Abstract
BACKGROUND
Bloodstream infections (BSIs) in hospitalized patients represent sentinel events requiring timely and responsive antimicrobial prescribing. These infections represent an attractive but seldom-evaluated stewardship opportunity.
METHODS
Retrospective pre-post study design, with review of patient charts 18 months before and after initiation of a hospital Bloodstream Infection Stewardship Program (BSISP). Pre-intervention, the ward and attending physician were notified of all positive blood cultures. Post-intervention, an infectious disease (ID) pharmacist collaborating with an ID consultant was also notified.
RESULTS
Two hundred twenty-six eligible BSIs were identified pre-intervention and 195 post-intervention. The urinary tract was the most common source of infection; most common bloodstream isolates were Escherichia coli, Staphylococcus aureus, beta-hemolytic streptococci, and Klebsiella pneumoniae; 71.7% of infections were community acquired. Empiric therapy was not given in 17.3% of cases and inadequate in 16.4% of patients. Therapy was altered on the basis of Gram stain results (‘directed therapy’) in 54.6% of episodes and was inadequate in 3.5%. Compared to pre-intervention, the post-intervention cohort received directed therapy on average 4.36 hours earlier (p = 0.003), was more likely to receive appropriate definitive therapy (99.0% post versus 79.1% pre, p <0.001), stepped down to oral therapy earlier (6.0 versus 8.0 days, p = 0.031), and received fewer directed prescriptions (214 per 100 cases post versus 260 per 100 cases pre; p = 0.001), including fewer prescriptions of quinolones and clindamycin.
CONCLUSIONS
A BSISP could be an effective strategy for improving antimicrobial prescribing in hospitalized patients with a BSI.
Keywords: antimicrobial stewardship, bloodstream infections
Abstract
HISTORIQUE
Chez les patients hospitalisés, les infections sanguines sont des événements sentinelles qui exigent des prescriptions antimicrobiennes opportunes et adaptées. Ces infections représentent une possibilité de gestion attrayante, mais rarement évaluée.
MÉTHODOLOGIE
Les chercheurs ont utilisé une méthodologie d’étude avant-après comportant l’analyse des dossiers des patients 18 mois avant et après un programme de gestion des infections sanguines (PGIS) en milieu hospitalier. Avant l’intervention, le médecin du service a été avisé de toutes les cultures sanguines positives. Après l’intervention, un pharmacien infectiologue qui collaborait avec un consultant en infectiologie a également été avisé.
RÉSULTATS
Au total, les chercheurs ont relevé 226 infections sanguines admissibles avant l’intervention et 195 après l’intervention. Les voies urinaires étaient la principale source d’infection et les principaux isolats sanguins, l’Escherichia coli, le Staphylococcus aureus, les streptocoques bêta-hémolytiques et le Klebsiella pneumoniae; 71,7 % des infections étaient d’origine communautaire. Dans 17,3 % des cas, les patients n’ont pas reçu de traitement empirique et chez 16,4 % des patients, le traitement n’était pas approprié. Il était modifié en fonction des résultats de la coloration de Gram de base (« thérapie dirigée ») dans 54,6 % des épisodes et n’était pas approprié dans 3,5 % des cas. Par rapport à celle d’avant l’intervention, la cohorte d’après l’intervention a reçu une thérapie dirigée en moyenne 4,36 heures plus tôt (p = 0,003) et était plus susceptible de recevoir un traitement définitif approprié (99,0 % après par rapport à 79,1 % avant, p <0,001), de passer à un traitement par voie orale plus rapidement (6,0 jours plutôt que 8,0, p = 0,031) et de recevoir moins d’ordonnances dirigées (214 sur 100 cas après, par rapport à 260 sur 100 cas avant; p = 0,001), y compris moins d’ordonnances de quinolones et de clindamycine.
CONCLUSIONS
Un PGIS pourrait être une stratégie efficace pour améliorer les prescriptions d’antimicrobiens chez des patients hospitalisés atteints d’une infection sanguine.
Mots-clés : gestion des antimicrobiens, infections sanguines
Bloodstream infections (BSIs) are associated with significant morbidity, mortality, and prolonged length of stay (1,2). Population-based data for the United States shows an increase in BSIs over time, with an estimated incidence of 536,000 to 628,000 cases per year, ranking among the top seven causes of death (3). Further increases in morbidity and mortality can be expected due to an increase in the relative proportion of BSIs caused by multi-drug resistant gram-negative bacilli in numerous jurisdictions (4,5).
Antimicrobial therapy for these infections requires a complex four-stage process of care beginning with the rapid initiation of an appropriate therapy (‘empiric therapy’), followed by re-evaluation of that therapy once preliminary microbiology results are made available (‘directed therapy’), with further modification once final identification and susceptibility results are obtained (‘definitive therapy’), and eventual transition to oral therapy in many cases (‘step-down therapy’). Facilitation of outpatient parenteral antimicrobial therapy is also a frequent requirement in this population.
BSIs are managed in most hospitals in North America by the attending physician, who is called by the microbiology laboratory with Gram stain results when a positive blood culture is first identified. Several studies have documented that 40%–50% of patients with BSI receive inadequate empiric antimicrobial therapy and 8%–20% still receive inadequate definitive therapy (6–10). ID consultation is only sought in a minority of patients despite data indicating that this leads to improvements in both the process and outcome of care for patients hospitalized with infectious diseases in general, as well as for specific patient subgroups (eg, Staphylococcus aureus bacteremia, enterococcal bacteremia, candidemia) (6,8–16).
Although many hospitals in North America monitor BSIs, most do not have an established team to respond to these events in a timely fashion on a dedicated basis. Given the time-sensitive and complex process of care required for optimal antimicrobial delivery in these patients, we developed a BSISP whereby an interdisciplinary team was called at the same time as the attending physician when positive blood cultures were first identified. The primary objective of this study was to measure the impact of a BSISP on the process of care in hospitalized patients with newly identified BSI.
Methods
Study population and design
The study population was comprised of patients hospitalized with BSI at The Moncton Hospital, a 380-bed tertiary care teaching hospital located in New-Brunswick, Canada. Patients were identified retrospectively by microbiology laboratory BSI records. The pre-intervention cohort consisted of BSI cases identified over an 18-month period from October 1, 2009 to March 31, 2011. The post-intervention cohort consisted of BSI cases identified from October 1, 2011 to March 31, 2013. Cases were excluded from both cohorts if the patient was palliative, died within 48 hours of admission or was less than 14 years of age. For patients with multiple separate BSIs, only the first BSI was included in the study. Patients were also excluded from the second cohort if they were not assessed by the BSISP team.
The two cohorts were compared using a pre-post study design, with a primary objective to explore the impact of a BSISP on processes of care. Six process of care outcomes were identified as primary outcomes: adequacy of directed antimicrobial therapy, time to first-dose of directed antimicrobial therapy, adequacy of definitive therapy, time to step-down to oral antimicrobial, duration of antimicrobial therapy, and total antimicrobial expenditures. Three outcomes of care were identified as secondary outcomes: duration of BSI, length of stay, and total and BSI-related mortality. Analysis of the impact of obtaining an ID consultation was added when it was found that the intervention increased the frequency of ID consultation.
The study was approved by the Research Ethics Board for the Horizon Health Network.
Intervention
The pre-intervention cohort was managed as per standard of care. Notification of all positive blood cultures were called to the patient’s ward and the attending physician, with an ID consultation at the discretion of the attending physician.
The intervention consisted of a BSISP interdisciplinary team (an ID pharmacist collaborating with an ID consultant, the medical laboratory technologist, and the medical microbiologist) that was active from 8:00 a.m.–4:00 p.m. Monday to Friday. Surveillance on Monday morning also included a review of any positive bloodstream cultures that were identified over the weekend.
Post-intervention, notification of positive blood cultures was called to the ward, the attending physician, and to the ID pharmacist, who reviewed the chart and laboratory data and interviewed the patient. Bedside assessment was carried out by the ID consultant if the case was not straight-forward, with a recommendation for full consultation in complex cases (eg, S. aureus bacteremia, risk factors for endocarditis or other deep space infection). A note was made describing the infectious syndrome with recommendations regarding investigation and treatment. If an immediate change in therapy or an ID consultation was indicated, then the attending physician was called to seek collaborative approval. Patients were generally followed by the BSISP team until their clinical status improved with a clear treatment plan delineated.
Definitions
BSI and source
BSI was defined as per Centers for Disease Control guidelines (17). A BSI was declared if a recognized pathogen was isolated from one or more blood culture vials or if a skin commensal was identified from at least two separate blood culture vials drawn at different time periods in association with sepsis symptoms or signs without an alternative focus. A bloodstream contaminant was designated if a skin commensal species was only identified in one set of blood cultures. Community acquired BSI was defined as any BSI identified ≤48 hours post-admission; hospital-acquired BSI was defined as any positive blood culture identified >48 hours post-admission. The source of infection was determined according to attending physician chart documentation supplemented by chart review by an ID consultant.
Microbiology laboratory processing
All blood cultures were processed using an automated system (BACT/ALERT 3D™; bioMérieux), with continuous agitation. Micro-organisms were identified using standard procedures. Clinical and Laboratory Standards Institute (CLSI) breakpoints were utilized for determining antimicrobial susceptibility. This study was performed prior to the introduction of molecular rapid diagnostic testing for bloodstream isolates in our laboratory.
Antimicrobial therapy
Empiric therapy was defined as antimicrobial therapy administered for fever or other signs of systemic infection less than 72 hours before the first positive blood culture was reported. Directed therapy was defined as any modification to empiric therapy less than 48 hour after the first positive blood culture was reported. Definitive therapy was defined as all antimicrobial therapy delivered more than 48 hours after the positive blood culture was first reported, by which point susceptibility results were usually available. Step-down therapy was defined as any switch from intravenous (IV) to oral therapy delivered for the BSI. Time to directed therapy was calculated from the time the positive blood culture was reported until directed therapy was given. Empiric and directed therapy were defined as adequate if the pathogen was susceptible to the chosen therapy regardless of other measures of appropriateness. Definitive therapy was defined as appropriate if the chosen therapy was active according to in vitro susceptibility testing, with an appropriate guideline-concordant spectrum, route, dose, and duration.
Antimicrobial resistance for gram-negative micro-organisms was defined as any resistance to aminoglycosides, third-generation cephalosporins, quinolones, extended spectrum penicillin or carbapenems. Antimicrobial resistance for gram-positive micro-organisms was defined as any resistance to oxacillin or vancomycin. Antimicrobial cost was calculated from the time of BSI notification until the time of discharge.
Statistical analysis
All tests of significance were two-tailed. For continuous variables, comparisons between the two cohorts were performed using the Welch’s t-test for symmetrically distributed variables and the Mann-Whitney U test for non-symmetrically distributed variables. For dichotomous variables, the Fisher exact test was used. For non-dichotomous qualitative variables, the chi-square was used (or its Monte Carlo simulated version if there were several cells with low frequencies).
The distribution of costs and of some time durations (eg, length of stay) were approximately lognormal (ie, the distribution of the logarithm of the values was approximately normal). In such cases, the geometric means (which can be interpreted as the median), as well as the ordinary (arithmetic) means are reported.
For lognormal variables, some analyses were carried out on the logarithm of the values. Where a p-value has been associated with two geometric means, it came from a Welch t-test applied to the log of the values.
Analysis of the number of antimicrobials for directed therapy was carried out using a Poisson regression on that number minus one.
Step-wise logistic regression was performed to explore the impact of multiple factors on mortality.
Results
During the pre-intervention study period, 371 BSI cases were identified with positive blood cultures. Seventy-two cases were excluded (36 patients were never admitted, 18 patients died or were deemed palliative within the first 48 hours, 12 charts were incomplete, 6 BSI cases were the patient’s second or later BSI). This left 226 patients with verified BSI and 73 bloodstream contaminants.
During the post-intervention period, 348 patients were identified with positive blood cultures. One hundred and five patients were excluded (37 were not seen by the BSISP due discharge prior to evaluation or stay less than 48 hours, 29 were not admitted, 24 died or were deemed palliative within the first 48 hours, 10 charts were incomplete, and 5 BSI cases were the patient’s second or later BSI). This left 195 patients with verified BSI and 48 bloodstream contaminants.
Cohort characteristics
There was no significant difference in baseline characteristics between the two cohorts (Table 1). BSI was community acquired in 71.7% of cases and 35.4% were either already in the ICU or required ICU admission at the time of their bacteremic event.
Table 1:
Baseline characteristics for pre- and post- implementation bloodstream infection cases
| Variable | Pre-intervention; n = 226 | Post-intervention; n = 195 | p-value |
|---|---|---|---|
| Age, y, mean (SD) | 66.3 (17.0) | 63.9 (17.1) | 0.49 |
| Male, no. (%) | 122 (54.0) | 110 (56.4) | 0.63 |
| Charlson Index, mean (SD) | 5.5 (3.4) | 5.0 (3.4) | 0.14 |
| ICU admission, no. (%) | 78 (34.5) | 71 (36.4) | 0.68 |
| ICU length-of-stay Mean days (SD) Geometric mean days (SD*) |
n = 78 20.0 (31.1) 9.7 (×/÷ 3.1) |
n = 71 12.9 (14.6) 8.0 (×/÷ 2.6) |
0.07 0.26 |
| Mechanical ventilation, no. (%) | 31 (13.7) | 26 (13.3) | 1.00 |
| Surgery, no. (%) | 52 (23.0) | 53 (27.2) | 0.37 |
| Dialysis, no. (%) | 8 (3.5) | 11 (5.6) | 0.35 |
| Community acquired, no. (%) | 167 (73.9) | 135 (69.2) | 0.33 |
*For a geometric mean, the variability is multiplicative; for example, 10 (×/÷2) indicates a range from 5 to 20, which would include approximatively 86% of the observations if the distribution is lognormal
There was no significant difference in the BSI source between cohorts (Table 2). The urinary tract was the most common source, occurring in 26.4% of cases.
Table 2:
Source of the bloodstream infection for pre-and post-intervention cases
| Infection source | Pre-intervention, no. (%); n = 226 | Post-intervention, no. (%); n = 195 | Total, no. (%); n = 421 | p-value |
|---|---|---|---|---|
| Urinary tract | 51 (22.6) | 60 (30.8) | 111 (26.4) | 0.08 |
| Intra-abdominal | 25 (11.1) | 29 (14.9) | 54 (12.8) | |
| Biliary | 31 (13.7) | 14 (7.2) | 45 (10.7) | |
| Endovascular | 19 (8.4) | 19 (9.7) | 38 (9.0) | |
| Skin/soft tissue | 18 (8.0) | 17 (8.7) | 35 (8.3) | |
| Line sepsis | 17 (7.5) | 17 (8.7) | 34 (8.1) | |
| Pneumonia | 20 (8.8) | 9 (4.6) | 29 (6.9) | |
| Neutropenic sepsis | 13 (5.8) | 8 (4.1) | 21 (5.0) | |
| Bone and joint | 7 (3.1) | 8 (4.1) | 15 (3.6) | |
| Surgical site | 5 (2.2) | 7 (3.6) | 12 (2.9) | |
| Central nervous system | 2 (0.9) | 2 (1.0) | 4 (1.0) | |
| Unknown | 15 (6.6) | 5 (2.6) | 20 (4.8) | |
| Miscellaneous | 3 (1.3) | 0 (0.0) | 3 (0.7) | |
| Total | 226 (100.0) | 195 (100.0) | 421 (100.0) |
There was no significant difference in BSI microbiology between cohorts (p = 0.078; Table 3). Escherichia coli, S. aureus, beta hemolytic streptococci, and Klebsiella pneumoniae were the most common bloodstream isolates.
Table 3:
Bloodstream infection microbiology
| Species | Pre-intervention, no. (%) | Post-intervention, no. (%) | Total, no. (%) | p-value |
|---|---|---|---|---|
| Escherichia coli | 78 (30.2) | 53 (23.8) | 131 (27.2) | 0.078 |
| Staphylococcus aureus | 42 (16.3) | 40 (17.9) | 82 (17.0) | |
| Beta-hemolytic streptococci | 21 (8.1) | 19 (8.5) | 40 (8.3) | |
| Klebsiella sp | 19 (7.4) | 18 (8.1) | 37 (7.7) | |
| Other Enterobacteriaceae | 11 (4.3) | 21 (9.4) | 32 (6.7) | |
| Enterococcus faecalis | 11 (4.3) | 13 (5.8) | 24 (5.0) | |
| Viridans group streptococci | 12 (4.7) | 11 (4.9) | 23 (4.8) | |
| Other enterococci | 5 (1.9) | 10 (4.5) | 15 (3.1) | |
| Pseudomonas aeruginosa | 12 (4.7) | 3 (1.3) | 15 (3.1) | |
| Coagulase negative staphylococci | 7 (2.7) | 8 (3.6) | 15 (3.1) | |
| Candida sp | 9 (3.5) | 5 (2.2) | 14 (2.9) | |
| Bacteroides sp | 8 (3.1) | 5 (2.2) | 13 (2.7) | |
| Other anaerobes | 4 (1.6) | 9 (4.0) | 13 (2.7) | |
| Streptococcus pneumoniae | 9 (3.5) | 2 (0.9) | 11 (2.3) | |
| Clostridium sp | 4 (1.6) | 1 (0.4) | 5 (1.0) | |
| Other | 6 (2.3) | 5 (2.2) | 11 (2.3) | |
| Total* | 258 (100.0) | 223 (100.0) | 481 (100.0) |
* Total is greater than number of cases due to polymicrobial infections
Polymicrobial infection was observed in 48 cases (pre: 12.4%; post: 10.3%; p = 0.809). Antimicrobial resistance was identified in 38 of the 192 Enterobacteriaceae cases (pre: 18.7%; post: 21.2%; p = 0.717), in 4 of the 82 S. aureus cases (pre :7.1%; post: 2.4%; p = 0.616), and in 29 of the 186 ‘other isolates’ cases (pre: 16.8%; post: 14.1%; p = 0.687).
BSISP activity
In the post-intervention group, the BSISP team made 373 therapeutic recommendations for 173 (88.7%) cases (Figure 1). Three hundred and seventy (99.2%) recommendations were accepted for 170 (98.3%) cases. The most frequent recommendation was to change the duration of therapy. The BSISP team initiated therapy in 14 bacteremic cases that were untreated when the positive culture was first identified. All patients in the post-intervention cohort received parenteral antimicrobial therapy at some point during treatment; seven bacteremic patients in the pre-therapy cohort received no parenteral antimicrobials (p = 0.02). The ID consultation rate was 25.9% higher post-intervention (pre: 84/226 [37.2%] post: 123/195 [63.1%]; p <0.001) even though the BSISP team recommended an ID consultation in only 24 (12.3%) of the cases.
Figure 1:
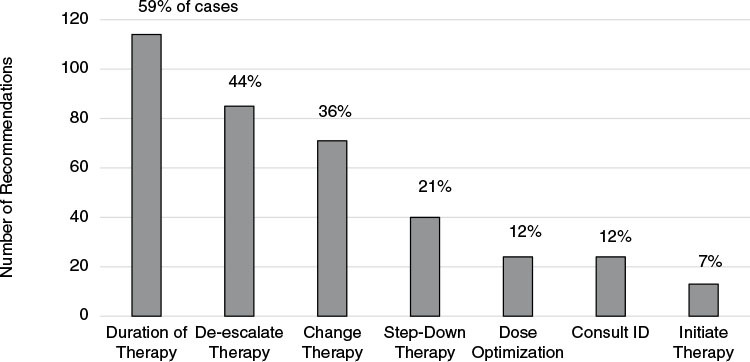
Frequency of recommendations from the bloodstream infection stewardship program team
Process of care outcomes
Administration and adequacy of empiric therapy was similar in both cohorts (Table 4). The post-intervention cohort received directed therapy on average 4.36 hours earlier than the pre-intervention cohort (p = 0.003) and was more likely to receive appropriate definitive therapy (99.0% versus 79.1%, p <0.001). For the 118 patients stepped down from IV therapy to oral therapy, patients in the post-intervention cohort were stepped down to oral therapy two days earlier (pre: 8.0 days; post: 6.0 days, p = 0.031); only one (0.5%) post-intervention patient stepped down after more than 12 days of IV therapy versus 11 (4.9%) patients pre-intervention.
Table 4:
Antimicrobial therapy
| Variable | Pre-intervention; n = 226 | Post-intervention; n = 195 | p-value |
|---|---|---|---|
|
Empiric therapy, no. (%) None Adequate Inadequate |
38 (16.8) 155 (68.6) 33 (14.6) |
35 (17.9) 136 (69.7) 24 (12.3) |
0.79 |
|
Directed therapy, no. (%) None Adequate Inadequate |
110 (48.7) 110 (48.7) 6 (2.7) |
81 (41.5) 112 (57.4) 2 (1.0) |
0.128 |
|
Time to first dose directed therapy, mean hours (SD) |
13.4 (12.5) |
9.0 (8.9) |
0.003 |
|
Appropriate definitive therapy, no. (%) |
178 (79.1) |
193 (99.0) |
<0.001 |
|
Parenteral antimicrobial therapy, no. (%) No IV therapy IV to oral step down No oral therapy |
7 (3.1) 67 (29.6) 152 (67.3) |
0 (0.0) 51 (26.2) 144 (73.8) |
0.02 |
|
Time to IV-PO step down, d Mean (SD) Geometric mean |
8.0 (6.7) 6.2 (×/÷ 2.1) |
6.0 (2.9) 5.3 (×/÷ 1.7) |
0.031 0.190 |
|
Antimicrobial duration, d Mean (SD) Geometric mean |
12.8 (10.3) 9.6 (×/÷ 2.1) |
15.0 (12.6) 11.7 (×/÷ 2.0) |
0.059 0.014 |
|
Total antimicrobial cost, CAN$ Mean (SD) Geometric mean |
323 (931) 117 (×/÷ 4.17) |
275 (515) 126 (×/÷ 3.53) |
0.50 0.21 |
|
Total antimicrobial cost after report of positive blood culture, CAN$ Mean (SD) Geometric mean |
306 (924) 83 (×/÷ 5.5) |
260 (511) 105 (×/÷ 4.3) |
0.516 0.121 |
While there was a trend toward longer antimicrobial duration for treatment of BSI post-intervention, treatment durations were shorter for bloodstream contaminants (5.4 days versus 1.9 days, p <0.0001).
For patients receiving directed therapy, the mean number of directed antimicrobial prescriptions was 214.0 per 100 cases post-intervention compared with 259.5 per 100 cases pre-intervention (p = 0.001 (Table 5); the decrease in geometric mean was 45.4%. The proportion of cases prescribed more than two directed antimicrobials was lower. There were remarkable decreases in prescription rates for quinolones (pre: 32.8 prescriptions per 100 cases; post: 16.7 prescriptions per 100 cases), beta-lactam/beta-lactamase inhibitors, aminoglycosides, and clindamycin.
Table 5:
Number of directed antimicrobials
| Antimicrobial prescription | Pre-intervention; n = 116 | Post-intervention; n = 114 | Change | ||
|---|---|---|---|---|---|
| Number | Number/100 cases | Number | Number/100 cases | Number/100 cases | |
| Penicillin | 40 | 34.5 | 41 | 36 | 1.5 |
| Beta-lactam/beta-lactamase inhibitor | 47 | 40.5 | 36 | 31.6 | –8.9 |
| Cephalosporin 1st generation | 25 | 21.6 | 28 | 24.6 | 3 |
| Cephalosporin 2nd generation | 5 | 4.3 | 0 | 0 | –4.3 |
| Cephalosporin 3rd generation | 34 | 29.3 | 40 | 35.1 | 5.8 |
| Quinolones | 38 | 32.8 | 19 | 16.7 | –16.1 |
| Sulfamethoxazole/trimethoprim | 1 | 0.9 | 3 | 2.6 | 1.8 |
| Carbapenem | 15 | 12.9 | 17 | 14.9 | 2 |
| Clindamycin | 12 | 10.3 | 3 | 2.6 | –7.7 |
| Aminoglycoside | 17 | 14.7 | 7 | 6.1 | –8.5 |
| Vancomycin | 40 | 34.5 | 36 | 31.6 | –2.9 |
| Macrolide | 1 | 0.9 | 0 | 0 | –0.9 |
| Fluconazole | 5 | 4.3 | 2 | 1.8 | –2.6 |
| Caspofungin | 3 | 2.6 | 3 | 2.6 | 0 |
| Metronidazole | 17 | 14.7 | 9 | 7.9 | –6.8 |
| Daptomycin | 1 | 0.9 | 0 | 0 | –0.9 |
| All antimicrobials | 301 | 259.5 | 244 | 214 | –45.4* |
* The total number of different antimicrobials prescribed per patient was less post-intervention than pre-intervention (p = 0.001)
Clinical outcomes
Post-intervention patients experienced a shorter duration of fever (pre: 2.9 days; post: 2.3 days, p = 0.043) (Table 6). The duration of fever was greater than 5 days for 13.4% of cases pre-intervention and 5.3% post-intervention.
Table 6:
Clinical outcomes
BSI = Bloodstream infection
|
Variable |
Pre-intervention; n = 226 |
Post-intervention; n = 195 |
p-value |
|---|---|---|---|
|
Presence of fever, no. (%) |
127 (56.7) |
152 (77.9) |
<0.001 |
|
Duration of fever, d Mean (SD) Geometric mean |
2.9 (2.6) 2.1 (×/÷ 2.1) |
2.3 (1.8) 1.8 (×/÷ 1.9) |
0.043 0.098 |
|
Presence of hypothermia, no. (%) |
107 (47.8) |
113 (57.9) |
0.040 |
|
14-day mortality attributed to BSI, no. (%) |
17 (7.5) |
11 (5.6) |
0.56 |
|
Overall mortality, no. (%) |
35 (15.5) |
28 (14.4) |
0.78 |
|
Length of stay, d Mean (SD) Geometric mean (SD) |
38.5 (68.2) 17.0 (×/÷ 3.3) |
42.8 (82.9) 19.5 (×/÷ 3.3) |
0.56 0.23 |
|
Length of stay: survivors only, d Mean (SD) Geometric mean (SD) |
n = 191 32.6 (56.7) 15.0 (×/÷ 3.2) |
n = 167 40.6 (85.7) 17.8 (×/÷ 3.3) |
0.307 0.180 |
The combined hospital mortality rate for the two cohorts was 22.7% (105/463), before excluding patients who were made palliative or died in the first 48 hours of the hospital stay. There was a trend toward an association of 14-day mortality with inadequate therapy in 13/17 (76.5%) of cases pre-intervention and 4/11 (36.4%, p = 0.05), post-intervention, mostly due to absent or inadequate empiric therapy.
Impact of ID consultation
The intervention led to a much higher ID consultation rate (pre: 37.2%; post: 63.1%). Accordingly, we explored the impact of ID consultation in more detail. Longer fever duration was associated with an increased likelihood of an ID consultation. When ID consultation was obtained, fever duration was relatively shorter post-intervention. Without an ID consultation, fever duration was similar pre- and post-intervention (Table 7).
Table 7:
Impact of ID consultation on duration of fever
| Parameter | Cohort | |||
|---|---|---|---|---|
| Pre-intervention | Post-intervention | |||
| n = 72 | n = 56 | |||
| Without | Duration of fever, days* mean (SD) | 1.94 (± 1.45) | 2.09 (± 1.40) | |
| Geometric mean (SD) | 1.59 (×/÷ 1.82) | 1.73 (×/÷ 1.82) | ||
| Duration of fever > 5 days†, n (%) | 4 (5.6%) | 2 (3.6%) | ||
| ID consultation | n = 55 | n = 96 | ||
| With | Duration of fever, days mean (SD) | 4.09 (± 3.28) | 2.44 (± 2.04) | |
| Geometric mean (SD) | 3.06 (×/÷ 2.19) | 1.89 (×/÷ 1.97) | ||
| Duration of fever > 5 days†, n (%) | 13 (23.6%) | 6 (6.2%) | ||
Notes:
* Linear regression on log scale: P < 0.0001; interaction: P < 0.001
† Logistic regression: Cohort: P = 0.005; ID consultation: P = 0.007
Tests * and† are not independent
For cases without ID consultation, total antimicrobial costs were similar pre- and post-intervention (Table 8). For cases with an ID consultation, total costs were lower post- than pre-intervention; (p = 0.025). Comparing cases with and without ID consultation, the ratio of geometric means is CAD$234.00/CAD$45.00 = 5.2 pre-intervention and CAD$152.00/CAD$57.00 = 2.7 post-intervention.
Table 8:
Impact of ID consultation on total antimicrobial costs
| Parameter | intervention | |||
|---|---|---|---|---|
| Pre-intervention | Post-intervention | |||
| n = 142 | n = 72 | |||
| Without | Total antimicrobial costs* mean (SD) | $104 (±187) | $126 (±260) | |
| Geometric mean (SD) | $45 (×/÷4.8) | $57 (×/÷3.6) | ||
| ID Consult | n = 84 | n = 123 | ||
| With | Total antimicrobial costs* mean (SD) | $649 (±1437) | $338 (±600) | |
| Geometric mean (SD) | $234 (×/÷ 4.0) | $152 (×/÷ 4.3) | ||
Notes:* Linear regression on log scale: pre versus post P = 0.093; ID Consultation With versus Without P < 0.0001; Interaction P = 0.025
Predictors of mortality
The impact of patient characteristics, acquisition setting (community versus hospital), microbiology, infection source, and therapy on mortality were analyzed by logistic regression (Table 9. Due to the presence of multicollinearity, a downward, step-wise selection procedure was applied to identify a set of mortality predictors. Patient age, Charlson class, mechanical ventilation, nosocomial acquisition, presence of MRSA, presence of antimicrobial resistant Enterobacteriaceae, and source of infection were retained in the model. Consultation with ID and adequacy of empiric or directed therapy were not retained, suggesting that these were not important predictors of mortality.
Table 9:
Logistic regression of mortality predictors
| Variable | df | p-value (chi-square) |
|---|---|---|
| Gender | 1 | 0.544 |
| Age class | 4 | 0.004 |
| Charlson class | 4 | 0.000 |
| Dialysis | 1 | 0.001 |
| Surgery | 1 | 0.004 |
| Mechanical ventilation | 1 | 0.000 |
| Acquisition environment (community or hospital) | 1 | 0.005 |
| Enterobacteriaceae (susceptible) | 1 | 0.328 |
| Enterobacteriaceae (resistant) | 1 | 0.502 |
| Other bloodstream isolates (susceptible) | 1 | 0.702 |
| Other bloodstream isolates (resistant) | 1 | 0.978 |
| MSSA | 1 | 0.427 |
| MRSA | 1 | 0.253 |
| Infection source | 12 | 0.002 |
| ID consultation obtained | 1 | 0.197 |
| Empiric therapy status | 2 | 0.177 |
| Directed therapy status | 2 | 0.438 |
MRSA = Methicillin-resistant S. aureus; MSSA = Methicillin-suseptible S. aureus
Discussion
The role of a comprehensive BSISP serving all hospitalized patients with BSI at all levels and stages of care has seldom been studied; only two similar comprehensive BSISP have been described (18,20). The first differed from our program, as blood cultures were only reviewed once per week, all initial management was made by the attending physician and bedside assessment did not occur. The second included pharmacist notification of all positive blood cultures but the intervention was only targeted at the appropriateness of directed therapy with no further follow-up. In addition, outcome data was only analysed for patient’s not receiving antimicrobial therapy at the time of notification of positive blood cultures. Most BSI stewardship research has focused on specific high-risk populations (eg, critically ill) (19), a specific intervention (eg, time to first antimicrobial dose) (20), or organism (eg, S. aureus) (13,14).
The present study targeted all admitted patients with BSI from the time of first positive blood culture as a stewardship intervention, with a primary objective to improve the process of care. This objective was met, with multiple process improvements identified. We identified a high rate of inadequate empiric therapy (not given or inadequate in 24% of patients), confirming previous research (8,12). A non-significant trend toward improvement in the adequacy of directed therapy was observed even while the number of antimicrobials deployed per case was significantly lower. A significant improvement may not have been observed because directed therapy was defined as adequate as long as the bloodstream isolate was susceptible, in order to capture the impact of ‘bug-drug’ mismatch. This minimal standard for adequacy and the low level of antimicrobial resistance in our locale likely explains why 95% of patients received adequate directed therapy pre-intervention.
The above contrasts with the significant improvement observed for definitive therapy, which was inappropriate in 21% of cases prior to the intervention but only 1% post.
Despite significant improvements in the process of care, there was no improvement observed in duration of antimicrobial therapy, length of stay, or antimicrobial cost. This may be due in part to the frequency of inappropriately short treatment durations observed in the pre-intervention cohort, accounting for inadequate definitive care. Routine ID or medical microbiology consultation for BSI has been associated with longer duration of therapy, with no decrease in length of stay or antimicrobial cost (6,14). Recommendations regarding treatment duration were the most common intervention made by the BSISP post-intervention. The lack of impact on antimicrobial cost may also be related to the fact that cost was influenced by multiple non-modifiable patient factors (age, comorbidity, illness severity), antimicrobial resistance and the frequency of intra-abdominal gram-negative septicemia, where source control determines cost and outcome. Costs were significantly reduced post-intervention when an ID consult was obtained.
Urosepsis and intra-abdominal infection were the most common sources of BSI, accounting for the predominance of Enterobacteriaceae as the most common isolates. This has been shown in other recent studies documenting a relative increase in BSI secondary to drug-resistant gram-negative rods and a decline in methicillin-resistant S. aureus (MRSA) (5,18,21).
Mortality was a secondary outcome in this study, with no difference identified in the two cohorts, a finding observed in a similar BSISP study (18). This may be due to the low 14-day mortality rate, observed in 28/421 (6.7%) of patients (excluding patients who died in the first 48 hours), reflecting the large proportion of patients with community-acquired urosepsis, which has low mortality risk. Lack of impact on 14-day mortality may also be related to the fact that empiric therapy tended to have a greater influence on mortality in this study. Our intervention would be expected to have no impact on empiric therapy but would have significant potential to impact directed and definitive therapy. This may explain why no process of care interventions were identified as risk factors for mortality by logistic regression analysis.
ID consultation was obtained more often in patients with longer duration of fever, a result consistent with the hypothesis that ID consultation occurred more often in complicated cases. Without an ID consultation, fever duration was similar pre- and post-intervention. When an ID consultation was obtained, fever duration was shorter post-intervention. This may indicate that the BSISP increased the effectiveness of an ID consultation by accelerating the response time. Accelerated ID consultation provided through a BSISP was also associated with reduced antimicrobial costs. The increased rate of ID consultation associated with this BSISP may have driven other differences in process of care post-intervention.
The inherent limitations of retrospective analyses apply to this study. Although there is always potential for period effect, there were no other major changes in the process of care, (aside from the intervention), or laboratory investigation in our hospital during the two time periods. Although this study was conducted 10 years ago, the criteria used in this study to define a bloodstream infection and its source, remain compliant with recent updates made by the National Healthcare Safety Network. Molecular rapid diagnostic testing was introduced by our microbiology laboratory after the study was completed, an advance which has improved our ability to make timely stewardship decisions. This does not diminish the relevance of this study, given that molecular rapid testing has only been effective at improving outcomes when paired with an antimicrobial stewardship program (22). Reviewer bias was minimized through use of well-defined, objective definitions of process and outcome; however, outcome analysis was not blinded. The impact of the intervention may be an under-estimate, given that the BSISP was not active during holidays and weekends and the study phases were limited to 18-month time periods. Thirty-seven patients were excluded post-intervention, as they were rapidly discharged before they could be seen by the BSISP. This population would not have been excluded in the pre-intervention cohort. This selection bias would have increased the complexity and length of stay in the post-intervention cohort, and again would tend to underestimate the impact of a BSISP.
Since complete cohorts were used, p-values can only be used as an indication of the impact of a BSISP in a larger similar context.
In conclusion, a hospital BSISP is associated with improvement in the process of care for patients admitted with BSI and could be an effective stewardship strategy, with significant potential to improve the management of BSI over the current standard of care.
Funding Statement
This work was supported by a grant from the MedBuy Research Education and Development Fund and the Friends of the Moncton Hospital Community Health Research and Education Endowment Fund.
Acknowledgments:
The authors are indebted to Mareika Dow and Jana Reddy for their work on chart review and data abstraction, Dr Magdalena Kuhn and Tammy Wilcox for their invaluable microbiology laboratory support, and the Health Records Department at the Moncton Hospital.
Ethics Approval:
The manuscript has been reviewed by the Horizon Research Ethics Review Board.
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Data Accessibility:
All data will not be made publicly available. Researchers who require access to the study data can contact the corresponding author for further information.
Funding:
This work was supported by a grant from the MedBuy Research Education and Development Fund and the Friends of the Moncton Hospital Community Health Research and Education Endowment Fund.
Disclosures:
T MacLaggan received an honorarium from the Canadian Society of Hospital Pharmacists for reviewing an antimicrobial stewardship CME. He is also a member and part of the secretariat for both New Brunswick provincial and Horizon Health Antimicrobial Stewardship Committees, and an infectious diseases pharmacist for Horizon Health in New Brunswick. The other authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. 10.1086/421946. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41(8):3655–60. 10.1128/JCM.41.8.3655-3660.2003. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–9. 10.1111/1469-0691.12195. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Coulter S, Roberts JA, Hajkowicz K, Halton K. The use of bloodstream infection mortality to measure the impact of antimicrobial stewardship interventions: Assessing the evidence. Infect Dis Rep. 2017;9(1):6849. 10.4081/idr.2017.6849. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson J, Elgohari S, Livermore DM, et al. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect. 2011;17(3):451–8. 10.1111/j.1469-0691.2010.03262.x. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Bouza E, Sousa D, Munoz P, Rodriguez-Creixems M, Fron C, Lechuz JG. Bloodstream infections: A trial of the impact of different methods of reporting positive blood culture results. Clin Infect Dis. 2004;39(8):1161–9. 10.1086/424520. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602. 10.1093/clind/24.4.584. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Fluckiger U, Zimmerli W, Sax H, Frei R, Widmer AF. Clinical impact of an infectious disease service on the management of bloodstream infection. Eur J Clin Microbiol Infect Dis. 2000;19(7):493–500. 10.1007/s100960000306. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Pulcini C, Botelho-Nevers E, Dyar OJ, Harbarth S. The impact of infectious disease specialists on antibiotic prescribing in hospitals. Clin Microbiol Infect. 2014;20(10):963–72. 10.1111/1469-0691.12751. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Gomez J, Conde Cavero SJ, Hernandez Cardona JL, et al. The influence of the opinion of an infectious disease consultant on the appropriateness of antibiotic treatment in a general hospital. J Antimicrob Chemother. 1996;38(2):309–14. 10.1093/jac/38.2.309. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Tang G, Huang L, Zong Z. Impact of infectious disease consultation on clinical management and outcome of patients with bloodstream infection: A retrospective cohort study. Sci Rep. 2017;7(1):12898. 10.1038/s41598-017-13055-2. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerremans JJ, Verbrugh HA, Vos MC. Frequency of microbiologically correct antibiotic therapy increased by infectious disease consultations and microbiological results. J Clin Microbiol. 2012;50(6):2066–8. 10.1128/JCM.06051-11. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsblom E, Ruotsalainen E, Ollgren J, Jarvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;56(4):527–35. 10.1093/cid/cis889. Medline: [DOI] [PubMed] [Google Scholar]
- 14.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123(7):631–7. 10.1016/j.amjmed.2010.01.015. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuichi M, Furuichi M, Horikoshi Y, Miyairi I. Infectious Diseases Consultation Improves Treatment and Decreases Mortality by Enterococcal Bacteremia in Children. Pediatr Infect Dis J. 2018. Sept;37(9):856–60. 10.1097/INF.0000000000001919. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Farmakiotis D, Kyvernitakis A, Tarrand JJ, Kontoyiannis DP. Early initiation of appropriate treatment is associated with increased survival in cancer patients with Candida glabrata fungaemia: A potential benefit from infectious disease consultation. Clin Microbiol Infect. 2015;21(1):79–86. 10.1016/j.cmi.2014.07.006. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. 10.1016/j.ajic.2008.03.002. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Maeda M, Takuma T, Seki H, et al. Effect of interventions by an antimicrobial stewardship team on clinical course and economic outcome in patients with bloodstream infection. J Infect Chemother. 2016;22(2):90–5. 10.1016/j.jiac.2015.11.004. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–55. 10.1378/chest.118.1.146. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Bias TE, Vincent WR, 3rd, Trustman N, Berkowitz LB, Venugopalan V. Impact of an antimicrobial stewardship initiative on time to administration of empirical antibiotic therapy in hospitalized patients with bacteremia. Am J Health Syst Pharm. 2017;74(7):511–9. 10.2146/ajhp160096. Medline: [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Bano J, Lopez-Prieto MD, Portillo MM, et al. Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clin Microbiol Infect. 2010;16(9):1408–13. 10.1111/j.1469-0691.2010.03089.x. Medline: [DOI] [PubMed] [Google Scholar]
- 22.Jeon YD, Seong H, Kim D, Ahn MY, et al. Impact of matrix-assisted laser desorption/ionization time of flight mass spectrometric evaluation on the clinical outcomes of patients with bacteremia and fungemia in clinical settings lacking an antimicrobial stewardship program: A pre-post quasi experimental study. BMC Infectious Diseases. 2018;18:385–90. 10.1186/s12879-018-3299-y. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will not be made publicly available. Researchers who require access to the study data can contact the corresponding author for further information.


