Yellow fever (YF), an acute viral hemorrhagic disease transmitted by infected mosquitoes, has the potential to spread rapidly and cause serious public health impact. The disease predominantly affects people in sub-Saharan Africa and tropical South America, where 40 countries are considered endemic and at high-risk for YF outbreaks1. Despite the availability of safe and effective vaccines since the 1930s, YF outbreaks continue to occur resulting in an estimated 109,000 severe cases and 51,000 deaths annually2. These figures are likely underestimates as most mild YF cases go undetected due to nonspecific symptoms and limited surveillance or laboratory diagnostic capacity in many at-risk regions.
Because of large explosive outbreaks in the last five years, YF has reemerged as a major international public health threat. In 2016, an explosive outbreak occurred in Angola, spreading to neighboring areas in the Democratic Republic of Congo and infecting expatriate workers, including at least 11 workers who returned to China while ill3. At the time of the outbreak in Angola, vaccination coverage and disease awareness were low as the last YF outbreak was in 1971. In addition, control measures, such as requiring a valid international certificate of vaccination for travelers, were not enforced4. Thirty million doses of YF vaccine were needed to stop the outbreak, which both outstripped the available global vaccine supply and led to the unprecedented use of fractional doses of the vaccine to prevent further disease spread5. In late 2016–2017, outbreaks of YF were also detected in coastal areas of Brazil where cases had not been reported since the 1940s and vaccination was not routinely recommended6. Again, fractional doses of the vaccine were needed to protect those residing in affected areas. Although fractional doses have been demonstrated to provide good short-term protection, questions remain if they will provide the same long-term protective immunity as a full dose7–9. Until these questions can be adequately answered, fractional doses should only be considered in emergency scenarios if there are insufficient doses of the vaccine to respond to active or imminent threats of large-scale amplification of YF10,11.
The YF outbreaks and resulting public health crises in Angola, the Democratic Republic of Congo, and Brazil underscored the need for a comprehensive, updated and intensified strategy to eliminate YF epidemics. To that end, the multi-partner global Eliminate Yellow Fever Epidemics (EYE) Strategy was established in 2017 to improve detection, outbreak preparedness, and response1. The EYE Strategy has 3 main objectives: (1) to protect at-risk populations through immunization; (2) to prevent international spread of the disease through vaccinating high-risk workers, enforcing International Health Regulations and supporting the development of resilient urban centers; and (3) to contain outbreaks rapidly by improving surveillance and timely access to validated diagnostic tests and emergency vaccines. The Strategy was endorsed by WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and launched in the African Region in 20181,12. Through engagement with the EYE Strategy at country, regional and global levels in three regions (Africa, North Africa-Middle East, and the Americas), there have been remarkable achievements. Close to 250 million people have been vaccinated against YF in Africa since the inception of the EYE Strategy. In 2021 alone, more than 65 million people were vaccinated in Africa through routine immunization (RI) program (18 million) and preventive (43.7 million), reactive (3.8 million), and catch-up (1 million) campaigns13. Advocacy work by partner agencies has resulted in increased YF vaccine supply and improved confidence in production channels14. Twenty years ago, there were only 20 million doses of YF vaccine produced annually15. By 2016, the number of doses had increased to 93 million and over >150 million doses were available in both 2019 and 202015,16. This improved supply has been instrumental to assure that surging demand for preventive mass vaccination campaigns (PMVCs) can be provided for in addition to the RI and outbreak response needs. The EYE Strategy has achieved stronger laboratory capacity for YF diagnostic testing, allowing for improved surveillance, faster diagnostic confirmations, and improving detection and response to outbreaks17. Innovative tools, such as simple-to-use national and subnational risk assessment tools, have been developed and implemented to allow for streamlined, transparent, and risk-informed vaccination allocations13,18.
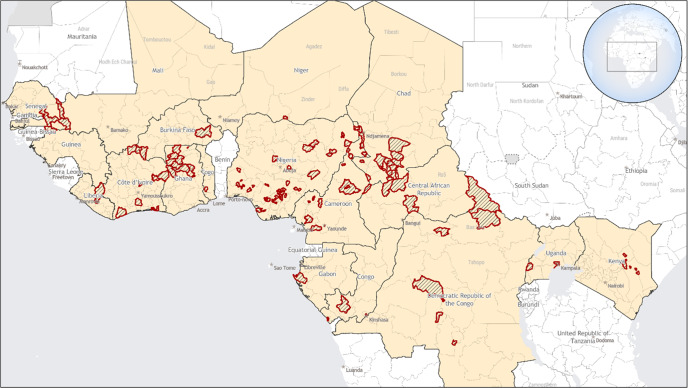
Despite these successes, YF outbreaks continue to occur. In late 2020, confirmed YF outbreaks were reported in Nigeria, Senegal, and Guinea19. In 2021 and early 2022, 12 countries across Africa (Cameroon, Chad, Central African Republic, Côte d’Ivoire, the Democratic Republic of Congo, Gabon, Ghana, Kenya, Niger, Nigeria, Republic of Congo, and Uganda) reported confirmed YF outbreaks (Fig. 1)20–22. An additional five countries (Burkina Faso, Ethiopia, Liberia, Sierra Leone, and Togo) reported probable YF cases during the same time frame.
Fig. 1. Yellow fever disease cases — Africa, September 2020-March 2022.
Areas with confirmed and probable yellow fever disease cases are outlined in red.
There are several factors that have likely contributed to the resurgence of YF in endemic areas. The global coronavirus disease-19 (COVID-19) pandemic and other events such as the Ebola outbreaks in West Africa, have had devasting impacts on healthcare delivery and access23. During the recent crises, RI programs have been unable to enhance the delivery of YF vaccines to children, resulting in declining RI coverages in west and central African countries with long-standing YF RI programs (72% in 2010 to 65% in 2020 and 2021)24. From 2020-2021, nine countries have an average YF RI coverage <60%, including Angola, Cameroon, Central African Republic, Chad, Democratic Republic of Congo, Equatorial Guinea, Gabon, Guinea, and Liberia24. Additionally, many affected countries are struggling with added vulnerabilities such as fragile governments and regional conflicts, that further limit capacities to prevent and respond to outbreaks and impede access to health services20. Finally, additional drivers of YF virus circulation, such as periodic increases in sylvatic transmission, climate and vector factors, and population movement likely have contributed to the recent increase20,21,25.
The specific factors that are driving the occurrence of YF cases vary by region and country. For instance, the decreasing RI coverage and the stress on existing health systems by competing public health priorities are likely important contributing factors to the increase in YF cases reported over the last two years in West and Central Africa20. Up until 2020, there had been minimal numbers of YF cases detected in West African countries following successful preventive mass vaccination campaigns previously conducted under the YF Initiative, the precursor to the EYE Strategy26. However, there have been several outbreaks detected since late 202019–22. Field investigations conducted in Guinea determined that many of the cases were among children who had not been vaccinated in the RI program and were born after the last large preventive campaign19. In Nigeria, confirmed outbreaks were noted in six states (Enugu, Ebonyi, Delta, Benue, Bauchi, and Borno) that had not yet undergone preventive campaigns; some of the areas had been scheduled for campaigns but timelines were delayed due to COVID-19 and other public health crises. Levels of population immunity also have declined due to the movement of susceptible populations in affected areas20. The outbreak in Senegal was centered in communities located along the border where there was fluid population movement and recent interruptions to primary health services19. In Ghana, an investigation found that many of the affected persons belonged to nomadic communities who had not been vaccinated during previous campaigns, suggesting challenges in equitable or sustained access to vaccination services20. In East Africa, the resurgence of YF cases is likely because of increased sylvatic activity that occurs in the region usually after prolonged periods of inactivity and lack of adequate vaccination25. Several high-risk countries in Eastern Africa (Ethiopia, South Sudan, and Uganda) have yet to introduce YF vaccine into their national RI programs or scale up existing programs19.
The current resurgence of YF is of particular concern as increased population mobility in today’s world amplifies the likelihood of new introductions and resulting local transmission in large population centers near currently affected areas, like Abidjan or Lagos, or to other non-endemic countries with Aedes aegypti populations27,28. Many areas of the world without YF virus do have Ae. aegypti-borne diseases and could theoretically be at risk for YF outbreaks3,4. This global threat has been illustrated by the recent spread of similar Ae. aegypti-borne viruses including chikungunya, dengue, and Zika29.
Although YF vaccination protects against infection within 7–10 days, implementation of emergency vaccination campaigns to contain rapidly expanding outbreaks is often hampered by limited in-country vaccine supplies, challenges with timely requests to access the emergency stockpile, and problems with vaccine delivery11,27. In addition, there continue to be competing vaccination priorities (e.g., COVID-19, polio, measles vaccine campaigns) and limitations in YF vaccine supplies as one of the four WHO prequalified manufacturers has paused production during the refurbishing of their facility and a second manufacturer is facing potential supply chain issues. However, additional manufacturers expected to come online by 203030. Effective YF control requires adequate and sustained population vaccination coverage. To achieve high vaccination coverage on a long-term basis, the optimal strategy is to incorporate YF vaccination into RI programs and implement supplementary immunization activities, including catch-up campaigns in older populations1,28.
If comprehensive healthcare services and immunization delivery continue to erode, we can expect more YF outbreaks to occur in additional countries where YF virus exists in sylvatic cycles, including in those with a history of previous campaigns where RI services are unable to sustain high vaccine coverages. Thus, it is of vital importance to close the existing immunity gaps to not lose what ground has been gained in the fight against YF. It is critical that the global public health community continue to provide essential support and encourage acceleration of these programs to protect against future outbreaks in established regions and prevent spread.
Acknowledgements
We would like to thank Jewgeni Bader at the World Health Organization for his assistance with the figure. There was no external source of funding to support this work.
Author contributions
N.P.L., J.H., A.D.T.B., M.D., O.T., M.V.H., H.Z., L.C., and J.E.S. contributed to the conception of the manuscript; N.P.L. and J.E.S. contributed to reviewing and the interpreting available literature; N.P.L., J.H., L.C., and J.E.S. contributed to drafting the manuscript; and N.P.L., J.H., A.D.T.B., M.D., O.T., M.V.H., H.Z., I.S.F., L.C., and J.E.S. contributed to reviewing and editing the manuscript. All authors approved the submitted version and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Competing interests
N.P.L., J.H., T.M., M.D., O.T., M.V.H., H.Z., L.C., and J.E.S. declare no competing interests. A.D.T.B. is Editor-in-Chief of npj Vaccines.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclosure: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and World Health Organization.
References
- 1.World Health Organization. Eliminate Yellow fever Epidemics (EYE): a global strategy, 2017–2026. Wkly Epidemiol. Rec. 2017;92:193–204. [PubMed] [Google Scholar]
- 2.Gaythorpe KA, et al. The global burden of yellow fever. eLife. 2021;10:e64670. doi: 10.7554/eLife.64670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Epidemic focus: yellow fever. Wkly Epidemiol. Rec. 2017;91:217–218. [Google Scholar]
- 4.Vasconcelos P. F., Monath T. P. Yellow fever remains a potential threat to public health. Vector-Borne and Zoonotic Diseases. 16. Published Online: 10.1089/vbz.2016.2031 (2016). [DOI] [PubMed]
- 5.Situation report: yellow fever, 28 October 2016. Geneva: World Health Organization. Available at: http://apps.who.int/iris/bitstream/10665/250661/1/yellowfeversitrep28Oct16-eng.pdf?ua=1).
- 6.Possas C, et al. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz. 2018;113:e180278. doi: 10.1590/0074-02760180278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey RM, et al. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak - final report. N. Engl. J. Med. 2019;381:444–454. doi: 10.1056/NEJMoa1710430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juan-Giner A, et al. Immunogenicity and safety of fractional doses of yellow fever vaccines: a randomised, double-blind, non-inferiority trial. Lancet. 2021;397:119–127. doi: 10.1016/S0140-6736(20)32520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Menezes Martins R, et al. Collaborative Group for Studies on Duration of Immunity from Yellow Fever Vaccine. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine. 2018;36:4112–4117. doi: 10.1016/j.vaccine.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Yellow fever vaccine: WHO position on the use of fractional doses – June 2017. Wkly Epidemiol. Rec. 2017;92:345–350. [PubMed] [Google Scholar]
- 11.Staples JE, Alvarez AR. Public health role for fractional dosage of yellow fever vaccine. Lancet. 2021;397:76–77. doi: 10.1016/S0140-6736(20)32707-0. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Eliminate Yellow Fever Epidemics: 2018 highlights. https://www.who.int/initiatives/eye-strategy#cms (2019).
- 13.World Health Organization. Eliminate Yellow Fever Epidemics: 2021 highlights. https://www.who.int/initiatives/eye-strategy#cms (2022).
- 14.World Health Organization. Yellow fever in Africa and the Americas, 2018. Wkly Epidemiol. Rec. 2019;94:365–378. [Google Scholar]
- 15.World Health Organization. Yellow fever urban outbreak in Angola and the risk of extension. Wkly Epidemiol. Rec. 2016;91:186–192. [PubMed] [Google Scholar]
- 16.World Health Organization. International Coordinating Group (ICG) on Vaccine Provision for Cholera, Meningitis, and Yellow Fever: Report of the Annual Meeting. https://www.who.int/publications/i/item/9789240029163 (2021).
- 17.Johnson BW, et al. Laboratory capacity assessments in 25 African countries at high risk of yellow fever, August-December 2018. Pan Afr. Med J. 2021;38:402. doi: 10.11604/pamj.2021.38.402.28886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Eliminate Yellow Fever Epidemics: 2020 highlights. https://www.who.int/initiatives/eye-strategy#cms (2021).
- 19.World Health Organization. Global yellow fever update, 2020. Wkly Epidemiol. Rec. 2021;96:377–392. [Google Scholar]
- 20.World Health Organization Africa. Yellow Fever in West and Central Africa. Weekly Bulletin on Outbreaks and Other Emergencies. Week 1: 27 December 2021 – 2 January 2022. https://apps.who.int/iris/bitstream/handle/10665/350967/OEW01-271202012022.pdf (2022).
- 21.World Health Organization. Yellow Fever —Kenya. Disease Outbreak News. March 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON361 (2022).
- 22.World Health Organization. Yellow Fever —Uganda. Disease Outbreak News. April 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON367 (2022).
- 23.Jafari H, et al. Rethinking public health campaigns in the COVID-19 era: a call to improve effectiveness, equity, and impact. BMJ Glob. Health. 2021;6:e006397. doi: 10.1136/bmjgh-2021-006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO/UNICEF Joint Estimates of National Immunization Coverage (WUENIC): Yellow Fever vaccination coverage. https://immunizationdata.who.int/pages/coverage/yfv.html (2022).
- 25.Ellis BR, Barrett AD. The enigma of yellow fever in East Africa. Rev. Med Virol. 2018;18:331–346. doi: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Yellow fever initiative: providing an opportunity of a lifetime. https://www.who.int/publications/i/item/WHO-HSE-GAR-ERI-2010-3 (2010).
- 27.Nomhwange T, et al. The resurgence of yellow fever outbreaks in Nigeria: a 2-year review 2017–2019. BMC Infect. Dis. 2021;21:1054. doi: 10.1186/s12879-021-06727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Vaccines and vaccination against yellow fever. WHO position paper—June 2013. Wkly Epidemiol. Rec. 2013;88:269–283. [PubMed] [Google Scholar]
- 29.Näslund J, et al. Emerging mosquito-borne viruses linked to Aedes aegypti and Aedes albopictus: global status and preventive strategies. Vector Borne Zoonotic Dis. 2021;21:731–746. doi: 10.1089/vbz.2020.2762. [DOI] [PubMed] [Google Scholar]
- 30.Montalvo Zurbia-Flores G, Rollier CS, Reyes-Sandoval A. Re-thinking yellow fever vaccines: fighting old foes with new generation vaccines. Hum. Vaccin Immunother. 2022;18:1895644. doi: 10.1080/21645515.2021.1895644. [DOI] [PMC free article] [PubMed] [Google Scholar]