Abstract
Background
Studies combining data from digital surveys and electronic health records (EHR) can be used to conduct comprehensive assessments on COVID-19 vaccine safety.
Methods
We conducted an observational study using data from a digital survey and EHR of children aged 5–11 years vaccinated with Pfizer-BioNTech COVID-19 mRNA vaccine across Kaiser Permanente Southern California during November 4, 2021-February 28, 2022. Parents/guardians who enrolled their children were sent a 14-day survey on reactions. Survey results were combined with EHR, and medical encounters were described for children whose parents or guardians indicated seeking medical care for vaccine-related symptoms. This study describes self-reported reactions (local and systemic) and additional symptoms (chest pain, tachycardia, and pre-syncope).
Results
The study recruited 7,077 participants aged 5–11 years who received the Pfizer-BioNTech COVID-19 mRNA vaccine. Of 6,247 participants with survey responses after dose 1, 2,176 (35 %) reported at least one systemic reaction, and 1,076 (32 %) of 3,401 respondents following dose 2 reported at least one systemic reaction. Local reactions were reported less frequently following dose 2 (1,113, 33 %) than dose 1 (3,140, 50 %). The most frequently reported reactions after dose 1 were pain at the injection site (48 %), fatigue (20 %), headache (12 %), myalgia (9 %) and fever (5 %). The most frequently reported symptoms after dose 2 were also pain at the injection site (30 %), fatigue (19 %), headache (13 %), myalgia (10 %) and fever (9 %). Post-vaccination reactions occurred most frequently-one day following vaccination. Chest pain or tachycardia were reported infrequently (1 %). EHR demonstrated that parents rarely sought care for post-vaccination symptoms, and among those seeking care, the most common symptoms documented in EHR were fever and nausea, comprising <0.5 % of children. No encounters were related to myocarditis.
Conclusion
While post-vaccination reactions to the Pfizer-BioNTech COVID-19 mRNA vaccine were common in children aged 5–11 years, our data showed that in most cases they were transient and did not require medical care.
Keyword: COVID-19, Vaccination, Adverse events, Surveillance, Vaccine safety
Abbreviations: CDC, Centers for Disease Control and Prevention; EHR, electronic health records; KPSEM, Kaiser Permanente Side Effect Monitor
1. Introduction
Global mass vaccination campaigns are underway to combat the ongoing COVID-19 pandemic, with almost 12.8 billion doses of COVID-19 vaccines having been administered as of October 2022. [1] The United States was one of the first countries to issue an Emergency Use Authorization to allow for the administration of the Pfizer-BioNTech COVID-19 mRNA vaccine among children aged 5–11 years. However, vaccine uptake in this age group has been low. Vaccine hesitancy may be related to safety concerns, particularly following recent reports of rare serious adverse events occurring mostly in young adults and adolescents such as thrombosis with thrombocytopenia syndrome and myocarditis [2], [3].
Robust vaccine safety surveillance systems are important to maintain public confidence in the vaccination program. Traditionally, post-marketing vaccine safety monitoring in the United States has relied on the Vaccine Adverse Event Reporting System (VAERS), a spontaneous reporting vaccine safety system, and the Vaccine Safety Datalink (VSD), which conducts near real time monitoring of Electronic Health Records (EHR) data from 8 integrated healthcare systems [4]. As part of the COVID-19 vaccine safety monitoring response, the Centers for Disease Control and Prevention (CDC) developed a novel smartphone-based tool that uses text messaging and secure web-based surveys to provide personalized health check-ins called ‘v-safe’ [5]. V-safe is an opt-in active surveillance system for any COVID-19 vaccine recipients in the United States (US). However, v-safe survey responses are not linked with the vaccinee’s EHR; thus, their clinical interpretation is limited [6].
The current study is the first to combine self-reported survey data from a new digital tool called ‘The Kaiser Permanente Side Effect Monitor’ (KPSEM), with individual-level EHR across all care settings from a large integrated healthcare system. This study describes reactions and symptoms in the two weeks following Pfizer-BioNTech COVID-19 vaccination among children aged 5–11 years by combining self-reported data collected from KPSEM and EHR-based healthcare utilization data from Kaiser Permanente Southern California (KPSC).
2. Methods
2.1. Study setting
KPSC, a contributing VSD site, is a large integrated healthcare system with more than 4·7 million members that covers 15 large medical center areas. Members are generally representative of the insured population of Southern California [7]. The EHR includes all clinical data such as diagnostic, pharmacy, laboratory, and vaccination history information across all settings of care, regardless of the location of care delivery within the KPSC network, as outside providers must submit detailed claims to KPSC for reimbursement by the health plan.
2.2. Study population
The study population consisted of all KPSC members aged 5–11 years that received a Pfizer-BioNTech COVID-19 mRNA vaccination at KPSC facilities between November 4, 2021, and February 28, 2022, for whom a parent or guardian completed at least one digital KPSEM survey in the 0–14 days following vaccination.
3. Study procedures
3.1. Recruitment
KPSEM is a voluntary digital system that uses text messaging and secure web-based surveys to monitor COVID-19 vaccine safety for common local or injection-site and systemic reactions. Two methods were used to recruit COVID-19 vaccinated patients into KPSEM: (1) passive recruitment of individuals at the time of vaccination via QR codes available on posters, banners, and study handouts provided at the vaccination clinic; and (2) electronic invitation via text message or email using contact information provided at the time of the vaccination appointment or from the EHR. Study materials were provided in Spanish and English languages, according to the patient preference expressed at recruitment or as indicated in their EHR. Online consent from parents or guardians was obtained at the time of recruitment.
3.2. Follow up
Following consent, parents or guardians were prompted to complete surveys via text message or email following vaccination. Survey prompts were sent daily for the first week (days 0–7), and on alternate days for the second week (days 8–14) following recruitment. The system resets automatically to the initial survey frequency after the EHR indicates that the participant has received an additional dose. We analyzed survey reports from days 0–14 following dose 1 and dose 2 vaccinations for local reactions, including pain, itching, redness or swelling around the injection site and systemic reactions, including fatigue, fever, headache, body aches or muscle or joint pain (myalgia), nausea or vomiting and rash.
In addition to the pre-specified local and systemic reactions, KPSEM collected additional symptoms that may be indicative of emerging safety concerns (hereafter referred to as ‘symptoms’). For example, information on tachycardia and chest pain were included as proxy indicators of myocarditis. KPSEM also included an option for parents to report other symptoms (outside the pre-selected symptoms) using a single checkbox on the survey.
Participants that indicated experiencing any of the above solicited reactions or symptoms were subsequently asked whether they sought medical care (yes/no). Survey questions and timings are described in further detail in the Appendix (Appendix A).
3.3. Physician chart reviews
Participants who reported receiving medical care in the 0–14 days following Pfizer-BioNTech COVID-19 mRNA vaccination with either dose, and for whom a documentation of a medical encounter within an emergency department (ED) or inpatient setting was identified in the 0–21 days following vaccination were flagged for a physician chart review. The purpose of the physician chart review was to confirm the diagnoses and to identify whether severe health events could have been plausibly related to the COVID-19 vaccination. Due to the focus on severe outcomes, outpatient encounters in the 0–21 days following vaccination were not assessed via chart review. Two co-investigators conducted independent chart reviews and any disagreements were discussed by the wider study team for a final determination. As a sensitivity analysis to assess whether any severe post-vaccine reactions or symptoms were missed by the KPSEM survey tool, the EHR of all study participants with an ED or hospitalization 0–21 days following receipt of Pfizer-BioNTech COVID-19 mRNA vaccine underwent chart review, regardless of KPSEM survey responses. The chart review form was developed with input from members of the VSD, CDC and study co-authors, which included pediatricians, epidemiologists, and public health researchers. The chart review form is available upon request.
3.4. Healthcare utilization
To assess overall healthcare utilization for reported systemic reactions or symptoms among KPSEM participants, EHR records across all care settings for all study participants occurring 0–21 days following receipt of Pfizer-BioNTech COVID-19 mRNA vaccine were searched for diagnosis codes related to their reported reactions. Diagnosis codes were categorized according to the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10). Each encounter was categorized as potentially related to the self-reported post-vaccine systemic reaction or symptom according to a pre-specified list of ICD-10 codes (Appendix B).
3.5. Statistical analysis
Descriptive statistics were calculated for participants' demographic characteristics (sex, age, and race or ethnicity, insurance type [government subsidized vs commercial] and prior SARS-CoV-2 infection [including self-reported and EHR-documented diagnoses or laboratory codes]). Categorical variables were summarized by frequency distributions and compared using chi-square tests or the Fisher’s exact test. The number and proportion of self-reported reactions or symptoms and related healthcare utilization were calculated by dose number and time since vaccination (days and weeks).
Analyses were completed using SAS (version 9.4) and graphical representations were generated using R (version 4.1.3). All study activities were reviewed by CDC and completed in accordance with applicable federal law and CDC policy. In addition, the study protocol was reviewed and approved by the KPSC institutional review board, which waived requirement for informed consent (#12769).
4. Results
4.1. KPSEM participation statistics
A total of 79,780 children aged 5–11 years received their first dose of Pfizer-BioNTech COVID-19 mRNA vaccine at KPSC facilities over the study period, of whom parents or guardians for 6,247 (7.8 %) completed at least one KPSEM survey in the 14 days following vaccination (Table 1; Appendix C). Compared with dose 1, participation was lower following dose 2, with 4.2 % (3,401/80,863) of parents or guardians of all eligible children completing at least one survey in the 0–14 days following the second dose of vaccination (Appendix C). Participation was slightly higher among parents or guardians of children of younger ages, of white race/ethnicity vs all other race/ethnicities (p = 0.021), and among children covered by commercial compared to state-subsidized (Medicaid) insurance plans (p = 0.021).
Table 1.
Demographic characteristics of the study population*, by dose.
| Characteristic | Overall | Dose 1 | Dose 2 |
|---|---|---|---|
| Total, N | 7,077 | 6,247 | 3,401 |
| Age, years | |||
| 5 | 1,010 (14 %) | 889 (14 %) | 497 (15 %) |
| 6 | 1,007 (14 %) | 902 (14 %) | 488 (14 %) |
| 7 | 995 (14 %) | 880 (14 %) | 476 (14 %) |
| 8 | 1,030 (15 %) | 909 (15 %) | 516 (15 %) |
| 9 | 1,022 (14 %) | 895 (14 %) | 478 (14 %) |
| 10 | 985 (14 %) | 882 (14 %) | 467 (14 %) |
| 11 | 1,028 (15 %) | 890 (14 %) | 479 (14 %) |
| Sex | |||
| Female | 3,498 49 %) | 3,090 (49 %) | 1,671 (49 %) |
| Male | 3,576 (51 %) | 3,154 (50 %) | 1,729 (51 %) |
| Race/Ethnicity | |||
| Hispanic | 2,817 (40 %) | 2,449 (39 %) | 1,393 (41 %) |
| Asian | 881 (12 %) | 797 (13 %) | 409 (12 %) |
| Black | 312 (4 %) | 272 (4 %) | 156 (5 %) |
| Other/Unknown | 433 (6 %) | 374 (6 %) | 201 (6 %) |
| White | 2,151 (30 %) | 1,923 (31 %) | 1,018 (30 %) |
| Insurance | |||
| Medicaid | 1,078 (15 %) | 958 (15 %) | 508 (15 %) |
| Commercial | 5,999 (85 %) | 5,289 (85 %) | 2,893 (85 %) |
| Prior COVID-19† | |||
| Yes | 733 (10 %) | 650 (10 %) | 345 (10 %) |
| No | 6,344 (90 %) | 5,597 (90 %) | 3,056 (90 %) |
* Study participants included children aged 5–11 years for whom a parent or guardian completed at least one KPSEM survey 0–14 days following vaccination.
Prior COVID-19 was defined as any documentation of SARS-CoV-2 positive laboratory result of COVID-19 diagnosis in electronic health records prior to the date of vaccination, or any self-reported prior COVID-19 infection prior to or at the time of vaccination.
4.2. Study population
Among all participants (N = 7,077), the median age was 8 years, 40 % were Hispanic, 31 % were White, and 15 % were on government subsidized health plans (Table 1 ). The study population consisted of approximately equal proportions of males and females. Approximately 10 % of all study participants had evidence of a previous SARS-CoV-2 infection, defined as either self-reported prior infection or documentation of a previous SARS-COV-2 diagnosis, or a positive SARS-CoV-2 laboratory test in the EHR. Characteristics of the study population were similar by dose number, with a large overlap of study participants between dose 1 and dose 2 (2,571/7,077, 36 %).
4.3. Self-reported post-vaccine reactions and symptoms
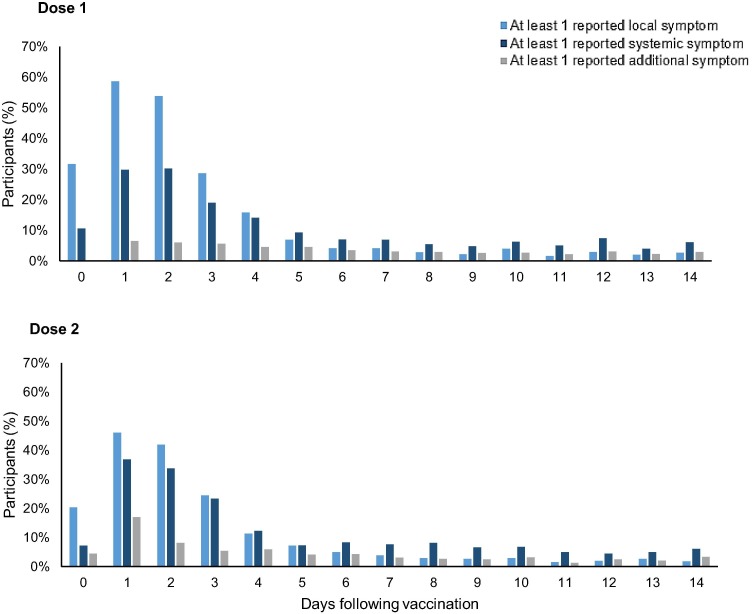
Within 14 days following vaccination, approximately half of participants receiving dose 1 (3,140/6,247; 50 %) and one third of participants receiving dose 2 (1,113/3,401; 33 %) reported experiencing local injection site reactions through KPSEM (Table 2 ). The most common reported local reaction was pain around the injection site (48 % and 30 % for dose 1 and 2, respectively). Systemic reactions were reported at similar rates following dose 1 (2,176/6,247; 35 %) and dose 2 (1,076/ 3,401; 32 %). Reactions and symptoms occurred most frequently-one day following vaccination for both doses (Fig. 1 ), with most reported symptoms occurring within the first 7 days (Appendix D & E).
Table 2.
KPSEM responses, by self-reported symptom or reaction and dose.
| Symptom |
Dose 1 |
Dose 2 |
||||||
|---|---|---|---|---|---|---|---|---|
|
KPSEM report |
Self-reported medical care† |
KPSEM report |
Self-reported medical care† |
|||||
| N | % | N | % | N | % | N | % | |
| All | 6,247 | 3,401 | ||||||
| Any symptom* | 3,934 | 63.0 | 129 | 2.1 | 1,601 | 47.1 | 59 | 1.7 |
| Local reaction | ||||||||
| Any local | 3,140 | 50.3 | 87 | 1.4 | 1,113 | 32.7 | 37 | 1.1 |
| Pain | 2,988 | 47.8 | 77 | 1.2 | 1,028 | 30.2 | 35 | 1.0 |
| Itching | 281 | 4.5 | 16 | 0.3 | 119 | 3.5 | 5 | 0.1 |
| Swelling | 213 | 3.4 | 8 | 0.1 | 110 | 3.2 | 7 | 0.2 |
| Redness | 195 | 3.1 | 12 | 0.2 | 102 | 3.0 | 8 | 0.2 |
| Systemic reaction | ||||||||
| Any systemic | 2,176 | 34.8 | 106 | 1.7 | 1,076 | 31.6 | 47 | 1.4 |
| Fatigue | 1,262 | 20.2 | 68 | 1.1 | 649 | 19.1 | 31 | 0.9 |
| Headache | 775 | 12.4 | 46 | 0.7 | 447 | 13.1 | 25 | 0.7 |
| Myalgia | 584 | 9.3 | 34 | 0.5 | 325 | 9.6 | 16 | 0.5 |
| Fever | 330 | 5.3 | 31 | 0.5 | 292 | 8.6 | 26 | 0.8 |
| Nausea | 288 | 4.6 | 32 | 0.5 | 168 | 4.9 | 13 | 0.4 |
| Rash | 268 | 4.3 | 21 | 0.3 | 125 | 3.7 | 9 | 0.3 |
| Chills | 220 | 3.5 | 27 | 0.4 | 155 | 4.6 | 16 | 0.5 |
| Additional symptom | 37 |
1.1 |
||||||
| Pre-syncope | 145 | 2.3 | 18 | 0.3 | 107 | 3.1 | 6 | 0.2 |
| Chest pain | 42 | 0.7 | 12 | 0.2 | 42 | 1.2 | 6 | 0.2 |
| Tachycardia | 37 | 0.6 | 10 | 0.2 | 26 | 0.8 | 3 | 0.1 |
| Other | 582 | 9.3 | 80 | 1.3 | 244 | 7.2 | 23 | 0.7 |
* Number of study participants whose parents or guardians reported any of the symptoms or reactions at least once in the 0–14 days following vaccination.
Number of study participants whose parents or guardians reported seeking medical care for any symptom or reaction with 0–14 days following vaccination.
Fig. 1.
Local, systemic and additional symptoms reported to KPSEM, by day following vaccination and dose.
The most common reported systemic reactions were fatigue (20 % and 19 %), followed by headache (12 % and 13 %), myalgia/body pain (9 % and 10 %) and fever (5 % and 9 %). Systemic reactions were documented more frequently among children of older ages after dose 1 compared with children of younger ages (p = 0.003, Table 3 ); however, parents or guardians of younger children reported seeking medical care for their post-vaccine reactions or symptoms at a higher frequency than older children. The proportion of participants with at least one reported systemic effect after dose 1 was higher among children of non-Hispanic Black race, with 131 (48 %) of 272 children of Black race experiencing at least one systemic effect compared with 674 (35 %) of 1,923 non-Hispanic White children (p < 0.001). Medicaid (government-subsidized) insurance was associated with increased reporting, with 428 (45 %) of 958 children on Medicaid subsidized insurance reporting experiencing at least one systemic reaction compared with 1,953 (37 %) of 5,289 children on commercial insurance plans (p < 0.001). The observed differences for the proportion of participants experiencing systemic reactions by race/ethnicity and insurance plans were similar following dose 2.
Table 3.
Proportion of study participants reporting systemic symptoms 0–14 days following COVID-19 vaccination and related healthcare utilization, by dose.
|
Characteristics |
Dose 1 |
Dose 2 |
||||||
|---|---|---|---|---|---|---|---|---|
|
KPSEM reports, row % |
Relevant medical encounter¶ |
KPSEM reports, row% |
Relevant medical encounter¶ | |||||
| Any systemic symptom* | P value† | Sought care§ | Any systemic symptom* | P value† | Sought care§ | |||
| Total N | 2,381 (38 %) | 129 (5 %) | 34 (1 %) | 1,198 (35 %) | 59 (5 %) | 23 (2 %) | ||
| Age, years | 0.003 | 0.171 | ||||||
| 5 | 306 (34 %) | 25 (8 %) | 6 (2 %) | 182 (37 %) | 14 (8 %) | 7 (4 %) | ||
| 6 | 327 (36 %) | 19 (6 %) | 5 (2 %) | 151 (31 %) | 6 (4 %) | 1 (1 %) | ||
| 7 | 318 (36 %) | 17 (5 %) | 3 (1 %) | 170 (36 %) | 8 (5 %) | 4 (2 %) | ||
| 8 | 370 (41 %) | 22 (6 %) | 7 (1 %) | 178 (34 %) | 8 (5 %) | 6 (3 %) | ||
| 9 | 353 (39 %) | 13 (4 %) | 6 (2 %) | 166 (35 %) | 10 (6 %) | 2 (1 %) | ||
| 10 | 352 (40 %) | 21 (6 %) | 4 (1 %) | 166 (36 %) | 6 (4 %) | 2 (1 %) | ||
| 11 | 355 (40 %) | 12 (3 %) | 3 (1 %) | 185 (39 %) | 7 (4 %) | 1 (1 %) | ||
| Sex | 0.007 | 0.649 | ||||||
| Female | 1,228 (40 %) | 64 (5 %) | 21 (2 %) | 598 (36 %) | 27 (5 %) | 11 (2 %) | ||
| Male | 1,152 (37 %) | 65 (6 %) | 13 (1 %) | 600 (35 %) | 32 (5 %) | 12 (2 %) | ||
| Race/Ethnicity | <0.001 | 0.025 | ||||||
| Hispanic | 1,030 (42 %) | 65 (6 %) | 23 (2 %) | 515 (37 %) | 26 (5 %) | 9 (2 %) | ||
| Asian | 257 (32 %) | 12 (5 %) | 3 (1 %) | 131 (32 %) | 6 (5 %) | 3 (2 %) | ||
| Black | 131 (48 %) | 8 (6 %) | 0 (-) | 68 (44 %) | 8 (5 %) | 5 (7 %) | ||
| Other/Unknown | 134 (36 %) | 8 (6 %) | 2 (1 %) | 61 (30 %) | 1 (2 %) | 0 (-) | ||
| White | 674 (35 %) | 30 (4 %) | 5 (1 %) | 347 (34 %) | 13 (4 %) | 3 (1 %) | ||
| Insurance | <0.001 | 0.004 | ||||||
| Medicaid | 428 (45 %) | 31 (7 %) | 7 (2 %) | 216 (43 %) | 14 (6 %) | 7 (3 %) | ||
| Commercial | 1,953 (37 %) | 98 (5 %) | 27 (1 %) | 982 (34 %) | 45 (5 %) | 16 (2 %) | ||
| Prior COVID-19 | ||||||||
| Yes | 273 (42 %) | <0.001 | 26 (10 %) | 7 (3 %) | 143 (41 %) | <0.001 | 12 (8 %) | 5 (3 %) |
| No | 1,903 (34 %) | 103 (5 %) | 22 (1 %) | 933 (31 %) | 47 (5 %) | 16 (2 %) | ||
*Persons were identified as ever having self-reported symptoms through KPSEM if their parent or guardian responded with ‘yes’ to KPSEM for any systemic reactions in the first two weeks following vaccination.
Differences in systemic adverse event reports were compared across demographic characteristics using chi-square tests or Fisher’s exact test.
Persons identified as self-reporting seeking advice from medical professionals in response to adverse events from the COVID-19 vaccine were defined as ever having responded ‘Yes’ to the survey question asking whether they sought care for any of their symptoms in the first two weeks following vaccination.
Persons were identified as having medically attended events related to their vaccine if their parent or guardian reported having reached out to their healthcare provider via KPSEM within 14 days of receiving a Pfizer BioNTech vaccination and had evidence of a diagnosis in their medical record within 21 days of receiving a Pfizer BioNTech vaccination corresponding to the relevant adverse event specified via KPSEM (Appendix B).
Parents or guardians of children with documented prior SARS-CoV-2 infection were also more likely to report their child experiencing at least one systemic reaction after doses 1 and 2 (p < 0.001). This apparent association between prior SARS-CoV-2 exposure and post-vaccine symptom reporting was also observed for local reactions and additional symptoms, but importantly, it did not appear to be dominated by a particular symptom (Appendix F).
4.4. Healthcare utilization for reported reactions
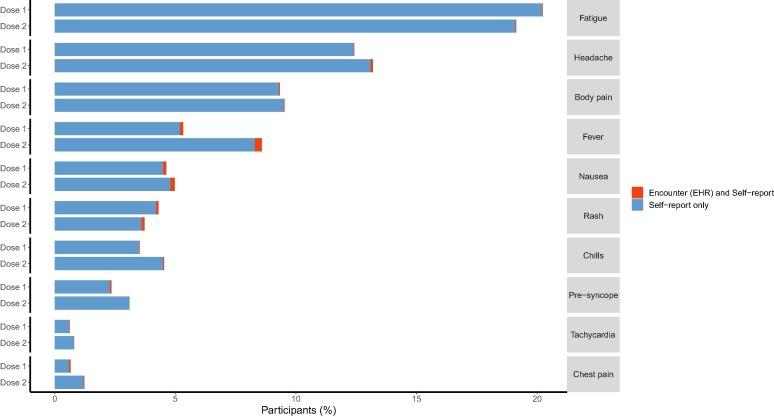
Parents or guardians reported seeking care for their child’s local or systemic reactions rarely (2 %): 129 following dose 1 and 59 following dose 2 (Table 2). Among those children reported as receiving care for their symptoms, the most common symptom documented in EMR was fever, followed by nausea, occurring in up to 0.3 % of all participants (Fig. 2 ).
Fig. 2.
Self-reported systemic reactions or additional symptoms and corresponding healthcare utilization within 14 days following BNT162b2 vaccination among children aged 5–11 years, by dose.
4.5. Physician chart reviews
Medical record review was conducted for a total of 20 unique children whose parents reported seeking medical care for solicited reactions and symptoms and for whom there was documentation of an ED or hospital admission within 21 days following vaccination to confirm that the encounter was related with the vaccine. Of these, 8 children were identified for whom the hospital encounter was possibly associated with the vaccine. These children’s diagnoses included non-specific nausea (N = 2), fever (N = 1), right lower quadrant pain (N = 1), gastroenteritis (N = 1), acute appendicitis (N = 1), allergy (N = 1), and abdominal pain (N = 1). Three (37.5 %) of these eight individuals had reported chest pain or tachycardia via KPSEM in the 0–14 days following vaccination, but their medical charts did not contain evidence for myocarditis or pericarditis, with the primary complaints being non-specific nausea (N = 2) and abdominal pain (N = 1). Two of these individuals had evidence of prior illness in their record, including prior infection and potential precordial catch syndrome.
Among all study participants receiving dose 1 of COVID-19 vaccine, 9.3 % (N = 582/6,247) reported experiencing ‘other’ symptoms outside of the pre-specified list of solicited symptoms, of whom 13.7 % (N = 80) reported seeking medical care for their post-vaccine symptoms (Table 2). After dose 2, 7.2 % (244/3,401) of children reported “other” symptoms; and 9.4 % (N = 23) reported seeking medical care. Appendix G provides the distribution of diagnosis codes among participants who reported seeking medical care and had a documented medical encounter with a diagnosis not included the pre-specified list in their EHR within 21 days following doses 1 and 2. Acute upper respiratory infection (N = 12), viral infection (N = 6), cough (N = 5), allergy (N = 4), and acute pharyngitis (N = 4) were the most frequent diagnoses after dose 1 and acute upper respiratory infection (N = 2), viral infection (N = 2), and acute pharyngitis (N = 2) were the most frequent diagnoses after dose 2.
From the sensitivity analysis of EHR diagnosis codes identified among all study participants with ED encounters or hospital admissions occurring in the 21 days following vaccination, an additional three hospital encounters were identified that were possibly related to the vaccine among individuals that did not register a self-report seeking medical care using KPSEM. The visits ranged from 1 to 7 days after receipt of vaccine, and the major presenting conditions included chest pain (N = 1, for whom the clinician did not suspect myocarditis/pericarditis upon examination), unspecified acute appendicitis (N = 1), and postvaccination fever (N = 1).
5. Discussion
The current study describes the self-reported reactogenicity and related healthcare utilization following administration of Pfizer-BioNTech COVID-19 mRNA vaccine to over 7,000 children aged 5–11 years in a large integrated US healthcare system. Using a combination of a digital survey and associated EHR data, we observed that reported reactions were generally mild and transient in nature, occurring most frequently the day after vaccination. The majority (99 %) of reported reactions did not result in medical encounters. These findings were generally consistent with those of the clinical trial data and findings from v-safe within this age group [8], [9].
Reports of local reactions were more common than systemic reactions. The most frequently reported post-vaccine local reaction was pain around the injection site (48 % and 30 % for dose 1 and 2, respectively), and the most common reported systemic reactions were fatigue (20 % and 19 %), followed by headache (12 % and 13 %), myalgia (9 % and 10 %) and fever (5 % and 9 %). These vaccine-associated reactions have been consistently observed in this order across many populations and age groups [9], [10], [11], [12], [13], [14], [15], [16]. In the current study, care givers of children of younger ages were more likely to report seeking care for their child’s post-vaccine symptoms. While it is possible that reactogenicity is more likely to require medical care in younger children compared with older children, parents of younger children have also expressed greater levels of concern around vaccine safety [17], and therefore may be more likely to seek medical care as a precaution rather than as a result of enhanced acuity of reactions.
Children in our study had an overall lower frequency of local reactions compared to published literature and clinical trial data. In the current study, approximately-one third of participants experienced any local reaction compared to over 70 % of children aged 5–11 years in clinical trials [8]. We also observed that local reactions were reported less frequently after the dose 2 than after dose 1, a finding which was not reported by v-safe. These inconsistencies could be the result of differences in population characteristics and/or differences in the data collection tool. Indeed, as observed in the current study and elsewhere, participation or interest in digital interventions varies substantially by demographic characteristics [18], [19]. For example, in KPSEM, we achieved participation rates above 7 % compared with <1 % for v-safe [9]. An important advantage of KPSEM compared with other digital tools is that we have been able to quantify representativeness, by patient characteristic, against the overall vaccinated population.
We observed higher reporting rates of reactions and symptoms among parents or guardians of children with documented SARS-CoV-2 infection prior to vaccination compared to those without documented prior SARS-CoV-2 infection. This finding has been reported previously, although in smaller studies, mostly among adults [20], [21], [22], [23]. This observation is thought to be the result of a phenomenon known as ‘immune priming’ whereby raised antibody levels following infection are similar to the levels observed following receipt of additional doses of vaccine [24].
KPSEM connects to healthcare utilization data across all care settings using the EHR, including prior medical history. This unique feature facilitates the linkage of survey responses with potentially associated medical care visits, the differentiation of incident and pre-existing outcomes, and long and short-term follow-up for health outcomes of interest. Further, most clinical trials and post-licensure digital surveillance systems have focused their safety assessments on a pre-specified list of solicited survey questions, which preclude the capture of previously unidentified adverse outcomes. In contrast, KPSEM includes an option for parents or guardians to report symptoms outside of the specified list of established post-vaccine reactions, and linkage to the EHR across all care settings provides an opportunity to further characterize potential new symptoms of concern. Our results show that among participants whose parents indicated they had experienced symptoms outside of the pre-specified list, subsequent medical encounters were rare, and mostly involved non-specific complaints, the most common being acute upper respiratory infection. Similarly, an additional advantage of KPSEM was the ability to rapidly introduce new survey questions to respond to emerging health concerns. For example, we were able to integrate and investigate self-reported chest pain or tachycardia to address emerging concerns about this potential post-vaccination symptom. We found that chest pain or tachycardia were infrequently reported (1 %), and there was no indication of myocarditis or pericarditis diagnoses in the EHR within the 21 days following vaccination among those reporting these symptoms. These findings were novel for the 5–11-year age group and are consistent with other real-world studies observing a low frequency of these adverse events with use of BNT162b2 in other age groups [25].
Despite evidence that the benefits to getting the COVID-19 vaccine far out-weigh any risks, parents or guardians continue to express hesitancy to vaccinate their children, for which perceived safety concerns may be a primary contributing factor [17]. This is particularly notable in the younger age groups, with <30 % of children aged 5–11 years having completed a primary series in the US as of June 2022 [26]. Therefore, by providing large, robust and complete data to complement existing safety studies, the findings presented in the current study may help to alleviate safety concerns among parents of young patients and healthcare providers regarding vaccine safety for this age group. Furthermore, by leveraging advances in healthcare technology to provide an additional source of patient data to a national surveillance system, the current study demonstrates the value of research collaborations between CDC and large healthcare organizations in post licensure epidemiologic evaluations of vaccine safety. Future uses of the KPSEM system include the potential to expand to other US vaccines or other US integrated healthcare systems (particularly those within the VSD network).
5.1. Limitations
The current study is subject to several limitations. First, it was not possible to establish background rates for the reported reactions or symptoms since there was no unvaccinated control population. This limitation affects other similar active surveillance systems. Many of the symptom complaints were non-specific and common in this age group regardless of receiving a vaccination, such as cough, nausea, and headache. Second, this study did not investigate healthcare utilization for local reactions following vaccination, since these are less specific and may not be accurately captured in the EHR. Third, KPSEM is a voluntary system and may be subject to a ‘healthy volunteer’ bias, which could result in an underestimate of vaccine reactions. However, we observed a high participation rate overall, even among population subgroups known to face barriers to participation in digital health interventions. Fourth, low numbers precluded specific investigation of safety following the 3rd dose of vaccination in this age group among individuals with immunocompromising conditions. Finally, participants may have experienced post-vaccine symptoms or sought healthcare after the study period, which would have been missed in the current study.
6. Conclusion
In conclusion, reactions following receipt of BNT162b2 among children aged 5–11 years were common, however most were transient. Most reactions (both systemic and local) occurred 1–2 days following vaccination. Requiring medical care for symptoms within 21 days of receiving vaccination was rare and the most common documented complaints included non-specific fever or nausea. These data may provide assurance to the public and healthcare professionals concerned about potential side effects of BNT162b2 among young children.
Funding/Support
This study was funded by US Centers for Disease Control and Prevention but was solely conducted at Kaiser Permanente Southern California.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Mention of a product or company name is for identification purposes only and does not constitute endorsement by CDC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank colleagues from Kaiser Permanente Digital and Kaiser Permanente National IT Delivery Team; clinicians and operational staff across KPSC vaccination sites; and the patients who contributed to these data. We also thank Cassandra Bezi and Scot Hickey for data processing support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.079.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. 2022 https://covid19.who.int/ [accessed October 8 2022].
- 2.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021;6(12):1446. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver S., Gargano J., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moro P.L., McNeil M.M. Successes of the CDC monitoring systems in evaluating post-authorization safety of COVID-19 vaccines. Expert Rev Vaccines. 2022;21(3):281–284. doi: 10.1080/14760584.2022.2019020. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim H., Liu X., Zariffa N., Morris A.D., Denniston A.K. Health data poverty: an assailable barrier to equitable digital health care. Lancet Digit Health. 2021;3(4):e260–e265. doi: 10.1016/S2589-7500(20)30317-4. [DOI] [PubMed] [Google Scholar]
- 6.Trogstad L., Robertson A.H., Mjaaland S., Magnus P. Association between ChAdOx1 nCoV-19 vaccination and bleeding episodes: Large population-based cohort study. Vaccine. 2021;39(40):5854–5857. doi: 10.1016/j.vaccine.2021.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebnick C., Langer-Gould A.M., Gould M.K., Chao C.R., Iyer R.L., Smith N., et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hause A.M., Shay D.K., Klein N.P., Abara W.E., Baggs J., Cortese M.M., et al. Safety of COVID-19 Vaccination in United States Children Ages 5 to 11 Years. Pediatrics. 2022;150(2) doi: 10.1542/peds.2022-057313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan J.D., McMillan D., Levi M.L. COVID-19 mRNA Vaccination, ABO Blood Type and the Severity of Self-Reported Reactogenicity in a Large Healthcare System: A Brief Report of a Cross-Sectional Study. Cureus. 2021;13(12):e20810. doi: 10.7759/cureus.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedel D.S., Peters I.J., Banderet F., Epple V., Egli S., et al. Smartphone-based active vaccine safety surveillance (SmartVax) at a Swiss adult vaccination clinic - a pilot study. Swiss Medical Weekly. 2021;151 doi: 10.4414/smw.2021.w30090. [DOI] [PubMed] [Google Scholar]
- 13.Hause A.M., Baggs J., Marquez P., Abara W.E., Olubajo B., Myers T.R., et al. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12-17 Years - United States, December 9, 2021-February 20, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):347–351. doi: 10.15585/mmwr.mm7109e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hause A.M., Baggs J., Marquez P., Myers T.R., Gee J., Su J.R., et al. COVID-19 Vaccine Safety in Children Aged 5-11 Years - United States, November 3-December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1755–1760. doi: 10.15585/mmwr.mm705152a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Addio F., Sabiu G., Usuelli V., Assi E., Abdelsalam A., et al. Immunogenicity and Safety of SARS-CoV-2 mRNA Vaccines in a Cohort of Patients With Type 1 Diabetes. Diabetes. 2022;71(8):1800–1806. doi: 10.2337/db22-0053. [DOI] [PubMed] [Google Scholar]
- 16.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suran M. Why parents still hesitate to vaccinate their children against COVID-19. JAMA. 2021;327(1):23–25. doi: 10.1001/jama.2021.21625. [DOI] [PubMed] [Google Scholar]
- 18.Office for National Statistics. Exploring the UK's digital divide. UK Government https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/articles/exploringtheuksdigitaldivide/2019-03-04 [accessed 8 October 2022].
- 19.Litchfield IA-O, Shukla D, Greenfield SA-O. Impact of COVID-19 on the digital divide: a rapid review. BMJ Open 2021;11(10):e053440. [DOI] [PMC free article] [PubMed]
- 20.Baldolli A., Michon J., Appia F., Galimard C., Verdon R., Parienti J.J. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–4413. doi: 10.1016/j.vaccine.2021.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raw R.K., Rees J., Kelly C.A., Wroe C., Chadwick D.R. Prior COVID-19 infection is associated with increased Adverse Events (AEs) after the first, but not the second, dose of the BNT162b2/Pfizer vaccine. Vaccine. 2022;40(3):418–423. doi: 10.1016/j.vaccine.2021.11.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tré-Hardy M., Cupaiolo R., Papleux E., Wilmet A., Horeanga A., Antoine-Moussiaux T., et al. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai C., Peng Y., Shen E., Huang Q., Chen Y., Liu P., et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther. 2021;29(9):2794–2805. doi: 10.1016/j.ymthe.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC COVID Data Tracker. 2022; Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.