Abstract
Inflammation including neuroinflammation is considered a protective response and is directed to repair, regenerate, and restore damaged tissues in the central nervous system. Persistent inflammation due to chronic stress, age related accrual of free radicals, subclinical infections or other factors lead to reduced survival and increased neuronal death. Circadian abnormalities secondary to altered sleep/wake cycles is one of the earliest signs of neurodegenerative diseases. Brain specific or global deficiency of core circadian trans-activator brain and muscle ARNT (Arylhydrocarbon Receptor Nuclear Translocator)-like protein 1 (BMAL1) or that of the transrepressor REV-ERBα, impaired neural function and cognitive performance in rodents. Consistently, transcripts of inflammatory cytokines and host immune responses have been shown to exhibit diurnal variation, in parallel with the disruption of the circadian rhythm. Glucocorticoids that exhibit both a circadian rhythm similar to that of the core clock transactivator BMAL1 and tissue specific ultradian rhythm are critical in the control of neuroinflammation and re-establishment of homeostasis. It is widely accepted that the glucocorticoids suppress nuclear factor-kappa B (NF-κB) mediated transactivation and suppress inflammation. Recent mechanistic elucidations suggest that the core clock components also modulate NF-κB mediated transactivation in the brain and peripheral tissues. In this review we discuss evidence for interactions between the circadian clock components, glucocorticoids and NF-κB signaling responses in the brain and propose glucocorticoid induced leucine zipper (GILZ) encoded by Tsc22d3, as a molecular link that connect all three pathways in the maintenance of CNS homeostasis as well as in the pathogenesis of neuroinflammation-neurodegeneration.
Keywords: CLOCK, glucocorticoids, NF-κB p65, GILZ
Introduction
Circadian or diurnal rhythms in gene expression occur in almost all animal tissues due to daily cycles of activity including eating, sleeping, and fasting. Such rhythms are driven by an internal clock system consisting of transcriptional-translational feedback loops (TTLs) that gives rise to gene networks generating self-sustained 24h cycle oscillations. The clock’s core transcriptional-translational feedback loops (TTL) consists of CLOCK (circadian locomotor output cycles kaput) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) transcriptional factors that function as heterodimers, bind the E-box cis elements, and activate transcription of other core clock components, including Period (PER1, 2, 3) and the Cryptochrome (CRY1, 2) genes. period (PER) and CRY proteins upon reaching a certain level in the cytoplasm form a heterodimeric complex, translocate to the nucleus, and inhibit CLOCK/BMAL1 transcription, forming a negative feedback transcriptional loop that maintains a 24h oscillation. In general, it is observed that BMAL1 peaks at or just before resting at around Zeitgeber Time (ZT0) in murine tissues in antiphase with PER1 and PER2. While PER1 has been shown to peak at ZT12, Per2 exhibits a gradual increase reaching a peak at ZT18 (Figure 1A). This core loop is assisted by accessory loops that provide additional levels of regulation to stabilize molecular oscillations and increase system robustness. Prominent accessory TTL genes that are also transactivated by CLOCK/BMAL1 heterodimers include the REV-ERBα/b (reverse erythroblastoma) and the RORα−γ (retinoic acid receptor-related orphan receptor α−γ). While the REV-ERB proteins inhibit BMAL1 transcription and exert negative feedback, the RORs are positive regulators of BMAL1 transcription (Fonken et al., 2015; Nader et al., 2009; Spencer et al., 2018). The daily expression rhythms in >80% of protein-coding genes constitutes a conserved regulatory mechanism that integrates diverse biochemical functions within and across cell types (Mure et al., 2018).
Figure 1.
Schematic representation of core clock mechanism. The molecular clock is an autoregulatory loop consisting of BMAL1/CLOCK heterodimer which binds the E-Box response elements and activates the transcription of Period (Per) and Cryptochrome (Cry) genes. PER and CRY proteins in the cytoplasm form a heterodimer, translocate to the nucleus, and inhibit CLOCK:BMAL1 forming the negative arm of the feedback loop. The accessory loop includes the RORs and REV-ERB factors with positive feedback by RORs transactivating BMAL1 and negative feedback by REV-ERB. (B) Schematic representation of GC interactions with the clock components (neurotoxic) and NF-κB p65 (neuroprotective). CLOCK protein acetylates GR protein preventing it from binding the GRE and inhibit the transactivation of anti-inflammatory genes. GR either tether or interfere with the DNA-bound p65 by recruiting transcription corepressor and by inhibiting the recruitment of transcriptional coactivators such as nuclear coactivator. (C) Schematic representation of NF-κB p65 interactions and molecular clock. NF-κB p65 directly interacts with BMAL1 via protein: protein interaction and prevents the later from binding the E-Box, thereby exerting a direct trans-repressing effect. CLOCK protein binds with the p65 protein or its co-regulators and enhance κB mediated transactivation of inflammatory cytokines.
The molecular clock regulates thousands of output genes that govern many physiological systems and cellular processes. The hypothalamic-pituitary axis and the glucocorticoids (GC) (corticosterone in rodents) feedback loop is one such system that exhibits strong circadian rhythm and influences cell growth, apoptosis, and inflammation (Spencer et al., 2018). The release of GC by the adrenal cortex peaks early in the active phase (morning for diurnal or evening for nocturnal species) to prepare the brain and the body for physical and cognitive activities and reaches nadir in the resting phase (evening for diurnal and morning for nocturnal species). In addition, the GC also exhibit hourly pulses (ultradian rhythm), the amplitude of which is modulated in an activity (daytime) dependent fashion (Qian et al., 2012; Stavreva et al., 2009).
At the cellular level, the GC act by binding two receptor subtypes, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) in the cytoplasm with the former exhibiting higher affinity for GC than the latter. Upon binding the ligand, the GC: receptor complex translocates to the nucleus, binds specific DNA sequences called the glucocorticoid response elements (GREs), to modulate transcription of responsive genes. The high affinity MR is occupied by GC most of the day. However, GR occupancy increases at higher GC concentrations, such as during the peak and ultradian pulses. Thus, GR is often the main GC receptor responsible for regulating the gene expression during circadian oscillation (Bekhbat et al., 2017; Spencer et al., 2018).
Cellular and animal experiments showed that the ultradian GC treatment induces pulsatile release of GR regulated genes by a process referred to as ‘gene pulsing’ (Stavreva et al., 2009). GR mRNA expression was two-fold higher in the morning than in the evening in human peripheral blood mononuclear cells in correlation with the GC rhythm. In parallel, genes transactivated by GR such as the dual specificity phosphatase 1 (DUSP1), glucocorticoid-inducible leucine zipper protein (GILZ) and the tristetraprolin exhibit rhythmic expression, being higher in the morning than in the evening (Charmandari et al., 2011). The low circulating GC and the reduced GR activity in the evening correlate with the increased expression of inflammatory genes (Fonken et al., 2016b).
Modulation of GR Transactivation Properties by Circadian Clock Genes
Considerable data suggest that the GC regulate the components of both the core and the accessary clock loops (Figure 1B). Significantly, it has been suggested that CLOCK/BMAL1 heterodimers function as limiting factors or feedback regulators of GR induced transcriptional activity. Consistently, it was observed that the hepatocytes isolated from dexamethasone treated young adult male mice entrained to a 12:12 light–dark photoperiodic cycle exhibited peak expression of GR mediated GILZ and FKBP5 transcripts at ZT00 in anti-phase with CLOCK/BMAL1. In human cell lines transfected with GC responsive reporter plasmid, overexpression of CLOCK/BMAL1 repressed GR-induced transcriptional activity independent of its E-box mediated transactivation. It was observed that BMAL1 tethering to DNA supported CLOCK mediated repression of GR activity, although the GR repressive ability of BMAL1 alone has also been reported. Multiple mechanisms have been proposed for CLOCK/BMAL1 mediated repression of GR activity. It has been suggested that the increased PER1/CRY1 complex within the nucleus by CLOCK/BMAL1 could exert strong negative influence on the glucocorticoid response element (GRE)-dependent target genes expression. Acetylation of multiple lysine residues in the hinge region of GR by CLOCK protein, attenuates the ability of GR to bind GREs and thus suppress target gene transactivation in a GC dependent fashion (Nader et al., 2009; Spencer et al., 2018).
In healthy humans, serum cortisol is 2–4 times higher in the morning than at night (Roelfsema et al., 2017; Yurtsever et al., 2019). Transcript analysis of human peripheral blood mononuclear cells showed that CLOCK and BMAL1 mRNA expressions was higher at 8 am than at night mimicking GC circadian rhythm. In contrast, the expression of PER1 transcript was higher at night than in the morning suggesting reciprocal circadian regulation as that of Clock/Bmal1. PER3 expression also exhibited rhythmic oscillations across day/night cycle in a reverse direction to that of the GC. Furthermore, following dexamethasone treatment, induction of GC responsive transcripts exhibited similar time of day rhythm as that of CLOCK and BMAL1 mRNA in human blood mononuclear cells (Charmandari et al., 2011). However, as opposed to the observations in human tissues, the expression of BMAL1 in murine tissues is anti-phasic to GC rhythm (Nader et al., 2009, 2010). While the GC induced transcripts of CLOCK, BMAL1 and PER1 involved binding ofe GRE in rodent tissues, expression of PER2 mRNA was mediated by the overlapping GRE and E-box (GE2) elements in a BMAL1 dependent manner. Glucocorticoid-mediated PER2 induction delayed the phase of the circadian rhythm (Cheon et al., 2013). Further, corticosterone treatment decreased the expression of the accessory clock genes REV-ERBα and RORα in a GR-dependent manner, supporting a direct trans-repressive effect (Gibbs et al., 2012; Griffin et al., 2019). The clock repressors CRY1 and CRY2 bind GR directly, interfere with its ability to bind GRE, and consequently modulate target gene expression (Lamia et al., 2011). While the pulsatile induction of PER1-2 transcription following ultradian GC exposure support the presence of functional GRE in the promoter region of PER genes, the relative role of GRE and E-box elements is yet to be elucidated (Spencer et al., 2018; Yurtsever et al., 2016).
Interactions Between GC, Clock Components and Nuclear Factor-κB
Extensive data also suggest that the GC and the clock components regulate inflammatory responses mediated by the transcription factor nuclear factor-kappa B (NF-κB) (Haimovich et al., 2010; Hong et al., 2018). The NF-κB family includes five reticuloendotheliosis viral oncogene (REL) homology domain proteins that function as homo or heterodimers and play critical roles in immune-inflammatory responses. In most cell types, NF-κB is represented mainly by the p65(rel avian reticuloendotheliosis viral oncogene homolog A [RELA])/p50 heterodimers. In resting cells, the p65:p50 heterodimer remains as an inactive complex with the inhibitory IκB proteins. Upon activation the p65:p50 dimer translocate to the nucleus where it binds specific DNA sequences called κB response elements and induce transactivation of target genes (Giridharan & Srinivasan, 2018). In-vivo tracking of activated p65 showed that the expression of κB responsive genes were significantly higher in animals that were challenged during the resting period (GC low) than during the active period (GC high) supporting the circadian influence on NF-κB p65 activation (Han et al., 2021). The profound anti-inflammatory effects of GC are largely attributed to GR mediated inhibition of NF-κB p65, by one of three methods. Firstly, GR binds GRE in the promoter and enhance trans-activation of anti-inflammatory proteins such as annexin 1, GILZ and IκBα (Bekhbat et al., 2017; Desmet & De Bosscher, 2017). GILZ is a leucine zipper protein that belongs to the transforming growth factor β-stimulated clone-22 (Tsc22d3) family of transcription factors. GILZ protein binds the transactivation domain of activated p65 and prevents its nuclear translocation (Cannarile et al., 2001; Di Marco et al., 2007). Although GILZ and IκBα have been shown to inhibit activated NF-κB in the cytoplasm, little is known about the synchronization or delays in their transcription and subsequent inhibitory functions. Secondly, GR tethers and interferes with the ability of p65 to bind DNA. Thirdly, GR interacts with the DNA-bound p65 by recruiting transcription repressor such as nuclear receptor corepressor and by inhibiting the recruitment of transcriptional coactivators such as nuclear coactivator (Bekhbat et al., 2017). In addition, time course analyzes suggested that the GR mediated NF-κB activity exhibits bimodal responses. While the inhibitory potential of GR was low at basal GC, elevated plasma cortisol in the early phase of sickness increased its inhibitory potential. Further, the suppressive potential was lost subsequently with the ensuing development of GC resistance(Bekhbat et al., 2017; Han et al., 2021).
Indeed, the core clock genes PER1, CRY2, D site of albumin promoter binding protein, and REV-ERBα, possess κB binding sequences downstream of the E-box motif in the promoter region. Pertinently, pronounced p65 binding in the κB response element in PER2 promoter and its trans-repression has been reported in the hepatocytes of saline injected mice (Hong et al., 2018). This suggests a tonic role for RELA in the control of the negative feedback arm of the clock transcription loop even in the unstimulated state. In-vitro cellular transfection studies showed that the overexpression of RELA reduced the transcription of PER and REV-ERB genes (Spengler et al., 2012). In endotoxin or tumor necrosis factor (TNF)-α challenged mice, the elevated RELA correlated with reduced D site of albumin promoter (albumin D-box) binding protein and PER transcription in the liver, lungs, and other tissues. Much like the canonical CRY1 repressors, increased RELA repressed circadian oscillations without affecting the cell viability (Shen et al., 2021). Intravenous administration of a bolus of endotoxin(2ng/kg) resulted in a profound suppression of CLOCK, CRY1-2, PER3, CSNK1ε, RORα and REV- ERB genes in peripheral blood monocytes with the nadir occurring between 3 to 6 h post-infusion. While the expression of PER1 and PER2 reached a nadir later at 12h post-infusion, CLOCK gene expression remained suppressed for up to 17 h, irrespective of the phase of the clock at the time of the endotoxin challenge. The cytokines IL-6 and TNF-α peaked within 2hrs post-infusion and returned to baseline within 6 h (Gibbs et al., 2012; Haimovich et al., 2010). Furthermore, elevated plasma IL-6 has been correlated with decreased CRY1 transcript during sepsis (Li et al., 2013). However, colocalization of CLOCK: BMAL1 within the E-box in the promoter of clock genes suggest that their binding is not mutually exclusive(Hong et al., 2018). Taken together, it is evident that CLOCK expression in peripheral blood monocytes is dramatically altered during periods of acute systemic inflammation. Mechanistically, a direct interaction is observed between the REL homology domain of p65 and the transactivation domain (TAD) of BMAL1. Since the clock repressors, CRY1-2 inhibit interactions between BMAL1-TAD and transcriptional coactivators, it has been suggested that the RELA potentially competes with the CRY and the coactivator CREB binding protein/p300 for binding BMAL1-TAD and mediates transcriptional repression of the clock feedback (Shen et al., 2021).
Interestingly, RELA has been shown to interact with the two proteins in CLOCK: BMALI1 heterodimer, with distinct effects. While interaction with BMAL1 suppressed the transactivation of clock repressors as discussed above, CLOCK protein enhances RELA mediated transactivation of κB-responsive genes. It was observed that the κB-responsive promoter activation and the p65 mediated inflammatory cytokine secretion was significantly higher in mice stimulated during the resting period as opposed to the active periods correlating with levels of CLOCK: BMAL1 expression (Spengler et al., 2012). Transfection of human embryonic kidney cells-293T cells with κB-Luc reporter along with different combinations of RELA, CLOCK and BMAL1 expressing plasmids, increasing CLOCK expression correlated with increased RELA activation (Hong et al., 2018). Mechanistically, CLOCK formed a protein complex with RELA and mediated strong κB promoter activation. Indeed, CLOCK-mediated increase in κB promoter activation was associated with a dose-dependent increase in phosphorylated p65, the active state of RELA (Spengler et al., 2012). Furthermore, in activated cells CLOCK/BMAL1 was shown to re-localize to p65 binding κB sites in inflammatory genes and enhance the transactivation. This ability of CLOCK to enhance RELA mediated transactivation was observed to be independent of BMAL1, although its co-expression partially countered the increased RELA responses (Hong et al., 2018). Consistently, knock down of CLOCK gene reduced nuclear p65 and related gene transcription in multiple tissues. Together, these data support the presence of tightly controlled feedback loops between NF-κB p65 and clock systems at the molecular level (Shen et al., 2021; Spengler et al., 2012).
Cross Talk Between GC, Molecular Clock, and NF-κB in Neuroinflammation and Neurodegeneration
A large body of evidence suggests that the brain homeostasis is dependent on balanced intrinsic inflammatory and repair processes that are often under circadian control. Indeed, while subthreshold inflammatory reactions termed euflammation has been shown to stimulate the immune system, mild activation prior to injury or infection is known to reduce the subsequent excessive inflammation and improve neuroprotective responses. Hence, a degree of neuroinflammation is beneficial under physiological conditions and does not involve damage to the blood brain barrier or cause neuropathology. In general, while the central nervous system can quickly resolve and normalize the effects of acute inflammation, ample evidence suggest that the disruption of the interdependent relationship between the circadian rhythm of clock genes, GC and NF-κB signaling sensitizes the brain to chronic inflammatory challenges and associated pathologies (de Kloet et al., 2018; Liu et al., 2016; Sochocka et al., 2017; Tarr et al., 2014; Wendeln et al., 2018).
GCs regulate the expressions of clock genes in many regions of the brain (Bonaconsa et al., 2013; Chen et al., 2021; Leliavski et al., 2014; Spencer et al., 2018). Using a microdialysis probe inserted in both the jugular vein and the hippocampus in adult rats, Qian et al. found that the free corticosterone in both compartments exhibited very similar rhythms (Qian et al., 2012). This suggests that the assessment of cortisol concentration in the plasma is a true representation of that in the brain. Consistently, diurnal variation in the clock repressor PER-2 that is entrained by circulating corticosterone has been observed in amygdala, dentate gyrus, and prefrontal cortex in mice (Girotti et al., 2009; Woodruff et al., 2016). In adult rats, robust 24h rhythmic PER1, PER2, and BMAL1 mRNA expression occurred in the paraventricular nucleus, anterior cingulate cortex, ventral orbital cortex, rostral agranular insula (insula), and basal lateral amygdala. BMAL1 expression was antiphase with that of PER1/PER2 mRNA and peaked at different time points in different regions of the brain. This is partly supported by the nycthemeral expression of PER1 and PER2 mRNA in the brain exhibiting two acrophases, in the mid-light phase and the early to mid-dark phase (Chun et al., 2015). At the cellular level, microglia from adult rats have been shown to exhibit rhythmic expression of PER1, PER2 and REV-ERBα with peak expression during the light or resting phase in the unstimulated state. Further, pulsatile corticosterone also induced cyclic PER1 transcription through a direct effect mediated by GR (Conway-Campbell et al., 2010). In murine astrocytes, light induced disruption of the circadian rhythm resulted in loss of detectable oscillations in BMAL1 (Lananna et al., 2018). Taken together, BMAL1 and the clock transrepressors are entrained by the GC rhythm, mediated by GR binding GRE in the promoter region of target genes (Chun et al., 2015; Yamamoto et al., 2005).
Age related changes in circulating cortisol have been variably reported in humans, with many studies suggesting higher plasma cortisol concentrations in older individuals (Echouffo-Tcheugui et al., 2018; Hatfeld et al., 2004; Pietrzak et al., 2017). The discrepancies in observations could be attributed to the methods of assessment and the number and frequency of samples assessed. Data community-based studies suggest that higher morning cortisol in humans correlated with worse cognitive performances, reduced brain volumes, microvascular damage, and alterations in brain microstructure (Echouffo-Tcheugui et al., 2018; Roelfsema et al., 2017). In addition, nycthemeral observations showed that the plasma cortisol in humans was higher even in the late evening and early night times in older than young individuals (Echouffo-Tcheugui et al., 2018). Pertinently, by associating the relative gene expression with the time of death, diurnal rhythmicity of BMAL1, PER1 and PER2 mRNA have been implicated in many regions in human brain (Chen et al., 2016; Li et al., 2013; Spencer et al., 2018). Similar time of death analysis in zeitgeber time (ZT) time scales of 146 postmortem human brain samples showed consistent age-related effects in the expression of canonical clock genes in the prefrontal cortex. The Broadmann’s area 47 (BA47) appeared to be strongly influenced by age since greater number of changes in patters of gene expression was observed in BA47 than in BA11. Specifically, in older individuals the acrophases of PER1 rhythms shifted from ZT5 (BA11) and ZT7 (BA47) to ZT1–ZT2 with a reduction in amplitude in both regions. Similar phase shifts in peak expression were observed for PER2, suggesting significantly disrupted temporal expression of PER1 and PER2 in older individuals(Chen et al., 2016). Comparably, in aged rats (24 months old) consistent with the anti-phaseBMAL1:GC relationship, the expression of clock transrepressors PER1 and PER2 in the hippocampal microglia was lower during the light/resting phase with significant attenuation in the CA1 and CA2 regions (Fonken et al., 2016a; Sellix et al., 2012). In contrast, the hippocampal microglia in young rats exhibit rhythmic expression of PER1 and PER2 transcripts(Fonken et al., 2016a).
Desynchronization of circadian clock and chronic GC elevation, common features frequently observed in the elderly individuals have been attributed to mediate neuroinflammation and neurodegeneration observed in dementia, depression, and other brain pathologies (Chen et al., 2016; de Kloet et al., 2018). While BMAL-1 expression exhibits regular circadian oscillations with peak in the early evening in healthy brain tissues, in early Alzheimer’s disease (AD) cortical tissues BMAL-1 exhibited aberrant methylation and abnormal transcription with peak expression in the morning (Cronin et al., 2017). Global or brain specific deletion of BMAL1 or CLOCK induced severe age dependent astrogliosis, inflammation, and oxidative stress in mice (Musiek et al., 2013). A potential mechanism by which BMAL1 could modulate neuroinflammation is by regulating the transcription of other clock components. Indeed, deletion of REV-ERBα/b has been shown to induce hippocampal microgliosis, elevated proinflammatory profile and neuronal damage in mice (Gibbs et al., 2012; Griffin et al., 2019). Ex-vivo corticosterone treatment of microglia isolated from adult rats during the middle of the dark phase exhibited dose dependent upregulation of PER1 and BMAL-1 up to 100nM but this was lost at higher corticosterone concentration. Further, the expression of anti-inflammatory markers CD200R and CX3CR1 exhibited a dose dependent decrease in corticosterone treated microglia isolated from adult rats during the middle of the light or dark phase. In contrast, the expression of NFκBIA, a negative regulator of NF-κB increased in a dose-dependent manner (Fonken et al., 2016b). In vivo observations showed that in aged rats the unstimulated microglia express higher TNF-α in the light/resting phase suggesting loss of phasic expression with GC rhythm observed in young rats. However, no age-related difference was observed in the ability of microglia to respond to exogenous corticosterone (Fonken et al., 2016a). In another study, endotoxin challenged adult rats exhibited distinct time of challenge dependent sickness responses. The pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in hippocampal tissues was markedly attenuated during the dark/active phase (GC high) as compared to the light/resting phase (GC low) (Fonken et al., 2015). Interestingly, in IL-6 deficient mice, the expressions of clock genes CRY1 and DEC2 were increased and that of REV-ERBβ was decreased in the hippocampus (Monje et al., 2017).
Han et al. monitored the signal trajectories and timing of NFκB-GR interplay in the brain in real time in a mouse model of time-dependent depressive behaviors (Han et al., 2021). GR mediated NF-κB inhibition was none to minimal in the early and the late phase, but most efficient in the middle phase. While the reduced inhibition in the early phase reflects the low GC at rest, the loss of inhibitory potential in the late phase could be largely attributed to the GC resistance and partly to GR: comodulator interactions that suppress positive GRE transactivation (Han et al., 2021). This temporal shift in the brain GR ability and the differential NF-κB signaling was attuned to the varying concentrations of GC (Charmandari et al., 2011). Furthermore, experiments with GR antagonists suggest a protective role for basal GC in the early brain inflammatory assault (de Kloet et al., 2018). Hence it would be logical to suggest that GILZ induction by GC:MR binding the GRE in its promoter contribute to the protective effects. However, it was observed that GILZ knock out protected mice against SCI induced inflammation characterized by reduced lymphocyte and granulocyte infiltration (Mazzon et al., 2014). Furthermore, depletion of GILZ in B cells prevented GC mediated apoptosis leading to the development of B cell lymphocytois (Bruscoli et al., 2015). Seemingly paradoxically, overexpression of GILZ in T-lymphocytes also protected mice against SCI induced inflammation characterized by reduced histological damage, decreased perilesional astrogliosis, reduced nuclear NF-κB p65 and suppressed neural apoptosis (Esposito et al., 2012). The injured SCI tissues expression of cytokines suggested elevated anti-inflammatory IL-10 cytokine under conditions of GILZ deficiency or T cell GILZ overexpression. Interestingly, while IL-10 has been shown to upregulate GILZ in T-cells, these observation in spinal cord injury suggests variable effect in glial cells that is perhaps modulated by the extent of proinflammatory cytokines in the tissue environment (Esposito et al., 2012; Mazzon et al., 2014).
GILZ at the Intersection of Circadian Glucocorticoid Rhythm and MR:GR Balance in the Regulation of Neuroinflammation
With six GRE in its promoter, GILZ protein is strongly induced by the GC with varying dynamics in different tissues suggesting that the expression is modulated by local environment with potential effects on cellular responses (Bruscoli et al., 2021). In human and rodents, GILZ is ubiquitously expressed in the brain and spinal cord (Ayyar et al., 2015; Mazzon et al., 2014). In situ hybridization showed that the highest expression of GILZ mRNA is observed in the thalamus and hippocampus in adult rats (George et al., 2017). Within the hippocampus, higher signal was observed in the CA2 and CA3 pyramidal cell layers compared to that in the CA1 region. Hybridization signal was also observed in the glial cells in stratum radiatum at the border between CA1 and dentate gyrus. In the thalamus, GILZ mRNA was highest in the ventral posteromedial thalamic nuclei and moderate in the cortex (van der Laan et al., 2008). In mice, the neuroanatomical distribution of GILZ was largely similar, except for more homogenous expression in the hippocampus (Yachi et al., 2007). In human brain, GILZ mRNA expression was observed in prefrontal cortex, hippocampus, central and medial amygdaloid(Pandey et al., 2013).
As a gene strongly upregulated by GC, GILZ expression is expected to exhibit time of day fluctuations (Ayyar et al., 2015; Yurtsever et al., 2019). Assessment of endogenous GILZ expression in adipose tissue obtained from normal mice housed under a tightly controlled 12-h light/dark cycles, showed that GILZ mRNA exhibits a robust circadian rhythm with peak expression in the dark period and a trough in the light period at 16h and 8 h after lights on respectively (Yurtsever et al., 2019). In murine subcutaneous fat, liver, and kidney tissues, GILZ transcript exhibited a peak at the beginning of the active phase much like serum corticosterone (Gimble et al., 2009; Pizarro et al., 2013). Furthermore, ultradian GC as determined by exposure to pulsatile corticosterone from 0 to 3h, showed that GILZ mRNA increased with every pulse and for extended periods of up to 5 hr (George et al., 2017). In humans, the expression of GILZ mRNA in peripheral blood mononuclear cells exhibited diurnal oscillations with a peak in the morning and a nadir at night. GILZ expression followed plasma cortisol levels with the highest positive correlations observed at a lag of + 30 min (Ryan & McLoughlin, 2019; van der Laan et al., 2008). In serum shocked HeLa cells GC induced GILZ transcript fluctuated in a circadian fashion mirroring CLOCK:BMAL1 mRNA oscillations. Knock down of CLOCK or BMAL1 upregulated GR mediated GILZ induction in HTC116 and Hela cells. While blocking of either CLOCK or BMAL1 exerted moderate effects, co-transfection of both CLOCK and BMAL1 siRNA synergistically enhanced dexamethasone induced GILZ mRNA expression(Kino, 2012; Kinouchi et al., 2021; Nader et al., 2009). Pertinently, loss of BMAL1 in T-lymphocytes or myeloid cells exacerbated inflammatory responses in mouse model of multiple sclerosis (Druzd et al., 2017; Sutton et al., 2017). The inflammatory response and pathology were time of day dependent potentially reflecting the low GC and hence presumably decreased GILZ.
Glucocorticoid resistance and reduced GR function have been reported in depressive disorders, suggesting that the reduced GILZ could increase susceptibility to inflammation (Perrin et al., 2019). Regional differences in GILZ expression were observed in the brains of teenage suicide victims as compared to normal subjects. GILZ mRNA was significantly decreased in the prefrontal cortex and central amygdaloid nuclei mimicking GR expression pattern (Pandey et al., 2013; Pandey et al., 2019). GILZ expression was observed to be lower in the hippocampus in transgenic mouse models of neuroinflammation (Witek et al., 2018). Microglial GILZ expression was also decreased in mice exposed to social defeat, an animal model of anxiety and depression (Wohleb et al., 2011). Interestingly, in humans diagnosed with major depressive disorder the reduced hippocampal volume has been correlated with lower GILZ expression in circulating lymphocytes (Frodl et al., 2012; Ryan & McLoughlin, 2019).
Structurally, GILZ protein has an amino terminal leucine zipper motif and a carboxy terminal proline rich region for protein-protein interactions. The leucine zipper motif is a characteristic of a DNA-binding transcriptional regulator that facilitates the ability of GILZ to modulate transcription. The carboxy terminal of GILZ has been shown to bind the transactivation domain of NF-κB p65 and prevent its nuclear translocation (Di Marco et al., 2007; Srinivasan & Janardhanam, 2011). Thus, GILZ protein may regulate NF-κB directed gene expression in multiple cellular processes (Di Marco et al., 2007). GILZ also inhibits signaling via kinases including the Raf1, Ras or PI3K/Akt pathways (Ayroldi et al., 2002). Consistently, data from in-vitro studies suggest that GILZ exhibits cell-context dependent functions ranging from inhibition of inflammation and immune responses to modulation of proliferation and cell death (Ayroldi et al., 2002; Bruscoli et al., 2021). In preclinical models of neuroinflammation, exogenous GILZ or GILZ mimetic suppressed NF-κB p65 mediated signaling responses (Srinivasan et al., 2016; Srinivasan et al., 2018). Taken together, differential expression and temporal alterations of GILZ in different tissues potentially modulate its functional effects. We propose a model that projects GILZ as a molecular modulator at the intersection of the circadian clock system, GC, and NF-κB pathways (Figure 2) and suggest that the individual specific homeostasis between the three systems play critical roles in central nervous system (CNS) health and disease.
Figure 2.
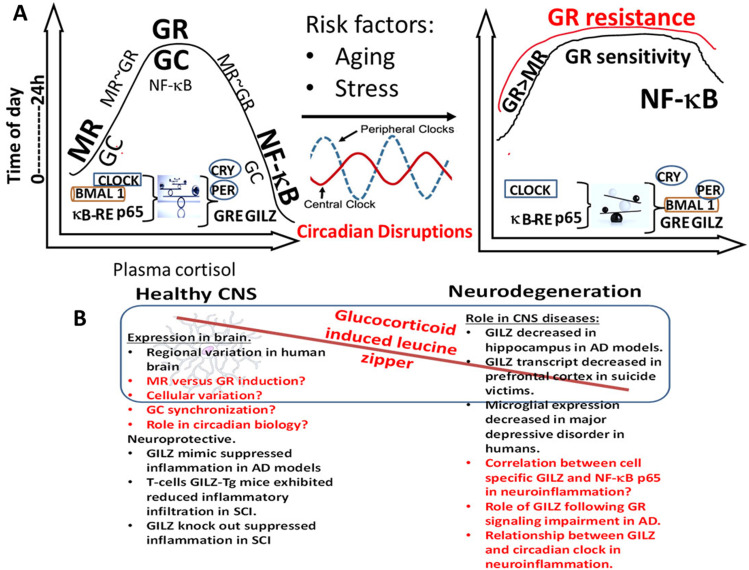
Cross talk between the GC, circadian clock and NF-κB in brain health and disease. (A). During health, release of GC is rhythmically in phase with CLOCK-BMAL1 transcripts with higher expression in the active phase (morning) and gradually decreasing reaching a nadir in the middle of the resting phase followed by gradual increase. MR is the predominant receptor at low GC concentration with increasing GR occupation as the GC concentration rises. While at high GC, NF-κB activation is minimal as needed for protection and cellular homeostasis, at low GC NF-κB p65 expression is higher increasing the susceptibility for inflammation. Disruption of this balance by the factors such as the age-related altered sleep cycle affect the autoregulatory feedback loop, precipitate GR resistance, promote persistent activation of NF-κB p65 and lead to exaggerated inflammatory responses. B) Hypothetical model of GILZ as a link between molecular clock, GCs and NF-κB in CNS health and disease. In health, the autoregulatory clock loop and the endogenous GC synergistically prime glial cells to patrol for danger signals and activate NF-κB p65 for optimal response. Key observations in the literature supporting the model and critical questions to be addressed are given in black and red texts respectively.
Perspectives and Future Directions
As discussed above, correlative associations between circadian timing, glucocorticoid secretion and neuroinflammation contribute to the pathogenesis of neurodegenerative diseases such as AD. In healthy adults, the fluctuation of cognitive performance during the day is similar to physiological rhythms, peaking shortly after waking and cycling with the circadian phase (Wright et al., 2006). Abrupt wakefulness during the usual rest phase is known to induce transient increases in circulating glucocorticoid levels, reset the phase of circadian rhythms (Duncan, 2020; Legan et al., 2015) and mediate changes in clock gene expression (Franken, 2013). Depletion of BMAL1 in astrocytes in APP-presenilin transgenic mice induced sustained astrogliosis (Furtado et al., 2020). Furthermore, in 3xTg AD mice microglial priming and hippocampal inflammatory cytokines exhibited increased responses during the light or resting phase as opposed to that in the active or dark phase (Sterniczuk et al., 2010). In humans, BMAL1 modulations due to a phase delay in wakefulness will suppress GR mediated transcription since clock activators are in phase with GC. Consequently, downregulation of GILZ and other GR mediated anti-inflammatory functions will shift the balance towards an inflammatory milieu.
In mice, GILZ expression has been observed to be modulated by endogenous and exogenous GC in brain tissues (Ayyar et al., 2015). In lipopolysaccharide induced neuroinflammation GILZ expression in activated microglia was reported to be inversely related to nuclear NF-κB p65 (Witek et al., 2018). Given the correlation between plasma and brain cortisol and GILZ expression in the periphery, it is conceivable that GILZ could represent a candidate molecule in regulating time-of-day dependent glucocorticoid and NF-κB responses in the CNS (Figure 2A,B). There are several gaps in knowledge for bridging the circadian, NF-κB and GILZ function in the brain. There is no direct evidence that GILZ expression in the brain affects cytokine secretion, gliosis, or neuronal function in health or in neuroinflammation-neurodegeneration. However, considerable evidence suggests that the absolute levels of corticosterone as well as the circadian rhythm and ultradian pulsing of GC modulate cellular responses (Dickmeis et al., 2013; Juszczak & Stankiewicz, 2018; Sorrells et al., 2009). Accordingly, in a model of disrupted normal GC rhythm induced by subcutaneous corticosterone pellet, nuclear translocation of GR and GILZ expression decreased in the CA1 region of hippocampus in conditions of flattened circulating cortisol. In contrast, basal GILZ expression was increased in conditions of increased circulating corticosterone with loss of pulsatility. Acute corticosterone exposure continued to decrease GILZ transcript in conditions of average circulating cortisol and had no effects in hypercortisolemia(Sarabdjitsingh et al., 2010). Taken together, both the concentration and pattern of glucocorticoid exposure modulated GILZ expression in the CA1 region of the hippocampus. It will of interest to compare the circadian oscillations of GILZ in the brain between health and neuroinflammation-neurodegeneration in animal models and clinical cohorts. Time of death studies could examine GILZ expression at the cellular level in human brain. Further, it will of interest to develop murine strains with CNS cell specific deletion or transgenic expression of GILZ to address its role in circadian biology of CNS. Characterization of GILZ in BMAL1/CLOCK knockout or overexpression models could critically evaluate its contribution to disease pathogenesis. Most research in rodents is performed during daytime (their inactive period), potentially compromising the available data on the interactions between the glucocorticoids, NF-κB and/or the clock system in CNS homeostasis. Experiments performed around the clock on rodents will provide better understanding of underlying mechanisms of neuroinflammation-neurodegeneration. However, since mouse and human tissues exhibit opposing patterns of expression of BMAL1 with respect to GC rhythm, extrapolating data from mouse models to human AD should be done with caution. Evaluating the role of GILZ in modulating inflammatory mediators in chronic jetlag represents another strategy to study the interrelationship between circadian rhythm, GILZ, and neuroinflammation (Chen et al., 2021; Shen et al., 2019).
In conclusion, considerable evidence supports GILZ as an anti-inflammatory molecule that inhibits NF-κB activation and prevents inflammatory cell infiltration, elucidation of its specific role in neurodegenerative processes will help developing novel therapeutics. In this context, GILZ has been suggested as an GC alternative with less potential for adverse effects such as osteoporosis and obesity (Ayroldi & Riccardi, 2009; Bruscoli et al., 2021).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging, (grant number R41AG053117).
ORCID iD: Mythily Srinivasan https://orcid.org/0000-0003-1431-8215
References
- Ayroldi E., Riccardi C. (2009). Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB Journal 23, 3649–3658. 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- Ayroldi E., Zollo O., Macchiarulo A., Di Marco B., Marchetti C., Riccardi C. (2002). Glucocorticoid-induced leucine zipper inhibits the raf-extracellular signal-regulated kinase pathway by binding to raf-1. Molecular and Cellular Biology 22, 7929–7941. 10.1128/MCB.22.22.7929-7941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyar V. S., Almon R. R., Jusko W. J., DuBois D. C. (2015). Quantitative tissue-specific dynamics of in vivo GILZ mRNA expression and regulation by endogenous and exogenous glucocorticoids. Physiological Reports 3 (6), e12382. 10.14814/phy2.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., Rowson S. A., Neigh G. N. (2017). Checks and balances: the glucocorticoid receptor and NFkB in good times and bad. Frontiers in Neuroendocrinology 46, 15–31. 10.1016/j.yfrne.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaconsa M., Colavito V., Pifferi F., Aujard F., Schenker E., Dix S., Grassi-Zucconi G., Bentivoglio M., Bertini G. (2013). Cell clocks and neuronal networks: Neuron ticking and synchronization in aging and aging-related neurodegenerative disease. Current Alzheimer Research 10, 597–608. 10.2174/15672050113109990004. [DOI] [PubMed] [Google Scholar]
- Bruscoli S., Biagioli M., Sorcini D., Frammartino T., Cimino M., Sportoletti P., Mazzon E., Bereshchenko O., Riccardi C. (2015). Lack of glucocorticoid-induced leucine zipper (GILZ) deregulates B-cell survival and results in B-cell lymphocytosis in mice. Blood 126, 1790–1801. 10.1182/blood-2015-03-631580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscoli S., Riccardi C., Ronchetti S. (2021). GILZ As a regulator of cell fate and inflammation. Cells 11(1), 122. 10.3390/cells11010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarile L., Zollo O., D'Adamio F., Ayroldi E., Marchetti C., Tabilio A., Bruscoli S., Riccardi C. (2001). Cloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced gene. Cell Death & Differentiation 8, 201–203. 10.1038/sj.cdd.4400798. [DOI] [PubMed] [Google Scholar]
- Charmandari E., Chrousos G. P., Lambrou G. I., Pavlaki A., Koide H., Ng S. S., Kino T. (2011). Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One 6, e25612. 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Logan R. W., Ma T., Lewis D. A., Tseng G. C., Sibille E., McClung C. A. (2016). Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America 113, 206–211. 10.1073/pnas.1508249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Weitzner A. S., McKennon L. A., Fonken L. K. (2021). Chronic circadian phase advance in male mice induces depressive-like responses and suppresses neuroimmune activation. Brain Behav Immun Health 17, 100337. 10.1016/j.bbih.2021.100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S., Park N., Cho S., Kim K. (2013). Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Research 41, 6161–6174. 10.1093/nar/gkt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun L. E., Woodruff E. R., Morton S., Hinds L. R., Spencer R. L. (2015). Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. Journal of Biological Rhythms 30, 417–436. 10.1177/0748730415598608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell B. L., Sarabdjitsingh R. A., McKenna M. A., Pooley J. R., Kershaw Y. M., Meijer O. C., de Kloet E. R., Lightman S. L. (2010). Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of Neuroendocrinology 22, 1093–1100. 10.1111/j.1365-2826.2010.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin P., McCarthy M. J., Lim A. S. P., Salmon D. P., Galasko D., Masliah E., De Jager P. L., Bennett D. A., Desplats P. (2017). Circadian alterations during early stages of Alzheimer's disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 13, 689–700. 10.1016/j.jalz.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E. R., Meijer O. C., de Nicola A. F., de Rijk R. H., Joels M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology 49, 124–145. 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Desmet S. J., De Bosscher K. (2017). Glucocorticoid receptors: Finding the middle ground. Journal of Clinical Investigation 127, 1136–1145. 10.1172/JCI88886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeis T., Weger B. D., Weger M. (2013). The circadian clock and glucocorticoids--interactions across many time scales. Molecular and Cellular Endocrinology 380, 2–15. 10.1016/j.mce.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Di Marco B., Massetti M., Bruscoli S., Macchiarulo A., Di Virgilio R., Velardi E., Donato V., Migliorati G., Riccardi C. (2007). Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: Role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Research 35, 517–528. 10.1093/nar/gkl1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd, D., Matveeva, O., Ince, L., Harrison, U., He, W., Schmal, C., Herzel, H., Tsang, A., Kawakami, N., Leliavski, A., Uhl, O., Yao, L., Erik Sander, L., Chen, C.-S., Kraus, K.,de Juan, A., Martina Hergenhan,S., Ehlers, M., Koletzko, B., … & Scheiermann, C. (2017). Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. 46, 120–132. 10.1016/j.immuni.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. J. (2020). Interacting influences of aging and Alzheimer's disease on circadian rhythms. European Journal of Neuroscience 51, 310–325. 10.1111/ejn.14358. [DOI] [PubMed] [Google Scholar]
- Echouffo-Tcheugui J., Conner S., Himali J., Maillard P., DeCarli C., Beiser A., Vasan R., Seshadri S. (2018). Circulating cortisol and cognitive and structural brain measures. The framingham heart study. Neurology 91, e1961–e1970. 10.1212/WNL.0000000000006549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E., Bruscoli S., Mazzon E., Paterniti I., Coppo M., Velardi E., Cuzzocrea S., Riccardi C. (2012). Glucocorticoid-induced leucine zipper (GILZ) over-expression in T lymphocytes inhibits inflammation and tissue damage in spinal cord injury. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics 9, 210–225. 10.1007/s13311-011-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken L. K., Frank M. G., Kitt M. M., Barrientos R. M., Watkins L. R., Maier S. F. (2015). Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behavior and Immunity 45, 171–179. 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken L. K., Kitt M. M., Gaudet A. D., Barrientos R. M., Watkins L. R., Maier S. F. (2016a). Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiology of Aging 47, 102–112. 10.1016/j.neurobiolaging.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken L. K., Weber M. D., Daut R. A., Kitt M. M., Frank M. G., Watkins L. R., Maier S. F. (2016b). Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 66, 82–90. 10.1016/j.psyneuen.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P. A. r. f. c. g. i. s. h. C. O. N. O. -d. j. c. E. (2013). A role for clock genes in sleep homeostasis. Current Opinion in Neurobiology 23, 864–872. 10.1016/j.conb.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Frodl T., Carballedo A., Hughes M. M., Saleh K., Fagan A., Skokauskas N., McLoughlin D. M., Meaney J., O'Keane V., Connor T. J. (2012). Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: High IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Translational Psychiatry 2, e88. 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado A., Astaburuaga R., Costa A., Duarte A., Gonçalves I., Cipolla-Neto J., Lemos M., Carro E., Relógio A., Santos C., Quintela T. (2020). The rhythmicity of clock genes is disrupted in the choroid plexus of the APP/PS1 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease: JAD 77, 795–798-796. 10.3233/JAD-200331 [DOI] [PubMed] [Google Scholar]
- George C. L., Birnie M. T., Flynn B. P., Kershaw Y. M., Lightman S. L., Conway-Campbell B. L. (2017). Ultradian glucocorticoid exposure directs gene-dependent and tissue-specific mRNA expression patterns in vivo. Molecular and Cellular Endocrinology 439, 46–53. 10.1016/j.mce.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. E., Blaikley J., Beesley S., Matthews L., Simpson K. D., Boyce S. H., Farrow S. N., Else K. J., Singh D., Ray D. W., Loudon A. S. (2012). The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America 109, 582–587. 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Ptitsyn A. A., Goh B. C., Hebert T., Yu G., Wu X., Zvonic S., Shi X. M., Floyd Z. E. (2009). Delta sleep-inducing peptide and glucocorticoid-induced leucine zipper: Potential links between circadian mechanisms and obesity? Obesity Reviews 10 Suppl 2, 46–51. 10.1111/j.1467-789X.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- Giridharan S., Srinivasan M. (2018). Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. Journal Of Inflammation Research 11, 407–419. 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M., Weinberg M. S., Spencer R. L. (2009). Diurnal expression of functional and clock-related genes throughout the rat HPA axis: System-wide shifts in response to a restricted feeding schedule. American Journal of Physiology. Endocrinology and Metabolism 296, E888–E897. 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, P., Dimitry, J. M., Sheehan, P. W., Lananna, B. V., Guo, C., Robinette, M. L., Hayes, M. E., Cedeno, M. R., Nadarajah, C. J., Ezerskiy, L. A., Colonna, M., Zhang, J., Bauer, A. Q., Burris, T. P., & Musiek, E. S. (2019). Circadian clock protein rev-erbalpha regulates neuroinflammation. Proceedings of the National Academy of Sciences of the United States of America 116, 5102–5107. 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Calvano J., Haimovich A. D., Calvano S. E., Coyle S. M., Lowry S. F. (2010). In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Critical Care Medicine 38, 751–758. 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. M., Kim, M. S., Jo, J., Shin, D., Kwon, S. H., Seo, J. B., Kang, D., Lee, B. D., Ryu, H., Hwang, E. M., Kim, J. M., Patel, P. D., Lyons, D. M., Schatzberg, A. F., & Her, S. (2021). Decoding the temporal nature of brain GR activity in the NFkappaB signal transition leading to depressive-like behavior. Molecular Psychiatry 26, 5087–5096. 10.1038/s41380-021-01016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfeld C., Herbert J., van Someren E., Hodges J., Hastings M. (2004). Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain 127, 1061–1074. 10.1093/brain/awh129 [DOI] [PubMed] [Google Scholar]
- Hong H. K., Maury E., Ramsey K. M., Perelis M., Marcheva B., Omura C., Kobayashi Y., Guttridge D. C., Barish G. D., Bass J. (2018). Requirement for NF-kappaB in maintenance of molecular and behavioral circadian rhythms in mice. Genes & Development 32, 1367–1379. 10.1101/gad.319228.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczak G. R., Stankiewicz A. M. (2018). Glucocorticoids, genes and brain function. Progress in Neuro-Psychopharmacology & Biological Psychiatry 82, 136–168. 10.1016/j.pnpbp.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Kino T. (2012). Circadian rhythms of glucocorticoid hormone actions in target tissues: Potential clinical implications. Science Signaling 5, pt4. 10.1126/scisignal.2003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi K., Mikami Y., Kanai T., Itoh H. (2021). Circadian rhythms in the tissue-specificity from metabolism to immunity: Insights from omics studies. Molecular Aspects of Medicine 80, 100984. 10.1016/j.mam.2021.100984. [DOI] [PubMed] [Google Scholar]
- Lamia K. A., Papp S. J., Yu R. T., Barish G. D., Uhlenhaut N. H., Jonker J. W., Downes M., Evans R. M. (2011). Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480, 552–556. 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lananna B. V., Nadarajah C. J., Izumo M., Cedeno M.R., Xiong D. D., Dimitry J., Tso C. F., McKee C. A., Griffin P., Sheehan P. W., Haspel J. P., Barres B. A., Liddelow S. A., Takahashi S. A., Karatsoreos I. N., Musiek E. S. (2018). Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep 25, 1–9, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan S. J., Peng X., Yun C., Duncan M. J. (2015). Effect of arousing stimuli on circulating corticosterone and the circadian rhythms of luteinizing hormone (LH) surges and locomotor activity in estradiol-treated ovariectomized (ovx + EB) Syrian hamsters. Hormones and Behavior. 28–38. 10.1016/j.yhbeh.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliavski A., Shostak A., Husse J., Oster H. (2014). Impaired glucocorticoid production and response to stress in arntl-deficient male mice. Endocrinology 155, 133–142. 10.1210/en.2013-1531. [DOI] [PubMed] [Google Scholar]
- Li C. X., Liang D. D., Xie G. H., Cheng B. L., Chen Q. X., Wu S. J., Wang J. L., Cho W., Fang X. M. (2013). Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Molecular Medicine Reports 7, 1117–1122. 10.3892/mmr.2013.1331. [DOI] [PubMed] [Google Scholar]
- Liu, X., Nemeth, D. P., Tarr, A. J., Belevych, N., Syed, Z. W., Wang, Y., Ismail, A. S., Reed, N. S., Sheridan, J. F., Yajnik, A. R., Disabato, D. J., Zhu, L., & Quan, N. (2016). Euflammation attenuates peripheral inflammation-induced neuroinflammation and mitigates immune-to-brain signaling. Brain Behavior and Immunity 54, 140–148. 10.1016/j.bbi.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon E., Bruscoli S., Galuppo M., Biagioli M., Sorcini D., Bereshchenko O., Fiorucci C., Migliorati G., Bramanti P., Riccardi C. (2014). Glucocorticoid-induced leucine zipper (GILZ) controls inflammation and tissue damage after spinal cord injury. CNS Neuroscience & Therapeutics 20, 973–981. 10.1111/cns.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje F. J., Cicvaric A., Acevedo Aguilar J. P., Elbau I., Horvath O., Diao W., Glat M., Pollak D. D. (2017). Disrupted ultradian activity rhythms and differential expression of several clock genes in interleukin-6-deficient mice. Frontiers in Neurology 8, 99. 10.3389/fneur.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure L., Le H., Benegiamo G., Chang M., Rios L., Jillani N., Ngotho M., Kariuki T., Dkhissi-Benyahya O., Cooper H., Panda S. (2018). Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science (New York, N.Y.) 359, 1232–1234. 10.1126/science.aao0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek, E. S., Lim, M. M., Yang, G., Bauer, A. Q., Qi, L., Lee, Y., Roh, J. H., Ortiz-Gonzalez, X., Dearborn, J. T., Culver, J. P., Herzog, E. D., Hogenesch, J. B., Wozniak, D. F., Dikranian, K., Giasson, B. I., Weaver, D. R., Holtzman, D. M., & Fitzgerald, G. A. (2013). Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. Journal of Clinical Investigation 123, 5389–5400. 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N., Chrousos G. P., Kino T. (2009). Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB Journal 23, 1572–1583. 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N., Chrousos G. P., Kino T. (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology and Metabolism 21, 277–286. 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G. N., Rizavi H. S., Bhaumik R., Ren X. (2019). Increased protein and mRNA expression of corticotropin-releasing factor (CRF), decreased CRF receptors and CRF binding protein in specific postmortem brain areas of teenage suicide subjects. Psychoneuroendocrinology 106, 233–243. 10.1016/j.psyneuen.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G. N., Rizavi H. S., Ren X., Dwivedi Y., Palkovits M. (2013). Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology 38, 2628–2639. 10.1016/j.psyneuen.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A. J., Horowitz M. A., Roelofs J., Zunszain P. A., Pariante C. M. (2019). Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Frontiers in Psychiatry 10, 423. 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak, R. H., Laws, S. M., Lim, Y. Y., Bender, S.J., Porter, T., Doecke, J., Ames, D., Fowler, C., Masters, C. L., Milicic, L., Rainey-Smith, S., Villemagne, V. L., Rowe, C. C., Martins, R. N., & Maruff, P. (2017). Plasma cortisol, brain amyloid-beta, and cognitive decline in preclinical Alzheimer's disease: A 6–year prospective cohort study. Biological Psychiatry 2, 45–52. 10.1016/j.bpsc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Pizarro A., Hayer K., Lahens N. F., Hogenesch J. B. (2013). CircaDB: A database of mammalian circadian gene expression profiles. Nucleic Acids Research 41, D1009–D1013. 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Droste S. K., Lightman S. L., Reul J. M., Linthorst A. C. (2012). Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology 153, 4346–4353. 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F., van Heemst D., Iranmanesh A., Takahashi P., Yang P., Veldhuis J. (2017). Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocrine Connections 6, 500–509. 10.1530/EC-17-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. M., McLoughlin D. M. (2019). Peripheral blood GILZ mRNA levels in depression and following electroconvulsive therapy. Psychoneuroendocrinology 101, 304–310. 10.1016/j.psyneuen.2018.12.234. [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh R. A., Isenia S., Polman A., Mijalkovic J., Lachize S., Datson N., de Kloet E. R., Meijer O. C. (2010). Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 151, 1177–1186. 10.1210/en.2009-1119 [DOI] [PubMed] [Google Scholar]
- Sellix M. T., Evans J. A., Leise T. L., Castanon-Cervantes O., Hill D. D., DeLisser P., Block G. D., Menaker M., Davidson A. J. (2012). Aging differentially affects the re-entrainment 15 response of central and peripheral circadian oscillators. Journal of Neuroscience 32, 16193–16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Wu J., Ni Y., Xie X., Yu C., Xiao Q. Z., J, Wang X., Fu Z. (2019). Exposure to jet lag aggravates depression-like behaviors and age-related phenotypes in rats subject to chronic corticosterone. Acta Biochimica et Biophysica Sinica 51, 834–844. 10.1093/abbs/gmz070 [DOI] [PubMed] [Google Scholar]
- Shen, Y., Endale, M., Wang, W., Morris, A. R., Francey, L. J., Harold, R. L., Hammers, D. W., Huo, Z., Partch, C. L., Hogenesch, J. B., Wu, Z. H., & Liu, A. C. (2021). NF-kappaB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genetics 17, e1009933. 10.1371/journal.pgen.1009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M., Diniz B. S., Leszek J. (2017). Inflammatory response in the CNS: friend or foe? Molecular Neurobiology 54, 8071–8089. 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S. F., Caso J. R., Munhoz C. D., Sapolsky R. M. (2009). The stressed CNS: When glucocorticoids aggravate inflammation. Neuron 64, 33–39. 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. L., Chun L. E., Hartsock M. J., Woodruff E. R. (2018). Glucocorticoid hormones are both a major circadian signal and major stress signal: how this shared signal contributes to a dynamic relationship between the circadian and stress systems. Frontiers in Neuroendocrinology 49, 52–71. 10.1016/j.yfrne.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Spengler M. L., Kuropatwinski K. K., Comas M., Gasparian A. V., Fedtsova N., Gleiberman A. S., Gitlin II, Artemicheva N. M., Deluca K. A., Gudkov A. V., Antoch M. P. (2012). Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proceedings of the National Academy of Sciences of the United States of America 109, E2457–E2465. 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Bayon B., Chopra N., Lahiri D. K. (2016). Novel nuclear factor-KappaB targeting peptide suppresses beta-amyloid induced inflammatory and apoptotic responses in neuronal cells. PLoS One 11, e0160314. 10.1371/journal.pone.0160314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Janardhanam S. (2011). Novel p65 binding glucocorticoid-induced leucine zipper peptide suppresses experimental autoimmune encephalomyelitis. Journal of Biological Chemistry 286, 44799–44810. 10.1074/jbc.M111.279257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Lahiri N., Thyagarajan A., Witek E., Hickman D., Lahiri D.K. (2018). Nuclear factor-kappa B: glucocorticoid-induced leucine zipper interface analogs suppress pathology in an Alzheimer's disease model. Alzheimers Dement (N Y) 4, 488–498. 10.1016/j.trci.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva D. A., Wiench M., John S., Conway-Campbell B. L., McKenna M. A., Pooley J. R., Johnson T. A., Voss T. C., Lightman S. L., Hager G. L. (2009). Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nature Cell Biology 11, 1093–1102. 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R., Dyck R., Laferla F., Antle M. (2010). Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain Research 1348, 139–148. 10.1016/j.brainres.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Sutton C., Finlay C., Raverdeau M., Early J., DeCourcey J., Zaslona Z., O'Neill L., Mills K., Curtis A. (2017). Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat commun. Nat Commun 8, 1923–1934. 10.1038/s41467-017-02111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr A. J., Liu X., Reed N. S., Quan N. (2014). Kinetic characteristics of euflammation: The induction of controlled inflammation without overt sickness behavior. Brain Behavior and Immunity 42, 96–108. 10.1016/j.bbi.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan S., Sarabdjitsingh R. A., Van Batenburg M. F., Lachize S. B., Li H., Dijkmans T. F., Vreugdenhil E., de Kloet E. R., Meijer O. C. (2008). Chromatin immunoprecipitation scanning identifies glucocorticoid receptor binding regions in the proximal promoter of a ubiquitously expressed glucocorticoid target gene in brain. Journal of Neurochemistry 106, 2515–2523. 10.1111/j.1471-4159.2008.05575.x. [DOI] [PubMed] [Google Scholar]
- Wendeln, A., Degenhardt, K., Kaurani, L., Gertig, M., Ulas, T., Jain, G., Wagner, J., Häsler, L., Wild, K., Skodras, A., Blank, T., Staszewski, O., Datta, M., Pena Centeno, T., Capece, V., Md. Rezaul Islam, Kerimoglu, C., Staufenbiel, M., Schultze, J. L., … & Neher, J. J. (2018). Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338. 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek E., Hickman D., Lahiri D. K., Srinivasan M. (2018). Glucocorticoid induced leucine zipper in lipopolysaccharide induced neuroinflammation. Frontiers in Aging Neuroscience 10, 432. 10.3389/fnagi.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E., Hanke M., Corona A., Powell N., Stiner L., Bailey M., Nelson R., Godbout J., Sheridan J. (2011). β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience 31, 6277–6288, PMID: 21525267. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff E. R., Chun L. E., Hinds L. R., Spencer R. L. (2016). Diurnal corticosterone presence and phase modulate clock gene expression in the male rat prefrontal cortex. Endocrinology 157, 1522–1534. 10.1210/en.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. P. J., Hull J. T., Hughes R. J., Ronda J. M., Czeisler C. A. (2006). Sleep and wakefulness out of phase with internal biological time impairs learning in humans. Journal of Cognitive Neuroscience. 18, 508–521. 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- Yachi K., Inoue K., Tanaka H., Yoshikawa H., Tohyama M. (2007). Localization of glucocorticoid-induced leucine zipper (GILZ) expressing neurons in the central nervous system and its relationship to the stress response. Brain Research 1159, 141–147. 10.1016/j.brainres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakahata Y., Tanaka M., Yoshida M., Soma H., Shinohara K., Yasuda A., Mamine T., Takumi T. (2005). Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. Journal of Biological Chemistry 280, 42036–42043. 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- Yurtsever T., Schilling T. M., Kolsch M., Turner J. D., Meyer J., Schachinger H., Schote A.B. (2016). The acute and temporary modulation of PERIOD genes by hydrocortisone in healthy subjects. Chronobiology International 33, 1222–1234. 10.1080/07420528.2016.1211668. [DOI] [PubMed] [Google Scholar]
- Yurtsever T., Streit F., Foo J. C., Trifonova S., Kumsta R., Muller C. P., Turner J. D., Meyer J., Schote A. B. (2019). Temporal dynamics of cortisol-associated changes in mRNA expression of glucocorticoid responsive genes FKBP5, GILZ, SDPR, PER1, PER2 and PER3 in healthy humans. Psychoneuroendocrinology 102, 63–67. 10.1016/j.psyneuen.2018.11.033. [DOI] [PubMed] [Google Scholar]