, Marzullo A, Annese T, Marinaccio C, Errede M, Susca FC, Senetta R, Cassoni P, Vacca A. et al. Translocation of the proto-oncogene Bcl-6 in human glioblastoma multiforme. Cancer Lett 2014;353:41–51. [DOI] [PubMed] [Google Scholar]
- Sadeghi H, Esmkhani S, Pirjani R, Amin-Beidokhti M, Gholami M, Azizi Tabesh G, Ghasemi MR, Gachkar L, Mirfakhraie R.. CREB-binding protein (CREBBP) and preeclampsia: a new promising target gene. Mol Biol Rep 2021;48:2117–2122. [DOI] [PubMed] [Google Scholar]
- Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, Pernis A, Pasqualucci L, Dalla-Favera R.. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell 2007;12:280–292. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927a;3:93–110.143. [PMC free article] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927b;14:422–469. [Google Scholar]
- Sansone AM, Hisrich BV, Young RB, Abel WF, Bowens Z, Blair BB, Funkhouser AT, Schammel DP, Green LJ, Lessey BA. et al. Evaluation of BCL6 and SIRT1 as non-invasive diagnostic markers of endometriosis. Curr Issues Mol Biol 2021;43:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam N, Murphy AA, Parthasarathy S.. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci 2002;955:183–198. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE.. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007;22:2903–2911. [DOI] [PubMed] [Google Scholar]
- Shen M, Child T, Mittal M, Sarodey G, Salim R, Granne I, Southcombe JH.. B cell subset analysis and gene expression characterization in mid-luteal endometrium. Front Cell Dev Biol 2021;9:709280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silini AR, Di Pietro R, Lang-Olip I, Alviano F, Banerjee A, Basile M, Borutinskaite V, Eissner G, Gellhaus A, Giebel B. et al. Perinatal derivatives: where do we stand? A roadmap of the human placenta and consensus for tissue and cell nomenclature. Front Bioeng Biotechnol 2020;8:610544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, Acharya G.. Differential placental gene expression in severe preeclampsia. Placenta 2009;30:424–433. [DOI] [PubMed] [Google Scholar]
- Smolarz B, Szyllo K, Romanowicz H.. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci 2021;22:10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober S, Reiman M, Kikas T, Rull K, Inno R, Vaas P, Teesalu P, Marti JML, Mattila P, Laan M.. Extensive shift in placental transcriptome profile in preeclampsia and placental origin of adverse pregnancy outcomes. Sci Rep 2015;5:13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki A, Yanez DC, Lau CI, Rowell J, Barbarulo A, Ross S, Sahni H, Crompton T.. The transcriptional repressor Bcl6 promotes pre-TCR-induced thymocyte differentiation and attenuates Notch1 activation. Development 2020;147:dev192203. [DOI] [PubMed] [Google Scholar]
- Sonderegger S, Pollheimer J, Knofler M.. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta 2010;31:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Wang Z, Kan P, Ma Z, Wang Y, Wu Q, Yao X, Zhang B.. Knockdown of BCL6 inhibited malignant phenotype and enhanced sensitivity of glioblastoma cells to TMZ through AKT pathway. Biomed Res Int 2018;2018:6953506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Fazleabas AT.. Endometrial organoids: a rising star for research on endometrial development and associated diseases. Reprod Sci 2021;28:1626–1636. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R.. Pre-eclampsia. Lancet 2010;376:631–644. [DOI] [PubMed] [Google Scholar]
- Sun C, Li S, Yang C, Xi Y, Wang L, Zhang F, Li D.. MicroRNA-187-3p mitigates non-small cell lung cancer (NSCLC) development through down-regulation of BCL6. Biochem Biophys Res Commun 2016;471:82–88. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Kotlyar AM, Flores VA.. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839–852. [DOI] [PubMed] [Google Scholar]
- Tejera E, Bernardes J, Rebelo I.. Co-expression network analysis and genetic algorithms for gene prioritization in preeclampsia. BMC Med Genomics 2013;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, Juhasz K, Bhatti G, Leavitt RJ, Gelencser Z. et al. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol 2018;9:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberi L, Bonnefont J, van den Ameele J, Le Bon SD, Herpoel A, Bilheu A, Baron BW, Vanderhaeghen P.. A BCL6/BCOR/SIRT1 complex triggers neurogenesis and suppresses medulloblastoma by repressing Sonic Hedgehog signaling. Cancer Cell 2014;26:797–812. [DOI] [PubMed] [Google Scholar]
- Trifonova EA, Gabidulina TV, Ershov NI, Serebrova VN, Vorozhishcheva AY, Stepanov VA.. Analysis of the placental tissue transcriptome of normal and preeclampsia complicated pregnancies. Acta Naturae 2014;6:71–83. [PMC free article] [PubMed] [Google Scholar]
- Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN.. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab 1996;81:1118–1122. [DOI] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017;19:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018;564:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco MY, Moffett A.. Development of the human placenta. Development 2019;146:dev163428. [DOI] [PubMed] [Google Scholar]
- Vaiman D, Calicchio R, Miralles F.. Landscape of transcriptional deregulations in the preeclamptic placenta. PLoS One 2013;8:e65498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallve-Juanico J, Houshdaran S, Giudice LC.. The endometrial immune environment of women with endometriosis. Hum Reprod Update 2019;25:564–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Donnez J.. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002;77:861–870. [DOI] [PubMed] [Google Scholar]
- Walker SR, Liu S, Xiang M, Nicolais M, Hatzi K, Giannopoulou E, Elemento O, Cerchietti L, Melnick A, Frank DA.. The transcriptional modulator BCL6 as a molecular target for breast cancer therapy. Oncogene 2015;34:1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SR, Nelson EA, Yeh JE, Pinello L, Yuan GC, Frank DA.. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol Cell Biol 2013;33:2879–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Weng W, Chen T, Xu M, Wei P, Li J, Lu L, Wang Y.. LINC00152 promotes tumor progression and predicts poor prognosis by stabilizing BCL6 from degradation in the epithelial ovarian cancer. Front Oncol 2020;10:555132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Z, Zeng X, Wang J, Zhang L, Song W, Shi Y.. Wnt/beta-catenin signaling pathway in severe preeclampsia. J Mol Histol 2018;49:317–327. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Xu MD, Weng WW, Wei P, Yang YS, Du X.. BCL6 is a negative prognostic factor and exhibits pro-oncogenic activity in ovarian cancer. Am J Cancer Res 2015;5:255–266. [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Levens ED, Stefansson L, Nieman LK.. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J Clin Endocrinol Metab 2010;95:5330–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Gao W, Wu Y, Ni B, Tian Y.. Mutual interaction between BCL6 and miRNAs contributing to the pathogenesis of various cancers. Clin Transl Oncol 2015;17:841–846. [DOI] [PubMed] [Google Scholar]
- Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, Haimov-Kochman R, Yeh RF, Overgaard MT, Varki A. et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology 2009;150:452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Cheng Y, Li X, Li Q, Xu J, Zhang J, Liu Y, Xing Q, Wang L, He L. et al. Up-regulated expression and aberrant DNA methylation of LEP and SH3PXD2A in pre-eclampsia. PLoS One 2013;8:e59753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zhao M, Huang S, Chen P, Wu WY, Huang J, Wu ZS, Wu Q.. Prolactin inhibits BCL6 expression in breast cancer cells through a microRNA-339-5p-dependent pathway. J Breast Cancer 2016;19:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Green MR.. Epigenetic programing of B-cell lymphoma by BCL6 and its genetic deregulation. Front Cell Dev Biol 2019;7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Meng T.. Long noncoding RNA in preeclampsia: transcriptional noise or innovative indicators? Biomed Res Int 2019;2019:5437621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R.. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 1993;262:747–750. [DOI] [PubMed] [Google Scholar]
- Yilmaz BD, Bulun SE.. Endometriosis and nuclear receptors. Hum Reprod Update 2019;25:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Zhou HJ, Lin C, Long L, Yang X, Zhang H, Taylor H, Min W.. CD34(+)KLF4(+) stromal stem cells contribute to endometrial regeneration and repair. Cell Rep 2019;27:2709–2724.e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW, Lessey BA.. KRAS activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep 2017;7:6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Lessey BA.. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med 2010;28:5–16. [DOI] [PubMed] [Google Scholar]
- Yu JM, Sun W, Hua F, Xie J, Lin H, Zhou DD, Hu ZW.. BCL6 induces EMT by promoting the ZEB1-mediated transcription repression of E-cadherin in breast cancer cells. Cancer Lett 2015;365:190–200. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wegenka UM, Lutticken C, Buschmann J, Decker T, Schindler C, Heinrich PC, Horn F.. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol 1994;14:1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariades E, Foster H, Goumenou A, Thomas P, Rand-Weaver M, Karteris E.. Expression of membrane and nuclear progesterone receptors in two human placental choriocarcinoma cell lines (JEG-3 and BeWo): Effects of syncytialization. Int J Mol Med 2011;27:767–774. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ.. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 1997;99:2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.