Abstract
Liver transplantation (LT) is the standard of care for end-stage liver failure and hepatocellular carcinoma. Over the years, immunosuppression regimens have improved, resulting in enhanced graft and patient survival. At present, the side effects of immunosuppressive agents are a significant threat to post-LT quality of life and long-term outcome. The role of personalized immunosuppression is to reach a delicate balance between optimal immunosuppression and minimal side effects. Today, immunosuppression in LT is more of an art than a science. There are no validated markers for overimmunosuppression and underimmunosuppression, only a few drugs have therapeutic drug monitoring and immunosuppression regimens vary from center to center. The immunosuppressive agents are broadly classified into biological agents and pharmacological agents. Most regimens use multiple agents with different modes of action to reduce the dosage and minimize the toxicities. The calcineurin inhibitor (CNI)–related toxicities are reduced by antibody induction or using mTOR inhibitor/antimetabolites as CNI sparing or CNI minimization strategies. Post-liver transplant immunosuppression has an intensive phase in the first three months when alloreactivity is high, followed by a maintenance phase when immunosuppression minimization protocols are implemented. Over time some patients achieve “tolerance,” defined as the successful stopping of immunosuppression with good graft function and no indication of rejection. Cell-based therapy using immune cells with tolerogenic potential is the future and may permit complete withdrawal of immunosuppressive agents.
Keywords: immunosuppression, liver transplantation, calcineurin inhibitors, everolimus, antimetabolites, basiliximab, tacrolimus, cyclosporine, mycophenolate mofetil
Abbreviations: AMR, Antibody-mediated rejection; APCs, Antigen-presenting cells; ATG, Anti-thymocyte globulin; CNI, Calcineurin inhibitors; CsA, Cyclosporine A; EVR, Everolimus; IL-2R, Interleukin 2 Receptor; LT, Liver transplantation; MMF, Mycophenolate mofetil; MPA, Mycophenolic acid; mTORi, mammalian targets of rapamycin inhibitor; SRL, Sirolimus; TAC, Tacrolimus; TCMR, T-cell-mediated rejection
Liver transplantation (LT) is the standard of care for end-stage liver failure and hepatocellular carcinoma (HCC).1 Post-liver transplant patient and graft survival has improved in the last 50 years due to improved surgical techniques, better perioperative care and increased efficacy of immunosuppressive drugs.1 Data from United Network for Organ Sharing (UNOS) database and liver registry with Organ Procurement and Transplantation Network (OPTN) showed that 1-year survival post-liver transplant improved from 66% in 1986 to 92% in 2015.2 At the same time, post-LT long-term survival has not improved mainly due to increased incidence of immunosuppression-related metabolic side effects, opportunistic infections, and malignancies.2
In the 1960s, corticosteroids and azathioprine (AZA) were the only immunosuppressive agents available for use in LT.3 This was followed by the introduction of cyclosporine (1983), tacrolimus (TAC) (1994), mycophenolate mofetil (MMF) (1995), anti-thymocyte globulin (ATG) (1998), basiliximab (1998), sirolimus (SRL) (1999), mycophenolate sodium (2004), and everolimus (EVR) (2010).3 The improved efficacy of immunosuppressive regimens came at the cost of long-term side effects. In long-term survivors of LT, mortality due to rejection was only 1.7%.2 Immunosuppression-related malignancy (16.4%) and opportunistic infections (10.5%) were the leading cause of death in long-term survivors.2 More potent immunosuppressive drugs also led to increased metabolic disorders, cardiovascular events, and renal dysfunction, with chronic kidney disease seen in 20% of patients surviving more than 5-years post-liver transplant.4
The aim of immunosuppression in LT is to prevent the host immune system from rejecting the allograft, at the same time, preserve the immune control over neoplasia and infections. Current immunosuppressive regimens in liver transplants use various combinations of calcineurin inhibitors (CNIs), corticosteroids, molecular target of rapamycin (mTOR) inhibitors, antimetabolites, and biological agents. The combination protocols permit the use of drugs at lower doses without increasing the risk for allograft rejection and concurrently reducing the toxicity of individual agents. These regimens allow a delicate balance between optimal immunosuppression and minimal toxicity. Each regimen is individualized, taking into account the patient's preoperative and perioperative risk profile and changed according to its efficacy, toxicity, and time from transplant.5
Immunology of liver transplantation and allograft rejection
The liver is an immune-privileged organ with less rejection rates than other solid organs.5 Unlike other organs, human leukocyte antigen (HLA) typing is not done in LT as matching does not affect post-LT outcomes.6 The possible explanations for the immune privilege are (1) transfer of passenger donor immune cells to the recipient, establishing a form of microchimerism, (2) production of soluble donor MHC class I molecules by the liver that block preformed antibodies in LT recipients and inhibit T-cell activation, and (3) resistance of liver to damage by rejection by its sheer size and regenerative capacity.7 However, LT recipients experience immunological rejection and requires long-term immunosuppressive medication, but at a much lower dose. With time, alloreactivity in LT recipients declines, and patients acquire “tolerance,” defined as the successful cessation of immunosuppression while maintaining graft function and avoiding rejection.8 Here, immune responses to the allograft occur but are kept in check by suppressive mechanisms, hence the term “operational tolerance.”8
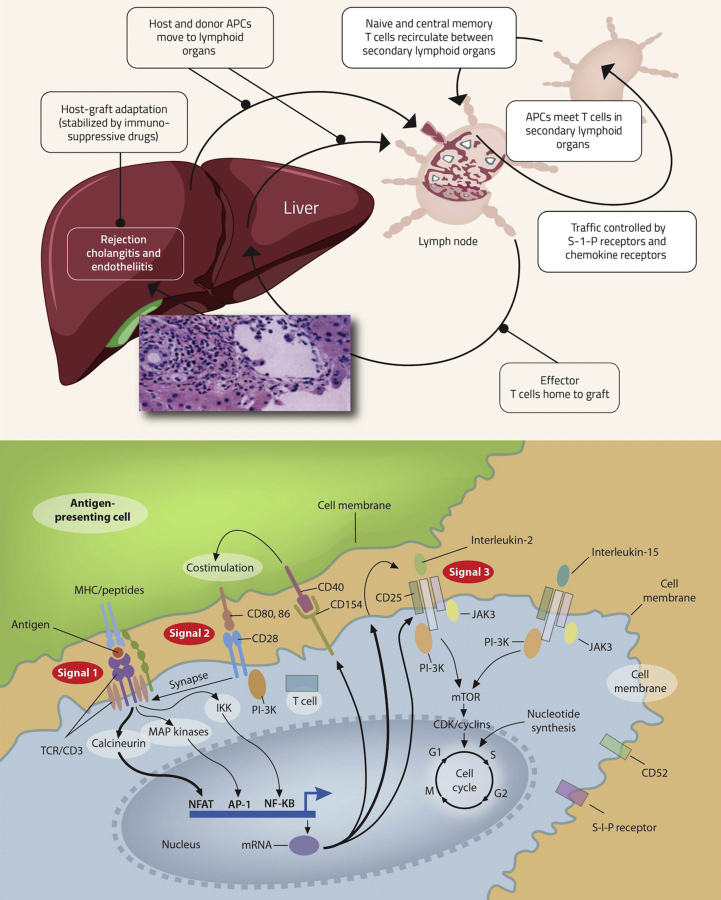
Hepatic allograft rejection is due to an adaptive immune response triggered by the mismatch of a major histocompatibility complex (MHC) between the donor and the recipient and innate responses initiated by surgical trauma and organ reperfusion injury.9 Interactions with foreign antigens activate antigen-presenting cells (APCs) from both the donor and the recipient present in the hepatic allograft.10 These activated antigen-bearing cells move to secondary lymphoid organs where they engage with T-cell receptors (TCRs; CD3 complex) on alloantigen reactive naive T-cells and memory cells to activate lymphocytes (Figure-1). This interaction between donor peptides presented by MHC molecules on the APCs and TCRs (CD3 complex) on alloantigen reactive naive T-cells is called signal-1.10 When CD80 and CD86 receptors on the APCs interact with CD28 receptors on T lymphocytes, co-stimulation or signal-2 for T-cell activation occurs.11 The signal-1 and signal-2 activate the transcription factors nuclear factor of activated T-cells, activating protein 1 (AP-1), and nuclear factor-kB (NF-kB) via three signaling pathways: calcium–calcineurin, mitogen-activated protein kinase, and protein kinase C–nuclear factor-kB (NF-kB), which in turn promotes transcription of CD154 (which further activates APCs), interleukin-2 receptor alpha chain (CD25), and interleukin-2. Interleukin-2 (IL-2) along with interleukin-15 (IL-15) initiate the cell replication cycle via growth signals (signal-3) through the phosphoinositide-3-kinase (PI-3K) pathway and the mTOR pathway. Lymphocytes require the synthesis of purine and pyrimidine nucleotides for replication. It is regulated by inosine monophosphate dehydrogenase (IMPDH) and dihydroorotate dehydrogenase, respectively. Lymphocyte proliferation generations large numbers of effector T- and B-cells in lymphoid organs, which migrate to the hepatic allograft and initiate an inflammatory response leading to allograft destruction.12 The understanding of these pathways helped develop newer drugs used in current immunosuppression regimens.
Figure-1.
A- Pathway of immune activation of the host by donor antigens processed through host antigen-presenting cell (APC) (indirect antigen presentation) and/or donor antigens present on donor APC (direct antigen presentation). Host and donor APCs migrates to a lymphoid organ (spleen or lymph node), whereby recipient T-cells are activated and they traffic back to the allograft. Cell adhesion receptors signal circulating alloreactive T-cells to bind and infiltrate the graft where targets of alloimmunity include the biliary epithelium and endothelial cells, causing bile duct injury and endothelitis, hallmarks of acute rejection. B- Model of three signal T-cell activation (adapted with permission from Halloran et al13 and Wiesner RH et al15).
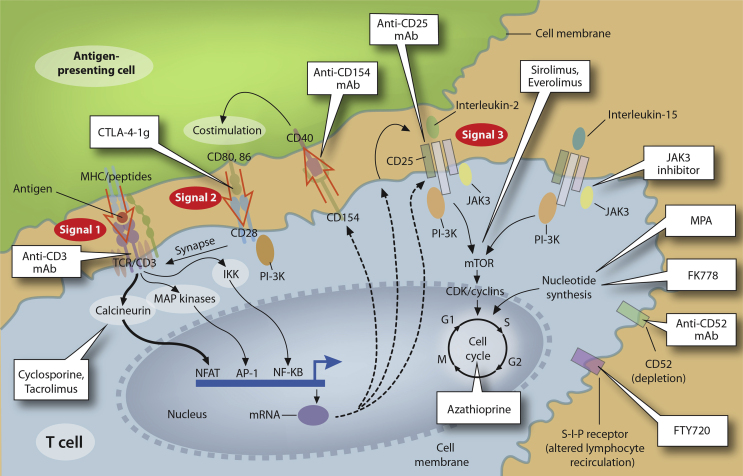
The various immunosuppressive drugs act by inhibiting small molecules downstream of signals 1, 2, and 3 (Table 1). CNIs bind to intracellular small molecules, “cyclophilin” for cyclosporine A (CsA) and “FK506-binding protein (FKBP12)” for TAC and inhibit calcium/calmodulin-dependent phosphatase, “calcineurin.”40 The calcineurin inhibition prevents IL-2 gene transcription, which inturn inhibits T-cell activation and proliferation. mTOR inhibitors bind intracellularly to FK506-binding protein (FKBP12) but inhibit the mammalian target of rapamycin complex instead of calcineurin.57,58,59 It, thus, blocks signal-3 of T-cell activation, thereby inhibiting IL-2 and IL-15 induced T-cell proliferation. The antimetabolite group of drugs inhibit T-cell proliferation by reversible inhibition of purine synthesis. MMF and its active metabolite, mycophenolic acid (MPA), have additional immunomodulatory actions. Corticosteroids have anti-inflammatory and immunomodulatory action by interacting with antigen-presenting dendritic cells, modulating IL-1 transcription, decreasing the number of circulating CD4+ T-cells, and inhibiting IL-1-dependent lymphocyte activation. The various biological agents act by either depleting T-cells and B-cells or inhibiting their proliferation by binding to respective receptors.
Table 1.
Classification and Action of Immunosuppressants.
| Immunosuppressant | Action |
|---|---|
| T-cell activation inhibitors | |
| Cyclosporine | Inhibits calcineurin via cyclophilin, blocking IL2 transcription |
| Tacrolimus | Inhibits calcineurin via FKBP12, blocking IL2 transcription |
| Belatacept | CTLA-4 homologue competing with CD28 for CD80/86 binding, inhibiting T-cell co-stimulation |
| T-cell depletion | |
| Anti-thymocyte globulin | Antibody preparation directed against lymphocytes |
| Alemtuzumab | Anti-CD52-specific antibody highly depletive of lymphocytes, as well as NK cells, monocytes and thymocytes |
| Muromonab-CD3 (OKT3) | Anti-CD3-specific antibody causing T-cell depletion |
| T-cell proliferation inhibitors | |
| MPA prodrugs | IMPDH inhibitor: enzyme required for de novo synthesis of guanosine nucleotides, required for lymphocyte proliferation |
| mTOR inhibitors | mTOR blockade prevents IL2-induced T-cell proliferation |
| Azathioprine | Inhibits purine synthesis, thereby blocking immune cell proliferation |
| IL2 receptor antibodies | Blocks IL2 engagement and resultant lymphocyte proliferation |
APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte antigen 4; FKBP12, FK-binding protein-12; IL2, interleukin 2; IMPDH, inosine-59-monophosphate dehydrogenase; LFA-1, leukocyte function-associated antigen-1; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; NK, natural killer.
Classification of immunosuppressive drugs
There are two types of immunosuppressive drugs: (1) pharmacological agents or small molecule agents and (2) biological agents (i.e., polyclonal and monoclonal anti-lymphocyte antibodies) (Figure 2).13,14,15 The pharmacological immunosuppressive agents act by inhibiting cytokine release (CNIs, corticosteroids) or inhibiting the cell cycle (anti-metabolites and mTOR inhibitors.14,15 Biological immunosuppressive are classified into lymphocyte-depleting immunosuppressive agents that deplete T-cells (ATG), B-cells (rituximab), or plasma cells (bortezomib) and non–lymphocyte-depleting agents (basiliximab) that inhibit T-cell proliferation without affecting lymphocyte populations.16,17,18,19 In LT, biological agents are used as antibody induction agents, in treating steroid-refractory rejection, in ABO-incompatible LT and management of antibody-mediated rejection (AMR).16,17,18,19 Immunosuppressive agents can also be classified based on their mechanism of action (Table 1).
Figure 2.
Individual immunosuppressive drugs and sites of action in the Three-Signal Model (adapted with permission from Halloran et al13).
Biological agents (polyclonal or monoclonal antibodies)
Antibodies that inhibit or deplete T-cells are used as induction agents or to treat steroid-refractory rejection in LT (Table 2).16,20, 21, 22 Antibody induction is commonly used in "steroid-free" protocols and as CNI sparing agents in LT.5,16,17 This "steroid-free" regime is beneficial in hepatitis C and non-alcoholic steatohepatitis (NASH)-related cirrhosis.5,16,17 Antibody induction permits delayed CNI introduction, thus protecting renal function in LT recipients.23,24,25 Overall, there is decreased acute rejection episodes and no increase in adverse side effects with antibody induction.16 However, they are costly. Biological agents used in induction therapy are classified into, T-cell-depleting agents [polyclonal – ATGs, monoclonal – alemtuzumab (Campath – 1H), muromonab-CD3 (OKT3)], and non-depleting agents [interleukin 2 receptor antagonists (IL- 2Ra), anti-CD28 inhibitor (belatacept)].16,26,27
Table -2.
Biological Agents in Liver Transplantation.
| Drug | Mechanism of action | Use | Comments |
|---|---|---|---|
| Muromonab-CD3 (OKT3) | T-cell-depleting monoclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | Withdrawn from the market. |
| Alemtuzumab (campath-1H) | T-cell-depleting monoclonal antibody | Induction of immunosuppression | Variable between centers, a single dose of 30 mg may be used in operating room. |
| ATG (thymoglobulin, ATGAM) | T-cell-depleting polyclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | Variable between centers, for induction 1.5 mg/kg per day iv for 3 d and for treatment of rejection 1.5 mg/kg per day iv for 5–7 d of thymoglobulin may be used. For ATGAM a higher dose of 15 mg/kg per day is usually used. |
| Daclizumab (Zenapax) | IL-2Ra, monoclonal antibody | Induction of immunosuppression, treatment of steroid-resistant rejection | Withdrawn from the market. |
| Basiliximab (Simulect) | IL-2Ra, monoclonal antibody | Induction of immunosuppression, treatment of steroid resistant rejection | For induction a 20 mg iv dose is administered within 6 h of reperfusion and another 20 mg on days 4 post Tx. |
| Belatacept | Anti-CD28 monoclonal antibody | CNI sparing agent | Not recommended in LT. |
ATG: Anti-thymocyte globulin, IL-2Ra Interleukin-2 receptor.
Polyclonal T-cell-depleting antibodies
Anti-thymocyte globulins
ATGs are polyclonal antibodies used to deplete circulating lymphocytes.28,29 ATG is mostly used to treat steroid resistance rejection and rarely as an induction agent in LT. Of the two preparations of ATG, ATGAM is from the horse, and Thymoglobulin is of rabbit origin.30 Thymoglobulin is superior to the ATGAM with fewer opportunistic infections, less serious adverse side effects and better efficacy.29,30 But More profound leucopenia is observed with thymoglobulin than ATGAM.28,29,30
A daily infusion of 2.5 mg/kg/d of Thymoglobulin for ten days is the standard procedure for ATG induction therapy. Shorter three-day courses have shown similar efficacy in LT with fewer adverse effects.28,31 Intermittent dosing of ATGAM, where subsequent doses of ATG are given only if CD3 count is above 20 cells/mm3 is also effective and has cost benefit.31 Infusion reactions, serum sickness, severe cytokine release syndrome are rare adverse effects of ATG infusion. Antihistamines and acetaminophen are recommended before ATG infusion to prevent infusion reactions. The risk of opportunistic infections, HCV recurrence and post-transplantation lymphoproliferative disorders (PTLD) are not increased with ATG induction compared with no induction.28,32
Monoclonal T-cell-depleting antibodies
Alemtuzumab (Campath- 1H)
Alemtuzumab (Campath- 1H) is a T-cell-depleting humanized monoclonal antibody against.
CD52 receptors on T-cells.26,33 It is potent but has an increased risk for opportunistic infections post-LT. There are reports of rapid progression of recurrent HCV in patients receiving alemtuzumab induction.34 Currently, Alemtuzumab has a limited role in LT because the risks out way benefit.26,33
Muromonab-CD3 (OKT3)
Muromonab-CD3 (OKT3) is a T-cell-depleting monoclonal antibody against CD3 receptors on peripheral T-cells. It showed benefit in steroid-resistant T-cell-mediated rejection (TCMR) and induction therapy. However, because of increased side effects, the production was discontinued in 2010.35
Non-depleting antibodies
Interleukin-2 Receptor Antibodies
Interleukin-2 and its receptor CD25 have a significant role in the activation and proliferation of T lymphocytes involved in cell-mediated immunity.7 Daclizumab is a fully humanized monoclonal antibody against CD25, while basiliximab is a chimeric antiCD25 monoclonal antibody.36,37 Both inhibit T-cell proliferation by binding to IL-2 receptor on activated T lymphocytes.7 In LT, interleukin-2 receptor antagonists (IL-2Ras) are used in induction therapy as steroid or CNI sparing agents. Daclizumab has been off the market since 2010 due to the increased incidence of inflammatory encephalitis.38 Studies in LT have shown IL-2Ra induction to have similar efficacy with fewer side effects than ATG induction.37 IL-2Ra induction was associated with better renal functions, fewer opportunistic infections and fewer metabolic complications than steroids.39 There is no increase in cytomegalovirus (CMV) infection, PTLD or HCV recurrence with IL-2Ra induction.36 There were fewer acute rejection episodes and better graft survival with IL-2Ra induction.36,37,39
CD28 Antibodies (Belatacept)
CD28 receptor on T-cell is involved in signal-2 of T-cell activation and proliferation. CD28 inhibitor (Belatacept) is a potent non-T-cell-depleting monoclonal antibody used in renal transplantation. More research is required before its use in LT.27
Pharmacologic agents
Calcineurin Inhibitors
The two available drugs in this class include CsA and TAC (Table 3). CNIs inhibit the calcium/calmodulin-dependent phosphatase, “calcineurin” by binding to intracellular small molecules, “cyclophilin” for CsA and “FKBP12” for TAC.40 The binding inhibits IL-2 gene transcription, preventing T-cell activation and proliferation.40 CNI discovery has significantly improved the graft and patient survival post-LT.7,41,42 TAC is 100 times more potent than CsA, with TAC displaying reduced acute cellular rejection, steroid-resistant rejection and improved patient and graft survival than cyclosporine.42,43
Table 3.
Pharmacological Immunosuppressive Agents.
| Drug | Dose | Half-life (Hours0 | Therapeutic range | Adverse effects |
|---|---|---|---|---|
| Tacrolimus | 0.1–0.15 mg/kg/daily divided in 2 doses, 12 h apart | 2–36 | General range: 5–12 ng/mL | Nephrotoxicity, neurotoxicity, diabetes, hyperkalemia, metabolic acidosis, hypertension, hyperlipidemia |
| Cyclosporine | 10–15 mg/kg/daily, divided in 2 doses, 12 h apart. | 5–8 | General range: 100–250 ng/mL | Nephrotoxicity, neurotoxicity, diabetes, hyperlipidemia1, hypertension1, hyperkalemia, metabolic acidosis, gingival hyperplasia, hypertrichosis |
| MMF | 500–1000 mg twice daily | 11–12 | Therapeutic monitoring not recommended | Myelosuppression, gastrointestinal side effects, viral infections (CMV, HSV), spontaneous abortions in pregnant women |
| Everolimus | 0.25–0.5 mg twice daily | 30 | General range: 3–8 ng/mL | Hyperlipidemia, myelosuppression, proteinuria, poor wound healing, pneumonitis, skin rash |
| Corticosteroids | 5 mg–10 mg/kg induction followed by tapering dose | 2.5–3.5 | Therapeutic monitoring not recommended | Diabetes, hypertension, obesity, osteoporosis, avascular necrosis, growth retardation, Cushingoid features, psychosis, poor wound healing, adrenal suppression, cataracts |
MMF: mycophenolate mofetil; CMV: cytomegalovirus; HSV: herpes simplex virus.
CsA and TAC have similar side effect profiles, including neurotoxicity, renal dysfunction, metabolic syndrome, and vasculopathy.44 TAC is more diabetogenic, while gingival hyperplasia and hypertrichosis are seen with CsA only. Hypertension, hyperlipidemia, and endothelial dysfunction leading to cardiovascular disease are more common with CsA.45 Both TAC and CsA increase the risk for opportunistic infections and malignancies. CNIs produce dose-dependent afferent renal arteriolar vasoconstriction that is often reversible.44 However, long-standing ischemic glomerular and tubular injuries occur, with 20% of patients on CNI developing chronic renal dysfunction by 5 years.4,44 The cytochrome P450 system metabolizes CNIs, and they have multiple drug interactions requiring the monitoring of drug levels (Table 4).7 In addition, foods that alter p-glycoprotein levels affect CNI absorption.32 Drug interactions with direct acting antiviral agents (DAAs) should be checked in patients with chronic hepatitis C who need DAA after LT (Table 5).
Table 4.
Drugs That Alter CNI and mTORi Levels.
| Drugs that increase CNI and mTORi levels |
| Macrolides: clarithromycin, erythromycin, azithromycin |
| Antifungals: fluconazole, itraconazole, ketoconazole, voriconazole, clotrimazole |
| Calcium channel blockers: verapamil, diltiazem, nifedipine |
| Others: cisapride, metaclopramide, amiodarone, cimetidine, protease inhibitors |
| Drugs that decrease CNI and mTORi levels |
| Antibiotics: rifabutin, rifampin |
| Anticonvulsants: carbamazepine, phenobarbital, phenytoin, fosphenytoin |
| Others: St. John's Wort |
CNI: Calcineurin Inhibitor, mTORi: Mammalian target of rapamycin inhibitor.
Table 5.
Drug Interactions Between DAA for CHC and Immunosuppressants.
| Sofosbuvir with or without daclatasvir, ledipasvir, or velpatasvir | Simeprevir or Elbasvir-grazoprevir | Paritaprevir/ritonavir plus ombitasvir plus dasabuvir | Ribavirin | |
|---|---|---|---|---|
| Calcineurin inhibitors | ||||
| Cyclosporine | No dose adjustment. | Not recommended. | When starting co-administration, give one fifth of the total daily dose of cyclosporine once daily. Therapeutic monitoring recommended. |
No dose adjustment. Monitor hemoglobin. |
| Tacrolimus | No dose adjustment | No dose adjustment Therapeutic monitoring suggested. |
When starting co-administration, administer 0.5 mg tacrolimus once every week. Therapeutic monitoring recommended. |
No dose adjustment. Monitor hemoglobin. |
| mTOR inhibitors | ||||
| Sirolimus | No dose adjustment | Sirolimus exposure may change. Therapeutic monitoring recommended. |
Sirolimus exposure may increase. A dose decrease may be needed for sirolimus. Therapeutic monitoring is recommended. |
No dose adjustment. Monitor hemoglobin. |
| Everolimus | Potential interaction with NS5A inhibitors. Therapeutic monitoring recommended. | Avoid if possible. Therapeutic monitoring recommended. Monitor for simeprevir toxicity. |
Not recommended | No dose adjustment. Monitor hemoglobin. |
| Antimetabolites | ||||
| Azathioprine | No dose adjustment | No dose adjustment | No dose adjustment | Not recommended |
| Mycophenolate | No dose adjustment | No dose adjustment | Mycophenolic acid exposures may increase. A reduction in dose may be needed. | No dose adjustment. Monitor hemoglobin. |
| Corticosteroids | ||||
| Prednisone | No dose adjustment | No dose adjustment | Prednisone exposure may increase. | No dose adjustment. |
DAA direct acting antivirals, CHC chronic hepatitis C. https://www.hep-druginteractions.org/.
TAC is currently the backbone of most post-LT immunosuppression regimes. TAC is started at a low oral dose (0.1–0.15 mg/kg daily in two divided doses 12 h apart) on the first-day post-LT, and the dose is titrated to achieve an adequate trough level. The usual C0 targets for TAC are 8–10 ng/mL in the initial 3 months and then 5–8 ng/mL after that.46 The CsA dose is 10–15 mg/kg daily in two divided doses 12 h apart. For CsA, the target C0 levels are 250 ng/mL in the initial three months and 150 ng/ml there after.47 If C2 (2 h after dose) monitoring is used for CsA, the recommended trough levels are 800–1400 ng/mL initially, then 600–1000 ng/mL for first year, and 500–700 ng/mL long term.47 Extended-release (TAC-ER) and prolonged-release (TAC-PR) once-daily formulations of TAC have better adherence and similar efficacy to conventional TAC.48
Antimetabolites
AZA, MMF, and mycophenolate sodium inhibit T-cell proliferation by reversible inhibition of purine synthesis.49,50 They block signal-3 of T-cell activation. MMF and its active metabolite MPA have additional immunomodulatory actions.49
Azathioprine
AZA is an antimetabolite with potent immunosuppressive action. But AZA is inferior to MMF in the LT setting, with randomized controlled trials showing a higher incidence of acute cellular rejection with AZA.50 Over the years, MMF has replaced AZA as the most used antimetabolite agent. Today, AZA is used when other agents are not tolerated or where finances are limited.7,32,40 AZA has significant hepatotoxicity and bone marrow toxicity.7,40
Mycophenolate mofetil
MPA is the active metabolite of MMF and mycophenolate sodium (EC-MPS).51 It inhibits the de novo synthesis of guanosine nucleotides by blocking inosine-5′-monophosphate dehydrogenase (IMPDH), thereby suppressing T-cell proliferation.51,52,53 MPAs lack renal toxicity and are used in combination to deescalate or discontinue CNIs.54 MPAs may also supplement immunosuppressive regimens in patients with signs of acute allograft rejection. Therapeutic drug monitoring is not recommended for MPAs as bioavailability is high. The average daily dose for MMF is 500 mg to 1 g twice daily (360–720 mg twice daily for EC-MPS).51,52,53
MPAs have significant gastrointestinal and bone marrow side effects.51,52 Diarrhea is a common dose-limiting side effect. Patients can also have abdominal pain, nausea, and vomiting.55 Rarely, MMF can cause inflammatory bowel disease (IBD) like colitis and graft-versus-host disease like enteritis.55,56 Gastrointestinal symptoms resolve with the withdrawal of the drug or switching to enteric-coated mycophenolate sodium.56 MPAs also predispose to opportunistic infections.49
Mammalian target of rapamycin inhibitors
EVR and SRL bind intracellularly to FK506 binding protein (FKBP12) but inhibits the mammalian target of rapamycin complex instead of calcineurin.57, 58, 59 It, thus, blocks signal-3 of T-cell activation, thereby inhibiting IL-2- and IL-15-induced T-cell proliferation.57, 58, 59 Even though they bind to the same receptor, mTORi and CNI do not compete but act synergistically.57,58 Like the CNIs, SRL and EVR metabolism is via the cytochrome P450 system, and therapeutic drug monitoring is recommended.58,59 Watson et al in 1999 first reported the effectiveness of SRL as an immunosuppressive agent.60 Excess hepatic artery thrombosis (HAT) and early graft failure in the de novo SRL therapy arm lead to early termination of subsequent large trials.61,62 This led FDA to issue a black box label warning about early post-transplant HAT with SRL use in LT.63 However, several later trials assessed the safety of mTORi introduced 30 days after LT and found no evidence of an elevated risk for HAT.63, 64, 65, 66, 67, 68, 69
EVR is a derivative of SRL, and compared with SRL, EVR has a shorter half-life, does not require a loading dose, and a relatively narrow therapeutic window (3–8 ng/mL).57, 58, 59 EVR showed good results when combined early with low-dose TAC in rejection rates and improved renal function.64 Early conversion to mTORi therapy improves renal function in patients with CNI-induced nephropathy (Table 6).63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 Patients receiving mTORi early (30 ± 5 days) or very early (≤10 days) post-LT have a renal function improvement of 8–12 mL/min per 1.73 m2 at 12 months after transplantation.64,66,68 Similar improvement is not seen with mTORi in patients with GFR less than 60 mL/min per 1.73 m2.70 Some studies have also shown that late introduction may worsen pre-existing renal disease and promote proteinuria.60,62,70 The 5-year real-life data from the Everolimus Liver registry (EVEROLIVER) an observational database of all liver transplant recipients receiving EVR across nine centers in France showed that CNI withdrawal was feasible in 57.7% of patients at month 60. At 36 months and 60 months, even individuals with chronic kidney disease (CKD) (eGFR <60 mL/min/1.73 m2) exhibited improvement in eGFR. Early conversion to EVR (<3 months) was related to a greater chance of eGFR improvement than late conversion (55% vs. 39.4%) in patients with CKD.75
Table-6.
Role of Everolimus in Liver Transplantation.
| Author | Year | Comparator | N | Duration | Results |
|---|---|---|---|---|---|
| Levy et al63 | 2006 | EVR versus placebo | 119 | 36 mo | CsA + EVR provides similar efficacy versus CsA + placebo |
| Masetti et al66 | 2010 | EVR monotherapy versus CsA-based immunosuppression | 78 | 12 mo | EVR monotherapy provides similar efficacy and better renal function |
| Fischer et al67 | 2012 | EVR after conversion from CNIs versus continued CNIs | 203 | 12 mo | EVR monotherapy provides similar efficacy and better renal function |
| Sterneck et al65 | 2014 | EVR and discontinue CNI, or continue their current posttransplant CNI-based regimen at 4 weeks | 81 | 3 y | Efficacy results and improved renal function maintained |
| Sterneck et al71 | 2016 | Extension of above study by Sterneck et al | 81 | 5 y | Compared with the CNI-based treatment, EVR-based CNI-free immunosuppression resulted in significantly better renal function and comparable patient and graft outcomes after 5-yr follow-up |
| De Simone et al68 | 2012 | (i) EVR initiation with TAC elimination; (ii) EVR initiation with reduced-exposure TAC; (iii) standard-exposure TAC |
719 | 12 mo | EVR + rTAC provides similar efficacy and better renal function |
| Saliba et al64 | 2013 | Extension of the above study by De Simone et al.18 | 719 | 24 mo | Early introduction of EVR with rTAC at 1 month after liver transplantation provided a significant benefit for renal function at 2 years posttransplant. |
| Nashan B et al72 | 2021 | Early initiation of EVR + rTAC or standard-exposure tacrolimus (sTAC) with steroids | 333 | 12 mo | Early use of EVR in combination with rTAC showed comparable efficacy, safety, and well-preserved renal function versus sTAC therapy at month 12. |
| Lee SG et al73 | 2021 | EVR + rTAC versus sTAC | 772 | 24 mo | EVR + rTAC versus sTAC showed comparable efficacy and safety with significantly better renal function, particularly in patients with normal/mildly decreased renal function (CKD stage 1/2) at randomization. |
| Gómez-Bravo M et al74 | 2022 | EVR + rTAC versus MMF + TAC | 211 | 12 mo | EVR+rTAC allows a safe reduction in tacrolimus exposure in de novo liver transplant recipients, with a significant improvement in eGFR but without significant differences in renal clinical benefit 1 year after liver transplantation. |
EVR everolimus, CsA Cyclosporine A, CNI calcineurin inhibitor, rTAC reduced dose tacrolimus, sTAC standard dose tacrolimus, MMF mycophenolate mofetil.
mTORi have antiproliferative effects, preclinical and clinical studies have shown that they may prevent HCC recurrence.59.50.62 They also reduce the risk of post-transplant de novo malignancies.60,62 Recent clinical studies on mTORi and post-transplant recurrence in patients with HCC show conflicting reports.76,77,78,79 One study on SIR-based immunosuppression in preventing HCC recurrence showed a beneficial effect only in patients meeting Milan criteria.80 Current data on the prevention of HCC recurrence is scanty, and mTORi may be tried in patients with increased risk for HCC recurrence.59 mTORi may also be beneficial in preventing de novo and recurrent extra-hepatic malignancies after LT, and its use is supported by retrospective, cohort, and registry data analysis.81,82,83
EVR introduced early permits CNI dose reduction. Complete withdrawal of CNI increases the risk of acute rejections, and a 10–20% risk of acute rejection is seen depending on time from LT.65,66,67,68 EVR and TAC have no interactions, and the TAC dose is reduced only after the EVR target trough level is reached. EVR can increase CsA trough levels. and the CsA dose should be decreased upon combining with EVR.62,63 Side effects of mTORi include anemia, thrombocytopenia, leukopenia, dyslipidemia, hypertension, poor wound healing, oral ulcers, interstitial pneumonia, proteinuria, and fluid retention and are dose dependent.62 Most side effects respond to dose reduction or discontinuation of the drug.64,68 The risk of EVR-induced proteinuria (>1 gm/d) is 3% at 3 years and responds to dose reduction.84 Severe neutropenia (<1000 mm3), leukopenia (<2000 mm3), or thrombocytopenia (<50,000 mm3) with EVR needs dose reduction or withdrawal.85,86 Inhibition of fibroblast growth factor by mTORi leads to poor wound healing and risk of incisional hernia.60,62 Interstitial pneumonitis is dose dependent and resolves when EVR is withdrawn.60,62 There is no increased risk of opportunistic infections with EVR-based immunosuppressive regimens.65,68 Currently, mTORi are used as CNI sparing agents in LT recipients with kidney dysfunction, HCC, and de novo neoplasms.59 Most centers introduce EVR between 15 and 30 days post-LT.
Corticosteroids
Corticosteroids have anti-inflammatory and immunomodulatory by interacting with antigen-presenting dendritic cells, modulating IL-1 transcription, decreasing the number of circulating CD4+ T-cells, and inhibiting IL-1-dependent lymphocyte activation.7,40,87 Currently in LT, corticosteroids are used for induction and maintenance of immunosuppression and treating acute cellular rejection.7,40 Corticosteroids are tapered and stopped by 3–6 months except in patients with autoimmune liver disease or prior rejection episodes.7,40 Concerns about the use of corticosteroids in HCV patients is unwarranted.88 Corticosteroids are associated with worsening metabolic syndrome, central obesity, diabetes mellitus, hypertension, and dyslipidaemia.7,40 Use of steroids in the long term is linked to osteoporosis, cushingoid features, avascular necrosis, poor wound healing, psychosis, adrenal suppression, and cataracts.7,40
Newer immunosuppressive agents
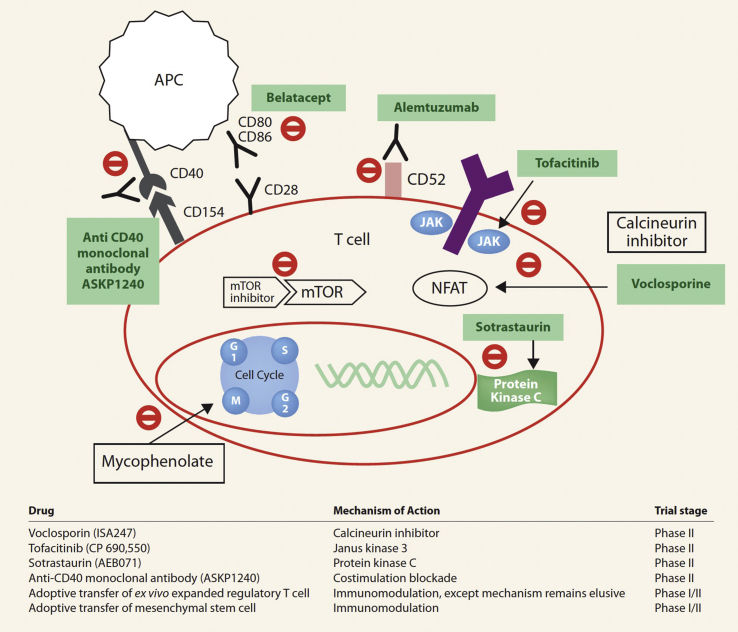
The newer immunosuppressive agents with success in non-LT, including Janus Kinase (JAK)-3 inhibitors (tofacitinib), protein kinase C inhibitors (sotrastaurin), costimulatory inhibitor [ASKP1240 (Human anti-CD 40 monoclonal antibody)], efalizumab [humanized anti-leukocyte function-associated antigen-1 (CD11a)], alefacept (LFA3-IgG1 fusion receptor protein), and voclosporin (novel semisynthetic analogue of cyclosporine) have failed to prove safety and efficacy in liver recipients (Figure 3).7,89 The real future in LT is cell therapy with dendritic cells, mesenchymal stem cells, novel macrophages, and regulatory T-cells that have tolerogenic properties.7,89 The advantage of using cell therapy is that cells can be made to downregulate responses to specific donor antigens and inhibit their migration to anatomical sites where they can exert locally suppressive effects.7,89 At present, cell therapy in LT is still experimental.
Figure-3.
Newer immunosuppressant drugs: Mechanism of action (adapted with permission from Wong TC et al89).
Immunosuppressive regimens in liver transplantation
The level of medical therapy that achieves stable allograft function with minimal suppression of systemic immunity is known as optimal transplant immunosuppression.90,91 Immunosuppression administration is more of an art than a science. Most transplant centers are moving from a protocol-based immunosuppression regimen to a personalized regimen. Current immunosuppressive regimens use multiple agents with different modes of action, allowing lower doses of each drug to achieve less toxicity and with better patient and graft survival.90,91 The immunosuppressive agents used vary with recipient profile, time from transplantation, the initial disease process, and graft behavior.
Immunosuppression in LT is divided into (1) induction phase, (2) maintenance phase, and (3) treatment of acute cellular rejection or TCMR.89,90 Antibody-mediated rejection (AMR), steroid-refractory rejection, and chronic rejection are rare in liver transplants and are beyond the scope of this article. The initial immunosuppressive regimen used in the first 30 days following LT when alloreactivity is at its height is known as the induction phase.90,91 A triple-drug therapy with a CNI (TAC, CsA), corticosteroid, and an antimetabolite [AZA or MMF] is the most common induction regimen.90,91 Antibody induction therapy [ATG, basiliximab] may be used as a steroid-sparing or CNI sparing regimen.92,93,94
Maintenance immunosuppression refers to an immunosuppressive regimen that is continued 30 days from transplantation and used indefinitely after that.91 Personalizing immunosuppression is seen as tailoring immunosuppression protocols considering recipient characteristics (renal function, metabolic syndrome), etiology of liver disease and extent of alloimmune activation. Our center uses a combination of TAC, corticosteroids, and MMF during the induction phase. Intravenous methylprednisolone 500 mg is administered during the an-hepatic phase and then tapered in the next five days to 20 mg per oral (PO) dose by postoperative day 6. At discharge, patients remain on low dose TAC, MMF, and prednisone 20 mg PO daily, tapered and stopped over 3 months. LT recipients with autoimmune liver disease, IBD, or episodes of TCMR remain on low dose maintenance of prednisolone.94 In patients with renal dysfunction, basiliximab 20 mg intravenous is administered on postoperative days 0 and 4; TAC is withheld until postoperative day 5. If there is no renal dysfunction, TAC 1 mg administered orally twice daily is started on postoperative day one, with further dose adjustments depending on a target trough level of 8–10 ng/mL for the first 3 months and 5–8 ng/mL after that.5,90,93 From the second year onward, trough levels of 3–6 ng/mL are considered adequate. MMF is given in a dose of 500 mg twice daily and is usually discontinued after 12 months in stable patients.5,90 In patients at risk for kidney injury or having HCC, MMF is substituted with EVR between postoperative days 15–30. EVR is started at a dose of 0.25–0.5 mg PO twice daily and subsequently adjusted based on the target trough level.5,95 By the second year, most patients are on monotherapy with TAC, and the TAC trough level is between 3–6 ng/mL.5,90,93,94 Long-term measurement of trough levels are unnecessary if the liver chemistry tests are normal.
Treatment of T cell–mediated rejection
Hepatic allograft rejection is an important cause of morbidity and graft loss after LT. Histological diagnosis and grading of TCMR is according to the Banff working group definitions and is characterized by portal inflammation, venous endothelial inflammation, and bile duct inflammation/damage.96,97 Liver biopsy is mandatory for the diagnosis of hepatic allograft rejection.96 Incidence of early TCMR is between 10% and 30%, and it occurs within 90 days of transplant.98 Mild cases of early TCMR are managed by increasing CNI levels and adding other drugs (antimetabolites or mTORi). Moderate to severe TCMR requires pulse steroid therapy (500–1000 mg methylprednisolone given daily for 3 days), higher CNI trough levels, and additional drugs.5,90,93 Patients who do not respond to steroid pulse therapy are treated with ATG.5,90,93 Currently, there is limited evidence for IL-2 receptor blockers in the management of TCMR.5 Patients on repeated pulse steroid therapy or ATG should receive prophylaxis against CMV, PCP, and fungus. Late TCMR is defined as allograft rejection occurring more than 90 days after LT and has an incidence of 7.5%–23%.99 Unlike early rejection, steroid resistance and progression to chronic rejection are more common in late TCMR, and can result in reduced graft survival.5,99,100 Treatment is similar to early TCMR.5,100
Chronic allograft rejection is seen in 1%–5% of adult LT and leads to the irreversible bile duct damage and vascular injury with graft loss.101 Diagnosis is made in patients with persistent cholestasis unresponsive to immunosuppression modifications and biopsy showing (1) bile duct loss in >50% of the portal tracts or (2) bile duct atrophy/pyknosis with or without duct loss in most portal tracts and/or (3) Foam cell obliterative arteriopathy.102 Chronic TCMR is difficult to treat and most patients will need retransplantation. Patients chronic TCMR on cyclosporine should be switched to TAC.102 AMR is seen in <1% of all LT and in <5% of sensitized individuals.103,104 Histologic evidence of microvascular C4d positivity, circulating donor-specific antibody (DSA), and exclusion of alternate causes are all needed for diagnosis of AMR.97 Mild acute AMR may respond to steroid pulses or lymphodepletion.5 Moderate to severe AMR is treated with DSA depleting therapy including plasmapheresis, intravenous immunoglobulin, anti-B-cell (rituximab), anti-plasma cell (Bortezomib), and anti-compliment (eculizumab) agents.105,106,107,108 Chronic AMR has no defined treatment strategy.97
Immunosuppression minimization protocols
Immunosuppression minimization protocols aim to identify the ideal drug and dose for inhibiting alloimmune responses while minimizing adverse effects.5 Its especially beneficial in patients with metabolic syndrome and NASH to prevent worsening obesity, systemic hypertension, diabetes mellitus, and dyslipidemia. It also helps to avoid long-term metabolic complications, cardiovascular events, renal dysfunction, malignancies, and opportunistic infections. Patients with (1) proven steroid-resistant rejection, (2) autoimmune etiology, (3) re-transplantation, and (4) who had an AMR are not considered for immunosuppression minimization.5 Immunosuppression minimization strategies are initiated 3 months after LT and in patients with stable graft function for the last 4 weeks preceding the protocol initiation. Most patients are off corticosteroids by 3 months after LT.109 Patients on dual therapy and have stable graft function may be switched to TAC monotherapy any time after 3 months.59,110,111 TAC minimization with EVR or MMF is done in patients with renal dysfunction. Complete withdrawal of TAC with EVR or MMF monotherapy increases the risk of rejection, and it is not recommended in the first year after transplantation.112 If TAC needs to be stopped for some reason, it is preferable to use two agents like MMF or low-dose corticosteroids and EVR. During follow-up, it is essential to keep a close watch on the graft function and if liver chemistry tests become abnormal anytime, then switch back to the standard immunosuppression regimen.113 Under close follow-up, immunization minimization is possible and safe, but complete immunosuppression withdrawal is limited to clinical trials.
Conclusion
Recent advancements in immunosuppressive medications have lowered the rate of acute rejections and considerably prolonged the graft life. This has come at a cost of increased morbidity and mortality associated with immunosuppressive drugs. The goal of optimum immunosuppression is to increase drug effectiveness while lowering the adverse effects in the hope of long-term graft and recipient survival with a good quality of life. Every person needs an immunosuppression regimen tailored to their: (1) age, (2) the co-morbid conditions, (3) transplantation indications, (4) behavior of the allograft, (5) complications related to immunosuppression, and (6) post-LT physiologic conditions. The ability to induce tolerance in transplant patients and immunosuppression withdrawal remains the ultimate objective. While this is not a common practice, it may become a viable option soon. Till then, immunosuppression minimization, defined as the lowest amount of immunosuppression compatible with a rejection-free state, should be the goal for all patients.
CREDIT AUTHORSHIP CONTRIBUTION STATEMENT
Dr Charles Panackel: Conceptualization, writing original draft, visualization.
Dr Joe Francis Mathew: Editing and reviewing the article, visualization.
Dr Mohamed Fawas N; Editing and reviewing the article, visualization.
Dr Mathew Jacob: Editing and reviewing the article, supervising.
Conflicts of interest
All authors have none to declare.
Funding
The authors have received no funding for the article.
References
- 1.Van Thiel D.H., Schade R.R., Starzl T.E., et al. Liver transplantation in adults. Hepatology. 1982 Sep-Oct;2:637–640. doi: 10.1002/hep.1840020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rana A., Ackah R.L., Webb G.J., et al. No gains in long-term survival after liver transplantation over the past three decades. Ann Surg. 2019 Jan;269:20–27. doi: 10.1097/SLA.0000000000002650. [DOI] [PubMed] [Google Scholar]
- 3.Tasdogan B.E., Ma M., Simsek C., Saberi B., Gurakar A. Update on immunosuppression in liver transplantation. Euroasian J Hepato-Gastroenterol. 2019 Jul-Dec;9:96–101. doi: 10.5005/jp-journals-10018-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojo A.O., Held P.J., Port F.K., et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M., Levitsky J., Aqel B., et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018 May;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 6.Navarro V., Herrine S., Katopes C., Colombe B., Spain C.V. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transplant. 2006 Apr;12:652–658. doi: 10.1002/lt.20680. [DOI] [PubMed] [Google Scholar]
- 7.Geissler E.K., Schlitt H.J. Immunosuppression for liver transplantation. Gut. 2009 Mar;58:452–463. doi: 10.1136/gut.2008.163527. [DOI] [PubMed] [Google Scholar]
- 8.Knechtle S.J., Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin Liver Dis. 2009 Feb;29:91–101. doi: 10.1055/s-0029-1192058. [DOI] [PubMed] [Google Scholar]
- 9.Choudhuri K., Wiseman D., Brown M.H., Gould K., van der Merwe P.A. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005 Jul 28;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 10.Martinez O.M., Rosen H.R. Basic concepts in transplant immunology. Liver Transplant. 2005 Apr;11:370–381. doi: 10.1002/lt.20406. [DOI] [PubMed] [Google Scholar]
- 11.Wang D., Matsumoto R., You Y., et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol. 2004 Jan;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesner R.H., Ludwig J., van Hoek B., Krom R.A. Current concepts in cell-mediated hepatic allograft rejection leading to ductopenia and liver failure. Hepatology. 1991 Oct;14(4 Pt 1):721–729. doi: 10.1016/0270-9139(91)90064-3. [DOI] [PubMed] [Google Scholar]
- 13.Halloran P.F. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004 Dec 23;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 14.Bush W.W. Overview of transplantation immunology and the pharmacotherapy of adult solid organ transplant recipients: focus on immunosuppression. AACN Clin Issues. 1999 May;10:253–269. doi: 10.1097/00044067-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner R.H., Fung J.J. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transplant. 2011 Nov;17(suppl 3):S1–S9. doi: 10.1002/lt.22410. [DOI] [PubMed] [Google Scholar]
- 16.Turner A.P., Knechtle S.J. Induction immunosuppression in liver transplantation: a review. Transpl Int. 2013 Jul;26:673–683. doi: 10.1111/tri.12100. [DOI] [PubMed] [Google Scholar]
- 17.Penninga L., Wettergren A., Wilson C.H., Chan A.W., Steinbrüchel D.A., Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database Syst Rev. 2014 Jun 5:CD010253. doi: 10.1002/14651858.CD010253.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav D.K., Hua Y.F., Bai X., et al. ABO-incompatible adult living donor liver transplantation in the era of rituximab: a systematic review and meta-analysis. Gastroenterol Res Pract. 2019;2019 doi: 10.1155/2019/8589402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C.F., Eldeen F.Z., Chan K.M., et al. Bortezomib is effective to treat acute humoral rejection after liver transplantation. Transplant Proc. 2012 Mar;44:529–531. doi: 10.1016/j.transproceed.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.G., Lee J., Lee J.J. Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes M.L., Lee Y.M., Sutedja D. Treatment of steroid-resistant acute liver transplant rejection with basiliximab. Transplant Proc. 2005;37:2179. doi: 10.1016/j.transproceed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary N.S., Saigal S., Bansal R.K., Saraf N., Gautam D., Soin A.S. Acute and chronic rejection after liver transplantation: what A clinician needs to know. J Clin Exp Hepatol. 2017;7:358–366. doi: 10.1016/j.jceh.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez C.B., Doria C., Frank A.M., et al. Completely steroid-free immunosuppression in liver transplantation: a randomized study. Clin Transplant. 2013;27:463–471. doi: 10.1111/ctr.12119. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger J.M., Mamelok R.D., Neuhaus P., et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. Am J Transplant. 2009;9:327–336. doi: 10.1111/j.1600-6143.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 25.Soliman T., Hetz H., Burghuber C., et al. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transplant. 2007;13:1039–1044. doi: 10.1002/lt.21185. [DOI] [PubMed] [Google Scholar]
- 26.Dhesi S., Boland B., Colquhoun S. Alemtuzumab and liver transplantation: a review. Curr Opin Organ Transplant. 2009 Jun;14:245–249. doi: 10.1097/MOT.0b013e32832b45d0. [DOI] [PubMed] [Google Scholar]
- 27.Perez C.P., Patel N., Mardis C.R., Meadows H.B., Taber D.J., Pilch N.A. Belatacept in solid organ transplant: review of current literature across transplant types. Transplantation. 2018 Sep;102:1440–1452. doi: 10.1097/TP.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt T.M., Phillips M., Sawyer R.G., et al. Anti-thymocyte globulin for the treatment of acute cellular rejection following liver transplantation. Dig Dis Sci. 2010;55:3224–3234. doi: 10.1007/s10620-010-1149-x. [DOI] [PubMed] [Google Scholar]
- 29.Langrehr J.M., Nüssler N.C., Neumann U., et al. A prospective randomized trial comparing interleukin-2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation. Transplantation. 1997;63:1772–1781. doi: 10.1097/00007890-199706270-00012. [DOI] [PubMed] [Google Scholar]
- 30.Brennan D.C., Flavin K., Lowell J.A., et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 31.Soliman T., Hetz H., Burghuber C., et al. Short-term versus long-term induction therapy with antithymocyte globulin in orthotopic liver transplantation. Transpl Int. 2007;20:447–452. doi: 10.1111/j.1432-2277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 32.Moini M., Schilsky M.L., Tichy E.M. Review on immunosuppression in liver transplantation. World J Hepatol. 2015 Jun 8;7:1355–1368. doi: 10.4254/wjh.v7.i10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magliocca J.F., Knechtle S.J. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006;19:705–714. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 34.Marcos A., Eghtesad B., Fung J.J., et al. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78:966–971. doi: 10.1097/01.tp.0000142674.78268.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon H., Gonwa T.A., Mor E., et al. OKT3 rescue for steroid-resistant rejection in adult liver transplantation. Transplantation. 1993 Jan;55:87–91. doi: 10.1097/00007890-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez C.B., Doria C., di Francesco F., Iaria M., Kang Y., Marino I.R. Basiliximab induction in adult liver transplant recipients with 93% rejection-free patient and graft survival at 24 months. Transplant Proc. 2006;38:3633–3635. doi: 10.1016/j.transproceed.2006.10.110. [DOI] [PubMed] [Google Scholar]
- 37.Uemura T., Schaefer E., Hollenbeak C.S., Khan A., Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti-thymocyte globulin, daclizumab, and corticosteroid. Transpl Int. 2011;24:640–650. doi: 10.1111/j.1432-2277.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu C.L., Fan S.T., Lo C.M., et al. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transplant. 2004;10:728–733. doi: 10.1002/lt.20144. [DOI] [PubMed] [Google Scholar]
- 39.Di Maira T., Little E.C., Berenguer M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol. 2020 Jun-Aug:46–47. doi: 10.1016/j.bpg.2020.101681. [DOI] [PubMed] [Google Scholar]
- 40.Pillai A.A., Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol. 2009 Sep 14;15:4225–4233. doi: 10.3748/wjg.15.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichlmayr R., Winkler M., Neuhaus P., et al. Three-year follow-up of the European multicenter tacrolimus (FK506) liver study. Transplant Proc. 1997;29:2499–2502. doi: 10.1016/s0041-1345(97)00464-8. [DOI] [PubMed] [Google Scholar]
- 42.Wiesner R.H. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493–499. doi: 10.1097/00007890-199808270-00014. [DOI] [PubMed] [Google Scholar]
- 43.O'Grady J.G., Burroughs A., Hardy P., Elbourne D., Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomized controlled trial. Lancet. 2002;360:1119–1125. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- 44.Issa N., Kukla A., Ibrahim H.N. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37:602–612. doi: 10.1159/000351648. [DOI] [PubMed] [Google Scholar]
- 45.Haddad E.M., McAlister V.C., Renouf E., Malthaner R., Kjaer M.S., Gluud L.L. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;4:CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodr guez-Per lvarez M., Germani G., Darius T., Lerut J., Tsochatzis E., Burroughs A.K. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12:2797–2814. doi: 10.1111/j.1600-6143.2012.04140.x. [DOI] [PubMed] [Google Scholar]
- 47.Villamil F., Pollard S. C2 monitoring of cyclosporine in de novo liver transplant recipients: the clinician's perspective. Liver Transplant. 2004;10:577–583. doi: 10.1002/lt.20112. [DOI] [PubMed] [Google Scholar]
- 48.Trunečka P. Once-daily tacrolimus in liver transplantation: a 'me-too drug', or a therapeutic advantage. Curr Opin Organ Transplant. 2017 Apr;22:118–122. doi: 10.1097/MOT.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 49.Staatz C.E., Tett S.E. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88:1351–1389. doi: 10.1007/s00204-014-1247-1. [DOI] [PubMed] [Google Scholar]
- 50.Wiesner R., Rabkin J., Klintmalm G., et al. A randomized double-blind comparative study of mycophenolate mofetil and Azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transplant. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 51.Stewart S.F., Hudson M., Talbot D., Manas D., Day C.P. Mycophenolate mofetil monotherapy in liver transplantation. Lancet. 2001;357:609–610. doi: 10.1016/s0140-6736(00)04065-4. [DOI] [PubMed] [Google Scholar]
- 52.Franklin T.J., Cook J.M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969;113:515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allison A.C., Eugui E.M. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF) Clin Transplant. 1996;10:77–84. [PubMed] [Google Scholar]
- 54.Barkmann A., Nashan B., Schmidt H.H., et al. Improvement of acute and chronic renal dysfunction in liver transplant patients after substitution of calcineurin inhibitors by mycophenolate mofetil. Transplantation. 2000;69:1886–1890. doi: 10.1097/00007890-200005150-00025. [DOI] [PubMed] [Google Scholar]
- 55.Parfitt J.R., Jayakumar S., Driman D.K. Mycophenolate mofetilrelated gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32:1367–1372. doi: 10.1097/pas.0b013e31816bf3fe. [DOI] [PubMed] [Google Scholar]
- 56.Al-Absi A.I., Cooke C.R., Wall B.M., Sylvestre P., Ismail M.K., Mya M. Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc. 2010;42:3591–3593. doi: 10.1016/j.transproceed.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 57.Halloran P.F. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004 Dec 23;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 58.Shihab F., Christians U., Smith L., Wellen J.R., Kaplan B. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014 Jun;31:22–32. doi: 10.1016/j.trim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 59.De Simone P., Fagiuoli S., Cescon M., et al. Consensus Panel Use of everolimus in liver transplantation: recommendations from a working group. Transplantation. 2017 Feb;101:239–251. doi: 10.1097/TP.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson C.J.E., Friend P.J., Jamieson N.V., et al. Sirolimus: a potent new Immunosuppressant for liver transplantation. Transplantation. 1999;67:505–509. doi: 10.1097/00007890-199902270-00002. [DOI] [PubMed] [Google Scholar]
- 61.Wiesner R., Klintmalm G., McDiarmid S. Rapamune Liver Transplant Study Group. Sirolimus immunotherapy results in reduced rates of acute rejection in de novo orthotopic liver transplant recipients. Am J Transplant. 2002;2(suppl 3):464. [abstract)] [Google Scholar]
- 62.Trotter J.F. Sirolimus in liver transplantation. Transplant Proc. 2003 May;35(3 suppl l):193S–200S. doi: 10.1016/s0041-1345(03)00234-3. [DOI] [PubMed] [Google Scholar]
- 63.Levy G., Schmidli H., Punch J., et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transplant. 2006;12:1640–1648. doi: 10.1002/lt.20707. [DOI] [PubMed] [Google Scholar]
- 64.Saliba F., De Simone P., Nevens F., et al. H2304 Study Group. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013 Jul;13:1734–1745. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 65.Sterneck M., Kaiser G.M., Heyne N., et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am J Transplant. 2014;14:701–710. doi: 10.1111/ajt.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masetti M., Montalti R., Rompianesi G., et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–2262. doi: 10.1111/j.1600-6143.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 67.Fischer L., Klempnauer J., Beckebaum S., et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation – PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 68.De Simone P., Nevens F., De Carlis L., et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Simone P., Metselaar H.J., Fischer L. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transplant. 2009;15:1262–1269. doi: 10.1002/lt.21827. [DOI] [PubMed] [Google Scholar]
- 70.Abdelmalek M.F., Humar A., Stickel F., et al. Sirolimus Liver Conversion Trial Study Group. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012 Mar;12:694–705. doi: 10.1111/j.1600-6143.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 71.Sterneck M., Kaiser G.M., Heyne N., et al. Long-term follow-up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clin Transplant. 2016 Jun;30:741–748. doi: 10.1111/ctr.12744. [DOI] [PubMed] [Google Scholar]
- 72.Nashan B., Schemmer P., Braun F., et al. Hephaistos Study Group Early everolimus-facilitated reduced tacrolimus in liver transplantation: results from the randomized HEPHAISTOS trial. Liver Transplant. 2022 Jun;28(6):998–1010. doi: 10.1002/lt.26298. 34525259. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee S.G., Jeng L.B., Saliba F., et al. Efficacy and safety of everolimus with reduced tacrolimus in liver transplant recipients: 24-month results from the pooled analysis of 2 randomized controlled trials. Transplantation. 2021 Jul 1;105:1564–1575. doi: 10.1097/TP.0000000000003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gómez-Bravo M., Prieto Castillo M., Navasa M., et al. Everolimus plus minimized tacrolimus on kidney function in liver transplantation: REDUCE, a prospective, randomized controlled study. Rev Esp Enferm Dig. 2022 Jun;114(6):335–342. doi: 10.17235/reed.2022.8549/2021. 35469409. In this issue. [DOI] [PubMed] [Google Scholar]
- 75.Saliba F., Dharancy S., Salamé E., et al. Time to conversion to an everolimus-based regimen: renal outcomes in liver transplant recipients from the EVEROLIVER registry. Liver Transplant. 2020 Nov;26:1465–1476. doi: 10.1002/lt.25879. [DOI] [PubMed] [Google Scholar]
- 76.Toso C., Merani S., Bigam D.L., et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 77.Cholongitas E., Mamou C., Rodriguez-Castro K.I., et al. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 78.Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–261. doi: 10.1586/14737140.9.2.247. [DOI] [PubMed] [Google Scholar]
- 79.Geissler E.K., Schnitzbauer A.A., Zülke C., et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yanik E.L., Chinnakotla S., Gustafson S.K., et al. Effects of maintenance immunosuppression with sirolimus after liver transplant for hepatocellular carcinoma. Liver Transplant. 2016;22:627–634. doi: 10.1002/lt.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guba M., von Breitenbuch P., Steinbauer M., et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 82.Kauffman H.M., Cherikh W.S., Cheng Y., et al. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 83.Piselli P., Serraino D., Segoloni G.P., et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur J Cancer. 2013;49:336–344. doi: 10.1016/j.ejca.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 84.Kasiske B.L., de Mattos A., Flechner S.M., et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant. 2008;8:1384–1392. doi: 10.1111/j.1600-6143.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- 85.Singh S., Watt K.D. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779–790. doi: 10.1016/j.mayocp.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fischer L., Saliba F., Kaiser G.M., et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 2015;99:1455–1462. doi: 10.1097/TP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 87.Starzl T.E., Marchioro T.L., Vonkaulla K.N., Hermann G., Brittain R.S., Waddell W.R. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 88.Vivarelli M., Burra P., La Barba G., et al. Influence of steroids on HCV recurrence after liver transplantation: a prospective study. J Hepatol. 2007;47:793–798. doi: 10.1016/j.jhep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 89.Wong T.C., Lo C.M., Fung J.Y. Emerging drugs for prevention of T-cell mediated rejection in liver and kidney transplantation. Expet Opin Emerg Drugs. 2017 Jun;22:123–136. doi: 10.1080/14728214.2017.1330884. [DOI] [PubMed] [Google Scholar]
- 90.Cillo U., De Carlis L., Del Gaudio M., et al. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group. Hepatol Int. 2020 Dec;14:930–943. doi: 10.1007/s12072-020-10091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiesner R.H., Fung J.J. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transplant. 2011 Nov;17(suppl 3):S1–S9. doi: 10.1002/lt.22410. [DOI] [PubMed] [Google Scholar]
- 92.Kirk A.D. Induction immunosuppression. Transplantation. 2006;82:593–602. doi: 10.1097/01.tp.0000234905.56926.7f. [DOI] [PubMed] [Google Scholar]
- 93.Choudhary N.S., Saigal S., Shukla R., Kotecha H., Saraf N., Soin A.S. Current status of immunosuppression in liver transplantation. J Clin Exp Hepatol. 2013 Jun;3:150–158. doi: 10.1016/j.jceh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saigal S., Shah S.R. Liver transplantation-economics in the less developed world. Indian J Gastroenterol. 2012;31:13–14. doi: 10.1007/s12664-011-0159-8. [DOI] [PubMed] [Google Scholar]
- 95.Greig P., Lilly L., Scudamore C., et al. Early steroid withdrawal after liver transplantation: the Canadian tacrolimus versus microemulsion cyclosporin A trial: 1-year follow-up. Liver Transplant. 2003;9:587–595. doi: 10.1053/jlts.2003.50102. [DOI] [PubMed] [Google Scholar]
- 96.Wiesner R.H., Demetris A.J., Belle S.H., et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 97.Demetris A.J., Bellamy C., Hubscher S.G., et al. Comprehensive update of the BanffWorking Group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16:2816–2835. doi: 10.1111/ajt.13909. 2016. [DOI] [PubMed] [Google Scholar]
- 98.Levitsky J., Goldberg D., Smith A.R., et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol. 2017;15:584–593. doi: 10.1016/j.cgh.2016.07.035. e2 e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mor E., Gonwa T.A., Husberg B.S., et al. Late-onset acute rejection in orthotopic liver transplantation—associated risk factors and outcome. Transplantation. 1992;54:821–824. doi: 10.1097/00007890-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 100.Thurairajah P.H., CarboneM, Bridgestock H., et al. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955–959. doi: 10.1097/TP.0b013e3182845f6c. [DOI] [PubMed] [Google Scholar]
- 101.Tannuri A.C., Lima F., Mello E.S., et al. Prognostic factors for the evolution and reversibility of chronic rejection in pediatric liver transplantation. Clinics. 2016;71:216–220. doi: 10.6061/clinics/2016(04)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blakolmer K., Jain A., Ruppert K., et al. Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression: long-term follow-up and evaluation of features for histopathological staging. Transplantation. 2000;69:2330–2336. doi: 10.1097/00007890-200006150-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O'Leary J.G., Kaneku H., Demetris A.J., et al. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transplant. 2014;20:218–227. doi: 10.1002/lt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim P.T., Demetris A.J., O'Leary J.G. Prevention and treatment of liver allograft antibody-mediated rejection and the role of the "two-hit hypothesis". Curr Opin Organ Transplant. 2016;21:209–218. doi: 10.1097/MOT.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 105.Schadde E., d'Alessandro A.M., Musat A.I., et al. Donor-specific HLAantibody- mediated humoral rejection in a liver transplant recipient fully reversed with plasmapheresis and immunoglobulin. Clin Transpl. 2006:479–482. [PubMed] [Google Scholar]
- 106.Paterno F., Shiller M., Tillery G., et al. Bortezomib for acute antibody mediated rejection in liver transplantation. Am J Transplant. 2012;12:2526–2531. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamar N., Lavayssiere L., Muscari F., et al. Early plasmapheresis and rituximab for acute humoral rejection after ABO-compatible liver transplantation. World J Gastroenterol. 2009;15:3426–3430. doi: 10.3748/wjg.15.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kulkarni S., Kirkiles-Smith N.C., Deng Y.H., et al. Eculizumab therapy for chronic antibody-mediated injury in kidney transplant recipients: a pilot randomized controlled trial. Am J Transplant. 2017;17:682–691. doi: 10.1111/ajt.14001. [DOI] [PubMed] [Google Scholar]
- 109.Lerut J.P., Pinheiro R.S., Lai Q., et al. Is minimal, [almost] steroid-free immunosuppression a safe approach in adult liver transplantation? Long-term outcome of a prospective, double blind, placebo-controlled, randomized, investigator-driven study. Ann Surg. 2014;260:886–891. doi: 10.1097/SLA.0000000000000969. ; discussion 891–882. [DOI] [PubMed] [Google Scholar]
- 110.Lerut J., Mathys J., Verbaandert C., et al. Tacrolimus monotherapy in liver transplantation: one-year results of a prospective, randomized, double blind, placebo-controlled study. Ann Surg. 2008;248:956–967. doi: 10.1097/SLA.0b013e31819009c9. [DOI] [PubMed] [Google Scholar]
- 111.Kriss M., Sotil E.U., Abecassis M., et al. Mycophenolate mofetil monotherapy in liver transplant recipients. Clin Transplant. 2011;25:E639–E646. doi: 10.1111/j.1399-0012.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 112.Lin M., Mittal S., Sahebjam F., et al. Everolimus with early withdrawal or reduced-dose calcineurin inhibitors improves renal function in liver transplant recipients: a systematic review and meta-analysis. Clin Transplant. 2017;31 doi: 10.1111/ctr.12872. [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez-Peralvarez M., Garcia-Caparros C., Tsochatzis E., et al. Lack of agreement for defining 'clinical suspicion of rejection' in liver transplantation: a model to select candidates for liver biopsy. Transpl Int. 2015;28:455–464. doi: 10.1111/tri.12514. [DOI] [PubMed] [Google Scholar]