Abstract
The occurrence of various liver diseases can lead to organ failure of the liver, which is one of the leading causes of mortality worldwide. Liver tissue engineering see the potential for replacing liver transplantation and drug toxicity studies facing donor shortages. The basic elements in liver tissue engineering are cells and biomaterials. Both mature hepatocytes and differentiated stem cells can be used as the main source of cells to construct spheroids and organoids, achieving improved cell function. To mimic the extracellular matrix (ECM) environment, biomaterials need to be biocompatible and bioactive, which also help support cell proliferation and differentiation and allow ECM deposition and vascularized structures formation. In addition, advanced manufacturing approaches are required to construct the extracellular microenvironment, and it has been proved that the structured three-dimensional culture system can help to improve the activity of hepatocytes and the characterization of specific proteins. In summary, we review biomaterials for liver tissue engineering, including natural hydrogels and synthetic polymers, and advanced processing techniques for building vascularized microenvironments, including bioassembly, bioprinting and microfluidic methods. We then summarize the application fields including transplant and regeneration, disease models and drug cytotoxicity analysis. In the end, we put the challenges and prospects of vascularized liver tissue engineering.
Keywords: biomaterials, extracellular matrix, liver, vasculature engineering
Graphical Abstract
Introduction
The liver is the largest gland in the human body and is responsible for a variety of critical biological functions in the body, including the metabolism of carbohydrates, proteins and lipids, bile production and detoxification [1]. It is well established that the liver has a strong regenerative capacity. However, liver fibrosis, viral infection and drug damage will reduce this regeneration ability and cause irreversible damage [2]. Hence, the manufacture of complex liver tissues with adequate functionality has become particularly important due to the high demand for organ transplantation and the inability to replace in vitro models required for new drug development and organ pathology research [3, 4]. Liver tissue engineering is considered the most promising alternative to mimic microstructure and maintain major function for liver implantation and drug screening [5–8]. Three-dimensional (3D) hepatic cell culture can generate complex cell structures, shapes and arrangements, which significantly increases the accuracy and reliability of experiments. It has significant advantages in constructing the heterogeneity and complexity of liver tissues. In order to culture hepatocytes in vitro for a long time and fully reproduce the liver functionalities, it is of great importance to reconstruct the unique liver vasculature system [9, 10].
Under physiological conditions, the human vasculature has essential biological functions, such as nutrient and gas exchange, metabolic waste removal and homeostasis maintenance. The diffusion limit of oxygen and nutrients is about 200 μm, which means that cells farther from the capillaries experience hypoxia and apoptosis [11–13]. The liver is one of the most vascularized organs in the body. Therefore, the constructed vascularized liver tissue will more favorably mimic the physiologically heterogeneous structure and cellular microenvironment, which can make it more convenient for biomedical applications in regenerative medicine and drug development [14].
Hepatic extracellular matrix (ECM) is a complex macromolecular network that not only provides cells with a natural microenvironment but also participates in the regulation of cellular functions [15, 16]. For example, the regulation of cell motility and differentiation is controlled by the surrounding physical environment and biochemical signals [17]. Biomaterials should then be able to recreate the key features of the extracellular microenvironment, including microarchitecture, mechanical strength, tissue-specific protein composition and pro-angiogenic properties to maintain cell morphology and growth [17–20]. Different types of biomaterials, which can be broadly classified into natural hydrogels and synthetic polymers, have been used in liver tissue engineering to reconstruct the liver ECM [21, 22].
Another important factor in liver tissue engineering is the cell sources. According to the differentiation degrees of cells, the fabrication methods for adapted liver tissues are also different. Undifferentiated cells have good biological activity and differentiation potential, and the co-culture of cells allows communication through paracrine factors and contributes to the formation of complex cellular structures with more functionalities [23–26]. By combining with highly biocompatible biomaterials, the microenvironment is used to control cells for the differentiation and regeneration of blood vessels. These printed liver tissues can deposit 3D microstructures more accurately by using extrusion or photocuring to build complex vascular networks with fixed patterns [27–29]. Combining biomaterials with stronger mechanical properties can ensure the stability of the spatial structure and increase the tightness of the connection between cells. In addition, the microfluidic-based organ-on-a-chip technology has the characteristics of dynamic perfusion culture [30, 31]. Nutrients and metabolites flow through the internal microchannels and diffuse between endothelial cells and hepatocyte layers, simulating the physiological microcirculation.
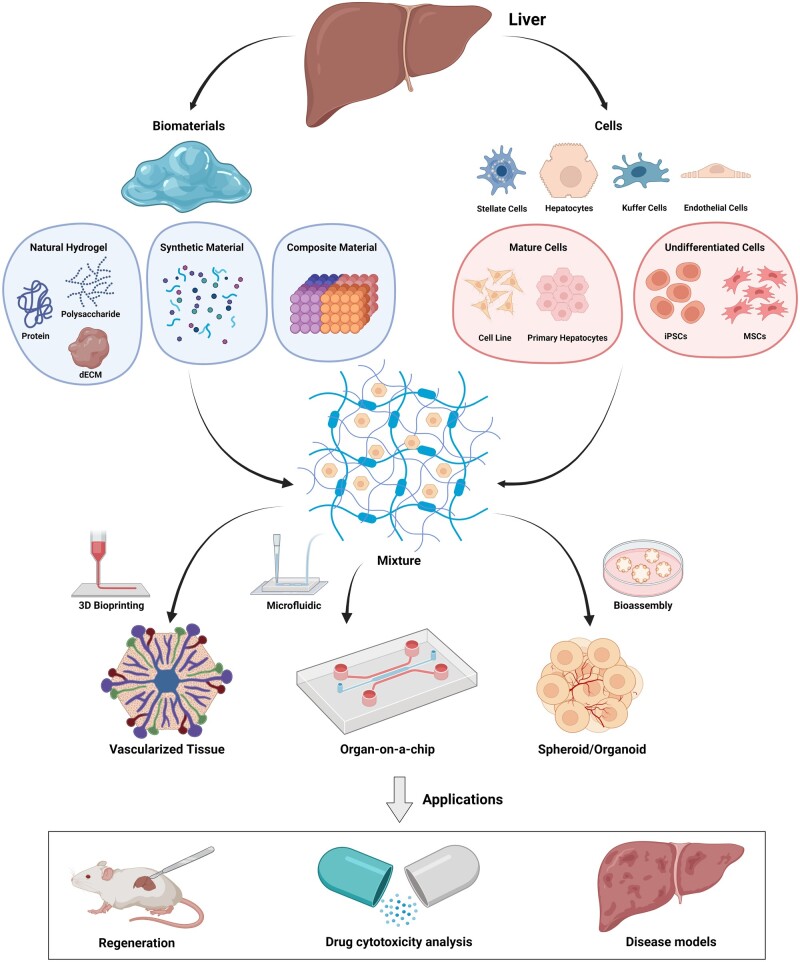
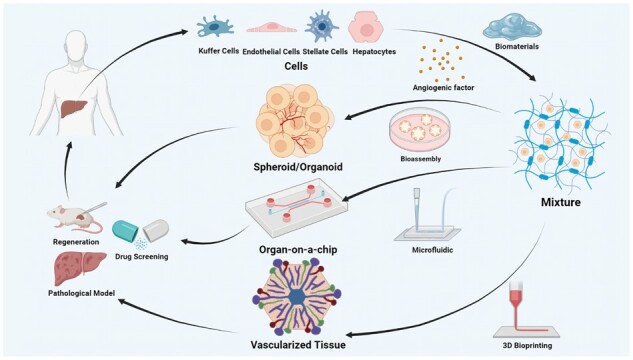
In this review, we discuss recent advances in tissue engineering of vascularized liver tissues, with a special focus on the biomaterials and cells matched to different liver extracellular matrices. In summary, we first describe the structure and function of the hepatic vasculature and then classify applicable biomaterials. Based on the degree of cell differentiation, it is roughly divided into differentiated cells and mature cells, thereby developing different strategies for constructing specific vascular systems in vitro. Then, the applications of the prepared vascularized liver tissues are discussed, such as drug discovery, disease model and liver regeneration. Finally, the current challenges and future perspectives of vascularized liver tissue engineering will be provided. The schematic of vascularized liver tissue engineering is summarized in Fig. 1.
Figure 1.
Schematic of vascularized liver tissue engineering (created with BioRender.com).
Structure of the liver
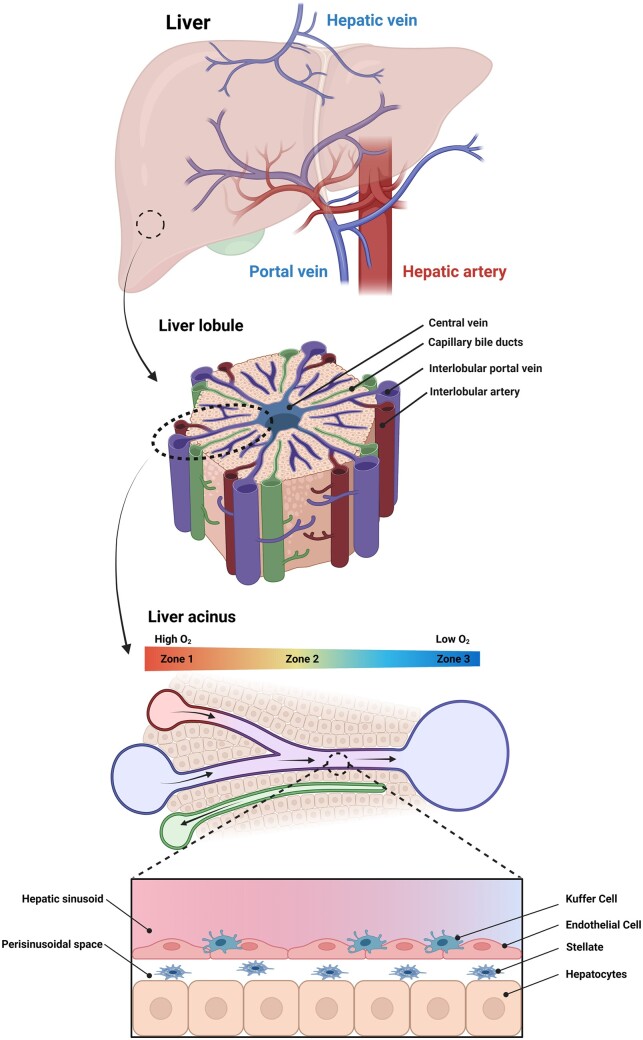
The liver is the largest gland in the human body with complex and diverse biochemical functions [32]. At the same time, the liver is rich in blood supply, the liver vascular system includes the inflow and outflow blood vessels, the former refers to the proper hepatic artery and the portal vein, and the latter refers to the hepatic vein [32]. After entering the liver, both the hepatic artery and portal vein branch repeatedly and become thinner and thinner, finally form interlobular arteries and interlobular veins, which pass through the hepatic plate and connect with the hepatic sinusoids to form a rich vascular network. Subsequently, the central vein of the hepatic lobule merges into the inferior lobular vein, which finally merges into the hepatic vein [33].
The unique feature of the liver vasculature is the dual blood supply. Its blood supply includes the portal vein and the hepatic artery. The portal vein mainly collects the blood flow of the gastrointestinal tract and the splenic vein, and transports nutrients and some toxic substances to the liver for metabolism, and the hepatic artery mainly provides oxygen [34].
Although the normal liver is mainly composed of parenchymal cells, the surrounding fibrous tissue is very limited in number, the extracellular microenvironment has significant effects on the physiological organization and organ function [17, 35]. Therefore, the ECM plays a crucial role in liver physiology and pathology, and any modification of the ECM will directly impact liver function [36]. The structure and elements of the liver can be seen in Fig. 2.
Figure 2.
Structure and elements of the liver (created with BioRender.com).
Basic units of the liver
Due to the different perspectives of studying the relationship between the structure and function of the liver, the essential components can be divided into two types: hepatic lobule and hepatic acinus [37].
Hepatic lobule
The hepatic lobules are polygonal. It has a central vein that runs along its long axis and is surrounded by radially arranged hepatic cords and sinusoids [38, 39]. Hepatocyte monolayers are arranged in an uneven plate-like structure called the liver plate. Adjacent liver plates are anastomosed and connected to form the hepatic cords [39]. The hepatic sinusoids are located between the hepatic plates and communicate with each other through pores in the hepatic plates, forming the smallest capillaries of the liver [40]. The plasma membrane on the adjacent side of the hepatocytes is partially recessed, forming tiny bile ducts. In this way, the hepatic plate, hepatic sinusoids and bile ducts form an independent and closely related complex network within the hepatic lobules [33]. The area between adjacent hepatic lobules is called the portal area. There are three to six portal areas around each hepatic lobule, in which three accompanying ducts can be seen, namely interlobular veins, interlobular arteries and interlobular bile ducts [37].
Hepatic sinusoids
The hepatic sinusoids are located between the hepatic plates, the cavity is large and irregular, and the sinus wall is surrounded by endothelial cells [41]. The blood from the interlobular arteries and the interlobular veins flows into the hepatic sinusoids [42, 43]. Due to the slow blood flow in the sinusoids, the plasma can have sufficient material exchange with the liver cells and then flow into the central vein [44, 45].
Perisinusoidal space
The perisinusoidal space is the narrow space between the hepatic sinusoidal endothelium and the hepatic plate, also known as the Disse space, where substances exchange between liver cells and blood [44]. Due to the high permeability of the hepatic sinusoid endothelium, the perisinusoidal space is filled with plasma, and a large number of microvilli on the blood perisinusoidal surface of the liver cells are soaked in the plasma, which can conduct sufficient and efficient material exchange [46].
Hepatic acinus
The hepatic acinus is smaller in size and resembles the shape of an olive [47]. It is a substantial block with the terminal branch of the portal canal as the central axis and the central vein as the boundary at both ends [48]. The blood flows from the portal area of the central axis into the interlobular vessels and passes through the hepatic plate to connect with the hepatic sinusoids. After sufficient material exchange, blood flows radially to the central veins at both ends, forming a complex anastomotic vascular network. Blood flow along the radial axis of the leaflet creates a gradient of oxygen, nutrients and hormones, creating a highly variable microenvironment [48–50]. According to the direction of blood flow and the order of nutrient acquisition, the hepatic acinus can be divided into three regions [51–54]. The periportal hepatocytes around the afferent vessels and the hepatocytes around the central vein around the efferent vessels show different metabolic capacities and subcellular structures, resulting in the metabolic division of hepatic acinus [52]. Hepatic microcirculation is closely related to the pathogenesis of pathology, and the metabolic zoning of hepatic acini can reasonably explain the pathogenesis of some liver lesions [6, 51, 55, 56].
Extracellular matrix of the liver
The ECM constitutes a complex protein network that enables tissue to form an integral structure and plays an essential role in regulating tissue homeostasis, remodeling and regeneration [21, 57, 58]. Its mechanical elastic properties can support cells to maintain their shape and structure, and the excellent biological properties provide a microenvironment that promotes cell survival, proliferation, differentiation and migration [16, 58]. In normal liver, the ECM occupies ∼3% of the relative area and 0.5% of the weight. Collagen types I, III, IV and V are the most abundant proteins in the intrahepatic matrix [59, 60]. Other components of the hepatic ECM are present in small amounts, mainly including glycoproteins such as laminin, fibronectin, tenascin, nestin and SPARC [59]. Proteoglycans include heparan, dermatan, chondroitin sulfate, perlecan, hyaluronic acid (HA), biglycan and decorin [60].
ECM of hepatocytes
Type I collagen and fibronectin are mainly found around hepatocytes. A small amount of ECM is better for cell adhesion and improves the mechanical coherence and resistance of the liver [60]. Meanwhile, the liver ECM also plays a role in several major biological functions such as cell proliferation, migration, differentiation and gene expression [36].
ECM of the vasculature
For the ECM of the vasculature in the hepatic lobules, laminin and collagen IV surround the bile duct, and collagen types I, III and V are mainly confined to the walls of the portal and central veins [15, 46]. Collagen type IV binds to laminin and entactin/nidogen to form a basement membrane-like substance along the sinus walls [36]. The low density of the ECM, accompanied by the abundant perisinusoidal endothelial cell windows, is ideal for facilitating the rapid bidirectional exchange of macromolecules that occurs between blood and hepatocytes, which is also critical for maintaining the differentiation of adjacent hepatocytes [17].
ECM in lesions
ECM also plays a significant role in pathological liver models, such as liver fibrosis. The composition of ECM is similar to that in normal liver, but the relative amount of the components is redistributed, and the amount increases to three to five times [35]. The most obvious change is the dilation of the ECM from the portal or central veins [61]. Specifically, type I collagen can better reflect the degree of liver fibrosis, and type III collagen is positively correlated with the degree of liver cirrhosis and is more closely related to liver inflammation. Type IV collagen is an essential component of the basement membrane. In the early stage of liver fibrosis, type IV collagen hyperplasia can form a basement membrane in the Disse space [21, 62, 63]. Although fibrosis is a major biological event, it is inextricably linked to other important mechanisms in the liver, such as hepatocyte regeneration and vascular redistribution.
Cells and biomaterials for vascularized liver tissue
Mixtures of cells and biomaterials are mainly used to construct liver tissue in vitro. Among them, cells mainly play the role of biological functions, while biomaterials can promote cell growth and maintain the shape of the overall structure.
Cell types and sources
The liver is mainly composed of hepatic parenchymal cells and hepatic non-parenchymal cells. Liver parenchymal cells are mainly hepatocytes, which perform the major metabolic and protein secretion functions of the liver. Non-parenchymal cells include Kupffer cells, endothelial cells and hepatic stellate cells (HSCs), which are mainly used to support hepatocytes and improve the functional structure of the liver [64]. In this section, we will introduce cells for 3D bioprinting according to these categories. Table 1 summarizes the main cell types and sources used in 3D bioprinting.
Table 1.
Cell type and source
| Cell type | Cell source | Functionality | References |
|---|---|---|---|
| Hepatocytes | Primary hepatocytes, from human | Metabolic and secretory functions of the liver | [65, 66] |
| HepG2 (hepatocellular carcinoma, cell line, from human) | Metabolic and secretory functions of the liver | [67–70] | |
| Huh7 (hepatocellular carcinoma, cell line, from human) | Metabolic and secretory functions of the liver | [71, 72] | |
| HepaRG (hepatic progenitor cells, from human) | Can differentiate into hepatocytes and bile duct cells to achieve liver metabolism and secretion | [73, 74] | |
| MSCs (mesenchymal stem cells, from human) | Has the potential to differentiate into a variety of cells, it can differentiate into hepatocytes for metabolism and secretion | [75–77] | |
| hiPSCs (human-induced pluripotent stem cells, from human) | Has the potential to differentiate into a variety of cells, it can differentiate into hepatocytes for metabolism and secretion | [25, 26, 78–80] | |
| Kupffer cells | Primary Kuffer cells, from human | Remove foreign bodies, monitor tumors | [66] |
| Kup5, (c-myc-immortalized Kupffer cells, cell line, from mouse) | Remove foreign bodies, monitor tumors | [81] | |
| Stellate cells | LX-2 (hepatic stellate cells, cell line, from human) | Involved in vitamin A metabolism and fat storage, producing extracellular matrix | [82] |
| Liver perisinusoidal endothelial cell | HUVEC (human umbilical vein endodermal cell, cell line, from human) | Reduce blood flow rate, promote substance interaction | [14, 81, 83] |
| Cholangiocytes | SV40SM (cholangiocytes, cell line, from mouse) | Formation of bile ducts | [84] |
| Cholangiocarcinoma cells, from human | Induced hepatocarcinogenesis | [85] |
Hepatocytes
Liver parenchyma cells are the most numerous and densest cell population in the liver, accounting for 80% of the total number of total liver cells [2, 86]. There are well-developed villi on the surface of the blood sinus and bile canaliculi of hepatocytes, which increase the surface area of the cells and promote the exchange of substances [87].
Such cells perform major hepatic metabolic and secretory functions. Primary cells have high metabolic activity and are the ideal cell source [86, 88, 89]. However, due to the lack of human primary hepatocytes, these cells can easily lose their phenotype, so the in vitro tissue construction based on primary hepatocytes is still difficult. To meet the research needs, researchers have developed a variety of cell lines with good proliferation and characterization. It is worth mentioning that although HepG2 are tumor cells, their expression functions are roughly consistent with those of normal liver cells [29]. At the same time, it has the ability of hypoxic respiration and high proliferation that normal liver cells do not have. Therefore, in previous research, these cells have been widely used in 3D bioprinting to construct disease models and test drug cytotoxicity [14, 28, 67, 68]. However, researchers are still working to improve liver tissue function in vitro. Since some progenitor cells or pluripotent stem cells can differentiate into fully functional hepatocytes in vitro, they have unparalleled advantages in constructing perfect structure and protein expression [90]. For example, HepaRG cells, which retain bipotent hepatic progenitor-like characteristics, are the most promising type for bioprinting to construct in vitro liver tissue [73, 74, 82]. Therefore, cells of this type are increasingly used in the latest research.
Endothelial cells
Endothelial cells have numerous fenestrations of varying sizes [91, 92]. Endothelial cells are loosely connected with wide intercellular spaces [93, 94]. There is no basement membrane outside the endothelium, and only a small amount of reticular fibrin is attached [91, 95]. Therefore, the hepatic sinusoidal endothelium has a high permeability, and various plasma components can enter the perisinusoidal space [93, 96–99]. In most research, hepatic sinusoidal endothelial cells can be replaced with human umbilical vein endothelial cells (HUVECs) [100–102]. This type of cell grows faster, can spread efficiently, and spontaneously induce directional induction based on endothelial growth factors to form pathways [103, 104]. Therefore, it is very suitable for building vascularized structures.
Kupffer cells
Liver sinusoids contain resident macrophages that attach to endothelial cells and can penetrate endothelial fenestrations and intercellular spaces deep into the peri-sinusoidal space [92, 105]. Due to the active phagocytic ability of cells, they play an important role in clearing the liver of antigenic foreign bodies, senescent blood cells, and monitoring tumors [97, 106, 107]. In the uninjured liver, Kupffer cells constitute the major population of inflammatory cells and are important for many homeostatic functions [108, 109].
Hepatic stellate cells
There are irregular fat-storing cells in the perisinusoidal space, also known as HSCs, which are mainly involved in the metabolism of vitamin A and the storage of fat in the liver [110, 111]. In addition, another function of HSCs is to produce ECM, from which the reticular fibers in the perisinusoidal space are produced [112–114]. Under pathological conditions, adipocytes are activated and abnormally proliferate, producing ECM and increasing intrahepatic fibrosis, which can finally lead to cirrhosis [110, 115, 116].
Interaction between hepatocytes and endothelial cells
The reciprocal relationship between the development of the liver and the vasculature has long been investigated and elucidated [117]. Early research studies questioned the fast regeneration of the liver, which pushed them to use partial hepatectomy models removed generally from rats [117]. These hepatectomy models allowed the discovery of reciprocal communication between liver sinusoidal endothelial cells (LSECs) and hepatocytes using vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) factors [118].
HGF was later found as the most potent simulator of hepatocytes which is produced by LSECs and HSCs. The binding of HGF and the tyrosine kinase receptor c-Met consequently activates several signaling pathways, including JAK/STAT3, phosphoinositide 3-kinase (PI3K)/Akt/NF-κB, Ras/Raf and mitogen-activated protein kinase (MAPK) cascades [119]. Each of these pathways has a specific role in liver regeneration, such as regulating cell growth, migration, differentiation and apoptosis [119]. The HGF/c-Met pathway has a vital role in maintaining a normal liver indeed. However, slight dysfunction of this pathway may cause diverse pathologies such as tumors [120, 121]. On the other hand, VEGF is the main glycoprotein responsible for vascular growth, including vasculogenesis and angiogenesis [98]. In the liver, the VEGF is produced by hepatocytes and HSCs [98].
Hepatectomy models have served as great proof of the crucial role that plays the endothelial cells in the liver. Therefore, the co-culture of endothelial cells and hepatocytes should offer a more accurate in vitro liver model, which has indeed been approved consequently. In an interesting, Wang et al. [122] used a co-culture system containing hepatocytes and ECs with different ratios. As a result, this co-culture system showed to improve the secretion of albumin and urea and the expressions of albumin, BYP3A4 and HNF4α. These findings have also been enhanced by Lee and Cho [123] using a more sophisticated dynamic liver model. The research team have used a one-step microextrusion-based bioprinting method to create a PCL-based liver-on-a-chip model. A dynamic co-culture model of hepatocytes and endothelial cells was shown to provide greater albumin secretion and urea synthesis with higher cell viability.
Biomaterials
Given that hepatocytes are anchorage-dependent cells and the ECM is required for their survival and functionality realization [124]. Therefore, in past studies, different kinds of biomaterials have been investigated for their successful cell cultures. By examining the cellular morphology and characterization of hepatocytes used in 3D bioprinted in vitro models, different biomaterials can be developed to mimic as much as possible the in vivo microenvironment as well as the physiological changes of cells in vitro, thus closer to the actual situation. The commonly used biomaterials for the construction of vascularized liver tissue are summarized in Fig. 3.
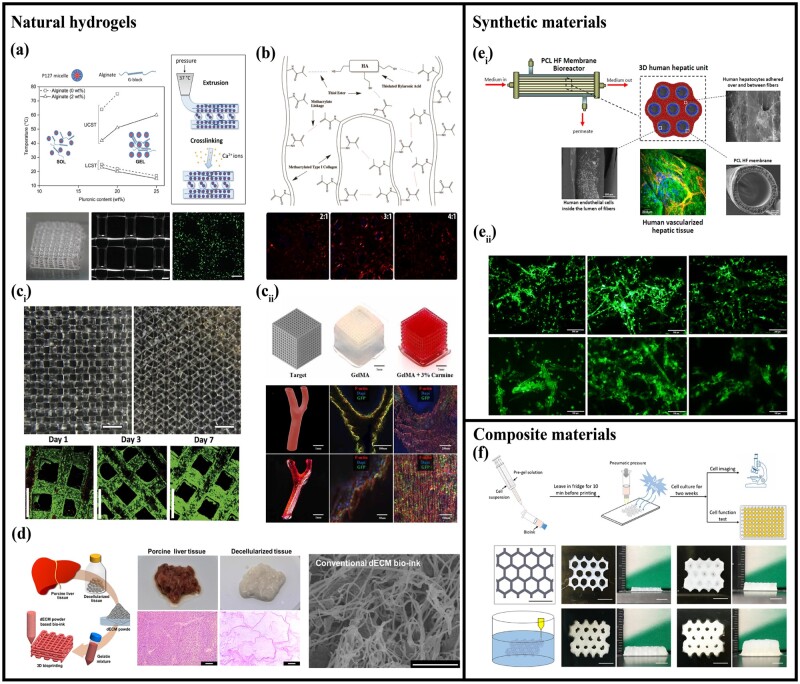
Figure 3.
Biomaterials used in the vascularized liver tissue engineering. (a) Pluronic and alginate with high applicability. Adapted with permission from Ref. [140]. (b) Collagen type I with hyaluronic acid. Adapted with permission from Ref. [149]. (c) Gelatin. (ci) Gelatin scaffolds. Adapted with permission from Ref. [71]. (cii) Photocrosslinking of GelMA, producing vascularized tissues with complex shapes. Adapted with permission from Ref. [147]. (d) dECM bioink with enhanced printability and mechanical properties. Adapted with permission from Ref. [151]. (e) Synthetic polymers. (ei) PCL mixed with biodegradable hollow fibers (HF). Adapted with permission from Ref. [156]. (eii) PLGA scaffolds. Adapted with permission from Ref. [157]. (f) Cellulose nanocrystals (CNC). Adapted with permission from Ref. [164].
Natural hydrogels
Hydrogels are water-swellable, highly cross-linked polymer networks that are soft, quasi-solid, and can support and protect cells. Since protein-based and polysaccharide-based ECM occupies most of the liver, this type of hydrogel has a significant promoting effect on the cell growth of liver tissue in vitro and is more conducive to restoring the cellular microenvironment in vivo and improving the metabolism effect of the liver cells, is commonly used to prepare scaffolds for liver regeneration. Table 2 summarizes some of the commonly used natural hydrogels.
Table 2.
Normal natural hydrogels for liver tissue engineering
| Type | Origin | Constituents | Crosslinking | Fabrication | Features | References |
|---|---|---|---|---|---|---|
| Alginate | From cell walls of algae/seaweed | Guluronic acid and mannuronic acid | Ionic (Ca2+, Ba2+, Mg2+) | Extrusion-based bioprinting; Inkjet-based bioprinting | With shear thinning properties, abundant sources, easy to crosslink | [125–127] |
| Agarose | Marine seaweed | 3,6-anhydro-L-galactopyranose and D-galactose | Temperature | Extrusion-based bioprinting | High propensity to gelation | [128] |
| Chitosan | Insects and crustacean exoskeleton | N-deacetylation of chitin and glycosaminoglycans | Ionic and covalent cross-link | Extrusion-based bioprinting | High viscosity fluids, resistant to bacteria and fungi and analgesia | [129, 130] |
| Hyaluronic Acid (HA) | ECM, all over the body | β1, 4D glucosamine and N-acetyl-D-glucosamine | Physical, light-controlled chem | Extrusion-based bioprinting | Hydrophilicity and biocompatibility, faster healing | [131] |
| Gelatin | Cow skin, pig skin, fish skin | Glycosaminoglycans | Covalent, enzymatic, temperature, physical | Extrusion-based bioprinting | It is sensitive to thermal environment, effective cell bonding | [132] |
| Gelatin MA | Semi-natural polymer | Gelatin and acrylates | Light (UV) | Digital light processing (DLP)-based 3D bioprinting; Extrusion-based bioprinting; Inkjet-based bioprinting | Facilitate attachment of cells with scaffold promoting adhesion and cell activity, have great mechanical properties | [133, 134] |
| Collagen | Nature-derived ECM, the tendon of rat tail, fish skin | Chains of polypeptide | pH, ionic cross-link, temperature, protein riboflavin | Extrusion-based bioprinting; Inkjet-based bioprinting | Enzyme catalyzed degradation, poor printability | [135, 136] |
| Fibrin | Distributed in plasma | Fibrous and non-globular glycoproteins | Enzymatic Crosslinking (Factors IIa, XIIIa and IV) | Extrusion-based bioprinting | Biodegradable with cell adhesion sites | [137, 138] |
| Decellularized extracellular matrix (dECM) | Extracellular matrix | Collagen, fibrin, gelatin | Light (UV), enzymatic | Digital light processing (DLP)-based 3D bioprinting; Extrusion-based bioprinting; | With complex natural ingredients and biomimetic structure | [139] |
Alginate
Alginate has been widely used in liver tissue engineering due to its good biocompatibility, abundant sources, easiness to cross-link and low price [126, 127]. Gori et al. [140] developed an in vitro 3D liver model using a composite hydrogel of the biologically inert materials Pluronic and alginate with high applicability in supporting the viability and metabolic activity of the HepG2 cell line (Fig. 3a). Taymour applied alginate and methylcellulose (algMC)-based bioinks for core-shell bioprinting and established a functional co-culture model with independently tunable compartments for different cell types which provides a liver-like microenvironment for more complex in vitro models [141]. However, pure alginate does not provide a cell adhesion site for the encapsulated cells. Researchers usually modified the alginate hydrogel with some cellular recognition motifs such as Arginylglycylaspartic (RGD), which is widely used in the engineered hydrogels to bind the cell adhesion protein integrins, hence significantly enhancing the cell adhesion and proliferation [142].
Hyaluronic acid
HA is the main component of the peri-sinusoidal space and has excellent biocompatibility and biodegradability, which plays an important role in cell proliferation and angiogenesis [131]. As in the process of liver fibrosis, HSCs are activated, resulting in the massive production of a hard matrix. In order to study the cellular mechanotransduction of HSCs in related diseases, Caliari et al. [143] used HA to prepare a hardened hydrogel model to simulate liver cirrhosis and proved the stiffening HA hydrogels could be a more faithful model for studying myofibroblast activation than traditional static substrates.
Gelatin
Gelatin and its derivatives are another widely used hydrogels for the construction of livers in vitro [132]. The triple helical structure of collagen can be lost by hydrolysis, so gelatin is more soluble in a hydrophilic medium and more convenient to prepare [144]. Lewis et al. [71] demonstrated the ability to precisely control the pore geometry of 3D printed gelatin scaffolds (Fig. 3ci). They showed high viability and proliferative capacity when seeded on 3D printed scaffolds of different geometries with an undifferentiated hepatocyte cell line (HUH7). However, due to the long cross-linking time and poor printability of gelatin, researchers gradually eliminated it in the fabrication of precise microstructures.
Gelatin Methacrylamide (GelMA) is a modified gelatin that crosslinks in seconds under UV light [133, 145]. Compared with other photocurable hydrogels, GelMA is widely favored by researchers due to its excellent biocompatibility and strong mechanical properties at the same time [146]. Sun et al. [147] precisely controlled the degree of photocrosslinking of GelMA, producing vascularized tissues with complex shapes, high precision, and controllable mechanical properties (Fig. 3cii). Roopesh et al. [148] made sandwiched liver parenchyma microtissues with GelMA. Monitoring of liver-specific function revealed that the 3D structure of liver tissue in the hydrogel sandwich was maintained while compared with it in suspension, albumin secretion, urea synthesis and CYP450 activity were enhanced. These researches all demonstrate the potential of GelMA for in vitro tissue construction.
Collagen
Collagen is the most abundant component in the liver ECM, especially type I collagen, but due to its low viscosity, collagen is less suitable for bioprinting [135]. Therefore, various methods are currently developed to improve the mechanical properties and printability of collagen. Mazzocchi et al. [149] printed 3D liver tissue structures containing primary human hepatocytes (PHHs) and HSCs using a mixture of collagen type I and HA and found that hepatocytes were able to express albumin and survive for more than 2 weeks while responding appropriately to acetaminophen, a common liver poison (Fig. 3b). Deng et al. [74] seeded cells in microporous platforms to form spheroids, which were then directly encapsulated in mixed hydrogels containing various collagen and protein, including collagen type I (COL1), collagen IV (COL4), fibronectin protein (FN) and laminin (LM). The results showed that different ECM components promoted the expression and secretion of hepatic markers of cell spheroids [74].
Decellularized extracellular matrix
The natural ECM has better biological properties and degradability, which can provide a scaffold for a 3D liver microenvironment with complex natural components and biomimetic structures, which is necessary for the development of better tissue models [124, 150]. However, its poor printability and weak mechanical properties remain a challenge. Kim et al. [151], developed a new gelatin-mixed decellularized ECM (dECM) bioink with enhanced printability and mechanical properties (Fig. 3d). In order to further solidify the acellular ECM to strengthen its mechanical properties, Mao et al. [152] developed a liver-specific bioink and encapsulated human-induced hepatocytes (hiHep cells) to form cell-laden bioinks and found that hepatocytes spread farther in this microtissue and had better hepatocyte-specific functions. In the research of biocompatibility, Sharma et al. [72] developed a hybrid liver-specific three-dimensional scaffold using gelatin with native decellularized liver matrix (DCL) and silk fibroin, providing a favorable microenvironment for enhanced hepatocyte differentiation and function. Minami et al. [26] successfully developed a novel artificial liver model using human-induced pluripotent stem cells and rat decellularized liver scaffolds and demonstrated its potential to promote functions characteristic of human livers. It can be seen that the high biocompatibility of acellular ECM has great advantages in inducing undifferentiated cells to maintain cell-specific functions and form in vitro tissues.
Synthetic materials
Although natural biomaterials have absolute advantages in biocompatibility, some problems, such as low viscosity, poor mechanical properties and insufficient sources, hamper their biomedical applications. Synthetic polymeric with better mechanical properties have become increasingly used in liver tissue engineering, especially for vessel structures requiring precise and better shape retention. It has high mechanical strength, which can be applied to certain research purposes. Meanwhile, to overcome the low binding affinity for cells, these synthetic polymers are often modified with biomolecules (e.g. proteins, polysaccharides, polypeptides) to improve their biocompatibility by adding cellular recognition motifs. Among the many 3D printable synthetic polymers, polyethylene glycol (PEG), poly(ε-caprolactone) (PCL) and poly(lactic-co-glycolic acid) (PLGA) are primarily used for bioprinting of liver structures [153, 154]. They have tunable mechanical properties that can provide a microenvironment to guide liver regeneration and remodeling. PCL has good biocompatibility and processability, softness and flexibility at body temperature [144]. Lee et al. [155] injected a bioink containing primary hepatocytes, HUVECs, and human lung fibroblasts into PCL scaffolds to induce capillary-like network formation and hepatocyte growth. A co-culture 3D microenvironment of these three types of cells was successfully established and maintained [155]. Salerno et al. [156] reported a bioink based on PCL mixed with biodegradable hollow fibers. After printing the liver tissue model, it was found that endothelial cells were massively integrated with the inner surface of individual PCL fibers to form a blood vessel-like structure, and hepatocytes completely covered the outer surface and the space between the fibers (Fig. 3ei) [156]. Furthermore, the tunable degradability and support properties of PLGA can be adapted to the regeneration process. Liu et al. [157] co-cultured mesenchymal stem cells (MSCs) and hepatocytes and demonstrated the stable differentiation ability of MSCs upon hepatocytes in PLGA scaffolds (Fig. 3eii).
Composite/multicomponent/hybrid materials
Although a single hydrogel is easy to prepare, it still has limitations in its multifunctional biocompatibility with multiple cell types. Therefore, in order to further reduce the components of the ECM, composite/multicomponent/hybrid materials are gradually used for liver construction.
Hybrid
Hybrid materials are generally a mixture of multiple collagens or extracellular matrices. The diversity of this material composition has a positive effect on the growth of hepatocytes. Clark et al. [158] described a novel bioink composed entirely of materials in the ECM of human tissue. This was achieved by incorporating gelatin nanoparticles into a base bioink made of collagen methacrylated and HA, with excellent mechanical properties and printability. Matrigel is a biomaterial that regulates the cellular microenvironment and contains various components such as cohesin, collagen IV, fibronectin and heparan sulfate [159, 160]. Tao et al. [161] supplemented macromolecules including Matrigel and polysaccharides at different concentrations into HepG2 spheroids to modulate the cellular microenvironment and observed the effect on cell viability.
Nanocelluloses
In the context of biomedical hydrogels for tissue engineering, one particular kind of synthetic nanomaterials called nanocelluloses share structural similarities to the ECM due to their porosity and interconnected framework within the structural hydrogel [162]. Meanwhile, the fiber morphology of cellulose fibrils is somewhat similar to that of collagen and fibronectin, which has attracted great attention. This provides better shape fidelity and print resolution for the stent. Wu et al. [163] mixed alginate and cellulose nanocrystals to prepare a hybrid bioink with shear-thinning rheological properties. Fibroblasts and hepatoma cells were then cultured together on the printed scaffolds and found that the viability of the cells was not affected. Subsequently, they designed a new bioink using alginate, cellulose nanocrystals and GelMA, which can directly print cell-laden structures by micro-extrusion. The ink exhibits excellent shear thinning behavior and solid-like properties, enabling high printability without obvious cell damage (Fig. 3f) [164]. The composite bioink of cellulose nanofiber hydrogels combined with alginate is an effective method for achieving cross-linking of printed scaffolds in the presence of Ca2+. The porous structures formed can help cells adhesion on the surface and inside of the scaffolds to improve cell viability. Zhang et al. [165] prepared a CNF-alginate-CLP (cellulose nanofiber hydrogel-alginate-spherical colloidal lignin nanoparticle) nanocomposite scaffold to which CLPs brought antioxidant properties and increased the viscosity of the hydrogel at low shear rates, HepG2 cells encapsulated remain high cell viability proved that this kind of nanocomposite is suitable for liver tissue engineering.
Biomanufacturing approaches for vascularized liver tissues
Various new strategies for vascularized liver tissue creation are being proposed as biomanufacturing technology advances. From the level of cell origin, it can be simply divided into two categories. One is progenitor cells or stem cells with differentiation potential. The majority of methods employing such cells for liver tissue development use scaffold-free approaches to generate spheroids or organoids without fixed structures and rely on cells’ differentiation ability to stimulate the creation of blood vessels. The other is mature cells, which do not have the ability to differentiate. Most of them use 3D bioprinting methods to construct hepatic lobular tissue with vascular structure or microfluidic-based methods to prepare liver-on-chips [166]. In the following paragraphs, we will briefly discuss different manufacturing strategies for using these two types of cells to construct vascularized liver tissue in vitro.
Bioassembled vascularized liver models
Organoids constructed from undifferentiated cells have the ability to self-renew, are closer to real tissues, and have highly similar histological functions. In general, strategies for vascularizing spheroids and organoids are carried out in two steps: First, organoids are constructed in vitro, and cells are induced to differentiate to form primary blood vessels. Then, transplantation into highly vascularized regions of the liver in vivo to further induce vascularized structures.
Spheroids
Various types of 3D cell aggregates, such as spheroids, organoids and tissue sprouts, have received increasing attention in regenerative medicine. Among them, spheroids break the limitation of traditional monolayer cell culture and make the connection between cells more closely, are widely used in high-throughput evaluation in vitro and tissue repair in vivo [167]. MSCs transplantation is a promising treatment for ischemia–reperfusion injury. Sun et al. [76] used 3D spheroid culture, enhanced the nutritional and anti-inflammatory properties of MSCs, while increasing the secretion of VEGF, which was helpful for transplantation. Cuvellier et al. [168] printed PHHs with GelMA and found that the spontaneous polarization of the cells produced hollow spheroids. These highly differentiated PHHs were then implanted in mice and showed the ability to be printed to the structures for vascularization. Park [77] investigated the use of photobiomodulation treatment to differentiate human adipose stem cells in spheroids and stimulate angiogenesis to improve the recovery of liver function. Such hepatic spheroids act as individual vascularized units, facilitating the development and functioning of new microvascular networks within the implanted tissue structure [77]. In terms of disease models, Suurmond et al. [81] proposed an in vitro model of nonalcoholic fatty liver disease formed by co-culture of hepatic progenitor cells (HepaRG), umbilical vein endothelial cells (HUVECs) and Kupffer cells into spheroids. Compared with those made from HepG2 cells, these spheroids made from HepaRG showed a closer trend to the functions in humans.
Organoids
Organoids are relatively simple to generate and have the ability to play a good role in damage repairment [169]. In addition to their application in clinical transplantation, liver organoids can be used as a salvage bridge in the transition from liver failure treatment to liver regeneration or as a supplement to extensive liver resection and temporary maintenance of the liver while awaiting transplantation. Based on this purpose, Yang et al. [73] used HepaRG to prepare liver tissue patches, and after 7 days of differentiation in vitro with DMSO, these patches were transplanted into liver-damaged mice and found that the survival time of the mice was significantly increased. New blood vessels began to form on Day 14 post-transplantation, and some common human-specific biomarkers were detected. In another research by Wu et al. [75], mesenchymal stromal cells (MSCs) aggregates were deposited and attached to decellularized liver scaffolds and transplanted into the omentum of liver-injured rats. This liver tissue had a stable structure, with completed functional expression and angiogenic capacity [75]. Bioinks containing a variety of hepatocyte ECM components will be used to fabricate complex liver organoids, which can promote the transformation of undifferentiated cells into hepatocytes and the expression of specific proteins. Janani et al. [170] utilized human adipose-derived MSCs, HUVECs and human HSCs and applied two bioinks to support parenchymal and non-parenchymal cells, hepatic lobular organoids were constructed with functional sinusoidal lumen-like networks in both horizontal and vertical orientations. The results showed that this co-cultured liver model exhibited enhanced albumin production, urea synthesis and cytochrome P450 (CPR) activity.
Bioprinted vascularized liver models
In contrast, the strategies used by mature cells to build vascularized liver tissue are more diverse. Since cells do not have the ability to differentiate, 3D bioprinting is more conducive to the construction of liver tissue with precise vascular networks that can grow according to a preset pattern that preserves structure well and promotes cell-to-cell interactions. 3D bioprinting involves mixing cells and hydrogels together to form bioinks, and then applying additive manufacturing techniques such as extrusion, photocuring and inkjet to precisely deposit the bioinks in vitro to construct in vitro tissues and organs [171, 172]. While microvascular induction uses prefabricated cells that can self-assemble to build corresponding structures [100, 173]. Both strategies are followed by a phase of organizational remodeling and maturation [174]. In Table 3, we summarize some biofabrication methods for liver model construction.
Table 3.
Biomanufacturing approaches for liver model construction
| Biomanufacturing approaches | Advantage | Disadvantage | References |
|---|---|---|---|
| Inkjet-based bioprinting | Small tissues and organs with high resolution requirements can be constructed, ability to print low-viscosity biomaterials | Inability to provide continuous flow, low vertical structure accuracy, low cell density | [175–178] |
| Extrusion-based bioprinting | Broad bioink compatibility, printable with multiple viscosities, good biocompatibility, printable with high-cell densities, continuous gradient printing is possible | Low resolution, slow print speed, only suitable for viscous liquids | [70, 175, 178, 179] |
| Photocuring-based bioprinting | High printing resolution; can construct more complex structures; high printing speed | Toxicity of UV light sources to cells, possible damage to cells with photo-initiators | [147, 178, 180–183] |
Inkjet-based bioprinting
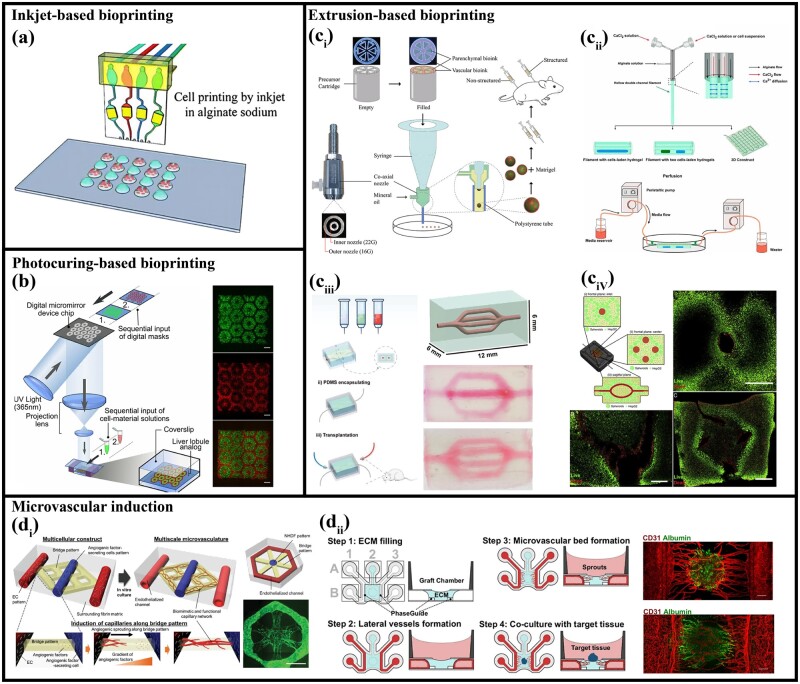
Inkjet-based bioprinting uses voltage to change the shape of piezoelectric materials and generate pressure and then eject droplets from nozzles to achieve the printing of small-scale structures, which can be widely used in materials such as living cells, biomolecules and biocompatible hydrogels [184]. Some attempts have been made to utilize inkjet-based bioprinting in vascularized liver tissue engineering. Zhang et al. [177] used inkjet as a cell-patterning method in microchips to form an integrated system that mimics vascularized structures by printing different kinds of cells at designed locations combined with corresponding microchannels (Fig. 4a). At the same time, the human hepatoma cell line HepG2 and the human glioma cell line U251 were co-cultured and subjected to drug metabolism and diffusion experiments. The experimental results showed that the drug was metabolized by HepG2, showing a significant anticancer effect on U251. Arai et al. [176] used inkjet printing to construct monolayer 3D hydrogel sheets for hepatocyte attachment. They used two gel sheets to create a sandwich structure to form parallel layers of liver cells. In this way, a hepatic cord structure can be achieved and has some potential for the formation of the vascular system. However, due to the slow printing efficiency and very few selection of low viscosity bioink of inkjet printing, also the applied voltage has the problem of affecting the specific function expression of cells, so the usage of inkjet bioprinting in liver tissue construction has limited.
Figure 4.
Bioprinted vascularized liver models. (a) Inkjet-based bioprinting. Adapted with permission from Ref. [177]. (b) Photocuring-based bioprinting. Adapted with permission from Ref. [189]. (c) Extrusion-based bioprinting. (ci) Pre-set micro-extrusion printing. Adapted with permission from Ref. [102]. (cii) Coaxial extrusion printing. Adapted with permission from Ref. [67]. (ciii, civ) Sacrificial printing. Adapted with permission from Ref. [69, 187]. (d) Microvascular induction. (di) Microvascular induction to generate liver organoids. Adapted with permission from Ref. [103]. (dii) Microvascular induction to generate hepatic spheroids. Adapted with permission from Ref. [196].
Extrusion-based bioprinting
The extrusion-based 3D bioprinting technologies extrude the material through a nozzle into continuous filaments and uses the movement of the nozzle or the receiving plate to build different 3D structures [18]. It has attracted much attention in the preparation of liver tissue due to its ease of use and potential to adapt to various bioink viscosities, offering high cell loading densities and minimal damage to cells [185]. Meanwhile, extrusion printing enables the construction of continuous gradient tissue. Liu et al. [179] printed a pattern of endothelialized tissue in which four sections loaded with human dermal fibroblasts (HDFs), HepG2 human hepatocytes, human MSCs (hMSCs) and cell-free bioinks were deposited on the bottom, respectively. Then a vasculature similar to encapsulating HUVECs was integrated on the top [179].
Pre-set
However, extrusion bioprinting has the disadvantages of low printing resolution and slow printing speed, so it is still a challenge for building vascular structures. In order to overcome these problems, Jin et al. [70] proposed the pre-set extrusion technology in 2018, with the principle that the fluid with a low Reynolds number is not easy to mix. They printed a complex multimaterial high-resolution structure containing HepG2 cells and endothelial cells [70]. Two years later, the team used the technique to print vascularized structures resembling liver lobules, including the central lumen and sinusoids [101]. After culture, endothelial cells were observed to spread well on the surface and inside the lumen. Recently, combined with microfluidic emulsification technology, hepatic lobular vascularized multicellular spheroids were successfully constructed on the scale of hundreds of micrometers. In these spheroids, endothelial cells were distributed on the outside, which ensures the integrity of the overall structure, and forms a radial vascular architecture similar to the liver lobule (Fig. 4ci) [102].
Coaxial extrusion printing
Coaxial extrusion printing has the characteristics of simple and high practicability and is widely used in the construction of the vascular network. Pi et al. [186] achieved a perfusable circumferential multilayer tissue structure using a digitally tunable multilayer coaxial nozzle. Besides, Yu et al. [67] used dual-channel filaments for extrusion bioprinting, successfully constructed vascular access and simulated the dynamic function between hepatocytes through sequential perfusion (Fig. 4cii).
Sacrificial printing
Because the scale of blood vessels is relatively small, and the precision of extrusion printing is generally not enough to construct the structure of microvessels, the sacrificial printing method to forming a hollow channel structure by heating and dissolving temperature-sensitive materials is proposed and widely used in the fabrication of vascular networks. Pimentel et al. [69] utilized sacrificial printing to construct a tissue with an intact 3D perfusable network and soft-tissue-scale stiffness. The obtained tissue constructs were cultured by perfusion using a custom-built fluidic platform, resulting in significantly prolonged survival (>14 days) (Fig. 4civ) [69]. Liu et al. [187] dissolved the fugitive inks Pluronic F127 to form channels and incubated endothelial cells (ECs) to form vascular beds (Fig. 4ciii). The printed constructs can be perfused through branched endothelial vasculature to support well-formed 3D capillary networks, which then mimic mature and functional liver tissue in terms of liver-specific protein synthesis.
Photocuring bioprinting
The photocurable bioprinting method uses light to cross-link the bioink, and build up layer by layer with the preset pattern, the most common used manner also known as digital light processing. This printing strategy, with the advantages of higher accuracy, faster printing speed and higher resolution, helps build more complex and complete microstructures within the hepatic lobules [188]. However, this method has potential phototoxicity, which may have a certain impact on the viability of cells. Ma et al. [189] encapsulated hepatocytes and endothelial- and mesenchymal-derived supporting cells in complementary patterns and restored the hepatic lobular structure by constructing vascular channels through a photopolymerization method of a hydrogel matrix (Fig. 4b). Grigoryan et al. [190] created 3D entangled multivascular networks using photopolymerizable hydrogels. Then, a more advanced vehicle was constructed that could deliver liver aggregates in native fibrin gels with vascular compartments that could be seeded with endothelial cells. Bernal et al. [180] build highly complex and unique structures by reducing scattering by refractive index matching of specific intracellular components, a development that enables high-resolution volumetric bioprinting. This research opens up the possibility of constructing sophisticated microvascular networks in the future. These findings demonstrate the close relationship between a fine structure produced by photocuring methods and the resulting biological function, further underscoring the potential of biofabrication for advanced tissue engineering.
Photosymbiotic tissue engineering
Tissue engineering offers the possibility of in vitro biofabrication of three-dimensional (3D) tissues, but due to the complexity of vascularized structures, ideal 3D tissue scaffolds are difficult to achieve [191]. To overcome this difficulty, the strategy of introducing oxygen in tissue engineering has been extensively explored [192]. Given that oxygen is produced by photosynthetic microorganisms such as microalgae and cyanobacteria in nature, and they have a symbiotic relationship with a variety of eukaryotic hosts including animals, a nascent photosymbiotic tissue engineering has gradually been widely studied [193]. It has to be mentioned that Maharjan et al. [194] used sacrificial printing to develop an in vitro vascularized tissue structure with sufficient oxygen supply. Among them, they utilized algae to act as natural photosynthetic oxygen generators in the liver tissue structure, support the viability and function of HepG2 cells in the surrounding GelMA matrix, and evenly distribute HUVEC layers to form endothelialization channels. This method can effectively avoid cell death due to hypoxia, providing a new idea for in vitro combinatorial construction.
Microvascular induction
Bioprinting is a well-established approach to generate large vessels embedded in liver tissues; however, the common bioprinting methods are not suitable to create microvascular due to the limitations of the printing resolution. Methods of microvascular induction target the formation of vascular channels by exploiting the tendency of cells to grow toward higher nutrient concentrations. This method can break through the limitations of printing resolution and enables the formation of microvascular systems that are closer to the physiological range [104, 195]. Following this strategy, Son et al. used angiogenic factor-secreting cells to create angiogenic factor gradients along a bridge pattern, using a method of biological self-assembly to form microvascular networks (Fig. 4di) [103]. However, although this method can build a network of blood vessels, the hepatocytes is not included around the network, which cannot simulate the direct interaction between blood flow and the cells. To compensate for this shortcoming, Bonanini et al. [196] established a vascular bed by inducing endothelial cells with endothelial growth factor. After transplantation, the hepatic microspheres spontaneously anastomosed with the microvascular bed to form a vascular network. And after 7 days of co-culture in the Disse-like structural space between hepatocytes and endothelium, endothelial cells were found to penetrate the liver microtissue and form stable, perfusable microvasculature (Fig. 4dii) [196]. Although this method of microvascular induction can construct fine capillary networks, it still has the disadvantages of small overall scale, long construction time and unstable directional formation ability.
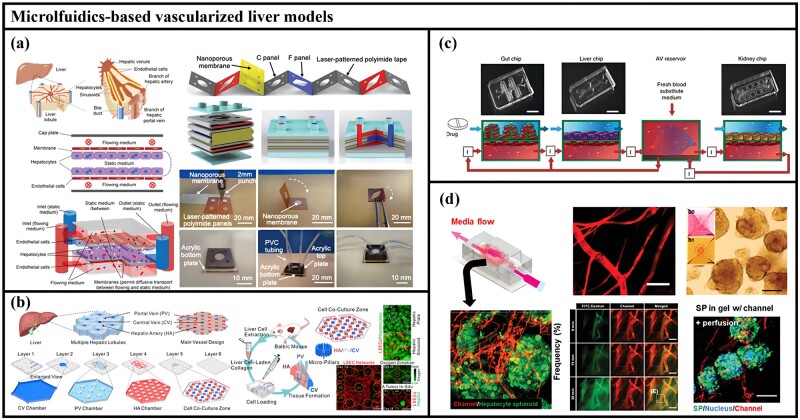
Microfluidics-based vascularized liver models
Organ-on-a-chip technology is a microfluidic-based technology, which is used to cultivate a variety of living cells in a microchamber under continuous perfusion conditions to form a biomimetic system of in vivo organ microenvironment [197]. Although various liver-on-chips technologies have been developed, it remains a great challenge to use this technology to simulate hepatic lobule structures that contain perfusable sinusoidal networks. Current liver-on-chips lack the ECM necessary for hepatocytes and the biliary system necessary for the excretion of bile. Lee et al. [198] constructed a multicell cultured 3D liver chip using liver dECM bioink. The chip has dual-flow hepatic vascular/biliary channels, overcoming the limitations of existing models and successfully observing the formation of the biliary system and enhanced liver function in the chip. Xie et al. [30] utilized a rigid polymer, and a soft porous membrane folded together to form a stack of three adjacent flow chambers separated by the membrane. Endothelial cells were seeded in the upper and lower chambers to simulate sinusoids, and hepatocytes were seeded in the middle chamber. Nutrients and metabolites flow through the simulated sinusoids and diffuse between the vascular channels and the hepatocyte layer, simulating physiological microcirculation (Fig. 5a) [30]. Ya et al. [199] developed a realistic biomimetic hepatic lobule-on-a-chip on which a perfusable sinusoidal network was realized using a microfluidic-guided angiogenesis approach. Furthermore, after self-assembly, the oxygen concentration was adjusted to mimic the physiological level of dissolution provided by actual hepatic arterioles and venules (Fig. 5b) [199]. These studies showed that, chips with vascularized structures can better simulate the biomimetic liver microstructure, have the higher metabolic capacity and longer-lasting hepatocyte functions.
Figure 5.
Microfluidics-based vascularized liver models. (a) Liver sinusoidal fold chip. Adapted with permission from Ref. [30]. (b) Bionic liver lobule chip. Adapted with permission from Ref. [199]. (c) Fluid-coupled multiorgan chip. Adapted with permission from Ref. [201]. (d) Nonalcoholic fatty liver organ chip. Adapted with permission from Ref. [202].
Due to the high-throughput characteristics of liver-on-chips, they are often used in disease models and drug metabolism studies [200]. Lasli et al. [83] presented an in vitro system to study nonalcoholic fatty liver disease by first co-culturing HepG2 cells and HUVECs into spheroids and then transferring the spheroids to a chip system with an array of interconnected hexagonal microwells. Proven to help monitor liver function by increasing the interaction of albumin secretion with HepG2-HUVEC and increasing the production of reactive oxygen species in adipose spheroids. Drugs typically pass through the endothelium-parenchymal tissue interface in the body, and the endothelium can contribute to drug-toxic behavior. Herland et al. [201] designed a fluid-coupled multiorgan chip to quantitatively demonstrate the pharmacokinetic and pharmacodynamic models of human physiological drugs through drug absorption, metabolism and excretion (Fig. 5c). Using the chip can simulate the drug transfer under physiological conditions between organs through the endothelial-lined vasculature and maintain long-term viability. Besides, Lee et al. [202] developed a new liver model aimed at enabling the implantation and maintenance of liver buds in perfusable 3D hydrogels in which a microvascular network develops within the 200 µm diffusion limit. This system replicates inflammation, lipid accumulation, and fibrosis processes in the progression of nonalcoholic fatty liver disease and predicts outcomes in mouse models (Fig. 5d) [202]. Recently, Chhabra et al. [203] developed a microfluidic chip platform called a structurally vascularized liver ensemble. It enables the control of hemodynamic changes to mimic those that occur during liver injury and regeneration and supports the management of biochemical inputs such as cytokines and paracrine interactions with endothelial cells. The model can provide information that cannot be gleaned from mouse or other animal studies, allowing scientists to more precisely track the processes involved in liver regeneration.
Vascularized liver tissue applications and evaluation
The liver has a complex and unique microenvironment with multiple cell–cell interactions and an internal vascular network. Tissues with abundant vascularization can highly restore the cellular microenvironment and help improve the secretion and metabolic functions of hepatocytes. This kind of highly biologically active in vitro tissue will be widely used in transplantation and regeneration. In addition, the development of clinically relevant vascularized liver models that can mimic the stimulation and induction of molecular pathways can help to elucidate disease mechanisms in biomedical research and applications in preclinical drug screening. It also provides a powerful platform for personalized liver toxicity screening [204], helping pharmaceutical companies to speed up drug development and drug toxicity analysis [3, 205]. For functional assessment of transplant regeneration, secretion of albumin and urea is the focus of attention. Cytochrome P450, transaminases and bilirubin are hallmark biomarkers in the presence of liver injury and are therefore frequently detected in disease modeling and drug screening [206]. As shown in Table 4, we exemplify the applications and evaluation indicators of some vascularized liver tissues.
Table 4.
Application and evaluation indicators
| Cell type | Culture mediuma | Structure | Applications | Biomarkers | References |
|---|---|---|---|---|---|
| HepaRG | High Glucose Dulbecco’s Modified Eagle Medium (DMEM) with dimethyl sulfoxide (DMSO) medium | Scaffold | Transplantation | Albumin, α-1 antitrypsin, factor VII, factor IX, CYP1A2, CYP3A4, alanine aminotransferase (ALT), aspartate aminotransferase (AST), direct bilirubin (DBIL), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), albumin/globulin ration (A/G) | [73] |
| HepG2, HUVEC | FBS and penicillin-streptomycin solution mixed in fresh DMEM | Hepatic lobule spheroids | Transplantation | MRP2, albumin, β-catenin, P-β-catenin, α-1 antitrypsin, urea, CYP3A4, CYP1A2, CYP2B6, CD31 | [102] |
| Mesenchymal stem cells (MSCs) | Heparinized acellular extracellular matrix and mesenchymal stem cell culture medium | Spheroids | Transplantation | albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TIBL), FOXA2, NR1I2, SLC27A5, INS2, CYP1A1, CYP3A9, GPX3, AKR1D1 | [75] |
| HepaRG, stellate cells (LX-2), HUVEC | William’s E medium with fetal bovine serum Hyclone III, penicillin, streptomycin, human insulin, l-glutamine, hydrocortisone hemisuccinate and DMSO | Scaffold | Liver fibrosis induction | Albumin, ACTA2, COL1A1, MMP2, TIMP1, Cytochrome P450 isoforms (CYP3A4, CYP2B6, CYP2E1, CYP2C9, CYP2C19) | [82] |
| Primary human hepatocytes, HSCs, HUVEC, Kuffer cells | DMSO was spiked into the TGF-β1 dosing solution and standard culture medium | Scaffold | Liver fibrosis induction | miR-122, albumin, urea, lactate dehydrogenase (LDH) | [207] |
| HepG2 | DMEM with fetal bovine serum | Hepatic lobule organoids | Hepatocellular carcinoma | Albumin, E-cadherin, matrix metalloproteinases (MMP2 MMP9), AFP, Twist-related protein 1 (TWIST1) | [208] |
| Primary hepatocellular carcinoma | DMEM/F12, penicillin/streptomycin, GlutaMAX, HEPES, B27, N2, N-acetyl-l-cysteine, nicotinamide, recombinant human (Leu15)-gastrin I, recombinant human EGF, recombinant human FGF10, recombinant human HGF, forskolin, A8301, Y27632, dexamethasone | Scaffold | Hepatocellular carcinoma | Ki-67, AFP, Raf-1, VEGFR1, VEGFR2 | [209] |
| HepaRG | William’s E medium with fetal bovine serum, l-Glutamine, recombinant human insulin, hydrocortisone hemisuccinate, penicillin/streptomycin, DMSO | Scaffold | Viral transcription | Albumin, CYP3A4, lactate dehydrogenase (LDH), adenoviral DNA | [210] |
| HepG2 | Minimum essential medium (MEM) α supplemented with FBS and antibiotic–antimycotic | Spheroid | Drug-induced hepatotoxicity | MPT, cytosolic calcium, caspase-3 | [28] |
| Hepatocytes, endothelial cells, Kupffer cells, and stellate cells | William’s E medium containing GlutaMAX, ITS+ Premix [human recombinant insulin, human transferrin, selenous acid, BSA, and linoleic acid], dexamethasone, ascorbic acid, fetal bovine serum, and penicillin/streptomycin | Organ-on-a-chip | Drug toxicity, predict drug-induced liver injury (DILI) | Cytochrome P450 isoforms (CYP1A, CYP2B, and CYP3A), MRP2, miR122, α-GST, keratin 18, ALT, AST, GLDH |
[66] |
| Human adipose-derived mesenchymal stem cells (ADMSCs), HUVEC, human hepatic stellate cells (HHSCs) | HiPer high glucose Dulbecco’s Modified Eagle Medium, containing fetal bovine serum, l-glutamine, basic fibroblast growth factor, endothelial cell growth supplement, stellate cell growth supplement | Hepatic lobule organoids | Drug toxicity, drug screening | Cytochrome P450, albumin, cytokeratin 18, CYP2E1, fibronectin, HNF4α markers, α-fetoprotein (AFP) | [170] |
| Human pluripotent stem cell (hPSC) | Advanced DMEM/F12 supplemented with GlutaMAX, HEPES, B27, BSA, N-acetyl-L-cysteine, [Leu15]-gastrin I human, A83–01, DAPT, 3 μM dexamethasone, FGF19, BMP7 and HGF. For suspension culture, media were supplemented with Matrigel | Spheroid | Drug-induced liver injury (DILI) | Cytochrome P450, albumin, urea | [5] |
| HepaRG | William’s E medium supplemented with L-Glutamine, fetal bovine serum, hydrocortisone hemisuccinate, recombinant human insulin, penicillin/streptomycin and DMSO | Scaffold | Drug toxicity | lactate dehydrogenase (LDH), albumin, urea | [211] |
The culture medium in Table 4 is all the components of the co-culture medium, among which DMSO is mostly used after 1–2 weeks to induce differentiation.
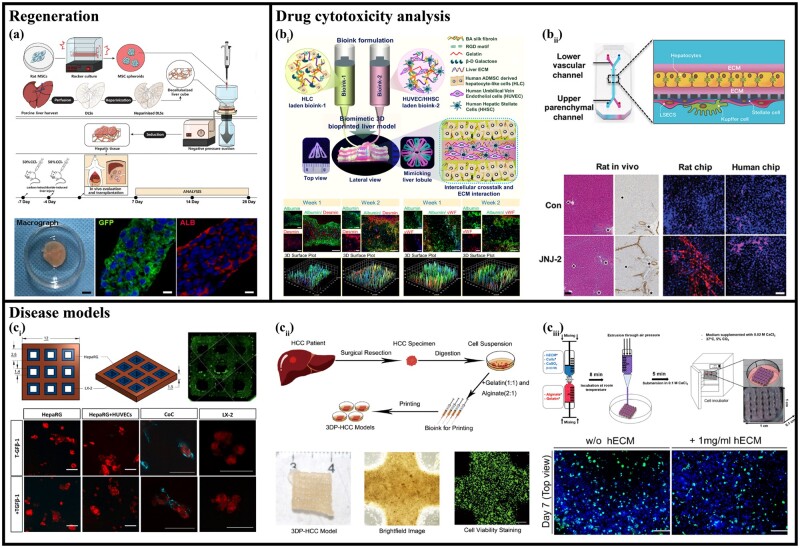
Transplant and regeneration
The shortage of organ donors is a key challenge in the treatment of end-stage organ failure, prompting the development of alternative strategies to generate organs in vitro. Hong et al. [102] subcutaneously injected structured microtissue spheroids mimicking hepatic lobules into nude mice and found the formation of functional blood vessels with structural integrity and stability. Although HepG2 is a suitable choice for in vitro studies of hepatic microtissues, the level of metabolic activity is low. Therefore, liver tissue composed of undifferentiated cells has better biological activity and weaker rejection response, which can improve the structural integrity and stability of implantation. Yang et al. [73] used HepaRG cells to construct liver organoids, acquired several liver functions after 7 days of differentiation, exhibited liver-specific protein synthesis after transplantation into liver-injured mice, and formed a functional vascular system, further supporting the substance transport function, significantly improved the survival rate of treated mice. Wu et al. [75] used MSCs to construct liver tissue and transplanted it into the omentum of liver-injured rats (Fig. 6a). The grafts were found to have hepatocyte-specific functions, exhibit strong proliferative activity in the ectopic liver system, and were able to anastomose the host vascular network efficiently and compatible with the host immune system.
Figure 6.
Applications of vascularized liver tissue. (a) Transplanted liver tissue into the liver-injured rats. Adapted with permission from Ref. [75]. (b) Drug cytotoxicity analysis. (bi) Vascularized hepatic lobular organoids for drug cytotoxicity analysis. Adapted with permission from Ref. [170]. (bii) Mechanism of action of several known hepatotoxic drugs and one experimental compound. Adapted with permission from Ref. [66]. (c) Disease models. (ci) Liver fibrosis model. Adapted with permission from Ref. [82] (cii) Cancer model [209]. (ciii) Virus transfection model. Adapted with permission from Ref. [210].
Disease models
The generation of various diseases is inseparable from the interaction between cell-cell and cell-ECM. Since the induction of diseases requires precise control of communication between cells, the interaction process between substances is essential. Therefore, 3D vascularized co-cultures have opened up opportunities for studying the development of hepatic disease models.
The construction of an in vitro hepatic fibrosis model provides a better way to study potential inducers and inhibitors of collagen expression and deposition. The findings of Norona et al. [207] demonstrated that Kupffer cells influence the biochemical and gene expression levels of different stages of the response and that their regulation of the injury/fibrotic response is context-dependent. Cuvellier et al. [82] precisely controlled cellular communication to induce liver fibrosis in 3D multicellular bioprinted constructs containing three types of cells: HepaRG, LX-2 and endothelial cells, and found fibrillar collagen deposition (Fig. 6ci). These results further demonstrate the utility of bioprinted human liver tissue for modeling and examining fibrotic events in vitro and understanding fibrotic injury.
In the study of liver cancer, in vitro liver tissue models can also play a huge role. Ma et al. [208] utilized a 3D biomimetic liver platform to study the behavior of various hepatoma cells in specific fibrotic settings. Xie et al. [209] established a patient-derived HCC (Hepatocellular carcinoma) model that retained the characteristics of parental HCC, including stable expression of biomarkers, genetic alterations and stable maintenance of expression profiles (Fig. 6cii). These models can visually and quantitatively demonstrate predicting patient-specific drug outcomes for personalized treatment. Another 3D model printed by Hiller et al. [210] supports efficient adenovirus replication, making them suitable for studying virus biology and developing new antiviral compounds (Fig. 6ciii).
Drug cytotoxicity analysis
Drug development is a long, expensive and risky process with a very low success rate. Due to portal absorption of oral drugs and their high bioactivation capacity, the liver is inadvertently exposed to high concentrations of drugs and bioactive metabolites, which are often the direct cause of drug-induced liver injury. For in situ quantitative assessment and high-content monitoring of drug toxicity, Hong et al. [28] developed a HepG2 liver spheroid culture model, a promising method for screening and characterizing drug-induced hepatotoxicity. To predict drug-induced liver injury, an in vitro microenvironment rich in vascularization was developed. Facilitating intercellular events and crosstalk by co-culturing parenchymal and non-parenchymal cells has great advantages in modeling the complexity and diversity of drug metabolism and drug toxicity pathways. Jang et al. [66] applied microengineered organ-on-a-chip technology to design rat, dog and human liver-on-chips containing species-specific primary hepatocytes, kupffer cells and HSCs linked to hepatic sinusoidal endothelial cells in physiological fluid culture flow, confirming the mechanism of action of several known hepatotoxic drugs and one experimental compound. The chip detected multiple phenotypes of hepatotoxicity, including hepatocyte damage, steatosis, cholestasis and fibrosis, as well as species-specific toxicity upon treatment with the tool compounds. Janani et al. [170] constructed multicellular co-cultured vascularized hepatic lobular organoids for subsequent assessment of cell viability and metabolic capacity by estimating DNA concentration and lactate dehydrogenase activity after exposure to different concentrations of hepatotoxic drugs (Fig. 6bi). Cytochrome P450 activity revealed dose-dependent clinically relevant hepatotoxicity. Since a complex liver microenvironment usually causes high metabolism of drugs and toxins, cells with differentiation ability will show good results in the detection of drug toxicity. Kim et al. [5] generated functionally characterized liver organoids as a high-fidelity model for drug safety assessment, including high CYP450 activity and apical drug transport capacity. Schmidt et al. [211] prepared HepaRG cultures using alginate-gelatin-Matrigel-based hydrogels to test the toxicity of aflatoxin B1 in vitro (Fig. 6bi). Such organoids that restore the extracellular microenvironment may provide a suitable alternative in vitro for chronic hepatotoxicity studies.
Challenges and future perspectives
Despite tremendous progress in liver tissue engineering over the past few decades, how to construct multiscale functional microvasculature with high precision and high resolution remains a challenge. Here, we summarize the current challenges and present ideas for corresponding implementations.
Firstly, in terms of vascularization accuracy, most of them are still at the millimeter level. The method of biological self-assembly can greatly improve the accuracy of formed capillary networks [104]. Micro-extrusion method can be applied to realize the alternating structure of liver tissue and vascular access and control the precision to the level of 100 microns. The in vitro vascularized tissue will be then formed by biological self-assembly to achieve a highly simplified liver sinusoid model. Such a capillary network, on the one hand, can promote the exchange of substances and improve the metabolic capacity of hepatocytes. On the other hand, it is also helpful for orthotopic transplantation and improves the efficiency of vascular system regeneration.
Secondly, in terms of the microenvironment, the liver has a rich variety of ECM, many types of cells, high density and relatively clear partitions, but the fabrication of a multimaterial, multicell co-culture model is a great challenge. Zhou et al. [212] proposed a multimaterial multiprocess fusion fabrication method that facilitates the construction of complex spatially heterogeneous structures. Combining with the embedded printing, it is possible to construct complex tissues with extra-low viscosity hydrogels such as collagen, which cannot be fabricated with traditional 3D extrusion-based bioprinting in the air [83]. A versatile embedded medium was proposed and had the ability to realize the coexistence of multiple cross-linking modes, which is helpful for the simultaneous construction of multiple materials at the same time [213, 214]. In addition, the sacrificial writing to functional tissue (SWIFT) method also helps to generate organ-specific tissue with high maturity [215]. Especially for liver tissue with high cell density and frequent material interaction, this is also an efficient construction method to create liver tissues with complex vasculatures.
Finally, in terms of functionalization, due to the lack of sources of primary hepatocytes and easy to lose functions during the cell culture process, liver tissue in vitro cannot restore the complete functional characterization in vivo. At the same time, the metabolic partitioning generated by hepatic acinus can rationally explain multiple disease models [40, 51, 54]. Therefore, microfluidic liver-on-a-chip technology can be integrated to carry out a long-term perfusion culture through the sequence of fluid flow so as to generate the gradient of metabolism, which can restore the metabolic gradient model of liver acinus. At the same time, adding more types of liver cells, such as HSCs, and Kuffer cells, can provide a more physiological model for the subsequent study of disease mechanisms and drug toxicity.
Conclusions
In recent years, the construction of vascularized liver tissue in vitro has shown a new direction, and the development of this field requires a wide range of cell sources and suitable biomaterials aimed at damage repairment and drug toxicity studies. In this review, we outline various cell sources and analyze the advantages of scaffold materials for assembled cells. We first introduce the components of scaffold materials, such as protein- and polysaccharide-based natural materials, acellular extracellular matrices, synthetic polymers and nanocellulose materials. We then outline common and novel strategies for preparing vascularized liver tissues based on different types of cells. With the in vitro fabricated vascularized liver tissues, many biomedical applications can be applied on these models, such as liver regeneration, disease model as well as drug screening. In terms of transplantation regeneration, prevascularized tissue can be anastomosed with the host’s vascular system, increasing the success rate of transplantation. At the same time, animal experiments are ultimately limited in terms of inducing disease models and studying drug toxicity, and vascularization models can provide suitable alternatives to more realistically understand human responses to drug testing, toxicological analysis or pathological models.
Acknowledgments
The authors would like to thank the National Key Research and Development Program of China (No. 2018YFA0703000), the National Natural Science Foundation of China (No. 52275294, No. 51875518 and No. 52105310).
Author contributions
W.L. and H.Z. did most writing for the manuscript and literature studying. A.A. assisted partially in writing and literature searching. X.X. and M.Y. provided the guidance and framework of the study. Y.Y.S.H. discussed and edited the manuscript. H.Y. discussed the study and provide the resources. L.M. conceived, supervised the manuscript process and edited the manuscript.
Conflicts of interest statement. The authors declare no conflict of interest.
Contributor Information
Weikang Lv, State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310058, China; School of Mechanical Engineering, Zhejiang University, Hangzhou 310058, China.
Hongzhao Zhou, State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310058, China; School of Mechanical Engineering, Zhejiang University, Hangzhou 310058, China.
Abdellah Aazmi, State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310058, China; School of Mechanical Engineering, Zhejiang University, Hangzhou 310058, China.
Mengfei Yu, The Affiliated Stomatologic Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China.
Xiaobin Xu, School of Materials Science and Engineering, Tongji University, Shanghai 201804, China.
Huayong Yang, State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310058, China; School of Mechanical Engineering, Zhejiang University, Hangzhou 310058, China.
Yan Yan Shery Huang, Department of Engineering, University of Cambridge, Cambridge CB2 1PZ, UK.
Liang Ma, State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310058, China; School of Mechanical Engineering, Zhejiang University, Hangzhou 310058, China.
References
- 1. Knolle PA, Limmer A.. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol 2001;22:432–7. [DOI] [PubMed] [Google Scholar]
- 2. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY.. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu SM, Zhang JW, Lin S, Zheng DF, Shen Y, Qin JL, Li YY, Wang SQ.. Recent advances in the development of in vitro liver models for hepatotoxicity testing. Biodes Manuf 2021;4:717–34. [Google Scholar]
- 4. Zhang YS, Khademhosseini A.. Engineering in vitro human tissue models through bio-design and manufacturing. Biodes Manuf 2020;3:155–9. [Google Scholar]
- 5. Kim H, Im I, Jeon JS, Kang EH, Lee HA, Jo S, Kim JW, Woo DH, Choi YJ, Kim HJ, Han JS, Lee BS, Kim JH, Kim SK, Park HJ.. Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials 2022;286:121575. [DOI] [PubMed] [Google Scholar]
- 6. Gough A, Soto-Gutierrez A, Vernetti L, Ebrahimkhani MR, Stern AM, Taylor DL.. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat Rev Gastroenterol Hepatol 2021;18:252–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, Chen S.. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev 2018;132:235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim KS, Baptista M, Moon S, Woodfield TBF, Rnjak-Kovacina J.. Microchannels in development, survival, and vascularisation of tissue analogues for regenerative medicine. Trends Biotechnol 2019;37:1189–201. [DOI] [PubMed] [Google Scholar]
- 9. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang R-R, Ueno Y, Zheng Y-W, Koike N, Aoyama S, Adachi Y, Taniguchi H.. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499:481–4. [DOI] [PubMed] [Google Scholar]
- 10. Takebe T, Zhang R-R, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H.. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 2014;9:396–409. [DOI] [PubMed] [Google Scholar]
- 11. Jian HL, Wang MY, Wang ST, Wang AH, Bai S.. 3D bioprinting for cell culture and tissue fabrication. Biodes Manuf 2018;1:45–61. [Google Scholar]
- 12. Rouwkema J, Khademhosseini A.. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol 2016;34:733–45. [DOI] [PubMed] [Google Scholar]
- 13. Aazmi A, Zhou HZ, Lv WK, Yu MF, Xu XB, Yang HY, Zhang YS, Ma L.. Vascularizing the brain in vitro. Iscience 2022;25:104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massa S, Sakr MA, Seo J, Bandaru P, Arneri A, Bersini S, Zare-Eelanjegh E, Jalilian E, Cha B-H, Antona S, Enrico A, Gao Y, Hassan S, Acevedo JP, Dokmeci MR, Zhang YS, Khademhosseini A, Shin SR.. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017;11:044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rezakhani S, Gjorevski N, Lutolf MP.. Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 2021;276:121020. [DOI] [PubMed] [Google Scholar]
- 16. Hastings JF, Skhinas JN, Fey D, Croucher DR, Cox TR.. The extracellular matrix as a key regulator of intracellular signalling networks. Br J Pharmacol 2019;176:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez-Hernandez A, Amenta PS.. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol 1993;423:1–11. [DOI] [PubMed] [Google Scholar]
- 18. Askari M, Afzali Naniz M, Kouhi M, Saberi A, Zolfagharian A, Bodaghi M.. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci 2021;9:535–73. [DOI] [PubMed] [Google Scholar]
- 19. Jain E, Damania A, Kumar A.. Biomaterials for liver tissue engineering. Hepatol Int 2014;8:185–97. [DOI] [PubMed] [Google Scholar]
- 20. Kedderis GL. Biochemical basis of hepatocellular injury. Toxicol Pathol 1996;24:77–83. [DOI] [PubMed] [Google Scholar]
- 21. Herrera J, Henke CA, Bitterman PB.. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 2018;128:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahadian S, Khademhosseini A.. A perspective on 3D bioprinting in tissue regeneration. Biodes Manuf 2018;1:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT.. Concise review: bioprinting of stem cells for transplantable tissue fabrication. Stem Cells Transl Med 2017;6:1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, Fukunishi T, Zhang H, Hibino N.. 3D bioprinting using stem cells. Pediatr Res 2018;83:223–31. [DOI] [PubMed] [Google Scholar]
- 25. Raasch M, Fritsche E, Kurtz A, Bauer M, Mosig AS.. Microphysiological systems meet hiPSC technology—new tools for disease modeling of liver infections in basic research and drug development. Adv Drug Deliv Rev 2019;140:51–67. [DOI] [PubMed] [Google Scholar]
- 26. Minami T, Ishii T, Yasuchika K, Fukumitsu K, Ogiso S, Miyauchi Y, Kojima H, Kawai T, Yamaoka R, Oshima Y, Kawamoto H, Kotaka M, Yasuda K, Osafune K, Uemoto S.. Novel hybrid three-dimensional artificial liver using human induced pluripotent stem cells and a rat decellularized liver scaffold. Regen Ther 2019;10:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lei X, Shao C, Shou X, Shi K, Shi L, Zhao Y.. Porous hydrogel arrays for hepatoma cell spheroid formation and drug resistance investigation. Biodes Manuf 2021;4:842–50. [Google Scholar]
- 28. Hong S, Song JM.. A 3D cell printing-fabricated HepG2 liver spheroid model for high-content in situ quantification of drug-induced liver toxicity. Biomater Sci 2021;9:5939–50. [DOI] [PubMed] [Google Scholar]
- 29. Jeon H, Kang K, Park SA, Kim WD, Paik SS, Lee SH, Jeong J, Choi D.. Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver 2017;11:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie X, Maharjan S, Kelly C, Liu T, Lang RJ, Alperin R, Sebastian S, Bonilla D, Gandolfo S, Boukataya Y, Siadat SM, Zhang YS, Livermore C.. Customizable microfluidic origami liver-on-a-chip (oLOC). Adv Mater Technol 2022;7:2100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grix T, Ruppelt A, Thomas A, Amler A-K, Noichl BP, Lauster R, Kloke L.. Bioprinting Perfusion-enabled liver equivalents for advanced organ-on-a-chip applications. Genes 2018;9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mardinoglu A, Boren J, Smith U, Uhlen M, Nielsen J.. Systems biology in hepatology: approaches and applications. Nat Rev Gastroenterol Hepatol 2018;15:365–77. [DOI] [PubMed] [Google Scholar]
- 33. Rappaport AM. The microcirculatory hepatic unit. Microvasc Res 1973;6:212–28. [DOI] [PubMed] [Google Scholar]
- 34. Snyder CD. Recent advances in knowledge of the liver. Physiol Rev 1942;22:54–73. [Google Scholar]
- 35. Gressner AM. Hepatic fibrogenesis: the puzzle of interacting cells, fibrogenic cytokines, regulatory loops, and extracellular matrix molecules. Z Gastroenterol 1992;30:5–16. [PubMed] [Google Scholar]
- 36. Bedossa P, Paradis V.. Liver extracellular matrix in health and disease. J Pathol 2003;200:504–15. [DOI] [PubMed] [Google Scholar]
- 37. McCuskey RS. The hepatic microvascular system in health and its response to toxicants. Anat Rec (Hoboken) 2008;291:661–71. [DOI] [PubMed] [Google Scholar]
- 38. Ekataksin W, Kaneda K.. Liver microvascular architecture: an insight into the pathophysiology of portal hypertension. Semin Liver Dis 1999;19:359–82. [DOI] [PubMed] [Google Scholar]
- 39. Matsumoto T, Kawakami M.. The unit-concept of hepatic parenchyma—a re-examination based on angioarchitectural studies. Acta Pathol Jpn 1982;32:285–314. [PubMed] [Google Scholar]
- 40. Gebhardt R, Matz-Soja M.. Liver zonation: novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol 2014;20:8491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver 2000;20:3–7. [DOI] [PubMed] [Google Scholar]
- 42. Mak KM, Shin DW.. Hepatic sinusoids versus Central veins: structures, markers, angiocrines, and roles in liver regeneration and homeostasis. Anat Rec (Hoboken) 2021;304:1661–91. [DOI] [PubMed] [Google Scholar]
- 43. Goltz D, Fischer H-P.. Liver sinusoids: pathology of endothelial findings. Pathologe 2020;41:471–7. [DOI] [PubMed] [Google Scholar]
- 44. Brunt EM, Gouw ASH, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, Clouston AD, Dienes HP, Goodman ZD, Roberts EA, Roskams T, Terracciano L, Torbenson MS, Wanless IR.. Pathology of the liver sinusoids. Histopathology 2014;64:907–20. [DOI] [PubMed] [Google Scholar]
- 45. Oda M, Yokomori H, Han JY.. Regulatory mechanisms of hepatic microcirculation. Clin Hemorheol Microcirc 2003;29:167–82. [PubMed] [Google Scholar]
- 46. Imai K, Sato T, Senoo H.. Adhesion between cells and extracellular matrix with special reference to hepatic stellate cell adhesion to three-dimensional collagen fibers. Cell Struct Funct 2000;25:329–36. [DOI] [PubMed] [Google Scholar]
- 47. Rappaport A, Borowy Z, Lougheed W, Lotto W.. Subdivision of hexagonal liver lobules into a structural and functional unit. Bole in hepatic physiology and pathology. Anat Rec 1954;119:11–33. [DOI] [PubMed] [Google Scholar]
- 48. Cunningham RP, Porat-Shliom N.. Liver zonation—revisiting old questions with new technologies. Front Physiol 2021;12:732929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ben-Moshe S, Itzkovitz S.. Spatial heterogeneity in the mammalian liver. Nat Rev Gastroenterol Hepatol 2019;16:395–410. [DOI] [PubMed] [Google Scholar]
- 50. Paris J, Henderson NC.. Liver zonation, revisited. Hepatology 2022;76:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kietzmann T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol 2017;11:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Katz NR. Metabolic heterogeneity of hepatocytes across the liver acinus. J Nutr 1992;122:843–9. [DOI] [PubMed] [Google Scholar]
- 53. Lamers WH, Hilberts A, Furt E, Smith J, Jonges GN, van Noorden CJ, Janzen JW, Charles R, Moorman AF.. Hepatic enzymic zonation: a reevaluation of the concept of the liver acinus. Hepatology 1989;10:72–6. [DOI] [PubMed] [Google Scholar]
- 54. Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK.. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie 2014;96:121–9. [DOI] [PubMed] [Google Scholar]
- 55. Macdonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DHG, Ramm GA.. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol 2001;16:599–606. [DOI] [PubMed] [Google Scholar]
- 56. Bhunchet E, Wake K.. The portal lobule in rat liver fibrosis: a re-evaluation of the liver unit. Hepatology 1998;27:481–7. [DOI] [PubMed] [Google Scholar]
- 57. Ford AJ, Rajagopalan P.. Extracellular matrix remodeling in 3D: implications in tissue homeostasis and disease progression. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2018;10:e1503. [DOI] [PubMed] [Google Scholar]
- 58. McQuitty CE, Williams R, Chokshi S, Urbani L.. Immunomodulatory role of the extracellular matrix within the liver disease microenvironment. Front Immunol 2020;11:574276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis 1990;10:1–10. [DOI] [PubMed] [Google Scholar]
- 60. Rojkind M, Giambrone MA, Biempica L.. Collagen types in normal and cirrhotic liver. Gastroenterology 1979;76:710–9. [PubMed] [Google Scholar]
- 61. Bataller R, Brenner DA.. Liver fibrosis. J Clin Invest 2005;115:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meurer SK, Karsdal MA, Weiskirchen R.. Advances in the clinical use of collagen as biomarker of liver fibrosis. Expert Rev Mol Diagn 2020;20:947–69. [DOI] [PubMed] [Google Scholar]
- 63. Baiocchini A, Montaldo C, Conigliaro A, Grimaldi A, Correani V, Mura F, Ciccosanti F, Rotiroti N, Brenna A, Montalbano M, D'Offizi G, Capobianchi MR, Alessandro R, Piacentini M, Schinina ME, Maras B, Del Nonno F, Tripodi M, Mancone C.. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS One 2016;11:e0151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fang ZQ, Ruan B, Liu JJ, Duan JL, Yue ZS, Song P, Xu H, Ding J, Xu C, Dou GR, Wang L.. Notch-triggered maladaptation of liver sinusoidal endothelium aggravates nonalcoholic steatohepatitis through endothelial nitric oxide synthase. Hepatology 2022;76:742–58. [DOI] [PubMed] [Google Scholar]
- 65. Huang D, Gibeley SB, Xu C, Xiao Y, Celik O, Ginsberg HN, Leong KW.. Engineering liver microtissues for disease modeling and regenerative medicine. Adv Funct Mater 2020;30:1909553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jang KJ, Otieno MA, Ronxhi J, Lim HK, Ewart L, Kodella KR, Petropolis DB, Kulkarni G, Rubins JE, Conegliano D, Nawroth J, Simic D, Lam W, Singer M, Barale E, Singh B, Sonee M, Streeter AJ, Manthey C, Jones B, Srivastava A, Andersson LC, Williams D, Park H, Barrile R, Sliz J, Herland A, Haney S, Karalis K, Ingber DE, Hamilton GA.. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med 2019;11:eaax5516. [DOI] [PubMed] [Google Scholar]
- 67. Yu Y, Xie R, He Y, Zhao F, Zhang Q, Wang W, Zhang Y, Hu J, Luo D, Peng W.. Dual-core coaxial bioprinting of double-channel constructs with a potential for perfusion and interaction of cells. Biofabrication 2022;14:035012. [DOI] [PubMed] [Google Scholar]
- 68. Kang HK, Sarsenova M, Kim D-H, Kim MS, Lee JY, Sung E-A, Kook MG, Kim NG, Choi SW, Ogay V, Kang K-S.. Establishing a 3D in vitro hepatic model mimicking physiologically relevant to in vivo state. Cells 2021;10:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pimentel CR, Ko SK, Caviglia C, Wolff A, Emneus J, Keller SS, Dufva M.. Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network. Acta Biomater 2018;65:174–84. [DOI] [PubMed] [Google Scholar]
- 70. Kang D, Ahn G, Kim D, Kang HW, Yun S, Yun WS, Shim JH, Jin S.. Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication 2018;10:035008. [DOI] [PubMed] [Google Scholar]
- 71. Lewis PL, Green RM, Shah RN.. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater 2018;69:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharma A, Rawal P, Tripathi DM, Alodiya D, Sarin SK, Kaur S, Ghosh S.. Upgrading hepatic differentiation and functions on 3D printed silk-decellularized liver hybrid scaffolds. ACS Biomater Sci Eng 2021;7:3861–73. [DOI] [PubMed] [Google Scholar]
- 73. Yang H, Sun L, Pang Y, Hu D, Xu H, Mao S, Peng W, Wang Y, Xu Y, Zheng Y-C, Du S, Zhao H, Chi T, Lu X, Sang X, Zhong S, Wang X, Zhang H, Huang P, Sun W, Mao Y.. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2021;70:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Deng S, Zhu Y, Zhao X, Chen J, Tuan RS, Chan HF.. Efficient fabrication of monodisperse hepatocyte spheroids and encapsulation in hybrid hydrogel with controllable extracellular matrix effect. Biofabrication 2022;14:015002. [DOI] [PubMed] [Google Scholar]
- 75. Wu Q, Li Y, Yang Z, Li L, Yang J, Zhu X, Liu Y, Bao J, Bu H.. Ectopic expansion and vascularization of engineered hepatic tissue based on heparinized acellular liver matrix and mesenchymal stromal cell spheroids. Acta Biomater 2022;137:79–91. [DOI] [PubMed] [Google Scholar]
- 76. Sun Y, Wang Y, Zhou L, Zou YZ, Huang GW, Gao G, Ting S, Lei X, Ding X.. Spheroid-cultured human umbilical cord-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury in rats. Sci Rep 2018;8:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Park IS. Synergistic effect of three-dimensional coculture and photobiomodulation therapy on vascularized liver spheroid formation by stem cells. J Cell Physiol 2021;236:5865–74. [DOI] [PubMed] [Google Scholar]
- 78. Lu D, Liu Y, Li W, Ma H, Li T, Ma X, Mao Y, Liang Q, Ma Z, Wang J.. Development and application of 3D bioprinted scaffolds supporting induced pluripotent stem cells. Biomed Res Int 2021;2021:4910816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Goulart E, de Caires-Junior LC, Telles-Silva KA, Silva Araujo BH, Rocco SA, Sforca M, de Sousa IL, Kobayashi GS, Musso CM, Assoni AF, Oliveira D, Caldini E, Raia S, Lelkes PI, Zatz M.. 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication 2019;12:015010. [DOI] [PubMed] [Google Scholar]
- 80. Wang J, Sun M, Liu W, Li Y, Li M.. Stem Cell-Based therapies for liver diseases: an overview and update. Tissue Eng Regen Med 2019;16:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Suurmond CAE, Lasli S, van den Dolder FW, Ung A, Kim HJ, Bandaru P, Lee K, Cho HJ, Ahadian S, Ashammakhi N, Dokmeci MR, Lee J, Khademhosseini A.. In vitro human liver model of nonalcoholic steatohepatitis by coculturing hepatocytes, endothelial cells, and kupffer cells. Adv Healthc Mater 2019;8:e1901379. [DOI] [PubMed] [Google Scholar]
- 82. Cuvellier M, Ezan F, Oliveira H, Rose S, Fricain J-C, Langouet S, Legagneux V, Baffet G.. 3D culture of HepaRG cells in GelMa and its application to bioprinting of a multicellular hepatic model. Biomaterials 2021;269:120611. [DOI] [PubMed] [Google Scholar]
- 83. Lasli S, Kim HJ, Lee K, Suurmond CE, Goudie M, Bandaru P, Sun W, Zhang S, Zhang N, Ahadian S, Dokmeci MR, Lee J, Khademhosseini A.. A human liver-on-a-Chip platform for modeling nonalcoholic fatty liver disease. Adv Biosyst 2019;3:e1900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lewis PL, Su J, Yan M, Meng FY, Glaser SS, Alpini GD, Green RM, Sosa-Pineda B, Shah RN.. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci Rep 2018;8:12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mao S, He J, Zhao Y, Liu T, Xie F, Yang H, Mao Y, Pang Y, Sun W.. Bioprinting of patient-derived in vitro intrahepatic cholangiocarcinoma tumor model: establishment, evaluation and anti-cancer drug testing. Biofabrication 2020;12:045014. [DOI] [PubMed] [Google Scholar]
- 86. Sun P, Zhang G, Su X, Jin C, Yu B, Yu X, Lv Z, Ma H, Zhang M, Wei W, Li W.. Maintenance of primary hepatocyte functions in vitro by inhibiting mechanical Tension-Induced Yap activation. Cell Rep 2019;29:3212–22 e4. [DOI] [PubMed] [Google Scholar]
- 87. Wei J, Qu Q.. Hepatocyte polarity: constitutive and functional characteristics and pathological appearance in liver diseases. Chin Bull Life Sci 2011;23:51–6. [Google Scholar]
- 88. Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB.. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One 2016;11:e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Bottger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Rowlands JC, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CEP, Gomez-Lechon MJ, Groothuis GMM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Haussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhutter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Park BK, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi JB, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EHK, Stieger B, Stober R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG.. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 2013;87:1315–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christ B, Stock P.. Mesenchymal stem cell-derived hepatocytes for functional liver replacement. Front Immunol 2012;3:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sorensen KK, Simon-Santamaria J, McCuskey RS, Smedsrod B.. Liver sinusoidal endothelial cells. Compr Physiol 2015;5:1751–74. [DOI] [PubMed] [Google Scholar]
- 92. Xing XK, Wu HY, Feng HG, Yuan ZQ.. Immune function of nonparenchymal liver cells. Genet Mol Res 2016;15(1):gmr15018524. [DOI] [PubMed] [Google Scholar]
- 93. Shetty S, Lalor PF, Adams DH.. Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol 2018;15:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang M, Zhang C.. The role of liver sinusoidal endothelial cells in cancer liver metastasis. Am J Cancer Res 2021;11:1845–60. [PMC free article] [PubMed] [Google Scholar]
- 95. Wisse E, Braet F, Luo D, De Zanger R, Jans D, Crabbe E, Vermoesen A.. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol 1996;24:100–11. [DOI] [PubMed] [Google Scholar]
- 96. Lao Y-X, He F-C, Jiang Y.. Physiological function and pathological mechanisms of liver sinusoidal endothelial cell. Zhongguo Shengwu Huaxue yu Fenzi Shengwu Xuebao 2012;28:609–16. [Google Scholar]
- 97. Samarasinghe DA, Farrell GC.. The Central role of sinusoidal endothelial cells in hepatic hypoxia-reoxygenation injury in the rat. Hepatology 1996;24:1230–7. [DOI] [PubMed] [Google Scholar]
- 98. DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest 2013;123:1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang L, Wang XD, Xie GH, Wang L, Hill CK, DeLeve LD.. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest 2012;122:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ma L, Wu Y, Li Y, Aazmi A, Zhou H, Zhang B, Yang H.. Current advances on 3D-bioprinted liver tissue models. Adv Healthc Mater 2020;9:e2001517. [DOI] [PubMed] [Google Scholar]
- 101. Kang D, Hong G, An S, Jang I, Yun WS, Shim JH, Jin S.. Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small 2020;16:1905505. [DOI] [PubMed] [Google Scholar]
- 102. Hong G, Kim J, Oh H, Yun S, Kim CM, Jeong YM, Yun WS, Shim JH, Jang I, Kim CY, Jin S.. Production of multiple cell-laden microtissue spheroids with a biomimetic hepatic-lobule-like structure. Adv Mater 2021;33:e2102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Son J, Hong SJ, Lim JW, Jeong W, Jeong JH, Kang H-W.. Engineering tissue-specific, multiscale microvasculature with a capillary network for prevascularized tissue. Small Methods 2021;5:2100632. [DOI] [PubMed] [Google Scholar]
- 104. Wan Z, Zhong AX, Zhang S, Pavlou G, Coughlin MF, Shelton SE, Nguyen HT, Lorch JH, Barbie DA, Kamm RD.. A robust method for perfusable microvascular network formation in vitro. Small Methods 2022;6:e2200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bouwens L. Structural and functional aspects of kupffer cells. Revis Biol Celular 1988;16:69–94. [PubMed] [Google Scholar]
- 106. Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE.. Kupffer cells in the liver. Compr Physiol 2013;3:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li W, Chang N, Li L.. Heterogeneity and function of kupffer cells in liver injury. Front Immunol 2022;13:940867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li L, Wang BE.. [Kupffer cells and liver fibrosis]. Zhonghua Gan Zang Bing Za Zhi 2007;15:559–60. [PubMed] [Google Scholar]
- 109. Elchaninov AV, Fatkhudinov TK, Vishnyakova PA, Lokhonina AV, Sukhikh GT.. Phenotypical and functional polymorphism of liver resident macrophages. Cells 2019;8:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hellerbrand C. Hepatic stellate cells—the pericytes in the liver. Pflugers Arch 2013;465:775–8. [DOI] [PubMed] [Google Scholar]
- 112. Thomas RJ, Bhandari R, Barrett DA, Bennett AJ, Fry JR, Powe D, Thomson BJ, Shakesheff KM.. The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs 2005;181:67–79. [DOI] [PubMed] [Google Scholar]
- 113. Asahina K. Hepatic stellate cell progenitor cells. J Gastroenterol Hepatol 2012;27(Suppl 2):80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chan KM, Fu YH, Wu TJ, Hsu PY, Lee WC.. Hepatic stellate cells promote the differentiation of embryonic stem cell-derived definitive endodermal cells into hepatic progenitor cells. Hepatol Res 2013;43:648–57. [DOI] [PubMed] [Google Scholar]
- 115. Senoo H. Structure and function of hepatic stellate cells. Med Electron Microsc 2004;37:3–15. [DOI] [PubMed] [Google Scholar]
- 116. Puche JE, Saiman Y, Friedman SL.. Hepatic stellate cells and liver fibrosis. Compr Physiol 2013;3:1473–92. [DOI] [PubMed] [Google Scholar]
- 117. Semela D, Shah V.. Interactions Between Hepatocytes and Liver Sinusoidal Endothelial Cells. UK: Cambridge University Press, 2007, 609–15. [Google Scholar]
- 118. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Organ SL, Tsao M-S.. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011;3:S7–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Coxon A, Rex K, Meyer S, Sun J, Sun J, Chen Q, Radinsky R, Kendall R, Burgess TL.. Soluble c-Met receptors inhibit phosphorylation of c-Met and growth of hepatocyte growth factor: c-Met-dependent tumors in animal modelsSoluble c-Met with antitumor activity. Mol Cancer Ther 2009;8:1119–25. [DOI] [PubMed] [Google Scholar]
- 121. Wang H, Rao B, Lou J, Li J, Liu Z, Li A, Cui G, Ren Z, Yu Z.. The function of the HGF/c-Met axis in hepatocellular carcinoma. Front Cell Dev Biol 2020;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang G, Zheng Y, Wang Y, Cai Z, Liao N, Liu J, Zhang W.. Co-culture system of hepatocytes and endothelial cells: two in vitro approaches for enhancing liver-specific functions of hepatocytes. Cytotechnology 2018;70:1279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee H, Cho D-W.. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016;16:2618–25. [DOI] [PubMed] [Google Scholar]
- 124. Kaur S, Tripathi DM, Venugopal JR, Ramakrishna S.. Advances in biomaterials for hepatic tissue engineering. Curr Opin Biomed Eng 2020;13:190–6. [Google Scholar]
- 125. Pahlevanzadeh F, Mokhtari H, Bakhsheshi-Rad HR, Emadi R, Kharaziha M, Valiani A, Poursamar SA, Ismail AF, RamaKrishna S, Berto F.. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials 2020;13:3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rastogi P, Kandasubramanian B.. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019;11:042001. [DOI] [PubMed] [Google Scholar]
- 127. Shams E, Barzad MS, Mohamadnia S, Tavakoli O, Mehrdadfar A.. A review on alginate-based bioinks, combination with other natural biomaterials and characteristics. J Biomater Appl 2022;37:355–72. [DOI] [PubMed] [Google Scholar]
- 128. Gopinathan J, Noh I.. Recent trends in bioinks for 3D printing. Biomater Res 2018;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li SL, Tian XH, Fan J, Tong H, Ao Q, Wang XH.. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines-Basel 2019;10:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shoueir KR, El-Desouky N, Rashad MM, Ahmed MK, Janowska I, El-Kemary M.. Chitosan based-nanoparticles and nanocapsules: overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int J Biol Macromol 2021;167:1176–97. [DOI] [PubMed] [Google Scholar]
- 131. Highley CB, Prestwich GD, Burdick JA.. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 2016;40:35–40. [DOI] [PubMed] [Google Scholar]
- 132. Lukin I, Erezuma I, Maeso L, Zarate J, Desimone MF, Al-Tel TH, Dolatshahi-Pirouz A, Orive G.. Progress in gelatin as biomaterial for tissue engineering. Pharmaceutics 2022;14:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A.. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015;73:254–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Noshadi I, Hong S, Sullivan KE, Shirzaei Sani E, Portillo-Lara R, Tamayol A, Shin SR, Gao AE, Stoppel WL, Black LD III, Khademhosseini A, Annabi N.. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater Sci 2017;5:2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lee JM, Suen SKQ, Ng WL, Ma WC, Yeong WY.. Bioprinting of collagen: considerations, potentials, and applications. Macromol Biosci 2021;21:e2000280. [DOI] [PubMed] [Google Scholar]
- 136. Osidak EO, Kozhukhov VI, Osidak MS, Domogatsky SP.. Collagen as bioink for bioprinting: a comprehensive review. Int J Bioprinting 2020;6:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shpichka A, Osipova D, Efremov Y, Bikmulina P, Kosheleva N, Lipina M, Bezrukov EA, Sukhanov RB, Solovieva AB, Vosough M, Timashev P.. Fibrin-based bioinks: new tricks from an old dog. Int J Bioprint 2020;6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. de Melo BAG, Jodat YA, Cruz EM, Benincasa JC, Shin SR, Porcionatto MA.. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater 2020;117:60–76. [DOI] [PubMed] [Google Scholar]
- 139. Dzobo K, Motaung K, Adesida A.. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 2019;20:4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gori M, Giannitelli SM, Torre M, Mozetic P, Abbruzzese F, Trombetta M, Traversa E, Moroni L, Rainer A.. Biofabrication of hepatic constructs by 3D bioprinting of a Cell-Laden thermogel: an effective tool to assess drug-induced hepatotoxic response. Adv Healthc Mater 2020;9:e2001163. [DOI] [PubMed] [Google Scholar]
- 141. Taymour R, Kilian D, Ahlfeld T, Gelinsky M, Lode A.. 3D bioprinting of hepatocytes: core-shell structured co-cultures with fibroblasts for enhanced functionality. Sci Rep 2021;11:5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Finosh GT, Jayabalan M.. Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering. RSC Adv 2015;5:38183–201. [Google Scholar]
- 143. Caliari SR, Perepelyuk M, Cosgrove BD, Tsai SJ, Lee GY, Mauck RL, Wells RG, Burdick JA.. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep 2016;6:21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Morais A, Vieira S, Zhao X, Mao Z, Gao C, Oliveira JM, Reis RL.. Advanced biomaterials and processing methods for liver regeneration: state-of-the-art and future trends. Adv Healthc Mater 2020;9:e1901435. [DOI] [PubMed] [Google Scholar]
- 145. Xie MJ, Zheng YT, Gao Q, He Y.. Facile 3D cell culture protocol based on photocurable hydrogels. Biodes Manuf 2021;4:149–53. [Google Scholar]
- 146. Chimene D, Lennox KK, Kaunas RR, Gaharwar AK.. Advanced bioinks for 3D printing: a materials science perspective. Ann Biomed Eng 2016;44:2090–102. [DOI] [PubMed] [Google Scholar]
- 147. Sun Y, Yu K, Nie J, Sun M, Fu J, Wang H, He Y.. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication 2021;13:035032. [DOI] [PubMed] [Google Scholar]
- 148. Roopesh RP, Muthusamy S, Velayudhan S, Sabareeswaran A, Anil Kumar PR.. High-throughput production of liver parenchymal microtissues and enrichment of organ-specific functions in gelatin methacrylamide microenvironment. Biotechnol Bioeng 2022;119:1018–32. [DOI] [PubMed] [Google Scholar]
- 149. Mazzocchi A, Devarasetty M, Huntwork R, Soker S, Skardal A.. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018;11:015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kabirian F, Mozafari M.. Decellularized ECM-derived bioinks: prospects for the future. Methods 2020;171:108–18. [DOI] [PubMed] [Google Scholar]
- 151. Kim MK, Jeong W, Lee SM, Kim JB, Jin S, Kang HW.. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020;12:025003. [DOI] [PubMed] [Google Scholar]
- 152. Mao Q, Wang Y, Li Y, Juengpanich S, Li W, Chen M, Yin J, Fu J, Cai X.. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl 2020;109:110625. [DOI] [PubMed] [Google Scholar]
- 153. Sarkar J, Kamble SC, Kashikar NC.. Polymeric bioinks for 3D hepatic printing. Chemistry (Basel) 2021;3:164–81. [Google Scholar]
- 154. Liu F, Chen QH, Liu C, Ao Q, Tian XH, Fan J, Tong H, Wang XH.. Natural polymers for organ 3D bioprinting. Polymers-Basel 2018;10:1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lee JW, Choi Y-J, Yong W-J, Pati F, Shim J-H, Kang KS, Kang I-H, Park J, Cho D-W.. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016;8:015007. [DOI] [PubMed] [Google Scholar]
- 156. Salerno S, Tasselli F, Drioli E, De Bartolo L.. Poly(epsilon-Caprolactone) hollow fiber membranes for the biofabrication of a vascularized human liver tissue. Membranes (Basel) 2020;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Liu M, Yang J, Hu W, Zhang S, Wang Y.. Superior performance of co-cultured mesenchymal stem cells and hepatocytes in poly(lactic acid-glycolic acid) scaffolds for the treatment of acute liver failure. Biomed Mater 2016;11:015008. [DOI] [PubMed] [Google Scholar]
- 158. Clark CC, Aleman J, Mutkus L, Skardal A.. A mechanically robust thixotropic collagen and hyaluronic acid bioink supplemented with gelatin nanoparticles. Bioprinting 2019;16:e00058. [Google Scholar]
- 159. Hughes CS, Postovit LM, Lajoie GA.. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 2010;10:1886–90. [DOI] [PubMed] [Google Scholar]
- 160. Kleinman HK, Martin GR.. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 2005;15:378–86. [DOI] [PubMed] [Google Scholar]
- 161. Tao FMY, Sayo K, Sugimoto K, Aoki S, Kojima N.. Development of a tunable method to generate various three-dimensional microstructures by replenishing macromolecules such as extracellular matrix components and polysaccharides. Sci Rep 2020;10:6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wang XJ, Wang QB, Xu CL.. Nanocellulose-based inks for 3D bioprinting: key aspects in research development and challenging perspectives in applications—a mini review. Bioengineering (Basel) 2020;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wu Y, Lin ZY, Wenger AC, Tam KC, Tang X.. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018;9:1–6. [Google Scholar]
- 164. Wu Y, Wenger A, Golzar H, Tang XS.. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci Rep 2020;10:20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhang X, Morits M, Jonkergouw C, Ora A, Valle-Delgado JJ, Farooq M, Ajdary R, Huan S, Linder M, Rojas O, Sipponen MH, Österberg M.. Three-dimensional printed cell culture model based on spherical colloidal lignin particles and cellulose nanofibril-alginate hydrogel. Biomacromolecules 2020;21:1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wu GH, Wu JG, Li ZH, Shi SY, Wu D, Wang XB, Xu H, Liu H, Huang YX, Wang RD, Shen J, Dong ZH, Wang SQ.. Development of digital organ-on-a-chip to assess hepatotoxicity and extracellular vesicle-based anti-liver cancer immunotherapy. Biodes Manuf 2022;5:437–50. [Google Scholar]
- 167. Heydari Z, Moeinvaziri F, Agarwal T, Pooyan P, Shpichka A, Maiti TK, Timashev P, Baharvand H, Vosough M.. Organoids: a novel modality in disease modeling. Biodes Manuf 2021;4:689–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Cuvellier M, Rose S, Ezan F, Jarry U, de Oliveira H, Bruyère A, Drieu La Rochelle C, Legagneux V, Langouët S, Baffet G.. In vitro long term differentiation and functionality of three-dimensional bioprinted primary human hepatocytes: application for in vivo engraftment. Biofabrication 2022;14:035021. [DOI] [PubMed] [Google Scholar]
- 169. Rawal P, Tripathi DM, Ramakrishna S, Kaur S.. Prospects for 3D bioprinting of organoids. Biodes Manuf 2021;4:627–40. [Google Scholar]
- 170. Janani G, Priya S, Dey S, Mandal BB.. Mimicking native liver lobule microarchitecture in vitro with parenchymal and non-parenchymal cells using 3D bioprinting for drug toxicity and drug screening applications. ACS Appl Mater Interfaces 2022;14:10167–86. [DOI] [PubMed] [Google Scholar]
- 171. Santoni S, Gugliandolo SG, Sponchioni M, Moscatelli D, Colosimo BM.. 3D bioprinting: current status and trends-a guide to the literature and industrial practice. Biodes Manuf 2022;5:14–42. [Google Scholar]
- 172. Zhang B, Gao L, Ma L, Luo YC, Yang HY, Cui ZF.. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering 2019;5:777–94. [Google Scholar]
- 173. Dellaquila A, Le Bao C, Letourneur D, Simon-Yarza T.. In vitro strategies to vascularize 3D physiologically relevant models. Adv Sci 2021;8:2100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, Yoo JJ.. Biofabrication strategies for 3D in vitro models and regenerative medicine (vol 3, pg 21, 2018). Nat Rev Mater 2018;3:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Holzl K, Lin SM, Tytgat L, Van Vlierberghe S, Gu LX, Ovsianikov A.. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016;8:032002. [DOI] [PubMed] [Google Scholar]
- 176. Arai K, Yoshida T, Okabe M, Goto M, Mir TA, Soko C, Tsukamoto Y, Akaike T, Nikaido T, Zhou K, Nakamura M.. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J Biomed Mater Res A 2017;105:1583–92. [DOI] [PubMed] [Google Scholar]
- 177. Zhang J, Chen F, He Z, Ma Y, Uchiyama K, Lin JM.. A novel approach for precisely controlled multiple cell patterning in microfluidic chips by inkjet printing and the detection of drug metabolism and diffusion. Analyst 2016;141:2940–7. [DOI] [PubMed] [Google Scholar]
- 178. Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M.. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 2018;3:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, Moises Alvarez M, Yang J, Li Y-C, Trujillo-de Santiago G, Miri AK, Zhu K, Khoshakhlagh P, Prakash G, Cheng H, Guan X, Zhong Z, Ju J, Zhu GH, Jin X, Shin SR, Dokmeci MR, Khademhosseini A.. Rapid continuous multimaterial extrusion bioprinting. Adv Mater 2017;29:1604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J.. Research on the printability of hydrogels in 3D bioprinting. Sci Rep 2016;6:29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bernal PN, Bouwmeester M, Madrid-Wolff J, Falandt M, Florczak S, Rodriguez NG, Li Y, Größbacher G, Samsom RA, van Wolferen M, van der Laan LJW, Delrot P, Loterie D, Malda J, Moser C, Spee B, Levato R.. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv Mater 2022;34:e2110054. [DOI] [PubMed] [Google Scholar]
- 182. Yu C, Ma X, Zhu W, Wang P, Miller KL, Stupin J, Koroleva-Maharajh A, Hairabedian A, Chen S.. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019;194:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. He Y, Yang FF, Zhao HM, Gao Q, Xia B, Fu JZ.. Research on the printability of hydrogels in 3D bioprinting. Sci Rep 2016;6:29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Li X, Liu B, Pei B, Chen J, Zhou D, Peng J, Zhang X, Jia W, Xu T.. Inkjet bioprinting of biomaterials. Chem Rev 2020;120:10793–833. [DOI] [PubMed] [Google Scholar]
- 185. Cooke ME, Rosenzweig DH.. The rheology of direct and suspended extrusion bioprinting. APL Bioeng 2021;5:011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, Robledo-Padilla F, Parra-Saldivar R, Hu N, Jia W, Xu C, Kang J, Hassan S, Cheng H, Hou X, Khademhosseini A, Zhang YS.. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater 2018;30:1706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Liu X, Wang X, Zhang L, Sun L, Wang H, Zhao H, Zhang Z, Liu W, Huang Y, Ji S, Zhang J, Li K, Song B, Li C, Zhang H, Li S, Wang S, Zheng X, Gu Q.. 3D liver tissue model with branched vascular networks by multimaterial bioprinting. Adv Healthc Mater 2021;10:e2101405. [DOI] [PubMed] [Google Scholar]
- 188. Sun Y, Yu K, Gao Q, He Y.. Projection-based 3D bioprinting for hydrogel scaffold manufacturing. Biodes Manuf 2022;5:633–9. [Google Scholar]
- 189. Ma X, Qu X, Zhu W, Li YS, Yuan S, Zhang H, Liu J, Wang P, Lai CS, Zanella F, Feng GS, Sheikh F, Chien S, Chen S.. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA 2016;113:2206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS.. BIOMEDICINE multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019;364:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA.. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 2016;113:3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Agarwal T, Costantini M, Maiti TK.. Recent advances in tissue engineering and anticancer modalities with photosynthetic microorganisms as potent oxygen generators. Biomed Eng Adv 2021;1:100005. [Google Scholar]
- 193. Maharjan S, Bonilla-Ruelas DP, Orive G, Zhang YS.. Photosymbiotic tissue engineering and regeneration. Prog Biomed Eng 2022;4:043001. [Google Scholar]
- 194. Maharjan S, Alva J, Camara C, Rubio AG, Hernandez D, Delavaux C, Correa E, Romo MD, Bonilla D, Santiago ML, Li WL, Cheng F, Ying GL, Zhang YS.. Symbiotic photosynthetic oxygenation within 3D-bioprinted vascularized tissues. Matter 2021;4:217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Heydari Z, Zarkesh I, Ghanian MH, Aghdaei MH, Kotova S, Zahmatkesh E, Farzaneh Z, Piryaei A, Akbarzadeh I, Shpichka A, Gramignoli R, Timashev P, Baharvand H, Vosough M.. Biofabrication of size-controlled liver microtissues incorporated with ECM-derived microparticles to prolong hepatocyte function. Biodes Manuf 2021;4:790–805. [Google Scholar]
- 196. Bonanini F, Kurek D, Previdi S, Nicolas A, Hendriks D, de Ruiter S, Meyer M, Clapes Cabrer M, Dinkelberg R, Garcia SB, Kramer B, Olivier T, Hu H, Lopez-Iglesias C, Schavemaker F, Walinga E, Dutta D, Queiroz K, Domansky K, Ronden B, Joore J, Lanz HL, Peters PJ, Trietsch SJ, Clevers H, Vulto P.. In vitro grafting of hepatic spheroids and organoids on a microfluidic vascular bed. Angiogenesis 2022;25:455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Aazmi A, Zhou HZ, Li YT, Yu MF, Xu XB, Wu YT, Ma L, Zhang B, Yang HY.. Engineered vasculature for organ-on-a-chip systems. Engineering 2022;9:131–47. [Google Scholar]
- 198. Lee H, Chae S, Kim JY, Han W, Kim J, Choi Y, Cho D-W.. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019;11:025001. [DOI] [PubMed] [Google Scholar]
- 199. Ya S, Ding W, Li S, Du K, Zhang Y, Li C, Liu J, Li F, Li P, Luo T, He L, Xu A, Gao D, Qiu B.. On-chip construction of liver lobules with self-assembled perfusable hepatic sinusoid networks. ACS Appl Mater Interfaces 2021;13:32640–52. [DOI] [PubMed] [Google Scholar]
- 200. Hassan S, Sebastian S, Maharjan S, Lesha A, Carpenter AM, Liu X, Xie X, Livermore C, Zhang YS, Zarrinpar A.. Liver-on-a-chip models of fatty liver disease. Hepatology 2020;71:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, Chalkiadaki A, Benson Chou D, Marquez S, Delahanty A, Jalili-Firoozinezhad S, Milton Y, Sontheimer-Phelps A, Swenor B, Levy O, Parker KK, Przekwas A, Ingber DE.. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng 2020;4:421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lee JB, Park JS, Shin YM, Lee DH, Yoon JK, Kim DH, Ko UH, Kim YT, Bae SH, Sung HJ.. Implantable vascularized liver chip for Cross-Validation of disease treatment with animal model. Adv Funct Mater 2019;29:1900075. [Google Scholar]
- 203. Chhabra A, Song HG, Grzelak KA, Polacheck WJ, Fleming HE, Chen CS, Bhatia SN.. A vascularized model of the human liver mimics regenerative responses. Proc Natl Acad Sci USA 2022;119:e2115867119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ma L, Barker J, Zhou CC, Li W, Zhang J, Lin BY, Foltz G, Kublbeck J, Honkakoski P.. Towards personalized medicine with a three-dimensional micro-scale perfusion-based two-chamber tissue model system. Biomaterials 2012;33:4353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Maharjan S, Bonilla D, Sindurakar P, Li HB, Li WL, Duarte S, Zarrinpar A, Zhang YS.. 3D human nonalcoholic hepatic steatosis and fibrosis models. Biodes Manuf 2021;4:157–70. [Google Scholar]
- 206. Zanger UM, Schwab M.. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103–41. [DOI] [PubMed] [Google Scholar]
- 207. Norona LM, Nguyen DG, Gerber DA, Presnell SC, Mosedale M, Watkins PB.. Bioprinted liver provides early insight into the role of kupffer cells in TGF-beta 1 and methotrexate-induced fibrogenesis. PLoS One 2019;14:e0208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ma X, Yu C, Wang P, Xu W, Wan X, Lai CSE, Liu J, Koroleva-Maharajh A, Chen S.. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018;185:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Xie F, Sun L, Pang Y, Xu G, Jin B, Xu H, Lu X, Xu Y, Du S, Wang Y, Feng S, Sang X, Zhong S, Wang X, Sun W, Zhao H, Zhang H, Yang H, Huang P, Mao Y.. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 2021;265:120416. [DOI] [PubMed] [Google Scholar]
- 210. Hiller T, Berg J, Elomaa L, Röhrs V, Ullah I, Schaar K, Dietrich A-C, Al-Zeer M, Kurtz A, Hocke A, Hippenstiel S, Fechner H, Weinhart M, Kurreck J.. Generation of a 3D liver model comprising human extracellular matrix in an alginate/Gelatin-Based bioink by extrusion bioprinting for infection and transduction studies. IJMS 2018;19:3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Schmidt K, Berg J, Roehrs V, Kurreck J, Al-Zeer MA.. 3D-bioprinted HepaRG cultures as a model for testing long term aflatoxin B1 toxicity in vitro. Toxicol Rep 2020;7:1578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zhou HZ, Liu P, Gao ZQ, Li Q, Lv WK, Yin J, Zhang B, Yang HY, Ma L.. Simultaneous multimaterial multimethod bioprinting. Biodes Manuf 2022;5:433–6. [Google Scholar]
- 213. Li Q, Jiang Z, Ma L, Yin J, Gao Z, Shen L, Yang H, Cui Z, Ye H, Zhou H.. A versatile embedding medium for freeform bioprinting with multi-crosslinking methods. Biofabrication 2022;14:035022. [DOI] [PubMed] [Google Scholar]
- 214. Li Q, Ma L, Gao Z, Yin J, Liu P, Yang H, Shen L, Zhou H.. Regulable supporting baths for embedded printing of soft biomaterials with variable stiffness. ACS Appl Mater Interfaces 2022;14:41695–711. [DOI] [PubMed] [Google Scholar]
- 215. Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA.. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv 2019;5:eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]