Abstract
Purpose
We conducted a retrospective study to compare transarterial chemoembolization (TACE) plus lenvatinib plus programmed death-1 (PD-1) inhibitors with TACE plus lenvatinib in patients with unresectable hepatocellular carcinoma (HCC).
Patients and methods
Patients with HCC were analyzed from January 2018 to January 2022 in three hospitals. Patients received TACE plus lenvatinib with or without PD-1 inhibitors (TACE+L+PD-1 or TACE+L, respectively). The baseline characteristics of the two groups were compared, and propensity score matching (PSM) was performed. Overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) of the two groups were compared. Adverse events in the two groups were analyzed.
Results
A total of 166 patients were evaluated (TACE+L+PD-1, n = 75; TACE+L, n = 91). Before PSM, OS was prolonged in the TACE+L+PD-1 group (p = 0.010), but PFS was similar between the two groups (p = 0.18). ORR was higher in the TACE+L+PD-1 group (p = 0.047). After PSM, estimated OS rates at 6, 12, and 24 months were 97.9%, 84.6%, and 74.1%, respectively, in the TACE+L+PD-1 group (n = 48) and 93.1%, 66.1%, and 43.4%, respectively, in the TACE+L group (n = 48). Estimated PFS rates at 3, 6, and 12 months were 81.9%, 61.8%, and 30.9%, respectively, in the TACE+L group and 95.7%, 82.1%, and 68.4%, respectively, in the TACE+L+PD-1 group. OS, PFS, and ORR were improved in the TACE+L+PD-1 group compared to the TACE+L group (p = 0.030; p = 0.027; p = 0.013). The safety of the TACE+L+PD-1 regimen was acceptable.
Conclusions
The addition of PD-1 inhibitors to TACE+L significantly improved clinical outcomes in patients with unresectable HCC. Side effects were manageable.
Keywords: hepatocellular carcinoma, lenvatinib, TACE, PD-1, unresectable
Introduction
Liver cancer is the fifth most common cancer worldwide and the second most common cause of cancer-related death (1). Hepatocellular carcinoma (HCC) comprises 80%–90% of primary liver cancers. Treatment options include surgical resection, image-guided ablation, liver transplantation, transarterial chemoembolization (TACE), sorafenib, and lenvatinib (2). Different treatments are indicated at particular clinical stages, and combination therapies are often used (2–4). Although multiple treatment options are available, the prognosis of unresectable HCC remains poor (5).
The REFLECT trial (6) disclosed that lenvatinib was non-inferior to sorafenib in prolonging overall survival (OS) in untreated advanced HCC. Furthermore, clinically meaningful improvement was observed in progression-free survival (PFS), objective response rate (ORR), and time to progression (TTP); consequently, both lenvatinib and sorafenib have emerged as first-line treatments for advanced HCC. Combination treatment with TACE and lenvatinib for advanced HCC has achieved satisfactory efficacy (7, 8).
Although the combination of programmed death-1 (PD-1) monoclonal antibodies with other modalities yields promising outcomes (9, 10), the efficacy of PD-1 inhibitors as monotherapy is unsatisfactory. In the KEYNOTE-240 trial, the addition of pembrolizumab to best supportive care as second-line therapy for advanced HCC did not improve OS and PFS per specified criteria (11). In the Check-Mate 459 trial of patients with advanced HCC, nivolumab did not improve OS and PFS when compared to sorafenib (12).
Combination therapies (including lenvatinib plus PD-1 inhibitors) have yielded promising results in patients with advanced HCC. A phase Ib study of lenvatinib plus pembrolizumab for unresectable HCC demonstrated an ORR of 46%, a median PFS of 9.3 months by modified Response Evaluation Criteria In Solid Tumors (mRECIST) criteria, and a median OS of 22 months (13). Based on the potential clinical benefits of combining lenvatinib with PD-1 inhibitors, combinations of TACE or hepatic arterial infusion chemotherapy (HAIC) with lenvatinib and PD-1 inhibitors have been studied for the treatment of advanced HCC and have demonstrated improved survival and tumor responses (14, 15).
However, the optimal combination therapy of unresectable HCC is undefined. To further evaluate the treatment of HCC, we designed a retrospective study to compare TACE plus lenvatinib plus PD-1 inhibitors (TACE+L+PD-1) with TACE plus lenvatinib (TACE+L) in patients with unresectable HCC.
Patients and methods
Study design and patient selection
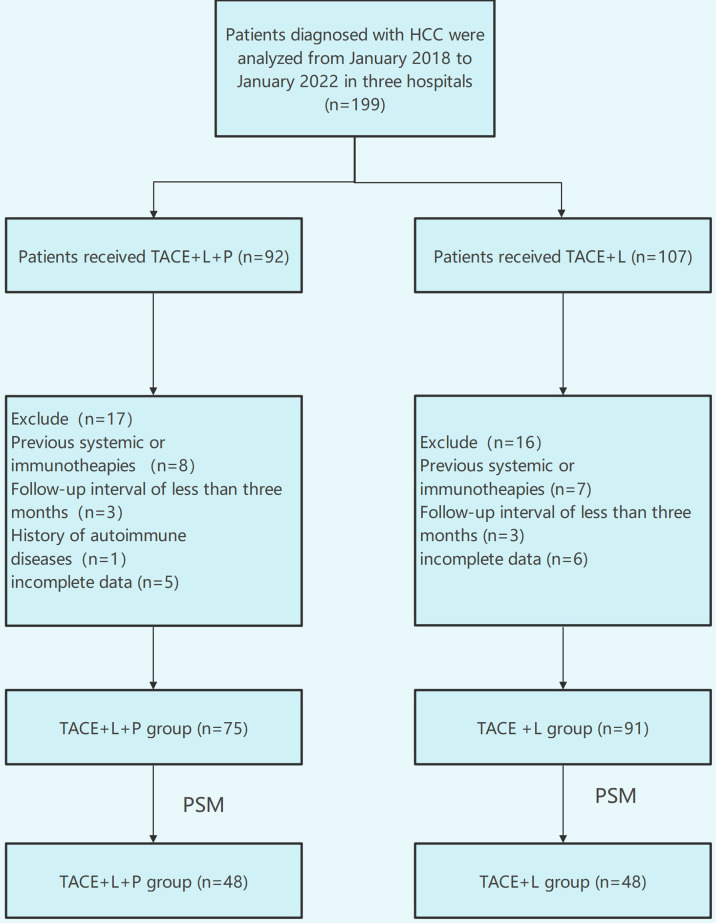
The Ethics Board of the Shanxi Bethune Hospital, Shanxi Provincial People’s Hospital, and Second Hospital of Shanxi Medical University approved this retrospective cohort study. An informed consent requirement was waived due to the retrospective study design and anonymized data. Patients diagnosed with HCC were analyzed from January 2018 to January 2022 in Shanxi Bethune Hospital, Shanxi Provincial People’s Hospital, and the Second Hospital of Shanxi Medical University. All patients were diagnosed with HCC by non-invasive criteria or pathological examination. According to the European Association for the Study of the Liver (EASL) guidelines (16), the criteria for the non-invasive diagnosis of HCC are as follows: 1) background history of cirrhosis, 2) lesion diameter ≥1 cm, and 3) hypervascularization in the arterial phase and washout in the venous or delayed phases of four-phase multi-detector computed tomography or dynamic magnetic resonance imaging (MRI). Eligible patients were older than 18 years, in Barcelona Clinic Liver Cancer (BCLC) stages B or C (unresectable HCC), had not received previous systemic therapy, had not received previous radiotherapy or TACE, had at least one measurable lesion, had a class A Child–Pugh score, and had an Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0 to 1. Exclusion criteria were as follows: 1) previous systemic or immunotherapies, 2) follow-up interval of less than 3 months, and 3) history of autoimmune diseases.
Baseline assessment
Baseline characteristics of age, sex, ECOG-PS, albumin–bilirubin (ALBI) grade, Child–Pugh class, tumor diameter, tumor number, BCLC stage, serum α-fetoprotein (AFP) level, presence of portal vein thrombosis, and presence of extrahepatic metastasis were compared between the two groups.
Transarterial chemoembolization
TACE was performed by interventional radiologists with more than 5 years of experience. After a successful femoral artery puncture, a catheter (5F, RH catheter; Cook, Bloomington, IN, USA) was selected for common hepatic artery angiography. If necessary, superior mesenteric artery and phrenic artery angiography was performed. After the tumor-feeding arteries were identified, a microcatheter (SP microcatheter; Terumo, Tokyo, Japan) was used for superselective arterial embolization performed with a mixed emulsion of lipiodol (Lipiodol; Guerbet, France) and epirubicin (50 mg/m2). TACE was repeated every 4–6 weeks until disease progression occurred, with a limit of six TACE treatments per patient.
Systemic therapy
Lenvatinib was administered in doses of either 12 mg (bodyweight > 60 kg) or 8 mg (bodyweight < 60 kg) orally once daily 1–3 days after TACE. PD-1 inhibitors (camrelizumab or sintilimab) were given intravenously at the standard dose (200 mg) within 7 days of the initiation of lenvatinib, with repeat dosing at 21-day intervals. PD-1 inhibitors were added to the regimens of patients who experienced tumor progression while on TACE+L treatment.
Follow-up
Follow-up was performed every 2 months after TACE for the first three visits and every 2–3 months thereafter. Follow-up included enhanced abdominal CT or MRI and laboratory tests. Laboratory examinations included bilirubin, albumin, prothrombin time, and serum AFP levels. Adverse events were analyzed based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (17).
Definitions
OS was defined as the time interval from the first TACE until death or loss to follow-up. PFS was defined as the time interval from the first TACE to disease progression or loss to follow-up. Tumor progression was defined as either an increase in tumor diameter of 25% from baseline, the appearance of new lesions, macrovascular invasion, or extrahepatic metastasis (18). Complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD) assessments were performed 1 month after the first TACE. ORR included CR and PR. PFS, CR, PR, SD, and PD were estimated by mRECIST criteria.
Statistical analysis
Categorical data are reported as number with percentage. Propensity score matching (PSM) was used to correct potential confounding biases between the two groups (a nearest-neighbor algorithm [1:1] was used to account for pre-treatment laboratory tests, imaging, and clinical covariates). The x 2 test or Fisher’s exact test was used to compare categorical data before PSM. The Cochran–Mantel–Haenszel test was used to evaluate differences in the categorical data after PSM. Primary endpoints were OS; secondary endpoints were PFS and ORR. The Kaplan–Meier curves were used to estimate OS and PFS. A comparison of survivals between the two groups was performed by using the log-rank test. All tests of significance were two-sided, and a p-value <0.05 was considered statistically significant. All statistical analyses were performed using EmpowerStats (www.empowerstats.com) and R software, version 3.6.2.
Results
Patient demographics
A total of 199 patients were evaluated for eligibility, comprising 107 patients in the TACE+L group and 92 patients in the TACE+L+PD-1 group. A total of 33 patients were excluded because they met the exclusion criteria, and 166 patients were analyzed in this study last ( Figure 1 ). Before PSM, sex, ECOG-PS, and Child–Pugh class were different between the two groups (p < 0.05). There were no significant differences in other indicators. After PSM, there were no significant intergroup differences in any of the baseline characteristics ( Table 1 ).
Figure 1.
Flow diagram of patient enrollment.
Table 1.
Baseline characteristics before and after PSM.
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| TACE+L n=91n, (%) | TACE+L+PD1 n=75n, (%) | P | TACE+L n=48n, (%) | TACE+L+PD1 n=48n, (%) | P | |
| Age | 0.263 | 0.593 | ||||
| ≤60 | 66 (72.5) | 60 (80.0) | 41 (85.4) | 38 (79.2) | ||
| >60 | 25 (27.5) | 15 (20.0) | 7 (14.6) | 10 (20.8) | ||
| Sex | 0.017 | 1.000 | ||||
| Female | 3 (3.3) | 10 (13.3) | 2 (4.2) | 1 (2.1) | ||
| Male | 88 (96.7) | 65 (86.7) | 46 (95.8) | 47 (97.9) | ||
| ECOG-PS | <0.001 | 1.000 | ||||
| 0 | 58 (63.7) | 69 (92.0) | 43 (89.6) | 43 (89.6) | ||
| 1 | 33 (36.3) | 6 (8.0) | 5 (10.4) | 5 (10.4) | ||
| ALBI grade | 0.292 | 0.320 | ||||
| 1 | 34 (37.4) | 37 (49.3) | 20 (41.7) | 25 (52.1) | ||
| 2 | 55 (60.4) | 37 (49.3) | 28 (58.3) | 22 (45.8) | ||
| 3 | 2 (2.2) | 1 (1.3) | 0 (0) | 1 (2.1) | ||
| Child-Pugh Class | 0.009 | 0.673 | ||||
| A | 78 (85.7) | 73 (97.3) | 44 (91.7) | 46 (95.8) | ||
| B | 13 (14.3) | 2 (2.7) | 4 (8.3) | 2 (4.2) | ||
| Tumor diameter (cm) | 0.863 | 0.527 | ||||
| ≤7 | 34 (37.4) | 29 (38.7) | 20 (41.7) | 16 (33.3) | ||
| >7 | 57 (62.6) | 46 (61.3) | 28 (58.3) | 32 (66.7) | ||
| Tumor number (n) | 0.697 | 0.173 | ||||
| ≤3 | 28 (30.8) | 21 (28.0) | 10 (20.8) | 17 (35.4) | ||
| >3 | 63 (69.2) | 54 (72.0) | 38 (79.2) | 31 (64.6) | ||
| BCLC stage | 0.727 | 0.511 | ||||
| B | 20(22.0) | 20 (26.7) | 12 (25.0) | 9 (28.8) | ||
| C | 71(78.0) | 55 (73.3) | 36 (75.0) | 39 (81.2) | ||
| AFP (ng/ml) | 0.086 | 0.674 | ||||
| ≤400 | 41 (45.1) | 24 (32.0) | 20 (41.7) | 17 (35.4) | ||
| >400 | 50 (54.9) | 51 (68.0) | 28 (58.3) | 31 (64.6) | ||
| Portal vein thrombosis | 0.622 | 0.671 | ||||
| NO | 33 (36.3) | 30 (40.0) | 19 (39.6) | 16 (33.3) | ||
| Yes | 58 (63.7) | 45 (60.0) | 29 (60.4) | 32 (66.7) | ||
| Extrahepatic metastasis | 0.207 | 0.538 | ||||
| NO | 42 (46.2) | 42 (56.0) | 28 (58.3) | 24 (50) | ||
| Yes | 49 (53.8) | 33 (44.0) | 20 (41.7) | 24 (50) | ||
ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Overall survival
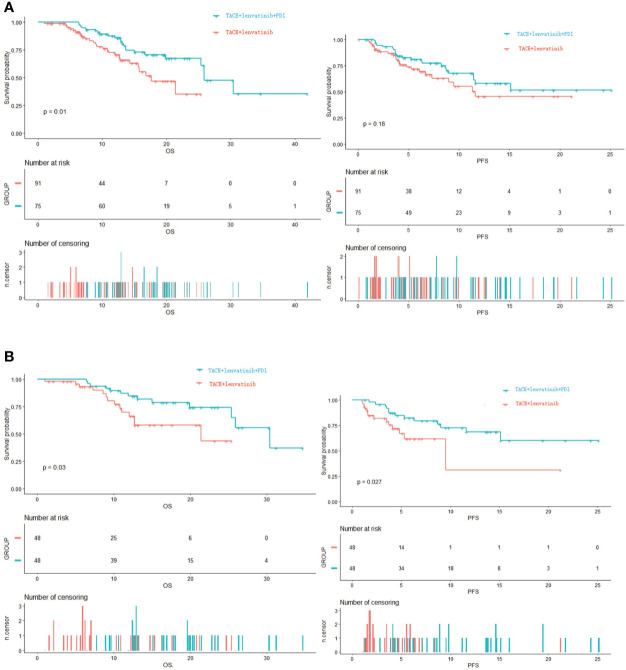
The median follow-up time was 14.7 months (95% CI: 12.2–17.1). Before PSM, the median OS in the TACE+L group was 17.6 months (95% CI: 12.9-22.3); estimated OS rates at 6, 12, and 24 months were 91.3%, 70.4%, and 35.1%, respectively. In the TACE+L+PD-1 group, the median OS was 25.9 months (95% CI: 21.8–30.0); estimated OS rates at 6, 12, and 24 months were 93.3%, 89.1%, and 61.0%, respectively. The Kaplan–Meier curves showed significant intergroup differences in OS rates (p = 0.010) ( Figure 2A ).
Figure 2.
(A) Kapan–Meier curves of OS and PFS before PSM. (B) Kapan–Meier curves of OS and PFS after PSM. OS, overall survival; PFS, progression-free survival; PSM, propensity score matching.
After PSM, the median OS was 21.4 months (95% CI: 12.7–not estimable) in the TACE+L group; estimated OS rates at 6, 12, and 24 months were 93.1%, 66.1%, and 43.4%, respectively. The median OS was 30.4 months (95% CI: 25.4–not estimable) in the TACE+L+PD-1 group; estimated OS rates at 6, 12, and 24 months were 97.9%, 84.6%, and 74.1%, respectively. The Kaplan–Meier curves showed significant intergroup differences in OS (p = 0.030, Figure 2B ).
Progression-free survival
Before PSM, the estimated PFS rates in the TACE+L group at 3, 6, and 12 months were 86.8%, 69.3%, and 45.5%, respectively, and 91.6%, 79.1%, and 57.9%, respectively, in the TACE+L+PD-1 group. Although the PFS rates of the TACE+L+PD-1 group were higher than those of the TACE+L group, the Kaplan–Meier curves were similar (p = 0.18, Figure 2A ).
Estimated PFS rates after PSM at 3, 6, and 12 months were 81.9%, 61.8%, and 30.9%, respectively, in the TACE+L group. In contrast, estimated PFS rates at the corresponding time points were 95.7%, 82.1%, and 68.4%, respectively, in the TACE+L+PD-1 group. The Kaplan–Meier curves showed significant intergroup differences in PFS (p = 0.027, Figure 2B ).
Objective response rate
In the TACE+L group, 3, 45, 20, and 12 patients developed CR, PR, SD, and PD, respectively. Four, 47, 11, and 23 patients in the TACE+L+PD-1 group developed CR, PR, SD, and PD, respectively. The ORR before PSM was 52.7% in the TACE+L group and 68.0% in the TACE+L+PD-1 group. The ORRs of the two groups were significantly different (p = 0.047) ( Table 2 ).
Table 2.
Tumor response before and after PSM.
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| TACE+L n=91n, (%) | TACE+L+PD-1 n=75n, (%) | P | TACE+L n=48n, (%) | TACE+L+PD-1 n=48n, (%) | P | |
| CR | 3 (3.3) | 4 (5.3) | 1 (2.1) | 2 (4.2) | ||
| PR | 45 (49.5) | 47 (62.7) | 18 (37.5) | 31 (64.6) | ||
| SD | 20 (22.0) | 11 (14.7) | 12 (25.0) | 7 (14.6) | ||
| PD | 23 (25.3) | 13 (17.3) | 17 (35.4) | 8 (16.7) | ||
| ORR | 48 (52.8) | 51 (68.0) | 0.046 | 19 (39.6) | 33 (66.8) | 0.013 |
PSM, propensity score matching; TACE, transarterial chemoembolization, L, lenvatinib; PD-1, anti-PD-1 blockade; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate.
After PSM, 1, 18, 12, and 17 patients in the TACE+L group experienced CR, PR, SD, and PD, respectively, while 2, 31, 7, and 8 patients in the TACE+L+PD-1 group developed CR, PR, SD, and PD, respectively. ORRs were 41.7% and 68.8% in the TACE+L and TACE+L+PD-1 groups, respectively, and were significantly different (p = 0.013).
Adverse events
Forty-five (49.5%) and 62 (82.7%) patients reported adverse events (AEs) of any grade in the TACE+L and TACE+L+PD-1 groups, respectively ( Table 3 ). Fatigue, hypertension, diarrhea, and decreased appetite were the most common AEs. Seven patients (7.7%) experienced grade 3 or higher AEs in the TACE+L group, while 23 patients (30.7%) developed grade 3 or higher AEs in the TACE+L+PD-1 group. Thirty-three (44.0%) and 9 (12.0%) patients in the TACE+lenvatinib+PD-1 group required dose reductions or discontinuations, respectively, because of treatment-related AEs. Treatments were reduced in 11 (12.1%) and discontinued in 2 (2.1%) patients in the TACE+L group because of treatment-related AEs. No treatment-related deaths occurred in either group.
Table 3.
Treatment-related adverse events.
| AEs | TACE+L n=91n, (%) | TACE+L+PD-1 n=75n, (%) | ||
|---|---|---|---|---|
| Any Grade | ≥ Grade 3 | Any Grade | ≥ Grade 3 | |
| Hypertension | 11 (12.1) | 3 (3.3) | 19 (25.3) | 6 (8.0) |
| Fatigue | 10 (10.1) | 2 (2.2) | 10 (13.3) | 4 (5.3) |
| Diarrhea | 7 (7.7) | 0 (0) | 7 (9.3) | 4 (5.3) |
| Decreased appetite | 8 (8.8) | 2 (2.2) | 8 (10.7) | 3 (4.0) |
| Nausea | 7 (7.7) | 0 (0) | 7 (9.3) | 3 (4.0) |
| Palmar-plantar Erythrodysesthesia syndrome | 1 (1.1) | 0 (0) | 4 (5.3) | 1 (1.3) |
| Proteinuria | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) |
| Abnormal liver function | 0 (0) | 0 (0) | 2 (2.6) | 1 (1.3) |
| Thrombocytopenia | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) |
| Abdominal pain | 0 (0) | 0 (0) | 1 (1.3) | 1 (1.3) |
| Hypothyroidism | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) |
| Rash | 1 (1.1) | 0 (0) | 1 (1.3) | 0 (0) |
TACE, transarterial chemoembolization; PD-1, anti-PD-1 blockade.
Discussion
TACE is the first-line treatment for mid-stage HCC according to the EASL guidelines and the BCLC treatment strategy. However, the efficacy of TACE monotherapy of unresectable and advanced HCC is unsatisfactory. In contrast, the combination of TACE with lenvatinib has yielded favorable results (8, 19, 20) and is better than TACE monotherapy. In the LAUNCH trial, a phase III and randomized clinical trial, TACE combined with lenvatinib vs. lenvatinib alone as first-line treatment for advanced hepatocellular carcinoma, the median OS and PFS were significantly longer in the TACE+L group than lenvatinib group (17.8 vs. 11.5 months; 10.6 vs. 6.4 months); patients in the TACE+L group had a significantly higher ORR according to the modified RECIST criteria (54.1% vs. 25.0%) (21). Furthermore, triple therapy consisting of TACE, lenvatinib, and PD-1 inhibitors has improved clinical outcomes while causing controllable side effects (7, 22).
In our study, our findings of higher OS and ORR before PSM and improved OS, PFS, and ORR after PSM in the TACE+L+PD-1 group compared to TACE+L recipients suggest that the addition of PD-1 inhibitors conferred significant survival benefits. The efficacy of triple therapy in our study was better than or similar to previously reported results. In a prospective cohort study of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone, the median OS was 23.9 months and the ORR was 67.9% in the TACE+L+PD-1 group (22). Another study of TACE combined with lenvatinib plus PD-1 inhibition for advanced HCC reported a median OS of 16.9 months and an ORR of 56.1% (23).
The majority of our patients presented with more than three tumors, which may suggest that TACE+L+PD-1 triple therapy improves the outcomes of multifocal HCC. The high prevalence of BCLC stage C (portal vein thrombosis and/or extrahepatic metastases) in our study population ( Table 1 ) may also indicate that patients with portal vein thrombosis or extrahepatic metastasis derive further clinical benefit from the addition of PD-1 inhibitors to TACE combined with lenvatinib.
The safety of TACE+lenvatinib+PD-1 was also acceptable. Although dose reductions and treatment discontinuations due to treatment-related AEs were indicated in 44.0% and 12.0%, respectively, in the TACE+L+PD-1 group, there were no treatment-related deaths.
Our study has several limitations. The first is its retrospective design, which may lead to selection bias. Second, because of the limited number of cases, no subgroup analyses were performed in either treatment group. After PSM, each group included a relatively small sample size of 48 patients. Consequently, our findings should be confirmed by a large-sample and randomized controlled study.
Conclusion
For unresectable HCC, TACE combined with lenvatinib and PD-1 inhibitors was associated with significantly better OS, PFS, and ORR than TACE combined with lenvatinib. Side effects were manageable.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by third hospital of Shanxi medical university, Shanxi provincial people´s hospital and second hospital of Shanxi medical university. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PG: paper writing,data collection and design. FG: data collection and follow-up. QL: data collection and follow-up. DL: data collection. WF: data collection. WC: paper writing and design. All authors contributed to the article and approved the submitted version.
Funding
This work has been financially supported by the foundation of ShanXi Academy of Medical Sciences (No. REMD03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. European Association for the Study of the Liver. L. European Association for the Study of the EASL Clinical Practice Guidelines . Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/s0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 3. Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res (2022) 52(8):721–9. doi: 10.1111/hepr.13773 [DOI] [PubMed] [Google Scholar]
- 4. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut (2014) 63(5):844–55. doi: 10.1136/gutjnl-2013-306627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau WY, Leung TW, Lai BS, Liew CT, Ho SK, Yu SC, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg (2001) 233(2):236–41. doi: 10.1097/00000658-200102000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 7. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol (2021) 148(8):2115–25. doi: 10.1007/s00432-021-03767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: Initial lenvatinib therapy with subsequent selective TACE. Liver Cancer (2019) 8(5):299–311. doi: 10.1159/000502905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology (2017) 66(6):1920–33. doi: 10.1002/hep.29360 [DOI] [PubMed] [Google Scholar]
- 10. Huang J, Guo Y, Huang W, Hong X, Quan Y, Lin L, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma (2022) 9:157–70. doi: 10.2147/JHC.S353956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 12. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5 [DOI] [PubMed] [Google Scholar]
- 13. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer (2021) 21(1):1126. doi: 10.1186/s12885-021-08858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol (2001) 35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 17. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol (2018) 69(1):60–9. doi: 10.1016/j.jhep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 18. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut (2020) 69(8):1492–501. doi: 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan P, Song J, Wang F, Zhu G, Chen B. Combination of TACE and lenvatinib as a promising option for downstaging to surgery of initially unresectable intrahepatic cholangiocarcinoma. Invest New Drugs (2022) 40(5):1125–32. doi: 10.1007/s10637-022-01257-z [DOI] [PubMed] [Google Scholar]
- 20. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol (2021) 11:783480. doi: 10.3389/fonc.2021.783480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol (2022) JCO2200392. doi: 10.1200/JCO.22.00392 [DOI] [PubMed] [Google Scholar]
- 22. Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: A prospective cohort study. Front Oncol (2022) 12:874473. doi: 10.3389/fonc.2022.874473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol (2022) 13:848387. doi: 10.3389/fimmu.2022.848387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.