Abstract
Purpose
Phosphorylated AKT1 (p-AKT1) at Ser473 is a functional isoform of AKT and a key component of the PI3K/mTOR/AKT pathway. This study aimed to evaluate the prognostic significance of p-AKT1 (Ser473) based on the molecular subtypes of breast cancer.
Methods
To investigate the prognostic value of p-AKT1 (Ser473), we performed a retrospective chart review of patients with breast cancer. Data on p-AKT1 (Ser473) positivity, hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) expression status, and other clinicopathological factors were obtained. Furthermore, the therapeutic effect of blocking p-AKT1 (Ser473) in breast cancer cells was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cell apoptosis assay, apoptosis protein array, and western blot analysis.
Results
A total of 3,044 patients were evaluated, and the median follow-up time was 43 (range: 0–125) months. In patients with HR-positive and HER2-positive disease, the p-AKT1 (Ser473)-positive group had worse disease-free survival (DFS) than the p-AKT1 (Ser473)-negative group (hazard ratio, 1.9; 95% confidence interval, 1.1–3.5; p = 0.024). In the multivariate analysis, p-AKT1 (Ser473) remained a significantly worse prognostic factor in patients with HR-positive/HER2-positive breast cancer (p = 0.03). There was no difference in DFS according to p-AKT1 (Ser473) status among patients with other breast cancer subgroups. In vitro analysis showed that blocking p-AKT1 (Ser473) levels enhanced trastuzumab-induced cell death in HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cells.
Conclusion
p-AKT1 (Ser473) is a prognostic marker for poor outcomes in patients with HR-positive/HER2-positive breast cancer and may have a potential value as a therapeutic target.
Keywords: AKT1 protein, Breast Neoplasms, Disease-Free Survival, Prognosis
INTRODUCTION
Since the introduction of endocrine therapy, breast cancer treatment has been a leading field in precision medicine. Studies to determine the prognostic and predictive markers have aimed to attain more specific treatment strategies for individual patients. The standard treatment strategies for each patient with breast cancer vary according to clinical or pathologic factors, such as menopausal status, tumor size, status of lymph node metastasis, expression of hormone receptors (HRs) and human epidermal growth factor receptor 2 (HER2), and gene expression assays. Because of these tailored therapies, patient outcomes have improved, and the likelihood of adverse events has been reduced. New targeted agents are typically tested based on the following four subtypes of breast cancer: HR-positive/HER2-negative (HR+/HER2−), HR-positive/HER2-positive, HR-negative/HER2-positive, and HR-negative/HER2-negative. In clinical trials, adding a new drug to the standard backbone therapy according to the breast cancer subtype has been a standard scheme for evaluating the efficacy of the drug.
In recent decades, many biological molecules in the PI3K/mTOR/AKT pathway have been investigated in preclinical and clinical settings. AKT is a key component of the PI3K/mTOR/AKT pathway and plays an important role in tumor growth, metastasis, apoptosis, and angiogenesis in breast cancer [1]. Few AKT inhibitors have been tested in phase I and II clinical trials in patients with breast cancer. The phase II FAKTION trial demonstrated that the addition of the pan-AKT inhibitor capivasertib to fulvestrant therapy significantly improved the outcomes of postmenopausal patients with aromatase inhibitor-resistant, HR-positive/HER2-negative advanced or metastatic breast cancer [2]. The median progression-free survival time was 10.3 months in the capivasertib group and 4.8 months in the placebo group (hazard ratio, 0.58; 95% confidence interval [CI], 0.39–0.84; p = 0.0044). In the LOTUS trial, a phase II study of the AKT inhibitor ipatasertib versus placebo in combination with paclitaxel was conducted in patients with previously untreated metastatic triple-negative breast cancer. The median progression-free survival time of the ipatasertib arm was 6.2 months, which was significantly better than that of the placebo arm at 4.9 months (stratified hazard ratio, 0.60; 95% CI, 0.37–0.98; log-rank p = 0.037) [3]. The PAKT trial investigated capivasertib in combination with paclitaxel as the first-line treatment for metastatic triple-negative breast cancer. The median progression-free survival time was 5.9 months in the capivasertib arm and 4.2 months in the placebo arm (hazard ratio, 0.74; 95% CI, 0.37–0.99; p = 0.04) [4].
To date, three isoforms of AKT have been identified: AKT1, AKT2, and AKT3 [5]. Although all three isoforms share over 85% homology in their catalytic domains, they perform different functions in cancer cells [1]. Of these, AKT1 is a key molecule in the pathway associated with cancer, especially breast cancer [6,7]. AKT1 is activated by phosphorylation of Thr308 and Ser473 residues, and full activation of AKT1 activity occurs through serine phosphorylation by mTORC2 at residue Ser473 [7]. In a study using proximity ligation assays, high levels of phosphorylated AKT1 (p-AKT1, Ser473) were significantly associated with poor prognosis [8,9,10,11]. Therefore, we hypothesized that p-AKT1 at residue Ser473 might be a prognostic marker in breast cancer patients, and because of its crosstalk with the estrogen receptor (ER) and/or HER2, the potential value of p-AKT1 (Ser473) as a prognostic marker might be different based on the subtypes. In addition, p-AKT1 (Ser473) may be a predictive marker of AKT inhibition in a specific subtype of breast cancer.
In this study, we evaluated the prognostic significance of p-AKT1 (Ser473) based on breast cancer subtypes. Additionally, the importance of p-AKT1 (Ser473) as a therapeutic target was investigated in a preclinical setting.
METHODS
Study design and patient selection
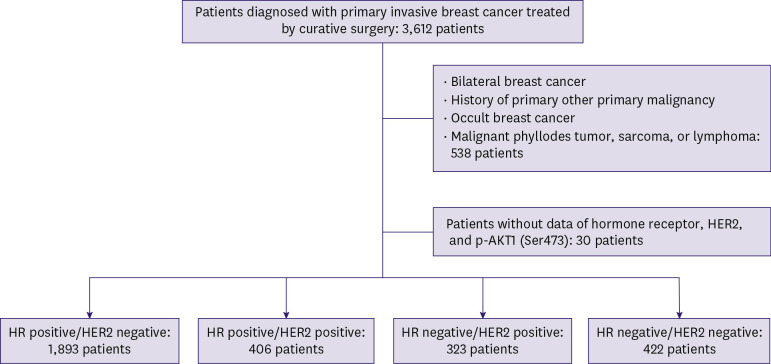
As shown in Figure 1, 3,612 patients who were newly diagnosed with primary invasive breast cancer between January 2010 and December 2018 and underwent curative surgery were analyzed in this study. Clinicopathological characteristics and survival data were obtained through a retrospective chart review. A total of 538 patients with bilateral breast cancer, history of other primary organ malignancies, occult breast cancer, malignant phyllodes tumor, sarcoma, or lymphoma were excluded. Thirty patients with incomplete data on HR, HER2, or p-AKT1 (Ser473) status were also excluded from this analysis. In total, 3,044 patients were included in the study. The TNM stage was classified according to the American Joint Committee on Cancer (AJCC) 8th edition anatomical staging. This study was approved by the Institutional Review Board (IRB number:2020-10-010) and was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
Figure 1. Patient selection.
HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; p-AKT1 = phosphorylated AKT1.
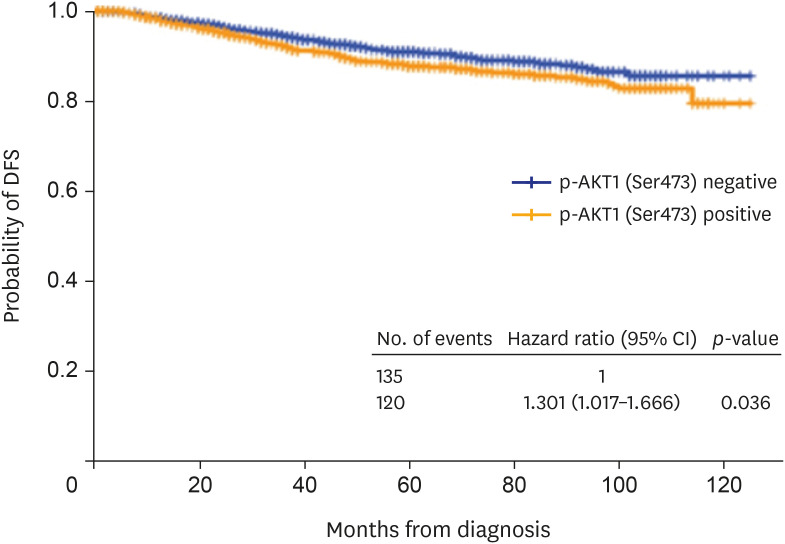
Figure 3. DFS of all patients according to p-AKT1 (Ser473) status.
DFS = disease-free survival; p-AKT1 = phosphorylated AKT1; CI = confidence interval.
Immunohistochemistry
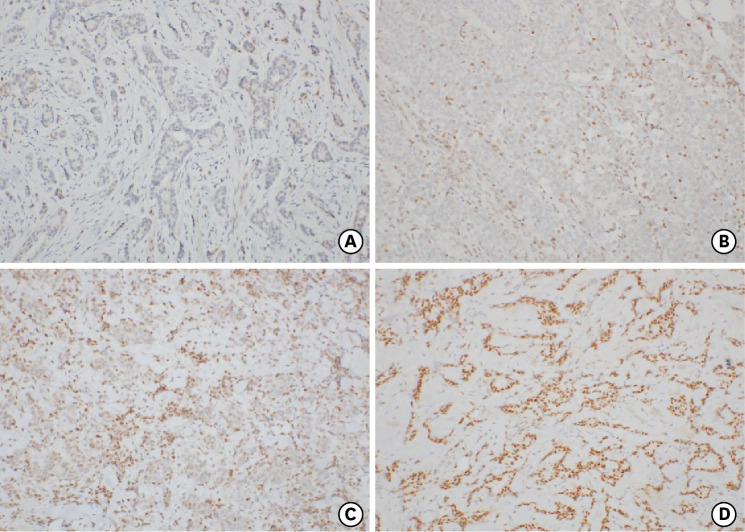
Positivity for ER, progesterone receptor (PR), HER2, Ki67, phosphorylated-S6K1 (p-S6K1), and p-AKT1 (Ser473) was evaluated by immunohistochemistry. All slides were reviewed by more than three pathologists at our institute, and a pathologist specializing in breast pathology confirmed the reading. ER or PR positivity was defined as the expression of ER or PR in > 1% of tumor cells. In cases where HER2 expression was classified as 2+, FISH or SISH was performed and reported according to the American Society of Clinical Oncology (ASCO) guidelines at the time of reporting after each surgery [12,13]. Cases where HER2 expression was equivocal on FISH or SISH were classified as negative. Because of the update in the ASCO guidelines, there was a correction in the interpretation of HER2 expression; however, it was confirmed by a pathologist that there was no difference in the data before and after the update. Negative p-S6K1 expression was assigned a score of 0 and positive expression a score of 1+ to 3+. Details of the procedures have been described in our previous report on the immunochemistry of p-S6K1 [14]. For p-AKT1 (Ser473) staining in the primary tumors, the sections were incubated with a primary antibody against p-AKT1(Ser473) (Invitrogen™, dilution 1:50; Thermo Fisher Scientific, Waltham, MA, USA), followed by anti-mouse immunoglobulin G (IgG) secondary antibody. The slides were then incubated in a tertiary antibody-horse peroxidase conjugate, followed by diaminobenzidine. After counterstaining with Meyer’s hematoxylin, the slides were dehydrated and mounted. p-AKT1 immunoreactivity was interpreted in a semi-quantitative manner using an intensity-proportion scoring system. The score was calculated as the sum of the intensity and proportion scores, which provided a score between 0 and 6. The proportion score was as follows: 0, no positive cells; +1, less than one-third positive tumor cells; +2, one-third to two-thirds positive tumor cells; and +3, more than two-thirds positive tumor cells. The intensity scores were assigned as follows: +1, weak staining; +2, intermediate staining; and +3, strong staining. A score of 0 was categorized as 0, score 2 as 1+, scores 3 and 4 as 2+, and scores 5 and 6 as 3+. The final scoring categories of 0 and 1+ were defined as negative p-AKT1 and 2+ and 3+ were defined as positive (Figure 2).
Figure 2. Representative immunohistochemical staining of p-AKT1 (Ser473).
(A) Tumor with a negative score, (B) tumor with a score of 1+, (C) tumor with a score of 2+, and (D) tumor with a score of 3+.
p-AKT1 = phosphorylated AKT1.
Cell culture and reagents
The breast cancer BT474 and trastuzumab-resistant BT474 (BT474-TR) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 (Welgene, Gyeongsan, Korea) containing 10% fetal bovine serum (FBS). MDA-MB-361 cells were kindly provided by Prof. Taeg Kyu Kwon (Department of Immunology, School of Medicine, Keimyung University, Daegu, Korea). Tamoxifen was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Perifosine, an AKT inhibitor, was purchased from Selleck Chemicals (Houston, TX, USA). SB3 (Samfenet™; Samsung Bioepis, Incheon, Korea), a trastuzumab biosimilar reagent, was provided by Daewoong Pharmaceutical (Seoul, Korea). For treatment, the cell medium was refreshed with RPMI-1640 supplemented with 10% charcoal-stripped FBS.
siRNA transfection
The cells were transfected with AKT1 siRNA or negative control siRNA using RNAiMax (Invitrogen™; Thermo Fisher Scientific), according to the manufacturer’s instructions. After transfection, the cells were incubated for 24 hours before further analysis. The siRNA against AKT1 mRNA corresponding to the coding sequence starting at position 667 (siRNA-5′-CACCUUCCAUGUGGAGACU-3′) relative to the cDNA sequence NM_005163.2 was synthesized (Bioneer, Daejeon, Korea). The AccuTarget™ negative control siRNA (SN-1001) was also purchased from Bioneer.
Western blot analysis
Total cell extracts were obtained using lysis buffer (Cell Signalling Technology, Beverly, MA, USA) supplemented with a protease inhibitor cocktail (Promega, Thermo Fisher Scientific) and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentrations were measured using the Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) for 1 hour at room temperature and incubated with specific primary antibodies, followed by horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG. The stained bands were visualized using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). The following primary antibodies were used: AKT, p-AKT1 (Ser473), cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase-3, survivin, Mcl-1, and p27/Kip1 obtained from Cell Signalling Technology, and β-actin obtained from Sigma-Aldrich.
Cell apoptosis assay
Cell apoptosis was assessed using the Muse™ Annexin V & Dead Cell Kit (Millipore; Merck KGaA), according to the manufacturer’s instructions. Briefly, a total of 1 × 105 cells were collected via centrifugation and washed with cold PBS. The cells were then resuspended in PBS with 1% BSA and stained with Muse™ Annexin V and Dead Cell Reagent for 20 minutes at room temperature in the dark. Finally, the stained cells were analyzed using the Muse™ Cell Analyzer.
Apoptosis protein array
The detection of multiple apoptosis-related proteins in cells was performed using a Human Apoptosis Proteome Profiler™ array (R&D Systems, Bio-Techne, Minneapolis, MN, USA), according to the manufacturer’s protocol.
Statistical analysis
The p-AKT1 (Ser473) status and clinicopathological parameters were evaluated using the chi-squared test. Disease-free survival (DFS) was defined as any breast cancer relapse, including locoregional recurrence, distant metastasis, contralateral breast cancer, primary malignancy in other organs, or death from any cause, without disease relapse. The Kaplan–Meier method with the log-rank test was used to analyze differences in DFS between the groups. Cox proportional hazards regression model was used to calculate hazard ratios and 95% CIs in the univariate and multivariate analyses.
In preclinical experiments, a paired sample t-test was used to compare the differences between the two populations. Statistical significance was set at p < 0.05. Data are expressed as mean ± standard deviation (SD).
RESULTS
Patient characteristics and p-AKT1 (Ser473) positivity distribution
A total of 3,044 patients were included in this study (Figure 1). Of these, 1,218 (40.0%) were classified into the p-AKT1 (Ser473)-positive group (Table 1). p-AKT1 (Ser473) positivity was associated with HR- and p-S6K1 positivity (Table 1).
Table 1. Patients’ demographics.
| Characteristics | p-AKT1 (Ser473) negative (n = 1,826) | p-AKT1 (Ser473) positive (n = 1,218) | p-value | |
|---|---|---|---|---|
| Age | 0.970 | |||
| < 50 years | 1,060 (58.1) | 706 (58.0) | ||
| ≥ 50 years | 766 (41.9) | 512 (42.0) | ||
| T stage | 0.091 | |||
| T1 | 1,128 (61.8) | 772 (63.4) | ||
| T2 | 639 (35.0) | 423 (34.7) | ||
| T3 | 54 (3.0) | 20 (1.6) | ||
| T4 | 5 (0.3) | 2 (0.2) | ||
| Unknown | 0 | 1 (0.1) | ||
| N stage | 0.807 | |||
| N0 | 1,243 (68.0) | 829 (68.1) | ||
| N1 | 418 (22.9) | 285 (23.4) | ||
| N2 | 107 (5.9) | 62 (5.1) | ||
| N3 | 53 (2.9) | 40 (3.3) | ||
| Unknown | 5 (0.3) | 2 (0.2) | ||
| Histologic grade | 0.002 | |||
| Grade 1 or 2 | 1,092 (59.8) | 756 (62.1) | ||
| Grade 3 | 664 (36.3) | 388 (31.9) | ||
| Unknown | 70 (3.8) | 74 (6.1) | ||
| Histologic type | 0.068 | |||
| Invasive ductal carcinoma | 1,621 (88.8) | 1,047 (86.0) | ||
| Invasive lobular carcinoma | 78 (4.3) | 67 (5.5) | ||
| Others | 127 (7.0) | 104 (8.5) | ||
| HR | 0.006 | |||
| ER and PR negative | 479 (26.2) | 266 (21.8) | ||
| ER or PR positive | 1,347 (73.8) | 952 (78.2) | ||
| HER2 | 0.527 | |||
| Negative | 1,396 (76.5) | 919 (75.5) | ||
| Positive | 430 (23.5) | 299 (24.5) | ||
| Ki67 | < 0.001 | |||
| < 20% | 885 (48.5) | 526 (43.2) | ||
| ≥ 20% | 601 (32.9) | 376 (30.9) | ||
| Unknown | 340 (18.6) | 316 (25.9) | ||
| p-S6K1 | < 0.001 | |||
| Negative | 787 (43.1) | 212 (17.4) | ||
| Positive | 1,031 (56.5) | 1,001 (82.2) | ||
| Unknown | 8 (0.4) | 5 (0.4) | ||
| Surgery | 0.992 | |||
| Breast conserving surgery | 1,257 (68.8) | 838 (68.8) | ||
| Mastectomy with or without reconstruction | 570 (31.2) | 380 (31.2) | ||
| Radiotherapy | 0.026 | |||
| Done | 1,402 (76.8) | 922 (75.7) | ||
| Not done | 370 (20.3) | 276 (22.7) | ||
| Unknown | 54 (3.0) | 20 (1.6) | ||
| Neoadjuvant or adjuvant chemotherapy | 0.014 | |||
| Taxane based | 573 (31.4) | 358 (29.4) | ||
| Non-taxane based | 634 (34.7) | 486 (39.9) | ||
| Not done or unknown | 619 (33.9) | 374 (29.4) | ||
| Adjuvant endocrine therapy | 0.135 | |||
| Aromatase inhibitor | 579 (31.7) | 402 (33.0) | ||
| Tamoxifen only | 674 (36.9) | 475 (39.0) | ||
| Not done | 573 (31.4) | 339 (27.8) | ||
| Adjuvant trastuzumab treatment | 0.556 | |||
| Done | 301 (16.5) | 191 (15.7) | ||
| Not done or unknown | 1,525 (83.5) | 1,027 (84.3) | ||
Values are presented as number (%).
p-AKT1 = phosphorylated-AKT1; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; p-S6K1 = phosphorylated-S6K1.
According to the subtypes, p-AKT1 (Ser473) was positive in 41.8%, 39.7%, 42.7%, and 30.3% of the HR-positive/HER2-negative, HR-positive/HER2-positive, HR-negative/HER2-positive, and HR-negative/HER2-negative patients, respectively (Table 2). Relatively high levels of p-S6K1 in the p-AKT1 (Ser473)-positive group were observed in all subtypes.
Table 2. Demographics based on subtype.
| Characteristics | HR-positive/HER2-negative (n = 1,893) | HR-positive/HER2-positive (n = 406) | HR-negative/HER2-positive (n = 323) | HR-negative/HER2-negative (n = 422) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-AKT1 (Ser473) negative (n = 1,102) | p-AKT1 (Ser473) positive (n = 791) | p-value | p-AKT1 (Ser473) negative (n = 245) | p-AKT1 (Ser473) positive (n = 161) | p-value | p-AKT1 (Ser473) negative (n = 185) | p-AKT1 (Ser473) positive (n = 138) | p-value | p-AKT1 (Ser473) negative (n = 294) | p-AKT1 (Ser473) positive (n = 128) | p-value | ||
| Age | 0.321 | 0.071 | 0.544 | 0.880 | |||||||||
| < 50 years | 499 (45.3) | 340 (43.0) | 95 (38.8) | 77 (47.8) | 48 (25.9) | 40 (29.0) | 124 (42.2) | 55 (43.0) | |||||
| ≥ 50 years | 603 (54.7) | 451 (57.0) | 150 (61.2) | 84 (52.2) | 137 (74.1) | 98 (71.0) | 170 (57.8) | 73 (57.0) | |||||
| T stage | 0.452 | 0.355 | 0.834 | 0.020 | |||||||||
| T1 | 735 (66.7) | 538 (68.0) | 142 (58.0) | 95 (59.0) | 101 (54.6) | 76 (55.1) | 150 (51.0) | 63 (49.2) | |||||
| T2 | 345 (31.3) | 241 (30.5) | 92 (37.6) | 63 (39.1) | 74 (40.0) | 57 (41.3) | 128 (43.5) | 62 (48.4) | |||||
| T3 | 20 (1.8) | 12 (1.5) | 9 (3.7) | 3 (1.9) | 9 (4.9) | 4 (2.9) | 16 (5.4) | 1 (0.8) | |||||
| T4 | 2 (0.2) | 0 | 2 (0.8) | - | 1 (0.5) | 1 (0.7) | - | 1 (0.8) | |||||
| Unknown | - | - | - | - | - | - | - | 1 (0.8) | |||||
| N stage | 0.197 | 0.507 | 0.200 | 0.074 | |||||||||
| N0 | 749 (68.0) | 557 (70.4) | 151 (61.6) | 95 (59.0) | 126 (68.1) | 81 (58.7) | 217 (73.8) | 96 (75.0) | |||||
| N1 | 261 (23.7) | 183 (23.1) | 67 (27.3) | 51 (31.7) | 33 (17.8) | 36 (26.1) | 57 (19.4) | 15 (11.7) | |||||
| N2 | 60 (5.4) | 27 (3.4) | 21 (8.6) | 10 (6.2) | 15 (8.1) | 15 (10.9) | 11 (3.7) | 10 (7.8) | |||||
| N3 | 28 (2.5) | 23 (2.9) | 6 (2.4) | 4 (2.5) | 11 (5.9) | 6 (4.3) | 8 (2.7) | 7 (5.5) | |||||
| Unknown | 4 (0.4) | 1 (0.1) | - | 1 (0.6) | - | - | 1 (0.3) | 0 | |||||
| Histologic grade | 0.097 | 0.598 | 0.155 | 0.272 | |||||||||
| Grade 1 or 2 | 863 (78.3) | 611 (77.2) | 117 (47.8) | 69 (42.9) | 47 (25.4) | 43 (31.2) | 65 (22.1) | 33 (25.8) | |||||
| Grade 3 | 199 (18.1) | 135 (17.1) | 109 (44.5) | 77 (47.8) | 132 (71.4) | 86 (62.3) | 224 (76.2) | 90 (70.3) | |||||
| Unknown | 40 (3.6) | 45 (5.7) | 19 (7.8) | 15 (9.3) | 6 (3.2) | 9 (6.5) | 5 (1.7) | 5 (3.9) | |||||
| Histologic type | 0.053 | 0.944 | 0.327 | 0.630 | |||||||||
| Invasive ductal carcinoma | 958 (86.9) | 656 (82.9) | 222 (90.6) | 146 (90.6) | 179 (96.8) | 135 (97.8) | 262 (89.1) | 110 (85.9) | |||||
| Invasive lobular carcinoma | 66 (6.0) | 61 (7.7) | 4 (1.6) | 2 (1.2) | 2 (1.1) | 0 | 6 (2.0) | 4 (3.1) | |||||
| Others | 78 (7.1) | 74 (9.4) | 19 (7.8) | 13 (8.1) | 4 (2.2) | 3 (2.2) | 26 (8.8) | 14 (10.9) | |||||
| Ki67 | < 0.001 | < 0.001 | 0.856 | 0.925 | |||||||||
| < 20% | 704 (63.9) | 437 (55.2) | 84 (34.3) | 33 (20.5) | 46 (24.9) | 32 (23.2) | 51 (17.3) | 24 (18.8) | |||||
| ≥ 20% | 187 (17.0) | 150 (19.0) | 91 (37.1) | 50 (31.1) | 117 (63.2) | 87 (63.0) | 206 (70.1) | 89 (69.5) | |||||
| Unknown | 211 (19.1) | 204 (25.8) | 70 (28.6) | 78 (48.4) | 22 (11.9) | 19 (13.8) | 37 (12.6) | 15 (11.7) | |||||
| p-S6K1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||
| Negative | 521 (47.3) | 149 (18.8) | 91 (37.1) | 28 (17.4) | 65 (35.1) | 19 (13.8) | 110 (37.4) | 16 (12.5) | |||||
| Positive | 579 (52.5) | 638 (80.7) | 152 (62.0) | 133 (82.6) | 117 (63.2) | 118 (85.5) | 183 (62.2) | 112 (87.5) | |||||
| Unknown | 2 (0.2) | 4 (0.5) | 2 (0.8) | 0 | 3 (1.6) | 1 (0.7) | 1 (0.3) | 0 | |||||
| Surgery | 0.585 | 0.346 | 0.144 | 0.071 | |||||||||
| Breast conserving surgery | 804 (73.0) | 586 (74.1) | 144 (58.8) | 87 (54.0) | 101 (54.6) | 64 (46.4) | 207 (70.4) | 101 (78.9) | |||||
| Mastectomy | 298 (27.0) | 205 (25.9) | 101 (41.2) | 74 (46.0) | 84 (45.4) | 74 (53.6) | 87 (29.6) | 27 (21.1) | |||||
| Radiotherapy | 0.021 | 0.068 | 0.385 | 0.056 | |||||||||
| Done | 879 (79.8) | 622 (78.6) | 168 (68.6) | 103 (64.0) | 129 (69.7) | 87 (63.3) | 226 (76.9) | 110 (85.9) | |||||
| Not done | 187 (17.0) | 157 (19.8) | 64 (26.1) | 55 (34.2) | 54 (29.2) | 48 (34.8) | 65 (22.1) | 16 (12.5) | |||||
| Unknown | 36 (3.3) | 12 (1.5) | 13 (5.3) | 3 (1.9) | 2 (1.1) | 3 (2.2) | 3 (1.0) | 2 (1.6) | |||||
| Neoadjuvant or adjuvant chemotherapy | 0.001 | 0.625 | 0.128 | 0.157 | |||||||||
| Taxane based | 283 (25.7) | 186 (23.5) | 81 (33.1) | 57 (35.4) | 59 (31.9) | 59 (42.8) | 150 (51.0) | 56 (43.8) | |||||
| Non-taxane based | 301 (27.3) | 279 (35.3) | 127 (51.8) | 85 (52.8) | 95 (51.4) | 61 (44.2) | 111 (37.8) | 61 (47.7) | |||||
| Not done or unknown | 518 (47.0) | 326 (41.2) | 37 (15.1) | 19 (11.8) | 31 (16.8) | 18 (13.0) | 33 (11.2) | 11 (8.6) | |||||
| Adjuvant endocrine therapy | 0.177 | 0.059 | 0.484 | - | |||||||||
| Aromatase inhibitor | 476 (43.2) | 348 (44.0) | 102 (41.6) | 52 (31.3) | 1 (0.5) | 2 (1.4) | - | - | |||||
| Tamoxifen only | 570 (51.7) | 388 (49.1) | 103 (42.0) | 87 (54.0) | 1 (0.5) | 0 | - | - | |||||
| Not done or unknown | 56 (5.1) | 55 (7.0) | 40 (16.3) | 22 (13.7) | 183 (99.0) | 136 (98.6) | - | - | |||||
| Adjuvant trastuzumab treatment | 0.267 | 0.345 | 0.451 | 0.599 | |||||||||
| Done | 16 (1.5) | 7 (0.9) | 153 (62.4) | 93 (57.8) | 128 (69.2) | 90 (65.2) | 4 (1.4) | 1 (0.8) | |||||
| Not done or unknown | 1,086 (98.5) | 784 (99.1) | 92 (37.6) | 68 (42.2) | 57 (30.8) | 48 (34.8) | 290 (98.6) | 127 (99.2) | |||||
HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; p-AKT1 = phosphorylated-AKT1; p-S6K1 = phosphorylated-S6K1.
Values are presented as number (%).
DFS based on p-AKT1 (Ser473) positivity
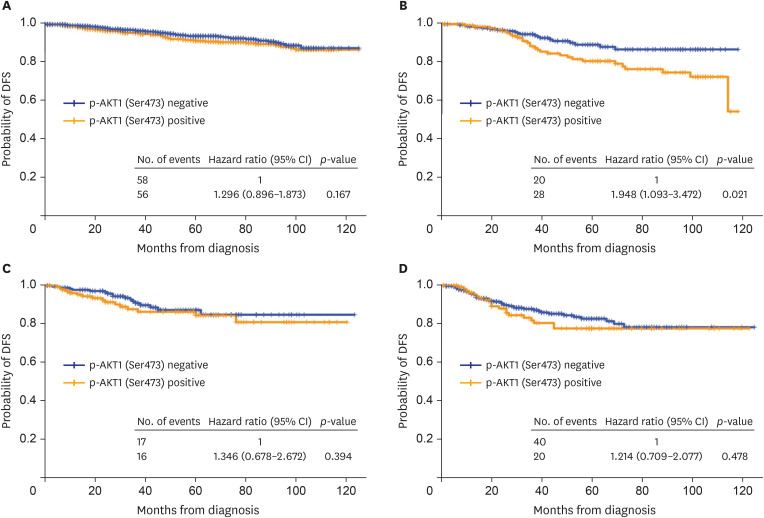
The median follow-up period was 43 months (range: 0-125 months). Among all patients, those with p-AKT1 (Ser473)-positive tumors had a significantly worse prognosis than those with p-AKT1 (Ser473)-negative tumors (hazard ratio, 1.3; 95% CI, 1.0–1.7; p = 0.036) (Figure 3). DFS was analyzed according to the subtypes (Figure 4). Among patients with HR-positive and HER2-positive disease, the p-AKT1 (Ser473)-positive group had worse DFS than the p-AKT1 (Ser473)-negative group (hazard ratio, 1.9; 95% CI, 1.1–3.5 p = 0.024) (Table 3). Otherwise, there was no difference in DFS according to p-AKT1 (Ser473) positivity among other subgroups of breast cancer. In multivariate analysis, p-AKT1 (Ser473) positivity remained a significantly worse prognostic factor for patients with HR+/HER2+ breast cancer (p = 0.03; Table 3).
Figure 4. DFS of patients based on subtypes.
Prognostic significance of p-AKT1 (Ser473) status for (A) HR-positive/HER2-negative patients, (B) HR-positive/HER2-positive patients, (C) HR-negative/HER2-positive patients, and (D) HR-negative/HER2-negative patients.
DFS = disease-free survival; p-AKT1 = phosphorylated AKT1; HR = hormone receptor; HER2 = human epidermal growth factor receptor 2.
Table 3. Univariate and multivariate analyses of DFS based on p-AKT1 (Ser473) in patients with HR positive/HER2-positive breast cancer.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| Age (≥ 50 years vs. < 50 years) | 0.8 (0.4–1.4) | 0.394 | |||
| T stage (vs. T1) | |||||
| T2 | 5.1 (2.6–9.9) | 3.7 (1.8–7.7) | |||
| T3 | 5.8 (1.6–20.7) | 6.4 (1.6–25.1) | |||
| T4 | 61.1 (7.4–503.6) | < 0.001 | 113.3 (11.2–1,143.5) | < 0.001 | |
| N stage (vs. N0) | |||||
| N1 | 2.2 (1.2–4.3) | 1.8 (0.7–4.5) | |||
| N2 | 4.4 (2.0–9.8) | 4.8 (1.5–15.1) | |||
| N3 | 11.5 (3.3–39.7) | < 0.001 | 8.0 (1.9–33.1) | 0.016 | |
| Histologic grade (Grade 3 vs. Grade 1 or 2) | |||||
| Grade 3 | 1.8 (1.0–3.4) | 0.052 | |||
| Ki67 (≥ 20% vs. < 20%) | 1.7 (0.7–4.2) | 0.266 | |||
| p-AKT1 (Ser473) (Positive vs. Negative) | 1.9 (1.1–3.5) | 0.024 | 2.0 (1.1–3.6) | 0.030 | |
| Surgery (Mastectomy vs. Breast conserving surgery) | 2.0 (1.1–3.6) | 0.019 | 0.7 (0.3–1.6) | 0.364 | |
| Radiotherapy (Not done vs. Done) | 2.1 (1.2–3.7) | 0.014 | 3.3 (1.3–8.5) | 0.015 | |
| Neoadjuvant or Adjuvant chemotherapy (vs. Taxane based) | |||||
| Non-taxane based | 0.8 (0.4–2.0) | 1.4 (0.4–4.5) | |||
| Not done or unknown | 0.5 (0.2–0.8) | 0.037 | 1.4 (0.6–3.5) | 0.709 | |
| Adjuvant endocrine therapy (vs. Aromatase inhibitor) | |||||
| Tamoxifen only | 1.2 (0.6–2.2) | ||||
| Not done | 1.3 (0.5–3.0) | 0.844 | |||
| Adjuvant trastuzumab treatment (Not done or unknown vs. Done) | 1.4 (0.8–2.6) | 0.226 | |||
DFS = disease-free survival; p-AKT1 = phosphorylated-AKT1; HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; CI = confidence interval.
Perifosine, an AKT inhibitor, enhances trastuzumab-induced cell death in HR- and HER2-positive breast cancer cells
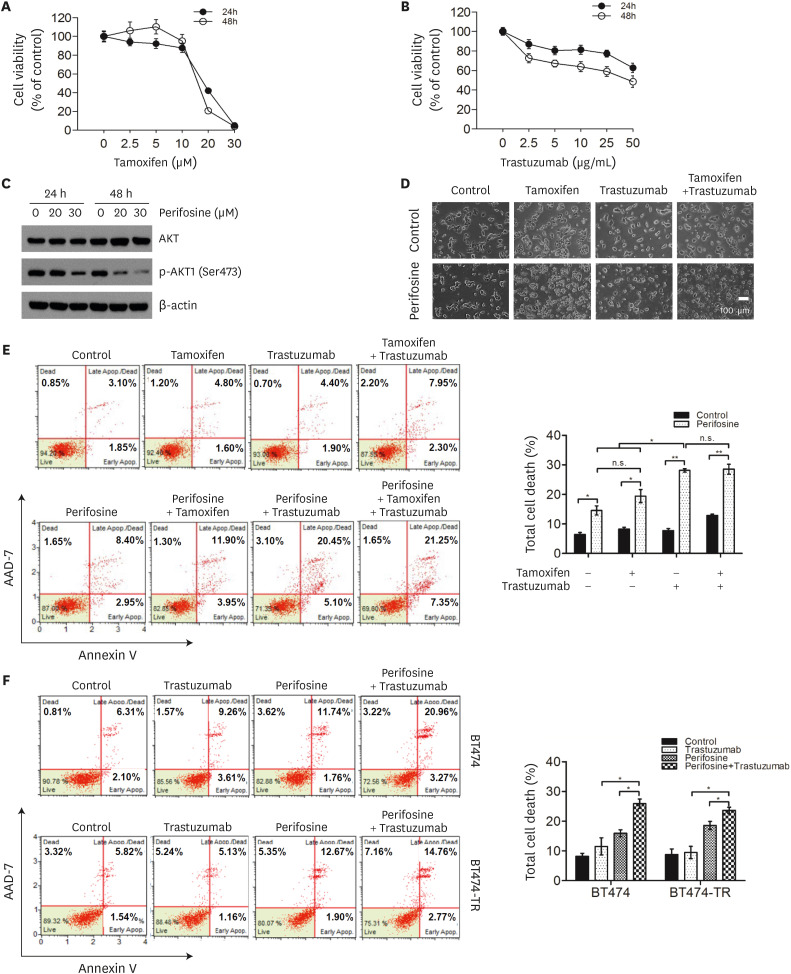
As p-AKT1 (Ser473) is a poor prognostic marker in patients with HR-positive/HER2-positive disease, we investigated whether inhibition of p-AKT1 (Ser473) would have a therapeutic effect when combined with tamoxifen and/or trastuzumab in the HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cell lines, BT474 and MDA-MB-361. First, we evaluated the effects of tamoxifen and trastuzumab on the growth of BT474 cells and MDA-MB-361. Cells were exposed to either tamoxifen or trastuzumab at different concentrations for 24 or 48 h, and cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Figure 5A and B, Supplementary Figure 1A and B). The IC50 values of tamoxifen in BT474 and MDA-MB-361 cells were approximately 16 and 17 μM, respectively, after 24 hours exposure. The IC50 values of tamoxifen in BT474 and MDA-MB-361 cells were approximately 18 and 12 μM, respectively, after 48 hours treatment. The IC50 value of trastuzumab in both BT474 and MDA-MB-361 cells at 48 hours was approximately 50 μg/mL.
Figure 5. AKT inhibitor enhanced trastuzumab-induced cell death in BT474 and BT474-TR cells.
The effects of tamoxifen (A) and trastuzumab (B) on cell viability were analyzed using the MTT assay (n = 3). (C) Protein levels of AKT and p-AKT (Ser473) in the perifosine-treated BT474 cells. Cells were treated with 20 or 30 μM perifosine, an AKT inhibitor, for 24 or 48 hours and then subjected to western blot analysis. β-Actin was used as an internal control. (D) BT474 cells were treated with or without 20 μM perifosine and co-treated with 10 μM tamoxifen and/or 2 μg/mL trastuzumab for 48 hours. Cell morphology was examined using a microscope. (E, F) Flow cytometric analysis of apoptotic cells using the Muse™ Annexin V & Dead Cell Kit. Control- or perifosine-treated BT474 cells were cotreated with 10 μM tamoxifen and/or 2 μg/mL trastuzumab for 48 hours (E). Control- or perifosine-treated BT474 and BT474-TR cells were cotreated with 2 μg/mL trastuzumab for 48 hours (F). All data in the graphs are presented as the mean ± standard deviation (n = 3). Images represent three independent experiments.
MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; p-AKT1 = phosphorylated AKT1; n.s. = non-significant.
*p < 0.05; **p < 0.01.
Next, we investigated the effects of p-AKT1 (Ser473) inhibition on tamoxifen-and/or trastuzumab-induced cell death in BT474 and MDA-MB-361 cells. Perifosine is an orally available alkyl phospholipid that inhibits AKT1 phosphorylation [15,16]. Western blot analysis showed that perifosine significantly reduced p-AKT1 (Ser473) levels in BT474 and MDA-MB-361 cells in a dose- and time-dependent manner, indicating the effective inhibition of AKT activity (Figure 5C, Supplementary Figure 1C). The cells were then subjected to single, dual, and triple treatments with tamoxifen, trastuzumab, and perifosine. As shown in Figure 5D, reduced cell viability was observed in BT474 cells treated with a combination of tamoxifen and trastuzumab when AKT1 activity was inhibited by perifosine. To clarify this result, the percentage of apoptotic BT474 and MDA-MB-361 cells was evaluated using annexin V/AAD-7 staining (Figure 5E, Supplementary Figure 1D). Inhibition of p-AKT1 (Ser473) by perifosine increased the number of apoptotic cells in untreated, tamoxifen-treated, and trastuzumab-treated BT474 cells (p < 0.05, p < 0.05, and p < 0.01, respectively) and MDA-MB-361 cells (p < 0.05, p < 0.05, and p < 0.05, respectively). Meanwhile, perifosine markedly enhanced the sensitivity of BT474 cells to trastuzumab compared with tamoxifen (Figure 5E). These data suggest that p-AKT1 (Ser473) blocking might be more sensitive to trastuzumab than tamoxifen in HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cells.
We further clarified the effect of blocking p-AKT1 (Ser473) on conferring sensitivity to trastuzumab in trastuzumab-resistant HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cells, BT474-TR (Figure 5F, Supplementary Figure 2). Indeed, perifosine significantly enhanced sensitivity to trastuzumab in trastuzumab-treated BT474-TR cells compared with perifosine or trastuzumab alone (p < 0.05 or p < 0.05; Figure 5F). Taken together, these results demonstrate that p-AKT1 (Ser473) inhibition with perifosine confers sensitivity to breast cancer therapy in HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cells. In addition, it seems that perifosine has a higher synergistic effect with trastuzumab than with tamoxifen in HR-positive/HER2-positive and p-AKT1 (Ser473)-positive breast cancer cell lines.
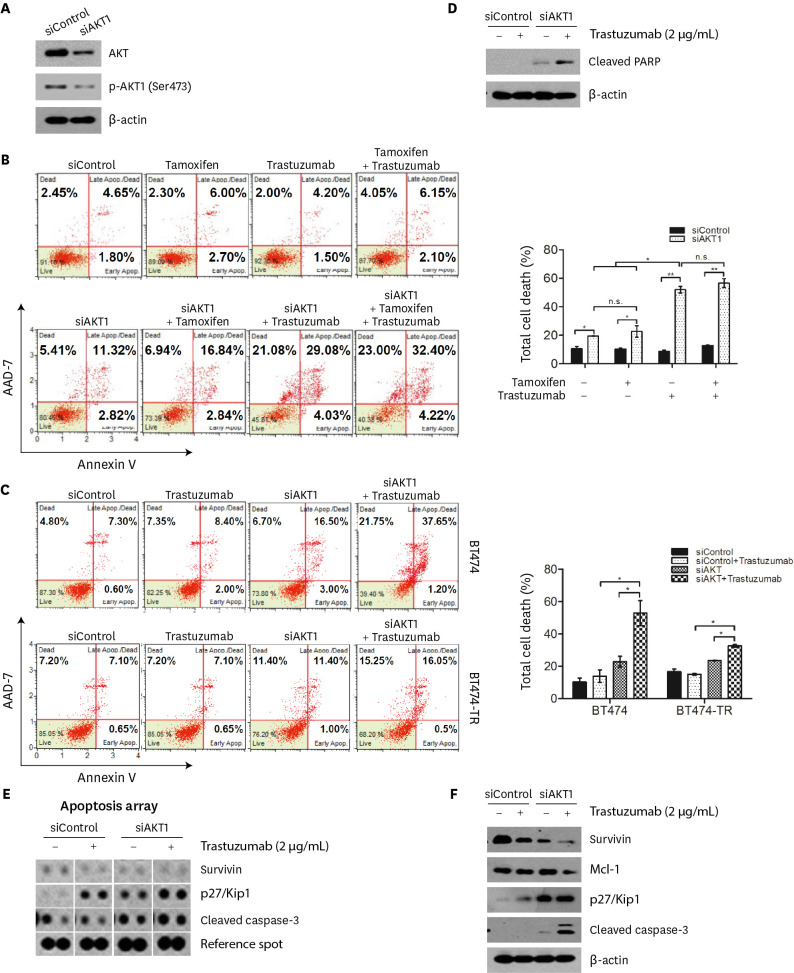
AKT1 knockdown enhances trastuzumab-induced cell death in HR- and HER2-positive breast cancer cells
To confirm the effect of p-AKT1 (Ser473) on trastuzumab-induced cell death, BT474 and MDA-MB-361 cells were transiently transfected with siRNA targeting AKT1, followed by treatment with tamoxifen and/or trastuzumab. AKT1 siRNA decreased total AKT and p-AKT1 (Ser473) protein expression in BT474 and MDA-MB-361 cells (Figure 6A, Supplementary Figure 3A). As expected, AKT1 knockdown enhanced the sensitivity of BT474 and MDA-MB-361 cells to trastuzumab (Figure 6B, Supplementary Figure 3B). We further confirmed that AKT1 knockdown significantly improved trastuzumab sensitivity of BT474-TR cells (Figure 6C). Furthermore, we confirmed that AKT1 inhibition by siRNA increased cleaved PARP expression in trastuzumab-treated BT474 cells (Figure 6D). Together, these results indicate that suppression of AKT1 activity significantly enhances trastuzumab sensitivity of HR-positive/HER2-positive breast cancer cells.
Figure 6. Knockdown of AKT1 by siRNA enhanced trastuzumab-induced cell death in BT474 and BT474-TR cells.
(A) Knockdown of AKT1 in BT474 cells. The cells were transfected with either control siRNA (siControl) or AKT1 siRNA (siAKT1) for 48 hours and then subjected to western blot analysis. β-Actin was used as the internal protein control. (B, C) Flow cytometric analysis of apoptotic cells with the Muse™ Annexin V & Dead Cell kit. Control siRNA- or AKT1 siRNA-transfected BT474 cells were cotreated with 10 μM tamoxifen and/or 2 μg/mL trastuzumab for 48 hours (B). Control siRNA- or AKT1 siRNA-transfected BT474 and BT474-TR cells were cotreated with 2 μg/mL trastuzumab for 48 hours (C). (D) The expression level of PARP in BT474 cells treated with a combination of siAKT1 and trastuzumab. (E) Analysis via proteome profiler array of BT474 cells treated with the combination of AKT1 knockdown and trastuzumab. The cells were transfected with 50 μM control siRNA or AKT1 siRNA for 48 hours and then treated with or without 2 μg/mL trastuzumab. (F) The expression levels of antiapoptotic proteins (survivin and Mcl-1) and proapoptotic proteins (p27/Kip1 and cleaved caspase-3) were measured by western blot analysis. β-Actin was used as the internal protein control. All data in graphs are presented as the means ± standard deviations. Images are representative of three independent experiments (n = 3).
PARP = poly(ADP-ribose) polymerase; n.s. = non-significant.
*p < 0.05; **p < 0.01.
The combination of AKT1 knockdown and trastuzumab enhanced cell death by regulating apoptosis-related proteins in HR- and HER2-positive breast cancer cells
To gain further insight into the differential expression of apoptosis-related proteins, we performed a human apoptosis proteome array in BT474 cells using a combination of AKT1 knockdown and trastuzumab treatment. The human apoptotic proteome profiler array showed that a combination of AKT1 knockdown and trastuzumab treatment significantly upregulated the pro-apoptotic proteins cleaved caspase-3 and p27/Kip1 in BT474 cells compared to cells with either AKT1 knockdown or trastuzumab treatment alone (Figure 6E). Furthermore, the expression of the antiapoptotic protein survivin was diminished in BT474 cells treated with the combination treatment. The differential expression patterns of apoptosis-related proteins were validated using western blot analysis (Figure 6F). Again, a combination of AKT1 knockdown and trastuzumab treatment increased cleaved caspase-3 and p27/Kip1 levels, whereas the combination decreased the expression of survivin and Mcl-1.
DISCUSSION
In this study, p-AKT1 (Ser473) positivity was an independent prognostic factor for patients with HR-positive/HER2-positive early breast cancer, while there was no significant difference in DFS based on p-AKT1 (Ser473) status in other subtypes. In our in vitro study, we also showed that AKT inhibition with perifosine, an AKT inhibitor, significantly enhanced the sensitivity to tamoxifen and trastuzumab therapy in the both HR-positive/HER2-positive and p-AKT1 (Ser473)-positive cell lines, BT474 and MDA-MB-361. We found that perifosine had a better synergistic effect with trastuzumab than with tamoxifen in BT474 and MDA-MB-361 cells. The synergistic effect of perifosine with trastuzumab was evident when the Annexin V/AAD-7 staining data showed that perifosine significantly improved the sensitivity of trastuzumab-resistant BT474 cells (BT474-TR). We obtained similar results using siRNA against AKT1 in BT474, MDA-MB-361, and BT474-TR cells. Indeed, AKT1 knockdown significantly enhanced the death of trastuzumab-treated cells by regulating the expression levels of apoptosis-related proteins, including cleaved PARP, cleaved caspase-3, p27/Kip1, survivin, and Mcl-1.
In terms of the poor prognosis of patients with p-AKT1 (Ser473) positivity, the results of our study are consistent with those of Spears et al. [8]. High levels of p-AKT1 (Ser473) were associated with large tumor size, older age, and PR negativity. High p-AKT1(Ser473) positivity was associated with significantly reduced distant relapse-free survival (hazard ratio, 1.45; 95% CI, 1.14–1.83; p = 0.002) and overall survival (hazard ratio, 1.42; 95% CI, 1.10–1.83; p = 0.007) [8]. Our results also showed that p-AKT1 (Ser473)-positive tumors were associated with worse DFS. Interestingly, when the analysis was conducted based on subtype, the poor prognostic effect of p-AKT1 (Ser473) positivity was confined to HR-positive/HER2-positive disease.
To our knowledge, no other study has explained the relationship between the specific breast cancer subtypes and p-AKT1. However, a correlation between the ER and p-Akt1 was reported by Gershtein et al. [17] in 2006. Furthermore, association between HER2 amplification and AKT has been reported by many researchers [18,19,20]. The findings of these studies can help understand the results of our study.
HR-positive/HER2-positive breast cancer can be treated with both endocrine and HER2-targeted therapies. However, it can become resistant to endocrine therapy or HER2-targeted therapies and can have a more complicated multifactorial resistance mechanism than the other subtypes. Several trials have demonstrated that HR-positive cancers with HER2 overexpression are relatively resistant to hormonal therapy in both early and advanced stages compared with HER2-negative cases [21]. Therefore, it would be beneficial to develop the novel targeted agents that can overcome the resistance to endocrine therapy and HER2-targeted therapy in HR-positive/HER2-positive breast cancer. However, these are relatively neglected compared with other molecular types in the field of new drug development. Consequently, while HER2-targeted therapy is the standard first-line treatment for patients with HER2-positive advanced or metastatic breast cancer, there are still no precise treatment guidelines for patients with HR-positive/HER2-positive disease, although this population accounts for more than 11% of newly diagnosed breast cancer cases [22]. As endocrine therapy or HER2-targeted therapy is more tolerable than cytotoxic chemotherapy, there may be an opportunity to add another targeted agent to these treatments. In this study, the combination of an AKT inhibitor and trastuzumab showed a better effect in terms of cell death than monotherapy with an AKT inhibitor, trastuzumab, or tamoxifen in HR-positive/HER2-positive breast cancer cell lines. Moreover, combined treatment confers trastuzumab sensitivity in trastuzumab-resistant HR-positive/HER2-positive breast cancer cells, indicating that the combination regimen with trastuzumab is a promising treatment for HR-positive/HER2-positive breast cancer. Thus, the results of this study could help in designing clinical trials using AKT inhibitors for patients with breast cancer and evaluating the efficacy of the combination regimen of AKT inhibitors with trastuzumab treatment based on p-AKT1(Ser473) status for patients with HR-positive/HER2-positive disease.
The limitation of this study was its retrospective design. Radiation therapy was observed as a negative factor in prognosis. However, it may be an unpredictable bias because of the retrospective research design. Furthermore, long-term follow-up data could not be obtained because of the retrospective nature of the study. However, this study included a relatively large number of patients treated consecutively at a single institute, which might provide data that are more in line with the real-world situations.
Perifosine is a pan-AKT inhibitor that targets all AKT isoforms [15,23]. Emerging reports have shown that several AKT inhibitors in clinical development are pan-AKT inhibitors; however, the results of these clinical trials are rarely introduced in the clinical arena because of the relatively severe adverse effects [24,25]. Identifying specific targets with new drugs could reduce the adverse effects of a drug. Therefore, we evaluated the effect of blocking AKT1, instead of inhibiting pan-AKT. We specifically blocked AKT1 using siRNA in BT474 cells and confirmed that trastuzumab-induced cell death was enhanced compared with perifosine treatment.
In conclusion, p-AKT1 (Ser473) is a prognostic marker in patients with HR-positive/HER2-positive early-stage breast cancer. p-AKT1 (Ser473) could be a therapeutic target in this population, and trastuzumab might be a good choice as the backbone partner of AKT inhibitors in these patients. In addition, the possibility of AKT1 inhibition should be considered in future studies. Thus, the results of this study could provide guidance for designing future clinical trials using AKT inhibitors for patients with breast cancer and for evaluating the efficacy of the combination regimen of AKT inhibitors with a trastuzumab backbone based on p-AKT1 (Ser473) status in patients with HR-positive/HER2-positive disease.
ACKNOWLEDGMENTS
We greatly appreciate Prof. Taeg Kyu Kwon (Keimyung University, Korea) for providing us with the MDA-MB-361 cell line. We also appreciate Daewoong Pharmaceutical for kindly providing SB3.
Footnotes
Funding: This study was supported by grants from the Korea Institute of Radiological and Medical Sciences funded by the Ministry of Science and ICT, Republic of Korea (50548-2022; 50531-2022).
Conflict of Interest: The authors declare that they have no competing interest.
- Conceptualization: Noh WC, Kim HA.
- Data curation: Kim JY, Jang SK, Seol H, Seong MK, Noh WC, Park IC, Kim HA.
- Formal analysis: Kim JY, Kim HA.
- Investigation: Kim JY, Park CS, Jang SK, Seol H, Park IC.
- Methodology: Kim JY, Jang SK, Seol H, Seong MK, Noh WC, Park IC, Kim HA.
- Supervision: Seol H, Seong MK, Kim HA.
- Visualization: Kim JY.
- Writing - original draft: Kim JY, Park CS, Kim HA.
- Writing - review & editing: Kim JY, Park CS, Jang SK, Seol H, Seong MK, Noh WC, Park IC, Kim HA.
SUPPLEMENTARY MATERIALS
AKT inhibitor enhanced trastuzumab-induced cell death in MDA-MB-361 cells.
Comparison of trastuzumab sensitivity in BT474 and BT474-TR cells.
Knockdown of AKT1 by siRNA enhanced trastuzumab-induced cell death in MDA-MB-361 cells.
References
- 1.Hinz N, Jücker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SB, Dent R, Im SA, Espié M, Blau S, Tan AR, et al. LOTUS investigators. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423–433. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Lambring CB. Akt isoforms: a family affair in breast cancer. Cancers (Basel) 2021;13:3445. doi: 10.3390/cancers13143445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbarzadeh Kaboli P, Salimian F, Aghapour S, Xiang S, Zhao Q, Li M, et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer - a comprehensive review from chemotherapy to immunotherapy. Pharmacol Res. 2020;156:104806. doi: 10.1016/j.phrs.2020.104806. [DOI] [PubMed] [Google Scholar]
- 7.Ortega MA, Fraile-Martínez O, Asúnsolo Á, Buján J, García-Honduvilla N, Coca S. Signal transduction pathways in breast cancer: the important role of PI3K/Akt/mTOR. J Oncol. 2020;2020:9258396. doi: 10.1155/2020/9258396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spears M, Cunningham CA, Taylor KJ, Mallon EA, Thomas JS, Kerr GR, et al. Proximity ligation assays for isoform-specific Akt activation in breast cancer identify activated Akt1 as a driver of progression. J Pathol. 2012;227:481–489. doi: 10.1002/path.4022. [DOI] [PubMed] [Google Scholar]
- 9.Xing Y, Lin NU, Maurer MA, Chen H, Mahvash A, Sahin A, et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019;21:78. doi: 10.1186/s13058-019-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 11.Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Allison KH, Harvey BE, McShane LM, Dowsett M. HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update summary. J Oncol Pract. 2018;14:437–441. doi: 10.1200/JOP.18.00206. [DOI] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 14.Kim EK, Kim HA, Koh JS, Kim MS, Kim KI, Lee JI, et al. Phosphorylated S6K1 is a possible marker for endocrine therapy resistance in hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2011;126:93–99. doi: 10.1007/s10549-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 15.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 16.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershtein ES, Scherbakov AM, Anurova OA, Krasilńikov MA, Kushlinsky NE. Phosphorylated Akt1 in human breast cancer measured by direct sandwich enzyme-linked immunosorbent assay: correlation with clinicopathological features and tumor VEGF-signaling system component levels. Int J Biol Markers. 2006;21:12–19. [PubMed] [Google Scholar]
- 18.Fujimoto Y, Morita TY, Ohashi A, Haeno H, Hakozaki Y, Fujii M, et al. Combination treatment with a PI3K/Akt/mTOR pathway inhibitor overcomes resistance to anti-HER2 therapy in PIK3CA-mutant HER2-positive breast cancer cells. Sci Rep. 2020;10:21762. doi: 10.1038/s41598-020-78646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Saenz A, Dreyer C, Campbell MR, Steri V, Gulizia N, Moasser MM. HER2 amplification in tumors activates PI3K/Akt signaling independent of HER3. Cancer Res. 2018;78:3645–3658. doi: 10.1158/0008-5472.CAN-18-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokunaga E, Kimura Y, Oki E, Ueda N, Futatsugi M, Mashino K, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–289. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- 21.Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–3025. doi: 10.1200/JCO.2005.04.3034. [DOI] [PubMed] [Google Scholar]
- 22.Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, et al. Breast cancer statistics in Korea in 2017: data from a Breast Cancer Registry. J Breast Cancer. 2020;23:115–128. doi: 10.4048/jbc.2020.23.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 25.Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, et al. Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (review) Int J Oncol. 2016;48:869–885. doi: 10.3892/ijo.2015.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AKT inhibitor enhanced trastuzumab-induced cell death in MDA-MB-361 cells.
Comparison of trastuzumab sensitivity in BT474 and BT474-TR cells.
Knockdown of AKT1 by siRNA enhanced trastuzumab-induced cell death in MDA-MB-361 cells.