Abstract
BACKGROUND
To provide the optimal milieu for implantation and fetal development, the female reproductive system must orchestrate uterine dynamics with the appropriate hormones produced by the ovaries. Mature oocytes may be fertilized in the fallopian tubes, and the resulting zygote is transported toward the uterus, where it can implant and continue developing. The cervix acts as a physical barrier to protect the fetus throughout pregnancy, and the vagina acts as a birth canal (involving uterine and cervix mechanisms) and facilitates copulation. Fertility can be compromised by pathologies that affect any of these organs or processes, and therefore, being able to accurately model them or restore their function is of paramount importance in applied and translational research. However, innate differences in human and animal model reproductive tracts, and the static nature of 2D cell/tissue culture techniques, necessitate continued research and development of dynamic and more complex in vitro platforms, ex vivo approaches and in vivo therapies to study and support reproductive biology. To meet this need, bioengineering is propelling the research on female reproduction into a new dimension through a wide range of potential applications and preclinical models, and the burgeoning number and variety of studies makes for a rapidly changing state of the field.
OBJECTIVE AND RATIONALE
This review aims to summarize the mounting evidence on bioengineering strategies, platforms and therapies currently available and under development in the context of female reproductive medicine, in order to further understand female reproductive biology and provide new options for fertility restoration. Specifically, techniques used in, or for, the uterus (endometrium and myometrium), ovary, fallopian tubes, cervix and vagina will be discussed.
SEARCH METHODS
A systematic search of full-text articles available in PubMed and Embase databases was conducted to identify relevant studies published between January 2000 and September 2021. The search terms included: bioengineering, reproduction, artificial, biomaterial, microfluidic, bioprinting, organoid, hydrogel, scaffold, uterus, endometrium, ovary, fallopian tubes, oviduct, cervix, vagina, endometriosis, adenomyosis, uterine fibroids, chlamydia, Asherman’s syndrome, intrauterine adhesions, uterine polyps, polycystic ovary syndrome and primary ovarian insufficiency. Additional studies were identified by manually searching the references of the selected articles and of complementary reviews. Eligibility criteria included original, rigorous and accessible peer-reviewed work, published in English, on female reproductive bioengineering techniques in preclinical (in vitro/in vivo/ex vivo) and/or clinical testing phases.
OUTCOMES
Out of the 10 390 records identified, 312 studies were included for systematic review. Owing to inconsistencies in the study measurements and designs, the findings were assessed qualitatively rather than by meta-analysis. Hydrogels and scaffolds were commonly applied in various bioengineering-related studies of the female reproductive tract. Emerging technologies, such as organoids and bioprinting, offered personalized diagnoses and alternative treatment options, respectively. Promising microfluidic systems combining various bioengineering approaches have also shown translational value.
WIDER IMPLICATIONS
The complexity of the molecular, endocrine and tissue-level interactions regulating female reproduction present challenges for bioengineering approaches to replace female reproductive organs. However, interdisciplinary work is providing valuable insight into the physicochemical properties necessary for reproductive biological processes to occur. Defining the landscape of reproductive bioengineering technologies currently available and under development for women can provide alternative models for toxicology/drug testing, ex vivo fertility options, clinical therapies and a basis for future organ regeneration studies.
Keywords: bioengineering, uterus, endometrium, myometrium, ovary, fallopian tubes, cervix, vagina, female reproduction, fertility restoration
Introduction
To provide the optimal milieu for implantation and fetal development, the female reproductive system must orchestrate uterine dynamics in response to ovarian hormones. Specifically, estradiol and progesterone are produced through the processes of follicle development and luteinization in the ovary, and respectively regulate the proliferative and secretory phases in the endometrium. After ovulation, mature oocytes may be fertilized in the fallopian tubes, and the resulting zygote is transported toward the uterus, where it can implant and continue developing (if the endometrium is in an adequately receptive state). Throughout pregnancy, the cervix acts as a physical barrier to protect the fetus from external microorganisms or foreign objects that may enter through the vagina. Fertility can be compromised by pathologies that affect any of these organs or processes, and therefore, being able to accurately model them or restore their function is of paramount importance in applied and translational research.
The study of human reproduction requires multidisciplinary approaches. While animal models provide many opportunities for translational discoveries, there are inherent limitations due to differences compared to human reproductive physiology. Similarly, 2D cell or tissue culture models can provide novel insights on aspects of reproductive biology, but these models are more static and simplified and therefore do not recapitulate the dynamic, complex in vivo biology. These limitations underscore the need for continued research along with development of dynamic and more complex in vitro platforms, ex vivo approaches and in vivo therapies. This need is being filled, in part, by rapid advancements in the field of bioengineering, which applies life science and engineering principles to develop biomaterials for restoring, maintaining and/or improving tissue functions. Indeed, bioengineering is leading the way to a new dimension in the study of female reproduction by providing a wide range of potential applications and approaches for discovery.
Proposed bioengineering approaches to repair and/or improve female reproductive potential have evolved in parallel with advances in scientific knowledge and technology. Based on our systematic search, current strategies can be classified into six major categories, and these can be applied synergistically to understand reproductive biology and solve related problems: scaffold-free systems, hydrogels, decellularized extracellular matrix (dECM) or polymer scaffolds, 3D bioprinting, organoids and microfluidic approaches. Scaffold-free approaches make use of cells’ ability to self-organize and synthesize their own matrices, generating structures that can be used as functional units or regenerative blocks (Hayama et al., 2014; Orabi et al., 2017; Kuramoto et al., 2018, 2020). Hydrogels (which, for the purposes of this review, are defined by their liquid/injectable original state) can include a variety of natural and synthetic components and offer innumerable options for encapsulating or loading drugs, molecules, cells or reproductive tissues (Zhu et al., 2016; Tavana et al., 2016a; Yang et al., 2021; Zhang et al., 2021b). Selecting the most suitable hydrogel requires knowing the necessary mechanical and physicochemical properties for a given application (Kedem et al., 2011; Shikanov et al., 2011b). For example, animal-derived hydrogels include commercial mixtures of extracellular matrix (ECM) components, such as Matrigel and Cultrex, which are purified basement membrane extracts secreted by mouse Engelbreth-Holm-Swarm tumor cells.
In contrast, dECM scaffolds derive from tissues and organs that were processed by physical, chemical and/or enzymatic methods (Hellström et al., 2014; Laronda et al., 2015; Campo et al., 2017; Pors et al., 2019; Li et al., 2021; Sargazi et al., 2021; Pennarossa et al., 2021a). These biocompatible scaffolds preserve the structure and biochemical milieu of the tissue of origin (in terms of ECM signaling and migration), minimizing the risk of immune rejection after transplantation (Raya-Rivera et al., 2014; Daryabari et al., 2019; Yao et al., 2020b; Padma et al., 2021b). Notably, to facilitate transplantation/implementation, these scaffolds are often solubilized and used in hydrogel format (López-Martínez et al., 2021a). Scaffolds can also be produced from other natural polymers (such as collagen and bacterial cellulose) or synthetic components (Young et al., 2003; Liu et al., 2007; Edwards et al., 2015). Taking the fabrication of cell-loaded or cell-free scaffolds one step further, 3D bioprinting creates materials with precise shapes, textures and porosities, and offers vast applications in regenerative medicine (Laronda et al., 2017; Souza et al., 2017; Acién et al., 2019; Tiboni et al., 2021; Wu et al., 2022).
Among more recent developments are organoids and microfluidics. Organoids are simplified organs or organ-like structures formed in 3D culture systems, which enable recreation of the architecture and physiology of most female reproductive tissues. Organoids provide models for healthy and diseased tissue phenotypes, making them ideal platforms for personalizing bioengineering and biomedicine through both in vitro and in vivo studies (Kessler et al., 2015; Turco et al., 2017; Lõhmussaar et al., 2021; Oliver et al., 2021). Microfluidic platforms, increasingly referred to as the ‘organ-on-a-chip’ concept, utilize properties of fluid dynamics in small-channelled platforms to facilitate study of the dynamic hormonal cycles and endocrine interactions that characterize the reproductive organs (Xiao et al., 2017).
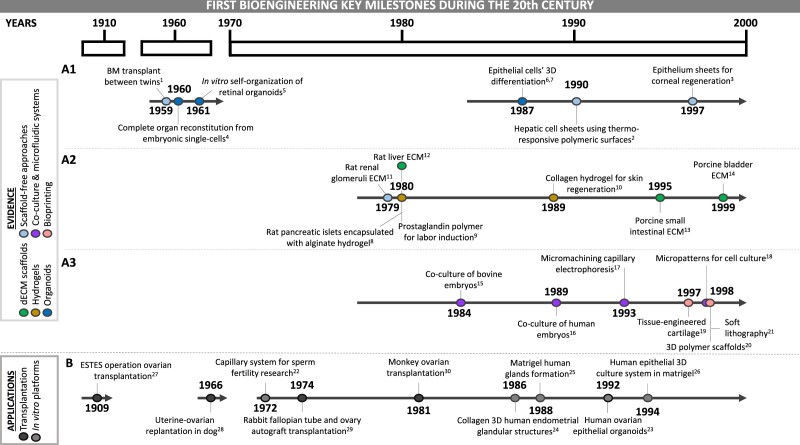
The majority of bioengineering studies date from the year 2000. However, innovative works from the 20th century built the foundation of this emerging field (Fig. 1). The groundwork for scaffold-free approaches included the first bone marrow transplant between twins (Thomas et al., 1959), and the generation of cell-sheets (Yamada et al., 1990) with regenerative potential (Pellegrini et al., 1997) (Fig. 1A1). Organoids were described as early as the 1960s, when single-cell suspensions completely reconstituted whole organs (Weiss and Taylor, 1960), retinal organoids self-organized in vitro (Stefanelli et al., 1961) and later, breast (Li et al., 1987) and alveolar (Shannon et al., 1987) epithelial cells aggregated to form 3D structures in Matrigel (Fig. 1A1).
Figure 1.
Key milestones during the 20th century forging the development of the bioengineering field. (A) Evidence. (A1) Advances such as the first bone marrow transplant between twins (1) (Thomas et al., 1959), the control of attachment and detachment of cultured cells (2) (Yamada et al., 1990) and the use of cell sheets (3) (Pellegrini et al., 1997) laid the groundwork for scaffold free-approaches. Concomitantly, in 1960, the reconstitution of a complete organ from single-cell suspensions (4) (Weiss and Taylor, 1960) opened an avenue to the present organoids. The in vitro self-organization of retina (5) (Stefannelli et al., 1961) and the 3D organization of breast (6) (Li et al., 1987) and alveolar (7) (Shannon et al., 1987) epithelial cells after culture with Matrigel moved this path further along. (A2) Some works from the 1980s reported the combination of hydrogels with different biological products such as pancreatic islets (8) (Lim and Sun, 1980), E2 (9) (Embrey et al., 1980) and epithelial cells (10) (Yannas et al., 1989), introducing these promising biomaterials for regenerative medicine. In parallel, obtaining ECM from renal glomeruli (11) (Hjelle et al., 1979), from liver connective tissue (12) (Rojkind et al., 1980), and a decade later, an intact acellular matrix from intestinal submucosa (13) (Badylak et al., 1995) and bladder (14) (Chen et al., 1999) provided the beginnings of the dECM scaffold approaches. (A3) The beginnings of co-culture systems are captured in two main works in which embryos were cultured together with trophoblastic vesicles (15) (Camous et al., 1984) and ampullary cells (16) (Bongso et al., 1989). Research that formed the basis of microfluidic systems was reported in the nineties; some examples are the emergence of on-chip capillary electrophoresis (17) (Harrison et al., 1993) and elastomeric microchannel networks for cell culture (18) (Folch and Toner, 1998). Works from the end of the century paved the way for bioprinting: creation of a tissue-engineered ear (19) (Cao et al., 1997), use of 3D printed substrates for cell adhesion (20) (Park et al., 1998) and introduction of soft lithography (21) (Xia and Whitesides, 1998). (B) Applications. The establishment of a capillary system for sperm samples (22) (Ulstein, 1972) and the culture of human ovarian epithelial organoids (23) (Kruk and Auersperg, 1992) were the beginnings of the development of in vitro screening platforms. The next generation in vitro platforms are based on studies like those from 1986 and 1988, which established endometrial epithelial cells were co-cultured with an ECM from glandular structures (24, 25) (Kirk and Alvarez, 1986; Rinehart et al., 1988) and a similar system also containing endometrial stromal cells (26) (Bentin-Ley et al., 1994). Finally, the development of the ESTES technique for dog ovarian transplantation (27) (Estes, 1909) in the early 20th century provided an excellent basis for a later dog uterus replantation (28) (Eraslan et al., 1966), a rabbit fallopian tube and ovary autograft transplantation (29) (Winston and Browne, 1974) and a primate ovarian transplantation (30) (Scott et al., 1981). BM, bone marrow; E2, estradiol; ECM, extracellular matrix; dECM, decellularized extracellular matrix.
Explorations in the 1980s and 1990s produced different types of in vitro co-culture systems (Fig. 1A2 and 3). In particular, the successful combination of hydrogels with different biological products, such as pancreatic islets (Lim and Sun, 1980), prostaglandins (Embrey et al., 1980) and epithelial cells (Yannas et al., 1989), encouraged the use of different biomaterials for regenerative medicine. In this regard, studies in which embryos were cultured together with trophoblastic vesicles (Camous et al., 1984) or ampullary cells (Bongso et al., 1989) inspired other co-culture systems. On the other hand, dECM scaffolds appeared after ECM was obtained from murine renal glomeruli (Hjelle et al., 1979), liver connective tissue (Rojkind et al., 1980), intact acellular matrix from porcine intestinal submucosa (Badylak et al., 1995) and bladder (Chen et al., 1999) (Fig. 1A2). Microfluidic platforms also emerged with micromachining capillary electrophoresis (Harrison et al., 1993), and microchannel networks for cell culture (Folch and Toner, 1998). Finally, bioprinting gained popularity with the first tissue-engineered ear (Cao et al., 1997), the use of 3D-printed substrates for cell adhesion (Park et al., 1998) and introduction of soft lithography (Xia and Whitesides, 1998); the latter encompasses a group of techniques for fabricating or replicating structures, channels or membranes by using soft polymeric material (usually polydimethylsiloxane) stamps or molds (Kim et al., 2018) (Fig. 1A3).
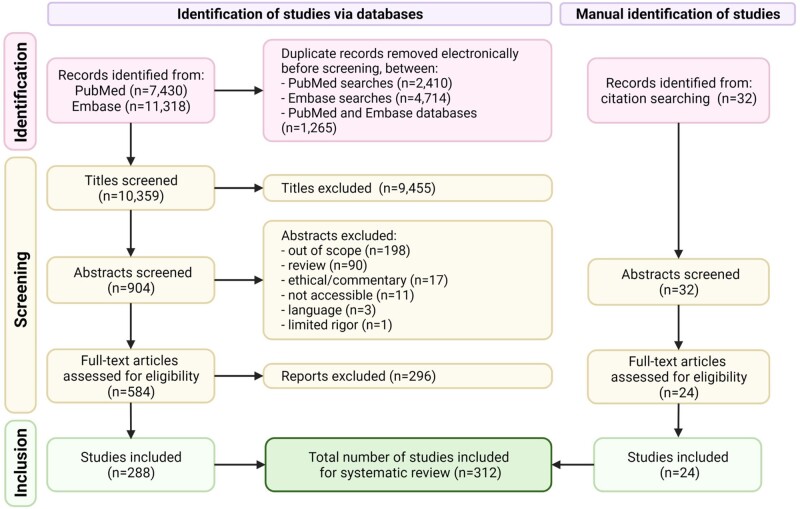
Figure 2.
PRISMA flow diagram. Exact terms used for each of the database searches are detailed in Supplementary Table SI. Template adapted from Page et al. (2021). Created with BioRender.com.
These six categories of bioengineering strategies promote four main translational and/or clinical applications: the development of next-generation in vitro platforms, or representative in vitro toxicology and drug screening models; the discovery of alternative therapies or new biomarkers; and improvement of tissue/organ regeneration and/or transplantation protocols (Fig. 1B). The establishment of a capillary system for sperm samples (Ulstein, 1972) is an excellent example of an innovative platform to improve ART, while the in vitro culture of human endometrial 3D glandular structures (Kirk and Alvarez, 1986; Rinehart et al., 1988), endometrial stromal cells embedded in a collagen matrix [and covered with epithelial cells (Bentin-Ley et al., 1994)] and ovarian epithelial organoids (Kruk and Auersperg, 1992) ensured the initial steps towards personalized in vitro screening platforms. Finally, the early development of the ESTES technique, where a portion of the ovary is transplanted into the uterus (Estes, 1909), provided a foundation for later progress in reproductive organ transplantation (Eraslan et al., 1966; Winston and Browne, 1974; Scott et al., 1981).
Since these initial discoveries paved the way, the bioengineering field has undergone rapid growth and expansion. Many engineered reproductive tissues and platforms are currently in different stages of clinical development; most models remain experimental, but others are in pre-clinical trials, and some are already being applied clinically. Given the quantity and heterogeneity of studies published within this specialty, the goal of this review was to systematically summarize the mounting evidence on bioengineering strategies, platforms and therapies, both currently available and under development, in the context of female reproductive medicine, including novel alternatives for fertility restoration.
Methods
Search strategy
PubMed and Embase were searched for relevant reports. The search strategy was limited to full-text articles, published in English, involving mammals or material derived therefrom, between January 2000 and September 2021. Combinations of the following keywords were used: bioengineering, reproduction, artificial, biomaterial, microfluidic, bioprinting organoid, hydrogel, scaffold, uterus, endometrium, ovary, fallopian tubes, oviduct, cervix, vagina, endometriosis, adenomyosis, uterine fibroids, chlamydia, Asherman’s syndrome (AS), intrauterine adhesions, uterine polyps, polycystic ovary syndrome and primary ovarian insufficiency. Specific queries used in each database are presented in Supplementary Table SI. Additional studies were identified by manually searching the references of the selected articles and of complementary reviews.
Study selection and eligibility criteria
Literature search results were exported to an MS Excel spreadsheet and duplicates were identified using electronic and manual methods (Fig. 2). Titles, abstracts and full texts were then screened independently and in duplicate by two authors (E.F.-H. and R.L.) using the following eligibility criteria: original, rigorous and accessible peer-reviewed work published in English, on female reproductive bioengineering techniques in preclinical (in vitro/in vivo/ex vivo) and/or clinical testing phases. Studies in which gels were developed for intravaginal delivery of hormones, bactericides, nucleic acids or contraceptive drugs were not considered in this review because of their pharmacological nature. Questions or disagreements were resolved by discussion (E.F.-H., R.L., A.P. and I.C.). The final list of included studies was approved by I.C.
Data extraction
Extracted data, including titles, authors, year of publication, reproductive organ (uterus, ovary, fallopian tube, cervix, vagina or full tract), bioengineering strategy, platform/biomaterial used, species, cell/tissue model, study type (in vitro, in/ex vivo, clinical) and main findings were compiled into a shared Google Sheets spreadsheet and revised by M.H., L.M.-G., S.H. and M.B.
Synthesis of results
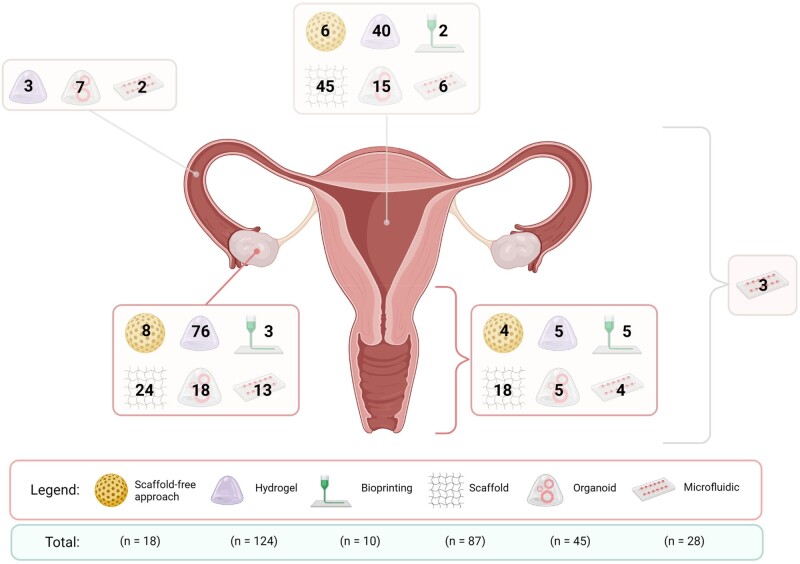
Relevant findings extracted from each study are summarized in Table I. Due to the inability to completely detail the many articles comprising this systematic review in Table I, a comparison of in vivo uterine regeneration parameters (e.g. immune tolerance, recovery of thickness and muscle layer, presence of glands, angiogenesis, implantation potential and maintenance of pregnancy) is provided in Supplementary Table SII, while specific outcomes of in vitro follicle growth (IVFG) studies (e.g. follicle survival, initial and final follicle size, steroidogenesis, oocyte maturation rates, developmental competence and/or fertility restoration) are detailed in Supplementary Table SIII.
Table I.
Main findings of bioengineering studies related to female reproductive organs.
| Strategy | Platform/biomaterial | Type of study (model) | Disease-related | Main findings | Reference |
|---|---|---|---|---|---|
| ENDOMETRIUM AND MYOMETRIUM | |||||
| Scaffold-free approaches | Cell sheet | In vivo (rat) |

|
Rat oral mucosal epithelial cell sheets prevented IUAs caused by endometrial damage and helped to maintain the uterine luminal structure. | Kuramoto et al. (2015) |

|
Multilayered rat endometrial epithelial and stromal cell sheet transplantation regenerated endometrial tissue, supporting pregnancy similar to normal endometrial tissue. | Kuramoto et al. (2018) | |||

|
Rat adipose-derived stem cell sheets transplanted into partially excised uteri promote regeneration of endometrial and muscle cells and stimulate angiogenesis. | Sun et al. (2018) | |||

|
Human UC-MSC sheets improved uterine incision repair in a rat hysterotomy model. | Kuramoto et al. (2020) | |||
| MicroTissues 3D Petri Dish micro-mold spheroids | In vitro | Generation of endometrial organoids with both epithelial and stromal cells of the human endometrium. | Murphy et al. (2019) | ||

|
Human endometrial organoids containing epithelial and stromal cells responded to androgens associated with PCOS. | Wiwatpanit et al. (2020) | |||
| Hydrogels | DC endometrium | ECM coating from synchronous DC rabbit endometrium achieved similar results to the gold standard embryo culture conditions. | Campo et al. (2019) | ||
| In vitro + in vivo (mouse) | Porcine endometrial ECM hydrogel supports in vitro culture of human endometrial cells in 2D and 3D conditions. Improved proliferation of EnSCs with respect to collagen and Matrigel. | López-Martínez et al. (2021a) | |||
| In vivo (mouse) |

|
Porcine endometrial ECM hydrogel loaded with growth factors enhanced tissue regeneration and restored fertility in a mouse model of endometrial injury. | López-Martínez et al. (2021b) | ||
| Collagen | In vitro | E2 stimulation of human Ishikawa cells induced functional changes in HUVECs within a collagen biomaterial. | Pence et al. (2015) | ||
| 3D collagen gel-embedded human endometrial tissue slices responded to ovarian steroid hormones over 3 weeks. | Muruganandan et al. (2020) | ||||
| A tissue-engineered human endometrial stroma manifests changes in morphology and biochemical markers of decidualization, and responds to steroid withdrawal. | Schutte and Taylor (2012) | ||||
| Human endometrial stromal cells acquired contractile ability by passive loading of cyclic tensile stretch. | Kim et al. (2020b) | ||||
| In vivo (rat) |

|
Collagen-binding VEGF restored fertility in a full-thickness injury model of rat scarred uterus. | Lin et al. (2012) | ||

|
Human UC-MSCs facilitated collagen scaffold degradation in rat uterine scars, promoting full-thickness wall regeneration and restoring fertility. | Xu et al. (2017c) | |||
| Clinical |

|
Improvement in endometrial proliferation, differentiation and neovascularization following allogeneic cell therapy using human UC-MSCs on collagen hydrogels in patients with IUAs. | Cao et al. (2018) | ||

|
Transplantation of human UC-MSCs on collagen hydrogels improved endometrial angiogenesis, proliferation and response to hormones in patients with AS. | Zhang et al. (2021b) | |||

|
Collagen-binding bFGF improved functional remodeling of scarred endometrium in infertile women. | Jiang et al. (2019) | |||
| Hydrogels | Collagen-Matrigel | In vitro | 3D culture model with human stromal and epithelial cells replicates the normal endometrium physiologically and morphologically, including stromal invasion of KLE cells. | Park et al. (2003) | |
| Co-culture model of human epithelial and stromal cells changed cytokine production, reducing inflammation and protease activity. | Schutte et al. (2015) | ||||

|
Development of a 3D spheroid human model of endometriosis where collagen I triggers directional migration, invasion and matrix remodeling of stroma cells. | Stejskalová et al. (2021) | |||
| Matrigel | Luminal and glandular epithelial cells covered the injured surface of human endometrial strips in a Matrigel-based in vitro model. | Stavreus-Evers et al. (2003) | |||
| Spheroid-based 3D cell culture system consisting in endometrial adenocarcinoma and EVT cell lines mimics early implantation events in humans. | Buck et al. (2015) | ||||
| In vivo (rat) |

|
Injectable human UC-MSC-laden Matrigel microspheres enhanced endometrial regeneration and improved fertility rates in a rat model with thin endometrium. | Xu et al. (2021) | ||
| HA | In vitro + in vivo (rat) |

|
Local injection of HA-danazol gel reduced size of endometrial cysts, without disrupting the estrous cycle in a rat model of endometriosis. | Nomura et al. (2006) | |
| In vivo (mouse) |

|
HA-fibrin-encapsulated murine dEMSCs repaired the damaged endometrium, with successful implantation and normal embryo development. | Kim et al. (2019) | ||
| In vitro + ex vivo + in vivo (rat) |

|
In situ administration of HA gel/human MSC-secretome treatment repaired endometrial injury, promoting pregnancy, in a rat model of AS. | Liu et al. (2019) | ||
| Collagen + HA + agar | In vitro + in vivo (mouse) |

|
Three-layered artificial endometrium (made from human EnSC, stromal and vessel cells) remained functional in vitro for 28 days and restored fertility (with successful pregnancy and LBs) in endometrial ablation mouse model. | Park et al. (2021) | |
| Dextrin | In vivo (pig) |

|
Using a dextrin-based adhesion barrier resulted in a higher percentage of adhesion-free sites compared with the controls after laparoscopy in a pig model. | Kai et al. (2018) | |
| Fibrin-agarose | In vitro | Human implantation is modeled by co-culturing human endometrial epithelial and stromal cells in a 3D system that allows JAR spheroid attachment. | Wang et al. (2012) | ||
| Human epithelial-stromal interaction enhanced prolactin expression in fibrin-agarose gel. JAR spheroids invaded the epithelium and embedded into the 3D matrix under decidualization conditions. | Wang et al. (2013) | ||||
| PEG | Clinical |

|
PEG-based sprayable adhesion barrier reduced adhesion tenacity, extent and incidence scores in patients undergoing myomectomy. | Mettler et al. (2004) | |

|
Resorbable PEG-based hydrogel reduced post-operative adhesions following myomectomy. | Mettler et al. (2008) | |||
| In vitro | Co-culture of human endometrial epithelial cells and stromal cells encapsulated in a PEG hydrogel with ECM-binding peptides remodel the synthetic matrix and display hormone-mediated differentiation. | Cook et al. (2017) | |||
| In vitro + in vivo (rat) |

|
l-phenylalanine-loaded PEBP/PEG hydrogel suppressed uterine fibrosis and promoted embryo implantation in a rat uterine curettage model. | Wang et al. (2021) | ||

|
Human AD-MSC exosome-hydrogel promoted neovascularization and endometrial regeneration in rats, facilitating LBs. | Lin et al. (2021a) | |||
| PVA/CMC | In vivo (rabbit) |

|
Reduced incidence, extent and severity of peritoneal adhesions following gynaecological surgery. | Müller et al. (2011) | |
| Pluronic F-12, Vitamin C | In vivo (rat) |

|
Pluronic F-12 hydrogel encapsulating vitamin C and rat bone marrow stromal cells promoted rat endometrial regeneration by restoring the endometrial membrane and reducing inflammation. | Yang et al. (2017) | |
| Hydrogels | Methacrylamide-functionalized gelatin | In vitro |

|
Human endometrial epithelial and stromal cells showed proangiogenic activity in response to E2 in gelatin hydrogels. | Pence et al. (2017) |
| HP | In vitro + in vivo (rat) |

|
ε-Polylysine-HP hydrogel encapsulating keratinocyte growth factor repaired the morphology of injured rat endometrium. | Xu et al. (2017a) | |

|
HP hydrogel loaded with keratinocyte growth factor facilitates the morphologic and functional recovery of injured rat uteri. | Xu et al. (2017b) | |||

|
HP-E2 hydrogel prolongs release of E2, improving both gland numbers and fibrotic area, in a IUA rat model. | Zhang et al. (2017b) | |||
| In vivo (rat) |

|
HP-E2 hydrogel facilitated the regeneration of injured endometrium, inhibiting cell apoptosis in a IUA rat model. | Zhang et al. (2020b) | ||
| Chitosan-heparin |

|
Treating injured rat endometrium with a stromal cell derived factor-1α-loaded chitosan-heparin hydrogel restored endometrial thickness, gland number and reduced fibrosis. | Wenbo et al. (2020) | ||
| Actamax adhesion barrier | Clinical |

|
Spraying a degradable hydrogel adhesion barrier during gynecologic laparoscopic abdominopelvic surgery reduced postoperative adhesion development. | Trew et al. (2017) | |
| Aloe poloxamer + DC uterus nanoparticles | In vitro + in vivo (rat) |

|
Aloe poloxamer with E2 encapsulated in DC rat uterus nanoparticles significantly recovers morphology and decreases uterine fibrosis in a IUA rat model. | Yao et al. (2020a) | |
| dECM and polymer scaffolds | DC uterus | Proof of concept | Comparison of three protocols for whole rat uterus decellularization. The sodium deoxycholate protocol gave rise to a scaffold that structurally and mechanically resembled native uterus. | Hellström et al. (2014) | |
| Proof of concept + in vitro | Whole pig uterus decellularization produced a cytocompatible scaffold. Recellularization with human EnSC resulted in organoid-like structure formation. | Campo et al. (2017) | |||
| In vivo (mouse) |

|
DC uterine matrix transplantation restored all the uterine layers and fertility. | Hiraoka et al. (2016) | ||
| In vitro + in vivo (rat) |

|
Xenogeneic crosslinked rabbit uterine ECM achieved rat uterus regeneration and was recellularized in vivo after 90 days. | Yao et al. (2020b) | ||
| Whole DC sheep uterus gave rise to biocompatible scaffolds with native-like biomechanical, structural and vascular properties that were recellularized in vivo. | Daryabari et al. (2019) | ||||

|
Engraftment of rat MSC-recellularized DC uterine matrix on partially excised uteri yielded functional uteri with pregnancy and fetus rates comparable to the control group. | Li et al. (2021) | |||

|
Perfusion-recellularized uterine matrix is able to partially regenerate and reconstruct the damaged rat uteri. | Miyazaki and Maruyama (2014) | |||

|
Recellularized uterine ECM patches repair a partially defective uterus and support pregnancy. | Hellström et al. (2016) | |||
| In vivo (rat) |

|
Both high hydrostatic pressure and detergent-based decellularization protocols can efficiently create rat uterine matrices for uterine regeneration. | Santoso et al. (2014) | ||

|
The orientation of a DC uterine scaffold determines the tissue topology and architecture of regenerated uterus in rats, without affecting pregnancy. | Miki et al. (2019) | |||
| Decellularization based on Triton-X100 and deionized water generated the lowest immune response after allogeneic transplantation of DC rat uterine scaffolds. | |||||
| Rat uterus decellularization with sodium deoxycholate revealed more ECM-related damage-associated molecular patterns, and resulting scaffolds induced pro-inflammatory cytokine responses. | Padma, Alsheikh, Song, et al. (2021b) | ||||
| dECM and polymer scaffolds | In vitro | Mouse uterine DC scaffolds proved to be an adequate natural niche for human MenMSCs differentiation toward uterus-specific cell lineages. | Arezoo et al. (2021) | ||
| Comparison of three protocols for whole sheep uterus decellularization, generating different ECM scaffolds that supported in vitro stem cell growth and proliferation. | Tiemann et al. (2020) | ||||
| Enzymatic preconditioning of sheep uterine ECM scaffolds improved recellularization compared with standard culture conditions and with the use of transwells alone. | Padma et al. (2021c) | ||||
| DC myometrium | Creation of allo- and xeno-neo-myometrium by culturing isolated myocytes into DC rat and human myometrial scaffolds. | Young and Goloman (2013) | |||
| DC endometrium | Human recellularized endometrium responded to a 28-day hormone treatment by expressing E2 and P4 receptors and secreting IGF binding protein-1 and prolactin. | Olalekan et al. (2017) | |||
| Comparison of different decellularization protocols for human endometrial fragments. | Sargazi et al. (2021) | ||||
| DC human amniotic membrane | In vivo (rat) |

|
Engineered rat oral mucosa epithelial cells prevented progression of IUA and improved endometrial epithelium regeneration. | Chen et al. (2019) | |

|
Human amniotic membrane and adipose stem cells improved regeneration, angiogenesis and receptivity in a rat IUA model. | Han et al. (2020) | |||
| Urinary bladder ECM |

|
Porcine urinary bladder matrix scaffolds improved endometrial regeneration in a rat model of intrauterine adhesions. | Zhang et al. (2020a) | ||
| HA/carboxymethylcellulose membrane |

|
Both melatonin and HA/carboxymethylcellulose membrane proved to be effective in prevention of adhesion formation in rats. | Demirbag et al. (2005) | ||
| HA + mitomycin C |

|
Mitomycin C-loaded crosslinked HA films and gels reduced formation of postoperative adhesions between uterine horns and with surrounding tissues and organs. | Liu et al. (2005) | ||
| Carbylan-SX (semisynthetic glycosaminoglycan) |

|
Carbylan-SX film and gel were efficacious in reducing postoperative intra-abdominal adhesion formation in cecum-abdominal wall and uterine horn in rats. | Liu et al. (2007) | ||
| GelMA and sodium-alginate |

|
Porous scaffold from droplet microfluidics loaded with bFGF had the ability to improve neovascularization and repair rat endometrium. | Cai et al. (2019) | ||
| Alginate | In vitro | Development of an embryo implantation model consisting of an alginate scaffold seeded with human epithelial cells to which JAR spheroids are able to adhere. | Stern-Tal et al. (2020) | ||
| Alginate-multivalent integrin α5β1 ligand | Alginate-multivalent integrin α5β1 ligand scaffolds enhanced human endometrial stromal cell growth under perfusion culture. | Li et al. (2011b) | |||
| Collagen | In vitro + in vivo (rat) |

|
Collagen scaffold loaded with human UC-MSCs promoted endometrial regeneration and restored fertility in a rat model. | Xin et al. (2019) | |
| In vivo (rat) |

|
Collagen scaffolds with human bFGF improved regeneration of rat uterine endometrium and muscular cells, vascularization and pregnancy outcomes. | Li et al. (2011a) | ||

|
Collagen/rat BM-MSCs system increased proliferation of endometrial and myometrial cells, enhanced angiogenesis and restored fertility. | Ding et al. (2014) | |||

|
Collagen scaffold loaded with human ESC-derived endometrium-like cells regenerated the structure and function of rat uterine horns. | Song et al. (2015) | |||

|
Collagen scaffold loaded with human endometrial perivascular cells overexpressing CYR61 promoted endometrial and myometrial regeneration and induced neovascular regeneration in injured rat uteri model. | Li et al. (2019) | |||

|
Collagen scaffold with human UC-MSCs improved IUAs in rats, by increasing endometrial glands and reducing firbosis. | Liu et al. (2020) | |||
| Clinical |

|
Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promoted functional endometrium reconstruction in patients with AS. | Zhao et al. (2017) | ||
| Gelatin-coated polyamide | In vitro | Human eMSCs were differentiated into smooth muscle cells or fibroblast-like cells to simulate fascial tissue composition, using an optimized gelatin-coated polyamide scaffold. | Su et al. (2014) | ||
| In vivo (rat) | Seeding human eMSCs in SC gelatin-coated polyamide mesh resulted in enhanced collagen growth and organization. | Edwards et al. (2015) | |||
| PGA-PLGA | In vivo (rabbit) |

|
Subtotal uterine excisions were reconstructed with autologous constructs made from endometrial and myometrial cells. Fetal development supported to term and LB. | Magalhaes et al. (2020) | |
| Poly(glycerol sebacate) | In vitro + in vivo (rat) |

|
Directional differentiation of rat BM-MSCs and restoration of morphology and function of wounded uteri. | Xiao et al. (2019) | |
| PLA + Pluronic F68 |

|
Novel PLA-Pluronic copolymer films prevented adhesion comparable to membranes of oxidized regenerated cellulose. | Yamaoka et al. (2001) | ||
| PLA-poly(ε-caprolactone)/gelatin nanofiber | In vivo (mouse) |

|
Degradable mesh with murine eMSCs promoted tissue integration and anti-inflammatory response after subcutaneous transplantation. | Mukherjee et al. (2019) | |
| PLA patch (“nanofilm”) | Ex vivo + in vivo (rabbit) |

|
PLA nanofilm sealed defects smaller than 3 mm in chorion-amnion and uterine membranes allowing intrauterine development in a rabbit model. | Pensabene et al. (2015) | |
| Polyglactin-910 mesh | In vitro | Human uterine smooth muscle myocytes proliferated and formed 3D tissues within 14 days. | Young et al. (2003) | ||
| Emulsion-templated porous polymers | 3D scaffolds enhanced differentiation of primary human endometrial epithelial and stromal cells resembling the in vivo architecture and function. | Eissa et al. (2018) | |||
| Scaffolds with fibronectin improved adhesion, infiltration and function of primary human endometrial stromal cells. | Richardson et al. (2019) | ||||
| PTFE | Novel hormone responsive in vitro model of the human uterine wall by co-culturing smooth muscle cells and endometrial epithelial and stromal cells on a synthetic membrane. | Kuperman et al. (2020) | |||
| Bioprinting | Gelatin + alginate | In vitro + in vivo (rat) |

|
3D-printed hydrogel scaffold loaded with human iPSC-derived MSCs promoted the regeneration of endometrial and endothelial cells, and improved endometrial receptivity in a rat model. | Ji et al. (2020) |
| Myometrial 3D cell rings | In vitro | Bioprinted uterine rings created with human myometrial cells show origin-dependent patterns of contractility and respond differently to uterine contractility inhibitors. | Souza et al. (2017) | ||
| Organoid | DC endometrium | Solubilized endometrial ECM from porcine uteri enhances proliferation rates of human endometrial organoids. | Francés-Herrero et al. (2021b) | ||
| Matrigel-based 3D culture platform |

|
Development of a functional C. trachomatis-murine endometrial organoids infection model system. | Bishop et al. (2020) | ||
| Establishment of a novel organotypic culture system that models the hormonal responses of the normal human endometrium (epithelia and stroma) in vitro. | Bläuer et al. (2005) | ||||

|
Generation of long-term, hormone-responsive human endometrial organoid cultures from healthy and cancerous tissue. | Turco et al. (2017) | |||
| Formation of human and murine organoid structures showing long-term expansion, and reproducing the molecular and histological phenotype of the endometrial epithelium. | Boretto et al. (2017) | ||||
| In vitro + in vivo (mouse) |

|
Establishment of human organoids from endometriotic, cancerous and pre-cancerous tissues showing disease diversity and original lesions in vivo. | Boretto et al. (2019) | ||
| In vitro | Derivation of human endometrial gland organoids from term placenta that express typical markers of glandular epithelia. | Marinić et al. (2020) | |||

|
Glandular organization, ultrastructural features, hormone responsiveness and glycodelin A expression make human organoids a powerful in vitro model for the endometrium-embryo cross-talk. | Luddi et al. (2020) | |||
| Organoid | Derivation of human endometrial organoids from menstrual flow, comparable to those derived from endometrial biopsies. | Cindrova-Davies et al. (2021) | |||
| Establishment of human endometrial assembloids, consisting of gland-like organoids and primary stromal cells, to model the impact of decidual senescence on embryo implantation. | Rawlings et al. (2021) | ||||
| Co-culture of human iPSC-ESFs with placenta-derived endometrial epithelial cells generated hormone-responsive organoids in a model of human decidua. | Cheung et al. (2021) | ||||
| In vitro + in vivo (rat) |

|
Human endometrial organoids containing H9-ESC induced into EEPCs and stromal components facilitated endometrial regeneration and angiogenesis in a rat model of AS. | Jiang et al. (2021) | ||
| Collagen | In vitro | Generation of a 3D collagen scaffold-based model of the human endometrium by co-culturing endometrial organoids and stromal cells. | Abbas et al. (2020) | ||
| Functionalized PEG-macromers | Derivation of human endometrial organoids with cell specificity and apicobasal polarity in fully synthetic matrices. | Hernandez-Gordillo et al. (2020) | |||
| Microfluidic | PDMS chip | Proof of concept | Development of a microfluidic device for single mouse embryo co-culture with murine endometrial cells. | Kimura et al. (2009) | |
| Resin-based porous membrane; PDMS | In vitro | Development of a microfluidic model of the human endometrium, compartmentalizing culture of perivascular stroma and endothelial cells. | Gnecco et al. (2017) | ||
| PDMS | Enhanced decidualization of human endometrial stromal cells via endothelial-derived prostaglandin E2 and prostacyclin due to the action of hemodynamic forces. | Gnecco et al. (2019) | |||
| Porous glass | Human endometrium on-a-chip revealed insulin- and glucose-induced alterations in the transcriptome and proteomic secretome. | De Bem et al. (2021) | |||
| PDMS + fibrin gel | Reconstitution of a three-layer, hormone-responsive, vascularized endometrium-on-a-chip on a 3D fibrin matrix using human HUVECs, Ishikawa and ESFs. | Ahn et al. (2021) | |||
| OVARY | |||||
| Scaffold-free approaches | Micro-molded agarose gel created with PDMS cast | In vitro | Human TCs self-assembled into complex spheroid, toroid and honeycomb microtissues. Artificial human ovary constructed at 72 h with TCs surrounding GC spheroids or COCs without stromal invasion or disruption. | Krotz et al. (2010) | |
| Ovary-like tissue | In vivo (mouse) |

|
Murine and rat PGCs and PGC-free gonadal cells can develop and reconstruct ovary-like tissue containing functional oocytes in an ectopic xenogeneic microenvironment. | Hayama et al. (2014) | |
| Qdot 655 ITK carboxyl QDs | QDs found in the ovaries do not affect mouse behavior or estrous cycles, but decreases IVF rate. QDs can downregulate FSH and LH receptors and decrease maturation rate. | Xu et al. (2016) | |||
| PDMS | In vitro + in vivo (rat) |

|
Spheroid human PD-MSCs likely prolonged ovarian function, produced more follicles, doubled E2 levels compared to 2D culture and increased Nanos3, Nobox and Lhx8 at 1 and 2 weeks. | Kim et al. (2018) | |
| PEG-PLA versus TiO2 nanoparticles | Ex vivo |

|
FSH/LH and IGF-1 supplementation rescued initial decrease of E2/P4 with PEG-PLA nanoparticles in rat ovaries. Neonatal exposure to TiO2 nanoparticles hindered FSH/IGF stimulation. | Scsukova et al. (2020) | |
| Chitosan-based nanoparticles | In vitro + in vivo (rat) |

|
Treatment based on curcumin-encapsulated, self-assembled nanoparticles showed positive effects in reverting the symptoms of PCOS in rats. | Raja et al. (2021) | |
| Hydrogels | Alginate | In vitro + in vivo (mouse) |

|
BMP4 increased number of developing porcine follicles, E2 secretion and GDF9/AMH. After xenotransplantation, hormone levels restored in ovariectomized mice and antral follicles developed. | Felder et al. (2019) |
| 1.5% alginate enhanced murine secondary follicle survival and oocyte maturation, supported normal IVF and resulted in LB after ET. | Xu et al. (2006) | ||||
| In vitro | Culturing multiple murine primary follicles together promoted follicle growth, rescued follicle integrity and increased transzonal projections and oocyte maturation. | Hornick et al. (2013) | |||
| Co-culturing murine primary-secondary follicles with MEFs for the whole 14-day period increased survival and growth. Primary follicles had lower oocyte maturation rates than >80 μm follicles. | Tagler et al. (2012) | ||||
| 90 µm murine follicles survived twice as much as 80 µm follicles and grew on average 29 µm more. | Tagler et al. (2013) | ||||
| Hydrogels | 70 μm murine follicles survived much more than 60 μm follicles. Ascorbic acid supplementation improved structural integrity via expression of ECM and cell adhesion molecules. | Tagler et al. (2014) | |||
| 0.7% alginate resulted in visible TC layer and approporiate steroidogenesis of murine follicles, as well as enhanced size, pseudoantrum rate and GVBD. | West et al. (2007) | ||||
| 0.5% alginate increased average murine follicle diameter. Antral follicles produced appropriate levels of E2+P4, a 34-fold increase in aromatase expression and elevated LH receptors. | West-Farrell et al. (2009) | ||||
| Cryopreservation (by slow freezing) produced murine follicles with similar survival, average follicle diameter, antral development, decreases in Cx-43 and Cx-37 expression and increases in P4/E2 and maturation rates. | Xu et al. (2009a) | ||||
| Cultured murine follicles had aromatase, inhibin βa, BMP15, KIT ligand, TGFβR2 expression downregulated relative to in vivo follicles, while their COCs had increased expression of Inhibin α and βa, decreased expression of BMP15, GDF9, KIT and similar expression of Figlα, JAG1, Mater. | Parrish et al. (2011) | ||||
| Murine secondary follicles mature, ovulate and luteinize in vitro. Progesterone agents (mifepristone and ulipristal acetate) significantly inhibited rupture. | Skory et al. (2015) | ||||
| Co-culture of mouse secondary follicles and ovarian cells in 0.5% alginate increased follicle survival, diameter and P4 production, while decreasing oocyte cortical granule abnormalities. | Jamalzaei et al. (2020) | ||||
| Normal OSE migrated and encapsulated wounded surfaces of mouse ovarian fragments. Direct effects of fetal bovine serum and bovine serum albumin on encapsulation and proliferation. | Jackson et al. (2009) | ||||
| In vivo (mouse) |

|
SC murine ovarian grafts with the least amount of follicles had the highest survival. SC sites produced more mature oocytes. Higher embryo development rates after IVF versus ICSI. MDA-MB-231 cells encapsulated with follicles did not produce metastatic lesions. | Rios et al. (2018) | ||
| In vitro | 0.25% alginate increased survival of rat preantral follicles, average follicle diameter, antral development, ovulation and oocyte maturation compared to 2D culture. | Zhang et al. (2019c) | |||
| Pre-antral canine follicles in 0.5% alginate grew faster, but had smaller diameters, and produced 5–10× less P4 than in 1.5% alginate. LH may be required to support TC differentiation and GC function. | Songsasen et al. (2011) | ||||
| 0.25% alginate produces larger and more morphologically abnormal caprine follicles but higher E2/P4, aromatase and 3βHSD, antrum formation, growth and oocyte maturation rates. | Brito et al. (2014) | ||||
| Ovine secondary follicles cultured in 1% alginate increased COC expansion, maturation rates, mitochondrial activity and ROS as well as upregulated TFAM, ATP6/8 and downregulated KHDC3, NLRP5. | Mastrorocco et al. (2020) | ||||
| Collecting rhesus monkey follicles during the follicular phase (versus luteal phase) significantly increased survival, and average follicle diameter. Follicles grew significantly more with FSH alone versus FSH and LH. | Xu et al. (2009c) | ||||
| By preserving follicle viability and growth better than ethylene glycol, dimethylsufoxide can safely be used to cryopreserve human primordial/primary follicles encapsulated in alginate. | Camboni et al. (2013) | ||||

|
E2, P4, inhibin A/B and activin A secretion patterns of human follicles in vitro mimicked in vivo serum levels. Individually cultured human primary–secondary follicles produced AMH approximately through the time of antrum formation. | Skory et al. (2015) | |||

|
Multilayered human secondary follicles continued to grow long term. E2/P4 positively correlated with follicle development whereas AMH transiently increased during early follicle development and then declined upon antrum formation. A total of 20% oocyte maturation and MII oocyte size was similar to germinal vesicle oocyte size. | Xiao et al. (2015) | |||

|
1% alginate supports survival (of oocytes and GCs) and development of small pre-antral human follicles from frozen-thawed OT for a week after enzymatic isolation. | Amorim et al. (2009) | |||

|
Human follicles in native OT remain viable for up to 24 h whereas isolated primordial follicles did not survive in 2% alginate. Encapsulating OT fragments supported antral development and surface epithelium, but not retention of follicle organization or basement membranes. | Laronda et al. (2014) | |||
| Hydrogels | Alginate versus collagen | Human MenMSCs increased early secondary follicle survival, diameter, antrum formation, E2/P4 production, oocyte maturation and expression of BMP15 and GDF9 but decreased expression of Mater. | Rajabi et al. (2018) | ||
| Collagen | 3, 5, 7 mg/ml hydrogels supported rat follicle survival, size, integrity and GVBD better than 1 mg/ml. | Joo et al. (2016) | |||
| In vivo (rat) |

|
Transplantation of rat AD-MSCs-laden collagen scaffolds improved restoration of ovarian function and fertility outcomes in a rat model of POI. | Su et al. (2016) | ||
| In vivo (mouse) |

|
Transplantation of human UC-MSCs into POF mice preserved ovarian function as well as increased E2, AMH, ovarian volume, number of antral follicles, GC proliferation and CD31 expression. | Yang et al. (2019) | ||
| Clinical |

|
Primordial follicles activated in vitro via phosphorylation of FOXO3a and FOXO1. Transplantation to the ovaries of patients with POF rescued overall ovarian function. CP achieved in patients with POF after transplantation of human UC-MSC or collagen/UC-MSCs. | Ding et al. (2018) | ||
| Alginate + PLO | In vivo (rat) |

|
Ovarian contructs (of rat OT, GCs, TCs) restored hormone levels, in ovariectomized rats, for 90 days after transplantation. May be used as an alternative and safe cell-based hormone replacement therapy. | Sittadjody et al. (2017) | |
| Alginate versus PEG-fibrinogen ± PTEN inhibitors | In vitro |

|
Alginate + bpV (pic) produced significantly more atretic follicles in human OT fragments than PEG-fibrinogen. Addition of 740Y-P (versus bpV(pic)) significantly increased follicle development and E2 levels. | Lerer-Serfaty et al. (2013) | |
| Alginate versus FA versus HA | FA increased survival, follicle size, antral development, oocyte maturation and embryo cleavage after fertilization but did not affect E2/P4 production. | Jin et al. (2010) | |||
| Grouping five caprine multilayered secondary follicles per bead improved antral development and oocyte maturation. Alginate was better than HA and FA. FA produced 8-cell parthenotes. Cultured follicles had similar Cx43, Cx37 and 3βHSD but higher aromatase gene expression compared to non-cultured. | Brito et al. (2016) | ||||
| Alginate in growth factor-reduced Matrigel and alginate lyase microspheres | In vitro + in vivo (mouse) |

|
After in vitro culture or grafting with murine ovarian cells, beads degraded, lost spherical shape and infiltrating blood capillaries could be observed in the grafted beads. CD34+ and CD45+ cells were found around and inside the matrix. | Vanacker et al. (2012) | |
| Alginate and/or Matrigel | In vitro |

|
Human secondary follicles survived and developed to the antral stage. Hormones produced from individual follicles were undetectable the first week. | Xu et al. (2009b) | |
| Human small pre-antral follicles were well preserved in both groups, but encapsulation before cryopreservation improved survival and follicle size compared to cryopreservation before encapsulation. | Vanacker et al. (2013) | ||||

|
Alginate significantly improved survival (after 1 week) and follicle development in human OT, compared to Matrigel, but did not affect E2 levels. | Kedem et al. (2011) | |||
| Matrigel | In vitro + in vivo (rat) |

|
Implantation of vascularized hydrogel with ovarian spheroids (made of rat GCs and TCs) in ovariectomized rats significantly aids the recovery of endocrine function, leading to full endometrial regeneration. | Yoon et al. (2021) | |
| In vitro + in vivo (mouse) |

|
Matrigel loaded with human UC-MSCs promote GC proliferation and ovarian vascularization in a mouse model of POI. | Zhou et al. (2021) | ||
| Agar versus Matrigel | In vitro | Agar substrate proved to be as suitable as Matrigel on growth and development of cryopreserved-thawed human follicles in OT culture. | Ghezelayagh et al. (2021) | ||
| Alginate versus VitroGel | VitroGel improved pseudoantrum formation, E2 production, COC recovery, oocyte maturation (normal spindle and chromosome alignment and low ROS and mitochondrial membrane potential), from murine pre-antral follicles, compared to alginate. | Kim et al. (2020a) | |||
| FA-IPN | FA-IPN supports murine secondary follicle survival, GC proliferation, antral formation, growth, appropriate E2/P4/Androstenedione production and improves oocyte maturation. | Shikanov et al. (2009) | |||
| Alginate content can be <0.25% with the IPN. Growing murine secondary follicles secrete proteases, which degrade fibrin (to reduce compressive forces), mimicking their naturally dynamic microenvironment in vivo. | Shikanov et al. (2011b) | ||||
| Hydrogels | Sodium alginate bioglass | In vitro + in vivo (mouse) |

|
Encapsulated human amniotic epithelial cells or its conditioned media can protecting GC function and enhance ovarian vascularization in chemotherapy-induced POF model. | Huang et al. (2021) |
| Sodium alginate, fibrin or fibrin-HBP-VEGF | In vivo (mouse) |

|
Murine hemi-ovaries in the fibrin-HBP-VEGF group had more primordial follicles, allowing mice to resume cyclicity earlier and conceived more rapidly. VEGF increased blood vessels at 3 weeks. | Shikanov et al. (2011c) | |
| HA | In vitro | HA increased murine secondary follicle survival and accelerated antral formation. Vitrified-warmed follicles encapsulated in HA had 54% MII compared to 57% in non-embedded follicles. | Desai et al. (2012) | ||
| In vivo (rat) |

|
Rats with HA+VEGF+bFGF-encapsulated ovaries maintained primordial follicles, but had shorter first estrous cycles, lower levels of E2 and c-Myc after autotransplantation. | Tavana et al. (2016b) | ||

|
OT encapsulation with HA can minimize ischemia-induced follicle loss, preserve the follicular pool, promote follicular survival, facilitate angiogenesis and restore hormone levels. | Tavana et al. (2016a) | |||

|
Autotransplanted vitrified OT encapsulated with HA had less intact follicles and lower FSH levels. | Taheri et al. (2016) | |||
| HA gel versus PLGA/MH sponge | In vitro + in vivo (mouse) |

|
Local delivery of human ESC-MPCs increased ovarian reserves, E2 and AMH levels, improving quality of oocytes, embryos and estrous cycle regularity in a POI model. | Shin et al. (2021) | |
| Fibrinogen-thrombin | In vivo (mouse) |

|
Exogenous murine endothelial cells revascularized human OT grafts, increasing their viability and follicle development. Cells engineered to constitutively express AMH preserved primordial follicle reserves. | Man et al. (2017) | |

|
F25/T4 and F12.5/T1 had similar vascular surface, CD45+ cells and supported murine preantral follicle recovery, survival and development. Isolated murine ovarian cells also survived and proliferated after grafting. | Luyckx et al. (2014) | |||
| More murine secondary (than primordial-primary) follicles were proliferating. After 1 week, follicles had higher viability with 5–6% of follicles reaching the next developmental stage. | Chiti et al. (2016) | ||||
| Dense fibrin network encapsulated murine primary follicles, maintained physiological and morphological features, improved blood vessels around secondary follicles, but not theca parameters. | Chiti et al. (2017) | ||||

|
Grafting of 10 or 100 human leukemic cells with ovarian stroma (artificial ovary) was insufficient to cause leukemia after 20 weeks, while grafting with 3 × 10(6) cells produced peritoneal masses at 4 weeks and systemic disease. | Soares et al. (2015) | |||
| Human STEMPRO AD-MSCs increased partial pressure of oxygen, surface area of human CD34+ vessels, follicle survival and decreased apoptosis after xenotransplantation. | Manavella et al. (2018) | ||||
| Human STEMPRO AD-MSCs protected follicle reserves by modulating the PI3K/Akt pathway to maintain quiescence of primordial follicles. | Cacciottola et al. (2021) | ||||

|
The combination of human ovarian graft embedding in fibrin clots and host treatment with simvastatin resulted in improved post-implantation outcomes in a mouse model. | Magen et al. (2020) | |||
| In vitro | F50/T50 best mimics native human OT (based on fiber thickness, porosity and rigidity). | Chiti et al. (2018) | |||
| F100/T4 had highest proliferation rate and least variable apoptosis, but F25/T4 and F12.5/T1 had uniform cell distribution, better homogeneity, human ovarian stromal cell density and reproducible fibrin degradation. | Luyckx et al. (2013) | ||||
| Fibrin | In vivo (mouse) |

|
Initial survival of murine primordial follicles decreased but follicles developed and ovulated. Ovarian function confirmed by reduction in FSH and daily vaginal cytology. | Smith et al. (2014) | |
| >75 μg/ml bFGF improved survival, increased proliferation and protected primordial follicles (but did not affect primary and secondary follicles), in murine hemi-ovaries, with increased revascularization. | Gao et al. (2013) | ||||
| Hydrogels | Cryopreserved human preantral follicles, isolated and encapsulated in fibrin matrices (with or without HA) survive and grow for 7 days after xenografting in mouse. | Paulini et al. (2016) | |||

|
Fibrin-collagen hydrogels with murine MSCs restored cyclicity earlier but delayed follicle development in mouse OT. VP-MSC increased expression of AMH, FSH receptor, GDF9 and VEGF while BM-MSC increased expression of Ptch1. | Mehdinia et al. (2020) | |||
| Laminin versus Matrigel | In vitro | Culturing human OT using laminin components of the native ovarian ECM enhanced follicle survival and proportion of secondary follicles compared to Matrigel. | Hao et al. (2020) | ||
| bFGF sheet | In vivo (mouse) |

|
Transplanting bFGF sheets (which released bFGF) with frozen-thawed human OT increased revascularization and follicle density (primordial and primary), but decreased fibrosis. | Tanaka et al. (2018) | |
| Chitosan-Silk fibroin | In vitro | Development of a novel in vitro model by encapsulating human ovarian stromal cells in chitosan-silk hydrogels. | Jafari et al. (2021) | ||
| PEG-VS | 5% hydrogel supported murine secondary follicle survival and antral development. Follicle morphology quickly diminished and deteriorated in >10% PEG solution. The YKNR plasmin substrate degraded rapidly, but supported antral formation and oocyte maturation. | Shikanov et al. (2011a) | |||
| >10% hydrogel supported murine antral formation, but reduced oocyte maturation (compared to 5–7.5% hydrogel). Parthenotes with highest pronuclear and blastocyst formation in 10% hydrogel. | Ahn et al. (2015) | ||||
| In vivo (mouse) |

|
Antral and mature preovulatory follicles, functional blood vessels and corpora lutea (indicated successful ovulation) after orthotopic transplant of murine primordial and primary follicles. A total of 60% of follicular reserve maintained at day 60. | Kim et al. (2016) | ||
| PEG-VS versus Dual PEG-VS versus TheraCyte | In vivo (mouse) |

|
Although it took twice as long, murine ovaries in Dual PEG capsules produced the greatest number of cycling mice (and functional tissue), in addition to preventing sensitization and lymphocytic infiltration. | Day et al. (2019) | |
| PEG + ECM sequestering peptides | In vitro | Sequestered cell-secreted ECM proteins loaded in PEG hydrogel improved murine early secondary follicle survival, growth and oocyte maturation. | Tomaszewski et al. (2021) | ||
| dECM and polymer scaffolds | DC ovary | Proof of concept | Comparison of three protocols of murine ovarian decellularization by agitation. | Alshaikh et al. (2019) | |
| Proof of concept + in vitro | Comparison of protocols for de- and re-cellularization of murine ovaries proposed sodium deoxycholate as the best detergent for this application. | Alshaikh et al. (2020) | |||
| Rapid cell adhesion and aggregation of homologous fibroblasts, consistent with porcine ovarian scaffold’s ability to sustain cell adherence, proliferation and differentiation. | Pennarossa et al. (2020) | ||||

|
SDS-T-A DC scaffolds had intact ECM components/microstructure, reduced residual DNA and supported fibroblast viability and recovery of murine preantral follicles. DC human cortex had smaller pores and denser collagen fibers compared to the bovine ovary. | Nikniaz et al. (2021) | |||
| In vitro + ex vivo + In vivo (rat) | DC porcine ovary caused minimal immunogenic response after SC xenotransplantation in rats and showed an improvement in E2 secretion ex vivo. | Liu et al. (2017) | |||
| In vitro | Comparison of protocols for decellularization of human ovary and successful recellularization with human endometrial mesenchymal cells. | Sistani et al. (2021) | |||
| Germline stem cells (isolated through magnetic activated cell sorting) can repopulate DC porcine ovarian scaffolds and differentiate into adult mature ovarian cells when stimulated. | Pennarossa et al. (2021b) | ||||
| Porcine ovarian ECM sustained in vitro cell survival and drove epigenetically-erased cell differentiation, fate and viability. | Pennarossa et al. (2021a) | ||||
| In vitro + in vivo (mouse) |

|
DC bovine/human OT scaffold recellularized with murine primary ovarian cells and transplanted to initiate puberty in mice that had been ovariectomized. | Laronda et al. (2015) | ||

|
Peritoneum-derived MSCs in human OT scaffolds can produce germ cell markers (DAZL) after 1 week in vitro, and GDF9+ follicle-like structures 1 month after transplantation. | Eivazkhani et al. (2019) | |||
| dECM and polymer scaffolds | In vivo (mouse) |

|
DC human OT recellularized with human ovarian stroma cells supported survival of human follicles and antral formation of murine follicles after transplantation. A total of 21–25% follicles recovered at 3 weeks. | Pors et al. (2019) | |
| In vivo (rat) |

|
Primary rat ovarian cells maintained viability and bioactivity as well as reconstructed follicle-like structures within DC human ovaries. Cells expressed steroid hormone receptors and GC markers and significantly increased E2/P4 in ovariectomized rats. | Hassanpour et al. (2018) | ||
| DC amniotic membrane | In vitro | Intact human amniotic membrane increased murine primary–secondary follicle survival, size, E2 production, survival index and expression of Cx37, GDF9 and BMP15. | Motamed et al. (2017) | ||
| Bovine ovary and uterus ‘tissue papers’ | Proof of concept + in vitro + ex vivo | DC ovarian ‘tissue paper’ supports murine follicle adhesion, viability and health in vitro, as well as maintains viability and hormonal function of primate and human OT ex vivo for 8 weeks postmortem. | Jakus et al. (2017) | ||
| AlloDerm | In vivo (mouse) + clinical |

|
Follicle development detected in human OT after 8–10 weeks and 6–8 antral follicle count achieved by 11–14 months. FSH normalized by 7 months. Embryos cryopreserved after 7–8 IVF cycles. Both patients achieved CPs after ET and one had LB. | Oktay et al. (2016) | |
| DC SIS | In vivo (rabbit) |

|
Using porcine SIS to reconstruct ovarian resection reduced adhesion score and improved ovarian volume and epithelization in rabbit. | Celik et al. (2009) | |
| Collagen versus SIS | In vivo (mouse) |

|
Human OT wrapped in human recombinant collagen improved grafting in mice, compared with porcine SIS. | Abir et al. (2020) | |
| hrVit versus SIS versus alginate scaffolds versus CollPlant | In vitro |

|
Primordial follicle growth in human OT not enhanced by LIF. Despite some significant differences among the four matrices, none appeared to have a clear advantage. | Younis et al. (2017) | |
| FA versus fibrin-collagen | In vivo (mouse) |

|
Fibrin encapsulation enhanced murine primordial-primary follicle survival, integration with the host tissue and resumption of estrous cycling. LBs achieved with follicles in VEGF-loaded fibrin beads. | Kniazeva et al. (2015) | |
| Bioprinting | ORMOCER versus SU8 | In vitro | ORMOCER did not improve doubling times or damage DNA, but forms gap junctions. Applying a two-photon polymerization to Ormocomp allows adherence to vertical/steep surfaces and layer formation after 3–4 days. | Ovsianikov et al. (2007) | |
| Porcine gelatin ‘ink’ | In vivo (mouse) |

|
30° and 60° scaffolds provide corners that surround murine multilayered secondary follicles on multiple sides while 90° scaffolds have an open porosity that limits follicle-scaffold interaction. Transplant restored ovarian function and LB achieved. | Laronda et al. (2017) | |
| GelMA | In vitro | Cell-laden 3D printing of artificial ovaries supported follicle development and produced MII oocytes after IVM. | Wu et al. (2022) | ||
| Organoid | Matrigel | Prolonged treatment of tumor necrosis factor alpha induced phenotypic changes of human OSE spheroids. | Kwong et al. (2009) | ||
| Generation of organoids from dissociated human female gonad cell suspensions in a three-layered Matrigel-based system. | Oliver et al. (2021) | ||||
| Microfluidic | Alginate | Static conditions produced larger primordials and supported primordial-primary dog follicle transition but decreased RNA and GDF9 in (abnormal) cat follicles. 10 µl/min flow systems supported primordial-primary cat follicle transition and initial growth (D0-3), and dog follicle growth (but not normality). Preantral dog follicles had the highest growth rate in normal alginate beads (antral follicles grew the least). | Nagashima et al. (2018) | ||
| Alginate versus collagen | Oxidized alginate does not support murine early secondary follicle survival. More antral follicles developed in collagen (versus alginate) core. | Choi et al. (2014) | |||
| PDMS |

|
Tp53R273H-mutated murine FTE (but not OSE) cells radially migrated out of cortical inclusion cysts. Number of invading cells and invasion distance enhanced by follicular fluid but worsened by collagen I. | Fleszar et al. (2018) | ||
| Germinal vesicle-stage murine COCs denuded by passing through a microchannel (without hyaluronidase). Dynamic conditions improve oocyte maturation and glutathione, developmental competence and blastocyst formation. | Sadeghzadeh Oskouei et al. (2016) | ||||
| Proof of concept | Murine COCs pass through a series of constriction–expansion units. No significant difference in fertilization or blastocyst rates after ICSI and IVF for oocytes denuded on chip versus denuded manually. | Weng et al. (2018) | |||
| FALLOPIAN TUBE | |||||
| Hydrogels | DC oviduct | In vitro | Rabbit embryos cultured on oviductal ECM hydrogel-coated wells presented a ‘quieter’ metabolism compared to embryos cultured under standard conditions. | Francés-Herrero et al. (2021a) | |
| Alginate | Co-culture of human FTE and murine secondary follicles revealed crosstalk in the reproductive cycle. | Zhu et al. (2016) | |||
| Ex vivo | 3D human fimbriae cultures retained tissue architecture and epithelial subtypes, responding to H2O2 and insulin exposure. | Eddie et al. (2015) | |||
| Organoid | Matrigel-based 3D culture platform | In vitro | Establishment of long-term organoid cultures from mouse FTE cells. | Xie et al. (2018) | |
| Establishment of long-term, stable 3D organoid cultures from human FTE that respond to E2 and P4 treatment in a physiological manner. | Kessler et al. (2015) | ||||
| Distal regions of human FTE showed increased organoid forming capacity, Wnt/inflammatory signaling and high-grade serous carcinoma signatures compared to proximal regions. | Rose et al. (2020) | ||||
| Co-culture of human FT-MSCs, HUVECs and FTE cells formed organoids that could be blocked by Wnt inhibitor DKK1. | Chang et al. (2020) | ||||
| Use of human iPSCs to establish a novel in vitro 3D human FTE organoid model. | Yucer et al. (2017) | ||||
| Mebiol | Murine FTE stem cells formed organoid colonies in a PEG-based 3D culture system, with some cells differentiating into secretory or ciliated cells. | Lin et al. (2021b) | |||
| Micro fluidic | PDMS + Nuclepore chip | Bovine oviduct-on-a-chip supported more physiological (in vivo-like) zygote genetic reprogramming than conventional IVF. | Ferraz et al. (2018) | ||
| CERVIX-VAGINA | |||||
| Scaffold free | Cell constructs | In vitro + in vivo (mouse) | Construction of a model of human vaginal mucosa with a capillary-like network that has the potential to become functional in vivo. | Jakubowska et al. (2020) | |
| Self-assembly | Human vaginal tissue was bioengineered using a self-assembly technique, which formed mature vaginal epithelium and matrix after in vivo animal implantation. | Orabi et al. (2017) | |||
| Air–liquid interface | In vitro | Generation of a 3D human cervical model using ectocervical epithelilum built on a cervical stromal equivalent (that resembles native ECM) . | De Gregorio et al. (2017) | ||

|
Generation of a 3D herpes simplex virus-2 infection model using human vaginal epithelial cells that reproduce basal and apical layers and shows pathological effects after virus inoculation. | Zhu et al. (2017) | |||
| Hydrogels | Silk-HA | Ex vivo | Development of an ex vivo pregnant-like tissue model (with human cervical tissue and fibroblasts) to assess silk-based hydrogels-mediated cervical augmentation. | Raia et al. (2020) | |
| Collagen derivative | In vivo (rat) |

|
Collagen derivative T16 hydrogel improved autologous collagen arrangement, cell proliferation and vaginal epithelium thickness in ovariectomy rat model. | You et al. (2020) | |
| Chitosan-thioglycolic acid | In vitro + in vivo (rat) |

|
Genistein-loaded chitosan-thioglycolic acid hydrogel has high mucoadhesive properties and partially recovered the epithelial thickness of atrophic murine vagina. | Yang et al. (2021) | |
| dECM and polymer scaffolds | DC vagina | In vivo (rat) |

|
Porcine acellular vagina matrix promoted tissue-engineered vagina reconstruction in a rat model of partial vaginectomy. | Zhang et al. (2017a) |
| In vitro | Generation of a porcine vaginal ECM scaffold that allows attachment and growth of AD-MSCs and vaginal epithelial cells. | Greco et al. (2018) | |||
| DC ectocervix | Development of three human ectocervical tissue models: (I) de- and recellularized ectocervix; (II) co-culture of ectocervical and ovarian explants; (II) cell-based ectocervix construct. | McKinnon et al. (2020) | |||
| dECM and polymer scaffolds | DC Porcine SIS | In vivo (rhesus monkey) |

|
Porcine SIS scaffold loaded with human UC-MSCs enhanced vaginal repair in ovariectomized rhesus monkey. | Ma et al. (2021) |
| Clinical |

|
Tissue-engineered autologous vaginal organs showed normal structural and functional variables in patients with MRKHS with a follow-up of up to 8 years. | Raya-Rivera et al. (2014) | ||

|
Porcine SIS graft used for successful cervicovaginal reconstruction in a patient with MRKHS. | Zhang et al. (2019b) | |||
| De-epidermized dermis | Proof of concept | Development of 3D human normal and cervical cancer tissue models via re-cellularization of dermal grafts. | Karolina Zuk et al. (2017) | ||
| RENOV | Clinical |

|
Vaginal reconstruction with acellular human cadaver dermal matrix proved to be a safe, effective and minimally invasive procedure that provided near-normal sexual function for patients with MRKH. | Zhang et al. (2017c) | |
| Collagen | In vitro | In vitro human vaginal epithelial cell model based on collagen-coated beads recapitulated in vivo structural and functional properties. | Hjelm et al. (2010) | ||
| In vivo (rat) |

|
Rat AD-MSCs-laden collagen scaffold promoted vaginal epithelial cell regeneration, vaginal tissue repair and improved vaginal stenosis and contracture on radiation-induced injury. | Ye et al. (2020) | ||
| Clinical |

|
Anterior colporrhaphy with bovine pericardium reinforcement slightly improved success rate over colporrhaphy alone. | Guerette et al. (2009) | ||

|
Successful creation of a neovagina in a patient with MRKHS using a bovine-derived dermis scaffold. | Noguchi et al. (2004) | |||
| PEG | Ex vivo + in vivo (mouse) | PEG-coated nanoparticles diffused through human cervicovaginal mucus ex vivo, and uniformly lined the mouse colorectal and vaginal epithelium in vivo. | Maisel et al. (2016) | ||
| Alginate + chitosan | In vitro | Development of an alginate/chitosan membrane that is stable in a simulated human vaginal environment and with the ability of releasing metronidazole over time. | Tentor et al. (2020) | ||
| Alvetex | Generation of 3D human endocervical model that responds to E2 and P4 during a 28-day culture. Treatment with mifepristone attenuated the inhibition of IL-1β and LIF secretion. | Arslan et al. (2015) | |||
| Silk | Treating human cervical-like constructs with P4 decreased collagen and increased the softness of the ECM over 28 days. | House et al. (2018) | |||
| PLA and compact polyurethane membrane | Proof of concept | Fibrin glue could successfully adhere a PLA and polyuretane bilayer membrane to human cervical tissues. The membrane provides a fluid barrier and can be inserted through the cervix. | Roman et al. (2018) | ||
| Oxidized cellulose | Clinical |

|
Vaginal reconstruction using oxidized cellulose proved to be a safe and effective procedure, with minimum complications and good success rates. | Dadhwal et al. (2010) | |
| Bioprinting | PACIENA prosthesis + Interceed |

|
Good anatomical and functional results were achieved using 3D printed PACIENA prosthesis for vaginoplasties without skin grafts. | Acién et al. (2019) | |
| DC vagina bioink | In vitro + in vivo (rat) |

|
Human BM-MSCs could differentiate in 3D vagina tissue printed with ECM bioink of DC porcine vagina, inducing vascularization and epithelization in vivo. | Hou et al. (2021) | |
| Polyetherurethane | In vitro | 3D-printed cervical implants supported HUVECs adhesion and growth, allowing for controlled loading and release of anti-human papillomavirus protein. | Zhao et al. (2020) | ||
| Polyurethane + clotrimazole | 3D-printed clotrimazole-loaded vaginal ring sustained drug release over 7 days and displayed a complete C. albicans growth inhibition after 5 days. | Tiboni et al. (2021) | |||
| Organoid | Matrigel | Derivation of human organoids from the squamocolumnar junction region of the uterine cervix, along with metaplastic squamous cells in the transformation zone. | Maru et al. (2020) | ||
| Generation of human and murine ecto- and endocervical organoids, which were used in the study of the mechanisms that maintain cervical epithelial junctions and the emergence of metaplasia. | Chumduri et al. (2021) | ||||
| Cultrex RGF-BME type 2 | In vitro + in vivo (mouse) |

|
Establishment of human ecto- and endocervical 3D organoids that stably recapitulate physiological and carcinogenic traits, growing as xenografts in mice. | Lõhmussaar et al. (2021) | |
| Micro fluidic | PDMS | In vitro | Development of an organ-on-chip of the cervical epithelial layer that can recapitulate the human ecto- and the endocervical epithelial regions. | Tantengco et al. (2021) | |
| FULL TRACT | |||||
| Microfluidic | Acrylic | Ex vivo | Development of a microfluidic system (with human FTE, endometrial cells, ectocervix explant and liver microtissues) that supports murine ovarian follicles and reproduces the human 28-day menstrual cycle hormone profile. | Xiao et al. (2017) | |
| PDMS | In vitro | Fabrication of a microwell-structured microfluidic device that allows single mouse oocyte trapping, IVF and embryo culture. | Han et al. (2010) | ||
| Human endometrial (EnSCs, HUVECs and stromal cells) and ovarian (GCs and TCs) components modeled the bidirectional crosstalk between the uterus and the ovary in a ‘dual reproductive organ-on-a-chip’ model, which could be used for testing reproductive toxicants. | Park et al. (2020) | ||||
Description of the main bioengineering findings in the female reproductive system in the last 21 years based on different strategies, platforms/biomaterials, type of study (including in vivo models) and gynaecological related-diseases.
To note: model in type of study column only refers to in vivo approach; studies with human cells/tissues are marked in bold, while clinical studies are highlighted in yellow; and pill icons indicate studies carried out with patients, animal models and biological samples with female reproduction-related diseases (established cell lines have not been taken into account).
3βHSD, 3β-Hydroxysteroid dehydrogenase; AD-MSC, adipose-derived mesenchymal stem cell; AMH, anti-Müllerian hormone; AS, Asherman’s syndrome; bFGF, basic fibroblast growth factor; BM-MSC; bone marrow-derived mesenchymal stem cell; BMP, bone morphogenic protein; C. albicans: Candida albicans; C. trachomatis: Chlamydia trachomatis; CD, cluster of differentiation; COC, cumulus-oocyte complex; Collplant, human recombinant virgin collagen bioengineered in tobacco plant lines; CP, clinical pregnancy; Cx37/43, connexin 37/43; CYR61: cysteine-rich angiogenic inducer 61; DAZL, deleted in azoospermia like; DC, decellularized; dECM, decellularized extracellular matrix; dEMSC, decidualized endometrial stromal cells; DKK, Dickkopf WNT Signaling Pathway Inhibitor 1; E2, estradiol; ECM, extracellular matrix; EEPC, endometrial epithelial progenitor cell; eMSC, endometrial mesenchymal stem cell; EnSC, endometrial stem cell; ESC, embryonic stem cell; ESC-MPC, embryonic stem cell-derived mesenchymal progenitor cell; ESF, endometrial stromal fibroblast; ET, embryo transfer; EVT, extravillous trophoblast; F_/T_, fibrinogen (mg/ml)/thrombin (IU/ml) concentration ratio; FA, fibrin-alginate; FA-IPN, fibrin-alginate interpenetrating network; FOXO1/3a, forkhead box protein O1/3a; FT-MSC, Fallopian tube mesenchymal stem cell; FTE, Fallopian tube epithelium; GC, granulosa cell; GDF9, growth differentiation factor 9; GelMA, gelatin-methacryloyl; GVBD, germinal vesicle breakdown; H9-ESC: human embryonic stem cell-9 line; HA, hyaluronic acid; HBP, heparin binding protein; HP, heparin poloxamer; hrVit, human recombinant vitronectin; HUVEC, human umbilical vein endothelial cell; IGF, insulin-like growth factor; iPSC, induced pluripotent stem cell; IUA, intrauterine adhesion; Jag1, jagged canonical Notch ligand 1; JAR spheroids: human choriocarcinoma (JAR) cells grown as multicellular spheroids; KHDC3, KH domain containing 3 like; LB, live birth; Lhx8, LIM homeobox 8; LIF, leukemia inhibitory factor; MEF, mouse embryonic fibroblast; MenMSC, menstrual blood mesenchymal stem cell; MH, magnesium hydroxide; MII, metaphase II; MRKH, Mayer-Rokitansky-Küster-Hauser; MSC, mesenchymal stem cell; Nanos3, Nanos C2HC-Type Zinc Finger 3; NLRP5, NLR Family pyrin domain containing 5; ORMOCER, Photosensitive organic-inorganic hybrid polymer (ORganically MOdified CERamics); OSE, ovarian surface epithelium; OT, ovarian tissue; P4, progesterone; PD-MSC, placenta-derived mesenchymal stem cell; PDMS, polydimethylsiloxane; PEBP, poly(ethylene glycol)-b-poly(l-phenylalanine); PEG, 4-polymeric poly-(ethylene glycol); PEG-PLA, polymeric poly(ethylene glycol)-block-polylactide methyl ether; PEG-VS, 4-arm poly-(ethylene glycol) tetravinyl sulfone; PGC, primordial germ cell; PLA, poly-l-lactic acid; PLGA, poly(d, l-lactide-co-glycolide); PLO, poly-l-ornithine; POF, premature ovarian failure; POI, premature ovarian insufficiency; PSC-ESF, pluripotent stem cells induced into endometrial stromal fibroblast; PTFE, polytetrafluoroethylene fluoropolymers; PVA/CMC, polyvinyl alcohol-carboxymethylcellulose; QD, quantum dots; RGF-BME, reduced growth factor-basement membrane extract; ROS, reactive oxygen species; SC, subcutaneous; SDS-T-A, sodium dodecyl sulfate-Triton-Ammonium; SIS, small intestine submucosa; SU8, negative photo-resistor based on epoxy components; TC, theca cell; TFAM, transcription factor A (mitochondrial); TGFβR2, transforming growth factor beta receptor 2; tHESC, hTERT-immortalized human endometrial stromal cell; TiO2, titanium dioxide; UC-MSC, umbilical cord-derived mesenchymal stem cell; VEGF, vascular endothelial growth factor; VP-MSC, visceral peritoneum mesenchymal stem cell.
Studies related to gynecological pathologies, both included in the initial search terms and different ones addressed by the selected articles (such as endometriosis, uterine fibroids, AS, intrauterine adhesions, polycystic ovary syndrome, primary ovarian insufficiency and Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome) were included in Table I, however owing to the extent of relevant studies applying bioengineering techniques to create novel ovarian, uterine and cervical cancer models (published between April 2014 and September 2021), the latter were grouped separately according to their application and organ in Supplementary Table SIV. Finally, throughout the entire review we classify hydrogels as originally softer/injectable materials regardless of whether they gelify afterwards (e.g. collagen solutions), and scaffolds as their more rigid counterparts (e.g. collagen membranes).
Results
Search results
The search queries yielded 10 390 results (from a total of 18 748 titles identified) after removal of duplicates. Titles and abstracts were screened for eligibility (based on exclusion criteria presented in Fig. 2) and 584 (5.6%) full-text papers were retrieved for detailed assessment. An additional 24 studies were retrieved from manual searching of citations. We classified studies by bioengineering strategy within each organ (Fig. 3), and by their main application(s). Finally, 312 articles were included for systematic review, including 18 (5.8%) studies on scaffold-free approaches, 124 (39.7%) discussing hydrogels, 87 (27.9%) related to dECM and polymer scaffolds, 10 (3.2%) using bioprinting, 45 (14.4%) implementing organoid cultures and 28 (8.9%) exploring microfluidic techniques (Table I and Supplementary Table SIV). The majority of the bioengineering studies included in vitro/ex vivo or in vivo work using animal models, with only a few studies reaching clinical stages (seven uterine, two ovarian and seven cervical).
Figure 3.
Organ-level overview of the bioengineering studies carried out between January 2000 and September 2021 and included in this systematic review. The studies involved the uterus, ovaries, fallopian tubes and cervix/vagina. The numbers reflect the number of studies included in Table I and Supplementary Table SIV. Created with BioRender.com.
Bioengineering tools in female reproductive medicine: systematic summary of the evidence
Below we summarize studies using the six bioengineering techniques in work related to female reproductive organs.
Scaffold-free approaches
This section mainly contemplates studies based on the non-matrix-assisted self-organizing capacity of cells to generate multicellular entities. Six studies presented scaffold-free approaches applied to bioengineering of the uterus and its tissues, including four in vivo studies based on cell sheets (Kuramoto et al., 2015, 2018, 2020; Sun et al., 2018) and two in vitro investigations based on the generation and study of epithelial and stromal organoids (Murphy et al., 2019; Wiwatpanit et al., 2020). Of the eight studies involving scaffold-free approaches in ovary, one used a cell-based method involving primordial germ cells to generate ovarian tissue (OT) in vivo (Hayama et al., 2014), four developed self-assembled spheroids (Krotz et al., 2010; Chowanadisai et al., 2016; Kim et al., 2018; Ward Rashidi et al., 2019), while toxicity of Qdot 655 ITK carboxyl quantum dots (Xu et al., 2016) and nanoparticles made with chitosan (Raja et al., 2021), polyethylene glycol (PEG) and polylactic acid or titanium dioxide (Scsukova et al., 2020), were tested in preclinical and ex vivo models, respectively. Scaffold-free approaches to develop bioengineered vaginal and cervical tissues were included in four studies, which applied self-assembled vaginal constructs in vivo (Orabi et al., 2017; Jakubowska et al., 2020) or air–liquid interface techniques to generate vaginal and cervical in vitro models (De Gregorio et al., 2017; Zhu et al., 2017).
Hydrogels
This review unveiled a plethora of different hydrogel-based research involving uterine cells or tissues. Of the 40 studies compiled, 65%, 30% and 5% used natural, synthetic or hybrid hydrogels, respectively. The most commonly used natural hydrogels were based on collagen (46.2%), Matrigel (24.4%) and hyaluronic acid (HA; 15.4%). Synthetic hydrogels were based predominantly on PEG (38.5%) and poloxamer (30.7%). Studies applied these approaches for in vitro modeling of disease, tissue cross-talk and differentiation (Schutte et al., 2015; Cook et al., 2017; Pence et al., 2017; Stejskalová et al., 2021), in vivo regeneration of the endometrium and myometrium (Lin et al., 2012; Yang et al., 2017; Li et al., 2019; Yoon et al., 2021; Lin et al., 2021a) and the treatment of intrauterine adhesions (Müller et al., 2011; Liu et al., 2020).
Encapsulation of follicles and tissue fragments is the most exploited hydrogel-based application in ovarian bioengineering. Alginate (alone or in combination with other materials) is the most commonly implemented hydrogel, with use in 47.14% of studies evaluating hydrogels for in vitro cultures of murine (Xu et al., 2006, 2009a; West et al., 2007; West-Farrell et al., 2009; Jackson et al., 2009; Jin et al., 2010; Parrish et al., 2011; Tagler et al., 2013, 2014; Skory et al., 2015; Zhang et al., 2019c), canine (Songsasen et al., 2011), caprine (Brito et al., 2014), ovine (Mastrorocco et al., 2020), primate (Xu et al., 2009c) and human (Amorim et al., 2009; Camboni et al., 2013; Laronda et al., 2014; Skory et al., 2015; Xiao et al., 2015) ovarian follicles and tissue. Natural and synthetic hydrogels such as laminin (Hao et al., 2020), collagen (Joo et al., 2016), fibrin (Smith et al., 2014; Paulini et al., 2016), Matrigel (Xu et al., 2009b; Kedem et al., 2011; Ghezelayagh et al., 2021), VitroGel (Kim et al., 2020a), HA (Desai et al., 2012) or PEG (Shikanov et al., 2011a; Lerer-Serfaty et al., 2013; Tomaszewski et al., 2021) are less frequently reported.
Twenty-four studies used hydrogels in preclinical models of IVF (Xu et al., 2006), allo-/xenotransplantation of ovarian cells, follicles and fragments (Vanacker et al., 2012) or ovarian function restoration (Su et al., 2016; Ding et al., 2018; Yang et al., 2019; Yoon et al., 2021) (Table I); seven applied hydrogels in oncological modeling and drug testing (Supplementary Table SIV).
Tissue-specific hydrogels based on rabbit oviductal dECM and alginate resulted, respectively, in in vitro models of embryo culture (Francés-Herrero et al., 2021a) and crosstalk between human epithelium and murine follicles (Zhu et al., 2016), or ex vivo models of the human fallopian tube fimbriae (Eddie et al., 2015). Hydrogels based on silk-HA, collagen derivatives and chitosan could recreate an ex vivo pregnant-like human cervical model (Raia et al., 2020), or treat vaginal atrophy in vivo (You et al., 2020; Yang et al., 2021).
Decellularized extracellular matrix and polymer scaffolds
Forty-five uterine-related studies evaluated dECM and polymer scaffolds in cytocompatibility and in vitro modeling experiments, as well as in vivo regeneration and anti-adhesions tests (Table I). Those based on decellularized (DC) matrices accounted for 46.6% of reports, while 33.3% and 20% used scaffolds based on purified natural or artificial polymers, respectively. Various studies isolated and evaluated tissue-specific ECM from whole uteri of rats (Hellström et al., 2014, 2016; Miyazaki and Maruyama, 2014; Santoso et al., 2014; Miki et al., 2019; Padma et al., 2021a,b), mice (Hiraoka et al., 2016), pigs (Campo et al., 2017), rabbits (Yao et al., 2020b) and sheep (Daryabari et al., 2019; Tiemann et al., 2020; Padma et al., 2021c); from rabbit (Campo et al., 2019), pig (López-Martínez et al., 2021a,b) and human endometrium (Olalekan et al., 2017; Sargazi et al., 2021); and from rat and human myometrium (Young and Goloman, 2013). Natural polymer scaffolds included alginate (Li et al., 2011b; Stern-Tal et al., 2020), HA (Demirbag et al., 2005; Liu et al., 2005) and gelatin-coated (Su et al., 2014; Edwards et al., 2015; Cai et al., 2019) materials, while artificial polymer scaffolds were created with polyglactin (Young et al., 2003), polylactide (Pensabene et al., 2015; Mukherjee et al., 2019), polyglycolic acid (Magalhaes et al., 2020), emulsion-templated highly porous materials (Eissa et al., 2018; Richardson et al., 2019) and polytetrafluoroethylene fluoropolymers (Kuperman et al., 2020).
In contrast, only dECM or natural polymers were used for 24 studies involving ovarian research. Several reports described the generation and in vitro/in vivo biocompatibility of DC murine (Alshaikh et al., 2019, 2020), porcine (Liu et al., 2017; Pennarossa et al., 2020, 2021a,b), ovine (Eivazkhani et al., 2019), bovine (Laronda et al., 2015; Nikniaz et al., 2021) and human (Hassanpour et al., 2018; Pors et al., 2019; Sistani et al., 2021) ovaries, as well as porcine small intestine submucosa (Celik et al., 2009; Abir et al., 2020) or human amniotic membrane (Motamed et al., 2017).
Of the 18 studies that assessed scaffolds for cervicovaginal applications, 33.3% were clinical studies of vaginal reconstruction using oxidized cellulose (Dadhwal et al., 2010), bovine collagen matrices (Noguchi et al., 2004; Guerette et al., 2009), human acellular dermis (Zhang et al., 2017c) or porcine small intestine submucosa (Raya-Rivera et al., 2014; Zhang et al., 2019b). The remaining studies consisted of either in vitro cervico-vagina models based on DC porcine vaginas (Greco et al., 2018), human cervical dECM (McKinnon et al., 2020) and scaffolds of collagen (Hjelm et al., 2010), alginate/chitosan (Tentor et al., 2020), silk (House et al., 2018), Alvetex (Arslan et al., 2015) and polylactide (Roman et al., 2018) or preclinical models of vaginal repair (Maisel et al., 2016; Zhang et al., 2017a; Ye et al., 2020; Ma et al., 2021).
Bioprinting
The scarcity of bioprinting implementation in reproductive studies reflects the novelty of this technique. Two reports applied 3D bioprinting technology to in vitro studies of uterine contractility (Souza et al., 2017) and in vivo endometrial regeneration using a gelatin-alginate hydrogel loaded with human induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (MSCs) (Ji et al., 2020). Similarly, three studies applied bioprinting with natural polymers for in vitro ovarian modeling (Ovsianikov et al., 2007), follicle culture (Wu et al., 2022) or fertility restoration in a preclinical model (Laronda et al., 2017). Finally, five groups bioprinted bactericidal vaginal rings (Tiboni et al., 2021), vaginal tissue with acellular bioink (Hou et al., 2021), PACIENA prosthesis for vaginoplasties (Acién et al., 2019), cervical implants (Zhao et al., 2020) or 3D models of cervical cancer (Gospodinova et al., 2021).
Organoids
We identified 15 studies generating endometrial organoids. Matrigel was primarily used as a supportive matrix, with (Francés-Herrero et al., 2021b) or without (78.5% of studies) endometrial dECM supplementation. Other studies used collagen (Abbas et al., 2020), or functionalized PEG matrices (Hernandez-Gordillo et al., 2020). Remarkably, in six studies, epithelial and stromal components were combined to create organoids (Murphy et al., 2019; Wiwatpanit et al., 2020; Jiang et al., 2021) or assembloids (Bläuer et al., 2005; Abbas et al., 2020; Rawlings et al., 2021), which simultaneously represent both endometrial populations. Ovarian spheroids or organoids were derived in 18 studies using Matrigel, Cultrex or agarose, together with diverse cell types, including ovarian epithelial cells (Kwong et al., 2009; Kopper et al., 2019), granulosa and theca cells (Yoon et al., 2021) or embryonic gonads (Oliver et al., 2021). Remarkably, 89% of the studies generated ovarian organoids, derived from patients with cancer, that reliably mimicked the original pathology (Maru et al., 2019). Similarly, in five studies, human organoids were derived from the squamocolumnar junction region of the uterine cervix (Maru et al., 2020) or ecto- and endocervical tissue using Matrigel or Cultrex (Lechanteur et al., 2017; Chumduri et al., 2021; Lõhmussaar et al., 2021; Tanaka et al., 2021). Fallopian tube organoids were generated from murine (Xie et al., 2018) and human (Kessler et al., 2015; Rose et al., 2020; Zhang et al., 2021a) fallopian tube epithelial cells used alone or co-cultured with umbilical vein endothelial cells and fallopian tube-derived MSCs (Chang et al., 2020), and from human iPSC (Yucer et al., 2017) or murine fallopian tube epithelial stem cells (Lin et al., 2021b). Culture was supported by Matrigel (in five of these six studies) or Mebiol (Lin et al., 2021b). Organoid studies accounted for 58%, 13% and 13% of bioengineering platforms reported in the fallopian tubes, ovary and uterus, respectively.
Microfluidic systems
Microfluidic platforms can be used to model static, passive (created by gravity) or active dynamic (with a determined flow rate) conditions. Six studies applied microfluidics to create advanced in vitro models of the human endometrium (Astolfi et al., 2016; Gnecco et al., 2017, 2019; Ahn et al., 2021; De Bem et al., 2021), or co-culture embryos with endometrial cells (Kimura et al., 2009). Of the 13 microfluidic-based studies in the ovarian field, two applied dynamic follicle culture in alginate and/or collagen matrices (Choi et al., 2014; Nagashima et al., 2018), while two studies evaluated the mechanical effect of flow on oocyte denudation and maturation (Sadeghzadeh Oskouei et al., 2016; Weng et al., 2018). The rest of the studies sought to recreate oncological models, evaluate drug effectiveness or elucidate therapeutic targets (as we discuss in detail in the applications sections). Finally, four studies applied microfluidics to model the human cervical epithelial layer (Lin et al., 2017; Aziz et al., 2020; Yang et al., 2020; Tantengco et al., 2021) and bovine (Ferraz et al., 2018) and canine (Ferraz et al., 2020) oviducts, while one study modeled human uterine and ovarian endocrine crosstalk (Park et al., 2020), and two studies recreated a complete female tract in microfluidic systems, combining endometrial, ovarian, oviductal, cervix and liver tissue to model the hormonal profile of a menstrual cycle (Xiao et al., 2017) or to manipulate, fertilize and culture embryos in a single device (Han et al., 2010).
Preclinical models and clinical applications: an update
Bioengineering approaches can elucidate the normo- and patho-physiology of female reproductive organs by developing next-generation in vitro/ex vivo platforms, creating representative models for toxicology/drug screening, developing alternative therapeutic strategies, discovering new biomarkers and improving tissue/organ regeneration and/or transplantation protocols.
Development of next-generation in vitro and ex vivo platforms
The creation of in vitro platforms that faithfully reproduce the physiological and pathological states of higher organisms is of paramount importance in applied and translational research. Bioengineering platforms have provided novel 3D models of follicle culture (see citations in Hydrogels section), human embryo implantation (Wang et al., 2012, 2013; Buck et al., 2015; Stern-Tal et al., 2020; Rawlings et al., 2021), a three-layered endometrium that remained functional for 28 days (Park et al., 2021), endometrial cancer invasion (Park et al., 2003) and wound healing (Stavreus-Evers et al., 2003), as well as bidirectional crosstalk between the uterus and the ovaries (Park et al., 2020). Moreover, collagen scaffolds loaded with human epithelial and endothelial cells (Pence et al., 2015) or tissue slices (Muruganandan et al., 2020) respond to ovarian hormones, while collagen-embedded human stromal cells demonstrate decidualization changes (Schutte and Taylor, 2012) and contractile ability (Kim et al., 2020b). Notably, endometrial cells encapsulated in a PEG hydrogel with ECM-binding peptides remodeled the synthetic matrix and displayed hormone-mediated differentiation (Cook et al., 2017).
In vitro follicle growth produced developmentally competent murine oocytes (Xu et al., 2006; Ahn et al., 2015) that led to live births (LBs) after embryo transfer (Xu et al., 2006). Most studies (94%) implemented individual follicle culture, while others successfully demonstrated that culturing multiple follicles together substantially improves follicle survival (>80% versus <29% with individual culture) and oocyte maturation (Hornick et al., 2013; Brito et al., 2016). Interestingly, IVFG benefits from the addition of ECM sequestering peptides (Tomaszewski et al., 2021), ascorbic acid [which increases expression of ECM and cell adhesion molecules (Tagler et al., 2014)], bone morphogenetic protein 4 (Felder et al., 2019), mouse embryonic fibroblasts (Tagler et al., 2012), ovarian cells (Jamalzaei et al., 2020) and human menstrual blood MSCs (Rajabi et al., 2018), but not denuded oocytes, oocyte-secreted factors, granulosa cells (Hornick et al., 2013) or leukaemia inhibitory factor (Younis et al., 2017). VitroGel, a novel animal-origin free hydrogel, also improves IVFG parameters, outperforming alginate and producing competent oocytes in a recent study by Kim et al. (2020a). Furthermore, when used exclusively, alginate concentrations ranged between 0.25% and 3% (Supplementary Table SIII); however, as a fibrin-alginate interpenetrating network, the concentration of alginate can be reduced below 0.25%, providing a more realistic environment for follicle growth and improving oocyte maturation (Shikanov et al., 2009, 2011b). Notably, combinations of 25 mg/ml fibrinogen and 4 IU/ml thrombin or 12.5 mg/ml fibrinogen and 1 IU/ml thrombin, are suggested as the best scaffolds for human ovarian stromal cells in vitro (Luyckx et al., 2013) and for murine follicle development in vivo (Luyckx et al., 2014), while 50 mg/ml fibrinogen and 50 IU/ml thrombin best mimics the rigidity of the native human ovarian cortex (Chiti et al., 2018).
DC models also offer important advances by maintaining unique tissue-specific ECM milieus, not only providing the most realistic scaffold for each organ’s endogenous cell types, but also remarkably acting as a biocompatible framework for cells from other tissues/species. For example, DC mouse uterine tissue is an adequate natural niche for human menstrual blood MSC differentiation toward uterus-specific cell lineages (Arezoo et al., 2021), DC sheep uterus stimulates rat fetal dorsal root ganglion regeneration and angiogenesis during chicken embryo development (Padma et al., 2021c) and solubilized porcine endometrial dECM enhances proliferation rates of human endometrial organoids (Francés-Herrero et al., 2021b).
The generation and increasing use of organoids are revolutionizing the field of reproductive medicine. Among other limitations, the primarily epithelial nature of these structures is noteworthy. To date, several investigations have already provided models of multicompartment tissues [i.e. endometrial and stromal compartments (Murphy et al., 2019; Rawlings et al., 2021)], ecto- and endo-cervical epithelial regions (Maru et al., 2020; Chumduri et al., 2021; Lõhmussaar et al., 2021) and heterogeneous tumors (see next paragraph and/or Supplementary Table SIV), in addition to research models for chlamydia (Bishop et al., 2020) and herpes (Zhu et al., 2017) infections. Remarkably, organoids are able to reproduce specific uterine (Boretto et al., 2019; Bishop et al., 2020; Hernandez-Gordillo et al., 2020; Luddi et al., 2020; Marinić et al., 2020), ovarian (described in detail below) and cervical (Karolina Zuk et al., 2017; Maru et al., 2020; Lõhmussaar et al., 2021) tissue phenotypes, as well as respond to hormones (Bläuer et al., 2005; Boretto et al., 2017; Turco et al., 2017; Wiwatpanit et al., 2020; Cheung et al., 2021). These models can be established from patient biopsies (Maru et al., 2019; Lõhmussaar et al., 2021), biological fluids (Cindrova-Davies et al., 2021) or cell lines (e.g. SKOV3, H08910, OVCAR3/4/8 used in oncological studies listed in Supplementary Table SIV). Further transplantation of spheroids or organoids may restore ovarian function (Kim et al., 2018) or promote endometrial regeneration (Jiang et al., 2021).
Next-generation platforms for oncological studies include the development of 3D ovarian cancer models using scaffolds of bacterial cellulose with chitosan (Ul-Islam et al., 2019), collagen (Zheng et al., 2015), poly-dl-lactide-coglycolide-PEG (Zhou et al., 2018) or RADA16-I peptide hydrogel (Song et al., 2020). Similarly, a novel 3D cervical cancer model was created with 3D-printing, using bioinks mixed with sodium alginate (Gospodinova et al., 2021). Dynamics of cancer progression can be modeled ex vivo in 3D (Ajeti et al., 2017; Fleszar et al., 2018; Loessner et al., 2019; Flont et al., 2020; Fan et al., 2021), utilizing multilayered microfluidic systems (Lin et al., 2017; Flont et al., 2020) and ovarian spheroids [to study macromolecular crowding (Bascetin et al., 2021)].
Unique ex vivo and in vitro proof of concept applications include, DC bovine ovarian and uterine ‘tissue papers’ (Jakus et al., 2017), an in vitro artificial human ovary (Krotz et al., 2010), a pregnant-like cervix (Raia et al., 2020), an endocervical model that responds to hormones during a 28-day cycle (Arslan et al., 2015), automated and reliable oocyte denudation on a chip (Weng et al., 2018) and the EVATAR platform that models the dynamics of the human menstrual cycle (Xiao et al., 2017).
Realistic in vitro toxicology and drug screening models
Bioengineered in vitro platforms enable evaluation of the biocompatibility of biomaterials (Xu et al., 2016; Scsukova et al., 2020), effects of chemical toxicants [such as doxorubicin (Zhou et al., 2015; Aziz et al., 2020), or dioxin (Park et al., 2020)] or response to cancer therapies (Supplementary Table SIV). For example, 3D tumor models in ring format currently support automated and rapid personalized drug screening (Phan et al., 2019). Other drug screening models include microdissected tumor tissues in microfluidic culture (Astolfi et al., 2016) or alginate hydrogels (Salas et al., 2020), organoids of small cell neuroendocrine carcinoma of the uterine cervix (Tanaka et al., 2021) and ovarian cancer organoids, which have proven to be excellent models to test chemotherapy drugs (Maru et al., 2019; de Witte et al., 2020; Maenhoudt et al., 2020). In fact, since endometrial and ovarian organoids can be derived from each patient’s biopsies (Kopper et al., 2019; Nanki et al., 2020; Bi et al., 2021; Chen et al., 2021; Espedal et al., 2021), they reflect specific tumor heterogeneity and are ideal for drug pre-screening and the development of personalized treatment regimens. Notably, ovarian cancer spheroids exhibited increased tumorigenicity and proportion of cancer stem cells after several passages (Ward Rashidi et al., 2019); chemoresistant cancer stem cells can also be generated with 3D culture of CD44+CD117+ cells (Chen et al., 2014). Fallopian tube organoids are similarly suitable for developing combination therapies for high-grade serous ovarian cancer (Zhang et al., 2021a) while multicellular spheroids derived from these cancer patients’ malignant effusions enable drug screening (Chen et al., 2020).
Further, recent applications of drug-loaded hydrogels (Jamal et al., 2018; Cabral-Romero et al., 2020) and microfluidic conditions (Ran et al., 2019; Saha et al., 2020; Yang et al., 2020) evaluated targeted cytotoxicity. HA-carboxymethyl cellulose scaffolds facilitate the study of ovarian cancer persistence (Picaud et al., 2014), and ovarian constructs enable evaluation of metastatic potential of leukemic cells that could have infiltrated OT (Soares et al., 2015).
New therapeutic biomarkers and clinical strategies
The organs and tissues of the female reproductive system are not only subject to pathologies that affect reproductive capacity, but also to those that can be life threatening, such as cancers. Via recent applications, microfluidic platforms and organoid/spheroid cultures are revealing diagnostic (Wang et al., 2015; Dorayappan et al., 2019; Zhang et al., 2019a; Chung et al., 2021), and prognostic (Chowanadisai et al., 2016; Ward Rashidi et al., 2019; Chung et al., 2021) biomarkers and/or gene signatures, in addition to elucidating drivers of tissue metaplasia (Chumduri et al., 2021). These approaches are helping to establish new and alternative cancer therapies. For example, endometrial organoids allowed the identification of a menin-mixed lineage leukemia inhibitor for endometrial cancer (Chen et al., 2021), while fallopian tube organoids provided a platform to test combination therapies for ovarian cancer (Zhang et al., 2021a). Locally injectable hydrogels, such as those made of PEG and polylactic-co-glycolic acid (Shin and Kwon, 2017), PEG and poly(ε-caprolactone) polymeric micelles (Xu et al., 2018), polypeptide PC10A and silver sulfide quantum dots (Jin et al., 2019) or light-cured glycol chitosan (Hyun et al., 2019), successfully sustained delivery of drugs to ovarian or cervical tumor models, while those made of HA-danazol reduced the size of endometriosis cysts (Nomura et al., 2006). Similarly, 3D cervical models supported testing the efficacy of PEGylated lipoplexes containing silencing RNAs targeting human papillomavirus lesions (Lechanteur et al., 2017), while gold nanorods can facilitate intracellular drug delivery (Yan et al., 2016). Another bioengineering strategy that can improve clinical workflow is the encapsulation of follicles in alginate (with or without Matrigel) before cryopreservation, which not only is more time efficient, but also affords a means of improving follicle survival and development (Camboni et al., 2013; Vanacker et al., 2013).
Tissue and organ regeneration or transplantation
The complete or partial regeneration of damaged tissues and organs is arguably the application for which bioengineering is most recognized. In the reproduction field, many in vivo studies have tested hydrogels and scaffolds for uterine regeneration (Supplementary Table SII). Among them, polylactide nanofilm can seal defects smaller than 3 mm in chorion-amnion and uterine membranes (Pensabene et al., 2015), while degradable polylactic acid-co-poly(ε-caprolactone)-gelatin nanofiber meshes with endometrial MSCs promote tissue integration via an anti-inflammatory response (Mukherjee et al., 2019). Heparin-poloxamer hydrogels (Xu et al., 2017a,b; Zhang et al., 2017b, 2020b), collagen hydrogels or scaffolds loaded with bone marrow MSCs (Ding et al., 2014), basic fibroblast growth factor [(bFGF; (Li et al., 2011a)], embryonic stem cell-derived endometrium-like cells (Song et al., 2015), vascular endothelial growth factor [VEGF (Lin et al., 2012)] or human umbilical cord-derived MSCs [UC-MSCs (Xin et al., 2019; Liu et al., 2020)] and stromal cell-derived factor-1α-loaded chitosan-heparin hydrogel (Wenbo et al., 2020) repaired morphology and restored the function of injured rat uteri. Further, improved uterine regeneration, and some restoration of fertility with successful implantations, pregnancies and LBs is achievable via transplantation of DC human amniotic membrane loaded with adipose stem cells (Han et al., 2020) or oral mucosal epithelial cells (Chen et al., 2019), DC uterine matrix (Santoso et al., 2014; Hellström et al., 2016; Hiraoka et al., 2016; Miki et al., 2019; Li et al., 2021), or DC endometrial ECM hydrogel loaded with growth factors (López-Martínez et al., 2021b) (Supplementary Table SII). Similarly, gelatin methacrylated and sodium-alginate scaffolds with bFGF (Cai et al., 2019), MSC-laden Matrigel microspheres (Xu et al., 2021), hydrogel-encapsulated decidualized endometrial stromal cells (Kim et al., 2019), HA hydrogels (Liu et al., 2019), HA-collagen hydrogels with endometrial stem cells, stromal cells and vessel cells (Park et al., 2021), PEG-based hydrogels (Wang et al., 2021) or poly(glycerol sebacate) scaffolds seeded with bone marrow-MSCs (Xiao et al., 2019) also successfully regenerated a damaged endometrium.
One reproductive disorder prompting a search for an effective tissue regeneration treatment is AS, an acquired iatrogenic disorder characterized by adhesions within the uterine cavity or cervix. To date, patients have received treatments using collagen hydrogels loaded with UC-MSCs (Cao et al., 2018; Zhang et al., 2021b), bFGF (Jiang et al., 2019) or bone marrow mononuclear cells (Zhao et al., 2017) to improve uterine response and function. In vivo studies in rats demonstrated that UC-MSCs facilitate collagen degradation, regenerate uterine wall thickness and restore fertility (Xu et al., 2017c), while organoids derived from human embryonic stem cells regenerate uteri of AS models (Jiang et al., 2021). Furthermore, HA-based hydrogels (Liu et al., 2019) and cell sheets made of rat endometrial cells (Kuramoto et al., 2018), rat oral mucosa epithelial cells (Kuramoto et al., 2015) or UC-MSCs (Kuramoto et al., 2020) also demonstrated utility in preventing and/or repairing uterine adhesions. Other biomaterials, such as AdSpray [based on dextrin (Kai et al., 2018)], Carbylan-SX (Liu et al., 2007), mitomycin C-loaded crosslinked HA films and gels (Liu et al., 2005), urinary bladder ECM (Zhang et al., 2020a), HA/carboxymethylcellulose membranes (Demirbag et al., 2005) and polylactic acid-pluronic copolymer (Yamaoka et al., 2001), also prevent post-operative adhesions, while hydrogels made of PEG with or without poly(l-phenylalanine) (Wang et al., 2021), aloe poloxamer with estradiol encapsulated in nanoparticulate DC uterus (Yao et al., 2020a) and stromal cell-derived factor-1α-loaded chitosan-heparin (Wenbo et al., 2020) prevent/reduce uterine fibrosis in preclinical models. Notably, commercial hydrogel-based adhesion barriers, such as PEG-based SprayGel [used for myomectomy patients (Mettler et al., 2004, 2008)] and Actamax Adhesion Barrier (Trew et al., 2017), have already proceeded to clinical use.
Research over the last two decades also yielded significant strides in reproductive organ transplantation. Although uterine and ovarian transplantation surgeries often are performed without the aid of bioengineering, recent approaches may provide benefit, particularly for some OT transplantation patients. For example, encapsulating human OT in Alloderm allowed two patients to conceive through ART (Oktay et al., 2016). In mouse models, transplanted HA-encapsulated vitrified ovaries compromises follicles and FSH production (Taheri et al., 2016), but encapsulating fresh ovaries with a HA-based hydrogel (with/without VEGF and bFGF) protects the follicular reserve and re-establishes endocrine function (Tavana et al., 2016a,b). Similarly, co-culture of human bone marrow- or visceral peritoneal-derived MSC hydrogels with mouse OT can restore endocrine function earlier after transplantation, but delays follicle development (Mehdinia et al., 2020). On the other hand, culturing OT fragments with laminin components of the native ovarian ECM enhances follicle survival and development to the secondary follicle stage (Hao et al., 2020), while encapsulating follicles in PEG vinyl-sulfone hydrogels maintains the reserves to day 60 and supports antral development and ovulation (Kim et al., 2016). In corroboration, encapsulating OT in TheraCyte or Dual-PEG capsules (which has a proteolytically degradable PEG vinyl-sulfone core with a non-degradable shell) restores ovarian function and follicle development after allotransplantation, without evoking an immune response (Day et al., 2019). Using hydrogels to sustain local release of bFGF decreases fibrosis in human OT, in addition to improving revascularization and follicle density after xenotransplantation (Tanaka et al., 2018); these findings corroborate prior work using fibrin-bFGF scaffolds, which protect murine follicular reserves and increase revascularization after transplantation (Gao et al., 2013). Similarly, exogenous mouse endothelial cells engineered to constitutively express anti-Müllerian hormone (AMH) (Man et al., 2017), or STEMPRO® adipose-derived MSCs (Manavella et al., 2018; Cacciottola et al., 2021), can preserve primordial follicles by promoting revascularization of OT encapsulated with fibrinogen-thrombin. A recent report describes improved ovarian cortex xenografting outcomes achieved by embedding OT in fibrin clots and treating mice with simvastatin (Magen et al., 2020). Furthermore, encapsulation of OT with an alginate hydrogel results in developmentally competent oocytes and protects against metastatic lesions [at least short term (Rios et al., 2018)].
One goal of reproductive bioengineering is to achieve artificial ovaries for alternative fertility preservation strategies. This goal remains somewhat out of reach, but initial work described the encapsulation of ovarian stromal cells in chitosan-silk hydrogels (Jafari et al., 2021). Further, primordial follicles in murine ovarian fragments encapsulated with fibrin modified with heparin-binding peptide, heparin and VEGF (Shikanov et al., 2011c; Kniazeva et al., 2015) and follicles transplanted in bioprinted scaffolds (Laronda et al., 2017) have also produced pups after natural mating. Fibrinogen and thrombin, which are clotting factors, are similarly used to encapsulate follicles (Chiti et al., 2016, 2017) or OT, with or without addition of stem cells or stromal cells. On the other hand, transplantation of granulosa and theca cell constructs restores hormone function, improving bone and uterine health as well as lowering body fat, compared to pharmacological hormone replacement therapy (Sittadjody et al., 2017).
Hydrogels and scaffolds provide some advantages in models of premature ovarian failure (POF) or premature ovarian insufficiency (POI). For example, human amniotic epithelial cells encapsulated within sodium alginate bioglass protect granulosa cell function and ovarian vascularization in a chemotherapy-induced POF model (Huang et al., 2021). Similarly transplant of human UC-MSCs embedded in Matrigel promotes granulosa cell proliferation and ovarian vascularization (Zhou et al., 2021), and adipose-derived stem cells in a collagen scaffold restore ovarian function in POI models (Su et al., 2016). Notably, local delivery of embryonic stem cell-derived mesenchymal progenitor cells in a HA gel increases the ovarian reserve, and estrogen and AMH levels, ultimately improving the quality of oocytes and embryos in mice that model POI (Shin et al., 2021).
Bioengineered materials and techniques can also be implemented during reconstructive gynecological surgeries. Recently, vaginal reconstruction was successful in a patient with MRKH syndrome, a rare congenital disorder characterized by abnormal uterine and vaginal development despite normal ovarian function and external genitalia; this approach used a DC porcine small intestine submucosa scaffold (Zhang et al., 2019b). Remarkably, this biomaterial achieves structural and functional vaginas for up to 8 years (Raya-Rivera et al., 2014). Similarly, vaginoplasty with an acellular dermal matrix (called RENOV) is safe and effective, and results in an anatomically correct vagina that provides near-normal sexual function (Zhang et al., 2017c). Neovaginas were also safely constructed using Surgicel (an oxidized cellulose scaffold) for 10 patients (Dadhwal et al., 2010), or bovine-derived dermis scaffold for another patient (Noguchi et al., 2004).
Discussion
Summary of the evidence: where do we stand?
The organs of the female reproductive system—the uterus, ovaries, fallopian tubes, cervix and vagina—work together to provide the hormonal and anatomical support necessary for the generation of offspring. As such, reproductive health is susceptible to a number of negative congenital or acquired factors, restricting fertility and quality of life. These concerns prompt a large field of research into the underlying biology as well as approaches for preventing or treating various pathologies. However, ethical and technical limitations around using and/or transplanting human tissues for research purposes requires that most studies are conducted in vitro or in vivo using animal models. While valuable, these approaches face inherent limitations in translatability, such as the complexity of recreating the anatomy, physiology and interactions of reproductive organs using classical 2D in vitro models, in addition to the differences between species. Thus, bioengineering has become indispensable for creating representative and reliable 3D models (for both in vitro and in vivo uses) as well as providing alternative applications for regenerative medicine. This review’s systematic compilation of the extensive bioengineering advances in the context of the female reproductive system since 2000, provides a global overview of the different techniques, their pre-clinical testing and/or clinical applications and the anticipation of future trends.
Uterus
The uterus, and in particular the endometrium, is fundamental for implantation and maintenance of pregnancy (Governini et al., 2021). As such, much research is devoted to the creation of functional endometrial models and combining endogenous endometrial cell populations in different formats and biomaterials (Table I). Notable among these are paracrine models of epithelial and stromal cell co-culture (Schutte et al., 2015; Park et al., 2021), as well as models of decidualization (Schutte and Taylor, 2012; Gnecco et al., 2019), implantation (Park et al., 2003; Wang et al., 2012, 2013; Buck et al., 2015), vascularization (Pence et al., 2017), ECM interactions (Cook et al., 2017) and uterine contractility (Kim et al., 2020b). In recent years, several groups attempted to recreate the complexity of these models with organoids or assembloids (Boretto et al., 2017; Turco et al., 2017; Murphy et al., 2019; Abbas et al., 2020; Rawlings et al., 2021), which offer an apparently unlimited potential to recreate the physiological and pathological states of the endometrium (Boretto et al., 2019). In fact, organoid technology is marking a turning point in endometrial-related research. Despite having been described only 5 years ago, more than 13% of the uterus-related articles reported in this study exploit this technology. Remarkably, although most biomaterials attempt to mimic ECM interactions in vitro, only a few studies notably implement native ECMs (Young and Goloman, 2013; Olalekan et al., 2017; Campo et al., 2019; Arezoo et al., 2021; López-Martínez et al., 2021a; Francés-Herrero et al., 2021b).
Absolute uterine factor infertility can be treated with uterine transplantation (UTx). Taking into account scientific (Brännström et al., 2021) and media reports, as well as personal communications, we currently estimate that more than 40 LBs have been achieved from over 80 UTx procedures that have been performed thus far. The surgical success rate (defined by a viable organ within 3 months, resumption of regular menstruations within a year, successful pregnancy and LB) was 78% and 64% for live and deceased donor UTx procedures, respectively, and the cumulative LB rates in surgically successful UTx procedures were estimated to be above 80%. Despite these promising success rates, this procedure involves an invasive surgery and associated risks. Bioengineering has been used to mitigate these risks by providing alternative clinical applications. Specifically, bioengineering techniques for the uterus focus predominantly on preventing/reducing adhesions, often associated with AS (Zhao et al., 2017; Cao et al., 2018; Zhang et al., 2021b) and related to uterine factor infertility. In these and other cases of endometrial damage, the main therapeutic objectives are to regenerate tissue structure (e.g. recover endometrial thickness, angiogenesis) and consequently restore function, which ultimately allows the uterus to support implantation and carry a pregnancy to term (Hellström et al., 2016; Kuramoto et al., 2018; Li et al., 2019; Liu et al., 2019; Wang et al., 2021). Toward this end, different hydrogels and scaffolds show potential in vivo, by regenerating injured uteri in rodent models (Supplementary Table SII). Emerging technologies, such as 3D bioprinting and microfluidics, remain under-utilized in research applied to uterine health, but promising possibilities exist for both in vitro modeling (Ahn et al., 2021; De Bem et al., 2021) and in vivo tissue regeneration (Ji et al., 2020).
Ovary
The ovaries exert two main functions, namely to tightly regulate folliculogenesis so as to avoid premature depletion of oocytes, and to produce sufficient sex hormones (e.g. estrogen and progesterone) to support decidualization, pregnancy, breast development for lactation and even bone health (Sittadjody et al., 2017). Developing new IVFG platforms opens opportunities for oncological patients who cannot benefit from current fertility preservation strategies (specifically, OT cryopreservation) due to risk of reintroducing malignancy upon autologous re-transplantation. Culturing follicles/OT in vitro ‘bypasses’ this risk and can produce mature oocytes faster than if the OT was xenografted into a murine model [usually in 8–12 days (Supplementary Table SIII) versus weeks-months (Oktay et al., 2016)], but does not have the potential to restore endocrine function. Most IVFG studies we included in this review successfully cultured secondary follicles to the antral stage, and some even recovered mature and competent oocytes (Supplementary Table SIII). Few groups have ventured into culturing primary follicles because these follicles tend to have lower survival and oocyte maturation rates (Tagler et al., 2012, 2014; Smith et al., 2014).
The success of IVFG is not only affected by initial follicle size, but also by the saturation of the biomaterial. Physiologically, the rigidity of the ovarian cortex and the ‘sponginess’ of the medulla play important roles in regulating folliculogenesis. In fact, the mechanical forces of the ovarian cortex ECM may maintain reserves of primordial follicles, only releasing a couple of follicles to grow in the medulla every menstrual cycle (Choi et al., 2014). Nonetheless, although softer/more flexible biomaterials, such as alginate, Matrigel and VitroGel, could facilitate follicle expansion, materials that are too soft (i.e. 1 mg/ml collagen, fibrin alone or HA-Matrigel, rapidly degrading YKNR plasmin substrate) cannot provide the necessary 3D support, causing granulosa cells to erroneously proliferate and migrate into their surroundings (Shikanov et al., 2009, 2011b; Desai et al., 2012; Joo et al., 2016). In contrast, saturated/rigid matrices [i.e. 1.5% alginate (West-Farrell et al., 2009)] hinder follicle growth. Although OT transplantation has led to more than 200 human LBs so far (Dolmans et al., 2021), encapsulating OT before transplantation may provide additional benefits by promoting revascularization, decreasing fibrosis, protecting follicles from “burn-out” (ischemia-induced death of follicles during the first couple of days after transplant), and ultimately, providing the best microenvironment for follicle development in vivo. However, in attempts to standardize OT transplantation or replacement and be able to offer these strategies to a broad population (e.g. oncological patients and/or those in need of hormone replacement therapy), the construction of an artificial ovary containing immature stimulable follicles is gaining momentum and could lead the way for the next decade. Another common ovarian bioengineering application with great potential is the development of heterogeneous and/or patient-derived organoid models to evaluate individual drug response and cancer dissemination (Supplementary Table SIV).
Fallopian tubes
Fallopian tubes (or oviducts) are the anatomical structures that connect the ovaries and the uterus, providing the space and physiological environment for fertilization and early embryo development. Few bioengineering methods exist to date to recapitulate fallopian tubes and their associated functions in vitro, despite their crucial supportive role during early embryo development. Derivation of human fallopian tube organoids from different cell types (Kessler et al., 2015; Lin et al., 2021a) provided an important breakthrough in the creation of functional in vitro models. Among the few other fallopian tube studies in the bioengineering field, some demonstrate the important cross-talk between the ovaries and the fallopian tubes (Zhu et al., 2016), or the direct effect of oviductal ECM molecules on embryonic metabolism (Francés-Herrero et al., 2021a). Microfluidic platforms, with their small channels, may be the most suitable for modeling the physiology and pathology (Ferraz et al., 2020) of this tubular organ. Indeed, the implementation of a bovine oviduct-on-a-chip led to improved IVF outcomes (Ferraz et al., 2018).
Cervix and vagina
The cervix and vagina play critical roles in reproduction by serving as an entryway for sperm during ovulation, physical barriers for infectious microorganisms and a pathway during childbirth. Bioengineering these tissues has provided novel multilayered organoid models to study herpes (Zhu et al., 2017) and cervical cancer (Tanaka et al., 2021), also enabling testing of their respective treatments. Although a functional vagina can be created by self-dilation of the vaginal dimple in a majority of patients with MRKH syndrome, vaginal scaffolds are used for reconstructive surgeries (Noguchi et al., 2004; Dadhwal et al., 2010; Zhang et al., 2017c, 2019b; Acién et al., 2019). Other bioengineering alternatives may prevent premature rupture of fetal membranes and incontinence (Roman et al., 2018), or test contractility inhibitors with bioprinted uterine rings (Souza et al., 2017). Moreover, hydrogels can be used as carriers for antibiotics, antivirals, antifungals, contraceptives and other drugs (Dos Santos et al., 2020).
Full tract
Female reproductive function is orchestrated by multiple autocrine, paracrine and endocrine dialogues, which so far have only been studied in vivo in model organisms that cannot accurately reproduce the human body. To overcome the limitations of these models, there exists the need to recreate a multiorgan environment that incorporates physical, mechanical and hormonal variables. Microfluidics offers the most promising bioengineering method, having already enabled the development of an organ system-on-a-chip that combines human liver spheroids, mouse ovarian explants, human fallopian tube epithelium, human endometrium and human cervix tissues to physiologically model a 28-day menstrual cycle (Xiao et al., 2017). Recently, the endocrine crosstalk between the uterus and the ovary has been modeled on-a-chip, to be able to evaluate the effects of reproductive toxicants (Park et al., 2020). Another application rarely exploited to date is the possibility of combining, in a single microfluidic platform, a major portion of the workflow in assisted reproduction clinics, thereby minimally altering the environmental conditions to which gametes and embryos are exposed (Han et al., 2010).
Future perspectives
New 3D in vitro models representing multiple cell types and/or tissue layers are not only helping to elucidate the physiological dynamics of complex biological processes within the reproductive tract (e.g. those that regulate folliculogenesis, ovulation, decidualization and cancer progression), but also improving personalized medicine (Stejskalová et al., 2021). In particular, organoids generated in 3D culture can adequately mimic healthy and diseased cell-cell and cell-ECM native tissue interactions, making them ideal models for evaluating individual drug response (for cancer, endometriosis, dysmenorrhea, hormone disorders or other related issues, bacterial/viral/fungal infection, etc.) or implantation potential (Wei et al., 2021). However, organoid models, especially endometrial ones, have unresolved issues, which the scientific community has started, and should continue, to investigate. Among others, the main limitations are: the lack of expandable organoid lines with stromal and immunological components; the inaccessibility to the organoid lumen; the lack of interactions with native ECM components; and the variability associated with patient tissue origin and culture handling. Automated ‘lab-on-a-chip’ technologies that can rapidly screen various bodily fluids (e.g. blood, ascites or pleural fluid, urine) for specific biomarkers, cancer cells, drugs or oocytes may also efficiently and reliably refine future clinical/therapeutic decisions. Since body-on-a-chip platforms have the potential to model hormone dynamics and systemic disease (e.g. PCOS, diabetes, cancer), combining them with organoid or organ culture and ECM-based environments may provide more robust 3D models for genetic/epigenetic and pharmacokinetics testing.
Much remains to be achieved for the field to create (and eventually offer) a completely artificial female reproductive system. Nevertheless, recent advances in the creation of an artificial ovary (Krotz et al., 2010; Chiti et al., 2016; Sittadjody et al., 2017; Jafari et al., 2021; Yoon et al., 2021; Wu et al., 2022), uterus (Souza et al., 2017; Ji et al., 2020; Li et al., 2021; Park et al., 2021), cervix (Arslan et al., 2015; De Gregorio et al., 2017; Zhao et al., 2020) and vagina (Orabi et al., 2017; Hou et al., 2021) have made promising headway toward this incredible goal. For example, the development of alternative, more natural, options for hormone replacement therapies offers promise for mitigating menopause-associated problems (Sittadjody et al., 2017; Yoon et al., 2021). In the race to manufacture transplantable tissues and organs, 3D bioprinting has played a discreet role so far, accounting for only 3% of the studies included in this review. Specifically, its relative novelty, limited accessibility among research groups worldwide and lack of standardized protocols and technology could be slowing down its take-off, making it an attractive and necessary niche for investment. Studies focused on bioengineering of the fallopian tubes are scarce, since their functions are bypassed in assisted reproduction clinics. However, recent work demonstrates that an artificial oviduct-on-a-chip may substantially improve IVF and early embryo culture systems by providing a more realistic microenvironment (Ferraz et al., 2018). Moreover, these anatomical structures are the target of numerous studies to develop alternative contraceptive methods. Among these, artificial hydrogels based on styrene maleic anhydride (Subramanian et al., 2019) and PEG (McLemore et al., 2005) offer promise as contraceptive approaches through successful testing in the fallopian tubes of rats and rabbits. Finally, we note the need for greater clinical translation in reproductive bioengineering. Despite the large number of proposals described at the preclinical level, only 5% of the studies compiled in this review are clinical. Advances at the legislative level, meta-analyses to establish optimal procedures, and stronger networks of collaboration between laboratories and medical centers, could be of value.
Limitations
This systematic review identified a wealth of bioengineering-related studies in the context of female reproduction. Nonetheless, it is possible that relevant studies were not found or were excluded because of the keyword selection, subjective nature of the filtering process or reference limit. We compiled the 312 articles that we considered the most significant and representative of the current state of the field. There is an additional limitation in terms of classification of the articles by biomaterial, since the literature lacks consensus in delineating certain hydrogels and scaffolds (e.g. collagen was reported as a hydrogel and scaffold), and some studies combined bioengineering techniques (e.g. organoid or culture with hydrogel/scaffold within a microfluidic system). Therefore, we classified articles, on a case-by-case basis, in a way we deemed most appropriate. Since the original Embase search identified numerous oncology-related studies, additional searches with keywords representing reproductive diseases were conducted to ensure appropriate coverage of the latter. Finally, due to different organs under consideration and divergences in study objectives and designs, the included studies exhibit wide heterogeneity that precluded meta-analysis of the results.
Conclusion
Female reproduction is regulated by complex networks of molecular, endocrine and tissue/organ interactions. As such, substituting the entire female reproductive tract will be challenging; however, interdisciplinary work provides novel insight into the physicochemical properties necessary to support and achieve these biological processes. Advances in reproductive bioengineering technologies have redefined the landscape of fertility-restoring strategies and therapeutic options that are, or soon could be, available to patients. These translational endeavors provide substantial promise for effective treatments for a wide range of reproductive system pathologies.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Supplementary Material
Contributor Information
Emilio Francés-Herrero, Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia, Valencia, Spain; Fundación IVI, IVI-RMA Global, Valencia, Spain.
Rosalba Lopez, Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia, Valencia, Spain; Fundación IVI, IVI-RMA Global, Valencia, Spain; Reproductive Medicine Research Group, IIS La Fe, Valencia, Spain.
Mats Hellström, Laboratory for Transplantation and Regenerative Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Obstetrics and Gynecology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Lucía de Miguel-Gómez, Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia, Valencia, Spain; Fundación IVI, IVI-RMA Global, Valencia, Spain.
Sonia Herraiz, Fundación IVI, IVI-RMA Global, Valencia, Spain; Reproductive Medicine Research Group, IIS La Fe, Valencia, Spain.
Mats Brännström, Laboratory for Transplantation and Regenerative Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Obstetrics and Gynecology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Stockholm IVF-EUGIN, Stockholm, Sweden.
Antonio Pellicer, Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia, Valencia, Spain; IVI Roma Parioli, IVI-RMA Global, Rome, Italy.
Irene Cervelló, Fundación IVI, IVI-RMA Global, Valencia, Spain; Reproductive Medicine Research Group, IIS La Fe, Valencia, Spain.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
Conceptualization: I.C., E.F.-H., R.L., M.H., L.d.M.-G., S.H., M.B. and A.P.; systematic literature search, selection and data curation: E.F.-H. and R.L.; data review: I.C., E.F.-H. and R.L.; manuscript and figure preparation: I.C., E.F.-H., R.L. and L.d.M.-G.; manuscript review: I.C., M.H., L.d.M.-G., S.H., M.B. and A.P. All authors have agreed to the published version of the manuscript.
Funding
This study was supported by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union (Fondo Social Europeo «El FSE invierte en tu futuro») [PI17/01039-PI21/00305-CP19/00149 (I.C.), CP19/00141 (S.H.)]; Spanish Ministry of Science, Innovation, and Universities [FPU18/06327 (E.F.-H.)]; Generalitat Valenciana [GRISOLIAP/2018/029 (R.L.), PROMETEO/2018/137 (I.C., S.H., L.d.M-G., and A.P.)].
Conflict of interest
The authors declare no conflict of interest.
References
- Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, Moffett A, Best S, Turco MY, Burton GJ. et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 2020;10:20190079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abir R, Stav D, Taieb Y, Gabbay-Benziv R, Kirshner M, Ben-Haroush A, Freud E, Ash S, Yaniv I, Herman-Edelstein M. et al. Novel extra cellular-like matrices to improve human ovarian grafting. J Assist Reprod Genet 2020;37:2105–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acién P, Nohales-Alfonso FJ, Sánchez-Ferrer M-L, Sánchez-Lozano M, Navarro-Lillo V, Acién M.. Clinical pilot study to evaluate the neovaginal PACIENA prosthesis® for vaginoplasty without skin grafts in women with vaginal agenesis. BMC Womens Health 2019;19:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Yoon M-J, Hong S-H, Cha H, Lee D, Koo HS, Ko J-E, Lee J, Oh S, Jeon NL. et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum Reprod 2021;36:2720–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JI, Kim GA, Kwon HS, Ahn JY, Hubbell JA, Song YS, Lee ST, Lim JM.. Culture of preantral follicles in poly(ethylene) glycol-based, three-dimensional hydrogel: a relationship between swelling ratio and follicular developments. J Tissue Eng Regen Med 2015;9:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajeti V, Lara-Santiago J, Alkmin S, Campagnola PJ.. Ovarian and breast cancer migration dynamics on laminin and fibronectin bidirectional gradient fibers fabricated via multiphoton excited photochemistry. Cell Mol Bioeng 2017;10:295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brännström M, Hellström M.. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res 2019;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brännström M, Hellström M.. Decellularization and recellularization of the ovary for bioengineering applications; studies in the mouse. Reprod Biol Endocrinol 2020;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim CA, Van Langendonckt A, David A, Dolmans M-M, Donnez J.. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod 2009;24:92–99. [DOI] [PubMed] [Google Scholar]
- Arezoo N, Mohammad H, Malihezaman M.. Tissue engineering of mouse uterus using menstrual blood stem cells (MenSCs) and decellularized uterine scaffold. Stem Cell Res Ther 2021;12:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan SY, Yu Y, Burdette JE, Pavone ME, Hope TJ, Woodruff TK, Kim JJ.. Novel three dimensional human endocervix cultures respond to 28-day hormone treatment. Endocrinology 2015;156:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi M, Péant B, Lateef MA, Rousset N, Kendall-Dupont J, Carmona E, Monet F, Saad F, Provencher D, Mes-Masson A-M et al Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Lab Chip 2016;16:312–325. [DOI] [PubMed] [Google Scholar]
- Aziz AUR, Yu X, Jiang Q, Zhao Y, Deng S, Qin K, Wang H, Liu B.. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol In Vitro 2020;62:104677. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C.. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res 1995;29:977–985. [DOI] [PubMed] [Google Scholar]
- Bascetin R, Laurent-Issartel C, Blanc-Fournier C, Vendrely C, Kellouche S, Carreiras F, Gallet O, Leroy-Dudal J.. A biomimetic model of 3D fluid extracellular macromolecular crowding microenvironment fine-tunes ovarian cancer cells dissemination phenotype. Biomaterials 2021;269:120610. [DOI] [PubMed] [Google Scholar]
- Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T.. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil 1994;101:327–332. [DOI] [PubMed] [Google Scholar]
- Bi J, Newtson AM, Zhang Y, Devor EJ, Samuelson MI, Thiel KW, Leslie KK.. Successful patient-derived organoid culture of gynecologic cancers for disease modeling and drug sensitivity testing. Cancers 2021;13:2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RC, Boretto M, Rutkowski MR, Vankelecom H, Derré I.. Murine endometrial organoids to model chlamydia infection. Front Cell Infect Microbiol 2020;10:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläuer M, Heinonen PK, Martikainen PM, Tomás E, Ylikomi T.. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod 2005;20:864–871. [DOI] [PubMed] [Google Scholar]
- Bongso A, Soon-Chye N, Sathananthan H, Lian NP, Rauff M, Ratnam S.. Improved quality of human embryos when co-cultured with human ampullary cells. Hum Reprod 1989;4:706–713. [DOI] [PubMed] [Google Scholar]
- Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A. et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017;144:1775–1786. [DOI] [PubMed] [Google Scholar]
- Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I. et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 2019;21:1041–1051. [DOI] [PubMed] [Google Scholar]
- Brännström M, Belfort MA, Ayoubi JM.. Uterus transplantation worldwide: clinical activities and outcomes. Curr Opin Organ Transplant 2021;26:616–626. [DOI] [PubMed] [Google Scholar]
- Brito I, Silva G, Sales A, Lobo C, Rodrigues G, Sousa R, Moura A, Calderón C, Bertolini M, Campello C. et al. Fibrin–alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod Dom Anim 2016;51:997–1009. [DOI] [PubMed] [Google Scholar]
- Brito IR, Silva CMG, Duarte ABG, Lima IMT, Rodrigues GQ, Rossetto R, Sales AD, Lobo CH, Bernuci MP, Rosa-E-Silva ACJS et al Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol Reprod Dev 2014;81:636–645. [DOI] [PubMed] [Google Scholar]
- Buck VU, Gellersen B, Leube RE, Classen-Linke I.. Classen-Linke I. Interaction of human trophoblast cells with gland-like endometrial spheroids: a model system for trophoblast invasion. Hum Reprod 2015;30:906–916. [DOI] [PubMed] [Google Scholar]
- Cabral-Romero C, Solís-Soto JM, Sánchez-Pérez Y, Pineda-Aguilar N, Meester I, Pérez-Carrillo E, Nakagoshi-Cepeda SE, Sánchez-Nájera RI, Nakagoshi-Cepeda MAA, Hernandez-Delgadillo R. et al. Antitumor activity of a hydrogel loaded with lipophilic bismuth nanoparticles on cervical, prostate, and colon human cancer cells. Anticancer Drugs 2020;31:251–259. [DOI] [PubMed] [Google Scholar]
- Cacciottola L, Courtoy GE, Nguyen TYT, Hossay C, Donnez J, Dolmans M-M.. Adipose tissue-derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation. J Assist Reprod Genet 2021;38:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wu F, Yu Y, Liu Y, Shao C, Gu H, Li M, Zhao Y.. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater 2019;84:222–230. [DOI] [PubMed] [Google Scholar]
- Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA.. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013;67:64–69. [DOI] [PubMed] [Google Scholar]
- Camous S, Heyman Y, Méziou W, Ménézo Y.. Cleavage beyond the block stage and survival after transfer of early bovine embryos cultured with trophoblastic vesicles. J Reprod Fertil 1984;72:479–485. [DOI] [PubMed] [Google Scholar]
- Campo H, Baptista PM, López-Pérez N, Faus A, Cervelló I, Simón C.. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol Reprod 2017;96:34–45. [DOI] [PubMed] [Google Scholar]
- Campo H, García-Domínguez X, López-Martínez S, Faus A, Vicente Antón JS, Marco-Jiménez F, Cervelló I.. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater 2019;89:126–138. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L. et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther 2018;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA.. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 1997;100:297–302; discussion 303–304. [DOI] [PubMed] [Google Scholar]
- Celik O, Esrefoglu M, Hascalik S, Gul M, Tagluk ME, Elter K, Aydin E.. Use of porcine small intestinal submucosa to reconstruct an ovarian defect. Int J Gynaecol Obstet 2009;106:218–222. [DOI] [PubMed] [Google Scholar]
- Chang Y-H, Chu T-Y, Ding D-C.. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J Biomed Sci 2020;27:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Yoo JJ, Atala A.. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 1999;54:407–410. [DOI] [PubMed] [Google Scholar]
- Chen H, Gotimer K, De Souza C, Tepper CG, Karnezis AN, Leiserowitz GS, Chien J, Smith LH.. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol Oncol 2020;157:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang J, Zhang Y, Chen D, Yang C, Kai C, Wang X, Shi F, Dou J.. Observation of ovarian cancer stem cell behavior and investigation of potential mechanisms of drug resistance in three-dimensional cell culture. J Biosci Bioeng 2014;118:214–222. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao L, Peng H, Dai S, Quan Y, Wang M, Wang J, Bi Z, Zheng Y, Zhou S. et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Ther 2021;28:112–125. [DOI] [PubMed] [Google Scholar]
- Chen X, Sun J, Li X, Mao L, Cui L, Bai W.. Transplantation of oral mucosal epithelial cells seeded on decellularized and lyophilized amniotic membrane for the regeneration of injured endometrium. Stem Cell Res Ther 2019;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Peng C-Y, Marinić M, Sakabe NJ, Aneas I, Lynch VJ, Ober C, Nobrega MA, Kessler JA.. Pluripotent stem cell-derived endometrial stromal fibroblasts in a cyclic, hormone-responsive, coculture model of human decidua. Cell Rep 2021;35:109138. [DOI] [PubMed] [Google Scholar]
- Chiti MC, Dolmans MM, Lucci CM, Paulini F, Donnez J, Amorim CA.. Further insights into the impact of mouse follicle stage on graft outcome in an artificial ovary environment. Mol Hum Reprod 2017;23:381–392. [DOI] [PubMed] [Google Scholar]
- Chiti MC, Dolmans M-M, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, Van Ruymbeke E, Champagne S-D, Donnez J, Amorim CA.. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet 2018;35:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti MC, Dolmans MM, Orellana R, Soares M, Paulini F, Donnez J, Amorim CA.. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum Reprod Oxf Engl 2016;31:427–435. [DOI] [PubMed] [Google Scholar]
- Choi JK, Agarwal P, Huang H, Zhao S, He X.. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014;35:5122–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowanadisai W, Messerli SM, Miller DH, Medina JE, Hamilton JW, Messerli MA, Brodsky AS.. Cisplatin resistant spheroids model clinically relevant survival mechanisms in ovarian tumors. PLoS ONE 2016;11:e0151089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumduri C, Gurumurthy RK, Berger H, Dietrich O, Kumar N, Koster S, Brinkmann V, Hoffmann K, Drabkina M, Arampatzi P. et al. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia. Nat Cell Biol 2021;23:184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-D, Liu T-H, Liang Y-L, Lin C-N, Hsu K-F, Lee G-B.. An integrated microfluidic platform for detection of ovarian clear cell carcinoma mRNA biomarker FXYD2. Lab Chip 2021;21:2625–2632. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Zhao X, Elder K, Jones CJP, Moffett A, Burton GJ, Turco MY.. Menstrual flow as a non-invasive source of endometrial organoids. Commun Biol 2021;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, Isaacson KB, Lauffenburger DA, Griffith LG.. Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol (Camb) 2017;9:271–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadhwal V, Ghosh B, Gupta B, Deka D, Mittal S.. Oxidized cellulose for epithelialization of neovagina in vaginal agenesis. J Gynecol Surg 2010;26:189–193. [Google Scholar]
- Daryabari SS, Kajbafzadeh A-M, Fendereski K, Ghorbani F, Dehnavi M, Rostami M, Garajegayeh BA, Tavangar SM.. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J Assist Reprod Genet 2019;36:1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JR, David A, Barbosa MGdM, Brunette MA, Cascalho M, Shikanov A.. Encapsulation of ovarian allograft precludes immune rejection and promotes restoration of endocrine function in immune-competent ovariectomized mice. Sci Rep 2019;9:16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bem THC, Tinning H, Vasconcelos EJR, Wang D, Forde N.. Endometrium on-a-chip reveals insulin- and glucose-induced alterations in the transcriptome and proteomic secretome. Endocrinology 2021;162:bqab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio V, Imparato G, Urciuolo F, Tornesello ML, Annunziata C, Buonaguro FM, Netti PA.. An engineered cell-instructive stroma for the fabrication of a novel full thickness human cervix equivalent in vitro. Adv Healthc Mater 2017;6. doi: 10.1002/adhm.201601199. [DOI] [PubMed] [Google Scholar]
- Demirbag S, Cetinkursun S, Tasdemir U, Ozturk H, Pekcan M, Yesildaglar N.. Comparison of hyaluronate/carboxymethylcellulose membrane and melatonin for prevention of adhesion formation in a rat model. Hum Reprod Oxf Engl 2005;20:2021–2024. [DOI] [PubMed] [Google Scholar]
- Desai N, Abdelhafez F, Calabro A, Falcone T.. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod Biol Endocrinol 2012;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Li X, Sun H, Su J, Lin N, Péault B, Song T, Yang J, Dai J, Hu Y.. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 2014;35:4888–4900. [DOI] [PubMed] [Google Scholar]
- Ding L, Yan G, Wang B, Xu L, Gu Y, Ru T, Cui X, Lei L, Liu J, Sheng X. et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci 2018;61:1554–1565. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, Liebenthron J, Pellicer A, Donnez J, Andersen CY.. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril 2021;115:1102–1115. [DOI] [PubMed] [Google Scholar]
- Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, Gogna R, Flannery MM, Hays J, Hansford DJ. et al. A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res 2019;79:3503–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos AM, Carvalho SG, Araujo VHS, Carvalho GC, Gremião MPD, Chorilli M.. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int J Pharm 2020;590:119867. [DOI] [PubMed] [Google Scholar]
- Eddie SL, Quartuccio SM, Zhu J, Shepherd JA, Kothari R, Kim JJ, Woodruff TK, Burdette JE.. Three-dimensional modeling of the human fallopian tube fimbriae. Gynecol Oncol 2015;136:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SL, Ulrich D, White JF, Su K, Rosamilia A, Ramshaw JAM, Gargett CE, Werkmeister JA.. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater 2015;13:286–294. [DOI] [PubMed] [Google Scholar]
- Eissa AM, Barros FSV, Vrljicak P, Brosens JJ, Cameron NR.. Enhanced differentiation potential of primary human endometrial cells cultured on 3D scaffolds. Biomacromolecules 2018;19:3343–3350. [DOI] [PubMed] [Google Scholar]
- Eivazkhani F, Abtahi NS, Tavana S, Mirzaeian L, Abedi F, Ebrahimi B, Montazeri L, Valojerdi MR, Fathi R.. Evaluating two ovarian decellularization methods in three species. Mater Sci Eng C Mater Biol Appl 2019;102:670–682. [DOI] [PubMed] [Google Scholar]
- Embrey MP, Graham NB, McNeill ME.. Induction of labour with a sustained-release prostaglandin E2 vaginal pessary. Br Med J 1980;281:901–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraslan S, Hamernik RJ, Hardy JD.. Replantation of uterus and ovaries in dogs, with successful pregnancy. Arch Surg 1966;92:9–12. [DOI] [PubMed] [Google Scholar]
- Espedal H, Berg HF, Fonnes T, Fasmer KE, Krakstad C, Haldorsen IS.. Feasibility and utility of MRI and dynamic 18F-FDG-PET in an orthotopic organoid-based patient-derived mouse model of endometrial cancer. J Transl Med 2021;19:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W. A method of implanting ovarian tissue in order to maintain ovarian function. Pennsylv Med J 1909;13:610–613. [Google Scholar]
- Fan Y, Sun Q, Li X, Feng J, Ao Z, Li X, Wang J.. Substrate stiffness modulates the growth, phenotype, and chemoresistance of ovarian cancer cells. Front Cell Dev Biol 2021;9:2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Masasa H, Orenbuch A, Levaot N, Shachar Goldenberg M, Cohen S.. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials 2019;205:11–22. [DOI] [PubMed] [Google Scholar]
- Ferraz MAMM, Nagashima JB, Venzac B, Le Gac S, Songsasen N.. A dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma. Sci Rep 2020;10:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz MAMM, Rho HS, Hemerich D, Henning HHW, van Tol HTA, Hölker M, Besenfelder U, Mokry M, Vos PLAM, Stout TAE. et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun 2018;9:4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleszar AJ, Walker A, Porubsky V, Flanigan W, James D, Campagnola PJ, Weisman PS, Kreeger PK.. The extracellular matrix of ovarian cortical inclusion cysts modulates invasion of fallopian tube epithelial cells. APL Bioeng 2018;2:031902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flont M, Jastrzębska E, Brzózka Z.. A multilayered cancer-on-a-chip model to analyze the effectiveness of new-generation photosensitizers. Analyst 2020;145:6937–6947. [DOI] [PubMed] [Google Scholar]
- Folch A, Toner M.. Cellular micropatterns on biocompatible materials. Biotechnol Prog 1998;14:388–392. [DOI] [PubMed] [Google Scholar]
- Francés-Herrero E, De Miguel-Gómez L, López-Martínez S, Campo H, Garcia-Dominguez X, Diretto G, Faus A, Vicente JS, Marco-Jiménez F, Cervelló I.. Development of decellularized oviductal hydrogels as a support for rabbit embryo culture. Reprod Sci 2021a;28:1644–1658. [DOI] [PubMed] [Google Scholar]
- Francés-Herrero E, Juárez-Barber E, Campo H, López-Martínez S, de Miguel-Gómez L, Faus A, Pellicer A, Ferrero H, Cervelló I.. Improved models of human endometrial organoids based on hydrogels from decellularized endometrium. JPM 2021b;11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J-M, Yan J, Li R, Li M, Yan L-Y, Wang T-R, Zhao H-C, Zhao Y, Yu Y, Qiao J.. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum Reprod 2013;28:2784–2793. [DOI] [PubMed] [Google Scholar]
- Ghezelayagh Z, Abtahi NS, Khodaverdi S, Rezazadeh Valojerdi M, Mehdizadeh A, Ebrahimi B.. The effect of agar substrate on growth and development of cryopreserved-thawed human ovarian cortical follicles in organ culture. Eur J Obstet Gynecol Reprod Biol 2021;258:139–145. [DOI] [PubMed] [Google Scholar]
- Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG.. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod 2019;34:702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, Osteen KG.. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng 2017;45:1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodinova A, Nankov V, Tomov S, Redzheb M, Petrov PD.. Extrusion bioprinting of hydroxyethylcellulose-based bioink for cervical tumor model. Carbohydr Polym 2021;260:117793. [DOI] [PubMed] [Google Scholar]
- Governini L, Luongo FP, Haxhiu A, Piomboni P, Luddi A.. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell 2021;73:101656. [DOI] [PubMed] [Google Scholar]
- Greco KV, Jones LG, Obiri-Yeboa I, Ansari T.. Creation of an acellular vaginal matrix for potential vaginal augmentation and cloacal repair. J Pediatr Adolesc Gynecol 2018;31:473–479. [DOI] [PubMed] [Google Scholar]
- Guerette NL, Peterson TV, Aguirre OA, VanDrie DM, Biller DH, Davila GW.. Anterior repair with or without collagen matrix reinforcement: a randomized controlled trial. Obstet Gynecol 2009;114:59–65. [DOI] [PubMed] [Google Scholar]
- Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L, Mitchelson K, Wang J, Huang G, Qiao J. et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip 2010;10:2848–2854. [DOI] [PubMed] [Google Scholar]
- Han X, Ma Y, Lu X, Li W, Xia E, Li T-C, Zhang H, Huang X.. Transplantation of human adipose stem cells using acellular human amniotic membrane improves angiogenesis in injured endometrial tissue in a rat intrauterine adhesion model. Cell Transplant 2020;29:963689720952055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Tuck AR, Prakash CR, Damdimopoulos A, Sjödin MOD, Lindberg J, Niklasson B, Pettersson K, Hovatta O, Damdimopoulou P.. Culture of human ovarian tissue in xeno-free conditions using laminin components of the human ovarian extracellular matrix. J Assist Reprod Genet 2020;37:2137–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A.. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993;261:895–897. [DOI] [PubMed] [Google Scholar]
- Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z.. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther 2018;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T, Yamaguchi T, Kato-Itoh M, Hamanaka S, Kawarai M, Sanbo M, Tamura C, Lee Y-S, Yanagida A, Murayama H. et al. Generation of mouse functional oocytes in rat by xeno-ectopic transplantation of primordial germ cells. Biol Reprod 2014;91:89. [DOI] [PubMed] [Google Scholar]
- Hellström M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Bäckdahl H, Johansson BR, Olausson M, Sumitran-Holgersson S, Brännström M.. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater 2014;10:5034–5042. [DOI] [PubMed] [Google Scholar]
- Hellström M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K, Maruyama T, Brännström M.. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril 2016;106:487–496.e1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gordillo V, Kassis T, Lampejo A, Choi G, Gamboa ME, Gnecco JS, Brown A, Breault DT, Carrier R, Griffith LG.. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 2020;254:120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka T, Hirota Y, Saito-Fujita T, Matsuo M, Egashira M, Matsumoto L, Haraguchi H, Dey SK, Furukawa KS, Fujii T. et al. STAT3 accelerates uterine epithelial regeneration in a mouse model of decellularized uterine matrix transplantation. JCI Insight 2016;1:e87591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelle JT, Carlson EC, Brendel K, Meezan E.. Biosynthesis of basement membrane matrix by isolated rat renal glomeruli. Kidney Int 1979;15:20–32. [DOI] [PubMed] [Google Scholar]
- Hjelm BE, Berta AN, Nickerson CA, Arntzen CJ, Herbst-Kralovetz MM.. Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biol Reprod 2010;82:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK.. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 2013;145:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Zheng J, Li Z, Qi X, Tian Y, Zhang M, Zhang J, Huang X.. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int J Biol Macromol 2021;180:177–186. [DOI] [PubMed] [Google Scholar]
- House M, Kelly J, Klebanov N, Yoshida K, Myers K, Kaplan DL.. Mechanical and biochemical effects of progesterone on engineered cervical tissue. Tissue Eng Part A 2018;24:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Ma Z, Kuang X, Zhang Q, Li H, Lai D.. Sodium alginate-bioglass-encapsulated hAECs restore ovarian function in premature ovarian failure by stimulating angiogenic factor secretion. Stem Cell Res Ther 2021;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H, Park MH, Jo G, Kim SY, Chun HJ, Yang DH.. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar Drugs 2019;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KS, Inoue K, Davis DA, Hilliard TS, Burdette JE.. Three-dimensional ovarian organ culture as a tool to study normal ovarian surface epithelial wound repair. Endocrinology 2009;150:3921–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari H, Dadashzadeh A, Moghassemi S, Zahedi P, Amorim CA, Shavandi A.. Ovarian cell encapsulation in an enzymatically crosslinked silk-based hydrogel with tunable mechanical properties. Gels 2021;7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowska W, Chabaud S, Saba I, Galbraith T, Berthod F, Bolduc S.. Prevascularized tissue-engineered human vaginal mucosa: in vitro optimization and in vivo validation. Tissue Eng Part A 2020;26:811–822. [DOI] [PubMed] [Google Scholar]
- Jakus AE, Laronda MM, Rashedi AS, Robinson CM, Lee C, Jordan SW, Orwig KE, Woodruff TK, Shah RN.. “Tissue Papers” from organ-specific decellularized extracellular matrices. Adv Funct Mater 2017;27:1700992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Shahzadi L, Ahtzaz S, Zahid S, Chaudhry AA, Rehman IU, Yar M.. Identification of anti-cancer potential of doxazocin: loading into chitosan based biodegradable hydrogels for on-site delivery to treat cervical cancer. Mater Sci Eng C Mater Biol Appl 2018;82:102–109. [DOI] [PubMed] [Google Scholar]
- Jamalzaei P, Valojerdi MR, Montazeri L, Baharvand H.. Effects of alginate concentration and ovarian cells on in vitro development of mouse preantral follicles: a factorial study. Int J Fertil Steril 2020;13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Hou B, Lin W, Wang L, Zheng W, Li W, Zheng J, Wen X, He P.. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater 2020;116:268–284. [DOI] [PubMed] [Google Scholar]
- Jiang P, Tang X, Wang H, Dai C, Su J, Zhu H, Song M, Liu J, Nan Z, Ru T. et al. Collagen-binding basic fibroblast growth factor improves functional remodeling of scarred endometrium in uterine infertile women: a pilot study. Sci China Life Sci 2019;62:1617–1629. [DOI] [PubMed] [Google Scholar]
- Jiang X, Li X, Fei X, Shen J, Chen J, Guo M, Li Y.. Endometrial membrane organoids from human embryonic stem cell combined with the 3D Matrigel for endometrium regeneration in Asherman syndrome. Bioact Mater 2021;6:3935–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang X, Zhao D, Hou X, Li C, Song X, Chen W, Wang Q, Zhao Y, Liu B.. An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale 2019;11:16080–16091. [DOI] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK.. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril 2010;93:2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S, Oh S-H, Sittadjody S, Opara EC, Jackson JD, Lee SJ, Yoo JJ, Atala A.. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed Mater 2016;11:065009. [DOI] [PubMed] [Google Scholar]
- Kai M, Maeda K, Tasaki M, Kira S, Nakamura S, Chino N, Hagiwara H, Nishida H, Kawanishi T.. Evaluation of a spray-type, novel dextrin hydrogel adhesion barrier under laparoscopic conditions in a porcine uterine horn adhesion model. J Minim Invasive Gynecol 2018;25:447–454. [DOI] [PubMed] [Google Scholar]
- Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, Dor J, Freud E, Felz C, Abir R.. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet 2011;28:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf H-J, Mangler M, Sehouli J. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 2015;6:8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Yang C, Lee J, Youm HW, Lee JR, Suh CS, Kim SH.. The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology 2020a;144:33–40. [DOI] [PubMed] [Google Scholar]
- Kim J, Perez AS, Claflin J, David A, Zhou H, Shikanov A.. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. NPJ Regen Med 2016;1:16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ushida T, Montagne K, Hirota Y, Yoshino O, Hiraoka T, Osuga Y, Furuakwa KS.. Acquired contractile ability in human endometrial stromal cells by passive loading of cyclic tensile stretch. Sci Rep 2020b;10:9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-H, Choi JH, Jun Y, Lim SM, Park S, Paek J-Y, Lee S-H, Hwang J-Y, Kim GJ.. 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Sci Rep 2018;8:15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Park K-H, Kim YJ, Kim MS, Liu HC, Rosenwaks Z, Ku S-Y.. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater 2019;89:139–151. [DOI] [PubMed] [Google Scholar]
- Kimura H, Nakamura H, Akai T, Yamamoto T, Hattori H, Sakai Y, Fujii T.. On-chip single embryo coculture with microporous-membrane-supported endometrial cells. IEEE Trans Nanobiosci 2009;8:318–324. [DOI] [PubMed] [Google Scholar]
- Kirk D, Alvarez RB.. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined media. In Vitro Cell Dev Biol 1986;22:604–614. [DOI] [PubMed] [Google Scholar]
- Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS, Shea LD.. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci Rep 2015;5:17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med 2019;25:838–849. [DOI] [PubMed] [Google Scholar]
- Krotz SP, Robins JC, Ferruccio T-M, Moore R, Steinhoff MM, Morgan JR, Carson S.. In vitro maturation of oocytes via the pre-fabricated self-assembled artificial human ovary. J Assist Reprod Genet 2010;27:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk PA, Auersperg N.. Human ovarian surface epithelial cells are capable of physically restructuring extracellular matrix. Am J Obstet Gynecol 1992;167:1437–1443. [DOI] [PubMed] [Google Scholar]
- Kuperman T, Gavriel M, Gotlib R, Zhang Y, Jaffa A, Elad D, Grisaru D.. Tissue-engineered multi-cellular models of the uterine wall. Biomech Model Mechanobiol 2020;19:1629–1639. [DOI] [PubMed] [Google Scholar]
- Kuramoto G, Hammad IA, Einerson BD, Allshouse AA, Debbink M, Grainger DW, Silver RM, Okano T.. Human mesenchymal stem cell sheets improve uterine incision repair in a rodent hysterotomy model. Am J Perinatol 2020. doi: 10.1055/s-0040-1721718. [DOI] [PubMed] [Google Scholar]
- Kuramoto G, Shimizu T, Takagi S, Ishitani K, Matsui H, Okano T.. Endometrial regeneration using cell sheet transplantation techniques in rats facilitates successful fertilization and pregnancy. Fertil Steril 2018;110:172–181.e4. [DOI] [PubMed] [Google Scholar]
- Kuramoto G, Takagi S, Ishitani K, Shimizu T, Okano T, Matsui H.. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum Reprod 2015;30:406–416. [DOI] [PubMed] [Google Scholar]
- Kwong J, Chan FL, Wong K, Birrer MJ, Archibald KM, Balkwill FR, Berkowitz RS, Mok SC.. Inflammatory cytokine tumor necrosis factor alpha confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia 2009;11:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK.. encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet 2014;31:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK.. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015;50:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN.. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 2017;8:15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechanteur A, Furst T, Evrard B, Delvenne P, Piel G, Hubert P.. Promoting vaginal distribution of E7 and MCL-1 siRNA-silencing nanoparticles for cervical cancer treatment. Mol Pharm 2017;14:1706–1717. [DOI] [PubMed] [Google Scholar]
- Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, Ben-Haroush A, Abir R.. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet 2013;30:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ.. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A 1987;84:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sun H, Lin N, Hou X, Wang J, Zhou B, Xu P, Xiao Z, Chen B, Dai J. et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 2011a;32:8172–8181. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Y, Ma R, Liu X, Song B, Duan Y, Guo J, Feng G, Cui T, Wang L. et al. Reconstruction of functional uterine tissues through recellularizing the decellularized rat uterine scaffolds by MSCs in vivo and in vitro. Biomed Mater 2021;16:035023. [DOI] [PubMed] [Google Scholar]
- Li Z, Kreiner M, Edrada-Ebel R, Cui Z, van der Walle CF, Mardon HJ.. Perfusion culture enhanced human endometrial stromal cell growth in alginate-multivalent integrin α5β1 ligand scaffolds. J Biomed Mater Res A 2011b;99:211–220. [DOI] [PubMed] [Google Scholar]
- Li Z, Yan G, Diao Q, Yu F, Li X, Sheng X, Liu Y, Dai Y, Zhou H, Zhen X. et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res Ther 2019;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Sun AM.. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980;210:908–910. [DOI] [PubMed] [Google Scholar]
- Lin J, Wang Z, Huang J, Tang S, Saiding Q, Zhu Q, Cui W.. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small 2021a;17:2007235. [DOI] [PubMed] [Google Scholar]
- Lin L, Lin X, Lin L, Feng Q, Kitamori T, Lin J-M, Sun J.. Integrated microfluidic platform with multiple functions to probe tumor–endothelial cell interaction. Anal Chem 2017;89:10037–10044. [DOI] [PubMed] [Google Scholar]
- Lin N, Li X, Song T, Wang J, Meng K, Yang J, Hou X, Dai J, Hu Y.. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials 2012;33:1801–1807. [DOI] [PubMed] [Google Scholar]
- Lin Y, Wei Y, Jiang M, Tang X, Huang F, Yang X.. Organoid culture of mouse fallopian tube epithelial stem cells with a thermo-reversible gelation polymer. Tissue Cell 2021b;73:101622. [DOI] [PubMed] [Google Scholar]
- Liu F, Hu S, Yang H, Li Z, Huang K, Su T, Wang S, Cheng K.. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of Asherman’s syndrome. Adv Healthc Mater 2019;8:e1900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W-Y, Lin S-G, Zhuo R-Y, Xie Y-Y, Pan W, Lin X-F, Shen F-X.. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng Part C Methods 2017;23:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cai J, Luo X, Wen H, Luo Y.. Collagen scaffold with human umbilical cord mesenchymal stem cells remarkably improves intrauterine adhesions in a rat model. Gynecol Obstet Invest 2020;85:267–276. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li H, Shu XZ, Gray SD, Prestwich GD.. Crosslinked hyaluronan hydrogels containing mitomycin C reduce postoperative abdominal adhesions. Fertil Steril 2005;83(Suppl 1):1275–1283. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shu XZ, Prestwich GD.. Reduced postoperative intra-abdominal adhesions using Carbylan-SX, a semisynthetic glycosaminoglycan hydrogel. Fertil Steril 2007;87:940–948. [DOI] [PubMed] [Google Scholar]
- Loessner D, Rockstroh A, Shokoohmand A, Holzapfel BM, Wagner F, Baldwin J, Boxberg M, Schmalfeldt B, Lengyel E, Clements JA. et al. A 3D tumor microenvironment regulates cell proliferation, peritoneal growth and expression patterns. Biomaterials 2019;190-191:63–75. [DOI] [PubMed] [Google Scholar]
- Lõhmussaar K, Oka R, Espejo Valle-Inclan J, Smits MHH, Wardak H, Korving J, Begthel H, Proost N, van de Ven M, Kranenburg OW et al Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 2021;28:1380–1396. e6. [DOI] [PubMed] [Google Scholar]
- López-Martínez S, Campo H, de Miguel-Gómez L, Faus A, Navarro AT, Díaz A, Pellicer A, Ferrero H, Cervelló I.. A natural xenogeneic endometrial extracellular matrix hydrogel toward improving current human in vitro models and future in vivo applications. Front Bioeng Biotechnol 2021a;9:639688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martínez S, Rodríguez-Eguren A, de Miguel-Gómez L, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Ferrero H, Cervelló I.. Bioengineered endometrial hydrogels with growth factors promote tissue regeneration and restore fertility in murine models. Acta Biomater 2021b;135:113–125. [DOI] [PubMed] [Google Scholar]
- Luddi A, Pavone V, Semplici B, Governini L, Criscuoli M, Paccagnini E, Gentile M, Morgante G, Leo V, Belmonte G. et al. Organoids of human endometrium: a powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells 2020;9:1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx V, Dolmans M-M, Vanacker J, Legat C, Fortuño Moya C, Donnez J, Amorim CA.. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril 2014;101:1149–1156. [DOI] [PubMed] [Google Scholar]
- Luyckx V, Dolmans M-M, Vanacker J, Scalercio SR, Donnez J, Amorim CA.. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J Ovarian Res 2013;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang Y, Chen J, Li L, Liu X, Zhang L, Ma C, Wang Y, Tian W, Song X. et al. Mesenchymal stem cell-based bioengineered constructs enhance vaginal repair in ovariectomized rhesus monkeys. Biomaterials 2021;275:120863. [DOI] [PubMed] [Google Scholar]
- Maenhoudt N, Defraye C, Boretto M, Jan Z, Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen E. et al. Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Reports 2020;14:717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A.. A tissue-engineered uterus supports live births in rabbits. Nat Biotechnol 2020;38:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen R, Shufaro Y, Daykan Y, Oron G, Tararashkina E, Levenberg S, Anuka E, Ben-Haroush A, Fisch B, Abir R.. Use of simvastatin, fibrin clots, and their combination to improve human ovarian tissue grafting for fertility restoration after anti-cancer therapy. Front Oncol 2020;10:598026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel K, Reddy M, Xu Q, Chattopadhyay S, Cone R, Ensign LM, Hanes J.. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine (Lond) 2016;11:1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man L, Park L, Bodine R, Ginsberg M, Zaninovic N, Man OA, Schattman G, Rosenwaks Z, James D.. Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci Rep 2017;7:8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavella DD, Cacciottola L, Desmet CM, Jordan BF, Donnez J, Amorim CA, Dolmans MM.. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: a potential way to improve ovarian tissue transplantation. Hum Reprod Oxf Engl 2018;33:270–279. [DOI] [PubMed] [Google Scholar]
- Marinić M, Rana S, Lynch VJ.. Derivation of endometrial gland organoids from term placenta. Placenta 2020;101:75–79. [DOI] [PubMed] [Google Scholar]
- Maru Y, Kawata A, Taguchi A, Ishii Y, Baba S, Mori M, Nagamatsu T, Oda K, Kukimoto I, Osuga Y. et al. Establishment and molecular phenotyping of organoids from the squamocolumnar junction region of the uterine cervix. Cancers 2020;12:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y, Tanaka N, Itami M, Hippo Y.. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol Oncol 2019;154:189–198. [DOI] [PubMed] [Google Scholar]
- Mastrorocco A, Cacopardo L, Martino NA, Fanelli D, Camillo F, Ciani E, Roelen BAJ, Ahluwalia A, Dell’Aquila ME.. One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitro maturation. PLoS One 2020;15:e0238812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon KE, Sensharma R, Williams C, Ravix J, Getsios S, Woodruff TK.. Development of human ectocervical tissue models with physiologic endocrine and paracrine signaling†. Biol Reprod 2020;103:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLemore R, Kim E-J, Brandon TA, Aerni G, Roy KH, Vernon BL.. Tubal sterilization with a waterborne polyethylene glycol in situ cross-linking material: a minimally invasive approach. Fertil Steril 2005;83(Suppl 1):1284–1292. [DOI] [PubMed] [Google Scholar]
- Mehdinia Z, Ashrafi M, Fathi R, Taheri P, Valojerdi MR.. Restoration of estrous cycles by co-transplantation of mouse ovarian tissue with MSCs. Cell Tissue Res 2020;381:509–525. [DOI] [PubMed] [Google Scholar]
- Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR.. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil Steril 2004;82:398–404. [DOI] [PubMed] [Google Scholar]
- Mettler L, Hucke J, Bojahr B, Tinneberg H-R, Leyland N, Avelar R.. A safety and efficacy study of a resorbable hydrogel for reduction of post-operative adhesions following myomectomy. Hum Reprod 2008;23:1093–1100. [DOI] [PubMed] [Google Scholar]
- Miki F, Maruyama T, Miyazaki K, Takao T, Yoshimasa Y, Katakura S, Hihara H, Uchida S, Masuda H, Uchida H. et al. The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in rats†. Biol Reprod 2019;100:1215–1227. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Maruyama T.. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 2014;35:8791–8800. [DOI] [PubMed] [Google Scholar]
- Motamed M, Sadr Z, Valojerdi MR, Moini A, Oryan S, Totonchi M, Ebrahimi B, Maroufizadeh S, Taghiabadi E, Fathi R.. Tissue engineered human amniotic membrane application in mouse ovarian follicular culture. Ann Biomed Eng 2017;45:1664–1675. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Darzi S, Rosamilia A, Kadam V, Truong Y, Werkmeister JA, Gargett CE.. Blended nanostructured degradable mesh with endometrial mesenchymal stem cells promotes tissue integration and anti-inflammatory response in vivo for Pelvic Floor application. Biomacromolecules 2019;20:454–468. [DOI] [PubMed] [Google Scholar]
- Müller SA, Weis C, Odermatt EK, Knaebel H-P, Wente MN.. A hydrogel for adhesion prevention: characterization and efficacy study in a rabbit uterus model. Eur J Obstet Gynecol Reprod Biol 2011;158:67–71. [DOI] [PubMed] [Google Scholar]
- Murphy AR, Wiwatpanit T, Lu Z, Davaadelger B, Kim JJ.. Generation of multicellular human primary endometrial organoids. J Vis Exp 2019. doi: 10.3791/60384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandan S, Fan X, Dhal S, Nayak NR.. Development of A 3D tissue slice culture model for the study of human endometrial repair and regeneration. Biomolecules 2020;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima JB, El Assal R, Songsasen N, Demirci U.. Evaluation of an ovary-on-a-chip in large mammalian models: species specificity and influence of follicle isolation status. J Tissue Eng Regen Med 2018;12:e1926–e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F. et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep 2020;10:12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikniaz H, Zandieh Z, Nouri M, Daei-farshbaf N, Aflatoonian R, Gholipourmalekabadi M, Jameie SB.. Comparing various protocols of human and bovine ovarian tissue decellularization to prepare extracellular matrix-alginate scaffold for better follicle development in vitro. BMC Biotechnol 2021;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Nakatsuka M, Sugiyama Y, Chekir C, Kamada Y, Hiramatsu Y.. Use of artificial dermis and recombinant basic fibroblast growth factor for creating a neovagina in a patient with Mayer-Rokitansky-Kuster-Hauser syndrome. Hum Reprod 2004;19:1629–1632. [DOI] [PubMed] [Google Scholar]
- Nomura K, Murakami K, Shozu M, Nakama T, Yui N, Inoue M.. Local application of danazol-loaded hyaluronic acid hydrogel to endometriosis in a rat model. Fertil Steril 2006;85(Suppl 1):1157–1167. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V.. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol 2016;214:94.e1–9–94.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ.. Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod 2017;96:971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E, Alves-Lopes JP, Harteveld F, Mitchell RT, Åkesson E, Söder O, Stukenborg J-B.. Self-organising human gonads generated by a Matrigel-based gradient system. BMC Biol 2021;19:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabi H, Saba I, Rousseau A, Bolduc S.. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl Res 2017;180:22–36. [DOI] [PubMed] [Google Scholar]
- Ovsianikov A, Schlie S, Ngezahayo A, Haverich A, Chichkov BN.. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J Tissue Eng Regen Med 2007;1:443–449. [DOI] [PubMed] [Google Scholar]
- Padma AM, Alshaikh AB, Song MJ, Akouri R, Akyürek LM, Oltean M, Brännström M, Hellström M.. Immune response after allogeneic transplantation of decellularized uterine scaffolds in the rat. Biomed Mater Bristol Engl 2021. a;16. [DOI] [PubMed] [Google Scholar]
- Padma AM, Alshaikh AB, Song MJ, Akouri R, Oltean M, Brännström M, Hellström M.. Decellularization protocol-dependent damage-associated molecular patterns in rat uterus scaffolds differentially affect the immune response after transplantation. J Tissue Eng Regen Med 2021b;15:674–685. [DOI] [PubMed] [Google Scholar]
- Padma AM, Carrière L, Krokström Karlsson F, Sehic E, Bandstein S, Tiemann TT, Oltean M, Song MJ, Brännström M, Hellström M.. Towards a bioengineered uterus: bioactive sheep uterus scaffolds are effectively recellularized by enzymatic preconditioning. NPJ Regen Med 2021c;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Wu B, Griffith LG.. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J Biomater Sci Polym Ed 1998;9:89–110. [DOI] [PubMed] [Google Scholar]
- Park DW, Choi DS, Ryu H-S, Kwon HC, Joo H, Min CK.. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett 2003;195:185–192. [DOI] [PubMed] [Google Scholar]
- Park S-R, Kim S-R, Im JB, Park CH, Lee H-Y, Hong I-S.. 3D stem cell-laden artificial endometrium: successful endometrial regeneration and pregnancy. Biofabrication 2021;13:045012. [DOI] [PubMed] [Google Scholar]
- Park SR, Kim SR, Lee JW, Park CH, Yu WJ, Lee SJ, Chon SJ, Lee DH, Hong IS.. Development of a novel dual reproductive organ on a chip: recapitulating bidirectional endocrine crosstalk between the uterine endometrium and the ovary. Biofabrication 2020;13:015001. [DOI] [PubMed] [Google Scholar]
- Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD.. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 2011;142:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulini F, Vilela JMV, Chiti MC, Donnez J, Jadoul P, Dolmans M-M, Amorim CA.. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod Biomed Online 2016;33:425–432. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M.. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- Pence JC, Clancy KBH, Harley BAC.. The induction of pro-angiogenic processes within a collagen scaffold via exogenous estradiol and endometrial epithelial cells. Biotechnol Bioeng 2015;112:2185–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence JC, Clancy KBH, Harley BAC.. Proangiogenic activity of endometrial epithelial and stromal cells in response to estradiol in gelatin hydrogels. Adv Biosys 2017;1:1700056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarossa G, De Iorio T, Gandolfi F, Brevini TAL.. Ovarian decellularized bioscaffolds provide an optimal microenvironment for cell growth and differentiation in vitro. Cells 2021a;10:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TAL.. Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering. J Assist Reprod Genet 2020;37:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TAL.. Creation of a bioengineered ovary: isolation of female germline stem cells for the repopulation of a decellularized ovarian bioscaffold. Methods Mol Biol 2021b;2273:139–149. [DOI] [PubMed] [Google Scholar]
- Pensabene V, Patel PP, Williams P, Cooper TL, Kirkbride KC, Giorgio TD, Tulipan NB.. Repairing fetal membranes with a self-adhesive ultrathin polymeric film: evaluation in mid-gestational rabbit model. Ann Biomed Eng 2015;43:1978–1988. [DOI] [PubMed] [Google Scholar]
- Phan N, Hong JJ, Tofig B, Mapua M, Elashoff D, Moatamed NA, Huang J, Memarzadeh S, Damoiseaux R, Soragni A.. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun Biol 2019;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud L, Thibault B, Mery E, Ouali M, Martinez A, Delord J-P, Couderc B, Ferron G.. Evaluation of the effects of hyaluronic acid-carboxymethyl cellulose barrier on ovarian tumor progression. J Ovarian Res 2014;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pors SE, Ramløse M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, Kristensen SG.. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod 2019;34:1523–1535. [DOI] [PubMed] [Google Scholar]
- Raia NR, Bakaysa SL, Ghezzi CE, House MD, Kaplan DL.. Ex vivo pregnant-like tissue model to assess injectable hydrogel for preterm birth prevention. J Biomed Mater Res B Appl Biomater 2020;108:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja MA, Maldonado M, Chen J, Zhong Y, Gu J.. Development and evaluation of curcumin encapsulated self-assembled nanoparticles as potential remedial treatment for PCOS in a female rat model. Int J Nanomed 2021;16:6231–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi Z, Yazdekhasti H, Noori Mugahi SMH, Abbasi M, Kazemnejad S, Shirazi A, Majidi M, Zarnani A-H.. Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod Biol 2018;18:122–131. [DOI] [PubMed] [Google Scholar]
- Ran R, Wang H-F, Hou F, Liu Y, Hui Y, Petrovsky N, Zhang F, Zhao C-X.. A microfluidic tumor-on-a-chip for assessing multifunctional liposomes’ tumor targeting and anticancer efficacy. Adv Healthc Mater 2019;8:e1900015. [DOI] [PubMed] [Google Scholar]
- Rawlings TM, Makwana K, Taylor DM, Molè MA, Fishwick KJ, Tryfonos M, Odendaal J, Hawkes A, Zernicka-Goetz M, Hartshorne GM. et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021;10:e69603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A.. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 2014;384:329–336. [DOI] [PubMed] [Google Scholar]
- Richardson SA, Rawlings TM, Muter J, Walker M, Brosens JJ, Cameron NR, Eissa AM.. Covalent attachment of fibronectin onto emulsion-templated porous polymer scaffolds enhances human endometrial stromal cell adhesion, infiltration, and function. Macromol Biosci 2019;19:e1800351. [DOI] [PubMed] [Google Scholar]
- Rinehart CA, Lyn-Cook BD, Kaufman DG.. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol 1988;24:1037–1041. [DOI] [PubMed] [Google Scholar]
- Rios PD, Kniazeva E, Lee HC, Xiao S, Oakes RS, Saito E, Jeruss JS, Shikanov A, Woodruff TK, Shea LD.. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng 2018;115:2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M, Gatmaitan Z, Mackensen S, Giambrone MA, Ponce P, Reid LM.. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol 1980;87:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman S, Hillary C, Narice B, Bullock AJ, Anumba DO, MacNeil S.. Visualisation of the insertion of a membrane for the treatment of preterm rupture of fetal membranes using a synthetic model of a pregnant uterus. J Biomater Appl 2018;33:234–244. [DOI] [PubMed] [Google Scholar]
- Rose IM, Bidarimath M, Webster A, Godwin AK, Flesken-Nikitin A, Nikitin AY.. WNT and inflammatory signaling distinguish human Fallopian tube epithelial cell populations. Sci Rep 2020;10:9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghzadeh Oskouei B, Pashaiasl M, Heidari MH, Salehi M, Veladi H, G, Pakdel F, Shahabi P, Novin MG.. Evaluation of mouse oocyte in vitro maturation developmental competency in dynamic culture systems by design and construction of a lab on a chip device and its comparison with conventional culture system. Cell J 2016;18:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Mathur T, Handley KF, Hu W, Afshar-Kharghan V, Sood AK, Jain A.. OvCa-Chip microsystem recreates vascular endothelium–mediated platelet extravasation in ovarian cancer. Blood Adv 2020;4:3329–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas A, López J, Reyes R, Évora C, de Oca FM, Báez D, Delgado A, Almeida TA.. Organotypic culture as a research and preclinical model to study uterine leiomyomas. Sci Rep 2020;10:5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, Osuga Y, Saito S, Ushida T, Furukawa KS.. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One 2014;9:e103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargazi Z, Zavareh S, Jafarabadi M, Salehnia M.. An efficient protocol for decellularization of the human endometrial fragments for clinical usage. Prog Biomater 2021;10:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte SC, James CO, Sidell N, Taylor RN.. Tissue-engineered endometrial model for the study of cell-cell interactions. Reprod Sci 2015;22:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte SC, Taylor RN.. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril 2012;97:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Keye WR, Poulson AM, Reynolds WA.. ovarian transplantation in the primate. Fertil Steril 1981;36:512–515. [DOI] [PubMed] [Google Scholar]
- Scsukova S, Mlynarcikova AB, Rollerova E.. The use of ex vivo ovary culture for assessment of alterations in steroidogenesis following neonatal exposure to poly(ethylene glycol)-block-polylactide methyl ether or titanium dioxide nanoparticles in Wistar rats. Endocr Regul 2020;54:53–63. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Mason RJ, Jennings SD.. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim Biophys Acta 1987;931:143–156. [DOI] [PubMed] [Google Scholar]
- Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD.. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011a;32:2524–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD.. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009;30:5476–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD.. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp 2011b;(49):2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD.. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A 2011c;17:3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Kwon GS.. Pre-clinical evaluation of a themosensitive gel containing epothilone B and mTOR/Hsp90 targeted agents in an ovarian tumor model. J Control Release 2017;268:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E-Y, Kim D-S, Lee MJ, Lee AR, Shim SH, Baek SW, Han DK, Lee DR.. Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells. Stem Cell Res Ther 2021;12:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistani MN, Zavareh S, Valujerdi MR, Salehnia M.. Characteristics of a decellularized human ovarian tissue created by combined protocols and its interaction with human endometrial mesenchymal cells. Prog Biomater 2021;10:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittadjody S, Saul JM, McQuilling JP, Joo S, Register TC, Yoo JJ, Atala A, Opara EC.. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun 2017;8:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory RM, Xu Y, Shea LD, Woodruff TK.. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod 2015;30:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD.. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A 2014;20:3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M, Saussoy P, Sahrari K, Amorim CA, Donnez J, Dolmans M-M.. Is transplantation of a few leukemic cells inside an artificial ovary able to induce leukemia in an experimental model? J Assist Reprod Genet 2015;32:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Cai G, Liang J, Ao D, Wang H, Yang Z.. Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials. J Nanobiotechnology 2020;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Zhao X, Sun H, Li X, Lin N, Ding L, Dai J, Hu Y.. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng Part A 2015;21:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songsasen N, Woodruff TK, Wildt DE.. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction 2011;142:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza GR, Tseng H, Gage JA, Mani A, Desai P, Leonard F, Liao A, Longo M, Refuerzo JS, Godin B.. Magnetically bioprinted human myometrial 3D cell rings as a model for uterine contractility. Int J Mol Sci 2017;18:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreus-Evers A, Hovatta O, Eriksson H, Landgren B-M.. Development and characterization of an endometrial tissue culture system. Reprod Biomed Online 2003;7:243–249. [DOI] [PubMed] [Google Scholar]
- Stefanelli A, Zacchei A, Ceccherini V.. Retinal reconstitution in vitro after disaggregation of embryonic chicken eyes. Acta Embryol Morphol Exper 1961;4:47–55. [Google Scholar]
- Stejskalová A, Fincke V, Nowak M, Schmidt Y, Borrmann K, von Wahlde M-K, Schäfer SD, Kiesel L, Greve B, Götte M.. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci Rep 2021;11:4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Tal D, Achache H, Jacobs Catane L, Reich R, Tavor Re'em T.. Tavor Re’em T. Novel 3D embryo implantation model within macroporous alginate scaffolds. J Biol Eng 2020;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Ding L, Cheng J, Yang J, Li X, Yan G, Sun H, Dai J, Hu Y.. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod 2016;31:1075–1086. [DOI] [PubMed] [Google Scholar]
- Su K, Edwards SL, Tan KS, White JF, Kandel S, Ramshaw JAM, Gargett CE, Werkmeister JA.. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater 2014;10:5012–5020. [DOI] [PubMed] [Google Scholar]
- Subramanian B, Rameshbabu AP, Ghosh K, Jha PK, Jha R, Murugesan S, Chattopadhyay S, Dhara S, Mondal KC, Basak P. et al. Impact of styrene maleic anhydride (SMA) based hydrogel on rat fallopian tube as contraceptive implant with selective antimicrobial property. Mater Sci Eng C Mater Biol Appl 2019;94:94–107. [DOI] [PubMed] [Google Scholar]
- Sun H, Lu J, Li B, Chen S, Xiao X, Wang J, Wang J, Wang X.. Partial regeneration of uterine horns in rats through adipose-derived stem cell sheets. Biol Reprod 2018;99:1057–1069. [DOI] [PubMed] [Google Scholar]
- Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD.. Supplemented αMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng 2013;110:3258–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Makanji Y, Tu T, Bernabé BP, Lee R, Zhu J, Kniazeva E, Hornick JE, Woodruff TK, Shea LD.. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng 2014;111:1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, Shea LD.. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A 2012;18:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri MA, Valojerdi MR, Ebrahimi B.. Intramuscular autotransplantation of vitrified rat ovary encapsulated with hyaluronic acid hydrogel. Biopreserv Biobank 2016;14:114–121. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Nakamura H, Tabata Y, Fujimori Y, Kumasawa K, Kimura T.. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J Obstet Gynaecol Res 2018;44:1947–1955. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kondo J, Kaneko K, Endo H, Onuma K, Coppo R, Masuda M, Kamiura S, Yoshino K, Ueda Y. et al. Heterogenous chemosensitivity of a panel of organoid lines derived from small cell neuroendocrine carcinoma of the uterine cervix. Hum Cell 2021;34:889–900. [DOI] [PubMed] [Google Scholar]
- Tantengco OAG, Richardson LS, Medina PMB, Han A, Menon R.. Organ-on-chip of the cervical epithelial layer: a platform to study normal and pathological cellular remodeling of the cervix. FASEB J 2021;35:e21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavana S, Azarnia M, Valojerdi MR, Shahverdi A.. Hyaluronic acid-based hydrogel scaffold without angiogenic growth factors enhances ovarian tissue function after autotransplantation in rats. Biomed Mater 2016a;11:055006. [DOI] [PubMed] [Google Scholar]
- Tavana S, Valojerdi MR, Azarnia M, Shahverdi A.. Restoration of ovarian tissue function and estrous cycle in rat after autotransplantation using hyaluronic acid hydrogel scaffold containing VEGF and bFGF. Growth Factors 2016b;34:97–106. [DOI] [PubMed] [Google Scholar]
- Tentor F, Siccardi G, Sacco P, Demarchi D, Marsich E, Almdal K, Bose Goswami S, Boisen A.. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery. J Mater Sci Mater Med. 2020;31:25. [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Cannon JH, Sahler OD, Ferrebee JW.. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest 1959;38:1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiboni M, Campana R, Frangipani E, Casettari L.. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int J Pharm 2021;596:120290. [DOI] [PubMed] [Google Scholar]
- Tiemann TT, Padma AM, Sehic E, Bäckdahl H, Oltean M, Song MJ, Brännström M, Hellström M.. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol Hum Reprod 2020;26:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski CE, DiLillo KM, Baker BM, Arnold KB, Shikanov A.. Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation. Acta Biomater 2021;132:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew GH, Pistofidis GA, Brucker SY, Krämer B, Ziegler NM, Korell M, Ritter H, McConnachie A, Ford I, Crowe AM. et al. A first-in-human, randomized, controlled, subject- and reviewer-blinded multicenter study of ActamaxTM Adhesion Barrier. Arch Gynecol Obstet 2017;295:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017;19:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul-Islam M, Subhan F, Islam SU, Khan S, Shah N, Manan S, Ullah MW, Yang G.. Development of three-dimensional bacterial cellulose/chitosan scaffolds: analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int J Biol Macromol 2019;137:1050–1059. [DOI] [PubMed] [Google Scholar]
- Ulstein M. Evaluation of a capillary tube sperm penetration method for fertility investigations. Acta Obstet Gynecol Scand 1972;51:287–292. [DOI] [PubMed] [Google Scholar]
- Vanacker J, Luyckx V, Amorim C, Dolmans M-M, Van Langendonckt A, Donnez J, Camboni A.. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril 2013;99:1363–1368. e2. [DOI] [PubMed] [Google Scholar]
- Vanacker J, Luyckx V, Dolmans M-M, Des Rieux A, Jaeger J, Van Langendonckt A, Donnez J, Amorim CA.. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012;33:6079–6085. [DOI] [PubMed] [Google Scholar]
- Wang B, Feng C, Dang J, Zhu Y, Yang X, Zhang T, Zhang R, Li J, Tang J, Shen C. et al. Preparation of fibroblast suppressive poly(ethylene glycol)-b-poly(l-phenylalanine)/poly(ethylene glycol) hydrogel and its application in intrauterine fibrosis prevention. ACS Biomater Sci Eng 2021;7:311–321. [DOI] [PubMed] [Google Scholar]
- Wang C-H, Weng C-H, Che Y-J, Wang K, Lee G-B.. Cancer cell-specific oligopeptides selected by an integrated microfluidic system from a phage display library for ovarian cancer diagnosis. Theranostics 2015;5:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bocca S, Anderson S, Yu L, Rhavi BS, Horcajadas J, Oehninger S.. Sex steroids regulate epithelial-stromal cell cross talk and trophoblast attachment invasion in a three-dimensional human endometrial culture system. Tissue Eng Part C Methods 2013;19:676–687. [DOI] [PubMed] [Google Scholar]
- Wang H, Pilla F, Anderson S, Martínez-Escribano S, Herrer I, Moreno-Moya JM, Musti S, Bocca S, Oehninger S, Horcajadas JA.. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol Hum Reprod 2012;18:33–43. [DOI] [PubMed] [Google Scholar]
- Ward Rashidi MR, Mehta P, Bregenzer M, Raghavan S, Fleck EM, Horst EN, Harissa Z, Ravikumar V, Brady S, Bild A. et al. Engineered 3D model of cancer stem cell enrichment and chemoresistance. Neoplasia 2019;21:822–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhang C, Fan G, Meng L.. Organoids as novel models for embryo implantation study. Reprod Sci 2021;28:1637–1643. [DOI] [PubMed] [Google Scholar]
- Weiss P, Taylor AC.. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proc Natl Acad Sci U S A 1960;46:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenbo Q, Lijian X, Shuangdan Z, Jiahua Z, Yanpeng T, Xuejun Q, Xianghua H, Jingkun Z.. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int J Biol Macromol 2020;143:163–172. [DOI] [PubMed] [Google Scholar]
- Weng L, Lee GY, Liu J, Kapur R, Toth TL, Toner M.. On-chip oocyte denudation from cumulus-oocyte complexes for assisted reproductive therapy. Lab Chip 2018;18:3892–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD.. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007;28:4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD.. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod 2009;80:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston RM, Browne JC.. Pregnancy following autograft transplantation of Fallopian tube and ovary in the rabbit. Lancet 1974;2:494–495. [DOI] [PubMed] [Google Scholar]
- de Witte CJ, Espejo Valle-Inclan J, Hami N, Lõhmussaar K, Kopper O, Vreuls CPH, Jonges GN, van Diest P, Nguyen L, Clevers H et al Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep 2020;31:107762. [DOI] [PubMed] [Google Scholar]
- Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, Kim JJ.. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab 2020;105:dgz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Gao YY, Su J, Tang XN, Chen Q, Ma LW, Zhang JJ, Wu JM, Wang SX.. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022;25:170–178. [DOI] [PubMed] [Google Scholar]
- Xia Y, Whitesides GM.. Soft Lithography. Angew Chem Int Ed Engl 1998;37:550–575. [DOI] [PubMed] [Google Scholar]
- Xiao B, Yang W, Lei D, Huang J, Yin Y, Zhu Y, You Z, Wang F, Sun S.. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv Healthc Mater 2019;8:e1801455. [DOI] [PubMed] [Google Scholar]
- Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017;8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK.. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 2015;5:17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Park E-S, Xiang D, Li Z.. Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube. Stem Cell Res 2018;32:51–60. [DOI] [PubMed] [Google Scholar]
- Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, Ma L, Gao C, Zhang S.. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater 2019;92:160–171. [DOI] [PubMed] [Google Scholar]
- Xu B, Cao Y, Zheng Z, Galan EA, Hu Z, Ge J, Xing X, Ma S.. Injectable mesenchymal stem cell-laden matrigel microspheres for endometrium repair and regeneration. Adv Biol 2021;5:e2000202. [DOI] [PubMed] [Google Scholar]
- Xu G, Lin G, Lin S, Wu N, Deng Y, Feng G, Chen Q, Qu J, Chen D, Chen S. et al. The reproductive toxicity of CdSe/ZnS quantum dots on the in vivo ovarian function and in vitro fertilization. Sci Rep 2016;6:37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H-L, Xu J, Shen B-X, Zhang S-S, Jin B-H, Zhu Q-Y, ZhuGe D-L, Wu X-Q, Xiao J, Zhao Y-Z.. Dual regulations of thermosensitive heparin–poloxamer hydrogel using ε-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl Mater Interfaces 2017a;9:29580–29594. [DOI] [PubMed] [Google Scholar]
- Xu H-L, Xu J, Zhang S-S, Zhu Q-Y, Jin B-H, ZhuGe D-L, Shen B-X, Wu X-Q, Xiao J, Zhao Y-Z.. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv 2017b;24:867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ding L, Wang L, Cao Y, Zhu H, Lu J, Li X, Song T, Hu Y, Dai J.. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther 2017c;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD.. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng 2009a;103:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK.. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009b;24:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK.. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006;12:2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB.. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009c;81:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Du X, Feng G, Zhang Y, Li J, Lin B, Yang L, Fu S, Wu J.. Efficient inhibition of cervical cancer by dual drugs loaded in biodegradable thermosensitive hydrogel composites. Oncotarget 2018;9:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y.. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chem, Rapid Commun 1990;11:571–576. [Google Scholar]
- Yamaoka T, Takahashi Y, Fujisato T, Lee CW, Tsuji T, Ohta T, Murakami A, Kimura Y.. Novel adhesion prevention membrane based on a bioresorbable copoly(ester-ether) comprised of poly-L-lactide and Pluronic: in vitro and in vivo evaluations. J Biomed Mater Res 2001;54:470–479. [DOI] [PubMed] [Google Scholar]
- Yan E, Cao M, Wang Y, Hao X, Pei S, Gao J, Wang Y, Zhang Z, Zhang D.. Gold nanorods contained polyvinyl alcohol/chitosan nanofiber matrix for cell imaging and drug delivery. Mater Sci Eng C Mater Biol Appl 2016;58:1090–1097. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu S, Feng R, Huang J, Liu L, Liu F, Chen Y.. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res Ther 2017;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I-H, Lin I-E, Chen T-C, Chen Z-Y, Kuan C-Y, Lin J-N, Chou Y-C, Lin F-H.. Synthesis, characterization, and evaluation of BDDE crosslinked chitosan-TGA hydrogel encapsulated with genistein for vaginal atrophy. Carbohydr Polym 2021;260:117832. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lei L, Wang S, Sheng X, Yan G, Xu L, Liu J, Liu M, Zhen X, Ding L. et al. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell Dev Biol Anim 2019;55:302–311. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu S, Chen C, Huang H, Tao L, Qian Z, Li W.. Microfluidic-enabled self-organized tumor model for in vitro cytotoxicity assessment of doxorubicin. Biomed Microdev 2020;22:70. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF.. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A 1989;86:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Zheng Y-W, Lan Q-H, Wang L-F, Huang Z-W, Chen R, Yang Y, Xu H-L, Kou L, Zhao Y-Z.. Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur J Pharm Sci 2020a;148:105316. [DOI] [PubMed] [Google Scholar]
- Yao Q, Zheng Y-W, Lin H-L, Lan Q-H, Huang Z-W, Wang L-F, Chen R, Xiao J, Kou L, Xu H-L. et al. Exploiting crosslinked decellularized matrix to achieve uterus regeneration and construction. Artif Cells Nanomed Biotechnol 2020b;48:218–229. [DOI] [PubMed] [Google Scholar]
- Ye M, Yu L, She Y, Wang S, Wang M, Zhao Q, Gu C, Bian L, Wen N, Gong J. et al. Healing effects of a protein scaffold loaded with adipose-derived mesenchymal stem cells on radiation-induced vaginal injury in rats. J Int Med Res 2020;48:300060520958826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H-J, Lee YJ, Baek S, Chung YS, Kim D-H, Lee JH, Shin YC, Shin YM, Ryu C, Kim H-S. et al. Hormone autocrination by vascularized hydrogel delivery of ovary spheroids to rescue ovarian dysfunctions. Sci Adv 2021;7:eabe8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Liu S, Dong X, Li H, Zhu Y, Hu L.. Intravaginal administration of human type III collagen-derived biomaterial with high cell-adhesion activity to treat vaginal atrophy in rats. ACS Biomater Sci Eng 2020;6:1977–1988. [DOI] [PubMed] [Google Scholar]
- Young RC, Goloman G.. Allo- and Xeno-reassembly of human and rat myometrium from cells and scaffolds. Tissue Eng Part A 2013;19:2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Schumann R, Zhang P.. Three-dimensional culture of human uterine smooth muscle myocytes on a resorbable scaffolding. Tissue Eng 2003;9:451–459. [DOI] [PubMed] [Google Scholar]
- Younis AJ, Lerer-Serfaty G, Stav D, Sabbah B, Shochat T, Kessler-Icekson G, Zahalka MA, Shachar-Goldenberg M, Ben-Haroush A, Fisch B. et al. Extracellular-like matrices and leukaemia inhibitory factor for in vitro culture of human primordial follicles. Reprod Fertil Dev 2017;29:1982–1994. [DOI] [PubMed] [Google Scholar]
- Yucer N, Holzapfel M, Jenkins Vogel T, Lenaeus L, Ornelas L, Laury A, Sareen D, Barrett R, Karlan BY, Svendsen CN.. Directed differentiation of human induced pluripotent stem cells into fallopian tube epithelium. Sci Rep 2017;7:10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang Q, Zhang J, Sheng F, Wu S, Yang F, Li W.. Urinary bladder matrix scaffolds improve endometrial regeneration in a rat model of intrauterine adhesions. Biomater Sci 2020a;8:988–996. [DOI] [PubMed] [Google Scholar]
- Zhang JK, Du RX, Zhang L, Li YN, Zhang ML, Zhao S, Huang XH, Xu YF.. A new material for tissue engineered vagina reconstruction: acellular porcine vagina matrix. J Biomed Mater Res A 2017a;105:1949–1959. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhou X, He M, Shang Y, Tetlow AL, Godwin AK, Zeng Y.. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng 2019a;3:438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Iyer S, Ran H, Dolgalev I, Gu S, Wei W, Foster CJR, Loomis CA, Olvera N, Dao F. et al. Genetically defined, syngeneic organoid platform for developing combination therapies for ovarian cancer. Cancer Discov 2021a;11:362–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-S, Xia W-T, Xu J, Xu H-L, Lu C-T, Zhao Y-Z, Wu X-Q.. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17β-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int J Nanomedicine 2017b;12:5643–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-S, Xu X-X, Xiang W-W, Zhang H-H, Lin H-L, Shen L-E, Lin Q, Lin F, Zhou Z-Y.. Using 17β-estradiol heparin-poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. FASEB J 2020b;34:446–457. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ding Y, Hua K, Liu S, Jia N.. Combined laparoscopic and vaginal cervicovaginal reconstruction using acellular porcine small intestinal submucosa graft in a patient with Mayer-Rokitansky-Küster-Hauser Syndrome (U5aC4V4). J Minim Invasive Gynecol 2019b;26:396–397. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang L, Tian Y, Xia Y, Yan L, Wu C, Zhang T, Zhu J.. Establishment of in-vitro three dimensional rat follicle culture system and validation of the applicability as an in vitro female reproductive toxicity testing system. Toxicol in Vitro 2019c;58:161–169. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu Z, Yang Y, Yao Y, Tao Y.. The clinical outcomes of vaginoplasty using tissue-engineered biomaterial mesh in patients with Mayer-Rokitansky-Küster-Hauser syndrome. Int J Surg 2017c;44:9–14. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi L, Lin X, Zhou F, Xin L, Xu W, Yu H, Li J, Pan M, Pan Y. et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther 2021b;12:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang Z, Hua C, Ji J, Zhou Z, Fang Y, Weng D, Lu L, Pang Y, Sun W.. Design, modeling and 3D printing of a personalized cervix tissue implant with protein release function. Biomed Mater 2020;15:045005. [DOI] [PubMed] [Google Scholar]
- Zhao G, Cao Y, Zhu X, Tang X, Ding L, Sun H, Li J, Li X, Dai C, Ru T. et al. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating ΔNp63 expression in Asherman’s syndrome. Sci China Life Sci 2017;60:404–416. [DOI] [PubMed] [Google Scholar]
- Zheng L, Hu X, Huang Y, Xu G, Yang J, Li L.. In vivo bioengineered ovarian tumors based on collagen, matrigel, alginate and agarose hydrogels: a comparative study. Biomed Mater 2015;10:015016. [DOI] [PubMed] [Google Scholar]
- Zhou H, Malik MA, Arab A, Hill MT, Shikanov A.. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS One 2015;10:e0140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Hu K, Guo Z, Zhang Q, Chen J, Zhang T, Gu N.. Thermo-sensitive PLGA-PEG-PLGA tri-block copolymer hydrogel as three-dimensional cell culture matrix for ovarian cancer cells. J Nanosci Nanotechnol 2018;18:5252–5255. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhou J, Xu X, Du F, Nie M, Hu L, Ma Y, Liu M, Yu S, Zhang J. et al. Matrigel/umbilical cord-derived mesenchymal stem cells promote granulosa cell proliferation and ovarian vascularization in a mouse model of premature ovarian failure. Stem Cells Dev 2021;30:782–796. [DOI] [PubMed] [Google Scholar]
- Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE.. Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod 2016;22:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yang Y, Guo J, Dai Y, Ye L, Qiu J, Zeng Z, Wu X, Xing Y, Long X. et al. Ex vivo 2D and 3D HSV-2 infection model using human normal vaginal epithelial cells. Oncotarget 2017;8:15267–15282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk K, Wen A, Dilworth X, Li S, Ghali D.. L. Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J Biomed Res 2017;31:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.