Abstract
BACKGROUND
The pandemic has affected hundreds of millions of people; early reports suggesting high rates of prolonged symptoms may be prone to selection bias.
METHODS
In a program caring for all SARS-CoV-2 positive inpatients and outpatients between March to October 2020, and offering universal 90-day follow-up, we compared those who died prior to 90 days, not responding to follow-up, declining, or accepting follow-up. Among those seen or declining follow-up, we determined the prevalence and predictors of persistent symptoms.
RESULTS
Among 993 patients, 21 (2.1%) died prior to 90 days, 506 (50.9%) did not respond, 260 (26.1%) declined follow-up because they were well, and 206 (20.7%) were fully assessed. Of 466 who responded to follow-up inquiry, 133 (28.5%) reported ≥1 persistent symptom, including constitutional (15.5%), psychiatric (14.2%), rheumatologic (13.1%), neurologic (13.1%), cardiorespiratory (12.0%), and gastrointestinal (1.7%). Predictors differed for each symptom type. Any persistent symptom was more common in older patients (adjusted odds ratio [aOR] 1.11, 95% CI 1.04 to 1.18/5 years), those diagnosed in hospital (aOR 2.03, 95% CI 1.24 to 3.33) and those with initial constitutional and rheumatologic symptoms. Patients not responding to follow-up were younger and healthier at baseline.
CONCLUSION
Persistent symptoms are common and diverse 3 months post-COVID-19 but are likely over-estimated by most reports.
Keywords: COVID-19, outpatients, persistent symptoms, post-COVID-19, prevalence
Abstract
HISTORIQUE
La pandémie touche des centaines de millions de gens. Les rapports précoces laissant croire à des symptômes prolongés pourraient être assujettis à un biais de sélection.
MÉTHODOLOGIE
Dans un programme de soins auprès de tous les patients ambulatoires et hospitalisés ayant reçu un résultat positif au SRAS-CoV-2 entre mars et octobre 2020, assorti d’un suivi universel de 90 jours, les chercheurs ont comparé les personnes qui ont succombé avant 90 jours, n’ont pas répondu au suivi ou ont décliné ou accepté le suivi. Chez celles qui ont été vues ou ont décliné le suivi, ils ont déterminé la prévalence et les prédicteurs de symptômes persistants.
RÉSULTATS
Chez les 993 patients, 21 (2,1 %) sont décédés avant les 90 jours, 506 (50,9 %) n’ont pas répondu, 260 (26,1 %) ont décliné le suivi parce qu’ils se sentaient bien et 206 (20,7 %) se sont soumis à une évaluation complète. Des 466 qui ont répondu à l’offre de suivi, 133 (28,5 %) ont signalé ressentir au moins un symptôme persistant, y compris d’ordre constitutionnel (15,5 %), psychiatrique (14,2 %), rhumatologique (13,1 %), neurologique (13,1 %), cardiorespiratoire (12,0 %) et gastro-intestinal (1,7 %). Les prédicteurs différaient en fonction de chaque type de symptômes. Les symptômes persistants étaient courants chez les personnes âgées (rapport de cotes corrigé [RCc] 1,11, IC à 95 %, 1,04 à 1,18/cinq ans), les personnes diagnostiquées à l’hôpital (RCc 2,03, IC à 95 %, 1,24 à 3,33) et celles dont les manifestations initiales comportaient des symptômes constitutionnels et rhumatologiques. Les patients qui ne répondaient pas au suivi étaient plus jeunes et en meilleure santé au départ.
CONCLUSION
Les symptômes persistants sont courants et diversifiés trois mois après la COVID-19, mais sont probablement surestimés dans la plupart des rapports.
Mots-clés : COVID-19, patients ambulatoires, post-COVID-19, prévalence, symptômes persistants
COVID-19 has affected more than 200 million people and resulted in more than 4 million deaths worldwide (1). In addition, COVID-19 has had a profound impact on health care systems and economies globally. In Canada, the first case of COVID-19 occurred in January 2020 (2), and since then there have been more than 2 million confirmed cases and over 30,000 deaths across the country (3).
Initial reports from China, where the pandemic originated, indicate that the vast majority of individuals affected by COVID-19 (81%) will experience mild symptoms. Approximately 14% develop a severe infection and 5% will experience critical illness (23). The predictors of severe outcomes during initial infection have been extensively studied, and include older age, medical comorbidities, obesity, and certain laboratory parameters such as lymphopenia and elevated inflammatory markers (4–6).
Although the manifestations and spectrum of acute COVID-19 have been well described, (7–10) very little is known about the intermediate and long-term sequela of COVID-19. Most studies of long-term follow-up and outcomes have focused on patients requiring hospitalization for severe COVID-19 and suggest that the majority of those individuals are still experiencing residual symptoms up to 6 months post-illness onset (8,11). However, less is known about the long-term prognosis across the full spectrum of acute COVID-19 illness, particularly those with mild- moderate infection who do not require hospitalization. As these individuals account for more than 80% of those affected by COVID-19, it is important to assess the longer-term effect of COVID-19 infection in this population.
Therefore, we conducted a cohort study to describe the prevalence and nature of COVID-19 symptoms 90 days post-infection, across the full spectrum of illness severity and to evaluate predictors of persistent constitutional, upper respiratory, lower respiratory, rheumatologic, neurologic, gastrointestinal, and psychiatric symptoms.
Methods
Study design and participants
This is a retrospective cohort study of all consecutive patients included in the COVID-19 Expansion to Outpatients (COVIDEO) program at Sunnybrook Health Science Centre (SHSC), from March 2020 to October 2020. SHSC is a 678-bed tertiary-care teaching hospital in Toronto, Ontario, Canada.
COVIDEO is an inter-disciplinary collaboration between Infectious Diseases, Microbiology, Infection Prevention and Control, and Public Health. A full description of the program has been previously published (12). Testing could have been performed at the COVID-19 assessment centre (CAC), emergency department (ED), occupational health and safety (OHS), inpatient ward, or any SHSC-affiliated outpatient clinic. Occasionally, patients and/or health care workers who were tested at a different facility were enrolled into the program based on referral by a SHSC-based physician or the OHS department. Assessments were conducted virtually (either by telephone or video). All patients were offered an initial baseline assessment by either a physician or nurse practitioner. Further follow-up during the acute phase of illness was at the discretion of the clinician doing the initial assessment, but 90-day in-person follow-up was offered to all patients. First, all COVIDEO patients were contacted after 90 days via email survey inquiring about ongoing complications requiring a clinical follow-up, current symptoms and return to functional baseline. Then, patients who replied that they were having symptoms or those who wanted a follow-up were contacted individually via phone call to schedule an appointment.
Data collection
We reviewed patients’ electronic medical records to collect data from baseline COVIDEO assessments and 90-day follow-up assessments, which includes information on demographics, exposure, household contacts, past medical history, laboratory tests, and physical examination. This initial assessment was essential to accurately determine the prevalence of persistent symptoms among the overall population of patients with COVID-19 (to avoid selection bias related to only capturing those requiring or requesting clinical follow-up).
Data analysis
Baseline characteristics for the cohort were described with medians and interquartile ranges (for continuous variable) or frequencies and percentages (for categorical variables). The prevalence of individual symptoms was described for both initial illness and 90-day follow-up. We then grouped individual symptoms into different systems to facilitate more detailed analyses, including: constitutional symptoms (fever, chills, lymphadenopathy, fatigue), upper respiratory (pharyngitis, rhinorrhea, otalgia, conjunctivitis), cardiorespiratory (chest pain, cough, sputum, hemoptysis, dyspnea), gastrointestinal (abdominal pain, nausea, vomiting, diarrhea), rheumatologic (myalgia, arthralgia, rash, hair loss), neurologic (anosmia, dysgeusia, headache, confusion), and psychiatric symptoms (depression, anxiety, insomnia, anorexia). For this analysis, patients were stratified as outpatients (patients tested at the CAC, OHS, dialysis, clinic, or Toronto Public Health) and in-hospital (tested in the ward or ED). We used medians and inter-quartile range for continuous variables, and frequency and percentages for categorical variables.
Bivariate analyses (chi-square test and unadjusted logistic regression) were used to assess potential predictors of persistent symptoms at 90-day follow-up. Predictors of interest included age, sex, presence of a cardiorespiratory comorbidity (asthma, cardiovascular diseases, or chronic lung disease), presence/absence of each symptom group at baseline assessment, location at time of test (outpatient versus in-hospital), and requirement for hospital admission at any time during the course of illness. These analyses were performed separately for the presence of any persistent individual symptom, and then again for the presence of persistent constitutional, upper respiratory, cardiovascular, gastrointestinal, rheumatologic, neurologic, and psychiatric symptoms.
We then performed multivariable logistic regression, incorporating these same pre-specified predictor variables. One multivariable logistic regression model was performed for the outcome of any persistent symptom at 90-day follow-up. Separate multivariable models were then carried out for the presence of persistent constitutional, upper respiratory, cardiovascular, gastrointestinal, rheumatologic, neurologic, and psychiatric symptoms. However, regression models were not conducted for outcomes with insufficient events. Models were exploratory across multiple symptom group outcomes and so we did not perform correction for multiple hypothesis testing. All analyses were performed in SAS Studio Version 5.2 (Cary, NC).
Results
Patients assessed by COVIDEO
Between March 2020 and October 2020, 993 patients were assessed by the COVIDEO program (Table 1). Approximately half of the patients were female (n = 514, 51.7%), and the median age was 38 years (IQR 27–57). Most of the patients assessed by COVIDEO were tested at the outpatient CAC (707, 71.1%). At initial presentation, the most commonly reported symptoms were constitutional symptoms in 629 (63.2%) of patients. 145 patients (14.5%) were hospitalized within 30 days of diagnosis, of which most (140, 96.6%) were already hospitalized at time of testing, 44 (4.4%) required ICU admission, and 34 (3.4%) required mechanical ventilation.
Table 1:
Baseline characteristics of patients with COVID-19 including those responding and not responding to follow-up invitation
| Demographics | No. (%) of patients* | ||||
|---|---|---|---|---|---|
| All patients; n = 993 | Seen at 90-day follow-up; n = 206 | Declined follow-up invitation; n = 260 | Never responded to follow-up; n = 506 | Died prior to 90 days; n = 21 | |
| Age (median, IQR) | 38 (27-57) | 51 (35–61) | 37 (26–54) | 34 (25–53) | 79 (71–88) |
| Sex (female) | 514 (51.7) | 122 (59.2) | 136 (52.3) | 246 (48.6) | 10 (43.5) |
| Pregnant | 20 (2.0) | 7 (3.4) | 5 (1.9) | 8 (1.6) | 0 (0) |
| Health care worker | 206 (20.7) | 66 (32.0) | 57 (21.9) | 83 (16.4) | 0 (0) |
| International travel (within 14 days of symptom onset) | 73 (7.3) | 21 (10.2) | 15 (5.7) | 36 (7.1) | 1 (4.4) |
| Known exposure to case with COVID-19 | 524 (52.7) | 96 (46.6) | 140 (53.8) | 272 (53.8) | 16 (69.6) |
| Household contacts (average per case) | 0.9 | 0.9 | 0.9 | 0.9 | 0.8 |
| Number of days from symptom onset to COVID-19 test | 4.2 (-5 to 42) | 4.4 (-3 to 37) | 4 (-1 to 31) | 4.4 (-4 to 42) | 3.1 (-5 to 11) |
| Location of COVID-19 test | |||||
| Assessment centre | 707 (71.1) | 106 (51.4) | 189 (72.7) | 411 (81.2) | 1 (4.4) |
| In-hospital | 219 (22.0) | 80 (38.8) | 60 (23.0) | 62 (12.3) | 17 (73.9) |
| Number of days from symptom onset to initial COVIDEO assessment | 6.5 (-2 to 48) | 7 (-2 to 48) | 6.5 (1 to 46) | 6.3 (-2 to 44) | 5.2 (2 to 17) |
| Initial symptoms grouped by system | |||||
| Constitutional | 629 (63.2) | 151 (73.3) | 159 (61.2) | 302 (59.7) | 17 (73.1) |
| Cardiorespiratory | 557 (56.0) | 134 (65.1) | 148 (57.0) | 257 (50.8) | 18 (78.3) |
| Upper respiratory | 468 (47.0) | 103 (50.0) | 112 (43.1) | 250 (49.4) | 3 (13.0) |
| Neurological | 527 (53.0) | 121 (58.7) | 141 (54.2) | 255 (50.4) | 10 (43.5) |
| Gastrointestinal | 296 (29.8) | 80 (38.8) | 77 (29.6) | 134 (26.5) | 5 (21.7) |
| Rheumatologic | 373 (37.5) | 96 (46.6) | 103 (39.6) | 173 (34.2) | 1 (4.4) |
| Psychiatric | 290 (29.2) | 79 (38.4) | 76 (29.2) | 133 (25.3) | 7 (30.4) |
| Comorbidities | |||||
| Cardiac disease | 66 (6.6) | 14 (6.8) | 19 (7.3) | 25 (4.9) | 8 (34.8) |
| Hypertension | 176 (17.6) | 57 (27.7) | 45 (17.3) | 61 (12.1) | 13 (56.5) |
| Diabetes | 93 (9.3) | 22 (10.7) | 21 (8.1) | 42 (8.3) | 8 (34.8) |
| Chronic lung disease | 29 (2.9) | 8 (3.9) | 4 (1.5) | 10 (2.0) | 7 (30.4) |
| Asthma | 89 (8.9) | 27 (13.1) | 22 (8.4) | 38 (7.5) | 2 (8.7) |
| Chronic kidney disease | 29 (2.9) | 5 (2.4) | 9 (3.5) | 11 (2.2) | 4 (17.4) |
| Chronic liver disease | 11 (1.1) | 2 (0.9) | 4 (1.5) | 4 (0.8) | 1 (4.4) |
| Malignancy | 62 (6.2) | 14 (6.8) | 19 (7.3) | 20 (4.0) | 9 (39.1) |
| Chronic hematologic disease | 23 (2.3) | 6 (2.9) | 4 (1.5) | 11 (2.2) | 2 (8.7) |
| Rheumatic disease | 17 (1.7) | 7 (3.4) | 5 (1.9) | 3 (0.6) | 2 (8.7) |
| HIV | 4 (0.4) | 0 (0) | 2 (0.8) | 1 (0.2) | 1 (4.6) |
| Chronic neurological disease | 60 (6.0) | 9 (4.3) | 16 (6.2) | 21 (4.2) | 14 (60.9) |
| Obesity | 49 (4.9) | 22 (10.7) | 15 (5.8) | 12 (2.4) | 0 (0) |
| Smoker | 66 (6.6) | 13 (6.3) | 22 (8.4) | 29 (5.7) | 2 (8.7) |
| Malnutrition | 8 (0.8) | 0 (0) | 1 (0.4) | 3 (0.6) | 4 (17.4) |
| Antibiotic therapy | |||||
| Beta-lactam | 96 (9.7) | 28 (13.6) | 31 (11.9) | 24 (4.7) | 13 (56.5) |
| Macrolides | 55 (5.5) | 17 (8.3) | 19 (7.3) | 14 (2.8) | 5 (21.7) |
| Additional household cases | 0.8 (0–6) | 0.8 (0–6) | - | - | - |
| Lymphocyte count | 1.2 (0.5) | 1.2 (0.2–3.3) | 1.1 (0–2.4) | 1.3 (0.3–5.0) | 1.2 (0.4–4.3) |
| Abnormal chest X-ray | 171 (17.1) | 59 (28.6) | 45 (17.3) | 49 (9.7) | 18 (78.3) |
| Hospitalization at or within 30 days of diagnosis | 145 (14.5) | 44 (21.4) | 46 (17.7) | 35 (6.9) | 20 (87.0) |
| Days from diagnosis to hospital admission | 1.4 (-61 to 74) | 2 (-3 to 11) | 3 (-31 to 74) | 0.9 (-61 to 70) | -2.5 (-34 to 6) |
| ICU admission within 30 days of diagnosis | 44 (4.4) | 15 (7.3) | 13 (5.0) | 9 (1.8) | 7 (30.4) |
| Mechanical ventilation within 30 days of diagnosis | 34 (3.4) | 11 (5.3) | 9 (3.5) | 7 (1.4) | 7 (30.4) |
| Duration of hospitalization, d | 1.7 (0–164) | 1.7 (0–23) | 1.6 (0–125) | 1.0 (0–164) | 17.8 (0–61) |
| Duration of mechanical ventilation, d | 6.7 (0–60) | 4.8 (0–21) | 5.8 (0–60) | 8.5 (0–36) | 9.5 (0–52) |
*Unless otherwise specified
Availability for follow-up assessment at 90 days
Among the cohort of patients, 21 (2.1%) died prior to 90 day follow up assessment, 506 (50.9%) did not respond to the offer for follow-up visit, 260 (26.1%) declined follow-up because they were feeling well, and 206 (20.7%) participated in a complete 90-day follow-up assessment. Therefore, information on follow-up symptoms was available for 466 (46.8%) of patients (Table 1). Notably, the patients that did not respond to the offer for follow-up assessment were younger, had fewer comorbidities, and were more likely to have been diagnosed in the outpatient setting (Table 1). Among the 206 patients seen in clinic for a complete 90-day assessment, 133 patients (64.5%) reported at least one residual symptom. Even though more than half of the patients reported at least one symptom, the vast majority (191/206, 93.2%) reported feeling ‘back to baseline’ during the 90-day assessment.
Baseline symptoms by test location
Baseline characteristics and outcomes of patients are described and categorized by test location in Table 2; overall rates of persistent symptoms are described in Figure 1. The total number of patients tested at the outpatient CAC and in-hospital (ED or ward) reporting at least one symptom at initial assessment was 246 (56.6%) and 133 (95%), respectively. Furthermore, the most common reported group of symptoms among those tested as outpatients or in-hospital were constitutional in 178 (40.9%) and 109 (77.9%) patients, respectively.
Table 2:
Initial symptoms and persistent symptoms at 90 days among patients diagnosed with COVID-19 in outpatient and inpatient settings
| Outpatients, no. (%); n = 326 | Inpatients, no. (%); n = 140 | |||
|---|---|---|---|---|
| Initial assessment | 90-day assessment | Initial assessment | 90-day assessment | |
| n (%) | n (%) | n (%) | n (%) | |
| Any symptom | 276 (84.7) | 74 (22.7) | 133 (95) | 59 (42.1) |
| Constitutional | 201 (61.7) | 38 (11.7) | 109 (77.9) | 34 (24.3) |
| Cardiorespiratory | 177 (54.3) | 27 (8.3) | 105 (75.0) | 29 (20.7) |
| Upper respiratory | 164 (50.3) | 10 (3.1) | 51 (36.4) | 11 (7.9) |
| Neurological | 189 (58.0) | 32 (9.8) | 73 (52.1) | 29 (20.7) |
| Gastrointestinal | 86 (26.4) | 3 (0.9) | 71 (50.7) | 5 (3.6) |
| Rheumatologic | 134 (41.1) | 28 (8.6) | 65 (46.4) | 33 (23.6) |
| Psychiatric | 92 (28.2) | 37 (11.4) | 63 (45.0) | 29 (20.7) |
| Fever | 101 (31.0) | 0 (0) | 76 (54.3) | 1 (0.7) |
| Pharyngitis | 95 (29.1) | 2 (0.6) | 22 (15.7) | 3 (2.1) |
| Rhinorrhea | 104 (31.9) | 6 (1.8) | 31 (22.1) | 6 (4.3) |
| Cough | 151 (46.3) | 13 (4.0) | 85 (60.7) | 7 (5.0) |
| Sputum | 24 (7.4) | 2 (0.6) | 25 (5.7) | 5 (3.6) |
| Hemoptysis | 1 (0.3) | 0 (0) | 3 (3.6) | 0 (0) |
| Dyspnea | 33 (10.1) | 10 (3.1) | 49 (35.0) | 16 (11.4) |
| Dyspnea at rest | 5 (1.5) | 2 (0.6) | 9 (6.4) | 3 (2.1) |
| Tachypnea | 9 (2.8) | 1 (0.3) | 12 (8.6) | 0 (0) |
| Breathless today | 11 (3.4) | 2 (0.6) | 12 (8.6) | 3 (2.1) |
| Chills | 76 (23.3) | 1 (0.3) | 44 (31.4) | 1 (0.7) |
| Chest pain | 43 (13.2) | 7 (2.2) | 28 (20.0) | 8 (5.7) |
| Wheezing | 7 (2.2) | 5 (1.5) | 8 (5.7) | 1 (0.7) |
| Otalgia | 15 (4.6) | 0 (0) | 7 (5.0) | 3 (2.1) |
| Conjunctivitis | 15 (4.6) | 4 (1.2) | 8 (5.7) | 0 (0) |
| Anosmia | 93 (28.5) | 18 (5.5) | 25 (17.9) | 10 (7.1) |
| Dysguesia | 107 (32.8) | 12 (3.7) | 22 (15.7) | 4 (2.9) |
| Myalgia | 123 (37.7) | 7 (2.2) | 62 (44.3) | 6 (4.3) |
| Arthralgia | 42 (12.9) | 5 (1.5) | 22 (15.7) | 7 (5.0) |
| Abdominal pain | 21 (6.4) | 3 (0.9) | 27 (19.3) | 2 (1.4) |
| Nausea/vomiting | 44 (13.5) | 0 (0) | 38 (27.1) | 2 (1.4) |
| Diarrhea | 46 (14.1) | 1 (0.3) | 39 (27.9) | 2 (1.4) |
| Lymphadenopathy | 4 (1.2) | 0 (0) | 0 (0) | 1 (0.7) |
| Rash | 6 (1.8) | 5 (1.5) | 2 (1.4) | 7 (5.0) |
| Fatigue | 165 (50.6) | 33 (10.1) | 88 (62.9) | 25 (17.9) |
| Headache | 134 (41.1) | 8 (2.5) | 55 (39.3) | 12 (8.6) |
| Confusion | 5 (1.5) | 17 (5.2) | 12 (8.6) | 14 (10.0) |
| Depression | 2 (0.6) | 12 (3.7) | 1 (0.7) | 9 (6.4) |
| Insomnia | 28 (8.6) | 17 (5.2) | 26 (18.6) | 17 (12.1) |
| Anorexia | 77 (23.6) | 2 (0.6) | 52 (37.1) | 5 (3.6) |
| Weight loss | - | 7 (2.2) | - | 14 (10.0) |
| Hair loss | - | 16 (4.9) | - | 22 (15.7) |
Figure 1:
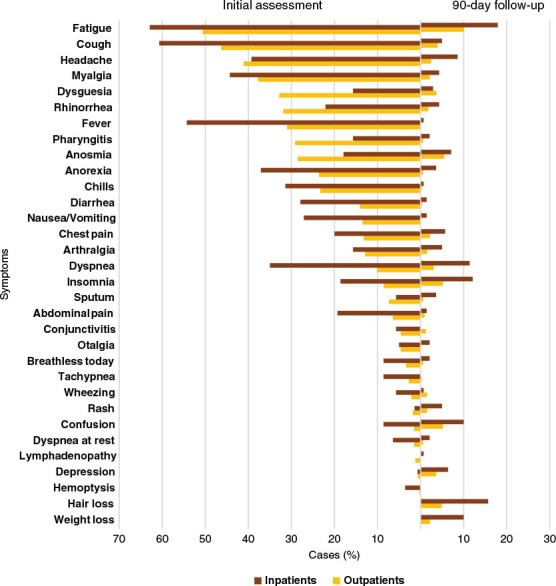
Symptom prevalence at initial assessment and 90-day follow-up among those diagnosed in the inpatient (red) and outpatient (yellow) settings
Prevalence of symptoms after 90 days
Long-term and persistent symptoms among patients with a 90-day assessment are summarized in Figure 1. Just under a third of patients reported persistent symptoms after 90 days from diagnosis (133/466, 28.5%). Constitutional, psychiatric, rheumatologic, and neurological symptoms were the most prevalent symptom groups after 90 days in 72/466 (15.5%), 66/466 (14.2%), 61/466 (13.1%), and 61/466 (13.1%) of patients, respectively. Among individual symptoms, the most common were fatigue (58/466, 12.5%), hair loss (38/466, 8.2%), insomnia (34/466, 7.3%), confusion (31/466, 6.7%), and anosmia (28/466, 6.0%) (Figure 1).
Associations with clinical outcomes after 90 days
Univariate predictors of persistent symptoms at 90 days are summarized in Table 3. Baseline cardiorespiratory comorbidity (asthma, cardiovascular diseases, or chronic lung disease) was significantly more common among those with any symptom than those without any persistent symptoms (25.6% versus 14.4%, p = 0.004). Cardiorespiratory comorbidities were also more common among people with persistent cardiorespiratory symptoms (33.9% versus 15.4%, p = 0.0006) and upper respiratory symptoms (52.4% versus 16.0%, p <0.0001) after 90 days of diagnosis. Similarly, reporting two or more comorbidities at baseline was significantly associated with any persistent symptoms (17.3% versus 9.3%, p = 0.015), as well as persistent constitutional (19.4% versus 10.2%, p = 0.02), gastrointestinal (37.5% versus 11.1% p = 0.02), and rheumatologic symptoms (23.0% versus 9.9%, p = 0.002) at 90-day follow-up.
Table 3:
Univariate predictors of persistent symptoms at 90 days, by symptom group
| Any symptoms at 90 d | Constitutional symptoms at 90 d | Cardiorespiratory symptoms at 90 d | Upper respiratory symptoms at 90 d | Neurological symptoms at 90 d | Gastrointestinal symptoms at 90 d | Rheumatologic symptoms at 90 d | Psychiatric symptoms at 90 d | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | |
| Mean age, y | 42.2 | 50.0 | <0.0001 | 43.0 | 52.3 | <0.0001 | 43.9 | 47.9 | 0.13 | 44.1 | 51.7 | 0.06 | 43.6 | 49.7 | 0.01 | 44.4 | 45.6 | 0.85 | 42.9 | 54.6 | <0.0001 | 43.7 | 48.6 | 0.04 |
| Sex | ||||||||||||||||||||||||
| Female | 53.2 | 60.9 | 0.12 | 54.3 | 61.1 | 0.28 | 54.4 | 62.5 | 0.25 | 54.2 | 81.0 | 0.01 | 52.8 | 72.1 | 0.004 | 55.2 | 62.5 | 0.68 | 54.6 | 60.7 | 0.37 | 54.3 | 62.1 | 0.23 |
| Comorbidities | ||||||||||||||||||||||||
| Cardiorespiratory | 14.4 | 25.6 | 0.004 | 16.5 | 23.6 | 0.14 | 15.4 | 33.9 | 0.0006 | 16.0 | 52.4 | <0.0001 | 16.3 | 26.2 | 0.05 | 17.3 | 37.5 | 0.13 | 16.3 | 26.3 | 0.05 | 16.8 | 22.7 | 0.23 |
| 1 comorbidity | 28.2 | 35.3 | 0.13 | 29.2 | 36.1 | 0.23 | 29.8 | 33.9 | 0.52 | 30.1 | 33.3 | 0.75 | 29.6 | 34.4 | 0.44 | 30.6 | 12.5 | 0.27 | 29.1 | 37.7 | 0.17 | 30.0 | 31.8 | 0.76 |
| 2 comorbidities | 9.3 | 17.3 | 0.015 | 10.2 | 19.4 | 0.02 | 11.0 | 16.1 | 0.26 | 11.2 | 19.1 | 0.27 | 11.1 | 14.8 | 0.4 | 11.1 | 37.5 | 0.02 | 9.9 | 23.0 | 0.002 | 10.8 | 16.7 | 0.16 |
| 3+ comorbidities | 9.3 | 11.3 | 0.51 | 9.4 | 12.5 | 0.41 | 9.3 | 14.3 | 0.23 | 9.2 | 23.8 | 0.02 | 9.6 | 11.5 | 0.65 | 9.8 | 12.5 | 0.8 | 9.4 | 13.1 | 0.36 | 9.8 | 10.6 | 0.82 |
| Test location | ||||||||||||||||||||||||
| CAC | 69.7 | 47.4 | <0.0001 | 66.0 | 48.6 | 0.004 | 66.6 | 39.3 | <0.0001 | 64.3 | 42.9 | 0.04 | 66.2 | 44.3 | 0.0009 | 63.8 | 37.5 | 0.12 | 67.2 | 37.7 | <0.0001 | 66.3 | 45.5 | 0.001 |
| In-hospital | 24.3 | 44.4 | <0.0001 | 26.9 | 47.2 | 0.0005 | 27.1 | 51.8 | 0.0002 | 29.0 | 52.4 | 0.02 | 27.4 | 47.5 | 0.0014 | 29.5 | 62.5 | 0.04 | 26.4 | 54.1 | <0.0001 | 27.8 | 43.9 | 0.007 |
| Hospital admission | 17.6 | 27.9 | 0.01 | 18.6 | 30.4 | 0.02 | 18.7 | 34.0 | 0.01 | 20.1 | 27.8 | 0.43 | 18.5 | 33.3 | 0.009 | 20.4 | 25.0 | 0.74 | 16.5 | 46.6 | <0.0001 | 18.8 | 30.2 | 0.03 |
| ICU admission | 7.0 | 14.4 | 0.03 | 7.9 | 15.7 | 0.07 | 7.4 | 24.2 | 0.001 | 8.2 | 30.8 | 0.005 | 8.2 | 14.3 | 0.17 | 9.4 | 0.0 | 0.43 | 6.5 | 25.0 | <0.0001 | 7.3 | 20.0 | 0.006 |
| Symptomatic initial | 85.0 | 94.7 | 0.003 | 86.8 | 93.1 | 0.13 | 86.6 | 96.4 | 0.03 | 87.2 | 100 | 0.08 | 86.4 | 96.7 | 0.02 | 87.6 | 100 | 0.28 | 86.7 | 95.1 | 0.06 | 87.0 | 92.4 | 0.21 |
| Constitutional | 60.1 | 82.7 | <0.0001 | 63.7 | 81.9 | 0.002 | 63.9 | 85.7 | 0.001 | 65.9 | 81.0 | 0.15 | 64.4 | 80.3 | 0.01 | 66.2 | 87.5 | 0.2 | 64.4 | 80.3 | 0.01 | 64.0 | 81.8 | 0.004 |
| Cardiorespiratory | 57.1 | 69.2 | 0.01 | 59.1 | 68.1 | 0.15 | 58.1 | 78.6 | 0.003 | 59.3 | 85.7 | 0.015 | 58.3 | 75.4 | 0.01 | 60.5 | 62.5 | 0.9 | 59.0 | 70.5 | 0.08 | 59.3 | 68.2 | 0.16 |
| Upper respiratory | 43.5 | 52.6 | 0.07 | 45.2 | 51.4 | 0.33 | 45.1 | 53.6 | 0.23 | 45.8 | 52.4 | 0.55 | 43.7 | 62.3 | 0.006 | 45.9 | 62.5 | 0.34 | 46.4 | 44.3 | 0.75 | 45.0 | 53.0 | 0.22 |
| Neurological | 53.2 | 63.9 | 0.03 | 55.1 | 62.5 | 0.24 | 54.9 | 66.1 | 0.11 | 55.7 | 66.7 | 0.32 | 53.8 | 72.1 | 0.007 | 56.1 | 62.5 | 0.71 | 55.6 | 60.7 | 0.45 | 55.0 | 63.4 | 0.19 |
| Gastrointestinal | 30.7 | 41.4 | 0.02 | 33.3 | 36.1 | 0.63 | 32.2 | 44.6 | 0.06 | 32.8 | 52.4 | 0.06 | 32.1 | 44.3 | 0.06 | 33.4 | 50.0 | 0.32 | 31.6 | 47.5 | 0.01 | 32.0 | 43.9 | 0.05 |
| Rheumatologic | 37.5 | 55.6 | 0.0004 | 40.9 | 52.8 | 0.06 | 40.2 | 60.7 | 0.003 | 42.3 | 52.4 | 0.35 | 39.8 | 62.3 | 0.0009 | 42.4 | 62.5 | 0.25 | 41.2 | 52.5 | 0.09 | 40.0 | 59.1 | 0.003 |
| Psychiatric | 29.4 | 42.9 | 0.005 | 31.2 | 44.4 | 0.02 | 30.2 | 55.4 | 0.0002 | 32.4 | 52.4 | 0.05 | 30.9 | 49.2 | 0.004 | 33.0 | 50.0 | 0.31 | 31.9 | 42.6 | 0.09 | 31.3 | 45.5 | 0.02 |
In hospital testing, as compared to outpatient testing, was associated with nearly all types of persistent symptoms at 90 days (Table 2). Initial constitutional symptoms were strongly associated with persistent symptoms at 90 days, including any symptoms (23.6%, p = <0.0001), constitutional (12.6%, p = 0.002), cardiorespiratory (10.3%, p = 0.001), neurological (10.5%, p = 0.01), rheumatologic (10.5%, p = 0.01), and psychiatric symptoms (11.5%, p = 0.004) (Table 3).
Multivariable predictors of clinical outcomes after 90 days
Among patients with a 90-day assessment, those reporting constitutional symptoms at initial assessment (OR 2.24, 95% CI 1.23 to 4.08), rheumatologic symptoms at initial assessment (OR 1.64, 95% CI 1.01 to 2.64), and those diagnosed in-hospital (OR 2.03, 95% CI 1.24 to 3.33) had increased odds of experiencing at least one persistent symptom (See Table 4).
Table 4:
Multivariate analysis of predictors of persistent symptoms at 90 days, by symptom group
| Odds ratio (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Any symptoms at 90 days | Constitutional symptoms at 90 days | Cardiorespiratory symptoms at 90 days | Upper respiratory symptoms at 90 days* | Neurological symptoms at 90 days | Gastrointestinal symptoms at 90 days* | Rheumatologic symptoms at 90 days | Psychiatric symptoms at 90 days | |
| Age | 1.11 (1.04 to 1.18) | 1.13 (1.04 to 1.22) | 0.99 (0.91 to 1.09) | - | 1.08 (0.99 to 1.18) | - | 1.13 (1.04 to 1.23) | 1.05 (0.97 to 1.13) |
| Sex | ||||||||
| Female | 1.46 (0.94 to 2.28) | 1.46 (0.85 to 2.51) | 1.35 (0.73 to 2.50) | - | 2.39 (1.28 to 4.48) | - | 1.43 (0.79 to 2.59) | 1.42 (0.81 to 2.48) |
| Comorbidities | ||||||||
| Cardiorespiratory | 1.50 (0.88 to 2.55) | 1.15(0.61 to 2.19) | 2.03 (1.06 to 3.89) | - | 1.32 (0.67 to 2.61) | - | 1.31 (0.66 to 2.60) | 1.10 (0.57 to 2.14) |
| Test location | ||||||||
| In-hospital | 2.03 (1.24 to 3.33) | 1.48 (0.81 to 2.70) | 2.37 (1.21 to 4.67) | - | 1.93 (1.01 to 3.7) | - | 1.74 (0.89 to 3.37) | 1.89 (1.02 to 3.51) |
| Hospital admission | 0.89 (0.49 to 1.61) | 1.21 (0.61 to 2.43) | 1.13 (0.53 to 2.42 | - | 1.58 (0.74 to 3.35) | - | 2.38 (1.18 to 4.77) | 1.25 (0.61 to 2.54) |
| Constitutional | 2.24 (1.23 to 4.08) | 2.12 (1.01 to 4.44) | 1.84 (0.75 to 4.5 | - | 1.00 (0.45 to 2.25) | - | 1.38 (0.62 to 3.08) | 1.68 (0.77 to 3.66) |
| Cardiorespiratory | 0.82 (0.50 to 1.36) | 0.82 (0.44 to 1.50) | 1.38 (0.65 to 2.91 | - | 1.05 (0.52 to 2.12) | - | 0.84 (0.43 to 1.65) | 0.78 (0.41 to 1.47) |
| Upper respiratory | 1.33 (0.82 to 2.16) | 1.38 (0.77 to 2.50) | 1.06 (0.54 to 2.07 | - | 1.99 (1.03 to 3.87) | - | 1.16 (0.6 to 2.22) | 1.29 (0.7 to 2.37) |
| Neurological | 1.12 (0.68 to 1.85) | 1.04 (0.57 to 1.90) | 1.00 (0.5 to 2.01 | - | 1.61 (0.8 to 3.24) | - | 1.09 (0.57 to 2.10) | 1.01 (0.54 to 1.89) |
| Gastrointestinal | 0.90 (0.55 to 1.49) | 0.65 (0.35 to 1.21) | 0.75 (0.38 to 1.46 | - | 0.83 (0.43 to 1.61) | - | 1.30 (0.68 to 2.51) | 0.96 (0.52 to 1.77) |
| Rheumatologic | 1.64 (1.01 to 2.64) | 1.40 (0.78 to 2.52) | 1.59 (0.82 to 3.07 | - | 1.93 (1.01 to 3.68) | - | 1.49 (0.78 to 2.83) | 1.80 (0.98 to 3.30) |
| Psychiatric | 1.03 (0.63 to 1.68) | 1.21 (0.67 to 2.18) | 1.99 (1.03 to 3.84) | - | 1.19 (0.63 to 2.26) | - | 0.83 (0.43 to 1.60) | 1.19 (0.65 to 2.19) |
* Insufficient events for multivariable modelling for those two outcomes
Experiencing initial constitutional symptoms (OR 2.12, 95% CI 1.01 to 4.44) was associated with increases odds of constitutional symptoms after 90 days. Some symptoms at diagnosis were predictive of other persistent symptom types at follow-up. For example, having initial rheumatologic symptoms was associated with increased odds of neurological symptoms (OR 1.93, 95% CI 1.01 to 3.68) and psychiatric symptoms (OR 1.8, 95% CI 0.98 to 3.3) after 90 days of diagnosis. Similarly, early upper respiratory symptoms (OR 1.99, 95% CI 1.03 to 3.87) were associated with neurological symptoms after 90 days.
Regarding the test location, being tested in hospital also showed greater odds of experiencing cardiorespiratory (OR 2.37, 95% CI 1.21 to 4.67), neurological (OR 1.93, 95% CI 1.01 to 3.71), and psychiatric (OR 1.89, 95% CI 1.02 to 3.51) symptoms after 90 days.
Presence of a cardiorespiratory comorbidity was associated with higher odds of having any cardiopulmonary symptoms after 90 days of diagnosis (OR 2.03, 95% CI 1.06 to 3.89).
Discussion
In this retrospective cohort study across the full spectrum of consecutive patients with COVID-19, a substantial proportion of patients (28.5%) reported at least one persistent symptom during their 90-day follow-up assessment. Fatigue was the most common persistent individual symptom, and constitutional symptoms were the most common persistent symptom group, but symptomatology in this group was diverse, with 10%–15% still experiencing some cardiorespiratory, neurologic, rheumatologic, and psychiatric symptoms. However, these rates of persistent symptoms, and those from other studies, likely over-estimate the burden of persistent illness, given that patients who did not respond to follow-up invitations tended to be younger, have less comorbidities, and be more likely to have been originally diagnosed in the outpatient setting. Further, even in the context of persistent symptoms, more than 90% of patients reported returning ‘back to baseline,’ suggesting that only the minority were experiencing symptoms severe enough to prevent resumption of daily activities, or that some of the persistent symptoms could have been related to other chronic disease conditions and pre-dated COVID-19 diagnosis.
Several studies have followed survivors of COVID-19 and found that many patients experience a protracted course. While some studies found a prevalence of symptoms of 50% or more, (11,13–16) our study aligns with findings of other groups detecting persistent symptoms in approximately 1/3 of patients (17,18). Our study also contradicts previous findings from a prevalence study in France were 30% of patients did not return to work after 6 months due to disabling consequences (6). Our study focused on the full spectrum of patients with COVID-19 illness, whereas most prior research has been limited to those with initial infection that was severe enough to require hospitalization. Similar to previous studies, fatigue, dyspnea, and anosmia (11,13–17,19) were among the most prevalent persistent symptoms.
Persistent symptoms at 90 days were associated with older patient age, comorbidity, in-hospital diagnosis, and extent of initial symptoms. Our findings support data from the United Kingdom, United States, Sweden, and France, in which patients with older age and more extensive initial symptoms were more likely to experience prolonged symptoms (11,20). Since older and sicker patients, and those with more significant initial infection are more likely to participate in follow-up visits, most long-term outcome studies will naturally over-estimate the prevalence of persistent symptoms. Unlike some other studies, we did not detect a higher rate of most post-infectious symptoms in women (only higher neurologic symptoms). However, our comparison of patients presenting versus not presenting to follow-up, did indicate that women were more likely than men to present for full assessment.
Some of the post-COVID-19 persistent symptoms likely represent direct sequelae of SARS-CoV-2 infection. For example, anosmia is a highly specific symptom of this virus (20) and has been attributed to invasion of the olfactory nerve and supportive tissues. Other SARS-CoV-2 specific effects may range from tachycardia due to autonomic dysfunction to fibrosis from lung injury and thrombosis (22). However, it is probable that most persistent symptoms following COVID-19 are not specific to the SARS-CoV-2 virus. One clear example is telogen effluvium, hair loss characterized by diffuse hair shedding, resulting from early entry of hair into the telogen phase. It is not specific to COVID-19 and can be triggered by any acute infection, systemic disease, stressful life event or other insults (23). Among patients with severe disease requiring intensive care unit and ventilator support, many of the persistent symptoms involve common sequelae of critical illness and acute respiratory distress syndrome, including deconditioning, muscle weakness, polyneuropathy, respiratory dysfunction, and post-traumatic stress (24). Even among patients with milder disease, many of the common post-COVID-19 symptoms are similar to post-infectious functional symptomatology seen after other routine viral infections (25).
As with most studies of long-term COVID-19 complications, our cohort is limited by potential selection bias given that not all patients returned for follow-up. However, unlike other studies, we are able to compare baseline characteristics among those with and without long-term follow-up, and in doing so we can infer that the rates of long-term complications are likely over-estimates rather than under-estimates—given that younger, healthier patients were less likely to return to clinic. Another reason that our study is likely to over-estimate long-term COVID-19 complication rates, as will other studies, is that it is difficult to determine whether some of the reported symptoms may be chronic, with onset pre-dating the COVID-19 infection. We established our symptom checklist at the onset of pandemic and maintained the same list throughout, and therefore we did not systematically capture some symptoms related to new and unexpected syndromes which became notable as the pandemic progressed, such as palpitations. Our follow-up was limited to 3 months, and so cannot extrapolate to later time points following infection. Lastly, our study was intentionally pragmatic and derived from routinely offered clinical care, and so does not provide comprehensive data on physiologic, laboratory and radiology outcomes. In contrast with most literature, our cohort was strengthened by inclusion of all consecutive patients, across the full spectrum of disease severity, with standardized assessments on initial assessment and again at 90 days.
In a pandemic unrivalled in the past century, hundreds of millions of patients have developed the same new virus infection over a short period of time. Our data suggest that among survivors we should expect that close to one-third may experience persistent symptoms at 3 months, but far fewer will have symptoms severe enough to limit their return to routine activities. Therefore, most studies likely over-estimate the burden of post-COVID-19 symptoms. Persistent symptoms will likely be more common among older patients, those with baseline comorbidity, and those hospitalized for their acute illness. Younger, healthier patients are more likely to be non-responders to follow-up offers, and so most studies likely over-estimate the prevalence of post-COVID-19 symptoms. Given the scope of the pandemic, we will require novel approaches to address COVID-19-specific pathology and to manage post-infectious functional impairment. A greater understanding of the mechanisms underpinning post-COVID-19 symptoms will also be needed to guide development of effective preventative and therapeutic strategies.
Ethics Approval:
This study was approved by the SHSC Research Ethics Board. Informed consent for collection of baseline data and 90-day assessment data (from in-person assessments) was not required as this was a retrospective analysis of data collected via review of clinical charts. However, verbal informed consent was obtained to gather 90-day symptom profile from patients that chose not to attend the 90-day follow-up clinic because for these patients the data collection was beyond their routine care.
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Data Accessibility:
All data will not be made publicly available. Researchers who require access to the study data can contact the corresponding author for further information.
Funding:
This work was supported by funding from the Sunnybrook COVID-19 Research Initiative.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
Funding Statement
This work was supported by funding from the Sunnybrook COVID-19 Research Initiative.
References
- 1.World Health Organization. COVID-19 Weekly Epidemiological Update. 2021. [Google Scholar]
- 2.Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395(10225):734. 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Government of Canada. Coronavirus disease (COVID-19): Outbreak update. 2021. [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. 10.1001/jama.2020.2648. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–7. 10.1093/cid/ciaa415. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–62. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S. Novel Wuhan (2019-nCoV) Coronavirus. Am J Respir Crit Care Med. 2020;201(4):P7-p8. 10.1164/rccm.2014P7. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl). 2020;133(9):1015–24. 10.1097/CM9.0000000000000722. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–7. 10.1002/jmv.25689. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosn J, Piroth L, Epaulard O, et al. French COVID cohort study and investigators groups. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: Results from a large prospective cohort. Clin Microbiol Infect. 2021; 27(7):1041.e1–1041.e4. 10.1016/j.cmi.2021.03.012. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam PW, Sehgal P, Andany N, et al. A virtual care program for outpatients diagnosed with COVID-19: A feasibility study. CMAJ Open. 2020;8(2):E407. 10.9778/cmajo.20200069. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6): 603–5. 10.1001/jama.2020.12603. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: A cohort study in Israeli patients. Clin Microbiol Infect. 2021;27(5):769–74. 10.1016/j.cmi.2021.02.008. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021; 27(9):1607–13. 10.1038/s41591-021-01433-3. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands—A longitudinal study among non-hospitalized patients. Clin Infect Dis. 2021;73(11)e4058–63. 10.1093/cid/ciaa1792. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehme M, Braillard O, Alcoba G, et al. COVID-19 symptoms: Longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021; 174(5):723–5. 10.7326/M20-5926. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830-e. 10.1001/jamanetworkopen.2021.0830. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peghin M, Palese A, Venturini M, et al. Post- COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507–13. 10.1016/j.cmi.2021.05.033. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31. 10.1038/s41591-021-01292-y. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–40. 10.1038/s41591-020-0916-2. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir Med. 2020;8(8):750–2. 10.1016/S2213-2600(20)30222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivetti N, Barruscotti S. Management of telogen effluvium during the COVID-19 emergency: Psychological implications. Dermatol Ther. 2020;33(4):e13648. 10.1111/dth.13648. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. NEJM. 2011;364(14):1293–304. 10.1056/NEJMoa1011802. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Burke MJ, del Rio C. Long COVID has exposed medicine’s blind-spot. Lancet Infect Dis. 2021;21(8):1062–4. 10.1016/S1473-3099(21)00333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will not be made publicly available. Researchers who require access to the study data can contact the corresponding author for further information.


