Abstract
Objectives
This review summarizes sex-based differences in aortic stenosis (AS) and identifies knowledge gaps that should be addressed by future studies.
Background
AS is the most common valvular heart disease in developed countries. Sex-specific differences have not been fully appreciated, as a result of widespread under diagnosis of AS in women.
Summary
Studies including sex-stratified analyses have shown differences in pathophysiology with less calcification and more fibrosis in women's aortic valve. Women have impaired myocardial perfusion reserve and different compensatory response of the left ventricle (LV) to pressure overload, with concentric remodeling and more diffuse fibrosis, in contrast to men with more focal fibrosis and more dilated/eccentrically remodeled LV.
There is sex difference in clinical presentation and anatomical characteristics, with women having more paradoxical low-flow/low-gradient AS, under-diagnosis and severity underestimated, with less referral to aortic valve replacement (AVR) compared to men. The response to therapies is also different: women have more adverse events with surgical AVR and greater survival benefit with transcatheter AVR. After AVR, women would have more favorable LV remodeling, but sex-related differences in changes in myocardial reserve flow need future research.
Conclusions
Investigation into these described sex-related differences in AS offers potential utility for improving prevention and treatment of AS in women and men. To better understand sex-based differences in pathophysiology, clinical presentation, and response to therapies, sex-specific critical knowledge gaps should be addressed in future research for sex-specific personalized medicine.
Keywords: Sex, Aortic stenosis, Cardiovascular disease
Highlights
-
•
Aortic stenosis (AS) has increasing incidence in women, but sex-specific differences have not been fully appreciated.
-
•
Identifying sex differences in aortic valve disease would potentially improve prevention and treatment of AS, both in women and men.
-
•
Future studies are needed to address sex-specific knowledge gaps and provide sex-specific personalized medicine.
1. Introduction
Aortic stenosis (AS) is the most common valvular heart disease in developed countries [1]. While AS has long been associated with aging, sex-specific differences have not been fully appreciated, as a result of widespread under diagnosis of AS in women [1].
However, more recent evidence suggests that the incidence of AS in older patients (>75 years of age) is in fact higher in women compared to men [2].
In AS, as in many cardiovascular disorders, women and men differ due in part to anatomical and physiological differences. Indeed, biological sex is known to impact cardiac remodeling and fibrosis in AS [3], [4]. Moreover, women have increased risk of adverse events after surgical aortic valve replacement (AVR) [5], and being woman is a risk factor in the commonly used Society of Thoracic Surgeons (STS) score [6].
The objective of this review is to summarize the current evidence of sex-based differences in AS and identify knowledge gaps that should be addressed by future studies.
2. Pathophysiology
Aortic stenosis is the late result of an inflammatory process leading to aortic valve calcification (AVC), fibrosis and changes in the myocardium in response to pressure overload. There are important differences between men and women in the anatomy and adaptive pathophysiology to AS which we summarize here.
2.1. Aortic valvular calcification
AVC is the primary pathophysiological mechanism of AS. The AVC score by Multislice Computed Tomography (MCT) correlates strongly with the calcium weight of aortic valve, being the gold standard method to measure it [7]. There are sex differences in AVC measured by MCT: sex-specific Agatston units thresholds for diagnosis of severe AS are lower in women (1300) compared to men (2000) [8]. Women tend to have less calcification and more fibrosis deposits on their aortic valve [9]. For similar amounts of AVC, women reach hemodynamically more severe AS, even after adjusting for smaller body surface area [9], [10], [11], [12]. This reflects the contribution of leaflet fibrosis and calcification to increased leaflet stiffness in women.
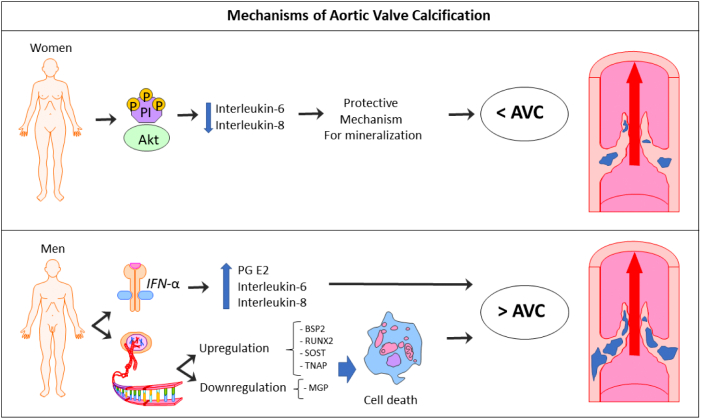
In the pathophysiology of AVC, inflammation, lipoprotein profiles, and matrix remodeling are the main factors involved in the calcification process [13]. The impact on sex is poorly understood, but the molecular mechanisms proposed for AVC underlying differences between sexes appear to be the following (Fig. 1):
-
•
IFN-α activity alone, and in combination with lipopolysaccharide, triggers higher inflammation and calcification in male aortic valve interstitial cells (VICs) compared to females, by a higher secretion of prostaglandin E2, Interleukin-6 and interleukin-8 [14], [15].
-
•
Female-specific phosphorylation of Akt — a kinase reported to play a role in aortic VICs calcification [13], [15] — lowers interleukin-6 secretion in female aortic VICs, protecting interstitial cells from mineralization [14].
-
•
Difference in gene expression profiles between men and women. To-date, 183 genes have been identified as being significantly different in male versus female aortic valve leaflets [16], [17]. These gene expressions are implicated in different biological processes linked to calcification, including cellular proliferation, apoptosis, migration, ossification, angiogenesis, inflammation, and extracellular matrix reorganization [16]. Males are associated with upregulation of bone sialoprotein 2 (BSP2), runt related transcription factor-2 (RUNX2), osteocyte marker sclerostin (SOST) and tissue nonspecific alkaline phosphatase (TNAP) genes, and downregulation of the mineralization inhibitor matrix-Gla protein (MGP) [14]. The effect of the different gene expression in male aortic VICs makes them more prone to apoptosis, with secondary dystrophic calcification [18], explaining the higher degree of AVC in men than in women found in clinical trials.
Fig. 1.
Mechanisms of aortic valve calcification (AVC).
(Abb.: Akt = kinase; PI = phosphorylation; IFN-α = interferon alfa; PG E2 = prostaglandin E2; BSP2 = bone sialoprotein 2; RUNX2 = runt-related transcription factor-2; SOST = osteocyte marker sclerotin; TNAP = tissue nonspecific alkaline phosphatase; MGP = mineralization inhibitor matrix-Gla protein.)
2.2. Aortic valvular fibrosis
In response to stress or injury, VICs (fibroblasts) become activated to myofibroblasts, which is associated with extracellular matrix remodeling and contributes to valve fibro-calcification [19]. As we mentioned before, compared to men, women have more fibrous collagen in their aortic valves [10], [11]. Mechanisms underlying sex-related differences in fibrosis include different gene expression profiles and phenotypes [16], causing elevation of α-smooth muscle actin (α-SMA) and increased myofibroblast activation in VICs of female aortic valves, compared to male aortic valves [20].
2.3. Left ventricular (LV) response to pressure overload
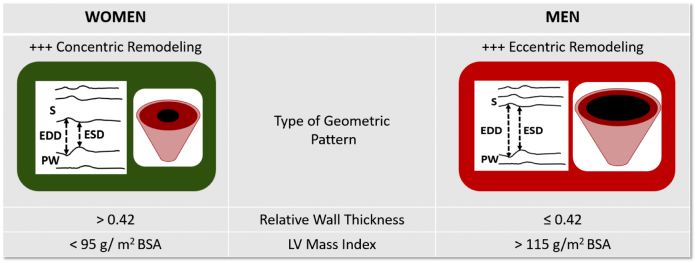
Men and women appear to develop different patterns of LV remodeling and myocardial fibrosis [3], [4]. Compared to men, even with the same degree of valvular stenosis, women tend to develop a more restrictive physiology pattern with concentrically remodeled and subsequent concentric LV hypertrophy and less dilated left ventricle, whereas men present with more dilated and eccentrically remodeled left ventricle [3], [4] (Fig. 2).
Fig. 2.
Sex-related differences in geometric patterns of LV response to pressure overload.
Patterns of cardiac remodeling according to relative wall thickness and LV mass index.
Each type of LV geometry is illustrated by lines representing M-mode images.
(Abb.: LV = left ventricle; S = septum; EDD = end-diastolic diameter; ESD = end-systolic diameter; PW = posterior wall.)
Women have greater LV relative wall thickness, smaller LV cavity volumes and dimensions, higher estimated LV filling pressures (related to reduced LV compliance), and more advanced LV diastolic dysfunction [4]. In addition, according to studies with CMR comparing LV ejection fraction (LVEF) between women and men in the general population, women had a higher LVEF compared to men; and the threshold value to define low LVEF was below 61 % in women and below 55 % in men [21]. This may affect thresholds used to make therapeutic decisions in asymptomatic patients with severe AS.
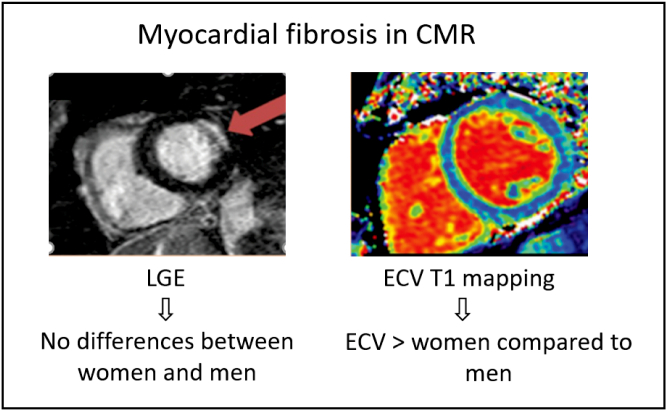
Depending on the stage of AS evaluated and the imaging modality used, variable sex-differences in AS have been reported. Studies with echocardiography in severe AS have shown that women have more concentric LV hypertrophy [4]. In contrast, studies with cardiovascular magnetic resonance (CMR) — a method that gives a more accurate assessment of LV chamber size and morphology and has the ability to identify myocardial fibrosis — have shown a trend toward less concentric LV hypertrophy, more concentric LV remodeling and lower LV mass index in women compared to men [3], [22]. Despite women having a smaller LV mass index, compared to men, they have larger extracellular volume (ECV) fraction (measured noninvasively by CMR T1 mapping) and similar non-infarct pattern of late gadolinium enhancement (LGE, also a noninvasive CMR measure), regardless of AS severity [22]. Whereas LGE represents irreversible focal fibrosis, ECV represents a potentially reversible diffuse pattern of interstitial fibrosis that occurs at an earlier stage of the disease [22] (Fig. 3). More work in this area is still needed, however, because while CMR-derived ECV correlates with collagen content [23], [24], it is possible that the larger ECV fraction in women could be related to others factors like greater capillary density [25]. This could explain why AS studies that have taken biopsies report different results than CMR derived ECV [24].
Fig. 3.
Sex differences in expansion of myocardial fibrosis in aortic stenosis.
Compared to men, women have similar amounts of replacement myocardial fibrosis (=LGE) and larger extent of diffuse myocardial fibrosis (=ECV).
(Abb.: CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement; ECV = extra cellular volume.)
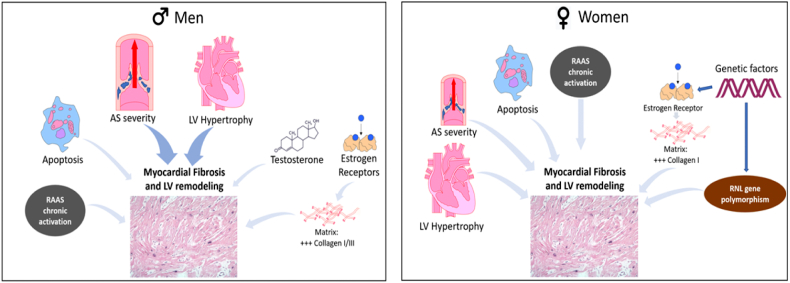
The molecular mechanisms underlying differences between sexes are not completely understood; differences in fibrosis regulatory pathways could partially explain sex-related differences (Fig. 4). In men myocardial fibrosis appears to be mainly driven by AS severity and extent of LV hypertrophy, whereas in women, the remodeling, hypertrophy, and fibrosis are more heterogeneous and multifactorial.
Fig. 4.
Molecular mechanisms underlying myocardial fibrosis and LV remodeling.
Myocardial fibrosis: in men, mainly driven by LV hypertrophy and AS severity, and more cardiomyocyte loss but in women the response to pressure overload is more heterogeneous.
(Abb: RAAS = renin-angiotensin-aldosterone system; RNL = renalase.)
In males' fibrosis is also associated with sex hormones: both testosterone [26] and 17β-estradiol, which mediates its effect via estrogen receptor activation, resulting in increased deposition of collagen I and III in men compared to females [27]. In addition, the renin-angiotensin-aldosterone system (RAAS) activation plays a greater role in males compared to females, since estrogen downregulates angiotensin 1 [28].
Response to AS overload in women appears to depend more on: 1) genetic factors as matrix-related gene expression [16], [29]; 2) a preferential transcriptional activation of collagen I over other extracellular matrix components in the myocardium [27], [29]; 3) polymorphism in the estrogen receptor in postmenopausal women with chronic RAAS activation [30]; and 4) a functional polymorphism of the renalase (RNL) gene — an enzyme which protects tissues of adrenergic activation, decreasing circulating catecholamines that promotes hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts [31].
Differences in sex-specific cardiac remodeling may also be explained by more cardiomyocyte loss (apoptosis) in males than females, resulting in more of an eccentric pattern of hypertrophy vs females who have more a concentric hypertrophic pattern [32], [33].
There are very limited data on the impact of the different types of remodeling on clinical outcomes and prognosis in AS. From the limited information available, concentric remodeling and hypertrophy appear to be independently associated with all-cause and cardiovascular mortality [34]. Indeed, among women with AS and preserved LVEF, the impact of concentric hypertrophy on prognosis is worse (60 % increased risk) than in men [34]. We have elaborated on adverse sex-specific relationships between patterns of left ventricular remodeling and clinical outcomes in women previously [35].
The exact mechanism linking concentric remodeling with worse outcomes in women remains incompletely understood. Subendocardial ischemia may represent one potential mechanism, due to oxygen supply/demand mismatch of the hypertrophied myocardium, reduced diastolic perfusion time, coronary microvascular dysfunction and low coronary perfusion pressure [36], [37], [38]. Indeed, basal blood flow is higher in the hypertrophied myocardium (i.e. increased baseline blood flow velocity), while hyperemic flow is reduced, resulting in a reduction of myocardial perfusion reserve (MPR) – a measure of microcirculatory function. Impaired MPR, as a marker of microvascular dysfunction, is an independent predictor for future cardiovascular events in AS [39], [40], [41], [42], and seems to be a key contributor to the transition from adaptive to maladaptive LV remodeling [42]. However, when using echocardiography to measure aortic valve area (AVA), CMR to assess LV mass, and positron emission tomography to quantify resting and hyperemic myocardial blood flow and coronary vasodilator reserve, a correlation between LV mass and MPR is not always found in AS [39], [43]. Likewise, LV mass did not relate to MPR when derived from stress CMR in patients with severe AS [40]. Interestingly, a correlation between impaired MPR and female sex, myocardial fibrosis and filling pressure was observed in this later investigation. However, these results were collected in a relatively small number of predominantly male participants. This correlation has not been much studied in a sex-specific manner in AS, emphasizing the need for further sex-specific investigation in this area.
3. Clinical presentation
Recently, description addressing sex differences in the clinical presentation of AS has increased [2], [44], [45], [46]. For the same aortic valve area and hemodynamic impairment, women are older at presentation, with lower body mass index, higher frailty score of 2 to 3, lower glomerular filtration rates and higher anemia rates [5], [44], [45], [46]. Compared to men, women have a higher prevalence of hypertension and diastolic dysfunction, less coronary artery disease [46], with an overall higher surgical risk profile [45], [46]. Obesity in AS is associated with increased mortality in women and men, although it is less of a factor in older women with AS who are more often lean [47], [48].
Women hearts and their aortic annuli/aortic roots tend to be smaller, and concomitant mitral and tricuspid valve disease is substantially more common [45], [46]. The older age and higher prevalence of hypertension in women both lead to reduced systemic arterial compliance. Lower systemic arterial compliance in AS is typically associated with older age and women, and is also independently associated with impaired prognosis [49]. Interestingly, although having a higher normal value for LVEF, women have a lower stroke volume index and a reduced flow rate across the valve [50], an entity called ‘paradoxical low-flow low-gradient AS’. This entity seems to be more prevalent in women than men [51]. Importantly, this AS entity has a worse prognosis with medical treatment, higher operative mortality and long-term postoperative mortality [52].
At the time of diagnosis of AS, women have more advanced New York Heart Association (NYHA) class symptoms [44], [53], [54]; with a shorter exercise duration and lower anaerobic threshold [55]. Women have a trend toward a greater symptomatic presentation with shortness of breath and dizziness/syncope [45], [46], [56]. Probably explained by their greater prevalence of microvascular dysfunction, higher frequency of concomitant tricuspid/mitral valvular disease, smaller LV cavity and lower LV mass with diastolic dysfunction.
4. Diagnosis of aortic stenosis
Compared to men, women with severe AS are older and with more atypical symptoms, such as dyspnea and dizziness. They tend to perceive their cardiac disease as less severe, they are more hesitant at the time to undergo a diagnostic procedure, and they are less referred to a specialist undergoing fewer diagnostic tests [57], [58].
Furthermore, the higher prevalence of hypertension, smaller aortic root, smaller LV cavity with smaller stroke volume index and lower flow rate [45], [46], [50], and higher prevalence of paradoxical low-flow low-gradient AS in women [51] contribute to the accuracy of AS diagnosis and severity grading. Besides, the lack of sex-specific cut-off values for identification of low stroke volume [21], make an accurate diagnosis of AS in women challenging.
Thus, these clinical challenges in women at the time of AS grading/diagnosis may explain the under diagnosis and underestimation of AS severity in women. This is a key reason for which women are referred later than men for intervention.
5. Treatment options and outcomes
Without treatment, severe symptomatic AS has a poor prognosis, with most patients dying 2–3 years after diagnosis [59], [60]. To date, no medical treatment has been shown to slow AS progression [61], [62], [63], [64], [65]. The only definitive treatment option for severe AS is the aortic valve replacement (AVR), either surgically or transcatheter approach [66], [67], [68]. Current guidelines recommend intervention in patients who are symptomatic or asymptomatic but in the presence of LV dysfunction or with symptoms/sustained fall in blood pressure in an exercise test [8], [69]. As we mentioned before, the normal reference values for LVEF are higher in women compared to men [21]. Thus, the threshold LVEF defining LV dysfunction in women may need to be revised when making clinical decisions about treatment in asymptomatic AS.
5.1. Selection of surgical aortic valve replacement (SAVR) vs transcatheter aortic valve replacement (TAVR)
Overall, selecting the optimal therapy for women with severe AS between SAVR and TAVR, depends on their anatomy and risk profile. Recent data has shown that the risk of 5-year mortality after diagnosis of severe AS was greater in women than in men, explained by a more conservative AS management in women [53]. Compared to men, women appear to be less frequent and later referred to AVR than men, being older and at a later stage of the disease [53], [70].
Women's representation in the most relevant interventional trials in AS is better than in cardiovascular disease clinical trials in general. However, women's inclusion in most of these studies is still under 50 % (Table 1).
Table 1.
Most relevant interventional trials in aortic stenosis.
| Trial | Risk | No. patients included | Median age (years) | Women included (%) |
|---|---|---|---|---|
| Partner B [54] | High | 358 | 83 | 54 |
| Partner A [81] | High | 694 | 83 | 43 |
| Core Valve U.S Pivotal High Risk [82] | High | 795 | 83 | 47 |
| Partner 2 [83] | Intermediate | 1032 | 81 | 46 |
| SURTAVI [84] | Intermediate | 660 | 79 | 43 |
| Partner 3 [85] | Low | 950 | 73 | 33 |
| Notion [86] | Low | 280 | 79 | 47 |
| Evolut Low Risk [87] | Low | 1403 | 74 | 36 |
Although women are at an increased risk for adverse events after SAVR [5], [6], [71], [72], [73], and have greater survival benefit with TAVR [44], [74], [75], [76], [77], [78], [79], [80], these results were never confirmed by a specific trial in women. Despite none of these trials did randomize on the basis of gender, women are more likely to undergo a TAVR procedure. This is confirmed by data from TVT Registry and European Registry, where women account for about 50 % of patients undergoing TAVR [78].
5.2. Short-term events and survival outcomes data
After TAVR, there are no differences in in-hospital and 30-day mortality rates between sexes [71], [77], [78]. However, as a result of having less aortic valve calcification and a smaller annular size, women are less likely to develop paravalvular regurgitation, which is an important determinant of prognosis following TAVR [88]. The procedure related complications, including bleeding and device related complications, strokes events as well as conversion to conventional SAVR, are more common in women [74], [75], [78], [79], [89]. These could be related to several factors, such as a smaller body area with smaller-caliber peripheral arteries, smaller aortic annulus and aortic root [90], higher rates of porcelain aorta and hormonal influences on vascular biology [91]. On the other hand, women with smaller aortic annulus, would have a possible benefit of TAVR over SAVR, due to less prosthesis-patient mismatch [92] — particularly in combination with paradoxical low flow, low gradient severe AS — which significantly increases the risk of mortality [93]. There are conflicting results of the sex differences on pacemaker implantation after TAVR [94]. According to a recent meta-analysis of 70,000 patients, the risk of post TAVR pacemaker implant is 10 % lower in women compared to men [95].
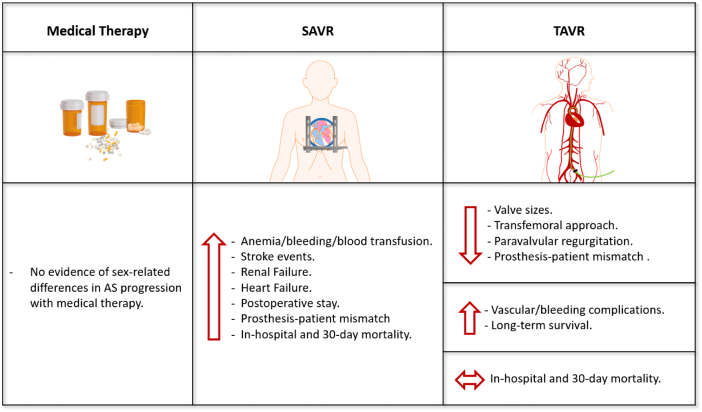
Alarmingly, studies assessing the impact of sex on outcomes of SAVR provide conflicting results suggesting that women have worse outcomes for mortality (in-hospital and 30-day mortality rates), stroke, and postoperative stay than men [6], [70], [96]. A recent review suggests that SAVR is associated with an increased risk of 30-day mortality in women compared to men [97]. The same as TAVR, anemia, vascular complications, bleeding or blood transfusion is also more common in women than men undergoing SAVR [44]. Women also have more renal and heart failure [71], and higher transvalvular gradients with higher prosthesis-patient mismatch [92] (Fig. 5).
Fig. 5.
Treatment options in severe aortic stenosis and women specific characteristics.
(Abb.: SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement; AS = aortic stenosis.)
More research with randomized clinical trials on the basis of gender is clearly needed to better understand the pathophysiologic mechanisms driving sex-specific outcomes.
5.3. Long-term events and survival outcomes
Women seem to have a better long-term survival after TAVR compared to men. Compared with women undergoing SAVR, female TAVR patients have lower major stroke and lower 1-year/2-year mortality [44], [97], [98].
Sex differences in reverse remodeling after AVR have been studied. Women with AS have more diffuse fibrosis, though they appear to respond more favorably to AVR than men. After both SAVR and TAVR, women have less myocardial fibrosis, more favorable LV remodeling and faster regression of LV hypertrophy than men [99], [100]. On the other hand, women with maladaptive LV hypertrophy have worse survival after AVR than women with adaptive LV hypertrophy; in contrast to men, where the pattern of LV hypertrophy did not affect survival [99]. However, sex-specific studies with CMR in this area are necessary to better asses LV mass post AVR and confirm these results.
Although an active area of investigation, lower expression of periostin [99] — a key regulator of cardiac fibrosis — and fast changes in protein synthesis [100], likely contribute to the lower fibrosis (linked to an adaptive LV hypertrophy) and regression of ventricular remodeling in female hearts after AVR.
Even following indications of treatment of AS by current guidelines, a significant proportion of patients, predominantly women, experiences persistent dyspnea after AVR. In the PARTNER II trial, 30 to 40 % of surviving patients remained in NYHA class II or more 2 years after TAVR or SAVR and can be considered as heart failure with preserved ejection [83]. Data from the WIN-TAVI Registry — the first “real world” all females registry examining outcomes following TAVR [101] — showed an increased incidence of hospitalizations for heart failure or valve-related symptoms during 1-year follow-up in women. They also demonstrated that 36.4 % of women remained in NYHA class II/IV 1 year after TAVR. While the exact mechanism for sex-specific symptoms in women remain incompletely understood, we hypothesize that the higher incidence of microvascular dysfunction and persistent diastolic dysfunction in women likely play an important role [38], [102]. Indeed, elevated interleukin-6 strongly predicted heart failure hospitalization and all-cause mortality in women with coronary microvascular dysfunction, suggesting that inflammation plays an important role in the pathogenesis [102]. Prospective serial CMR anatomical, perfusion, and T1 imaging with serial inflammatory biomarkers analyzing sex-related differences are therefore needed to determine unresolved mechanisms contributing to persistent symptoms, reduced quality of life, and frequent hospitalization in patients after TAVR.
6. Summary and identification of knowledge gaps
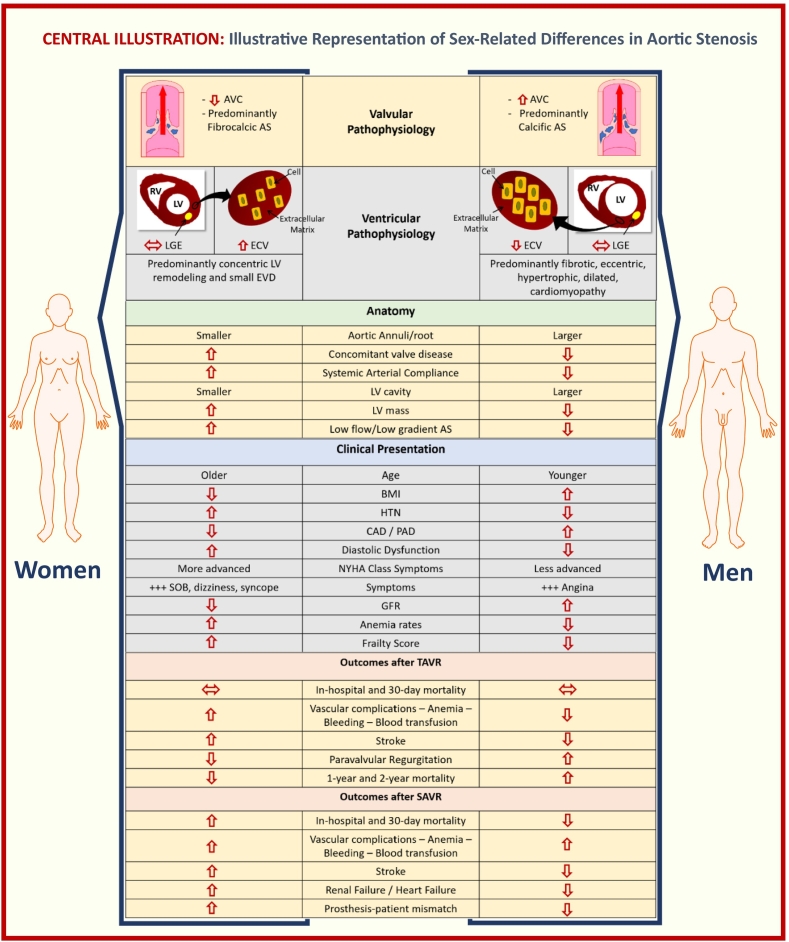
A better understanding of sex-related differences in AS could lead to improved risk stratification schemes, optimized timing of intervention, and formulation of sex-specific prevention and treatment plans. The Central illustration summarizes most relevant sex-related differences in AS, including pathophysiology, anatomy, clinical presentation and clinical outcomes after treatment. As demonstrated in Table 1, women are underrepresented in most interventional AS trials, and the extrapolation of these results to women could be inappropriate. This highlights the need of further research specifically in women, like the currently ongoing RHEIA (Randomized researcH in womEn all Comers Aortic stenosis) Trial [103], evaluating safety and efficacy between TAVR and SAVR in female patients with severe symptomatic AS.
Central illustration.
Illustrative representation of sex-related differences in aortic stenosis.
(Abb.: AVC = aortic valve calcification; AS = aortic stenosis; RV = right ventricle; LV = left ventricle; LGE = late gadolinium enhancement; ECV = extra cellular volume; EDV = end diastolic volume; BMI=body mass index; HTN=hypertension; CAD=coronary artery disease; PAD=peripheral artery disease; SOB=shortness of breath; GFR = glomerular filtration rate.)
Investigation into these described sex-related differences in AS offers potential utility for improving prevention and treatment of AS in women and men. To better understand sex-based differences in pathophysiology, clinical presentation, and response to therapies, the knowledge gaps summarized in Table 2 should be addressed in the future research for sex-specific personalized medicine.
Table 2.
Sex-specific critical knowledge gaps.
|
Updating prior articles on this topic [80], [104], this review article of sex-related differences in important aspects of the aortic stenosis highlights the lack of existing evidence and knowledge gaps, identifying needs for sex-specific investigation and clinical trials.
Funding
This work was supported by the National Institutes of Health R01HL124649, U54 AG065141, the Eli and Edythe Broad Foundation and the Constance Austin Women's Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, DC, the Linda Joy Pollin Women's Heart Health Program, the Erika Glazer Women's Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. C. Noel Bairey Merz, serves as Board of Director for iRhythm, fees paid through CSMC from Abbott Diagnostics and Sanofi. Dr. Janet Wei served on an advisory board for Abbott Vascular. All other authors have no other conflicts of interest to report.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Toyofuku M., Taniguchi T., Morimoto T., Yamaji K., Furukawa Y., Takahashi K., Tamura T., Shiomi H., Ando K., Kanamori N., et al. Sex differences in severe aortic stenosis- clinical presentation and mortality. Circ J. 2017;81:1213–1221. doi: 10.1253/circj.CJ-16-1244. [DOI] [PubMed] [Google Scholar]
- 3.Treibel T.A., Kozor R., Fontana M., Torlasco C., Reant P., Badiani S., Espinoza M., Yap J., Diez J., Hughes A.D., et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc. Imaging. 2018;11:962–973. doi: 10.1016/j.jcmg.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramariuc D., Rieck A.E., Staal E.M., Wachtell K., Eriksen E., Rossebø A.B., Gerdts E. Factors influencing left ventricular structure and stress-corrected systolic function in men and women with asymptomatic aortic valve stenosis (a SEAS Substudy) Am. J. Cardiol. 2008;101:510–515. doi: 10.1016/j.amjcard.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs C., Mascherbauer J., Rosenhek R., Pernicka E., Klaar U., Scholten C., Heger M., Wollenek G., Czerny M., Maurer G., et al. Gender differences in clinical presentation and surgical outcome of aortic stenosis. Heart. 2010;96:539–545. doi: 10.1136/hrt.2009.186650. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien S.M., Shahian D.M., Filardo G., Ferraris V.A., Haan C.K., Rich J.B., Normand S.L., DeLong E.R., Shewan C.M., Dokholyan R.S., et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann. Thorac. Surg. 2009;88:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Messika-Zeitoun D., Aubry M.C., Detaint D., Bielak L.F., Peyser P.A., Sheedy P.F., Turner S.T., Breen J.F., Scott C., Tajik A.J., et al. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110:356–362. doi: 10.1161/01.Cir.0000135469.82545.D0. [DOI] [PubMed] [Google Scholar]
- 8.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., III, Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. 2020 ACC/AHA guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 9.Simard L., Côté N., Dagenais F., Mathieu P., Couture C., Trahan S., Bossé Y., Mohammadi S., Pagé S., Joubert P., et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis. Circ. Res. 2017;120:681–691. doi: 10.1161/circresaha.116.309306. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S.R., Clavel M.A., Messika-Zeitoun D., Cueff C., Malouf J., Araoz P.A., Mankad R., Michelena H., Vahanian A., Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ. Cardiovasc. Imaging. 2013;6:40–47. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 11.Thaden J.J., Nkomo V.T., Suri R.M., Maleszewski J.J., Soderberg D.J., Clavel M.A., Pislaru S.V., Malouf J.F., Foley T.A., Oh J.K., et al. Sex-related differences in calcific aortic stenosis: correlating clinical and echocardiographic characteristics and computed tomography aortic valve calcium score to excised aortic valve weight. Eur. Heart J. 2016;37:693–699. doi: 10.1093/eurheartj/ehv560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linde L., Carter-Storch R., Christensen N.L., Øvrehus K.A., Diederichsen A.C.P., Laursen K., Jensen P.S., Rasmussen L.M., Møller J.E., Dahl J.S. Sex differences in aortic valve calcification in severe aortic valve stenosis: association between computer tomography assessed calcification and valvular calcium concentrations. Eur. Heart J. Cardiovasc. Imaging. 2021;22:581–588. doi: 10.1093/ehjci/jeaa096. [DOI] [PubMed] [Google Scholar]
- 13.El Husseini D., Boulanger M.C., Fournier D., Mahmut A., Bosse Y., Pibarot P., Mathieu P. High expression of the pi-transporter SLC20A1/Pit1 in calcific aortic valve disease promotes mineralization through regulation of Akt-1. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra-Izquierdo I., Castanos-Mollor I., Lopez J., Gomez C., San Roman J.A., Sanchez Crespo M., Garcia-Rodriguez C. Calcification induced by type I interferon in human aortic valve interstitial cells is larger in males and blunted by a janus kinase inhibitor. Arterioscler. Thromb. Vasc. Biol. 2018;38:2148–2159. doi: 10.1161/ATVBAHA.118.311504. [DOI] [PubMed] [Google Scholar]
- 15.El Husseini D., Boulanger M.C., Mahmut A., Bouchareb R., Laflamme M.H., Fournier D., Pibarot P., Bosse Y., Mathieu P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through akt: implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014;72:146–156. doi: 10.1016/j.yjmcc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 16.McCoy C.M., Nicholas D.Q., Masters K.S. Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarajlic P., Plunde O., Franco-Cereceda A., Bäck M. Artificial intelligence models reveal sex-specific gene expression in aortic valve calcification. JACC Basic Transl. Sci. 2021;6:403–412. doi: 10.1016/j.jacbts.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torre M., Hwang D.H., Padera R.F., Mitchell R.N., VanderLaan P.A. Osseous and chondromatous metaplasia in calcific aortic valve stenosis. Cardiovasc. Pathol. 2016;25:18–24. doi: 10.1016/j.carpath.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Grim J.C., Aguado B.A., Vogt B.J., Batan D., Andrichik C.L., Schroeder M.E., Gonzalez-Rodriguez A., Yavitt F.M., Weiss R.M., Anseth K.S. Secreted factors from proinflammatory macrophages promote an osteoblast-like phenotype in valvular interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2020;40:e296–e308. doi: 10.1161/atvbaha.120.315261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguado B.A., Walker C.J., Grim J.C., Schroeder M.E., Batan D., Vogt B.J., Gonzalez Rodriguez A., Schwisow J.A., Moulton K.S., Weiss R.M., et al. Genes that escape X chromosome inactivation modulate sex differences in valve myofibroblasts. Circulation. 2022 doi: 10.1161/circulationaha.121.054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung A.K., Das S.R., Leonard D., Peshock R.M., Kazi F., Abdullah S.M., Canham R.M., Levine B.D., Drazner M.H. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas heart study. Circulation. 2006;113:1597–1604. doi: 10.1161/circulationaha.105.574400. [DOI] [PubMed] [Google Scholar]
- 22.Tastet L., Kwiecinski J., Pibarot P., Capoulade R., Everett R.J., Newby D.E., Shen M., Guzzetti E., Arsenault M., Bedard E., et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc. Imaging. 2020;13:699–711. doi: 10.1016/j.jcmg.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Azevedo C.F., Nigri M., Higuchi M.L., Pomerantzeff P.M., Spina G.S., Sampaio R.O., Tarasoutchi F., Grinberg M., Rochitte C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 24.Bull S., White S.K., Piechnik S.K., Flett A.S., Ferreira V.M., Loudon M., Francis J.M., Karamitsos T.D., Prendergast B.D., Robson M.D., et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickander J., Themudo R., Sigfridsson A., Xue H., Kellman P., Ugander M. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males - an adenosine stress cardiovascular magnetic resonance study. Sci. Rep. 2020;10:10380. doi: 10.1038/s41598-020-67196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwadlo C., Schmidtmann E., Szaroszyk M., Kattih B., Froese N., Hinz H., Schmitto J.D., Widder J., Batkai S., Bähre H., et al. Antiandrogenic therapy with finasteride attenuates cardiac hypertrophy and left ventricular dysfunction. Circulation. 2015;131:1071–1081. doi: 10.1161/circulationaha.114.012066. [DOI] [PubMed] [Google Scholar]
- 27.Dworatzek E., Mahmoodzadeh S., Schriever C., Kusumoto K., Kramer L., Santos G., Fliegner D., Leung Y.K., Ho S.M., Zimmermann W.H., et al. Sex-specific regulation of collagen I and III expression by 17β-estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc. Res. 2019;115:315–327. doi: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickenig G., Strehlow K., Wassmann S., Bäumer A.T., Albory K., Sauer H., Böhm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- 29.Villar A.V., Llano M., Cobo M., Exposito V., Merino R., Martin-Duran R., Hurle M.A., Nistal J.F. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J. Mol. Cell. Cardiol. 2009;46:526–535. doi: 10.1016/j.yjmcc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Nordstrom P., Glader C.A., Dahlen G., Birgander L.S., Lorentzon R., Waldenstrom A., Lorentzon M. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J. Intern. Med. 2003;254:140–146. doi: 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Orlowska-Baranowska E., Gadomska Vel Betka L., Gora J., Baranowski R., Pedzich-Placha E., Zakrzewski D., Dlugosz A., Kossowska H., Zebrowska A., Zakoscielna E., et al. Functional polymorphism of the renalase gene is associated with cardiac hypertrophy in female patients with aortic stenosis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oneglia A., Nelson M.D., Merz C.N.B. Sex differences in cardiovascular aging and heart failure. Curr. Heart Fail. Rep. 2020;17:409–423. doi: 10.1007/s11897-020-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fliegner D., Schubert C., Penkalla A., Witt H., Kararigas G., Dworatzek E., Staub E., Martus P., Ruiz Noppinger P., Kintscher U., et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Phys. Regul. Integr. Comp. Phys. 2010;298:R1597–R1606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 34.Capoulade R., Clavel M.A., Le Ven F., Dahou A., Thebault C., Tastet L., Shen M., Arsenault M., Bedard E., Beaudoin J., et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging. 2017;18:1378–1387. doi: 10.1093/ehjci/jew288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bairey Merz C.N., Nelson M.D., Cheng S., Wei J. Sex differences and the left ventricle: morphology matters. Eur. Heart J. Cardiovasc. Imaging. 2020;21:991–993. doi: 10.1093/ehjci/jeaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elboudwarej O., Wei J., Darouian N., Cook-Wiens G., Li Q., Thomson L.E.J., Petersen J.W., Anderson R.D., Mehta P., Shufelt C., et al. Maladaptive left ventricular remodeling in women: an analysis from the Women's ischemia syndrome evaluation-coronary vascular dysfunction study. Int. J. Cardiol. 2018;268:230–235. doi: 10.1016/j.ijcard.2018.03.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S.M., Wei J., Cook-Wiens G., Nelson M.D., Thomson L., Berman D., Handberg E., Petersen J., Anderson D., Pepine C.J., et al. Left ventricular concentric remodelling and functional impairment in women with ischaemia with no obstructive coronary artery disease and intermediate coronary flow reserve: a report from the WISE-CVD study. Eur. Heart J. Cardiovasc. Imaging. 2019;20:875–882. doi: 10.1093/ehjci/jez044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson M.D., Wei J., Bairey Merz C.N. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur. Heart J. 2018;39:850–852. doi: 10.1093/eurheartj/ehx818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajappan K., Rimoldi O.E., Camici P.G., Bellenger N.G., Pennell D.J., Sheridan D.J. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. doi: 10.1161/01.CIR.0000074211.28917.31. [DOI] [PubMed] [Google Scholar]
- 40.Steadman C.D., Jerosch-Herold M., Grundy B., Rafelt S., Ng L.L., Squire I.B., Samani N.J., McCann G.P. Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis. JACC Cardiovasc. Imaging. 2012;5:182–189. doi: 10.1016/j.jcmg.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Singh A., Greenwood J.P., Berry C., Dawson D.K., Hogrefe K., Kelly D.J., Dhakshinamurthy V., Lang C.C., Khoo J.P., Sprigings D., et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic importance of MIcrovascular dysfunction in aortic stenosis (PRIMID AS) study. Eur. Heart J. 2017;38:1222–1229. doi: 10.1093/eurheartj/ehx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W., Sun Y.P., Divakaran S., Bajaj N.S., Gupta A., Chandra A., Morgan V., Barrett L., Martell L., Bibbo C.F., et al. Association of Myocardial Blood Flow Reserve with Adverse Left Ventricular Remodeling in patients with aortic stenosis: the microvascular disease in aortic stenosis (MIDAS) study. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajappan K., Rimoldi O.E., Dutka D.P., Ariff B., Pennell D.J., Sheridan D.J., Camici P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–476. doi: 10.1161/hc0402.102931. [DOI] [PubMed] [Google Scholar]
- 44.Williams M., Kodali S.K., Hahn R.T., Humphries K.H., Nkomo V.T., Cohen D.J., Douglas P.S., Mack M., McAndrew T.C., Svensson L., et al. Sex-related differences in outcomes after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: insights from the PARTNER trial (Placement of aortic transcatheter Valve) J. Am. Coll. Cardiol. 2014;63:1522–1528. doi: 10.1016/j.jacc.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 45.Steeds R.P., Messika-Zeitoun D., Thambyrajah J., Serra A., Schulz E., Maly J., Aiello M., Rudolph T.K., Lloyd G., Bortone A.S. IMPULSE: the impact of gender on the presentation and management of aortic stenosis across Europe. Open Heart. 2021:8. doi: 10.1136/openhrt-2020-001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bienjonetti-Boudreau D., Fleury M.A., Voisine M., Paquin A., Chouinard I., Tailleur M., Duval R., Magnan P.O., Beaudoin J., Salaun E., et al. Impact of sex on the management and outcome of aortic stenosis patients. Eur. Heart J. 2021;42:2683–2691. doi: 10.1093/eurheartj/ehab242. [DOI] [PubMed] [Google Scholar]
- 47.Larsson S.C., Wolk A., Håkansson N., Bäck M. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur. Heart J. 2017;38:2192–2197. doi: 10.1093/eurheartj/ehx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogge B.P., Cramariuc D., Lønnebakken M.T., Gohlke-Bärwolf C., Chambers J.B., Boman K., Gerdts E. Effect of overweight and obesity on cardiovascular events in asymptomatic aortic stenosis: a SEAS substudy (Simvastatin ezetimibe in aortic Stenosis) J. Am. Coll. Cardiol. 2013;62:1683–1690. doi: 10.1016/j.jacc.2013.04.081. [DOI] [PubMed] [Google Scholar]
- 49.Bahlmann E., Cramariuc D., Saeed S., Chambers J.B., Nienaber C.A., Kuck K.H., Lønnebakken M.T., Gerdts E. Low systemic arterial compliance is associated with increased cardiovascular morbidity and mortality in aortic valve stenosis. Heart. 2019;105:1507–1514. doi: 10.1136/heartjnl-2018-314386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll J.D., Carroll E.P., Feldman T., Ward D.M., Lang R.M., McGaughey D., Karp R.B. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 51.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 52.Clavel M.A., Dumesnil J.G., Capoulade R., Mathieu P., Senechal M., Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012;60:1259–1267. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 53.Tribouilloy C., Bohbot Y., Rusinaru D., Belkhir K., Diouf M., Altes A., Delpierre Q., Serbout S., Kubala M., Levy F., et al. Excess mortality and undertreatment of women with severe aortic stenosis. J. Am. Heart Assoc. 2021;10 doi: 10.1161/jaha.120.018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., Tuzcu E.M., Webb J.G., Fontana G.P., Makkar R.R., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 55.Legget M.E., Kuusisto J., Healy N.L., Fujioka M., Schwaegler R.G., Otto C.M. Gender differences in left ventricular function at rest and with exercise in asymptomatic aortic stenosis. Am. Heart J. 1996;131:94–100. doi: 10.1016/s0002-8703(96)90056-3. [DOI] [PubMed] [Google Scholar]
- 56.Singh A., Chan D.C.S., Greenwood J.P., Dawson D.K., Sonecki P., Hogrefe K., Kelly D.J., Dhakshinamurthy V., Lang C.C., Khoo J.P., et al. Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. JACC Cardiovasc. Imaging. 2019;12:96–105. doi: 10.1016/j.jcmg.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Bach D.S., Radeva J.I., Birnbaum H.G., Fournier A.A., Tuttle E.G. Prevalence, referral patterns, testing, and surgery in aortic valve disease: leaving women and elderly patients behind? J. Heart Valve Dis. 2007;16:362–369. [PubMed] [Google Scholar]
- 58.Nau D.P., Ellis J.J., Kline-Rogers E.M., Mallya U., Eagle K.A., Erickson S.R. Gender and perceived severity of cardiac disease: evidence that women are "tougher". Am. J. Med. 2005;118:1256–1261. doi: 10.1016/j.amjmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Genereux P., Stone G.W., O'Gara P.T., Marquis-Gravel G., Redfors B., Giustino G., Pibarot P., Bax J.J., Bonow R.O., Leon M.B. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J. Am. Coll. Cardiol. 2016;67:2263–2288. doi: 10.1016/j.jacc.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 60.Rosenhek R., Zilberszac R., Schemper M., Czerny M., Mundigler G., Graf S., Bergler-Klein J., Grimm M., Gabriel H., Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- 61.Cowell S.J., Newby D.E., Prescott R.J., Bloomfield P., Reid J., Northridge D.B., Boon N.A., Scottish Aortic S., Lipid Lowering Trial IoRI A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 62.Chan K.L., Teo K., Dumesnil J.G., Ni A., Tam J., Investigators A. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 63.Rossebo A., Pedersen T., Boman K. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 64.van der Linde D., Yap S., van Dijk A. Effects of rosuvastatin on progression os stenosis in adults patients with congenital aortic stenosis (PROCAS Trial) Am. J. Cardiol. 2011;108:265–271. doi: 10.1016/j.amjcard.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 65.Chockalingam A., Venkatesan S. T. S. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: Symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic Stenosis (SCOPE-AS) Am. Heart J. 2004;147 doi: 10.1016/j.ahj.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Pai R.G., Kapoor N., Bansal R.C., Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann. Thorac. Surg. 2006;82:2116–2122. doi: 10.1016/j.athoracsur.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 67.Kang D.H., Park S.J., Rim J.H., Yun S.C., Kim D.H., Song J.M., Choo S.J., Park S.W., Song J.K., Lee J.W., et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502–1509. doi: 10.1161/CIRCULATIONAHA.109.909903. [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi T., Morimoto T., Shiomi H., Ando K., Kanamori N., Murata K., Kitai T., Kawase Y., Izumi C., Miyake M., et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J. Am. Coll. Cardiol. 2015;66:2827–2838. doi: 10.1016/j.jacc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D., Conradi L., De Bonis M., De Paulis R., et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021;2021 doi: 10.1093/ejcts/ezab389. [DOI] [Google Scholar]
- 70.Chaker Z., Badhwar V., Alqahtani F., Aljohani S., Zack C.J., Holmes D.R., Rihal C.S., Alkhouli M. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J. Am. Heart Assoc. 2017;6 doi: 10.1161/jaha.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onorati F., D'Errigo P., Barbanti M., Rosato S., Covello R.D., Maraschini A., Ranucci M., Santoro G., Tamburino C., Grossi C., et al. Different impact of sex on baseline characteristics and major periprocedural outcomes of transcatheter and surgical aortic valve interventions: results of the multicenter italian OBSERVANT registry. J. Thorac. Cardiovasc. Surg. 2014;147:1529–1539. doi: 10.1016/j.jtcvs.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 72.Duncan A.I., Lin J., Koch C.G., Gillinov A.M., Xu M., Starr N.J. The impact of gender on in-hospital mortality and morbidity after isolated aortic valve replacement. Anesth. Analg. 2006;103:800–808. doi: 10.1213/01.ane.0000231890.95212.12. [DOI] [PubMed] [Google Scholar]
- 73.Cho L., Kibbe M.R., Bakaeen F., Aggarwal N.R., Davis M.B., Karmalou T., Lawton J.S., Ouzounian M., Preventza O., Russo A.M., et al. Cardiac surgery in women in the current era: what are the gaps in care? Circulation. 2021;144:1172–1185. doi: 10.1161/circulationaha.121.056025. [DOI] [PubMed] [Google Scholar]
- 74.Kodali S., Williams M.R., Doshi D., Hahn R.T., Humphries K.H., Nkomo V.T., Cohen D.J., Douglas P.S., Mack M., Xu K., et al. Sex-specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: a cohort study. Ann. Intern. Med. 2016;164:377–384. doi: 10.7326/M15-0121. [DOI] [PubMed] [Google Scholar]
- 75.Humphries K.H., Toggweiler S., Rodes-Cabau J., Nombela-Franco L., Dumont E., Wood D.A., Willson A.B., Binder R.K., Freeman M., Lee M.K., et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2012;60:882–886. doi: 10.1016/j.jacc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Buja P., Napodano M., Tamburino C., Petronio A.S., Ettori F., Santoro G., Ussia G.P., Klugmann S., Bedogni F., Ramondo A., et al. Comparison of variables in men versus women undergoing transcatheter aortic valve implantation for severe aortic stenosis (from italian multicenter CoreValve registry) Am. J. Cardiol. 2013;111:88–93. doi: 10.1016/j.amjcard.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 77.Hayashida K., Morice M.C., Chevalier B., Hovasse T., Romano M., Garot P., Farge A., Donzeau-Gouge P., Bouvier E., Cormier B., et al. Sex-related differences in clinical presentation and outcome of transcatheter aortic valve implantation for severe aortic stenosis. J. Am. Coll. Cardiol. 2012;59:566–571. doi: 10.1016/j.jacc.2011.10.877. [DOI] [PubMed] [Google Scholar]
- 78.Chandrasekhar J., Dangas G., Yu J., Vemulapalli S., Suchindran S., Vora A.N., Baber U., Mehran R., Registry S.A.T. Sex-based differences in outcomes with transcatheter aortic valve therapy: TVT registry from 2011 to 2014. J. Am. Coll. Cardiol. 2016;68:2733–2744. doi: 10.1016/j.jacc.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 79.Conrotto F., D'Ascenzo F., Presbitero P., Humphries K.H., Webb J.G., O'Connor S.A., Morice M.C., Lefevre T., Grasso C., Sbarra P., et al. Effect of gender after transcatheter aortic valve implantation: a meta-analysis. Ann. Thorac. Surg. 2015;99:809–816. doi: 10.1016/j.athoracsur.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 80.Shan Y., Pellikka P.A. Aortic stenosis in women. Heart. 2020;106:970–976. doi: 10.1136/heartjnl-2019-315407. [DOI] [PubMed] [Google Scholar]
- 81.Smith C.R., Leon M.B., Mack M.J., Miller D.C., Moses J.W., Svensson L.G., Tuzcu E.M., Webb J.G., Fontana G.P., Makkar R.R., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 82.Adams D.H., Popma J.J., Reardon M.J., Yakubov S.J., Coselli J.S., Deeb G.M., Gleason T.G., Buchbinder M., Hermiller J., Kleiman N.S., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014;370:1790–1798. doi: 10.1056/nejmoa1400590. [DOI] [PubMed] [Google Scholar]
- 83.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K., Thourani V.H., Tuzcu E.M., Miller D.C., Herrmann H.C., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 84.Reardon M.J., Van Mieghem N.M., Popma J.J., Kleiman N.S., Søndergaard L., Mumtaz M., Adams D.H., Deeb G.M., Maini B., Gada H., et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 85.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., Kapadia S.R., Malaisrie S.C., Cohen D.J., Pibarot P., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 86.Thyregod H.G., Steinbrüchel D.A., Ihlemann N., Nissen H., Kjeldsen B.J., Petursson P., Chang Y., Franzen O.W., Engstrøm T., Clemmensen P., et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J. Am. Coll. Cardiol. 2015;65:2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 87.Popma J.J., Deeb G.M., Yakubov S.J., Mumtaz M., Gada H., O'Hair D., Bajwa T., Heiser J.C., Merhi W., Kleiman N.S., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 88.Ferrante G., Pagnotta P., Petronio A.S., Bedogni F., Brambilla N., Fiorina C., Giannini C., Mennuni M., De Marco F., Klugmann S., et al. Sex differences in postprocedural aortic regurgitation and mid-term mortality after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2014;84:264–271. doi: 10.1002/ccd.25377. [DOI] [PubMed] [Google Scholar]
- 89.O'Connor S.A., Morice M.C., Gilard M., Leon M.B., Webb J.G., Dvir D., Rodes-Cabau J., Tamburino C., Capodanno D., D'Ascenzo F., et al. Revisiting sex equality with transcatheter aortic valve replacement outcomes: a collaborative, patient-level meta-analysis of 11,310 patients. J. Am. Coll. Cardiol. 2015;66:221–228. doi: 10.1016/j.jacc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed B., Lischke S., Holterman L.A., Straight F., Dauerman H.L. Angiographic predictors of vascular complications among women undergoing cardiac catheterization and intervention. J. Invasive Cardiol. 2010;22:512–516. [PubMed] [Google Scholar]
- 91.Schwertz D.W., Penckofer S. Sex differences and the effects of sex hormones on hemostasis and vascular reactivity. Heart Lung. 2001;30:401–426. doi: 10.1067/mhl.2001.118764. quiz 427–408. [DOI] [PubMed] [Google Scholar]
- 92.Pibarot P., Weissman N.J., Stewart W.J., Hahn R.T., Lindman B.R., McAndrew T., Kodali S.K., Mack M.J., Thourani V.H., Miller D.C., et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort–a analysis. J. Am. Coll. Cardiol. 2014;64:1323–1334. doi: 10.1016/j.jacc.2014.06.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tasca G., Brunelli F., Cirillo M., DallaTomba M., Mhagna Z., Troise G., Quaini E. Impact of valve prosthesis-patient mismatch on left ventricular mass regression following aortic valve replacement. Ann. Thorac. Surg. 2005;79:505–510. doi: 10.1016/j.athoracsur.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 94.Pighi M., Piazza N., Martucci G., Lachapelle K., Perrault L.P., Asgar A.W., Lauck S., Webb J.G., Popma J.J., Kim D.H., et al. Sex-specific determinants of outcomes after transcatheter aortic valve replacement. Circ. Cardiovasc. Qual. Outcomes. 2019;12 doi: 10.1161/circoutcomes.118.005363. [DOI] [PubMed] [Google Scholar]
- 95.Ravaux J.M., Di Mauro M., Vernooy K., Van't Hof A.W., Veenstra L., Kats S., Maessen J.G., Lorusso R. Do women require less permanent pacemaker after transcatheter aortic valve implantation? A meta-analysis and meta-regression. J. Am. Heart Assoc. 2021;10 doi: 10.1161/jaha.120.019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barreto-Filho J.A., Wang Y., Dodson J.A., Desai M.M., Sugeng L., Geirsson A., Krumholz H.M. Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA. 2013;310:2078–2085. doi: 10.1001/jama.2013.282437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caponcello M.G., Banderas L.M., Ferrero C., Bramlage C., Thoenes M., Bramlage P. Gender differences in aortic valve replacement: is surgical aortic valve replacement riskier and transcatheter aortic valve replacement safer in women than in men? J. Thorac. Dis. 2020;12:3737–3746. doi: 10.21037/jtd-20-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vaturi M., Shapira Y., Rotstein M., Adler Y., Porter A., Birnbaum Y., Vered Z., Sagie A. The effect of aortic valve replacement on left ventricular mass assessed by echocardiography. Eur. J. Echocardiogr. 2000;1:116–121. doi: 10.1053/euje.2000.0014. [DOI] [PubMed] [Google Scholar]
- 99.Petrov G., Dworatzek E., Schulze T.M., Dandel M., Kararigas G., Mahmoodzadeh S., Knosalla C., Hetzer R., Regitz-Zagrosek V. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc. Imaging. 2014;7:1073–1080. doi: 10.1016/j.jcmg.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Petrov G., Regitz-Zagrosek V., Lehmkuhl E., Krabatsch T., Dunkel A., Dandel M., Dworatzek E., Mahmoodzadeh S., Schubert C., Becher E., et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122:S23–S28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- 101.Chieffo A., Petronio A.S., Mehilli J., Chandrasekhar J., Sartori S., Lefevre T., Presbitero P., Capranzano P., Tchetche D., Iadanza A., et al. 1-year clinical outcomes in women after transcatheter aortic valve replacement: results from the first WIN-TAVI registry. JACC Cardiovasc. Interv. 2018;11:1–12. doi: 10.1016/j.jcin.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 102.AlBadri A., Lai K., Wei J., Landes S., Mehta P.K., Li Q., Johnson D., Reis S.E., Kelsey S.F., Bittner V., et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored women's ischemia syndrome evaluation (WISE) PLoS One. 2017;12 doi: 10.1371/journal.pone.0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eltchaninoff H., Bonaros N., Prendergast B., Nietlispach F., Vasa-Nicotera M., Chieffo A., Pibarot P., Bramlage P., Sykorova L., Kurucova J., et al. Rationale and design of a prospective, randomized, controlled, multicenter study to evaluate the safety and efficacy of transcatheter heart valve replacement in female patients with severe symptomatic aortic stenosis requiring aortic valve intervention (Randomized researcH in womEn all comers wIth aortic stenosis [RHEIA] trial) Am. Heart J. 2020;228:27–35. doi: 10.1016/j.ahj.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 104.Mihos C.G., Klassen S.L., Yucel E. Sex-specific considerations in women with aortic stenosis and outcomes after transcatheter aortic valve replacement. Curr. Treat. Options Cardiovasc. Med. 2018;20:52. doi: 10.1007/s11936-018-0651-x. [DOI] [PubMed] [Google Scholar]