Summary
Background
Introgression of genetic material from species of the insect bacteria Wolbachia into populations of Aedes aegypti mosquitoes has been shown in randomised and non-randomised trials to reduce the incidence of dengue; however, evidence for the real-world effectiveness of large-scale deployments of Wolbachia-infected mosquitoes for arboviral disease control in endemic settings is still scarce. A large Wolbachia (wMel strain) release programme was implemented in 2017 in Rio de Janeiro, Brazil. We aimed to assess the effect of this programme on the incidence of dengue and chikungunya in the city.
Methods
67 million wMel-infected mosquitoes were released across 28 489 locations over an area of 86·8 km2 in Rio de Janeiro between Aug 29, 2017 and Dec 27, 2019. Following releases, mosquitoes were trapped and the presence of wMel was recorded. In this spatiotemporal modelling study, we assessed the effect of the release programme on the incidence of dengue and chikungunya. We used spatiotemporally explicit mathematical models applied to geocoded dengue cases (N=283 270) from 2010 to 2019 and chikungunya cases (N=57 705) from 2016 to 2019.
Findings
On average, 32% of mosquitoes collected from the release zones between 1 month and 29 months after the initial release tested positive for wMel. Reduced wMel introgression occurred in locations and seasonal periods in which cases of dengue and chikungunya were historically high, with a decrease to 25% of mosquitoes testing positive for wMel during months in which disease incidence was at its highest. Despite incomplete introgression, we found that the releases were associated with a 38% (95% CI 32–44) reduction in the incidence of dengue and a 10% (4–16) reduction in the incidence of chikungunya.
Interpretation
Stable establishment of wMel in the geographically diverse, urban setting of Rio de Janeiro seems to be more complicated than has been observed elsewhere. However, even intermediate levels of wMel seem to reduce the incidence of disease caused by two arboviruses. These findings will help to guide future release programmes.
Funding
Bill & Melinda Gates Foundation and the European Research Council.
Introduction
Dengue virus continues to circulate endemically across tropical and subtropical regions worldwide, causing an estimated 50 million symptomatic infections per year.1 In addition, large-scale outbreaks of chikungunya virus, spread by the same Aedes mosquitoes that transmit dengue virus, have become increasingly common. In Brazil, more than 10·1 million cases of dengue and 1·2 million cases of chikungunya were reported across the country between 2014 and 2021.2 Both viruses are still circulating, with cases being consistently reported in 2022. Sustainable methods to reduce the transmission of both viruses are urgently needed.
Mosquitoes infected with species of the Wolbachia genus of intracellular bacteria (wMel strain) are a promising technology to reduce viral transmission.3 wMel-infected mosquitoes have a reduced ability to harbour and transmit different arboviruses, including dengue and chikungunya viruses.4, 5, 6, 7, 8 wMel can be stably inserted into Aedes aegypti mosquitoes,4 and when released the infected mosquitoes mate with the wild-type population. The offspring of an infected female all contain wMel and the offspring of an infected male and a wild-type female are non-viable, driving the introgression of wMel into the mosquito population. Results of field trials in multiple countries have shown successful establishment of wMel in the local A aegypti population following a release period of several weeks or months.9, 10, 11, 12, 13 In a cluster randomised controlled trial in which wMel-infected A aegypti were deployed in Yogyakarta, Indonesia, wMel quickly reached greater than 90% penetration, which was followed by a significant reduction in the incidence of dengue and in dengue-related hospitalisations in intervention areas.10, 14 wMel has also been shown to reduce the incidence of both dengue and chikungunya in Niterói, Brazil.15
Research in context.
Evidence before this study
We searched Google Scholar and PubMed from Jan 1, 2020, to Dec 31, 2021, for studies published in English on the effect of wMel introgression on the incidence of dengue and chikungunya using the following keywords: “(wmel) AND (dengue OR chikungunya) AND (effect* OR effic* OR assess* OR impact OR trial)”. As of January, 2022, in vitro studies have shown that wMel-infected mosquitoes have a lower potential to transmit dengue and chikungunya viruses than do uninfected mosquitoes. These studies were followed by trials in communities, which showed that wMel can successfully become established in a wild-type mosquito population after wMel-infected mosquitoes are released. A randomised controlled trial in Yogyakarta, Indonesia showed that local replacement of wild-type Aedes aegypti by wMel-infected Aedes aegypti was followed by a significant reduction in the incidence of dengue. wMel has also been shown to reduce the incidence of both dengue and chikungunya in Niterói, Brazil.
Added value of this study
We studied the effect of a large wMel release programme in Rio de Janeiro, Brazil, on the case occurrence of dengue and chikungunya. We used detailed geolocated case data to inform spatiotemporal modelling approaches to quantify the effect of the programme. Unlike in other studies conducted in different locations, wMel did not fully establish in this setting, providing an important opportunity to examine the durability of wMel and the effect on case incidence when full introgression does not occur. Further, the fine spatial resolution of both the case data and the entomological data enabled us to estimate the effect of different intermediate levels of introgression on the incidence of dengue and chikungunya. The wMel release programme in Rio de Janeiro that we study here, and the previous release programme in Niterói, also provide estimates of the effect of wMel on the incidence of chikungunya, as previous studies have focused on dengue.
Implications of all the available evidence
We show that wMel is a promising technology that can reduce the public health burden of different arboviruses within the same community, even when full introgression does not occur. A major challenge remains achieving the complete establishment of wMel in complex urban communities such as Rio de Janeiro. Originally designed to curb the transmission of dengue virus, our work also shows the potential of wMel to reduce the transmission of chikungunya virus.
Because Aedes mosquitoes are a vector for many human pathogens, vector-oriented interventions have the potential to reduce the transmission of several pathogens at the same time. Typical interventions that target the vector, such as the spraying of pesticide, are often implemented in reaction to ongoing reported circulation of the virus and are not a long-term solution as they give rise to resistant strains of vectors. However, wMel-infected mosquitoes can be released pre-emptively. Further, if implemented successfully, the population of these mosquitoes seems able to self-maintain,9, 10, 11, 12, 13 making the technology comparatively cost-efficient and sustainable.
Except in extreme scenarios in which case numbers decrease to almost zero, understanding the effect of spatially targeted interventions is complicated because cases of dengue and chikungunya vary substantially over space and time, driven by local variations in immunity, human behaviours, mosquito density, population density, building constructions, and climate, among other factors.16, 17, 18 Further, the movement of people outside a release zone means that local residents can still become infected even if the intervention is 100% effective. Additionally, most infections are not detected, because they result in few symptoms or because health care is not sought.19, 20
As wMel is deployed at increasing scale, a robust understanding of the effect of these releases on the incidence of arboviral disease in a range of epidemiological settings is needed. In this Article we studied the effect of wMel-infected mosquito releases in Rio de Janeiro, Brazil, on dengue and chikungunya case occurrence in the city. Entomological results 1 year after the end of releases in the first two release areas in Rio de Janeiro showed successful wMel introgression overall, but with heterogeneity in the prevalence of wMel at the neighbourhood level.21 We made use of the public health systems of Rio de Janeiro, in which dengue and chikungunya cases from all hospitals and health clinics throughout the city have been systematically geocoded since 2010 for dengue and 2016 for chikungunya. We developed spatially explicit mathematical models to fit the timing and location of cases and estimated the effect of wMel on the occurrences of dengue and chikungunya.
Methods
Setting and wMel field implementation
Our spatiotemporal modelling study was set in Rio de Janeiro, the second largest city in Brazil, with 6·7 million inhabitants over 1260 km2. The city is a patchwork of highly dense, flat urban areas and uninhabited mountains covered with tropical forest. The wMel release programme started in the northwest of the city in August, 2017.21 The release area was subdivided into five zones (RJ1, RJ2, RJ3.1, RJ3.2, and RJ3.3), covering a total area of 86·8 km2 with around 890 000 inhabitants, and releases were phased through the different zones.22
A pilot test in the city found that insecticide resistance was widespread in wild-type A aegypti, which can hinder the successful establishment of wMel in an area, as the original wMel mosquitoes would be more susceptible to insecticides used in the release zones. The release programme therefore crossed female wMel-infected mosquitoes with wild-type males, which allowed for a better matching of genetic profiles between wMel-infected and field mosquitoes.23 All releases used this newly developed, locally matched, insecticide-resistant mosquito strain. Releases started on Aug 29, 2017, and continued until Dec 27, 2019. Release points were distributed every 50 m in five release zones. Around 100 wMel-infected mosquitoes were released each time. A network of 1168 BG-Sentinel traps (Biogents, Regensburg, Germany) was used to monitor wMel introgression. The traps were regularly distributed throughout the release area with an average distance between two adjacent traps of 250 m. A trap was set 4 weeks after the first release in the area and mosquitoes were collected every two weeks until Dec 30, 2019. Up to ten A aegypti mosquitoes (male and female) per collection were tested individually for wMel using quantitative PCR. This testing regime gives an estimate of the overall proportion of mosquitoes that were infected in a given trap at a given time.
Case data
Every suspected case of dengue or chikungunya in Rio de Janeiro in a patient who presents to a health-care facility must be recorded in Brazil's national surveillance system database, known as the Notifiable Diseases Information System. Suspected dengue cases are defined as patients who present with fever and at least two of the following manifestations: nausea or vomiting, rashes, myalgia or arthralgia, headache or retro-orbital pain, petechiae or a positive tourniquet test, and leukocytopenia. Suspected chikungunya cases are defined as patients with fever and arthralgia or arthritis.24 A differential diagnosis between chikungunya and dengue is based on the duration of fever (up to 7 days for dengue and up to 3 days for chikungunya) and the intensity of arthralgia, which is more intense in chikungunya. Lymphocytopenia is also frequent in chikungunya, whereas it is uncommon in dengue.25 A subset of cases were confirmed by laboratory testing. Cases of dengue were confirmed by PCR (38 870 [11·1%] of 350 102 cases), whereas cases of chikungunya were mainly confirmed through IgM serology (14 688 [23·5%] of all 62 415 suspected cases).
All dengue and chikungunya cases are systematically geolocated by the Rio de Janeiro city health department using information on the home address of the patient where possible. A subset of notified cases could not be geolocated (66 832 [19·1%] of 350 102 cases of dengue and 4710 [7·5%] of 62 415 cases of chikungunya), and were therefore not included in the analysis. This study was approved by the Brazilian National Institutional Review Board (CONEP; 59175616.2.0000.0008).
Spatial model
For our modelling approach, we divided the project area into 500 × 500 m cells (N=465 cells). We then counted the number of dengue and chikungunya cases that occurred in each 30-day period within each cell between Jan 1, 2010, and Sept 25, 2019, for dengue and between Jan 1, 2016, and Sept 25, 2019, for chikungunya (N=117 time periods, resulting in 54 405 total space–time units). We used WorldPop data to obtain detailed estimates of the distribution of the underlying population throughout the project area.26
We constructed Poisson regression models to separately fit the number of dengue cases and chikungunya cases for each space–time unit throughout the project area during the period 2010–19 for dengue and 2016–19 for chikungunya. To incorporate the spatial correlation in the location of dengue and chikungunya cases, we used integrated nested Laplace approximation, as implemented in R-INLA.27 This approach enabled us to introduce a spatial correlation term that explicitly incorporates the spatial dependence between locations. Additionally, to account for temporal correlation in the timing of cases, we used a temporally structured random effect by using an order one autoregressive model to the monthly time variable. We used the log of population size within the cell as an offset to the model.
To estimate the effect of the wMel release programme, we considered three separate measures. First, we used a binary variable for which each space–time unit was coded as 1 if wMel was detected in A aegypti mosquitoes across the traps within that location and within that month, and 0 if it was not. Second, we used the actual proportion of Aedes mosquitoes that were infected by wMel across these traps, using non-overlapping bins (0·0%, 0·1–10·0%, 10·1–20·0%, 20·1–30·0%, 30·1–40·0%, 40·1–50·0%, 50·1–60·0%, >60·0%). Finally, we considered wMel as a continuous variable and estimated the reduction in incidence of chikungunya and dengue for each unit increase in wMel. In all models, space–time units before the initiation of releases were given a value of 0. Space–time units with no traps and those in which no mosquitoes were caught were removed from the main analysis.
Sensitivity analysis
To assess the robustness of our approach, we conducted a range of sensitivity analyses. To test whether the released A aegypti mosquitoes were being recaptured in the traps (leading to falsely high estimates of wMel) and potentially driving our estimates, we conducted a sensitivity analysis in which we excluded space–time units that occurred within a short amount of time following releases. Two different exclusion criteria were designed. First, we excluded space–time units within which releases occurred, which resulted in the exclusion of 57% of space–time units with a non-zero wMel value. The second, more exclusive criterion was to exclude from the analysis space–time units both during the month of the release and the subsequent month. This approach resulted in 67% of space–time units with non-zero wMel values being excluded.
We then repeated the analysis using an augmented dataset that inferred the percentage of wMel-infected A aegypti (defined as %wMel) captured in places where data were missing. Missing %wMel estimates occurred either in places where no traps were set up despite releases having already occurred in the area or when traps did not capture any mosquitoes. We used a separate spatial regression model to estimate the number of wMel-positive mosquitoes within each space–time unit. The model to estimate %wMel can be summarised by Ni(s,t)|η(s,t) ~ Poisson(Nt(s,t) × η(s,t)), with η(s,t)=u(s) + v(t), where Ni(s,t) is the number of infected A aegypti captured in a given cell, Nt(s,t) is the total number of A aegypti captured in the same cell, u(s) is a spatially structured random effect, v(t) is a temporally structured random effect, s is the spatial unit index, and t is time. Both random effects were defined in the same way as for the case-count model (appendix p 2). We fit the model using the observed mosquito count and %wMel data. We then replaced the space–time locations that had missing %wMel estimates with values predicted by this wMel model, leading to an increase in the size of the dataset of 23%. We also ran a sensitivity analysis in which the wMel dataset was entirely replaced by values predicted by the wMel prediction model—not just in the space–time locations that had missing %wMel estimates.
Model fit
We examined model fit by comparing predicted case counts within space–time units with observed case counts. We split the dataset into training and testing sets using two different methods. The first was to randomly split the case dataset into two equal parts, with each space–time unit having an equal probability of being within each set. The second was to split up large spatiotemporal regions, consisting of 20% of the global dataset, as the testing dataset. We then fit the model using the training set only and predicted the number of cases per space–time unit in the testing set. In estimating the case count in larger spatiotemporal regions, the model was asked to predict in places it had no information about over a whole year and across a wide area.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
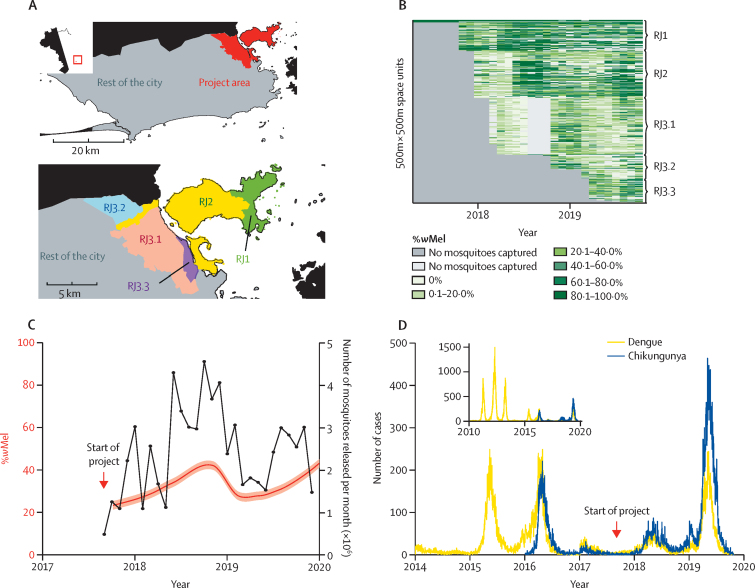
Between Aug 29, 2017, and Dec 27, 2019, an estimated 67 million wMel-infected A aegypti mosquitoes were released in 658 179 individual release events at 28 489 different locations in the five release zones (figure 1A, B, appendix p 3). The mean average number of releases per month was 24 377. A total of 36 894 mosquito trap collection events were recorded, of which 23 071 contained at least one A aegypti mosquito. The overall proportion of trapped mosquitoes that were wMel-positive was 33·8% (95% CI 33·4–34·2; figure 1C), although differences were seen across the release region and over time, with a higher prevalence in release zone RJ1 (52·3% [51·2–53·3]) and a lower prevalence in release zone RJ3.1 (19·8% [19·4–20·3]; figure 1B).
Figure 1.
Details of the wMel release programme and the incidence of dengue and chikungunya in Rio de Janeiro, Brazil
(A) Map of Rio de Janeiro with a detailed view of the project area, showing the different project subareas. The top-left inset shows the location of Rio de Janeiro within Brazil. (B) Proportion of wMel-infected Aedes aegypti mosquitoes found in traps in each spatiotemporal cell. (C) Number of mosquitoes released and the level of introgression. The black line shows the total number of mosquitoes released per month. The red line shows the proportion of A aegypti captured in traps that were infected by wMel (mean and 95% CI). (D) Number of cases of dengue and chikungunya reported in Rio de Janeiro between 2014 and 2020, including both the project area and the rest of the city. The inset shows the number of cases of these diseases recorded between Jan 1, 2010, and Sept 25, 2019, for dengue and between Jan 1, 2016, and Sept 25, 2019, for chikungunya.
Between 2010 and 2019, 283 270 cases of dengue were reported in Rio de Janeiro's health-care centres, with an average of 28 327 cases per year. Chikungunya was designated a notifiable disease in 2016 and has been continuously reported since, with an average of 14 426 cases per year (57 705 total cases since 2016; figure 1D). Cases of both chikungunya and dengue have been reported throughout the city (appendix p 4). Before the start of the project, the temporal evolution of cases of both diseases in the project area followed the same trend as in the rest of the city. In 2019, we observed a decrease in the number of reported cases of both diseases in the project area compared with the rest of the city (appendix p 5).
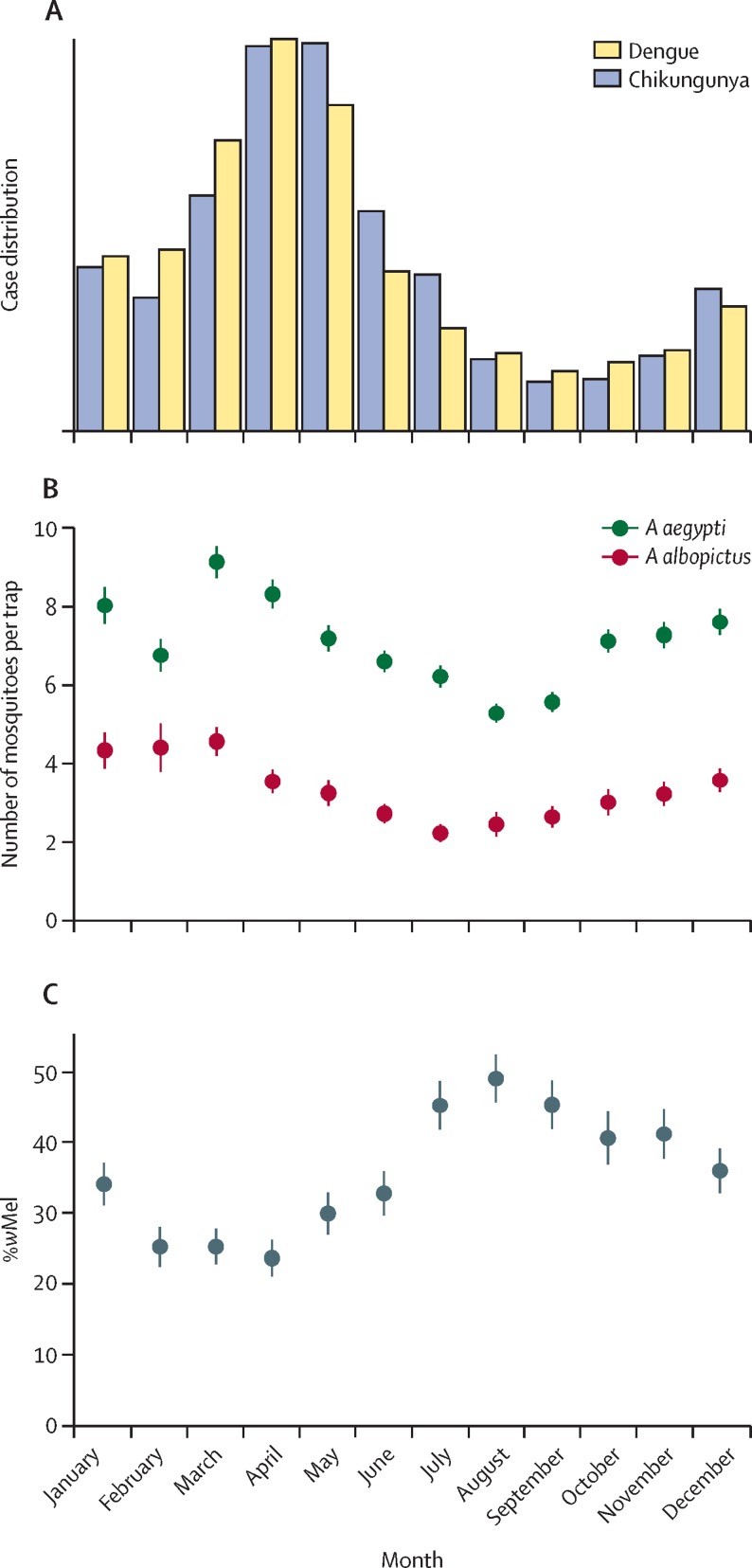
We observed strong seasonality in the case data and the mosquito data. For both dengue and chikungunya, we found that transmission peaked during March and April (figure 2A). The number of A aegypti and Aedes albopictus mosquitoes trapped as part of the study also followed a similar seasonal trend, peaking in March (figure 2B, appendix p 6). By contrast, the proportion of mosquitoes that were infected with wMel was inversely correlated with the number of cases (Spearman's r=−0·82), decreasing to around 25% in March and April and peaking at 49% in August, when the incidence of disease is lowest (figure 2C). The seasonal signal is still marked when removing subareas RJ3.2 and RJ3.3, where releases occurred later than in the other areas (appendix p 7). The correlation of wMel with the number of releases by month was less substantial (Spearman's r=−0·34).
Figure 2.
Seasonal pattern of dengue and chikungunya cases and mosquito levels
(A) Monthly distribution of dengue cases (2010–19) and chikungunya cases (2016–19). (B) Monthly distribution of Aedes aegypti and Aedes albopictus mosquitoes found in traps (average values from 2017–19). Data are mean and 95% CI. (C) Proportion of trapped female A aegypti mosquitoes that were infected with wMel by month across the project area (average values from 2017–19). Data are mean and 95% CI.
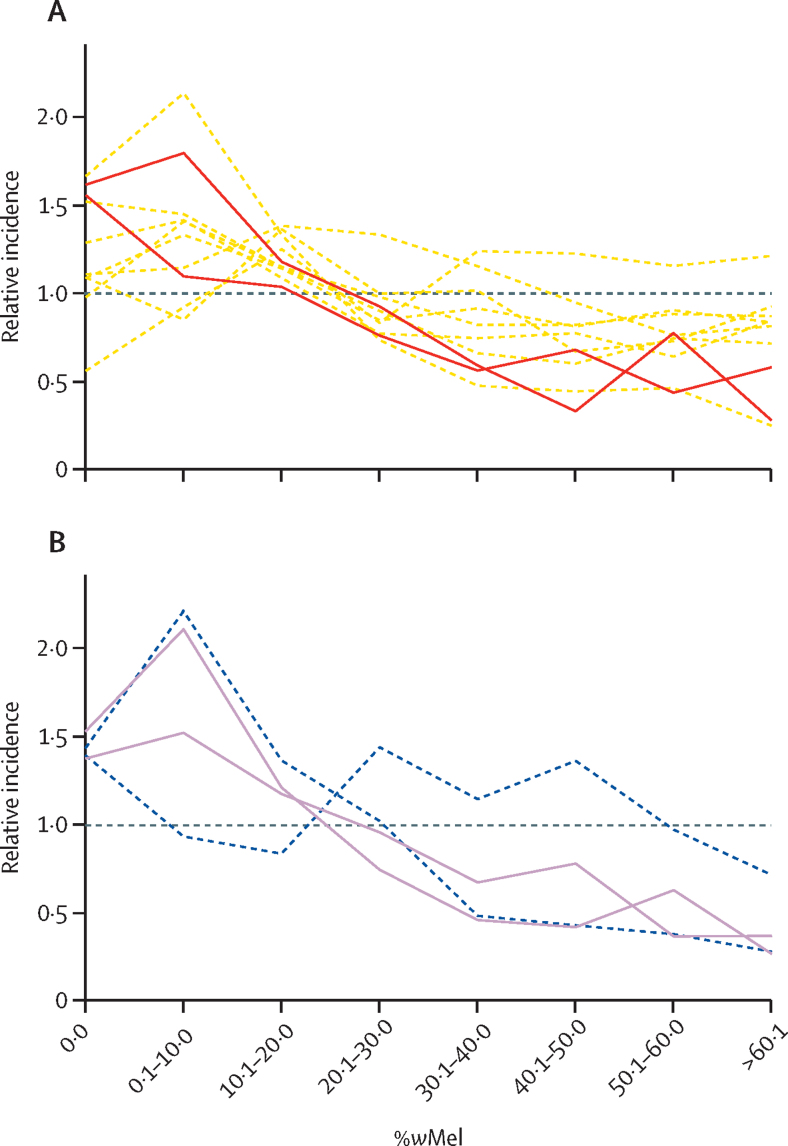
To explore the overall relationship between wMel introgression and case incidence both before and after the release programme, we initially compared the prevalence of wMel in each space–time unit with the number of cases reported in the same cell and time period across different years (figure 3). We looked at case incidence in the same time period as the wMel data and in the equivalent time period in previous years. These comparisons showed that wMel introgression was more successful in months and locations in which dengue and chikungunya case incidence tended to be lower each year.
Figure 3.
Introgression success as a function of historical and future dengue and chikungunya case incidence
The average monthly introgression of wMel within a 500 m × 500 m cell and the standardised incidences of dengue (A) and chikungunya (B) in that location and month. Each line represents the incidence from a different year. Dashed lines are years before the start of the release programme (2010–17 for dengue 2016–17 for chikungunya). Solid lines represent the years 2018 and 2019, which are after the release programme started. We standardised the incidence of dengue and chikungunya by dividing by the overall mean incidence in that year; this method enables us to compare years with large disease outbreaks with years with smaller disease outbreaks on the same plot.
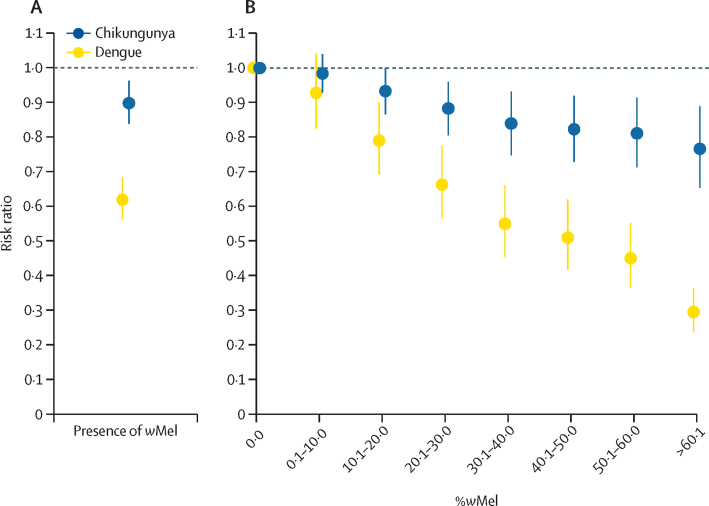
To estimate the effect of the intervention accounting for underlying heterogeneities in space and time in both the case data and the mosquito data, we fit a spatially explicit model to locations in which cases were found within the project area. 2209 space–time units (34% of the project area) had no trapped A aegypti mosquitoes and were therefore excluded from the main analysis as we could not identify the proportion of wMel in those locations. The presence of wMel in local A aegytpi mosquitoes had a strong effect on the number of cases of dengue and chikungunya in that location at that time (figure 4). The mean average incidence of dengue within a space–time unit in which wMel was detected was 0·62 (95% CI 0·56–0·68) times the incidence in space–time units in which wMel was not detected; for chikungunya, this value was 0·90 (95% CI 0·84–0·96; figure 4A). The model estimated that spatial correlation between the location of dengue cases within any month extended to 1460 m (95% CI 1071–1815), and to 2081 m (95% CI 1594–2610) for chikungunya cases.
Figure 4.
Results of spatiotemporal models
(A) Estimated overall relative incidence of dengue and chikungunya in space–time units in which wMel was recorded compared with those in which wMel was not recorded. (B) Relative incidence of dengue and chikungunya in space–time units as a function of the proportion of Aedes aegypti mosquitoes that were infected with wMel. Space–time units within the study area in which no wMel was detected are the reference. Error bars show 95% CI.
We next fit separate models that explored the relationship between the observed proportion of A aegypti mosquitoes within each space–time unit that were infected with wMel and the incidence of dengue and chikungunya in that space–time unit. We found that space–time units in which 0·1–10·0% of the mosquitoes were infected with wMel had 0·93 (95% CI 0·83–1·04) times the incidence of dengue and 0·98 (95% CI 0·93–1·04) times the incidence of chikungunya compared with locations in which no wMel was detected (figure 4B). In areas with a wMel prevalence of greater than 60%, these values decreased to 0·29 (95% CI 0·24–0·36) for dengue and 0·77 (95% CI 0·65–0·89) for chikungunya. In separate models that considered wMel as a continuous variable, we estimated that each 10% increase in the prevalence of wMel was associated with 0·85 (95% CI 0·83–0·87) times the incidence of dengue and 0·96 (95% CI 0·94–0·97) times the incidence of chikungunya (appendix p 8).
To ensure that our results were not affected by the recapture of recently released mosquitoes, we repeated our analysis on a dataset in which data for all locations where a release event had occurred within a previous month had been removed. Additionally, because traps were not placed in all locations at all times, leading to missing estimates of %wMel in some space–time units, we also conducted a separate sensitivity analysis in which we initially predicted the %wMel in all space–time locations of the study region (appendix p 2). In both of these sensitivity analyses, the effect of wMel on the incidence of both dengue and chikungunya was consistent with our previous results (appendix p 9).
To assess the performance of our model, we repeatedly removed randomly selected space–time locations from the model (termed held-out locations) and predicted the number of cases in those locations using model fit on the remaining data. We then compared our estimates with the observed number of cases in that location. Good correlation was observed between the predicted and observed number of cases (appendix p 10), including when 50% of randomly selected space–time units were held out (appendix p 10), and when randomly selected spatially clustered cells (5 km2 in area) were held out for one year at a time (appendix p 10).
Discussion
In this study, we have critically assessed the effect of a wMel release programme on the incidence of dengue and chikungunya in a diverse, urban setting. By December, 2019, 29 months after the phased releases began, the prevalence of wMel in local A aegypti mosquitoes in the five release areas in Rio de Janeiro was between 27% and 60%. Using a spatially and temporally explicit modelling framework we have shown that, despite varying prevalences of wMel across the study area, wMel releases still resulted in lower incidence of both dengue and chikungunya viruses during the first 2 years after the intervention, highlighting the potential of this technology.
Quantifying the protective effect of low-to-moderate wMel prevalence has not previously been possible, because rapid establishment has generally been observed following wMel deployments in other settings9, 10, 13 and arboviral case notification data are not commonly available at a high spatial resolution.15 Here we report a dose–response relationship between wMel prevalence and relative reductions in dengue and chikungunya case incidence. A small but important protective effect against dengue was seen even at very low local wMel prevalence (≤10%), and for locations in which the prevalence of wMel was greater than 60%, the protective effect was 76% (95% CI 64–71), which is comparable to previous results (using different methods) from the neighbouring municipality of Niterói and from Indonesia.10, 15
Why wMel was unable to become quickly established in Rio de Janeiro despite large numbers of releases is unclear. The underlying incidence of dengue and chikungunya in the city is highly heterogeneous. We found that wMel introgression was lower in areas that have a high annual incidence of disease. Seasonal fluctuations in wMel introgression were also observed, with lower levels between February and May, the hottest period of the year. High temperatures have been linked to lower wMel acquisition in laboratory studies.28 The release programme also made fewer releases during the summer months, which could contribute to this observed seasonal effect. The areas of the city with persistently high dengue and chikungunya incidence could have other factors that complicate the wMel release programme, including large, heterogeneously distributed baseline mosquito populations, or be in areas that are hard to access, such as favela communities. A albopictus also circulates in Rio de Janeiro but has not been implicated as being involved in dengue and chikungunya incidence in this city.29 A role for A albopictus in affecting wMel introgression in A aegypti remains unclear.
We observed less of an effect of wMel on chikungunya than on dengue. The reasons for this difference are unclear, although A albopictus could have a role. Although A albopictus has not directly been implicated in the transmission of chikungunya virus in Rio de Janeiro, this species has been an important vector for the virus elsewhere, and is more rarely involved in the transmission of dengue virus.30, 31 Even ongoing low levels of chikungunya virus transmission by A albopictus would result in a seemingly reduced effect of the release programme on the transmission of this virus. Alternatively, there could be underlying biological differences in the relative transmissibility of the dengue and chikungunya viruses in wMel-positive A aegypti mosquitoes.
Cluster randomised trials—such as that in Yogyakarta, Indonesia, and the trial currently being conducted in Belo Horizonte, Brazil32 (NCT04514107)—provide a gold-standard measure of the effectiveness of wMel on disease incidence. However, these large trials might not always be feasible, especially when resources are scarce. Our study highlights how the systematic geocoding of cases provides a valuable resource to understand where incidence is concentrated that can also act as a reference point to evaluate the effect of spatially targeted interventions. However, we have shown that, alongside detailed data, we need to use structured models to appropriately measure these datasets. For example, our finding that wMel introgression in a location was correlated with the incidence of dengue and chikungunya in that same location in the years before the intervention highlights the complexity of using observational case data to understand the effect of an intervention of which penetration is itself spatially and temporally uneven. Only through the use of spatiotemporally structured models can we disentangle these different correlation structures to identify the underlying effect of the intervention.
Our study considers data to the end of 2019. Extending the analysis period by including additional years would provide important insight into the durability of the intervention. Unfortunately, the COVID-19 pandemic has had substantial effects on the mosquito release programme and the city health department. In particular, the systematic geocoding of dengue and chikungunya cases by the city health department largely stopped in 2020. By the end of 2021, additional ovitrapping data showed that the average introgression level across the project area was more than 50%, suggesting that complete introgression might be possible. The study also has other limitations. In particular, arboviral cases are notified on the basis of the patient's place of residence, but people move around and beyond the city and patients might have acquired their infection elsewhere. Further, the majority of the cases are diagnosed on the basis of clinical presentation alone, meaning that some cases could have been misdiagnosed. If we include only confirmed cases in our model (128 dengue cases, 1078 chikungunya cases), the results are consistent with those that included all reported cases; however, the number of confirmed dengue cases is too small to obtain precise estimates (appendix p 11). We also note the good correlation between the spatiotemporal distribution of unconfirmed cases and that of confirmed cases, suggesting that there is no substantial bias caused by the lack of case confirmation (appendix p 12). In the event that many of the unconfirmed cases were caused by other pathogens, such as influenza, that would not be affected by the mosquito release programme, our estimates of the effect of wMel would be biased towards the null and the true effect would be greater.
Our results provide further evidence that wMel can considerably reduce the public health burden from different arboviruses within the same community. The establishment of wMel in complex urban communities such as Rio de Janeiro is a major challenge, and understanding why the introgression of wMel into A aegypti populations is faster and more homogeneous in some locations than in others will help to underpin its future success.
We developed a flexible analytical framework that successfully measured, at a fine scale, the effect of a spatially and temporally targeted intervention on the transmission of pathogens with complex dynamics. Our model identified the relationship between the level of introgression and the effect on disease transmission. This framework could be used to assess the efficiency of other spatiotemporally targeted interventions.
Data sharing
All code and data used in these analyses are available at https://github.com/pdgcam/wMel_Rio_dengue_chik.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was funded by the Bill & Melinda Gates Foundation and the European Research Council (grant 804744).
Contributors
GRdS visualised the project and did the formal analysis. GRdS and HS did the investigation and developed the method. VS, TISR, and SBP curated the data. BD and KLA validated the data and the method. GRdS wrote the initial draft, and SBP, KLA, LAM, and HS reviewed and edited the manuscript. BD, SBP, KLA, and LAM were involved in project administration, HS acquired funding, and LAM and HS supervised the project.
Supplementary Material
References
- 1.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan American Health Organization Chikungunya weekly report. Jan 17, 2019. https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html
- 3.Hoffmann AA, Montgomery BL, Popovici J, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 4.Walker T, Johnson PH, Moreira LA, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 5.Pereira TN, Rocha MN, Sucupira PHF, Carvalho FD, Moreira LA. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci Rep. 2018;8:6889. doi: 10.1038/s41598-018-25236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Hurk AF, Hall-Mendelin S, Pyke AT, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye YH, Carrasco AM, Frentiu FD, et al. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill SL, Ryan PA, Turley AP, et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2019;2:36. doi: 10.12688/gatesopenres.12844.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indriani C, Tantowijoyo W, Rancès E, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020;4:50. doi: 10.12688/gatesopenres.13122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, et al. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt TL, Barton NH, Rašić G, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15:e2001894. doi: 10.1371/journal.pbio.2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan PA, Turley AP, Wilson G, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2020;3:1547. doi: 10.12688/gatesopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utarini A, Indriani C, Ahmad RA, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto SB, Riback TIS, Sylvestre G, et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: a quasi-experimental study. PLoS Negl Trop Dis. 2021;15:e0009556. doi: 10.1371/journal.pntd.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salje H, Lessler J, Endy TP, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA. 2012;109:9535–9538. doi: 10.1073/pnas.1120621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salje H, Lessler J, Paul KK, et al. How social structures, space, and behaviors shape the spread of infectious diseases using chikungunya as a case study. Proc Natl Acad Sci USA. 2016;113:13420–13425. doi: 10.1073/pnas.1611391113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bavia L, Melanda FN, de Arruda TB, et al. Epidemiological study on dengue in southern Brazil under the perspective of climate and poverty. Sci Rep. 2020;10:2127. doi: 10.1038/s41598-020-58542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salje H, Cummings DAT, Rodriguez-Barraquer I, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018;557:719–723. doi: 10.1038/s41586-018-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gesto JSM, Pinto S, Dias FBS, et al. Large-scale deployment and establishment of Wolbachia into the Aedes aegypti population in Rio de Janeiro, Brazil. Front Microbiol. 2021;12:711107. doi: 10.3389/fmicb.2021.711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durovni B, Saraceni V, Eppinghaus A, et al. The impact of large-scale deployment of Wolbachia mosquitoes on dengue and other Aedes-borne diseases in Rio de Janeiro and Niterói, Brazil: study protocol for a controlled interrupted time series analysis using routine disease surveillance data. F1000Res. 2019;8:1328. doi: 10.12688/f1000research.19859.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia GA, Sylvestre G, Aguiar R, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis. 2019;13:e0007023. doi: 10.1371/journal.pntd.0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notifiable Diseases Information System Sinan Dengue/Chikungunya. March 7, 2016. http://portalsinan.saude.gov.br/sinan-dengue-chikungunya in Portuguese.
- 25.Ministry of Health Surveillance guide in health. Vol 1, 3rd edn. 2019. https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf (in Portuguese)
- 26.Worldpop WorldPop. https://hub.worldpop.org/geodata/summary?id=44874
- 27.Lindgren F, Rue H. Bayesian spatial modelling with R-INLA. J Stat Softw. 2015;63:1–25. [Google Scholar]
- 28.Ulrich JN, Beier JC, Devine GJ, Hugo LE. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl Trop Dis. 2016;10:e0004873. doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segura NA, Muñoz AL, Losada-Barragán M, et al. Epidemiological impact of arboviral diseases in Latin American countries, arbovirus–vector interactions and control strategies. Pathog Dis. 2021;79:ftab043. doi: 10.1093/femspd/ftab043. [DOI] [PubMed] [Google Scholar]
- 30.Weaver SC, Chen R, Diallo M. Chikungunya virus: role of vectors in emergence from enzootic cycles. Annu Rev Entomol. 2020;65:313–332. doi: 10.1146/annurev-ento-011019-025207. [DOI] [PubMed] [Google Scholar]
- 31.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 32.Collins MH, Potter GE, Hitchings MDT, et al. EVITA Dengue: a cluster-randomized controlled trial to EValuate the efficacy of Wolbachia-InfecTed Aedes aegypti mosquitoes in reducing the incidence of Arboviral infection in Brazil. Trials. 2022;23:185. doi: 10.1186/s13063-022-05997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code and data used in these analyses are available at https://github.com/pdgcam/wMel_Rio_dengue_chik.