ABSTRACT
Background
Teduglutide use in pediatric patients with short bowel syndrome can aid in the achievement of enteral autonomy, but with a price of >$400,000 per y.
Objective
The current study evaluated the cost-effectiveness of using teduglutide in conjunction with offering intestinal transplantation in US pediatric patients with short bowel syndrome.
Design
A Markov model was used to evaluate the costs (in US dollars) and effectiveness [in quality-adjusted life years (QALYs)] of using teduglutide compared with offering intestinal transplantation. Parameters were estimated from published data where available. The primary effect modeled was the probability of weaning from parenteral nutrition while on teduglutide. Sensitivity analyses were performed on all model parameters.
Results
Compared with offering only intestinal transplantation, adding teduglutide cost 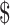 124,353/QALY gained. Reducing the cost of the medication by 16% allowed the cost to reach the typical benchmark of
124,353/QALY gained. Reducing the cost of the medication by 16% allowed the cost to reach the typical benchmark of 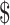 100,000/QALY gained. Probabilistic sensitivity analysis favored transplantation without offering teduglutide in 68% of iterations at a
100,000/QALY gained. Probabilistic sensitivity analysis favored transplantation without offering teduglutide in 68% of iterations at a  100,000/QALY threshold. Never using teduglutide created an opportunity cost of over
100,000/QALY threshold. Never using teduglutide created an opportunity cost of over  100,000 per patient.
100,000 per patient.
Conclusions
At its current price, teduglutide does not provide a cost-effective addition to transplantation in the treatment of pediatric short bowel syndrome. Further work should look to identify cost-reducing strategies, including alternative dosing regimens.
Keywords: intestinal failure, glucagon-like peptide 2, intestinal rehabilitation, clinical decision analysis, home parenteral nutrition, intestinal transplant
Introduction
Short bowel syndrome in children represents a heterogeneous group of disorders often present from the neonatal period. Many of these children require lifelong parenteral nutrition (PN), leading to substantial morbidity and mortality (1). Some patients will go on to require intestinal transplantation, which has a 65% success rate in achieving enteral autonomy, i.e., freedom from PN (2). Previous work has demonstrated that intestinal transplantation provides a cost-effective treatment in adult patients with intestinal failure, and these results have been extrapolated to children (3).
Glucagon-like peptide 2 (GLP-2) promotes intestinal adaptation via an increase in villus height and crypt depth (4–6). Teduglutide, an FDA-approved GLP-2 analog for short bowel syndrome treatment, has reduced PN requirements, with some patients achieving enteral autonomy (7–10). The high cost of teduglutide, estimated to be ∼$400,000 per patient per y, prohibits widespread use (11).
We recently published an economic analysis of teduglutide in adults with short bowel syndrome showing that, although teduglutide was effective, its cost exceeded the benefits, according to traditional cost-effectiveness criteria (12). Our results mirrored those of unpublished reports from the UK National Institute for Health and Care Excellence (NICE) and the Scottish Medicine Consortium (13, 14). However, children with short bowel syndrome constitute a population with a large potential for gain. Moreover, our previous study did not consider the potential for intestinal transplantation, which is used with some frequency in children. The current study evaluates the cost-effectiveness of using teduglutide in pediatric patients with short bowel syndrome.
Methods
Model Structure
We built a Markov model to evaluate the costs (in US dollars) and effectiveness [in quality-adjusted life years (QALYs)] of treatments for pediatric short bowel syndrome. The simulated cohort was presumed to begin at age 5 y, at which point spontaneous adaptation without teduglutide could be considered negligible. The cohort was simulated over a 5-y period, based on the lack of data beyond that time. The results were then adjusted for the expected QALYs lost by cohort members who died during the simulation. A healthcare perspective was taken, evaluating only direct medical costs. Future costs and QALYs were discounted at 3% per y.
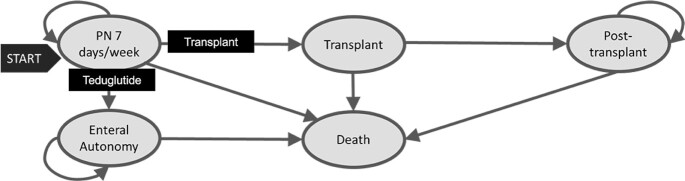
All patients started on PN for 7 d/wk. Figure 1 depicts the model health states. We compared teduglutide and no teduglutide as well as intestinal transplant and no intestinal transplant, testing the following 4 combinations of strategies: no teduglutide/no transplant, no teduglutide/transplant (the reference case), teduglutide/transplant, and teduglutide/no transplant. Patients could achieve enteral autonomy only through strategies in which teduglutide was used; strategies labeled as no teduglutide assumed patients had completed natural adaptation without achieving enteral autonomy. Transplant strategies provided the opportunity for a small percentage of patients on PN to undergo intestinal transplant. As an example, in the teduglutide/transplant strategy, patients on PN 7 d/wk would receive teduglutide, allowing them the possibility of achieving enteral autonomy, whereas a small percentage of the patients remaining on PN would undergo intestinal transplant and no longer continue teduglutide.
FIGURE 1.
State diagram of health states in the Markov model. Arrows represent transition probabilities between states. The transition probability of the arrow labeled Teduglutide is 0 for strategies not including teduglutide. The transition probability of the arrow labeled Transplant is 0 for strategies not including transplant. PN, parenteral nutrition.
During each monthly cycle, patients either remained in the same state, obtained enteral autonomy, died, or received an intestinal transplant. Unlike the adult model, the pediatric model did not have health states to account for number of days per week on PN as, in our practice, children often remain on daily support and instead wean the amount of PN received per day. We assumed that children obtaining enteral autonomy were never restarted on PN and that after 3 mo of enteral autonomy, they would have their central line removed. Until that time, they would be at risk of developing a central line–associated bloodstream infection (CLABSI), which was incorporated by use of an estimated incidence rate and an estimated cost (15). Once the central line was removed, we assumed that mortality returned to the baseline mortality for age (16). The primary effect modeled was the increased likelihood of achieving enteral autonomy when using teduglutide, leading to greater quality of life and lower costs.
Parameter Estimation
Parameter values are listed in Table 1. Transition probabilities were calculated primarily from the reported cohort of the Pediatric Intestinal Failure Consortium (PIFCon) (1). The model was calibrated to the reported cumulative incidence of death, transplant, and enteral autonomy over 6 y. The probability of achieving enteral autonomy while on teduglutide was obtained from a single 12-wk open-label study by taking that study's probability of achieving enteral autonomy on the optimal dose (20%) and assuming a constant probability over time (10). The probability of achieving enteral autonomy was extended beyond the duration of the study on the basis of adult data that suggest sustained teduglutide effects (17).
TABLE 1.
Parameter values used in the model1
| Base case | Range | Distribution | |
|---|---|---|---|
| Probability of stopping PN over 1 mo with teduglutide (10) | 7.2% | 0.3—24.5% | β |
| Rate of CLABSI (per 1000 catheter d) (Institutional) | 4 | 0—12 | Uniform |
| Monthly probability of death on PN (1) | 1.6% | 0.5—3.4% | β |
| Monthly probability of death off PN (1) | 0.5% | 0.3—0.7% | β |
| Monthly probability of transplant (1) | 1.7% | 0.5—3.5% | β |
| Monthly probability of death after transplant (1, 2) | 0.5% | 0—3.1% | β |
| Utilities | |||
| Enteral autonomy (18) | 0.82 | 0.76—0.88 | Normal |
| 7 d PN/wk (18) | 0.36 | 0.26—0.46 | Normal |
| Costs | |||
| Teduglutide cost/wk (11) | $7799 | $3500 - 13,700 | γ |
| Parenteral nutrition cost/d (23, 24) | $280 | $238–390 | Triangular |
| PN-related lab cost/wk (20) | $63 | $29—110 | γ |
| Office visits (21) | $115 | $71—169 | γ |
| Cost per CLABSI (15) | $30,094 | $6690—138,760 | Lognormal |
| Intestinal transplant (22) | $104,991 | $54,408—172,346 | γ |
| Care after transplant/mo | |||
| First 2 y posttransplant (25) | $7868 | $6553—9669 | γ |
| 3+ y posttransplant (25) | $5816 | $4553—7572 | γ |
Columns represent the base case model, the range over which sensitivity analyses were performed, and the assumed shape of the statistical distribution used in probabilistic sensitivity analysis for each parameter. References in parentheses. CLABSI, central line associated bloodstream infection; PN, parenteral nutrition.
Because of the lack of quality of life utility data in children, utility values were extrapolated from adult data (18) and adjusted for age. Utility values ranged from 0 to 1, with 0 representing death and 1 representing a healthy child. QALYs are the product of health-state utility and duration. Based on the limited quality of life data available, we assumed no disutility from teduglutide use (19). Children who received an intestinal transplant were assumed to have a utility value of 0 during the initial hospital stay, which was presumed to be 1 mo. On the basis of data from the pediatric report of the International Intestinal Transplant Registry, we assumed that 35% of patients would still require PN after transplant, whereas the remaining 65% would obtain enteral autonomy (2). We used a weighted average of these states to calculate the utility of the cohort posttransplant.
Teduglutide cost was obtained from the Federal Supply Schedule (11). Centers for Medicare and Medicaid Services (CMS) data were used to identify costs of physician office visits, laboratory tests, and intestinal transplant procedures (20–22). Prior to transplant, office visit frequency was assumed as monthly while on PN and quarterly once off PN, as is standard practice in our institution. Laboratory monitoring frequency was based on institutional practice. Home PN cost was estimated from a literature report of customized PN cost (23, 24). Costs posttransplantation included the costs of rehospitalization and immunosuppression, as well as PN cost for those patients unable to achieve enteral autonomy (25). All costs were adjusted to 2018 values by use of the US Consumer Price Index.
Analysis Methods
TreeAge Pro Healthcare 2019 (TreeAge Software) was used to evaluate the model. The primary outcome was the incremental cost-effectiveness ratio (ICER) of the tested strategies. Secondary outcomes included life expectancy and transplant frequency. Probabilistic sensitivity analysis was performed over 5000 iterations, allowing all probabilities, costs, and utilities to vary. A willingness-to-pay threshold of $100,000/QALY gained, a commonly cited benchmark in the United States, was used as the cost-effectiveness criterion (26).
Value-of-information analysis combines techniques of decision analysis and econometrics to give value to the importance of knowing more information about a decision (27). We calculated the expected value of perfect information (EVPI) as the average amount that was lost by choosing the overall preferred strategy, rather than a preferred strategy for an individual iteration. For example, in our simulation, if in a single iteration the preferred strategy was teduglutide/transplant, but the overall preferred strategy was no teduglutide/transplant, then the loss for that iteration would be the difference between choosing these 2 strategies. The EVPI would then be the average of these losses over all probabilistic sensitivity-analysis iterations. This can be interpreted as the average dollar amount saved per patient if the ideal strategy for each patient was perfectly known. We also calculated the expected value of partial perfect information (EVPPI), a component of the EVPI, which is the average loss that can be attributed to a single parameter. For example, the EVPPI for the probability of weaning off PN while using teduglutide is the difference between the strategy preferred, on average for the cohort, and the predicted perfect strategy for each individual in the cohort, given that value of that parameter. Recent work suggested that EVPPI can be estimated by linear regression, the method used in our calculations (28), which were performed in Microsoft Excel 2016 using the aforementioned willingness-to-pay threshold of $100,000/QALY gained.
Results
Primary analysis
In the base case analysis, when added to transplant alone, teduglutide resulted in an additional 9 QALYs with an additional cost of 1.6 million dollars, resulting in an ICER of $124,353 per QALY gained. Table 2 shows the accumulated costs and difference in effectiveness between the tested strategies; 5-y-old patients with irreversible intestinal failure from short bowel syndrome had a 20% 5-y mortality with teduglutide, compared with a 30% 5-y mortality without teduglutide if they continued to achieve enteral autonomy at the rate reported in the PIFCON data. Assuming no ability to achieve enteral autonomy without teduglutide resulted in an estimated 50% 5-y mortality rate, which is the assumption used for all analyses.
TABLE 2.
Costs and relative effectiveness of each strategy1
| Strategy | Cost | Incremental cost | Incremental effectiveness (QALYs) | ICER |
|---|---|---|---|---|
| No teduglutide, transplant (reference) | $439,728 | — | — | |
| Teduglutide, transplant | $1,553,225 | $1,113,496 | +8.95 | $124,353/QALY |
| Teduglutide, no transplant | $1,735,213 | $181,988 | +0.17 | $1,094,249/QALY |
1The strategy of no transplant and no teduglutide was excluded as it was more expensive and less effective than the reference strategy. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Uncertainty Analysis
Figure 2 shows the results of 1-way sensitivity analyses, displayed as a tornado diagram. Four parameters could be plausibly adjusted to a range in which teduglutide achieved the typical ICER threshold of $100,000/QALY. These parameters included decreasing medication cost, increasing mortality on PN, increasing transplant cost, and increasing CLABSI cost. Table 3 shows the threshold values at which it becomes cost-effective to use teduglutide for each of these parameters. Figure 3 shows a 1-way sensitivity analysis of the cost of the medication, showing the thresholds at which each strategy becomes cost-effective. Teduglutide cost would need to be reduced by 16% for teduglutide added to transplant to be cost-effective. It would need to be reduced by 75% for it to be cost-effective to use only teduglutide and not transplant.
FIGURE 2.
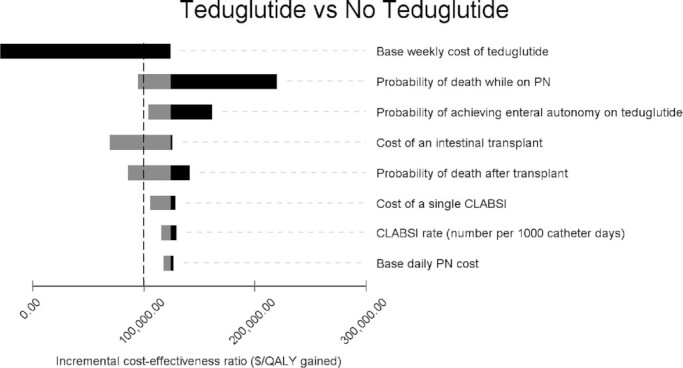
Tornado diagram of 1-way sensitivity analyses comparing teduglutide to no teduglutide in the presence of transplant. Only parameters with an effect on the ICER are pictured. Gray bars represent increasing the value of a parameter, and black bars represent decreasing the value a parameter. The vertical dashed line represents $100,000 per QALY gained. CLABSI, central line associated bloodstream infection; ICER, incremental cost-effectiveness ratio; PN, parenteral nutrition; QALY, quality-adjusted life-year.
TABLE 3.
Threshold at which teduglutide becomes <$100,000 per quality-adjusted life year gained for each parameter1
| Parameter | Base case | Threshold value | Change |
|---|---|---|---|
| Cost of medication | $407,000/y | $342,000/y | Decrease by 16% |
| Probability of death on PN | 18%/y | 35%/y | Increase by 94% |
| Cost of transplant | $104,991 | $940,325 | Increase by 796% |
| Cost per CLABSI admission | $30,094 | $169,497 | Increase by 463% |
1CLABSI, central line-associated bloodstream infection; PN, parenteral nutrition; QALY, quality-adjusted life year.
FIGURE 3.
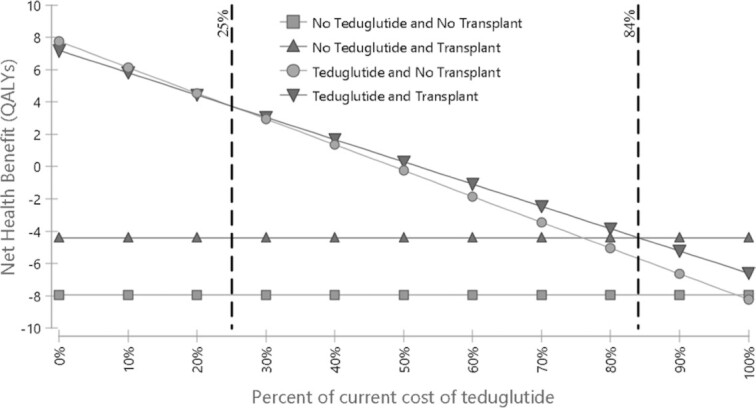
One-way sensitivity analysis of the cost of teduglutide. QALY, quality-adjusted life-year.
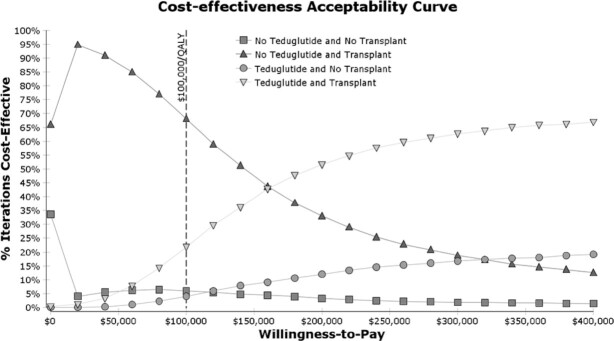
Figure 4
shows the results of the probabilistic sensitivity analysis. At the willingness-to-pay threshold of 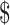 100,000/QALY gained, 68% of iterations favored the base strategy of offering transplant but not teduglutide, and 22% of simulations favored adding teduglutide to pretransplant care. Adding teduglutide resulted in a mean cost of
100,000/QALY gained, 68% of iterations favored the base strategy of offering transplant but not teduglutide, and 22% of simulations favored adding teduglutide to pretransplant care. Adding teduglutide resulted in a mean cost of 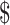 1.03 million and an additional 7 QALYs, which resulted in a mean ICER of
1.03 million and an additional 7 QALYs, which resulted in a mean ICER of 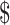 147,955 per QALY gained.
147,955 per QALY gained.
FIGURE 4.
Cost-effectiveness acceptability curve derived from probabilistic sensitivity analysis. QALY, quality-adjusted life-year.
Value-of-Information Analysis
The EVPI or opportunity cost of never using teduglutide was $103,383 per patient. Estimating EVPPI for all parameters in the model produced nonzero values for only 5 parameters. The value of information for each of these can be found in Table 4.
TABLE 4.
The expected value of partial perfect information for each parameter1
| Parameter | EVPPI |
|---|---|
| Cost of teduglutide | $43,507/patient |
| Cost of CLABSI | $1549/patient |
| Probability of death while on PN | $5678/patient |
| Probability of death post-transplant | $12,894/patient |
| Probably of achieving enteral autonomy on teduglutide | $16,855/patient |
1Only nonzero values were included in the table. CLABSI, central line associated bloodstream infection; EVPPI, expected value of partial perfect information; PN, parenteral nutrition.
Discussion
Using a Markov model adapted to pediatric patients, we found that teduglutide can be an effective treatment for short bowel syndrome with intestinal failure. However, assessing costs over a 5-y period, transplant alone becomes more cost-effective than adding teduglutide using a traditional $100,000/QALY gain willingness-to-pay threshold. Replacing transplant entirely with teduglutide provides a slight quality of life advantage, but with an even greater cost relative to the benefit. This reinforces the cost-effectiveness of intestinal transplant in this population even as new treatments emerge.
The current study focused on a 5-y period over which costs were accumulated so as not to overextrapolate the effect of teduglutide. This similarly limited the cost burden of teduglutide, as current data suggest that teduglutide may need to be continued indefinitely to maintain its effect (29). These assumptions thus favored teduglutide in the analysis.
Rates of death and transplantation used in the model were obtained from the PIFCon data, which were collected prior to many advances in intestinal rehabilitation. This time frame also corresponds to the peak of intestinal transplantation. Thus, the rate of intestinal transplantation and the mortality rates used in this analysis are likely overestimates of current rates. However, in sensitivity analysis, overestimating these parameters actually favored teduglutide. Thus, rather than using newer single-center data sources showing lower rates of intestinal transplantation and mortality for those on PN, we used the multicenter PIFCon data that overestimate mortality on PN as another assumption favoring teduglutide use. This also explains the high mortality seen in the simulated cohort.
The rate of achieving enteral autonomy used in the analysis was derived from the 12-wk open-label study. Since that time, the results of the full 24-wk study have been published 8. In this report, no additional patients achieved enteral autonomy in the additional 12 wk of the study. Again, the estimate extrapolated from the 12-wk study may overestimate enteral autonomy achievement and tend to favor teduglutide.
Despite all these favorable assumptions, teduglutide remained outside traditional cost-effectiveness criterion. This criterion may have its own limitations, however. The value of 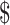 100,000/QALY has not been well-supported in the literature, with the true estimate of an individual's value for a QALY in the US likely coming closer to
100,000/QALY has not been well-supported in the literature, with the true estimate of an individual's value for a QALY in the US likely coming closer to 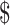 200,000/QALY. Moreover, the estimate does not include other societal benefits for using the medication, such as decreasing caregiver burden and improvement in caregiver quality of life. Teduglutide may also meet the more traditional criterion using alternative dosing strategies. For example, if teduglutide dosing could be decreased to lower frequency in the maintenance phase, it might significantly improve the cost-effectiveness.
200,000/QALY. Moreover, the estimate does not include other societal benefits for using the medication, such as decreasing caregiver burden and improvement in caregiver quality of life. Teduglutide may also meet the more traditional criterion using alternative dosing strategies. For example, if teduglutide dosing could be decreased to lower frequency in the maintenance phase, it might significantly improve the cost-effectiveness.
Uncertainty analyses identified 4 parameters that could be reasonably adjusted to reach the traditional criterion. Of these, base case mortality in the model (18%/y) likely already represents an overestimate and is unlikely to approach the threshold value (35%/y). Transplant and CLABSI admission costs would need to be much higher than the available estimates used in the model. This transplant cost may be more similar to estimates derived from public databases, although the actual value estimated in these studies may represent a different entity than the more standardized reimbursement from CMS. CLABSI admission cost can have great variability if a child requires care in an intensive care unit, as these costs would be much higher. The rate at which these children require this type of support may be influenced by individual patient factors and local preferences. Moreover, the current model did not account for the costs of a child presenting with fever and being observed in the hospital for 48 h; although, as previous reports estimate that 50% of fever presentations result in confirmed bacteremia in this population, we feel that our sensitivity analysis, which examined up to 3 times the rate of CLABSI, would cover this difference (30, 31). The ideal target for making teduglutide cost-effective would be medication cost itself, particularly because a reduction of only 16% is necessary to reach the traditional criterion.
Markov cohort models assume that a population has a given probability of experiencing an outcome. This assumption limits the ability to identify subpopulations of patients that may experience a greater benefit from teduglutide and thus improve its cost-effectiveness. These differences may be due to anatomy or diagnosis, as has been shown with natural adaptation (32, 33). However, in sensitivity analysis over a wide range of teduglutide effectiveness values, we found no effectiveness for which teduglutide would be favored. Similarly, patients with differing diagnoses or baseline anatomy likely have differential rates of intestinal transplantation, but transplantation rates also did not meaningfully impact the results.
An important part of our analyses focused on the benefit of obtaining additional information before deciding whether to use teduglutide. The opportunity cost of never using teduglutide and having only transplant available is 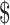 103,383. Thus, it is not appropriate to use this analysis to exclude teduglutide. Rather, it is important to carefully consider appropriate patients and use teduglutide effectively and to consider research to support these decisions, as this may provide an overall cost savings to the health system. Notably, the majority of the value was attributed to knowing the cost of the medication (
103,383. Thus, it is not appropriate to use this analysis to exclude teduglutide. Rather, it is important to carefully consider appropriate patients and use teduglutide effectively and to consider research to support these decisions, as this may provide an overall cost savings to the health system. Notably, the majority of the value was attributed to knowing the cost of the medication (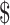 43,507 per patient) compared to knowing the likelihood of weaning off PN while on teduglutide (
43,507 per patient) compared to knowing the likelihood of weaning off PN while on teduglutide (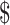 16,855 per patient). Together, this information suggests that clinical trials addressing the effectiveness of teduglutide or attempting to identify responders may be superseded by trials to identify alternative dosing schedules that may have a greater impact on medication cost while still allowing widespread use.
16,855 per patient). Together, this information suggests that clinical trials addressing the effectiveness of teduglutide or attempting to identify responders may be superseded by trials to identify alternative dosing schedules that may have a greater impact on medication cost while still allowing widespread use.
In summary, teduglutide is highly effective but not cost-effective in the treatment of pediatric patients with short bowel syndrome. As such, it should continue to be used, given its effectiveness, but particular attention should be paid to how it is used, and priority should be given to evaluating strategies to lower its cost per patient.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—VKR, JAR, KJS: designed research; VKR, KJS: conducted research; VKR: analyzed data and performed statistical analysis; VKR: wrote the paper; VKR: had primary responsibility for final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Data described in the manuscript, code book, and analytic code will be made available upon request pending written request and approval.
Abbreviations used: CLABSI, central line–associated bloodstream infection; CMS, Centers for Medicare and Medicaid Services; EVPI, expected value of perfect information; EVPPI, expected value of partial perfect information;GLP-2, glucagon-like peptide 2; ICER, incremental cost-effectiveness ratio; NICE, National Institute for Health and Care Excellence; PIFCon, Pediatric Intestinal Failure Consortium; PN, parenteral nutrition; QALY, quality-adjusted life year.
Contributor Information
Vikram Kalathur Raghu, Departments of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Jeffrey A Rudolph, Departments of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Kenneth J Smith, Departments of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
- 1. Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161(4):723. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raghu VK, Beaumont JL, Everly MJ, Venick RS, Lacaille F, Mazariegos GV. Pediatric intestinal transplantation: analysis of the intestinal transplant registry. Pediatr Transplant. 2019;23(8):e13580. doi: 10.1111/petr.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roskott AM, Groen H, Rings EH, Haveman JW, Ploeg RJ, Serlie MJ, Wanten G, Krabbe PF, Dijkstra G. Cost-effectiveness of intestinal transplantation for adult patients with intestinal failure: a simulation study. Am J Clin Nutr. 2015;101(1):79–86. [DOI] [PubMed] [Google Scholar]
- 4. Thymann T, Stoll B, Mecklenburg L, Burrin DG, Vegge A, Qvist N, Eriksen T, Jeppesen PB, Sangild PT. Acute effects of the glucagon-like peptide 2 analogue, teduglutide, on intestinal adaptation in short bowel syndrome. J Pediatr Gastroenterol Nutr. 2014;58(6):1. doi: 10.1097/MPG.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 5. Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naberhuis JK, Deutsch AS, Tappenden KA. Teduglutide-stimulated intestinal adaptation is complemented and synergistically enhanced by partial enteral nutrition in a neonatal piglet model of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2017;41(5):853–65. [DOI] [PubMed] [Google Scholar]
- 7. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kocoshis SA, Merritt RJ, Hill S, Protheroe S, Carter BA, Horslen S, Hu S, Kaufman SS, Mercer DF, Pakarinen MP et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. Journal of Parenteral and Enteral Nutrition. 2020. doi: 10.1002/jpen.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'Keefe S J, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 10. Carter BA, Cohran VC, Cole CR, Corkins MR, Dimmitt RA, Duggan C, Hill S, Horslen S, Lim JD, Mercer DF et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr. 2017;181:102. doi: 10.1016/j.jpeds.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 11. Internet : https://www.va.gov/opal/docs/nac/fss/vaFssPharmPrices.xls (accessed January 15, 2019).
- 12. Raghu VK, Binion DG, Smith KJ. Cost-effectiveness of teduglutide in adult patients with short bowel syndrome: Markov modeling using traditional cost-effectiveness criteria. Am J Clin Nutr. 2020. doi: 10.1093/ajcn/nqz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Internet: www.nice.org.uk/guidance/indevelopment/gid-ta10048 (accessed May 30, 2019).
- 14. Internet: www.scottishmedicines.org.uk/medicines-advice/teduglutide-revestive-fullsubmission-113916/ (accessed April 9, 2018).
- 15. Raphael BP, Hazekamp C, Samnaliev M, Ozonoff A. Analysis of healthcare institutional costs of pediatric home parenteral nutrition central line infections. J Pediatr Gastroenterol Nutr. 2018;67(4):e77–81. [DOI] [PubMed] [Google Scholar]
- 16. Arias E, Xu J United States Life Tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–66. [PubMed] [Google Scholar]
- 17. Seidner DL, Fujioka K, Boullata JI, Iyer K, Lee HM, Ziegler TR. Reduction of parenteral nutrition and hydration support and safety with long-term teduglutide treatment in patients with short bowel syndrome-associated intestinal failure: STEPS-3 Study. Nutr Clin Pract. 2018;33(4):520–7. [DOI] [PubMed] [Google Scholar]
- 18. Ballinger R, Macey J, Lloyd A, Brazier J, Ablett J, Burden S, Lal S. Measurement of utilities associated with parenteral support requirement in patients with short bowel syndrome and intestinal failure. Clin Ther. 2018;40(11):1878. doi: 10.1016/j.clinthera.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 19. Jeppesen PB, Pertkiewicz M, Forbes A, Pironi L, Gabe SM, Joly F, Messing B, Loth S, Youssef NN, Heinze H et al. Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue teduglutide–analyses from a randomised, placebo-controlled study. Clin Nutr. 2013;32(5):713–21. [DOI] [PubMed] [Google Scholar]
- 20. Internet : https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/19CLABQ1.zip(accessed January 15, 2019).
- 21. Internet : https://www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx(accessed January 15, 2019).
- 22. Internet : https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2016(accessed May 30, 2019.
- 23. Howard L. Home parenteral nutrition: survival, cost, and quality of life. Gastroenterology. 2006;130(2 Suppl 1):S52–9. doi: 10.1053/j.gastro.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 24. Turpin RS, Canada T, Liu FX, Mercaldi CJ, Pontes-Arruda A, Wischmeyer P. Nutrition therapy cost analysis in the US: pre-mixed multi-chamber bag vs compounded parenteral nutrition. Appl Health Econ Health Policy. 2011;9(5):281–92. doi: 10.2165/11594980-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sudan D. Cost and quality of life after intestinal transplantation. Gastroenterology. 2006;130(2 Suppl 1):S158–62. doi: 10.1053/j.gastro.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 26. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 27. Wilson EC. A practical guide to value of information analysis. Pharmacoeconomics. 2015;33(2):105–21. doi: 10.1007/s40273-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 28. Jalal H, Goldhaber-Fiebert JD, Kuntz KM. Computing expected value of partial sample information from probabilistic sensitivity analysis using linear regression metamodeling. Med Decis Making. 2015;35(5):584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Compher C, Gilroy R, Pertkiewicz M, Ziegler TR, Ratcliffe SJ, Joly F, Rochling F, Messing B. Maintenance of parenteral nutrition volume reduction, without weight loss, after stopping teduglutide in a subset of patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2011;35(5):603–9. [DOI] [PubMed] [Google Scholar]
- 30. Szydlowski EG, Rudolph JA, Vitale MA, Zuckerbraun NS. Bloodstream infections in patients with intestinal failure presenting to a pediatric emergency department with fever and a central line. Pediatr Emerg Care. 2017;33(12):e140–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisenberg M, Monuteaux MC, Fell G, Goldberg V, Puder M, Hudgins J. Central line-associated bloodstream infection among children with intestinal failure presenting to the emergency department with fever. J Pediatr. 2018;196:237. doi: 10.1016/j.jpeds.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 32. Quiros-Tejeira RE, Ament ME, Reyen L, Herzog F, Merjanian M, Olivares-Serrano N, Vargas JH. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145(2):157–63. [DOI] [PubMed] [Google Scholar]
- 33. Khan FA, Squires RH, Litman HJ, Balint J, Carter BA, Fisher JG, Horslen SP, Jaksic T, Kocoshis S, Martinez JA et al. Predictors of enteral autonomy in children with intestinal failure: a multicenter cohort study. J Pediatr. 2015;167(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]