Abstract
Background:
Biliary atresia (BA) is a progressive obstructive hepatic disease that requires early diagnosis and the prompt initiation of treatment. Although portoenterostomy (PES) is usually performed as the initial surgical procedure, the liver damage may subsequently progress, such that liver transplantation (LTx) may be required. In this study, we comprehensively evaluated the histopathology of liver samples collected during PES and retrospectively evaluated its relationship with prognosis.
Methods:
Forty-seven patients with BA who underwent PES between 2002 and 2021 were included. Their biopsy samples were semi-quantitatively graded according to the severity of liver fibrosis, bile duct proliferation, cholestasis, ductal plate malformation, and inflammatory cell infiltration; and the expression of cluster of differentiation (CD)3, CD20, human leukocyte antigen II-DR, and α-smooth muscle actin (α-SMA). The relationships of each with the prevalence of survival with native liver (SNL) were evaluated to identify prognostic markers.
Results:
The median postoperative duration of follow-up was 11.8 years (maximum, 18.0 years; minimum, 3.5 years). There were no deaths during this period, but LTx was performed in 31 patients and the final prevalence of SNL was 34.0% (16/47). There were negative correlations of liver fibrosis and α-SMA with SNL, and a positive correlation between CD20 and SNL. Multivariate analysis using a proportional hazards regression model showed that only CD20 expression was significant.
Conclusions:
Comprehensive histopathological analysis of liver biopsy samples obtained at the time of PES showed a positive correlation between CD20 expression and SNL, suggesting that this may represent a useful prognostic marker.
Level of evidence:
III
Keywords: Biliary atresia, prognosis, histopathology, survival with native liver, cluster of differentiation 20, graft versus host disease
Introduction
Biliary atresia (BA) is a pediatric disease that causes progressive hepatic damage because of cholestasis, which, if left untreated, can lead to liver cirrhosis, liver failure, and death within 2 years.1 The early diagnosis of BA and initiation of treatment are necessary to save lives, and the portoenterostomy (PES) technique first described by Kasai2 is widely performed as the initial surgical treatment. In many cases, liver damage progresses even after PES, but the degree of damage varies among patients. Some patients develop liver failure at an early stage and require liver transplantation (LTx), others develop liver failure more gradually and require LTx after several months to several years, and a third group develop little liver damage and do not require LTx, even in adulthood.3
The histopathology of liver biopsies collected during PES is considered to be helpful in determining the prognosis of BA.4 Features such as liver fibrosis, cholestasis, and inflammatory cell infiltration (ICI) have been reported to be markers of the prognosis of BA, but most previous studies included relatively few parameters and were not of high quality5; therefore, no definitive conclusions have been reached. Identifying which of the various parameters is most important is not only necessary for predicting the prognosis of BA, but also has the potential to provide important insights into the etiology of BA. In the present study, we performed a comprehensive histopathological analysis of hepatic wedge biopsies obtained at the time of PES in patients with BA, and retrospectively determined the relationships between histopathological findings and the prognosis following PES.
Methods
Participants
All patients with BA who had undergone PES in our institution between August 2002 and July 2018 were included in the present study. To assess the long-term prognosis of BA, we excluded eligible patients who were not followed up for more than 3 years as of July 2021. A wedge biopsy of the liver had been performed at the time of PES in all participants. Informed consent was obtained from the parents of all of the participants for tissue collection and evaluation. Ethics approval was obtained from the Institutional Review Board of our institution (Approval number: 1857). LTx was performed in participants who experienced irreversible liver failure, including those whose jaundice did not improve after PES, those who experienced repeated episodes of postoperative cholangitis, and those who developed symptoms associated with portal hypertension.
Histopathological and immunohistochemical assessments
The biopsy tissue was formalin-fixed, paraffin-embedded, and thinly sectioned, prior to staining with hematoxylin and eosin (HE), Azan, or Masson’s trichrome (MT). In addition, immunostaining was performed on 4-μm-thick sections for cluster of differentiation (CD)3 (monoclonal mouse, Clone PS1, Code 713 241, dilution; Nichirei, Tokyo, Japan), CD20 (monoclonal mouse, Clone L26, Code 722 441, dilution; Nichirei), cytokeratin 7 (CK7) (monoclonal mouse, Clone OV-TL 12/30, Code M7018, 1:100 dilution; Dako, Glostrup, Denmark), anti-human leukocyte antigen class II DRB1 beta-chain antibody (HLA-DR) (monoclonal rabbit, Clone EPR6148, Code ab133578, 1:200 dilution; Abcam, Cambridge, UK), and alpha-human smooth muscle actin (α-SMA) (monoclonal mouse, Clone 1A4, Code M0851, 1:200 dilution; Dako) expression.
Diagnostic criteria
The histological changes in the liver tissue (liver fibrosis, bile duct proliferation [BDP], cholestasis, ductal plate malformation [DPM], and ICI) were evaluated. To make the assessments more objective, we used the same scoring systems for histopathological and immunohistochemical findings that were used in previous studies of the prognosis of BA.6 -9 We needed to create novel grading systems because there have been no previous assessments on CD3, CD20, HLA-DR and α-SMA. We created these systems as follows. First, for each parameter, we identified which areas of the liver tissue had the most positive cells. Cases that had a small number of positive cells in that region were defined as Grade 1. Second, cases in which positive cells spread to adjacent areas were defined as Grade 2. Finally, cases in which positive cells spread to the entire adjacent area or with clustering were defined as Grade 3 (Table 1).
Table 1.
Grading and definition of histopathological and immunohistochemical findings.
| Fibrosis grade | Severity | Definition (Method of Weerasooriya et al)6 |
|---|---|---|
| Grade 1 | mild | portal fibrous expansion to bridging fibrosis, involving <50% of the portal areas |
| Grade 2 | moderate | portal fibrous expansion to bridging fibrosis, involving <50% of the portal areas |
| Grade 3 | severe | bridging fibrosis involving >50% of the portal areas, with nodular architectural changes |
| BDP | Severity | Definition (Method of Zhang et al)7 |
| Grade 1 | mild | mild small bile duct hyperplasia in the portal areas and at the edge of the lobules, discontinuous distribution |
| Grade 2 | moderate | marked hyperplasia in the portal areas and at the edge of the lobules, with a continuous distribution and lobular extension, and the formation of a two-to-three-layer structures |
| Grade 3 | severe | same lesions as Grade 2, but with more than three layers or net-like structures |
| Cholestasis | Severity | Definition (Method of Roy et al)8 |
| Grade 0 | absent | no accumulation |
| Grade 1 | mild | bile accumulation in centrilobular hepatocytes |
| Grade 2 | moderate | bile accumulation in centrilobular and periportal hepatocytes or in the portal areas |
| Grade 3 | severe | with the presence of bile infarcts |
| DPM | Severity | Definition (Method of Desmet)9 |
| Grade 0 | absent | DPM is absent |
| Grade 1 | present | DPM is present |
| ICI | Severity | Definition (Method of Roy et al)8 |
| Grade 1 | mild | inflammatory cells across less than one third of the portal areas |
| Grade 2 | moderate | inflammatory cells across one-to-two thirds of the portal areas |
| Grade 3 | severe | dense packing of inflammatory cells across more than two thirds of the portal areas |
| CD3 | Level of expression | Definition |
| Grade 1 | mild | positive cells are scattered along the margins of the portal areas |
| Grade 2 | moderate | positive cells are found in the portal areas and/or in less than half of the lobules |
| Grade 3 | severe | highly-expressing cells in the portal areas and/or in more than half of the lobules |
| CD20 | Level of expression | Definition |
| Grade 1 | mild | few positive cells are found |
| Grade 2 | moderate | positive cells at the margins of the portal areas |
| Grade 3 | severe | positive cells throughout the portal areas, clustered in some cases |
| HLA II-DR | Level of expression | Definition |
| Grade 1 | mild | some positive cells in the lobules |
| Grade 2 | moderate | some positive cells in the portal areas and the lobules, clustered in some cases |
| Grade 3 | severe | positive cells in the portal areas and the lobules, with clustering |
| α-SMA | Level of expression | Definition |
| Grade 1 | mild | single layer of linear staining around vessels and bile ducts and at the edges of the portal areas |
| Grade 2 | moderate | multiple layers of positive cells covering approximately half of the edges of the portal areas |
| Grade 3 | severe | multiple layers of positive cells at the edges of the portal areas and others within the portal areas |
Abbreviations: BDP, bile duct proliferation; CD, cluster of differentiation; DPM, ductal plate malformation; HLA, human leukocyte antigen; ICI, inflammatory cell infiltration; α-SMA, α-smooth muscle actin.
Histopathological and immunohistochemical findings were graded on scales comprising 2 to 4 categories. To make the assessments more objective, we used the same scoring systems that were used in previous studies of the prognosis of BA. We created novel grading systems for parameters that had not been assessed in the past.
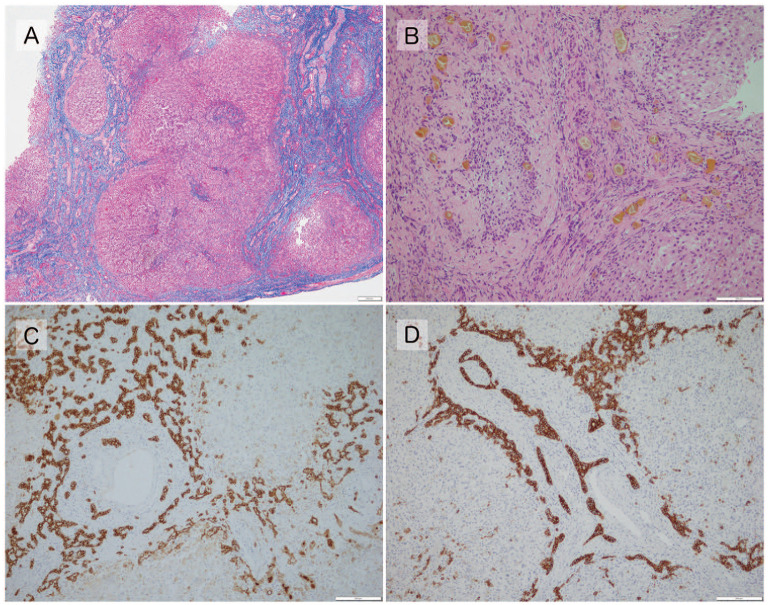
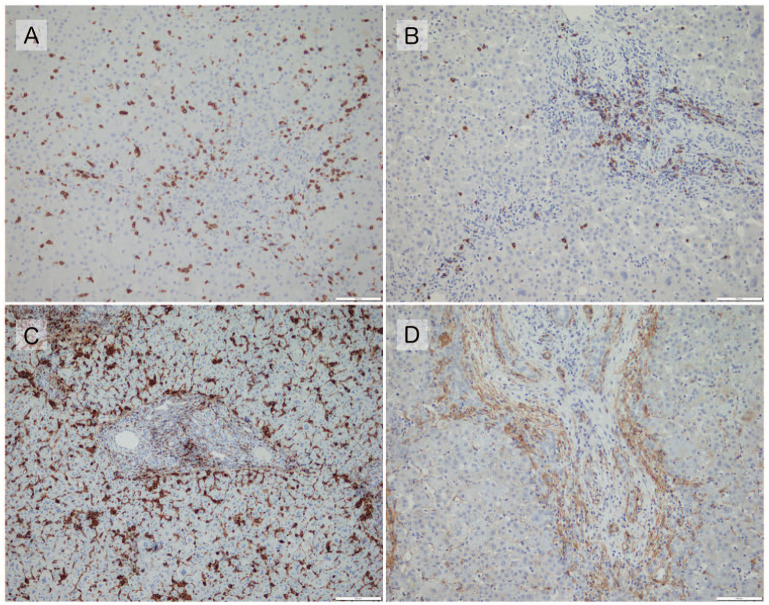
Each of these factors was graded semi-quantitatively by a single investigator who was not informed about the clinical course or prognosis of the participants, and these grades were analyzed (Figures 1 and 2).
Figure 1.
(A) Fibrosis grade 3, with fibrotic extension of the portal area and the formation of nodules (pseudo-lobules) (Masson’s trichrome, ×40). (B) Cholestasis grade 3, with severe cholestasis in the hepatocyte and in the small bile ducts at the margins of the portal area (hematoxylin and Eosin, ×200). (C) BDP Grade 3, with marked hyperplasia of the bile ducts at the margins of the portal area, forming multiple structures of 3 or more layers (anti-cytokeratin 7, ×100). (D) DPM present, with concentrically-arranged bile ducts in the portal area (anti-cytokeratin 7, ×100).
Figure 2.
(A) CD3 grade 3. Positive cells are present in the portal regions and hepatic lobules (×200). (B) CD20 grade 3. Positive cells are present throughout the portal regions, with some clustering (×200). (C) HLA II-DR grade 3. Positive cells are present throughout the portal regions and in the lobules (×200). (D) α-SMA grade 3, with multiple layers of positive areas at the margins of the portal areas and positive areas within (×200).
Statistical analysis of prognosis
To evaluate survival with native liver (SNL), LTx during the follow-up period was set as an endpoint, indicative of a poor prognosis, and survival was analyzed using the log-rank test for each parameter. The significance level was set at 0.05. Multivariate analysis using a proportional hazards regression model was performed to reduce confounding bias, including the parameters that were found to have P < .1 in univariate analysis. The statistical analyses were performed using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 4.01 (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R commander designed to add statistical functions that are frequently used in biostatistics.10
Results
Characteristics of the participants
The characteristics of the participants are shown in Table 2. PES was performed in 47 patients with BA during the study period (31 girls and 16 boys). No patients were lost to follow-up within 3 years of surgery, and none were excluded from the present study. The mean age of the participants at the time of PES was 76.2 ± 34.2 days, and the median duration of follow-up was 11.8 years (maximum, 18.0 years; minimum, 3.5 years). There were no deaths during the study period, but LTx was performed in 31 participants, resulting in a prevalence of SNL of 34.0% (16/47). The median length of time between PES and LTx was 241 days (maximum, 3668 days; minimum, 32 days). A reoperation of PES was performed in 7 patients, and one patient had a reoperation 3 times. The most common postoperative complication of PES was cholangitis, which occurred in 26 patients. In addition, esophageal varices were found in 19 patients, bile lake in 9 patients, cytomegalovirus infection in 4 patients, gastrointestinal bleeding in 3 patients, and ileus in 3 patients.
Table 2.
Characteristics of the participants.
| Sex | |
|---|---|
| Male | 31 |
| Female | 16 |
| Mean age at the time of PES | 76.2 ± 34.2 days |
| Median duration of follow-up | 11.8 year |
| Postoperative course of PES | |
| LTx | 31 |
| SNL | 16 |
| Deaths | 0 |
| Reoperation of PES | |
| 3 times | 1 |
| 1 time | 6 |
| None | 40 |
| Complications after PES | |
| Cholangitis | 26 |
| Esophageal varices | 19 |
| Bile lake | 9 |
| Cytomegalovirus infection | 4 |
| Gastrointestinal bleeding | 3 |
| Ileus | 3 |
Relationships between pathological findings and prognosis
Each histopathological parameter was graded and their relationships with the prognosis are shown in Table 3.
Table 3.
Relationships between each parameter and the prevalence of SNL.
| Parameter | Grade | n | Median survival time (years) | 95% CI | P-value |
|---|---|---|---|---|---|
| Fibrosis | 1 | 16 | NA | 1.10-NA | .03* |
| 2 | 21 | 0.73 | .42-1.36 | ||
| 3 | 10 | 1.38 | .21-6.06 | ||
| BDP | 1 | 11 | NA | 1.09-NA | .08 |
| 2 | 17 | 1.27 | 0.44-NA | ||
| 3 | 10 | 0.73 | .35-5.14 | ||
| Cholestasis | 0 | 0 | NA | NA | .90 |
| 1 | 9 | 1.09 | 0.46-NA | ||
| 2 | 18 | 1.36 | 0.56-NA | ||
| 3 | 17 | 1.32 | .35-10.04 | ||
| DPM | 0 | 31 | 1.49 | 0.66–NA | .30 |
| 1 | 16 | 1.12 | .35-7.26 | ||
| ICI | 1 | 17 | 1.49 | 0.43-10.04 | .32 |
| 2 | 21 | 1.36 | 0.56-NA | ||
| 3 | 9 | 0.67 | 0.27-NA | ||
| CD3 | 1 | 11 | NA | 0.56–NA | .08 |
| 2 | 21 | 1.20 | .40-5.14 | ||
| 3 | 15 | 1.03 | 0.53-NA | ||
| CD20 | 1 | 18 | 0.71 | 0.35-1.23 | .01* |
| 2 | 17 | 1.49 | .66-10.04 | ||
| 3 | 12 | NA | 0.50-NA | ||
| HLA II-DR | 1 | 13 | 1.20 | 0.27-NA | .81 |
| 2 | 18 | 1.42 | 0.87-NA | ||
| 3 | 16 | 1.24 | 0.50-NA | ||
| α-SMA | 1 | 14 | NA | 1.09-NA | .04* |
| 2 | 10 | 1.11 | .33-7.26 | ||
| 3 | 23 | 0.73 | .43-1.49 |
Abbreviations: CI, confidence interval.
*Significant difference.
We evaluated the relationships of the grades of liver fibrosis, BDP, cholestasis, DPM, ICI, CD3, CD20, HLA II-DR, and α-SMA expression with the prevalence of SNL using the log-rank test. There were negative relationships for liver fibrosis and α-SMA, and a positive relationship for CD20.
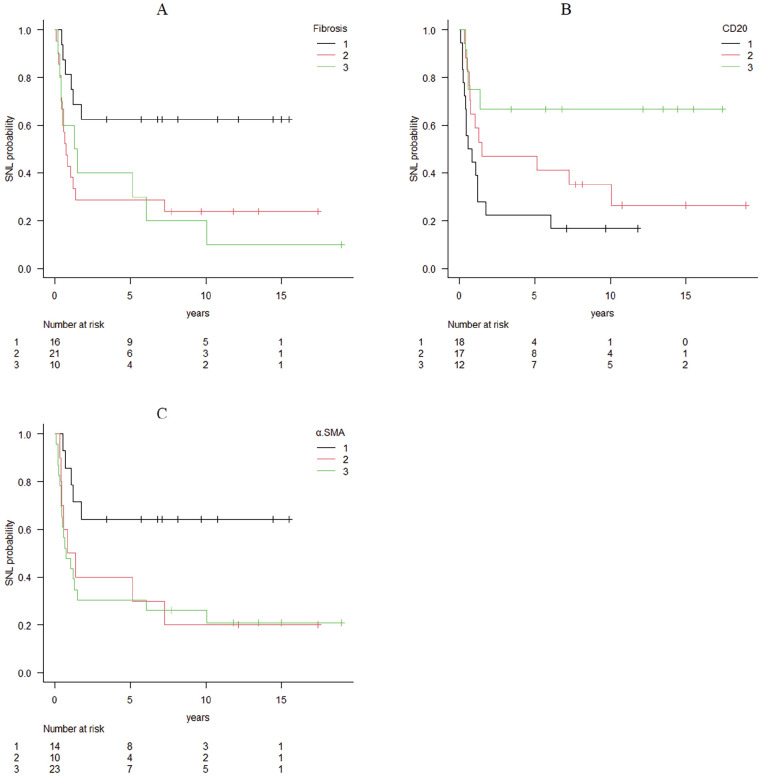
There was a negative relationship between the liver fibrosis grade and the prevalence of SNL, which indicated that SNL was less likely to occur as fibrosis became worse (P = .03, Figure 3). There were no relationships of the grades of BDP, cholestasis, DPM, or ICI with the prevalence of SNL.
Figure 3.
(A) Survival curve for fibrosis. (B) Survival curve for CD20 expression. (C) Survival curve for α-SMA expression. Survival curves for the 3 parameters that showed significant correlations in the log rank tests of the relationships between each histopathological and immunohistochemical parameter and the prevalence of SNL. Liver fibrosis and α-SMA expression showed negative correlations with SNL, whereas CD20 showed a positive correlation. There were no significant relationships of other parameters with SNL.
Immunostaining showed a negative relationship between α-SMA expression and the prevalence of SNL (P = .04, Figure 3), whereas CD20, a cell surface marker of B-cell lineage, showed a positive relationship with the prevalence of SNL (P = .02, Figure 3). This finding indicated that the prevalence of SNL increased with a higher CD20 expression. There were no relationships between the expression of the other target proteins and the prevalence of SNL.
We next performed a multivariate analysis, including liver fibrosis, α-SMA, CD20, BDP, and CD3, all of which had P values of <.1 in the univariate analysis, using a proportional hazards regression model. This analysis showed a significant relationship for CD20 alone (P = .01, Table 4).
Table 4.
Results of multivariate analysis performed using variables with P < .1 in univariate analysis.
| Parameter | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Fibrosis | 1.24 | 0.70-2.23 | .47 |
| BDP | 1.14 | 0.58-2.22 | .71 |
| CD3 | 1.34 | 0.71-2.52 | .37 |
| CD20 | 0.44 | 0.24-0.80 | .008* |
| α-SMA | 1.21 | 0.67-2.21 | .53 |
Abbreviations: CI, confidence interval.
*Significant difference.
A proportional hazards regression model was used. Only CD20 was found to be a significant predictor.
Discussion
Many patients with BA show progression of their disease following PES, and there have been many previous studies of the potential use of histopathological and immunohistochemical parameters as prognostic markers.6,8,11 -31 Liver fibrosis is the clearest histopathological change in the livers of patients with BA and has been the most frequently studied parameter to date.6,8,11 -23 Several previous studies, including one that included 43 patients,11 showed that the prognosis of the patients became worse with a higher grade of hepatic fibrosis.6,8,12 -15 However, Obayashi et al found no relationship between liver fibrosis and the prognosis in their study of 32 patients,16 and a number of other studies showed similar findings.17 -23 However, the scoring systems used for the evaluation of histopathological findings and the outcomes set varied among the previous studies, and thus they cannot be easily compared. Therefore, the studies that did show relationships tended to include a relatively long follow-up period, whereas the majority that did not show a relationship included a follow-up period of <1 year. This finding suggests that liver fibrosis is related to the long-term prognosis of patients following PES. In the present study, the median duration of follow-up was long (11.8 years). Additionally, the length of the follow-up period was associated with the prognosis in the univariate analysis, but this relationship disappeared in the multivariate analysis. Therefore, a higher degree of liver fibrosis at the time of PES may indicate that postoperative bile excretion is more disadvantageous. This possibility indicates that the extent of liver fibrosis is a useful means of predicting the prognosis at the time of the initial surgery.
Among the previous studies of the relationships of BDP and cholestasis with the prognosis,8,11,12,16 -19,21 -23 more than half showed no relationship of either.8,17,19,21 -23 El-Guindi et al found that BDP had the highest accuracy (91.7) for the differentiation of patients with BA who had a good or poor prognosis.32 However, although BDP is a characteristic finding in BA, its expression was not related to the prognosis in the present study.
DPM was defined by Desmet as “the excessive presence of juvenile bile duct structures due to the absence of ductal plate remodeling that should differentiate into mature bile ducts during fetal life.”9 DPM is the cause of many congenital diseases, such as congenital liver fibrosis and autosomal dominant polycystic kidney disease.33 Since Low et al first reported a relationship between DPM and the prognosis of BA in 2001,25 many other studies have shown similar findings.8,11,12,16,17,24,25 However, we found no significant relationship between DPM and the prognosis of BA in the present study, and the Japanese BA guidelines state that “We conclude that DPM has no significant effect on SNL.”5 Although there have been some studies of ICI,8,11,19,21 few have shown relationships with the prognosis,8,11,21 and no relationship between ICI and the prognosis of BA was found in the present study.
A recent study by Gürünlüoğlu et al evaluated the relationships between histological findings and the prognosis.23 They compared a group of patients who underwent PES within 60 days of birth with a good prognosis with a group of patients who underwent PES after 60 days with a poor prognosis. While histopathological parameters indicating the severity of parenchymal damage were not different between the 2 groups, electron microscopic examinations showed more severe signs of intracellular damage to the liver in the worse prognosis group. They speculated that liver damage did not exceed the irreversible threshold in the early-operated groups in which the liver’s regeneration capacity may have been maintained. Although electron microscopic examinations were not performed in the present study, our findings of no associations between the prognosis and histopathological parameters, except for immunohistochemical findings, are consistent with those in Gürünlüoğlu et al’s study.23 Their study is interesting because it showed that subtle intracellular structural changes may affect the prognosis of BA.
α-SMA is a marker of activated hepatic stellate cells (HSCs), and it is expressed when HSCs differentiate into myofibroblasts in response to hepatic injury. Suominen et al stated that HSCs are a major cause of liver fibrosis in BA.13 In the present study, we found a significant relationship between α-SMA expression and SNL, and similar relationships have been identified in previous relevant studies.13,14,26 This finding implies that HSCs play an important role in liver fibrosis and thus influence the prognosis of BA.
HLA-class II antigens, and especially the DR antigen, are specifically and abnormally expressed in the bile duct epithelium of patients with BA.34 This expression plays a major role in the progression of hepatobiliary tissue destruction induced by cytotoxic T cells,35 but we found no relationship between antigen expression and the prognosis of BA in the present study.
In recent years, immunological factors have been proposed to play an important role in the etiology of BA. Therefore, in the present study, we characterized the relationships of the distributions of T and B cells in the liver with the prognosis of BA, using CD3 and CD20 as the respective cell surface markers. CD20 expression was the only significant predictor identified in the multivariate analysis, which showed that the prognosis of BA improved with increasing CD20 expression.
Muraji claimed that the etiology of BA is maternal microchimerism, which is mediated via the umbilical vein.36 They also proposed that GvHD caused by maternally derived effector T cells impairs the function of antigen-presenting cells in the bile duct epithelium and hepatocytes. In addition, some studies have shown that GvHD causes B-cell depletion and functional abnormalities via Fas ligand.37,38 These findings suggest the hypothesis that maternally derived effector T cells cause GvHD in the bile duct epithelium and hepatocytes of patients, resulting in a reduction in the number of B cells. This possibility is consistent with the reduction in the number of CD20-positive cells identified in the present study. Therefore, high CD20 expression in liver tissue may be indicative of mild GvHD, caused by maternally derived effector T cells. Further studies are required to confirm this possibility, but this hypothesis may be helpful in clarifying the etiology of BA.
The limitations of the present study include the small number of participants and the fact that it was performed at a single center, which may have created type I statistical errors in the analysis.
In the present analysis of the relationships between a comprehensive list of histopathological findings and the prognosis of BA, we found that high expression of CD20 is an independent predictor of a favorable prognosis. Therefore, CD20 may represent a useful predictor of SNL after PES, and GvHD may be involved in the etiology of BA.
Conclusion
Previous studies have only assessed the potential utility of a few histopathological parameters for the prediction of the prognosis of BA. In the present study, we performed a comprehensive histopathological examination of liver biopsy samples obtained from patients with BA at the time of PES, and graded these semi-quantitatively. In a multivariate analysis, we found a significant relationship between CD20 and the prevalence of SNL. The prevalence of SNL decreased as the expression of CD20 increased, which suggests that the etiology of BA involves GvHD caused by maternal microchimerism.
Acknowledgments
We would like to thank the patients and parents who participated in this study. We thank the clinical laboratory technicians at the National Center for Child Health and Development who assisted with the specimen preparation. We also thank Mark Cleasby, PhD from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Author Contributions: All authors contributed to the study conception and design. Data acquisition were performed by Takako Yoshioka, Yutaka Kanamori, Akihiro Fujino. Analysis and data interpretation were performed by Atsushi Higashio. The first draft of the manuscript was written by Atsushi Higashio, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Superina R. Liver transplantation for biliary atresia: does the insurance type really make a difference? Liver Transpl. 2013;19:470-471. [DOI] [PubMed] [Google Scholar]
- 2. Kasai M. Advances in treatment of biliary atresia. Jpn J Surg. 1983;13:265-276. [DOI] [PubMed] [Google Scholar]
- 3. Nio M, Sasaki H, Wada M, Kazama T, Nishi K, Tanaka H. Impact of age at Kasai operation on short- and long–term outcomes of type III biliary atresia at a single institution. J Pediatr Surg. 2010;45:2361-2363. [DOI] [PubMed] [Google Scholar]
- 4. Lopez RN, Ooi CY, Krishnan U. Early and peri-operative prognostic indicators in infants undergoing hepatic portoenterostomy for biliary atresia: a review. Curr Gastroenterol Rep. 2017;19:16. [DOI] [PubMed] [Google Scholar]
- 5. Ando H, Inomata Y, Iwanaka T, et al. Clinical practice guidelines for biliary atresia in Japan: A secondary publication of the abbreviated version translated into English. J Hepatobiliary Pancreat Sci. 2021;28:55-61. [DOI] [PubMed] [Google Scholar]
- 6. Weerasooriya VS, White FV, Shepherd RW. Hepatic fibrosis and survival in biliary atresia. J Petiatr. 2004;144:123-125. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Wu Y, Liu Z, Tao Q, Huang J, Yang W. Hepatic pathology of biliary atresia: A new comprehensive evaluation method using liver biopsy. Turk J Gastroenterol. 2016;27:257-263. [DOI] [PubMed] [Google Scholar]
- 8. Roy P, Chatterjee U, Ganguli M, Banerjee S, Chatterjee SK, Basu AK. A histopathological study of liver and biliary remnants with clinical outcome in cases of extrahepatic biliary atresia. Indian J Pathol Microbiol. 2010;53:101-105. [DOI] [PubMed] [Google Scholar]
- 9. Desmet VJ. Ludwig symposium on biliary disorders-part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73:80-89. [DOI] [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muthukanagarajan SJ, Karnan I, Srinivasan P, Sadagopan P, Manickam S. Diagnostic and prognostic significance of various histopathological features in extrahepatic biliary atresia. J Clin Diagn Res. 2016;10:EC23-EC27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukhopadhyay SG, Roy P, Chatterjee U, et al. A histopathological study of liver and biliary remnants in the long-term survivors (>10 years) of cases of biliary atresia. Indian J Pathol Microbiol. 2014;57:380-385. [DOI] [PubMed] [Google Scholar]
- 13. Suominen JS, Lampela H, Heikkilä P, Lohi J, Jalanko H, Pakarinen MP. Myofibroblastic cell activation and neovascularization predict native liver survival and development of esophageal varices in biliary atresia. World J Gastroenterol. 2014;20:3312-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong R, Luo Y, Zheng S. α-SMA overexpression associated with increased liver fibrosis in infants with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:653-656. [DOI] [PubMed] [Google Scholar]
- 15. Karrer FM, Lilly JR, Stewart BA, Hall RJ. Biliary atresia registry, 1976 to 1989. J Pediatr Surg. 1990;25:1076-NaN80; discussion 1081. [DOI] [PubMed] [Google Scholar]
- 16. Obayashi J, Tanaka K, Ohyama K, et al. Relation between amount of bile ducts in portal canal and outcomes in biliary atresia. Pediatr Surg Int. 2016;32:833-838. [DOI] [PubMed] [Google Scholar]
- 17. Vuković J, Grizelj R, Bojanić K, et al. Ductal plate malformation in patients with biliary atresia. Eur J Pediatr. 2012;171:1799-1804. [DOI] [PubMed] [Google Scholar]
- 18. Santos JL, Kieling CO, Meurer L, et al. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J Pediatr Surg. 2009;44:695-701. [DOI] [PubMed] [Google Scholar]
- 19. Azarow KS, Phillips MJ, Sandler AD, Hagerstrand I, Superina RA. Biliary atresia: should all patients undergo a portoenterostomy? J Pediatr Surg. 1997;32:168-NaN72; discussion 172. [DOI] [PubMed] [Google Scholar]
- 20. Altman RP, Lilly JR, Greenfeld J, Weinberg A, van Leeuwen K, Flanigan L. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia: twenty-five years of experience from two centers. Ann Surg. 1997;226:348-NaN53; discussion 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang N, Davenport M, Driver M, Howard ER. Hepatic histology and the development of esophageal varices in biliary atresia. J Pediatr Surg. 1993;28:63-66. [DOI] [PubMed] [Google Scholar]
- 22. Vazquez-Estevez J, Stewart B, Shikes RH, Hall RJ, Lilly JR. Biliary atresia: early determination of prognosis. J Pediatr Surg. 1989;24:48-50; discussion 50. [DOI] [PubMed] [Google Scholar]
- 23. Semra Gürünlüoğlu S, Gül M, Zararsiz G, et al. Ultra-structural and histopathological features of liver biopsy taken during laparotomy to confirm the diagnosis of biliary atresia. Indian J Pathol Microbiol. 2022;65:572-580. [DOI] [PubMed] [Google Scholar]
- 24. Shimadera S, Iwai N, Deguchi E, et al. Significance of ductal plate malformation in the postoperative clinical course of biliary atresia. J Pediatr Surg. 2008;43:304-307. [DOI] [PubMed] [Google Scholar]
- 25. Low Y, Vijayan V, Tan CE. The prognostic value of ductal plate malformation and other histologic parameters in biliary atresia: an immunohistochemical study. J Pediatr. 2001;139:320-322. [DOI] [PubMed] [Google Scholar]
- 26. Shteyer E, Ramm GA, Xu C, White FV, Shepherd RW. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42:93-99. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi H, Puri P, O’Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997;32:590-593. [DOI] [PubMed] [Google Scholar]
- 28. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [DOI] [PubMed] [Google Scholar]
- 29. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [DOI] [PubMed] [Google Scholar]
- 30. Lee WS, Looi LM. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J Gastroenterol. 2009;15:5326-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinugasa Y, Nakashima Y, Matsuo S, Shono K, Suita S, Sueishi K. Bile ductular proliferation as a prognostic factor in biliary atresia: an immunohistochemical assessment. J Pediatr Surg. 1999;34:1715-1720. [DOI] [PubMed] [Google Scholar]
- 32. El-Guindi MA, Sira MM, Sira AM, et al. Design and validation of a diagnostic score for biliary atresia. J Hepatol. 2014;61:116-123. [DOI] [PubMed] [Google Scholar]
- 33. Awasthi A, Das A, Srinivasan R, Joshi K. Morphological and immunohistochemical analysis of ductal plate malformation: correlation with fetal liver. Histopathology. 2004;45:260-267. [DOI] [PubMed] [Google Scholar]
- 34. Muraji T, Nishijima E, Tsugawa C, et al. Increased expression of HLA-DR antigens on biliary epithelial cells in biliary atresia. J Jpn Soc Pediatr Surg. 1988;24:793-796. [Google Scholar]
- 35. Saidman SL, Duquesnoy RJ, Zeevi A, Fung JJ, Starzl TE, Demetris AJ. Recognition of major histocompatibility complex antigens on cultured human biliary epithelial cells by alloreactive lymphocytes. Hepatology. 1991;13:239-246. [PMC free article] [PubMed] [Google Scholar]
- 36. Muraji T. Maternal microchimerism in biliary atresia: are maternal cells effector cells, targets, or just bystanders? Chimerism. 2014;5:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115:5401-5411. [DOI] [PubMed] [Google Scholar]
- 38. Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265-2274. [DOI] [PubMed] [Google Scholar]