Abstract
Aims
Microinflammation and malnutrition are common in individuals with type 2 diabetes mellitus (T2DM). We aimed to validate whether prognostic nutritional index (PNI) may increase the risk of diabetic kidney disease (DKD) and all-cause mortality in T2DM patients.
Methods
This retrospective cohort study was based on the National Health and Nutrition Examination Survey (NHANES) and National Death Index (NDI) 2013–2018 database. A total of 14,349 eligible subjects were included, and 2720 of them were with T2DM. PNI was assessed by the 5 × lymphocyte count (109/L) + serum albumin (g/L). The Logistic and Cox regression analyses were conducted to investigate the risk factors of DKD and mortality in T2DM patients.
Results
For 14,349 participants represented 224.7 million noninstitutionalized residents of the United State, the average PNI was 53.72 ± 0.12, and the prevalence of T2DM was 14.89%. T2DM patients had a lower level of PNI and dietary protein intake, a higher risk of mortality, kidney injury, anemia, arterial hypertension and hyperuricemia, compared with non-T2DM subjects. DKD occurred in 35.06% of diabetic participants and a higher PNI was independently related with a lower risk of DKD (OR 0.64, 95% CI 0.459–0.892, p = 0.01) in T2DM after multivariate adjustment. During a median follow-up of 46 person-months (29–66 months), a total of 233 T2DM individuals died from all causes (mortality rate = 8.17%). Subjects with T2DM who had a higher PNI showed a lower risk of all-cause mortality (HR 0.60, 95% CI 0.37–0.97, p = 0.036).
Conclusions
PNI, as a marker of immunonutrition, correlated with the incidence of DKD, and was an independent predictor for all-cause mortality in participants with T2DM. Thus, PNI may conduce to the risk stratification and timely intervention of T2DM patients.
Keywords: Prognostic nutritional index, Immunonutrition, Type 2 diabetes, Diabetic kidney disease, All-cause mortality
Introduction
The past few years have witnessed a dramatically increase in type 2 diabetes mellitus (T2DM) worldwide. It has become global public health problem with an estimation of 537 million adults in 2021 [1]. Despite the milestone progress has been made in the treatment and prognosis of diabetes with the advent of sodium glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1(GLP-1) receptor agonists [2–4], quite a few patients with T2DM still develop to diabetic kidney disease (DKD) and the risk of death remains high, which has become one of the top 10 causes of death worldwide [5]. It is of great importance to identify more risk factors for timely intervention of diabetic individuals, so as to delay diabetes progression and reduce the diabetes-related mortality.
Prognostic nutritional index (PNI), as a marker of immunonutrition, reflects the chronic inflammation, immune status and nutrition of the individual [6]. It was first established in 1984 and used to evaluate the prognosis of cancer initially [7]. Recent studies have suggested that the PNI is also related with the prognosis of cardiovascular disease, COVID-19, and chronic kidney disease (CKD) [8–10]. T2DM has been proved to be a low-grade inflammation state, in which the immune disorders and malnutrition are also not uncommon [11, 12]. However, the evidence regarding the relationships of PNI with DKD and mortality risk among diabetes is very limited. Besides, whether age, race, obesity, smoking and diet protein intake could modify the relationship of interest is also unclear.
To fill these knowledge gaps, we intended to prospectively investigate the relationships of PNI with DKD prevalence and all-cause mortality risk in a nationally representative sample with T2DM in the USA.
Materials and methods
Study population
This study was conducted in 29,400 participants from the National Health and Nutrition Examination Survey (NHANES) 2013–2018 in the USA performed by the Centers for Disease Control (CDC) and Prevention of the USA. The related mortality data were extracted for the National Death Index (NDI) database of CDC. The interview, examination, dietary, laboratory data in NHANES were collected from a complex, multistage and stratified probability sample representative of noninstitutionalized U.S. civilians [13, 14]. Mortality information in the NDI was from the date of survey participation through 31 December 2019. All data in this study are publicly available at https://www.cdc.gov/nchs (accessed on 4 July 2022). NHANES was approved by the institutional review board of the National Center of Health Statistics and the written informed consent was obtained from all participants.
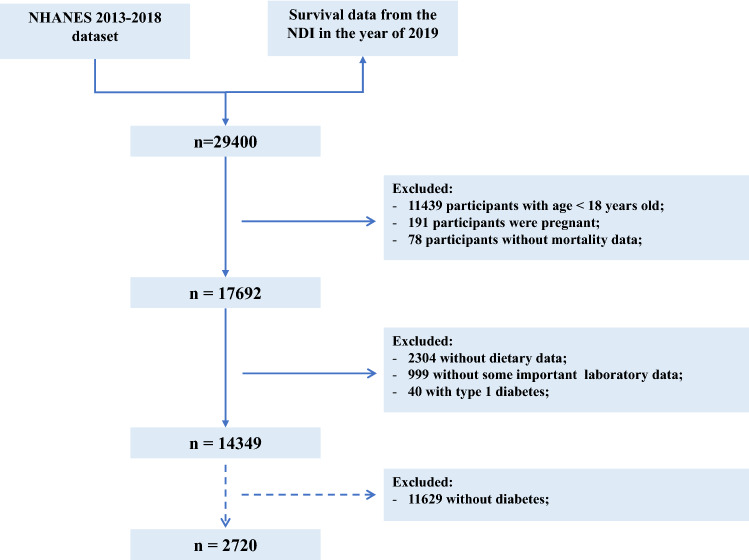
In this current study, individuals with age ≥ 18 years old were eligible. The exclusion criteria were as follows (Fig. 1): (1) participants with age < 18 years old; (2) participants were pregnant; (3) participants without mortality data; (4) participants without dietary data; (5) participants without some important laboratory data, such as HbA1c, eGFR, blood lymphocyte count, et al.; (6) participants with type 1 diabetes, (defined as a diagnosis of diabetes at age < 30 years and the insulin use as the only hypoglycemic agent). Besides, 2720 participants with T2DM were selected to evaluate the associations of PNI with DKD and all-cause mortality.
Fig.1.
The flow chart of individual inclusion and exclusion in this study
Measurement of serum albumin and blood lymphocyte count
A complete blood count (CBC) is a routine blood test used to estimate the contribution of diet and other factors to whole blood levels of nutrients and immunologic function. The methods used to derive CBC parameters are based on the Beckman Coulter methodology of counting and sizing [15]. Serum albumin level is frequently used to evaluate the nutritional status and was measured using the dye bromcresol purple (BCP) method in NHANES database. The PNI was calculated by 5 × lymphocyte count(109/L) + serum albumin (g/L) in this study.
Clinical and laboratory data
Laboratory methods for measurements of serum creatinine, blood urea nitrogen, total serum uric acid, total cholesterol, HbA1c, UACR, et al. are reported in detail elsewhere [16]. Diabetes was defined as a self-reported diabetes diagnosis, use of diabetes medication or insulin, HbAlc ≥ 6.5%, a plasma glucose level ≥ 7.0 mmol/L or a random blood glucose (mmol/l) ≥ 11.1 mmol/L [13]. The CKD was defined as urinary albumin-to-creatinine ratio (UACR) > 30 mg/g and/or estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 according to the “KDIGO 2021 Guidelines” [17]. The DKD was defined as the CKD combined with diabetes. The CKD_EPI_Scr equation was used to calculate eGFR [17].
Smoking status was classified as never smoker (defined as smoking less than 100 cigarettes in life), former smoker (defined as smoking more than 100 cigarettes in life and smoking not at all now) and now smoker (defined as smoking moth than 100 cigarettes in life and smoking some days or every day). Arterial hypertension was defined as a self-reported hypertension diagnosis, use of anti-hypertensive medication, average systolic blood pressure (SBP) > 140 mmHg or average diastolic blood pressure (DBP) > 90 mmHg [18]. The dietary inflammatory index (DII) was evaluated using 24 h dietary recall data in the “dietary” section and based on the calculation protocols established by Nitin Shivappa et al. [19] BMI was calculated as weight in kilograms divided by height in meters squared, and was divided into normal (BMI < 25 kg/m2), overweight (25 ≤ BMI ≤ 30 kg/m2) and obesity (BMI > 30 kg/m2). Diet protein intake (DPI) was calculated as total dietary protein in per day divided by weight in kilograms. The diagnosis of metabolic syndrome (MetS) was based on the modified National Cholesterol Education Program Adult Treatment Panel 3 criteria [20]. The hypoalbuminemia was defined as serum albumin < 35 g/L. Hyperuricemia was defined as serum uric acid ≥ 420 μmol/L for male and ≥ 360 μmol/L for female. The anemia was defined as hemoglobin levels < 130 g/L for male and < 120 g/L for female [17].
Statistical Analyses
All analyses were conducted in R version 4.1.3 (http://www.Rproject.org, the survey package). Weighted samples and the stratification and clustering of the design were considered in all analyses based on the CDC guidelines to derive estimates that were applicable to the general population in the U.S.[15]The 6-year sample weight was calculated by taking one third for the considered 2-year weight for each person in 2013–2018 to supply estimates for the entire 6 years. Continuous variables are presented as the weighted means ± Standard Error (SE) and categorial variables are shown as estimated proportions, A weighted t-test for continuous variables and chi-square test for categorical variables were applied to evaluate the differences between groups. The association of PNI and mortality in all the individuals was evaluated by the cubic spline curve initially, and then the cutoff value of PNI to predication for DKD or mortality in diabetic subjects was assessed by the receiver operating characteristic (ROC) curve. The relationships between PNI and some important clinical features were assessed by linear regression. In addition, the association of PNI with DKD prevalence was assessed by logistic regressions, while that between PNI and all-cause mortality among diabetic participants was investigated by Cox regression analysis. A two-way p < 0.05 was considered significant.
Results
Baseline features of all the participants
A total of 14,349 NHANES participants were included in this study, which represented 224.7 million noninstitutionalized residents of the USA. The baseline features of all the eligible participants are presented in Table 1. Among them, the average age was 47.26 ± 0.37 years old, the male accounted for 48.92%, and the weighted prevalence of T2DM was 14.89%. The mean PNI was 53.72 ± 0.12, and the individuals with T2DM tended to have lower PNI (52.53 ± 0.19 vs. 53.92 ± 0.12), lower energy intake (1945.72 ± 31.30 vs. 2175.34 ± 11.83 kcal) and protein intake (77.01 ± 1.43 vs. 84.06 ± 0.65 g/d) relative to those without T2DM. We also identified higher DII, BMI, UACR, serum creatinine, triglyceride, white blood cell (WBC), neutrophil lymphocyte ratio (NLR) and red cell distribution width (RDW), and lower eGFR in T2DM individuals, while there was no material difference in lymphocyte count. The overall prevalence rates of arterial hypertension, obesity, CKD, hyperuricemia, anemia and hypoalbuminemia were 38.42%, 39.07%, 14.8%, 17.85%, 6.9% and 1.32%, respectively, and people with T2DM seemed to have a higher risk of them. During a median follow-up of 46 person-months (29–66 months), the total estimated mortality was 3.4%, and T2DM individuals had a higher mortality than those without diabetes (8.17% vs.2.57%).
Table 1.
Clinical features of the participants with or without T2DM
| Valuable | All (n = 14,349) |
Non-T2DM (n = 11,629) |
T2DM (n = 2720) |
p-value |
|---|---|---|---|---|
| PNI | 53.72 ± 0.12 | 53.92 ± 0.12 | 52.53 ± 0.19 | < 0.0001 |
| Age (years) | 47.26 ± 0.37 | 45.11 ± 0.37 | 59.55 ± 0.43 | < 0.0001 |
| Gender (male, %) | 48.92 | 48.50 | 51.29 | 0.13 |
| Race | 0.01 | |||
| Mexican American | 9.53 | 9.40 | 10.24 | |
| Other hispanic | 6.22 | 6.25 | 6.02 | |
| Non-hispanic white | 64.03 | 64.61 | 60.72 | |
| Non-hispanic black | 10.79 | 10.41 | 12.93 | |
| Non-hispanic asian | 5.62 | 5.53 | 6.13 | |
| Other race | 3.82 | 3.80 | 3.96 | |
| Smoking (%) | 42.31 | 41.09 | 49.28 | < 0.0001 |
| Non-smoker | 57.69 | 58.91 | 50.73 | |
| Former smoker | 24.36 | 22.51 | 34.97 | |
| Current smoker | 17.95 | 18.58 | 14.31 | |
| DII | 1.42 ± 0.04 | 1.37 ± 0.05 | 1.66 ± 0.06 | < 0.001 |
| DII < 0 | 24.52 | 25.25 | 20.32 | 0.002 |
| DII > 0 | 75.48 | 74.75 | 79.68 | |
| Energy intake (kcal/d) | 2141.15 ± 11.24 | 2175.34 ± 11.83 | 1945.72 ± 31.30 | < 0.0001 |
| Protein intake (g/d) | 83.01 ± 0.60 | 84.06 ± 0.65 | 77.01 ± 1.43 | < 0.0001 |
| Hypertension (%) | 38.42 | 32.72 | 70.97 | < 0.0001 |
| BMI (kg/m2) | 29.36 ± 0.14 | 28.69 ± 0.13 | 33.18 ± 0.25 | < 0.0001 |
| BMI category | < 0.0001 | |||
| Normal (< 25) | 28.18 | 31.62 | 10.14 | |
| Overweight (25–30) | 31.78 | 33.10 | 26.29 | |
| Obese (≥ 30) | 39.07 | 35.28 | 63.56 | |
| CKD (%) | 14.80 | 11.25 | 35.06 | < 0.0001 |
| eGFR(ml/min/1.73m2) | 94.70 ± 0.51 | 96.57 ± 0.52 | 84.02 ± 0.67 | < 0.0001 |
| UACR (mg/g) | 33.50 ± 1.98 | 19.54 ± 1.09 | 113.28 ± 11.66 | < 0.0001 |
| Serum creatinine (umol/L) | 77.86 ± 0.41 | 76.87 ± 0.39 | 83.54 ± 1.18 | < 0.0001 |
| BUN (mmol/L) | 5.09 ± 0.04 | 4.97 ± 0.04 | 5.80 ± 0.07 | < 0.0001 |
| Hyperuricemia (%) | 17.85 | 16.49 | 25.62 | < 0.0001 |
| MetS (%) | 26.90 | 20.43 | 63.85 | < 0.0001 |
| Triglyceride (mmol/L) | 1.71 ± 0.02 | 1.62 ± 0.02 | 2.24 ± 0.06 | < 0.0001 |
| Total cholesterol (mmol/L) | 4.97 ± 0.02 | 5.00 ± 0.02 | 4.76 ± 0.03 | < 0.0001 |
| HDL-C (mmol/L) | 1.39 ± 0.01 | 1.42 ± 0.01 | 1.23 ± 0.01 | < 0.0001 |
| HbA1c (%) | 5.64 ± 0.01 | 5.39 ± 0.01 | 7.07 ± 0.04 | < 0.0001 |
| Anemia (%) | 6.90 | 6.08 | 11.57 | < 0.0001 |
| Hypoalbuminemia (%) | 1.32 | 0.98 | 3.23 | < 0.001 |
| WBC (109/L) | 7.42 ± 0.04 | 7.33 ± 0.04 | 7.94 ± 0.07 | < 0.0001 |
| Lymphocyte (109/L) | 2.21 ± 0.02 | 2.21 ± 0.02 | 2.22 ± 0.03 | 0.84 |
| Neutrophils (109/L) | 4.36 ± 0.03 | 4.28 ± 0.03 | 4.82 ± 0.06 | < 0.0001 |
| NLR | 2.16 ± 0.02 | 2.11 ± 0.02 | 2.43 ± 0.04 | < 0.0001 |
| RDW (%) | 13.63 ± 0.02 | 13.55 ± 0.02 | 14.04 ± 0.04 | < 0.0001 |
| Decreased (%) | 3.40 | 2.57 | 8.17 | < 0.0001 |
Data is presented as the mean ± standard error (SE) or percentages
PNI, prognostic nutritional index; DII, dietary inflammatory index; BMI, body mass index; CKD, chronic kidney disease; e-GFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio; BUN, blood urea nitrogen; MetS, metabolic syndrome; HDL-C, high density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin A1c; WBC, white blood cell; NLR, Neutrophil lymphocyte ratio; RDW, red cell distribution width
The relationships of PNI with T2DM, CKD and mortality
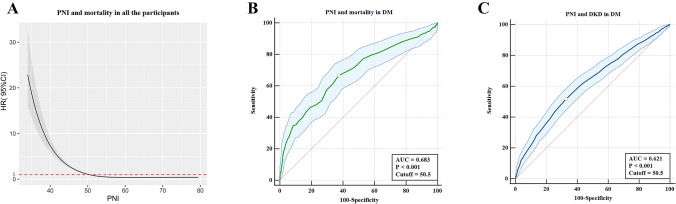
Estimated association between PNI and all-cause mortality outcome in total study population was shown from nonlinear spline model (Fig. 2A), and we estimated the mortality risk to reach a nadir at PNI > 50.5. In addition, the optimal cutoff value for PNI to predict the mortality and DKD in DM subjects evaluated with ROC curve was also 50.5 (Fig. 2B–C). Therefore, we divided the total cohort into two groups: higher PNI group (> 50.5) and lower PNI group (≤ 50.5).
Fig.2.
The cutoff of PNI for the prediction of DKD and mortality. a The spline curve of PNI to predict mortality in all the individuals using cox regression analysis. b The prediction of PNI for mortality in DM subjects evaluated with receiver operating characteristic (ROC) curve. c The prediction of PNI for DKD in DM subjects evaluated with receiver operating characteristic (ROC) curve. HR: hazard ratio
Table 2 showed the results of regression analyses evaluating the associations of PNI with T2DM, CKD and mortality in the total population. In the unadjusted model (Model 1), we identified that higher PNI was significantly related with risk of T2DM (OR 0.64, 95% CI 0.54–0.76, p < 0.0001, CKD (OR 0.47, 95% CI 0.42–0.54, p < 0.0001) and mortality (OR 0.34, 95% CI 0.27–0.44, p < 0.0001). These results remained stable when we adjusted for age, sex, and race for CKD and mortality, while not for T2DM. After adjustment for more potential covariates (Model 3), the people with higher PNI still showed a decreased likelihood of CKD (OR 0.75, 95% CI 0.65–0.86, p < 0.001) and mortality (OR 0.72, 95% CI 0.57–0.91, p = 0.007).
Table 2.
The relationships between higher PNI and T2DM, CKD and mortality
| Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| T2DM | 0.64 (0.54,0.76) | < 0.0001 | 1.03 (0.86,1.23) | 0.76 | – | – |
| CKD | 0.47 (0.42,0.54) | < 0.0001 | 0.75 (0.65,0.86) | < 0.001 | 0.75 (0.65,0.86) | < 0.001 |
| Mortality | 0.34 (0.27,0.44) | < 0.0001 | 0.65 (0.51,0.84) | 0.002 | 0.72 (0.57,0.91) | 0.007 |
aModel 1, unadjusted; bModel 2, adjusted for age, gender, and race; cModel 3, adjusted for age, gender, race, protein intake(g/d), smoking, BMI, hypertension, hyperuricemia and hypoalbuminemia. OR, odds ratio
Baseline features of the participants with T2DM
The baseline features of participants with T2DM were stratified by higher PNI and lower PNI and were shown in Table 3. A total of 2720 participants with a DKD prevalence of 35.06% were evaluated. Among them, the mean age was 59.55 ± 0.43 years old, and 51.29% were male. The individuals with higher PNI were younger, and had lower BMI, UACR, serum creatinine, NLR and RDW, higher eGFR, triglyceride, total cholesterol and lymphocyte count in comparison with those with lower PNI. Besides, the people with higher PNI had a lower prevalence of DKD, anemia, hypoalbuminemia and insulin use. We did not find a difference in gender, race, smoking, DII, energy intake, protein intake, DPI, arterial hypertension, hyperuricemia and HbA1c between the two groups.
Table 3.
Clinical features of the participants with T2DM based on PNI level
| Valuable | Higher PNI (> 50.5, n = 1640) |
Lower PNI (≤ 50.5, n = 1080) |
p-value |
|---|---|---|---|
| PNI | 55.57 ± 0.16 | 47.33 ± 0.15 | < 0.0001 |
| Age (years) | 57.71 ± 0.55 | 62.71 ± 0.60 | < 0.0001 |
| Gender (male, %) | 51.34 | 51.19 | 0.96 |
| Race | 0.07 | ||
| Mexican American | 10.78 | 9.31 | |
| Other hispanic | 6.14 | 5.82 | |
| Non-hispanic white | 59.23 | 63.27 | |
| Non-hispanic black | 12.35 | 13.94 | |
| Non-hispanic asian | 7.24 | 4.24 | |
| Other race | 4.27 | 3.41 | |
| Smoking (%) | 50.5 | 47.18 | 0.1 |
| Non-smoker | 49.50 | 52.82 | |
| Former smoker | 34.27 | 36.17 | |
| Current smoker | 16.23 | 11.01 | |
| DII | 1.59 ± 0.08 | 1.79 ± 0.10 | 0.11 |
| DII < 0 | 22.47 | 16.65 | 0.03 |
| DII > 0 | 77.53 | 83.35 | |
| Energy intake (kcal/d) | 1927.11 ± 35.49 | 1977.53 ± 47.73 | 0.35 |
| Protein intake (g/d) | 77.83 ± 1.78 | 75.60 ± 2.16 | 0.41 |
| DPI (g/kg/d) | 0.871 ± 0.017 | 0.830 ± 0.023 | 0.16 |
| < 0.6(g/kg/d) | 30.684 | 35.165 | 0.272 |
| 0.6–0.8 (g/kg/d) | 20.049 | 20.492 | |
| 0.8–1.0 (g/kg/d) | 20.538 | 15.271 | |
| 1.0–1.2 (g/kg/d) | 10.171 | 10.854 | |
| ≥ 1.2 (g/kg/d) | 18.557 | 18.219 | |
| Hypertension (%) | 69.43 | 73.60 | 0.1 |
| BMI (kg/m2) | 32.59 ± 0.21 | 34.21 ± 0.49 | 0.002 |
| BMI category | 0.05 | ||
| Normal (< 25) | 9.11 | 11.91 | |
| Overweight (25–30) | 28.05 | 23.28 | |
| Obese (≥ 30) | 62.83 | 64.82 | |
| DKD (%) | 29.34 | 44.85 | < 0.0001 |
| eGFR(ml/min/1.73m2) | 86.86 ± 0.83 | 79.18 ± 1.05 | < 0.0001 |
| UACR (mg/g) | 62.14 ± 6.94 | 200.69 ± 27.18 | < 0.0001 |
| Serum creatinine (umol/L) | 79.38 ± 0.94 | 90.66 ± 2.42 | < 0.0001 |
| BUN (mmol/L) | 5.53 ± 0.08 | 6.27 ± 0.13 | < 0.0001 |
| Hyperuricemia (%) | 26.63 | 23.90 | 0.22 |
| MetS (%) | 66.31 | 59.64 | 0.03 |
| Triglyceride (mmol/L) | 2.41 ± 0.07 | 1.95 ± 0.06 | < 0.0001 |
| Total cholesterol (mmol/L) | 4.87 ± 0.04 | 4.59 ± 0.05 | < 0.001 |
| HDL-C (mmol/L) | 1.23 ± 0.02 | 1.23 ± 0.01 | 0.75 |
| HbA1c (%) | 7.01 ± 0.05 | 7.17 ± 0.07 | 0.06 |
| Anemia (%) | 7.46 | 18.58 | < 0.0001 |
| Hypoalbuminemia (%) | 0.06 | 8.65 | < 0.0001 |
| WBC (109/L) | 8.37 ± 0.08 | 7.20 ± 0.11 | < 0.0001 |
| Lymphocyte (109/L) | 2.54 ± 0.04 | 1.67 ± 0.03 | < 0.0001 |
| Neutrophils (109/L) | 4.90 ± 0.07 | 4.69 ± 0.09 | 0.06 |
| NLR | 2.07 ± 0.04 | 3.06 ± 0.07 | < 0.0001 |
| RDW (%) | 13.86 ± 0.04 | 14.36 ± 0.07 | < 0.0001 |
| Insulin use (%) | 13.23 | 22.54 | < 0.001 |
| OHA (%) | 67.95 | 62.13 | 0.05 |
| Decreased (%) | 5.398 | 12.903 | < 0.001 |
Data is presented as the mean ± standard error (SE) or percentages
PNI, prognostic nutritional index; DII, dietary inflammatory index; DPI, diet protein intake; BMI, body mass index; DKD, diabetic kidney disease; e-GFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio; BUN, blood urea nitrogen; MetS, metabolic syndrome; HDL-C, high density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin A1c; WBC, white blood cell; NLR, Neutrophil lymphocyte ratio; RDW, red cell distribution width; OHA, Oral hypoglycemic agents
The correlations of PNI and clinical characteristics
The results of regression analyses evaluating the associations of PNI with clinical characteristics (Table 4) demonstrated that PNI was positively correlated with eGFR(β = 0.27 (0.08, 0.46), p = 0.01), and negatively related with UACR(β = − 15.93(− 24.26,− 7.59), p < 0.001),HbA1c (β = − 0.0142(− 0.028, − 0.0004), p = 0.044), DII(β = − 0.023(− 0.045,− 0.002), p = 0.036), NLR (β = − 0.1(− 0.13, − 0.07), p < 0.0001), RDW (β = −0.04(− 0.06, − 0.03), p < 0.0001) and hsCRP (β = − 0.005(− 0.008, − 0.002), p = 0.001) after adjustment for age, gender and race.
Table 4.
The corrections between PNI and clinical features
| PNI | ||
|---|---|---|
| β* (95% CI) | P value | |
| UACR (mg/g) | − 15.93 (− 24.26, − 7.59) | < 0.001 |
| eGFR (ml/min/1.73 m2) | 0.27 (0.08, 0.46) | 0.01 |
| HbA1c (%) | − 0.0142 (− 0.0280, − 0.0004) | 0.044 |
| DII | − 0.023 (− 0.045, − 0.002) | 0.036 |
| NLR | − 0.1 (− 0.13, − 0.07) | < 0.0001 |
| RDW (%) | − 0.04 (− 0.06, − 0.03) | < 0.0001 |
| hsCRP(mg/L) (n = 1895) | − 0.005 (− 0.008, − 0.002) | 0.001 |
UACR, urinary albumin-to-creatinine ratio; e-GFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin A1c; DII, dietary inflammatory index; NLR, Neutrophil lymphocyte ratio; RDW, red cell distribution width; hsCRP, high-sensitivity C-reactive protein; β, regression coefficient; CI, confidence interval
*Adjustment for age, sex and race
PNI and DKD in individuals with T2DM
The logistic regression analysis was used to explore the relationships between the PNI and DKD in T2DM participants (Table 5). We observed that the age, smoking, arterial hypertension, DII > 0, energy intake, DPI, hyperuricemia, HbA1c, triglyceride, hypoalbuminemia, anemia, lymphocyte and higher PNI (OR 0.51, 95% CI 0.40–0.65, p < 0.0001) were significantly related with the incidence of DKD in T2DM participants in univariate analysis. In multivariate analysis, people with higher PNI had 36% decreased incidence of DKD (OR 0.64, 95% CI 0.459–0.892, p = 0.01), while the hypoalbuminemia and lymphocyte count were not related with the risk of DKD.
Table 5.
The relationship between PNI and DKD using Logistic regression analysis
| Variables | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (yrs) | 1.05 (1.04,1.06) | < 0.0001 | 1.05 (1.04,1.06) | < 0.0001 |
| Gender (Male) | 1.04 (0.76,1.42) | 0.79 | 1.09 (0.79,1.50) | 0.60 |
| Non-Hispanic White (yes vs. no) | 1.23 (0.98,1.55) | 0.07 | 1.09 (0.85,1.39) | 0.48 |
| Smoking (yes vs. no) | 1.27 (1.01,1.59) | 0.04 | 1.232 (0.958,1.584) | 0.101 |
| Hypertension (yes vs. no) | 2.24 (1.69,2.96) | < 0.0001 | 1.466 (1.048,2.049) | 0.027 |
| BMI (kg/m2) | 1.01 (0.99,1.02) | 0.42 | – | – |
| DII group (DII > 0) | 1.74 (1.42,2.15) | < 0.0001 | 1.53 (1.16,2.02) | 0.004 |
| Energy intake (kcal/kg/d) | 0.984 (0.972,0.996) | 0.013 | 1.008 (0.988,1.027) | 0.429 |
| DPI (g/kg/d) | 0.62 (0.47,0.82) | 0.001 | 0.632 (0.395,1.009) | 0.054 |
| Hyperuricemia (yes vs. no) | 2.30 (1.85,2.86) | < 0.0001 | 2.088 (1.662,2.622) | < 0.0001 |
| HbA1c (%) | 1.27 (1.18,1.35) | < 0.0001 | 1.344 (1.237,1.459) | < 0.0001 |
| Triglyceride (mmol/L) | 1.13 (1.05,1.23) | < 0.001 | 1.139 (1.055,1.228) | 0.002 |
| Total cholesterol (mmol/L) | 1.02 (0.95,1.10) | 0.51 | – | – |
| Hypoalbuminemia (yes vs. no) | 2.73 (1.76,4.25) | < 0.0001 | 1.499 (0.834,2.695) | 0.169 |
| Anemia (yes vs. no) | 2.47 (1.84,3.33) | < 0.0001 | 1.879 (1.357,2.602) | < 0.001 |
| Lymphocyte (109/L) | 0.83 (0.72,0.96) | 0.01 | 1.021 (0.880,1.183) | 0.779 |
| PNI group (Higher PNI) | 0.51 (0.40,0.65) | < 0.0001 | 0.640(0.459,0.892) | 0.01 |
aadjustment for age, gender, race, smoking, hypertension, DII group, energy intake, DPI, hyperuricemia, HbA1c, triglyceride, hypoalbuminemia, anemia, lymphocyte and PNI group. OR, odds ratio; 95% CI, 95% confidence interval
PNI and mortality in individuals with T2DM
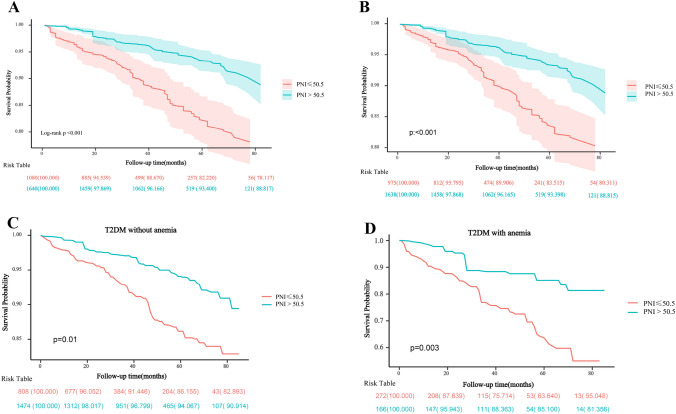
The Kaplan–Meier survival curve for all-cause mortality in the T2DM participants stratified by PNI groups was presented in Fig. 3A. We observed the mortality was lower in individuals with a higher PNI than in those with a lower PNI (log-rank p < 0.001). In addition, Similar results were also identified among subgroups of T2DM individuals without hypoalbuminemia (Fig. 3B), without anemia (Fig. 3C) or with anemia (Fig. 3D). The results of Cox regression analysis were shown in Table 6. In univariate analysis, higher PNI (HR 0.37, 95% CI 0.24–0.58, p < 0.0001), lymphocyte count and eGFR were negatively associated with the all-cause mortality rate, while age, non-hispanic white, smoking, arterial hypertension, hyperuricemia, hypoalbuminemia and anemia, were positive with the risk of mortality. After adjustment for the above factors, the higher PNI was still significantly related with the decreased risk of mortality (HR 0.60, 95% CI 0.37–0.97, p = 0.036), while age, Non-Hispanic Caucasian, smoking, arterial hypertension, hypoalbuminemia and anemia tended to be risk factors for increased risk of all-cause mortality.
Fig.3.
The prediction of PNI for DKD and mortality in DM individuals. a Kaplan–Meier curves of survival rate in DM with different PNI levels. b Kaplan–Meier curves of survival rate in DM subjects without hypoalbuminemia in the two PNI groups.c Kaplan–Meier curves of survival rate in DM subjects without anemia in the two PNI groups. d Kaplan–Meier curves of survival rate in DM subjects with anemia in the two PNI groups
Table 6.
The relationship between PNI and mortality in participants with T2DM
| Variables | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95% CI) | P value | |
| Age (yrs) | 1.09 (1.06,1.11) | < 0.0001 | 1.07 (1.04,1.09) | < 0.0001 |
| Gender (Male) | 0.93 (0.65,1.35) | 0.72 | 0.89 (0.63,1.26) | 0.51 |
| Non-hispanic white (yes vs. no) | 2.06 (1.44,2.94) | < 0.0001 | 1.68 (1.16,2.42) | 0.01 |
| Smoking (yes vs. no) | 1.94 (1.33,2.84) | < 0.001 | 2.07 (1.45,2.95) | < 0.0001 |
| Hypertension (yes vs. no) | 3.83 (2.50,5.85) | < 0.0001 | 2.11 (1.31,3.42) | 0.002 |
| BMI (kg/m2) | 1.00 (0.97,1.02) | 0.76 | – | – |
| DII group (DII > 0) | 1.24 (0.68,2.24) | 0.48 | – | – |
| Energy intake (kcal/kg/d) | 0.99 (0.97,1.02) | 0.68 | – | – |
| DPI(g/kg/d) | 0.86 (0.41,1.81) | 0.69 | – | – |
| Hyperuricemia (yes vs. no) | 2.05 (1.39,3.03) | < 0.001 | 1.36 1.36 (0.96,1.93) | 0.08 |
| HbA1c (%) | 1.03 (0.94,1.13) | 0.53 | – | – |
| Triglyceride (mmol/L) | 1.04 (0.95,1.14) | 0.39 | – | – |
| Total cholesterol (mmol/L) | 1.06 (0.87,1.29) | 0.57 | – | – |
| Hypoalbuminemia (yes vs. no) | 5.43 (3.26,9.05) | < 0.0001 | 3.48 (1.76,6.88) | < 0.001 |
| Anemia (yes vs. no) | 3.57 (2.41,5.28) | < 0.0001 | 2.15 (1.34,3.44) | 0.001 |
| UACR (mg/g) | 1.00 (1.00,1.00) | < 0.0001 | 1.00 (1.00,1.00) | 0.698 |
| eGFR (ml/min/1.73m2) | 0.97 (0.96,0.98) | < 0.0001 | 0.99(0.98,1.00) | 0.245 |
| Lymphocyte (109/L) | 0.70 (0.50,0.97) | 0.03 | 1.15 (0.93,1.42) | 0.19 |
| PNI group (Higher PNI) | 0.37 (0.24,0.58) | < 0.0001 | 0.60 (0.37,0.97) | 0.036 |
aadjustment for age, gender, race, smoke, hypertension, hyperuricemia, hypoalbuminemia, anemia, UACR, eGFR, lymphocyte and PNI group. HR, hazard ratio; 95% CI, 95% confidence interval
Discussion
As far as we know, this was the first cohort study to investigate whether the PNI was related with mortality and DKD prevalence in T2DM individuals. After adjustment for confounders, we identified that the higher PNI was significantly correlated with CKD prevalence and mortality risk in all the participants. Besides, we also found the PNI was positively correlated with eGFR, and negatively related with UACR, HbA1c, NLR, RDW and hsCRP, of which the last three are indicators of chronic inflammation. More importantly, higher PNI had an independently protective effect for incidence of DKD and mortality risk in T2DM patients.
PNI is a lymphocyte and albumin-based index that was developed to robustly reflects the immunonutrition status of individuals. Emerging evidence have proved that PNI could predict the prognosis of several types of cancer [6, 7, 21, 22], cardiovascular diseases [8, 23] and acute kidney injury [24]. Furthermore, PNI was also related with the increased risk of mortality in children [25] and older patients with CKD [10]. In this study, we also demonstrated that PNI was significantly related with CKD and all-cause mortality in total study population. More importantly, we further found that PNI was an independent predictor for all-cause mortality and DKD prevalence in diabetes.
Interestingly, hypoalbuminemia and anemia, as the markers of malnutrition, have been proved to be involved in adverse outcomes in diabetes [26, 27]. In this study, the PNI was still a significant predictor for all-cause mortality risk even after adjustment for anemia, hypoalbuminemia and lymphocytes in diabetes. Besides, PNI was also associated with DKD prevalence after adjustment for confounders, while the hypoalbuminemia was not. Furthermore, in the subgroups of T2DM patients without hypoalbuminemia and anemia, higher PNI still was related with a decreased in all-cause mortality rate. It is worth mentioning that we also identified the PNI was significantly related with the inflammation markers, such as NLR and hsCRP [28]. In line with the above findings, we may speculate on the reasons why PNI was a better predictor for mortality in subjects of different clinical settings. This might be due to PNI is a more comprehensive marker which integrates immune, nutrition, and chronic inflammation [23], all of which are important disease drivers. The chronic inflammatory could accelerate immune disorder and malnutrition, which in turn promote inflammation, forming a vicious cycle to contribute to disease progression [29–31].
In particular, relatively few studies evaluated DII as a prognostic marker in diabetes. One recent study demonstrated that the diabetic individuals adhering to a proinflammatory diet (DII > 0) were an independent risk factor for all-cause mortality based on the population from NHANES 2009–2014 [32]. In this study, we found that DII > 0 was significantly related with the increased prevalence of DKD in people with T2DM, and was negatively correlated with PNI after adjustment for age, gender and race. However, we did not observe a relationship between DII > 0 and all-cause mortality in T2DM patients in this study, most likely due to the shift in dietary attitudes and different inclusion and exclusion criteria for the research.
Diet protein intake plays a fundamental role in prevention and management of malnutrition in T2DM [33], and is closely related with PNI. In this study, we found the T2DM participants had lower energy intake, protein intake and PNI than those without diabetes. Besides, the DPI and PNI were negatively associated with the incidence of DKD, albeit DPI not emerging as an independent factor. However, the results of cox regressions demonstrated that the DPI was not related with the mortality risk in diabetes. Whether the diabetic individuals with renal injury should have a low protein diet (LPD) of 0.6–0.8 g/kg/day to [34]delay the renal disease progression and improve the prognosis remains controversial. One recent study [35] demonstrated that it was not higher dietary protein intake (> 163 g/day) but lower dietary protein intake (< 92 g/day) that affect the renal function decline in T2DM participants, which was to some extent consistent with our results. The exact relationship of DPI and DKD still awaits more studies to validate.
The blood lymphocyte count, as another factor of PNI, was related with DKD prevalence and mortality risk in diabetes in this study, although not being as an independent predictor. Relatively limited studies performed regarding to the relationship of lymphocyte with DKD and mortality. Cardoso et al. [28]. observed that lymphocyte count was not related with the DKD risk, but was an independent predictor for all-cause mortality in a type 2 diabetes cohort study. Other studies were conducted in individuals without diabetes, and showed that absolute lymphocytes were a prognostic marker for all-cause mortality in cardiovascular diseases [36]. The underlying mechanism might be a decreased lymphocytes contributing to a decline in immune defense, which makes individuals inadequate resistance to severe disease [28].
The strengths of this study are as follows. Firstly, to our knowledge, this was the first study to investigate the associations of PNI with DKD and mortality risk in diabetes. Secondly, we afford comparative evidence on the predictive value of anemia, hypoalbuminemia, lymphocyte count and PNI for the prevalence of DKD and mortality risk in clinical practice. Thirdly, PNI is a noninvasive index and convenient for clinical application.
The limitations of this study are also worth mentioning. Firstly. The DKD diagnosis was based on the results of a single test for eGFR and UACR, rather than three months of observation [17]. But the results were consistent with DKD prevalence, hence, the results tended to be reliable. Secondly, the association of PNI with DKD was based on a cross-sectional study, as such cannot clarify the causal relationship between PNI and DKD. Thirdly, some possible confounders, such as medicine intervention and combined diseases were not adjusted for, which may interfere with the results of this study.
In summary, we provided evidence that PNI could reflect and was relevant in the incidence of DKD and risk of all-cause mortality. This study demonstrated that the immunonutrition was very important for the T2DM patients, and its clinical utilization will help clinicians better manage diabetic patients. However, further studies are needed to validate the exact effect of PNI to DKD and mortality in T2DM.
Acknowledgements
Thanks to Jing Zhang (Shanghai Tongren Hosptial) for this work on the NHANES database.
Abbreviations
- PNI
Prognostic nutritional index
- T2DM
Type 2 diabetes mellitus
- DKD
Diabetic kidney disease
- NHANES
National health and nutrition examination survey
- NDI
National death index
- SGLT2i
Sodium glucose cotransporter 2 inhibitors
- GLP-1
Glucagon-like peptide-1
- CKD
Chronic kidney disease
- CDC
Centers for disease control
- CBC
Complete blood count
- BCP
Dye bromcresol purple
- DII
Dietary inflammatory index
- BMI
Body mass index
- e-GFR
Estimated glomerular filtration rate
- UACR
Urinary albumin-to-creatinine ratio
- BUN
Blood urea nitrogen
- MetS
Metabolic syndrome
- HDL-C
High density lipoprotein cholesterol
- HbA1c
Glycosylated hemoglobin A1c
- WBC
White blood cell
- NLR
Neutrophil lymphocyte ratio
- RDW
Red cell distribution width
- OHA
Oral hypoglycemic agents
- hsCRP
High-sensitivity C-reactive protein
- OR
Odds ratio
- HR
Hazard ratio
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted in accordance with the ethical standards laid down in an appropriate version of the 1964 Declaration of Helsinki, and all the NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junlin Zhang and Yao Chen Contributed equally to this work as Co-first authors.
References
- 1.Diabetes around the world in 2021. The IDF Diabetes Atlas 10th Edition [Internet] ed: International Diabetes Federation. p. Available from https://diabetesatlas.org/.
- 2.Block TJ, Batu D, Cooper ME. Recent advances in the pharmacotherapeutic management of diabetic kidney disease. Expert Opin Pharmacother. 2022;23:791–803. doi: 10.1080/14656566.2022.2054699. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 4.Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: a pooled analysis of SUSTAIN 6 and LEADER trials. Circulation. 2021;145:575–585. doi: 10.1161/CIRCULATIONAHA.121.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO’s Global Health Estimates (GHE) [Internet]. pp. Available from https://www.who.int/data/global-health-estimates/.
- 6.Wang D, Hu X, Xiao L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25:421–427. doi: 10.1007/s11605-019-04492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 8.Zencirkiran Agus H. Kahraman S Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020;75:450–455. doi: 10.1080/00015385.2019.1661139. [DOI] [PubMed] [Google Scholar]
- 9.Wei W, Wu X, Jin C, et al. Predictive significance of the prognostic nutritional index (PNI) in patients with severe COVID-19. J Immunol Res. 2021;2021:9917302. doi: 10.1155/2021/9917302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barutcu Atas D, Tugcu M, Asicioglu E, et al. Prognostic nutritional index is a predictor of mortality in elderly patients with chronic kidney disease. Int Urol Nephrol. 2022;54:1155–1162. doi: 10.1007/s11255-021-03002-6. [DOI] [PubMed] [Google Scholar]
- 11.Shim K, Begum R, Yang C, et al. Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World J Diabetes. 2020;11:1–12. doi: 10.4239/wjd.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesch GH. Diabetic nephropathy—is this an immune disorder? Clin Sci. 2017;131:2183–2199. doi: 10.1042/CS20160636. [DOI] [PubMed] [Google Scholar]
- 13.Wan Z, Guo J, Pan A, et al. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. 2020;44:350–357. doi: 10.2337/dc20-1485. [DOI] [PubMed] [Google Scholar]
- 14.Centers for disease control and prevention (2017): National Health and Nutrition Examination Survey (NHANES) US Department of health and human services; 2017 Available from https://wwwncdcgov/nchs/nhanes/continuousnhanes/default aspx?BeginYear=2017.
- 15.Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Internal Med. 2007;146:486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism clin Exp. 2021;121:154752. doi: 10.1016/j.metabol.2021.154752. [DOI] [PubMed] [Google Scholar]
- 17.KDIGO Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;2021(100):S1–s276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 21.Mirili C, Yılmaz A, Demirkan S, et al. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24:1301–1310. doi: 10.1007/s10147-019-01461-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22:8002. doi: 10.3390/ijms22158002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Cao Q, Wang H, et al. Prognostic nutritional index predicts acute kidney injury and mortality of patients in the coronary care unit. Exp Ther Med. 2021;21:123. doi: 10.3892/etm.2020.9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X, Wang B, Chen S, et al. Association between prognostic nutritional index and contrast-associated acute kidney injury in patients complicated with chronic kidney disease and coronary artery disease. J Interv Cardiol. 2021;2021:2274430. doi: 10.1155/2021/2274430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Tao Y, Wang Z, et al. Evaluation of nutritional status and prognostic impact assessed by the prognostic nutritional index in children with chronic kidney disease. Medicine. 2019;98:e16713. doi: 10.1097/MD.0000000000016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403. doi: 10.1681/ASN.2005030226. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang R, Wang Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res. 2019;2019:7825804. doi: 10.1155/2019/7825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardoso CRL, Leite NC. Salles GF importance of hematological parameters for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol. 2021;20:133. doi: 10.1186/s12933-021-01324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang S, Huang Y, Zhan X, et al. (2022) The relationship between the prognostic nutritional index and new-onset pneumonia in peritoneal dialysis patients. Int Urol Nephrol 1–8. [DOI] [PMC free article] [PubMed]
- 30.Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients. 2017;9:208. doi: 10.3390/nu9030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickey FB. Martin F diabetic kidney disease and immune modulation. Curr Opin Pharmacol. 2013;13:602–612. doi: 10.1016/j.coph.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Tan J, Liu N, Sun P, et al. A proinflammatory diet may increase mortality risk in patients with diabetes mellitus. Nutrients. 2022;14:2011. doi: 10.3390/nu14102011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merino J. Precision nutrition in diabetes: when population-based dietary advice gets personal. Diabetologia. 2022;65:1839–1848. doi: 10.1007/s00125-022-05721-6. [DOI] [PubMed] [Google Scholar]
- 34.Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76:S1–s107. doi: 10.1053/j.ajkd.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Oosterwijk MM, Groothof D, Navis G, et al. High-normal protein intake is not associated with faster renal function deterioration in patients with type 2 diabetes: a prospective analysis in the DIALECT cohort. Diabetes Care. 2022;45:35–41. doi: 10.2337/dc21-1211. [DOI] [PubMed] [Google Scholar]
- 36.Warny M, Helby J, Nordestgaard BG, et al. Incidental lymphopenia and mortality: a prospective cohort study. CMAJ. 2020;192:E25–e33. doi: 10.1503/cmaj.191024. [DOI] [PMC free article] [PubMed] [Google Scholar]