Abstract
The human gut virome is comprised of diverse commensal and pathogenic viruses. The colonization by these viruses begins right after birth through vaginal delivery, then continues through breastfeeding, and broader environmental exposure. Their constant interaction with their bacterial hosts in the body shapes not only our microbiomes but us. In addition, these viruses interact with the immune cells, trigger a broad range of immune responses, and influence different metabolic pathways. Besides its key role in regulating the human gut homeostasis, the intestinal virome contributes to disease development in distant organs, both directly and indirectly. In this review, we will describe the changes in the gut virome through life, health, and disease, followed by discussing the interactions between the virome, the microbiome, and the human host as well as providing an overview of their contribution to gut disease and disease of distant organs.
Keywords: virome, microbiome, gastrointestinal tract, phageome, immune response, Auxiliary metabolic genes
Interactions between virome, microbiome, and the human host, and their contribution to gut disease and beyond.
Introduction
The human virome is the assembly of viruses that can be found in the human body (Zarate et al. 2018). These viruses can be divided into eukaryotic viruses, which mostly infect human cells; prokaryotic viruses, otherwise known as bacteriophages or phages (Zarate et al. 2018); and archaeal viruses (Matijašić et al. 2020) (Table 1). Most archaeal viruses isolated so far have been double-stranded DNA (dsDNA) viruses (Prangishvili, Forterre and Garrett 2006). The interactions between archaeal viruses and their hosts as well as their impact on human health are still largely unexplored (Matijašić et al. 2020), and thus, will not be further discussed in this review. Eukaryotic viruses inhabiting the human body are the most studied compared to other members of the virome, as organisms that cause disease, and thus play a more obvious role in human health (Zarate et al. 2018). However, the majority of the viruses in the body are reported to be phages (Liang and Bushman 2021), which in the gut have an abundance of up to 108 virus-like-particles (VLPs) per millilitre of faecal matter (Dion, Oechslin and Moineau 2020). It has been suggested that phages play a significant role in maintaining gut homeostasis through regulating bacterial abundance, diversity, and metabolism (Mills et al. 2013).
Table 1.
Virus communities in the human gut. Please see Online Supplement Material for References in the table.
| Virus type | Genome | Infant | Adult |
|---|---|---|---|
| Bacteriophages | Double-stranded DNA | Myoviridae, Podoviridae, Siphoviridae, Corticoviridae, Tectiviridae, Amandaviridae, Sisseviridae, Picoviridae, Skunaviridae, β-crassviridae, Jeppeviridae, Alberteviridae, Hannahviridae, Flandersviridae, Evaviridae | Myoviridae, Podoviridae, Siphoviridae, crAssphages |
| Single-stranded DNA | Inoviridae, Microviridae, Gokushoviridae, Alpaviridae, Inesviridae, Almaviridae, Noraviridae, Circoviridae | Inoviridae, Microviridae | |
| Eukaryotic viruses | Double-stranded DNA | Adenoviridae *, Polyomaviridae* | Adenoviridae * , Herpesviridae * , Iridoviridae * , Marseilleviridae, Mimiviridae * , Papillomaviridae * , Polyomaviridae * , Poxviridae * |
| Single-stranded DNA | Anelloviridae * , Circoviridae, Geminiviridae, Nanoviridae, Parvoviridae * , Genomoviridae * | Anelloviridae * , Circoviridae, Parvoviridae * | |
| Double-stranded RNA | Picobirnaviridae * , Chrysoviridae, Reoviridae * | Picobirnaviridae * , Reoviridae * | |
| Single-stranded RNA | Caliciviridae * , Astroviridae * , Virgaviridae, Picornaviridae * , Alphaflexiviridae, Tombusviridae | Caliciviridae * , Astroviridae * , Virgaviridae, Picornaviridae * , Retroviridae * , Togaviridae * , Alphaflexiviridae, Bromoviridae, Luteoviridae | |
| Archaeal viruses | Lipothrixviridae |
Eukaryotic viruses reported to be associated with human diseases.
Here, we will mainly describe the phages and eukaryotic viruses living in the human gut, changes in their community structure through life, the influencing factors that shape their composition, and their interactions with the human hosts, as well as their role in human health and disease. We believe that our understanding of the complex interactions between the gut virome and the human host remains insufficient, especially regarding the contribution of gut virome to pathogenesis and disease progression in distant organs.
Gut virome through life, health and disease
The gastrointestinal virome contains a higher abundance of Caudovirales or tailed phages compared to other virus families (Carding, Davis and Hoyles 2017). Amongst them, crAssphages, which infect Bacteroides intestinalis, are the most common phages in the human gut virome (Townsend et al. 2021, Beller et al. 2022), followed by their close relatives Gubaphages (Camarillo-Guerrero et al. 2021). Other members of the human virome include phages belonging to the families Podoviridae and Siphoviridae, all of which are dsDNA phages (Clooney et al. 2019), and are mostly temperate (Shkoporov et al. 2019), as well as the single-stranded DNA (ssDNA) Microviridae, which are mostly lytic (Minot et al. 2013). Our understanding of phage diversity is limited, as most phage genomes show no homology to existing viral databases (Aggarwala, Liang and Bushman 2017), and cannot be linked to a bacterial host (Fitzgerald et al. 2021). Therefore, composition estimates of the gut virome are difficult and are complicated further by bias introduced during sample processing (Kim and Bae 2011).
Gut virome composition and the role of its key regulators
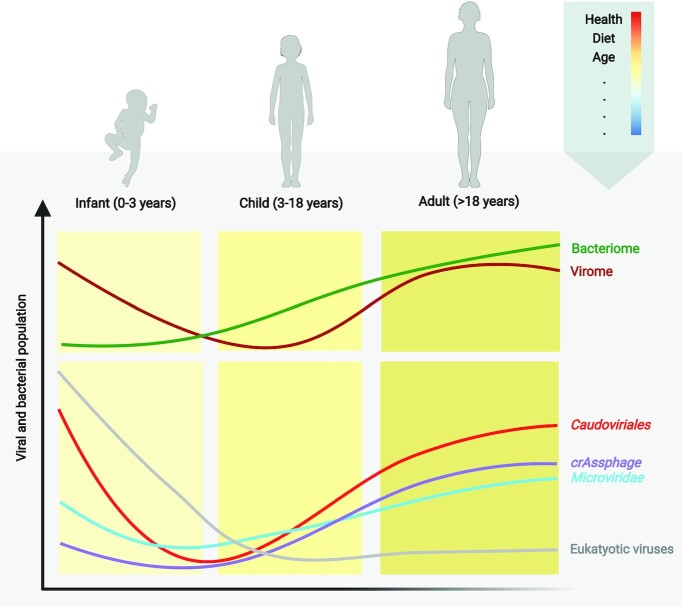
Together with diet and health-status, age is one of the main factors influencing gut virome composition, starting from birth (Gregory et al. 2020, Liang and Bushman 2021) (Fig 1). The quantification of VLPs shows very few to no particles in the meconium, the first infant stool samples. However, the number of VLPs increases to 109 VLPs per gram of faecal matter after one month of life (Liang et al. 2020). Birth by vaginal or caesarean delivery has a significant impact on alpha and beta diversity of the infant virome at 12 months whilst no significant difference was observed in bacterial diversity (McCann et al. 2018). There is some evidence that the earliest phages in the infant gut are induced prophages originating from the first colonizing bacteria (Liang et al. 2020), such as Proteobacteria, Actinobacteria, Bacteroidetes and Firmicutes (Baumann-Dudenhoeffer et al. 2018), which themselves are acquired during vaginal birth (Shamash and Maurice 2022). For example, Bifidobacteria containing prophages have been proven to be transmitted from mother to child via breastfeeding (Duranti et al. 2017). The induced bifidophages were shown to belong to Caudovirales (Lugli et al. 2016) which dominate the early infant phageome (Sharon et al. 2013), which only contains a small percentage of crAssphage (Shamash and Maurice 2022). CrAssphages start to appear between 1 and 3 months after Bacteroides colonize the infant gut (Gregory et al. 2015). Similarly to the colonization by Bifidobacteria and their phages, crAssphages from the infant gut have been shown to have up to 99% genomic similarity to crAssphages from the maternal gut (Siranosian et al. 2020). With age, the eukaryotic viral richness increases whilst phage richness and diversity decrease in an inverse relationship to bacterial richness and diversity (Lim et al. 2015). The Caudovirales dominated phageome shifts to Microviridae dominance in early childhood (Lim et al. 2015). In adulthood, crAssphages become the most abundant phages in the human gut virome (Guerin et al. 2018, Shkoporov et al. 2018, Beller et al. 2022)
Figure 1.
Changes in bacterial and viral populations inside the human gut across the lifespan of healthy individuals. Impact of different factors is illustrated: Health status, diet, and age are the most influential factors. Figure is adapted from (Gregory et al. 2020).
Longitudinal studies (Reyes et al. 2010, Lim et al. 2015, Shkoporov et al. 2019) have shown that the individual virome is highly stable. Virome intrapersonal variation is less than 5% within a year in healthy adult individuals (Reyes et al. 2010), compared to the highly diverse and changeable infant gut (Garmaeva et al. 2019).
Diet also influences the gut virome from birth. Infants fed with breast milk have a significantly different virome at 4 months when compared to infants fed with formula. Within the breast-milk-fed cohort, there was very little presence of eukaryotic viruses in the gut virome, and a high abundance in phages infecting Bifidobacterium and Lactobacillus (Liang et al. 2020). Later in life, diet still has an impact on gut virome. Dietary intervention studies (Minot et al. 2011, Garmaeva et al. 2021) showed that changes in diet, although moderate, had an effect on the gut virome, with the richness and diversity returning to pre-diet-intervention levels post-diet. However, recent studies have shown that common dietary ingredients such as coffee, cinnamon and oregano, amongst others (Boling et al. 2020), and orally taken medications, like NSAIDs (Sutcliffe et al. 2021), can induce prophages which could in turn alter the dynamics and composition of the gut microbiome.
Diet interconnects with ethnicity and geography as shown by a recent study of the gut virome in six different Chinese ethnic groups in both urban and rural environments. Ethnicity-specific diet had a significant effect on the gut virome composition between ethnic groups in the same environment. However, the most significant effect was between rural and urbanized environments, with higher urbanization being associated with a loss in virome diversity (Zuo et al. 2020). This has also been found in studies looking at the global abundance of crAssphages, finding that abundance was low in adults from traditional hunter-gatherer societies compared to modern industrialized populations (Honap et al. 2020). Similarly, in a study about the effect of breast milk and formula feeding on the infant gut, African–American cohorts were compared to a Botswanan cohort, which showed that in the Botswana cohort breast-milk-fed infants had a high number of Enteroviruses in their stool, which was not the case in the African–American cohorts (Liang et al. 2020). Other studies (Desai et al. 2020) confirmed that children in resource-poor regions have a higher diversity of enteric viral pathogens compared to those in resource-high regions.
Gut virome association with gut disease
Viral gastroenteritis is one of the most common illnesses globally as well as a leading cause of mortality, causing 2 to 3 million deaths annually (Oude Munnink and van der Hoek 2016). Interestingly, the main causative agent for viral gastroenteritis differs between adults and children. While children are primarily infected by Rotavirus, Sapovirus and Adenovirus (Eckardt and Baumgart 2011), adults are usually infected with Norovirus which accounts for 90% of viral gastroenteritis cases and roughly 50% of all viral gastroenteritis outbreaks worldwide (Patel et al. 2009). However, in addition to causing acute infections, eukaryotic viruses are also estimated to cause approximately 8–12 chronic viral infections per person throughout their lifetime, most of which are asymptomatic (Virgin, Wherry and Ahmed 2009). One example is Anellovirus, which is abundant in infants but reduced in children older than 18 months (Carding, Davis and Hoyles 2017), and whilst it persists in many sites of the human body, its role in human physiology and disease is largely unknown (Virgin, Wherry and Ahmed 2009). Similarly, Circovirus is abundant in the human gut virome without being linked to any specific disease. (Rascovan, Duraisamy and Desnues 2016).
Increased viral diversity can also be found in colorectal cancer patients, mostly for eukaryotic viruses. Epstein-Barr virus (Coughlan et al. 2021) was reported to cause gastric cancer (Norman et al. 2015), while both Epstein-Barr and the human papilloma viruses have been linked to colorectal cancer (Prendergast et al. 2015, Aftab, Shah and Hashmi 2016, Prendergast and Kelly 2016, Ma et al. 2021). Gut phages are not considered direct causative agents of disease in humans, but are driving factors that modulate bacterial communities. Global shifts in the phageome are linked to the development of diseases including environmental enteric dysfunction (EED) (Reyes et al. 2015), inflammatory bowel disease (IBD) (Coughlan et al. 2021), which includes Crohn's disease (CD) (Norman et al. 2015) and ulcerative colitis (UC) (Clooney et al. 2019), irritable bowel syndrome (IBS), coeliac disease and oesophageal adenocarcinoma (Emlet, Ruffin and Lamendella 2020) (Table 2). These findings reflect the association of phages with the development of disease and imply that phages are actively involved in pathogenesis through their modulation of intestinal inflammation and bacterial communities (Norman et al. 2015, Nakatsu et al. 2018, Zuo et al. 2019). Therefore, they have the potential to act as biomarker for dysbiosis-associated diseases. In addition, phage genomes carry evidence of interactions between phages and their bacterial hosts, like a ‘time capsule’, which remains to be explored (Scanlan et al. 2011, Koskella and Brockhurst 2014). Many of these genes may play a role in bacterial metabolism and pathogenicity, thus contributing to different diseases (Scanlan et al. 2011, Koskella and Brockhurst 2014).
Table 2.
Gut viral and bacterial population alterations in human diseases. Please see Online Supplement Material for References in the table.
| Disease | Major gut virome alteration | Major gut bacterial microbiome alteration |
|---|---|---|
| Diseases in the gut | ||
| IBD (Crohn's disease and ulcerative colitis) | Increased Caudovirales abundance; Increased Caudovirales richness; Increased ratio of Caudovirales to Microviridae; Increased Hepadnaviridae and Hepeviridae, reduced Polydnaviridae, Tymoviridae and Virgaviridae | Reduced bacterial diversity and richness; Reduced Bacteroides, Ruminococcus, and Blautia, increased Haemophilus and Streptococcus |
| Ulcerative colitis | Increased Caudovirales, phage and bacteria virulence functions, and loss of viral–bacterial correlations | |
| Crohn's disease | Moderate alterations; The virulent phages are replaced with temperate phages | Reduced bacterial alpha diversity |
| Colorectal cancer | Increased viral diversity | Reduced bacterial diversity |
| Coeliac disease autoimmunity | Increased enteroviruses Viral dysbiosis: increased Human polyomavirus 2, Enterobacteria phage mEpX1, and Enterobacteria phage mEpX2, reduced Lactococcus phages ul36 and Streptococcus phage Abc2 | |
| Cystic fibrosis | Different beta-diversity, reduced Myoviridae, Faecalibacterium phage FP Taranis and unclassified Gokushovirinae | Bacterial dysbiosis |
| Enteric graft-versus-host disease | Increased picobirnaviruses | Increased Lactobacillales spp,decreased Clostridiales spp. |
| Oesophageal adenocarcinoma | Abundant and rare phage communities in the gut may contribute to the progress of oesophageal carcinogesis | |
| Disease in distant organs | ||
| Type 1 diabetes | Reduced viral diversity;Increased E. coli phage/E. coli ratio | Bacterial dysbiosis; Depletion of amyloid-producing E. coli |
| Type 1 diabetes during pregnancy | Increased picobirnaviruses and tobamoviruses | |
| Type 2 diabetes | Increased putative phage scaffolds, complex core interaction between bacteria and phages | |
| Obesity and type 2 diabetes | Reduced viral richness and diversity, weakened viral-bacterial correlations | Significant change in bacterial communities |
| AIDS | Increased enteric adenoviruses | Shift from Bacteroides to Prevotella dominance, increased Enterobacteriaceae spp |
| Gulf War Illness | Increased viral richness and alpha diversity; DNA bacteriophage dysbiosis | Bacterial dysbiosis |
| Parkinson's disease | Reduced virus abundance; Increased Lactococcus phage, (increased phage/bacteria ratio in lactic acid bacteria) | Increased Verrucomicrobiaceae (Akkermansia muciniphila) and unclassified Firmicutes, reduced Prevotellaceae (Prevotella copri) and Erysipelotrichaceae (Eubacterium biforme); Bacterial dysbiosis, depletion of Lactococcus spp. |
| Coronary heart disease | Virgaviridae and Microviridae dominate in the enteric virome | |
| Hypertension | Erwinia phage ΦEaH2 and Lactococcus phage 1706 may be associated with hypertension, increased pervasive virus-bacteria linkages | |
| Atherosclerotic cardiovascular disease | Increased Enterobacteriaceae- and Streptococcus phages | Increased Enterobacteriaceae and Streptococcus spp. |
| Pulmonary Arterial Hypertension | Increased Enterococcal phage, reduced Lactococcal phage | Alteration of Enterococcus associacted with Enterococcal phage, increased Lactococcus |
| Pneumonia | Reduced Lactococcus phage, increased Staphylococcus- and Enterococcus phages | Loss of obligately anaerobic bacteria, increased Staphylococcus and Enterococcus spp |
| Pancreatic cancer | Cancer stage was positively correlated with relative abundances of Cyprinivirus | Bacterial dysbiosis, increased Klebsiella pneumoniae and Alistipes purtredinis |
| Alcohol-associated liver disease | Increased viral diversity, increased Escherichia-, Enterobacteria-, and Enterococcus phages and Parvoviridae and Herpesviridae | Reduced bacteria diversity |
| Non-alcoholic fatty liver disease | Reduced viral diversity, proportionately fewer phages, decreased Lactococcus- and Leuconostoc phages, increased Lactobacillus phage phiAT3 | Reduced bacterial diversity, low Lactococcus abundance |
| Cirrhosis | Different beta-diversity, altered phage and bacterial linkages centered on Streptococcus spp | Decreased bacterial alpha/beta diversity |
| Rheumatoid arthritis | Streptococcaceae, Bacteroidaceae, and Lachnospiraceae phages | Increased Lachnospiraceae |
Mechanisms of Host–Virome interactions
Impact of gut phageome on host through gut microbiota modulation
Phage-mediated microbiota modulation in the gut
Phages are able to significantly impact and regulate the bacterial communities within the human body. They exert immense evolutionary pressure, which drives bacteria to evolve numerous resistance mechanisms (Barr, 2017).
Bacteriophages indirectly influence human health and disease through their contributions to the gut microbiome composition, structure, and function (Manrique, Dills and Young 2017,Tisza and Buck 2021). Shifts in the gut virome are linked to bacterial dysbiosis and intestinal inflammation in patients with IBD: either Crohn's disease or UC (Norman et al. 2015). Observations of increased abundance of Caudovirales bacteriophages have also been reported in patients with IBD, although the viruses responsible for the microbiome shifts in each disease were different, suggesting specific virome signatures for each disease (Norman et al. 2015). Moreover, transplantation of phages from patients with UC alters the composition of the gut microbiome and increases colitis severity in human microbiota associated (HMA) mice (Levy et al. 2017, Sinha et al. 2022).
In addition to lysing their host bacteria, virulent phages can directly or indirectly shape the non-host bacterial community. For instance, lytic Enterococcus faecalis phages could inhibit non-host bacteria by inducing E. faecalis type VII secretion system upon their infection (Chatterjee et al. 2021). Moreover, virulent phages can cause shifts in gut bacteria through a cascading effect (Hsu et al. 2019), in which the phage-induced bacterial modulation impacts the gut metabolome, with reduced production of neurotransmitters and altered bile acid metabolism (Hsu et al. 2019), all of which are involved in interactions between the gut and distant organs (Sun and Chang 2014, Agus, Planchais and Sokol 2018). However, this study (Hsu et al. 2019) was performed in vivo with a consortium of 10 gut bacterial species, and hence further investigations are needed to determine the knock-on consequences of phage infection on the gut microbiome and human host.
Commensal bacteria such as Faecalibacterium prausnitzii are generally depleted in IBD patients (Cornuault et al. 2018). Previous studies reported an increase in the abundance or prevalence of F. prausnitzii phages in the faecal samples of IBD patients compared to healthy controls, suggesting a temperate-phage mediated killing of the bacteria that aggravates intestinal inflammation and promotes dysbiosis (Cornuault et al. 2018). Comparably to virome shifts in IBD patients, IBS patients have been shown to have significantly lower alpha diversity of phages compared to healthy controls (Coughlan et al. 2021). However this occurs without the switch in core phage lifecycles from lysogenic to lytic as has been described in IBD, where an individual-specific shift towards induced temperate phages replaced the healthy core virome, and their induction was presumably due to environmental stress factors associated with the inflamed gut (Clooney et al. 2019). Furthermore, gut inflammation and enteric disease were shown to trigger induction of the Salmonella Typhimurium (S.Tm) prophage, SopEΦ (Diard et al. 2017). This is a consequence of the bacterial SOS response being triggered by inflammation, which promotes prophage induction, leading to bacterial lysis and increased dysbiosis (Diard et al. 2017).
Translocation of gut viruses to distant organs
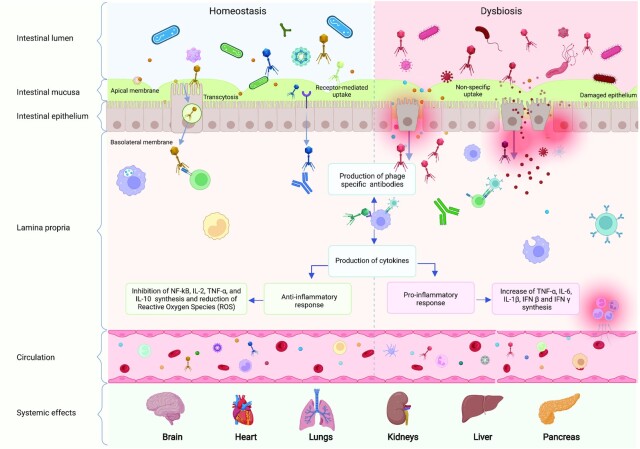
Some gut viruses can reach distant organs through multiple pathways. Specifically, gut phages are capable of adhering to the mucosal surfaces and crossing the epithelial cells by specific uptake, in which viruses are recognized by the epithelial cell receptors, which allows their crossing and migration into the body (Bomsel and Alfsen 2003, Cavarelli and Scarlatti 2014, Fu et al. 2017, Gilsdorf 2019, Good, Wells and Coyne 2019, Oechslin, Moradpour and Gouttenoire 2020), or nonspecific transcytosis (Nguyen et al. 2017), including macropinocytosis (Miernikiewicz and Dąbrowska 2022) as shown in Fig. 2. They can spread throughout the human body to reach blood and lymph, as well as major organs such as the liver, kidneys, lungs, urinary tract, and even the brain (Keller and Engley 1958, Weber-Dabrowska, Dabrowski and Slopek 1987, Barr 2017, Nguyen et al. 2017). In the lymphatic system, phages interact with macrophages and circulating dendritic cells (DCs), which triggers the humoral immune responses and stimulates cytokine production (Miernikiewicz et al. 2013, Van Belleghem et al. 2017) (Fig. 2). The presence of phage DNA was found inside extracellular bacterial membrane vesicles (MV) in circulation, suggesting another route for phage transport and dissemination outside of the gut (Champagne-Jorgensen et al. 2021: 1).
Figure 2.
Direct interactions between phages and the immune system in homeostasis compared to dysbiosis. Phages cross the intestinal epithelium via specific transcytosis and receptor-mediated uptake in homeostatic conditions; or non-specific and non-controlled uptake in the case of a damaged epithelium. Once they have crossed the intestinal epithelium, phages interact with circulating immune cells and can elicit different responses and modulate inflammation. Phages can then drain into lymph nodes and disseminate to different organs throughout the body.
Additionally, viruses can pass through the epithelium at sites of cellular damage and punctured vasculature into the interstitial matrix via a phenomenon characterized by increased intestinal permeability called ‘Leaky Gut’ (Handley et al. 2012, Karimi et al. 2016) (Fig. 2). Evidently, this phenomenon complicates underlying diseases; for example, gut dysbiosis-associated inflammation could allow small molecules to bypass the epithelium and enter the body where they can affect various organs (Hartmann, Chen and Schnabl 2012). It is still unclear whether gut leakage is a causative factor of gut diseases or simply occurs in association, however more studies support the latter hypothesis (Camilleri 2019).
Once these viruses enter the interstitial matrix and drain into the lymphatic system, they become circulating viruses, and can then access the regional lymph nodes and disseminate to organs throughout the body (Wiig, Keskin and Kalluri 2010, Choi, Lee and Hong 2012) according to their host tropism and receptor distribution (Jang et al. 2009, Jiao et al. 2014, Op de Beeck and Eizirik 2016, Majer, McGreevy and Booth 2020, Oechslin, Moradpour and Gouttenoire 2020).
Impact of gut phages on distant organs
Changes in gut phage composition and increased abundance of intestinal pathobionts and their infecting phages influence the development of diseases in distant organs, including pulmonary arterial hypertension (Kim et al. 2020), Parkinson's disease (Baizabal-Carvallo and Alonso-Juarez 2020), non-alcoholic fatty liver disease (Lang et al. 2020), type 1 diabetes (T1D) (Cinek et al. 2017; Zhao et al. 2017) and type 2 diabetes (T2D) (Chen et al. 2021) (Table 2). Increased gut permeability can result from phage-induced microbiota alterations (Tetz and Tetz 2016) and can trigger or intensify diseases in distant organs (Bosi et al. 2006; Baizabal-Carvallo and Alonso-Juarez 2020). Increased intestinal permeability has been linked to leakage of substances produced by gut microbiota into the central nervous system, which accelerates CNS inflammation and degeneration in neurodegenerative diseases (e.g. Parkinson's disease) (Baizabal-Carvallo and Alonso-Juarez 2020).
Gut phages can also positively impact distant organs. Increased levels of Caudovirales and specifically the Siphoviridae virus family in humans were linked to improved functioning in verbal memory and executive processes, while subjects with increased Microviridae levels exhibited impaired executive abilities (Mayneris-Perxachs et al. 2022). Comparably, Siphoviridae-rich microbiota transplantations from human donors improved memory function in both mice and Drosophila (Mayneris-Perxachs et al. 2022). Adding to the positive effect of gut phages on distant organs; applying lytic phages from Picovirinae subfamily to target cytolytic E. faecalis in the mammalian gut decreased cytolysin expression and attenuated alcoholic liver disease (Duan et al. 2019).
Temperate E. coli phages have a protective effect from autoimmune reactions like T1D, in which they reduce E. coli abundance and induce bacterial amyloids, which could function as antigens for plasmacytoid dendritic cells (pDCs) and trigger disease progression (Tetz et al. 2019). These examples validate the important role that gut phages play in modulating disease progression in distant organs. Further research is required in order to assess the exact role phages play in distant disease pathogenesis.
Auxiliary metabolic genes
Phages regulate bacterial community structures in most ecosystems both by predation and horizontal gene transfer. They contribute to host virulence, colonization, replication, and transmission (Hampton, Watson and Fineran 2020). Through phage-mediated horizontal gene transfer (HGT), phages supply their hosts with functional genes that were acquired during ancestral infections and allow fitness advantages to their host (Mara et al. 2020).
One example of this is auxiliary metabolic genes (AMGs). These are genes that have originated in bacteria and are mainly described for marine phages (Chevallereau et al. 2022). AMGs are involved in reprogramming the host metabolism, which indirectly influences several biogeochemical cycles important to living organisms, including carbon, nitrogen and sulphur) (Hurwitz, Hallam and Sullivan 2013, Anantharaman et al. 2014, Kieft et al. 2021a).
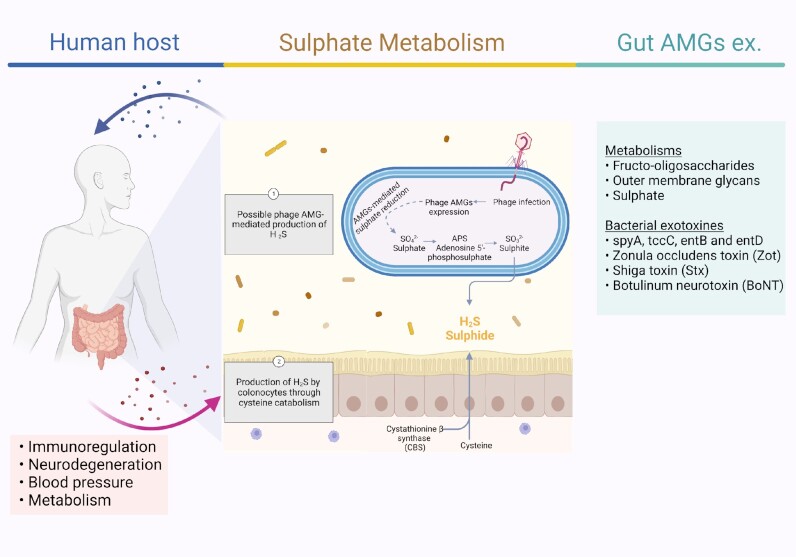
Dissimilatory Sulphur Metabolism (DSM) genes are encoded by ‘sulphur phages’ within the order Caudovirales and more specifically, the Myoviridae, Siphoviridae and Podoviridae families in marine environments (Kieft et al. 2021b). However, these AMGs are involved in the production of hydrogen sulphide (H₂S), a gas that mediates various biological processes, such as metabolism, neurodegeneration, regulation of inflammation, and blood pressure (Fike, Bradley and Rose 2015, Hellmich and Szabo 2015, Kieft et al. 2021a). The main sources for H₂S production in the human gut are 1) cysteine catabolism by colonocytes and 2) sulphate reduction by sulphate-reducing bacteria (Guo et al. 2016, Blachier et al. 2021). Increased levels of H₂S were found in cancer cells of various tissue types including colorectal cancer (CRC) (Shackelford et al. 2021). Additionally, the resistance of colon cancer cells to chemotherapy is linked to an increase in H₂S synthesis, while inhibition of H₂S synthesis increases the sensitivity of the cancer cells to chemotherapeutic agents (Shackelford et al. 2021) (Fig. 3). Although to date, DSM genes have only been described in marine phages, based on their considerable contribution to sulphur metabolism (Kieft et al. 2021b) and the important role that sulphate-reducing bacteria play in H₂S synthesis in the human gut (Guo et al. 2016), it is highly likely that these genes are also carried by gut phages, yet this remains to be explored.
Figure 3.
Overview of Hydrogen Sulphate (H2S) production pathways: Cysteine catabolism inside human colonocytes and potential phage AMG-mediated Sulphate reduction within Sulphate-reducing bacteria (SRB) and its effect on human health. H2S regulates DNA replication, metabolism, oxidative stress and inflammation in various organs of the body (e.g. brain, liver, heart, kidneys…). Other examples of AMGs in humans include genes that are involved in metabolisms of Fructo-oligosaccharides, Outer membrane glycans, etc., and bacterial exotoxins such as Zonula occludens toxin (Zot), Shiga toxin (Stx), and Botulinum neurotoxin (BoNT).
However, AMGs are found to be prevalent in the genomes of many gut phages identified by metagenomic analyses (Mathieu et al. 2020, de Jonge et al. 2021, Ma et al. 2021, Nayfach et al. 2021, Pratama et al. 2021). For example, individuals at risk of developing rheumatoid arthritis (RA) have been shown to harbour distinct gut phages compared to their healthy counterparts. In addition, these phages encode AMGs associated with immunomodulation and disease progression, including genes involved in outer membrane glycan metabolism (e.g. LPS) (Mangalea et al. 2021). Glycoside hydrolase family 32 (GH32), an AMG found in Bacillus subtilis phages, is involved in the metabolism of fructo-oligosaccharides (FOS) (Maaroufi and Levesque 2015, Ozaki et al. 2017), which retain intestinal permeability and tight junctions (Carvalho et al. 2021, Tanno et al. 2021).
AMGs not only assist in microbial cellular processes, but also in extracellular virulence by encoding bacterial exotoxins. These exotoxins are one of the most described virulence properties encoded by phages (Wagner and Waldor 2002). For example, bacterial exotoxins such as spyA, tccC, entB, and entD encoded by phages are found to be associated with the development of oesophageal diseases (Ma et al. 2021). In addition, CTXφ-encoded zonula occludens toxin (Zot) in Vibrio cholerae, that resembles the activity of zonulin structurally and functionally, can regulate the intestinal permeability and cause multiple diseases in distant organs (Fasano 2011, Pérez-Reytor et al. 2018).
The effects of AMGs can be significantly expanded through phage-mediated horizontal gene transfer (Tyler et al. 2013, Fasano 2002, Muniesa and Schmidt 2014, Mai-Prochnow et al. 2015). Considering the high abundance of prophages in the gut, we expect AMGs to play a significant role in human health and disease. Therefore, more studies are warranted to explore the function of phage-encoded genes in gut bacteria, especially those that are unknown.
Interactions between the gut virome and the mammalian immune system
Interactions between bacteriophages and the immune system
Bacteriophages are important components of the virome and have a great potential to shape and regulate mammalian immunity. In vitro incubation of purified Staphylococcus aureus and Pseudomonas aeruginosa phages with peripheral blood monocytes could induce immune responses, such as increasing the transcription of IL-1, IL-6, and tumour necrosis factor (TNF) (Van Belleghem et al. 2017). The oral treatment of germ-free mice with purified and lipopolysaccharide(LPS)-free E. coli phages resulted in the expansion of IFN-γ-producing CD4+ T cells and of CD8+ T cells in the Peyer's patches of the treated mice (Gogokhia et al. 2019) (Fig. 2). M13 phages could trigger interferon production and protect mice from tail lesions caused by vaccinia virus (Mori et al. 1996), and the Staphylococcus aureus phage A20/R induced the production of pro-inflammatory cytokine IL-6 (Zimecki et al. 2003). Lactobacillus, Escherichia, and Bacteroides phages were capable of exacerbating colitis via their activation of IFN-γ through a toll-like receptor 9 (TLR9)-dependent pathway (Gogokhia et al., 2019). Moreover, altered viral signatures in mice were correlated with the release of pro-inflammatory cytokines as well as decreased production of neurogenesis markers (Seth et al. 2019).
Nevertheless, phages are weak immunomodulatory agents, and they generally have poor pro-inflammatory effects. Research on the effects of T4 phages on the immune system showed that LPS-induced reactive oxygen species (ROS) production by peripheral blood polymorphonuclear leukocytes (PMNs) in response to bacterial infections was reduced when the cells were treated with purified phages with low endotoxin levels (Miedzybrodzki et al. 2008). Additionally, phages are capable of inhibiting human T-cell activation and proliferation as well as obstructing other pathogenic viral infections when present in the medium by inhibiting nuclear transcription factor NF-κB activation in response to the viral attack (Górski et al. 2006). However, it is important not to oversimplify the nature of interactions between phages and the immune system, because their effect on the immune system is not exclusively suppressive, as mentioned above.
Phages are therefore double-edged immunomodulators; they can trigger both pro- and anti-inflammatory immunological pathways, which further solidifies their role as modulators of disease development, either through their regulation of the microbiome or through stimulation of immune responses, the extent to which is still obscure and requires further research.
Interactions between eukaryotic viruses and the immune system
The immune system plays an essential role in regulating the intestinal microbiota via its control of the density and composition of the resident microbial and viral communities (Salzman et al. 2010, Duerkop and Hooper 2013). Host cells can recognize the invasion of viruses through the detection of viral components such as genomic material or viral proteins. The innate immune system first detects pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) (Akira, Uematsu and Takeuchi 2006).
Viruses can be beneficial to human health and can act as mutualistic symbionts, conferring advantages to their host instead of being strictly harmful parasites. For example, latent infection with herpesvirus increases resistance to Listeria monocytogenes and Yersinia pestis through the production of IFN-γ. Systemic activation of macrophages (Barton et al. 2007) and natural killer (NK) cells in mice results in higher protection against tumour grafts (White et al. 2010).
Consistent with the positive effects of the virome on host health, commensal viruses in healthy mice were shown to contribute to the development of intraepithelial lymphocytes (IELs) which are a key component of the defence system of the mammalian gut (Lee and Baldridge 2019). Conversely, an altered gut virome leads to increased intestinal inflammation and permeability as well as interleukin 6 (IL-6), IL-1β and interferon gamma (IFN-γ) production (Seth et al. 2019).
Gut eukaryotic viruses contribute to the recruitment of immunosuppressive regulator T cells (Treg) and the decrease of T cell activation (Pearson et al. 2019), which means that the immunomodulatory role of viruses is likely heavily influenced by the complex equilibrium between Tregs and affected cell populations in order to maintain immune tolerance to commensal viruses and prevent inflammation (Li, Handley and Baldridge 2021).
Viral infectivity of poliovirus and reovirus was reduced in antibiotic treated mice, suggesting a potential role that bacteria play in increasing viral pathogenicity (Kuss et al. 2011). These findings suggest that enteric viruses exploit intestinal microbes for increased pathogenicity and infectivity (Robinson and Pfeiffer 2014).
Viruses can encode host antigen-like proteins to elicit autoimmune responses, such as viral tyrosine phosphatase (IA-2) encoded by enterovirus and rotaviruses causing cross-reactive immune responses against β-cells (Härkönen et al. 2002, Honkanen et al. 2017). Additionally, host-like hormones and enzymes encoded by viruses can function similarly to those produced by the host. For example, Iridoviridae viruses produce insulin/insulin growth factor (IGF)1-like peptides in the gut, which can interact with human and murine insulin and IGF1 receptors, activate cell proliferation and increase glucose uptake (Altindis et al. 2018, Huang, Kahn and Altindis 2019).
Influenza-induced type-I IFN production in the lungs promotes the reduction of obligate anaerobic bacteria and the increase of Proteobacteria in the gut, leading to a dysbiotic intestinal microenvironment (Deriu et al. 2016). A recent study found that viral dsRNA induces NLRP6 inflammasome activation, which is involved in anti-microbial defence in the intestine and liver (Shen et al. 2021). Influenza infection also limits antimicrobial and inflammatory responses to Salmonella-induced colitis in the gut, thus escalating Salmonella intestinal colonization and systemic dissemination (Deriu et al. 2016). Conversely, the commensal microbiota in the intestine can regulate the production of CD4+ and CD8+ T cells and antibody responses following influenza virus infection and this can lead to a higher viral replication in the lung (Ichinohe et al. 2011).
These findings confirm the complexity of interactions between viruses and the human host, both locally and in distant organs.
Concluding remarks
Given the complexity of virome studies, from the known unknowns to the unknown unknowns, many challenges need to be overcome in order to reach a comprehensive understanding of the direct and indirect interactions between the virome and its hosts.
It seems promising to focus more efforts into looking at the associations between the gut and the rest of the human body, because, as the Greek physician Hippocrates said in the 5th century BCE: “All diseases begin in the gut.”
‘Omics’ approaches, including metagenomics, meta-transcriptomics and metabolomics, are specifically helpful for understanding the mechanisms by which the gut virome affects distant organs by providing unprecedented resolution of the interactions between viruses and their hosts. Therefore, we expect an inevitable transition from mono-omics to multi-omics in virome research in the near future to address the current shortcomings with virome analyses in revealing the mechanistic link between the virome and human health.
Funding
This work was funded by the German Research Foundation (DFG Emmy Noether program, Proj. no. 273124240, SFB 1371, Proj. no. 395357507), and the European Research Council Starting Grant (ERC StG 803077) awarded to L.D.
Supplementary Material
Acknowledgments
The authors thank Sophie E. Smith for proofreading the manuscript. Figures were created using BioRender (https://biorender.com).
Contributor Information
Kawtar Tiamani, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Shiqi Luo, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Sarah Schulz, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Jinling Xue, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Rita Costa, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Mohammadali Khan Mirzaei, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Li Deng, Institute of Virology, Helmholtz Centre Munich — German Research Centre for Environmental Health, 85764 Neuherberg, Germany; Chair of Microbial Disease Prevention, School of Life Sciences, Technical University of Munich, 85354 Freising, Germany.
Author contributions
All authors listed have contributed significantly to this work and approved it for publication.
Conflicts of interest statement
There are no interests to declare.
References
- Aftab A, Shah AA, Hashmi AM.. Pathophysiological Role of HERV-W in Schizophrenia. J Neuropsychiatry Clin Neurosci. 2016;28:17–25. [DOI] [PubMed] [Google Scholar]
- Aggarwala V, Liang G, Bushman FD.. Viral communities of the human gut: metagenomic analysis of composition and dynamics. Mobile DNA. 2017;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host & Microbe. 2018;23:716–24. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O.. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- Altindis E, Cai W, Sakaguchi Met al. Viral insulin-like peptides activate human insulin and IGF-1 receptor signaling: A paradigm shift for host-microbe interactions. Proc Natl Acad Sci. 2018;115:2461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman K, Duhaime Melissa B, Breier John Aet al. Sulfur Oxidation Genes in Diverse Deep-Sea Viruses. Science. 2014;344:757–60. [DOI] [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, Alonso-Juarez M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson's Disease. Neuroscience. 2020;432:160–73. [DOI] [PubMed] [Google Scholar]
- Barr JJ. A bacteriophages journey through the human body. Immunol Rev. 2017;279:106–22. [DOI] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JSet al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–9. [DOI] [PubMed] [Google Scholar]
- Baumann-Dudenhoeffer AM, D'Souza AW, Tarr PIet al. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 2018;24:1822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller L, Deboutte W, Vieira-Silva Set al. The virota and its transkingdom interactions in the healthy infant gut. Proc Natl Acad Sci. 2022;119:e2114619119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F, Andriamihaja M, Larraufie Pet al. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am J Physiol-Gastroin Liver Physiol. 2021;320:G125–35. [DOI] [PubMed] [Google Scholar]
- Boling L, Cuevas DA, Grasis JAet al. Dietary prophage inducers and antimicrobials: toward landscaping the human gut microbiome. Gut Microbes. 2020;11:721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Alfsen A.. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol. 2003;4:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi E, Molteni L, Radaelli MGet al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7. [DOI] [PubMed] [Google Scholar]
- Camarillo-Guerrero LF, Almeida A, Rangel-Pineros Get al. Massive expansion of human gut bacteriophage diversity. Cell. 2021;184:1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding SR, Davis N, Hoyles L.. Review article: the human intestinal virome in health and disease. Aliment Pharmacol Ther. 2017;46:800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho PLA, Andrade MER, Trindade LMet al. Prophylactic and therapeutic supplementation using fructo-oligosaccharide improves the intestinal homeostasis after mucositis induced by 5- fluorouracil. Biomed Pharmacother. 2021;133:111012. [DOI] [PubMed] [Google Scholar]
- Cavarelli M, Scarlatti G.. HIV-1 Infection: The Role of the Gastrointestinal Tract. Am J Reprod Immunol. 2014;71:537–42. [DOI] [PubMed] [Google Scholar]
- Champagne-Jorgensen K, Jose TA, Stanisz AMet al. Bacterial membrane vesicles and phages in blood after consumption of Lacticaseibacillus rhamnosus JB-1. Gut Microbes. 2021;13:1993583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Willett JLE, Dunny GMet al. Phage infection and sub-lethal antibiotic exposure mediate Enterococcus faecalis type VII secretion system dependent inhibition of bystander bacteria. PLos Genet. 2021;17:e1009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma X, Li Cet al. Enteric phageome alterations in patients with type 2 diabetes. Front Cell Infect Microbiol. 2021;10:575084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallereau A, Pons BJ, van Houte Set al. Interactions between bacterial and phage communities in natural environments. Nat Rev Microbiol. 2022;20:49–62. [DOI] [PubMed] [Google Scholar]
- Choi I, Lee S, Hong Y-K.. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2012;2:a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinek O, Kramna L, Lin Jet al. Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: Parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr Diabetes. 2017;18:588–98. [DOI] [PubMed] [Google Scholar]
- Clooney AG, Sutton TDS, Shkoporov ANet al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host & Microbe. 2019;26:764–778. [DOI] [PubMed] [Google Scholar]
- Cornuault JK, Petit MA, Mariadassou Met al. Phages infecting Faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome. 2018;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan S, Das A, O'Herlihy Eet al. The gut virome in Irritable Bowel Syndrome differs from that of controls. Gut Microbes. 2021;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge PA, Wortelboer K, Scheithauer TPM. et al. Members of a Highly Widespread Bacteriophage Family Are Hallmarks of Metabolic Syndrome Gut Microbiomes. Microbiology, 2021. [Google Scholar]
- Deriu E, Boxx GM, He Xet al. Influenza virus affects intestinal microbiota and secondary Salmonella infection in the gut through type I interferons. PLoS Pathog. 2016;12:e1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C, Handley SA, Rodgers Ret al. Growth velocity in children with Environmental Enteric Dysfunction is associated with specific bacterial and viral taxa of the gastrointestinal tract in Malawian children. PLoS NeglTrop Dis. 2020;14:e0008387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diard M, Bakkeren E, Cornuault JKet al. Inflammation boosts bacteriophage transfer between Salmonella spp. Science. 2017;355:1211–5. [DOI] [PubMed] [Google Scholar]
- Dion MB, Oechslin F, Moineau S.. Phage diversity, genomics and phylogeny. Nat Rev Microbiol. 2020;18:125–38. [DOI] [PubMed] [Google Scholar]
- Duan Y, Llorente C, Lang Set al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerkop BA, Hooper LV.. Resident viruses and their interactions with the immune system. Nat Immunol. 2013;14:654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti S, Lugli GA, Mancabelli Let al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt AJ, Baumgart DC.. Viral gastroenteritis in adults. Rec Patents Anti-Infect Drug Discov. 2011;6:54–63. [DOI] [PubMed] [Google Scholar]
- Emlet C, Ruffin M, Lamendella R.. Enteric virome and carcinogenesis in the gut. Dig Dis Sci. 2020;65:852–64. [DOI] [PubMed] [Google Scholar]
- Fasano A. Toxins and the gut: role in human disease. Gut. 2002;50:iii9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–75. [DOI] [PubMed] [Google Scholar]
- Fike DA, Bradley AS, Rose CV.. Rethinking the ancient sulfur cycle. Annu Rev Earth Planet Sci. 2015;43:593–622. [Google Scholar]
- Fitzgerald CB, Shkoporov AN, Upadrasta Aet al. Probing the “Dark Matter” of the human gut phageome: culture assisted metagenomics enables rapid discovery and host-linking for novel bacteriophages. Front Cell Infect Microbio. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhang L, Zhang Fet al. Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection. PLoS Pathog. 2017;13:e1006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmaeva S, Gulyaeva A, Sinha Tet al. Stability of the human gut virome and effect of gluten-free diet. Cell Rep. 2021;35:109132. [DOI] [PubMed] [Google Scholar]
- Garmaeva S, Sinha T, Kurilshikov Aet al. Studying the gut virome in the metagenomic era: challenges and perspectives. BMC Biol. 2019;17:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf JR. Acute flaccid myelitis: lessons from polio. Journal of the Pediatric Infectious Diseases Society. 2019;8:550–3. [DOI] [PubMed] [Google Scholar]
- Gogokhia L, Buhrke K, Bell Ret al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host & Microbe. 2019;25:285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C, Wells AI, Coyne CB.. Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci Adv. 2019;5:eaau4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górski A, Kniotek M, Perkowska-Ptasińska Aet al. Bacteriophages and transplantation tolerance. Transplant Proc. 2006;38:331–3. [DOI] [PubMed] [Google Scholar]
- Gregory AC, Zablocki O, Zayed AAet al. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe. 2020;28:724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KE, LaPlante RD, Shan Get al. Mode of birth influences preterm infant intestinal colonization with bacteroides over the early neonatal period. Adv Neonatal Care. 2015;15:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin E, Shkoporov A, Stockdale SRet al. Biology and taxonomy of crass-like bacteriophages, the most abundant virus in the human gut. Cell Host Microbe. 2018;24:653–64. [DOI] [PubMed] [Google Scholar]
- Guo F-F, Yu T-C, Hong Jet al. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front Physiol. 2016;7, DOI: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton HG, Watson BNJ, Fineran PC.. The arms race between bacteria and their phage foes. Nature. 2020;577:327–36. [DOI] [PubMed] [Google Scholar]
- Handley S, Thackray LB, Zhao Get al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härkönen T, Lankinen H, Davydova Bet al. Enterovirus infection can induce immune responses that cross-react with β-cell autoantigen tyrosine phosphatase IA-2/IAR. J Med Virol. 2002;66:340–50. [DOI] [PubMed] [Google Scholar]
- Hartmann P, Chen W-C, Schnabl B.. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich MR, Szabo C.. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honap TP, Sankaranarayanan K, Schnorr SLet al. Biogeographic study of human gut-associated crAssphage suggests impacts from industrialization and recent expansion. PLoS One. 2020;15:e0226930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen H, Oikarinen S, Nurminen Net al. Detection of enteroviruses in stools precedes islet autoimmunity by several months: possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia. 2017;60:424–31. [DOI] [PubMed] [Google Scholar]
- Hsu BB, Gibson TE, Yeliseyev Vet al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host & Microbe. 2019;25:803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Kahn CR, Altindis E.. Viral Hormones: Expanding Dimensions in Endocrinology. Endocrinology. 2019;160:2165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz BL, Hallam SJ, Sullivan MB.. Metabolic reprogramming by viruses in the sunlit and dark ocean. Genome Biol. 2013;14:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Yet al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci. 2011;108:5354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Boltz DA, Webster RGet al. Viral Parkinsonism. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 2009;1792:714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X-Y, Guo L, Huang D-Yet al. Distribution of EV71 receptors SCARB2 and PSGL-1 in human tissues. Virus Res. 2014;190:40–52. [DOI] [PubMed] [Google Scholar]
- Karimi M, Mirshekari H, Moosavi Basri SMet al. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv Drug Deliv Rev. 2016;106:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Engley FB.. Fate of bacteriophage particles introduced into mice by various routes. Exp Biol Med. 1958;98:577–80. [DOI] [PubMed] [Google Scholar]
- Kieft K, Breister AM, Huss Pet al. Virus-associated organosulfur metabolism in human and environmental systems. Cell Rep. 2021a;36:109471. [DOI] [PubMed] [Google Scholar]
- Kieft K, Zhou Z, Anderson REet al. Ecology of inorganic sulfur auxiliary metabolism in widespread bacteriophages. Nat Commun. 2021b;12. DOI: 10.1038/s41467-021-23698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Bae J-W.. Amplification methods bias metagenomic libraries of uncultured single-stranded and double-stranded DNA Viruses▿. Appl Environ Microbiol. 2011;77:7663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Rigatto K, Gazzana MBet al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskella B, Brockhurst MA.. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev. 2014;38:916–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CAet al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Demir M, Martin Aet al. Intestinal Virome Signature Associated With Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2020;159:1839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Baldridge MT.. Viruses RIG up intestinal immunity. Nat Immunol. 2019;20:1563–4. [DOI] [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CAet al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–32. [DOI] [PubMed] [Google Scholar]
- Li Y, Handley SA, Baldridge MT.. The dark side of the gut: Virome–host interactions in intestinal homeostasis and disease. J Exp Med. 2021;218:e20201044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Bushman FD.. The human virome: assembly, composition and host interactions. Nat Rev Microbiol. 2021;19:514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Zhao C, Zhang Het al. The stepwise assembly of the neonatal virome is modulated by breastfeeding. Nature. 2020;581:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ES, Zhou Y, Zhao Get al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli GA, Milani C, Turroni Fet al. Prophages of the genus Bifidobacterium as modulating agents of the infant gut microbiota. Environ Microbiol. 2016;18:2196–213. [DOI] [PubMed] [Google Scholar]
- Ma T, Ru J, Xue Jet al. Differences in gut virome related to barrett esophagus and esophageal adenocarcinoma. Microorganisms. 2021;9. DOI: 10.3390/microorganisms9081701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaroufi H, Levesque RC.. Glycoside hydrolase family 32 is present in Bacillus subtilis phages. Virology Journal. 2015;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai-Prochnow A, Hui JGK, Kjelleberg Set al. Big things in small packages: the genetics of filamentous phage and effects on fitness of their host. FEMS Microbiol Rev. 2015;39:465–87. [DOI] [PubMed] [Google Scholar]
- Majer A, McGreevy A, Booth TF.. Molecular pathogenicity of enteroviruses causing neurological disease. Front Microbiol. 2020;11:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalea MR, Paez-Espino D, Kieft Ket al. Individuals at risk for rheumatoid arthritis harbor differential intestinal bacteriophage communities with distinct metabolic potential. Cell Host Microbe. 2021;29:726–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique P, Dills M, Young MJ.. The human gut phage community and its implications for health and disease. Viruses. 2017;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara P, Vik D, Pachiadaki MGet al. Viral elements and their potential influence on microbial processes along the permanently stratified Cariaco Basin redoxcline. ISME J. 2020;14:3079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu A, Dion M, Deng Let al. Virulent coliphages in 1-year-old children fecal samples are fewer, but more infectious than temperate coliphages. Nat Commun. 2020;11:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matijašić M, Meštrović T, Čipčić Paljetak Het al. Gut microbiota beyond bacteria—mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int J Mol Sci. 2020;21:2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayneris-Perxachs J, Castells-Nobau A, Arnoriaga-Rodríguez Met al. Caudovirales bacteriophages are associated with improved executive function and memory in flies, mice, and humans. Cell Host Microbe. 2022. DOI: 10.1016/j.chom.2022.01.013. [DOI] [PubMed] [Google Scholar]
- McCann A, Ryan FJ, Stockdale SRet al. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ. 2018;6:e4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedzybrodzki R, Switala-Jelen K, Fortuna Wet al. Bacteriophage preparation inhibition of reactive oxygen species generation by endotoxin-stimulated polymorphonuclear leukocytes. Virus Res. 2008;131:233–42. [DOI] [PubMed] [Google Scholar]
- Miernikiewicz P, Dąbrowska K, Piotrowicz Aet al. T4 phage and its head surface proteins do not stimulate inflammatory mediator production. PLoS One. 2013;8:e71036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernikiewicz P, Dąbrowska K.. Endocytosis of bacteriophages. Curr Opin Virol. 2022;52:229–35. [DOI] [PubMed] [Google Scholar]
- Mills S, Shanahan F, Stanton Cet al. Movers and shakers. Gut Microbes. 2013;4:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot S, Bryson A, Chehoud Cet al. Rapid evolution of the human gut virome. Proc Natl Acad Sci. 2013;110:12450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot S, Sinha R, Chen Jet al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 2011;21:1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kubo T, Kibayashi Yet al. Anti-vaccinia virus effect of M13 bacteriophage DNA. Antiviral Res. 1996;31:79–86. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Schmidt H.. Shiga toxin-encoding phages: multifunctional gene ferries. 2014. [Google Scholar]
- Nakatsu G, Zhou H, Wu WKKet al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology. 2018;155:529–41. [DOI] [PubMed] [Google Scholar]
- Nayfach S, Páez-Espino D, Call Let al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nature Microbiology. 2021;6:960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S, Baker K, Padman BSet al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. Racaniello VR (ed). mBio. 2017;8. DOI: 10.1128/mBio.01874-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JM, Handley SA, Baldridge MTet al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin N, Moradpour D, Gouttenoire J.. Hepatitis E virus finds its path through the gut. Gut. 2020;69:796–8. [DOI] [PubMed] [Google Scholar]
- Op de Beeck A, Eizirik DL.. Viral infections in type 1 diabetes mellitus — why the β cells?. Nat Rev Endocrinol. 2016;12:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink BB, van der Hoek L. Viruses causing gastroenteritis: the known, the new and those beyond. Viruses. 2016;8:E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Abe N, Kimura Ket al. Genomic analysis of Bacillus subtilis lytic bacteriophage ϕNIT1 capable of obstructing natto fermentation carrying genes for the capsule-lytic soluble enzymes poly-γ-glutamate hydrolase and levanase. Biosci Biotechnol Biochem. 2017;81:135–46. [DOI] [PubMed] [Google Scholar]
- Patel MM, Hall AJ, Vinjé Jet al. Noroviruses: a comprehensive review. J Clin Virol. 2009;44:1–8. [DOI] [PubMed] [Google Scholar]
- Pearson JA, Tai N, Ekanayake-Alper DKet al. Norovirus changes susceptibility to type 1 diabetes by altering intestinal microbiota and immune cell functions. Front Immunol. 2019;10:2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Reytor D, Jaña V, Pavez Let al. Accessory toxins of vibrio pathogens and their role in epithelial disruption during infection. Front Microbiol. 2018;9:2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prangishvili D, Forterre P, Garrett RA.. Viruses of the Archaea: a unifying view. Nat Rev Microbiol. 2006;4:837–48. [DOI] [PubMed] [Google Scholar]
- Pratama AA, Bolduc B, Zayed AAet al. Expanding standards in viromics: in silico evaluation of dsDNA viral genome identification, classification, and auxiliary metabolic gene curation. PeerJ. 2021;9:e11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast AJ, Humphrey JH, Mutasa Ket al. Assessment of environmental enteric dysfunction in the SHINE trial: methods and challenges. Clin Infect Dis. 2015;61:Suppl 7:S726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast AJ, Kelly P.. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr Opin Infect Dis. 2016;29:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovan N, Duraisamy R, Desnues C.. Metagenomics and the Human Virome in Asymptomatic Individuals. Annu Rev Microbiol. 2016;70:125–41. [DOI] [PubMed] [Google Scholar]
- Reyes A, Blanton LV, Cao Set al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci. 2015;112:11941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Haynes M, Hanson Net al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Pfeiffer JK.. Viruses and the Microbiota. Ann Rev Virol. 2014;1:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai Det al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan PD, Hall AR, Lopez-Pascua LDCet al. Genetic basis of infectivity evolution in a bacteriophage. Mol Ecol. 2011;20:981–9. [DOI] [PubMed] [Google Scholar]
- Seth RK, Maqsood R, Mondal Aet al. Gut DNA virome diversity and its association with host bacteria regulate inflammatory phenotype and neuronal immunotoxicity in experimental gulf war illness. Viruses. 2019;11:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford RE, Mohammad IZ, Meram ATet al. Molecular functions of hydrogen sulfide in cancer. Pathophysiology. 2021;28:437–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash M, Maurice CF.. Phages in the infant gut: a framework for virome development during early life. ISME J. 2022;16:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon I, Morowitz MJ, Thomas BCet al. Time series community genomics analysis reveals rapid shifts in bacterial species, strains, and phage during infant gut colonization. Genome Res. 2013;23:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Li R, Negro Ret al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell. 2021;184. DOI: 10.1016/j.cell.2021.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoporov AN, Clooney AG, Sutton TDSet al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. 2019;26:527–41. [DOI] [PubMed] [Google Scholar]
- Shkoporov AN, Khokhlova EV, Fitzgerald CBet al. ΦCrAss001 represents the most abundant bacteriophage family in the human gut and infects Bacteroides intestinalis. Nat Commun. 2018;9:4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Li Y, Mirzaei MKet al. Transplantation of bacteriophages from ulcerative colitis patients shifts the gut bacteriome and exacerbates the severity of DSS colitis. Microbiome. 2022;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siranosian BA, Tamburini FB, Sherlock Get al. Acquisition, transmission and strain diversity of human gut-colonizing crAss-like phages. Nat Commun. 2020;11:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chang E.. Exploring gut microbes in Human health and disease: pushing the envelope. Genes Diseases. 2014;1. DOI: 10.1016/j.gendis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe SG, Shamash M, Hynes APet al. Common oral medications lead to prophage induction in bacterial isolates from the human gut. Viruses. 2021;13:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno H, Fujii T, Hirano Ket al. Characterization of fructooligosaccharide metabolism and fructooligosaccharide-degrading enzymes in human commensal butyrate producers. Gut Microbes. 2021;13:1869503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G, Brown SM, Hao Yet al. Type 1 diabetes: an association between autoimmunity, the dynamics of gut amyloid-producing E. coli and their phages. Sci Rep. 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G, Tetz V.. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathogens. 2016;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisza MJ, Buck CB.. A catalog of tens of thousands of viruses from human metagenomes reveals hidden associations with chronic diseases. Proc Natl Acad Sci. 2021;118. DOI: 10.1073/pnas.2023202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EM, Kelly L, Muscatt Get al. The human gut phageome: origins and roles in the human gut microbiome. Front Cell Infect Microbiol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J, Beeri K, Reynolds Jet al. Prophage induction is enhanced and required for renal disease and lethality in an EHEC Mouse Model. PLoS Pathog. 2013;9. DOI: 10.1371/journal.ppat.1003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belleghem JD, Clement F, Merabishvili Met al. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep. 2017;7:8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R.. Redefining chronic viral infection. Cell. 2009;138:30–50. [DOI] [PubMed] [Google Scholar]
- Wagner PL, Waldor MK.. Bacteriophage control of bacterial virulence. Infect Immun. 2002;70:3985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Dabrowska B, Dabrowski M, Slopek S.. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch Immunol Ther Exp (Warsz). 1987;35:563–8. [PubMed] [Google Scholar]
- White DW, Keppel CR, Schneider SEet al. Latent herpesvirus infection arms NK cells. Blood. 2010;115:4377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Keskin D, Kalluri R.. Interaction between the extracellular matrix and lymphatics: consequences for lymphangiogenesis and lymphatic function. Matrix Biol. 2010;29:645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate S, Taboada B, Yocupicio-Monroy Met al. The human virome. Arch Med Res. 2018;48. DOI: 10.1016/j.arcmed.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Zhao G, Vatanen T, Droit Let al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc Natl Acad Sci. 2017;114:E6166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimecki M, Weber-Dabrowska B, Łusiak-Szelachowska Met al. Bacteriophages provide regulatory signals in mitogen-induced murine splenocyte proliferation. Cell Mol Biol Lett. 2003;8:699–711. [PubMed] [Google Scholar]
- Zuo T, Lu X-J, Zhang Yet al. Gut mucosal virome alterations in ulcerative colitis. Gut. 2019;68:1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Sun Y, Wan Yet al. Human-Gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host Microbe. 2020;28:741–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.