Abstract
Respiratory syncytial virus (RSV) infection is known to predispose children to otitis media and sinusitis due to bacteria such as nontypeable Haemophilus influenzae (NTHI). In this study, we investigated the role of NTHI surface outer membrane protein P5-homologous fimbriae (P5-fimbriae) in attachment to RSV-exposed A549 epithelial cells. Analysis by fluorescence flow cytometry showed that a live P5-fimbriated NTHI strain (NTHIF+) attached to a higher proportion of RSV-exposed A549 cells than to control cells (mean, 68% for RSV versus 29% for control; P = 0.008), while attachment of the P5-fimbriae-deficient isogenic mutant strain (NTHIF−) was significantly lower than in control cells and rose only slightly following RSV exposure (mean, 17% for RSV versus 10% for control, P = 0.229). Attachment of NTHIF+ did not correlate with the amount of RSV antigen expressed by A549 cells. Furthermore, paraformaldehyde-fixed NTHIF+ also demonstrated an enhanced binding to RSV-exposed cells. Observations by transmission electronic microscopy showed that the mean number of bacteria attached per 100 RSV-exposed A549 cells was higher for NTHIF+ than NTHIF− (99 versus 18; P < 0.001). No intracellular bacteria were identified. UV-irradiated conditioned supernatants collected from RSV-infected A549 cultures (UV-cRSV) also enhanced the attachment of NTHIF+ to A549, suggesting the presence of a preformed soluble mediator(s) in UV-cRSV that enhances the expression of receptors for P5-fimbriae on A549 cells. In summary, RSV infection significantly enhances NTHI attachment to respiratory epithelial cells. P5-fimbria is the critical appendage of NTHI that participates in this attachment. In clinical settings, blocking of the P5-fimbria-mediated attachment of NTHIF+ by passive or active immunity may reduce the morbidity due to NTHI during RSV infection.
Nontypeable Haemophilus influenzae (NTHI) is a gram-negative, unencapsulated bacterium that frequently colonizes the nasopharyngeal mucosa of healthy children (14, 17). NTHI also accounts for a large proportion of bacterial conjunctivitis, otitis media, sinusitis, bronchitis, and pneumonia (22). Among the respiratory viruses, respiratory syncytial virus (RSV) is the most common virus associated with the development of otitis media due to NTHI in children (5, 6, 15, 29). Similar associations have been described for sinusitis and pneumonia (18, 19, 32). Furthermore, we have previously demonstrated in experimental animals that RSV infection enhances the nasopharyngeal colonization with NTHI (23).
Recent studies show that a surface fimbria structure of NTHI, known as outer membrane protein P5-homologous fimbria (P5-fimbria), is involved in the attachment to mucus and respiratory epithelial cells (2, 3, 21). The P5-fimbriae are approximately 2.4 nm in diameter, are long and flexible in appearance, and mediate attachment and not hemagglutination (1, 2). Immune response against the P5-fimbriae has been shown to reduce nasopharyngeal colonization as well as occurrence of otitis media in experimental animals (3, 30). However, the role of P5-fimbriae in attachment of NTHI to virus-infected airway epithelium has not been studied.
In the present study, we hypothesized that RSV infection enhances the attachment of NTHI to respiratory epithelial cells and that the enhancement is primarily mediated by the bacterial surface P5-fimbriae. This hypothesis was evaluated in vitro in A549 cells (type II-like alveolar epithelial cell carcinoma), as they have been used for studies of RSV infection (24) as well as bacterial attachment (13).
MATERIALS AND METHODS
Cell cultures.
A549 cells were obtained from the American Type Culture Collection (Rockville, Md.) and grown in minimal essential medium (MEM) containing 8% fetal bovine serum (Sigma, St. Louis, Mo.) and 2 mM l-glutamine. Maintenance MEM during RSV incubation contained 1% fetal calf serum (FCS). The media did not contain any antibiotics in order to allow study of live bacteria interactions.
RSV preparations.
The human Long strain of RSV (A2) was grown in HEp-2 cells (human laryngeal epithelial carcinoma; American Type Culture Collection) and purified by polyethylene glycol precipitation followed by centrifugation on a 35 to 60% discontinuous sucrose gradient (21). The titer of the purified RSV (pRSV) pool was 109.25 PFU/ml. Monolayers of A549 cells grown in 24-well tissue culture plates were incubated with pRSV at a multiplicity of infection (MOI) of 1 in MEM supplemented with 1% FCS and then incubated at 37°C in 5% CO2. When the cells exhibited extensive cytopathic effect at 48 h, the supernatant was aspirated and centrifuged at 5,000 × g to remove large cellular debris. This preparation is referred to as the conditioned RSV (cRSV) pool. UV-inactivated conditioned supernatants from pRSV-infected A549 cells (UV-cRSV) were prepared as previously described (30). Control supernatants were prepared similarly without RSV infection.
NTHI preparations.
P5-fimbriated NTHI strain 1128 (NTHIF+) was isolated from a child with otitis media and was used to construct a nonfimbriated mutant (NTHIF−) at the Otological Research Laboratories, The Ohio State University, Columbus (1, 30). NTHI were grown on chocolate agar plates at 37°C in 5% CO2 overnight. Two to three colonies were mixed in Haemophilus test medium (Becton Dickinson Microbiology Systems, Cockeysville, Md.) at 37°C overnight. The next day, 1-ml aliquots of bacteria suspensions were transferred to fresh Haemophilus test medium and recultured under the same conditions for about 4 h (mid-log phase). Bacteria were washed with phosphate-buffered saline (PBS) and concentrated to approximately 2 × 109 CFU/ml as estimated by optical density and confirmed by quantitative plate colony counts. For some experiments, bacteria were also fixed by being incubated with 1% paraformaldehyde for 15 min and washed three times with PBS. The nonviability of the paraformaldehyde-fixed NTHI was confirmed by plate culture.
NTHI attachment assay by culture.
A549 cells were grown as confluent monolayers in 24-well tissue culture plates. The cells were then inoculated with pRSV (MOI = 1) and incubated for 24 h at 37°C in 5% CO2. The monolayers were then washed twice, and 1 ml of Hanks’ balanced salt solution (HBSS; Sigma) containing NTHI was inoculated into each well (A549:NTHI = 1:0.5). After incubation for 3 h at 37°C, the monolayers were washed gently three times with prewarmed HBSS. A549 cells were then detached with trypsin-EDTA (Sigma), washed twice with HBSS, and plated on chocolate agar for quantitative colony counts.
NTHI attachment assay by flow cytometry.
Equivalent numbers of NTHIF+ and NTHIF−, as determined by optical density and confirmed by quantitative plate colony count, were used to compare bacterial attachment by flow cytometry. NTHI suspensions were labeled with PKH-26, a red fluorescent dye (General Linker kit; Sigma). In brief, approximately 2 × 109 CFU of NTHI was suspended in 1 ml of diluent and mixed with 1 ml of PKH-26 linker solution. The mixture was incubated at 25°C for 5 min. Equal volumes of FCS were then added to stop the labeling reaction. Bacteria were washed three times with PBS. Viability of the labeled bacteria was tested by quantitative cultures. Preliminary studies showed that the levels of PKH-26 labeling were comparable for NTHIF+ and NTHIF−.
Confluent monolayers of A549 cells were inoculated with pRSV or control medium and incubated for 24 h at 37°C in 5% CO2. The monolayers were then detached by incubation with 0.1 M EDTA (pH 7.5) for 10 min and washed twice with PBS. For some experiments, 5 × 105 cells were labeled with fluorescein isothiocyanate-conjugated anti-RSV polyclonal antibody (Chemicon, Temecula, Calif.) at 37°C in 5% CO2 for 1 h and then washed three times with PBS. PKH-26-labeled bacteria were mixed with RSV-exposed or control A549 cell suspensions at various ratios and incubated at 37°C on an orbital shaker for 30 min. The cells were washed three times by differential centrifugation to remove unattached bacteria and then fixed in 1% paraformaldehyde prior to analysis by two-color fluorescence flow cytometry (FACScan analyzer; Becton Dickinson, San Jose, Calif.).
NTHI attachment assay by transmission electron microscopy.
A549 monolayers grown in 24-well tissue culture plates were inoculated with pRSV (MOI = 1) or control medium for 24 h. NTHI suspensions were then added to the wells (A549:NTHI = 1:100) and incubated for 1 h. Unattached NTHI was removed by washing three times with prewarmed HBSS. The monolayers were then fixed with 2.5% cacodylate-buffered glutaraldehyde and postfixed in 1% osmium tetroxide. The cells were detached by gentle scraping with a needle, dehydrated, and embedded by standard techniques. The cells were visualized under a transmission electron microscope by an examiner who was unaware of the sample treatments. The sections were surveyed by using a square grid, counting only those without holes or obstacles (en face cuts excluded). Counts progressed back and forth across a section until at least 100 cells had been counted. Only the cells with complete nuclei were counted. Mitotic and degenerative cells were excluded. The number of attached bacteria were then counted and were categorized as tightly or loosely attached to the cell body or tightly attached to microvilli.
Statistics.
Nonparametric data were evaluated by Mann-Whitney rank sum test, while parametric data were evaluated by Student’s t test. Analysis of proportions was evaluated by z test. P values of <0.05 were considered statistically significant.
RESULTS
Analysis of NTHI attachment by culture.
Initial NTHI attachment studies were conducted by quantitative culture of bacteria that remained attached to washed monolayers of A549 cells. The number of NTHIF+ attached to RSV-exposed A549 cells was significantly higher than for control A549 cells (Fig. 1). The culture technique, however, could not exclude the possibility that the bacteria were loosely attached to the cells merely by virtue of gravity or were attached to the inner plastic surface of the well.
FIG. 1.
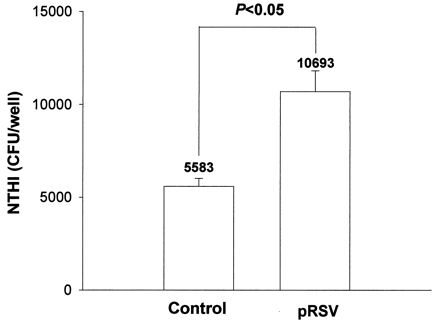
Effect of pRSV exposure (MOI = 1, 24 h) or control medium on binding of NTHIF+ to A549 cell monolayers (A549:NTHI = 1:0.5), as determined by the culture method. The values are means of experiments performed in triplicate. Here and in Fig. 3, 4, 7, and 8, error bars indicate standard deviations.
Role of NTHI P5-fimbriae in attachment.
We further evaluated the role of P5-fimbriae in attachment by the fluorescence flow cytometry method (Fig. 2). This method overcame some of the problems of the culture method since the NTHI-A549 coincubation was performed under rotational motion followed by vigorous washing. The results showed that NTHIF+ attached to a larger proportion of pRSV-exposed than control A549 cells (mean, 68% for RSV versus 29% for control; P = 0.008) while the attachment of NTHIF− was only slightly higher for pRSV-exposed cells than for control cells (mean, 17% for RSV versus 10% for control; P = 0.229) (Fig. 3). The relative density of NTHI attached to both control and pRSV-exposed A549 cells, as determined by mean fluorescence intensity, was also significantly higher for NTHIF+ than NTHIF− for both control and RSV-exposed A549 cells (Fig. 4). Initial live RSV infection was required as exposure of A549 cells to UV-irradiated pRSV did not result in any enhancement of NTHI attachment (data not shown).
FIG. 2.
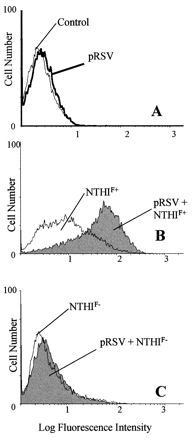
Histographic representation of number of A549 cells (y axis) expressing red fluorescence of PKH-26-labeled NTHI (x axis), as determined by flow cytometry (A549:NTHI = 1:100). (A) Background fluorescence of pRSV-exposed (MOI = 1, 24 h) or control A549 cells; (B and C) fluorescence of NTHIF+ and NTHIF− attached to control or pRSV-exposed A549 cells.
FIG. 3.
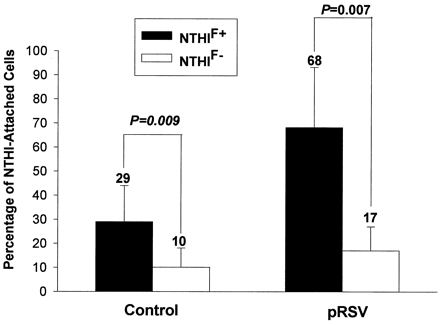
Percentage of pRSV-exposed (MOI = 1, 24 h) or control A549 cells demonstrating attached NTHIF+ or NTHIF− (A549:NTHI = 1:100), as determined by flow cytometry. The values are means of six experiments.
FIG. 4.
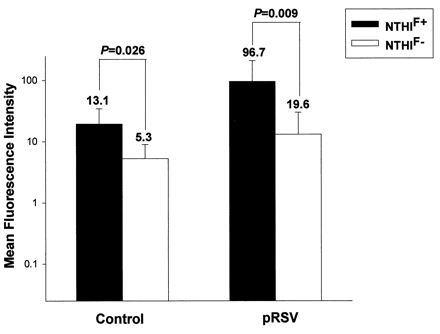
Mean fluorescence intensity of PKH-26-labeled NTHIF+ or NTHIF− to 5 × 105 pRSV-exposed (MOI = 1, 24 h) or control A549 cells (A549:NTHI = 1:100), as determined by flow cytometry. The values are means of six experiments.
As with live NTHIF+, paraformaldehyde-prefixed NTHIF+ attachment to pRSV-exposed A549 cells was higher than that to control cells (Fig. 5). The slightly higher attachment for live NTHI than prefixed NTHI was directly related to the ongoing bacterial replication of live NTHI during the assay (data not shown). These results suggested that activation or modification of P5-fimbriae proteins during contact with A549 cells is not required for enhanced attachment.
FIG. 5.
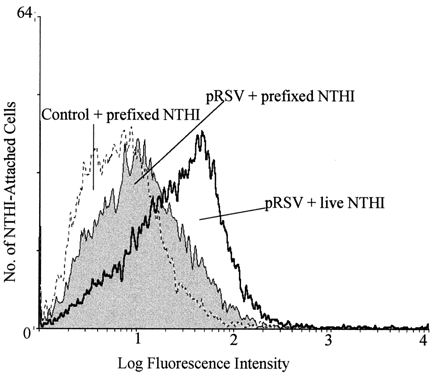
Comparison of attachment of live or prefixed (1% paraformaldehyde) NTHIF+ to pRSV-exposed (MOI = 1, 24 h) or control A549 cells (A549:NTHI = 1:100), as determined by flow cytometry.
The attachment of NTHI was further evaluated by transmission electron microscopy. The results showed that only a few NTHIF+ and NTHIF− were seen attached to the cell membrane of control cells (Table 1). Following pRSV exposure, however, >5-fold more NTHIF+ than NTHIF− attached to the cells, as evaluated by all parameters—tight or loose attachment to the cell body, and tight attachment to microvilli (99 NTHIF+ versus 18 NTHIF− per 100 A549 cells [Table 1]). Nonetheless, the attachment of NTHIF− was slightly but significantly higher for RSV-exposed than control cells (3.5 NTHIF+ versus 18 NTHIF− per 100 A549 cells), suggesting that adhesins other than P5-fimbriae may also play a role, albeit a very small one, in attachment. A549 cells exhibited electron-dense areas underlying attached NTHI and microvilli projections attaching to NTHI (Fig. 6). No intracellular NTHI was seen, suggesting that the enhanced association between NTHI and RSV-exposed A549 cells was purely at the surface membrane level.
TABLE 1.
Quantitative analysis of NTHI attachment to pRSV-exposed or control A549 cells by transmission electron microscopy
| Determination | Control cells
|
pRSV-exposed cells
|
||
|---|---|---|---|---|
| NTHIF+ | NTHIF− | NTHIF+ | NTHIF− | |
| A549 cell count | 172 | 157 | 148 | 141 |
| NTHI attachment | ||||
| Tight | 2 | 0 | 90 | 23 |
| Loose | 4 | 4 | 51 | 1 |
| Tight to microvilli | 0 | 0 | 6 | 1 |
| No. of NTHI attached/100 A549 cellsa | 3.5 a | 2.5 b | 99 c | 18 d |
Significance of a versus b, P = 0.076; significance of c versus d, P < 0.001; (calculated by z test with Yates correction).
FIG. 6.
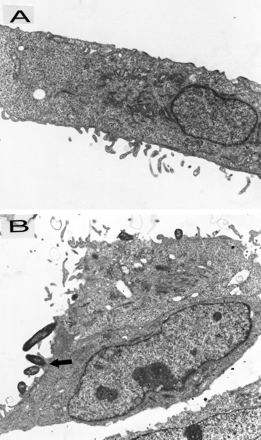
Transmission electron micrographs of A549 cells incubated with NTHIF+ (A549:NTHI = 1:100). (A) Control cells (magnification, ×14,300), which do not show any attached NTHI. (B) pRSV-exposed (MOI = 1, 24 h) cells (magnification, ×10,725); the arrow points to a microvillus projection adhering to NTHI with an the electron-dense area underlying the attached NTHI.
Overall, the results of flow cytometry and electron microscopy studies suggested that the P5-fimbriae are critical for NTHI attachment to control A549 cells. The increase in attachment to pRSV-exposed cells is also dependent on the P5-fimbriae.
Relationship between NTHI attachment and RSV antigen expression.
The relationship between NTHI attachment and cellular RSV antigen expression was analyzed to determine whether the bacterial attachment was directly influenced by viral replication within the cell. At 24 and 48 h of pRSV exposure, studies by two-color flow cytometry showed that while NTHIF+ attachment to A549 cells increased over time, there was no statistically significant difference in attachment to RSV antigen-positive or -negative cells (Fig. 7). Furthermore, studies by transmission electron microscopy revealed very few RSV particles in pRSV-exposed cells, with no clear association between the presence of cell-associated virus and NTHI attachment.
FIG. 7.
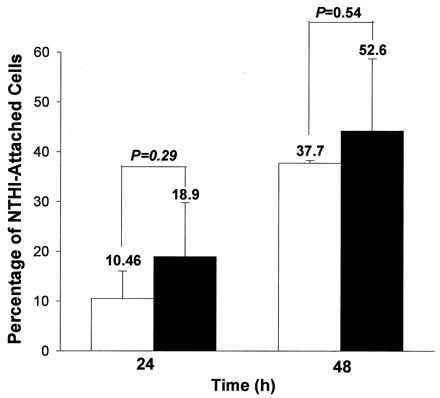
Comparison of attachment of NTHIF+ (A549:NTHI = 1:100) to RSV antigen-positive (□) or -negative (■) A549 cells exposed to pRSV (MOI = 1, 24 h), as determined by two color flow cytometry. The values are means of three experiments.
Role of soluble mediators in NTHI attachment.
As there was no relationship between RSV antigen expression and NTHI attachment, we next postulated that soluble mediators released by RSV-infected cells could induce, in an autocrine or paracrine manner, the synthesis or activation of NTHI-binding surface receptors on RSV-uninfected A549 cells. Conditioned supernatants from pRSV-exposed A549 cultures were rendered noninfectious by UV irradiation and subsequently incubated with fresh A549 cells (diluted 1:2 with fresh culture medium, 1 ml/well). After 24 h, NTHI attachment to suspended A549 cells was analyzed by flow cytometry. The results showed that the effect of UV-cRSV exposure on attachment of NTHIF+ and NTHIF− was comparable with that of live pRSV exposure (Fig. 8).
FIG. 8.
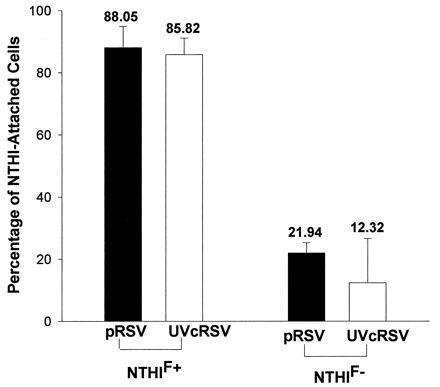
Percentages of pRSV-exposed (MOI = 1, 24 h) and UV-cRSV-exposed (1:2 dilution, 24 h) A549 cells demonstrating attached NTHIF+ or NTHIF− (A549:NTHI = 1:100), as determined by flow cytometry. The values are means of four experiments.
DISCUSSION
NTHI commonly exists in an asymptomatic nasopharyngeal colonization state. Respiratory viral infection is the commonest factor associated with the spread of NTHI contiguously in the respiratory tract to produce disease. We have previously shown that RSV infection enhances NTHI nasopharyngeal colonization in experimental animals (23). Also, Raza et al. have shown that binding of H. influenzae type b and Neisseria meningitidis to epithelial cells is enhanced by RSV infection in HEp-2 cells (26). Several adhesins of NTHI have been described, but their significance in the virus-enhanced disease state has not been studied. In this respect, we report here that the specific NTHI interaction with RSV-exposed respiratory epithelial cell in vitro involves enhanced attachment of NTHI that is primarily mediated by its P5-fimbriae.
In the present study, NTHIF− bound less avidly to normal as well as RSV-exposed A549 cells, suggesting that for the two NTHI strains used in the experiments, other adhesins of NTHI played a minor role in attachment. The role of P5-fimbriae is further strengthened by the previous observations that 100% of clinical strains are fimbriated (2). Furthermore, in a chinchilla model of otitis media, the P5-fimbriae have been shown to be important for adhesion and a proven virulence factor (2, 3). The role of other NTHI adhesins in clinical settings appears to be more variable. Approximately 32% of strains express hemagglutinating pili, and nonpiliated strains adhere just as well as piliated strains (13). Approximately 20 to 30% of NTHI isolates lack the HMW1/HMW2 family of adhesins (32). Another adhesin, Hia, is universally absent from NTHI that expresses HMW1 or HMW2 (32). Nonetheless, the roles of these adhesins, singly or in combination, in virus-enhanced attachment of NTHI need to be explored. Furthermore, our observations need to be confirmed in vitro, using respiratory epithelial cells of diverse origin, as well as in vivo.
We determined by transmission electron microscopy that the NTHI interaction with epithelial cells was similar to that described by Holmes and Bakaletz (16). A549 cells exhibited electron-dense areas underlying the attached NTHI and microvilli projections attaching to NTHI. The present study also showed that NTHI was not internalized by A549 cells. In contrast, Gilsdorf et al. (13) showed that H. influenzae type b is internalized by A549 cells. However, only 4 to 5% of NTHI was internalized, as determined by the gentamicin survivability test, and no electron microscopic observations were made. Studies by St. Geme and Falkow (31) demonstrated intracellular NTHI in Chang (human conjunctival) epithelial cells by electron microscopy. Nonetheless, our studies suggest that in A549 cells, the intracellular invasion is probably an infrequent event and that it is not enhanced by RSV infection. In vivo though, it is possible that NTHI exists in an intracellular quiescent stage in respiratory tissues such as the adenoids (10).
Since paraformaldehyde-fixed bacteria also adhered in enhanced numbers to RSV-exposed cells, our results suggest that de novo bacterial protein synthesis was not required for the P5-fimbriae to interact with the cells. The modest increase in adherence of live compared with fixed bacteria seen in our study, and possibly those seen in the studies of St. Geme and Falkow (31), could be due to the ongoing replication of live bacteria during the bacterium-epithelial cell coincubation stage. On the other hand, initial live RSV infection of epithelial cells was required to enhance the adhesiveness of the cells, since incubation with UV-irradiated purified RSV did not result in enhancement of NTHI attachment. The effect of live RSV infection was probably mediated by a soluble mediator(s) produced by RSV-infected cells. The identity of the bacterial attachment-enhancing mediator(s) has not been determined, but potential candidates include RSV-induced soluble cytokines by epithelial cells, such as interleukin-1α, interleukin-1β, and tumor necrosis factor alpha (23). These cytokines are known to activate several nuclear transcription factors that regulate the synthesis of a variety of proteins.
Elahmer et al. have shown that RSV up-regulates the attachment of N. meningitidis to HEp-2 cells via increased cellular expression of CD14 (8). The nature of the NTHI P5-fimbriae-binding receptor(s) expressed on the epithelial cells exposed to RSV has not been studied. Previous studies have shown that NTHI binds to sialylated oligosaccharides of normal epithelial cells. NTHI also binds to oligosaccharide-containing mucin secreted by epithelial cells (7, 9, 20). Specifically, Fakih et al. have shown that NTHI fimbriae bind to sialylated gangliosides on HEp-2 epithelial cells (9). Whether RSV infection enhances the P5-fimbria-mediated attachment of NTHI by increasing the expression of gangliosides on epithelial cells needs further investigation. The enhancement of attachment of bacteria by a variety of viruses may be also explained by the fact that many bacterial species use oligosaccharide receptors on epithelial cells and in mucin (25).
In summary, the present study shows that RSV enhances attachment of NTHI to epithelial cells which is largely mediated by NTHI P5-fimbria-binding receptors. In clinical settings, blocking of P5-fimbria-mediated attachment of NTHIF+ by passive or active immunity may reduce the disease incidence and severity during RSV infection.
ACKNOWLEDGMENTS
This work was supported by NIDCD grant DC-02129.
We thank Sandra Fuentes for secretarial assistance.
REFERENCES
- 1.Bakaletz L O, Ahmed M A, Forney I J, Kolatukudy P F, Lim D J. Cloning and sequence analysis of a pilin-like gene from an otitis media isolate of nontypable Haemophilus influenzae (strain # 1128) J Infect Dis. 1992;165:S201–S203. doi: 10.1093/infdis/165-supplement_1-s201. [DOI] [PubMed] [Google Scholar]
- 2.Bakaletz L O, Tallan B M, Hoepf T, DeMaria T F, Birck H G, Lim D J. Frequency of fimbriation of nontypeable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun. 1988;56:331–335. doi: 10.1128/iai.56.2.331-335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakaletz L O, Tallan B M, Andrzejewski W J, DeMaria H G, Lim D J. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins on nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3226–3229. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton C C, Jr, Carter M J, Derber D B, Kar S, Kramarik J A, Wood S W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis J. 1989;8:S54–S61. [PubMed] [Google Scholar]
- 5.Chonmaitree A, Heikkinen T. Role of viruses in middle-ear disease. Ann NY Acad Sci. 1997;830:143–157. doi: 10.1111/j.1749-6632.1997.tb51886.x. [DOI] [PubMed] [Google Scholar]
- 6.Chonmaitree T, Owen M J, Patel J A, Hedgpeth D, Horlick D, Howie V M. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120:856–862. doi: 10.1016/s0022-3476(05)81950-x. [DOI] [PubMed] [Google Scholar]
- 7.Davies J, Carlstedt I, Nilsson A, Hakansson A, Sabharwal H, Alphen L V, Ham M V, Svanborg C. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect Immun. 1995;63:2485–2492. doi: 10.1128/iai.63.7.2485-2492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elahmer O R, Raza N W, Ogilvie M M, Blackwell C C, Weir D M, Elton R A. The effect of respiratory syncytial virus infection on expression of cell surface antigens associated with binding of potentially pathogenic bacteria. In: Kahane I, Ofek I, editors. Toward anti-adhesion therapy for microbial diseases. New York, N.Y: Plenum Press; 1996. pp. 169–177. [DOI] [PubMed] [Google Scholar]
- 9.Fakih M G, Murphy T F, Pattoli M A, Berenson C S. Specific binding of Haemophilus influenzae to minor gangliosides of human respiratory epithelial cells. Infect Immun. 1997;65:1695–1700. doi: 10.1128/iai.65.5.1695-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus- and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 11.Ghafoor A, Nomanl N K, Ishaq Z, Sohail Z Z, Anwar F, Burney M I, Qureshi W, Ahmad S A. Diagnoses of acute lower respiratory tract infections in children in Rawalpindi and Islamabad, Pakistan. Rev Infect Dis. 1990;12:S907–S914. doi: 10.1093/clinids/12.supplement_8.s907. [DOI] [PubMed] [Google Scholar]
- 12.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilsdorf J R, Tucci M, Marrs C F. Role of pili in Haemophilus influenzae adherence to, and internalization by, respiratory cells. Pediatr Res. 1996;39:343–348. doi: 10.1203/00006450-199602000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D Tonawanda/Williamsville Pediatrics. Nasopharyngeal colonozation with nontypeable Haemophilus influenzae and recurrent otitis media. J Infect Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- 15.Hietella J, Uhari M, Tuokko H, Leionen M. Mixed bacterial and viral infections are common in children. Pediatr Infect Dis J. 1989;8:683–686. doi: 10.1097/00006454-198910000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Holmes K A, Bakaletz L O. Adherence of nontypable Haemophilus influenzae promotes reorganization of the actin cytoskeleton in human or chinchilla epithelial cells in vitro. Microb Pathog. 1997;23:157–166. doi: 10.1006/mpat.1997.0145. [DOI] [PubMed] [Google Scholar]
- 17.Ingvarsson L, Lundgren K, Ursing J. The bacterial flora in nasopharynx in healthy children. Acta Otolaryngol. 1982;386:S94–S96. [Google Scholar]
- 18.John T J, Cherian T, Steinhoff M C, Simoes E A F, John M. Etiology of acute respiratory infections in children in tropical southern India. Rev Infect Dis. 1991;13:S463–S469. doi: 10.1093/clinids/13.supplement_6.s463. [DOI] [PubMed] [Google Scholar]
- 19.Korppi M, Leinonen M, Koskela M, Makela H, Launiala K. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr Infect Dis J. 1989;8:687–692. doi: 10.1097/00006454-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Marieke Van Ham S, Alphen L, Mool F R, Putten J P M. Contribution of the major and minor subunits to fimbria-mediated adherence of Haemophilus influenzae to human epithelial cells and erythrocytes. Infect Immun. 1995;63:4883–4889. doi: 10.1128/iai.63.12.4883-4889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto N, Bakaletz L O. Selective attachment of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 22.Murphy T F, Apicella M A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Patel J A, Faden H, Sharma S, Ogra P L. Effect of respiratory syncytial virus on attachment, colonization, and immunity of nontypable Haemophilus influenzae: implication in otitis media. Int J Pediatr Otorhinolaryngol. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]
- 24.Patel J A, Kunimoto M, Garofalo R, Sim T C, Ruuskanen O, Chonmaitree T, Ogra P L, Schmalstieg F. IL-1 mediates enhanced expression of ICAM-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 25.Plotkowski M C, Bajolet-Laudinat O, Puchelle E. Cellular and molecular mechanisms of bacterial adhesion to respiratory mucosa. Eur Respir J. 1993;6:903–916. [PubMed] [Google Scholar]
- 26.Raza M W, Ogilvie M M, Blackwell C C, Stewart J, Elton R A, Weir D M. Effect of respiratory syncytial virus infection on binding of Neisseria meningitidis and Haemophilus influenzae type b to a human epithelial cell line (Hep-2) Epidemiol Infect. 1993;110:339–347. doi: 10.1017/s095026880006828x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read R C, Wilson R, Rutman A, Lund V, Todd H C, Brain A R, Jeffery P K, Cole P J. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 28.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyneal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruuskanen O, Heikkinen T. Viral-bacterial interaction in acute otitis media. Pediatr Infect Dis J. 1994;13:1047–1049. doi: 10.1097/00006454-199411000-00034. [DOI] [PubMed] [Google Scholar]
- 30.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St. Geme J W, III, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St. Geme J W, III, Kumar V V, Cutter D, Barenkamp S J. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun. 1998;66:364–368. doi: 10.1128/iai.66.1.364-368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueba O. Respiratory syncytial virus concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–272. [PubMed] [Google Scholar]


