Abstract
NK lysin is a 9-kDa polypeptide that was originally isolated from porcine intestinal tissue based on its antibacterial activity. It is produced by cytolytic lymphocytes and is cytolytic against a number of different types of tumor cells. Here we report the binding of NK lysin to lipopolysaccharide (LPS) and its anti-LPS activity. NK lysin binds to matrix-coated LPS from Escherichia coli, Pseudomonas aeruginosa, and different strains of Salmonella enterica. Lipid A and polymyxin B inhibited the binding, demonstrating a preferential interaction of NK lysin with the lipid part of LPS. Chromium-labeled lymphoma cells were lysed by NK lysin, and LPS dose-dependently inhibited the cytolysis at equimolar amounts. In the same manner, NK lysin inhibited certain LPS-stimulated effects on mouse bone marrow cells as well as LPS binding to mouse granulocytes. These results suggest that NK lysin may be a another natural LPS-binding protein from lymphocytes that may participate in the endogenous defense response associated with elevated concentrations of LPS.
Gram-negative bacterial infections can result in severe pathological changes, including fever, hypotension, shock, disseminated intravascular coagulation, multisystem organ failure, and death (11). The outer membranes of gram-negative bacteria contain a glycolipid lipopolysaccharide (LPS) or endotoxin (29) which when released into the circulation triggers a cascade of host-effector events. The release of effector molecules, notably tumor necrosis factor alpha (TNF-α), is thought to mediate the lethal effects of endotoxemia (34).
Two homologous LPS-binding proteins that regulate the biological activity of LPS in mammals have been characterized. LPS-binding protein (LBP) is produced by hepatocytes and enhances the inflammatory response to LPS (33). LPS complexed to LBP binds to the cell surface protein CD14 and stimulates various monocyte responses (40, 41) more potently than LPS alone. Other receptors for LPS have also been proposed (10, 38). In contrast, bactericidal/permeability-increasing protein, an antibacterial protein produced by neutrophils, neutralizes the effects of LPS (8).
Porcine T and NK cells produce a cationic polypeptide, NK lysin (NKL), that most likely is involved in the lytic machinery of cytolytic lymphocytes (2). It was isolated from intestinal tissue, and the peptide kills certain gram-negative bacteria. Direct antimicrobial activity has been noticed for NK and CTL cells (19), and NKL may be part of this mechanism. NKL also lyses certain tumor cells but not erythrocytes. Human cytolytic lymphocytes produce a counterpart to NKL, granulysin (27), that recently was shown to have antibacterial activity (36). Both peptides can be released after cell stimulation (3, 27), which suggests possible extracellular functions. The peptides have a motif in common that also is found in saposin-like proteins (SAPLIP) (1, 24). These proteins conduct a variety of functions associated with the binding or interaction of lipids. The family includes, among others, galactosylceramide and glucosylceramide-binding peptides called saposins and a lipase, acyloxyacyl hydrolase, that deacylates bacterial LPS. The structure of NKL was recently determined by nuclear magnetic resonance and represents the first model member of this family (20). The peptide folds in a compact structure that is composed of five amphipathic alpha-helices placed around a hydrophobic cavity. Membranotropic activities for NKL in artificial liposomes have been demonstrated (31) and even if the mechanism of bacterial killing is not fully understood, binding to membrane components and disruption of membrane integrity are likely to be important.
Identifications of endogenous molecules that bind to and regulate LPS activity are of clinical relevance. Recent approaches to neutralize LPS toxicity have explored the use of peptides that bind to the lipid A part of it. These include CAP-18 (17), MBI-27 and -28 (12), BPI (22), and synthetic peptides from the Limulus antilipopolysaccharide factor (30). Although many substances bind to lipid A, only a few antiendotoxin agents have been identified, and the clinical use of them can be limited by their toxicity (12, 13, 37). Other endotoxin-neutralizing strategies include the use of antibodies towards LPS and other downstream factors responsible for the outcome of sepsis (11). In this report, we show that NKL binds to lipid A, the more conserved anionic glycolipid region of LPSs, and that some effects of LPS can be neutralized.
MATERIALS AND METHODS
LPS binding assay: NKL binding to immobilized LPS.
Microtiter plates (96 well; CEB) were coated at 4 μg/ml (50 μl) with different LPSs in phosphate-buffered saline (PBS) (pH 7.4) for 2 h at 37°C. The materials used were Escherichia coli O111:B4 LPS and Salmonella enterica serovar Typhimurium LPS (Difco), serovar Abortus equi LPS and polysaccharides (PSs), serovar Paratyphi A LPS and PS, and serovar Riogrande LPS and PS (R. Girard and G. Bordenave). The PSs were obtained by acid hydrolysis of the corresponding LPSs as described by A. M. Staub (35). Pseudomonas aeruginosa was from Sigma. Milk powder-coated wells were included on each plate to determine nonspecific binding. Plates were washed three times in pyrogen-free PBS containing 0.05% Tween 20 (Sigma). Assay plates were blocked for 4 h at room temperature with 5% milk powder in PBS (or overnight at 4°C). NKL samples were diluted in PBS–1% bovine serum albumin (fraction V; ICN) and when indicated, various concentrations of LPS, polysaccharides, polymyxin B (Sigma), or lipid A (Diphosphoryl; S. enterica serovar Minnesota Re-595 [Sigma]) were added. Plates were incubated at 4°C overnight, washed three times in washing buffer, and then developed with polyclonal rabbit anti-NKL immunoglobulin G (IgG) (2) coupled to biotin (d-biotinoyl-ɛ-amidocaproic acid; Boehringer Mannheim, GmbH) at 1:5,000 (4 h at room temperature) followed by streptavidin-peroxidase (horseradish peroxidase; Southern Biotech Association Inc.) at 1:3,000 (1 h at room temperature). The binding was developed by o-phenylenediamine dihydrochloride-H2O2 (Sigma), and absorbances were read at 492 nm on a microplate reader (Multiscan MS; Labsystem).
Fluorescence experiments were carried out in PBS at 25°C with a Shimadzu RF-510LC fluorescence spectromonitor (7). Monodansylcadaverine (Sigma) was dissolved in MeOH and diluted to 50 μM (final concentration). LPS (E. coli O111:B4) was dissolved at 2 mg/ml and added to a final concentration of 5 μM. NKL was added from a stock solution to a final concentration between 0.01 and 10 μM. Samples were incubated for 30 min and excited at 340 nm. Emission was read at 525 nm with a bandpass of 5 nm. The concentration of NKL was determined by amino acid analysis.
NKL cytotoxicity assay.
EL4 mouse lymphoma cells were maintained in RPMI 1640 (BIO Whittaker) supplemented with 2 mM glutamine, 1 mM pyruvate, 10 mM HEPES (Gibco), 10% fetal calf serum, and antibiotics (100 IU of penicillin/ml and 100 mg of streptomycin/ml). The medium was routinely changed twice a week. For cytotoxicity studies, cells were centrifuged and taken up in fresh medium at 5 × 106 cells/ml. Cells (400 μl) were mixed with 100 μl of Na2–51CrO4 (2 mCi/ml) and incubated at 37°C for 1 h. Labeled cells were washed three times in a medium containing 3% fetal calf serum and resuspended at 0.4 × 106 cells/ml in PBS. In an assay, microtiter wells (96-well U-form microtiter plates; Costar) were loaded with 10 μl of NKL in PBS and 50 μl of PBS plus the indicated additives. The incubation was started by adding 50 μl of cells, giving a total of 20,000 cells/well. Plates were incubated at 37°C for 2 h (in 5% CO2 in air) and centrifuged for 4 min at 100 × g. Assays were run in triplicate, 75 μl was removed, and counts were determined.
LPS activity assay.
LPS activity was assayed as previously described (10). Briefly, incubation of LPS (10 ng/ml) with fresh mouse bone marrow cells for 18 h upregulates LPS binding sites on granulocytes. It has been suggested that LPS binding sites on bone marrow cells and granulocytes represent distinct subpopulations of receptors in each case. Different concentrations of NKL were coincubated with LPS during the stimulation of bone marrow cells. The cells were washed at the end of the incubation period and then incubated with LPS-fluorescein isothiocyanate (FITC) (0.25 μg/ml). The amount of LPS-FITC binding sites was measured in the granulocyte gated fraction with a fluorescence-activated cell sorter (FACS) flow cytometer (FACScan; Becton-Dickinson, Mountain View, Calif.) and Cell Quest software. In a separate experiment, NKL was premixed with LPS-FITC before addition to bone marrow cells stimulated by LPS without NKL.
LPS-induced sepsis.
Galactosamine (0.2 ml; 90 mg/ml) was injected intraperitoneally (i.p.) into C3H/HeOU mice (IFFA-Credo) (9). Within 1 h, 0.2 ml of samples was injected intravenously (i.v.), and the number of dead mice was recorded after 72 h. LPS (50 ng; S. enterica serovar Typhimurium) was preincubated in the presence or absence of NKL (1 μg) for 30 min at 37°C before injection.
RESULTS
NKL binding to LPS.
Microtiter wells coated with E. coli O111:B4 or S. enterica serovar Typhimurium LPS bound NKL in a dose-dependent manner (Fig. 1A). Plates coated with polyclonal NKL antisera were used as a positive control and background levels of NKL binding were kept low by using milk powder as a blocking agent. Coating of microtiter wells with different strains of S. enterica LPS (e.g., serovars Abortus equi, Paratyphi A, and Riogrande) or P. aeruginosa LPS gave similar results (data not shown). The binding of NKL to E. coli LPS was inhibited when LPS was preincubated with polymyxin B before coating the wells or if NKL was preincubated with LPS before addition to LPS-coated wells. This shows that the binding of NKL to microtiter wells is mediated through LPS (Fig. 1B). To define the site of LPS interaction, NKL was preincubated with lipid A or different polysaccharides generated from S. enterica strains before addition to LPS-coated wells (Fig. 1C). Results show that lipid A abolishes the binding of NKL to LPS, while polysaccharides have only a low inhibitory effect on NKL binding to LPS. This suggests that lipid A is responsible for the major part of the LPS-NKL interaction.
FIG. 1.
NKL binding to LPS immobilized on microtiter plates. After coating and blocking nonspecific residual binding, plates were incubated with NKL plus additives overnight at 4°C. Plates were washed, and bound NKL was developed by incubating with a polyclonal anti-NKL IgG conjugated with biotin and visualized by streptavidin-peroxidase–o-phenylenediamine. (A) Plates were coated with anti-NKL IgG (•), E. coli LPS (■), S. enterica serovar Typhimurium LPS (□), or blocking solution only (○). (B) Plates were coated with E. coli LPS (□, •, ■) or E. coli LPS premixed with polymyxin B (▵). NKL was thereafter incubated either alone (□) or in the presence of 1 μg (•) or 10 μg (■) of E. coli LPS per ml. (C) Plates were coated with E. coli LPS, and NKL was incubated in the presence of lipid A (■) or polysaccharides from S. enterica serovar Abortus equi (▵), S. enterica serovar Riogrande (▴), or S. enterica serovar Paratyphi A (○). Data are means ± standard deviations of triplicate values. O.D., optical density.
Dansylcadaverine (DC) has previously been used as a fluorescence displacement probe to obtain relative affinities of LPS-binding substances (7). The addition of LPS to a solution of DC results in an enhancement in the emission spectrum of DC. Addition of NKL displaces bound DC, leading to a quenching of fluorescence. The displacement curve was analyzed as a function of added ligand concentration by using a four-parameter logistic equation and the ALLFIT program (25). The 50% infective dose value computed for NKL was 0.7 μM.
LPS inhibition of NKL cytolysis.
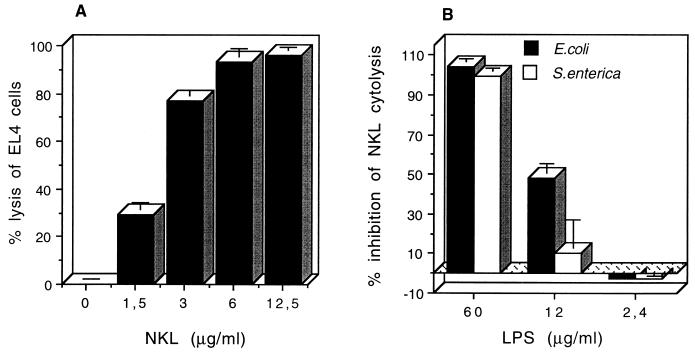
NKL is a cytolytic peptide against mouse lymphoma EL4 cells. 51Cr-labeled EL4 cells were dose-dependently lysed with NKL (Fig. 2A), and 100% lysis was achieved at approximately 10 μg of peptide/ml. LPS dose-dependently inhibited the cytolysis of 12.5 μg of NKL/ml (Fig. 2B). In this assay, 12 μg of LPS/ml from either E. coli or S. enterica serovar Typhimurium inhibited 15 to 50% of NKL cytolysis, which would correspond to a more than 75% reduction of the NKL concentration. This suggests that inhibition occurs at a 1:1 molar ratio of peptide to LPS, assuming a molecular mass of 10,000 Da for LPS. The presence of up to 5% fetal calf serum or 1% bovine serum albumin did not affect the cytolytic activity of 12.5 μg of NKL/ml, while 10% fetal calf serum reduced the lysis by 20% (not shown).
FIG. 2.
NKL cytolysis of EL4 cells and its inhibition by LPS. Chromium-labeled EL4 cells were seeded in microtiter plates at 20,000 cells/well. Cells were incubated with different concentrations of NKL (A) or with a fixed concentration of 12.5 μg of NKL/ml (B) in the presence of the indicated concentration of E. coli LPS or S. enterica serovar Typhimurium LPS. Data are means ± standard deviations of triplicate values.
Inhibition of LPS activity by NKL.
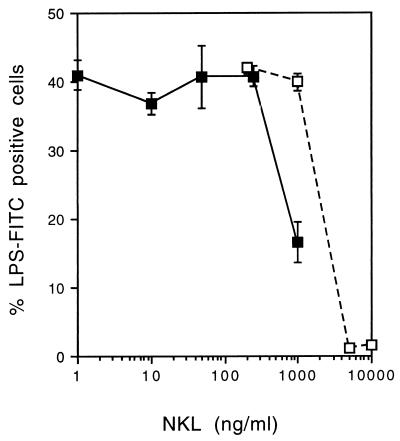
Inhibition in vitro was tested in two ways. After 18 h of incubation with LPS, upregulated LPS binding on granulocytes was measured by flow cytometry. Figure 3 shows that more than 250 ng of NKL/ml is required for inhibition and that 60% inhibition is found with 100× excess of NKL. Alternatively, NKL could directly inhibit the binding of LPS-FITC (0.25 μg/ml) to granulocytes, and a complete inhibition was found with 20× excess of peptide versus LPS (Fig. 3). This also shows that the reduction of upregulated LPS binding sites at 1,000 ng of peptide/ml is not a function of residual NKL inhibiting LPS-FITC binding.
FIG. 3.
Neutralization of LPS binding. In a first experiment (filled squares), bone marrow cells were incubated with unlabeled LPS (10 ng/ml) in the presence of increasing concentrations of NKL. The expression of inducible LPS binding sites was analyzed by FACS after 18 h. In a second experiment (open squares), bone marrow cells already expressing LPS binding sites were incubated with LPS-FITC in the presence of 10% fetal calf serum and different concentrations of NKL. The binding of the labeled LPS was analyzed by FACS. Data are means ± standard deviations.
Inhibition of LPS-induced sepsis.
LPS causes sepsis when injected (i.v. or i.p.) into galactosamine-sensitized mice (9). LPS (500 ng/ml) was mixed with equal volume buffer ± 20× the molar excess by weight of NKL (10 μg/ml) for 30 min at 37°C, and then 50 ng of LPS was injected i.v. into a mouse. Results show that 20% of the mice survived in the group of mice injected with the LPS alone. When NKL was premixed with LPS, LPS-induced death was blocked and 100% of the mice survived (Table 1). Administration of galactosamine alone had no observable effect over a 5-day monitoring period. However, this inactivation of LPS by the NKL peptide was carried out in vitro. To check whether NKL was also able to inactivate LPS in vivo, we injected LPS and NKL (2 μg), separately, in this or in the reverse order, into groups of eight mice which were sensitized with galactosamine. We found that in this model, NKL failed to protect mice from the toxic effects of LPS (data not shown).
TABLE 1.
In vitro NKL interaction with LPS protects galactosamine-treated C3H/HeOU micea
| Amt of NKL (μg/mouse) | Amt of LPS (μg/mouse) | No. of mice dead (% surviving) |
|---|---|---|
| 0 | 0 | 0/5 (100) |
| 0 | 0.05 | 4/5 (20) |
| 1 | 0.05 | 0/5 (100) |
Galactosamine was injected i.p. (18 mg/mouse). Thereafter, a mixture of NKL and S. enterica serovar Typhimurium LPS (0.2 ml; preincubated at 37°C for 30 min) was injected i.v.
DISCUSSION
Porcine cytolytic T and NK cells produce an antibacterial and tumorolytic peptide, NKL, that binds to LPS and inhibits certain LPS responses. Binding to LPS from E. coli, P. aeruginosa, and different strains of S. enterica occurs, indicating that this is not a species-specific effect. We note that NKL binds to LPS from both S. enterica serovar Typhimurium and P. aeruginosa, although none of these bacteria are killed by NKL (2). It appears that if NKL binding to LPS leads to a disordered outer membrane of the bacteria, this is not sufficient for further killing of P. aeruginosa and Salmonella. Polymyxin B has a high affinity (about 0.5 μM) for LPS and lipid A (7, 32) and can be used as a marker of lipid A binding. The binding of NKL to LPS is completely inhibited by polymyxin and also by lipid A itself, suggesting that the NKL interaction involves the lipid A part of LPS (Fig. 1). Displacement of an LPS-binding fluorescence probe by NKL gives an apparent dissociation constant of 0.7 μM for NKL, as computed by the ALLFIT program. The NKL-LPS interaction is less influenced by the carbohydrate moiety. The LPS binding is strong enough to inhibit the cytolytic activity of NKL, and the inhibitory concentrations needed indicate that LPS-NKL binding occurs at around 1:1 molar ratio. Moderate concentrations of fetal calf serum or bovine serum albumin do not effect the cytolytic activity. Thus, although serum factors influence the activity of NKL, it is reasonable to believe that LPS binding is not totally abrogated in biological fluids.
LPS exhibits toxicity through a complex series of responses of host cells, in which the initial events are a cellular stimulation by LPS (11). Lipid A is believed to be a principal mediator of the LPS toxicity (4), and it is a common constituent of gram-negative bacteria. In vitro incubation of LPS and NKL protects against LPS-induced sepsis in galactosamine-sensitized mice. This neutralizing effect of NKL is most likely mediated by its binding to LPS.
To our knowledge, NKL is one of the few cationic peptides produced by lymphocytes that binds to LPS. Many other cationic peptides and proteins that have LPS binding capacity are produced by leukocytes (39), and some of these molecules also have antibiotic activities. This includes BPI (8, 22), CAP37 (28), and CAP18/LL-37 (17). No preferential secondary structure in these sequences is responsible for the binding to LPS. Rather, a common denominator seems to be an amphipathic motif with positively charged residues. Thus, initial LPS interactions are likely to involve electrostatic interactions but LPS binding to the hydrophobic lipid tail may also occur.
NKL is a member of the family of SAPLIP which are thought to have a conserved three-dimensional structure (1, 20). NKL, granulysin, and peptides from the protozoan parasite Entamoeba histolytica (amoebapores) have antibiotic and lytic activities (2, 18, 36) while other peptides and proteins in the family have nonlytic functions. The saposins in the SAPLIP family bind glycolipids, and it has been suggested that each peptide has a hydrophobic pocket that potentially could bind the lipid (26). Acyloxyacyl hydrolase is a lipase that plays a role in LPS detoxification (23). It has two protein subunits of which one subunit contains a SAPLIP domain, and is required for catalysis, suggesting interaction with LPS. The SAPLIP domain might have evolved early in evolution based on lipid binding criteria. Different forms have then diverged to different specific functions.
The physiological relevance of NKL binding to LPS, besides antibiotic activity, is not known. Two proteins have been shown to regulate the binding of LPS to cell surface receptors. LBP stimulates the activity of LPS and facilitates the binding to membrane-bound CD14. Interestingly, it has been shown that LBP, which is important for induction of an inflammatory response to small amounts of LPS, is not important for the clearance of LPS in mice (15). BPI, a 50-kDa protein produced and stored in lysosomal granules in neutrophils (8), has high sequence homology with LBP and inhibits the activity of LPS (38).
LPS stimulates macrophages to release TNF-α, which is a key effector of the inflammatory response. Also, gamma interferon has been shown to be an important regulator (5) and is believed to be a potentiating factor in sepsis (6), possibly by enhancing the macrophage production of TNF-α (16). The main producers of gamma interferon are activated NK and T cells, and recent data suggest that NK cells are the most important source (14). Activated NK or T cells also have elevated levels of NKL (2) which, like granulysin, is localized to secretory granules and released from cells when stimulated (3, 27). Direct antimicrobial activity of NK and CTL cells has been noticed (19), and LPS may directly or indirectly stimulate NK cell activity (21) to produce proteins involved in the cytolytic machinery. It has recently been shown that part of the antimicrobial activity in human CTL cells is mediated by granulysin (36).
In conclusion, NKL is an LPS binding peptide from NK and T cells that may contribute to the defense against bacterial infections and LPS toxicity.
ACKNOWLEDGMENTS
This work was supported by a postdoctoral fellowship from INSERM (to M.A.) and by Karolinska Institutet, Magnus Berwall’s Foundation, and the Swedish Medical Research Council.
REFERENCES
- 1.Andersson M, Curstedt T, Jörnvall H, Johansson J. An amphipathic helical motif common to tumourolytic polypeptide NK-lysin and pulmonary surfactant polypeptide SP-B. FEBS Lett. 1995;362:328–332. doi: 10.1016/0014-5793(95)00268-e. [DOI] [PubMed] [Google Scholar]
- 2.Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Sillard R, Jörnvall H, Mutt V, Olsson B, Wigzell H, Dagerlind Å, Boman G H, Gudmundsson H G. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14:1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, M., and B. Olsson. Unpublished data.
- 4.Brade L, Brandenburg K, Kuhn H M, Kusumoto S, Macher I, Rietschel E, Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect Immun. 1987;55:2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Ague O, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowdery J S, Chace J H, Yi A-K, Krieg A M. Bacterial DNA induces NK cells to produce INF-gamma in vivo and increase the toxicity of lipopolysaccharides. J Immunol. 1997;156:4570–4575. [PubMed] [Google Scholar]
- 7.David S A, Balasubramanian K A, Mathan V I, Balaram P. Analysis of the binding of polymyxin B to endotoxic lipid A and core glycolipid using a fluorescent displacement probe. Biochim Biophys Acta. 1992;1165:147–152. doi: 10.1016/0005-2760(92)90180-4. [DOI] [PubMed] [Google Scholar]
- 8.Elsbach P, Weiss J. Prospects for use of recombinant BPI in the treatment of gram-negative bacterial infections. Infect Agents Dis. 1995;4:102–109. [PubMed] [Google Scholar]
- 9.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard R, Pedron T, Chaby R. Endotoxin-induced expression of endotoxin binding sites on murine bone marrow cells. J Immunol. 1993;150:4504–4513. [PubMed] [Google Scholar]
- 11.Glauser M P, Heumann D, Baumgartner J D, Cohen J. Pathogenesis and potential strategies for prevention and treatment of septic shock: an update. Clin Infect Dis. 1994;18:S205–S216. doi: 10.1093/clinids/18.supplement_2.s205. [DOI] [PubMed] [Google Scholar]
- 12.Gough M, Hancock R E W, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 14.Heremans H, Dillen C, van Damme J, Billiau A. Essential role for natural killer cells in lethal lipopolysaccharide-induced shwartzman-like reaction in mice. Eur J Immunol. 1994;24:1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
- 15.Jack R S, Fan X, Bernheiden M, Rune G, Ehlers M, Weber A, Kirsch G, Mentel R, Furll B, Freudenberg M, Schmitz G, Stelter F, Schutt C. Lipopolysaccharide-binding protein is required to combat a murine Gram-negative bacterial infection. Nature. 1997;389:742–745. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 16.Jin F-Y, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 17.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leippe M. Ancient weapons: NK-lysin is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell. 1995;83:17–18. doi: 10.1016/0092-8674(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 19.Levitz S M, Mathews H L, Murphy J W. Direct antimicrobial activity of T cells. Immunol Today. 1995;16:387–391. doi: 10.1016/0167-5699(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 20.Liepinsh E, Andersson M, Ruysschaert J-M, Otting G. Saposin fold revealed by the NMR structure of NK-lysin. Nat Struct Biol. 1997;4:793–795. doi: 10.1038/nsb1097-793. [DOI] [PubMed] [Google Scholar]
- 21.Lindemann R A. Bacterial activation of human killer cells: role of cell surface lipopolysaccharide. Infect Immun. 1988;56:1301–1308. doi: 10.1128/iai.56.5.1301-1308.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little R G, Kelner D N, Lim E, Burke D J, Conlon P J. Functional domains of recombinant bacterial/permeability increasing protein (rBPI23) J Biol Chem. 1994;269:1865–1872. [PubMed] [Google Scholar]
- 23.Munford R S, Hall C L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 24.Munford R S, Sheppard P O, O’Hara P J. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J Lipid Res. 1995;36:1653–1663. [PubMed] [Google Scholar]
- 25.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien J S, Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991;5:301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- 27.Pena S V, Hanson D A, Carr B A, Goralski T J, Krensky A M. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small lytic, granule proteins. J Immunol. 1997;158:2680–2688. [PubMed] [Google Scholar]
- 28.Pereira H A, Erdem I, Pohl J, Spitznagel J K. Synthetic bacterial peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein. Proc Natl Acad Sci USA. 1993;90:4733–4737. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raetz C R H. Biochemistry of endotoxin. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 30.Ried C, Wahl C, Miethke T, Wellnhofer G, Landgraf C, Schneider-Mergener J, Hoess A. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J Biol Chem. 1996;271:28120–28127. doi: 10.1074/jbc.271.45.28120. [DOI] [PubMed] [Google Scholar]
- 31.Ruysschaert J-M, Goormaghtigh E, Homblé F, Andersson M, Liepinsh E, Otting G. Lipid membrane binding of NK-lysin. FEBS Lett. 1998;425:341–344. doi: 10.1016/s0014-5793(98)00261-0. [DOI] [PubMed] [Google Scholar]
- 32.Schindler M, Osborn M J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18:4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- 33.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 34.Sherry B, Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988;107:1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staub A M. Somatic degraded polysaccharide of gram-negative bacteria. In: Whistler R L, editor. Methods in carbohydrate chemistry. New York, N.Y: Academic Press; 1965. pp. 93–95. [Google Scholar]
- 36.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C H, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, Porcelli S A, Bloom B R, Krensky A M, Modlin R L. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 37.Storm D R, Rosenthal K S, Swanson P E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–745. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 38.Su G L, Simmons R L, Wang S C. Lipopolysaccharide binding proteins participate in cellular activation by LPS. Crit Rev Immunol. 1995;15:201–214. doi: 10.1615/critrevimmunol.v15.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 39.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright S D, Ramos R D, Hermanowski-Vosatka A, Rockwell P, Dimers P A. Activation of adhesive capacity of CR3 on neutrophils by endotoxin: dependence on LPS binding protein and CD14. J Exp Med. 1991;173:1281–1285. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright S D, Ramos R D, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of LPS and LPS binding protein. Science. 1990;249:1431–1436. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]