Abstract
Aims/hypothesis
Nordic dietary patterns that are high in healthy traditional Nordic foods may have a role in the prevention and management of diabetes. To inform the update of the EASD clinical practice guidelines for nutrition therapy, we conducted a systematic review and meta-analysis of Nordic dietary patterns and cardiometabolic outcomes.
Methods
We searched MEDLINE, EMBASE and The Cochrane Library from inception to 9 March 2021. We included prospective cohort studies and RCTs with a follow-up of ≥1 year and ≥3 weeks, respectively. Two independent reviewers extracted relevant data and assessed the risk of bias (Newcastle–Ottawa Scale and Cochrane risk of bias tool). The primary outcome was total CVD incidence in the prospective cohort studies and LDL-cholesterol in the RCTs. Secondary outcomes in the prospective cohort studies were CVD mortality, CHD incidence and mortality, stroke incidence and mortality, and type 2 diabetes incidence; in the RCTs, secondary outcomes were other established lipid targets (non-HDL-cholesterol, apolipoprotein B, HDL-cholesterol, triglycerides), markers of glycaemic control (HbA1c, fasting glucose, fasting insulin), adiposity (body weight, BMI, waist circumference) and inflammation (C-reactive protein), and blood pressure (systolic and diastolic blood pressure). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of the evidence.
Results
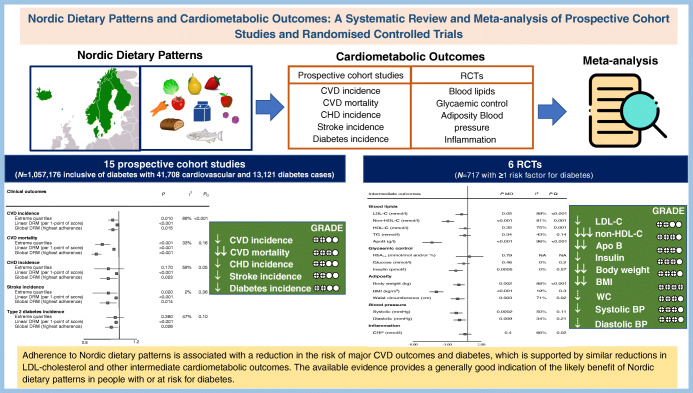
We included 15 unique prospective cohort studies (n=1,057,176, with 41,708 cardiovascular events and 13,121 diabetes cases) of people with diabetes for the assessment of cardiovascular outcomes or people without diabetes for the assessment of diabetes incidence, and six RCTs (n=717) in people with one or more risk factor for diabetes. In the prospective cohort studies, higher adherence to Nordic dietary patterns was associated with ‘small important’ reductions in the primary outcome, total CVD incidence (RR for highest vs lowest adherence: 0.93 [95% CI 0.88, 0.99], p=0.01; substantial heterogeneity: I2=88%, pQ<0.001), and similar or greater reductions in the secondary outcomes of CVD mortality and incidence of CHD, stroke and type 2 diabetes (p<0.05). Inverse dose–response gradients were seen for total CVD incidence, CVD mortality and incidence of CHD, stroke and type 2 diabetes (p<0.05). No studies assessed CHD or stroke mortality. In the RCTs, there were small important reductions in LDL-cholesterol (mean difference [MD] −0.26 mmol/l [95% CI −0.52, −0.00], pMD=0.05; substantial heterogeneity: I2=89%, pQ<0.01), and ‘small important’ or greater reductions in the secondary outcomes of non-HDL-cholesterol, apolipoprotein B, insulin, body weight, BMI and systolic blood pressure (p<0.05). For the other outcomes there were ‘trivial’ reductions or no effect. The certainty of the evidence was low for total CVD incidence and LDL-cholesterol; moderate to high for CVD mortality, established lipid targets, adiposity markers, glycaemic control, blood pressure and inflammation; and low for all other outcomes, with evidence being downgraded mainly because of imprecision and inconsistency.
Conclusions/interpretation
Adherence to Nordic dietary patterns is associated with generally small important reductions in the risk of major CVD outcomes and diabetes, which are supported by similar reductions in LDL-cholesterol and other intermediate cardiometabolic risk factors. The available evidence provides a generally good indication of the likely benefits of Nordic dietary patterns in people with or at risk for diabetes.
Registration
Funding
Diabetes and Nutrition Study Group of the EASD Clinical Practice.
Graphical abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-022-05760-z.
Keywords: Cardiovascular disease, Meta-analysis, Nordic diet, Prospective cohort, Randomised controlled trial, Systematic review
Introduction
Several dietary patterns high in plant foods have shown advantages for managing cardiometabolic risk. Evidence from prospective cohort studies and RCTs has shown that adherence to the Mediterranean [1–9], Dietary Approaches to Stopping Hypertension (DASH) [10], Portfolio [11–19] and healthy vegetarian [20, 21] dietary patterns is associated with a lower risk of CVD and a reduction in intermediate cardiometabolic risk factors in adults with and without diabetes. The applicability of these dietary patterns to northern European countries is limited by cultural values and preferences and the availability/costs of specific foods [22–24]. Nordic dietary patterns, known variably as the Nordic diet [25], New Nordic Diet [26], healthy Nordic diet [27, 28] and Baltic Sea diet [29], include foods that are typically consumed as part of traditional Nordic diets and that are consistent with Nordic dietary guidelines [26, 30]. These foods include whole-grain cereals (especially rye, oats and barley), berries, other temperate fruits (especially apples and pears), vegetables (especially root and cruciferous vegetables), legumes, fish/shellfish, nuts and canola oil/rapeseed oil (as primary fat sources) and low-fat dairy foods [26, 27, 29, 31].
The benefits of Nordic dietary patterns have been recognised in major clinical practice guidelines on obesity [32], and diabetes [33–36]. The EASD has not reviewed the evidence or made specific recommendations on Nordic dietary patterns. Although existing systematic reviews and meta-analyses of RCTs support the benefits of Nordic dietary patterns [37–40], these syntheses did not include prospective cohort studies or assess the certainty of the evidence. To update the EASD clinical practice guidelines for nutrition therapy, the Diabetes Nutrition Study Group commissioned a systematic review and meta-analysis of prospective cohort studies and randomised trials of Nordic dietary patterns and cardiometabolic outcomes, including an assessment of the certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Methods
Design
We followed the Cochrane handbook for systematic reviews of interventions [41], with results reported according to Meta-analyses of Observational Studies in Epidemiology (MOOSE) [42] and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [43] guidelines (see electronic supplementary material [ESM] Table 1). The protocol was registered at ClinicalTrials.gov (NCT04094194).
Data sources and searches
We searched MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials from inception to 9 March 2021 (ESM Table 2). Manual searches supplemented these database searches.
Eligibility criteria
We included prospective cohort studies of Nordic dietary patterns and CVD and diabetes outcomes with a duration of ≥1 year, and RCTs of Nordic dietary patterns and intermediate cardiometabolic outcomes with a duration of ≥3 weeks. Populations had to have diabetes but be free of CVD for assessment of CVD outcomes, be free of diabetes for assessment of diabetes outcomes, and have diabetes or risk factors for diabetes for assessment of intermediate outcomes. Prospective cohort studies were excluded if they did not report outcome data by level of exposure using an index or scale. RCTs were excluded if they lacked a suitable comparator diet (non-isocaloric). If more than one report was available for the same study, then the report with the longest follow-up was used. There were no language restrictions (ESM Tables 3 and 4).
Data extraction
Two reviewers (PM and EV, AJG, LC or AZ) independently extracted the data. The reviewers extracted RRs and 95% CIs for the most adjusted model from prospective cohort studies and mean differences (MDs) and SEMs from RCTs. MDs for change were preferred over end values. Missing SEMs were derived from available data using published formulae [44]. Ritz et al [45] were contacted for missing data. All disagreements were reconciled by consensus or arbitration by a senior reviewer (TAK, JLS)
Outcomes
The primary outcome was total CVD incidence in prospective cohort studies and LDL-cholesterol in RCTs. Secondary outcomes were CVD mortality, CHD incidence and mortality, stroke incidence and mortality and type 2 diabetes incidence in prospective cohort studies, and other established lipid targets (non-HDL-cholesterol, HDL-cholesterol, triglycerides [TG], apolipoprotein B [ApoB]), markers of glycaemic control (HbA1c, fasting blood glucose, fasting insulin), adiposity (body weight, BMI, waist circumference [WC]) and inflammation (C-reactive protein [CRP]) and blood pressure in RCTs.
Risk of bias
Two reviewers independently assessed risk of bias. The Newcastle–Ottawa Scale (NOS) [46] was used to assess the risk of bias in prospective cohort studies. Studies with a score of ≥6 out of 9 were considered to be of high quality. The Cochrane risk of bias tool was used to assess the risk of bias in RCTs [47] across five domains (sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting).
Statistical analysis
Pairwise meta-analyses were conducted using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). All other analyses were performed using Stata 16.1 (StataCorp LP, College Station, TX, USA).
Data were pooled using DerSimonian and Laird random-effects models. RR estimates were obtained from natural log-transformed RRs and their SEMs comparing the highest with the lowest diet scores in the most adjusted models; MDs were obtained by pooling MDs and SEMs. Hazard ratios and odds ratios were treated as RRs [48]. Paired analyses were conducted for crossover trials (correlation coefficient=0.5) [49]. Fixed-effects models were used when fewer than five comparisons were available [50].
Interstudy heterogeneity was estimated using the Cochran Q test and quantified by the I2 statistic. An I2≥50% and pQ<0.1 were considered evidence of substantial heterogeneity. Sources of heterogeneity were explored in sensitivity and subgroup analyses. Sensitivity analyses were conducted by the systematic removal of each study and recalculation of the pooled estimate. Sensitivity analyses by energy control were conducted by restricting analyses to ad libitum (free intake without strict energy control) trials for adiposity outcomes. If ten or more comparisons were available, subgroup analyses were performed by meta-regression. A priori subgroup analyses were conducted by follow-up, sex, risk of bias and funding source for prospective cohort studies, and by study design, follow-up, comparator, baseline values, risk of bias, diabetes duration and funding source for RCTs.
We performed dose–response meta-analyses (DRMs) using one-stage random effects [51–53]. Nordic dietary pattern scores were scaled or standardised to the Nordic diet score, with a range between 0 and 6 [54]. Linear DRM was expressed per 1-point score, and global DRM was assessed using the non-linear association at the highest global population adherence. DRMs were planned for RCTs when adherence scores were available for six or more study comparisons.
If ten or more comparisons were available, publication bias was assessed by visual inspection of funnel plots and formal testing with the Begg and Egger tests [55, 56], with significance set at p<0.1. The Duval and Tweedie trim-and-fill method was used to adjust for funnel plot asymmetry [57].
GRADE assessment
The GRADE approach [58–73] was used to assess the certainty of the evidence. The evidence from prospective cohort studies was classified initially as being of low certainty and that from RCTs was classified initially as being of high certainty, with the evidence downgraded or upgraded based on prespecified criteria. Criteria for downgrading included study limitations (high risk of bias), inconsistency (substantial unexplained interstudy heterogeneity: I2>50% and pQ<0.10), indirectness (presence of factors that limit generalisability), imprecision (95% CIs cross prespecified minimally important differences [MIDs]) and publication bias (detection of small-study effects). Criteria for upgrading included a dose–response gradient, large magnitude of effect (RR≥2 or RR≤0.5; prospective cohort studies only) and attenuation by plausible confounding. We interpreted the magnitude of the effect/association [74] based on prespecified criteria using MIDs and adapted GRADE thresholds using the following language: ‘trivial’, ‘small important’, ‘moderate’, ‘large’ and ‘very large’. At the request of the referees, we also performed a post hoc assessment of the certainty of the evidence using NutriGrade [75].
Results
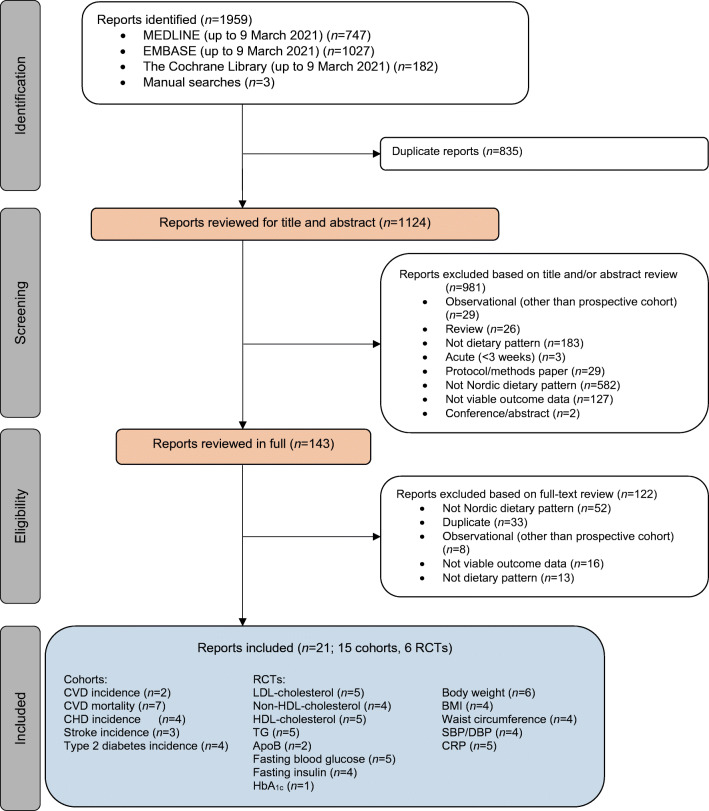
Figure 1 shows the results of the literature search. We identified 1959 reports, of which 21 met the eligibility criteria: 15 prospective cohort studies (n=1,057,176, with 41,708 cardiovascular events and 13,121 diabetes cases) [76–90] and six RCTs (n=717) [28, 91–95].
Fig. 1.
PRISMA flowchart showing the literature search
Prospective cohort studies
Study characteristics
Table 1 and ESM Tables 5 and 6 show the characteristics of the prospective cohort studies included. All studies were conducted in European adult populations. The median age of participants was 49–57 years. All of the prospective cohort studies included individuals with diabetes except for the four studies (six comparisons) assessing the association of Nordic dietary patterns with type 2 diabetes incidence [82, 87–89]. The median follow-up was 13.5–17.65 years. Adherence to Nordic dietary patterns was assessed using six scores: Healthy Nordic Food Index (eight studies [76, 77, 79–81, 83, 85, 88]), diet quality index (DQI) that assesses adherence to the 2005 Swedish Nutrition Recommendations (SNR) (DQI-SNR; three studies [78, 84, 89]), Nordic diet score (one study [82]), Baltic Sea Diet Score (one study [87]), modified Baltic Sea Diet Score (one study [90]) and Danish food-based dietary guidelines (one study [86]). All studies were funded by government, university or not-for-profit sources (agency funding) except one [87], which received agency and industry funding.
Table 1.
Summary of characteristics of the included cohorts
| Outcome | Study, year | Countries, n | Incident cases (range) | Median (range) age, years | Median (range) follow-up, years | Dietary intake assessments (at baseline), n | Nordic diet exposure assessments, n | Method of outcome assessment, n | Fundinga |
|---|---|---|---|---|---|---|---|---|---|
| CVD incidence (two cohorts, three comparisons) |
Malmö Diet and Cancer cohort [78] Swedish Women’s Lifestyle and Health cohort [80] |
Sweden 2 | 10,179 (703–8383) | 49 (29–73) | 17.65 (14–21.3) | 7-day food records 2, FFQ 2, diet history interview 1 | Healthy Nordic Food Index 1, DQI-SNR 1 | Medical record linkage 3 | Agency 2 |
| CVD mortality (seven cohorts, eight comparisons) |
Swedish Women’s Lifestyle and Health cohort [79] Swedish Mammography Cohort [81] Western Norway B-vitamin Intervention Trial [83] Malmö Diet and Cancer cohort [84] EPIC [85] Copenhagen General Population Study [86] Kuopio Ischaemic Heart Disease Risk Factor Study [90] |
Denmark 2, France 1, Greece 1, Germany 1, Italy 1, Norway 2, Spain 1, Sweden 5, the Netherlands 1, UK 1 | 11,146 (171–3761) | 53.75 (25–85) | 14.2 (7.5–26.3) | FFQ 6, 7-day records 4, 24h recall 1 | Healthy Nordic Food Index 4, DQI-SNR 2, modified Baltic Sea Diet Score 1, Danish food-based dietary guidelines 2 | Medical record linkage 5, records of death 1 | Agency 7 |
| CHD incidence (four cohorts, five comparisons) |
Danish Diet, Cancer and Health cohort [76] Swedish Women’s Lifestyle and Health cohort [80] EPIC-Potsdam [82] Western Norway B-vitamin Intervention Trial [83] |
Denmark 1, Germany 1, Norway 1, Sweden 1 | 3960 (307–1669) | 53.25 (29–85) | 15.3 (10.8–21.3) | FFQ 4, 7-day food records 1 | Nordic diet score, 1, Healthy Nordic Food Index 3 | Medical record linkage 4 | Agency 4 |
| Stroke incidence (three cohorts, three comparisons) |
Danish Diet, Cancer and Health cohort [77] Swedish Women’s Lifestyle and Health cohort [80] EPIC-Potsdam [82] |
Denmark 1, Germany 1, Sweden 1 | 3302 (321–2283) | 50 (29–65) | 13.5 (10.8–21.3) | FFQ 3, 7-day food records 1 | Nordic diet score 1, Healthy Nordic Food Index 2 | Medical records linkage 2, self-report 1 | Agency 3 |
| Τype 2 diabetes incidence (four cohorts, six comparisons) |
Danish Diet, Cancer and Health cohort [88] EPIC-Potsdam [82] Malmö Diet and Cancer cohort [89] Helsinki Birth Cohort Study, Health 2000 Survey [87] |
Denmark 2, Sweden 2, Finland 1, Germany 1 | 13,121 (541–4097) | 57 (25–85) | 15.3 (10.8–17) | FFQ 6, 7-day records in subsample 2, diet history 2 | Healthy Nordic Food Index 2, DQI-SNR 2, Baltic Sea Diet Score 1, Nordic diet score 1, Danish food-based dietary guidelines 1 | Medical record linkage 5, medical record linkage and self-report 1 | Agency 5, agency and industry 1 |
aAgency funding is that received from government, university or not-for-profit sources. Industry funding is that received from trade organisations that obtain revenue from the sale of products
EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, food frequency questionnaire
ESM Table 7 shows the confounding variables included in the most adjusted model for each of the cohorts included. The median (range) number of variables in the most adjusted model was 12 (7–17).
Risk of bias
ESM Table 8 shows the NOS scores for the cohorts included. All studies were of high quality (NOS≥6).
Primary outcome
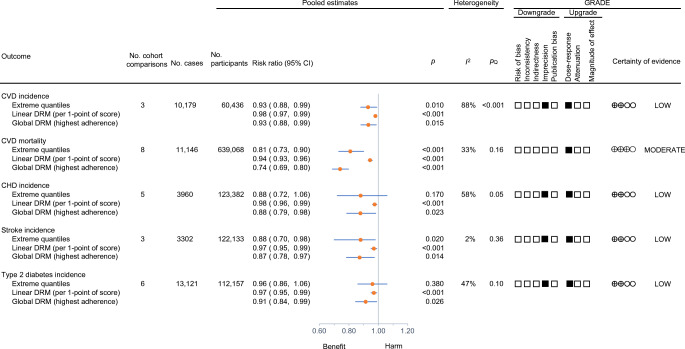
Figures 2, 3a and ESM Fig. 1 show the extreme quantiles and DRMs for the association between Nordic dietary patterns and the primary outcome, total CVD incidence. Nordic dietary patterns were associated with a lower incidence of CVD (RR, 0.93 [95% CI 0.88, 0.99], p=0.01; substantial heterogeneity: I2=88%, pQ<0.001) comparing participants with the highest adherence with those with the lowest adherence. There was an inverse linear dose–response gradient for adherence to Nordic dietary patterns and a decrease in total CVD incidence of 2% per increase in unit of the Nordic diet score (RR 0.98 [95% CI 0.97, 0.99], p<0.001), with global DRM showing that adherence to Nordic dietary patterns over the global range of scores was associated with a reduction in incidence of CVD of 7% (RR 0.93 [95% CI 0.88, 0.99]).
Fig. 2.
Summary plot of the association between Nordic dietary patterns and CVD, CHD, stroke and type 2 diabetes incidence and CVD mortality in prospective cohort studies. Pooled risk estimates are represented by the orange circles. The p values are for generic inverse variance random-effects models. Between-study heterogeneity was assessed using the Cochran Q statistic, where p<0.10 is considered statistically significant, and quantified by the I2 statistic, where I2≥50% is considered evidence of substantial heterogeneity [37]. Evidence from prospective cohort studies is rated as being of low certainty according to the GRADE approach and can be downgraded in five domains and upgraded in three domains. The filled black squares indicate where outcomes were downgraded and/or upgraded
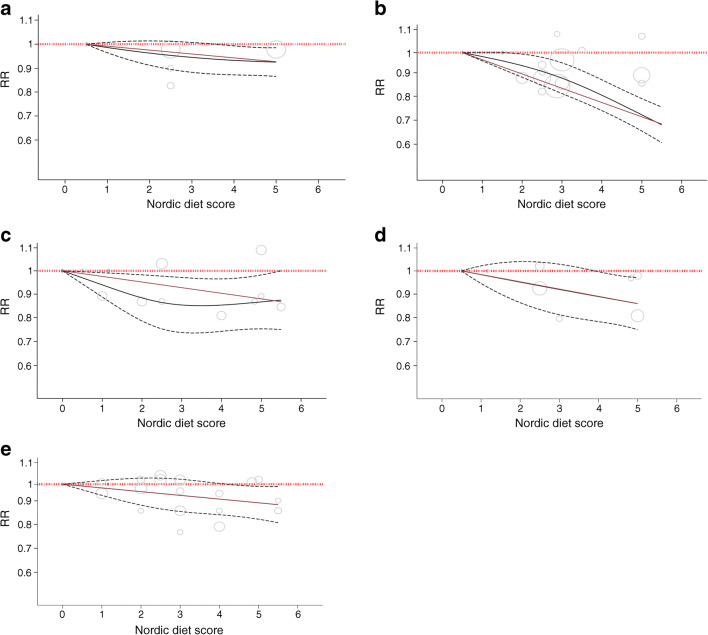
Fig. 3.
Dose–response relation between the Nordic diet score and (a) incidence of CVD (RRper-diet-score 0.98 [95% CI 0.97, 0.99], plinear<0.001, pdeparture-from-linearity=0.60), (b) CVD mortality (RRper-diet-score 0.94 [95% CI 0.93, 0.96], plinear<0.001, pdeparture-from-linearity=0.11), (c) incidence of CHD (RRper-diet-score 0.98 [95% CI 0.96, 0.99], plinear<0.001, pdeparture-from-linearity=0.13), (d) incidence of stroke (RRper-diet-score 0.97 [95% CI 0.95, 0.99], plinear<0.001, pdeparture-from-linearity=0.97) and (e) incidence of type 2 diabetes (RRper-diet-score 0.97 [95% CI 0.95, 0.99], plinear<0.001, pdeparture-from-linearity=1.00). The red lines represent the linear models and the black lines represent the non-linear models. The dotted lines represent the 95% CIs for the non-linear models
Secondary outcomes
Figures 2, 3b–e and ESM Figs 2–5 show the extreme quantiles and DRMs for the association between Nordic dietary patterns and the secondary cardiometabolic outcomes. Nordic dietary patterns were associated with lower CVD mortality (RR 0.81 [95% CI 0.73, 0.90], p<0.001; no substantial heterogeneity: I2=33%; pQ=0.16) and stroke incidence (RR 0.88 [95% CI 0.70, 0.98], p=0.02; no substantial heterogeneity: I2=2%; pQ=0.36) comparing participants with the highest adherence with those with the lowest adherence. There was an inverse linear dose–response relationship for adherence to Nordic dietary patterns (pdeparture-from-linearity≥0.05) for all secondary outcomes, with global DRM showing that adherence to Nordic dietary patterns over the global range of scores was associated with reductions of 26% in CVD mortality (RR 0.74 [95% CI 0.69, 0.80]) and reductions of 12%, 13% and 9% in the incidence of CHD (RR 0.88 [0.79, 0.98]), stroke (RR 0.87 [0.78, 0.97]) and type 2 diabetes (RR 0.91 [0.84, 0.99]), respectively. No studies reported CHD or stroke mortality outcomes.
Sensitivity analyses
ESM Table 9 shows the results of the influence analyses. The systematic removal of seven individual cohort comparisons altered several findings: the significance of the summary estimate changed from non-significant to significant for CHD incidence; the evidence of substantial heterogeneity was partially or fully explained for incidence of CVD, CHD and type 2 diabetes; and the significance of the summary estimate was lost for incidence of CVD and stroke.
Subgroup analyses
No subgroup analyses were undertaken as fewer than ten cohort comparisons were available per outcome.
Publication bias
No publication bias analyses were undertaken as fewer than ten cohort comparisons were available per outcome.
Randomised controlled trials
Study characteristics
Table 2 and ESM Table 10 show the characteristics of the six RCTs included [28, 91–95]. All of the trials were conducted in Europe and had a parallel design. Participants had a median age of 47.65–53.7 years and one or more risk factor for diabetes (overweight or obese [three trials], the metabolic syndrome [one trial], dyslipidaemia [one trial] or high cardiovascular risk [one trial]). Median follow-up was 12–48 weeks. Nordic dietary pattern interventions varied and comprised the New Nordic Diet (one trial [94]), new Nordic recommendations (two trials [91, 93]), Danish official dietary guidelines (one trial [92]) and healthy Nordic diet (two trials [28, 95]). The control diets also varied and comprised general healthy eating recommendation (one trial [93]), usual Western diet (one trial [28]) and usual/habitual Nordic diet (four trials [91, 92, 94, 95]). Feeding control ranged from the provision of dietary advice to the provision of some meals. Three trials received agency funding and three received agency and industry funding.
Table 2.
Summary of characteristics of the included RCTs
| Cardiometabolic risk factor | Total no. of trials | Total n | Median (range) sample size | Metabolic phenotypes: no. of trials | Median (range) age, yearsa | Median (range) follow-up, weeks | Trial design: no. of trials | Countries: no. of trials | Interventions: no. of trials | Comparators: no. of trials | Energy control: no. of trials | Feeding/compliance: no. of trials | Fundingb | Overall ROB: no. of trials |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDL-cholesterol (mmol/l) | 5 [28, 91, 92, 94, 95] | 606 | 134 (73–166) | OB 3, OW 1, MetS 1, HC 1, IHD risk 4 | 50.5 (18–66) | 24 (6–26) | Pa 5 | Denmark 2, Sweden 1, Iceland 1, Finland 1 | NNR 1, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, HD 1 | Ad lib 4, isocaloric 1 | Supp 2, Supp to ND 1, DA 2 | A+I 3, A 2 | Low 3, unclear 2 |
| Non-HDL-cholesterol (mmol/l) | 4 [28, 91, 92, 95] | 374 | 93 (49–166) | OB 3, OW 1, MetS 1, HC 1 | 47.65 (18–66) | 24 (6–26) | Pa 4 | Denmark 3, Sweden 1 | NNR 1, NND 1, HND 1, OD 1 | ADD 2, UWD 1, HD 1 | Ad lib 2, isocaloric 1, negative 1 | Supp 2, Supp to ND 1, DA 1 | A+I 2, A 2 | Low 2, unclear 2 |
| HDL-cholesterol (mmol/l) | 5 [28, 91, 92, 94, 95] | 606 | 134 (73–166) | OB 2, OW 1, MetS 1, mild HC 1; IHD risk factor 1 | 50.5 (18–66) | 24 (6–26) | Pa 5 | Denmark 4, Sweden 2, Iceland 1, Finland 1 | NNR 1, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, HD 1 | Ad lib 4, isocaloric 1 | Supp 2, Supp to ND 1, DA 2 | A+I 3, A 2 | Low 3, unclear 2 |
| TG (mmol/l) | 5 [28, 91, 92, 94, 95] | 606 | 134 (73–166) | OB 2, OW 1, MetS 1, mild HC 1, IHD risk factor 1 | 49.2 (18–66) | 24 (6–26) | Pa 5 | Denmark 3, Sweden 2, Iceland 1, Finland 1 | NNR 1, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, HD 1 | Ad lib 4, isocaloric 1 | Supp 2, Supp to ND 1, DA 2 | A+I 3, A 2 | Low 3, unclear 2 |
| ApoB (mmol/l) | 2 [28, 95] | 252 | 126 (86–166) | MetS 1, HC 1 | 53.7 (25–65) | 12 (6–18) | Pa 2 | Denmark 1, Sweden 2, Iceland 1, Finland 1 | HND 2 | UND 1, UWD 1 | Ad lib 1, isocaloric 1 | Supp 1, Supp to ND 1, DA 1 | A+I 1, A 1 | Low 1, unclear 1 |
| Fasting blood glucose (mmol/l) | 5 [28, 91, 92, 94, 95] | 606 | 134 (73–166) | OB 2, OW 1, MetS 1, HC 1, IHD risk 1 | 49.2 (18–66) | 24 (6–26) | Pa 5 | Denmark 5, Sweden 2, Iceland 1, Finland 1 | NNR 1, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, HD 1 | Ad lib 4, isocaloric 1 | Supp 3, Supp to ND 1, DA 3 | A+I 3, A 2 | Low 3, unclear 2 |
| Fasting insulin (pmol/l) | 4 [28, 91, 92, 94] | 440 | 110 (73–147) | OB 2, OW 1, HC 1, IHD risk 1 | 49.2 (27.3–51.8) | 24 (6–26) | Pa 4 | Denmark 3, Sweden 1 | NNR 1, NND 1, HND 1, OD 1 | ADD 2, UWD 1, HD 1 | Ad lib 4 | Supp 2, Supp to ND 1, DA 1 | A+I 2, A 2 | Low 3, unclear 1 |
| HbA1c (mmol/mol and/or %) | 1 [92] | 145 | 145 | IHD risk 1 | 50.5 (30–65) | 48 | Pa 1 | Denmark 1 | OD 1 | HD 1 | Ad lib 1 | DA 1 | A 1 | Low 1 |
| Body weight (kg) | 6 [28, 91–95] | 706 | 117 (86–166) | OB 2, OW 2, MetS 1, mild HC 1, IHD risk factor 1 | 49.2 (18–66) | 24 (6–48) | Pa 6 | Denmark 4, Sweden 3, Iceland 1, Finland 1 | NNR 2, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, GHE 1, HD 1 | Ad lib 4, isocaloric 1, negative 1 | Supp 2, Supp to ND 1, DA 4 | A+I 3, A 3 | Low 4, unclear 2 |
| BMI (kg/m2) | 4 [28, 91–93] | 393 | 93 (49–100) | OB 1, OW 2, mild HC 1, IHD risk factor 1 | 40.9 (18–65) | 18 (6–24) | Pa 4 | Denmark 2, Sweden 2 | NNR 2, HND 1, OD 1 | ADD 1, UWD 1, GHE 1, HD 1 | Ad lib 3, negative 1 | Supp 1, Supp to ND 1, DA 2 | A+I 1, A 3 | Low 3, unclear 1 |
| Waist circumference (cm) | 4 [28, 91–93] | 454 | 96.8 (92.3–105.3) | OB 2, OW 2, IHD risk factor 1 | 40.9 (18–66) | 24 (12–26) | Pa 4 | Denmark 3, Sweden 1 | NNR 2, NND 1, OD 1 | ADD 2, GHE 1, HD 1 | Ad lib 3, negative 1 | Supp 1, Supp to ND 1, DA 3 | A+ I 1, A 3 | Low 3, unclear 1 |
| SBP (mmHg) | 4 [28, 92, 94, 95] | 533 | 140.5 (86–166) | OB 1, MetS 1, mild HC 1, IHD risk factor 1 | 52.2 (25–65) | 21 (6–26) | Pa 4 | Denmark 2, Sweden 2, Iceland 1, Finland 1 | NND 1, HND 2, OD 1 | ADD 1, UND 1, UWD 1, HD 1 | Ad lib 3, isocaloric 1 | Supp 1, Supp to ND 2, DA 1 | A+I 2, A 2 | Low 3, unclear 1 |
| DBP (mmHg) | 4 [28, 92, 94, 95] | 533 | 140.5 (86–166) | OB 1, MetS 1, mild HC 1, IHD risk factor 1 | 52.2 (25–65) | 21 (6–26) | Pa 4 | Denmark 2, Sweden 2, Iceland 1, Finland 1 | NND 1, HND 2, OD 1 | ADD 1, UND 1, UWD 1, HD 1 | Ad lib 3, isocaloric 1 | Supp 1, Supp to ND 2, DA 1 | A+I 2, A 2 | Low 3, unclear 1 |
| CRP (nmol/l)c | 5 [28, 91, 92, 94, 95] | 606 | 134 (86–166) | OB 2, OW 1, MetS 1, mild HC 1, IHD risk factor 1 | 50.5 (6–24) | 24 (6–26) | Pa 5 | Denmark 4, Sweden 2, Iceland 1, Finland 1 | NNR 1, NND 1, HND 2, OD 1 | ADD 2, UND 1, UWD 1, HD 1 | Ad lib 4, isocaloric 1 | Supp 1, Supp to ND 2, DA 5 | A+I 3, A 2 | Low 3, unclear 2 |
aThe range represents the range of the mean age in the trials
bAgency funding is that received from government, university or not-for-profit sources. Industry funding is that received from trade organisations that obtain revenue from the sale of products
cFive out of six studies reported high-sensitivity CRP levels
A, agency; ADD, average Danish diet; Ad lib, ad libitum; DA, dietary advice; DODG, Danish official dietary guidelines; GHE, general healthy eating; HC, hypercholesterolaemia; HD, habitual diet; HND, healthy Nordic diet; I, industry; IHD, ischaemic heart disease; MetS, the metabolic syndrome; ND, Nordic diet; NND, New Nordic Diet; NNR, Nordic nutrition recommendations; OB, obese; OW, overweight; Pa, parallel design; ROB, risk of bias; Supp, supplemented; UND, usual Nordic diet; UWD, usual Western diet
Risk of bias
ESM Fig. 6 shows the summary and individual Cochrane risk of bias assessments for the included RCTs. Most RCTs were judged as having a low or unclear risk of bias across the five domains. Although three trials were rated as having a high risk of bias in one of the five domains, overall there was no evidence of serious risk of bias.
Primary outcome
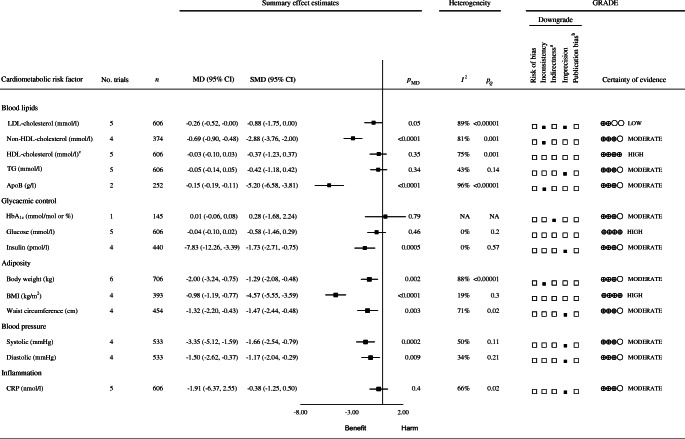
Figure 4 and ESM Fig. 7 show the effects of Nordic dietary patterns on the intermediate primary outcome LDL-cholesterol. Adherence to Nordic dietary patterns was associated with a reduction in LDL-cholesterol compared with control diets (MD −0.26 mmol/l [95% CI −0.52, −0.00], pMD=0.05; substantial heterogeneity: I2=89%, pQ<0.001). Linear or non-linear dose–responses could not be assessed.
Fig. 4.
Summary plot of the effect of Nordic dietary patterns on cardiometabolic risk factors in RCTs. Data are expressed as weighted MDs with 95% CIs using the generic inverse variance method modelled by random effects ( five trials available) or fixed effects (fewer than five trials available). To allow the pooled effect estimates for each endpoint to be displayed on the same axis, MDs were transformed to standardised mean differences (SMDs). The pseudo 95% CI for each transformed SMD was derived directly from the original MD and 95% CI. Between-study heterogeneity was assessed by the Cochran Q statistic, where p<0.10 is considered statistically significant, and quantified by the I2 statistic, where I2≥50% is considered evidence of substantial heterogeneity [61]. Evidence from RCTs is rated as being of high certainty according to the GRADE approach and can be downgraded in five domains. The filled black squares indicate where outcomes were downgraded. aAlthough all studies were conducted in Nordic countries and in those who were overweight or obese, we did not downgrade the evidence in this domain as there is no biological reason why the results would differ in other populations. bUnable to assess publication bias because of fewer than ten studies per outcome. cBecause of the difference in directionality of HDL-cholesterol compared with the other outcomes with regard to the signal for benefit or harm, the signs for the MD and SMD were changed. To convert total cholesterol, LDL-cholesterol and HDL-cholesterol to mg/dl, multiply by 38.67; to convert TG to mg/dl, multiply by 88.57; to convert blood glucose to mg/dl, multiply by 18.02; to convert CRP to mg/l, multiply by 0.105. NA, not available
Secondary outcomes
Figure 4 and ESM Figs 8–20 show the effects of Nordic dietary patterns on the intermediate secondary outcomes. Compared with control diets, Nordic dietary patterns were associated with reductions in non-HDL-cholesterol (−0.69 mmol/l [95% CI −0.9, −0.48], pMD <0.01; substantial heterogeneity: I2=81%, pQ<0.01), ApoB (−0.15 g/l [95% CI −0.19, −0.11], pMD<0.01; substantial heterogeneity: I2=96%, pQ<0.01), body weight (−2.00 kg [95% CI −3.24, −0.75], pMD=0.002; substantial heterogeneity: I2=88%, pQ<0.01), BMI (−0.98 kg/m2 [95% CI −1.19, −0.77], pMD<0.01; no substantial heterogeneity: I2=19%, pQ=0.3); WC (−1.32 cm [95% CI −2.20, −0.43], pMD=0.003; substantial heterogeneity: I2=71%, pQ=0.02); insulin (−7.83 pmol/l [95% CI −12.26, −3.39], pMD<0.01; no substantial heterogeneity: I2=0%, pQ=0.57), systolic blood pressure (SBP; −3.35 mmHg [−5.12, −1.59], pMD<0.01; substantial heterogeneity: I2=50%, pQ=0.11) and diastolic blood pressure (DBP; −1.50 mmHg [−2.62, −0.37], pMD=0.009; no substantial heterogeneity: I2=34%, pQ=0.21). There were no effects on the other intermediate cardiometabolic outcomes. Linear or non-linear dose–responses could not be assessed.
Sensitivity analyses
ESM Table 11 shows selected sensitivity analyses in which systematic removal of individual trials altered the results. Systematic removal of individual trials resulted in the following: loss of significance for LDL-cholesterol, ApoB and WC, although the pooled effect estimates still favoured Nordic diets; change in the pooled effect estimate from non-significant to a significant decrease for TG; and explanation of the substantial heterogeneity for LDL-cholesterol, non-HDL-cholesterol, TG, HDL-cholesterol, BMI, WC, DBP and CRP.
ESM Fig. 21 shows the results of the sensitivity analysis including only ad libitum trials. Removal of two trials (those by Uusitupa et al [95] [isocaloric trial] and Huseinovic et al [93] [negative energy balance trial]) resulted in reductions in body weight (MD −2.16 kg [95% CI −3.51 to −0.82 mmol/l], pMD=0.002; substantial heterogeneity: I2=82%, pQ=0.001), BMI (MD −0.85 kg/m2 [95% CI −1.31 to −0.40 mmol/l], pMD<0.01; no substantial heterogeneity: I2=37%, pQ=0.20) and WC (−1.32 cm [95% CI −3.49, 0.84], pMD=0.23; substantial heterogeneity: I2=78%, pQ=0.01) without changing the significance or the magnitude of the effect, except for BMI, for which the magnitude of the reduction was decreased.
Subgroup analyses
No subgroup analyses were undertaken as fewer than ten trial comparisons were available for any outcome.
Publication bias
No publication bias analyses were undertaken as fewer than ten trial comparisons were available for any outcome.
Medication use
Of the six trials, only two reported medication use [94, 95]. In one trial, one participant in the New Nordic Diet group and one in the average Danish diet group began antihypertensive medication during the study [94], while in the other trial no changes in dosage of antihypertensive and lipid-lowering medication were allowed during the study [95].
Adverse events
Two trials assessed adverse events [28, 92]. No adverse events were reported.
Acceptability
No trials assessed the acceptability of Nordic dietary patterns. Four trials assessed adherence to the Nordic dietary pattern, which was reported as satisfactory or high [28, 91, 94, 95].
GRADE assessments
ESM Table 12 summarises the GRADE assessments of the associations between Nordic dietary patterns and CVD outcomes in prospective cohorts. The certainty of evidence for the primary outcome, total CVD incidence (small important reduction), was graded as low owing to downgrades for imprecision and inconsistency and an upgrade for dose–response gradient. For the secondary outcomes, the certainty of evidence was graded as moderate for CVD mortality (moderate reduction) owing to an upgrade for dose–response gradient and no downgrades, and low for CHD (small important reduction), stroke (small important reduction) and type 2 diabetes (small important reduction) owing to downgrades for imprecision and upgrades for dose–response gradient in all cases. NutriGrade assessments gave the same ratings as the GRADE approach for four out of five (80%) outcomes, and a lower rating for the remaining outcome (20%) (ESM Table 13).
ESM Table 14 shows the GRADE assessments conducted for the effect of Nordic dietary patterns on cardiometabolic risk factors in RCTs. The certainty of evidence for the primary outcome, LDL-cholesterol (small important reduction), was graded as low owing to downgrades for inconsistency and imprecision. The certainty of evidence for the secondary outcomes was graded as high for HDL-cholesterol (no effect), BMI (moderate reduction) and blood glucose (no effect) in the absence of downgrades, moderate for non-HDL-cholesterol (large reduction), ApoB (moderate reduction) and body weight (moderate reduction) owing to downgrades for inconsistency, and moderate for TG (no effect), insulin (small important reduction), WC (trivial reduction), SBP (small important reduction), DBP (trivial reduction) and CRP (no effect) owing to downgrades for imprecision. Compared with the GRADE approach, NutriGrade assessments gave the same ratings for one of the 14 (7%) outcomes and lower ratings for 13 of 14 (93%) outcomes (ESM Table 15).
Discussion
We conducted a comprehensive systematic review and meta-analysis of Nordic dietary patterns and cardiometabolic outcomes, including 15 prospective cohort studies (n=1,057,176 with 41,708 cardiovascular events and 13,121 diabetes cases) and six RCTs (n=717). We observed that Nordic dietary patterns were associated with a small important reduction in the primary clinical outcome of CVD incidence (7% by global DRM), with similar or greater reductions in the secondary clinical outcomes of CVD mortality (26% by global DRM), CHD incidence (12% by global DRM) and stroke incidence (13% by global DRM) in adults with diabetes, and type 2 diabetes incidence (9% by global DRM) in adults without diabetes. These reductions were supported by reductions in intermediate cardiometabolic outcomes in adults with one or more risk factor for diabetes. Nordic dietary patterns resulted in small important reductions in the primary intermediate outcome LDL-cholesterol (−0.26 mmol/l) and similar or greater reductions in the secondary intermediate outcomes of non-HDL-cholesterol (−0.69 mmol/l), ApoB (−0.15 g/l), body weight (−2 kg), insulin (−7.83 pmol/l) and SBP (−3.35 mmHg). Other secondary outcomes showed trivial reductions or no effect.
Findings in the context of the literature
We are not aware of any previous systematic reviews and meta-analyses of prospective cohort studies of Nordic dietary patterns; however, our findings agree with those of previous systematic reviews and meta-analyses of key foods that are emphasised as being part of Nordic dietary patterns. Systematic reviews and meta-analyses of prospective cohort studies have demonstrated that total fruit and vegetable intake is associated with reductions in CVD, CHD and stroke incidence and/or mortality, with the greatest benefits found for certain root vegetables (i.e. carrots) and cruciferous vegetables (including cabbage) [96]. Other key components of Nordic dietary patterns, including whole grains (oats and rye) [97, 98], fish [99] and legumes [100], have been associated with reductions in CVD, stroke and cardiovascular mortality and/or incidence of type 2 diabetes.
Our findings also agree with and expand on those of several previous systematic reviews and meta-analyses of RCTs of Nordic dietary patterns and intermediate cardiometabolic outcomes. Three systematic reviews and meta-analyses of RCTs found reductions in SBP associated with Nordic dietary patterns [37, 101, 102]. One systematic review and meta-analysis of RCTs showed similar reductions in blood insulin levels but not blood glucose levels [38]. A second showed similar reductions in body weight but not BMI and WC [39, 103]. A third showed no effect of Nordic dietary patterns on inflammation, which agrees with our finding of no effect on CRP [40], although a single RCT did report interleukin-1 receptor antagonism [95].
There are several possible explanations for the observed benefits of Nordic dietary patterns. One is the concordance of Nordic dietary patterns with Mediterranean, DASH, Portfolio, healthy vegetarian and low glycaemic index/load dietary patterns, which are associated with improvements in clinical cardiometabolic outcomes and intermediate cardiometabolic outcomes [3, 10–12, 104–107]. Key foods shared with these other dietary patterns [27, 30] have also been shown to improve intermediate cardiometabolic outcomes. These foods include viscous fibres [108, 109] from oats and barley [110, 111], temperate fruit and berries [112, 113], nuts [103, 114–116] and legumes [117–125]. Another possible explanation for the observed benefits of Nordic dietary patterns is because of weight loss induced by the interventions. Most of the included RCTs, however, adjusted for weight [28, 94] or BMI [91, 93], indicating that effects were largely independent of weight loss.
Strengths and limitations
The present systematic review and meta-analysis has several strengths. It provides a comprehensive synthesis of the currently available evidence on the potential role of Nordic dietary patterns in both patient-important and surrogate CVD outcomes. We used a systematic search strategy to capture all pertinent prospective cohort studies and RCTs. We explored dose–responses in prospective cohorts, which highlighted a significant linear protective association of Nordic dietary patterns with CVD outcomes. We assessed the certainty of the evidence using the GRADE approach and performed a post hoc analysis using NutriGrade (although the latter was not used to inform our assessment of the certainty of the evidence as it was not prespecified or endorsed by the guidelines committee).
Several limitations were identified in the available evidence. First, there was evidence of serious inconsistencies. We observed substantial unexplained heterogeneity in the primary outcomes, total CVD incidence in the prospective cohort studies and LDL-cholesterol in the RCTs, and in several of the secondary outcomes in the RCTs. Second, there was evidence of serious imprecision. We observed imprecision in the primary outcomes, total CVD incidence in the prospective cohort studies and LDL-cholesterol in the RCTs, and in several of the secondary outcomes. Finally, there was some evidence of indirectness. Different criteria were used to define Nordic dietary patterns, which may have contributed to the heterogeneity observed. We downgraded the evidence for HbA1c because of serious indirectness, as data were available from only a single RCT of a single Nordic dietary pattern (Danish official dietary guidelines), limiting generalisability to other Nordic dietary patterns. We did not, however, downgrade the evidence for other outcomes because of the use of different definitions of Nordic dietary patterns, as the evidence appeared robust to the different definitions. Another potential source of indirectness was the inability to isolate the effects/associations in diabetes. Prospective cohort studies did not provide subgroup data by diabetes status, and none of the RCTs included individuals with diabetes. We did not downgrade for indirectness here, as the prospective cohort studies did include a representative proportion of individuals with diabetes and the RCTs included individuals at risk for diabetes. The key components of Nordic dietary patterns have also been shown individually to lower cardiometabolic risk factors reliably in people with diabetes [108–126].
Implications
Dietary interventions remain the cornerstone of type 2 diabetes and CVD prevention and management [127–129]. Clinical practice guidelines for obesity, type 2 diabetes and CVD have shifted from focusing on single nutrients to focusing on dietary patterns [127–129]. Nordic dietary patterns ( ≥25% energy as whole grains, ≥175g/day of temperate fruits, ≥150–200g/day of berries, ≥175g/day of vegetables, legumes and canola oil, three or more servings/week of fatty fish, two or more servings/day of low-fat dairy products) [95] have important similarities (with some differences [130, 131]) to other established dietary patterns that are high in plant foods such as the Mediterranean, DASH, Portfolio and vegetarian dietary patterns. Population intakes of Nordic countries, as well as other European countries, Canada and the USA, do not meet the targets for these other dietary patterns [125, 132–135]. Nordic dietary patterns may provide a promising alternative to help individuals in Nordic countries and elsewhere achieve the cardiometabolic benefits of dietary interventions. This approach may have impacts beyond health [136, 137]. As pointed out by the WHO Regional Office for Europe, the Nordic nutrition recommendations will be the first nutrition recommendations integrating environmental health with personal health [137].
Conclusions
Adherence to Nordic dietary patterns is associated with generally small important reductions in major CVD outcomes and incidence of diabetes and similar or greater reductions in LDL-cholesterol and other intermediate cardiometabolic outcomes. Our confidence in the evidence is generally low to moderate, with the evidence for reductions in clinical outcomes from prospective cohort studies supported by reductions in intermediate cardiometabolic outcomes from RCTs. Although there is a need for more long-term RCTs using standard definitions of Nordic dietary patterns that assess the effects on clinical outcomes and intermediate cardiometabolic outcomes (especially HbA1c) in diabetes, the available evidence provides a generally good indication of the likely benefit of Nordic dietary patterns in adults with or at risk for diabetes.
Supplementary information
(PDF 991 kb)
Acknowledgements
Aspects of this work were presented at the 36th International Symposium on Diabetes and Nutrition, Opatija, Croatia, 27–29 June 2018, and at Nutrition 2020 Live Online, 1–4 June 2020. With the exception of the Diabetes and Nutrition Study Group of the Clinical Practice Guidelines Expert Committee for Nutrition Therapy, none of the sponsors had a role in any aspect of the study, including design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript or decision to publish.
Authors’ relationships and activities
AZ is a part-time research associate at INQUIS Clinical Research (formerly Glycemic Index Laboratories), a contract research organisation, and a consultant for the Glycemic Index Foundation. AJG has received consulting fees from Solo GI Nutrition and an honorarium from the Soy Nutrition Institute. LC was a Mitacs Elevate postdoctoral fellow jointly funded by the Government of Canada and the Canadian Sugar Institute. She was previously employed as a casual clinical coordinator at INQUIS Clinical Research. TAK has received research support from the CIHR, the International Life Science Institute (ILSI) and the National Honey Board. He has been an invited speaker at the Calorie Control Council Annual Meeting for which he received an honorarium. EMC reports grants from the Natural Sciences and Engineering Research Council of Canada and the CIHR while this study was being conducted, has received research support from Lallemand Health Solutions and Ocean Spray, and has received consultant fees and speaker and travel support from Danone and Lallemand Health Solutions (all are outside this study). DR is director of Vuk Vrhovac University Clinic for Diabetes, Endocrinology and Metabolic Diseases at Merkur University Hospital, Zagreb, Croatia. He is the president of the Croatian Society for Diabetes and Metabolic Disorders of the Croatian Medical Association. He serves as an Executive Committee member of the Croatian Endocrine Society, Croatian Society of Obesity and Croatian Society for Endocrine Oncology. He was a board member and secretary of IDF Europe and is currently the chair of the IDF Young Leaders in Diabetes (YLD) Programme. He has served as an Executive Committee member of the Diabetes and Nutrition Study Group of the EASD and currently serves as an Executive Committee member of the Diabetes and Cardiovascular Disease Study Group of the EASD. He has served as principal investigator or co-investigator in clinical trials for AstraZeneca, Eli Lilly, MSD, Novo Nordisk, Sanofi Aventis, Solvay and Trophos. He has received travel support, speaker fees and honoraria for advisory board engagements and/or consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Belupo, Boehringer Ingelheim, Eli Lilly, LifeScan – Johnson & Johnson, the International Sweeteners Association, Krka, Medtronic, Mediligo, Mylan, Novartis, Novo Nordisk, MSD, Pfizer, Pliva, Roche, Salvus, Sandoz, Solvay, Sanofi Aventis and Takeda. HK is Director of Clinical Research at the Physicians Committee for Responsible Medicine, a non-profit organisation that provides nutrition education and research. JS-S reports serving on the board of and receiving grant support through his institution from the International Nut and Dried Fruit Council (INC) and the Eroski Foundation. He reports serving on the Executive Committee of the Instituto Danone Spain. He reports receiving research support from the Instituto de Salud Carlos III, Spain; Ministerio de Educación y Ciencia, Spain; the Departament de Salut Pública de la Generalitat de Catalunya, Catalonia, Spain; the European Commission; the California Walnut Commission, USA; Patrimonio Comunal Olivarero, Spain; La Morella Nuts, Spain; and Borges, Spain. He reports receiving consulting fees or travel expenses from Danone, the California Walnut Commission, the Eroski Foundation, the Instituto Danone Spain, Nuts for Life, the Australian Nut Industry Council, Nestlé, Abbot and Font Vella y Lanjarón. He is on the Clinical Practice Guidelines Expert Committee of the EASD and served on the Scientific Committee of the Spanish Agency for Food Safety and Nutrition and the Spanish Federation of the Scientific Societies of Food, Nutrition and Dietetics. He is a member of the International Carbohydrate Quality Consortium (ICQC) and an Executive Board Member of the Diabetes and Nutrition Study Group of the EASD. CWCK has received grants or research support from the Advanced Food and Materials Network, Agriculture and Agri-Food Canada (AAFC), the Almond Board of California, Barilla, the CIHR, the Canola Council of Canada, the International Nut and Dried Fruit Council, the International Tree Nut Council Nutrition Research and Education Foundation, Loblaw Brands, the Peanut Institute, Pulse Canada and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, the California Walnut Commission, Kellogg Canada, Loblaw Brands, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, WhiteWave Foods/Danone. He has received travel support and/or honoraria from Barilla, the California Walnut Commission, the Canola Council of Canada, General Mills, the International Nut and Dried Fruit Council, the International Pasta Organization, Lantmannen, Loblaw Brands, the Nutrition Foundation of Italy, the Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organisation, McCormick Science Institute and Oldways Preservation Trust. He is a founding member of the ICQC and an Executive Board Member of the Diabetes and Nutrition Study Group of the EASD, is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, the Ontario Research Fund, the Province of Ontario Ministry of Research, Innovation and Science, the CIHR, Diabetes Canada, the American Society for Nutrition (ASN), the International Nut and Dried Fruit Council Foundation, the National Honey Board (US Department of Agriculture [USDA] honey ‘Checkoff’ programme), the Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America), Pulse Canada, the Quaker Oats Center of Excellence, the United Soybean Board (USDA soy ‘Checkoff’ programme), the Tate and Lyle Nutritional Research Fund at the University of Toronto, the Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (established by the Alberta Pulse Growers), the Plant Protein Fund at the University of Toronto (which has received contributions from IFF) and the Nutrition Trialists Fund at the University of Toronto (established by an inaugural donation from the Calorie Control Council). He has received food donations to support RCTs from the Almond Board of California, the California Walnut Commission, the Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from the ASN, Danone, Dairy Farmers of Canada, FoodMinds, Nestlé, Abbott, General Mills, the Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, the International Food Information Council (IFIC), the Calorie Control Council and the International Glutamate Technical Committee. He has or has had ad hoc consulting arrangements with Perkins Coie, Tate & Lyle, Phynova and INQUIS Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and former member of the Soy Nutrition Institute Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, the EASD, the Canadian Cardiovascular Society and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid member of the Board of Trustees and an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Carbohydrates Committee of the IAFNS. He is a member of the ICQC, an Executive Board Member of the Diabetes and Nutrition Study Group of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. PM, EV, SBM, VC, US, UR, MU, A-MA, KH and IT declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
US, UR, MU, A-MA, KH, IT, DR, HK, JSS, CWCK, SBM and JLS were responsible for the conception and design of the study. PM, EV, AJG, LC and AZ acquired the data. PM, AZ and TAK performed the statistical analyses. PM, AZ, AJG, TAK, LC, SBM, VC and JLS drafted the article. All authors had access to the data and participated in the analysis and interpretation of the data, provided critical revision of the article for important intellectual content and approved the final version for publication. JLS is the guarantor. He accepts full responsibility for the conduct of the study, integrity of the data, accuracy of the data analyses and decision to publish.
Abbreviations
- ApoB
Apolipoprotein B
- CRP
C-reactive protein
- DASH
Dietary Approaches to Stopping Hypertension
- DBP
Diastolic blood pressure
- DQI-SNR
Diet quality index (DQI) that assesses adherence to the 2005 Swedish Nutrition Recommendations (SNR)
- DRM
Dose–response meta-analysis
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- MD
Mean difference
- MID
Minimally important difference
- NOS
Newcastle–Ottawa Scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- SBP
Systolic blood pressure
- TG
Triglycerides
- WC
Waist circumference
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. The Diabetes and Nutrition Study Group of the EASD commissioned this systematic review and meta-analysis and provided funding and logistical support for meetings as part of the development of the EASD clinical practice guidelines for nutrition therapy. This work was also supported by the Canadian Institutes of Health Research (CIHR; reference no. 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3D) Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the conduct of this work. PM was funded by a Connaught Fellowship, an Onassis Foundation Fellowship and a Peterborough KM Hunter Charitable Foundation Scholarship. AZ was funded by a Toronto3D Postdoctoral Fellowship Award and a Banting and Best Diabetes Centre (BBDC) Fellowship in Diabetes Care. AJG was funded by a Nora Martin Fellowship in Nutritional Sciences, the Banting & Best Diabetes Centre Tamarack Graduate Award in Diabetes Research, the Peterborough K. M. Hunter Charitable Foundation Graduate Award and an Ontario Graduate Scholarship. LC was funded by a Mitacs Elevate Postdoctoral Fellowship Award. TAK was funded by a Toronto 3D Postdoctoral Fellowship Award. EMC held the Lawson Family Chair in Microbiome Nutrition Research at the Lawson Centre for Child Nutrition, Temerty Faculty of Medicine, University of Toronto. JS-S is partially supported by the Catalan Institution for Research and Advanced Studies (ICREA) under the ICREA Acadèmia programme. JLS was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Canadian Diabetes Association Clinician Scientist Award, CIHR Institute of Nutrition, Metabolism and Diabetes (INMD)/Canadian Nutrition Society (CNS) New Investigator Partnership Prize and BBDC Sun Life Financial New Investigator Award.
Data availability
All data generated or analysed during this study are included in this published article (and its ESM information files).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ursula Schwab, Email: ursula.schwab@uef.fi.
John L. Sievenpiper, Email: john.sievenpiper@utoronto.ca
References
- 1.Pinto X, Fanlo-Maresma M, Corbella E, et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J Nutr. 2019;149(11):1920–1929. doi: 10.1093/jn/nxz147. [DOI] [PubMed] [Google Scholar]
- 2.Babio N, Toledo E, Estruch R, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186(17):E649–E657. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becerra-Tomas N, Blanco Mejia S, Viguiliouk E, et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(7):1207–1227. doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 4.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab. 2010;12(3):204–209. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 5.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Int Med. 2009;151(5):306–314. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200–1208. doi: 10.1038/ejcn.2014.243. [DOI] [PubMed] [Google Scholar]
- 7.Salas-Salvado J, Bullo M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas-Salvado J, Bullo M, Babio N, et al. Erratum. Reduction in the Incidence of Type 2 Diabetes With the Mediterranean Diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14-19. Diabetes Care. 2018;41(10):2259–2260. doi: 10.2337/dc18-er10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 10.Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiavaroli L, Nishi SK, Khan TA, et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-analysis of Controlled Trials. Prog Cardiovasc Dis. 2018;61(1):43–53. doi: 10.1016/j.pcad.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Glenn AJ, Hernandez-Alonso P, Kendall CWC, et al. Longitudinal changes in adherence to the portfolio and DASH dietary patterns and cardiometabolic risk factors in the PREDIMED-Plus study. Clin Nutr. 2021;40(5):2825–2836. doi: 10.1016/j.clnu.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Glenn AJ, Lo K, Jenkins DJA, et al. Relationship Between a Plant-Based Dietary Portfolio and Risk of Cardiovascular Disease: Findings From the Women's Health Initiative Prospective Cohort Study. J Am Heart Assoc. 2021;10(16):e021515. doi: 10.1161/JAHA.121.021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Chiavaroli L, Wong JM, et al. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. CMAJ. 2010;182(18):1961–1967. doi: 10.1503/cmaj.092128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Jones PJ, Lamarche B et al (2011) Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA 306(8):831–839. 10.1001/jama.2011.1202 [DOI] [PubMed]
- 16.Jenkins DJ, Kendall CW, Faulkner D, et al. A dietary portfolio approach to cholesterol reduction: combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism. 2002;51(12):1596–1604. doi: 10.1053/meta.2002.35578. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins DJ, Kendall CW, Faulkner DA, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr. 2006;83(3):582–591. doi: 10.1093/ajcn.83.3.582. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins DJ, Kendall CW, Marchie A, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52(11):1478–1483. doi: 10.1016/s0026-0495(03)00260-9. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins DJ, Kendall CW, Marchie A et al (2003) Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 290(4):502–510. 10.1001/jama.290.4.502 [DOI] [PubMed]
- 20.Glenn AJ, Viguiliouk E, Seider M, et al. Relation of Vegetarian Dietary Patterns With Major Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Front Nutr. 2019;6:80. doi: 10.3389/fnut.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viguiliouk E, Kendall CW, Kahleova H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1133–1145. doi: 10.1016/j.clnu.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Tong TYN, Imamura F, Monsivais P, et al. Dietary cost associated with adherence to the Mediterranean diet, and its variation by socio-economic factors in the UK Fenland Study. Br J Nutr. 2018;119:685–694. doi: 10.1017/S0007114517003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore SE, Mcevoy CT, Prior L et al (2018) Barriers to adopting a Mediterranean diet in Northern European adults at high risk of developing cardiovascular disease. J Hum Nutr Diet 31:451–462. 10.1111/jhn.12523 [DOI] [PubMed]
- 24.Papadaki A, Scott JA. The impact on eating habits of temporary translocation from a Mediterranean to a Northern European environment. Eur J Clin Nutr. 2002;56:455–467. doi: 10.1038/sj.ejcn.1601337. [DOI] [PubMed] [Google Scholar]
- 25.Slimani N, Fahey M, Welch AA, et al. Diversity of dietary patterns observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr. 2002;5(6B):1311–1328. doi: 10.1079/PHN2002407. [DOI] [PubMed] [Google Scholar]
- 26.Mithril C, Dragsted LO, Meyer C, Blauert E, Holt MK, Astrup A. Guidelines for the New Nordic Diet. Public Health Nutr. 2012;15(10):1941–1947. doi: 10.1017/S136898001100351X. [DOI] [PubMed] [Google Scholar]
- 27.Adamsson V, Reumark A, Cederholm T, Vessby B, Risérus U, Johansson G (2012) What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res 56. 10.3402/fnr.v56i0.18189 [DOI] [PMC free article] [PubMed]
- 28.Adamsson V, Reumark A, Fredriksson IB, et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: A randomized controlled trial (NORDIET) J Int Med. 2011;269:150–159. doi: 10.1111/j.1365-2796.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Mannisto S. The Baltic Sea Diet Score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr. 2014;17(8):1697–1705. doi: 10.1017/S1368980013002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker W, Lyhne N, Pedersen AN, et al. Nordic Nutrition Recommendations 2004-integrating nutrition and physical activity. Scand J Nutr. 2004;48(4):178–187. doi: 10.1080/1102680410003794. [DOI] [Google Scholar]
- 31.Brader L, Uusitupa M, Dragsted L, Hermansen K. Effects of an isocaloric healthy Nordic diet on ambulatory blood pressure in metabolic syndrome: a randomized SYSDIET sub-study. Eur J Clin Nutr. 2013;68:57–63. doi: 10.1038/ejcn.2013.192. [DOI] [PubMed] [Google Scholar]
- 32.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diabetes Canada Clinical Practice Guidelines Expert Committee, Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL (2018) Nutrition Therapy. Can J Diabetes 42(Suppl 1):S64–S79. 10.1016/j.jcjd.2017.10.009 [DOI] [PubMed]
- 34.Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35(5):541–547. doi: 10.1111/dme.13603. [DOI] [PubMed] [Google Scholar]
- 35.Helsedirektoratet (2016) 1. Diagnostikk av diabetes, risikovurdering ognoppfølging av personer med høy risiko for å utvikle diabetes. [1. Diagnosis of diabetes, risk assessment and follow-up of people at high risk of developing diabetes]. In: Diabetes – nasjonal faglig retningslinje [Diabetes – national professional guideline]. Helsedirektoratet, Oslo.
- 36.Laakso M, Groop L, Isomaa B, et al. Update on Current Care Guideline. Diabetes. Duodecim. 2016;132(9):881–882. [PubMed] [Google Scholar]
- 37.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr (Bethesda, Md) 2016;7:76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimorovat A, Mohammadi M, Ramezani-Jolfaie N, Salehi-Abargouei A. The healthy Nordic diet for blood glucose control: a systematic review and meta-analysis of randomized controlled clinical trials. Acta Diabetol. 2020;57(1):1–12. doi: 10.1007/s00592-019-01369-8. [DOI] [PubMed] [Google Scholar]
- 39.Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A (2019) Effects of a healthy Nordic diet on weight loss in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Eat Weight Disord 25:1141–1150. 10.1007/s40519-019-00773-x [DOI] [PubMed]
- 40.Sakhaei R, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A. The healthy Nordic dietary pattern has no effect on inflammatory markers: A systematic review and meta-analysis of randomized controlled clinical trials. Nutrition. 2019;58:140–148. doi: 10.1016/j.nut.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed]
- 43.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 151(4):264–269, W264. 10.7326/0003-4819-151-4-200908180-00135 [PMC free article] [PubMed]
- 44.Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions. 2. Chichester, UK: Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritz C, Astrup A, Larsen TM, Hjorth MF. Weight loss at your fingertips: personalized nutrition with fasting glucose and insulin using a novel statistical approach. Eur J Clin Nutr. 2019;73(11):1529–1535. doi: 10.1038/s41430-019-0423-z. [DOI] [PubMed] [Google Scholar]
- 46.Wells GA, Shea B, O’Connell D et al (2014) The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 28 Oct 2019
- 47.Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed]
- 48.Zhang J, Yu KF (1998) What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280(19):1690–1691. 10.1001/jama.280.19.1690 [DOI] [PubMed]
- 49.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 50.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid-Based Healthcare. 2015;13(3):196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 51.Orsini N, Bellocco R, Greenland S. Generalized Least Squares for Trend Estimation of Summarized Dose–response Data. Stata J. 2006;6(1):40–57. doi: 10.1177/1536867x0600600103. [DOI] [Google Scholar]
- 52.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–1596. doi: 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- 54.Roswall N, Eriksson U, Sandin S, et al. Adherence to the healthy Nordic food index, dietary composition, and lifestyle among Swedish women. Food Nutr Res. 2015;59:26336. doi: 10.3402/fnr.v59.26336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 56.Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed]
- 57.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66:140–150. doi: 10.1016/j.jclinepi.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence - Imprecision. J Clin Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence - Indirectness. J Clin Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence - Inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 62.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence - Publication bias. J Clin Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing Summary of Findings tables - Binary outcomes. J Clin Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence - Study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles - Continuous outcomes. J Clin Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Kaminski-Hartenthaler A, Gartlehner G, Kien C et al (2013) GRADE-Leitlinien: 11. Gesamtbeurteilung des Vertrauens in Effektschätzer für einen einzelnen Studienendpunkt und für alle Endpunkte [article in German]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 107:638–645. 10.1016/j.zefq.2013.10.033 [DOI] [PubMed]
- 68.Langendam M, Carrasco-Labra A, Santesso N, et al. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. J Clin Epidemiol. 2016;74:19–27. doi: 10.1016/j.jclinepi.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: Detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28–39. doi: 10.1016/j.jclinepi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Santesso N, Glenton C, Dahm P, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Schünemann HJ, Higgins JPT, Vist GE et al (2019) Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, et al (eds) Cochrane handbook for systematic reviews of interventions, 2nd edn. Wiley, Chichester, UK
- 73.Schünemann HBJ, Guyatt G, Oxman A (2013) GRADE Handbook. Available from https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 20 June 2022
- 74.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187–1197. doi: 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Schwingshackl L, Knüppel S, Schwedhelm C, et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. doi: 10.3945/an.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunge VB, Andersen I, Kyrø C, et al. Adherence to a healthy Nordic food index and risk of myocardial infarction in middle-aged Danes: The diet, cancer and health cohort study. Eur J Clin Nutr. 2017;71:652–658. doi: 10.1038/ejcn.2017.1. [DOI] [PubMed] [Google Scholar]
- 77.Hansen CP, Overvad K, Kyrø C, et al. Adherence to a Healthy Nordic Diet and Risk of Stroke: A Danish Cohort Study. Stroke. 2017;48:259–264. doi: 10.1161/STROKEAHA.116.015019. [DOI] [PubMed] [Google Scholar]
- 78.Hlebowicz J, Drake I, Gullberg B, et al. A High Diet Quality Is Associated with Lower Incidence of Cardiovascular Events in the Malmö Diet and Cancer Cohort. PLoS One. 2013;8:e71095. doi: 10.1371/journal.pone.0071095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roswall N, Sandin S, Löf M, et al. Adherence to the healthy Nordic food index and total and cause-specific mortality among Swedish women. Eur J Epidemiol. 2015;30:509–517. doi: 10.1007/s10654-015-0021-x. [DOI] [PubMed] [Google Scholar]
- 80.Roswall N, Sandin S, Scragg R, et al. No association between adherence to the healthy Nordic food index and cardiovascular disease amongst Swedish women: A cohort study. J Int Med. 2015;278:531–541. doi: 10.1111/joim.12378. [DOI] [PubMed] [Google Scholar]
- 81.Warensjo Lemming E, Byberg L, Wolk A, Michaelsson K. A comparison between two healthy diet scores, the modified Mediterranean diet score and the Healthy Nordic Food Index, in relation to all-cause and cause-specific mortality. Br J Nutr. 2018;119(7):836–846. doi: 10.1017/S0007114518000387. [DOI] [PubMed] [Google Scholar]
- 82.Galbete C, Kroger J, Jannasch F, et al. Nordic diet, Mediterranean diet, and the risk of chronic diseases: the EPIC-Potsdam study. BMC Med. 2018;16(1):99. doi: 10.1186/s12916-018-1082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Puaschitz NG, Assmus J, Strand E et al (2019) Adherence to the Healthy Nordic Food Index and the incidence of acute myocardial infarction and mortality among patients with stable angina pectoris. J Hum Nutr Diet 32(1):86–97. 10.1111/jhn.12592 [DOI] [PubMed]
- 84.Drake I, Gullberg B, Sonestedt E, et al. Scoring models of a diet quality index and the predictive capability of mortality in a population-based cohort of Swedish men and women. Public Health Nutr. 2013;16:468–478. doi: 10.1017/S1368980012002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lassale C, Gunter MJ, Romaguera D, et al. Diet quality scores and prediction of all-cause, cardiovascular and cancer mortality in a pan-european cohort study. PLoS One. 2016;11:e0159025. doi: 10.1371/journal.pone.0159025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ewers B, Marott JL, Schnohr P, Nordestgaard BG, Marckmann P (2020) Non-adherence to established dietary guidelines associated with increased mortality: the Copenhagen General Population Study. Eur J Prev Cardiol: 2047487320937491. 10.1177/2047487320937491 [DOI] [PubMed]
- 87.Kanerva N, Rissanen H, Knekt P, Havulinna AS, Eriksson JG, Mannisto S. The healthy Nordic diet and incidence of Type 2 Diabetes--10-year follow-up. Diabetes Res Clin Pract. 2014;106(2):e34–e37. doi: 10.1016/j.diabres.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 88.Lacoppidan SA, Kyrø C, Loft S, et al. Adherence to a Healthy Nordic Food Index Is Associated with a Lower Risk of Type-2 Diabetes--The Danish Diet, Cancer and Health Cohort Study. Nutrients. 2015;7:8633–8644. doi: 10.3390/nu7105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandalazi E, Drake I, Wirfalt E, Orho-Melander M, Sonestedt E. A High Diet Quality Based on Dietary Recommendations Is Not Associated with Lower Incidence of Type 2 Diabetes in the Malmo Diet and Cancer Cohort. Int J Mol Sci. 2016;17(6):901. doi: 10.3390/ijms17060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tertsunen HM, Hantunen S, Tuomainen TP, Virtanen JK. Healthy Nordic diet and risk of disease death among men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr. 2020;59(8):3545–3553. doi: 10.1007/s00394-020-02188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88(5):1232–1241. doi: 10.3945/ajcn.2007.25695. [DOI] [PubMed] [Google Scholar]
- 92.Gotfredsen JL, Hoppe C, Andersen R et al (2020) Effects of substitution dietary guidelines targeted at prevention of IHD on dietary intake and risk factors in middle-aged Danish adults: the Diet and Prevention of Ischemic Heart Disease: a Translational Approach (DIPI) randomised controlled trial. Br J Nutr: 1–15. 10.1017/S0007114520005164 [DOI] [PubMed]
- 93.Huseinovic E, Bertz F, Leu Agelii M, Hellebo Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr. 2016;104(2):362–370. doi: 10.3945/ajcn.116.135673. [DOI] [PubMed] [Google Scholar]
- 94.Poulsen SK, Due A, Jordy AB, et al. Health effect of the new nordic diet in adults with increased waist circumference: A 6-mo randomized controlled trial. Am J Clin Nutr. 2014;99:35–45. doi: 10.3945/ajcn.113.069393. [DOI] [PubMed] [Google Scholar]
- 95.Uusitupa M, Hermansen K, Savolainen MJ, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome - a randomized study (SYSDIET) J Int Med. 2013;274:52–66. doi: 10.1111/joim.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zurbau A, Au-Yeung F, Blanco Mejia S, et al. Relation of Different Fruit and Vegetable Sources With Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2020;9(19):e017728. doi: 10.1161/JAHA.120.017728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4(8):e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol. 2015;115(5):625–629. doi: 10.1016/j.amjcard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 99.Reynolds AN. Associations of fats and carbohydrates with cardiovascular disease and mortality-PURE and simple? Lancet. 2018;391(10131):1676. doi: 10.1016/S0140-6736(18)30845-6. [DOI] [PubMed] [Google Scholar]
- 100.Bazzano LA, He J, Ogden LG, et al. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med. 2001;161(21):2573–2578. doi: 10.1001/archinte.161.21.2573. [DOI] [PubMed] [Google Scholar]
- 101.Gibbs J, Gaskin E, Ji C, Miller MA, Cappuccio FP. The effect of plant-based dietary patterns on blood pressure: a systematic review and meta-analysis of controlled intervention trials. J Hypertens. 2021;39(1):23–37. doi: 10.1097/HJH.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 102.Sukhato K, Akksilp K, Dellow A, Vathesatogkit P, Anothaisintawee T. Efficacy of different dietary patterns on lowering of blood pressure level: an umbrella review. Am J Clin Nutr. 2020;112(6):1584–1598. doi: 10.1093/ajcn/nqaa252. [DOI] [PubMed] [Google Scholar]
- 103.Viguiliouk E, Kendall CW, Blanco Mejia S, et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;9(7):e103376. doi: 10.1371/journal.pone.0103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008) Adherence to Mediterranean diet and health status: meta-analysis. BMJ (Clinical research ed) 337. 10.1136/bmj.a1344 [DOI] [PMC free article] [PubMed]
- 105.Appel LJ, Moore TJ, Obarzanek E et al (1997) A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 117–124. 10.1016/s0022-1910(97)00049-8 [DOI] [PubMed]
- 106.Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr. 2011;141(4):639–644. doi: 10.3945/jn.110.131375. [DOI] [PubMed] [Google Scholar]
- 107.Kahleova H, Salas-Salvado J, Rahelic D, Kendall CW, Rembert E, Sievenpiper JL. Dietary Patterns and Cardiometabolic Outcomes in Diabetes: A Summary of Systematic Reviews and Meta-Analyses. Nutrients. 2019;11(9):2209. doi: 10.3390/nu11092209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ho HVT, Jovanovski E, Zurbau A, et al. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr. 2017;105(5):1239–1247. doi: 10.3945/ajcn.116.142158. [DOI] [PubMed] [Google Scholar]
- 109.Ho HV, Sievenpiper JL, Zurbau A et al (2016) Erratum: A systematic review and meta-analysis of randomized controlled trials of the effect of barley beta-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reduction(i-iv). Eur J Clin Nutr 70(11):1340. 10.1038/ejcn.2016.129 [DOI] [PubMed]
- 110.Hou Q, Li Y, Li L, et al. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2015;7(12):10369–10387. doi: 10.3390/nu7125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jovanovski E, Khayyat R, Zurbau A, et al. Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results From a Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2019;42(5):755–766. doi: 10.2337/dc18-1126. [DOI] [PubMed] [Google Scholar]
- 112.Heneghan C, Kiely M, Lyons J, Lucey A (2018) The effect of berry-based food interventions on markers of cardiovascular and metabolic health: a systematic review of randomized controlled trials. Mol Nutr Food Res 62(1). 10.1002/mnfr.201700645 [DOI] [PubMed]
- 113.Choo VL, Viguiliouk E, Blanco Mejia S et al (2018) Food sources of fructose-containing sugars and glycaemic control: systematic review and meta-analysis of controlled intervention studies. BMJ 363:k4644. 10.1136/bmj.k4644 [DOI] [PMC free article] [PubMed]
- 114.Blanco Mejia S, Kendall CW, Viguiliouk E, et al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4(7):e004660. doi: 10.1136/bmjopen-2013-004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishi SK, Viguiliouk E, Blanco Mejia S, et al. Are fatty nuts a weighty concern? A systematic review and meta-analysis and dose-response meta-regression of prospective cohorts and randomized controlled trials. Obes Rev. 2021;22(11):e13330. doi: 10.1111/obr.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170(9):821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 117.Li SS, Blanco Mejia S, Lytvyn L, et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2017;6(12):e006659. doi: 10.1161/JAHA.117.006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blanco Mejia S, Messina M, Li SS, et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J Nutr. 2019;149(6):968–981. doi: 10.1093/jn/nxz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ha V, Sievenpiper JL, de Souza RJ, et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. CMAJ. 2014;186(8):E252–E262. doi: 10.1503/cmaj.131727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jayalath VH, de Souza RJ, Sievenpiper JL, et al. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am J Hypertens. 2014;27(1):56–64. doi: 10.1093/ajh/hpt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jenkins DJA, Blanco Mejia S, Chiavaroli L, et al. Cumulative Meta-Analysis of the Soy Effect Over Time. J Am Heart Assoc. 2019;8(13):e012458. doi: 10.1161/JAHA.119.012458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim SJ, de Souza RJ, Choo VL, et al. Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2016;103(5):1213–1223. doi: 10.3945/ajcn.115.124677. [DOI] [PubMed] [Google Scholar]
- 123.Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52(8):1479–1495. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 124.Viguiliouk E, Blanco Mejia S, Kendall CW, Sievenpiper JL. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann N Y Acad Sci. 2017;1392(1):43–57. doi: 10.1111/nyas.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Viguiliouk E, Stewart SE, Jayalath VH, et al. Effect of Replacing Animal Protein with Plant Protein on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2015;7(12):9804–9824. doi: 10.3390/nu7125509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viguiliouk E, Kendall CW, Blanco Mejia S, et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;9(7):e103376. doi: 10.1371/journal.pone.0103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson TJ, Gregoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32(11):1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 128.Diabetes Canada Clinical Practice Guidelines Expert Committee, Lipscombe L, Butalia S et al (2020) Pharmacologic Glycemic Management of Type 2 Diabetes in Adults: 2020 Update. Can J Diabetes 44(7):575–591. 10.1016/j.jcjd.2020.08.001 [DOI] [PubMed]
- 129.Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL. Nutrition therapy. Can J Diabetes. 2018;42:S64–S79. doi: 10.1016/j.jcjd.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 130.Krznarić Ž, Karas I, Kelečić DL, Bender DV (2021) The Mediterranean and Nordic diet: a review of differences and similarities of two sustainable, health-promoting dietary patterns. Front Nutr 8. 10.3389/fnut.2021.683678 [DOI] [PMC free article] [PubMed]
- 131.Hemler EC, Hu FB. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr Atheroscler Rep. 2019;21(5):18. doi: 10.1007/s11883-019-0779-5. [DOI] [PubMed] [Google Scholar]
- 132.Mudryj AN, Aukema HM, Yu N. Intake patterns and dietary associations of soya protein consumption in adults and children in the Canadian Community Health Survey, Cycle 2.2. Br J Nutr. 2015;113(2):299–309. doi: 10.1017/S0007114514003638. [DOI] [PubMed] [Google Scholar]
- 133.Klingberg S, Andersson H, Mulligan A, et al. Food sources of plant sterols in the EPIC Norfolk population. Eur J Clin Nutr. 2008;62(6):695–703. doi: 10.1038/sj.ejcn.1602765. [DOI] [PubMed] [Google Scholar]
- 134.Klingberg S, Ellegård L, Johansson I, et al. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr. 2008;87(4):993–1001. doi: 10.1093/ajcn/87.4.993. [DOI] [PubMed] [Google Scholar]
- 135.Jenkins DJ, Kendall CW, Popovich DG, et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metab Clin Exp. 2001;50(4):494–503. doi: 10.1053/meta.2001.21037. [DOI] [PubMed] [Google Scholar]
- 136.Bere E, Brug J. Towards health-promoting and environmentally friendly regional diets–a Nordic example. Public Health Nutr. 2009;12(1):91–96. doi: 10.1017/S1368980008001985. [DOI] [PubMed] [Google Scholar]
- 137.World Health Organization (2021) Making diets environmentally friendly: Nordic countries lead the way. Available from https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/news/news/2021/10/making-diets-environmentally-friendly-nordic-countries-lead-the-way. Accessed 20 June 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 991 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its ESM information files).