Abstract
Type II topoisomerases (TOP2) are conserved regulators of chromatin topology that catalyze reversible DNA double-strand breaks (DSBs) and are essential for maintaining genomic integrity in diverse dynamic processes such as transcription, replication, and cell division. While controlled TOP2-mediated DSBs are an elegant solution to topological constraints of DNA, DSBs also contribute to the emergence of chromosomal translocations and mutations that drive cancer. The central importance of TOP2 enzymes as frontline chemotherapeutic targets is well known; however, their precise biological functions and impact in cancer development are still poorly understood. In this review, we provide an updated overview of TOP2A and TOP2B in the regulation of chromatin topology and transcription, and discuss the recent discoveries linking TOP2 activities with cancer pathogenesis.
From TADs to topoisomes, recent studies unfold the roles of TOP2A and TOP2B in genome regulation and carcinogenesis.
INTRODUCTION
The double-helical structure of DNA is fundamental for its compaction and precise replication, yet it holds an inherent risk of topological constraints in the genome. Rotation of the DNA double-helix during transcription, replication, chromatin condensation, and other cellular processes produces super-helical stress in DNA that poses threat to genome stability. In eukaryotic cells, DNA topoisomerase II (TOP2) enzymes resolve topological constraints by transiently nicking both DNA strands to create double-strand breaks (DSBs) that allow passage of the intact DNA segment (1). However, TOP2 activities can be intrinsically harmful as a failure to reseal the TOP2-linked DSBs introduces cytotoxic lesions, genome instability, and cell death and is associated with the emergence of chromosomal translocations and mutations that contribute to oncogenesis (2, 3). It is therefore important to understand the spatial and temporal context of TOP2 functions in the genomes of healthy cells, and the mechanisms that contribute to TOP2-mediated genomic transactions in cancer initiation and progression.
Vertebrates encode two TOP2 isoforms, TOP2A and TOP2B. In addition to their functions in cell nucleus, both TOP2A and TOP2B also localize to and are required in the nuclei and mitochondria (Fig. 1A). While the individual molecular functions of TOP2A and TOP2B, their implications to development and human disease, and the mechanistic interactions with chemotherapeutic, environmental, and dietary substances are subjects of active research, much still remains unknown. Here, we discuss the current progress and recent advances in the field of TOP2. We specifically focus on the genome biology, microscopy and chromatin landscape studies, and mechanistic investigations to understand the role of TOP2-mediated DNA breaks and their significance in transcription and cancer.
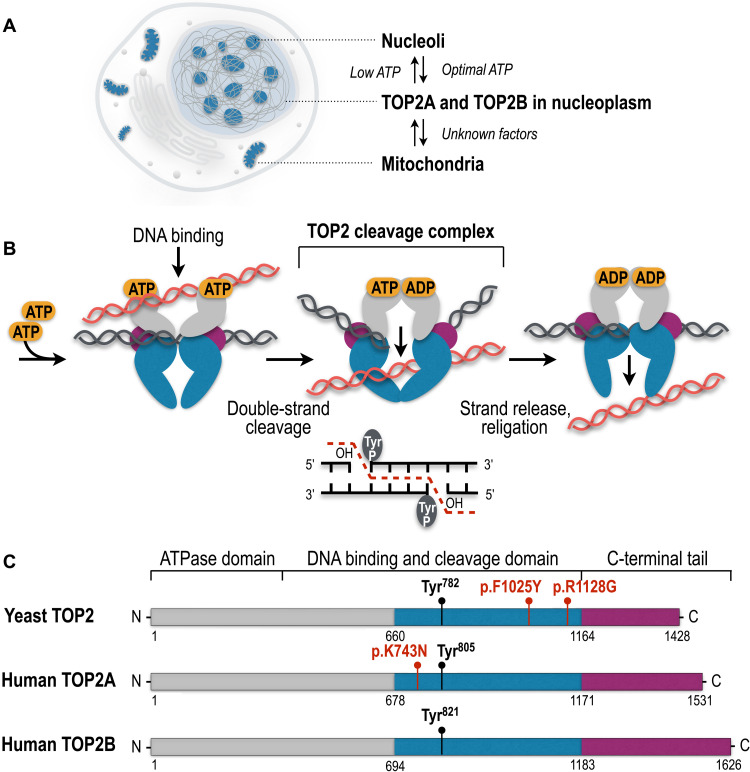
Fig. 1. Cellular localization, catalytic cycle, and evolutionary conservation of TOP2 enzymes.
(A) TOP2A and TOP2B are detected in the nucleoplasm, nucleoli, and mitochondria in the cell. (B) Eukaryotic TOP2 binds helix-helix crossovers of DNA as a homodimer that cleaves both strands of duplex DNA with 5′-four base overhangs and forms the TOP2cc by covalently attaching the catalytic tyrosine (Tyr) to the 5′-ends of the cleaved DNA. TOP2cc allows the passage of the uncleaved helix and the subsequent seamless religation of the DNA breaks. (C) Protein domains of yeast and human TOP2 enzymes. Colors show homologous regions of the enzymes. The N-terminal domain is responsible for ATP binding and hydrolysis. The central domain harbors tyrosine (Tyr) active sites to form covalent bonds with DNA and cleave and religate the DNA helix. The C-terminal domain shows the largest extent of evolutionary diversity and is involved in the association and dissociation of TOP2 with the DNA. TOP2 mutations associated with the self-poisoning phenotype and short insertions and deletions are shown in red.
BASIC STRUCTURE AND FUNCTION OF TOP2
The structure and function of TOP2 are shared across the tree of life and found in organisms in all phylogenetic domains including eukaryotes, prokaryotes, and archaea (4, 5). TOP2 acts as a homodimer that catalyzes cleavage and religation of DNA DSBs using adenosine triphosphate (ATP) hydrolysis. Each monomer of the TOP2 homodimer cleaves one DNA strand and forms a gate to pass the uncleaved DNA segment (Fig. 1B). TOP2 attaches temporarily to 5′ ends of cleaved DNA via covalent phospho-tyrosyl bonds, known as TOP2-DNA cleavage complexes (TOP2ccs), that protect the cleaved DNA ends from being recognized by cellular DNA damage response (DDR) pathways. Once the strand passage is completed, TOP2ccs are quickly and seamlessly religated and TOP2 dissociates from DNA (Fig. 1B) (1).
TOP2 enzymes include key structural and functional domains (Fig. 1C). The N-terminal domain is required for the conformational changes of TOP2 that are mediated by ATP binding and hydrolysis. The central breakage-reunion domain harbors an active tyrosine site that is required to establish covalent complexes between TOP2 and the 5′ termini of the DNA DSB ends (6, 7). The C-terminal domain contains nuclear localization motifs and is subject to extensive posttranslational modifications that regulate the catalytic activity, protein-protein interactions, and DNA binding properties of TOP2 (7–9).
Vertebrates have two paralogs of TOP2 genes, TOP2A and TOP2B, that originate from a whole-genome duplication event in early chordate evolution (Fig. 1C) (10, 11). While the catalytic and structural properties of TOP2A and TOP2B are similar, they are not functionally redundant and show characteristic expression patterns and distinct yet overlapping functions (7, 12).
TOP2A is essential for cell division, is highly expressed during mitosis, and is required for mitotic chromosome condensation and segregation (13–16). Genetic deletion of Top2a in mice leads to severe defects in nuclear division and blastocyst lethality at the four-cell stage (12, 17, 18). TOP2A is also important for structural maintenance of chromosomes, as acute auxin-inducible degradation of TOP2A during cell cycle disrupted the mitotic chromosomal structure and led to premature mitotic exit (19). The loss of TOP2A cannot be compensated by TOP2B despite the conserved structure and catalytic activity of the two topoisomerases (12, 20). Conversely, the residual DSB activity that has been observed in TOP2B knockout cells reflects some functional compensation through redundant activity of TOP2A (21, 22).
TOP2B is expressed in both dividing and nondividing cells and contributes to transcriptional regulation (16, 23–27). Top2b knockout mice develop in utero but die shortly after birth because of respiratory deficiencies caused by failure to innervate the diaphragm and other developmental defects in cerebral corticogenesis and motor and sensory neurons (28, 29). Besides its role in neurogenesis (30), TOP2B is required for ovulation (31), retinal development (32), B cell development (33), natural killer cell development (34), and replication of the human papillomavirus genome (35).
The functional differences of TOP2A and TOP2B are believed to originate, in part, from their distinct C-terminal domains (36, 37) that for both proteins are classified as intrinsically disordered domains (38, 39). Proteomic studies have also revealed a myriad of posttranslational modifications at this domain, the vast majority of which have not been ascribed a function (40). For instance, C-terminal domains of TOP2A and TOP2B contribute to their interactions with DNA and strand passage activity (9, 39, 41, 42), are involved in regulating the decatenation checkpoint, and contribute to the drug sensitivity of TOP2A and TOP2B (43–45). The C-terminal domains of TOP2A and TOP2B also contain regions that regulate their nucleolar accumulation and binding to RNA (46, 47). In addition, the C-terminal region of TOP2A, but not TOP2B, has a unique chromatin tether (ChT) domain that promotes interactions with chromosomes and localization to mitotic centromeres (48). Exploring the differential structure and function of the C-terminal domain of TOP2A and TOP2B represent new opportunities for paralog-specific drug development.
TOP2 AS A CANCER CHEMOTHERAPY TARGET
Chemotherapeutic targeting of TOP2 enzymes is routinely used to treat solid tumors and hematological malignancies including leukemia, lymphoma, sarcoma, neuroblastoma, germ-cell malignancies, and breast, lung, gastric, bone, and bladder cancers (49). The two major classes of drugs include TOP2 poisons and catalytic inhibitors. These compounds disrupt the catalytic cycle of TOP2 through distinct mechanisms of action and lead to accumulation of torsional stress, triggering of DDR pathways, and apoptosis primarily in rapidly proliferating cancer cells (50–52).
TOP2 poisons such as etoposide and doxorubicin form ternary complexes of TOP2 and DNA that trap TOP2 to 5′ termini of DNA DSBs and inhibit the religation of broken DNA strands (Fig. 2A) (50, 51). Etoposide is a poor DNA intercalator; however, extensive interactions of etoposide with DNA are promoted and stabilized when a complex is formed with TOP2 (44). These “poisoned” TOP2ccs obstruct normal cellular functions and activate DDR and repair pathways in which the DNA ends with trapped topoisomerases are processed, leading to the accumulation of protein-free “naked” DSBs that become cytotoxic if not seamlessly religated (52, 53). The TOP2 poisons currently in therapeutic use interact with both TOP2A and TOP2B. This lack of specificity has been associated with the emergence of severe side effects including cardiotoxicity, chromosomal translocations, and secondary malignancies (54–56).
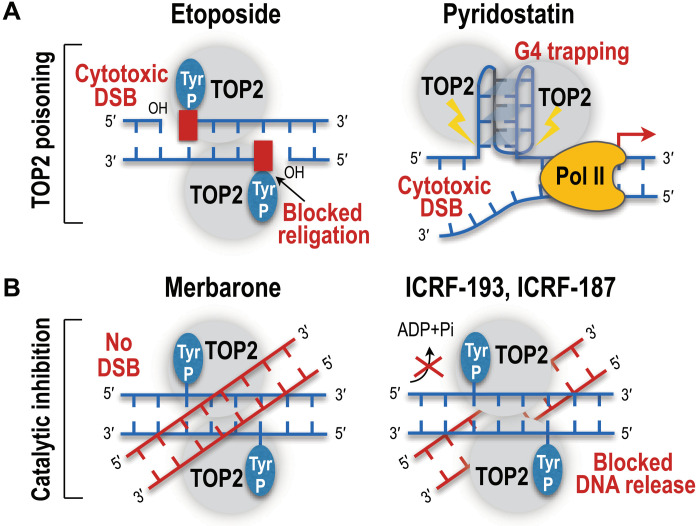
Fig. 2. Mechanism of action of TOP2 poisons and inhibitors in chemotherapy.
(A) TOP2 poisons such as etoposide trap TOP2 to 5′ termini of DNA DSBs, block religation of breaks, and cause accumulation of TOP2cc-blocked DSBs. In contrast, the small-molecule ligand pyridostatin traps TOP2A at the G-quadruplex (G4) structures and induces DNA damage and cell cycle arrest. (B) Merbarone inhibits TOP2 catalytically, stabilizes noncovalent TOP2-DNA complexes, and inhibits the formation of enzyme-mediated DSBs. The TOP2 inhibitors ICRF-193 and ICRF-187 decrease the ATPase activity of TOP2 and lock TOP2 in a closed clamp conformation that holds the cleaved and uncleaved DNA duplexes.
TOP2 inhibitors impair the enzymatic activity of TOP2 by disrupting the noncovalent catalytic steps in the TOP2 reaction cycle leading to different biochemical and cellular consequences (Fig. 2B) (50, 53). For instance, merbarone substantially decreases the catalytic activity of TOP2 by specifically blocking TOP2 cleavage of its substrate DNA helix (57). The compounds ICRF-193 and ICRF-187 reduce the ATP hydrolysis step of the TOP2 catalytic cycle, prevent the reopening of the adenosine triphosphatase (ATPase) domain, and keep the protein in the dimerized form that blocks enzyme turnover and further catalytic cycles (58, 59). ICRF-187 is also used in the clinic as combination therapy with doxorubicin or other anthracyclines to reduce cardiotoxicity and heart failure in patients receiving anthracycline chemotherapy (50).
Recent screens of genotoxic agents have identified pyridostatin (PDS) and CX5461 as highly potent TOP2 poisons (60–62). Both are small-molecule G4 ligands that can stabilize four-stranded G-quadruplex and R-loop DNA secondary structures by trapping TOP2 and provoking rapid induction of DNA DSBs and cytotoxicity specifically at the highly transcribed G-rich regions of the genome (Fig. 2A) (60–62). Compared to canonical TOP2 poisons that nonspecifically target both TOP2A and TOP2B, the cytotoxic and DNA damaging effects of PDS are specifically driven via TOP2A poisoning while CX5461 preferentially targets TOP2B (62, 63). The cytotoxicity of PDS primarily depends on the transcription of RNA polymerase II (Pol II); however, CX5461 treatment affects both RNA Pol I transcription of ribosomal DNA (rDNA) genes in the nucleolus and RNA Pol II transcription in the nucleus (62). Therefore, PDS and CX5461 are attractive candidates for anticancer drug development, with CX5461 currently undergoing phase 1 clinical trials for treatment of breast, ovary, pancreas, and prostate cancers (clinicaltrials.gov).
RESOLVING TRAPPED TOP2CC
Trapped TOP2ccs block essential metabolic transactions of DNA and activate DDR signaling and end processing of the protein-linked DSBs. Unblocking of the protein-linked DSBs is primarily mediated by either proteolytic degradation of trapped TOP2 followed by cleavage of the 5′ phosphotyrosyl bond with DNA, nucleolytic resection, or SUMOylation-induced direct hydrolysis of phosphotyrosyl bonds between TOP2cc and DNA (64, 65). In the first case, TOP2 enzymes are modified by ubiquitin for subsequent 26S proteasome degradation that removes stalled TOP2 from the DSB ends (66, 67). The remaining TOP2 peptides from the 5′ DNA cleavage sites are hydrolyzed by tyrosyl-DNA phosphodiesterase 2 (TDP2), resulting in protein-free DNA ends suitable for religation (68, 69). In the second case, trapped TOP2ccs are subjected to nucleolytic resection coordinated by DNA DSB repair proteins such as MRE11, CtIP, BRCA1, and others (70–74). A third mechanism involves proteasome-independent resolution of TOP2-DNA cross-links by E3 SUMO ligase ZNF451 (aka ZATT) and TDP2 (75). Recent study demonstrated that ZNF451 interaction with TOP2cc induces conformational changes that facilitate TDP2 access to the phosphotyrosyl bonds between TOP2 and DNA. Whereas ZNF451-mediated SUMOylation of TOP2ccs amplifies the interactions between TOP2ccs and TDP2, and stimulates phosphodiesterase activity of TDP2 for direct reversal of TOP2-DNA cross-links (75).
Once the trapped TOP2 enzymes are removed from DSBs, protein-free naked DNA ends are religated and repaired by two major DSB repair pathways, the cell cycle–independent nonhomologous end joining (NHEJ) pathway and the homologous recombination (HR) pathway; however, the latter only functions during DNA replication in proliferating cells (76).
TOP2A AND TOP2B IN CARCINOGENESIS
Inappropriate processing of trapped TOP2ccs and incorrect religation of the protein-free DSBs can trigger chromosomal rearrangements that drive certain hematological and solid tumors such as leukemia and prostate cancer (77–79). These translocations are associated with adverse side effects of TOP2 poisoning chemotherapy. For instance, approximately 2 to 15% of patients treated with TOP2 poison etoposide develop therapy-induced acute myeloid leukemia (t-AML) that typically involves recurrent translocations of the Mixed-lineage leukemia gene (MLL or KMT2A) at 11q23 and its numerous fusion partner genes (78, 80, 81).
Notably, up to 80% of infant leukemias carry the analogous de novo 11q23 fusions that are found in adult chemotherapy-treated t-AML patients (78). The presence of these translocations in umbilical cord and newborn blood, the high concordance among identical twins, and the early onset of disease have provided molecular proof of in utero origin of leukemia-initiating translocations (82). Furthermore, epidemiologic studies suggest that maternal exposure to TOP2-poisoning dietary compounds (e.g., bioflavonoids in green tea and soya) or environmental chemicals (e.g., metabolites of benzene in cigarette smoke and reactive oxygen species) may damage DNA and induce translocations by affecting the cleavage and religation of TOP2 DNA breaks in developing embryos (49).
Considering the chromosomal rearrangements at the MLL 11q23 locus that independently arise in both therapy-induced and infant leukemias, Cowell and colleagues (80) proposed a model in which illegitimate fusion events of MLL with its potential fusion partner genes are promoted by their transcription-mediated proximity. The study suggests that trapping of TOP2B-linked DSBs in MLL and its translocation partners that are transcribed in the common transcription factory allows incorrect end-joining (80). This idea was further supported by Gothe et al. (22) who measured the translocation frequencies and distances between MLL and its fusion partners (e.g., ENL, AF4, AF6, and AF9) in individual cells treated with etoposide using the high-throughput C-Fusion three-dimensional (3D) imaging method. Although broken by a similar frequency, the study demonstrated that the closer spatial proximity of MLL and ENL favored the formation of MLL-ENL fusions twice as often than other fusions, indicating that opportunistic fusions are more frequently enabled by a proximal genome structure.
TOP2B-mediated DSBs are also linked with the emergence of de novo TMPRSS2-ERG fusions found in >50% of prostate cancer genomes (77, 83). Transcription of TMPRSS2, ERG, and other androgen-responsive genes in prostate tissue is regulated by androgen signaling that co-recruits TOP2B and androgen receptor to the androgen-responsive genes. The spatial proximity of TMPRSS2 and ERG is induced by transcription in the common transcription factory (77, 84). Therefore, incomplete TOP2B cleavage at TMPRSS2 and ERG translocation breakpoints and the physical proximity of the broken ends may increase the probability for TMPRSS2-ERG translocations.
Trapping of TOP2B has long been implicated as the main source of DNA damage and genomic rearrangements in leukemia and prostate cancer (78, 80). However, experiments in TOP2B-depleted cells treated with etoposide demonstrated that TOP2A can also contribute to DNA DSBs and translocations at the 11q23 MLL locus (21, 22, 85). It is likely that the variation in the expression levels of TOP2A and TOP2B in different cell types and cell cycle phases, and specific interactions with TOP2 trapping compounds contributes to these processes.
CHARTING THE TOP2-OME: DNA-PROTEIN AND PROTEIN-PROTEIN INTERACTIONS OF TOP2
Next-generation sequencing and other high-throughput techniques allow us to explore the genome- and proteome-wide interactions of TOP2A and TOP2B and have revealed novel insights into their molecular functions and regulation. Several studies have now mapped genome-wide binding sites for TOP2A and TOP2B (21, 86–92), catalytically active TOP2ccs (85, 88, 93), global TOP2-induced DNA breakomes (21, 22, 88, 89, 94–96), and proximal-protein interactions of TOP2B (Table 1) (86). These studies have analyzed endogenous TOP2 functions under unperturbed conditions and mapped etoposide-induced TOP2-DNA interactions to uncover genomic regions as hotspots of TOP2-mediated DNA damage upon chemotherapy treatment (Table 1). Genome-wide TOP2 activities have been mapped in primary mouse hepatocytes, thymocytes, neurons, B cells, T cells, and mouse embryonic fibroblasts (MEFs). However, the current knowledge of TOP2 activities in human cells mostly originates from cell lines of cancer origin, while no data are available for TOP2 binding or DSBs in primary human tissues (Table 1).
Table 1. Overview of studies and high-throughput techniques to describe endogenous and etoposide-induced TOP2-DNA interactions and DNA DSBs in human and mouse.
| Endogenous TOP2 functions | Etoposide-directed TOP2 functions | ||
| TOP2A binding | TOP2B binding | ||
| ChIP-seq | Reference | ChIP-seq | Reference |
| •Mouse MEFs | (21) | •Human MCF10A-AsiSI cells | (89) |
| •Mouse thymocytes | (90) | •Human glioblastoma cell lines, tumors | (151) |
| •Human RPE-1 cells | (92) | ||
| TOP2B binding | TOP2cc maps | ||
| ChIP-seq | TOP2Acc-mapping | ||
| •Mouse primary neurons | (97) | •Human K562 cells | (85) |
| •Human MCF7 cells | (87, 91) | CC-seq | |
| •Mouse liver | (86) | •Human RPE-1 cells | (93) |
| •Mouse MEFs | (21, 88) | DSB maps | |
| •Mouse thymocytes | (90) | DSB-seq, SSB-seq | |
| •Human MCF10A-AsiSI cells | (89) | •Human HCT116 cells | (95) |
| •Human glioblastoma cell lines, tumors | (151) | END-seq | (21, 88, 96) |
| •Mouse B cells, T cells, MEFs, primary neurons | |||
| ChIP-exo | |||
| •Mouse liver | (86) | •Human MCF7, Nalm6, HCT116 cells | |
| DSB maps | |||
| BLISS | BLISS, sBLISS | ||
| •Human MCF10A-AsiSI cells | (89) | •Human TK6, K562, CD34+ cells | (22) |
| DSBCapture | DSBCapture | ||
| •Human HeLa cells | (94) | •Human HeLa cells | (94) |
| TOP2B protein-protein interactions | |||
| BioID | |||
| •Human HeLa cells | (86) | ||
TOP2 at transcriptionally active genes and regulatory elements
The genome-wide binding patterns of TOP2A and TOP2B, and DSB activity is consistently found at transcriptionally active regions and gene-regulatory elements. DNA binding sites of TOP2A and TOP2B are enriched at the promoters, enhancers, and gene bodies of actively transcribed genes. These also coincide with RNA Pol II occupancy, deoxyribonuclease I hypersensitivity, and posttranslational histone modifications characteristic of open chromatin (14, 21, 85–92, 97, 98). Similarly, TOP2-associated DNA DSBs frequently accumulate at transcriptionally active regions and are enriched at promoters, 5′ splice sites, and active enhancers (89, 94).
Open-chromatin regions and active regulatory elements are also more vulnerable to DNA damage induced by TOP2-targeting chemotherapy. Cells treated with the TOP2 poison etoposide undergo significantly higher levels of DNA breaks at the promoters and transcription start sites (TSS) of actively transcribed genes compared to less-transcribed genomic regions (22, 88, 89, 93, 95). This is explained by transcription-driven collisions of trapped TOP2ccs and elongating RNA Pol II that overwhelm the cellular DDR pathways and cause genome-wide accumulation of cytotoxic DSBs. Catalytic inhibition of RNA Pol II before TOP2 poisoning prevents formation of DSBs, further confirming the cooperation between TOP2 and RNA Pol II in transcription regulation (22). Cell type–specific transcription programs are therefore important determinants of cellular vulnerabilities to DNA damage driven by TOP2 poisons.
When assessing the role of topoisomerases in vivo versus during cancer treatment, it is important to consider the different cellular effects that are caused by covalent trapping of topoisomerases on the chromatin compared to genetic loss of function of topoisomerases. For example, chemotherapeutic poisoning and inhibition of TOP1 and TOP2B has shown to down-regulate the expression of very long genes (>200 kb), particularly in neuronal cells (27). However, subsequent studies in Top1 conditional knockout mice have demonstrated that only a fraction of long genes are differentially expressed, suggesting that the consequences of genetic deletion of TOP1 are much smaller than the effects of TOP1 poison topotecan treatment (99), and it is reasonable to expect similar results for genetic deletion of Top2b.
The TOP2-CTCF-cohesin connection
Genome-wide mapping of TOP2B binding has revealed that its occupancy overlaps with the binding sites of chromatin architectural regulators CTCF and the cohesin complex (21, 86, 88, 90, 91, 97). DNA binding of cohesin is an important determinant involved in the recruitment of TOP2B to the genomic sites of its activity. For instance, depletion of the cohesin subunit SMC3 in MEFs not only abolished cohesin binding but also proportionally reduced the genome-wide occupancy of TOP2B and etoposide-induced DSBs (88). On the other hand, depletion of the cohesin-releasing factor WAPL stabilized chromatin-cohesin interactions and simultaneously increased TOP2B occupancy and the overall frequency of DSBs at cohesin binding sites upon etoposide treatment of the cells (88).
Strong association of TOP2B with CTCF and cohesin has also been exploited by computational models developed to predict the global DNA occupancy of TOP2B and TOP2-induced DSBs (88, 91). These models use chromatin features for which genome-wide data are widely available, and may help make predictions for TOP2B function in cell types and species where assaying TOP2B occupancy or TOP2ccs has not been possible for technical reasons.
TOP2 at topologically associating domain boundaries
Topologically associating domains (TADs) are self-associating loop-like structures of the 3D genome that contain interacting cis-regulatory elements and target genes (100–103) and are formed by CTCF and cohesin-mediated chromatin loop extrusion (104–106). Approximately 50% of CTCF and cohesin binding sites at TAD boundaries are co-occupied by TOP2B (21, 86, 88, 90, 91, 97), a phenomenon that is especially evident at the chromatin loop anchors conserved in multiple species and cell types, suggesting potential constitutive function of TOP2B in genome organization and its contribution to evolutionary dynamics (86). TAD boundary regions are also hotspots for etoposide-induced DNA damage (21, 22, 88, 96).
It has been shown that DNA supercoiling at TSS decreases in the presence of TOP2 and TOP1 inhibitors, such as ICRF-193 and camptothecin, in a transcription-dependent manner (107). The supercoiling domain boundaries also coincide with CTCF sites, suggesting that topoisomerases, especially TOP2B, may function at TAD boundaries (86). In support of this, computational modeling of chromatin loop extrusion has shown that TOP2B-mediated DNA breaks at the TAD boundaries are needed to relax transcription-induced supercoiling that pushes cohesins from the source of transcription toward the TAD boundaries (108). TAD boundaries at clusters of enhancer loci, also known as stretch or super-enhancers, are particularly prone to TOP2B-mediated DSBs because of asymmetrical loop extrusion, indicating the robust presence and activity of TOP2B at the TAD boundaries during this process (96).
The involvement of TOP2A in torsional stress management at TAD boundaries is less characterized. Experiments in TOP2B-depleted cell lines have shown that TOP2A binding and DSBs also occur at CTCF and cohesin binding sites at TAD boundaries, similarly to TOP2B (21, 22). Therefore, TOP2A and TOP2B may function redundantly under certain circumstances in regulating topological stress at TAD boundaries that likely depends on their expression in different cell types. However, while the presence and purpose of TOP2B, and potentially TOP2A, at topological and supercoiling domain boundaries makes intuitive sense, the biological role of these interactions in vivo remains to be established. Given that DNA supercoiling at TAD boundaries is also affected by TOP1 catalytic inhibition (86, 107), the role of TOP1 in torsional stress management at TAD boundaries should also be considered.
Because TOP2A and TOP2B are potentially functionally redundant and yet essential for cell viability, dissecting their exact functions during establishment of chromatin contacts is challenging. In a recent study, TOP2 involvement in reestablishing the interphase chromosomal structures after cell division was addressed using human colorectal cancer cell lines with a full TOP2B knockout through auxin-inducible degradation of TOP2A (109). Unexpectedly, regardless of tremendous chromatin changes occurring from M to G1 transition, the Hi-C chromatin conformation maps revealed only ~600 lost or gained chromatin loops in the auxin-treated G1 reentry cells and slightly increased size of condition-specific loops, whereas chromosome compartments and trans contacts remained unchanged (109). Because auxin-induced degradation eliminated ~70% of TOP2A, it is still possible that the remaining TOP2A in these cells contribute to the resolution of chromatin supercoiling resulting from loop extrusion. The role of TOP1 in reestablishing interphase chromatin loops from M to G1 transition should also be examined as it may compensate for decreased TOP2 activities given that TOP1 interacts with RNA Pol II on mitotic chromosomes and promotes mitotic transcription by resolving transcription-mediated supercoiling (110).
Overall, the question whether TOP2 is directly involved in chromatin loop interactions requires further clarification. Given the null and conditional knockout phenotypes of Top2b in mice, it is possible that TOP2B binding at TAD boundaries and other cohesin-interacting sites allows it to function rapidly at specific times and in cell types experiencing topological challenges, without playing a central role in loop extrusion.
TOP2 at nucleolar rDNA loci
TOP2A and TOP2B localization and functions have also been studied in the nucleoli where they regulate RNA Pol I transcription (Fig. 1A). Nucleoli are dynamic biomolecular condensates involved in ribosomal biogenesis that form around actively transcribed rDNA loci (111). rDNA loci are among the most heavily transcribed regions of the genome, whereas the number of rDNA repeats and transcribed rRNA genes vary considerably between cell types, individuals, and disease states.
The shuffling of TOP2A and TOP2B between the nucleoplasm and the nucleolus is regulated by cellular ATP (Fig. 1A) (112, 113). TOP2A and TOP2B rapidly translocate to the nucleolus in response to suboptimal cellular levels of ATP, whereas the restoration of optimal ATP levels relocates the topoisomerases back to the nucleoplasm. A function of TOP2A in nucleoli is to alter rDNA topology at the rRNA core promoter, and it is required for the assembly of the functional RNA Pol I preinitiation complex and transcription initiation at the rDNA promoters in human cells (114). TOP2B binding to the rDNA spacer promoter and the transcribed rDNA regions suggests its role in resolving DNA supercoiling during RNA Pol I elongation (86).
Notably, the increased number of nucleoli, hyperactivation of RNA Pol I–mediated rDNA transcription, and elevated ribosome production are common features of cancer cells (115). While the role of TOP2A and TOP2B in oncogenic hypertranscription is not yet directly addressed, there is evidence about their contribution in the response to cancer chemotherapy via modulation of RNA Pol I transcription. It has been shown that nucleolar localization of TOP2 proteins significantly increases the tumor sensitivity to RNA Pol I inhibitors such as MBH-21, whereas the nuclear localization of TOP2 significantly decreases the effectiveness of the same drug (113). In addition, high rates of RNA Pol I transcription and the proportion of transcriptionally active to inactive rDNA repeats are important determinants for cancer cell sensitivity to CX5461 (116), a chemotherapy compound that targets TOP2B activity in RNA Pol I transcription in the nucleoli (62, 63). Thus, TOP2 subnuclear localization and RNA Pol I activity could help identify cancer patients who could potentially benefit from CX5461 and RNA Pol I inhibitors, and provide alternative avenues for cancer therapeutics development to specifically target nuclei in tumor cells.
TOP2A and TOP2B in mitochondria
Mitochondria are semi-autonomous organelles with their own circular double-stranded genomes that require topoisomerase activity to resolve topological problems (117). TOP1MT is the only vertebrate topoisomerase exclusively functioning in the mitochondria. TOP1MT introduces single-strand breaks to mitigate negative supercoiling during mitochondrial transcription, replication, and translation (118, 119). Although encoded in the nuclear genome, both TOP2A and TOP2B also translocate to the mitochondria where they function in a tissue- and growth condition–dependent manner (Fig. 1A) (120, 121). TOP2B participates in the regulation of mitochondrial DNA (mtDNA) replication and transcription by resolving positive supercoiling of mtDNA (121). TOP2A cleavage complexes have been detected at both ends of triple-stranded noncoding D-loop region that controls mtDNA replication, suggesting the role of TOP2A in decatenating mtDNA circles during replication, and possibly protecting D-loop ends from degradation (120). TOP2A knockdown in HeLa cells did not affect the topology or copy number of mtDNA (121, 122). This raises the question of whether TOP2B could compensate in mitochondria for the loss of TOP2A. So far, mitochondrial targeting sequences of TOP2 have not been identified, and it is still unknown how and when TOP2A and TOP2B are located to mitochondria and what is the significance of this in vivo (123).
Most TOP2-targeting anticancer compounds are intended to function in the nuclei of highly proliferating cancer cells; however, they have also been implicated in mitochondrial dysfunction and damage in postmitotic cells. For instance, a common side effect of doxorubicin treatment involves off-target poisoning of TOP2B in the mitochondria of cardiomyocytes that induces DSBs and transcriptional changes and results in mitochondrial dysfunction and cardiotoxicity (56). In addition, treatment with TOP2-inhibiting fluoroquinolone antibiotics such as ciprofloxacin causes accumulation of positive supercoiling of mtDNA that arrests mitochondrial transcription and replication. Depletion of mtDNA copy number blocks cell proliferation and differentiation, explaining side effects associated with fluoroquinolone antibiotics (121). It has been shown that nuclear TDP2 and the mitochondria-specific short isoform of TDP2 (TDP2s), both transcribed in the nucleus, facilitate the removal of abortive TOP2ccs in the mitochondria, while the deletions of TDP2 and TDP2s sensitize cells for TOP2-targeting poisons (124). Therefore, further clarification on the effects of TOP2-targeting drugs on mtDNA maintenance and stability, as well as mitochondria-specific poisoning of TOP2 in chemotherapy-resistant cancer cells is of high therapeutic importance. As for the nuclear genome, the ubiquitous presence of TOP2B in the mitochondrial genome also leads to notable phenotypes in the context of TOP2 poisons, yet its biological function under normal conditions will be more nuanced and difficult to discern.
Protein-protein interactions of TOP2A and TOP2B
Interacting proteins of TOP2A and TOP2B have been detected in previous proteomics studies and detailed experiments, and are curated across different species in open-access databases [e.g., BioGRID (125)]. In addition, proteome-wide proximal-protein interactions of TOP2B have been mapped in HeLa cells (86).
Analysis of protein-protein interaction networks has provided invaluable insights into the molecular functions of TOP2B. Besides its interactions with CTCF (126), TOP2B also interacts with subunits of the cohesin complex (RAD21, STAG1, STAG2, and SMC1A) and cohesin loading factors (NIPBL, PDS5A, and PDS5B), supporting its functions at TAD anchors (86). In accordance with its localization to nucleolus and rDNA loci, TOP2B interacts with nucleolar proteins that are involved in rDNA gene regulation, such as DDX18, DDX31, SDAD1, and RRP15 (86). Future studies should identify the interaction partners of TOP2A and TOP2B in the nucleolus to further explain their functions and shuffling between nucleoplasm and nucleolus. TOP2B also interacts with ZNF451, an E3 SUMO-protein ligase that facilitates the processing of trapped TOP2ccs independently of the proteasome (75, 86). In addition, TOP2B interacts with TOP2A and TOP1 (86, 127–129) and their concerted functions during transcription regulation in eukaryotes have been addressed recently (27, 89, 92, 94, 130). TOP2A-TOP2B heterodimers have been described in HeLa cells (129), potentially explaining the substantial interactions of the two proteins (86). However, the context of activity and specialized tasks of TOP2A-TOP2B heterodimers, their potential role in fine-tuning the transcriptional output and chromatin dynamics, or resolving particular DNA secondary structures remains to be characterized.
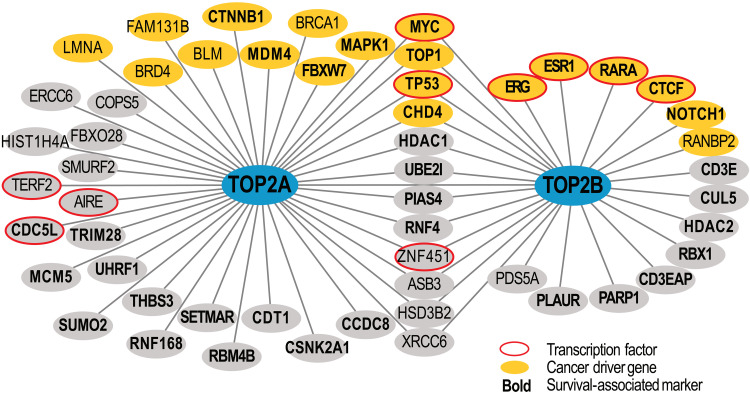
To summarize the high-confidence protein-protein interactions of TOP2A and TOP2B, we reviewed data available in the BioGRID database (thebiogrid.org, version 4.4.205). We selected high-confidence interactions that were confirmed in two or more independent studies or experimental approaches (Fig. 3). This analysis revealed 41 proteins interacting with TOP2A and 27 proteins interacting with TOP2B, including 12 proteins interacting with both topoisomerases (MYC, TOP1, TP53, CHD4, HDAC1, UBE2I, PIAS4, RNF4, ZNF451, ASB3, HSD3B2, and XRCC6; Fig. 3). Notably, >30% of TOP2A- and TOP2B-interacting proteins are encoded by frequently mutated cancer driver genes, including tumor suppressor genes (BLM, BRCA1, TP53, FBXW7, CTCF, ESR1, and NOTCH1) and oncogenes (MAPK1, MDM4, CHD4, and MYCN). Several known cancer genes are also involved in translocations and amplifications (BRD4, CTNNB1, MYC, ERG, ESR1, RARA, NOTCH1, TOP1, FAM131B, LMNA, and RANBP2) according to the COSMIC Cancer Gene Census database (v92) (131). Furthermore, most of interaction partners of TOP2A and TOP2B associate with cancer patient survival in various cancer types [The Cancer Genome Atlas (TCGA); Fig. 3].
Fig. 3. High-confidence protein-protein interactions of TOP2A and TOP2B.
Known protein-protein interactions were retrieved from the BioGRID database (v4.4.205) and the recent studies (75, 86, 130). Interactions were filtered to only include interactions detected in at least two independent studies or experimental approaches. Orange nodes indicate known cancer driver genes (from COSMIC Cancer Gene Census database), red circles highlight human transcription factors [from (156)], and cancer patient survival–associated markers are shown in bold [data from The Cancer Genome Atlas (TCGA)].
Together, genome-wide, proteome-wide, and detailed biochemical and functional analyses have been instrumental to our understanding of the shared and distinct functions and regulatory mechanisms of TOP2A and TOP2B, and their contributions to genome dynamics and stability. A deeper understanding of topoisomerase biology is fundamental to improving our knowledge of existing chemotherapies and the development of novel strategies for cancer treatments.
TOP2 IN RNA POL II TRANSCRIPTION
Transcription-associated supercoiling accumulates on both sides of RNA Pol II as it translocates along DNA, and is mitigated by combined activities of TOP2 and TOP1 enzymes (Fig. 4). While TOP1 preferentially resolves the positive supercoiling favoring the RNA Pol II elongation (132), a growing body of evidence indicates isoform-specific functions of TOP2A and TOP2B in mitigation of positive and negative DNA supercoiling in RNA Pol II pause release and transcriptional elongation.
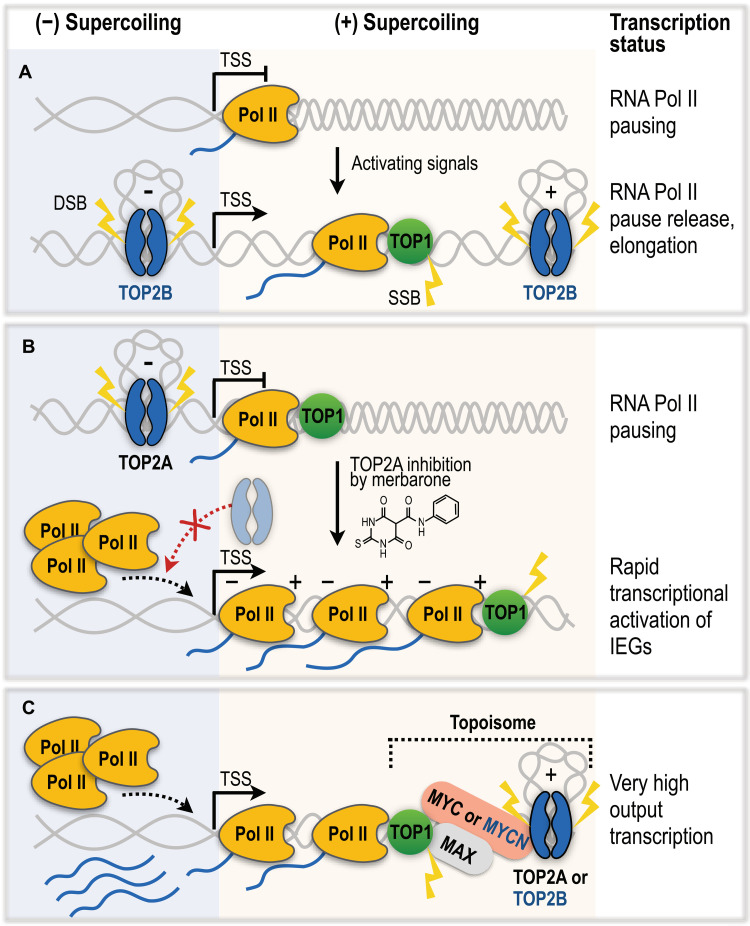
Fig. 4. The roles of TOP2A, TOP2B, and TOP1 in RNA Pol II transcription regulation.
(A) The breaking model. TOP2B catalyzes DNA DSBs to promote permissive chromatin structures and facilitate RNA Pol II pause release for rapid activation of transcription. TOP1 removes DNA supercoils of elongating RNA Pol II via single-strand breaks (SSBs). (B) The nonbreaking model of transcriptional activation of immediate early genes (IEGs). Catalytic inhibition of TOP2A induces RNA Pol II promoter-proximal pause release independently of formation of DNA breaks. Negative supercoiling at TSS facilitates separation of DNA molecules, allowing tandem recruitment and transcription of multiple RNA Pol II complexes. Topological feedback loop of transcribing polymerases cancels negative and positive supercoiling. (C) Topoisomes assembled by MYC or MYCN transcription factors unite TOP1 and TOP2A or TOP2B, respectively. Topoisomes stimulate enzymatic activities of topoisomerases to overcome topological challenges resulting from high-output transcription of MYC/MYCN-regulated genes.
Breaking model of RNA Pol II pause release
An emerging model of the function of TOP2B in transcription regulation can be referred to as the “breaking model” whereby TOP2B-mediated DSBs are proposed to be key regulators that promote permissive chromatin structures and facilitate RNA Pol II pause release for rapid transcription in the nonproliferating cells (Fig. 4A). On the basis of this model, TOP2B DSBs are required for efficient transcription initiation of a subset of stimulus-induced genes, including the targets of estrogen and androgen receptors (26, 77), glucocorticoid receptor targets (133), neuronal early response genes (97), and serum-induced immediate early response genes (134).
While TOP2-mediated DNA breaks are considered short-lived and protected by covalent bonds between TOP2 and DNA, normal cells proficient for p53-mediated apoptosis and senescence are shown to accumulate persistent DSBs that are correlated with TOP2B activity at the sites of RNA Pol II pause release (89, 94). DSBs at paused promoters activate DDR that halts cell cycle progression and recruit NHEJ repair proteins such as XRCC4 and PARP1 to broken sites (26, 77, 89, 97, 134). Further supporting the formation of DNA breaks during RNA Pol II pause release, a functional relationship between TOP2B-induced breaks and poly(adenosine diphosphate–ribose) polymerase (PARP) enzymes in chromatin reorganization is apparent in developing mouse spermatids (135). When activated by DNA DSBs, PARP1 and PARP2 bind to the TOP2B-induced break sites, undergo structural changes, and start to synthesize PAR polymer that initiates removal of TOP2B-PARP complexes from DNA and their further degradation (135). The cycles of PAR formation and degradation therefore provide a mechanism that allows chromatin decondensation and transcription initiation through controlled TOP2B breaks, whereas genetic or pharmacological inhibition of PARP leads to increased TOP2B activity in mouse spermatids (135). Overall, RNA Pol II pause release in response to intra- or extracellular activating signals could serve as a mechanism that generates cell type–specific and stimulus-induced distributions of DSBs in the genome.
Nonbreaking model of RNA Pol II pause release
An alternative model of RNA Pol II pause release through repressive functions of TOP2A has recently been proposed that challenges the accepted mechanism of TOP2B-induced DSBs in the transcriptional regulation of paused genes (Fig. 4B) (92). By investigating the genome-wide effects of merbarone, a TOP2 catalytic inhibitor that prevents formation of TOP2cc (Fig. 2B), Herrero-Ruiz et al. (92) observed a rapid and robust up-regulation of immediate early genes (IEGs) in human retinal pigment epithelial 1 (RPE-1) cells independently of the formation of TOP2A- or TOP2B-induced DSBs. Genetic depletion experiments further showed that the activation of IEGs was primarily caused by TOP2A inhibition and accumulation of negative supercoiling at the TSS that caused promoter-proximal pause release of RNA Pol II, suggesting that TOP2A catalytic activity favors promoter-proximal pausing of RNA Pol II that minimizes the expression of IEGs under basal conditions (92).
The study proposes that robust up-regulation of IEGs upon TOP2A inhibition could be achieved by tandem recruitment and elongation of multiple RNA Pol II complexes, and DNA supercoiling management in the topological feedback loop where negative supercoiling behind the first transcribing RNA Pol II absorbs the positive supercoiling generated by the next approaching Pol II complex (Fig. 4B). In contrast to the DNA breakage model, this study favors a scenario in which transcriptional up-regulation does not entail a risk for genome integrity, and instead suggests that the observed TOP2-induced DSBs during transcription are consequential rather than causal of transcriptional up-regulation (92). However, future studies should determine the mechanisms that endogenously control TOP2A inhibition in RNA Pol II pause release, in addition to the reported pharmacological inhibition. Last, it is also possible that the different regulation of RNA Pol II pause release and activation of IEGs by TOP2A and TOP2B depends on their protein abundances in different cell types and the competition of the two proteins in binding the specific gene promoters.
Topoisomes
To support the rapid bursts of transcription and supercoiling, the activity of TOP2 and TOP1 at highly transcribed regions can be stimulated through the formation of specialized complexes with transcription factors. Using biochemical and genomic approaches, Das et al. (130) demonstrated that in proliferating cells, a MYC-MAX transcription factor dimer recruits TOP2A and TOP1 and forms ternary “topoisome” complexes that are critical for resolving DNA topological issues associated with high-output transcription of MYC-regulated promoters (Fig. 4C). In postmitotic cells such as neurons where TOP2A and MYC are not expressed, the MYCN-MAX transcription factors instead interact with TOP2B and TOP1 to assemble the topoisome and manage the high-level torsional stress arising from expression of MYCN-regulated genes (130). In each topoisome, MYC or MYCN directly stimulates the activities of TOP1 and either TOP2A or TOP2B, respectively. TOP2A and TOP2B are thought to be mutually exclusive in these complexes, with TOP2A only interacting with MYC and TOP2B with MYCN to support transcriptional bursts of MYC/MYCN promoters (130).
The function of topoisomes in amplifying the activity of topoisomerases at the high-output promoters raises a question whether other transcription factors and oncogenes besides MYC and MYCN can also assemble similar topoisome complexes, and how these complexes could contribute to carcinogenesis. Because MYC as well as TOP2A, TOP2B, and TOP1 translocate to the nucleolus and contribute to the regulation of rDNA genes (86, 112–114, 136, 137), it is also possible that specialized topoisomes play an important role in ribosome biogenesis, providing potential novel strategies to block cancer cell growth via nucleolar-specific targeting of topoisomes.
MECHANISTIC INSIGHTS INTO TOP2-INDUCED CARCINOGENESIS
Stabilization of TOP2ccs by chemotherapeutic, dietary, and environmental compounds and unfaithful resolution of trapped TOP2-DNA adducts is a known source for DNA damage and malignant translocations in the noncancerous cells (Fig. 5). However, emerging evidences show that impaired TOP2 cleavage and religation that contributes to potential cancer driver mutations and oncogenesis also occurs under physiological conditions in the absence of TOP2 poisons (Fig. 5). TOP2 positioning at promoters, enhancers, and CTCF and cohesin binding sites therefore suggests mechanisms by which tissue-specific DNA damage and oncogenesis could be imparted.
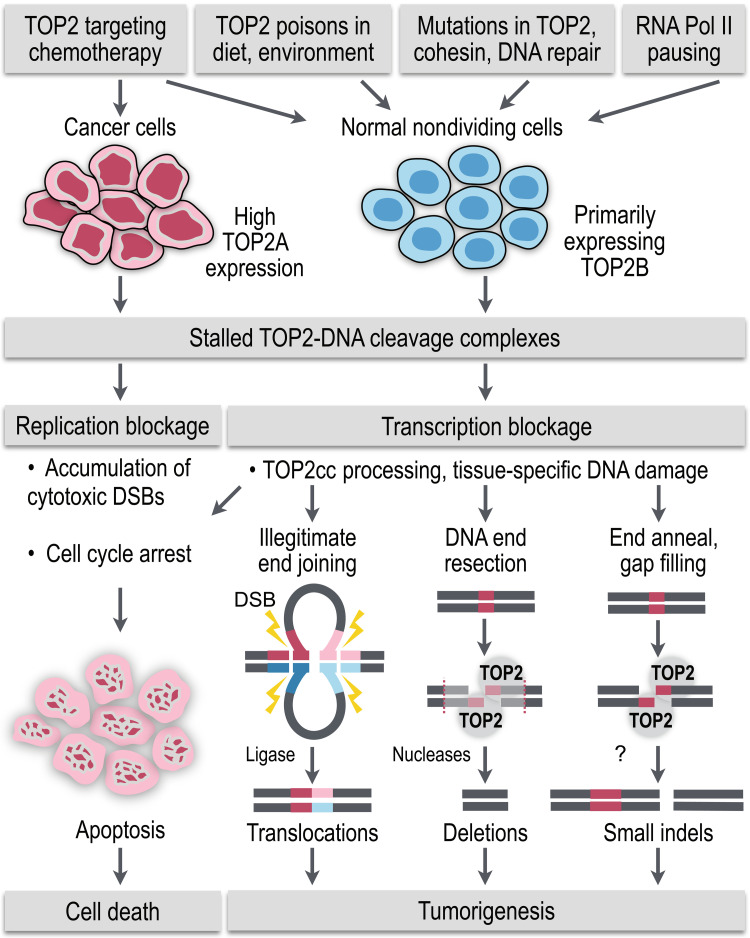
Fig. 5. TOP2 role in tumorigenesis.
Normally short-lived TOP2-DNA cleavage intermediates become stalled and trapped to the chromatin by (i) chemotherapeutic, dietary, and environmental TOP2 poisons, (ii) mutations in TOP2, cohesin, and DNA repair enzymes, and (iii) endogenous cellular processes that prolong TOP2-DNA interactions, such as RNA Pol II pausing. In proliferating cancer cells, trapped TOP2ccs block the essential steps in DNA replication and cell division, which overwhelms the DNA damage repair mechanisms and leads to accumulation of cytotoxic DNA lesions and apoptosis of the cancer cell. However, encounter of the stalled TOP2ccs with the transcription machinery in the normal nondividing cells and rapid processing of TOP2-DNA intermediates into protein-free DSBs provide the means for emergence of tissue-specific DNA damage and mutagenesis, including tumorigenic translocations, deletions, and short indels.
Impact of transcription and chromatin architecture on TOP2-mediated translocations
The principles of TOP2 cleavage in translocation process have been recently explored using high-throughput genome-wide translocation sequencing (88), whole-chromosome fluorescence in situ hybridization (FISH) (138), and high-throughput C-Fusion 3D approaches (22). Collectively, these studies have revealed that TOP2-linked translocations occur at actively transcribed sites and near TAD boundaries (21, 22, 88, 96, 138), supporting the role of intrinsic cell- and tissue-specific selection processes and mechanisms in generation of genomic rearrangements (139).
Emergence of TOP2-induced DSBs and translocations at highly transcribed loci is often associated with cell exposure to TOP2 poisoning compounds that trap TOP2ccs to the genome. At the sites where trapped TOP2ccs block RNA Pol II elongation, a rapid DDR and processing of trapped TOP2ccs takes place (22, 138). While this process is crucial to convert the trapped TOP2ccs into protein-free DSBs for further repair, it also enables the formation of illegitimate fusions, especially when the broken DNA ends of the two loci become into a close proximity (Fig. 5) (22). On the contrary, most of the trapped TOP2ccs at transcriptionally inactive loci remain intact and, upon removal of TOP2 trapping compound, are reversed by TOP2 without the generation of protein-free DSBs (88). Thus, the risk of therapy-induced chromosomal translocations is determined by the rate at which trapped TOP2ccs are processed at the transcriptionally active regions and the spatial proximity of the fusion partners.
Cancer-associated translocations also arise from endogenous TOP2-induced DNA breaks that occur during RNA Pol II pause release (Fig. 5) (89). By analyzing DSBs in the MCF10A breast cancer cell line and chromosomal rearrangements detected in breast cancer patients, Dellino et al. (89) observed that intragenic DSBs were enriched at 5′ splice sites of promoter-associated long introns and active enhancers within gene-body introns. Supporting the role of endogenous DSBs and release of paused RNA Pol II in translocation process, the study demonstrated that only the introns bound by TOP2B, DSB repair protein XRCC4, and RNA Pol II pS5 that marks transcriptional elongation were associated with translocation breakpoints of breast cancer. Furthermore, while RNA Pol II pause release directs the formation of tissue-specific endogenous DSBs, the proximity to the loop anchors further increases their probability to undergo translocations (89). Thus, TOP2-induced breaks during RNA Pol II pause release that could be triggered by various intra- and extracellular stimuli may predispose for cell type–specific chromosomal translocations.
Error-prone resolution of stalled TOP2ccs
Removal of trapped TOP2 adducts and repair of the resulting DSBs is a complex cellular challenge that involves coordinated activities of different enzymes and pathways and serves as a crucial mechanism for tumor suppression and genome stability (140). However, the cellular mechanisms of resolving trapped TOP2ccs may also contribute to the emergence of translocations and other mutations in cancer genomes (Fig. 5). Systematic analysis of these mechanisms in normal and cancer cells will ultimately help understand their role in cancer pathogenesis and identify novel targets for therapeutic intervention.
Somatic mutations and transcriptional depletion of genes responsible for processing and removing the stalled TOP2 adducts have important roles in tumorigenesis. For example, germline loss-of-function mutations in the tumor suppressor ATM, a kinase that activates responses to facilitate the removal of trapped TOP2ccs and further DNA DSBs repair, predispose patients for lymphoid and thymic malignancies (90, 141). Inactivating mutations in the tumor suppressor genes BRCA1 and BRCA2 predispose individuals for breast and ovarian cancers due to switch from the HR pathway to the more erroneous NHEJ pathway for DSB repair (142). It has been shown that BRCA1 promotes the removal of estrogen- and etoposide-induced TOP2 adducts from DSB ends, whereas cells that are deficient for BRCA1 have higher risk for TOP2cc-dependent DNA damage especially at the estrogen-regulated promoters (74). Because breast and ovary tissues rely on estrogens for their proliferation, the mutations in BRCA1/2 that compromise the fidelity of TOP2 adducts repair at estrogen-responsive promoters therefore explain the role of these mutations in initiation of breast and ovarian cancers.
A growing body of evidence demonstrates that the activity of DNA repair proteins could be prone to errors and increase the probability for inaccurate processing of TOP2-linked DSBs. For example, Sciascia and colleagues (143) showed that inhibiting proteasome-mediated degradation of stalled TOP2ccs before etoposide treatment prevented a robust DDR and minimized the occurrence of DSBs and chromosomal translocations that would otherwise have been expected. Thus, if not degraded, trapped TOP2ccs retain their enzymatic competency and, upon etoposide washout, can reseal the protein-linked DSBs in an error-free manner without invoking DDR signaling (143).
Nucleolytic resection is another potentially error-prone mechanism that resolves trapped TOP2ccs (73). For example, MRE11 endonuclease that exhibits 5′ and 3′ nucleolytic activity causes loss of short sequences at the processed DNA ends before religation (3, 72). Hence, MRE11 and other nucleases that remove additional nucleotides from the DNA ends can lead to aberrant repair of trapped TOP2ccs but may also give rise to potentially tumorigenic somatic mutations and deletions in the genome (Fig. 5).
Last, inaccurate religation of TOP2-mediated DSBs provokes gene fusions. On the basis of a comprehensive analysis of more than 2500 cancer genomes, most structural variants have no sequence homology at their breakpoint junctions and are therefore likely the consequence of NHEJ rather than HR-mediated rejoining of broken DNA ends (139). An essential core factor of NHEJ pathway that reseals the TOP2-mediated DSBs, the ligase 4 (LIG4) enzyme, however, is also responsible for illegitimate end joining of these breaks and resulting chromosomal rearrangements (138). Although LIG4 knockout cells become hypersensitive to etoposide and accumulate DSBs, the genome-wide FISH analysis of LIG4 knockout cells instead revealed significant decrease of chromosomal translocations in LIG4−/− cells compared to wild types (138). Thus, LIG4-mediated rejoining of TOP2-induced DNA breaks may result in errors that provoke fusions downstream of TOP2 proteolysis and DSB end processing (138).
Rewiring of TOP2 breakome in cancer
Proteins interacting with TOP2, such as CTCF and cohesin subunits (RAD21, SMC1A, STAG1, and STAG2), as well as the binding sites of CTCF and cohesin are subject to frequent somatic mutations in different cancer types (144–147). These mutations are linked with disruptions in TAD organization, gene-enhancer interactions, and transcriptional deregulation of cancer genes (148). However, mutations that disrupt the chromatin binding or functions of cohesin and CTCF may also interfere with the activities of TOP2B (21, 22, 88).
For instance, mutations in the cohesin complex or its regulatory subunits that either decrease or increase cohesin residence time on DNA also reduce or prolong TOP2-DNA interactions (88), and thus have potential to alter the frequency of TOP2-induced DSBs and translocations, and affect the outcome of TOP2-targeting chemotherapy. Also, single-nucleotide polymorphisms at CTCF and cohesin binding sites may alter the frequency of TOP2-induced DSBs or rewire the DSBs to novel sites. It has been shown that single-nucleotide polymorphisms that reduced the binding of CTCF in mouse B cells also reduced the etoposide-induced DSBs at the same sites (21). In addition, mutations at the conserved bases of the CTCF DNA binding motif that prevented CTCF binding to the TAD anchor overlapping the t-AML translocation hotspot of MLL in hematopoietic cells also substantially reduced etoposide-induced DSBs at this site compared to parental cells (22). It is therefore possible that TOP2 activities at chromatin loop anchors may interact with somatic mutational processes in cancer genomes that disrupt existing CTCF binding sites or create new binding motifs and TOP2 DSB sites. Overall, encoding mutations of CTCF and cohesin, and their binding site mutations, could alter TAD structures, cause TOP2-mediated chromatin fragility, and affect the transcription of genes involved in oncogenesis or tumor suppression.
Copy number and somatic mutations in TOP2
The copy number and expression of TOP2 is often altered in human cancers and associated with poor prognosis and shorter overall patient survival, likely due to the ability of TOP2 to support transcription and replication of cancer cells (TCGA) (149, 150). For instance, systematic analysis of >24,000 solid tumors demonstrated that TOP2A is amplified in approximately 4% of all tumors, with the highest rate of amplifications found in >10% gallbladder and gastro-esophageal malignancies (149). TOP2A coamplification with oncogenic HER2 tyrosine kinase located ~700 kb centromeric from TOP2A is frequent in patients with breast, ovarian, gastroesophageal, and pancreatic cancers (149). As another example, overexpression of TOP2B in a subset of gliomas has shown to modulate transcription of MYC and PDGFRA oncogenes and promote proliferation of glioma cells (151).
Unlike CTCF and cohesin genes that are frequently mutated in cancer, surprisingly little is currently known about the somatic mutations of TOP2A or TOP2B and their roles in oncogenesis. However, recent analyses of whole-genome sequencing datasets of human cancers have revealed a rare somatic mutation in p.K743N of TOP2A that occurs in less than 0.1% of the tumors analyzed and has been identified in cases of gastric, pancreatic, and prostate cancers and cholangiocarcinoma (Fig. 1C) (152, 153). This mutation affects TOP2A DNA cleavage domain and causes a specific pattern of insertion-deletion (indel; ID) mutagenesis entitled as ID_TOP2A (Fig. 5) (152, 153). To date, 18 indel mutational signatures have been described in human cancers comprising distinctive set of mutations caused by different mutation processes (152, 154). On the basis of this information, ID_TOP2A that gives rise to 2– to 4–base pair (bp) de novo duplications matches with previously reported indel signature 17 (ID17), whereas the deletions of 2, 3, 4, or ≥5 bp (mostly 6 to 8 bp in size) observed in ID_TOP2A in the nonrepetitive genomic sequences are associated with ID8 signature (152, 153). Correlation of a higher mutational activity and higher expression of genes indicates that the ID_TOP2A signature likely exhibits transcription-associated DNA damage at TOP2A cleavage sites (152, 153). Furthermore, orthologous TOP2A-p.K743N mutation introduced in the genome of budding yeast (Saccharomyces cerevisiae) induced higher cleavage activity of TOP2 and led to emergence of 2- to 4-bp duplications similar to ID17 signature (153). These results demonstrate a potential oncogenic role of mutations of TOP2 genes.
A markedly similar indel mutator phenotype to human ID_TOP2A (and ID17) has also been described in the yeast p.F1025Y and p.R1128G Top2 double-mutant (Fig. 1C). Functional characterization of this Top2 double-mutant revealed self-poisoning and stabilized cleavage intermediates that are trapped to DNA, similarly to TOP2 poisoning with etoposide (155). Elevated DNA cleavage observed in double-mutant yeast cells was independent of cellular ATP and was associated with up to 80-fold increase of de novo 2- to 4-bp duplications compared to wild-type yeast strains (155). These duplications arise from blunt end gap filling of the protein-free DNA ends by DNA polymerase and ligation by the NHEJ. Notably, both p.F1025Y and p.R1128G mutations map to the C-terminal dimerization region of the yeast Top2 protein and are therefore divergent from the TOP2A p.K743N mutation associated with ID_TOP2A signature in human cancer genomes (Fig. 1C) (155).
While more evidence regarding the TOP2 potential mutator phenotypes will become available in the future as more human cancer genomes are sequenced, the current observations suggest that elevated expression and mutations in TOP2 are potential drivers of mutational processes in cancer in addition to their more established involvement with therapy-related carcinogenesis.
CONCLUDING REMARKS
The localization of TOP2A and TOP2B to the most essential parts of the vertebrate genome makes them crucial cellular assets for solving topological challenges. While the essential role of TOP2A in cell division has been well established, the in vivo functions of TOP2B remain more elusive. Evidence to date supports a model where TOP2B is positioned in standby mode, waiting to participate in solving extreme topological challenges that occur during normal development, cellular differentiation and stimulation, and reproduction. To fully untangle the role of TOP2A and TOP2B, more work is needed to (i) define the essential molecular events and cellular processes that require TOP2B and (ii) identify the structural features that govern the unique expression patterns and interactions of the two topoisomerases.
In terms of carcinogenesis, it is becoming clear that endogenous cellular processes that prolong TOP2-DNA interactions can lead to structural rearrangements and mutations that have the potential to drive cancer. A better understanding of TOP2 functions in different subcellular locations (e.g., the nucleus, nucleolus, and mitochondria) is an exciting area of topoisomerase research with direct relevance to cancer. While the efficacious use of TOP2 poisons as frontline chemotherapeutics clearly illustrates how we can exploit the known features of TOP2-chromatin interactions in the clinic, the recurrent secondary malignancies and cardiotoxicity underscore the need for continued innovation in the field. For example, identifying TOP2-targeting compounds with different modes of action and propensity for poisoning of TOP2A over TOP2B or perhaps even reducing TOP2B levels before treatment have clinical potential. Continued research on the basic mechanisms of TOP2 function in vitro and in vivo will be essential for understanding genome regulation in health and cancer.
Acknowledgments
We thank J. Reimand and E. Ling for critical reading and input on this manuscript. We apologize to our colleagues whose work was not discussed here due to space and citation limitations.
Funding: L.U.-R. is supported by the Next Generation of Scientist Scholarship (PIN25558) from the Cancer Research Society. M.D.W. is supported by a tier II Canada Research Chair.
Author contributions: L.U.-R. reviewed the literature and conceptualized the structure for the article, created the figures and tables, wrote the manuscript, and edited it before submission. M.D.W. contributed to discussion of content and reviewed and edited the manuscript before submission.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.Champoux J. J., DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Pommier Y., Sun Y., Huang S.-Y. N., Nitiss J. L., Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 17, 703–721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashour M. E., Atteya R., El-Khamisy S. F., Topoisomerase-mediated chromosomal break repair: An emerging player in many games. Nat. Rev. Cancer 15, 137–151 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Forterre P., Gribaldo S., Gadelle D., Serre M.-C., Origin and evolution of DNA topoisomerases. Biochimie 89, 427–446 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Corbett K. D., Berger J. M., Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu. Rev. Biophys. Biomol. Struct. 33, 95–118 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Berger J. M., Gamblin S. J., Harrison S. C., Wang J. C., Structure and mechanism of DNA topoisomerase II. Nature 379, 225–232 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Nitiss J. L., DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 9, 327–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotz C., Lamour V., The interplay between DNA topoisomerase 2α post-translational modifications and drug resistance. Cancer Drug Resist. 3, 149–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilroy K. L., Austin C. A., The impact of the C-terminal domain on the interaction of human DNA topoisomerase II α and β with DNA. PLOS ONE 6, e14693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh Y. S., Moore D. D., Linkage of the nuclear hormone receptor genes NR1D2, THRB, and RARB: Evidence for an ancient, large-scale duplication. Genomics 57, 289–292 (1999). [DOI] [PubMed] [Google Scholar]
- 11.McLysaght A., Hokamp K., Wolfe K. H., Extensive genomic duplication during early chordate evolution. Nat. Genet. 31, 200–204 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Carpenter A. J., Porter A. C. G., Construction, characterization, and complementation of a conditional-lethal DNA topoisomerase IIalpha mutant human cell line. Mol. Biol. Cell 15, 5700–5711 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capranico G., Tinelli S., Austin C. A., Fisher M. L., Zunino F., Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim. Biophys. Acta 1132, 43–48 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Thakurela S., Garding A., Jung J., Schübeler D., Burger L., Tiwari V. K., Gene regulation and priming by topoisomerase IIα in embryonic stem cells. Nat. Commun. 4, 2478 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Ali Y., Abd Hamid S., Human topoisomerase II alpha as a prognostic biomarker in cancer chemotherapy. Tumour Biol. 37, 47–55 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Woessner R. D., Mattern M. R., Mirabelli C. K., Johnson R. K., Drake F. H., Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 2, 209–214 (1991). [PubMed] [Google Scholar]
- 17.Akimitsu N., Kamura K., Toné S., Sakaguchi A., Kikuchi A., Hamamoto H., Sekimizu K., Induction of apoptosis by depletion of DNA topoisomerase IIalpha in mammalian cells. Biochem. Biophys. Res. Commun. 307, 301–307 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Dovey M., Patton E. E., Bowman T., North T., Goessling W., Zhou Y., Zon L. I., Topoisomerase II alpha is required for embryonic development and liver regeneration in zebrafish. Mol. Cell. Biol. 29, 3746–3753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen C. F., Zhang T., Barisic M., Kalitsis P., Hudson D. F., Topoisomerase IIα is essential for maintenance of mitotic chromosome structure. Proc. Natl. Acad. Sci. U.S.A. 117, 12131–12142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grue P., Grässer A., Sehested M., Jensen P. B., Uhse A., Straub T., Ness W., Boege F., Essential mitotic functions of DNA topoisomerase IIalpha are not adopted by topoisomerase IIbeta in human H69 cells. J. Biol. Chem. 273, 33660–33666 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Canela A., Maman Y., Jung S., Wong N., Callen E., Day A., Kieffer-Kwon K.-R., Pekowska A., Zhang H., Rao S. S. P., Huang S.-C., Mckinnon P. J., Aplan P. D., Pommier Y., Aiden E. L., Casellas R., Nussenzweig A., Genome organization drives chromosome fragility. Cell 170, 507–521.e18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gothe H. J., Bouwman B. A. M., Gusmao E. G., Piccinno R., Petrosino G., Sayols S., Drechsel O., Minneker V., Josipovic N., Mizi A., Nielsen C. F., Wagner E.-M., Takeda S., Sasanuma H., Hudson D. F., Kindler T., Baranello L., Papantonis A., Crosetto N., Roukos V., Spatial chromosome folding and active transcription drive DNA fragility and formation of oncogenic MLL translocations. Mol. Cell 75, 267–283.e12 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Calderwood S. K., A critical role for topoisomerase IIb and DNA double strand breaks in transcription. Transcription 7, 75–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madabhushi R., The roles of DNA topoisomerase iiβ in transcription. Int. J. Mol. Sci. 19, 1917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin C. A., Cowell I. G., Khazeem M. M., Lok D., Ng H. T., TOP2B’s contributions to transcription. Biochem. Soc. Trans. 49, 2483–2493 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Ju B.-G., Lunyak V. V., Perissi V., Garcia-Bassets I., Rose D. W., Glass C. K., Rosenfeld M. G., A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006). [DOI] [PubMed] [Google Scholar]
- 27.King I. F., Yandava C. N., Mabb A. M., Hsiao J. S., Huang H.-S., Pearson B. L., Calabrese J. M., Starmer J., Parker J. S., Magnuson T., Chamberlain S. J., Philpot B. D., Zylka M. J., Topoisomerases facilitate transcription of long genes linked to autism. Nature 501, 58–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X., Li W., Prescott E. D., Burden S. J., Wang J. C., DNA topoisomerase IIbeta and neural development. Science 287, 131–134 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Lyu Y. L., Wang J. C., Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc. Natl. Acad. Sci. U.S.A. 100, 7123–7128 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyu Y. L., Lin C.-P., Azarova A. M., Cai L., Wang J. C., Liu L. F., Role of topoisomerase IIbeta in the expression of developmentally regulated genes. Mol. Cell. Biol. 26, 7929–7941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.-L., Yu C., Ji S.-Y., Li X.-M., Zhang Y.-P., Zhang D., Zhou D., Fan H.-Y., TOP2β is essential for ovarian follicles that are hypersensitive to chemotherapeutic drugs. Mol. Endocrinol. 27, 1678–1691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Hao H., Tzatzalos E., Lin R.-K., Doh S., Liu L. F., Lyu Y. L., Cai L., Topoisomerase IIbeta is required for proper retinal development and survival of postmitotic cells. Biol. Open 3, 172–184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broderick L., Yost S., Li D., McGeough M. D., Booshehri L. M., Guaderrama M., Brydges S. D., Kucharova K., Patel N. C., Harr M., Hakonarson H., Zackai E., Cowell I. G., Austin C. A., Hügle B., Gebauer C., Zhang J., Xu X., Wang J., Croker B. A., Frazer K. A., Putnam C. D., Hoffman H. M., Mutations in topoisomerase IIβ result in a B cell immunodeficiency. Nat. Commun. 10, 3644 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broderick L., Clay G. M., Blum R. H., Liu Y., McVicar R., Papes F., Booshehri L. M., Cowell I. G., Austin C. A., Putnam C. D., Kaufman D. S., Disease-associated mutations in topoisomerase IIβ result in defective NK cells. J. Allergy Clin. Immunol. 149, 2171–2176.e3 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Kaminski P., Hong S., Kono T., Hoover P., Laimins L., Topoisomerase 2β induces DNA breaks to regulate human papillomavirus replication. MBio 12, e00005-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linka R. M., Porter A. C. G., Volkov A., Mielke C., Boege F., Christensen M. O., C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 35, 3810–3822 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S. H., Chan N.-L., Hsieh T., New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 82, 139–170 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Piovesan D., Necci M., Escobedo N., Monzon A. M., Hatos A., Mičetić I., Quaglia F., Paladin L., Ramasamy P., Dosztányi Z., Vranken W. F., Davey N. E., Parisi G., Fuxreiter M., Tosatto S. C. E., MobiDB: Intrinsically disordered proteins in 2021. Nucleic Acids Res. 49, D361–D367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanden Broeck A., Lotz C., Drillien R., Haas L., Bedez C., Lamour V., Structural basis for allosteric regulation of Human Topoisomerase IIα. Nat. Commun. 12, 2962 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin C. A., Lee K. C., Swan R. L., Khazeem M. M., Manville C. M., Cridland P., Treumann A., Porter A., Morris N. J., Cowell I. G., TOP2B: The first thirty years. Int. J. Mol. Sci. 19, 2765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meczes E. L., Gilroy K. L., West K. L., Austin C. A., The impact of the human DNA topoisomerase II C-terminal domain on activity. PLOS ONE 3, e1754 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendorff T. J., Schmidt B. H., Heslop P., Austin C. A., Berger J. M., The structure of DNA-bound human topoisomerase II alpha: Conformational mechanisms for coordinating inter-subunit interactions with DNA cleavage. J. Mol. Biol. 424, 109–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kozuki T., Chikamori K., Surleac M. D., Micluta M. A., Petrescu A. J., Norris E. J., Elson P., Hoeltge G. A., Grabowski D. R., Porter A. C. G., Ganapathi R. N., Ganapathi M. K., Roles of the C-terminal domains of topoisomerase IIα and topoisomerase IIβ in regulation of the decatenation checkpoint. Nucleic Acids Res. 45, 5995–6010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C.-C., Li T.-K., Farh L., Lin L.-Y., Lin T.-S., Yu Y.-J., Yen T.-J., Chiang C.-W., Chan N.-L., Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 333, 459–462 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Wu C.-C., Li Y.-C., Wang Y.-R., Li T.-K., Chan N.-L., On the structural basis and design guidelines for type II topoisomerase-targeting anticancer drugs. Nucleic Acids Res. 41, 10630–10640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda K., Kato Y., Ikeda S., Kawano S., Regulation of catalytic activity and nucleolar localization of rat DNA topoisomerase IIα through its C-terminal domain. Genes Genet. Syst. 95, 291–302 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Onoda A., Hosoya O., Sano K., Kiyama K., Kimura H., Kawano S., Furuta R., Miyaji M., Tsutsui K., Tsutsui K. M., Nuclear dynamics of topoisomerase IIβ reflects its catalytic activity that is regulated by binding of RNA to the C-terminal domain. Nucleic Acids Res. 42, 9005–9020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M., Liang C., Chen Q., Yan H., Xu J., Zhao H., Yuan X., Liu J., Lin S., Lu W., Wang F., Histone H2A phosphorylation recruits topoisomerase IIα to centromeres to safeguard genomic stability. EMBO J. 39, e101863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vann K. R., Oviatt A. A., Osheroff N., Topoisomerase II poisons: Converting essential enzymes into molecular scissors. Biochemistry 60, 1630–1641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nitiss J. L., Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pommier Y., Leo E., Zhang H., Marchand C., DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delgado J. L., Hsieh C.-M., Chan N.-L., Hiasa H., Topoisomerases as anticancer targets. Biochem. J. 475, 373–398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pommier Y., Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 8, 82–95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy M. B., Mercer S. L., Deweese J. E., Chapter Five—Inhibitors and poisons of mammalian type II topoisomerases. Adv. Mol. Toxicol. 11, 203–240 (2017). [Google Scholar]
- 55.Bjornsti M.-A., Kaufmann S. H., Topoisomerases and cancer chemotherapy: Recent advances and unanswered questions. [version 1; peer review: 3 approved]. F1000Res. 8, F1000 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S., Liu X., Bawa-Khalfe T., Lu L.-S., Lyu Y. L., Liu L. F., Yeh E. T. H., Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Fortune J. M., Osheroff N., Merbarone inhibits the catalytic activity of human topoisomerase IIalpha by blocking DNA cleavage. J. Biol. Chem. 273, 17643–17650 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Roca J., Ishida R., Berger J. M., Andoh T., Wang J. C., Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc. Natl. Acad. Sci. U.S.A. 91, 1781–1785 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris S. K., Baird C. L., Lindsley J. E., Steady-state and rapid kinetic analysis of topoisomerase II trapped as the closed-clamp intermediate by ICRF-193. J. Biol. Chem. 275, 2613–2618 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Olivieri M., Cho T., Álvarez-Quilón A., Li K., Schellenberg M. J., Zimmermann M., Hustedt N., Rossi S. E., Adam S., Melo H., Heijink A. M., Sastre-Moreno G., Moatti N., Szilard R. K., McEwan A., Ling A. K., Serrano-Benitez A., Ubhi T., Feng S., Pawling J., Delgado-Sainz I., Ferguson M. W., Dennis J. W., Brown G. W., Cortés-Ledesma F., Williams R. S., Martin A., Xu D., Durocher D., A genetic map of the response to DNA damage in human cells. Cell 182, 481–496.e21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno P. M., Lu M., Dennis K. A., Inam H., Moore C. J., Sheehe J., Elledge S. J., Hemann M. T., Pritchard J. R., The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning. Proc. Natl. Acad. Sci. U.S.A. 117, 4053–4060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bossaert M., Pipier A., Riou J.-F., Noirot C., Nguyên L.-T., Serre R.-F., Bouchez O., Defrancq E., Calsou P., Britton S., Gomez D., Transcription-associated topoisomerase 2α (TOP2A) activity is a major effector of cytotoxicity induced by G-quadruplex ligands. eLife 10, e65184 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan M., Wright W. C., Chapple R. H., Zubair A., Sandhu M., Batchelder J. E., Huddle B. C., Low J., Blankenship K. B., Wang Y., Gordon B., Archer P., Brady S. W., Natarajan S., Posgai M. J., Schuetz J., Miller D., Kalathur R., Chen S., Connelly J. P., Babu M. M., Dyer M. A., Pruett-Miller S. M., Freeman B. B., Chen T., Godley L. A., Blanchard S. C., Stewart E., Easton J., Geeleher P., The chemotherapeutic CX-5461 primarily targets TOP2B and exhibits selective activity in high-risk neuroblastoma. Nat. Commun. 12, 6468 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serrano-Benítez A., Cortés-Ledesma F., Ruiz J. F., “An end to a means”: How DNA-end structure shapes the double-strand break repair process. Front. Mol. Biosci. 6, 153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riccio A. A., Schellenberg M. J., Williams R. S., Molecular mechanisms of topoisomerase 2 DNA-protein crosslink resolution. Cell. Mol. Life Sci. 77, 81–91 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y., Miller Jenkins L. M., Su Y. P., Nitiss K. C., Nitiss J. L., Pommier Y., A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci. Adv. 6, eaba6290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao Y., Desai S. D., Ting C. Y., Hwang J., Liu L. F., 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J. Biol. Chem. 276, 40652–40658 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Gao R., Schellenberg M. J., Huang S.-Y. N., Abdelmalak M., Marchand C., Nitiss K. C., Nitiss J. L., Williams R. S., Pommier Y., Proteolytic degradation of topoisomerase II (Top2) enables the processing of Top2·DNA and Top2·RNA covalent complexes by tyrosyl-DNA-phosphodiesterase 2 (TDP2). J. Biol. Chem. 289, 17960–17969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortes Ledesma F., El Khamisy S. F., Zuma M. C., Osborn K., Caldecott K. W., A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461, 674–678 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Nakamura K., Kogame T., Oshiumi H., Shinohara A., Sumitomo Y., Agama K., Pommier Y., Tsutsui K. M., Tsutsui K., Hartsuiker E., Ogi T., Takeda S., Taniguchi Y., Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLOS Genet. 6, e1000828 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K. C., Padget K., Curtis H., Cowell I. G., Moiani D., Sondka Z., Morris N. J., Jackson G. H., Cockell S. J., Tainer J. A., Austin C. A., MRE11 facilitates the removal of human topoisomerase II complexes from genomic DNA. Biol. Open 1, 863–873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoa N. N., Shimizu T., Zhou Z. W., Wang Z.-Q., Deshpande R. A., Paull T. T., Akter S., Tsuda M., Furuta R., Tsutsui K., Takeda S., Sasanuma H., Mre11 is essential for the removal of lethal topoisomerase 2 covalent cleavage complexes. Mol. Cell 64, 580–592 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Aparicio T., Baer R., Gottesman M., Gautier J., MRN, CtIP, and BRCA1 mediate repair of topoisomerase II-DNA adducts. J. Cell Biol. 212, 399–408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasanuma H., Tsuda M., Morimoto S., Saha L. K., Rahman M. M., Kiyooka Y., Fujiike H., Cherniack A. D., Itou J., Callen Moreu E., Toi M., Nakada S., Tanaka H., Tsutsui K., Yamada S., Nussenzweig A., Takeda S., BRCA1 ensures genome integrity by eliminating estrogen-induced pathological topoisomerase II-DNA complexes. Proc. Natl. Acad. Sci. U.S.A. 115, E10642–E10651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schellenberg M. J., Lieberman J. A., Herrero-Ruiz A., Butler L. R., Williams J. G., Muñoz-Cabello A. M., Mueller G. A., London R. E., Cortés-Ledesma F., Williams R. S., ZATT (ZNF451)-mediated resolution of topoisomerase 2 DNA-protein cross-links. Science 357, 1412–1416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chapman J. R., Taylor M. R. G., Boulton S. J., Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 47, 497–510 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Haffner M. C., Aryee M. J., Toubaji A., Esopi D. M., Albadine R., Gurel B., Isaacs W. B., Bova G. S., Liu W., Xu J., Meeker A. K., Netto G., De Marzo A. M., Nelson W. G., Yegnasubramanian S., Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pendleton M., Lindsey R. H., Felix C. A., Grimwade D., Osheroff N., Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 1310, 98–110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gómez-Herreros F., DNA double strand breaks and chromosomal translocations induced by DNA topoisomerase II. Front. Mol. Biosci. 6, 141 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cowell I. G., Sondka Z., Smith K., Lee K. C., Manville C. M., Sidorczuk-Lesthuruge M., Rance H. A., Padget K., Jackson G. H., Adachi N., Austin C. A., Model for MLL translocations in therapy-related leukemia involving topoisomerase IIβ-mediated DNA strand breaks and gene proximity. Proc. Natl. Acad. Sci. U.S.A. 109, 8989–8994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Felix C. A., Secondary leukemias induced by topoisomerase-targeted drugs. Biochim. Biophys. Acta 1400, 233–255 (1998). [DOI] [PubMed] [Google Scholar]
- 82.Marcotte E. L., Spector L. G., Mendes-de-Almeida D. P., Nelson H. H., The prenatal origin of childhood leukemia: Potential applications for epidemiology and newborn screening. Front. Pediatr. 9, 639479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar-Sinha C., Tomlins S. A., Chinnaiyan A. M., Recurrent gene fusions in prostate cancer. Nat. Rev. Cancer 8, 497–511 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mani R.-S., Tomlins S. A., Callahan K., Ghosh A., Nyati M. K., Varambally S., Palanisamy N., Chinnaiyan A. M., Induced chromosomal proximity and gene fusions in prostate cancer. Science 326, 1230 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu X., Davenport J. W., Urtishak K. A., Carillo M. L., Gosai S. J., Kolaris C. P., Byl J. A. W., Rappaport E. F., Osheroff N., Gregory B. D., Felix C. A., Genome-wide TOP2A DNA cleavage is biased toward translocated and highly transcribed loci. Genome Res. 27, 1238–1249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uusküla-Reimand L., Hou H., Samavarchi-Tehrani P., Rudan M. V., Liang M., Medina-Rivera A., Mohammed H., Schmidt D., Schwalie P., Young E. J., Reimand J., Hadjur S., Gingras A.-C., Wilson M. D., Topoisomerase II beta interacts with cohesin and CTCF at topological domain borders. Genome Biol. 17, 182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manville C. M., Smith K., Sondka Z., Rance H., Cockell S., Cowell I. G., Lee K. C., Morris N. J., Padget K., Jackson G. H., Austin C. A., Genome-wide ChIP-seq analysis of human TOP2B occupancy in MCF7 breast cancer epithelial cells. Biol. Open 4, 1436–1447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Canela A., Maman Y., Huang S.-Y. N., Wutz G., Tang W., Zagnoli-Vieira G., Callen E., Wong N., Day A., Peters J.-M., Caldecott K. W., Pommier Y., Nussenzweig A., Topoisomerase II-induced chromosome breakage and translocation is determined by chromosome architecture and transcriptional activity. Mol. Cell 75, 252–266.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dellino G. I., Palluzzi F., Chiariello A. M., Piccioni R., Bianco S., Furia L., De Conti G., Bouwman B. A. M., Melloni G., Guido D., Giacò L., Luzi L., Cittaro D., Faretta M., Nicodemi M., Crosetto N., Pelicci P. G., Release of paused RNA polymerase II at specific loci favors DNA double-strand-break formation and promotes cancer translocations. Nat. Genet. 51, 1011–1023 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Álvarez-Quilón A., Terrón-Bautista J., Delgado-Sainz I., Serrano-Benítez A., Romero-Granados R., Martínez-García P. M., Jimeno-González S., Bernal-Lozano C., Quintero C., García-Quintanilla L., Cortés-Ledesma F., Endogenous topoisomerase II-mediated DNA breaks drive thymic cancer predisposition linked to ATM deficiency. Nat. Commun. 11, 910 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martínez-García P. M., García-Torres M., Divina F., Terrón-Bautista J., Delgado-Sainz I., Gómez-Vela F., Cortés-Ledesma F., Genome-wide prediction of topoisomerase IIβ binding by architectural factors and chromatin accessibility. PLOS Comput. Biol. 17, e1007814 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herrero-Ruiz A., Martínez-García P. M., Terrón-Bautista J., Millán-Zambrano G., Lieberman J. A., Jimeno-González S., Cortés-Ledesma F., Topoisomerase IIα represses transcription by enforcing promoter-proximal pausing. Cell Rep. 35, 108977 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gittens W. H., Johnson D. J., Allison R. M., Cooper T. J., Thomas H., Neale M. J., A nucleotide resolution map of Top2-linked DNA breaks in the yeast and human genome. Nat. Commun. 10, 4846 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh S., Szlachta K., Manukyan A., Raimer H. M., Dinda M., Bekiranov S., Wang Y.-H., Pausing sites of RNA polymerase II on actively transcribed genes are enriched in DNA double-stranded breaks. J. Biol. Chem. 295, 3990–4000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baranello L., Kouzine F., Wojtowicz D., Cui K., Przytycka T. M., Zhao K., Levens D., DNA break mapping reveals topoisomerase II activity genome-wide. Int. J. Mol. Sci. 15, 13111–13122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vian L., Pękowska A., Rao S. S. P., Kieffer-Kwon K.-R., Jung S., Baranello L., Huang S.-C., El Khattabi L., Dose M., Pruett N., Sanborn A. L., Canela A., Maman Y., Oksanen A., Resch W., Li X., Lee B., Kovalchuk A. L., Tang Z., Nelson S., Di Pierro M., Cheng R. R., Machol I., Hilaire B. G. S., Durand N. C., Shamim M. S., Stamenova E. K., Onuchic J. N., Ruan Y., Nussenzweig A., Levens D., Aiden E. L., Casellas R., The energetics and physiological impact of cohesin extrusion. Cell 175, 292–294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Madabhushi R., Gao F., Pfenning A. R., Pan L., Yamakawa S., Seo J., Rueda R., Phan T. X., Yamakawa H., Pao P.-C., Stott R. T., Gjoneska E., Nott A., Cho S., Kellis M., Tsai L.-H., Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161, 1592–1605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tiwari V. K., Burger L., Nikoletopoulou V., Deogracias R., Thakurela S., Wirbelauer C., Kaut J., Terranova R., Hoerner L., Mielke C., Boege F., Murr R., Peters A. H. F. M., Barde Y.-A., Schübeler D., Target genes of topoisomerase IIβ regulate neuronal survival and are defined by their chromatin state. Proc. Natl. Acad. Sci. U.S.A. 109, E934–E943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mabb A. M., Simon J. M., King I. F., Lee H.-M., An L.-K., Philpot B. D., Zylka M. J., Topoisomerase 1 regulates gene expression in neurons through cleavage complex-dependent and -independent mechanisms. PLOS ONE 11, e0156439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rao S. S. P., Huntley M. H., Durand N. C., Stamenova E. K., Bochkov I. D., Robinson J. T., Sanborn A. L., Machol I., Omer A. D., Lander E. S., Aiden E. L., A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dixon J. R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J. S., Ren B., Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lieberman-Aiden E., van Berkum N. L., Williams L., Imakaev M., Ragoczy T., Telling A., Amit I., Lajoie B. R., Sabo P. J., Dorschner M. O., Sandstrom R., Bernstein B., Bender M. A., Groudine M., Gnirke A., Stamatoyannopoulos J., Mirny L. A., Lander E. S., Dekker J., Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beagan J. A., Phillips-Cremins J. E., On the existence and functionality of topologically associating domains. Nat. Genet. 52, 8–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanborn A. L., Rao S. S. P., Huang S.-C., Durand N. C., Huntley M. H., Jewett A. I., Bochkov I. D., Chinnappan D., Cutkosky A., Li J., Geeting K. P., Gnirke A., Melnikov A., McKenna D., Stamenova E. K., Lander E. S., Aiden E. L., Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. U.S.A. 112, E6456–E6465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fudenberg G., Imakaev M., Lu C., Goloborodko A., Abdennur N., Mirny L. A., Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y., Shi Z., Zhang H., Finkelstein I. J., Yu H., Human cohesin compacts DNA by loop extrusion. Science 366, 1345–1349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naughton C., Avlonitis N., Corless S., Prendergast J. G., Mati I. K., Eijk P. P., Cockroft S. L., Bradley M., Ylstra B., Gilbert N., Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat. Struct. Mol. Biol. 20, 387–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Racko D., Benedetti F., Dorier J., Stasiak A., Transcription-induced supercoiling as the driving force of chromatin loop extrusion during formation of TADs in interphase chromosomes. Nucleic Acids Res. 46, 1648–1660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S., Übelmesser N., Josipovic N., Forte G., Slotman J. A., Chiang M., Gothe H. J., Gusmao E. G., Becker C., Altmüller J., Houtsmuller A. B., Roukos V., Wendt K. S., Marenduzzo D., Papantonis A., RNA polymerase II is required for spatial chromatin reorganization following exit from mitosis. Sci. Adv. 7, eabg8205 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiegard A., Kuzin V., Cameron D. P., Grosser J., Ceribelli M., Mehmood R., Ballarino R., Valant F., Grochowski R., Karabogdan I., Crosetto N., Lindqvist A., Bizard A. H., Kouzine F., Natsume T., Baranello L., Topoisomerase 1 activity during mitotic transcription favors the transition from mitosis to G1. Mol. Cell 81, 5007–5024.e9 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Panov K. I., Hannan K., Hannan R. D., Hein N., The ribosomal gene loci-the power behind the throne. Genes (Basel) 12, 763 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christensen M. O., Larsen M. K., Barthelmes H. U., Hock R., Andersen C. L., Kjeldsen E., Knudsen B. R., Westergaard O., Boege F., Mielke C., Dynamics of human DNA topoisomerases IIα and IIbeta in living cells. J. Cell Biol. 157, 31–44 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morotomi-Yano K., Yano K.-I., Nucleolar translocation of human DNA topoisomerase II by ATP depletion and its disruption by the RNA polymerase I inhibitor BMH-21. Sci. Rep. 11, 21533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ray S., Panova T., Miller G., Volkov A., Porter A. C. G., Russell J., Panov K. I., Zomerdijk J. C. B. M., Topoisomerase IIα promotes activation of RNA polymerase I transcription by facilitating pre-initiation complex formation. Nat. Commun. 4, 1598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hein N., Hannan K. M., George A. J., Sanij E., Hannan R. D., The nucleolus: An emerging target for cancer therapy. Trends Mol. Med. 19, 643–654 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Sanij E., Hannan K. M., Xuan J., Yan S., Ahern J. E., Trigos A. S., Brajanovski N., Son J., Chan K. T., Kondrashova O., Lieschke E., Wakefield M. J., Frank D., Ellis S., Cullinane C., Kang J., Poortinga G., Nag P., Deans A. J., Khanna K. K., Mileshkin L., McArthur G. A., Soong J., Berns E. M. J. J., Hannan R. D., Scott C. L., Sheppard K. E., Pearson R. B., CX-5461 activates the DNA damage response and demonstrates therapeutic efficacy in high-grade serous ovarian cancer. Nat. Commun. 11, 2641 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menger K. E., Rodríguez-Luis A., Chapman J., Nicholls T. J., Controlling the topology of mammalian mitochondrial DNA. Open Biol. 11, 210168 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H., Barceló J. M., Lee B., Kohlhagen G., Zimonjic D. B., Popescu N. C., Pommier Y., Human mitochondrial topoisomerase I. Proc. Natl. Acad. Sci. U.S.A. 98, 10608–10613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baechler S. A., Factor V. M., Dalla Rosa I., Ravji A., Becker D., Khiati S., Miller Jenkins L. M., Lang M., Sourbier C., Michaels S. A., Neckers L. M., Zhang H. L., Spinazzola A., Huang S. N., Marquardt J. U., Pommier Y., The mitochondrial type IB topoisomerase drives mitochondrial translation and carcinogenesis. Nat. Commun. 10, 83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H., Zhang Y.-W., Yasukawa T., Dalla Rosa I., Khiati S., Pommier Y., Increased negative supercoiling of mtDNA in TOP1mt knockout mice and presence of topoisomerases IIα and IIβ in vertebrate mitochondria. Nucleic Acids Res. 42, 7259–7267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hangas A., Aasumets K., Kekäläinen N. J., Paloheinä M., Pohjoismäki J. L., Gerhold J. M., Goffart S., Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of topoisomerase 2. Nucleic Acids Res. 46, 9625–9636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nicholls T. J., Nadalutti C. A., Motori E., Sommerville E. W., Gorman G. S., Basu S., Hoberg E., Turnbull D. M., Chinnery P. F., Larsson N.-G., Larsson E., Falkenberg M., Taylor R. W., Griffith J. D., Gustafsson C. M., Topoisomerase 3α is required for decatenation and segregation of human mtDNA. Mol. Cell 69, 9–23.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goffart S., Hangas A., Pohjoismäki J. L. O., Twist and turn-topoisomerase functions in mitochondrial DNA maintenance. Int. J. Mol. Sci. 20, 2041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang S.-Y. N., Dalla Rosa I., Michaels S. A., Tulumello D. V., Agama K., Khiati S., Jean S. R., Baechler S. A., Factor V. M., Varma S., Murai J., Miller Jenkins L. M., Kelley S. O., Pommier Y., Mitochondrial tyrosyl-DNA phosphodiesterase 2 and its TDP2S short isoform. EMBO Rep. 19, e42139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oughtred R., Rust J., Chang C., Breitkreutz B.-J., Stark C., Willems A., Boucher L., Leung G., Kolas N., Zhang F., Dolma S., Coulombe-Huntington J., Chatr-Aryamontri A., Dolinski K., Tyers M., The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Witcher M., Emerson B. M., Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol. Cell 34, 271–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wan C., Borgeson B., Phanse S., Tu F., Drew K., Clark G., Xiong X., Kagan O., Kwan J., Bezginov A., Chessman K., Pal S., Cromar G., Papoulas O., Ni Z., Boutz D. R., Stoilova S., Havugimana P. C., Guo X., Malty R. H., Sarov M., Greenblatt J., Babu M., Derry W. B., Tillier E. R., Wallingford J. B., Parkinson J., Marcotte E. M., Emili A., Panorama of ancient metazoan macromolecular complexes. Nature 525, 339–344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Havugimana P. C., Hart G. T., Nepusz T., Yang H., Turinsky A. L., Li Z., Wang P. I., Boutz D. R., Fong V., Phanse S., Babu M., Craig S. A., Hu P., Wan C., Vlasblom J., Dar V.-N., Bezginov A., Clark G. W., Wu G. C., Wodak S. J., Tillier E. R. M., Paccanaro A., Marcotte E. M., Emili A., A census of human soluble protein complexes. Cell 150, 1068–1081 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Biersack H., Jensen S., Gromova I., Nielsen I. S., Westergaard O., Andersen A. H., Active heterodimers are formed from human DNA topoisomerase II alpha and II beta isoforms. Proc. Natl. Acad. Sci. U.S.A. 93, 8288–8293 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Das S. K., Kuzin V., Cameron D. P., Sanford S., Jha R. K., Nie Z., Rosello M. T., Holewinski R., Andresson T., Wisniewski J., Natsume T., Price D. H., Lewis B. A., Kouzine F., Levens D., Baranello L., MYC assembles and stimulates topoisomerases 1 and 2 in a “topoisome”. Mol. Cell 82, 140–158.e12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tate J. G., Bamford S., Jubb H. C., Sondka Z., Beare D. M., Bindal N., Boutselakis H., Cole C. G., Creatore C., Dawson E., Fish P., Harsha B., Hathaway C., Jupe S. C., Kok C. Y., Noble K., Ponting L., Ramshaw C. C., Rye C. E., Speedy H. E., Stefancsik R., Thompson S. L., Wang S., Ward S., Campbell P. J., Forbes S. A., COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baranello L., Wojtowicz D., Cui K., Devaiah B. N., Chung H.-J., Chan-Salis K. Y., Guha R., Wilson K., Zhang X., Zhang H., Piotrowski J., Thomas C. J., Singer D. S., Pugh B. F., Pommier Y., Przytycka T. M., Kouzine F., Lewis B. A., Zhao K., Levens D., RNA polymerase II regulates topoisomerase 1 activity to favor efficient transcription. Cell 165, 357–371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trotter K. W., King H. A., Archer T. K., Glucocorticoid receptor transcriptional activation via the BRG1-dependent recruitment of TOP2β and Ku70/86. Mol. Cell. Biol. 35, 2799–2817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bunch H., Lawney B. P., Lin Y.-F., Asaithamby A., Murshid A., Wang Y. E., Chen B. P. C., Calderwood S. K., Transcriptional elongation requires DNA break-induced signalling. Nat. Commun. 6, 10191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meyer-Ficca M. L., Lonchar J. D., Ihara M., Meistrich M. L., Austin C. A., Meyer R. G., Poly(ADP-ribose) polymerases PARP1 and PARP2 modulate topoisomerase II beta (TOP2B) function during chromatin condensation in mouse spermiogenesis. Biol. Reprod. 84, 900–909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown I. N., Lafita-Navarro M. C., Conacci-Sorrell M., Regulation of nucleolar activity by MYC. Cells 11, 574 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muller M. T., Pfund W. P., Mehta V. B., Trask D. K., Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 4, 1237–1243 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olmedo-Pelayo J., Rubio-Contreras D., Gómez-Herreros F., Canonical non-homologous end-joining promotes genome mutagenesis and translocations induced by transcription-associated DNA topoisomerase 2 activity. Nucleic Acids Res. 48, 9147–9160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Roberts N. D., Wala J. A., Shapira O., Schumacher S. E., Kumar K., Khurana E., Waszak S., Korbel J. O., Haber J. E., Imielinski M., Weischenfeldt J., Beroukhim R., Campbell P. J., Patterns of somatic structural variation in human cancer genomes. Nature 578, 112–121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aparicio T., Baer R., Gautier J., DNA double-strand break repair pathway choice and cancer. DNA Repair (Amst.) 19, 169–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Álvarez-Quilón A., Serrano-Benítez A., Lieberman J. A., Quintero C., Sánchez-Gutiérrez D., Escudero L. M., Cortés-Ledesma F., ATM specifically mediates repair of double-strand breaks with blocked DNA ends. Nat. Commun. 5, 3347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prakash R., Zhang Y., Feng W., Jasin M., Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 7, a016600 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sciascia N., Wu W., Zong D., Sun Y., Wong N., John S., Wangsa D., Ried T., Bunting S. F., Pommier Y., Nussenzweig A., Suppressing proteasome mediated processing of topoisomerase II DNA-protein complexes preserves genome integrity. eLife 9, e53447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Katainen R., Dave K., Pitkänen E., Palin K., Kivioja T., Välimäki N., Gylfe A. E., Ristolainen H., Hänninen U. A., Cajuso T., Kondelin J., Tanskanen T., Mecklin J.-P., Järvinen H., Renkonen-Sinisalo L., Lepistö A., Kaasinen E., Kilpivaara O., Tuupanen S., Enge M., Taipale J., Aaltonen L. A., CTCF/cohesin-binding sites are frequently mutated in cancer. Nat. Genet. 47, 818–821 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Guo Y. A., Chang M. M., Huang W., Ooi W. F., Xing M., Tan P., Skanderup A. J., Mutation hotspots at CTCF binding sites coupled to chromosomal instability in gastrointestinal cancers. Nat. Commun. 9, 1520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Umer H. M., Cavalli M., Dabrowski M. J., Diamanti K., Kruczyk M., Pan G., Komorowski J., Wadelius C., A significant regulatory mutation burden at a high-affinity position of the CTCF motif in gastrointestinal cancers. Hum. Mutat. 37, 904–913 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Lee C. A., Abd-Rabbo D., Reimand J., Functional and genetic determinants of mutation rate variability in regulatory elements of cancer genomes. Genome Biol. 22, 133 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu H., Uusküla-Reimand L., Isaev K., Wadi L., Alizada A., Shuai S., Huang V., Aduluso-Nwaobasi D., Paczkowska M., Abd-Rabbo D., Ocsenas O., Liang M., Thompson J. D., Li Y., Ruan L., Krassowski M., Dzneladze I., Simpson J. T., Lupien M., Stein L. D., Boutros P. C., Wilson M. D., Reimand J., Candidate cancer driver mutations in distal regulatory elements and long-range chromatin interaction networks. Mol. Cell 77, 1307–1321.e10 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Heestand G. M., Schwaederle M., Gatalica Z., Arguello D., Kurzrock R., Topoisomerase expression and amplification in solid tumours: Analysis of 24,262 patients. Eur. J. Cancer 83, 80–87 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ren L., Liu J., Gou K., Xing C., Copy number variation and high expression of DNA topoisomerase II alpha predict worse prognosis of cancer: A meta-analysis. J. Cancer 9, 2082–2092 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gonzalez-Buendia E., Zhao J., Wang L., Mukherjee S., Zhang D., Arrieta V. A., Feldstein E., Kane J. R., Kang S. J., Lee-Chang C., Mahajan A., Chen L., Realubit R., Karan C., Magnusson L., Horbinski C., Marshall S. A., Sarkaria J. N., Mohyeldin A., Nakano I., Bansal M., James C. D., Brat D. J., Ahmed A., Canoll P., Rabadan R., Shilatifard A., Sonabend A. M., TOP2B enzymatic activity on promoters and introns modulates multiple oncogenes in human gliomas. Clin. Cancer Res. 27, 5669–5680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexandrov L. B., Kim J., Haradhvala N. J., Huang M. N., Tian Ng A. W., Wu Y., Boot A., Covington K. R., Gordenin D. A., Bergstrom E. N., Islam S. M. A., Lopez-Bigas N., Klimczak L. J., McPherson J. R., Morganella S., Sabarinathan R., Wheeler D. A., Mustonen V.; PCAWG Mutational Signatures Working Group, Getz G., Rozen S. G., Stratton M. R., PCAWG Consortium, The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boot A., Liu M., Stantial N., Shah V., Yu W., Nitiss K. C., Nitiss J. L., Jinks-Robertson S., Rozen S. G., Recurrent mutations in topoisomerase IIα cause a previously undescribed mutator phenotype in human cancers. Proc. Natl. Acad. Sci. U.S.A. 119, e2114024119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Forbes S. A., Beare D., Boutselakis H., Bamford S., Bindal N., Tate J., Cole C. G., Ward S., Dawson E., Ponting L., Stefancsik R., Harsha B., Kok C. Y., Jia M., Jubb H., Sondka Z., Thompson S., De T., Campbell P. J., COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 45, D777–D783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stantial N., Rogojina A., Gilbertson M., Sun Y., Miles H., Shaltz S., Berger J., Nitiss K. C., Jinks-Robertson S., Nitiss J. L., Trapped topoisomerase II initiates formation of de novo duplications via the nonhomologous end-joining pathway in yeast. Proc. Natl. Acad. Sci. U.S.A. 117, 26876–26884 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lambert S. A., Jolma A., Campitelli L. F., Das P. K., Yin Y., Albu M., Chen X., Taipale J., Hughes T. R., Weirauch M. T., The human transcription factors. Cell 172, 650–665 (2018). [DOI] [PubMed] [Google Scholar]