Abstract
Stimuli-responsive peptide-based biomaterials are increasingly gaining interest for various specific and targeted treatments, including drug delivery and tissue engineering. Among all stimuli, pH can be especially useful because endogenous pH changes are often associated with abnormal microenvironments. pH-Responsive amino acids and organic linkers can be easily incorporated into peptides that self-assemble into various nanostructures. Thus, these largely biocompatible and easily tunable platforms are ideal candidates for drug release and as fibrous materials capable of mimicking the native extracellular matrix (ECM). In this review, we highlight common design motifs and mechanisms of pH-responsiveness in self-assembling peptide-based biomaterials, focusing on recent advances of these biomaterials applied in drug delivery and tissue engineering. Finally, we suggest future challenges and areas for potential development in pH-responsive self-assembling peptide-based biomaterials.
Keywords: pH-responsive, cell-penetrating peptides, cell-adhesive peptides, tumor-targeting, cancer therapy, tissue regeneration, wound-healing
Graphical Abstract
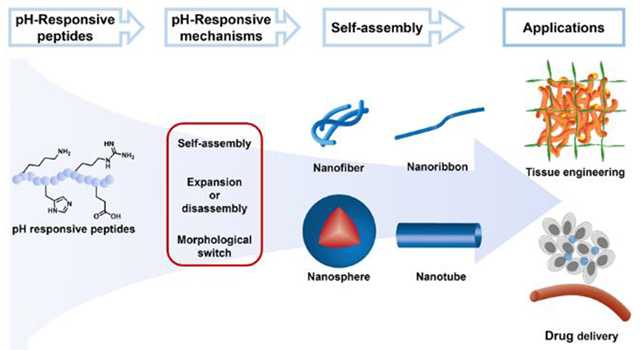
1. Introduction
Peptides, polyamides derived from two or more amino acids,1 perform a wide range of functions in biological systems. While serving as communication signals between cells, peptides regulate blood pressure2, reduce inflammation-related disease,3 and slow the aging process,4 among many other functions. With decades of development on solid-phase peptide synthesis (SPPS)5–6 and advanced purification methods like HPLC,7–8 peptides and peptide-based structures can be easily chemically synthesized, and some are used as active pharmaceutical ingredients (APIs).9 More recently, progress in the field of nanotechnology has sparked considerable interest in self-assembling peptide-based biomaterials. According to the different characteristics of the amino acid side chain groups,10 such as hydrophilicity, hydrophobicity, and aromaticity, abundant directional interactions between amino acid residues allow carefully designed peptides to self-assemble into a wide variety of complex nanostructures. These non-covalent interactions include electrostatic interactions, hydrogen bonds, hydrophobic interactions, aromatic-aromatic interactions, and van der Waals forces. Clever peptide design can also endow peptide-based biomaterials with the ability to respond to environmental stimuli, such as osmotic pressure, light, ultrasound, and local environmental factors, such as temperature, redox state, or pH.11–12 Among these, pH is commonly used to design stimuli-responsive peptide-based drug delivery vehicles and biomaterials due to natural pH variation within the human body.13
A particularly difficult problem in drug delivery that may benefit from pH-responsive peptides, taking advantage of natural pH variation, is drug targeting to specific sites in the body. A major challenge in peptide-based drug delivery systems is in targeting specific tissues, evidenced by the fact that few peptide delivery agents have been approved for clinical application while over 100 peptide drugs (i.e., APIs) are currently available on the market in the U.S., Europe, and Japan.14 In this context, pH-responsive peptides are a type of drug delivery vehicle under investigation for new therapeutic possibilities, which can be designed to target specific sites in the body. For example, oral drug delivery systems can take advantage of pH changes ranging from highly acidic in the stomach (pH 1-2) to more basic in the intestines (pH 5-8). Furthermore, tumor tissue is slightly acidic extracellularly (pH 6.5-7.2) compared to normal pH of 7.4, in which case some acid-responsive peptides can be designed for delivery of chemotherapeutics (Figure 1).15 The different pH levels can be used to direct drug delivery toward certain tissues or in some cases cellular compartments.
Figure 1.
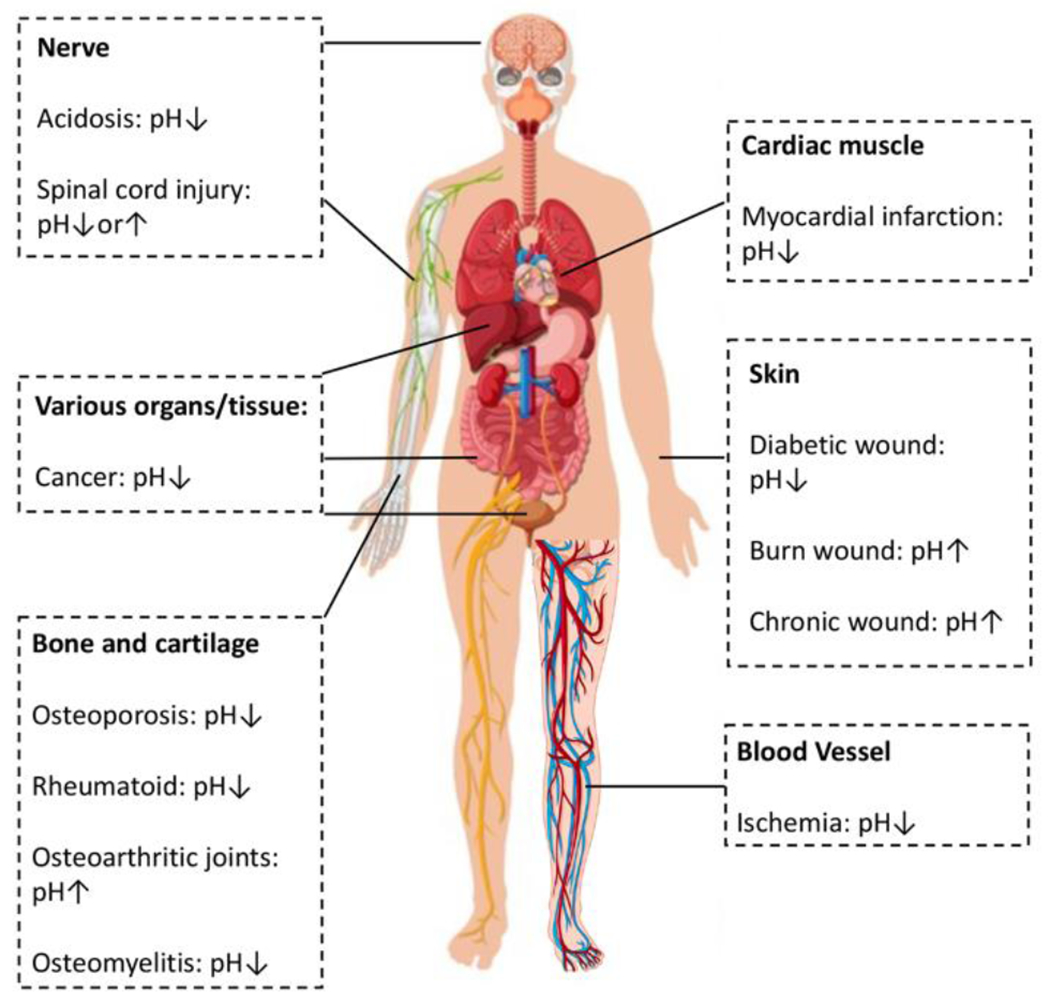
The pH change of common diseases and disorders of the human body. Inspired by and reproduced with permission from reference 19. Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Human body image designed by Freepik.
Besides precise drug delivery, pH-responsive self-assembling peptides are also popularly applied in tissue engineering, particularly as materials that mimic the native extracellular matrix (ECM),13 in wound dressings,16 and as tissue regeneration materials.17–18 Changes in the size, morphology, and self-assembly/disassembly can be triggered when the pH of the microenvironment changes, providing a dynamic and supportive scaffold for delivered and recruited cells in tissue engineering. For example, the pH of the tumor microenvironment changes from normal pH of 7.4 to acidic pH, while the pH in the microenvironment of chronic wounds can range from mildly acidic to mildly basic, depending on the specific wound type. Regenerative medicine may also benefit from this approach, allowing natural variations in pH to control a material response.
The rich self-assembly characteristics of peptides, combined with pH-responsiveness in peptide-based biomaterials, offer a powerful approach for precise drug delivery and efficient tissue regeneration. Additionally, compared with fully synthetic materials including many polymers, the biocompatibility in many contexts and biodegradability of peptide-based biomaterials reduce side effects and potentially toxic byproducts. Furthermore, the tunable but easy synthesis and flexible modification of peptides provide nearly endless possibilities for discovery. This review discusses recent developments (primarily since 2015) in the design and self-assembly behavior of pH-responsive self-assembling peptides, with a particular focus on their potential applications in drug delivery and tissue engineering.
2. Design and self-assembling behavior of pH responsive peptide-based biomaterials
2.1. Design of pH-responsive peptides
Peptides have been heavily studied in the medicinal chemistry and bioengineering fields. There are 21 commonly used amino acids as the main component of peptides, which we refer to throughout by their 1-letter codes after their introduction below. The 21 canonical amino acids are distinguished by their side chains and can be categorized into five different groups: amino acids with electrically charged side chains [arginine (R), histidine (H), lysine (K), aspartic acid (D), and glutamic acid(E)]; with polar uncharged side chains [serine (S), threonine (T), asparagine (N), and glutamine (Q)] with hydrophobic side chains [alanine (A), valine (V), isoleucine (I), leucine (L), methionine (M), phenylalanine (F), tyrosine (Y), and tryptophan (W)]; and other special cases [cysteine (C), selenocysteine (U), glycine (G), and proline (P)]. Among all amino acids, those with charged side chains are most important in designing pH-responsive peptides due to the changes in hydro/hydrophilicity that take place upon protonation or deprotonation. However, a single pKa value for an amino acid is often not sufficient for predicting at what pH a morphology change might occur. For example, incorporation of different numbers of same-charged amino acids in close proximity influences their pKa values. This effect was demonstrated by Williams and coworkers in a series of short peptides with increasing numbers of negatively charge D residues, where the overall pKa decreased as the number of D residues increased.20 In more complex systems with multiple amino acids with the same type of charge but with different pKa values, the overall pKa will be different as well, as estimated by Henderson-Hasselbalch equation. Additionally, for peptides with both basic and acidic residues, the pH responsiveness will be influenced as well.21
Because changes in abnormal cellular and tissue activities can trigger pH changes in the surrounding microenvironment (Figure 1), pH-responsive peptides can be designed for specific conditions and targeted treatments. Thus, in this section, we discuss the design of pH-responsive peptides focusing on 1) amino acid selection, and 2) attaching cleavable functional groups through pH-sensitive linkages.
2.1.1. pH-Responsiveness by Amino Acids
The charged amino acids can be categorized as basic amino acids (positively charged) and acidic amino acids (negatively charged). The basic amino acids (R, H, and K) are frequently incorporated into pH-responsive self-assembling peptide systems (Figure 2A), as the acidic microenvironment leads to protonation of the side chains, which can further trigger changes in structure, morphology, and drug release rate.22–23 -For example, Xu and coworkers designed a pH-responsive system containing KKGRGDS as a hydrophilic and acid-responsive head, and VVVVVV as a hydrophobic tail. This amphiphilic peptide self-assembled into micelles at neutral pH (pH 7.0), but at pH 5.0 the micelles disassembled due to repulsive electrostatic interactions among the mostly protonated K residues. After loading the anti-tumor drug doxorubicin (Dox) in the amphiphilic peptide micelles at pH 7.0, continuous and fast drug release was observed at pH 5.0, inhibiting tumor growth.24 Another basic amino acid, H, is popularly applied in the design of pH-responsive cell penetrating peptides (CPPs), which have been used in various pH-dependent peptides for tumor targeting.25–28 The imidazole side chain on H residues has a pKa near 6, enabling a change in charge status at physiologically relevant pH values. For example, Lei at al. recently designed a pH-responsive, self-assembled lignin-H nanoparticle that was loaded with the drug 10-hydroxycamptothecin (HCPT).29 The 30-40 nm nanoparticles were an ideal size for passing through cell membranes and disassembled under the acidic conditions of the tumor microenvironment upon protonation of the imidazole group, releasing the drug (Figure 3). These two examples highlight how pH-responsiveness can be incorporated into peptide-based drug delivery systems for cancer therapy through the judicious use of basic amino acids.
Figure 2.
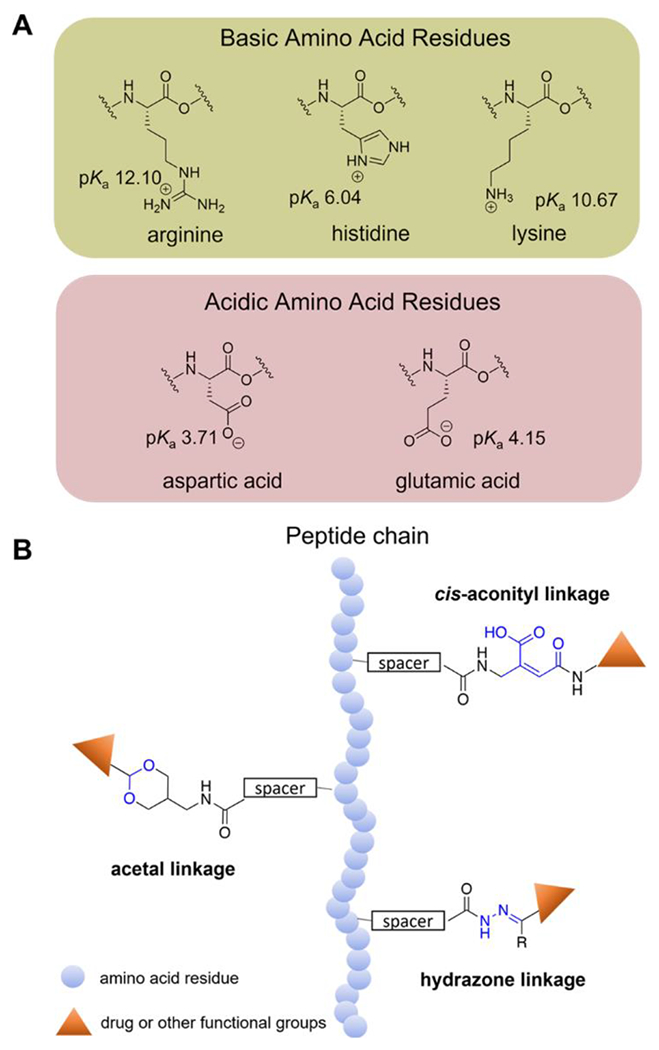
A) Chemical structures and pKa values (at 25 °C) of amino acid residues with electrically charged side chains. B) Structures of common pH-sensitive linkages.
Figure 3.
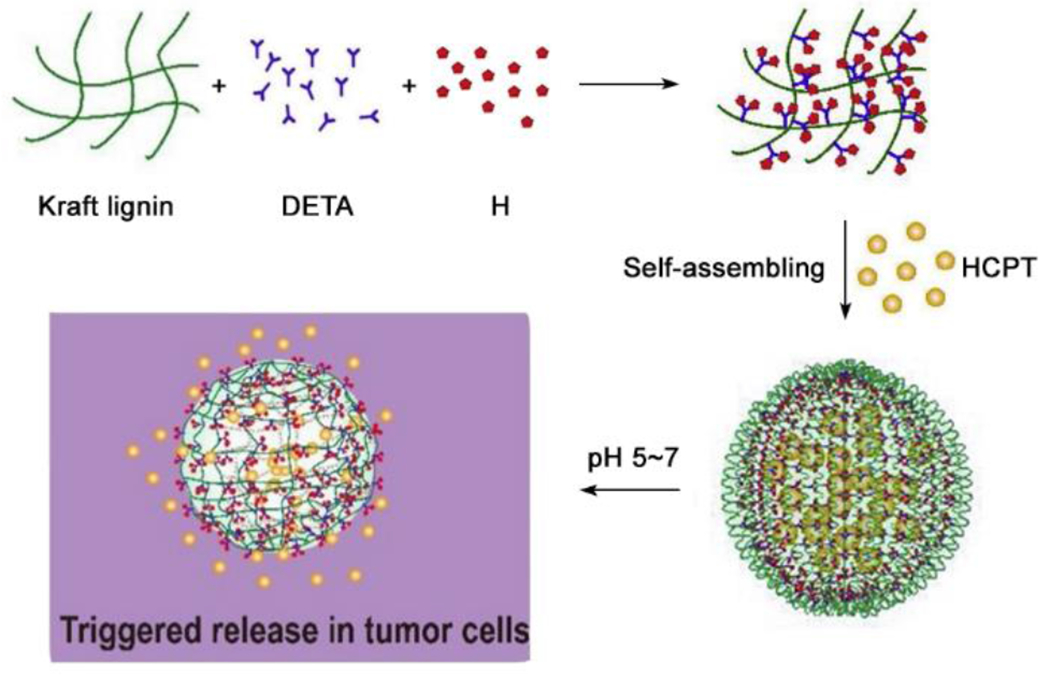
Schematic illustration of the construction of AL-His/HCPT nanoparticles via self-assembly. Reproduced with permission from reference 29. Copyright 2020 Elsevier.
Besides basic amino acids, acidic amino acids are also commonly used in the design of pH-responsive self-assembled materials. Moreover, the acidic and basic amino acids are often combined together to achieve a charge conversion system design.30–31 For example, Kim and coworkers utilized ABC triblock copolymers of the structure poly(ethylene imine) (PEI)–Kn–Em (PKE polymers) for gene delivery.32 As a pH-responsive charge conversion polymer, the Em block helped buffer the mildly acidic endosomal pH. Therefore, they designed and synthesized PKEs with various numbers of E residues (7, 9 or 13 units) to study their self-assembly and buffering ability. The results suggested that PK5E9 self-assembled into nanoparticles and improved the transfection efficiency in the tumor environment compared to PEI only. The in vitro and transfection experiments indicated that these PK5E9 polyplexes effectively escaped the endosome and improved cellular uptake by 1.5-2.5 times and transfection efficiency up to 50-fold at pH 6.0 compared to pH 7.4 due to pH-responsive charge-conversion, highlighting how pH-responsiveness can be utilized in the design of gene-delivery systems.
2.1.2. pH-responsiveness by Functional Groups
Besides tuning pH-responsiveness via carefully choosing amino acids, installing functional groups that have pH-cleavable linkages affords additional design space. These linkages are stable under normal physiological pH but undergo cleavage or structural changes under acidic or basic conditions. This approach has been recently widely applied in the field of pH-responsive self-assembling peptide biomaterials, as the non-functional molecule becomes functional when the pH changes, providing a specific and “smart” target treatment. Various pH-labile linkages such as hydrazones,33 cis-aconityls,34 dimethylmaleic amides,35 and acetals36 (Figure 2B) are commonly used in pH-responsive systems. For example, Balci and Top designed a pH-responsive drug delivery system by conjugating mPEG-peptide with Dox via an acid cleavable hydrazone bond to evaluate drug release under normal and acidic conditions.37 The conjugated peptide, AT1, included a pH-responsive H6 domain. They compared drug release and cell viability of mPEG conjugated directly to Dox through a hydrazone (mPEG-HYD-Dox) and the hydrazone-linked mPEG-peptide conjugate (mPEG-AT1-Dox). Results showed that mPEG-AT1-Dox released around 3-fold more Dox at pH 5.0 at 72 h and displayed higher cytotoxicity compared to mPEG-HYD-Dox. This example shows that the pH-responsive hydrazone bond, while useful in triggering drug release, can be enhanced by including other pH-responsive peptides in the delivery system design.
Recently, Weil and coworkers incorporated the pH-sensitive boronic acid-salicylhydroxamic (SHA) group in a novel peptide complex designed to undergo a multistage transformation inside cells.38 The design included a cell-penetrating TAT peptide conjugated with modified depsipeptides, which hydrolyzed in an acidic environment due to the acid-sensitive SHA group. The resulting boronic acid headgroup was then cleaved by intracellular hydrogen peroxide inside cancer cells, leading to a subsequent O→N-acyl shift, which further formed peptides that co-assembled into fibrillar networks inside A549 cells (Figure 4). The novel multistage transformation induced by the acidic microenvironment inside cancer cells provided a platform for precisely programming cell death. Additionally, this work suggests future directions of this approach focusing on incorporating other functional groups with pH-responsiveness and specific responses to metabolites within self-assembled nanostructures as a method for customized medicine.
Figure 4.
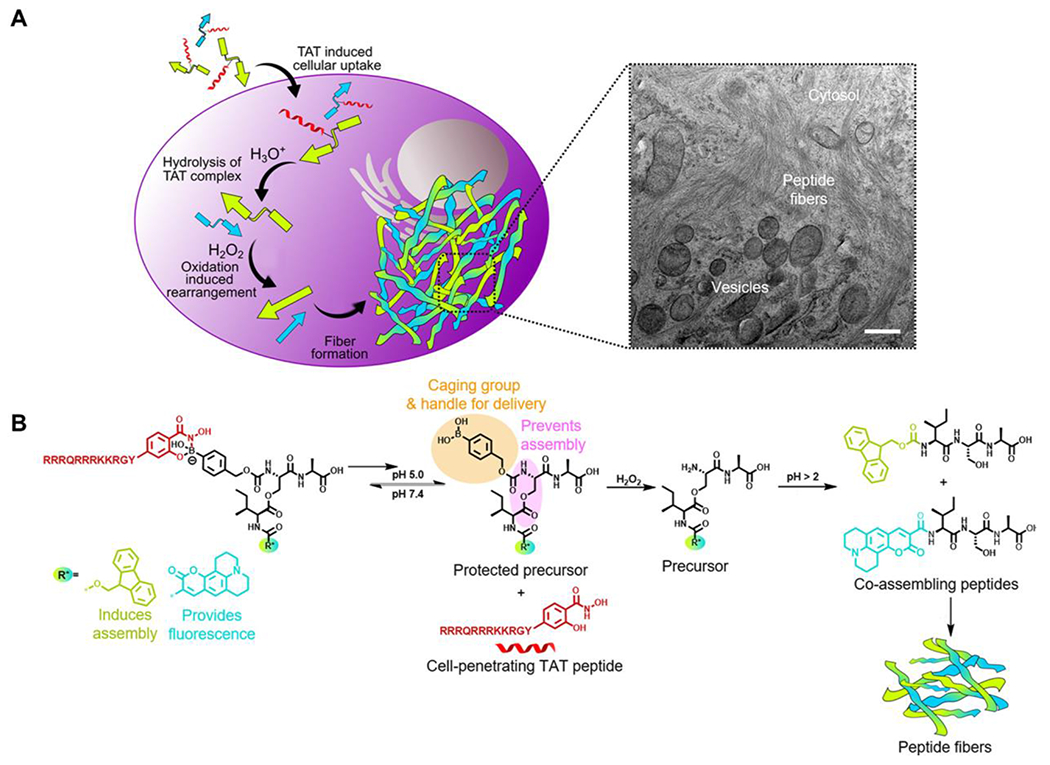
A) The overall scheme of intracellular co-assembly of peptides. Visible fibrillar networks were formed in the final step and observed by transmission electron microscopy (TEM, scale bar 500 nm). B) Scheme of chemical reactions that happened inside cells. Reproduced with permission from reference 38. Copyright 2020 American Chemical Society.
2.2. Response Mechanism
In pH-responsive peptides, it is typically the ionization state of the peptide side chain that dictates the hydrophilicity or hydrophobicity of the segments and therefore the chemical and/or morphological response of the systems. But it is important to consider that the peptide side chains are not ionized all at once. With the assumption that the acid–base properties of the peptide side chain functional groups are independent of each other, the overall pKa can be estimated based on the Henderson–Hasselbalch equation. In this case, when enough side chain functional groups become ionized or deionized, a self-assembled morphology change may occur depending on the amphiphilicity of not only the pH-responsive segment(s) but the entire peptide. Predicting at what pH a morphology change might occur becomes more difficult when amino acids with ionizable side chains have interdependent pKa values, i.e., when they are close to each other in the primary sequence. However, in most applications of pH-responsive peptides in drug delivery and tissue engineering, peptides become less charged upon a shift in pH, leading to self-assembly. Peptides can also become more charged upon a shift in pH, leading to either expansion or complete disassembly of a self-assembled morphology. Finally, a pH-shift can also cause a morphological switching between multiple self-assembled nanostructures. Among these three types of response mechanisms (self-assembly, expansion/disassembly, and morphology switching), the pH-triggered self-assembly mechanism is the most straightforward—peptides are initially molecularly dissolved, then a pH change gives rise to a shift in the ionization state and hydrophilic/hydrophobic balance, inducing self-assembly into nanostructures. The expansion/disassembly and morphological switching mechanisms can be more complex. Commonly, peptide-based pH-responsive systems self-assemble into a nanostructure in a minimally charged state. With changes in the environmental pH, some segments in peptide-based pH-responsive systems may gain positive or negative charges, becoming more hydrophilic. This shift causes expansion due to charge repulsion and can lead to eventual disassembly into molecularly dissolved components. Alternatively, peptides can be designed to self-assemble but contain segments that lose charge upon a change in pH to become more hydrophobic; these systems usually undergo a morphological switching responsive mechanism. Regardless of whether the pH-responsive peptide undergoes disassembly or morphological switching, if the ionization state is the only change, then these pH-responsive systems can exhibit reversible switching behavior, although there may be a large hysteresis. However, if a morphological switch is triggered by a chemical change such as bond-breaking, that switch is typically not reversible. Considering that reversibility is not required in most applications in drug delivery and tissue engineering, we focus here on these three types of switching mechanisms without discussing their (potential) reversibility.
2.2.1. pH-triggered self-assembly
In the pH-triggered self-assembly mechanism, peptides usually molecularly dissolve with partial charge on the side chains. A pH change then triggers protonation or deprotonation to decrease the total amount of negative or positive charge, which reduces electrostatic repulsion between peptides. Finally, hydrogen bonding, aromatic stacking, and other intermolecular interactions lead to self-assembly. For example, Zhang and coworkers recently reported a peptide sequence, KRRFFRRK, that underwent a pH-triggered self-assembling mechanism.39 This peptide remained in the unimeric state or as small, poorly defined aggregates at pH 7.4, where both the K and R residues were mostly protonated. When the pH was increased to 9.4, the K and R residues became partially deprotonated, inducing self-assembly into nanofibers driven by the reduced electrostatic repulsion and increased hydrophobic interactions. Another example was reported by Montenegro, Granja, and coworkers, who designed a cyclic peptide (CP1) that polymerized into nanotubes after the addition of base (Figure 5A).40 The key component of this cyclic peptide was two H residues and a K residue. When dissolved in acidic aqueous media, the positively charged H and K side chains on CP1 prevented aggregation due to cationic electrostatic repulsion. Neutralization of the aqueous media to pH > 8 gave rise to deprotonation of the H side chains, triggering self-assembly of CP1 into peptide nanotubes (Figure 5B). Further addition of base led to deprotonation of more H and K side chains, inducing self-assembly into hierarchical nanotubular networks and finally large fibers stabilized by H residue hydrogen-bonding.
Figure 5.
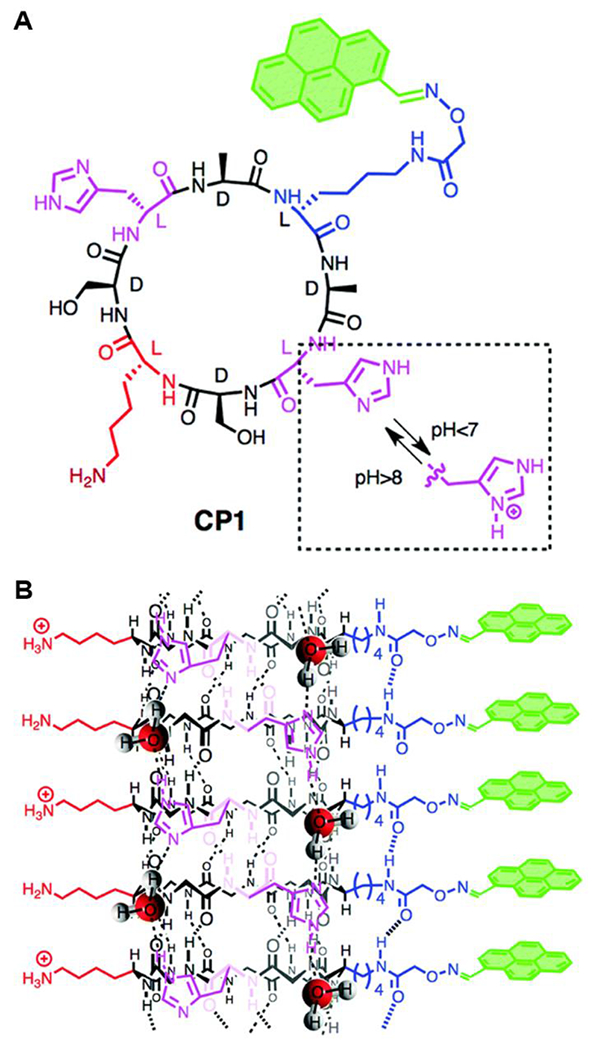
A) Structure of CP1 cyclic peptide. B) Self-assembly of CP1 into single peptide nanotubes. Reproduced with permission from reference 40. Copyright 2018 Royal Society of Chemistry.
2.2.2. pH-triggered Expansion and Disassembly
In response to a change in pH, self-assembled peptide morphologies can expand (swell) and disassemble (Figure 6A). In these situations, with a pH change the total amount of charge increases due to protonation or deprotonation, which then increases repulsive interactions between molecules, leading to expansion and disassembly. Kim and coworkers reported a series of micelle-forming, pH-responsive synthetic copolymers consisting of a poly(N-isopropylacrylamide) block with a DP of 55 and an Hn block with different DPs (n = 50, 75, 100, 125).41 The Hn block became protonated under acidic pH, resulting in stronger electrostatic repulsion, which led to swelling of the micelle structures. Dynamic light scattering data of different copolymers revealed that micelles maintained the same size in solution within a pH range from 6.5 to 10. However, when the pH of the solution decreased to 5.6, the size increased dramatically. With continuous lowering of the solution pH as low as pH 2, bigger micelles were observed. The size of the micelles grew at a similar rate with decreasing pH regardless of Hn block size, which was attributed to the similar percentage levels of protonated H residues as the pH decreased.
Figure 6.
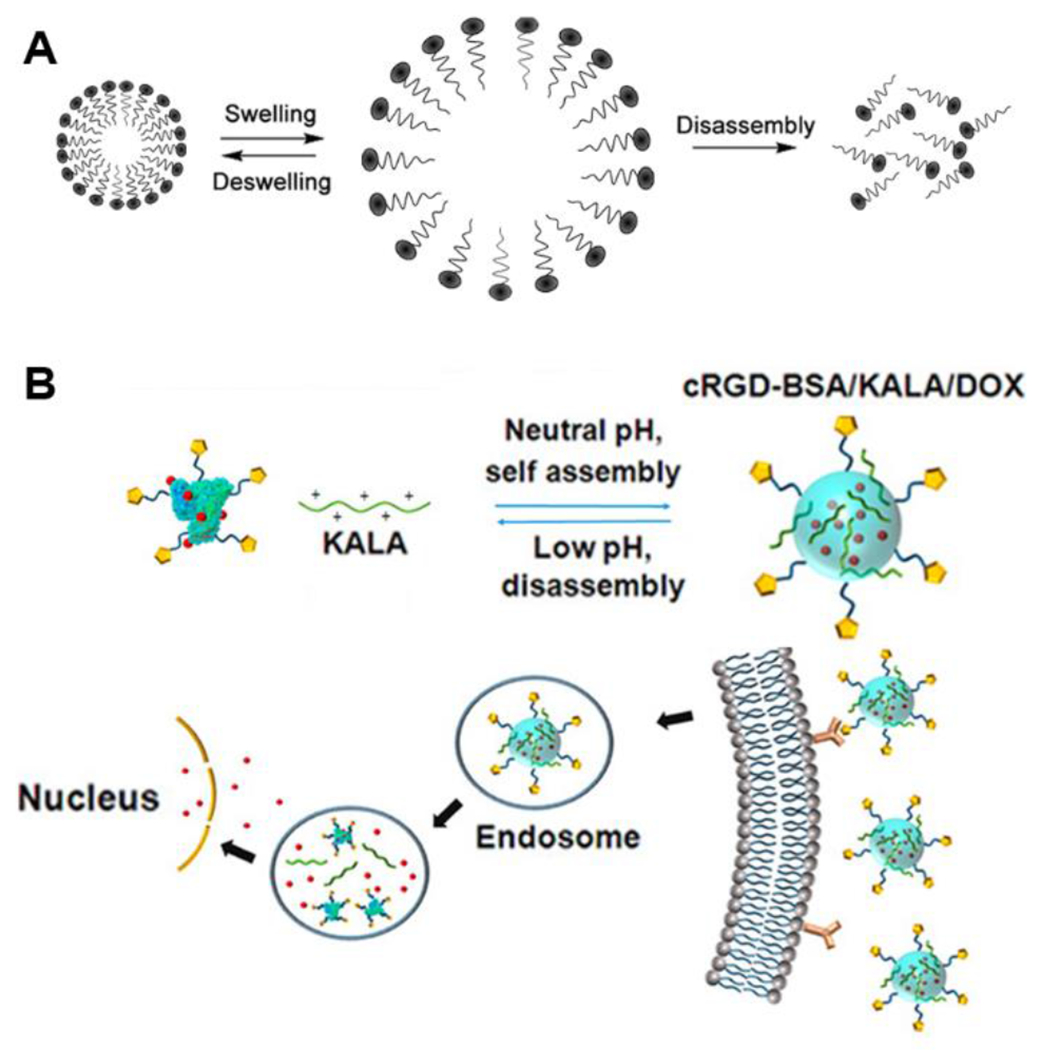
A) A general scheme describing the expansion and disassembly responsive mechanism triggered by a pH change. B) The self-assembly/disassembly behavior of cRGD-BSA/KALA/Dox nanoparticles and the drug delivery route of Dox to cancer cell nuclei. Reproduced with permission from reference 42. Copyright 2015 American Chemical Society.
Self-assembling peptides have been designed to disassemble in one or several steps in response to different pH values due to ionization or pH-sensitive bond cleavage. One example of ionization-induced disassembly was designed by Cheng and coworkers.42 They reported a bilayer nanoparticle drug delivery system with a positively charged KALA peptide that self-assembled to form the outer layer and a cyclic Arg-Gly-Asp (cRGD) peptide that was conjugated to negatively charged bovine serum albumin (BSA) and loaded with Dox to form small nanoparticle nuclei as the core (Figure 6B). With a decrease in pH, electrostatic interactions between BSA and KALA decreased, leading to the expansion and disassembly of the outer layer of the nanoparticles. Additionally, as more groups in the cRGD-BSA core were protonated at low pH, their interactions with positively charged Dox molecules weakened, causing disassembly of the nuclei to release the Dox. An example of pH-triggered, bond cleavage-induced disassembly is a poly(ethylene glycol)-block-poly(aspartate-hydrazone-Dox) block copolymer (PEG-PAHD) designed by Kataoka and coworkers.43 The block copolymer formed a spherical core-shell structure with a diameter of ~65 nm. The PEG-PAHD copolymer was stable at physiological pH, whereas it disassembled rapidly at pH 4-6 due to the cleavage of hydrazone bonds and release of the hydrophobic Dox units.
2.2.3. pH-triggered morphological switch
Transitions between two or more different self-assembled morphologies can be another response mechanism. In recent years, scientists have made many advances in synthesizing nanomaterials whose morphology can be switched due to pH variation, leading to changes in material properties.44 For example, Zhang and coworkers synthesized three flexible peptides [P1: (stearyl-GRGDG)2KG; P2: (Fmoc-GRGDG)2KG; and P3: (acetyl-GRGDG)2KG] that self-assembled into various morphologies at different pHs, including nanofibers, nanospheres, and lamellar structures.45 The RGD sequence was introduced as the hydrophilic backbone and also one of the simplest peptides segments that has both basic and acidic residues capable of responding to changes in pH. P1 aggregated into nanofibers at pH 3.0, then self-assembled into nanospheres at pH 6.0 and further changed into lamellar structures when the pH value was further increased to 10.0 (Figure 7A). P2 self-assembled into an entwined network structure at pH 3, then switched into nanospheres, lamellar structures, and vesicles with increasing pH values. Compared to P1 and P2, the lack of a large hydrophobic group on P3 resulted in no well-defined nanostructures for this specific peptide.
Figure 7.
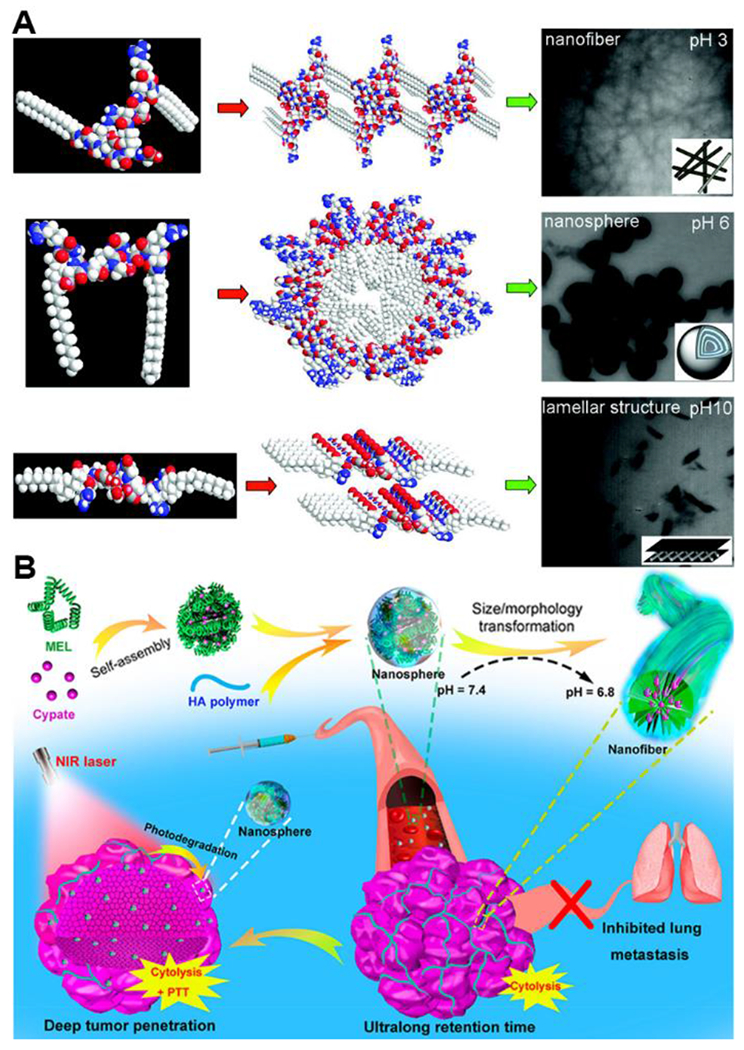
A) Self-assembly scheme and TEM images of P1 peptide under different pH values. Reproduced with permission from reference 45. Copyright 2012 American Chemical Society. B) Schematic representation of the fabrication of MEL/Cypate@HA complexes and their successive size/morphology transformations triggered by the mildly acidic tumor microenvironment and NIR laser irradiation. Reproduced with permission from reference 46. Copyright 2019 American Chemical Society.
A more recent example highlighting a morphology transformation was reported by Wu and coworkers. In this work, the authors fabricated a tumor acidity-responsive nano-agent from a combination of the cytolytic peptide melittin (MEL), the NIR-absorbing molecule cypate, and hyaluronic acid (HA).46 The MEL/Cypate@HA constructs physically self-assembled into nanospheres and further transformed into nanofibers in response to decreasing pH, then back into nanospheres in response to NIR treatment (Figure 7B). These examples highlight how pH-responsiveness can go beyond assembly/disassembly to enable complex changes in self-assembled nanostructures, a property that could be applied widely in biomedical applications in the future.
3. Drug Delivery
Self-assembled, peptide-based biomaterials have been widely used to develop new strategies for drug delivery. Due to significant therapeutic potential in various areas, such as systemic controlled release, in vivo theranostics, combined drug delivery, APIs with extended circulation time, and the capacity for multiple functions (e.g., targeting, imaging, cell penetration, morphology changes), innovative drug delivery systems built upon self-assembled peptide-based biomaterials have recently gained significant attention. Furthermore, incorporating one or more stimuli-responsive units into these constructs promises systems that can bypass biological barriers and achieve targeted intracellular drug delivery.47 Among many known environmental stimuli, pH gradients have been widely used to design responsive self-assembled peptide-based biomaterials. For example, pH values vary significantly in different tissues or organs, such as the stomach and liver, as well as in disease states including ischemia,48 infection,49 inflammation,50 and tumorigenesis.51 Commonly, pH-responsive peptides may be designed to deliver drugs at the organ level, the tissue level, and/or the cellular level. For example, at the cellular level the pH drops rapidly below 6.0 upon internalization due to endosomal acidification after endocytosis, which can induce the pH responsiveness of self-assembled peptides. However, in most drug delivery applications, pH-responsive self-assembling peptides take advantage of both cellular-level and tissue-level targeting. In contrast, drug delivery at the organ level typically focuses on self-assembled biomaterials capable of releasing one or more drugs at specific locations along the gastrointestinal (GI) tract.52–53 At the tissue level, self-assembled peptide-based biomaterials have been designed to exploit pH gradients that exist in tumor microenvironments to achieve high local drug concentrations at cancer sites.54–55
3.1. Oral drug delivery
Considering its convenience, potential for high patient compliance, and cost-effectiveness, oral delivery is an attractive drug delivery route. However, each segment of the GI tract maintains its own characteristic pH level, from the most acidic stomach lumen (pH 1 to 3 for digestion),56 to the weakly acidic duodenum where the pH is around 5, to the ileum where the pH nears neutrality at around 6.6 to 7.5.57–58 Orally delivered drugs are exposed to strong gastric acid and enzymes first in the GI tract, often resulting in degradation and leading to poor drug delivery efficiency. Therefore, achieving adequate and consistent bioavailability for sensitive orally administered drugs can be a challenge. One way to protect drugs through the harshest conditions in the GI tract is using chitosan as the drug carrier, which provides a barrier toward degradation under strongly acidic conditions in the stomach. Recent developments in nanodrug delivery systems include tunable compositions with pH-responsive mechanisms to deliver drugs to specific sites along GI tract.
The bacterium Helicobacter pylori is a gram-negative pathogen that colonizes the deep mucus layer near the stomach epithelium, where the pH is around 6.59 Recent work by Mi and coworkers successfully realized targeted release of amoxicillin, a traditionally used antibiotic for H. pylori infections,60 in a model of infected gastric mucosa.61 The drug delivery system included a zwitterionic, pH-responsive polypeptide, poly(γ-glutamic acid)-graft-arginine (γ-PGAn-g-Rm), which self-assembled into nanoparticles when loaded with amoxicillin in simulated gastric fluid (pH 2.5). The nanoparticles swelled and subsequently disassembled when the pH increased past 3.5, releasing the drug. In order to release the drug at a higher pH level, chitosan was used as a barrier layer on the surface of these pH-responsive nanoparticles, forming a polyelectrolyte complex that stabilized the system over a wide pH range (pH 2–6) (Figure 8). When the pH increased above 6, deprotonation of chitosan occurred, weakening electrostatic interactions and leading to exposure of γ-PGAn-g-Rm nanoparticles, which immediately swelled and disassembled to release the amoxicillin.
Figure 8.
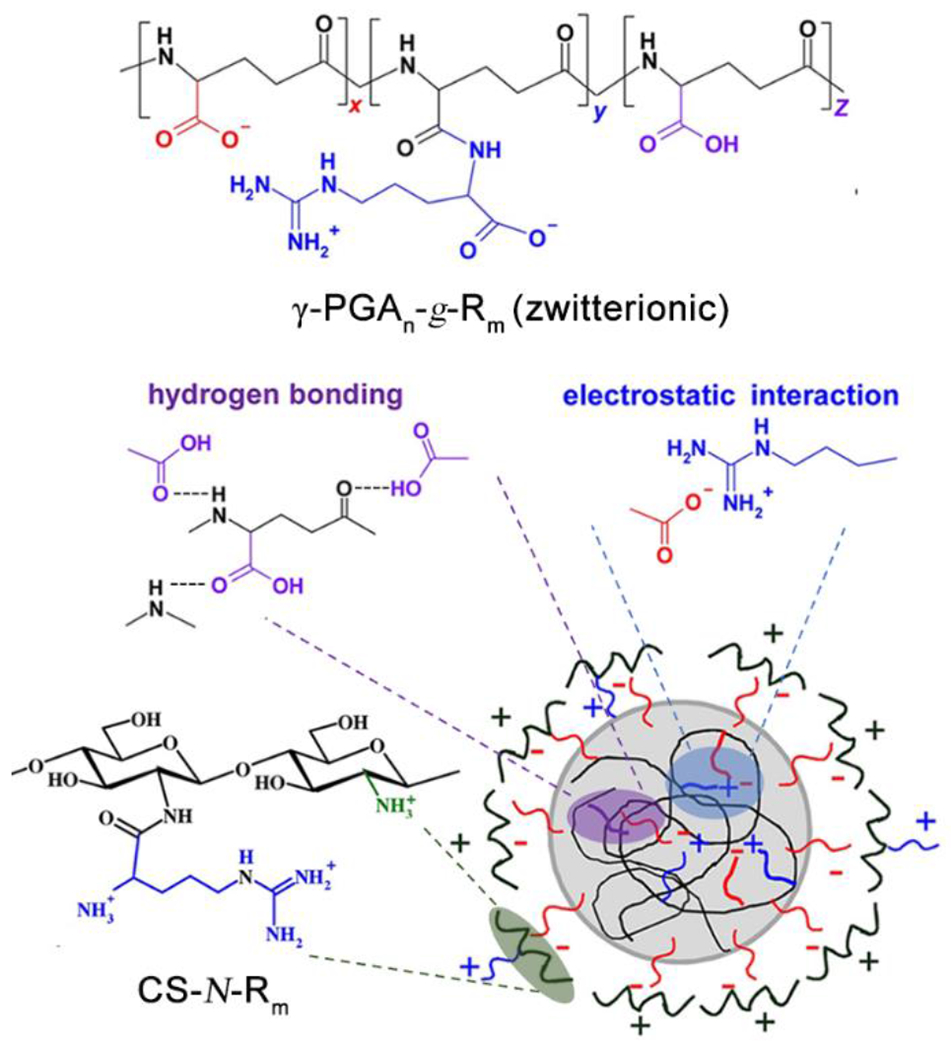
Schematic diagram of self-assembled CS-N-Rm/γ-PGAn-g-Rm complex nanoparticles. Reproduced with permission from reference 61. Copyright 2016 Elsevier.
In a related study, γ-PGA, tripolyphosphate, MgSO4, and chitosan were combined to fabricate multi-ion-crosslinked self-assembled peptide-based nanoparticles.62 The nanoparticles encapsulated insulin, a peptide hormone,63 with the goal of protecting it in the acidic stomach. As in the example above, chitosan protected the insulin under strongly acidic conditions by preventing nanoparticle destabilization. When the pH increased above 6, the multi-ion-crosslinked nanoparticles released insulin, delivering significantly more insulin than particles lacking tripolyphosphate and MgSO4, suggesting that multi-ion-crosslinked nanoparticles are a promising carrier for transmucosal delivery of insulin in the small intestine.
3.2. Tumor targeting
Due to elevated rates of glucose uptake but decreased rates of oxidative phosphorylation, rapidly growing tumor cells accumulate lactic acid, leading mildly acid tumor microenvironments (pH 5.7 to 6.8).64 Known as Warburg’s effect, this persistence of high lactate production by tumors in oxygen-abundant environments provides a growth advantage for tumor cells in vivo.65 Additionally, most tumors suffer from insufficient blood supply and poor lymphatic drainage, which also contribute to the acidic microenvironment at a tumor site.66 The acidic pH can be exploited to allow drug delivery vehicles to achieve high local drug concentrations at tumor sites while minimizing overall systematic drug exposure compared with free drugs.67 To achieve pH-dependent tumor targeting and drug release, self-assembled peptide-based biomaterials are commonly used and have been designed and fabricated with a tumor targeting group and a pH-responsive scaffold. Tumor-recognizing or targeting groups including cell-penetrating peptides (CPPs), cell-adhesive peptides (CAPs), and folic acid, among others, are thought to enhance delivery to a tumor site through active targeting.68 Complementing targeting ligands are pH-responsive components in nanostructures that can induce a morphological switch, assembly/disassembly, or hydrogel formation in response to the environmental pH change near tumor sites.22, 69
CPPs and CAPs have been widely used to increase cellular uptake of various drug carriers in drug delivery.26, 70–73 CPPs aim to overcome cellular obstacles such as membranes via direct penetration or endocytosis,74 while CAPs specifically bind to a cell receptor responsible for cell adhesion, raising the cell affinity of carriers.75 However, the effectiveness of CPPs or CAPs as drug delivery systems has been limited due to a lack of in vivo site-specificity, such as tumor selectivity. To realize tumor targeting, pH-sensitive CPPs and CAPs have been designed to exploit the acidic tumor pH. H residues are used widely in pH-responsive CPPs. In particular, the imidazole functional group on H residues is useful because it is protonated at mild acidic tumor pH but remains neutral under physiological conditions.76
Zhang and coworkers recently fabricated a tumor-specific drug delivery system termed Dox-PSL-H7K(R2)2, where a K residue was linked to an H7 sequence via its ε-amine and total of 4 R residues were attached to the N- and C-termini of this branching K residue. Thus, this design combined a pH-responsive peptide sequence, H7, and a branched CPP sequence, K(R2)2, with Dox-containing pH-sensitive liposomes (Dox-PSL) derived from dioleoylphosphatidylethanolamine and cholesteryl hemisuccinate.77 This construct formed spherical vesicles under neutral pH with an average diameter of 92 nm. At neutral pH, the H7K(R2)2 peptide anchored to the surface of the Dox-PSLs through H-bonding interactions in the H7 segment. With the pH decreasing from 7.4 to 6.5, the H7 segment became charged and stretched out, allowing the K(R2)2 unit to induce cell internalization. When pH decreased further to 5.5 in the endosome, this pH-responsive Dox carrier became unstable and released Dox. Compared with non-pH-responsive Dox-containing liposomes, this pH-responsive construct released 2-fold more Dox in C6 tumor cells and U87-MG glioma cells and exhibited better anti-tumor activity. This pH-responsive and cell-penetrating H7K(R2)2 unit highlights how clever peptide design can enable both cell adhesion and drug release.
Chen and coworkers also designed and fabricated a peptide amphiphile drug delivery system with the structure stearyl-H16R8-NH2, termed NP1 (Figure 9A).78 The goal of this nanocarrier system was to deliver siRNA capable of cancer cell destruction. The pH-responsive H16 component was mostly deprotonated above pH ~6, providing hydrogen bonding interactions among peptides and enhancing the formation of nanostructures. When the pH dropped below 6, the protonated H16 segment triggered disassembly due to the strong repulsive force induced and loss of hydrogen bonding between imidazole side chains. Due the high pKa of the guanidino groups on R residues, the R8 sequence is positively charged from mildly basic pH to acidic pH. It not only enhanced binding to the negatively charged plasma membrane via electrostatic interactions but also helped limit nanostructure growth and possibly drove formation of the spherical structures instead of fibers. With the synergistic function of the R8 CPP sequence and the H16 pH-responsive sequence, ellipticine (EPT) was successfully loaded into the micelle nanostructures via the self-assembly of NP1 and released inside cancer cells directly by a through-membrane translocation pathway followed by disassembly of the micelles, which avoided the EPT being trapped in the endosome. In vitro studies on A549 cells and CHO-K1 cells revealed that the NP1-EPT drug delivery system killed more than ~85% of the cancer cells, which was ~4 times higher than the same amount of EPT itself (Figure 9C).
Figure 9.
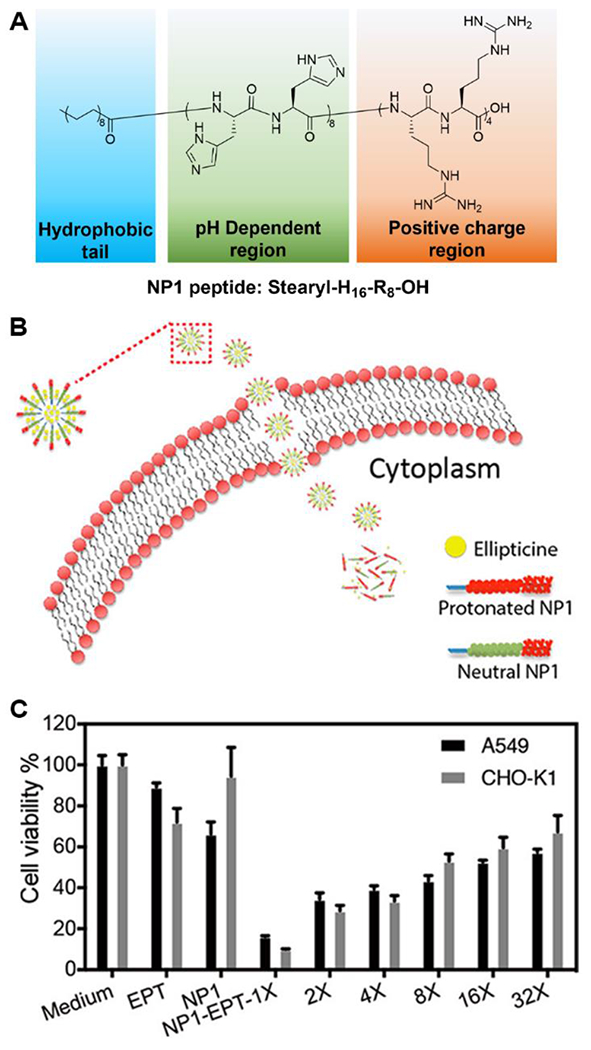
A) Molecular structure of NP1 peptide. B) Schematic representation of the process of how NP1-EPT penetrates the cell membrane. C) Cell cytotoxicity of EPT, NP1, and different-times diluted NP1-EPT on A549 and CHO-K1 cells. Reproduced with permission from reference 78. Copyright 2019 American Chemical Society.
In addition to CPPs, CAPs are also widely used to target drug delivery systems to tumors. One of the most commonly used CAP sequences is RGD. Its interaction with many known integrins like αvβ3 and αvβ5, which are overexpressed on the surface of malignant tumor cells and proliferating neovascular endothelial cells, makes RDG a tumor targeting sequence.79–81 Quan and coworkers reported an amphiphilic peptide (RGDSE10K(stearyl)) as a potential anti-cancer drug delivery vehicle.82 In pH 7.4 medium, the pH-sensitive E10 sequence was mostly deprotonated, where electrostatic repulsion between the carboxylate groups led to a random coil secondary structure. Driven by hydrophobic interactions among the stearyl groups, these peptide amphiphiles sequestered Dox as they self-assembled into micelles. With the pH decreasing to 5.0, protonation of the pH-responsive E10 sequence decreased the solubility of the E10 component, causing the peptide segment to adopt a β-sheet conformation, disrupting the micellar structure, and leading to release of Dox. Studies on HeLa cells revealed that this Dox-loaded peptide amphiphile system killed ~5 times more cells than the unloaded micelles, but the authors did not study a control amphiphilic peptide without a pH-responsive unit.
Besides examples involving micelle assembly/disassembly mentioned above, RGD-containing peptide conjugates can also have other pH-responsive mechanisms like hydrogel formation or dissolution. For example, Zhong and coworkers added an RGD segment and a pH-responsive H residue to a naphthyl-GFFY (Nap-GFFY) peptide, a widely used supramolecular hydrogelator.83 The Nap-GFFYRGDH aromatic peptide amphiphile self-assembled into nanofibers and formed a hydrogel at pH 7.0 capable of encapsulating Dox. Decreasing the pH to that of a typical tumor environment (pH ~6.5) protonated the imidazole group, thereby disrupting the balance of hydrophobicity and hydrophilicity. This protonation event led to nanofiber dissociation from the hydrogel and internalization into tumor cells via binding with integrin receptors. Finally, the mildly acidic endosome environment (pH ~5.5) triggered complete collapse of the nanofibers and also protonated the amine group on Dox, increasing the repulsive interactions between Dox and the aromatic peptide amphiphile, thereby leading to the release of Dox for cancer therapy.
These examples highlight synergistic effects between CPPs/CAPs and pH-responsive units in self-assembled peptides in cancer therapy. With the help of CPPs/CAPs, drug delivery systems can be internalized and accumulate toxic drugs in cancer cells. In addition, pH-responsive units control drug release in a certain pH range. Conjugates that include both CPPs/CAPs and pH-responsive units can therefore precisely target and achieve high local drug concentration at tumor sites at levels greater than either one of the components working alone.
Besides CPPs and CAPs, other tumor-recognizing functional groups or peptide segments that interact with overexpressed proteins and surface antigens on target cells can also achieve localized drug treatments. Folic acid (FA) is a popular targeting group and plays an essential role by binding with folate receptor, a membrane-anchored protein that is often overexpressed in cancer cells.84 An early example of a self-assembled FA-peptide drug delivery system by Yang and coworkers showed reduced side effects in Taxol anti-cancer chemotherapy compared to free drug due to assembly formation, but this system lacked pH-responsiveness.85 More recently, Wang and coworkers designed and synthesized a pH-responsive, FA-modified peptide prodrug with the structure FA-EEYSV.68 This work involved the short peptide segment YSV, which itself acts as an anticancer drug that can induce apoptosis and necrosis, as well as the pH-responsive EE sequence (Figure 10A). The EE sequence provided water solubility at neutral pH while also acting as a pH-responsive component. At pH 7.0, with both E residue carboxylic acid groups deprotonated, this peptide prodrug self-assembled into micelles with an average diameter of 20 nm. Decreasing the pH from 7.0 to 5.0 partially protonated the carboxylic acid groups and weakened electrostatic repulsion between these groups, inducing a morphological switch from spherical micelles into nanofibers with an average diameter of 10 nm. The nanofibers exhibited a higher internalization efficiency for HeLa cells than the spherical micelles and showed better anti-tumor activity.
Figure 10.
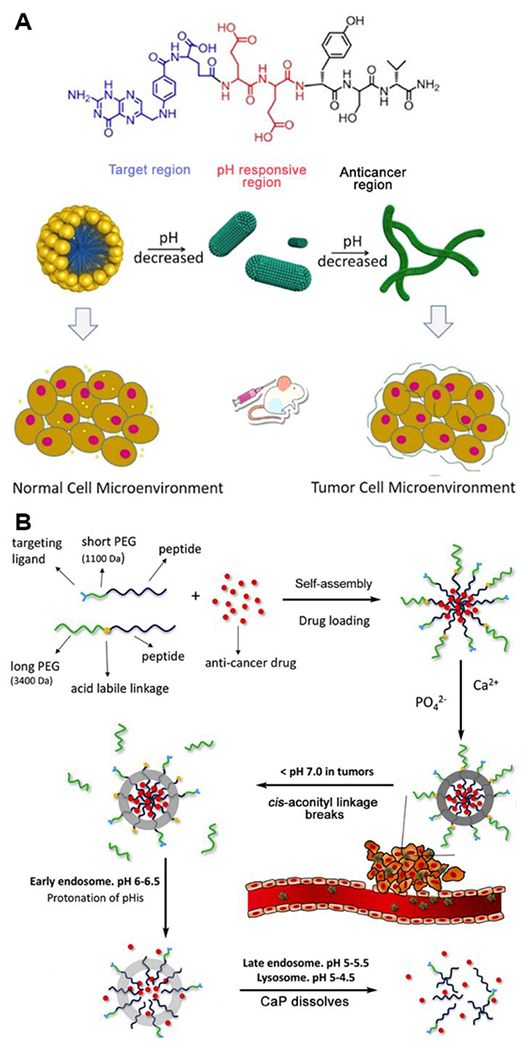
A) Molecular design and cartoons showing the properties of molecular assemblies of FA-EEYSV under different pH conditions. Reproduced with permission from reference 68. Copyright 2021 American Chemical Society. B) Formation of Dox-loaded nanoparticles with CaP mineralization layer and structural transition of nanoparticles under different in vivo conditions. Reproduced with permission from reference 36. Copyright 2017 Elsevier.
A particularly interesting pH-responsive system reported by Sun and coworkers involved multiple pH-sensitive components that maximized structural stability in the bloodstream, minimized drug loss before reaching target sites, and enhanced accumulation in tumor tissue.36 The multifunctional, pH-responsive self-assembling peptide-based biomaterials consisted of a protective layer of calcium phosphate (CaP), a biocompatible mineral that is abundant in human hard tissues, and two different amphiphilic sequences, PEG3400-cis-aconityl-E15H10L10 and LyP1-PEG1100-E15H10L10 (LyP1 is a cyclic peptide that binds to breast cancer cells86–88). Three triggers—the CaP layer, the cis-aconityl linkage, and the H10 sequence—worked in a sequential and hierarchical fashion to prevent leakage of encapsulated Dox during delivery. As shown in Figure 10B, with the protection of the PEG layer and the CaP layer, Dox-loaded micelles could circulate in the bloodstream where the pH is close to neutral. When the drug delivery vehicle reached the tumor environment with a mildly acidic pH, the cis-aconityl linkage, the first pH-responsive trigger, broke, shrinking the micelles and exposing the targeting group, LyP-1. Once the cancer cells engulfed the micelles through receptor-mediated endocytosis, the micelles directly entered the endosome and were exposed to a pH of 6-6.5. At this stage, the H10 sequence on the polypeptide became largely protonated, leading to swelling of the spheres. Then with the pH continuously decreasing in the late endosome and lysosome, the CaP layer dissolved, and the spherical structure collapsed, causing the release of Dox. This intelligent design provides a novel method to stabilize drug cargo based on self-assembled peptide-based biomaterials in a physiological environment and enhances the targeting ability to specific cancer cells.
Both in orally delivered and injectable formulations, pH-responsive peptide-based biomaterials are becoming increasingly important components in drug delivery. In oral drug delivery, chitosan plays a key role in protecting sensitive peptides in the highly acidic and protease-rich stomach. Once the drug delivery system reaches the mildly acidic intestines, pH-responsive units in peptides can trigger delivery to specific areas of the GI tract, improving drug targeting. Thus, these types of materials hold the potential to overcome bioavailability challenges that limit oral administration of sensitive therapeutics such as peptides or small molecules that lack acid stability. In injectable tumor-targeting drug delivery systems, many pH-responsive, self-assembling peptides have already been developed with spherical or fibrous morphologies. Many achieve higher tumor selectivity and anti-cancer activity than free drugs, especially with the aid of CPPs, CAPs, and other targeting functional segments. In the future, we expect to see systems with even higher targeting capacity and better stability using multiple types of pH-responsive units. Thus, pH-responsive, self-assembled peptides will continue to enable more precise delivery of small molecules and biologics to more effectively treat cancers.
4. Tissue Engineering
In addition to the broad applications of pH-responsive, self-assembling peptides in drug delivery systems, these biomimetic peptides have been widely exploited in the field of tissue engineering because of their easy modification, flexible design, and biocompatibility in many contexts. Tissue engineering scaffolds play an important role in providing cells with a supportive surrounding microenvironment for them to grow, migrate, differentiate, proliferate, and communicate with other cells.89 The tunable side chains and various combinations of amino acids make self-assembling peptides promising candidates for constructing scaffolds with molecular-level control triggered by pH changes. Since the pH of abnormal tissue microenvironments is different from the normal physiological microenvironment (pH 7.4), these pH-responsive peptide materials can be applied according to physiological pH changes to improve precision treatment to the ECM for tissue engineering. pH-Responsive peptides therefore hold promise as responsive materials in tissue engineering, where self-assembling peptide hydrogels have become popular in recent years, in large part to due to their structural and chemical similarity to natural ECM microenvironments.90 With both pH-responsiveness and self-assembly properties, these peptide-based materials provide an advanced platform in tissue engineering. In this section, we discuss how pH-responsiveness in self-assembling peptides can be harnessed to provide stimuli-responsive tissue engineering constructs.
4.1. Extracellular Matrix Scaffolds
The extracellular matrix is a natural, three-dimensional microenvironment that primarily provides structural support to cells but also includes chemical signals. It is composed of fiber-forming proteins including collagens, laminin, elastin, fibronectin, and fibrillin,91 as well as non-fiber-forming proteins, such as proteoglycans, glycosaminoglycans (GAG), and various soluble factors.92 The ECM is highly heterogeneous, and its composition varies from tissue to tissue, providing adhesive points for cells while also triggering cellular responses such as migration, proliferation, and differentiation using both chemical and physical cues.93 As the native ECM is a highly complex material that is practically impossible to reproduce in the lab, researchers construct simplified synthetic materials that are capable of mimicking certain aspects of spatiotemporal dynamics and functions of native ECM. Self-assembling peptides are one such material capable of mimicking native ECM because of their easy modification to include peptide-based chemical cues. Inclusion of pH-responsive units in self-assembling peptides used as ECM mimics has gained interest since the pH of an abnormal tissue microenvironments is often higher or lower than normal physiological pH (pH 7.4). For example, malignant melanoma tissues have a pH of ~6.9,94 while the pH in the microenvironment of chronic wounds can range from mildly acidic to mildly basic depending on the specific type of wound, the stage of the healing process, and the presence of infection.95 Self-assembling peptides designed to mimic the native ECM proteins collagen and laminin are of particular interest and importance.
4.1.1. Collagen-like Peptides
Collagen, the most abundant protein in the human body and the main structural component in the ECM, is an obvious starting point for ECM-mimicking materials. As a natural fibrous material, collagen is essential in providing structural and connective support, as well as regulating cell proliferation and migration.96 Collagen consists of three polypeptide α-chains supercoiled around a central axis and forming a triple helix structure. The composition of these three polypeptide chains is a repeating tripeptide of the sequence GXY, where X and Y are usually proline and 4-hydroxyproline (O), respectively.97 Collagen-like peptides with this type of triple helix structure have been considered as a method to mimic the structural and bio-functional properties of natural collagen.98–99 One example of a pH-responsive collagen-like peptide was reported by Mazzenga and coworkers.100 They synthesized the peptide amphiphile palmitic-KTTKS, of which the cationic pentapeptide headgroup is derived from pro-collagen I. They found that the peptide amphiphiles formed flat. tape-like structures at pH 7, while with the pH decreasing, they self-assembled into twisted fibrils at pH 4 and back to flat tape-like structures at pH 3, finally transforming into spherical micelles at pH 2. This work shows the dramatic influence that pH can have on collagen-like peptides even well below the pKa of the responsive groups.
The Wennemers group has also developed pH-responsive, self-assembling peptides as collagen mimics with a focus on pH-responsive proline derivatives.101–102 For example, they introduced γ-azaproline (γ-azPro) as a pH-responsive and functionalizable proline analogue for mimicking collagen.101 The amine in the γ-position of γ-azPro provides pH-responsiveness and can also be functionalized by acylation (Figure 11). They have designed two collagen model peptides (CMPs), one with γ-azPro in the X position in the GXY repeat (4X) and the other with γ-azPro in the Y position (4Y). Both the 4X and 4Y CMPs formed triple helices under acidic pH (pH 1.9) and basic pH (pH 10.1) conditions. Additionally, thermal denaturation studies indicated that the triple helices disassembled at pH ~4. This work shows how small chemical design features in CMPs can endow materials with pH-responsiveness.
Figure 11.
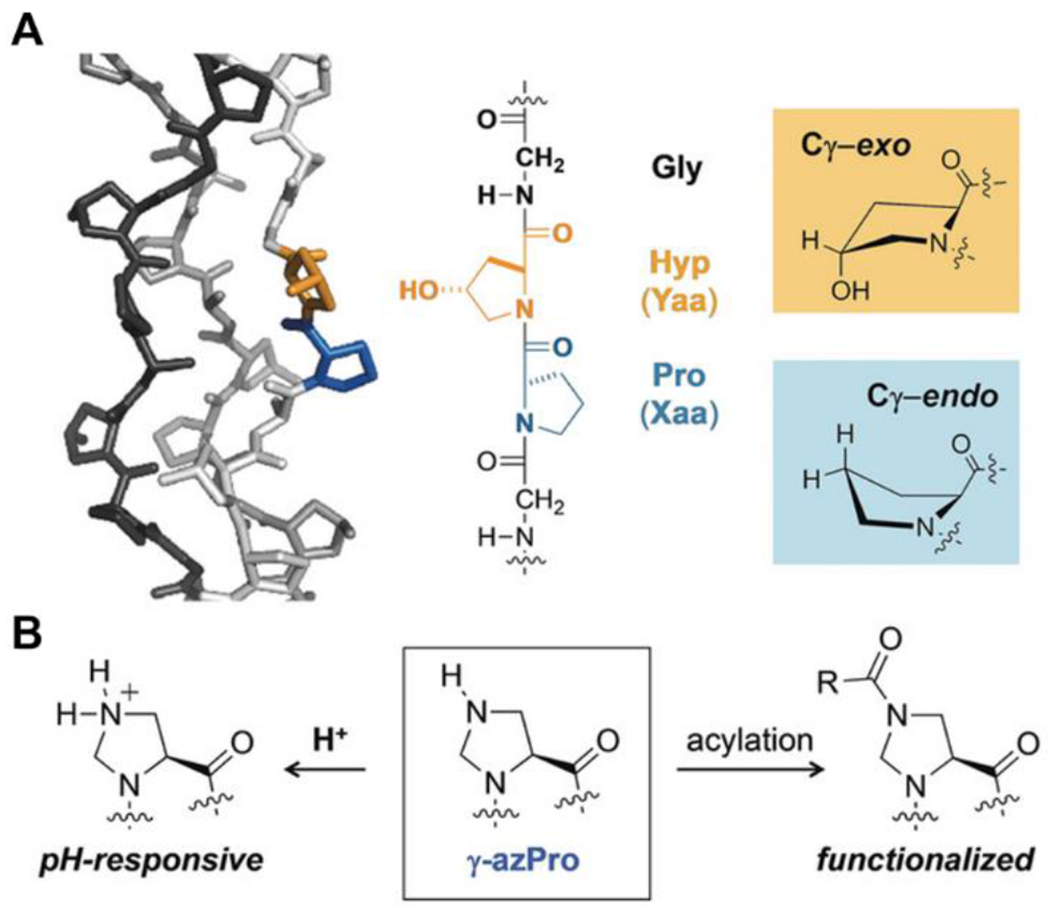
A. Structure and components of collagen. B. γ-azPro for pH responsiveness and functionalization. Reproduced with permission from reference 101. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
4.1.2. Laminin-like Peptides
In 1999, Coelho-Sampaio and Freire first reported self-assembly of natural mouse laminin under acidic conditions (pH 4) at low concentrations,103 which laid the foundation for studies on synthetic peptides derived from ECM components such as laminin and their pH-triggered self-assembly. Laminin is one of the major components of the ECM and is involved in several cell activities, such as cell differentiation, migration, and adhesion.96 The key sequence in laminin is the pentapeptide IKVAV, which itself does not have pH-responsiveness in a physiologically relevant range. In laminin-derived peptides, pH-responsiveness can be achieved by introducing various functional groups, which can also provide self-assembling properties.
Williams and coworkers reported a methodology to tune the pKa of a laminin-inspired peptide by simply incorporating acidic residues, shifting the pH at which self-assembly occurs from ~11.5 to 7.4, which would be suitable for cell culture condition.20 They synthesized a library of IKVAV containing peptides with 1, 2, or 3 D residues appended to the N-terminus of an Fmoc-IKVAV pentamer, creating the peptides Fmoc-IKVAV (pKa 6.42), Fmoc-DIKVAV (pKa 3.1), Fmoc-DDIKVAV (pKa 2.92), and Fmoc-DDDIKVAV (pKa 2.82). The number of D residues also changed the pH at which gelation occurred from pH 11 for Fmoc-IKVAV, to pH 9 for Fmoc-DIKVAV and pH 6 for Fmoc-DDDIKVAV. Specifically, Fmoc-DDIKVAV yielded a rigid hydrogel with a nanofibrous architecture at physiological pH. This work provided a method to optimize peptide self-assembly conditions toward physiological pH by simply tuning the sequence of the amino acids.
In related work, He and coworkers studied how cation types, pH, and assembly time influenced self-assembly behaviors of ECM-mimetic peptides.104 They appended RADA 16-I, a 16-residue self-assembling peptide, with positively charged IKVAV, as well as negatively charged RGD and YIGSR. Both IKVAV and YIGSR epitopes are laminin derivatives, while RGD is a cell-adhesion ligand derived from fibronectin. With increasing pH (from pH 4 to pH 7) and assembly times (from 0 h to 72 h), the β-sheet content increased, forming longer fibers. Their study summarized the factors that influenced the self-assembling mechanism, and how the introduction of different motifs can influence pH-responsive self-assembly. This work provides useful information in constructing nanofibrous, bioactive hydrogels at physiological pH by RADA 16-I-based self-assembling peptides, which can further serve as ECM mimics in tissue engineering.
Besides studying the mechanism and behaviors of self-assembling peptides, several groups have applied pH-responsive peptides in biomaterials applications. For example, Wang and coworkers developed dual-responsive, peptide-based nanoparticles that mimic natural laminin fibrillogenesis.105 The goal was to take advantage of mildly acidic pH in blood vessels in tumors to block blood flow and inhibit tumor growth in a selective manner. They designed a laminin-mimetic peptide with a hydrophobic unit and three peptide components: 1) the hydrogen-bonding sequence KLVFF for fibril formation, 2) the fibrin-binding sequence CREKA, and 3) the pH-responsive sequence H6. The final construct also included an oligoethylene glycol segment to improve solubility (Figure 12). In the acidic microenvironment of a tumor, the peptide nanoparticles switched into a fibrillar morphology upon protonation of the H6 sequence. Binding of the CREKA sequence in the blood vessels to fibrin, a protein involved in blood clotting, caused fast occlusion in the tumor blood vessels, reminiscent of a microthrombus. This led to a decrease in hemoglobin levels, inhibiting tumor growth. This work showed a specific in vivo system for inhibiting tumor cell growth by a pH-responsive, laminin-mimetic peptide. This drug-free, physical occlusion method of restricting tumor growth provides a unique biomimetic strategy for cancer treatment.
Figure 12.
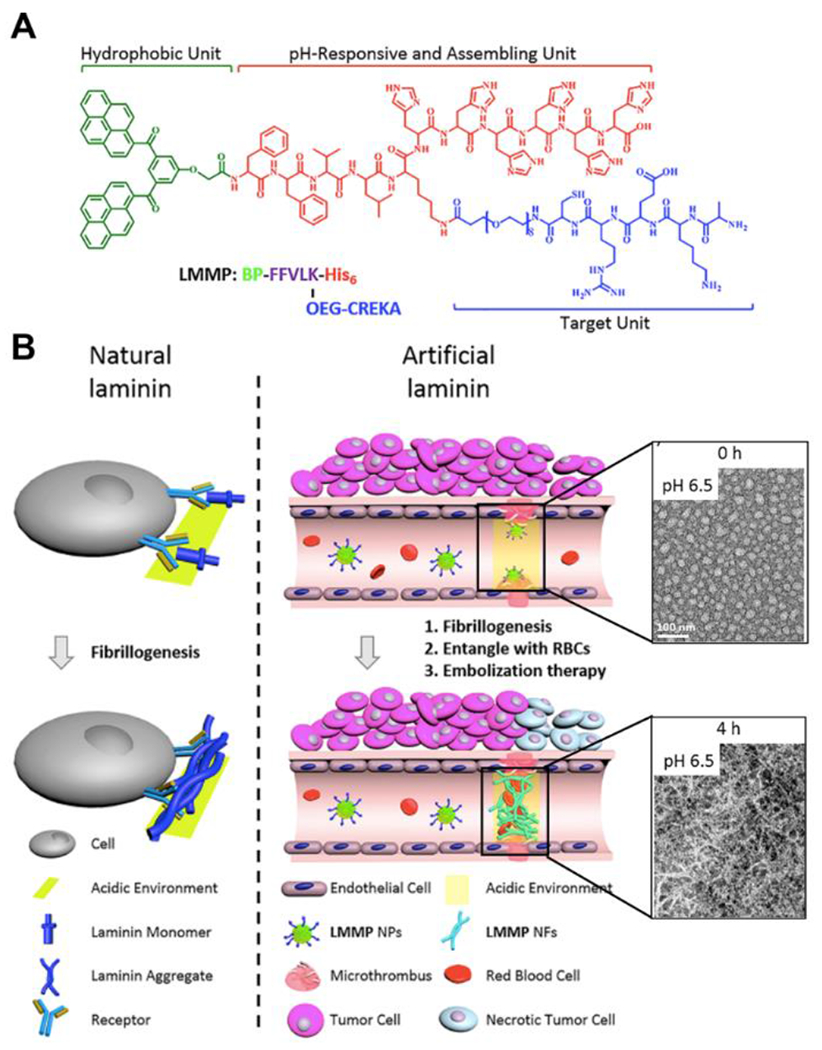
A) Chemical structure of laminin-mimetic peptide for tumor blood vessel occlusion. B) Schematic illustration of the fibrillogenesis of natural laminin and artificial laminin. TEM images show the morphological transformation at pH 6.5 at different time points. Scale bar = 100 nm. Reproduced with permission from reference 105. Copyright 2020 American Chemical Society.
4.2. Tissue Regeneration
Beyond applications as synthetic ECMs, biomimetic self-assembling peptides have been increasingly explored as artificial scaffolds in tissue regeneration.106 Among all the stimuli-responsive systems and scaffolds, pH-responsive self-assembling peptide hydrogels have gained interests in recent years. Peptide hydrogels are typically biocompatible and biodegradable in many aspects,107 and because of their high water absorption capacity, self-assembling peptide-based hydrogels provide cells with a similar 3D microenvironment to native tissue. Furthermore, the flexibility and functions of self-assembling peptides can be easily modified by different bioactive peptide motifs to achieve certain biological activities, mechanical properties, and other aspects. Additionally, the swelling behavior and morphology changes of pH-responsive peptide hydrogels can be affected by environmental pH, which can be specifically designed for different targets. In this section, we highlight recent progress in applying pH-responsive, self-assembling peptides in two important tissue regeneration applications: wound healing and nerve regeneration.
4.2.1. Wound Healing
Skin is the largest organ in the human body, protecting the body from bacteria, temperature, chemicals, and other external factors. Skin is also fragile and easy to injure. In 2018, the estimated costs for treatments of acute and chronic wounds and related infections ranged from $28-97 billion in the United States alone.108 Wound healing is a complex and slow biological process, but it is critical for tissue repair and regeneration.109 Thus, it is essential to develop treatments that promote wound healing that are low-cost and effective. The pH of healthy skin and wound skin are different: the pH of healthy and intact skin is mildly acidic, around 4.0-6.0;110 while the pH of acute and chronic wounds can be either acidic or basic.95 Therefore, pH-responsive, self-assembling peptide hydrogels are compelling candidates for wound healing.
Åkerfeldt, Linse, and coworkers reported a pH-responsive peptide GSFSIQYTYHV derived from human semenogelin I (SgI), and studied the self-assembling properties from pH 3.3 to pH 11.2.111 Below pH 6 and above pH 10, the peptide system formed a low viscosity fluid. At neutral pH, the peptide self-assembled into nanofibers composed of extended β-sheets, forming a transparent hydrogel. A highly viscous gel composed of rigid peptide fibrils formed between pH 7.0 and 9.0, which is within the wound pH range, suggesting that this SgI-derived, pH-responsive self-assembling peptide hydrogel may further be applied in wound dressings and in the delivery of anti-inflammatory drugs. In related work, Zhong and coworkers designed a dual-repairing factor system based on the peptide Nap-GFFKH (Nap = naphthalene), which instantly self-assembled into a hydrogel at pH ~6.16 The authors constructed a 4-component hydrogel from alginate and the Nap-GFFKH peptide that was loaded with both recombinant bovine basic fibroblast growth factor (FGF-2) and an antibacterial agent (Figure 13). The well-defined hydrogel formed within 5 s after pH adjustment to 6.0, and this dual-loaded, pH-responsive peptide hydrogel system showed good antibiotic activity in vitro and improved the wound healing process in animal studies. Additionally, the peptide hydrogel mechanical properties could be tuned by changing the ratios of the components.
Figure 13.
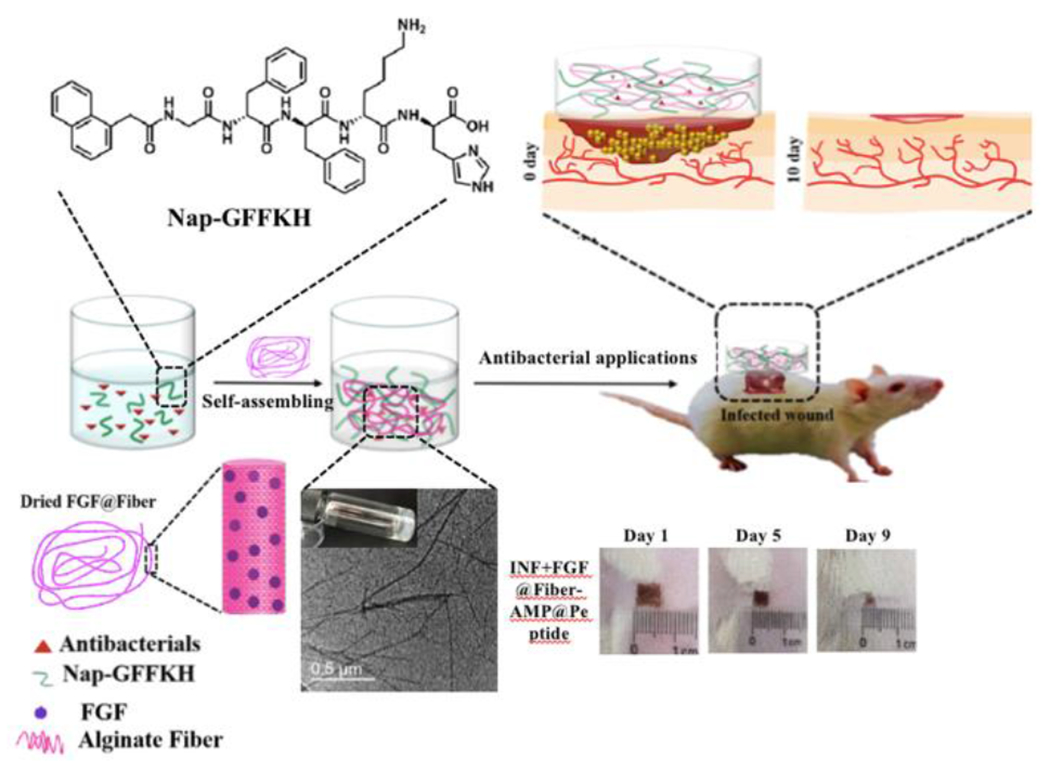
Schematic illustration of the self-assembly and in vivo antibacterial application of a dual-drug delivery, pH-responsive hydrogel. Reproduced with permission from reference 16. Copyright 2020 American Chemical Society.
Li and coworkers recently reported a pH-switchable antimicrobial hydrogel with nanofiber networks for chronic wound treatment.112 This unique dual-drug-loaded hydrogel was based on the octapeptide IKFQFHFD, which is electrically neutral at pH 7.4, but becomes cationic and antimicrobial under acidic conditions (pH 5.5) (Figure 14). Additionally, the peptide could self-assemble into hydrogels under neutral conditions while disassembling under acidic conditions, which provided a system to load wound healing drugs and control release in the wound bed of infected wounds with pH ~5.5. To highlight this capability, the authors encapsulated cypate (a photothermal dye that generates heat under near-infrared light) and proline (a procollagen component) to eradicate biofilm and promote ECM formation to improve the healing process, respectively. Both in vitro and in vivo experiments showed a decrease in biofilm content and improved wound healing in this hydrogel system. This pH-reversible, self-assembling peptide hydrogel system shows how pH-responsive peptides can be applied in treating infected wounds, taking advantage of natural pH variations in the wound bed.
Figure 14.
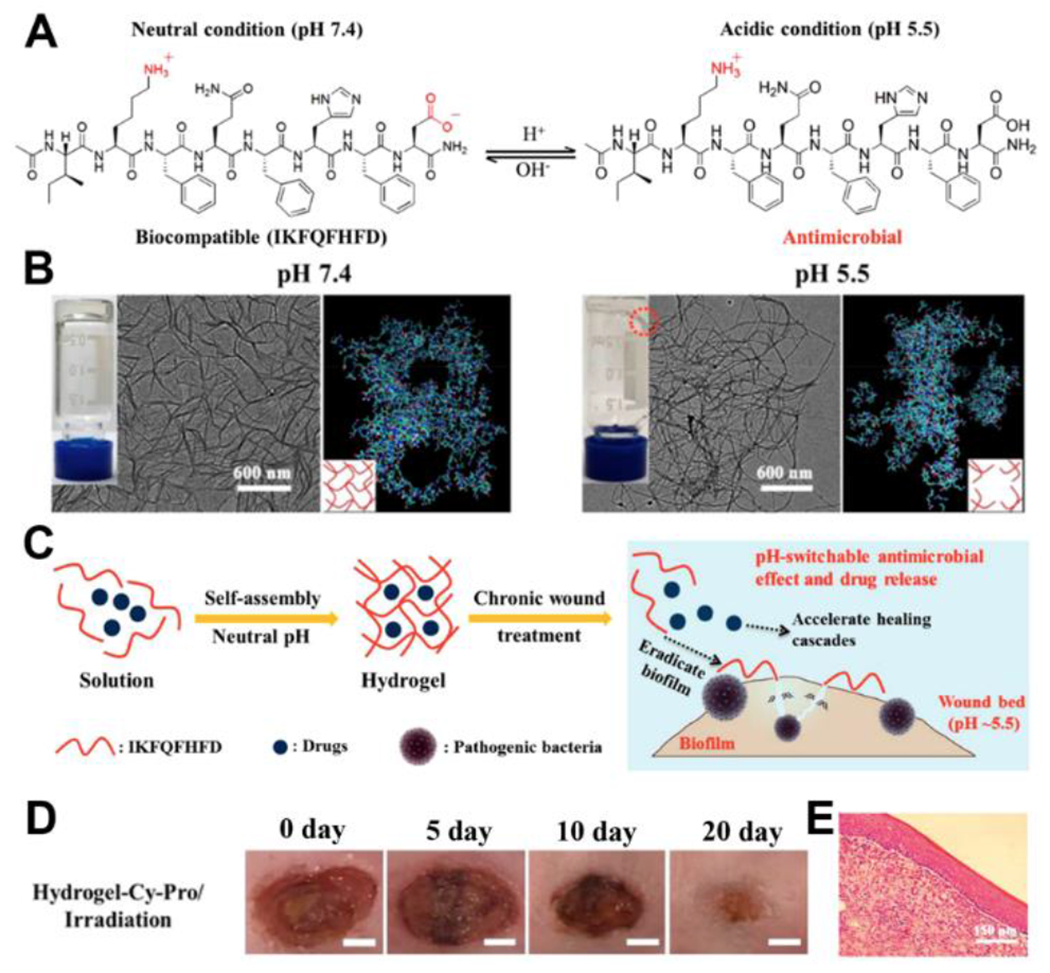
A) Chemical structures of IKFQFHFD under neutral and acidic conditions. B) Pictures and TEM images of the hydrogels under different pH conditions (Scale bar = 600 nm), as well as molecular dynamic simulations of the aggregation of IKFQFHFD in water at different pH values. C) Schematic illustration of pH-responsive self-assembled IKFQFHD peptide for biofilm eradication and rescuing stalled healing in chronic wounds. D) Representative photographs of diabetic mice wounds infected with MRSA biofilm treated with hydrogel-Cy-Pro/irradiation over 20 days. E) Representative histological (H&E staining) images of the skin tissue of MRSA biofilm-infected diabetic mice wounds with hydrogel-Cy-Pro/irradiation on the 20th day of treatment. Reproduced with permission from reference 112. Copyright 2019 American Chemical Society.
4.2.2. Nerve Regeneration
The nervous system is a complex system that regulates and coordinates normal physiological activities. It consists of two major parts, the central nervous system (CNS) and the peripheral nervous system. The nervous system is fragile, and damage can lead to severe neurological injuries and even death. The high complexity and limited regenerative capacity of the nervous system make tissue repair especially difficult. Biomimetic, self-assembling peptide hydrogels provide a potential pathway for nerve regeneration.113–116 Peptide hydrogels can provide a similar 3D microenvironment to native tissue, as well as promote regeneration of damaged tissue by using bioactive motifs. Laminin-like peptides are often applied in the design of nerve regeneration materials because laminin regulates important physiological and pathological functions in the nervous system.117 Common laminin-derived peptide sequences used in pH-responsive self-assembling hydrogels in nerve regeneration include YIGSR and IKVAV.116
For example, Wu, He and coworkers designed two oppositely charged peptides, RADA16-RGD [Ac-(RADA)4-DGDRGDS] and RADA16-IKVAV [Ac-(RADA)4-RIKVAV], to promote regeneration in nerve injury models.118 The appended motifs RGD and IKVAV can improve cell adhesion and neurite growth, respectively. The authors discovered that the self-assembling RADA 16-I-IKVAV/-RGD peptide formed a stable hydrogel at neutral pH, which supported differentiation of neural progenitor cells/stem cells (NPCs/NSCs) in the 3D environment. Interestingly, the unmodified peptide Ac-(RADA)4-CONH2 created acidic conditions that are damaging to the growth of sensitive cell types in the CNS. In contrast, the two modified RADA peptides were mixed and self-assembled into a stable and viscous hydrogel at neutral pH. NPCs and NSCs showed good survival results when fully embedded in the RADA16-IKVAV/RGD nanofiber hydrogel, but did not survive in hydrogels lacking the RGD and IKVAV sequences. Additionally, NPCs/NSCs grown in these hydrogels differentiated into neurons and glial cells even without adding growth factors (Figure 15). Compared to non-pH responsive materials, this system is more tunable and flexible for various applications.
Figure 15.
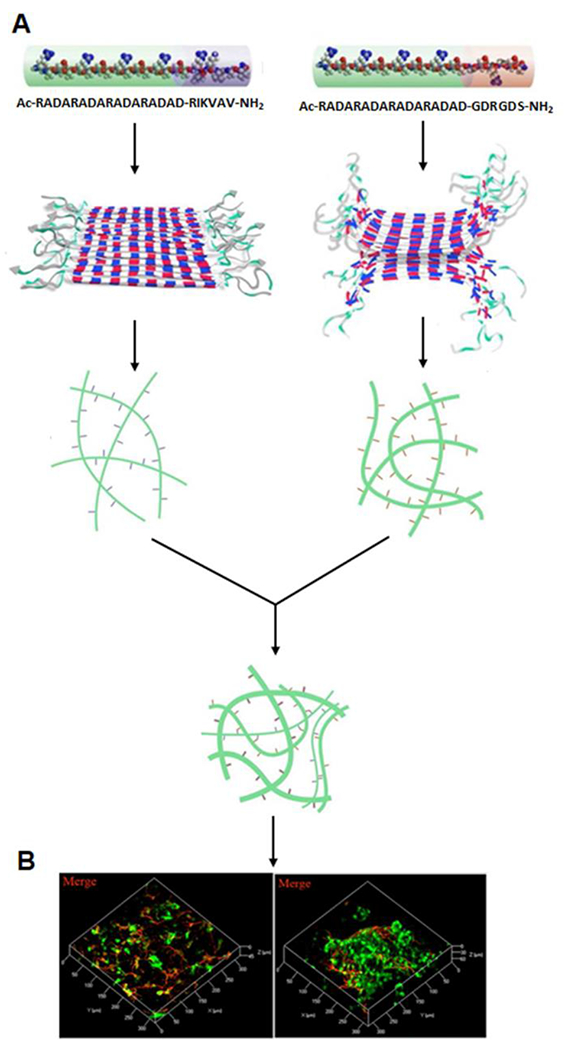
A) Schematic illustration of the self-assembly of RADA16-IKVAV and RADA16-RGD as well as a combination of these two self-assembled peptides. B) Differentiation of NPCs/NSCs from GFP-transgenic rats within RADA 16-IKVAV/RADA 16-RGD hydrogel. Reproduced with permission from reference 118. Copyright 2016 American Chemical Society.
5. Conclusions and Future Outlook
Exciting progress has been made in the design and application of pH-responsive, self-assembling peptides in the past few decades. Advances have been largely driven by designing peptides with well-known pH-responsive chemical motifs including charged amino acids and pH-responsive organic linkages (e.g., hydrazones, cis-aconityls). These pH-responsive units can induce self-assembly or disassembly, cause changes in self-assembled morphology, or lead to release of drugs or a shift in bioactivity of the peptide itself. The ease of modification and typically high biocompatibility of peptide-based biomaterials continue to motivate researchers to drive this field forward in an effort to translate these discoveries and designs to clinical drug delivery and tissue engineering applications.
Although abundant experimental data have established a steady foundation, challenges remain in the field of pH-responsive self-assembling peptide-based biomaterials. First, current pH-responsive systems sometimes rely on materials with batch-to-batch variability. For example, chitosan is used as a protective layer in oral drug delivery, where drugs must navigate extreme changes in pH in the GI tract. Although chitosan is FDA approved, its bioactivity and biocompatibility are still not fully understood due in large part to heterogeneity in polymer characteristics such as acetylation degree, molecular weight, and purity. A synthetic replacement in the form of a pH-responsive peptide could alleviate these concerns, but a peptide that resists degradation in the acidic and enzyme-rich stomach would be needed.
Another challenge lies in improving tumor targeting specificity. Most pH-responsive, self-assembling peptides respond to pH changes through a simple change in ionization state, which limits their tumor targeting ability. CPPs or CAPs can improve targeting of pH-responsive drug delivery systems, but ideal drug delivery systems should have additional functionalities that improve their targeting ability selectively to diseased cells as well as provide diagnostic capabilities. There is also a need for more precise pH-driven changes in nanostructure than those currently available that typically require a shift of ~1 pH unit or more; however, the level of precision required will depend on the type of microenvironments, tissues/organs, and diseases. Though narrower pH tuning (a shift in ~0.2 pH units) might be critical to study the deeper mechanism, it is unclear whether such a narrow response is clinically necessary. Because the difference in pH between normal and abnormal microenvironments is normally over 0.5 pH units (for example, the physiological pH is 7.4 and the pH of malignant melanoma tissues is ~6.9), this very narrow pH tuning might not be necessary for biological applications. Perhaps more critically, researchers must make sure that peptide-based materials are actually responsive at the desired site. We believe comprehensive in vivo studies in this aspect could benefit the applications of these materials toward specific treatments. Additionally, the effect of how encapsulated drugs influence the pH responsiveness of a given peptide system is still unclear. More discoveries in this aspect could help improve the design and specificity in the target treatment; for example, the amount of encapsulated drug could potentially be calculated to tune a switch at a desired pH value. Finally, to improve the specificity of the response, we think some simulation work could be done ahead of experiments to predict structural changes shifts in pH, which can further enhance peptide sequence design for precise control.
Beyond understanding the behavior of these materials, a major challenge lies in simplifying complex systems for ease of regulatory approval, where scrutiny of each component adds exponentially to the complexity and cost of testing. For example, full degradation studies and long-term side effects of peptide-based biomaterials are usually ignored in research but required for eventual clinical applications. All peptides as well as any conjugated small functional molecules must be evaluated in safety studies. Systems with only the needed design features and nothing extraneous are most likely to prevail. However, even with simple systems, full degradation studies and long-term side effects of peptide-based biomaterials are not often conducted in academic labs but are required for eventual clinical applications. Safety testing remains a hurdle toward clinical translation, where toxicity and immunogenicity concerns can slow translational efforts.
Although challenges remain in pH-responsive self-assembling peptide-based biomaterials, this field is developing fast as studies focus on real-world applications. Future work focusing on precise treatments and improved delivery efficiency will require continued tuning of peptide structures, including the amino acid sequences themselves and any additional pH-responsive units. With continued collaboration between chemists, biologists, pharmacologists, and others, we believe pH-responsive, self-assembling peptide-based biomaterials can be applied and expanded in the clinical market for various treatments.
Acknowledgements
We thank the National Institutes of Health (R01GM123508), the National Science Foundation (DMR- 1454754), and the Binational Science Foundation (2016096) for their support of our work in this area.
Reference:
- 1.Moss GP; Smith PAS; Tavernier D, Glossary of Class Names of Organic-Compounds and Reactive Intermediates Based on Structure. Pure Appl. Chem 1995, 67, 1307–1375. [Google Scholar]
- 2.Pripp AH, Effect of Peptides Derived from Food Proteins on Blood Pressure: a Meta-analysis of Randomized Controlled Trials. Food Nutr. Res 2008, 52, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Manna S; Di Natale C; Florio D; Marasco D, Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci 2018, 19, 2714–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldyrev AA; Gallant SC; Sukhich GT, Carnosine, the Protective, Anti-aging Peptide. Biosci. Rep 1999, 19, 581–587. [DOI] [PubMed] [Google Scholar]
- 5.Merrifield RB, Solid Phase Peptide Synthesis. 1. Synthesis of a Tetrapeptide. J. Am. Chem. Soc 1963, 85, 2149-&. [Google Scholar]
- 6.Coin I; Beyermann M; Bienert M, Solid-phase Peptide Synthesis: From Standard Procedures to the Synthesis of Difficult Sequences. Nat. Protoc 2007, 2, 3247–3256. [DOI] [PubMed] [Google Scholar]
- 7.Regnier FE, Hplc of Proteins, Peptides, and Polynucleotides. Anal. Chem 1983, 55, 1298-&. [DOI] [PubMed] [Google Scholar]
- 8.Pfannkoch E; Regnier FE, Preparative Hplc of Proteins and Peptides. Fed. Proc 1981, 40, 1642–1642. [Google Scholar]
- 9.Humphrey MJ; Ringrose PS, Peptides and Related Drugs - a Review of Their Absorption, Metabolism, and Excretion. Drug Metab. Rev 1986, 17, 283–310. [DOI] [PubMed] [Google Scholar]
- 10.Collantes ER; Dunn WJ, Amino-Acid Side-Chain Descriptors for Quantitative Structure-Activity Relationship Studies of Peptide Analogs. J. Med. Chem 1995, 38, 2705–2713. [DOI] [PubMed] [Google Scholar]
- 11.Shah A; Malik MS; Khan GS; Nosheen E; Iftikhar FJ; Khan FA; Shukla SS; Akhter MS; Kraatz HB; Aminabhavi TM, Stimuli-responsive Peptide-based Biomaterials as Drug Delivery Systems. Chem. Eng. J 2018, 353, 559–583. [Google Scholar]
- 12.Dehsorkhi A; Castelletto V; Hamley IW, Self-assembling Amphiphilic Peptides. J. Pept. Sci 2014, 20, 453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weerakkody D; Moshnikova A; El-Sayed NS; Adochite R-C; Slaybaugh G; Golijanin J; Tiwari RK; Andreev OA; Parang K; Reshetnyak YK, Novel pH-Sensitive Cyclic Peptides. Sci. Rep 2016, 6, 31322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau JL; Dunn MK, Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorganic Med. Chem. Lett 2018, 26, 2700–2707. [DOI] [PubMed] [Google Scholar]
- 15.Schmaljohann D, Thermo- and pH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev 2006, 58, 1655–1670. [DOI] [PubMed] [Google Scholar]
- 16.Cui TY; Li XP; He SY; Xu DH; Yin L; Huang XL; Deng SW; Yue WQ; Zhong WY, Instant Self-Assembly Peptide Hydrogel Encapsulation with Fibrous Alginate by Microfluidics for Infected Wound Healing. Acs Biomater. Sci. Eng 2020, 6, 5001–5011. [DOI] [PubMed] [Google Scholar]
- 17.Ma ML; Kuang Y; Gao Y; Zhang Y; Gao P; Xu B, Aromatic-Aromatic Interactions Induce the Self-Assembly of Pentapeptidic Derivatives in Water To Form Nanofibers and Supramolecular Hydrogels. J. Am. Chem. Soc 2010, 132, 2719–2728. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z; Liang G; Ma M; Gao Y; Xu B, In vitro and in vivo Enzymatic Formation of Supramolecular Hydrogels Based on Self-assembled Nanofibers of a beta-Amino Acid Derivative. Small 2007, 3, 558–62. [DOI] [PubMed] [Google Scholar]
- 19.Qu MY; Jiang X; Zhou XW; Wang OR; Wu QZ; Ren L; Zhu JX; Zhu SS; Tebon P; Sun WJ; Khademhosseini A, Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater 2020, 9, 1901714–1901733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez AL; Parish CL; Nisbet DR; Williams RJ, Tuning the amino acid sequence of minimalist peptides to present biological signals via charge neutralised self assembly. Soft Matter 2013, 9, 3915–3919. [Google Scholar]
- 21.Chen LL; Chen T; Fang WX; Wen Y; Lin SL; Lin JP; Cai CH, Synthesis and pH-Responsive “Schizophrenic” Aggregation of a Linear-Dendron-Like Polyampholyte Based on Oppositely Charged Polypeptides. Biomacromolecules 2013, 14, 4320–4330. [DOI] [PubMed] [Google Scholar]
- 22.Singh PK; Chibh S; Dube T; Chauhan VS; Panda JJ, Arginine-alpha, beta-dehydrophenylalanine Dipeptide Nanoparticles for pH-Responsive Drug Delivery. Pharm. Res 2018, 35, 35–46. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen CT; Webb RI; Lambert LK; Strounina E; Lee EC; Parat MO; McGuckin MA; Popat A; Cabot PJ; Ross BP, Bifunctional Succinylated epsilon-Polylysine-Coated Mesoporous Silica Nanoparticles for pH-Responsive and Intracellular Drug Delivery Targeting the Colon. Acs Appl. Mater. Inter 2017, 9, 9470–9483. [DOI] [PubMed] [Google Scholar]
- 24.Liang J; Wu WL; Xu XD; Zhuo RX; Zhang XZ, pH Responsive micelle self-assembled from a new amphiphilic peptide as anti-tumor drug carrier. Colloid Surface B 2014, 114, 398–403. [DOI] [PubMed] [Google Scholar]
- 25.Lee ES; Oh KT; Kim D; Youn YS; Bae YH, Tumor pH-Responsive Flower-Like Micelles of Poly(L-lactic acid)-b-Poly(ethylene glycol)-b-Poly(L-histidine). JCR 2007, 123, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T; Zhang Z; Zhang Y; Lv H; Zhou J; Li C; Hou L; Zhang Q, Dual-functional Liposomes Based on pH-Responsive Cell-penetrating Peptide and Hyaluronic Acid for Tumor-targeted Anticancer Drug Delivery. Biomaterials 2012, 33, 9246–58. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W; Song JJ; Zhang BZ; Liu LW; Wang KR; Wang R, Design of Acid-Activated Cell Penetrating Peptide for Delivery of Active Molecules into Cancer Cells. Bioconjugate Chem. 2011, 22, 1410–1415. [DOI] [PubMed] [Google Scholar]
- 28.Makovitzki A; Fink A; Shai Y, Suppression of human solid tumor growth in mice by intratumor and systemic inoculation of histidine-rich and pH-dependent host defense-like lytic peptides. Cancer Res. 2009, 69, 3458–63. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J; Zheng D; Tao Y; Li Y; Wang L; Liu J; He J; Lei J, Self-assembled pH-responsive polymeric nanoparticles based on lignin-histidine conjugate with small particle size for efficient delivery of anti-tumor drugs. Biochem. Eng. J 2020, 156, 107526. [Google Scholar]
- 30.Chen J; Dong X; Feng TS; Lin L; Guo ZP; Xia JL; Tian HY; Chen XS, Charge-conversional zwitterionic copolymer as pH-sensitive shielding system for effective tumor treatment. Acta Biomater. 2015, 26, 45–53. [DOI] [PubMed] [Google Scholar]
- 31.Tian HY; Guo ZP; Lin L; Jiao ZX; Chen J; Gao SQ; Zhu XJ; Chen XS, pH-responsive zwitterionic copolypeptides as charge conversional shielding system for gene carriers. JCR 2014, 174, 117–125. [DOI] [PubMed] [Google Scholar]
- 32.Ryu K; Lee MK; Park J; Kim TI, pH-Responsive Charge-Conversional Poly(ethylene imine)-Poly(L-lysine)-Poly(L-glutamic acid) with Self-Assembly and Endosome Buffering Ability for Gene Delivery Systems. Acs Appl. Bio. Mater 2018, 1, 1496–1504. [DOI] [PubMed] [Google Scholar]
- 33.Wu H; Zhu L; Torchilin VP, pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X; Liao W; Xie Z; Chen D; Zhang CY, A pH-responsive prodrug delivery system self-assembled from acid-labile doxorubicin-conjugated amphiphilic pH-sensitive block copolymers. Mater. Sci. Eng. C 2018, 90, 27–37. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JM; Lin WJ; Yang LJ; Zhang AJ; Zhang YM; Liu JJ; Liu JF, Injectable and pH-responsive self-assembled peptide hydrogel for promoted tumor cell uptake and enhanced cancer chemotherapy. Biomater. Sci 2022, 10, 854–862. [DOI] [PubMed] [Google Scholar]
- 36.Wang TW; Yeh CW; Kuan CH; Wang LW; Chen LH; Wu HC; Sun JS, Tailored design of multifunctional and programmable pH-responsive self-assembling polypeptides as drug delivery nanocarrier for cancer therapy. Acta Biomater. 2017, 58, 54–66. [DOI] [PubMed] [Google Scholar]
- 37.Balci B; Top A, PEG and PEG-peptide based doxorubicin delivery systems containing hydrazone bond. J. Polym. Res 2018, 25, 1–12. [Google Scholar]
- 38.Pieszka M; Han S; Volkmann C; Graf R; Lieberwirth I; Landfester K; Ng DYW; Weil T, Controlled Supramolecular Assembly Inside Living Cells by Sequential Multistaged Chemical Reactions. J. Am. Chem. Soc 2020, 142, 15780–15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen ZW; Guo Z; Zhou LM; Wang YJ; Zhang JJ; Hu J; Zhang Y, Biomembrane induced in situ self-assembly of peptide with enhanced antimicrobial activity. Biomater. Sci 2020, 8, 2031–2039. [DOI] [PubMed] [Google Scholar]
- 40.Mendez-Ardoy AM; Granja JR; Montenegro J, pH-Triggered self-assembly and hydrogelation of cyclic peptide nanotubes confined in water micro-droplets. Nanoscale Horiz. 2018, 3, 391–396. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RP; Jeong YI; John JV; Chung CW; Kang DH; Selvaraj M; Suh H; Kim I, Dual Stimuli-Responsive Poly(N-isopropylacrylamide)-b-poly(L-histidine) Chimeric Materials for the Controlled Delivery of Doxorubicin into Liver Carcinoma. Biomacromolecules 2013, 14, 1434–1443. [DOI] [PubMed] [Google Scholar]
- 42.Chen B; He XY; Yi XQ; Zhuo RX; Cheng SX, Dual-Peptide-Functionalized Albumin-Based Nanoparticles with pH-Dependent Self-Assembly Behavior for Drug Delivery. Acs Appl. Mater. Inter 2015, 7, 15148–15153. [DOI] [PubMed] [Google Scholar]
- 43.Bae Y; Fukushima S; Harada A; Kataoka K, Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem., Int. Ed. Engl 2003, 42, 4640–4643. [DOI] [PubMed] [Google Scholar]
- 44.Zhang WB; Gao CY, Morphology transformation of self-assembled organic nanomaterials in aqueous solution induced by stimuli-triggered chemical structure changes. J. Mater. Chem. A 2017, 5, 16059–16104. [Google Scholar]
- 45.Qin SY; Xu SS; Zhuo RX; Zhang XZ, Morphology Transformation via pH-Triggered Self-Assembly of Peptides. Langmuir 2012, 28, 2083–2090. [DOI] [PubMed] [Google Scholar]
- 46.Jia HR; Zhu YX; Liu XY; Pan GY; Gao G; Sun W; Zhang XD; Jiang YW; Wu FG, Construction of Dually Responsive Nanotransformers with Nanosphere-Nanofiber-Nanosphere Transition for Overcoming the Size Paradox of Anticancer Nanodrugs. Acs Nano 2019, 13, 11781–11792. [DOI] [PubMed] [Google Scholar]
- 47.Lowik DWPM; Leunissen EHP; van den Heuvel M; Hansen MB; van Hest JCM, Stimulus responsive peptide based materials. Chem. Soc. Rev 2010, 39, 3394–3412. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman WE; Charbel FT; Edelman G; Hannigan K; Ausman JI, Brain tissue oxygen pressure, carbon dioxide pressure and pH during ischemia. Neurol. Res 1996, 18, 54–56. [DOI] [PubMed] [Google Scholar]
- 49.Mcclure MO; Sommerfelt MA; Marsh M; Weiss RA, The Ph Independence of Mammalian Retrovirus Infection. J. Gen. Virol 1990, 71, 767–773. [DOI] [PubMed] [Google Scholar]
- 50.Blacklock NJ; Beavis JP, The response of prostatic fluid pH in inflammation. Br. J. Urol 1974, 46, 537–42. [DOI] [PubMed] [Google Scholar]
- 51.Tannock IF; Rotin D, Acid Ph in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res 1989, 49, 4373–4384. [PubMed] [Google Scholar]
- 52.Verma G; Hassan PA, Self assembled materials: design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys 2013, 15, 17016–17028. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y; Wang SP; Wang YT; Wang XH; Wang Q; Chen MW, Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv 2014, 32, 1301–1316. [DOI] [PubMed] [Google Scholar]
- 54.Li Y; Zheng XM; Cao ZH; Xu WR; Zhang JN; Gong M, Self-assembled peptide (CADY-1) improved the clinical application of doxorubicin. Int. J. Pharm 2012, 434, 209–214. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y; Ji TJ; Wang H; Li SP; Zhao YL; Nie GJ, Self-assembled Peptide Nanoparticles asTumor Microenvironment Activatable Probes for Tumor Targeting and Imaging. JCR 2014, 177, 11–19. [DOI] [PubMed] [Google Scholar]
- 56.Dressman JB; Berardi RR; Dermentzoglou LC; Russell TL; Schmaltz SP; Barnett JL; Jarvenpaa KM, Upper Gastrointestinal (Gi) Ph in Young, Healthy-Men and Women. Pharm. Res 1990, 7, 756–761. [DOI] [PubMed] [Google Scholar]
- 57.Read NW; Sugden K, Gastrointestinal Dynamics and Pharmacology for the Optimum Design of Controlled-Release Oral Dosage Forms. Crit. Rev. Ther. Drug Carr. Syst 1988, 4, 221–263. [PubMed] [Google Scholar]
- 58.Kararli TT, Comparison of the Gastrointestinal Anatomy, Physiology, and Biochemistry of Humans and Commonly Used Laboratory-Animals. Biopharm. Drug Dispos 1995, 16, 351–380. [DOI] [PubMed] [Google Scholar]
- 59.Parsonnet J, The Incidence of Helicobacter-Pylori Infection. Aliment. Pharm. Therap 1995, 9, 45–51. [PubMed] [Google Scholar]
- 60.Todd PA; Benfield P, Amoxicillin Clavulanic Acid - an Update of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Use. Drugs 1990, 39, 264–307. [DOI] [PubMed] [Google Scholar]
- 61.Su YR; Yu SH; Chao AC; Wu JY; Lin YF; Lu KY; Mi FL, Preparation and properties of pH-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloids Surf., A Physicochem. Eng. Asp 2016, 494, 9–20. [Google Scholar]
- 62.Lin YH; Sonaje K; Lin KM; Juang JH; Mi FL; Yang HW; Sung HW, Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. JCR 2008, 132, 141–9. [DOI] [PubMed] [Google Scholar]
- 63.Wilcox G, Insulin and insulin resistance. Clin. Biochem. Rev 2005, 26, 19–39. [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JW; Dang CV, Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006, 66, 8927–8930. [DOI] [PubMed] [Google Scholar]
- 65.Christofk HR; Vander Heiden MG; Harris MH; Ramanathan A; Gerszten RE; Wei R; Fleming MD; Schreiber SL; Cantley LC, The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–274. [DOI] [PubMed] [Google Scholar]
- 66.Brahimi-Horn MC; Pouyssegur J, Oxygen, a source of life and stress. FEBS Lett. 2007, 581, 3582–3591. [DOI] [PubMed] [Google Scholar]
- 67.Lee ES; Gao ZG; Bae YH, Recent progress in tumor pH targeting nanotechnology. JCR 2008, 132, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang D; Fan ZH; Zhang XC; Li H; Sun YW; Cao MW; Wei GC; Wang JQ, pH-Responsive Self-Assemblies from the Designed Folic Acid-Modified Peptide Drug for Dual-Targeting Delivery. Langmuir 2021, 37, 339–347. [DOI] [PubMed] [Google Scholar]
- 69.Raza F; Zhu Y; Chen L; You X; Zhang J; Khan A; Khan MW; Hasnat M; Zafar H; Wu J; Ge L, Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci 2019, 7, 2023–2036. [DOI] [PubMed] [Google Scholar]
- 70.Fei L; Yap LP; Conti PS; Shen WC; Zaro JL, Tumor targeting of a cell penetrating peptide by fusing with a pH-sensitive histidine-glutamate co-oligopeptide. Biomaterials 2014, 35, 4082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu YP; Wang Q; Liu YC; Xie Y, Molecular basis for the targeted binding of RGD-containing peptide to integrin alphaVbeta3. Biomaterials 2014, 35, 1667–75. [DOI] [PubMed] [Google Scholar]
- 72.Temming K; Schiffelers RM; Molema G; Kok RJ, RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updates 2005, 8, 381–402. [DOI] [PubMed] [Google Scholar]
- 73.Rizvi SFA; Mu S; Zhao CY; Zhang HX, Fabrication of self-assembled peptide nanoparticles for in vitro assessment of cell apoptosis pathway and in vivo therapeutic efficacy. Microchim Acta 2022, 189, 1–14. [DOI] [PubMed] [Google Scholar]
- 74.Madani F; Lindberg S; Langel U; Futaki S; Graslund A, Mechanisms of cellular uptake of cell-penetrating peptides. Biophys. J 2011, 2011, 414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huettner N; Dargaville TR; Forget A, Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [DOI] [PubMed] [Google Scholar]
- 76.Wolf J; Aisenbrey C; Harmouche N; Raya J; Bertani P; Voievoda N; Suss R; Bechinger B, pH-Dependent Membrane Interactions of the Histidine-Rich Cell-Penetrating Peptide LAH4-L1. Biophys. J 2017, 113, 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y; Ren W; Zhong T; Zhang S; Huang D; Guo Y; Yao X; Wang C; Zhang WQ; Zhang X; Zhang Q, Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. JCR 2016, 222, 56–66. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L; Xu J; Wang F; Din Y; Wang T; Jin G; Martz M; Gui ZZ; Ouyang PK; Chen P, Histidine-Rich Cell-Penetrating Peptide for Cancer Drug Delivery and Its Uptake Mechanism. Langmuir 2019, 35, 3513–3523. [DOI] [PubMed] [Google Scholar]
- 79.Chen JX; Xu XD; Chen WH; Zhang XZ, Multi-Functional Envelope-Type Nanoparticles Assembled from Amphiphilic Peptidic Prodrug with Improved Anti-Tumor Activity. Acs Appl. Mater. Inter 2014, 6, 593–598. [DOI] [PubMed] [Google Scholar]
- 80.Huang P; Song H; Wang W; Sun Y; Zhou J; Wang X; Liu J; Liu J; Kong D; Dong A, Integrin-targeted zwitterionic polymeric nanoparticles with acid-induced disassembly property for enhanced drug accumulation and release in tumor. Biomacromolecules 2014, 15, 3128–38. [DOI] [PubMed] [Google Scholar]
- 81.Fang ZH; Sun YP; Xiao H; Li P; Liu M; Ding F; Kan WS; Miao RS, Targeted osteosarcoma chemotherapy using RGD peptide-installed doxorubicin-loaded biodegradable polymeric micelle. Biomed. Pharmacother 2017, 85, 160–168. [DOI] [PubMed] [Google Scholar]
- 82.Chang C; Liang PQ; Chen LL; Liu JF; Chen SH; Zheng GH; Quan CY, pH-responsive nanoparticle assembly from peptide amphiphiles for tumor targeting drug delivery. J. Biomater. Sci. Polym. Ed 2017, 28, 1338–1350. [DOI] [PubMed] [Google Scholar]
- 83.Mei L; Xu K; Zhai Z; He S; Zhu T; Zhong W, Doxorubicin-reinforced supramolecular hydrogels of RGD-derived peptide conjugates for pH-responsive drug delivery. Org. Biomol. Chem 2019, 17, 3853–3860. [DOI] [PubMed] [Google Scholar]
- 84.Henne WA; Doorneweerd DD; Hilgenbrink AR; Kularatne SA; Low PS, Synthesis and activity of a folate peptide camptothecin prodrug. Bioorganic Med. Chem. Lett 2006, 16, 5350–5355. [DOI] [PubMed] [Google Scholar]
- 85.Wang HM; Yang CH; Wang L; Kong DL; Zhang YJ; Yang ZM, Self-assembled nanospheres as a novel delivery system for taxol: a molecular hydrogel with nanosphere morphology. ChemComm 2011, 47, 4439–4441. [DOI] [PubMed] [Google Scholar]
- 86.Laakkonen P; Akerman ME; Biliran H; Yang M; Ferrer F; Karpanen T; Hoffman RM; Ruoslahti E, Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 9381–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karmali PP; Kotamraju VR; Kastantin M; Black M; Missirlis D; Tirrell M; Ruoslahti E, Targeting of Albumin-Embedded Paclitaxel Nanoparticles to Tumors. Nanomed.: Nanotechnol. Biol. Med 2017, 5, 647–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong YA; Meng FH; Deng C; Zhong ZY, Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [DOI] [PubMed] [Google Scholar]
- 89.Zhu J; Hu J; Marchant RE, Biomimetic Biomaterials: Structure and Applications. [Online] ed.; Elsevier Science: 2013. [Google Scholar]
- 90.Chen J; Zou X, Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater 2019, 4, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frantz C; Stewart KM; Weaver VM, The extracellular matrix at a glance. J. Cell Sci 2010, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theocharis AD; Skandalis SS; Gialeli C; Karamanos NK, Extracellular matrix structure. Adv. Drug Deliv. Rev 2016, 97, 4–27. [DOI] [PubMed] [Google Scholar]
- 93.Wade RJ; Burdick JA, Engineering ECM signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar]
- 94.Ashby BS, pH Studies in Human Malignant Tumours. Lancet 1966, 288, 312–315. [DOI] [PubMed] [Google Scholar]
- 95.Schneider LA; Korber A; Grabbe S; Dissemond J, Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res 2007, 298, 413–20. [DOI] [PubMed] [Google Scholar]
- 96.Zhang W; Yu X; Li Y; Su Z; Jandt KD; Wei G, Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci 2018, 80, 94–124. [Google Scholar]
- 97.Luo J; Tong YW, Self-Assembly of Collagen-Mimetic Peptide Amphiphiles into Biofunctional Nanofiber. Acs Nano 2011, 5, 7739–7747. [DOI] [PubMed] [Google Scholar]
- 98.O’Leary LER; Fallas JA; Bakota EL; Kang MK; Hartgerink JD, Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem 2011, 3, 821–828. [DOI] [PubMed] [Google Scholar]
- 99.Sarkar B; O’Leary LER; Hartgerink JD, Self-Assembly of Fiber-Forming Collagen Mimetic Peptides Controlled by Triple-Helical Nucleation. J. Am. Chem. Soc 2014, 136, 14417–14424. [DOI] [PubMed] [Google Scholar]
- 100.Dehsorkhi A; Castelletto V; Hamley IW; Adamcik J; Mezzenga R, The effect of pH on the self-assembly of a collagen derived peptide amphiphile. Soft Matter 2013, 9, 6033–6036. [Google Scholar]
- 101.Aronoff MR; Egli J; Menichelli M; Wennemers H, gamma-Azaproline Confers pH Responsiveness and Functionalizability on Collagen Triple Helices. Angew. Chem., Int. Ed. Engl 2019, 58, 3143–3146. [DOI] [PubMed] [Google Scholar]
- 102.Aronoff MR; Egli J; Schmitt A; Wennemers H, Alkylation of gamma-Azaproline Creates Conformationally Adaptable Proline Derivatives for pH-Responsive Collagen Triple Helices. Chemistry 2020, 26, 5070–5074. [DOI] [PubMed] [Google Scholar]
- 103.Freire E; Coelho-Sampaio T, Self-assembly of laminin induced by acidic pH. J Biol. Chem 2000, 275, 817–822. [DOI] [PubMed] [Google Scholar]
- 104.Sun Y; Zhang Y; Tian L; Zhao Y; Wu D; Xue W; Ramakrishna S; Wu W; He L, Self-assembly behaviors of molecular designer functional RADA16-I peptides: influence of motifs, pH, and assembly time. Biomed. Mater. (Bristol) 2016, 12, 15007. [DOI] [PubMed] [Google Scholar]
- 105.Zhang K; Yang P-P; He P-P; Wen S-F; Zou X-R; Fan Y; Chen Z-M; Cao H; Yang Z; Yue K; Zhang X; Zhang H; Wang L; Wang H, Peptide-Based Nanoparticles Mimic Fibrillogenesis of Laminin in Tumor Vessels for Precise Embolization. Acs Nano 2020, 14, 7170–7180. [DOI] [PubMed] [Google Scholar]
- 106.Pugliese R; Gelain F, Peptidic Biomaterials: From Self-Assembling to Regenerative Medicine. Trends Biotechnol. 2017, 35, 145–158. [DOI] [PubMed] [Google Scholar]
- 107.Qian Y; Altamimi A; Yates SA; Sarkar S; Cochran M; Zhou M; Levi-Polyachenko N; Matson JB, H2S-releasing amphiphilic dipeptide hydrogels are potent S. aureus biofilm disruptors. Biomater. Sci 2020, 8, 2564–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sen CK, Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.A ES; Paul M; Marjana T-C, Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med 2014, 6, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones EM; Cochrane CA; Percival SL, The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frohm B; DeNizio JE; Lee DSM; Gentile L; Olsson U; Malm J; Åkerfeldt KS; Linse S, A peptide from human semenogelin I self-assembles into a pH-responsive hydrogel. Soft Matter 2015, 11, 414–421. [DOI] [PubMed] [Google Scholar]
- 112.Wang J; Chen X-Y; Zhao Y; Yang Y; Wang W; Wu C; Yang B; Zhang Z; Zhang L; Liu Y; Du X; Li W; Qiu L; Jiang P; Mou X-Z; Li Y-Q, pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. Acs Nano 2019, 13, 11686–11697. [DOI] [PubMed] [Google Scholar]
- 113.Ai J; Kiasat-Dolatabadi A; Ebrahimi-Barough S; Ai A; Lotfibakhshaiesh N; Norouzi-Javidan A; Saberi H; Arjmand B; Aghayan HR, Polymeric Scaffolds in Neural Tissue Engineering: A Review. Arch. Neurosci 2014, 1, 15–20. [Google Scholar]
- 114.He B; Yuan X; Jiang D, Molecular self-assembly guides the fabrication of peptide nanofiber scaffolds for nerve repair. RSC Adv. 2014, 4, 23610–23621. [Google Scholar]
- 115.Black KA; Lin BF; Wonder EA; Desai SS; Chung EJ; Ulery BD; Katari RS; Tirrell MV, Biocompatibility and Characterization of a Peptide Amphiphile Hydrogel for Applications in Peripheral Nerve Regeneration. Tissue Eng. Part A 2015, 21, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu L; Wang K; Ma T; Huang L; Xia B; Zhu S; Yang Y; Liu Z; Quan X; Luo K; Kong D; Huang J; Luo Z, Noncovalent Bonding of RGD and YIGSR to an Electrospun Poly(epsilon-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv. Healthc. Mater 2017, 6, 1600860–1600874. [DOI] [PubMed] [Google Scholar]
- 117.Nirwane A; Yao Y, Laminins and their receptors in the CNS. Biol. 2019, 94, 283–306. [DOI] [PubMed] [Google Scholar]
- 118.Sun Y; Li W; Wu X; Zhang N; Zhang Y; Ouyang S; Song X; Fang X; Seeram R; Xue W; He L; Wu W, Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. Acs Appl. Mater. Inter 2016, 8, 2348–2359. [DOI] [PubMed] [Google Scholar]


