Abstract
OBJECTIVE
To investigate serum triglycerides in relation to all-cause, cardiovascular, and non-cardiovascular mortality in an elderly Chinese population.
METHODS
The study participants (n = 3565) were elderly (≥ 60 years) community dwellers living in a suburban town of Shanghai. Hypertriglyceridemia was defined as a serum triglycerides concentration ≥ 2.30 mmol/L (definite) and ≥ 1.70 mmol/L (borderline), respectively.
RESULTS
The prevalence of definite and borderline hypertriglyceridemia at baseline was 7.5% and 29.5%, respectively. It was higher in women (n = 1982, 9.0% and 33.8%, respectively) than men (n = 1583, 6.2% and 27.9%, respectively), in obese and overweight participants (n = 1566, 10.5% and 36.4%, respectively) than normal weight participants (n = 1999, 5.6% and 27.1%, respectively), and in diabetic participants (n = 177, 11.9% and 39.0%, respectively) than non-diabetic participants (n = 3388, 7.5% and 30.8%, respectively). During a median of 7.9 years follow-up, all-cause, cardiovascular and non-cardiovascular deaths occurred in 529, 216 and 313 participants, respectively. In analyses according to the quintile distributions of serum triglycerides concentration, the sex- and age-standardized mortality rate was lowest in the middle quintile for all-cause, cardiovascular and non-cardiovascular mortality (18.6, 7.8 and 11.9 per 1000 person-years, respectively, versus 21.5, 10.5 and 12.7 per 1000 person-years, respectively, in the two lower quintiles and 21.7, 9.5 and 14.0 per 1000 person-years, respectively, in the two higher quintiles). The fully adjusted hazard ratios (95% CI) for the middle quintile versus the combined two lower with two higher quintiles were 0.85 (95% CI: 0.67–1.07, P = 0.17), 0.81 (95% CI: 0.54–1.19, P = 0.28) and 0.87 (95% CI: 0.64–1.17, P = 0.35) for all-cause, cardiovascular and non-cardiovascular mortality, respectively.
CONCLUSIONS
Our study showed high prevalence of hypertriglyceridemia, especially when defined as borderline and in obese and overweight participants, and mildly but non-significantly elevated risks of cardiovascular mortality relative to the middle level of serum triglycerides.
Serum triglycerides as a cardiovascular risk factor is revived, with the success of a recent trial on an efficacious triglycerides lowering agent, icosapent ethyl, a highly purified eicosapentaenoic acid ethyl ester.[1] This fish oil product orally taken twice daily significantly reduced serum triglycerides concentration by 0.44 mmol/L and the risk of fatal and non-fatal ischaemic cardiovascular events by 25%.[1] However, there is no consensus on the role of hypertriglyceridemia in the risk and prevention of cardiovascular disease (CVD) and mortality, not only with regard to the fish oil intake,[2] but also the use of fibrates.[3] In fact, even observational studies produced conflicting results.[4–8] Some,[4,5] but not other,[6–8] studies demonstrated significant association of serum triglycerides or hypertriglyceridemia with the risk of CVD.
With the recent changes in lifestyle in the Chinese population, the prevalence of dyslipidemia, especially hypertriglyceridemia, increased substantially.[9,10] According to a series of China national surveys in 2002, 2012 and 2015, serum triglycerides concentration increased from 1.12 mmol/L to 1.41 mmol/L and to 1.47 mmol/L, respectively; and the prevalence of hypertriglyceridemia (serum triglycerides ≥ 2.26 mmol/L) increased from 5.7% to 13.6% and to 15.0%, respectively.[9] The health consequences of this emerging epidemic for the Chinese population, however, is uncertain.
We recently performed an elderly population-based study in a suburban town of Shanghai. In the present analysis, we investigated the association between serum triglycerides and all-cause, cardiovascular, and non-cardiovascular mortality in this elderly Chinese population sample.
METHODS
Study Population
Our study was conducted in the framework of the Chronic Disease Detection and Management in the Elderly (≥ 60 years of age) Program supported by the municipal government of Shanghai.[11–14] In a newly urbanized suburban town 30 kilometers from the city centre, we invited all residents of at least 60 years of age to participate in comprehensive examinations of CVD and risk. The Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine approved the study protocol. All study participants provided written informed consent.
A total of 3830 subjects (participation rate: 90%) were enrolled in the period from 2006 to 2011 and followed up for vital status and cause of death till June 2015. Of them, 261 participants were excluded from the present analysis, because of extreme (≥ 10.0 mmol/L, n = 17) or missing values of serum triglycerides (n = 248), leaving 3565 participants in the analysis.
Serum Lipids and Other Biochemical Measurements and Definition of Dyslipidemia and Diabetes Mellitus
Venous blood samples were drawn after overnight fasting for the measurement of serum triglycerides, total cholesterol and plasma glucose. Serum lipids were measured using the enzymatic method. Dyslipidemia was defined according to the 2016 Chinese guideline for the management of dyslipidemia in adults.[15] Hypertriglyceridemia was defined as a serum triglycerides concentration ≥ 2.30 mmol/L (definite) and ≥ 1.70 mmol/L (borderline), respectively; and hypercholesterolemia as a serum total cholesterol concentration ≥ 6.20 mmol/L. Diabetes mellitus (DM) was defined as a plasma glucose level of at least 7.0 mmol/L, while fasting or 11.1 mmol/L at any time or use of antidiabetic agents.
Blood Pressure and Anthropometry
One experienced physician measured each participant’s blood pressure (BP) three times consecutively using a validated Omron 7051 oscillometric BP monitor (Omron, Kyoto, Japan), after the subjects had rested for at least 5 min in the sitting position. These three BP readings were averaged for analysis. Hypertension was defined as a sitting BP of at least systolic BP with 140 mmHg or diastolic BP with 90 mmHg or use of antihypertensive drugs.
A trained technician performed anthropometric measurements, including body height and body weight. Body mass index (BMI) was calculated as the body weight in kilograms divided by the body height in meters squared. Obesity and overweight were defined as a BMI ≥ 28 kg/m² and of 24–27.9 kg/m², respectively.[16]
Follow-up
Information on vital status and the cause of death was obtained from the official death certificate, with further confirmation by the local Community Health Centre and family members of the deceased people. The International Classification of Diseases 10th Revision was used to classify the cause of death. Cardiovascular mortality included deaths attributable to stroke, myocardial infarction, and other CVDs. Deaths other than cardiovascular reasons were considered as non-cardiovascular mortality.
Statistical Analysis
Statistical analysis was performed using the SAS 9.4 (SAS Institute, Cary, NC, USA). Means and proportions were compared by the unpaired Student’s t-test and the Pearson’s chi-squared test, respectively. Continuous measurements with skewed distribution were expressed as median (interquartile range). Logistic regression analysis was performed to analyze the associated factors of hypertriglyceridemia. The sex- and age-standardized mortality rate was calculated using the direct method. Multivariable Cox regression analysis was performed to compute adjusted hazard ratios (95% CI) for mortality according to the quintile distributions of serum triglycerides concentration. The cutoffs for the quintile distributions of serum triglyceride concentration were 1.15 mmol/L, 1.50 mmol/L, 1.65 mmol/L and 1.88 mmol/L, respectively.
RESULTS
Characteristics of the Study Participants
The study participants included 1583 men and 1982 women. Men and women significantly differed (P ≤ 0.05) in most of the characteristics at baseline, except for systolic BP, the prevalence of hypertension and DM and serum creatinine. None of the study participants were treated for hypertriglyceridemia or with a statin (P ≥ 0.14, Table 1).
Table 1. Characteristics of the study population at baseline.
| Characteristics | Men (n = 1583) | Women (n = 1982) | P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). Preexisting cardiovascular disease included myocardial infarction, stroke and cardiac failure. | |||
| Age, yrs | 68.2 ± 7.0 | 69.1 ± 7.4 | 0.0004 |
| Body mass index, kg/m2 | 23.5 ± 3.5 | 23.8 ± 3.7 | 0.053 |
| Systolic blood pressure, mmHg | 138.3 ± 19.6 | 139.0 ± 20.1 | 0.36 |
| Diastolic blood pressure, mmHg | 81.9 ± 10.8 | 80.5 ± 10.6 | < 0.0001 |
| Pulse rate, beat/min | 74.7 ± 11.9 | 78.1 ± 11.5 | < 0.0001 |
| Hypertension | 713 (45.0%) | 921 (46.5%) | 0.40 |
| Use of antihypertensive drugs | 600 (37.9%) | 806 (40.7%) | 0.052 |
| Diabetes mellitus | 69 (4.4%) | 108 (5.5%) | 0.14 |
| Use of antidiabetic drugs | 27 (1.7%) | 26 (1.3%) | 0.03 |
| Current smoking | 871 (55.0%) | 48 (2.4%) | < 0.0001 |
| Alcohol intake | 579 (36.6%) | 29 (1.5%) | < 0.0001 |
| Preexisting cardiovascular disease | 20 (1.3%) | 14 (0.7%) | 0.09 |
| Blood biochemistry | |||
| Plasma fasting glucose, mmol/L | 5.1 (4.7–5.7)* | 5.3 (4.8–5.8)* | < 0.0001 |
| Serum total cholesterol, mmol/L | 5.5 ± 1.4 | 5.7 ± 1.4 | 0.006 |
| Serum triglycerides, mmol/L | 1.56 (1.19–1.73)* | 1.60 (1.31–1.80)* | < 0.0001 |
| Serum creatinine, μmol/L | 91.2 (79.0–105.8)* | 91.5 (76.9–106.1)* | 0.16 |
| Serum uric acid, μmol/L | 271 (221–340)* | 259 (218–320)* | < 0.0001 |
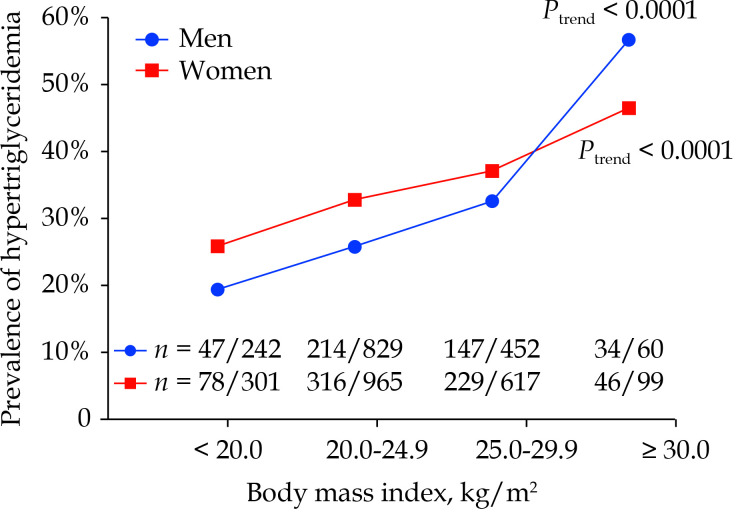
Serum triglycerides concentration was significantly lower in men than women (1.56 mmol/L vs. 1.60 mmol/L, P < 0.0001). The prevalence of hypertriglyceridemia also significantly differed (P ≤ 0.002) between men and women for both definite (6.2% vs. 9.0%) and borderline (27.9% vs. 33.8%). In addition, the prevalence of hypertriglyceridemia was higher with increasing BMI from the participants with a BMI < 20 kg/m², to 20–25 kg/m², to 25–30 kg/m² and to ≥ 30 kg/m² in men (19.4%, 25.8%, 32.5% and 56.7%, respectively; Ptrend < 0.0001) as well as women (25.9%, 32.8%, 37.1% and 46.5%, respectively; Ptrend < 0.0001; Figure 1).
Figure 1.
Prevalence of fasting hypertriglyceridemia (≥ 1.7 mmol/L) by sex and body mass index.
The number of patients with hypertriglyceridemia/participants is given for each group at the top and for each body mass index subgroup at the bottom of the figure. The total number of participants and the P-value for trend are also given for men and women separately.
Associated Factors of Serum Triglycerides Concentration
The multivariable stepwise logistic regression analyses identified gender, age, BMI, DM and alcohol intake as associated factors of definite hypertriglyceridemia (P ≤ 0.09) and additionally the presence of hypertension as an associated factor of borderline hypertriglyceridemia (P = 0.14, Table 2). As the strongest determinant, BMI alone explained 2.2% of the variance for the prevalence of definite and borderline hypertriglyceridemia.
Table 2. Logistic regression analysis on the associated factors of hypertriglyceridemia.
| Serum triglycerides ≥ 1.7 mmol/L | Serum triglycerides ≥ 2.3 mmol/L | ||||
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | ||
| In a logistic regression model, we forced sex and age and considered body mass index, hypertension, diabetes mellitus, current smoking and alcohol intake for entry and stay at a significance of P ≤ 0.15. | |||||
| Female | 1.41 (1.19–1.66) | < 0.0001 | 1.61 (1.19–2.19) | 0.002 | |
| Age, yrs | 1.04 (0.92–1.16) | 0.55 | 1.54 (1.28–1.85) | < 0.0001 | |
| Body mass index, kg/m2 | 1.07 (1.05–1.09) | < 0.0001 | 1.13 (1.09–1.17) | < 0.0001 | |
| Diabetes mellitus | 1.30 (0.94–1.78) | 0.11 | 1.52 (0.94–2.47) | 0.09 | |
| Alcohol intake | 0.78 (0.63–0.97) | 0.03 | 0.65 (0.44–0.96) | 0.03 | |
| Hypertension | 1.12 (0.96–1.30) | 0.14 | – | – | |
Associated Between Serum Triglycerides Concentration and Mortality
During a median follow-up of 7.9 years (interquartile range: 6.9–8.8 years), the cumulated number of person-years was 25,979, and all-cause, cardiovascular and non-cardiovascular deaths occurred in 529, 216 and 313 participants, respectively. The corresponding incidence rates were 20.4, 8.3 and 12.1 per 1000 person-years, respectively.
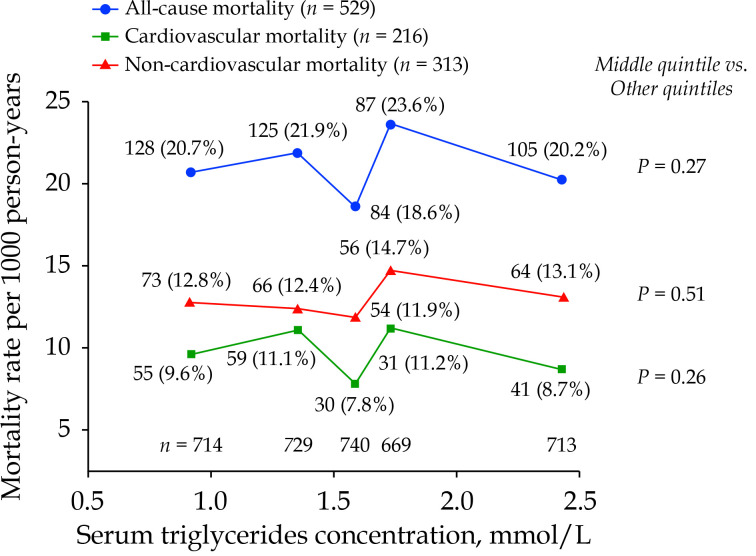
Analyses according to the quintile distributions of serum triglycerides concentration showed that the sex- and age-standardized mortality rate was lowest in the middle quintile for all-cause, cardiovascular and non-cardiovascular mortality (18.6, 7.8 and 11.9 per 1000 person-years, respectively, versus 21.5, 10.5 and 12.7 per 1000 person-years, respectively, in the two lower quintiles and 21.7, 9.5 and 14.0 per 1000 person-years, respectively, in the two higher quintiles; Figure 2).
Figure 2.
Sex- and age-standardized mortality rate according to the quintile distributions of fasting serum triglycerides concentration.
The age and sex-standardized mortality rates were calculated for all-cause, cardiovascular, and non-cardiovascular mortality per 1000 person-years. The number of participants is given for each quintile at the bottom of the figure. The number of deaths (rate per 1000 person-years) is given for each quintile alongside the symbols. The P-value for the middle quintile versus all the other quintiles is also given.
After adjustment for the abovementioned identified associated factors and preexisting CVD, the adjusted hazard ratios (95% CI) for mortality with the middle quintile of serum triglycerides concentration at baseline as reference were not statistically significant for either the two lower or two higher quintiles (P ≥ 0.07, Table 3). The adjusted hazard ratios (95% CI) for the middle quintile versus the combined two lower with two higher quintiles were 0.85 (95% CI: 0.67–1.07, P = 0.17), 0.81 (95% CI: 0.54–1.19, P = 0.28) and 0.87 (95% CI: 0.64–1.17, P = 0.35) for all-cause, cardiovascular and non-cardiovascular mortality, respectively.
Table 3. Adjusted hazard ratios (95% CI) for mortality according to quintile distributions of serum triglycerides concentration at baseline.
| Serum triglycerides concentration, mmol/L | |||||
| Quintile 1 (0.09–1.15) |
Quintile 2 (1.16–1.50) |
Quintile 3 (1.51–1.65) |
Quintile 4 (1.66–1.88) |
Quintile 5 (1.89–9.98) |
|
| Adjusted for age, sex, body mass index, hypertension, diabetes mellitus, preexisting cardiovascular disease, current smoking and alcohol intake, and serum total cholesterol. | |||||
| All-cause mortality | |||||
| All participants | 1.14 (0.85–1.52) | 1.20 (0.91–1.60) | Reference | 1.22 (0.91–1.65) | 1.16 (0.87–1.56) |
| Men | 1.35 (0.90–2.02) | 1.28 (0.85–1.91) | Reference | 1.47 (0.97–2.22) | 1.34 (0.88–2.03) |
| Women | 0.92 (0.60–1.42) | 1.11 (0.74–1.65) | Reference | 0.97 (0.63–1.50) | 0.96 (0.64–1.45) |
| Cardiovascular mortality | |||||
| All participants | 1.13 (0.71–1.80) | 1.44 (0.92–2.25) | Reference | 1.37 (0.83–2.27) | 1.22 (0.76–1.97) |
| Men | 1.31 (0.69–2.49) | 1.41 (0.74–2.67) | Reference | 1.67 (0.85–3.28) | 1.15 (0.58–2.30) |
| Women | 0.91 (0.45–1.83) | 1.42 (0.76–2.69) | Reference | 1.04 (0.49–2.22) | 1.13 (0.58–2.22) |
| Cardiovascular mortality | |||||
| All participants | 1.18 (0.81–1.71) | 1.10 (0.76–1.58) | Reference | 1.22 (0.84–1.77) | 1.16 (0.80–1.67) |
| Men | 1.40 (0.83–2.35) | 1.26 (0.75–2.12) | Reference | 1.47 (0.87–2.48) | 1.47 (0.87–2.48) |
| Women | 0.92 (0.53–1.60) | 0.90 (0.53–1.52) | Reference | 0.97 (0.57–1.65) | 0.88 (0.52–1.47) |
DISCUSSION
Our study in elderly Chinese showed high prevalence of hypertriglyceridemia, especially defined as borderline and in those obese and overweight participants. Hypertriglyceridemia, in general, did not confer risk of mortality. However, middle levels of serum triglycerides concentration trended to be associated with a slightly but non-significantly lower risk of cardiovascular mortality than those with hyper- or hypotriglyceridemia.
The prevalence of definite hypertriglyceridemia in our elderly Chinese population is similar to that was reported in the third chronic non-communicable disease and risk factor surveillance in China in 2010.[17] Indeed, among the surveillant population over 60 years of age (n = 19,973), the prevalence of hypertriglyceridemia (serum triglycerides ≥ 2.26 mmol/L) was 10.8%. It was higher (P < 0.01) in women (n = 10,457, 12.9%) than men (n = 9516, 8.7%) and in urban (n = 8290, 12.9%) than rural areas (n = 11,683, 10.1%).[17] If the borderline hypertriglyceridemia is compared between our study and studies in the Korean and American surveys in those over 60 years of age, the prevalence is also quite similar. In men (n = 12,417) and women (n = 9091) over 60 years of age, who were enrolled in the Korea National Health and Nutrition Examination Survey in 2013–2015, the prevalence of borderline hypertriglyceridemia was 31.5% and 27.0%, respectively.[18] Similarly, in men (n = 1509) and women (n = 1499) over 60 years of age, who were enrolled in the National Health and Nutrition Examination Survey in 2009–2012, the prevalence of hypertriglyceridemia (triglycerides ≥ 1.7 mmol/L) was 24.8% and 30.9%, respectively.[19]
Our observation on the association between hypertriglyceridemia and BMI suggests that calorie intake might have contributed to the increase in the prevalence of these two related metabolic disorders. Similar associations were observed in the recent chronic non-communicable disease and risk factor surveillance in China.[20] The odds ratio for obese participants (n = 6835) versus normal weight participants (n = 44,943) was 1.97 (95% CI: 1.80–1.97, P < 0.0001) for any dyslipidemia in elderly Chinese.[20] There is also evidence that dietary interventions for weight loss may reduce serum triglycerides concentration. Indeed, in a randomized controlled trial in 811 overweight participants, dietary weight loss interventions for two years reduced body weight by 4 kg and serum triglyceride concentration by 12%–17%.[21]
Hypertriglyceridemia has never been properly established as a cardiovascular risk factor. In some,[22,23] but not many other,[24,25] studies, serum triglycerides concentration was associated with clinical outcomes. Nonetheless, there is some observational evidence that hypertriglyceridemia may be associated with higher risks of cardiovascular events in low risk populations,[26] or in longer term follow-up studies.[27] In an Italian low risk population followed up for 3.2 years, the odds ratios (95% CI) for serum triglycerides concentration of 150–500 mg/dL versus < 150 mg/dL was 1.49 (95% CI: 1.36–1.63, P < 0.001) for all-cause mortality and 1.61 (95% CI: 1.43–1.82, P < 0.001) for cardiovascular events.[26] In a much longer term follow-up (mean: 19 years) study, initiated in the 1970s, the incidence of cardiovascular events increased from 4.09% in quartile 1 to 5.37%, 6.68% and 10.68% in quartiles 2, 3 and 4, respectively. The adjusted hazard ratio for quartile 4 versus quartile 1 was 1.40 (95% CI: 1.07–1.82, P = 0.004).[27]
There is also some interventional evidence that serum triglycerides lowering may be beneficial in cardiovascular prevention. In diabetic patients in the Action to Control Cardiovascular Risk in Diabetes study, reducing serum triglycerides concentration with fenofibrate did not reduce the risk of non-fatal myocardial infarction and stroke and cardiovascular mortality. However, in patients with a serum triglycerides concentration ≥ 204 mg/dL and serum high-density lipoprotein cholesterol ≤ 34 mg/dL, fenofibrate reduced the incidence of cardiovascular events (12.4% vs. 17.3% on placebo, P = 0.057).[28] In the recently published trial on serum triglycerides lowering with a highly purified eicosapentaenoic acid ethyl ester icosapent ethyl, serum triglycerides concentration was reduced by 0.44 mmol/L and the risk of fatal and non-fatal ischaemic cardiovascular events by 25%.[1]
The lower risk of cardiovascular mortality in the middle quintile is incompletely understood. Our study was population-based, and did not apply any exclusion criteria. Those with lower serum triglycerides may have other unmeasured risk factors for CVD.
LIMITATIONS
Our study should be interpreted within the context of its limitations. Firstly, our study had a relatively small sample size, few measurements on serum lipids and limited information on socioeconomic status and lifestyle. Secondly, we collected sufficient information on fatal but not non-fatal cardiovascular events. We were unable to perform analysis about the association between serum triglycerides and the risk of fatal combined with non-fatal cardiovascular events. Last but not least, our study was conducted in an elderly Chinese population living in the suburban town of Shanghai. The results of our study probably cannot be extrapolated to younger populations, other ethnic groups, or even Chinese people with a different diet.
CONCLUSIONS
Our study showed high prevalence of hypertriglyceridemia in elderly Chinese, especially when defined as borderline and in those obese and overweight participants, and mildly but non-significantly elevated risks of cardiovascular mortality relative to the middle level of serum triglycerides. With the substantial increase in the prevalence of hypertriglyceridemia and in the availability of triglycerides lowering treatment, the health consequences of hypertriglyceridemia need to be further addressed in future observational and interventional studies.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No.91639203 & No.82070435), the Ministry of Health (2016YFC0900902), the Shanghai Commissions of Science and Technology (19DZ2340200), the Clinical Research Program, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (2018CR010), and the Shanghai Commissions of Health (“Three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System” GWV-10.1-XK05 and a special grant for “leading academics”). All authors had no conflicts of interest to disclose.
References
- 1.Bhatt DL, Steg PG, Miller M, et al Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Lincoff AM, Garcia M, et al Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keech A, Simes RJ, Barter P, et al Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Varbo A Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 5.Puri R, Nissen SE, Shao M, et al Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36:2220–2228. doi: 10.1161/ATVBAHA.116.307601. [DOI] [PubMed] [Google Scholar]
- 6.Di Angelantonio E, Sarwar N, Perry P, et al Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal S, Buring JE, Rifai N, et al Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Nordestgaard BG, Benn M, Schnohr P, et al Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 9.Song PK, Man QQ, Li H, et al Trends in lipids level and dyslipidemia among Chinese adults, 2002–2015. Biomed Environ Sci. 2019;32:559–570. doi: 10.3967/bes2019.074. [DOI] [PubMed] [Google Scholar]
- 10.Li JH, Wang LM, Li YC, et al [Epidemiologic characteristics of dyslipidemia in Chinese adults 2010] Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:414–418. doi: 10.3760/cma.j.issn.0253-9624.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Sheng CS, Li Y, Huang QF, et al Pulse waves in the lower extremities as a diagnostic tool of peripheral arterial disease and predictor of mortality in elderly Chinese. Hypertension. 2016;67:527–534. doi: 10.1161/HYPERTENSIONAHA.115.06666. [DOI] [PubMed] [Google Scholar]
- 12.Sheng CS, Li Y, Li LH, et al Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124–1130. doi: 10.1161/HYPERTENSIONAHA.114.04063. [DOI] [PubMed] [Google Scholar]
- 13.Sheng CS, Liu M, Zeng WF, et al Four-limb blood pressure as predictors of mortality in elderly Chinese. Hypertension. 2013;61:1155–1160. doi: 10.1161/HYPERTENSIONAHA.111.00969. [DOI] [PubMed] [Google Scholar]
- 14.Sheng CS, Liu M, Kang YY, et al Prevalence, awareness, treatment and control of hypertension in elderly Chinese. Hypertens Res. 2013;36:824–828. doi: 10.1038/hr.2013.57. [DOI] [PubMed] [Google Scholar]
- 15.Joint Committee for Guideline Revision 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang M, Zhao Z, et al Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 national chronic disease and risk factor surveillance. Ann Intern Med. 2020;172:291–293. doi: 10.7326/M19-0477. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZH, Wang LH, Li YC, et al [Current status of diabetes, hypertension and dyslipidemia among older Chinese adults in 2010] Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:922–926. doi: 10.3760/cma.j.issn.0253-9624.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Kwon YJ, Lee JW, Kang HT Secular trends in lipid profiles in Korean adults based on the 2005–2015 KNHANES. Int J Environ Res Public Health. 2019;16:2555. doi: 10.3390/ijerph16142555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll M, Kit B, Lacher D Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS Data Brief. 2015;198:198. [PubMed] [Google Scholar]
- 20.Qi SG, Wang ZH, Li ZX, et al [The epidemiological characteristics of obesity among the Chinese elderly population and its attributable fractions for chronic diseases] Chin J Geriatr. 2018;37:919–923. doi: 10.3760/cma.j.issn.0254-9026.2018.08.021. [DOI] [Google Scholar]
- 21.Sacks FM, Bray GA, Carey VJ, et al Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsberg HN, Elam MB, Lovato LC, et al Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M, Cannon CP, Murphy SA Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Boden WE, Probstfield JL, Anderson T, et al Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MH, Rosenson RS, Maki KC, et al Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST) Arterioscler Thromb Vasc Biol. 2014;34:1298–1306. doi: 10.1161/ATVBAHA.113.302926. [DOI] [PubMed] [Google Scholar]
- 26.Arca M, Veronesi C, D’Erasmo L, et al Association of hypertriglyceridemia with all-cause mortality and atherosclerotic cardiovascular events in a low-risk Italian population: the TG-REAL retrospective cohort analysis. J Am Heart Assoc. 2020;9:e015801. doi: 10.1161/JAHA.119.015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu HH, Wu Y, Li Y, et al [Relationship between serum triglyceride and the risk of atherosclerotic cardiovascular disease: a prospective study] Chin Circ J. 2019;34:122–127. doi: 10.3969/j.issn.1000-3614.2019.02.003. [DOI] [Google Scholar]
- 28.Ginsberg HN, Elam MB, Lovato LC, et al Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]