Abstract
Müller glial cells (MGCs), the main glial component of the retina, play an active role in retinal homeostasis during development and pathological processes. They strongly monitor retinal environment and, in response to retinal imbalance, activate neuroprotective mechanisms mainly characterized by the increase of glial fibrillary acidic protein (GFAP). Under these circumstances, if homeostasis is not reestablished, the retina can be severely injured and GFAP contributes to neuronal degeneration, as they occur in several proliferative retinopathies such as diabetic retinopathy, sickle cell retinopathy and retinopathy of prematurity. In addition, MGCs have an active participation in inflammatory responses releasing proinflammatory mediators and metalloproteinases to the extracellular space and vitreous cavity. MGCs are also involved in the retinal neovascularization and matrix extracellular remodeling during the proliferative stage of retinopathies. Interestingly, low-density lipoprotein receptor-related protein 1 (LRP1) and its ligand α2-macroglobulin (α2M) are highly expressed in MGCs and they have been established to participate in multiple cellular and molecular activities with relevance in retinopathies. However, the exact mechanism of regulation of retinal LRP1 in MGCs is still unclear. Thus, the active participation of MGCs and LRP1 in these diseases, strongly supports the potential interest of them for the design of novel therapeutic approaches. In this review, we discuss the role of LRP1 in the multiple MGCs activities involved in the development and progression of proliferative retinopathies, identifying opportunities in the field that beg further research in this topic area.
Summary Statement
MGCs and LRP1 are active players in injured retinas, participating in key features such as gliosis and neurotoxicity, neovascularization, inflammation, and glucose control homeostasis during the progression of ischemic diseases, such as proliferative retinopathies.
Keywords: alpha -2 macroglobulin, diabetes, hypoxia, glia, lipoproteins, neurodegeneration, retina
Müller Glial Cells: The Key Players in the Retina
Müller glial cells (MGCs) play an active role in retinal development and homeostasis through their ability to interact with vessels, neurons and astrocytes (Sorrentino et al., 2016, Subirada et al., 2018). Moreover, MGCs are involved in the blood–retinal barrier (BRB) maintenance together with endothelial cells (ECs) and pericytes, which protect against harmful molecules or pathogens that affect the retina (Loukovaara et al., 2015). Under subtle changes in the retinal environment, MGCs activate intracellular mechanisms addressed to produce neuroprotection mainly characterized by cellular proliferation as well as the expression and synthesis of glial fibrillary acidic protein (GFAP) and vimentin (Coorey et al., 2012). This reactive process called gliosis is considered an indicator of a bad prognosis if it persists over time as in certain chronic pathologies, such as diabetic retinopathy (DR) (Liu et al., 2016). In addition, MGCs have active participation in inflammatory responses during retinal injuries releasing proinflammatory mediators, including cytokines, growth factors, and mediators and metalloproteinases (MMPs) to the extracellular space and vitreous cavity (Coughlin et al., 2017). In proliferative retinopathies, MGCs also release vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) involved in the formation of retinal neovascularization and extracellular matrix remodeling during proliferative stages (Coughlin et al., 2017; Lorenc et al., 2015; Lorenc et al., 2017). In the last years, it has been demonstrated that the low-density lipoprotein receptor-related protein 1 (LRP1) and its ligand α2-macroglobulin (α2M) are associated with enhanced activity of MMP-2 and MMP-9 in retinas with ischemia-induced neovascularization, where LRP1 is highly expressed in MGCs (Sánchez et al., 2006). Furthermore, LRP1 has been abundantly detected in retinas of individuals with proliferative retinopathies (Sánchez et al., 2007; Barcelona et al., 2010; Hollborn et al., 2004). Thus, the active participation of MGCs and LRP1 in neovascular retinal diseases strongly supports the potential interest of them as therapeutical targets in these dysfunctions. In this review, we discuss the role of LRP1 in the multiple MGCs activities, with particular focus on gliosis and neurotoxicity, neovascularization, inflammation, and glucose homeostasis involved in the development and progression of proliferative retinopathies.
LRP1 in MGCs: Structure and Functions
LRP1, also termed α2M-receptor or CD91, is a type I transmembrane protein belonging to the low-density lipoprotein receptor (LDL-R) family (Herz and Strickland, 2001). This receptor is expressed in various types of cells such as MGCs, neurons, epithelial cells, fibroblasts, monocytes, macrophages, hepatocytes, adipocytes, vascular smooth muscle cells (VSMCs), and tumor cells (Actis Dato and Chiabrando, 2018). LRP1 is synthesized as a precursor glycoprotein of 600 kDa, and processed by furin in the middle Golgi network to produce two subunits: (i) the extracellular α-subunit of 515 kDa, containing four extracellular ligand-binding domains and (ii) the transmembrane and intracellular β-subunit of 85 kDa that possesses the YxxL, di-Leucine (LL), and NPxY motifs that are essential for endocytosis and intracellular signaling activation of LRP1 (Boucher and Herz, 2011). LRP1 recognizes more than 40 unrelated ligands, including the α2M-protease complex (α2M*), tissue-plasminogen activator (tPA), MMPs, lactoferrin, and aggregated LDL (Actis Dato and Chiabrando, 2018). These ligands are internalized by clathrin-mediated endocytosis and then degraded by the lysosome pathway, whereas LRP1 is recycled to the plasma membrane by different intracellular traffic routes (Jaldín-Fincati et al., 2019, Actis Dato et al., 2018). In addition, LRP1 regulates the cellular functions and activities of other receptors and membrane proteins such as platelet-derived growth factor receptor (PDGFR) (Zhou et al., 2009), urokinase-plasminogen activator receptor (uPAR) (Sahores et al., 2008), membrane type 1-MMP (MT1-MMP) (Barcelona et al., 2013), β1-integrin (Rabiej et al., 2016, Ferrer et al., 2017), insulin receptor (Actis Dato et al., 2018, Liu et al., 2015), insulin-like growth factor receptor-1 (Fernandez et al., 2017; Hernandez-Garzón et al., 2016; Actis Dato et al., 2021) and glucose transporter type 4 (GLUT4) (Jaldin-Fincati et al., 2017). This ability of LRP1 to control membrane processes as well as intra and extracellular mechanisms seems critical for the cellular and metabolic function of MGCs. It has been demonstrated that α2M* induces the endocytosis and endocytic recycling of LRP1 through regulated exocytosis promoting the increase of this receptor at the MGCs plasma membrane (Jaldín-Fincati et al., 2019). In these cells, the α2M*-LRP1 interaction also triggers dynamic intracellular trafficking of protein membranes and vesicles tracked by MAPK-ERK1/2 and PI3K/Akt signaling activation. By endocytosis, both ligand and receptor are rapidly accumulated in early endosomes, from which α2M* follows a degradative lysosomal route, whereas the receptor is transported to LRP1-storage vesicles (LSVs) together with other membrane proteins including sortilin and VAMP-2 (Actis Dato et al., 2018). From LSVs, LRP1 is trafficked to the plasma membrane by the activation of the small GTPase Rab8A and Rab10. Apparently, the traffic of LRP1 induced by α2M* is essential for the MGCs motility, since the protein silencing of Rab8A and Rab10 is enough for the full inhibition of α2M*-induced cell migration (Jaldín-Fincati et al., 2019). In addition, the α2M*-LRP1 endocytosis also promotes the intracellular traffic and function of MT1-MMP in MGCs, which is critical for the extracellular remodeling mediated by MMP-2 activation and cell migration (Barcelona et al., 2013). In this case, MT1-MMP is translocated to the plasma membrane by Rab11 activation from recycling endosomes. Thus, these mechanisms highlight the central role of LRP1 in MGCs during the progression of extracellular remodeling that occur in proliferative retinopathies.
LRP1 and MGCs in Gliosis and Neurotoxicity
The first response of MGCs to an external detrimental stimulus is reactive gliosis, which is mainly characterized by the upregulation of GFAP (Lewis and Fisher, 2003). Under normal conditions, GFAP is very low expressed or undetectable in MGCs, but its expression is highly increased in several neurodegenerative retinal diseases, including DR and glaucoma (Shi et al., 2008, Mizutani et al., 1998). GFAP belongs to a heterogeneous group of proteins termed intermediate filaments that form 10 nm-diameter filaments and are highly stable cytoskeletal components in various types of cells such as astrocytes in central nervous system and MGCs in retina (Middeldorp and Hol, 2011). Through in vitro studies in MIO-M1 cells, a human immortalized Müller glial-derived cell line, it has been demonstrated that LRP1 mediates an increase in GFAP expression induced by α2M*, involving the JAK/signal transducer and activator of transcription (STAT) signaling pathway, in particular STAT3 activation (Barcelona et al., 2011). In the same way, retinas of mice intravitreally injected with α2M*, at a similar concentration to those reported in the vitreous of diabetic patients, have shown increased levels of STAT3 phosphorylation in ganglion-cell layer (GCL) and inner nuclear layer (INL) suggesting that the positive cells for p-STAT3 in the INL are in fact MGCs. Therefore, these findings suggest that the α2M*-LRP1 interaction may be responsible for the enhanced GFAP expression observed in MGCs of injured retinas.
It is well established that chronic hyperglycemia induces dysfunction and reduced thickness of retinal layers with substantial loss of glia–neuron interactions (Nawaz et al., 2019). Under this pathological condition, MGCs also promote a considerable gliotic response (Picconi et al., 2019). In this complex scenario, MGCs take special relevance due to their latent potential as stem cell, positioning them as an excellent target for regenerative therapies (Couturier et al., 2021). Under in vitro appropriate circumstances, MGCs can improve the ability to migrate in vivo toward retinal injured sites (Zhao et al., 2014, Lawrence et al., 2007). In this way, the motility of MGCs could be induced by several factors, including α2M*, IGF-1 and sphingosine-1-phosphate (S1P) (Lorenc et al., 2015, Barcelona et al., 2013, Simón et al., 2015). Although it is not known if LRP1 is mediating the migratory action of IGF-1 and S1P, it has been shown that α2M* stimulates the MGCs migration, promoting cell motility and extracellular remodeling by MT1-MMP and pro-MMP2 activation (Jaldín-Fincati et al., 2019, Barcelona et al., 2013).
Persistent gliosis can impair the recycling of neurotransmitters such as glutamate. A major function of MGCs is the involvement in the glutamate/glutamine cycle to control and protect the neural retina from excitotoxicity (Lieth et al., 2001). In this cycle, glutamate, from neurons is converted to glutamine by the enzyme glutamine synthetase (GS) in MGCs, and returned subsequently to neurons (Shank and Aprison, 1981). Under pathological conditions such as proliferative retinopathies, the MGCs dysfunction can lead to an abnormal accumulation of glutamate by a significant lower expression of GS (Cheng et al., 2019, Ridano et al., 2017). In addition, increased levels of α2M acts as an exacerbating factor in neurodegeneration by glutamate-induced excitotoxicity (Hayashi et al., 2012). On the other hand, an increased expression and secretion of apolipoprotein (apo) E-containing lipoproteins (E-LPs) in MGCs has been reported as neuronal support and protection in response to injury (Mahley, 2016). Recently, it has been demonstrated that E-LPs reduced the α2M expression and secretion in cultures of MGCs (Hayashi et al., 2021). In addition, the pretreatment with receptor-associated protein (RAP), a protein that inhibits the binding of E-LPs to LRP1, as well as with LRP1 silencing techniques significantly prevented the α2M reduction, indicating that this process was mediated by LRP1. Finally, E-LPs increased the phosphorylation level of STAT3 in MGCs, which was significantly attenuated by a STAT3 inhibitor, highlighting the importance of LRP1/STAT3 signaling pathway, which might play an important role in neuron survival in the retina. The apparently contradictory observations suggest that STAT3 may have opposite functions in early or advanced stages of proliferative retinopathies, initially promoting survival conditions but inducing gliosis and neurotoxicity at later stages of the disease.
LRP1 and MGCs in Neovascularization
At the retinal level, it has been demonstrated that LRP1 plays a central role in the development and progression of ischemic neovascular diseases (Barcelona et al., 2010, Mao et al., 2016, Mao et al., 2017). However, the function of LRP1 seems to be selective, acting as a proangiogenic or antiangiogenic factor, depending on the cell type where this receptor is expressed (Sánchez et al., 2006, Barcelona et al., 2013, Mao et al., 2016). In this way, it has been demonstrated that LRP1 regulates poly(ADP-ribose) polymerase-1 (PARP-1), promoting an increased activation of retinoblastoma protein (Rb) and cyclin-dependent kinase-2 (CDK2), which negatively regulates EC proliferation and neovascularization in the hypoxic retina (Mao et al., 2016). High levels of LRP1 have been detected in neural retinas of diabetic and sickle cell patients, suggesting that this receptor is involved in ischemic neovascular diseases (Barcelona et al., 2010). In this way, by immunohistochemistry analysis it has been shown that LRP1 is highly expressed in MGCs and astrocytes in a rat model of oxygen-induced retinal neovascularization (Sánchez et al., 2006). In addition, by zymographic analysis increased activity of MMP-2 and MMP-9 in hypoxic neural retinas was also observed, suggesting that LRP1 in MGCs is able to modulate retinal neovascularization by regulating extracellular proteolytic activities. As stated above, the LRP1-α2M* interaction induces MT1-MMP endocytic recycling to the plasma membrane and cell surface activity in MGCs, which is critical for the activation of pro-MMP-2 to the active MMP-2 in the extracellular milieu (Barcelona et al., 2013). Interestingly, vitreous samples of patients with proliferative DR exhibited increased α2M concentration together with enhanced levels of pro-MMP-2, pro-MMP-9 and active MMPs (Sánchez et al., 2007). It is well known that under hypoxic conditions the hypoxia-induced factor-1 (HIF-1), the master regulator of the expression of hypoxia-regulated angiogenic stimulators, is upregulated in the retina (Cheng et al., 2017). In this regard, it has been established that HIF-1 modulates the gene expression of VEGF and angiopoietin-like 4 (ANGPTL-4) in hypoxic MGCs in vitro and in the ischemic inner retina in vivo (Xin et al., 2013). An additional study has reported that LRP1 through a negative regulation of the Wnt/β-catenin pathway can regulate the expression of angiogenic and proinflammatory factors, including HIF-1, VEGF and ICAM-I in diabetic retinas and promotes multitarget effects in the regulation of retinal vascular abnormalities, such as ECM remodeling and BRB integrity (Hossain et al., 2017). Collectively, these data suggest that LRP1 in MGCs plays a key role in the control and regulation of neovascularization, hallmark of several ischemic retinal diseases such as DR and Retinopathy of Prematurity.
LRP1 and MGCs in Inflammation
It has been demonstrated that inflammation is a process that contributes to the development and progression of DR (Kern, 2007). In this way, several proinflammatory cytokines and growth factors are highly detected in vitreous and aqueous humor from diabetic patients with DR, which include IL-1β, IL-6, MCP-1, MCP-2, TNF-α, ICAM-1, and IGF-1 (Rübsam et al., 2018). In the retina, MGCs are the source of several of these inflammatory modulators during the progression of proliferative retinopathies (Yoshida et al., 2004). Moreover, under high-glucose conditions, MGCs are also activated and acquire the capacity to initiate inflammation (Silva et al., 2013). However, the cellular and signaling mechanism involved in initiating of this response remains less clear. Different family members of toll-like receptors (TLRs), such as TLR2, TLR3, TLR4 and TLR5, are highly expressed by MGCs, which suggests that these cells may be involved in innate and adaptative immune responses via TLR activation in the eye (Lin et al., 2013). In this regard, it has been demonstrated that lipopolysaccharide (LPS) and PAM3, two agonists of TLR2 and TLR4, induced the gene expression and release of IL-6 and the chemokine MIP-2/CXCL2 in murine MGCs.
Several studies have demonstrated that LRP1 is involved in atherogenic plaque formation in the subendothelial arterial spaces, mainly in carotid and coronary arteries (Boucher and Herz, 2011, Xian et al., 2017). In macrophages, LRP1 is highly expressed and it regulates cell proliferation and migration (Cáceres et al., 2010, Bonacci et al., 2007) as well as foam cell formation through the binding and internalization of aggregated LDL (Bornachea et al., 2020). However, in vivo and in vitro models have shown that LRP1 helps to suppress the TLR-induced inflammation in macrophages based on the fact that macrophage-specific Lrp1 gene deletion induced the production of proinflammatory factors such as TNF-α, IL-6, and CCL2 (Mantuano et al., 2016). This suppressive action exerted by LRP1 involves crosstalk induced by LPS-activated TLR, mainly TLR-2 and TLR-4, which promotes the β-subunit phosphorylation of LRP1, subsequent recruitment of Rab8a/PI3K complex, activation of Akt/mTOR signaling and release of anti-inflammatory cytokines (Luo et al., 2018). In addition, it has been demonstrated that this inflammatory suppressive crosstalk between LRP1 and TLRs can be potentiated by certain ligands of LRP1, such as α2M and tPA (Mantuano et al., 2016). By contrast, another group of LRP1 ligands, including RAP and lactoferrin, abrogated the LPS-induced LRP1-TLR crosstalk and induced an inflammatory response. For this reason, LRP1 ligands that induce anti-inflammatory response are termed agonists, whereas those ligands that promote pro-inflammatory profiles are considered antagonists. In this way, RAP and lactoferrin induce nuclear-factor κ B (NFκB) activation, which downstream activates the gene expressions of TNFα and IL6, among other proinflammatory factors (Mantuano et al., 2016). Thus, LRP1 might regulate inflammatory processes in retina through the crosstalk and suppression of TLRs signaling. In particular, activation of TLR3 signaling pathway has been involved in the pro-inflammatory response in rat models of oxygen-induced retinopathy (Cai et al., 2015). Interestingly, in these retinas LRP1 was up-regulated in MGCs concomitantly with α2M (Sánchez et al., 2006), which might play an anti-inflammatory role during the progression of retinopathy. However, it has been demonstrated that the inflammation mediated by TLRs can involve the activation of the JAK-STAT3 pathway in proliferative retinopathies (Ren et al., 2018, Li et al., 2021). In this way, considering that the α2M-LRP1 interaction activates the STAT3 phosphorylation in MGCs, it cannot be discarded a pro-inflammatory role. Thus, additional studies are required to develop sufficient understanding about the LRP1 roles in retinal MGCs during inflammatory processes.
LRP1 and MGCs in Retinal Glucose Homeostasis
It is well established that the retina is a tissue with a high glycolytic activity where the glucose that enters from systemic circulation is converted into lactate by aerobic glycolysis (Winkler et al., 2008). In DR, enhanced levels of glucose can promote advance glycation end products and induce oxidative stress in retinal cells (You et al., 2017). In contrast, low glucose levels can lead to photoreceptor loss affecting more rods than cones (Swarup et al., 2019). A large number of observations have consistently shown that the glucose transporter 1 (GLUT1) is the main mediator of glucose uptake in different cells of the retina, including retinal pigment epithelium, photoreceptors, endothelial BRB and MGCs (Actis Dato et al., 2021, Swarup et al., 2019, DeBosch et al., 2001). GLUT1 is a member of the gene family of glucose transporters (GLUTs1–5) and can be activated through different extracellular factors, hormones, and glucose levels (Mueckler and Thorens, 2013). In this way, both IGF-1 and insulin exert a synergic action on the GLUT1 traffic from endosomes to the plasma membrane in brain astrocytes (Fernandez et al., 2017), whereas in MGCs the GLUT1 function is specifically regulated by IGF-1 (Actis Dato et al., 2021). In this study, IGF-1 induced GLUT1 traffic to plasma membrane and promoted glucose uptake, involving the intracellular signaling activation of MAPK/ERK and PI3K/Akt pathways. In addition, IGF-1-induced signaling activation also produced the intracellular dissociation of LRP1 and GLUT1, mediating GLUT1 translocation from endosomes to plasma membranes. Remarkably, MGCs treated with specific siRNA for LRP1 impaired the GLUT1 expression on the plasma membrane and glucose uptake induced by IGF-1, suggesting that LRP1 plays an important role in the regulation of the glucose homeostasis in MGCs (Actis Dato et al., 2021). Thus, the prominent IGF-1 expression in the retina of patients with DR, should be considered as a protective action to induce GLUT1 activity and glucose uptake mediated by LRP1 in these cells.
Conclusions and Perspectives
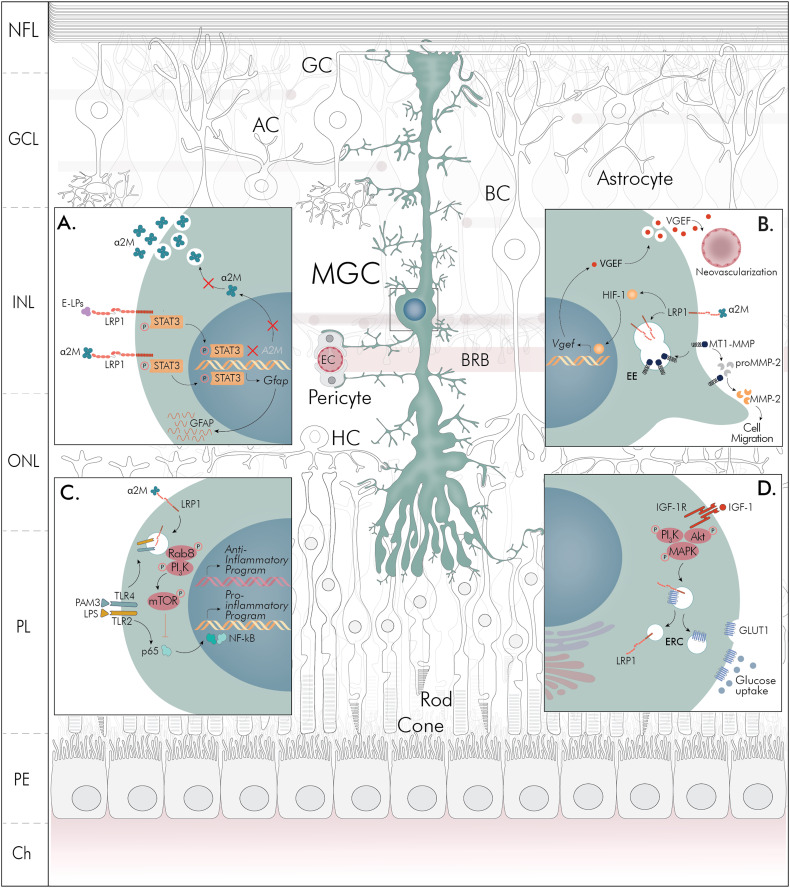
MGCs have gained progressively more attention over the last years and detailed molecular and functional characterization has indicated that these cells are active players in the retina. In Figure 1 are summarized the main multitarget activities of LRP1 and MGCs involved in the development and progression of proliferative retinopathies: gliosis and neurotoxicity (A), neovascularization (B), inflammation (C), and glucose control (D). In panel A, we showed that α2M induces the GFAP expression via LRP1 through the STAT3 activation, which could constitute the main mechanism of reactive gliosis observed in retinopathies. In addition, the exacerbated levels of α2M expression are suppressed by ApoE-enriched lipoproteins (E-LPs), in a process also mediated by STAT3 activation downstream of LRP1, which represents a neuroprotective and antigliotic effect in MGCs. In Panel B, the main activities of LRP1 and MGCs during retinal neovascularization ocurred in proliferative retinopathies are characterized. Here, the supressive action of LRP1 on Wnt/β-catenin pathway promotes the intracellular activation and nuclear translocation of HIF-1, which induces VEGF expression, as the main angiogenic factor. Next, the α2M-induced MGC motility by the endocytosis of LRP1 is also shown, where the MT1-MMP and proMMP-2 activation is triggered as an essential action for the extracellular remodeling during the ischemic-induced neovascularization. In Panel C, we hypothetize that the retinal inflammation generated by TLR activation may be supressed by α2M*/LRP1 interaction in MGCs, promoting an anti-inflammatory profile, through a mechanism demonstrated in macrophages. Related to this, the LRP1 endocytosis recruits Rab8/PI3K complexes in endosomes together with TLRs and subsequent mTOR activation, which downstream inhibit both NFκB activation and pro-inflammatory factors gene expression. Finally, in Panel D it is represented the glucose control homeostasis exerted by IGF-1-induced GLUT1 activation where LRP1 plays a critical role in the intracellular trafficking of this glucose transporter toward the plasma membrane of MGCs. In this mechanism, the intracelular signaling activation of PI3K/Akt and MAPK pathways is promoted by IGF-1. Considering that IGF-1 is also involved in neovascularization and inflammation during proliferative retinopathies, further studies should inquire about how IGF-1-regulated glucose control may be affected during these retinal disorders.
Figure 1.
Schematic representation of multitarget activities of LRP1 and MGCs in retinal processes associated with gliosis and neurotoxicity (A), neovascularization (B), inflammation (C) and glucose control homeostasis (D). NFL = nerve fiber layer; GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer; PL = plexiform layer; PE = pigmentary epithelium; Ch = choroid. GC = ganglion cell; AC = amacrine cell; BC = bipolar cell; MGC = Muller glial cell; BRB = blood–retinal barrier; HC = horizontal cell; EC = endothelial cell; α2M = α2-macroglobulin; E-LPs = apolipoprotein (apo) E-containing lipoproteins; LRP1 = low-density lipoprotein receptor-related protein 1; STAT3 = signal transducer and activator of transcription 3; GFAP = glial fibrillary acidic protein; VEGF = vascular endothelial growth factor; HIF-1 = hypoxia inducible factor 1; Wnt/βCat = Wingless e Int/β-Catenin; EE = early endosome; MT1-MMP = membrane type 1 matrix metalloproteinase; MMP2 = matrix metalloproteinase 2; Rab8 = small GTPase Rab8A; PI3K = phosphatidylinositol 3-kinases; mTOR = mammalian target of rapamycin; TLR = toll-like receptor; LPS = lipopolysaccharide; PAM3 = synthetic agonist of TLR; NFκB = nuclear factor κB; p65 = NF-κΒ p65 subunit; IGF-1 = insulin-like growth factor 1; IGF-1R = insulin-like growth factor 1 receptor; PKB = protein kinase B; MAPK = mitogen-activated protein kinase; ERC = endocytic recycling compartment; GLUT1 = glucose transporter type 1.
Presumably, the diversity of activities of LRP1 and MGCs seem to be critical in proliferative retinopathies, although they occur at different times during the progression of this pathology. For this reason, new studies are necessary to establish the chronology of occurrence of these activities, in order to determine which are related to actions of neuroprotection or neurodegeneration in the injured retina. Moreover, these multitarget activities of LRP1 and MGCs could be key features for new therapeutic interventions against the development and progression of proliferative retinopathies.
Acknowledgments
The authors are grateful to darwid.illustration for the design and drawing of the figure. The authors thank Dr. Paula V. Subirada for her technical contribution and language assistance to this work.
Author Contributions: MCS and GAC equally contributed in the conceptualization, writing original draft, writing—review & editing.
Conflict of Interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by grants from FonCyT (PICT 2020-01586), the Argentinean National Research Council (CONICET) (PIP 11220200100830), and the Secretary of Science and Technology, Universidad Nacional de Cordoba, (SECYT-UNC), Argentina (to M.C.S.). This work was also funded by FonCyT (PICT 2017–4497 and PICT 2019-01166); CONICET (PIP 11220200102450CO), and SECYT-UNC, Argentina (to G.A.C). M.C.S. and G.A.C. were members of the Research Career of CONICET
ORCID iD: Gustavo A. Chiabrando https://orcid.org/0000-0001-8902-6693
References
- Actis Dato V., Chiabrando G. (2018). The role of low-density lipoprotein receptor-related protein 1 in lipid metabolism, glucose homeostasis and inflammation. International Journal of Molecular Sciences, 19(6), 1780. 10.3390/ijms19061780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis Dato V., Grosso R. A., Sánchez M. C., Fader C. M., Chiabrando G. A. (2018). Insulin-induced exocytosis regulates the cell surface level of low-density lipoprotein-related protein-1 in Müller glial cells. Biochemical Journal, 475(9), 1669–1685. 10.1042/BCJ20170891 [DOI] [PubMed] [Google Scholar]
- Actis Dato V., Sánchez M. C., Chiabrando G. A. (2021). LRP1 mediates the IGF-1-induced GLUT1 expression on the cell surface and glucose uptake in Müller glial cells. Scientific Reports, 11(1). 10.1038/s41598-021-84090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelona P. F., Jaldin-Fincati J. R., Sanchez M. C., Chiabrando G. A. (2013). Activated alpha2-macroglobulin induces Muller glial cell migration by regulating MT1-MMP activity through LRP1. The FASEB Journal, 27(8), 3181–3197. http://www.ncbi.nlm.nih.gov/pubmed/23640058. 10.1096/fj.12-221598 [DOI] [PubMed] [Google Scholar]
- Barcelona P. F., Luna J. D., Chiabrando G. A., Juarez C. P., Bhutto I. A., Baba T., et al. (2010). Immunohistochemical localization of low density lipoprotein receptor-related protein 1 and α2-macroglobulin in retinal and choroidal tissue of proliferative retinopathies. Experimental Eye Research, 91(2), 264–272. 10.1016/j.exer.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelona P. F., Ortiz S. G., Chiabrando G. A., Sánchez M. C. (2011). α2-macroglobulin induces glial fibrillary acidic protein expression mediated by low-density lipoprotein receptor-related protein 1 in müller cells. Investigative Opthalmology & Visual Science, 52(2), 778. 10.1167/iovs.10-5759 [DOI] [PubMed] [Google Scholar]
- Bonacci G. R., Cáceres L. C., Sánchez M. C., Chiabrando G. A. (2007). Activated α2-macroglobulin induces cell proliferation and mitogen-activated protein kinase activation by LRP-1 in the J774 macrophage-derived cell line. Archives of Biochemistry and Biophysics, 460(1), 100–106. 10.1016/j.abb.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Bornachea O., Benitez-Amaro A., Vea A., Nasarre L., de Gonzalo-Calvo D., Escola-Gil J. C., et al. (2020). Immunization with the Gly1127-Cys1140 amino acid sequence of the LRP1 receptor reduces atherosclerosis in rabbits. Molecular, immunohistochemical and nuclear imaging studies. Theranostics, 10(7), 3263–3280. 10.7150/thno.37305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher P., Herz J. (2011). Signaling through LRP1: Protection from atherosclerosis and beyond. Biochemical Pharmacology, 81(1), 1–5. 10.1016/j.bcp.2010.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres L. C., Bonacci G. R., Sánchez M. C., Chiabrando G. A. (2010). Activated α2 macroglobulin induces matrix metalloproteinase 9 expression by low-density lipoprotein receptor-related protein 1 through MAPK-ERK1/2 and NF-κB activation in macrophage-derived cell lines. Journal of Cellular Biochemistry, 111(3), 607–617. 10.1002/jcb.22737 [DOI] [PubMed] [Google Scholar]
- Cai M., Zhang X., Li Y., Xu H. (2015). Toll-like receptor 3 activation drives the inflammatory response in oxygen-induced retinopathy in rats. British Journal of Ophthalmology, 99(1), 125–132. 10.1136/bjophthalmol-2014-305690 [DOI] [PubMed] [Google Scholar]
- Cheng L., Yu H., Yan N., Lai K., Xiang M. (2017). Hypoxia-inducible factor-1α target genes contribute to retinal neuroprotection. Frontiers in Cellular Neuroscience, 11, 20. 10.3389/fncel.2017.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Zhang T., Zheng J., Ding W., Wang Y., Li Y., et al. (2019). Betulinic acid derivatives can protect human Müller cells from glutamate-induced oxidative stress. Experimental Cell Research, 383(1), 111509. 10.1016/j.yexcr.2019.111509 [DOI] [PubMed] [Google Scholar]
- Coorey N. J., Shen W., Chung S. H., Zhu L., Gillies M. C. (2012). The role of glia in retinal vascular disease. Clinical and Experimental Optometry, 95(3), 266–281. 10.1111/j.1444-0938.2012.00741.x [DOI] [PubMed] [Google Scholar]
- Coughlin B. A., Feenstra D. J., Mohr S. (2017). Müller cells and diabetic retinopathy. Vision Research, 139, 93–100. 10.1016/j.visres.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier A., Blot G., Vignaud L., Nanteau C., Slembrouck-Brec A., Fradot V., et al. (2021). Reproducing diabetic retinopathy features using newly developed human induced-pluripotent stem cell-derived retinal Müller glial cells. Glia, 69(7), 1679–1693. 10.1002/glia.23983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBosch B. J., Baur E., Deo B. K., Hiraoka M., Kumagai A. K. (2001). Effects of insulin-like growth factor-1 on retinal endothelial cell glucose transport and proliferation. Journal of Neurochemistry, 77(4), 1157–1167. 10.1046/j.1471-4159.2001.00325.x [DOI] [PubMed] [Google Scholar]
- Fernandez A. M., Hernandez-Garzón E., Perez-Domper P., Perez-Alvarez A., Mederos S., Matsui T., et al. (2017). Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes, 66(1), 64–74. 10.2337/db16-0861 [DOI] [PubMed] [Google Scholar]
- Ferrer D. G., Dato V. A., Fincati J. R. J., Lorenc V. E., Sánchez M. C., Chiabrando G. A. (2017). Activated α2-macroglobulin induces mesenchymal cellular migration of Raw264.7 cells through low-density lipoprotein receptor-related protein 1. Journal of Cellular Biochemistry, 118(7), 1810–1818. 10.1002/jcb.25857 [DOI] [PubMed] [Google Scholar]
- Hayashi H., Eguchi Y., Fukuchi-Nakaishi Y., Takeya M., Nakagata N., Tanaka K., et al. (2012). A potential neuroprotective role of apolipoprotein E-containing lipoproteins through low density lipoprotein receptor-related protein 1 in normal tension glaucoma. Journal of Biological Chemistry, 287(30), 25395–25406. 10.1074/jbc.M112.370130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Mori M., Harashima M., Hashizume T., Furiya M., Mukaigaito C., et al. (2021). Apolipoprotein E-containing lipoproteins and LRP1 protect from NMDA-induced excitotoxicity associated with reducing α2-macroglobulin in Müller Glia. Investigative Ophthalmology & Visual Science, 62(13), 23. 10.1167/iovs.62.13.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Garzón E., Fernandez A. M., Perez-Alvarez A., Genis L., Bascuñana P., de la Rosa R F., et al. (2016). The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia, 64(11), 1962–1971. 10.1002/glia.23035 [DOI] [PubMed] [Google Scholar]
- Herz J., Strickland D. K. (2001). LRP: A multifunctional scavenger and signaling receptor. Journal of Clinical Investigation, 108(6), 779–784. 10.1172/JCI200113992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollborn M., Krausse C., Iandiev I., Yafai Y., Tenckhoff S., Bigl M., et al. (2004). Glial cell expression of hepatocyte growth factor in vitreoretinal proliferative disease. Laboratory Investigation, 84(8), 963–972. 10.1038/labinvest.3700121 [DOI] [PubMed] [Google Scholar]
- Hossain A., Tauhid L., Davenport I., Huckaba T., Graves R., Mandal T., et al. (2017). LRP-1 Pathway targeted inhibition of vascular abnormalities in the retina of diabetic mice. Current Eye Research, 42(4), 640–647. 10.1080/02713683.2016.1203441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaldin-Fincati J. R., Pavarotti M., Frendo-Cumbo S., Bilan P. J., Klip A. (2017). Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends in Endocrinology and Metabolism, 28(8), 597–611. 10.1016/j.tem.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Jaldín-Fincati J. R., Actis Dato V., Díaz N. M., Sánchez M. C., Barcelona P. F., Chiabrando G. A. (2019). Activated α2-macroglobulin regulates LRP1 levels at the plasma membrane through the activation of a Rab10-dependent exocytic pathway in retinal Müller glial cells. Scientific Reports, 9(1), 13234. http://www.nature.com/articles/s41598-019-49072-6. 10.1038/s41598-019-49072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern T. S. (2007). Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Experimental Diabetes Research, 2007, 95103. 10.1155/2007/95103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. M., Singhal S., Bhatia B., Keegan D. J., Reh T. A., Luthert P. J., et al. (2007). MIO-M1 Cells and similar müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells (Dayton, Ohio), 25(8), 2033–2043. 10.1634/stemcells.2006-0724 [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Fisher S. K. (2003). Up-Regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. International Review of Cytology, 230, 263–290. 10.1016/s0074-7696(03)30005-1 [DOI] [PubMed] [Google Scholar]
- Li J., Yu S., Lu X., Cui K., Tang X., Xu Y., et al. (2021). The phase changes of M1/M2 phenotype of microglia/macrophage following oxygen-induced retinopathy in mice. Inflammation Research, 70(2), 183–192. 10.1007/s00011-020-01427-w [DOI] [PubMed] [Google Scholar]
- Lieth E., LaNoue K. F., Berkich D. A., Xu B., Ratz M., Taylor C., et al. (2001). Nitrogen shuttling between neurons and glial cells during glutamate synthesis. Journal of Neurochemistry, 76(6), 1712–1723. 10.1046/j.1471-4159.2001.00156.x [DOI] [PubMed] [Google Scholar]
- Lin X., Fang D., Zhou H., Su S. B. (2013). The expression of Toll-like receptors in murine Müller cells, the glial cells in retina. Neurological Sciences, 34(8), 1339-1346. doi: 10.1007/s10072-012-1236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li C. P., Wang J. J., Shan K., Liu X., Yan B. (2016). RNCR3 knockdown inhibits diabetes mellitus-induced retinal reactive gliosis. Biochemical and Biophysical Research Communications, 479(2), 198–203. 10.1016/j.bbrc.2016.09.032 [DOI] [PubMed] [Google Scholar]
- Liu C. C., Hu J., Tsai C. W., Yue M., Melrose H. L., Kanekiyo T., et al. (2015). Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. Journal of Neuroscience, 35(14), 5851–5859. 10.1523/JNEUROSCI.5180-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenc V. E., Jaldín-Fincati J. R., Luna J. D., Chiabrando G. A., Sánchez M. C. (2015). IGF-1 regulates the extracellular level of active MMP-2 and promotes Müller glial cell motility. Investigative Opthalmology & Visual Science, 56(11), 6948. 10.1167/iovs.15-17496 [DOI] [PubMed] [Google Scholar]
- Lorenc V. E., Subirada Caldarone P. V., Paz M. C., Ferrer D. G., Luna J. D., Chiabrando G. A., et al. (2017). IGF-1R Regulates the Extracellular Level of Active MMP-2, Pathological Neovascularization, and Functionality in Retinas of OIR Mouse Model. Molecular Neurobiology, 55(2), 1123–1135. 10.1007/s12035-017-0386-9 [DOI] [PubMed] [Google Scholar]
- Loukovaara S., Nurkkala H., Tamene F., Gucciardo E., Liu X., Repo P., et al. (2015). Quantitative proteomics analysis of vitreous humor from diabetic retinopathy patients. Journal of Proteome Research, 14(12), 5131–5143. 10.1021/acs.jproteome.5b00900 [DOI] [PubMed] [Google Scholar]
- Luo L., Wall A. A., Tong S. J., Hung Y., Xiao Z., Tarique A. A., et al. (2018). TLR Crosstalk Activates LRP1 to Recruit Rab8a and PI3Kγ for Suppression of Inflammatory Responses. Cell Reports, 24(11), 3033–3044. 10.1016/j.celrep.2018.08.028 [DOI] [PubMed] [Google Scholar]
- Mahley R. W. (2016). Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(7), 1305–1315. 10.1161/ATVBAHA.116.307023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E., Brifault C., Lam M. S., Azmoon P., Gilder A. S., Gonias S. L. (2016). LDL receptor-related protein-1 regulates NFκB and microRNA-155 in macrophages to control the inflammatory response. Proceedings of the National Academy of Sciences, 113(5), 1369–1374. 10.1073/pnas.1515480113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Lockyer P., Davin Townley-Tilson W. H., Xie L., Pi X. (2016). LRP1 regulates retinal angiogenesis by inhibiting PARP-1 activity and endothelial cell proliferation. Arteriosclerosis Thrombosis and Vascular Biology, 36(2), 350–360. 10.1161/ATVBAHA.115.306713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H., Lockyer P., Li L., Ballantyne C. M., Patterson C., Xie L., et al. (2017). Endothelial LRP1 regulates metabolic responses by acting as a co-activator of PPARÎ 3. Nature Communications, 8, 14960. 10.1038/ncomms14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J., Hol E. M. (2011). GFAP In health and disease. Progress in Neurobiology, 93(3), 421–443. 10.1016/j.pneurobio.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Mizutani M., Gerhardinger C., Lorenzi M. (1998). Muller Cell changes in human diabetic retinopathy. Diabetes, 47(3), 445–449. 10.2337/diabetes.47.3.445 [DOI] [PubMed] [Google Scholar]
- Mueckler M., Thorens B. (2013). The SLC2 (GLUT) family of membrane transporters. Molecular Aspects of Medicine, 34(2-3), 121–138. 10.1016/j.mam.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz I. M., Rezzola S., Cancarini A., Russo A., Costagliola C., Semeraro F., et al. (2019). Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Progress in Retinal and Eye Research, 72, 100756. 10.1016/j.preteyeres.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Picconi F., Parravano M., Sciarretta F., Fulci C., Nali M., Frontoni S., et al. (2019). Activation of retinal müller cells in response to glucose variability. Endocrine, 65(3), 542–549. 10.1007/s12020-019-02017-5 [DOI] [PubMed] [Google Scholar]
- Rabiej V. K., Pflanzner T., Wagner T., Goetze K., Storck S. E., Eble J. A., et al. (2016). Low density lipoprotein receptor-related protein 1 mediated endocytosis of β1-integrin influences cell adhesion and cell migration. Experimental Cell Research, 340(1), 102–115. 10.1016/j.yexcr.2015.11.020 [DOI] [PubMed] [Google Scholar]
- Ren J. L., Yu Q. X., Liang W. C., Leung P. Y., Ng T. K., Chu W. K., et al. (2018). Green tea extract attenuates LPS-induced retinal inflammation in rats. Scientific Reports, 8(1), 429. 10.1038/s41598-017-18888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridano M. E., Subirada P. V., Paz M. C., Lorenc V. E., Stupirski J. C., Gramajo A. L., et al. (2017). Galectin-1 expression imprints a neurovascular phenotype in proliferative retinopathies and delineates responses to anti-VEGF. Oncotarget, 8(20), 32505. 10.18632/oncotarget.17129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsam A., Parikh S., Fort P. E. (2018). Role of inflammation in diabetic retinopathy. International Journal of Molecular Sciences, 19(4), 942. 10.3390/ijms19040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahores M., Prinetti A., Chiabrando G., Blasi F., Sonnino S. (2008). uPA binding increases UPAR localization to lipid rafts and modifies the receptor microdomain composition. Biochimica et Biophysica Acta Biomembranes, 1778(1), 250–259. 10.1016/j.bbamem.2007.09.030 [DOI] [PubMed] [Google Scholar]
- Sánchez M. C., Barcelona P. F., Luna J. D., Ortiz S. G., Juarez P. C., Riera C. M., et al. (2006). Low-density lipoprotein receptor-related protein-1 (LRP-1) expression in a rat model of oxygen-induced retinal neovascularization. Experimental Eye Research, 83(6), 1378–1385. 10.1016/j.exer.2006.07.016 [DOI] [PubMed] [Google Scholar]
- Sánchez M. C., Luna J. D., Barcelona P. F., Gramajo A. L., Juarez P. C., Riera C. M., et al. (2007). Effect of retinal laser photocoagulation on the activity of metalloproteinases and the α2-Macroglobulin proteolytic state in the vitreous of eyes with proliferative diabetic retinopathy. Experimental Eye Research, 85(5), 644–650. 10.1016/j.exer.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Shank R. P., Aprison M. H. (1981). Present status and significance of the glutamine cycle in neural tissues. Life Sciences, 28(8), 837–842. 10.1016/0024-3205(81)90044-8 [DOI] [PubMed] [Google Scholar]
- Shi Z., Rudzinski M., Meerovitch K., Lebrun-Julien F., Birman E., di Polo A., et al. (2008). α2–macroglobulin is a mediator of retinal ganglion cell death in glaucoma. Journal of Biological Chemistry, 283(43), 29156–29165. 10.1074/jbc.M802365200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K. C., Rosales M. A. B., Hamassaki D. E., Saito K. C., Faria A. M., Ribeiro P. A. O., et al. (2013). Green tea is neuroprotective in diabetic retinopathy. Investigative Opthalmology & Visual Science, 54(2), 1325. 10.1167/iovs.12-10647 [DOI] [PubMed] [Google Scholar]
- Simón M. V., Prado Spalm F. H., Politi L. E., Rotstein N. P. (2015). Sphingosine-1-phosphate is a crucial signal for migration of retina Müller glial cells. Investigative Opthalmology & Visual Science, 56(10), 5808. 10.1167/iovs.14-16195 [DOI] [PubMed] [Google Scholar]
- Sorrentino F. S., Allkabes M., Salsini G., Bonifazzi C., Perri P. (2016). The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sciences, 162, 54–59. 10.1016/j.lfs.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Subirada P. V., Paz M. C., Ridano M. E., Lorenc V. E., Vaglienti M. V., Barcelona P. F., et al. (2018). A journey into the retina: Müller glia commanding survival and death. European Journal of Neuroscience, 47(12), 1429–1443. 10.1111/ejn.13965 [DOI] [PubMed] [Google Scholar]
- Swarup A., Samuels I. S., Bell B. A., Han J. Y. S., Du J., Massenzio E., et al. (2019). Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: Impact on photoreceptors and Müller glial cells. American Journal of Physiology-Cell Physiology, 316(1), C121–C133. 10.1152/ajpcell.00410.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler B. S., Arnold M. J., Brassell M. A., Puro D. G. (2008). Energy metabolism in human retinal Muller cells. Investigative Opthalmology & Visual Science, 49(10), 4613. 10.1167/iovs.08-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X., Ding Y., Dieckmann M., Zhou L., Plattner F., Liu M., et al. (2017). LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. Elife, 6, e29292. 10.7554/eLife.29292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X., Rodrigues M., Umapathi M., Kashiwabuchi F., Ma T., Babapoor-Farrokhran S., et al. (2013). Hypoxic retinal Müller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proceedings of the National Academy of Sciences of the United States of America, 110(36), E3425–E3434. 10.1073/pnas.1217091110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Yoshida A., Ishibashi T. (2004). Induction of IL-8, MPC-1, and bFGF by TNF-α in retinal glial cells: Implications for retinal neovascularization during post-ischemic inflammation. Graefe’s Archive for Clinical and Experimental Ophthalmology, 242(5), 409–413. 10.1007/s00417-004-0874-2 [DOI] [PubMed] [Google Scholar]
- You Z. P., Zhang Y. L., Shi K., Shi L., Zhang Y. Z., Zhou Y., et al. (2017). Suppression of diabetic retinopathy with GLUT1 siRNA. Scientific Reports, 7(1), 7437. 10.1038/s41598-017-07942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. J., Ouyang H., Luo J., Patel S., Xue Y., Quach J., et al. (2014). Induction of retinal progenitors and neurons from mammalian müller glia under defined conditions. Journal of Biological Chemistry, 289(17), 11945–11951. 10.1074/jbc.M113.532671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Takayama Y., Boucher P., Tallquist M. D., Herz J. (2009). LRP1 Regulates architecture of the vascular wall by controlling PDGFRβ-dependent phosphatidylinositol 3-kinase activation. PLoS One, 4(9), e6922. 10.1371/journal.pone.0006922 [DOI] [PMC free article] [PubMed] [Google Scholar]