Abstract
Background
We conducted this study to assess the diagnostic test properties of point of care ultrasonography (POCUS) of lung and cardiovascular system in prediction of mortality in COVID-19 patients.
Methods
This is a cross-sectional study of 178 Covid-19 patients; POCUS was performed within one hour of admission to the ICU. We estimated sensitivity, specificity, positive predictive value, negative predictive value for prediction of mortality.
Results
The mean (SD) age of these patients was 57.3 (12.8) years. The findings were on cardiac ultrasonography were: mild pericardial effusion (45%), chamber dilatation (15%), hypokinesia (11%), and low ejection fraction (8%). In our study, 30 patients (17%) had died. A cut-off score of > to 13 (for lung ultrasound score [LUS]) had high sensitivity for mortality (93.3%, 95% CI: 77.9–99.2%). However, low ejection fraction (92.3%, 95% CI: 86,6–96.1%), and thrombosis in either vein (96.5%, 95% CI: 92.0–98.9%) were specific for mortality. A combination of LUS > =13 or low ejection fraction or thrombosis or spontaneous echo contrast (slow flow) improved sensitivity for mortality to 96.7% (95% CI: 82.8–99.9%). The agreement between LUS of > =13 and CT score of moderate/severe was 85.7% (95% CI: 62.8–100%). The interrater agreement between these two parameters was 0.82 (95% CI: 0.68, 0.97).
Conclusions
Multi-organ POCUS is effective in diagnosis, prognosis, and management of COVID-19 patients. Rather than just lung ultrasound, clinicians should use multiorgan POCUS for early identification of severe lung involvement and thrombotic changes; it may help reduce mortality in these patients.
Keywords: COVID-19 patient; Point of care ultrasonography; Lung; Cardiovascular system; Diagnostic test properties, mortality
1. Introduction
The World Health Organization (WHO) declared coronavirus disease of 2019 (COVID-19) to be an infection of concern and a global pandemic in March 2020 [1]. Thus, the effect of the virus is just not be limited to lungs but may have multiorgan involvement. Some of these include cardiac manifestations such as myocarditis, cardiomyopathy, arrhythmias, or coagulopathy leading to deep vein thrombosis, and pulmonary microthrombosis. [2], [3], [4], [5], [6]. A computed tomography (CT) scan has been identified as screening tool for COVID-19 pneumonia as well as in diagnosis and triage of patients [7], [8], [9]. However, there may be limitations of this procedure such as limited availability (particular in resource constrained settings, rural and tribal areas), risk of transmission of infection to health care workers during the procedure, risk of transportation, and radiation hazards, risk of deterioration of patient illness. Moreover, for mass-application, such as in cases of epidemic-pandemic situations, availability of such high end investigations may not be practically possible [10]. On the other hand, ultrasound has many advantages like easy availability and portability without any risk of radiation [11].
Thus, Point of Care Ultra-sonography (POCUS) may be useful to evaluate patients with COVID-19 infection in intensive care units (ICU) [12], [13]. Its most important role is to assess the degree of pneumonia using bedside lung ultrasound with good sensitivity especially in critical care units [14]. However, reports have suggested that in addition to critical care, POCUS is also useful for musculoskeletal, cardiac, emergency settings, and in anaesthesia and pain management [15]. With the increased involvement of the myocardium in such patients, POCUS planned cardiac ultrasound is the most useful tool to assess these cardiac features of COVID-19 infection [16], [17]. Fatal complications like myocarditis, stress cardiomyopathy, myocardial infarction could be interpreted in an ICU setting. The other important application of ultrasonography is to evaluate the venous system. Patients with COVID-19 infection have dual risk of deep venous thrombosis due to the coagulopathy related to COVID-19 infection and due to in-hospital state of the patient admitted to ICU setting [18], [19].
Lung ultrasound can be used to assess COVID-19 patients as it can detect classic lesions of COVID-19 [20]. Thus, this procedure can be used to monitor progression of COVID-19 pneumonia [7]. A meta-analysis found that lung ultrasound has good sensitivity and specificity in diagnosis of pneumonia [14]. However, all the studies included in this meta-analysis were from high-income settings. The role of lung ultrasound will particularly be useful in low and low-middle income settings, where access to health care diagnostics may be limited – particularly in rural settings. Studies have suggested that lung ultrasound may be useful in COVID-19 patients [11], [21], [22]. Indeed, a study found that the sensitivity and specificity of POCUS examination of lungs was good for diagnosis of non-critical COVD-19 infection [23]. However, as discussed earlier, due to the multi-system nature of the infection, POCUS can also be used to for examination of other organs – particularly the cardiovascular system.
With this background, we conducted the present study to examine the utility and assess the diagnostic test properties of POCUS (lung and cardiovascular system) in prediction of mortality in COVID-19 patients.
2. Material and methods
The current study was a cross-sectional analysis secondary data from 178 COVID-19 patients admitted to a private tertiary care centre in Mumbai, India (April 2020 to September 2020).
2.1. Study site and population
The data were collected from the intensive care unit (ICU) of a private tertiary care centre in Mumbai, India. Mumbai. Data from consecutive patients admitted patients were included for the present study. These patients were admitted to the hospital via the emergency room. The inclusion criteria were: age > 18 years; positive for nucleic acid of SARS CoV-2 detected by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR). Patients who had a history of interstitial lung disease or tuberculosis were excluded from the present study. We excluded four patients from the present analysis; these had extensive subcutaneous emphysema and we could not do the lung ultrasound.
2.2. Study data and procedures
Point Of care ultrasonography (POCUS) was performed within one hour of admission to the ICU and repeated later as per clinical need of the patient. It was performed by WINFOCUS certified intensivists with more than six years of experience in performing point of care ultrasound. Philips CX-50 portable machine was used for bedside ultrasonography along with phased array probe (1–5 MHz) and Linear probe (3–12 MHz). The machine and probes were covered with a protective sleeve cleaned by disinfectants approved for COVID-19 infection after every use.
Point of care ultrasound including lung, cardiac, and deep vein analysis was performed. The bedside lung ultrasound in emergency (BLUE) protocol was applied while performing lung ultrasonography and included total four insonation points on each lung. Each lung was divided into upper blue point, lower blue point, phrenic point, and lower prone point. The following features of lung involvement were assessed during sonography examination: 1) Pleural line: presence of sliding/thickening/irregularity of pleural line 2) Presence of ‘B′ lines: They were classified as B7: 7 mm apart, B3: 3 mm apart and Biroulleau pattern: Confluent B lines. 3) Presence of consolidation: It was characterized by presence of shred sign/tissue like sign with static or dynamic air bronchograms or subpulmonic consolidation (‘C′ Lines). 4) Pleural effusion. Each examination point was scored as follows: ‘A′ profile: 0; B′7 profile: 1 point; B′3 profile: 2 points; Biroulleau profile: 3 points; and C′ profile: 4 points. These profiles were defined as per BLUE protocol [24]. Such scoring systems have been used in literature [25]. The total score was calculated by adding individual score from all the eight points. The final score was called the ‘lung ultrasound score’ (LUS).
Cardiac sonography was done using the focus-assessed transthoracic echocardiography (FATE) [26] protocol and included parasternal long axis, parasternal short axis, apical four chamber and subcostal views. Each view was examined for any regional wall abnormality, chamber dilatation, and ejection fraction. Presence of pericardial effusion or any chamber clot was also looked for. Ejection fraction was evaluated with eyeballing method. [27] The size of pericardial effusion on two-dimensional echocardiography was qualitatively assessed by the end-diastolic distance of the echo-free space between the epicardium and parietal pericardium: small (<10 mm), moderate (10–20 mm), large (>20 mm) [28]. The chamber dilatation was assessed and reported by eye balling. Lower limb deep vein analysis was done using linear probe (5–12 MHz) and two-point compression sonography method [29]. The common femoral and popliteal veins were examined bilaterally for collapsibility, presence of any thrombus, or spontaneous echo contrast. We used these data for the present analysis.
2.3. Clinical data and other investigations
A detailed COVID-19 specific history along with examination and national early warning score (NEWS) 2 score reporting was done at admission to the critical care unit. Blood investigations including complete blood count, arterial blood gas, liver & renal function tests, coagulation profile, other laboratory parameters (quantitative Troponin I, d-Dimer, N terminal Pro BNP, Procalcitonin) and inflammatory panel including Interleukin-6 levels, C Reactive Protein, ferritin, and lactate dehydrogenase were carried out. A portable chest radiograph was done within two hours of admission. A high-resolution computed tomography of the chest or CT pulmonary angiography was done only in selected cases as per the clinical scenario of each patient. We abstracted all these data for the present analysis.
2.4. Statistical methods
We estimated the means and standard deviations (SD), or median and interquartile range (IQR) for continuous variables. We estimated the proportions for categorical variables. The means between two groups were compared using the t-test and Mann Whitney U test was used for non-parametric data. The proportions were compared using the chi square test or Fisher’s exact test for low expected cell counts. We used the receiver operator characteristic (ROC) curves to identify the optimal cut-off for the lung ultrasound score for mortality in these patients. After identifying the optimal cut-off, we estimated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) for prediction of mortality in cases of COVID-19 for this score. We also estimated these parameters for other cardiovascular findings on the ultrasound (low ejection fraction [yes/no], slow flow in either the common femoral vein or popliteal vein [yes/no], thrombosis in either the common femoral vein or popliteal vein [yes/no]). Furthermore, we combined the lung and cardiovascular features for identifying optimal sensitivity, specificity, PPV, and NPV for mortality in these patients.
We also did a sub-group analysis of comparing the severity scores as obtained by CT scans (49 patients) and the lung USG scores. We estimated the Gwet’s AC interrater agreement between these two scores [30], [31], [32].
A p value of < 0.05 was considered statistically significant. Data were analysed using Stata Version 15.1 (© StataCorp, College Station, Texas, USA).
The study was approved by the local Institutional Review Board as a secondary data analysis of clinical data.
3. Results
The mean (SD) age of these patients was 57.3 (12.9) years. About 71% of the study participants were males and 29% were females; there was no significant difference in the age of males and females (58.0 [14.1] vs 57.0 [12.4]; p = 0.65). The most common complaints were fever (85%), dyspnoea (75%), and cough (57%). The median (IQR) duration of symptoms to admission was 1 (1, 2) days. The common morbidities in these patients were hypertension (53%) and diabetes mellitus (39%). Additional demographic and clinical findings are presented in Table 1. The median (IQR) NEWS 2 score at the time of admission was 7 (5, 9); about 40% of participants were severe, 42% were medium, and 19% were in the low category. The median (IQR) sequential organ failure assessment (SOFA) score was 3 (2,4), and the mean (SD) P/F ratio was 196.5 (97.3). In our study population, 30 patients (17%) had died. Baseline demographic, clinical characteristics, and laboratory parameters have been presented in Table 1.
Table 1.
Table showing the demographic and clinical features in 178 COVID-19 patients, India.
| Variable | Total | Death - Yes | Death - No | p value |
|---|---|---|---|---|
| N (%) | n (%) | n (%) | ||
| 178 (100) | 30 (17) | 148 (83) | ||
| Demographics | ||||
| Age (Mean [SD]) | 57.3 (12.8) | 61.8 (14.2) | 56.3 (12.4) | 0.03 |
| Gender | ||||
| Female | 51 (29) | 9 (18) | 42 (82) | 0.86 |
| Male | 127 (71) | 21 (17) | 106 (83) | |
| History | ||||
| Contact | 34 (19) | 5 (15) | 29 (85) | 0.70 |
| Travel | 5 (3) | 1 (20) | 4 (80) | > 0.99 |
| Complaints | ||||
| Fever | 151 (85) | 23 (15) | 128 (85) | 0.17 |
| Cough | 101 (57) | 10 (10) | 91 (90) | 0.005 |
| Dyspnoea | 134 (75) | 20 (21) | 106 (79) | 0.012 |
| Sore Throat | 11 (6) | 1 (9) | 10 (91) | 0.48 |
| Anosmia | 1 (1) | 0 (0) | 1 (100) | > 0.99 |
| Ageusia | 6 (3) | 0 (0) | 6 (100) | 0.59 |
| Co-morbidities | ||||
| Diabetes mellitus | 70 (39) | 12 (17) | 58 (83) | 0.93 |
| Hypertension | 94 (53) | 17 (18) | 77 (82) | 0.64 |
| Ischemic heart disease | 29 (16) | 5 (17) | 24 (83) | 0.95 |
| Obesity | 6 (3) | 0 (0) | 6 (100) | 0.59 |
| Other | 43 (24) | 6 (14) | 37 (86) | 0.56 |
| SOFA Score | 3 (2, 4) | 7.5 (5, 9) | 2 (2, 3) | < 0.001 |
| P/F Ratio (Mean [SD]) | 196.5 (97.3) | 114.2 (43.8) | 213.3 (96.7) | < 0.001 |
| Laboratory parameters | ||||
| Haemoglobin (g/dl) (Mean [SD]) | 12.7 (1.9) | 12.9 (1.7) | 12.7 (1.9) | 0.46 |
| Total WBC (per cumm) (Median [IQR]) | 9185 (5930, 12680) | 11170 (8740, 17410) | 8860 (5750, 11590) | 0.003 |
| N/L ratio (Median [IQR]) | 8 (4.5, 12.8) | 10.8 (8.0, 18.6) | 7.2 (4.4, 12.0) | 0.005 |
| C-reactive protein (mg/L) (Median [IQR]) | 68 (34.6, 158.0) | 67.3 (43.5, 165.0) | 69.4 (33.3, 157.3) | 0.98 |
| S. ferritin (ng/ml) (Median [IQR]) | 469 (213, 1000) | 538.5 (332, 1410) | 411 (196, 988) | 0.06 |
| Lactate dehydrogenase (U/L) (Median [IQR]) | 379.5 (298.5, 513) | 561 (436, 709) | 357 (292 457) | < 0.001 |
| Interleukin – 6 (pg/ml) (Median [IQR]) | 72.3 (22.9, 156) | 106 (47.1, 228.6) | 67.0 (15.8, 135.5) | 0.03 |
| d-Dimer (mg/L) (Median [IQR]) | 0.85 (0.48, 2.30) | 2.34 (0.72, 8.06) | 0.75 (0.46, 1.73) | 0.002 |
| NT-pro-BNP (pg/ml) (Median [IQR]) | 305.5 (128, 753) | 602.5 (305.5, 1640) | 282.5 (97.5, 679.0) | 0.003 |
| Troponin (ng/ml) (Median [IQR]) | 0.012 (0.012, 0.012) | 0.022 (0.012, 0.132) | 0.012 (0.012, 0.012) | 0.0001 |
| Procalcitonin (ng/ml) (Median [IQR]) | 0.15 (0.10, 0.47) | 0.41 (0.10, 0.80) | 0.11 (0.10, 0.44) | 0.013 |
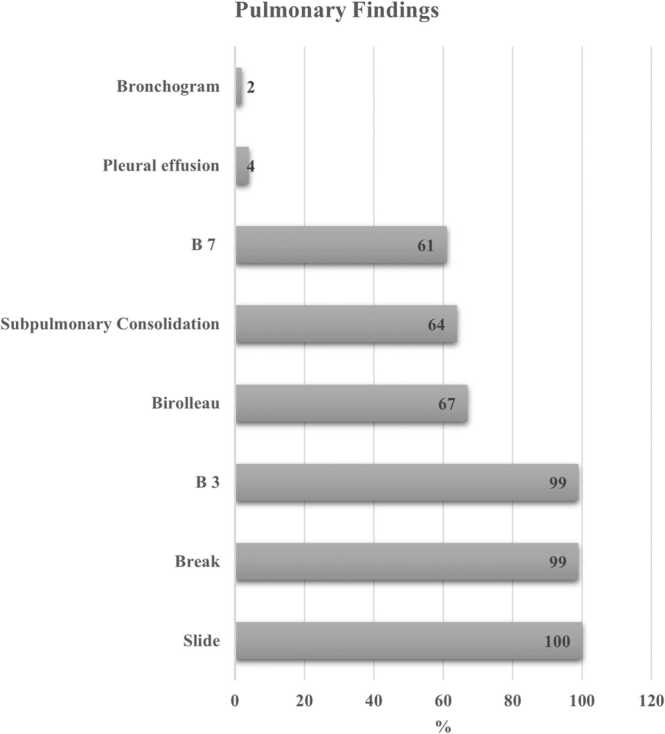
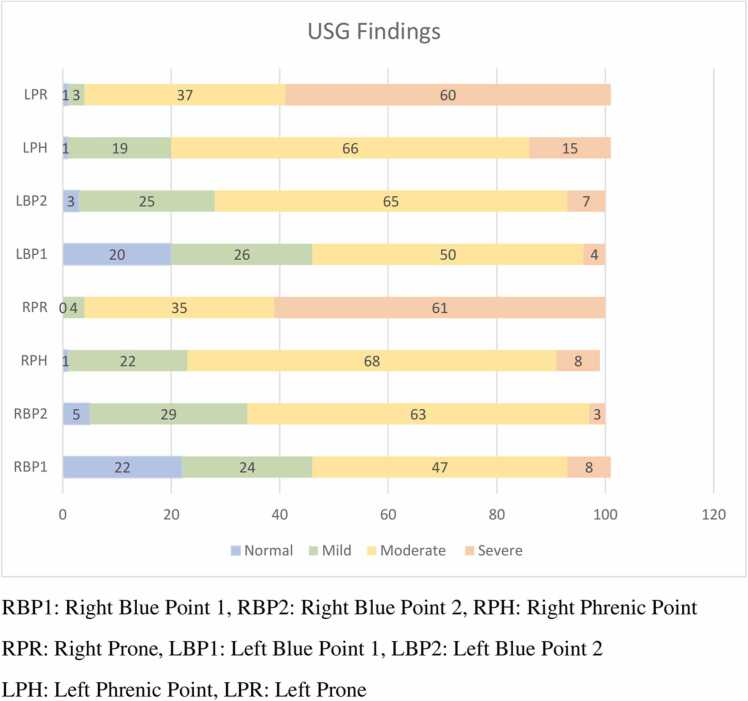
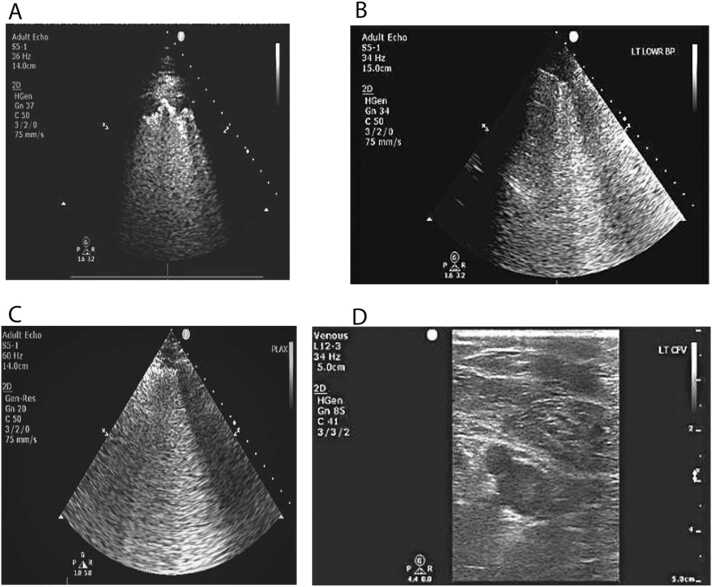
The most common findings seen on the USG of lungs (including all the zones) were: sliding (100%); irregular pleura (99%); B3 (99%); Biroulleau (67%); subpulmonic consolidation (64%); and B7 (61%). The less common features were: pleural effusion (4%) and air bronchogram (2%) (Fig. 1). The severity of the USG findings in various zones (blue point 1, blue point 2, phrenic point, and prone position of the right and left lungs are presented in Fig. 2). As seen in the figure, severe findings were noted in the left and right prone position (60% and 61% respectively). We have also highlighted other USG features - B3 lines, sub pleural consolidation, and irregular pleura (break) - in Fig. 3 A, B, and C. A figure of spontaneous echo contrast (slow venous flow) in the common femoral vein is shown in Fig. 3 D.
Fig. 1.
Graph showing the common findings seen on the ultrasonography of lungs in COVID-19 patients, Mumbai, India.
Fig. 2.
Graph showing the severity of findings in multiple zones of the lung as seen on the ultrasonography of the lung in COVID-10 patients, India.
Fig. 3.
A: Image showing Sub Pleural Consolidation. Fig. 3 B: Image showing irregular pleura at Left Blue Point. Fig. 3 C: Image showing B3 Lines. Fig. 3 D: Image showing Slow Flow in Common Femoral Vein.
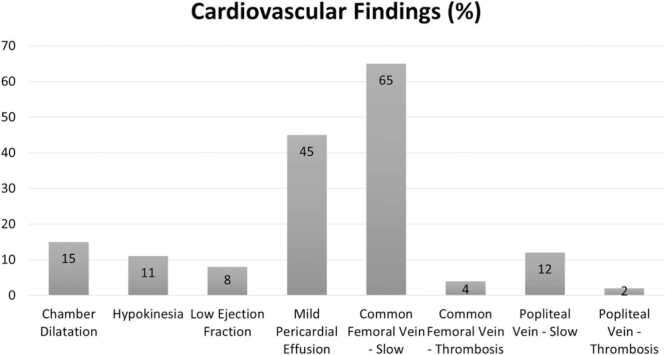
The common sonographic findings of the cardiovascular system were: spontaneous echo contrast (slow venous flow) in common femoral vein (65%); pericardial effusion (45%); chamber dilatation (15%); slow venous flow in the popliteal vein (12%); hypokinesia (11%); and low ejection fraction (8%) (Fig. 4). In our study all the pericardial effusions were of small size. None of the patients were diagnosed as ‘myocarditis’. In general, there was no association between slow venous flow or thrombosis with the d- Dimer value or NEWS 2 score. We found that a lower proportion of individuals who had ‘spontaneous echo contrast’ (slow venous flow) in the popliteal vein were in the ‘severe category’ of NEWS 2 score compared with those who did not have slow flow; the difference in proportions, however, was not statistically significant (19% vs 43%; p = 0.06). In general, there was no association between vascular parameters and severity of lung findings. Finally, we found that mortality was higher in patients who had features of thrombosis in the common femoral vein compared with those who did not have a thrombosis (50% vs 16%; p = 0.03). Even though, the proportion of deaths was higher in those with a thrombosis in the popliteal vein compared with those who did not, the difference was not statistically significant (67% vs 16%; p = 0.08). Detailed proportions for association between spontaneous echo contrast (slow venous flow) and thrombosis, and d- Dimer values, NEWS 2 score, lung USG score, and mortality are presented in Table 2.
Fig. 4.
Graph showing the common findings seen on the ultrasonography of cardiovascular system in COVID-19 patients, Mumbai, India.
Table 2.
Table showing the association between Cardiovascular findings and other outcomes in COVID-19 patients, India.
| D Dimer category | NEWS 2 Score (Admission) | Lung USG Score | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Common Femoral Vein | High | Normal | Low | Medium | Severe | 0–12 | > =13 | No | Yes |
| Slow Flow | |||||||||
| Yes | 81 (74) | 28 (26) | 46 (42) | 46 (42) | 18 (16) | 20 (17) | 96 (83) | 94 (85) | 17 (15) |
| No | 39 (71) | 16 (29) | 22 (36) | 25 (41) | 14 (23) | 18 (29) | 44 (71) | 49 (79) | 13 (21) |
| p value | 0.78 | 0.52 | 0.07 | 0.40 | |||||
| Thrombosis | |||||||||
| Yes | 6 (75) | 2 (25) | 0 (0) | 4 (50) | 4 (50) | 0 (0) | 8 (100) | 4 (50) | 4 (50) |
| No | 114 (73) | 42 (27) | 32 (20) | 67 (41) | 64 (39) | 38 (22) | 132 (78) | 139 (84) | 26 (16) |
| p value | > 0.99 | 0.46 | 0.21 | 0.03 | |||||
| Popliteal Vein | |||||||||
| Slow Flow | |||||||||
| Yes | 11 (58) | 8 (42) | 7 (33) | 10 (48) | 4 (19) | 2 (9) | 20 (91) | 18 (86) | 3 (14) |
| No | 109 (75) | 36 (25) | 25 (17) | 61 (41) | 64 (43) | 36 (23) | 120 (77) | 126 (82) | 27 (18) |
| 0.17 | 0.06 | 0.17 | > 0.99 | ||||||
| Thrombosis | |||||||||
| Yes | 2 (67) | 1 (33) | 0 (0) | 2 (67) | 1 (33) | 0 (0) | 3 (100) | 1 (33) | 2 (67) |
| No | 118 (73) | 43 (27) | 32 (19) | 69 (41) | 1 (33) | 38 (22) | 137 (78) | 142 (84) | 28 (16) |
| > 0.99 | > 0.99 | > 0.99 | 0.08 | ||||||
Using the ROC analysis, we found that a cut-off score of greater than or equal to 13 (for LUS) had a high sensitivity for mortality (93%, 95% CI: 77.9–99.2%). However, low ejection fraction (92.3%, 95% CI: 86,6–96.1%) and thrombosis in either vein (96.5%, 95% CI: 92.0–98.8%) were specific for mortality. A combination of LUS > =13 or low ejection fraction or thrombosis or spontaneous echo contrast (slow flow) had improved the sensitivity for mortality to 96.7% (95% CI: 82.8–99.9%). We have presented estimates and their 95% confidence intervals for sensitivity, specificity, positive predictive value, and negative predictive value for various ultrasound findings in Table 3.
Table 3.
Table showing the diagnostic test properties of various USG findings in COVID-19 patients, India.
| Test | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| Lung USG Score > =13 | 93.3 (77.9, 99.2) | 24.5 (17.7, 32.4) | 20.6 (14.1, 28.4) | 94.6 (81.8, 99.3) |
| Low Ejection Fraction | 10.3 (2.2, 27.4) | 92.3 (86.6, 96.1) | 21.4 (4.7, 50.8) | 83.4 (76.7, 88.9) |
| Slow flow (CFV or Popliteal) | 60.0 (40.6, 77.3) | 34.0 (26.5, 42.7) | 16.1 (9.8, 24.2) | 80.3 (68.2, 89.4) |
| Thrombosis (CFV or Popliteal) | 16.7 (6.0, 34.7) | 96.5 (92.0, 98.9) | 50.0 (18.7, 81.3) | 84.7 (78.2, 89.8) |
| Low ejection fraction or Thrombosis | 26.7 (12.3, 45.9) | 88.8 (82.5, 93.5) | 33.3 (15.6, 55.3) | 85.2 (78.5, 90.5) |
| Lung USG Score > =13 or Low ejection fraction or Thrombosis | 93.0 (77.9, 99.2) | 21.0 (14.6, 28.6) | 19.9 (13.6, 27.4) | 93.8 (79.2, 99.2) |
| Lung USG Score > =13 or Low ejection fraction or Thrombosis or Slow flow | 96.7 (82.8, 99.9) | 10.5 (5.9, 16.7) | 18.5 (12.7, 25.4) | 93.8 (69.8, 99.8) |
The mean (SD) P/F ratio was lower in those with a LUS of > =13 compared with those who had a lower score; though the difference was not statistically significant (190.3 [83.6] vs 219.1 [135.3]; p = 0.11). We identified pneumothorax and pulmonary embolism in 6% of the study population. All patients with pneumothorax were treated with intercostal drainage. Patients with hemodynamically unstable pulmonary embolism along with signs of right ventricular dysfunction on POCUS were thrombolysed with intravenous tissue plasminogen activator.
As indicated in the methods, we did a sub-group analysis of 49 patients in whom the CT scan reports were available. The median (IQR) CT score in these patients was 18 (15, 21); five (10.2%) were classified as mild, 14 (28.6%) were classified as moderate, and 30 were classified as severe (61.2%). CT pulmonary angiography was done in nine (18.4%) of these patients. We found that the agreement between USG score of > =13 and CT score of moderate/severe was 85.7% (95% CI: 62.8–100%). The interrater agreement between these two parameters was 0.82 (95% CI: 0.68, 0.97).
4. Discussion
The present study on the use of point-of-care ultrasonography of lungs and the cardio-vascular system (COVID-19 POCUS protocol) provides useful data on its utility in management of COVID-19 patients. The most common features seen on lung ultrasound were presence of sliding, irregular pleura, and B3 lines (B prime profile). In our study, severe findings were noted in the left and right prone positions - suggestive of a predominant lower lobe subpleural involvement. We also recorded spontaneous echo contrast and thrombosis in blood vessels. We also observed that mortality was higher in patients who had thrombosis in these veins. A lung ultrasound severity score of 13 was an optimal cut-off for prediction of mortality. A combination of lung and CVS findings improved the sensitivity. There was good agreement between the severity of lung ultrasound scores and CT scan scores.
POCUS is an important diagnostic tool in diagnosis and management of COVID-19. It has been reported that sonographic changes may be present even before development of hypoxemia [33]. Some common features seen on lung ultrasound are irregular pleura, multiple patterns of B lines, consolidations, or pleural thickening [34], [35], [36], [37], [38]. We also found these patterns of B prime profile and C profile in our study population. Even though, pleural effusion was seen in our population, the proportion was low (4%). Other authors have also reported that pleural effusion is an uncommon feature on the lung ultrasound in COVID-19 patients [34], [39]. In fact, in our study majority of the patients (75%) with pleural effusion had cardiac changes as well (pericardial effusion or low ejection fraction), probably suggesting a cardiac cause rather than a pulmonary cause. Though pleural effusion may be seen in patients with COVID-19, authors have argued that it may be uncommon and other causes should also be investigated [40], [41], [42]. Based on our findings, we also suggest that in the presence of pleural effusion on USG, the clinician should actively search for other causes (non-pulmonary) as well. Even though, we predominantly reported lower lobe involvement of the lungs, other authors have reported more involvement of the upper lateral and posterior zones [43].
The role of cardiac ultrasonography has also been discussed in literature. Indeed, findings suggestive of cardiomyopathy and acute cardiac injury have been reported by various authors [44], [45]. Cameli and colleagues [46] have presented a review of cardiac findings in the COVID-19 patients. Some of the important presentations are left ventricular dilatation (as a feature of acute heart failure), abnormalities of ventricular walls (features suggestive of acute coronary syndrome), or pericardial effusion (suggestive of tamponade) [46]. The most common finding in our study was mild pericardial effusion followed by chamber dilatation. Though, we had cases of mild pericardial effusion, there was no case of cardiac tamponade suggesting that it may be a primary feature of COVID-19 involvement of the pericardium. Nonetheless, these cardiac findings had high specificity for prediction of mortality. Previous studies have found that the prevalence of venous thrombosis ranges from 25% to as high as 69% in moderate to severe COVID-19 cases [18], [47], [48], [49]; however, the proportion of venous thrombosis in our study was about 6%. This may be due an actual low prevalence of thrombosis in our population, or due to universal USG in all patients (irrespective of the severity of the disease), we may have picked up the cases relatively early on in the disease. This may help us initiate antithrombotic treatment early and perhaps, reduce the mortality in these patients. Spontaneous echo contrast was initially reported by Dugar and colleagues in patients with COVID-19 in whom POCUS was done. They found a high proportion of this feature in their patients and it was also associated with high inflammatory markers and increased incidence of deep vein thrombosis [47]. Another study found that patients with spontaneous echo contrast had hyper viscosity and thrombotic events [50]. In general, there was no association between spontaneous echo contrast with the D Dimer value or NEWS 2 score. This further reaffirms the fallacy of using d- Dimer based anticoagulation and adds value to include the COVID-19 POCUS protocol to identify thrombosis of common femoral or popliteal veins.
The diagnostic and prognostic attributes of POCUS in COVID-19 have been discussed in the literature [51]. A study by Castelao and colleagues [25] found that severity of lungs in the anterior region was significantly associated non-invasive respiratory support (NIRS) Furthermore, they also found that an overall score of 19 was an optimal cut-off for NIRS. Another study by Dargent and co-workers [52] described the role of lung ultrasound score in management of COVID-19 patients. They found that it was associated with disease progression and early diagnosis of ventilator associated pneumonia. We found that a cut-off of 13 for the lung score had a good sensitivity and negative predictive value for mortality. Thus, this score can be used for active monitoring and management of COVID-19 patients. In addition, patients with pneumothorax can also be monitored and managed appropriately. As seen in our study, thrombosis and low ejection fraction had a high specificity and negative predictive value for mortality. Thus, individuals who did not have these features had a low probability of death. Furthermore, presence of a thrombus also helps in early initiation of antithrombotic management in these patients. It has been highlighted that multi-organ sonography is relatively easy compared with a CT scan [33]. This POCUS of the lungs and cardiovascular system is not only useful in diagnosis of COVID-19 but also in management of complications [53] and prognosis.
The study had its limitations. We did not repeat the COVID-19 POCUS protocol to monitor the progress of ultrasound changes and the clinical course during the stay in the hospital. This would have helped in monitoring the progress of the changes and its association with disease progression in these patients. Furthermore, we used mortality as the main outcome in our study. Previous authors have used NIRS and other intermediate outcomes. We could have added the upper prone point & the PLAPS to make the lung ultrasound severity score more robust, but it would have added to the insonation time.
Nonetheless, despite these limitations the study is a useful addition to the literature on the role of point of care ultrasonography. We have included both pulmonary and cardiovascular sonography (COVID-19 POCUS protocol) in our study. We found that a combination of pulmonary (>= 13 score on lung ultrasound) and cardiovascular findings (low ejection fraction, thrombosis, and slow flow) were sensitive for prediction of mortality in COVID-19 patients. More specifically, pulmonary findings had a higher sensitivity and cardiovascular findings had a higher specificity. The advantages of ultrasonography - such as portability, bed-side procedure, detection of silent cases (in contacts), and exposure to fewer health care workers– has been discussed in literature [35], [54]. Our study finds that multi-organ POCUS is effective in diagnosis, prognosis, and management of COVID-19 patients since COVID-19 does not involve the lung alone but other organs as well. Rather than just lung ultrasound, clinicians should use a multiorgan POCUS with a predefined protocol like ours in COVID-19 patients. This will help in early identification of severe lung involvement and thrombotic changes, and may help to reduce the mortality in these patients. It will be useful in rural areas of low and low-middle income countries (such as India, which was affected by stronger second wave of COVID-19 infection). In rural areas, access to radio-diagnostics such as CT scans is limited, and POCUS may be a good alternative.
4.1. Ethics
The study was approved by the Wockhardt Hospitals Institutional Review Board. It was a secondary data analysis of clinical data.
Availability of data and raw material
Data can be made available on suitable request to the authors and after approval by the local Ethics Committee. Requests for data can be made to Dr. P Rindani, CEO Wockhardt Hospitals, Mumbai, email: prindani@wockhardt.com.
Funding
The study was not funded.
CRediT authorship contribution statement
All the listed authors have contributed to conceptualisation of the study, management of patients, data collection, data entry and analysis, writing the manuscript, and providing feedback on the manuscript. Kedar Toraskar conceptualised the study and worked on the draft, Ravindra R. Zore supervised the study, helped with data, and provided feedback on the manuscript. Gaurav A. Gupta helped with project administration and data collection, Bhooshan Gondse and Gurudas Pundpal helped with investigations and data curation. Shirishkumar Kadam and Sachin Pawaskar helped with data collection and managing resources for the study. Maninder Singh Setia helped with data analysis and manuscript preparation.
Competing Interest
The authors declare that they do not have any competing interest.
Acknowledgement
We would like to acknowledge Dr. Parag Rindani for all the support. We would like to acknowledge Dr. Mahesh Dhokare, Dr. Ekta Vedak, Dr. Tooba Ansari, and Dr. Naaz Fatima Ansari for help with the study. We would also like to thank Dr. Bhawan Paunipagar for inputs of radiology. We would like to thank the reviewers of this manuscript. Their comments have helped us improve the manuscript.
Consent
The present study is a secondary data analysis of clinical data.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altamimi H., Abid A.R., Othman F., Patel A. Cardiovascular manifestations of COVID-19. Heart Views. 2020;21(3):171–186. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_150_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nandy S., Wan S.H., Brenes-Salazar J. Cardiovascular manifestations of COVID-19. Curr Cardiol Rev. 2020. [DOI] [PMC free article] [PubMed]
- 4.Sharma Y.P., Agstam S., Yadav A., Gupta A., Gupta A. Cardiovascular manifestations of COVID-19: An evidence-based narrative review. Indian J. Med Res. 2021;153(1 & 2):7–16. doi: 10.4103/ijmr.IJMR_2450_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic-a focused review for clinicians. Clin. Microbiol Infect. 2020;26(7):842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esakandari H., Nabi-Afjadi M., Fakkari-Afjadi J., Farahmandian N., Miresmaeili S.M., Bahreini E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online. 2020;22:19. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiala M.J. Ultrasound in COVID-19: a timeline of ultrasound findings in relation to CT. Clin. Radio. 2020;75(7):553–554. doi: 10.1016/j.crad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Homsi M., Chung M., Bernheim A., et al. Review of chest CT manifestations of COVID-19 infection. Eur. J. Radio. Open. 2020;7 doi: 10.1016/j.ejro.2020.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishfaq A., Yousaf Farooq S.M., Goraya A., et al. Role of High Resolution Computed Tomography chest in the diagnosis and evaluation of COVID -19 patients -A systematic review and meta-analysis. Eur. J. Radio. Open. 2021;8 doi: 10.1016/j.ejro.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A Preliminary Study on the Ultrasonic Manifestations of Peripulmonary Lesions of Non-Critical Novel Coronavirus Pneumonia (COVID-19) (February 26, 2020). 2020. https://ssrn.com/abstract=3544750.
- 11.Moore S., Gardiner E. Point of care and intensive care lung ultrasound: A reference guide for practitioners during COVID-19. Radiogr. (Lond. ) 2020;26(4):e297–e302. doi: 10.1016/j.radi.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pezzutti D.L., Wadhwa V., Makary M.S. COVID-19 imaging: Diagnostic approaches, challenges, and evolving advances. World J. Radio. 2021;13(6):171–191. doi: 10.4329/wjr.v13.i6.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhoi S., Sahu A.K., Mathew R., Sinha T.P. Point-of-care ultrasound in COVID-19 pandemic. Post. Med J. 2021;97(1143):62–63. doi: 10.1136/postgradmedj-2020-137853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez M.A., Shams N., Ellington L.E., et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir. Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joan Toth. Utility of Point-of-Care Ultrasound Across Clinical Applications Spurs Continued Growth. Imaging Technology News 2019; https://www.itnonline.com/article/utility-point-care-ultrasound-across-clinical-applications-spurs-continued-growth. Accessed 12 August, 2021.
- 16.Kogan E.A., Kukleva A.D., Berezovskiy Y.S., et al. [Clinical and morphological characteristics of SARS-CoV-2-related myocarditis proven by the presence of viral RNA and proteins in myocardial tissue] Arkh Patol. 2021;83(4):5–13. doi: 10.17116/patol2021830415. [DOI] [PubMed] [Google Scholar]
- 17.Finsterer J., Scorza F.A., Scorza C.A., Fiorini A.C. Extrapulmonary onset manifestations of COVID-19. Clin. (Sao Paulo) 2021;76 doi: 10.6061/clinics/2021/e2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allegorico E., Buonerba C., Bosso G., et al. The use of chest ultrasonography in suspected cases of COVID-19 in the emergency department. Future Sci. OA. 2020;7(1):FSO635. doi: 10.2144/fsoa-2020-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mort D.O., Limbu D., Nunan J., Walden A. Abnormal Lung Point-of-Care Ultrasound (POCUS) in Suspected Cases of COVID-19 pneumonia with Normal Plain Chest Radiographs - A Case Series. Acute Med. 2020;19(3):162–167. [PubMed] [Google Scholar]
- 22.Seibel A., Heinz W., Greim C.A., Weber S. [Lung ultrasound in COVID-19] Anaesthesist. 2021;70(2):146–154. doi: 10.1007/s00101-020-00883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil-Rodrigo A., Llorens P., Martinez-Buendia C., Luque-Hernandez M.J., Espinosa B., Ramos-Rincon J.M. Diagnostic yield of point-of-care ultrasound imaging of the lung in patients with COVID-19. Emergencias. 2020;32(5):340–344. [PubMed] [Google Scholar]
- 24.Lichtenstein D., Mezière G. The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit. Ultrasound J. 2011;3:109–110. [Google Scholar]
- 25.Castelao J., Graziani D., Soriano J.B., Izquierdo J.L. Findings and Prognostic Value of Lung Ultrasound in COVID-19 Pneumonia. J. Ultrasound Med. 2020 doi: 10.1002/jum.15508. [DOI] [PubMed] [Google Scholar]
- 26.Oveland N., Bogale N., Waldron B., Bech K., Sloth E. Focus assessed transthoracic echocardiography (FATE) to diagnose pleural effusions causing haemodynamic compromise. Case Rep. Clin. Med. 2013;2(3):189–193. [Google Scholar]
- 27.Lee Y., Shin H., Kim C., Lee I., Choi H.J. Learning curve-cumulative summation analysis of visual estimation of left ventricular function in novice practitioners: A STROBE-compliant article. Med. (Baltim. ) 2019;98(14) doi: 10.1097/MD.0000000000015191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler Y., Charron P., Imazio M., et al. ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2015. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.H., Lee S.H., Yun S.J. Comparison of 2-point and 3-point point-of-care ultrasound techniques for deep vein thrombosis at the emergency department: A meta-analysis. Med. (Baltim. ) 2019;98(22) doi: 10.1097/MD.0000000000015791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gwet K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br. J. Math. Stat. Psychol. 2008;61(Pt 1):29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 31.Walsh P., Thornton J., Asato J., et al. Approaches to describing inter-rater reliability of the overall clinical appearance of febrile infants and toddlers in the emergency department. PeerJ. 2014;2 doi: 10.7717/peerj.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wongpakaran N., Wongpakaran T., Wedding D., Gwet K.L. A comparison of Cohen's Kappa and Gwet's AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Med. Res. Method. 2013;13:61. doi: 10.1186/1471-2288-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldati G., Smargiassi A., Inchingolo R., et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound Med. 2020;39(7):1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Convissar D.L., Gibson L.E., Berra L., Bittner E.A., Chang M.G. Application of Lung Ultrasound During the COVID-19 Pandemic: A Narrative Review. Anesth. Analg. 2020;131(2):345–350. doi: 10.1213/ANE.0000000000004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buonsenso D., Piano A., Raffaelli F., Bonadia N., de Gaetano Donati K., Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur. Rev. Med Pharm. Sci. 2020;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 36.Thomas A., Haljan G., Mitra A. Lung ultrasound findings in a 64-year-old woman with COVID-19. CMAJ. 2020;192(15) doi: 10.1503/cmaj.200414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomoro P., Verde F., Zerboni F., et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur. J. Radio. Open. 2020;7 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jajodia A., Ebner L., Heidinger B., Chaturvedi A., Prosch H. Imaging in corona virus disease 2019 (COVID-19)-A Scoping review. Eur. J. Radio. Open. 2020;7 doi: 10.1016/j.ejro.2020.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin Y.H., Cai L., Cheng Z.S., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussein M., Haq I.U., Hameed M., et al. Pleural effusion as an isolated finding in COVID-19 infection. Respir. Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahad A.M., Al-Khalidi H.A., Abdulhameed Alhaideri Y.A., Majeed Altimimi Y.Q., Alshewered A.S. Pleural effusion in a patient with COVID-19 pneumonia and lung cancer: A case report. Respir. Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aujayeb A. Clarification on Pleural effusions in Covid-19. Radiol.: Cardiothorac. Imaging. 2020;2 doi: 10.1148/ryct.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosso G., Allegorico E., Pagano A., et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern Emerg. Med. 2021;16(2):471–476. doi: 10.1007/s11739-020-02512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capotosto L., Nguyen B.L., Ciardi M.R., Mastroianni C., Vitarelli A. Heart, COVID-19, and echocardiography. Echocardiography. 2020;37(9):1454–1464. doi: 10.1111/echo.14834. [DOI] [PubMed] [Google Scholar]
- 45.Abrams E.R., Rose G., Fields J.M., Esener D. Point-of-care ultrasound in the evaluation of COVID-19. J. Emerg. Med. 2020;59(3):403–408. doi: 10.1016/j.jemermed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameli M., Pastore M.C., Soliman Aboumarie H., et al. Usefulness of echocardiography to detect cardiac involvement in COVID-19 patients. Echocardiography. 2020;37(8):1278–1286. doi: 10.1111/echo.14779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dugar S., Duggal A., Bassel A., Soliman M., Moghekar A. Spontaneous echo contrast in venous ultrasound of severe COVID-19 patients. Intensive Care Med. 2020;46(8):1637–1639. doi: 10.1007/s00134-020-06094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connor-Schuler R., Daniels L., Coleman C., Harris D., Herbst N., Fiza B. Presence of Spontaneous Echo Contrast on Point-of-Care Vascular Ultrasound and the Development of Major Clotting Events in Coronavirus Disease 2019 Patients. Crit. Care Explor. 2021;3(1) doi: 10.1097/CCE.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perrone T., Soldati G., Padovini L., et al. A New Lung Ultrasound Protocol Able to Predict Worsening in Patients Affected by Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. J. Ultrasound Med. 2020 doi: 10.1002/jum.15548. [DOI] [PubMed] [Google Scholar]
- 52.Dargent A., Chatelain E., Kreitmann L., et al. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galluccio F., Ergonenc T., Yamak Altinpulluk E., et al. Role of point of care ultrasound in COVID-19 pandemic: what lies beyond the horizon? Med Ultrason. 2020;22(4):461–468. doi: 10.11152/mu-2614. [DOI] [PubMed] [Google Scholar]
- 54.Boero E., Schreiber A., Rovida S., Vetrugno L., Blaivas M. The role of lung ultrasonography in COVID-19 disease management. J. Am. Coll. Emerg. Physicians Open. 2020 doi: 10.1002/emp2.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]