Abstract
Objective
To evaluate the effects of one anastomosis gastric bypass (OAGB), Roux-en-Y gastric bypass (RYGB), and sleeve gastrectomy (SG) on cardiometabolic risk factors (CMRFs) in patients with severe obesity.
Methods
This retrospective cohort study included patients with severe obesity who had undergone OAGB, RYGB, or SG between 2015 and 2017 and follow-up assessments over 12-months.
Results
Among 485 included patients, anthropometric measurements, body composition, fasting blood glucose (FBG), lipid profile, and comorbidities were significantly improved for all three procedures throughout the follow-up period. Weight, % total weight loss (%TWL), body mass index, fat mass and fat mass to fat-free mass ratio improvements were higher with RYGB and OAGB than SG. There were no significant differences between procedures in all other variables. A significant trend toward remission rate of dyslipidemia and type 2 diabetes mellitus was observed with all three procedures, with no significant difference between the three groups. %TWL statistically correlated with fat mass, FBG, and triglycerides.
Conclusions
OAGB, RYGB, and SG had a beneficial impact on CMRFs and comorbidities during 12 months of follow-up. Of note, RYGB and OAGB may result in better outcomes, particularly anthropometric and body composition indices. Further large-sample, long-term follow-up studies are required to expand on the present findings.
Keywords: Bariatric surgery, obesity, dyslipidemia, type 2 diabetes mellitus, comorbidities, weight loss
Introduction
Obesity has become a major public health problem with drastic social and economic consequences requiring the development of effective treatment strategies to control this complex disease and its comorbidities.1 Severe obesity, diagnosed as body mass index (BMI) ≥ 40, or BMI ≥ 35 with obesity-related comorbidity, is generally accepted to be a main cardiometabolic risk factor (CMRF) for chronic diseases, such as type 2 diabetes mellitus (type 2 diabetes), cardiovascular disease (CVD), non-alcoholic fatty liver disease (NAFLD) and certain cancers.2,3 Thus, ascertaining the most efficient strategies to treat severe obesity and control CMRFs may enhance clinical and public health outcomes in this population.
Bariatric surgery has been proposed as the most effective therapy for achieving and maintaining weight loss in the long term, and has been shown to have significant therapeutic effects on obesity-related comorbidities, such as dyslipidemia, type 2 diabetes, CVD events, and mortality.3–5 Moreover, the long-term improvement in CMRFs following bariatric surgery is proposed to be partially attributable to the reduction of body fat mass.6
Among the variety of bariatric surgery procedures currently available, sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are the most widely used worldwide.7 SG is a restrictive surgical procedure with high safety and effectiveness, leading to weight loss due to favorable hormonal changes, caloric restriction, and appetite suppression; while RYGB has been regarded as the gold standard for treating metabolic abnormalities in patients with severe obesity.8 However, the one anastomosis gastric bypass (OAGB) procedure has attracted considerable recent attention. This procedure was first introduced by Rutledge in 1997 and was reported to have higher efficiency and fewer complications versus other bariatric surgeries.9,10 Despite all the available bariatric surgery procedures and their positive outcomes, few studies have compared the impact of different bariatric surgeries on weight loss and CMRF. Moreover, the limited research conducted to date has yielded contradictory findings.11,12 Of note, not only the procedure but also the patient’s geographical region appears to play a pivotal role in the effectiveness of bariatric surgery,13 and most research has been conducted in the USA and Europe, with the effects on populations from other areas of the world remaining under-investigated. Therefore, the main aim of the present study was to evaluate the effects of OAGB, RYGB, and SG on CMRFs (weight loss, body composition, blood glucose, and lipid profile) during 12 months of follow-up in Iranian patients with severe obesity. A further study aim was to evaluate the correlation between the percentage of total weight loss (%TWL) and changes in CMRFs.
Patients and methods
Study population
Patients with severe obesity were sequentially recruited from Doctors’ referrals and from The Governmental Hospital of Tehran University of Medical Science, Tehran, Iran. The inclusion criteria were as follows: 18–60 years of age, undergoing bariatric surgery for the first time, and having a BMI ≥ 40 or BMI ≥ 35 with obesity-related comorbidities. Exclusion criteria comprised taking any medicines that may affect study variables, including glucose-lowering and anti-obesity agents, corticosteroids, and lipid-lowering medications. Additionally, patients with known CVD, hypothyroidism, hyperthyroidism, cancer, liver disease, renal failure, or who were diagnosed with type 1 or type 2 diabetes mellitus, or dyslipidemia prior to the study, or other diseases that affect lipid and glucose metabolism, were excluded.
Study design
This non-randomized retrospective cohort trial included patients with severe obesity who had undergone bariatric surgery between 2015 and 2017 and had been followed-up for 12 months. Patients were referred to a medical team that included the surgeon, endocrinologist, nutritionist, and psychologist, for bariatric surgery as well as for routine consultation and assessment.
At 1 month prior to the surgery, all patients underwent a 1200–1500 Kcal/day diet, based on weight and physical activity level, to attain a weight loss of 5–10%. During the first month following surgery, patients were educated about having a high protein, low-calorie liquid diet (600–800 Kcal/day). In the second month, solid ingredients were gradually added to their diet (800–1000 Kcal/day). After that, participants were on a low fat, low carbohydrate, and high protein diet (1000–1200 Kcal/day).14 A trained nutritionist provided nutritional counseling and education for bariatric surgery, based on American Society for Metabolic and Bariatric Surgery (ASMBS) guidelines.15,16 Patients received an oral multivitamin, daily, for 3 months and were supplemented with iron, folate, vitamin B12, and vitamin D according to ASMBS guidelines and their requirements. Additionally, patients were asked to undertake walking exercise for 1 month after surgery and to gradually raise their speed as tolerated. According to the American College of Sports Medicine (ACSM) recommendations, participants were first asked to walk 150 min/week and to increase their total walking time to 150–200 min spread over 3–5 days/week after 2 months.17
All participants who met the eligibility criteria were invited to the nutrition clinic for evaluation of demographic information, dietary intake, physical activity, biochemical assessments, comorbidities, anthropometric measurement, and body composition immediately prior to surgery. Follow-up visits were scheduled at 1, 3, 6, and 12 months after surgery. Written informed consent was obtained from all participants at the beginning of the study. Ethics approval for the study was obtained from the Shahid Beheshti University of Medical Science and the study was conducted in accordance with the declaration of Helsinki.
Data collection
Anthropometric measurement and body composition
Measurements were obtained at baseline (prior to surgery) and during the 12-month follow-up period. Weight, fat mass (FM), and fat-free mass (FFM) were obtained in light clothes and without shoes utilizing an Inbody 370 body impedance analyzer (Biospace; Urbandale, IA, USA) following standard guidelines.18 Patients were asked to fast for 4–5 h, avoid alcohol for at least 24 h, avoid exercise for at least 12 h, maintain balanced hydration, and lie down for at least 5 min prior to measurement. Height was measured without shoes by a stadiometer (Seca; Hamburg, Germany) to the nearest 0.5 cm. BMI was determined as weight (kg) divided by height squared (kg/m2). Fat mass to fat-free mass ratio (F2FFMR) was calculated as FM (kg) divided by FFM (kg). The % excess weight loss (EWL) and %TWL was calculated by the following formulas:
Physical activity
To assess physical activity levels, an in-person interview was conducted to obtain information from the daily activity log books at baseline (prior to surgery) and at 12 months after bariatric surgery. Exercise intensity was evaluated using the Borg Scale, a relative scale that ranges from 6 to 20, in which 6–11 is categorized as ‘low’, 12–16 as ‘moderate’, and 17–20 as ‘high’ intensity.19
Biochemical assessments
Blood samples (5 ml) were collected in the early morning after 12 hours of fasting, at baseline, and postoperatively at 6 and 12 months. Serum was separated from whole blood by centrifugation at 1533 g for 10 min, and serum samples were stored at –80 °C prior to use. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) were determined by standard enzymatic methods using Pars Azmoon test kits (Parsazmoon, Tehran, Iran), according to the manufacturer’s instructions. Fasting blood glucose (FBG) was determined by a glucose oxidase–peroxidase method using a Pars Azmoon test kit (Parsazmoon).
Comorbidities
Type 2 diabetes was defined as FBG ≥ 126 mg/dl, and FBG < 100 mg/dl was defined as healthy. Dyslipidemia was defined as the presence of the following criteria: TG > 150 mg/dl, LDL-C > 130 mg, and HDL-C < 40 mg/dl in men and < 50 mg/dl in women.20
Statistical analyses
Data were analyzed using SPSS software, version 20 (IBM; Armonk, NY, USA). The normality of data distribution was assessed by Kolmogorov–Smirnov test. Descriptive statistics are presented using mean ± SD and frequency (%) for numeric and categorical data, respectively. Baseline characteristics were compared between the three study groups using analysis of variance (ANOVA) for continuous variables and χ2-test for categorical variables. Pairwise between-group comparisons were performed using Tukey’s honestly significant difference (HSD) test. One-way repeated measures ANOVA or Friedman test was used to compare the mean of markers between different time categories. Bonferroni test was used for pairwise comparisons between time-points. Baseline means of markers were compared between different bariatric surgery procedures using ANOVA. The linear mixed-effect model was used to evaluate the impact of operation type on markers over the study period. The relationship between %TWL and CMRF was assessed by Spearman’s correlation coefficient. A P-value < 0.05 was considered to be statistically significant.
Results
Patient recruitment and baseline characteristics
Among 647 patients who underwent initial screening, 162 patients were excluded from the study due to incomplete records, not meeting the inclusion criteria, or having undergone other types of bariatric surgery. Consequently, a total of 485 patients (394 female and 91 male) were included in the present study, and 12-month follow-up records were complete for all patients.
Baseline characteristics are summarised in Table 1. The overall mean age was 41.66 years, and the mean BMI was 46.06 kg/m2. Of the three different types of bariatric surgery procedures included in the study, 244 patients (50.3%) underwent SG, 95 (19.6%) underwent RYGB, and 146 (30.1%) underwent OAGB. There were no statistically significant differences in demographic and clinical variables between the surgery groups at baseline, except patients who underwent SG had lower weight (P = 0.002), BMI (P = 0.01), and FFM (P = 0.006) versus patients in the RYGB and OAGB groups.
Table 1.
Baseline characteristics of patients with severe obesity who underwent SG, RYGB or OAGB.
| Surgery group |
||||
|---|---|---|---|---|
| Variable | SG | RYGB | OAGB | Statistical significance |
| Sex, male | 38 (15.6) | 18 (18.9) | 35 (24.0) | NS |
| Age, years | 41.42 ± 11.77 | 43.27 ± 9.57 | 41.54 ± 11.20 | NS |
| Height, m | 1.62 ± 0.09 | 1.63 ± 0.09 | 1.63 ± 0.10 | NS |
| Weight, kg | 118.88 ± 18.97a | 126.90 ± 20.58 | 122.97 ± 18.87 | P = 0.002 |
| BMI, kg/m2 | 45 ± 5.71a | 47.07 ± 5.06 | 46 ± 6.15 | P = 0.010 |
| FM, kg | 58.31 ± 11.44 | 61.46 ± 12.12 | 59.57 ± 11.93 | NS |
| FFM, kg | 60.56 ± 11.87a | 65.43 ± 13.36 | 63.40 ± 15.46 | P = 0.006 |
| F2FFMR | 0.98 ± 0.19 | 0.96 ± 0.26 | 0.98 ± 0.24 | NS |
| FBG, mg/dl | 113.54 ± 36.17 | 110.40 ± 29.91 | 112.36 ± 32.77 | NS |
| TG, mg/dl | 160.54 ± 76.66 | 149.67 ± 59.50 | 168.54 ± 98.19 | NS |
| TC, mg/dl | 200.15 ± 37.17 | 197.24 ± 36.50 | 201.75 ± 40.25 | NS |
| LDL-C, mg/dl | 110.28 ± 29.46 | 110.44 ± 30.92 | 114.22 ± 30.04 | NS |
| HDL-C, mg/dl | 49.40 ± 10.58 | 49.65 ± 12.04 | 49.30 ± 11.58 | NS |
| Type 2 diabetes (%) | 51 (20.9) | 17 (17.9) | 35 (24.0) | NS |
| Dyslipidemia (%) | 15 (6.14) | 4 (4.21) | 11 (7.5) | NS |
| S/w of physical activity | 2.13 ± 1.07 | 2.23 ± 1.27 | 2.31 ± 1.11 | NS |
| Min/day of physical activity | 18.15 ± 7.86 | 16.84 ± 7.47 | 17.74 ± 7.61 | NS |
| Intensity of physical activity (%) | ||||
| Low | 187 (76.64) | 69 (72.63) | 101 (69.18) | NS |
| Moderate | 57 (23.36) | 26 (27.37) | 45 (30.82) | |
| High | 0 (0) | 0 (0) | 0 (0) | |
Data presented as mean ± SD or n (%) prevalence.
SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OAGB, one anastomosis gastric bypass; BMI, body mass index; FM, fat mass; FFM, fat-free mass; F2FFMR, fat mass to fat-free mass ratio; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; S/w, sessions per week; Min/day, minutes per day.
Differences in means between the three groups were assessed by analysis of variance. All pairwise between-group comparisons were performed using Tukey's honestly significant difference (HSD) test.
aP < 0.05, SG versus RYGB.
NS, no statistically significant between-group differences (P > 0.05).
Anthropometric and clinical characteristics of all patients who underwent bariatric surgery
Compared with baseline, all patients who underwent bariatric surgery experienced significant reductions in anthropometric and body composition measurements at 12 months following surgery, including weight, BMI, %TWL, %EWL, FM, FFM, and F2FFMR (P < 0.001; Table 2). Furthermore, lipid profile and FBG significantly improved at 12 months after bariatric surgery compared with baseline (P < 0.001; Table 2).
Table 2.
Demographic and clinical characteristics at baseline (prior to surgery), and different follow-up time-points in patients with severe obesity who underwent gastric bypass surgery.
| Variable | Time (month) |
Statistical significance | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 12 | ||
| Weight, kg | 121.68 ± 19.48a | 109.64 ± 17.08b | 98.83 ± 15.35c | 88.81 ± 13.38d | 81.81 ± 12.17e | P < 0.001 |
| BMI, kg/m2 | 45.73 ± 5.77a | 41.20 ± 5.13b | 37.14 ± 4.69c | 33.39 ± 4.15d | 30.76 ± 3.76e | P < 0.001 |
| FM, kg | 59.31 ± 11.76a | 51.68 ± 11.08b | 43.92 ± 9.93c | 35.56 ± 9.37d | 29.57 ± 8.57e | P < 0.001 |
| FFM, kg | 62.37 ± 13.44a | 57.95 ± 12.97b | 54.97 ± 11.20c | 53.24 ± 10.70d | 52.27 ± 10.09e | P < 0.001 |
| F2FFMR | 0.97 ± 0.22a | 0.92 ± 0.26b | 0.82 ± 0.23c | 0.69 ± 0.23d | 0.58 ± 0.21e | P < 0.001 |
| %TWL | – | 9.78 ± 2.91a | 18.63 ± 3.89b | 26.75 ± 4.85c | 32.41 ± 5.74d | P < 0.001 |
| %EWL | – | 22.51 ± 8.45a | 42.63 ± 10.69b | 61.05 ± 12.37c | 73.77 ± 13.64d | P < 0.001 |
| S/w of physical activity | 2.20 ± 1.12a | – | – | 2.89 ± 0.97b | 2.99 ± 1.13b,c | P < 0.001 |
| Min/day of physical activity | 17.77 ± 7.71a | – | – | 29.53 ± 11.20b | 29.08 ± 10.81b,c | P < 0.001 |
| FBG, mg/dl | 112.57 ± 33.97a | – | – | 92.92 ± 16.74b | 88.50 ± 10.88c | P < 0.001 |
| TG, mg/dl | 160.82 ± 81.05a | – | – | 117.28 ± 46.17b | 99.04 ± 31.94c | P < 0.001 |
| TC, mg/dl | 200.06 ± 37.95a | – | – | 184.06 ± 33.76b | 175.21 ± 30.70c | P < 0.001 |
| LDL-C, mg/dl | 111.50 ± 29.93a | – | – | 108.43 ± 27.72b | 98.75 ± 28.66c | P < 0.001 |
| HDL-C, mg/dl | 49.42 ± 11.16a | – | – | 52.73 ± 11b | 56.36 ± 10.46c | P < 0.001 |
Data presented as mean ± SD.
BMI, body mass index; FM, fat mass; FFM, fat-free mass; F2FFMR, fat mass to fat-free mass ratio; %EWL, % excessive weight loss; %TWL, % total weight loss; S/w, session per week; Min/day, minute per day; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
The Bonferroni test was used for pairwise comparisons between times.
aP < 0.05 versus b, c, d, and e; bP < 0.05 versus a, c, d, and e; cP < 0.05 versus a, b, d, and e; dP < 0.05 versus a, b, c, and e.
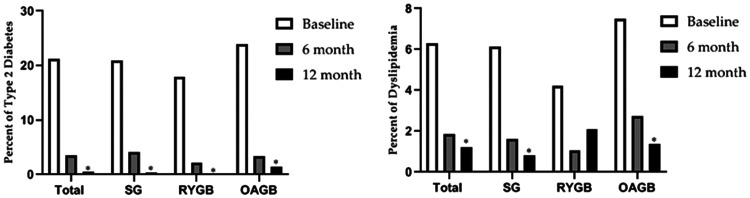
Regarding clinical characteristics, 103 patients (21.23%) had type 2 diabetes at baseline, which dropped to 3 (0.6%) after 12 months. Additionally, 30 patients (6.2%) had dyslipidemia at baseline, which reduced to 6 (1.23%) at the 12-month time-point (Figure 1). Thus, bariatric surgery significantly improved hyperglycemia and dyslipidemia after 12 months of follow-up (P < 0.001).
Figure 1.
Comparison of the proportion of patients with type 2 diabetes mellitus or dyslipidemia following sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), or one anastomosis gastric bypass (OAGB) over a 12-month follow-up. Type 2 diabetes mellitus was defined as fasting blood glucose > 126 mg/dl; Dyslipidemia was defined as triglycerides > 150 mg/dl, low-density lipoprotein cholesterol > 130 mg/dl, and high-density lipoprotein cholesterol > 40 mg/dl in men and > 50 mg/dl in women. *P < 0.05, statistically significant difference versus baseline in each group.
The duration, intensity, and the number of physical activity sessions per week were significantly increased after 12 months (P < 0.05), although this improvement was not significantly different between the groups. These results remained significant after adjustment for sex, age, and physical activity.
Changes in anthropometric and clinical characteristics after different bariatric surgery procedures
Analyses of the alterations in anthropometric and body composition variables are summarized in Table 3, as well as lipid profile and FBG, between SG, RYGB, and OAGB groups at 12 months following surgery. Reductions in mean weight, BMI, FM, and F2FFMR at the 12-month follow-up were significantly different between the SG group versus the RYGB and OAGB groups (P < 0.05). Only FM was significantly different between RYGB and OAGB (P = 0.032). Additionally, %TWL was significantly higher following RYGB than SG and OAGB procedures. The beneficial effects of bariatric surgery procedures on lipid profile and FBG were not significantly different between procedures. Furthermore, the proportions of patients with remission of dyslipidemia (SG, 2 [0.81%]; RYGB, 2 [2.1%]; and OAGB, 2 [1.36%]) and type 2 diabetes (SG, 1 [0.4%]; RYGB,0 [0%]; and OAGB, 2 [1.36%]) were not significantly different between the bariatric surgery types (Figure 1). The differences remained statistically significant after adjustment for sex, age, and physical activity.
Table 3.
Comparison of cardiometabolic risk factors at a 12-month follow-up between patients with severe obesity who underwent OAGB, RYGB, or SG.
| Statistical significance |
||||||
|---|---|---|---|---|---|---|
| Variable | SG versus RYGB | SG versus OAGB | RYGB versus OAGB | SG versus RYGB | SG versus OAGB | RYGB versus OAGB |
| Weight, kg | 5.74 ± 1.34 | 4.24 ± 1.16 | –1.49 ± 1.46 | P < 0.001 | P < 0.001 | NS |
| BMI, kg/m2 | 1.87 ± 0.45 | 1.43 ± 0.39 | –0.43 ± 0.49 | P < 0.001 | P < 0.001 | NS |
| FM, kg | 5.69 ± 1.17 | 2.94 ± 2.94 | –2.75 ± 1.28 | P < 0.001 | P = 0.004 | P = 0.032 |
| FFM, kg | –0.05 ± 0.91 | 1.30 ± 0.78 | 1.35 ± 0.99 | NS | NS | NS |
| F2FFMR | 0.09 ± 0.02 | 0.04 ± 0.01 | –0.04 ± 0.02 | P < 0.001 | P = 0.029 | NS |
| %EWL | –1.62 (1.16) | –1.85 (1.01) | –0.22 (1.26) | NS | NS | NS |
| %TWL | –2.33 ± 3.51 | –0.68 ± 0.60 | 1.65 ± 0.74 | P = 0.001 | NS | P = 0.028 |
| FBG, mg/dl | –1.38 ± 3.51 | –1.19 ± 3.04 | 0.19 ± 3.83 | NS | NS | NS |
| TG, mg/dl | –11.71 ± 8.52 | 5.68 ± 7.38 | 17.40 ± 9.29 | NS | NS | NS |
| TC, mg/dl | –5.62 ± 4.38 | –4.06 ± 3.79 | 1.55 ± 4.77 | NS | NS | NS |
| LDL-C, mg/dl | –5.18 ± 4.03 | 0.39 ± 3.48 | 5.57 ± 4.39 | NS | NS | NS |
| HDL-C, mg/dl | –1.14 ± 1.57 | –1.84 ± 1.35 | –0.69 ±1.71 | NS | NS | NS |
Data presented as mean ± SD.
SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OAGB, one anastomosis gastric bypass; BMI, body mass index; FM, fat mass; FFM, fat-free mass; F2FFMR, fat mass to fat-free mass ratio; %EWL, % excessive weight loss; %TWL, % total of weight loss; FBG, fasting blood glucose; TG, triglyceride, TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
NS, no statistically significant between-group difference (P >0.05). Adjusted parameter estimates of operation type using linear mixed effects model. All parameters adjusted by age and sex.
Correlations between %TWL and CMRFs
High %TWL was significantly related to reductions in FM (r = 0.120, P = 0.008), FBG (r = 0.106, P = 0.019), and TG (r = 0.095, P = 0.037) in the total study population at 12 months following bariatric surgery (Table 4). In the SG group, statistically significant correlations were observed between %TWL and FM (r = 0.151, P = 0.018), FFM (r = 0.154, P = 0.016), and FBG (r = 0.155, P = 0.015). No statistically significant relationships were found between %TWL and other variables.
Table 4.
Correlation between %TWL and cardiometabolic risk factors in patients with severe obesity at 12 months following bariatric surgery.
| Variable | Total |
SG |
RYGB |
OAGB |
||||
|---|---|---|---|---|---|---|---|---|
| r | Statistical significance | r | Statistical significance | r | Statistical significance | r | Statistical significance | |
| FM | –0.120 | P = 0.008 | –0.151 | P = 0.018 | 0.165 | NS | –0.042 | NS |
| FFM | 0.024 | NS | –0.154 | P = 0.016 | –0.064 | NS | 0.085 | NS |
| F2FFMR | –0.079 | NS | –0.065 | NS | 0.139 | NS | –0.086 | NS |
| FBG | –0.106 | P = 0.019 | –0.155 | P = 0.015 | –0.194 | NS | 0.011 | NS |
| TG | –0.095 | P = 0.037 | –0.070 | NS | –0.196 | NS | –0.154 | NS |
| TC | –0.041 | NS | –0.083 | NS | 0.090 | NS | –0.113 | NS |
| LDL-C | 0.042 | NS | –0.041 | NS | 0.134 | NS | –0.071 | NS |
| HDL-C | 0.009 | NS | 0.122 | NS | –0.078 | NS | –0.141 | NS |
SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OAGB, one anastomosis gastric bypass; FM, fat mass; FFM, fat-free mass; F2FFMR, fat mass to fat-free mass ratio; %TWL, % total weight loss; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
NS, no statistically significant correlation (P > 0.05; Spearman’s correlation coefficient).
Discussion
To the best of our knowledge, this study is the first to compare the efficacy of OAGB, RYGB, and SG procedures in the management of CMRFs in Middle Eastern populations. The study revealed that bariatric surgery procedures may improve obesity-related risk factors, at least partially, via modulation of anthropometric and body composition indices, as well as improvement in blood glucose and dyslipidemia. RYGB and OAGB were shown to be more effective at reducing weight, BMI, FM, and F2FFMR compared with SG. Moreover, RYGB led to greater changes in FM and %TWL than the OABG procedure. Additionally, %TWL was shown to be significantly correlated with FM, FBS, and TG over 12 months following bariatric surgery.
At 12 months following surgery, the mean %TWL was increased by 32% in the overall study population. Additionally, the mean weight, BMI and FM were significantly reduced in the RYGB and OAGB groups compared with SG at the 12-month follow-up. The findings of the current study are consistent with several studies that showed RYGB and OAGB may lead to a more prominent weight loss than SG. For instance, significantly greater improvements in %EWL have been reported after RYGB (77.5%) than SG (74.8%) after 12 months of follow-up.21 Furthermore, %TWL has been shown to be significantly higher following OAGB (38.7%) than following SG (35.7%).22 Despite the increasing use of different bariatric surgery procedures, the main mechanisms behind their effectiveness have not yet been fully clarified.23 Malabsorption and calorie intake restriction are not the only effects of bariatric surgery on weight loss.24,25 Changes in eating behaviors, appetite, taste, olfactory senses, food aversion, and hormonal changes may cause energy restriction, which is an important factor in weight loss after surgery.26,27 However, according to the present results, RYGB and OAGB were more effective compared with SG in terms of anthropometric and body composition, including weight loss, BMI, and FM, which may be due to the malabsorption effects of these two procedures.11,28
The present study also showed that bariatric surgery could significantly reduce F2FFMR, and this decrease was greater in patients who underwent RYGB and OAGB compared with SG. Moreover, reductions in F2FFMR were close to statistical significance between RYGB and OAGB (P = 0.061). Because there were no significant differences in FFM changes between bariatric surgery procedures, improvements in F2FFMR may be related to FM decrements. Indeed, RYGB was more effective at decreasing FM that the other surgery types, which may explain potentially greater improvements in F2FFMR. Importantly, emerging evidence indicates that the fat-to-muscle ratio may predict the risk of diabetes.29 Consequently, the drop in F2FFMR after bariatric surgery may improve hyperglycemia remission rates.
In the present study, blood glucose concentration decreased significantly over 12 months of follow-up. In contrast to our expectations, no statistically significant differences were observed between the three procedures. There is much evidence to support the view that, regardless of procedure type, bariatric surgery is an important strategy for treating diabetes and glycemic control in patients with severe obesity. In an investigation of 150 patients with diabetes, glycemic control in patients who underwent bariatric surgery was observed to be significantly greater than in those who received medical interventions.30 Additionally, no significant difference in glycemic control was found between SG and RYGB.30 In a study of 498 patients, no significant differences were found between SG, RYGB, and OAGB in terms of diabetes remission after a 5-year follow-up.22 In contrast to the present findings, there is evidence to show that OAGB may be more beneficial to glucose control than other procedures.11,31,32 Results of a study showed that OAGB had higher efficiency in diabetes treatment and blood sugar control compared with RYGB after a 6-year follow-up,32 and in another study, OAGB was more efficient than SG in terms of diabetes remission after 1 year.31 These discrepancies may be associated with population type, study design, local pattern of procedure and duration of follow-up.
The question here is whether improvement in blood glucose concentrations is related to weight loss. According to the present results, %TWL and FBG improvement were significantly correlated, indicating that weight loss has a potential effect on the improvement of glucose control. Evidence suggests that weight loss may improve glucose control and insulin sensitivity, but other mechanisms are implicated. Indeed, postoperative changes in the gastrointestinal tract anatomy may reduce blood glucose by activating several gastrointestinal-dependent mechanisms.33
Dyslipidemia is well-established as a common feature of patients with severe obesity, and is considered to be the main CMRF.34 The results of the present study suggested that lipid profile levels improved significantly at 12 months after surgery. However, no significant differences were observed between the three groups. Consistent with the present outcomes, the YOMEGA clinical trial, that investigated the efficiency of OAGB and RYGB, showed no significant difference between RYGB and OAGB in terms of lipid profile.12 Moreover, similar dyslipidemia remission rates have been shown between SG and RYGB after 12 months.35 However, previous studies have indicated that various bariatric surgery procedures have different effects on lipid profiles, where malabsorptive procedures considerably affect LDL-C and TC more than restrictive procedures.36,37 Indeed, malabsorptive procedures may reduce TC and LDL-C concentrations by decreasing the absorption area and increasing the cholesterol turnover.37,38 Furthermore, malabsorptive procedures have been claimed not to affect HDL-C and to even reduce it.39 These procedures have been proposed to reduce the synthesis of apolipoprotein A1 by increasing the circulation of bile acids and bypassing the duodenal and part of jejunum.39
It should be noted that improvements in glucose hemostasis and lipid profile may be partially influenced by physical activity. However, given that physical activity was similar between the three procedures in the current study, the probability of bias by this factor is low. In the present study, %TWL was found to have an inverse correlation with FM, FBG, and TG, indicating that weight loss is a potential mechanism in remission of dyslipidemia in these patients. Besides weight loss, improved glycemic control is another important factor presumed to improve the lipid profile in the present study. Changes in bile acid metabolism and intestinal microbiome composition are the other suggested mechanisms of this improvement.27
The results of the current study may be limited by several factors. First, the retrospective study design prevented access to relevant data, such as hemoglobin A1C, dietary intake, and blood pressure. Secondly, patients were non-randomly divided into groups, which may have introduced selection bias. Thirdly, consecutive sampling led to inconsistency of the proportions between the groups. However, the present investigation also had several strengths. First, patients' physical activity was controlled during the follow-up period, and these confounding factors did not affect the results of the study. Secondly, the patients participating in the study did not take glucose- or lipid-lowering medications, which reduces potential bias in the study results.
Conclusion
This study showed that bariatric surgery resulted in significant improvements in weight loss, body composition, blood glucose, lipid profile, and comorbidities at 12 months following the procedure. SG, RYGB and OAGB were all found to be effective and durable strategies for weight loss and treatment of comorbidities in severely obese patients. However, RYGB and OAGB procedures may be related to better outcomes in anthropometric and body composition indices than SG, and consequently may be considered a favorable option for patients with severe obesity. Further studies are required with large sample sizes and long-term follow-up to evaluate the efficiency of these procedures and their outcomes.
Footnotes
The authors declare that there is no conflict of interest.
Funding: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data accessibility
Data are available upon email request to the corresponding authors.
ORCID iD
Makan Cheraghpour https://orcid.org/0000-0003-4459-4528
References
- 1.Hosseini SA, Aghamohammadi V, Ashtary-Larky D, et al. Are young Iranian women with metabolically healthy obesity at increased risk of CVD incidence? J Vasc Bras 2020; 19: e20190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Ambrosi J, Moncada R, Valentí V, et al. Cardiometabolic profile related to body adiposity identifies patients eligible for bariatric surgery more accurately than BMI. Obes Surg 2015; 25: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 3.Hong YR, Kelly AS, Johnson-Mann C, et al. Degree of cardiometabolic risk factor normalization in individuals receiving bariatric surgery: evidence from NHANES 2015–2018. Diabetes care. 2021; 44: e57–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batsis JA, Miranda WR, Prasad C, et al. Effect of bariatric surgery on cardiometabolic risk in elderly patients: a population‐based study. Geriatr Gerontol Int 2016; 16: 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasani M, Mirahmadian M, Taheri E, et al. The effect of laparoscopic gastric plication surgery on body composition, resting energy expenditure, thyroid hormones, and physical activity in morbidly obese patients. Bariatric Surgical Practice and Patient Care 2015; 10: 173–179. [Google Scholar]
- 6.Gil S, Goessler K, Dantas WS, et al. Constraints of weight loss as a marker of bariatric surgery success: an exploratory study. Front Physiol 2021; 12: 640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melissas J, Stavroulakis K, Tzikoulis V, et al. Sleeve gastrectomy vs Roux-en-Y gastric bypass. Data from IFSO-European chapter center of excellence program. Obes Surg 2017; 27: 847–855. [DOI] [PubMed] [Google Scholar]
- 8.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 1994; 4: 353–357. [DOI] [PubMed] [Google Scholar]
- 9.Carbajo MA, Luque-de-León E, Jiménez JM, et al. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg 2017; 27: 1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 2001; 11: 276–280. [DOI] [PubMed] [Google Scholar]
- 11.Castro MJ, Jimenez JM, Carbajo MA, et al. Long-term weight loss results, remission of comorbidities and nutritional deficiencies of sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB) and one-anastomosis gastric bypass (OAGB) on type 2 diabetic (T2D) patients. Int J Environ Res Public Health 2020; 17: 7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert M, Espalieu P, Pelascini E, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet 2019; 393: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 13.Admiraal WM, Celik F, Gerdes VE, et al. Ethnic differences in weight loss and diabetes remission after bariatric surgery: a meta-analysis. Diabetes Care 2012; 35: 1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabesh MR, Maleklou F, Ejtehadi F, et al. Nutrition, physical activity, and prescription of supplements in pre-and post-bariatric surgery patients: a practical guideline. Obes Surg 2019; 29: 3385–3400. [DOI] [PubMed] [Google Scholar]
- 15.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9: 159–191. [DOI] [PubMed] [Google Scholar]
- 16.Parrott J, Frank L, Rabena R, et al. American Society for Metabolic and Bariatric Surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis 2017; 13: 727–741. [DOI] [PubMed] [Google Scholar]
- 17.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 18.Ellis KJ, Bell SJ, Chertow GM, et al. Bioelectrical impedance methods in clinical research: a follow-up to the NIH Technology Assessment Conference. Nutrition 1999; 15: 874–880. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). NIH publication no. 02-5215, September 2002. Bethesda, MD: National Heart, Lung, and Blood Institute. [Google Scholar]
- 21.Khalaj A, Tasdighi E, Hosseinpanah F, et al. Two-year outcomes of sleeve gastrectomy versus gastric bypass: first report based on Tehran obesity treatment study (TOTS). BMC surgery 2020; 20: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soong TC, Lee MH, Lee WJ, et al. Long-term efficacy of bariatric surgery for the treatment of super-obesity: comparison of SG, RYGB, and OAGB. Obes Surg 2021; 31: 3391–3399. [DOI] [PubMed] [Google Scholar]
- 23.Manning S, Pucci A, Carter NC, et al . Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc 2015; 29: 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr 2010; 92: 704–713. [DOI] [PubMed] [Google Scholar]
- 25.Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol 2011; 5: 1263–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakeri R, Batterham RL. Potential mechanisms underlying the effect of bariatric surgery on eating behaviour. Curr Opin Endocrinol Diabetes Obes 2018; 25: 3–11. [DOI] [PubMed] [Google Scholar]
- 27.Pucci A, Batterham R. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest 2019; 42: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iannelli A, Anty R, Schneck AS, et al. Evolution of low-grade systemic inflammation, insulin resistance, anthropometrics, resting energy expenditure and metabolic syndrome after bariatric surgery: a comparative study between gastric bypass and sleeve gastrectomy. J Visc Surg 2013; 150: 269–275. [DOI] [PubMed] [Google Scholar]
- 29.Gamboa-Gómez CI, Simental-Mendía LE, Rodríguez-Morán M, et al. The fat-to-lean mass ratio, a novel anthropometric index, is associated to glucose metabolic disorders. Eur J Intern Med 2019; 63: 74–78. [DOI] [PubMed] [Google Scholar]
- 30.Schauer PR, Bhatt DL, Kirwan JP, et al . Bariatric surgery versus intensive medical therapy for diabetes–5-year outcomes. N Engl J Med 2017; 376: 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musella M, Apers J, Rheinwalt K, et al . Efficacy of bariatric surgery in type 2 diabetes mellitus remission: the role of mini gastric bypass/one anastomosis gastric bypass and sleeve gastrectomy at 1 year of follow-up. A European survey. Obes Surg 2016; 26: 933–940. [DOI] [PubMed] [Google Scholar]
- 32.Markopoulos G, Skroubis G, Kalfarentzos F, et al . Comparison of one anastomosis gastric bypass versus standard Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion, in a case-matched, non-superobese population: 6 years of follow-up. Prz Gastroenterol 2022; 17: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miras AD, Kamocka A, Pérez-Pevida B, et al. The effect of standard versus longer intestinal bypass on GLP-1 regulation and glucose metabolism in patients with type 2 diabetes undergoing Roux-en-Y gastric bypass: The Long-Limb Study. Diabetes Care 2021; 44: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010; 363: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Climent E, Benaiges D, Pedro-Botet J, et al. Atherogenic dyslipidemia remission 1 year after bariatric surgery. Obes Surg 2017; 27: 1548–1553. [DOI] [PubMed] [Google Scholar]
- 36.Heffron SP, Parikh A, Volodarskiy A, et al. Changes in lipid profile of obese patients following contemporary bariatric surgery: a meta-analysis. Am J Med 2016; 129: 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benetti A, Del Puppo M, Crosignani A, et al. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes care 2013; 36: 1443–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffo E, Cotugno M, Nosso G, et al. Effects of sleeve gastrectomy and gastric bypass on postprandial lipid profile in obese type 2 diabetic patients: a 2-year follow-up. Obes Surg 2016; 26: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 39.Genua I, Ramos A, Caimari F, et al. Effects of bariatric surgery on HDL cholesterol. Obes Surg 2020; 30: 1793–1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon email request to the corresponding authors.