Abstract
Kappa-opioid receptor (KOR) agonists have been studied as potential treatments for pain, pruritus, and substance-use disorders, but prototypical KOR agonists produce side-effects like dysphoria and sedation. Atypical KOR agonists that exhibit G-protein biased signaling at the KOR have been reported to produce therapeutic-like effects with fewer or reduced side-effects relative to prototypical KOR agonists. In the current report, behavioral profiles were determined using a behavioral scoring system that was modified to quantify drug-induced behaviors in nonhuman primates (NHPs). Profiles were determined for a prototypical and two biased KOR agonists, alone and combined with the mu-opioid receptor (MOR) agonist, oxycodone. Five adult male rhesus monkeys implanted with intravenous catheters were administered a range of doses of the KOR agonist, U50–488H (0.01–0.1 mg/kg) and the biased KOR agonists, nalfurafine (0.0001–0.001 mg/kg) and triazole 1.1 (0.32–1.0 mg/kg), alone and combined with the MOR agonist, oxycodone (0.01–0.32 mg/kg). In addition, the largest triazole 1.1 dose tested (1.0 mg/kg) was administered in time-course determinations (0–56 min), alone and combined with oxycodone (0.1 mg/kg). U50–488H and nalfurafine produced sedative-like and motor-impairing effects. Triazole 1.1 had a milder side-effect profile, in some instances producing sedative-like effects but to a lesser degree compared with the other KOR agonists, particularly for lip droop and rest/sleep posture. All KOR agonists reduced oxycodone-induced scratch, but nalfurafine produced behavior-disrupting and sedative-like effects when combined with oxycodone that were not observed with triazole 1.1. The duration of triazole 1.1’s behavioral effects were relatively short, dissipating entirely by 56 min. Our results suggest that KOR agonists with comparable pharmacology to triazole 1.1 may be useful therapeutics with reduced side-effect profiles, and the mechanisms conferring these benefits may be attributed to factors other than G-protein bias.
Keywords: Kappa-opioid receptor, Mu-opioid receptor, Rhesus monkey, Observable behavior
1. Introduction
Kappa-opioid receptor (KOR) agonists have been studied as potential treatments for a variety of disorders including pain, pruritus, and substance-use disorder (Aldrich and McLaughlin, 2009; Kivell and Prisinzano, 2010; Prisinzano et al., 2005), but the prototypical compounds of this drug class produce untoward effects (e.g., dysphoria, psychotomimesis, sedation). Historically, these side effects have hampered the development of KOR agonists for clinical use (Mores et al., 2019; Pfeiffer et al., 1986). However, recent advances in drug discovery have identified a number of KOR agonists that produce therapeutic-like effects (antinociception, anti-pruritus), reduce the abuse-related effects of drugs of abuse, but do not produce KOR-typical side effects such as dysphoria and psychotomimesis (Mores et al., 2019). One of these KOR agonist, nalfurafine, has advanced to clinical use in Japan for the treatment of intractable pruritus (Remitch®; Inui, 2012), making it the first and so far only KOR agonist to be prescribed as a therapeutic in humans.
Investigations of the molecular pharmacology of atypical KOR agonists such as nalfurafine have identified unique signaling properties at the KOR that may account for their diminished side effects. Specifically, these compounds appear to have a higher potency to activate the G-protein signaling pathway relative to the recruitment of ß-arrestin-2 at the KOR, leading to the description of these compounds as “biased” KOR agonists (Dogra and Yadav, 2015; Mores et al., 2019; Zhou et al., 2022). Some have interpreted the observed relation between signaling bias and improved side-effect profiles to be an indication that the G-protein signaling pathway is responsible for therapeutic-like effects (e.g., antinociception) while ß-arrestin-2 recruitment mediates adverse effects such as dysphoria and sedation (Bedini et al., 2020; Brust et al., 2016; Mores et al., 2019). However, this dichotomy has not held up across all studies (Dogra and Yadav, 2015; Mores et al., 2019; White et al., 2015). Moreover, cell-signaling results that are used to quantify “bias factors” for test ligands vary between approaches and reports, which precludes precise scaling of drugs along the continuum of bias (Brust et al., 2016; Dunn et al., 2019; Dunn et al., 2018; Kaski et al., 2019). Nevertheless, the preclinical behavioral data collected with these compounds strongly support the notion that KOR agonists can be structurally modified to significantly improve their therapeutic selectivity (Brust et al., 2016; Spetea et al., 2017; White et al., 2015).
One compound that is arguably the most atypical of the G-protein biased KOR agonists is triazole 1.1. Structurally introduced in 2013, triazole 1.1 was reported to exhibit high KOR selectivity relative to MOR and delta opioid receptor binding and to be a full-efficacy agonist in vitro at the KOR (Brust et al., 2016; Zhou et al., 2013). Triazole 1.1 produced antinociception in mice at doses that did not cause sedation, suppress operant behavior, or affect brain dopamine concentrations (Brust et al., 2016; Zhou et al., 2013). Moreover, triazole 1.1 is the only KOR agonist reported to reverse pain-decreased behavior, an approach thought to distinguish antinociceptive effects of drugs from nonspecific suppressive effects on behavior (e.g., via sedation; Brust et al., 2016). We recently reported that triazole 1.1, when combined as a mixture with oxycodone, blocked oxycodone self-administration and enhanced oxycodone-induced thermal antinociception in rats, suggesting that formulations of KOR agonists from the triazole 1.1 chemical series with mu-opioid receptor (MOR) agonists like oxycodone could lead to improved pain medications with reduced abuse liability (Zamarripa et al., 2021). Moreover, we and others have demonstrated that the behavioral effects of triazole 1.1 in rodents are fully blocked by pretreatment with the KOR antagonist, norbinaltorphimine, thus demonstrating the KOR-specificity of triazole 1.1’s behavioral effects (Brust et al., 2016; Zamarripa et al., 2021).
While previous studies have investigated behavioral effects of KOR agonists in nonhuman primates (NHPs; Butelman et al., 2009; Butelman et al., 2010; Dykstra et al., 1987), only one study (Huskinson et al., 2020) has investigated triazole 1.1 in a NHP model. In that report, we quantified a number of behavioral effects of prototypical (U50–488H, salvinorin A) and biased (nalfurafine, triazole 1.1) KOR agonists, alone or combined with a select dose of oxycodone, in male rhesus monkeys. U50–488H and salvinorin A produced sedative-like and motor-impairing effects, and nalfurafine was similar to these KOR agonists on most outcomes. Conversely, triazole 1.1 did not produce sedative-like or motor-impairing effects at the doses tested, suggesting that it does not produce certain KOR-typical adverse effects. Lastly, all KOR agonists blocked oxycodone-induced scratching.
In our previous report (Huskinson et al., 2020), effects of KOR agonists were tested in combination with only one dose of oxycodone, which leaves unanswered the question of how oxycodone’s dose-response relations across the observed behaviors are affected by co-treatment with KOR agonists. Moreover, the dose selection for triazole 1.1 was constrained by solubility challenges that have since been improved. Lastly, there is currently no information on duration of effect for triazole 1.1 at a behaviorally active dose in NHPs. As such, the goal of the current report was to systematically compare the effects of a wider dose range of triazole 1.1 and other KOR agonists, alone or combined with multiple doses of oxycodone, on observable behavior in rhesus monkeys, including a time-course assessment for triazole 1.1 on the reversal of oxycodone-induced scratch and other observable behaviors.
2. Methods
All procedures were approved by the University of Mississippi Medical Center’s Institutional Animal Care and Use Committee and were conducted in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (8th edition, 2011).
2.1. Subjects
Five male adult rhesus monkeys (Macaca mulatta, weighing 10.5–13.3 kg) served as subjects. At the start of the experiment, all subjects had experimental histories. Subjects 1265, 0099, and 5286 had only served in our previous observation study with KOR agonists, oxycodone, cocaine, and ketamine (Huskinson et al., 2020), and subjects 105–2008, 1010, and 5286 had previous experience in food and drug self-administration, most recently with cocaine and benzodiazepine combinations (105–2008; Huskinson et al., 2019), cocaine, oxycodone, KOR agonists, and food (1010; Zamarippa et al., 2020; unpublished data), and with cocaine vs. food choice (5286; unpublished data). Subjects were individually housed in stainless steel enrichment-style cages (each unit: 0.76 m X 0.76 m X 0.86 m; Carter2 Systems, Inc., Beaverton, OR) that allowed visual and olfactory interaction with other monkeys. Cages were changed bi-weekly on Wednesdays. Subjects received water ad lib and were fed standard biscuits (Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI) twice daily at approximately the same time each day, in an amount sufficient to maintain healthy body weights. Fruits or vegetables and foraging materials were provided daily, after observation sessions were completed. Rooms were maintained on a 12-hr light/12-hr dark schedule (lights on at 0600 hr).
2.2. Surgery and Apparatus
The surgical protocol and apparatus have been described previously (Huskinson et al., 2020). Briefly, prior to surgery, subjects were given atropine sulfate (0.04 mg/kg, i.m.) and ketamine hydrochloride (10–20 mg/kg, i.m.) followed by inhaled isoflurane and preoperative antibiotics (cefazolin; 20–25 mg/kg, i.m.) and analgesics (carprofen, 2–4 mg/kg, s.c. and/or buprenorphine SR, 0.05 mg/kg, s.c.). Under aseptic conditions, a single lumen silicon catheter (Cole-Parmer; ID: 0.76 mm, OD: 1.65 or 2.46 mm) was implanted into a major vein with the tip terminating near the right atrium. The distal end of the catheter was passed subcutaneously to the mid-scapular region, where it exited the subject’s back. After surgery, subjects were fit with a jacket, and the catheter was threaded through a tether and connected to a single-lumen swivel (Lomir Biomedical, Inc., Malone, NY). Catheter material was attached to the swivel from the exterior of the home cage and connected to a 0.22 μm Millipore filter and plastic syringe that was housed in a custom-made box. Postoperative analgesics (carprofen 4 mg/kg, p.o) were given daily for 3 days, and antibiotics (usually Keflex, 22.2 mg/kg, p.o. or i.m.; Eli Lilly & Company, Indianapolis, IN) were given when recommended by veterinary staff. Catheters were flushed daily with heparinized saline (40–100 U/ml). If a catheter became nonfunctional, it was removed, and a new catheter was implanted once health was verified by veterinary staff.
2.3. Procedure
2.3.1. General behavioral observation procedure
The general observation procedure was identical to that described previously (Huskinson et al., 2020). A single session was conducted daily at the same time each day, using the focal animal behavioral scoring system described by Novak and colleagues (1992, 1998) and modified to include drug-induced behaviors (Duke et al., 2018, 2021; Huskinson et al., 2020; Platt et al., 2002; Rueedi-Bettschen et al., 2013). Observers met a 90% inter-observer reliability criterion prior to the experiments and were blind to drug treatments and hypotheses. Behaviors (see Table 1) were scored by recording the presence of each behavior in 15-s intervals during 5-min sessions. Sedation measures were scored as in our previous experiment (Huskinson et al., 2020). Scores were calculated as the number of intervals in which a behavior occurred, and the maximum possible score for each behavior was 20.
Table 1.
Behavioral categories, abbreviations, and definitions.
| Behavior | Brief Description |
|---|---|
| Passive Visual | Animal is standing or sitting motionless with eyes open |
| Locomotion | At least two directed steps in the horizontal and/or vertical plane |
| Self-Groom | Picking, scraping, spreading or licking of an animal’s own hair |
| Tactile/Oral Exploration | Any tactile or oral manipulation of the cage or environment |
| Scratch | Vigorous strokes of the hair with fingers or toenails |
| Stereotypy | Any repetitive, ritualized pattern of behavior that serves no obvious function |
| Forage | Sweeping and/or picking through wood chip substrate |
| Vocalization | Species-typical sounds emitted by monkey (not differentiated into different types) |
| Threat/Aggress | Multifaceted display involving one or more of the following: Open mouth stare with teeth partially exposed, eyebrows lifted, ears flattened or flapping, rigid body posture, piloerection, attack (e.g., biting, slapping) of inanimate object or other monkey |
| Cage Shake | Any vigorous shaking of the cage that may or may not make noise |
| Yawn | To open mouth wide and expose teeth |
| Body Spasm | An involuntary twitch or shudder of the entire body; also “wet dog” shake |
| Present | Posture involving presentation of rump, belly, flank, and/or neck to observer or other monkey |
| Drink | Mouth contact to fluid delivery sipper |
| Facial Rub | Excessive wiping of nose or chin on the home cage or with hand or arm |
| Fear Grimace | Grin-like facial expression involving the retraction of the lips exposing clenched teeth; may be accompanied by flattened ears, stiff, huddled body posture, screech/chattering vocalizations |
| Lip Smack | Pursing the lips and moving them together to produce a smacking sound, often accompanied by moaning |
| Lip Droop | Bottom lip drooping, showing bottom teeth |
| Vomit/Retch | Expulsion of food or fluid through mouth or nose or making the sound or movement of vomiting |
| Tremor/Jerk | A tremor or jerk of a part of the body (e.g., head, two limbs) |
| Observable Ataxia | Any slip, trip, fall, loss of balance. |
| Rest/Sleep Posture | Idiosyncratic posture adopted by monkeys during rest or sleep, easily roused; eyes open <3 s after stimulus |
| Moderate Sedation | Atypical loose-limbed posture (e.g., propped on the cage by the body or a limb), eyes closed, delayed response to external stimuli (> 3 s) |
| Deep Sedation | Atypical loose-limbed posture, eyes closed, does not respond to external stimuli |
Behavioral profiles for each drug and experimental condition were determined after subjects habituated to the presence of observers (at least 2 weeks). Vehicles and drugs were given intravenously (i.v.) by an experimenter who was not blinded to the drug condition or hypotheses. Injections were delivered by hand with a 3-ml syringe containing the vehicle or drug solution followed by an additional 3-ml injection of heparinized saline. Injections were delivered as quickly as possible, taking care to push at a rate that prevented a catheter line from coming disconnected at the swivel or other areas along the system (typically within a 15 to 30-s period). During cage-change weeks (bi-weekly), Mondays and Thursdays were considered baseline days, and one or two saline injections were administered prior to observation sessions at the same times that drugs and/or vehicles were scheduled to be administered on the following test day (details below). During these weeks, Tuesdays and Fridays were considered test days, and details are described below for each condition: dose-response determinations of single drugs, dose-response determinations of drug mixtures, and time-course assessments of triazole 1.1. During weeks that did not have a cage change, Tuesdays and Thursdays were considered baseline days, and Mondays, Wednesdays, and Fridays were considered test days.
2.3.2. Dose-response determinations of single drugs and drug mixtures.
Behavioral profiles for single drugs and drug mixtures were determined in four subjects (1265, 0099, 5286, 105–2008) following administration of saline, an 80% propylene glycol vehicle, and a range of doses of each drug and drug mixture. Single-drug dose-response determinations were completed for the MOR agonist, oxycodone (0.01–0.32 mg/kg); one prototypical KOR agonist, U50–488H (0.01–0.1 mg/kg); and two biased KOR agonists, nalfurafine (0.0001–0.001 mg/kg) and triazole 1.1 (0.32–1.0 mg/kg). On test days, vehicle or drug was administered i.v., 10 min before observation sessions. Each vehicle and each oxycodone dose were administered twice, and each KOR agonist dose was administered once. Behavioral profiles also were determined for each oxycodone dose mixed with each dose of KOR agonist that had been determined during the dose-response determination for the single drugs. Each mixture was administered once.
2.3.3. Time-course assessments of triazole 1.1.
In a previous experiment, time-course assessments were completed for oxycodone, U50–488H, nalfurafine, and triazole 1.1 (Huskinson et al., 2020). However, in that experiment, triazole 1.1 was evaluated at a smaller dose range (0.01–0.32 mg/kg) than in the current experiments (0.32–1.0 mg/kg). When administered as a single drug in the previous study, during dose-response determinations and during the time-course assessment at 0.32 mg/kg, triazole 1.1 had no reliable effects on behavior. As a result, a new time course was completed for triazole 1.1 using the largest dose tested in the current experiment (1.0 mg/kg) with four subjects (1265, 0099, 105–2008, 1010). For subjects that completed the dose-response testing for single drugs and drug mixtures (1265, 0099, 105–2008), time-course testing was completed after dose-response testing was complete. Subject 1010 only served in the time-course conditions. In addition, time-course testing had been initiated in subject 5286, however, this subject’s catheter became nonfunctional in the middle of testing, and no veins suitable for catheterization remained in this subject. None of 5286’s data were included in the analyses of time-course assessments for triazole 1.1.
In addition to evaluating the time course of triazole 1.1 alone, we evaluated the time course of triazole 1.1 in the context of oxycodone administration. On test days and depending on the condition, 1.0 mg/kg of triazole 1.1 or saline were administered i.v., 5.6, 10, 18, 32, or 56 minutes prior to the start of the observation session. A second injection also was administered on test days. Depending on the condition, 0.1 mg/kg of oxycodone or saline were administered 10 minutes prior to the start of the observation session. This dose of oxycodone was selected because it produced the greatest amount of scratching compared to other doses tested in the dose-response determinations of the single drugs. Therefore, four separate conditions were conducted for time-course assessments: triazole 1.1 time course + saline (10 min), triazole 1.1 time course + 0.1 mg/kg oxycodone (10 min), saline time course + saline (10 min), saline time course + 0.1 mg/kg oxycodone (10 min). Each subject experienced each timepoint and each condition once. On baseline days, two separate saline injections were administered at the same pretreatment times as was scheduled to occur on the following test day.
2.4. Drugs
Oxycodone hydrochloride was provided by the National Institute on Drug Abuse drug supply program (Rockville, MD). U50–488H and nalfurafine hydrochloride were provided by Dr. Thomas Prisinzano (University of Kentucky, Lexington, KY). Triazole 1.1 [2-(4-(furan-2-ylmethyl)-5-((4-methyl- 3-(trifluoromethyl)benzyl)thio)-4H-1,2,4-triazol-3-yl)pyridine] was provided by Dr. Bruce Blough (Research Triangle Institute, Research Triangle Park, NC). Oxycodone, nalfurafine, and U50–488H were dissolved in 0.9% sterile saline, and triazole 1.1 was dissolved in propylene glycol and diluted in 0.9% sterile saline to a final concentration of 80% propylene glycol/20% saline. Each injection (3 ml) was passed through a 0.22 μm Millipore filter prior to administration.
2.5. Data Analysis
For dose-response determinations, saline and 80% propylene glycol were not statistically different for any behavior and were combined in the overall analyses. For each drug alone (oxycodone, U50,488H, nalfurafine, and triazole 1.1) and each behavior, a separate repeated-measures one-way analysis of variance (ANOVA) was conducted with dose as a within-subjects variable. Dunnett’s multiple comparisons were used to compare each dose to vehicle. Similarly, for each drug mixture and each behavior, a separate repeated-measures one-way ANOVA was conducted for each KOR agonist dose with oxycodone dose as a within-subjects variable. Dunnett’s multiple comparisons were used to compare each oxycodone dose + a single KOR agonist dose to vehicle. To compare oxycodone alone to oxycodone mixed with each KOR agonist dose, a separate two-way ANOVA was conducted for each KOR agonist and each behavior with KOR agonist dose and oxycodone dose as separate within-subjects variables. Dunnett’s multiple comparisons were used to compare oxycodone alone to the corresponding oxycodone dose mixed with each KOR agonist dose. For triazole 1.1 time-course assessments, a separate two-way repeated-measures ANOVA was conducted for each behavior with condition [1.0 mg/kg of triazole 1.1 time course + saline (10 min), 1.0 mg/kg of triazole 1.1 time course + 0.1 mg/kg of oxycodone (10 min), saline time course + saline (10 min), saline time course + 0.1 mg/kg of oxycodone (10 min)] and timepoint (5.6, 10, 18, 32, 56 min) as within-subjects factors. Bonferroni’s multiple comparisons were used to compare each condition to vehicle [saline time course + saline (10 min)] and to compare each condition to oxycodone [saline time course + 0.1 mg/kg oxycodone (10 min)].
3. Results
3.1. Dose-response determinations of single drugs
Figure 1 shows average scores for dose-response determinations for scratch (A), facial rub (B), species-typical behavior (C), passive visual (D), lip droop (E), and rest/sleep posture (F) for oxycodone and each KOR agonist. Shaded areas represent the mean and SEM following vehicle administration. Species-typical activity is a global measure that includes tactile/oral exploration, locomotion, foraging, and self-groom (note the different y-axis for this measure). This measure was used to capture the individual differences in the types of species-typical activities that subjects displayed during baseline and subsequent disruption following drug administration. Facial rub is a measure purported to indicate gastrointestinal distress and lip droop is purported to indicate muscle relaxation (Weerts et al., 1998). Data for other behaviors from Table 1 were not statistically significant and are not presented. Table 2 presents statistical outcomes from the overall analyses for administration of the single drugs for each of these behaviors.
Figure 1. Dose-Response Determinations for Oxycodone and each KOR Agonist.
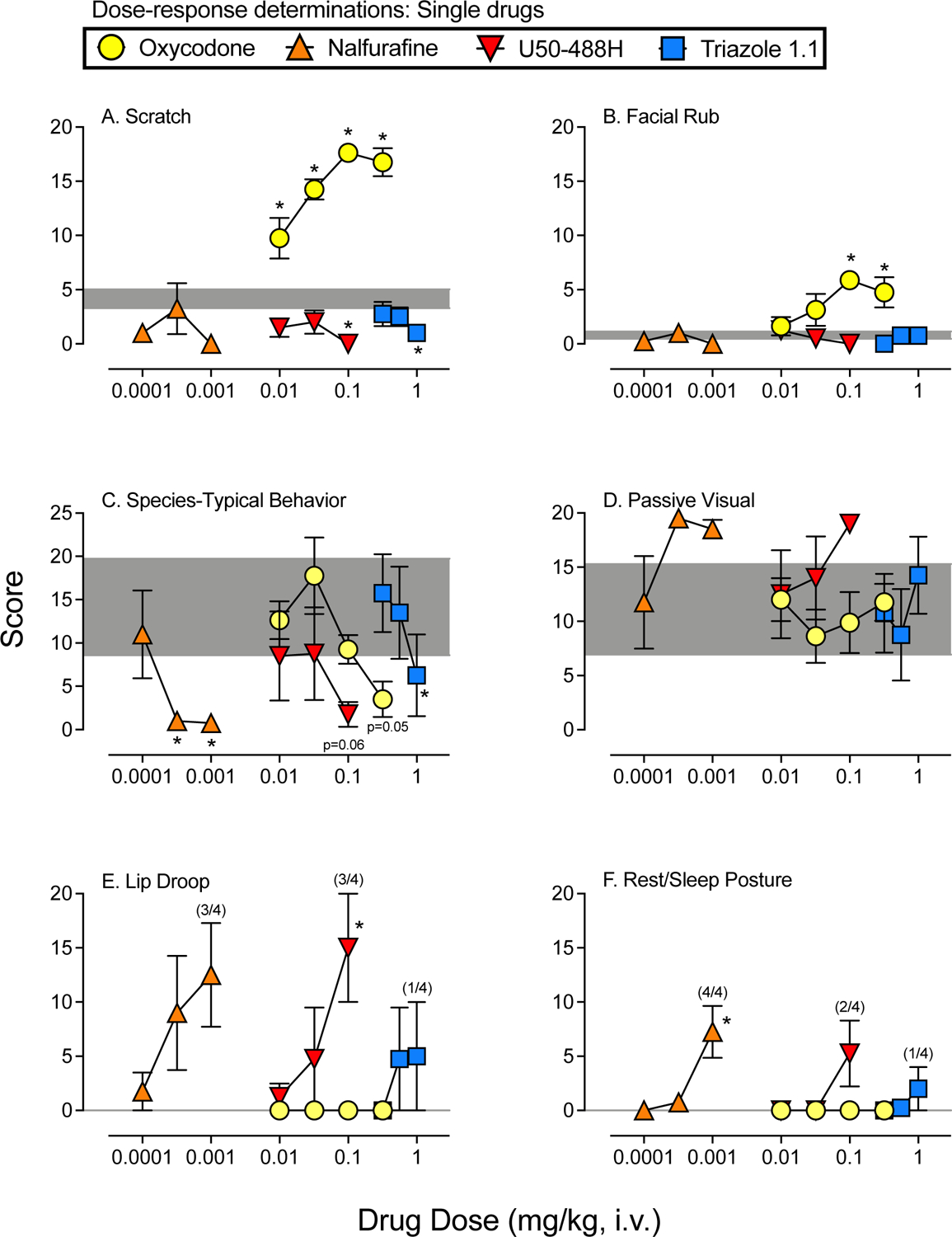
Mean (+SEM) score following administration of oxycodone (circles), nalfurafine (upward triangles), U50–488H (downward triangles), and triazole 1.1 (squares). All sessions lasted 5 min and began 10 min following vehicle or drug administration. Scores are shown in separate panels for scratch (A), facial rub (B), species-typical behavior (C), passive visual (D), lip droop (E), and rest/sleep posture (F). The shaded area represents the mean (+/−SEM) following vehicle administration. Asterisks represent doses of each drug that were statistically different from vehicle (p’s<0.05). Note: oxycodone alone and vehicle represent the same data in Figures 1–3.
Table 2.
Statistical outcomes from overall analyses. Values in bold-face font met statistical significance at p<0.05
| Dose-Response Determinations of Single Drugs: Main Effects of Dose | ||||||
|---|---|---|---|---|---|---|
| Drug | Scratch | Facial Rub | Species-Typical | Passive Visual | Lip Droop | Rest/Sleep Posture |
| Oxycodone mu-opioid agonist | F(4,12)=18.3, p<0.05 | F(4,12)=4.8, p<0.05 | F(4,12)=4.0, p<0.05 | F(4,120)=0.3, p=0.87 | Zero instances, ANOVA not conducted | Zero instances, ANOVA not conducted |
| U50–488H prototypical KOR agonist | F(3,9)=4.9, p<0.05 | F(3,9)=5.9, p<0.05 | F(3,9)=2.5, p=0.13 | F(3,9)=2.3, p=0.15 | F(3,9)=4.8, p<0.05 | F(3,9)=3.0, p=0.09 |
| Nalfurafine biased KOR agonist | F(3,9)=2.2, p=0.15 | F(3,9)=2.2, p=0.15 | F(3,9)=5.3, p<0.05 | F(3,9)=3.3, p=0.07 | F(3,9)=2.8, p=0.10 | F(3,9)=8.4, p<0.05 |
| Triazole 1.1 biased KOR agonist | F(3,9)=3.0, p=0.09 | F(3,9)=0.8, p=0.55 | F(3,9)=7.6, p<0.05 | F(3,9)=1.4, p=0.31 | F(3,9)=1.0, p=0.44 | F(3,9)=0.9, p=0.48 |
Oxycodone significantly increased scratch and facial rub behaviors (Figure 2, panels A and B), and in post-hoc analyses, all doses resulted in significant increases in scratch compared with vehicle, and the two largest doses tested (0.1 and 0.32 mg/kg) increased facial rub compared with vehicle. Conversely, U50–488H significantly decreased scratch and facial rub according to the overall analyses. In post-hoc comparisons, only the largest U50–488H dose tested (0.1 mg/kg) significantly reduced scratch compared to vehicle, and none of the U50–488H doses tested resulted in significant differences in facial rub compared with vehicle. The biased KOR agonists, nalfurafine and triazole 1.1, had no significant effects on scratch or facial rub behaviors in the overall analyses, however, the largest dose of triazole 1.1 tested (1.0 mg/kg) resulted in a significant decrease in scratch compared with vehicle. All of the single drugs tested, except U50–488H, resulted in significant reductions in species-typical behavior (Figure 2, panel C). When compared with vehicle, significant or nearly significant reductions were obtained at the largest doses of all drugs that were tested. With nalfurafine, a reduction in species-typical behavior also occurred at the second largest dose tested (0.00032 mg/kg). None of the single drugs tested resulted in significant changes in passive visual behavior (Figure 2, panel D), and there were zero instances of lip droop or rest/sleep posture with oxycodone at all doses tested (Figure 2, panels E and F). U50–488H significantly increased lip droop behavior, and the largest dose tested (0.1 mg/kg) was statistically different from vehicle. Conversely, rest/sleep posture was not significantly increased by administration of U50–488H, and while 2 of 4 subjects displayed some level of rest/sleep posture at the largest dose tested, this outcome was not statistically different from vehicle levels. The biased KOR agonist, nalfurafine, resulted in some instances of lip droop and rest/sleep posture that were present in most of the subjects, however, only rest/sleep posture was significantly increased by nalfurafine, and the largest dose tested (0.001 mg/kg) was statistically different from vehicle. Finally, one of the subjects displayed some lip droop following administration of triazole 1.1 at the two largest doses tested (0.56 and 1.0 mg/kg), and a different subject displayed some rest/sleep posture following administration of the largest dose of triazole 1.1 (1.0 mg/kg), but none of the outcomes with triazole 1.1 were statistically significant.
Figure 2. Dose-Response Determinations for Oxycodone Alone and Mixed with each KOR Agonist.
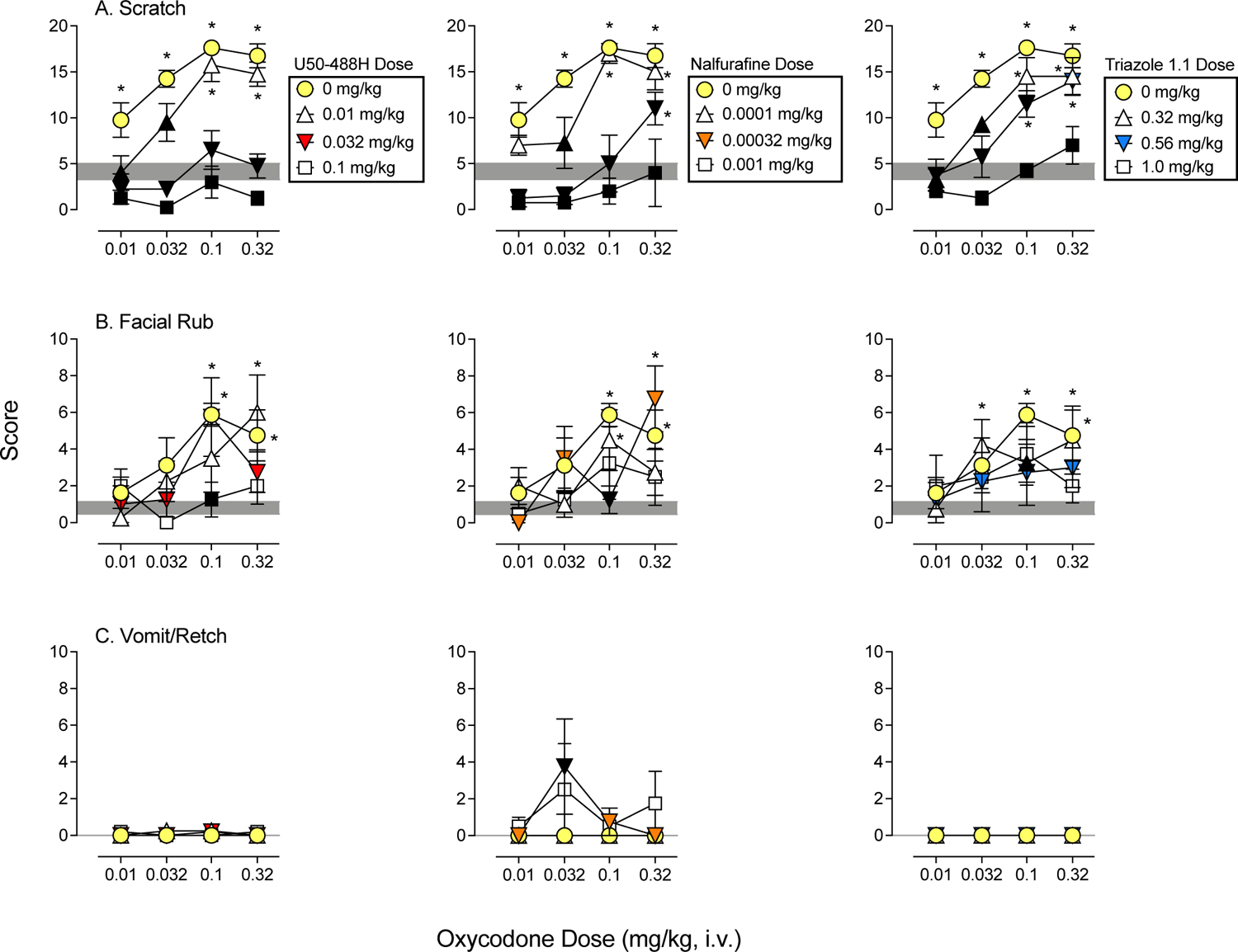
Mean (+SEM) score following administration of oxycodone (circles), oxycodone mixed with three doses of U50–488H (left column), nalfurafine (middle column), and triazole 1.1 (right column). Sessions lasted 5 min and began 10 min following vehicle or drug administration. Scores are shown in separate panels for scratch (A), facial rub (B), and vomit/retch (C). The shaded area represents the mean (+/−SEM) following vehicle administration. Asterisks represent oxycodone doses and drug mixtures that were statistically different from vehicle (p’s<0.05), and black symbols represent mixtures that were statistically different from oxycodone alone at the corresponding oxycodone dose. Note: oxycodone alone and vehicle represent the same data in Figures 1–3.
3.2. Dose-response determinations of drug mixtures
Figure 2 shows average scores for dose-response determinations for oxycodone mixed with U50–488H (left column), nalfurafine (middle column), and triazole 1.1 (right column) for scratch (A), facial rub (B), and vomit/retch (C), and Figure 3 shows average scores for dose-response determinations for oxycodone mixed with U50–488H (left column), nalfurafine (middle column), and triazole 1.1 (right column) for species-typical behavior (A), passive visual (B), lip droop (C), and rest/sleep posture (D). Shaded areas represent the mean and SEM following vehicle administration. Black symbols indicate significant differences compared with oxycodone alone (circles), and asterisks indicate significant differences compared with vehicle. Data for other behaviors from Table 1 were not statistically significant and are not presented. Table 3 shows statistical outcomes from the overall analyses for administration of oxycodone mixed with each KOR agonists. Main effects in each column are first shown for oxycodone dose and then shown for KOR agonist dose. Analyses for vomit/retch are not shown, because this behavior was only observed in some cases with oxycodone mixed with nalfurafine and did not occur with other oxycodone + KOR agonist mixtures.
Figure 3. Dose-Response Determinations for Oxycodone Alone and Mixed with each KOR Agonist.
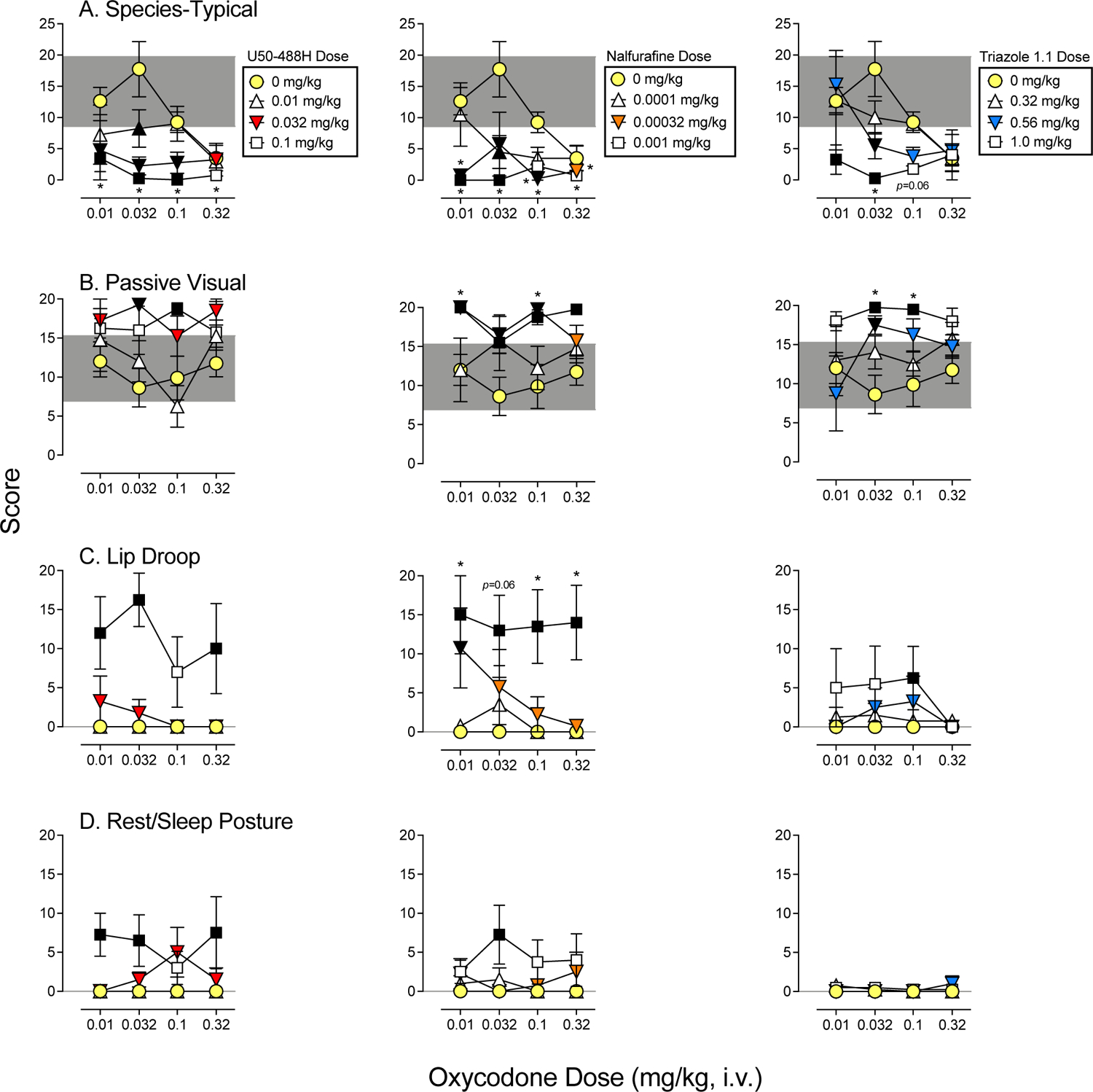
Mean (+SEM) score following administration of oxycodone (circles), oxycodone mixed with three doses of U50–488H (left column), nalfurafine (middle column), and triazole 1.1 (right column). All sessions lasted 5 min and began 10 min following vehicle or drug administration. Scores are shown in separate panels for species-typical (A), passive visual (B), lip droop (C), and rest/sleep posture (D). The shaded area represents the mean (+/−SEM) following vehicle administration. Asterisks represent oxycodone doses and drug mixtures that were statistically different from vehicle (p’s<0.05), and black symbols represent mixtures that were statistically different from oxycodone alone at the corresponding oxycodone dose. Note: oxycodone alone and vehicle represent the same data in Figures 1–3.
Table 3.
Statistical outcomes from overall analyses. Values in bold-face font met statistical significance at p<0.05
| Dose-Response Determinations of Drug Mixtures: Main Effects Oxycodone (first) or KOR Agonist (second) Dose | ||||||
|---|---|---|---|---|---|---|
| Drug | Scratch | Facial Rub | Species-Typical | Passive Visual | Lip Droop | Rest/Sleep Posture |
| Oxycodone + U50–488H |
F(3,9)=12.4, p<0.05 F(3,9)=83.8, p<0.05 |
F(3,9)=5.1, p<0.05 F(3,9)=3.0, p=0.09 |
F(3,9)=1.7, p=0.24 F(3,9)=8.2, p<0.05 |
F(3,9)=0.9, p=0.48 F(3,9)=8.7, p<0.05 |
F(3,9)=1.1, p=0.38 F(3,9)=35.4, p<0.05 |
F(3,9)=0.04, p=0.99 F(3,9)=6.7, p<0.05 |
| Oxycodone + Nalfurafine |
F(3,9)=14.6, p<0.05 F(3,9)=47.0, p<0.05 |
F(3,9)=3.7, p=0.06 F(3,9)=2.3, p=0.14 |
F(3,9)=3.0, p=0.09 F(3,9)=14.6, p<0.05 |
F(3,9)=0.4, p=0.78 F(3,9)=19.8, p<0.05 |
F(3,9)=1.6, p=0.25 F(3,9)=10.4, p<0.05 |
F(3,9)=0.3, p=0.99 F(3,9)=4.4, p<0.05 |
| Oxycodone + Triazole 1.1 |
F(3,9)=20.4, p<0.05 F(3,9)=32.8, p<0.05 |
F(3,9)=8.4, p<0.05 F(3,9)=1.4, p=0.31 |
F(3,9)=1.7, p=0.24 F(3,9)=10.3, p<0.05 |
F(3,9)=0.3, p=0.80 F(3,9)=12.9, p<0.05 |
F(3,9)=1.3, p=0.34 F(3,9)=1.2, p=0.35 |
F(3,9)=0.4, p=0.76 F(3,9)=0.8, p=0.54 |
All oxycodone and KOR agonist mixtures significantly reduced oxycodone-induced scratch (Figure 2, A panels), indicated by a downward shift in the oxycodone dose-response determinations as a function of increasing KOR agonist dose. For U50–488H and nalfurafine, the two larger doses tested, and for triazole 1.1, the largest dose tested significantly reduced scratching compared with oxycodone alone at all oxycodone doses and to levels that were not significantly different from vehicle in many cases (Figure 2, black symbols without asterisks). As described above for the single drugs, oxycodone increased facial rub, and this general pattern persisted when oxycodone was mixed with each KOR agonist (Figure 2, B panels; note the different y-axes for facial rub). However, at least one dose of each KOR agonist reduced facial rub when mixed with 0.1 mg/kg of oxycodone. Vomit/retch was rarely or never observed when oxycodone was mixed with U50–488H or triazole 1.1, but some vomit/retch did occur when oxycodone was mixed with nalfurafine (Figure 2, C panels; note the different y-axes for vomit/retch). Specifically, 0.032 mg/kg of oxycodone mixed with 0.00032 mg/kg of nalfurafine resulted in vomit/retch that was significantly greater compared with oxycodone alone.
All oxycodone and KOR agonist mixtures significantly reduced species-typical behavior (Figure 3, A panels), indicated by a downward shift in the oxycodone dose-response determinations as a function of increasing KOR agonist dose. Reductions in species-typical behavior compared with vehicle (asterisks) or compared with oxycodone alone (black symbols) were most pronounced following administration of the largest KOR agonist doses tested, but some reductions also occurred with the middle and lower doses tested with U50–488H and nalfurafine, and with the middle dose of triazole 1.1. Reductions in species-typical behavior were accompanied by increases in passive visual, and for U50–488H and nalfurafine, were also accompanied by increases in lip droop and rest/sleep posture. For U50–488H combined with oxycodone, passive visual was significantly increased compared with oxycodone alone at 0.032 mg/kg oxycodone + 0.032 mg/kg U50–488H and at 0.1 mg/kg oxycodone + 0.1 mg/kg U50–488H. Lip droop and rest/sleep posture also were significantly greater when the largest U50–488H dose tested (0.1 mg/kg) was mixed with 0.01, 0.032, and 0.32 mg/kg of oxycodone compared with the respective doses of oxycodone alone. Likewise, nalfurafine combined with oxycodone increased in passive visual behavior at all oxycodone doses mixed with 0.001 mg/kg of nalfurafine and at 0.01, 0.032, and 0.1 mg/kg of oxycodone mixed with 0.00032 mg/kg of nalfurafine compared with oxycodone alone. When compared to vehicle, passive visual was significantly increased following 0.01 mg/kg oxycodone + 0.00032 mg/kg nalfurafine and following 0.1 mg/kg oxycodone + 0.00032 mg/kg nalfurafine. For lip droop, the largest dose of nalfurafine tested (0.001 mg/kg) combined with all oxycodone doses resulted in significant, or in one case nearly significant (p=0.06), increases compared with vehicle and compared with oxycodone alone. A significant increase in lip droop also occurred with 0.01 mg/kg oxycodone + 0.00032 mg/kg nalfurafine compared with oxycodone alone. Conversely, only oxycodone 0.032 mg/kg + nalfurafine 0.001 mg/kg significantly increased rest/sleep posture compared with oxycodone alone. Finally, triazole 1.1 mixed with oxycodone also resulted in increases in passive visual behavior at the largest dose tested (1.0 mg/kg) combined with 0.032 and 0.1 mg/kg of oxycodone compared to oxycodone alone and compared to vehicle. Oxycodone (0.032 mg/kg) mixed with triazole 1.1 (0.56 mg/kg) also increased passive visual compared with oxycodone alone. Unlike U50–488H and nalfurafine, triazole 1.1 resulted in less lip droop and almost no rest/sleep posture when it was combined with oxycodone alone. The only significant effect occurred with 1.0 mg/kg triazole 1.1 + 0.1 mg/kg oxycodone, where lip droop was increased following administration of the combination compared with oxycodone alone.
3.3. Time-course assessments of triazole 1.1.
Figure 4 shows average scores for time-course assessments of triazole 1.1 for scratch (A), facial rub (B), species-typical behavior (C), passive visual (D), lip droop (E), and rest/sleep posture (F) for each condition: saline time course + saline (10 min; circles), saline time course + oxycodone (10 min; upward triangles), triazole 1.1 time course + saline (10 min; downward triangles), and triazole 1.1 time course + oxycodone (10 min; squares). Data for other behaviors from Table 1 were not statistically significant and are not presented. Table 4 presents statistical outcomes from the overall analyses for main effects of time point, main effects of condition, and for the interaction for each of these behaviors.
Figure 4. Time-Course Assessments of Triazole 1.1.
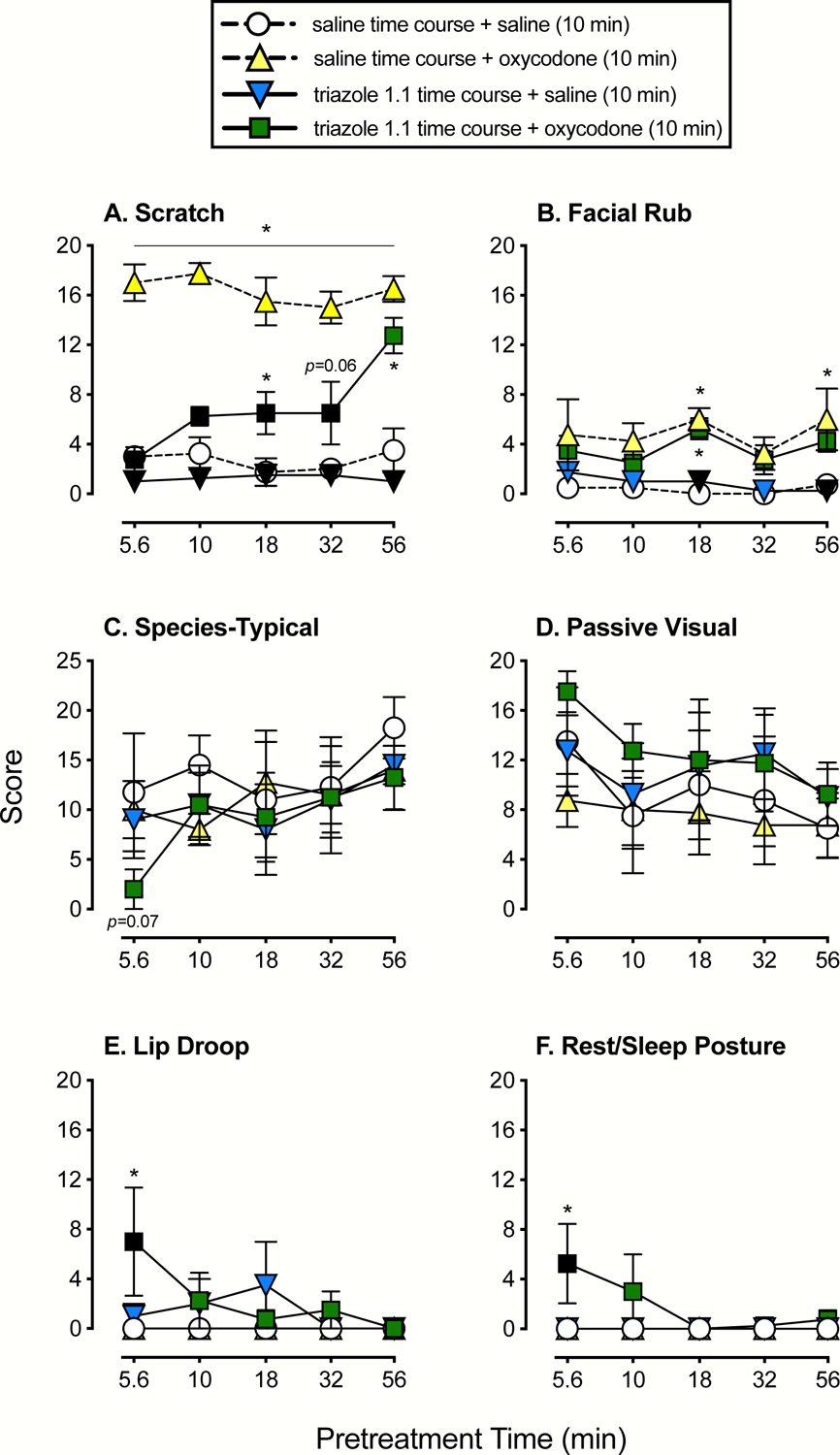
Mean (+SEM) score following administration of saline at various time points + saline administered 10 min prior to the start of the session (circles), saline at various time points + 0.1 mg/kg of oxycodone administered 10 min prior to the session start (upward triangles), 1.0 mg/kg of triazole 1.1 at various time points + saline administered 10 min prior to the session start (downward triangles), and 1.0 mg/kg of triazole 1.1 at various time points + 0.1 mg/kg of oxycodone administered 10 min prior to the session start (squares). Scores are shown in separate panels for scratch (A), facial rub (B), species-typical (C), passive visual (D), lip droop (E), and rest/sleep posture (F). Asterisks represent time points that were statistically different from saline time course + saline (10 min) or the vehicle condition (p’s<0.05), and black symbols represent time points that were statistically different from saline time course + oxycodone (10 min) or the oxycodone alone condition.
Table 4.
Statistical outcomes from overall analyses. Values in bold-face font met statistical significance at p<0.05
| Time-Course Assessments of Triazole 1.1 | ||||||
|---|---|---|---|---|---|---|
| Statistic | Scratch | Facial Rub | Species-Typical | Passive Visual | Lip Droop | Rest/Sleep Posture |
| Main Effect Time Point | F(4,12)=6.3, p<0.05 | F(4,12)=2.1, p=0.14 | F(4,12)=3.5, p<0.05 | F(4,12)=2.9, p=0.07 | F(4,12)=1.7, p=0.21 | F(4,12)=1.5, p=0.26 |
| Main Effect Condition | F(3,9)=205.6, p<0.05 | F(3,9)=26.1, p<0.05 | F(3,9)=0.4, p=0.78 | F(3,9)=0.7, p=0.57 | F(3,9)=1.6, p=0.26 | F(3,9)=2.6, p=0.12 |
| Time Point X Condition Interaction | F(12,36)=2.7, p<0.05 | F(12,36)=0.5, p=0.93 | F(12,36)=0.7, p=0.75 | F(12,36)=0.3, p=0.98 | F(12,36)=1.9, p=0.07 | F(12,36)=1.5, p=0.16 |
For scratch (Figure 4, panel A), when saline was administered at various timepoints and 0.1 mg/kg of oxycodone was always administered 10 min prior to the start of the session (upward triangles; hereafter referred to as oxycodone alone), scratch was significantly increased at all timepoints compared to the saline time course + saline administered 10 min prior to the start of the session (circles; hereafter referred to as vehicle alone). Similarly, when 1.0 mg/kg of triazole 1.1 was administered at various timepoints and saline was always administered 10 min prior to the start of the session (downward triangles; hereafter referred to as triazole 1.1. alone), scratching was significantly lower than oxycodone alone, and was similar to vehicle alone. Finally, when 1.0 mg/kg of triazole 1.1 was administered at various timepoints and 0.1 mg/kg of oxycodone was always administered 10 min prior to the start of the session (squares), oxycodone induced scratching was blocked or reduced at all timepoints except 56 min. When triazole 1.1 was administered at 5.6 and 10 min and oxycodone was administered at 10 min, scratching was significantly lower than oxycodone alone and was not statistically different from vehicle alone. At 18 and 32 minutes, triazole 1.1 continued to significantly reduce oxycodone-induced scratching, however, this behavior began to trend upward and also was significantly higher compared with vehicle alone. Finally, at 56 minutes, the effects of triazole 1.1 on oxycodone-induced scratching were no longer apparent. Scratch reached levels that were not significantly different from oxycodone alone but were significantly greater compared with vehicle alone.
Oxycodone resulted in some increases in facial rub (Figure 4, panel B) that were significantly different than vehicle at 18 and 56 minutes. The same general pattern was observed when triazole 1.1 was administered at various time points and oxycodone was administered 10 min prior to the start of the session, and only the 18-min time point resulted in a significant increase in facial rub compared with vehicle. Conversely, facial rub remained low and similar to vehicle alone following administration of triazole 1.1 across time points; when compared with oxycodone alone, significant differences were obtained at 18 and 56 minutes. Species-typical (Figure 4, panel C) and passive visual (Figure 4, panel D) behaviors were variable and largely unaffected by any of the treatments. There was, however, an overall main effect of time point with species-typical behavior, and when 1.0 mg/kg of triazole 1.1 was administered at 5.6 min plus 0.1 mg/kg of oxycodone at 10 min, there was a trend towards a significant reduction in species-typical behaviors compared with vehicle alone (p=0.07). For lip droop (Figure 4, panel E) and rest/sleep posture (Figure 4, panel F), none of the overall analyses were significant. Again, at the 5.6-min time point following administration of 1.0 mg/kg of triazole 1.1 and 0.1 mg/kg of oxycodone administered 10 min prior to the session, lip droop and rest/sleep posture were significantly increased compared with oxycodone or vehicle alone. No other conditions or time points resulted in statistically significant outcomes.
4. Discussion
The current study expands on our previous work studying the observable behavioral effects of KOR agonists, alone and combined with oxycodone administration, in male rhesus monkeys (Huskinson et al., 2020). The new findings from the current dataset were obtained by expanding the dose ranges for triazole 1.1 relative to what was tested in Huskinson et al. (2020), testing all oxycodone doses in combination with all KOR agonist doses, by conducting a time-course assessment with a dose of triazole 1.1 that is behaviorally active when delivered alone, and conducting a time-course assessment of triazole 1.1’s effects on oxycodone-induced scratch (and other behaviors). Overall, triazole 1.1 produced some, but not all, of the effects that were observed after administration of the KOR agonists, U50–488H and nalfurafine. In cases where effects were observed, they were generally of lesser magnitude for triazole 1.1 relative to the other KOR agonists, particularly for lip droop and rest/sleep posture. This trend held when the KOR agonists were co-administered with oxycodone. A time-course assessment for triazole 1.1 as a blocker of oxycodone-induced scratch indicated a relatively short duration of effect (approximately 30 min) that is consistent with what has been reported for triazole 1.1 as an antinociceptive compound in mice (Brust et al., 2016).
Dose-response comparisons of the KOR agonists alone rendered outcomes that were largely consistent with our previous report (Huskinson et al., 2020). U50–488H and nalfurafine produced sedation-like and motor-impairing effects when administered alone. Both decreased species-typical behavior, U50–488H increased lip droop, and nalfurafine increased rest/sleep posture. Similarly, triazole 1.1 dose-dependently decreased species-typical behavior, an effect that was not obtained previously with a lower dose range (Huskinson et al., 2020). However, triazole 1.1 administration did not produce lip droop or rest/sleep posture, behaviors that serve as proxies for muscle relaxation and sedation, respectively. This apparent lack of sedation-like effects for triazole 1.1 is consistent with previous findings in mice (Brust et al., 2016), though trends in lip droop and rest/sleep posture at the highest dose tested for triazole 1.1 do suggest that these KOR-typical effects may occur at higher doses.
Oxycodone, when administered alone, dose-dependently increased scratch and facial rub and reduced species-typical behavior. Previously, when a single oxycodone dose (0.1 mg/kg) was tested as a mixture with select KOR agonist doses (Huskinson et al., 2020), oxycodone mixed with U50–488H, nalfurafine, or triazole 1.1 resulted in significant reductions in oxycodone-induced scratching, which is consistent with previous reports studying KOR agonists as potential anti-pruritic agents (Beck et al., 2019; Inui, 2012; Ko and Husbands, 2009; Wakasa et al., 2004). In the current report, all KOR agonists reduced oxycodone-induced scratching and, for lower doses of oxycodone, were able to do so at KOR-agonist doses that did not produce behavior-disrupting or sedation-like effects on their own. At the highest oxycodone dose (0.32 mg/kg), where scratching frequency appeared to be the most difficult to diminish, all KOR agonists reduced scratching to control levels in a dose-dependent manner. However, the doses of U50–488H and triazole 1.1 required to produce this effect (0.032 and 1.0 mg/kg, respectively) caused minimal changes to species-typical behavior when combined with any oxycodone dose. Conversely, the smallest dose of nalfurafine (0.00032 mg/kg) required to reduce scratching when combined with the highest oxycodone dose reduced species-typical behavior when combined with all oxycodone doses. Similar findings were observed with the proxy measure for muscle relaxation (lip droop). Specifically, the dose of nalfurafine required to reverse scratching with the highest oxycodone dose produced robust increases in lip droop across all oxycodone doses. In contrast, the functionally-matched doses of U50–488H (0.032 mg/kg) and triazole 1.1 (1.0 mg/kg) produced minimal effects on lip droop across oxycodone doses. Effects on rest/sleep posture were minimal for all KOR agonists, with nalfurafine being the only KOR agonist to produce rest/sleep posture at the functionally-matched dose, and that only occurred with one dose of oxycodone in the middle of the range. Thus, when the doses of the KOR agonists were functionally matched according to their ability to reverse scratching at the highest oxycodone dose, G-protein bias did not predict outcomes on these “side effect” measures.
In time-course determinations, 1.0 mg/kg of triazole 1.1 administered alone produced no significant effects on any measured behavior at any timepoint relative to vehicle. However, triazole 1.1 reduced oxycodone-induced scratching to levels similar to vehicle at 5.6 and 10 min following triazole 1.1 administration and to levels significantly lower than oxycodone but greater than vehicle at 18 and 32 min. By 56 min following triazole 1.1 administration, oxycodone-induced scratching returned to levels similar to oxycodone alone. When taken together with previous tests of antinociception in mice (Brust et al., 2016), the duration of behavioral effects for triazole 1.1 appear to be less than one hour and similar in rodents and NHPs.
5. Conclusions
The observations from this report indicate that the relative potency of triazole 1.1 to produce anti-pruritic effects relative to behavior-disrupting and sedative-like effects is higher than nalfurafine but comparable to U50–488H. Thus, in this case, G-protein bias does not appear to predict therapeutic “windows” relating anti-pruritic effects to behavioral disruption and sedation-like effects. It should be noted, however, that the chief side effects of KOR agonists (i.e., dysphoria, psychotomimesis) were not modeled in this report. These effects are known to occur with prototypical KOR agonists (Mores et al., 2019; Pfeiffer et al., 1986) and are absent with nalfurafine in humans (Inui, 2012, 2015) and purportedly absent with triazole 1.1 in mice (Brust et al., 2016). Moreover, other therapeutic effects of MOR/KOR-agonist combinations are antinociception and abuse-deterrence (Zamarripa et al., 2020; Zamarripa et al., 2021), and the potency relations described here with behavioral observations may not be quantitatively similar to the potency relations obtained in antinociception and self-administration assays. That is, the ordering of therapeutic windows for the KOR agonists may change depending on the therapeutic and adverse effects being compared. Triazole 1.1 has yet to be tested in more naturalistic models of pain (e.g., neuropathy, injury), and conclusions about its potential as an analgesic (either alone or as a combination with MOR agonists) will require testing with more translational models. In general, triazole 1.1 produced a milder side-effect profile compared with U50–488h and nalfurafine, both alone and when combined with oxycodone and this warrants future studies with triazole 1.1, and compounds with similar profiles, in a wider array of assays.
Acknowledgements
The authors would like to thank Josh Woods, Kandace Farmer, Jessica Howard, Jemma Cook, Lais Berro, Tanya Pareek, and Zachary Smith for their technical assistance. Morgan Brasfield is now at William Carey University in Hattiesburg, MS 39401. This work was supported by the National Institute on Drug Abuse [DA039167 to K.B.F.; DA018151 to T.E.P; DA045011 to S.L.H; DA048586 to C.A.Z.] and the National Institute on Alcohol Abuse and Alcoholism [AA029023 to D.M.P.].
References
- Aldrich JV, McLaughlin JP, 2009. Peptide kappa opioid receptor ligands: potential for drug development. Aaps j 11(2), 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck TC, Hapstack MA, Beck KR, Dix TA, 2019. Therapeutic Potential of Kappa Opioid Agonists. Pharmaceuticals (Basel) 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedini A, Di Cesare Mannelli L, Micheli L, Baiula M, Vaca G, De Marco R, Gentilucci L, Ghelardini C, Spampinato S, 2020. Functional Selectivity and Antinociceptive Effects of a Novel KOPr Agonist. Front Pharmacol 11, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM, 2016. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9(456), ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ, 2009. Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in nonhuman primates: fast onset and entry into cerebrospinal fluid. J Pharmacol Exp Ther 328(2), 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Prisinzano TE, Kreek MJ, 2010. The discriminative effects of the kappa-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology (Berl) 210(2), 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Yadav PN, 2015. Biased agonism at kappa opioid receptors: Implication in pain and mood disorders. Eur J Pharmacol 763(Pt B), 184–190. [DOI] [PubMed] [Google Scholar]
- Dunn AD, Reed B, Erazo J, Ben-Ezra A, Kreek MJ, 2019. Signaling Properties of Structurally Diverse Kappa Opioid Receptor Ligands: Toward in Vitro Models of in Vivo Responses. ACS Chem Neurosci 10(8), 3590–3600. [DOI] [PubMed] [Google Scholar]
- Dunn AD, Reed B, Guariglia C, Dunn AM, Hillman JM, Kreek MJ, 2018. Structurally Related Kappa Opioid Receptor Agonists with Substantial Differential Signaling Bias: Neuroendocrine and Behavioral Effects in C57BL6 Mice. Int J Neuropsychopharmacol 21(9), 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH, 1987. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther 242(2), 413–420. [PubMed] [Google Scholar]
- Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, Freeman KB, 2020. Quantification of observable behaviors induced by typical and atypical kappa-opioid receptor agonists in male rhesus monkeys. Psychopharmacology (Berl) 237(7), 2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S, 2012. Nalfurafine hydrochloride for the treatment of pruritus. Expert Opin Pharmacother 13(10), 1507–1513. [DOI] [PubMed] [Google Scholar]
- Inui S, 2015. Nalfurafine hydrochloride to treat pruritus: a review. Clin Cosmet Investig Dermatol 8, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaski SW, White AN, Gross JD, Trexler KR, Wix K, Harland AA, Prisinzano TE, Aube J, Kinsey SG, Kenakin T, Siderovski DP, Setola V, 2019. Preclinical Testing of Nalfurafine as an Opioid-sparing Adjuvant that Potentiates Analgesia by the Mu Opioid Receptor-targeting Agonist Morphine. J Pharmacol Exp Ther 371(2), 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE, 2010. Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 210(2), 109–119. [DOI] [PubMed] [Google Scholar]
- Ko MC, Husbands SM, 2009. Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther 328(1), 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mores KL, Cummins BR, Cassell RJ, van Rijn RM, 2019. A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists. Front Pharmacol 10, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM, 1986. Psychotomimesis mediated by kappa opiate receptors. Science 233(4765), 774–776. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Tidgewell K, Harding WW, 2005. Kappa opioids as potential treatments for stimulant dependence. Aaps j 7(3), E592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M, Eans SO, Ganno ML, Lantero A, Mairegger M, Toll L, Schmidhammer H, McLaughlin JP, 2017. Selective kappa receptor partial agonist HS666 produces potent antinociception without inducing aversion after i.c.v. administration in mice. Br J Pharmacol 174(15), 2444–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasa Y, Fujiwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, Nagase H, 2004. Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life sciences 75(24), 2947–2957. [DOI] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL, 2015. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther 352(1), 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Naylor JE, Huskinson SL, Townsend EA, Prisinzano TE, Freeman KB, 2020. Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psychopharmacology (Berl) 237(5), 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Pareek T, Schrock HM, Prisinzano TE, Blough BE, Sufka KJ, Freeman KB, 2021. The kappa-opioid receptor agonist, triazole 1.1, reduces oxycodone self-administration and enhances oxycodone-induced thermal antinociception in male rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM, 2013. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem 288(51), 36703–36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Freeman K, Setola V, Cao D, Kaski S, Kreek MJ, Liu-Chen LY, 2022. Preclinical Studies on Nalfurafine (TRK-820), a Clinically Used KOR Agonist. Handb Exp Pharmacol 271, 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


