Abstract
Leukocytes activated by endotoxin or enterotoxins release proinflammatory cytokines, thereby contributing to the cascade of events leading to septic shock. In the present studies, we analyzed the effects of in vivo administration of a soluble immunomodulator, β-(1,6)-branched β-(1,3)-glucan (soluble β-glucan), on toxin-stimulated cytokine production in monocytes and lymphocytes isolated from treated mice. In vitro stimulation of lymphocytes isolated from soluble β-glucan-treated mice with lipopolysaccharide (LPS) resulted in enhanced production of interleukin-6 (IL-6) and suppressed production of tumor necrosis factor alpha (TNF-α), while stimulation of these cells with staphylococcal enterotoxin B (SEB) or toxic shock syndrome toxin 1 (TSST-1) resulted in enhanced production of gamma interferon (IFN-γ) and suppressed production of IL-2 and TNF-α compared to that in cells isolated from untreated mice. In vitro stimulation of monocytes isolated from soluble β-glucan-treated mice with LPS also resulted in suppressed TNF-α production, while stimulation of these cells with SEB or TSST-1 resulted in suppressed IL-6 and TNF-α production compared to that in cells isolated from untreated mice. Thus, the overall cytokine pattern of leukocytes from soluble β-glucan-treated mice reflects suppressed production of proinflammatory cytokines, especially TNF-α. Taken together, our results suggest that treatment with soluble β-glucan can modulate the induction cytokines during sepsis, resulting in an overall decrease in host mortality.
Sepsis caused by gram-negative bacilli or gram-positive cocci represents a major source of morbidity and mortality in medical facilities today (17, 34, 53, 71). The reasons for the high incidence of bacterial sepsis are probably related to several key factors. First, increased bacterial virulence and drug resistance have complicated treatment and led to problems in tracking disease-causing pathogens (50, 69). Second, the host defense capacity of many patients has been compromised by the increased use of immunosuppressive therapies (33, 35, 38). Finally, the incidence of opportunistic infections has grown rapidly due to the worldwide AIDS epidemic (33, 38, 60). To combat the problems of drug resistance, a significant amount of research has focused on the development of antibiotics that counteract resistance (50, 69). However, it will be some time before these agents become available, and, as has occurred in the past, the introduction of these agents may eventually lead to the emergence of resistant pathogens (29, 50). Thus, there is clearly a need to develop and characterize alternative anti-infective substances as adjuvants to classical antibiotic therapies.
One of the most promising recent alternatives to classical antibiotic treatment is the use of immunomodulators for enhancing host defense responses (5–7). Several types of immunomodulators have been identified, including mammalian proteins such as gamma interferon (IFN-γ) (22, 48), granulocyte colony-stimulating factor (49), and granulocyte-macrophage colony-stimulating factor (4, 45, 49), as well as substances isolated and purified from microorganisms (12, 14, 31, 37, 59). The latter type of immunomodulators typically induces nonspecific stimulation of the immune system. For example, β-(1,3)-glucans purified from fungi and yeast have been shown to have broad anti-infective activities (reviewed in references 11 and 80). These polysaccharide compounds have been shown to bind to receptors on leukocytes and stimulate a number of immune responses, such as cytokine release (1, 19, 57), generation of reactive oxygen species (27, 62), generation of nitric oxide (61), and release of arachidonic acid metabolites (15, 16, 19, 56). However, because of the poor solubility and direct leukocyte-activating action of β-(1,3)-glucans, these compounds have limited clinical usefulness.
Recently, several soluble derivatives of β-(1,3)-glucan that show potent immunomodulatory activity have been developed. Aminated β-(1,3)-glucan has been shown to induce resistance to bacterial infection (67) and cause regression of solid tumors in mice (66). In addition, a combination of IFN-γ with aminated β-(1,3)-glucan resulted in better inhibition of the growth of mouse liver metastases than did either of these agents alone (73). Soluble β-(1,6)-branched β-(1,3)-glucan (soluble β-glucan) has also been shown to enhance microbicidal activities of neutrophils and macrophages but, in contrast to aminated β-(1,3)-glucan (19), has been reported to have no direct activating effect on neutrophil or monocyte functions (11, 55). Rather, soluble β-glucan appears to prime leukocytes for an enhanced host defense response when they are exposed to a secondary stimulus, such as phorbol myristate acetate, N-formylated chemotactic peptide, or opsonized bacteria (11, 13, 51, 76, 79). Thus, soluble β-glucan represents a potentially useful immunomodulator, and it has been shown to reduce postoperative infection rates and reduce the length of hospitalization in clinical trials (5, 6).
Although glucan-derived substances have been extensively studied as immunomodulators for treatment of a number of bacterial, fungal, parasitic, and viral infections (reviewed in reference 11), less is known about how these substances exert their biological effects. Aminated β-(1,3)-glucan has been shown to stimulate the production of interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and prostaglandin E2 by human monocytes (19) and murine macrophages (68). Soluble β-glucan has been shown to prime the oxidative burst and microbicidal activity of human neutrophils (42, 77) and appears to bind to a unique β-glucan receptor that recognizes soluble β-glucan in these cells (77, 78). Soluble β-glucan has also been shown to increase total leukocyte numbers, enhance the clearance of bacteria from the blood, and reduce mortality in rat sepsis models (13, 51, 76). In more recent studies, Liang et al. (40) showed that soluble β-glucan enhanced the clearance of a multidrug-resistant strain of Staphylococcus aureus in a rat intra-abdominal sepsis model and found that this effect was accompanied by increased leukocyte numbers in blood and enhancement of the oxidative burst.
Because of the potential usefulness of β-(1,3)-glucan derivatives as immunomodulators, it is of interest to further characterize the mechanism(s) whereby soluble β-glucan modulates the host defense response. Therefore, we analyzed the effects of in vivo administration of soluble β-glucan on in vitro cytokine production by endotoxin- and enterotoxin-stimulated lymphocytes and monocytes. Production of the most important cytokines involved in the pathogenesis of septic shock (IL-2, IL-6, IFN-γ, and TNF-α) in cells isolated from untreated and soluble β-glucan-treated mice was analyzed. In addition, the level of apoptosis in lymphocytes and monocytes isolated from these mice was examined. Our results show that toxin-stimulated cytokine release and leukocyte apoptosis can be modulated by in vivo administration of soluble β-glucan in a mouse model.
MATERIALS AND METHODS
Animals.
Pathogen-free female BALB/cby inbred mice, aged 6 to 8 weeks, were obtained from the Animal Resource Center, Montana State University. All the mice were housed in accordance with approved guidelines and were provided with food and water ad libitum. All animal use was approved by the Montana State University Animal Care and Use Committee.
Drug treatment.
For each experiment, soluble β-glucan (Alpha-Beta Technology, Worcester, Mass.) was administered intramuscularly to 10 mice at a dose of 1 mg/kg of body weight. An equal number of mice served as controls and received no injection. The cells from each group of mice were then isolated, pooled, and analyzed as described below. Three independent experiments were performed to obtain the data presented.
Leukocyte isolation.
Spleen lymphocytes and bone marrow monocytes from both experimental groups were isolated aseptically 24 h after in vivo soluble β-glucan administration. To isolate spleen lymphocytes, the spleens were stripped of fat and cut into small pieces. Single-cell suspensions were made by pressing spleen pieces through 70-μm-mesh cell strainers (Becton Dickinson) into RPMI 1640 (Cellgro). After removal of erythrocytes by hypotonic lysis, the cell suspension was depleted of macrophages and monocytes by adherence to plastic tissue culture dishes for 40 min at 37°C. Purified cell suspensions were washed three times in complete RPMI 1640 supplemented with 10% fetal calf serum and used for in vitro cytokine and apoptosis experiments. Analysis of the isolated cells by light microscopy and by flow cytometry showed that >95% of the cells were lymphocytes.
Bone marrow monocytes were harvested by washing mouse femurs with Dulbecco’s phosphate-buffered saline by standard methods (21). The cells were then centrifuged and resuspended in RPMI 1640. The mononuclear cells were separated by density gradient centrifugation on Histopaque gradients (18, 21). Purified cell suspensions were washed three times in complete RPMI 1640 supplemented with 10% fetal calf serum and used for in vitro cytokine and apoptosis experiments. Analysis of the isolated cells by flow cytometry with lineage-specific antibodies showed that >93% of the cells were monocytes while the remaining cells were lymphocytes.
The viability of the cells used throughout was ≥95%. Note that the reagents and labware used in all experiments were lipopolysaccharide (LPS) free.
Measurement of cytokine production in vitro.
Aseptically isolated lymphocytes and monocytes were diluted at 5 × 106 cells/ml in complete RPMI 1640 and incubated separately with 10 μg of soluble β-glucan per ml, Toxic shock syndrome toxin 1 (TSST-1) (10 μg/ml; Toxin Technologies, Sarasota, Fla.), staphylococcal enterotoxin B (SEB) (10 μg/ml; Toxin Technologies), or LPS from E. coli K-235 (10 μg/ml; Sigma Chemical Co., St. Louis, Mo.) for the indicated times at 37°C under 5% CO2. After incubation, the cells were removed by centrifugation and the collected supernatants were stored at −20°C until analyzed.
Cytokine levels in culture supernatants were determined by a standard sandwich enzyme-linked immunosorbent assay technique (2). Briefly, Nunc MaxiSorp (Nalge Nunc International, Roskilde, Denmark) plates were coated for 12 h at 4°C with rat anti-murine IL-2, IL-6, IFN-γ, or TNF-α monoclonal antibodies (PharMingen, San Diego, Calif.), the plates were blocked, supernatant samples were added, and the plates were incubated overnight at 4°C. The plates were then washed and incubated for 90 min at room temperature with biotin-conjugated rat anti-murine IL-2, IL-6, TNF-α, or IFN-γ monoclonal antibodies followed by an alkaline phosphatase-conjugated goat anti-biotin monoclonal antibody (Vector Laboratories, Inc., Burlingame, Calif.). The fluorescent substrate for alkaline phosphatase, 4-methylumbelliferyl phosphate dicyclohexylammonium salt (Molecular Probes, Eugene, Oreg.), was used to develop the assay, and fluorescence was measured with a Bio-Tek Instruments FL 500 microtiter plate reader, using excitation and emission wavelengths of 360 and 460 nm, respectively. To quantify the amount of cytokine present in test samples, values were extrapolated from standard curves established by analyzing different dilutions of recombinant murine IL-2, IL-6, IFN-γ, and TNF-α. The values shown represent the mean ± standard error of the mean (SEM) of three independent experiments (triplicate samples in each experiment). The detection limits for IL-2, IL-6, IFN-γ, and TNF-α were 200, 200, 200, and 30 pg/ml, respectively.
Detection of apoptotic cells.
Apoptosis was analyzed with an APO DIRECT kit (Pharmingen), which is based on a modification of the TUNEL method (28, 30). Briefly, lymphocyte and monocyte samples, diluted at 106 cells/100 μl, were stained for fragmentation of the genomic DNA by terminal deoxynucleotidyltransferase-mediated nick end labeling (TUNEL) with fluorescein isothiocyanate-conjugated dUTP, and the cells were analyzed by flow cytometry. All samples were analyzed in duplicate.
Statistical analysis.
Unless otherwise indicated, the results are expressed as the mean ± SEM of data obtained from triplicate experiments. Statistical analysis was performed by a paired Student t test. Differences at P < 0.05 were considered statistically significant.
RESULTS
Endotoxin-induced cytokine release.
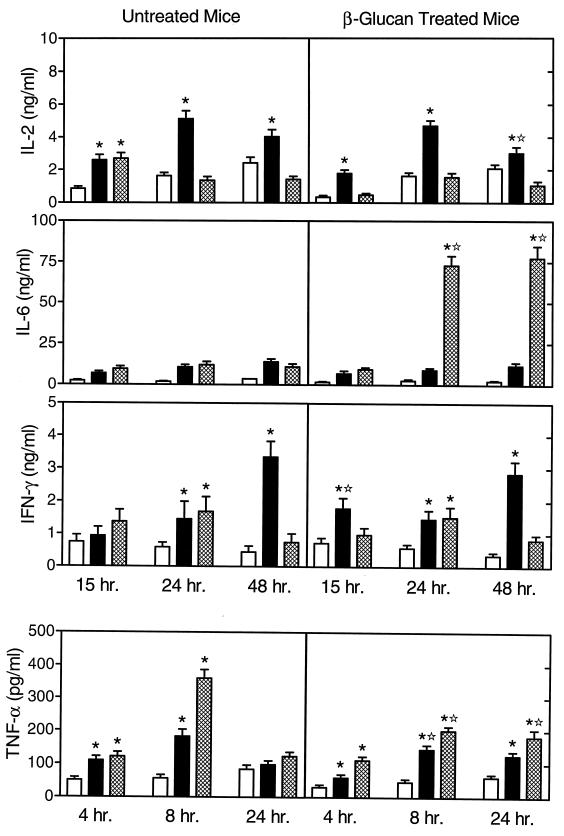
To investigate the effects of in vivo soluble β-glucan treatment on lymphocyte cytokine production in response to endotoxin or soluble β-glucan in vitro, we treated mice with soluble β-glucan and isolated spleen lymphocytes as described in Materials and Methods. As shown in Fig. 1, lymphocytes isolated from untreated and soluble β-glucan-treated mice exhibited some significant differences in cytokine production.
FIG. 1.
Endotoxin-stimulated cytokine production by lymphocytes isolated from untreated and soluble β-glucan-treated mice. Spleen lymphocytes were stimulated in vitro for the indicated times with 10 μg of soluble β-glucan (solid bars) or LPS (hatched bars) per ml. Control cells (open bars) were incubated in culture medium alone. The cytokine levels in the culture supernatants were measured by enzyme-linked immunosorbent assay with paired cytokine-specific antibodies, and the amounts of cytokine are expressed in nanograms or picograms per milliliter, standardized against mouse recombinant cytokines. The results represent the mean ± SEM of three independent experiments. ∗, statistically significant values (P < 0.05) compared to the control. ✫, statistically significant values (P < 0.05) compared to cells isolated from untreated mice.
Primary in vitro stimulation of lymphocytes with soluble β-glucan or LPS resulted in a modest but significantly higher release of IL-2 at 15 h compared with that from controls, whereas lymphocytes isolated from soluble β-glucan-treated mice produced increased levels of IL-2 only after soluble β-glucan stimulation but not after LPS stimulation. At 24 and 48 h, spleen lymphocytes from both untreated and soluble β-glucan-treated mice produced significantly higher levels of IL-2 only in response to in vitro stimulation by soluble β-glucan. Overall, the pattern of IL-2 production was similar for cells isolated from untreated and soluble β-glucan-treated mice.
The levels of IL-6 production by soluble β-glucan- or LPS-stimulated lymphocytes isolated from untreated mice were not significantly greater than those in control cells (Fig. 1). However, in lymphocytes isolated from soluble β-glucan-treated mice, LPS stimulation in vitro resulted in a large increase in IL-6 production in cell supernatants from 24- and 48-h incubations.
In lymphocytes isolated from untreated and soluble β-glucan-treated mice, soluble β-glucan and LPS both stimulated an increase in IFN-γ production at 24 h (Fig. 1). At 48 h, soluble β-glucan stimulated even higher levels of IFN-γ production while the levels of IFN-γ produced by LPS-stimulated cells had decreased to baseline. Overall, the pattern of IFN-γ production in cells isolated from soluble β-glucan-treated mice was similar to that observed in cells from untreated mice.
Lymphocytes isolated from untreated and soluble β-glucan-treated mice produced significant levels of TNF-α at 4 and 8 h in response to stimulation with either soluble β-glucan or LPS, although the response to LPS was, in general, higher than that to soluble β-glucan (Fig. 1). However, at 24 h, TNF-α production by cells from untreated mice stimulated with either agent had returned to background levels. Interestingly, in cells isolated from soluble β-glucan-treated mice, the peak level of stimulated TNF-α production at 8 h was significantly lower than in comparable samples from untreated mice (Fig. 1). In addition, cells from soluble β-glucan-treated mice were still producing a significant amount of TNF-α at 24 h of LPS stimulation while lymphocytes from untreated mice produced only minimal levels of TNF-α at 24 h. Thus, in vivo treatment with soluble β-glucan not only suppresses the magnitude of lymphocyte TNF-α production but also may act to prolong the production of low levels of this cytokine.
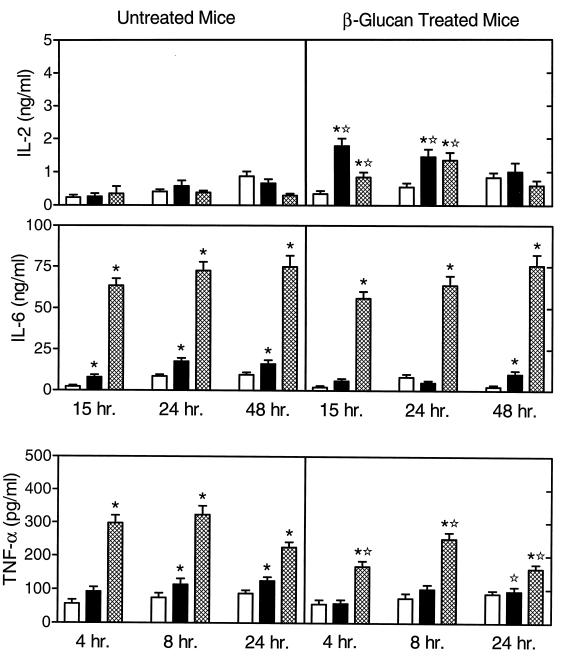
We also analyzed the effects of in vivo soluble β-glucan treatment on monocyte cytokine profiles in response to in vitro stimulation. As shown in Fig. 2, monocytes isolated from soluble β-glucan-treated mice produced increased levels of IL-2 after soluble β-glucan or LPS stimulation for 15 and 24 h, compared to cells from untreated mice. However, the levels of IL-2 measured here were very low (10 times lower than those produced by spleen lymphocytes) and may represent IL-2 produced by residual T cells (normally 5 to 10% of the cells) in our monocyte preparation.
FIG. 2.
Endotoxin-stimulated cytokine production by monocytes isolated from untreated and soluble β-glucan-treated mice. Bone marrow monocytes were stimulated in vitro for the indicated times with 10 μg of soluble β-glucan (solid bars) or LPS (hatched bars) per ml. Control cells (open bars) were incubated in culture medium alone. The cytokine levels in the culture supernatants were measured by ELISA with paired cytokine-specific antibodies, and the amounts of cytokine are expressed in nanograms or picograms per milliliter standardized against mouse recombinant cytokines. The results represent the mean ± SEM of three independent experiments. ∗, statistically significant values (P < 0.05) compared to the control; ✫, statistically significant values (P < 0.05) compared to cells isolated from untreated mice.
Monocytes isolated from untreated mice produced increased levels of IL-6 when stimulated in vitro with soluble β-glucan or LPS, although the response to LPS was three- to fourfold higher. In contrast, monocytes isolated from soluble β-glucan-treated mice produced high levels of IL-6 after in vitro stimulation with LPS but not after stimulation with soluble β-glucan (Fig. 2). Overall, the pattern of IL-6 production in cells isolated from soluble β-glucan-treated mice was similar to that observed in cells from untreated mice.
As shown in Fig. 2, monocytes isolated from both untreated and soluble β-glucan-treated mice produced significant levels of TNF-α after 4, 8, and 24 h of stimulation with LPS, although the levels produced by cells from soluble β-glucan-treated mice were significantly lower than in comparable cells from untreated mice. Soluble β-glucan stimulation of monocytes from untreated mice for 8 and 24 h resulted in the production of low levels of TNF-α, whereas no TNF-α was produced in response to in vitro stimulation by soluble β-glucan. Consistent with our lymphocyte analyses (Fig. 1), these results show that in vivo treatment with soluble β-glucan also suppresses the magnitude of endotoxin-stimulated TNF-α production in monocytes.
Enterotoxin-induced cytokine release.
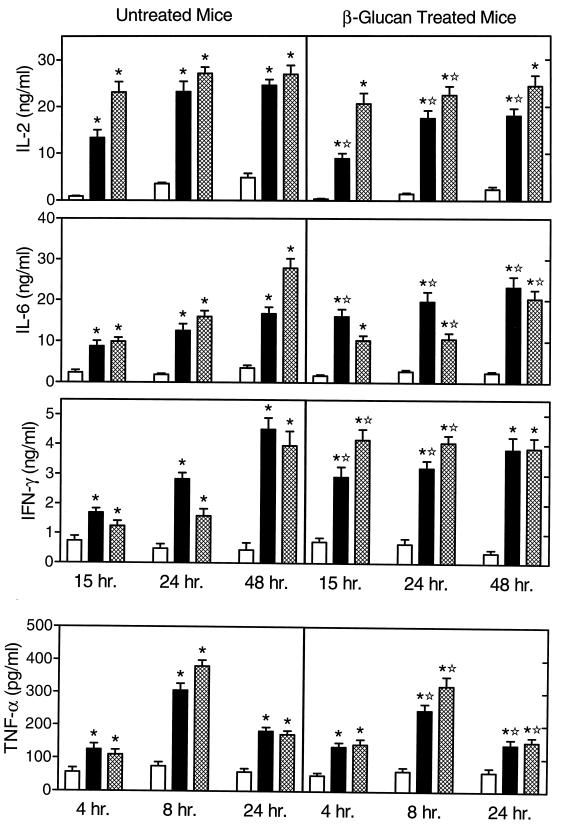
Enterotoxins (also known as superantigens) from gram-positive bacteria play an important role in septic shock by activating T cells, resulting in the release of inflammatory cytokines (46, 47, 72). Therefore, we also analyzed the effects of in vivo soluble β-glucan treatment on lymphocyte cytokine profiles in response to enterotoxins, such as SEB and TSST-1. As shown in Fig. 3, lymphocytes isolated from untreated and soluble β-glucan treated mice also exhibited some important differences in cytokine production, and the most significant changes were associated with IL-2, IFN-γ, and TNF-α. Lymphocytes isolated from untreated mice released significant amounts of IL-2 after stimulation with SEB or TSST-1 (Fig. 3). In contrast, in vitro stimulation of cells isolated from soluble β-glucan-treated mice with SEB or TSST-1 resulted in a modest but significantly decreased production of IL-2 (Fig. 3).
FIG. 3.
Enterotoxin-stimulated cytokine production by lymphocytes isolated from untreated and soluble β-glucan-treated mice. Spleen lymphocytes were stimulated in vitro for the indicated times with 10 μg of SEB (solid bars) or TSST-1 (hatched bars) per ml. Control cells (open bars) were incubated in culture medium alone. The cytokine levels in the culture supernatants were measured by ELISA with paired cytokine-specific antibodies, and the amounts of cytokine are expressed in nanograms or picograms per milliliter, standardized against mouse recombinant cytokines. The results represent the mean ± SEM of three independent experiments. ∗, statistically significant values (P < 0.05) compared to the control; ✫, statistically significant values (P < 0.05) compared to cells isolated from untreated mice.
In lymphocytes isolated from untreated and soluble β-glucan-treated mice, SEB and TSST-1 both stimulated IL-6 production at 15, 24, and 48 h. Although the responses to SEB and TSST-1 were significantly different at 24 and 48 h in cells isolated from the treated mice (Fig. 3), the overall pattern of IL-6 production was fairly similar between cells isolated from soluble β-glucan-treated and untreated mice.
Lymphocytes isolated from untreated mice also produced significant levels of IFN-γ when stimulated with SEB or TSST-1. In contrast to the IL-2 response, however, cells from soluble β-glucan-treated mice exhibited enhanced IFN-γ production at 15 and 24 h compared to the production after similar incubation times for cells from untreated mice (Fig. 3). At 48 h, similar levels of IFN-γ were produced by cells from both treated and untreated mice.
Lymphocytes isolated from untreated and soluble β-glucan-treated mice produced significant levels of TNF-α at 4, 8, and 24 h (with a peak at 8 h) in response to stimulation with either SEB or TSST-1, although the responses were significantly lower in cells from soluble β-glucan-treated mice than in cells from untreated mice (Fig. 3). Again, these results demonstrate that in vivo treatment with soluble β-glucan suppresses the magnitude of toxin-stimulated lymphocyte TNF-α production.
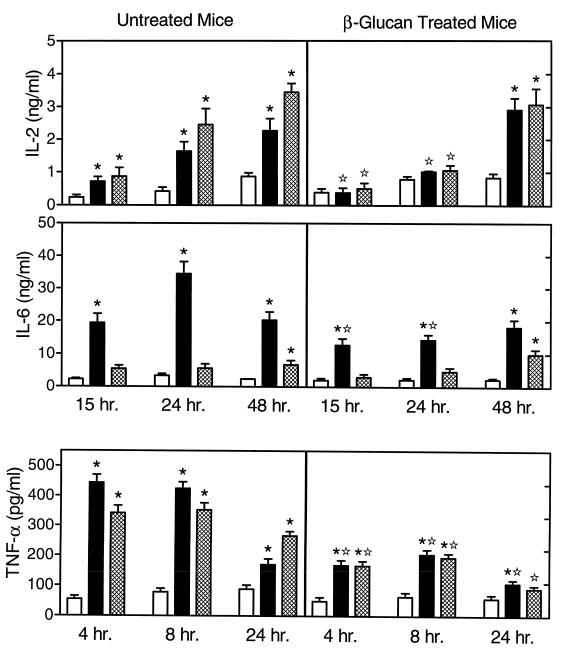
We also analyzed the effects of in vivo soluble β-glucan treatment on monocyte cytokine profiles in response to enterotoxin. As shown in Fig. 4, monocytes isolated from untreated mice produced higher levels of IL-2 than did control cells at 15, 24, and 48 h after stimulation with SEB or TSST-1. In contrast, the response of monocytes isolated from soluble β-glucan-treated mice was significantly lower at 15 and 24 h (no significant increase over controls) and significant levels of IL-2 were observed only after 48 h of incubation with the toxins. Again, the levels of IL-2 measured here were very low and may represent IL-2 produced by residual T cells in our monocyte preparation.
FIG. 4.
Enterotoxin-stimulated cytokine production by monocytes isolated from untreated and soluble β-glucan-treated mice. Bone marrow monocytes were stimulated in vitro for the indicated times with 10 μg of SEB (solid bars) or TSST-1 (hatched bars) per ml. Control cells (open bars) were incubated in culture medium alone. The cytokine levels in the culture supernatants were measured by ELISA with paired cytokine-specific antibodies, and the amounts of cytokine are expressed in nanograms or picograms per milliliter, standardized against mouse recombinant cytokines. The results represent the mean ± SEM of three independent experiments. ∗, statistically significant values (P < 0.05) compared to the control; ✫, statistically significant values (P < 0.05) compared to cells isolated from untreated mice.
Both monocytes from untreated and soluble β-glucan-treated mice produced IL-6 after stimulation with SEB; however, the responses were, in general, significantly lower in cells isolated from the treated mice (Fig. 4).
As shown in Fig. 4, monocytes isolated from both untreated and soluble β-glucan-treated mice produced significant levels of TNF-α after stimulation with SEB or TSST-1, although the levels produced by cells isolated from soluble β-glucan-treated mice were significantly lower at all time points than were those produced by comparable cells from untreated mice. Again, these results show that in vivo treatment with soluble β-glucan suppresses the magnitude of stimulated TNF-α production in monocytes.
Apoptosis.
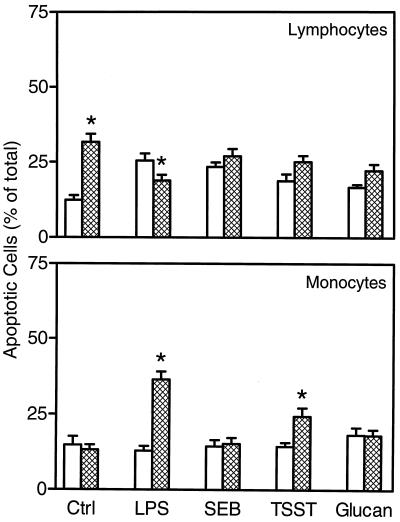
Spleen lymphocytes isolated from untreated mice and stimulated in vitro with LPS, SEB, TSST-1, or soluble β-glucan showed slight increases in the percentage of apoptotic cells at 12 h (Fig. 5) and 24 h (results not shown) compared to control unstimulated cell populations. In general, lymphocytes isolated from soluble β-glucan-treated mice showed a higher level of apoptosis than did cells isolated from untreated mice, especially in control cell populations (Fig. 5). One key exception was in response to LPS, where a significantly lower level of apoptosis was observed in cells isolated from soluble β-glucan-treated mice. We observed a similar pattern of responses after 24 h of incubation, although the overall level of apoptosis was increased (data not shown).
FIG. 5.
Analysis of monocyte and lymphocyte apoptosis in cells isolated from untreated and soluble β-glucan-treated mice. Toxin-stimulated apoptosis was analyzed in lymphocytes and monocytes from untreated (open bars) and soluble β-glucan-treated (hatched bars) mice at 12 h after isolation. The results are expressed as the percentage of total cells and represent the mean ± SEM of two independent experiments. ∗, statistically significant values (P < 0.05) compared to cells isolated from untreated mice.
Analysis of monocytes isolated from untreated mice showed a slightly lower overall level of apoptosis at 12 h (Fig. 5) and 24 h (results not shown) compared to lymphocytes isolated from the same mice, and none of the agents tested induced an increase in the level of apoptosis (Fig. 5). In contrast, LPS and TSST-1 were able to induce increased levels of apoptosis in monocytes isolated from soluble β-glucan-treated mice (Fig. 5). Again, we observed a similar pattern of responses after 24 h of incubation, although the overall level of apoptosis was increased (data not shown).
DISCUSSION
The mechanism(s) of modulation of the immune response by immunomodulators, such as soluble β-glucan, is not well understood. In previous studies, soluble β-glucan has been shown to enhance host defense mechanisms in animal infection models (13, 39, 40, 76) as well as in humans (5, 6). Considering the key role that leukocyte cytokine production plays in the pathogenesis of septic shock (76), we have examined how in vivo administration of soluble β-glucan could modulate in vitro primary and secondary cytokine responses in endotoxin- and enterotoxin-treated cells.
Primary stimulation of lymphocytes and monocytes isolated from untreated mice with LPS, SEB, or TSST-1 resulted in cytokine profiles characteristic for the induction of septic shock (24, 34, 47); i.e., these cells released high levels of all of the most important proinflammatory cytokines. In contrast, lymphocytes and monocytes isolated from mice treated in vivo with soluble β-glucan produced a significantly different cytokine response, which was generally characterized by enhanced IFN-γ production and suppressed production of TNF-α, suggesting that immunomodulation with soluble β-glucan might act to depress the inflammatory cytokine response. Interestingly, we observed that soluble β-glucan itself stimulated modest cytokine production by leukocytes isolated from untreated and soluble β-glucan-treated mice, while others have reported previously that some forms of soluble β-glucan did not induce cytokine production in vitro by human leukocytes (55, 77) or cultured murine BMC2.3 cells (3). In addition, Liang et al. (40) reported that soluble β-glucan did not induce the production of IL-1β or TNF-α in vivo in soluble β-glucan-treated rats. Possible explanations for these differences are related to the type of cells analyzed or differences in assay sensitivity; however, further studies are necessary to investigate this issue.
Currently, several experimental approaches are used in the treatment of bacterial sepsis (53). For example, antibodies against CD14, LPS-binding protein, or LPS itself have been used for the treatment of endotoxemia (41, 44, 75). Other reported treatments involve the use of soluble receptors (23) or monoclonal antibodies (25, 54, 63) against TNF-α for treatment of septic shock. In the present studies, we show that in vivo administration of soluble β-glucan suppressed TNF-α production and elevated IFN-γ production; these changes may play an important protective role in the outcome of septic shock. This idea is further supported by studies by Barton and Jackson (9), who found that pretreatment with an antibody to TNF-α protected mice from LPS-induced septic shock and that mortality was reduced even more by treatment with a combination of recombinant IL-6 and a low dose of the anti-TNF-α antibody. Thus, based on our present studies, we conclude that stimulation of the reticuloendothelial system by soluble β-glucans (11) acts, in part, to modulate the production of proinflammatory and anti-inflammatory cytokines during sepsis, resulting in an overall decrease in host mortality (13, 76).
Regulation of apoptosis has also been proposed to play an important role in the modulation of the inflammatory response (64, 65, 70). It appears, however, that the level of apoptosis is regulated by a complex interplay of cytokines encountered by the cell (43, 81). For example, the major outcome of exposure to endotoxin or enterotoxins is the production of a cascade of proinflammatory cytokines (24, 47), where TNF-α can regulate the production of IL-1β and IL-6 (10, 26), and both TNF-α and IL-6 play key roles in the modulation of apoptosis (81). Cytokines not only modulate apoptosis but also can enhance the capacity of the macrophage to ingest apoptotic cells. For example, the proinflammatory cytokines granulocyte-macrophage colony-stimulating factor, IL-1β, TNF-α, and IFN-γ can dramatically upregulate the capacity of human monocyte-derived macrophages to phagocytose apoptotic neutrophils (58, 74). In the present studies, we found that in vivo application of soluble β-glucan enhanced spontaneous lymphocyte apoptosis (Fig. 5). Thus, part of the anti-inflammatory effects of soluble β-glucan treatment in vivo might result from the enhanced apoptosis of a portion of the activated lymphocyte population (64, 65, 70). In support of this idea, Jimenez et al. (36) recently reported that delayed apoptosis contributed to postoperative systemic inflammatory response syndrome. Clearly, further studies are necessary to determine if soluble β-glucan interacts with specific lymphocyte subpopulations and if soluble β-glucan-induced apoptosis plays a physiologically important role in resolving inflammatory disease. In contrast to lymphocytes, there was no evidence for increased spontaneous apoptosis of monocytes. However, stimulation of monocytes isolated from soluble β-glucan-treated mice with LPS or TSST-1 showed a significantly increased level of apoptosis. These results suggest a possible role of soluble β-glucan in enhancing the removal of inflammatory cells from the sites of inflammation. Clearly, the balance between phagocyte apoptosis and necrosis in inflamed tissues seems to play an important role in the resolution and/or control of inflammation (32, 36, 65).
In summary, our results demonstrate that cytokine release induced by toxin stimulation of target cells can be manipulated by in vivo administration of soluble β-glucan in a mouse model. A comparison of the cytokine profiles of lymphocytes and monocytes isolated from soluble β-glucan-treated mice, compared to cells from untreated mice, is summarized in Table 1. In general, the results show a suppressed production of proinflammatory cytokines. Previous studies have demonstrated that part of the anti-inflammatory activity of glucocorticosteroids results from modulation of proinflammatory cytokine levels (8, 20); however, glucocorticosteroids also have serious side effects on the body. Thus, it is possible that the use of immunomodulators, such as soluble β-glucan, to manipulate the production of proinflammatory cytokines by activated lymphocytes and monocytes will represent a safer alternative for treating sepsis. These results contribute to the possible practical application of glucan-derived substances in reducing the severity of septic shock, especially in situations where application of anti-cytokine treatment may exacerbate systemic infection or worsen the outcome in a patient with sepsis (52).
TABLE 1.
Summary of endotoxin- and enterotoxin-stimulated cytokine responses of leukocytes isolated from soluble β-glucan-treated mice and untreated mice
| Cell type and cytokine | Cytokine response to stimulation by:
|
|
|---|---|---|
| Endotoxin | Enterotoxin | |
| Lymphocytes | ||
| IL-2 | No major change | Decreased levels |
| IL-6 | Increased levels | Decreased levels |
| IFN-γ | No major change | Increased levels |
| TNF-α | Decreased levels | Decreased levels |
| Monocytes | ||
| IL-2 | Increased levels | Decreased levels |
| IL-6 | No major change | Decreased levels |
| TNF-α | Decreased levels | Decreased levels |
ACKNOWLEDGMENTS
This work was supported in part by USDA/NRICGP grant 9502274, an Arthritis Foundation biomedical science grant, NIH grant S10 RR11877, NSF equipment grant DBI-9604797, a grant from the M. J. Murdock Charitable Trust, USDA Animal Health Formula Funds, the Montana State University Agricultural Experimental Station, and a grant from the Slovak Science Grant Agency VEGA (2-5012-98). M.T.Q. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Abel G, Czop J K. Stimulation of human monocyte beta-glucan receptors by glucan particles induces production of TNF-alpha and IL-1 beta. Int J Immunopharmacol. 1992;14:1363–1373. doi: 10.1016/0192-0561(92)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Abrams J S. Immunoenzymetric assay of mouse and human cytokines using NIP-labeled anti-cytokine antibodies. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 6.20.1–6.20.11. [DOI] [PubMed] [Google Scholar]
- 3.Adams D S, Pero S C, Petro J B, Nathans R, Mackin W M, Wakshull E. PGG-glucan activates NF-κB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J Leukocyte Biol. 1997;62:865–873. doi: 10.1002/jlb.62.6.865. [DOI] [PubMed] [Google Scholar]
- 4.Aviles A, Guzman R, Garcia E L, Talavera A, Diaz-Maqueo J C. Results of a randomized trial of granulocyte colony-stimulating factor in patients with infection and severe granulocytopenia. Anticancer Drugs. 1996;7:392–397. doi: 10.1097/00001813-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Babineau T J, Hackford A, Kenler A, Bistrian B, Forse R A, Fairchild P G, Heard S, Keroack M, Caushaj P, Benotti P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994;129:1204–1210. doi: 10.1001/archsurg.1994.01420350102014. [DOI] [PubMed] [Google Scholar]
- 6.Babineau T J, Marcello P, Swails W, Kenler A, Bistrian B, Forse R A. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994;220:601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballow M, Nelson R. Immunopharmacology: immunomodulation and immunotherapy. JAMA. 1997;278:2008–2017. doi: 10.1001/jama.278.22.2008. [DOI] [PubMed] [Google Scholar]
- 8.Barnes P J, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 9.Barton B E, Jackson J V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beutler B, van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 11.Bleicher P, Mackin W. Betafectin PGG-glucan: a novel carbohydrate immunomodulator with anti-infective properties. Annu Rev Pharmacol Toxicol. 1995;37:143–166. [Google Scholar]
- 12.Chihara G. Recent progress in immunopharmacology and therapeutic effects of polysaccharides. Dev Biol Stand. 1992;77:191–197. [PubMed] [Google Scholar]
- 13.Cisneros R L, Gibson F C, Tzianabos A O. Passive transfer of poly-(1-6)-beta-glucotriosyl-(1-3)-beta-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect Immun. 1996;64:2201–2205. doi: 10.1128/iai.64.6.2201-2205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 15.Czop J K, Austen K F. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc Natl Acad Sci USA. 1985;82:2751–2755. doi: 10.1073/pnas.82.9.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czop J K, Puglisi A V, Miorandi D Z, Austen K F. Perturbation of beta-glucan receptors on human neutrophils initiates phagocytosis and leukotriene B4 production. J Immunol. 1988;141:3170–3176. [PubMed] [Google Scholar]
- 17.Danner R L, Elin R J, Hosseini J M, Wesley R A, Reilly J M, Parillo J E. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 18.Davis A R, Mascolo P L, Bunger P L, Sipes K M, Quinn M T. Cloning and sequencing of the bovine flavocytochrome b subunit proteins, gp91-phox and p22-phox: comparison with other known flavocytochrome b sequences. J Leukoc Biol. 1998;64:114–123. doi: 10.1002/jlb.64.1.114. [DOI] [PubMed] [Google Scholar]
- 19.Doita M, Rasmussen L T, Seljelid R, Lipsky P E. Effect of soluble animated beta-1,3-d-polyglucose on human monocytes: stimulation of cytokine and prostaglandin E2 production but not antigen-presenting function. J Leukoc Biol. 1991;49:342–351. doi: 10.1002/jlb.49.4.342. [DOI] [PubMed] [Google Scholar]
- 20.Eigler A, Sinha B, Hartmann G, Endres S. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today. 1997;18:487–492. doi: 10.1016/s0167-5699(97)01118-3. [DOI] [PubMed] [Google Scholar]
- 21.Falk L A. Isolation of bone marrow derived macrophages. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 14.1.1–14.1.9. [Google Scholar]
- 22.Farrar M A, Schreiber R D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 23.Fisher C J J J, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M H, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 24.Fong Y, Moldawer L L, Marano M, Wei H, Tatter S B, Clarick R H, Santhanam U, Sherris D, May L T, Sehgal P B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989;142:2321–2324. [PubMed] [Google Scholar]
- 25.Fong Y, Tracey K J, Moldawer L L, Hesse D G, Manogue K B, Kenney J S, Lee A T, Kuo G C, Allison A C, Lowry S F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukumoto T, Torigoe N, Kawabata S, Murakami M, Uede T, Nishi T, Ito Y, Sugimura K. Peptide mimics of the CTLA4-binding domain stimulate T-cell proliferation. Nat Biotechnol. 1998;16:267–270. doi: 10.1038/nbt0398-267. [DOI] [PubMed] [Google Scholar]
- 27.Gallin E K, Green S W, Patchen M L. Comparative effects of particulate and soluble glucan on macrophages of C3H/HeN and C3H/HeJ mice. Int J Immunopharmacol. 1992;14:173–183. doi: 10.1016/0192-0561(92)90028-j. [DOI] [PubMed] [Google Scholar]
- 28.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold H S, Moellering R C., Jr Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 30.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 31.Goren M B. Immunoreactive substances of mycobacteria. Am Rev Respir Dis. 1982;125:50–69. doi: 10.1164/arrd.1982.125.3P2.50. [DOI] [PubMed] [Google Scholar]
- 32.Haslett C, Savill J S, Whyte M K B, Stern M, Dransfield I, Meagher L C. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc London Ser B. 1994;345:327–333. doi: 10.1098/rstb.1994.0113. [DOI] [PubMed] [Google Scholar]
- 33.Hedderwick S, Kauffman C A. Opportunistic fungal infections: superficial and systemic candidiasis. Geriatrics. 1997;52:50–59. [PubMed] [Google Scholar]
- 34.Horn D L, Opal S M, Lomastro E. Antibiotics, cytokines, and endotoxin: a complex and evolving relationship in gram-negative sepsis. Scand J Infect Dis Suppl. 1996;101:9–13. [PubMed] [Google Scholar]
- 35.Isoniemi H. New trends in maintenance immunosuppression. Ann Chir Gynaecol. 1997;86:164–170. [PubMed] [Google Scholar]
- 36.Jimenez M F, Watson R W, Parodo J, Evans D, Foster D, Steinberg M, Rotstein O D, Marshall J C. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–1269. doi: 10.1001/archsurg.1997.01430360009002. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko Y, Chihara G. Potentiation of host resistance against microbial infections by lentinan and its related polysaccharides. Adv Exp Med Biol. 1992;319:201–215. doi: 10.1007/978-1-4615-3434-1_21. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman C A, Hedderwick S. Opportunistic fungal infections: filamentous fungi and cryptococcosis. Geriatrics. 1997;52:40–49. [PubMed] [Google Scholar]
- 39.Kernodle D S, Gates H, Kaiser A B. Prophylactic anti-infective activity of poly-[1-6]-beta-d-glucopyranosyl-[1-3]-beta-d-glucopryanose glucan in a guinea pig model of staphylococcal wound infection. Antimicrob Agents Chemother. 1998;42:545–549. doi: 10.1128/aac.42.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, J., D. Melican, L. Cafro, G. Palace, L. Fisette, R. Armstrong, and M. L. Patchen. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-Glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. Int. J. Pharmacol., in press. [DOI] [PubMed]
- 41.Lynn, W. A. 1998. Anti-endotoxin therapeutic options for the treatment of sepsis. J. Antimicrob. Chemother. 41(Suppl. A):71–80. [DOI] [PubMed]
- 42.Mackin W, Brunke-Reese D, Crotty C, Cafro L, Daunais M, Fisette L, Bleicher P. Betafectin PGG-Glucan potentiates the microbicidal activities of human leukocytes measured ex vivo in healthy human volunteers. FASEB J. 1995;9:A521. . (Abstract.) [Google Scholar]
- 43.Mangan D F, Wahl S M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 44.Mayeux P R. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997;51:415–435. doi: 10.1080/00984109708984034. [DOI] [PubMed] [Google Scholar]
- 45.Meropol N J, Petrelli N J, Lipman B J, Rodriguez-Bigas M, Hicks W, Douglass H O J, Smith J L, Rasey M, Blumenson L E, Vaickus L, Hayes F A, Agosti J M. Granulocyte-macrophage colony-stimulating factor as infection prophylaxis in high-risk oncologic surgery. Am J Surg. 1996;172:299–302. doi: 10.1016/s0002-9610(96)00106-7. [DOI] [PubMed] [Google Scholar]
- 46.Miethke T, Wahl C, Gaus H, Heeg K, Wagner H. Exogenous superantigens acutely trigger distinct levels of peripheral T cell tolerance/immunosuppression: dose-response relationship. Eur J Immunol. 1994;24:1893–1902. doi: 10.1002/eji.1830240827. [DOI] [PubMed] [Google Scholar]
- 47.Miethke T, Wahl C, Regele D, Gaus H, Heeg K, Wagner H. Superantigen mediated shock: a cytokine release syndrome. Immunobiology. 1993;189:270–284. doi: 10.1016/S0171-2985(11)80362-1. [DOI] [PubMed] [Google Scholar]
- 48.Murray, H. W. 1996. Current and future clinical applications of interferon-gamma in host antimicrobial defense. Intensive Care Med. 22(Suppl. 4):S456–S461. [DOI] [PubMed]
- 49.Nemunaitis J. A comparative review of colony-stimulating factors. Drugs. 1997;54:709–729. doi: 10.2165/00003495-199754050-00004. [DOI] [PubMed] [Google Scholar]
- 50.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1072. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 51.Onderdonk A B, Cisneros R L, Hinkson P, Ostroff G. Anti-infective effect of poly-beta-1-6-glucotriosyl-beta-1-3-glucopyranose glucan in vivo. Infect Immun. 1992;60:1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opal S M, Cross A S, Jhung J W, Young L D, Palardy J E, Parejo N A, Donsky C. Potential hazards of combination immunotherapy in the treatment of experimental septic shock. J Infect Dis. 1996;173:1415–1421. doi: 10.1093/infdis/173.6.1415. [DOI] [PubMed] [Google Scholar]
- 53.Pearl, R. G. 1998. Treatment of shock—1998. Anesth. Analg. 1998(Suppl.):75–84.
- 54.Pennington, J. E. 1993. Therapy with antibody to tumor necrosis factor in sepsis. Clin. Infect. Dis. 17(Suppl. 2):S515–S519. [DOI] [PubMed]
- 55.Poutsiaka D D, Mengozzi M, Vannier E, Sinha B, Dinarello C A. Cross-linking of the beta-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. Blood. 1993;82:3695–3700. [PubMed] [Google Scholar]
- 56.Pretus H A, Browder I W, Lucore P, McNamee R B, Jones E L, Williams D L. Macrophage activation decreases macrophage prostaglandin E2 release in experimental trauma. J Trauma. 1989;29:1152–1156. doi: 10.1097/00005373-198908000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Rasmussen L T, Seljelid R. Novel immunomodulators with pronounced in vivo effects caused by stimulation of cytokine release. J Cell Biochem. 1991;46:60–68. doi: 10.1002/jcb.240460110. [DOI] [PubMed] [Google Scholar]
- 58.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154:2366–2374. [PubMed] [Google Scholar]
- 59.Reynolds J A, Kastello M D, Harrington D G, Crabbs C L, Peters C J, Jemski J V, Scott G H, Di Luzio N R. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980;30:51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roilides E. Host defense abnormalities as causes of increased susceptibility to infections in children with HIV infection. Int J Med Microbiol Virol Parasitol Infect Dis. 1994;280:433–438. doi: 10.1016/s0934-8840(11)80501-3. [DOI] [PubMed] [Google Scholar]
- 61.Sakurai T, Kaise T, Yadomae T, Matsubara C. Different role of serum components and cytokines on alveolar macrophage activation by soluble fungal (1→3)-beta-d-glucan. Eur J Pharmacol. 1997;334:255–263. doi: 10.1016/s0014-2999(97)01191-6. [DOI] [PubMed] [Google Scholar]
- 62.Sakurai T, Suzuki I, Kinoshita A, Oikawa S, Masuda A, Ohsawa M, Yadomae T. Effect of intraperitoneally administered beta-1,3-glucan, SSG, obtained from Sclerotinia sclerotiorum IFO 9395 on the functions of murine alveolar macrophages. Chem Pharm Bull (Tokyo) 1991;39:214–217. doi: 10.1248/cpb.39.214. [DOI] [PubMed] [Google Scholar]
- 63.Saravolatz L D, Wherry J C, Spooner C, Markowitz N, Allred R, Remick D, Fournel M, Pennington J E. Clinical safety, tolerability, and pharmacokinetics of murine monoclonal antibody to human tumor necrosis factor-alpha. J Infect Dis. 1994;169:214–217. doi: 10.1093/infdis/169.1.214. [DOI] [PubMed] [Google Scholar]
- 64.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 65.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–393. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 66.Seljelid R. A water-soluble aminated β-1,3-d-glucan derivative causes regression of solid tumors in mice. Biosci Rep. 1986;6:845–851. doi: 10.1007/BF01117108. [DOI] [PubMed] [Google Scholar]
- 67.Seljelid R, Bogwald J, Hoffman J, Larm O. A soluble beta-1,3-d-glucan derivative potentiates the cytostatic and cytolytic capacity of mouse peritoneal macrophages in vitro. Immunopharmacology. 1984;7:69–73. doi: 10.1016/0162-3109(84)90009-2. [DOI] [PubMed] [Google Scholar]
- 68.Seljelid R, Figenschau Y, Bogwald J, Rasmussen L T, Austgulen R. Evidence that tumor necrosis induced by aminated beta 1-3d-polyglucose is mediated by a concerted action of local and systemic cytokines. Scand J Immunol. 1989;30:687–694. doi: 10.1111/j.1365-3083.1989.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 69.Service R E. Antibiotics that resist resistance. Science. 1995;270:724–727. doi: 10.1126/science.270.5237.724. [DOI] [PubMed] [Google Scholar]
- 70.Squier M K T, Sehnert A J, Cohen J J. Apoptosis in leukocytes. J Leukoc Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 71.Stevens D L. The toxic shock syndromes. Infect Dis Clin North Am. 1996;10:727–746. doi: 10.1016/s0891-5520(05)70324-x. [DOI] [PubMed] [Google Scholar]
- 72.Stevens D L. Superantigens: their role in infectious diseases. Immunol Invest. 1997;26:275–281. doi: 10.3109/08820139709048933. [DOI] [PubMed] [Google Scholar]
- 73.Sveinbjornsson B, Rushfeldt C, Seljelid R, Smedsrod B. Inhibition of establishment and growth of mouse liver metastases after treatment with interferon gamma and β-1,3-d-glucan. Hepatology. 1998;27:1241–1248. doi: 10.1002/hep.510270509. [DOI] [PubMed] [Google Scholar]
- 74.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-alpha. Int Immunol. 1993;5:691–694. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 75.Tobias P S, Gegner J, Tapping R, Orr S, Mathison J, Lee J D, Kravchenko V, Han J, Ulevitch R J. Lipopolysaccharide dependent cellular activation. J Periodontal Res. 1997;32:99–103. doi: 10.1111/j.1600-0765.1997.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 76.Tzianabos A O, Cisneros R L. Prophylaxis with the immunomodulator PGG glucan enhances antibiotic efficacy in rats infected with antibiotic-resistant bacteria. Ann N Y Acad Sci. 1996;797:285–287. doi: 10.1111/j.1749-6632.1996.tb52980.x. [DOI] [PubMed] [Google Scholar]
- 77.Wakshull, E., D. Brunke-Reese, J. Lindermuth, L. Fisette, R. S. Nathans, J. J. Crowley, J. C. Tufts, J. Zimmerman, W. Mackin, and D. S. Adams. PGG-glucan, a soluble β-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-κB-like factor in human PMN: evidence for a glycosphingolipid b-(1,3)-glucan receptor. Immunopharmacology, in press. [DOI] [PubMed]
- 78.Wakshull E, Lindermuth J, Zimmerman J. Characterization of PGG-Glucan binding to a β-glucan receptor on human leukocytes. FASEB J. 1996;10:A1338. . (Abstract.) [Google Scholar]
- 79.Washburn W K, Otsu I, Gottschalk R, Monaco A P. PGG-glucan, a leukocyte-specific immunostimulant, does not potentiate GVHD or allograft rejection. J Surg Res. 1996;62:179–183. doi: 10.1006/jsre.1996.0192. [DOI] [PubMed] [Google Scholar]
- 80.Williams D L, Mueller A, Browder W. Glucan-based macrophage stimulators: a review of their anti-infective potential. Clin Immunother. 1998;5:392–399. [Google Scholar]
- 81.Wollenberg G K, DeForge L E, Bolgos G, Remick D G. Differential expression of tumor necrosis factor and interleukin-6 by peritoneal macrophages in vivo and in culture. Am J Pathol. 1993;143:1121–1130. [PMC free article] [PubMed] [Google Scholar]