Abstract
Low protein (LP) diet during pregnancy leads to reduced plasma insulin levels in rodents, but the underlying mechanisms remain unclear. Glucose is the primary insulin secretagogue, and enhanced glucose-stimulated insulin secretion (GSIS) in beta cells contributes to compensation for insulin resistance and maintenance of glucose homeostasis during pregnancy. In this study, we hypothesized that plasma insulin levels in pregnant rats fed LP diet are reduced due to disrupted GSIS of pancreatic islets. We first confirmed reduced plasma insulin levels, then investigated in vivo insulin secretion by glucose tolerance test and ex vivo GSIS of pancreatic islets in the presence of glucose at different doses, and KCl, glibenclamide, and L-arginine. Main findings include (1) plasma insulin levels were unaltered on day 10, but significantly reduced on days 14–22 of pregnancy in rats fed LP diet compared to those of control (CT) rats; (2) insulin sensitivity was unchanged, but glucose intolerance was more severe in pregnant rats fed LP diet; (3) GSIS in pancreatic islets was lower in LP rats compared to CT rats in the presence of glucose, KCl, and glibenclamide, and the response to L-arginine was abolished in LP rats; and (4) the total insulin content in pancreatic islets and expression of Ins2 were reduced in LP rats, but expression of Gcg was unaltered. These studies demonstrate that decreased GSIS in beta cells of LP rats contributes to reduced plasma insulin levels, which may lead to placental and fetal growth restriction and programs hypertension and other metabolic diseases in offspring.
Keywords: insulin secretion, glucose, low protein diet, pregnancy, rat
Summary Sentence
Decreased glucose-stimulated insulin secretion of pancreatic islets in pregnant rats fed a low diet is responsible for the reduced plasma insulin levels and consequently enhanced glucose intolerance.
Introduction
Normal pregnancy requires not only the status of insulin resistance to ensure glucose supply for rapid fetal growth [1], but also enhanced insulin levels to compensate for insulin resistance [1–4]. Enhanced insulin levels in circulation are achieved by increased beta cell proliferation [3,4] and glucose-stimulated insulin secretion (GSIS) from beta cells in pregnant humans and rodents [5]. In rodents, beta cell proliferation peaks in mid-pregnancy, declines thereafter, and returns to basal levels before partum [3,4], while plasma insulin levels continuously increase and peak in late pregnancy [2]; thus, beta cell insulin secretion contributes largely to fine tuning of plasma insulin levels during late pregnancy when insulin resistance is robust [2,6,7].
There are a variety of regulators of insulin secretion from beta cells of pancreatic islets. Among them, glucose is a potent stimulator which has been studied extensively. Glucose stimulates insulin secretion primarily by the ATP-sensitive potassium channel (KATP)-dependent pathway [8]. Briefly, glucose is transported into beta cells in pancreatic islets via glucose transporter 2 (Alias GLUT2; gene symbol SLC2A2) in rodents, and in addition GLUT1 (SLC2A1) in humans. Following glucose metabolism, ATP/ADP ratio is increased, which closes KATP channels and activates a cascade of signaling, including depolarization of plasma membrane, activation of voltage-dependent calcium channels (Cav), calcium influx, and increases in cytosolic calcium concentrations, resulting in rapid insulin exocytosis [9]. According to the current consensus model [10,11], activities of ion channels and glucose metabolism are two major regulators of GSIS. In addition, recently KATP- and Ca2+-independent GSIS has been proposed and via this mechanism, metabolic amplification factors such as ATP, GTP, glutamate, malonyl-CoA, hormones including glucagon like peptide-1 and other incretins, free fatty acids, and neuropeptides augment insulin secretion (reviewed in [10]). To date, ex vivo insulin secretion from pancreatic islets is the main approach to study the function of beta cells, in which KCl, glibenclamide, and L-arginine have been widely applied to explore the GSIS pathway as these agents target different players in the GSIS pathway [12]. Besides glucose, many pregnancy-associated factors affect insulin release in pregnant subjects, including placental lactogen, progesterone and estrogen, and insulin resistance, which make the regulation of insulin secretion during pregnancy more complicated than nonpregnant subjects.
Accumulating evidence indicates that dietary macronutrient components also affect insulin secretion during pregnancy. A low protein (LP) diet leads to reduced plasma insulin levels in pregnant rats [13–15]. Simultaneously, the LP diet during pregnancy not only causes fetal and placental growth restriction, but also predisposes hypertension, obesity, and diabetes in offspring by a process termed fetal programming [16–18], although the underlying mechanisms have not been understood completely. Insulin is emerging as an important factor in fetal programming, due to its critical role in regulating glucose homeostasis and energy metabolism during pregnancy, and also its capability of promoting placental and fetal growth [19,20].
In this study, we hypothesized that plasma insulin levels in pregnant rats fed LP diet are reduced due to the disrupted glucose GSIS of pancreatic islets. We first confirmed the reduced plasma insulin levels in pregnant rats fed LP diet, and then investigated in vivo insulin secretion by glucose tolerance test (GTT), in vivo insulin sensitivity by insulin tolerance test (ITT), and ex vivo GSIS of pancreatic islets in the presence of low and high concentration of glucose, and three plasma membrane depolarization reagents, KCl, glibenclamide, and L-arginine. In addition, to clarify the role of ion channels in reduced insulin secretion, other potential regulators in insulin secretion were investigated including insulin gene expression and total insulin content in pancreatic islets.
Materials and methods
Diets and animals
The isocaloric low (6% casein) and normal (20% casein) protein diets were purchased from Harlan Teklad (Cat. TD.90016 and TD.91352, respectively, Madison, WI, USA). More information about these diets was described in detail in our recent publication [21].
All procedures were approved by the Animal Care and Use Committee at Baylor College of Medicine and were in accordance with those published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Virgin female Sprague-Dawley rats weighing between 175 and 225 g and male rats weighing between 225 and 249 g were purchased from Harlan Laboratories (Indianapolis, IN, USA). These rats were housed in a room with a controlled light-dark cycle (light phase: 0600 to 2000 and dark phase: 2000 to 0600). Animals were allowed acclimation to our housing conditions for 1 week before breeding. Two virgin female rats and one male rat were kept in a cage overnight, and vaginal smears were checked under microscope next morning. The presence of sperm in vaginal smear indicated positive pregnancy status, and this day was designated as day 1 of pregnancy. Pregnant rats were housed individually, randomly divided into two dietary groups, and received ad libitum either control (CT) or LP diet until they were sacrificed.
Plasma insulin levels under fed status
Rats were anesthetized by carbon dioxide inhalation on day 10, 14, 18, 19, 21, or 22 of pregnancy. The whole blood was collected by left ventricle puncture using a 10-mL syringe and an 18-G needle, partially injected into BD Vacutainer blood collection tube containing heparin (Cat.367874, BD, Franklin Lakes, NJ, USA) and centrifuged at 3000 g for 15 min at 4°C. Aliquots of plasma were stored at –80°C until insulin and glucose analyses. Insulin concentration in the plasma was measured by ELISA, according to instructions of the assay kit (Cat. 10–1250-01, Mercodia, Uppsala, Sweden).
Intraperitoneal insulin tolerance test
On day 19 of pregnancy, rats were fasted for 6 h (6:00–12:00 pm), and then blood glucose was measured after puncturing lateral saphenous vein in the left leg (0 min). Insulin (1 U/Kg body weight; Cat. 002–8215-01, Lilly USA, LLC, Indianapolis, IN) was injected intraperitoneally (n = 5/diet group). Blood glucose was measured from lateral saphenous vein at 5, 15, 30, 60, 120, and 180 min after injection by an ACCU-CHEK Nano glucose meter with SmartView strips (Roche, Indianapolis, IN). The total area under the curve for glucose over 180 min was calculated.
Intraperitoneal glucose tolerance test
On day 19 of pregnancy, rats were fasted for 16 h (6:00 pm–10:00 pm), and then blood glucose was measured after puncturing lateral saphenous vein in the left leg (0 min). After collection of 100 μl of a blood sample at time 0 min, glucose solution (2 g glucose/kg body weight) was injected intraperitoneally (n = 5/diet group). Blood glucose levels were measured by an ACCU-CHEK Nano glucose meter with SmartView strips, immediately after puncturing lateral saphenous vein at 5, 15, 30, 60, 120, and 180 min after glucose injection, and then 100 μl of blood was collected for plasma preparation and insulin ELISA. The total area under the curve for glucose over 180 min was calculated.
Ex vivo glucose stimulated insulin secretion
On day 19 of pregnancy, pancreatic islets were isolated following collagenase digestion as described by previous reports [22–24]. Briefly, collagenase solution (0.7 mg/mL) was injected through common bile duct, and then the inflated pancreas was dissected and incubated at 37°C water bath for 27 min. After sedimentation and wash, islets were purified by gradient centrifuge in Histopaque -1077 (Sigma-Aldrich), followed by recovery in culture medium [RPMI-1640 (Cat. 11 875–093, Gibco) with 10% FBS, 10 mM HEPES, penicillin (100 U/mL)/streptomycin (100 μg/mL; Cat. 15 140–122, Gibco)] at 37°C for 6 h. Islets were transferred to Secretion Assay Buffer (SAB; KH buffer supplemented with 2.5% BSA, and 10 mM HEPES) with 2.8 mM glucose and incubated at 37°C for 60 min. Ten islets in medium size were seeded to each well of 12-well plate and incubated in SAB with 2.8 mM glucose for 30 min. After 50 μl buffer was removed, glucose solution was added to increase glucose in buffer to 16.7 mM, and then KCl (30 mM), L-arginine HCl (L-Arg) (30 mM), and glibenclamide (15 μM) were added to different wells, as determined by Yechoor et al. [12]. After incubation for 30 min, SAB and islets were collected for insulin determination and DNA measurement, respectively. Insulin concentration in SAB was measured by ELISA, and total insulin secretion in each treatment was normalized to the total DNA of pancreatic islets.
Pancreatic islet insulin and DNA measurement
Total insulin content in pancreatic islets was extracted with acid-ethanol, as described previously [12]. Briefly, islets were sonicated in water. Aliquots of tissue lysates were mixed with 70% ethanol/0.2N HCl, incubated overnight with shaking, followed by centrifuging at 17 000 g for 30 min at 4°C. The supernatant was neutralized with Tris buffer (0.4 M, pH 8.0), and then used for insulin ELISA. Aliquots of tissue lysates were used for DNA measurement by NanoDrop 1000 (Thermo Scientific, Wilmington, DE). Total insulin content in islets was normalized to the total DNA.
RNA extraction, RT-PCR, and quantitative real-time PCR
Total mRNAs were extracted from around 100 islets, using MiRNeasy micro kit (Cat. 217 004; Qiagen Inc., Valencia, CA), followed by DNA cleanup with RNA free DNase I (Cat. 79 254; Qiagen) and reverse transcription with miScript II RT Kit (Cat. 218 160; Qiagen). In all these procedures, manufacturers’ instructions were followed.
Real-time PCR detection was performed on a CFX96Real-Time PCR Detection System (Cat. 184–5096; Bio-Rad, Hercules, California). iTaq Universal Probes Supermix (Cat. 1 725 135; Bio-Rad) was used for amplification of proinsulin 2 (Ins2), glucagon (Gcg), and Actb. Sequences of primers for Ins2 and Actb were described previously [21,25]. Sequences of primers for Gcg are 5΄-CATTCACAGGGCACATTCAC-3΄ (Forward) and 5΄-TGACGTTTGGCAATGTTGTT-3΄ (Reverse). The size of Gcg PCR product is 119 bp. The reaction mixture was incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 s and 60°C for 1 min for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity. The relative gene expression was calculated by use of the threshold cycle (CT) Actb/CTIns2 or Gcg.
Statistical analysis
All quantitative data were subjected to least-squares analysis of variance (ANOVA) using the general linear models procedures of the Statistical Analysis System (Version 9.4., SAS Institute, Cary, NC). Data on gene expression and abundance of proteins were analyzed for the effect of diet treatment. Data on plasma levels of insulin during pregnancy, intraperitoneal insulin and GTTs, and ex vivo insulin secretion were analyzed for the effect of diet between the two diet groups. Log transformation of variables was performed when the variance of data was not homogenous among treatment groups, as assessed by the Levene test. A P-value ≤ 0.05 was considered significant. Data were presented as least-squares means with overall standard errors (SE).
Results
Plasma levels of insulin in fed status
Plasma insulin levels were significantly lower (P < 0.05) in the LP compared to the CT group during mid-late pregnancy (1.6-, 4.5-, 2.0-, 2.3-, 6.9-fold lower, on days 14, 18, 19, 21, and 22 of pregnancy, respectively), but not different on day 10 of pregnancy (Figure 1). Thus, the reduction of plasma insulin levels occurs in late pregnancy.
Figure 1.
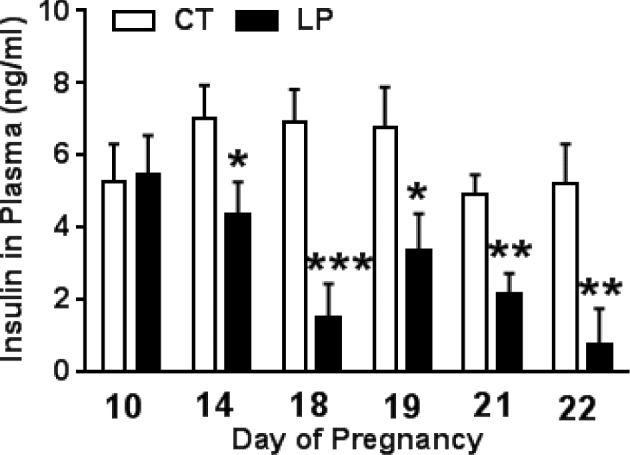
Plasma levels of insulin in pregnant rats fed a low protein diet. CT: control; LP, low protein. The error bar represents the mean ± SEM (n = 8–10 rats/diet group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Blood glucose levels in intraperitoneal insulin tolerance test
To determine whether insulin sensitivity, one of the factors affecting plasma glucose levels is altered in LP rats, intraperitoneal ITT was conducted in both LP and CT rats on day 19 of pregnancy. Blood glucose levels were decreased (P < 0.001) in both CT and LP rats at 15 min after injection of insulin, and remained low until 180 min. However, these values were similar in CT and LP rats at all time points investigated in this study (Figure 2), and consequently, the area under the curve of glucose was similar in CT and LP rats (data not shown). These observations suggest that insulin sensitivity in LP rats is unaltered and is similar to that in CT rats during late pregnancy.
Figure 2.
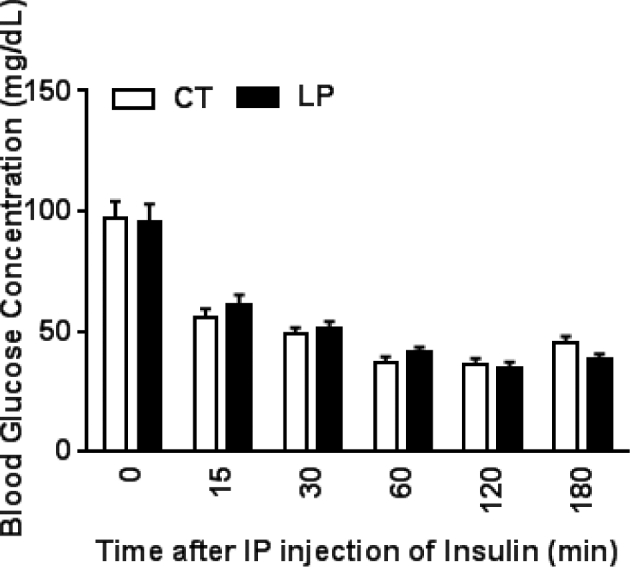
Insulin tolerance test on day 19 of pregnancy. Blood glucose concentrations after injection of insulin. CT: control; LP, low protein. The error bar represents the mean ± SEM (n = 5 rats/diet group).
Blood glucose and plasma insulin levels in intraperitoneal glucose tolerance test
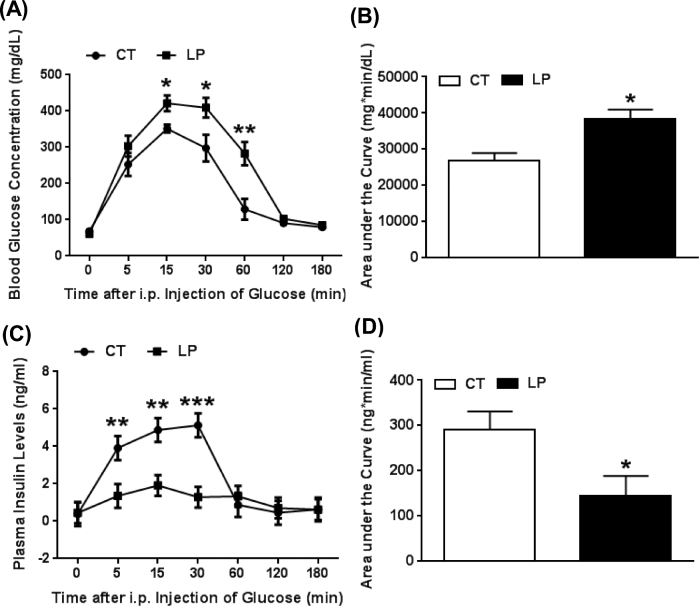
We assessed the ability of pregnant rats to regulate blood glucose levels following intraperitoneal glucose administration (GTT) to both LP and CT rats on day 19 of pregnancy. Blood glucose levels were increased following glucose injection in both CT and LP rats and returned to baseline levels by 120 min (Figure 3A) and the area under the curve of blood glucose levels was 1.43-fold higher (P < 0.05) in LP compared to CT rats (Figure 3B). Interestingly, blood glucose levels in LP rats were higher than CT rats up to 60 min after glucose injection [1.57-fold (P < 0.01), 1.23-fold (P < 0.05), 1.38-fold (P < 0.05), and 2.19-fold (P < 0.01) higher in LP rats at 5, 15, 30, and 60 min, respectively] (Figure 3A). In contrast, the increases in plasma insulin levels following glucose injections were significantly lower in LP compared to that in CT rats. The area under the curve of insulin increase was significantly lower (P < 0.05) in LP compared to CT rats (Figure 3D), although in both CT and LP rats these levels returned to baseline levels by 60 min after glucose injection (Figure 3C). Plasma insulin levels were lower in LP rats up to 30 min after glucose injection [2.89-fold (P < 0.01), 2.55-fold (P < 0.01), and 3.99-fold (P < 0.001) lower in LP rats, respectively], despite the elevated glucose levels in this period.
Figure 3.
Glucose tolerance test on day 19 of pregnancy. (A) Blood glucose concentrations after injection of glucose. (B) The area under the curve of glucose. (C) Plasma insulin levels after injection of glucose. (D) The area under the curve of insulin. CT: control; LP, low protein. The error bar represents the mean ± SEM (n = 4–5 rats/diet group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Insulin secretion in the presence of high and low glucose concentrations
To investigate if the sensitivity of pancreatic islets to the changes in glucose levels is altered in LP rats, we measured insulin secretion from pancreatic islets in the presence of low and high glucose concentrations which mimic the blood glucose concentration in the fasting and fed status, respectively [26]. In the presence of both 2.8 and 16.7 mM glucose concentrations, insulin secretion from isolated pancreatic islets from LP rats was significantly lower [2.33-fold (P < 0.05) and 2.70-fold (P < 0.001) lower in LP rats compared to CT rats, respectively)]. In CT rats, insulin secretion was 7.72-fold higher (P < 0.001) in the presence of 16.7 than 2.8 mM glucose. Similarly, in LP rats, insulin secretion was 6.67-fold higher (P < 0.001) in the presence of 16.7 than 2.8 mM glucose (Figure 4).
Figure 4.
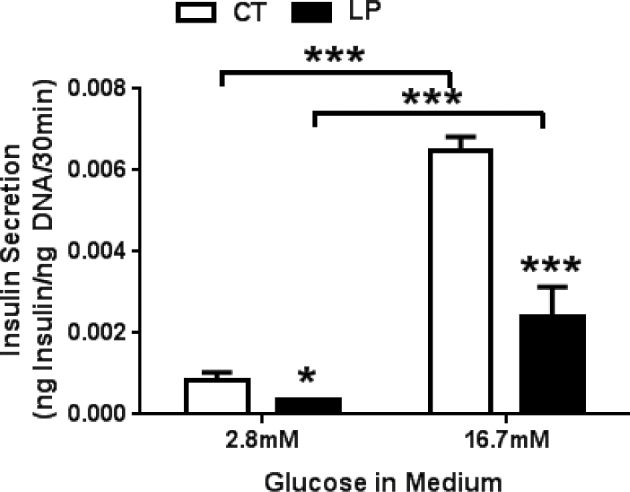
Glucose-stimulated insulin secretion in pancreatic islets from pregnant rats fed a low protein diet in the presence of 2.8 and 16.7 mM glucose. CT: control; LP, low protein. The error bar represents the mean ± SEM expressed as the ratio of amount of insulin secreted in medium during 30-min incubation to total amount of DNA in pancreatic islets (n = 4–5 rats/diet group). ***, P < 0.001.
Insulin secretion in the presence of KCl, glibenclamide, and L-Arg
To investigate the underlying mechanisms for reduced insulin secretion in pancreatic islets in LP rats, insulin secretion from islets was measured in the presence of three commonly used chemicals: KCl, glibenclamide, and L-Arg. In islets from CT rats, insulin secretion in the presence of KCl, glibenclamide, and L-Arg was increased by 4.18-fold (P < 0.001), 3.04-fold (P < 0.001), 1.71-fold (P < 0.05), respectively, compared to NONE treatment (vehicle treatment without any of the three chemicals). In islets from LP rats, insulin secretion in the presence of KCl, glibenclamide, and L-Arg was increased by 5.66-fold (P < 0.001), 2.27-fold (P < 0.001), 1.01-fold (not significant), respectively, compared to NONE treatment. Insulin secretion in islets of LP rats was 2.33-fold (P < 0.05), 1.73-fold (P < 0.05), 3.12-fold (P < 0.001), and 3.95-fold (P < 0.001) lower than that of CT rats in the presence of NONE, KCl, glibenclamide, and L-Arg, respectively (Figure 5). Thus, membrane depolarization, KATP activities, and L-Arg-related ER stress may be altered in pancreatic islets in LP rats.
Figure 5.
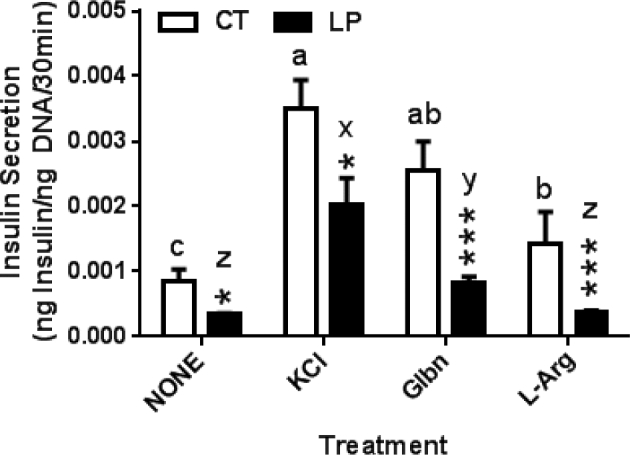
Effect of KCl, glibenclamide, and L-Arg on glucose-stimulated insulin secretion of pancreatic islets in the presence of 2.8 mM glucose. The error bar represents the mean ± SEM expressed as the ratio of amount of insulin secreted in medium during 30-min incubation to total amount of DNA in pancreatic islets (n = 4–5 rats/diet group). CT: control; LP, low protein. NONE: culture medium without supplementation of ghrelin and its antagonist; Glbn: glibenclamide; L-Arg: L-arginine HCl. Asterisks denote the statistical difference in each treatment between CT and LP rats. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Different letters denote statistical differences among all treatments within CT (a–c) or LP (x–z) group, P < 0.05.
Total insulin content in pancreatic islets
Total insulin content in pancreatic islets may affect insulin secretion. To determine whether reduced total insulin content also contributes to lower insulin secretion in LP rats, the total insulin content of pancreatic islets was measured and normalized to the total DNA amount. Total insulin content in pancreatic islets was 1.6-fold lower (P < 0.05) in LP compared to CT rats on day 19 of pregnancy (Figure 6). This indicates that the reduced insulin content in islets may also contribute to the impaired insulin secretion.
Figure 6.
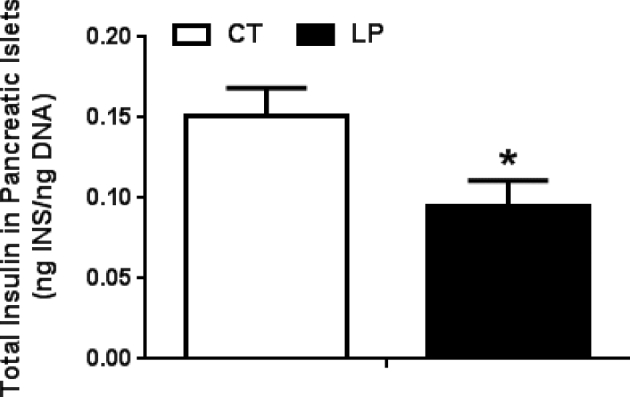
Total insulin content in pancreatic islets on day 19 of pregnancy. CT: control; LP, low protein. INS: insulin. The error bar represents the mean ± SEM expressed as the ratio of total amount of insulin in pancreatic islets to total amount of DNAs (ng/ng; n = 4–5 rats/diet group). *, P < 0.05.
Expression of proinsulin in pancreatic islets
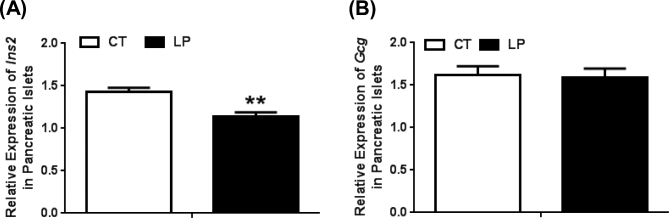
Besides insulin secretion, the insulin content in pancreatic islets could be affected by insulin expression which is regulated at transcriptional, translational, and post-translational levels. We investigated whether mRNA expression of insulin (Ins) and glucagon (Gcg) is affected in LP rats. The expression of Ins2 in pancreatic islets was 1.30-fold lower (P < 0.05) in LP compared to CT rats at day 19 of pregnancy (Figure 7A), indicating that insulin expression levels in beta cells are also altered by the LP diet in late pregnancy. In contrast, expression of Gcg was unaltered in LP rats (Figure 7B), indicating that the effect of LP diet on Ins expression in beta cells does not occur to Gcg expression in alpha cells.
Figure 7.
Quantitative real-time PCR analysis of expression of Ins2 (A) and Gcg (B) in pancreatic islets on Day 19 of pregnancy. CT: control; LP, low protein. The error bar represents the mean ± SEM expressed as relative units of mRNA standardized against Actb (n = 5 rats/diet group). **, P < 0.01.
Discussion
In normal pregnancy, enhanced plasma insulin levels are not only required to compensate for insulin resistance [1–4], but also contribute to placental and fetal growth [19,20]. However, pregnant rats fed low protein diet, a widely accepted model for studies on fetal programming of hypertension and diabetes [27], demonstrate reduced plasma insulin levels [13–15]. The current study confirmed significantly reduced plasma insulin levels in pregnant rats fed LP diet during mid and late pregnancy (Figure 1) and for the first time, to our knowledge, found the enhanced glucose intolerance (Figure 3) and the reduced GSIS from pancreatic islets in pregnant rats fed LP diet (Figure 4). More importantly, this study revealed that impaired KATP channel activities in pancreatic islets (Figure 5) and reduced insulin gene expression (Figures 6 and 7) contributed to the reduced insulin secretion from pancreatic islets. As a consequence to the reduced insulin secretion, glucose metabolism is impaired in addition to fetal and placental growth restriction [28–31], in pregnant rats fed LP diet. Thus, this study sheds a new light on the research of fetal programming in response to gestational protein insufficiency.
Low protein diet causes the reduced plasma insulin levels during mid-late pregnancy. The reduced plasma insulin levels during late pregnancy in response to LP diet have been reported by others [13–15]. It is noteworthy that previous studies focused on rats not later than day 15 of pregnancy when insulin resistance has not occurred [15]. In this study, the unaltered plasma insulin levels in LP rats on day 10 of pregnancy but reduced values during mid and late pregnancy (Figure 1) indicates that some factors related to the progression of pregnancy that regulates insulin secretion are yet to be defined. For instance, our previous studies showed that the diet intake demonstrates biphasic changes with the progression of pregnancy, with greater in early pregnancy, but less diet intake in late pregnancy in LP compared to control rats and that the body weight gain was similar in early pregnancy between these two groups, but it was less in mid and late pregnancy in LP compared to CT groups [21,25]. However, how the changes in diet intake and body compositions in pregnant rats in response to the LP diet affect insulin secretion remains unclear. In addition, the control and LP diet in our studies are isocaloric and reduced protein components are compensated with excess sucrose; thus, the higher contents of sucrose may contribute to insulin resistance as shown in literature [32,33]. Intriguingly, the effect of excess sucrose in LP diet on insulin resistance is not revealed by unaltered insulin sensitivity in LP rats in this study (Figure 2).
Impaired insulin secretion causes the reduced plasma insulin levels in pregnant rats fed LP diet during late pregnancy. This causal relationship is supported by both in vivo and ex vivo experiments in this study. In GTT, both blood glucose levels and area under the curve of glucose were higher in LP rats compared to CT rats (Figure 3). More remarkably, at 60 min after glucose injection, plasma insulin levels are similar in CT and LP rats, but plasma glucose levels were still 2.19-fold higher in LP rats. These results clearly demonstrated more severe glucose intolerance in LP than CT rats which develop glucose intolerance naturally after day 15 of pregnancy [2]. Moreover, the enhanced glucose intolerance is due to the reduced insulin secretion, but not impaired insulin sensitivity, because in ITT, blood glucose levels were similar in CT and LP groups at all time points after administration of insulin (Figure 2), suggesting that insulin sensitivity in LP rats is similar to that in CT rats during late pregnancy. In ex vivo GSIS of pancreatic islets, in the presence of both low and high glucose, islets of LP rats release less amount of insulin than those of CT rats (Figure 4), consistent with other studies [15]. The lower GSIS in the presence of low glucose in LP rats is parallel with the lower plasma insulin levels in the fed status, investigated during 8–10 am on different days of pregnancy in this study (Figure 1). It is noteworthy that another study demonstrated reduced GSIS in LP rats in response to higher dose of glucose but not lower dose [34]; however, this previous study was conducted on day 15 of pregnancy and diet contents and secreted insulin experiment were different from ours, and therefore a direct comparison between this previous study and ours is not possible. In addition, it is noteworthy that ITT was done after 6-h fasting, so the basal glucose levels were lower than the fed status. ITT in this study only reflects the capacity of insulin in handling lower blood glucose, but not high blood glucose situations. Taken together, the reduction of plasma insulin levels is mainly attributed to impaired insulin secretion in rats fed LP diet during late pregnancy, although reduced diet intake may affect postprandial plasma insulin levels [21].
This study for the first time, to our knowledge, explored the underlying mechanisms of reduced GSIS in pregnant rats fed LP diet. According to the current consensus model on GSIS [10,11], glucose metabolism and activities of ion channels in beta cells are two key regulators in GSIS. In this study, we did not study glucose metabolism in beta cells, but mainly investigated potential changes in ion channels (primarily KATP) in beta cells by applying three chemicals, KCl, glibenclamide, and L-Arg, in GSIS. These three chemicals target on different players in GSIS, which have been widely used to study the GSIS pathway [12]. High dose of extracellular KCl depolarizes plasma membrane of beta cell via various potassium channels, and activates voltage-dependent calcium channels (Cav). Glibenclamide binds to sulfonylurea receptor, a subunit of KATP channel [35], leading to closure of KATP and depolarization of plasma membrane [36]. The mechanism of arginine-induced insulin release remains unclear to date, although plasma membrane depolarization [37] and the involvement of the cAMP pathway [38] and Gαi2 [39] have been suggested. The responsiveness of pancreatic islets to KCl was lower in LP rats compared to control rats (Figure 5), suggesting the blunt depolarization of plasma membrane and/or disruption of following events in beta cells, while the reduced responsiveness to glibenclamide specifically suggested the disrupted activity of KATP channels in LP rats (Figure 5). In addition to KATP, other K+ channels may be also altered in beta cells of LP rats. The outward of potassium mainly via Kv2.1 attenuates membrane excitability and consequently suppresses calcium influx and insulin release [40]. Kv2.1 is the major Kv channel subtype present in beta cells and contributes up to 85% of the total Kv current [41,42]. More importantly, unlike KATP, Kv2.1 expression and currents could be modulated by glucose in a dose-dependent manner [43], and also by other factors such as ghrelin [44]. To support this, plasma ghrelin levels was significantly elevated in LP rats [21]. More intriguingly, pancreatic islets of LP rats did not respond to extracellular L-Arg, but islets of CT rats did. Recently, a novel mechanism mediated by endoplasmic reticulum (ER) has been proposed, in which insulin release from ER is achieved by arginine target factors, functional complexes located on ER [45]. Another recent study found that arginine stimulates ER stress in isolated pancreatic islet [46]. Thus, in pregnant rats fed LP diet, arginine-induced ER stress may not only impair insulin secretion in a short term, but also affect insulin translation and post-translational modification in a long term as these procedures occur in ER. Synergistically with the reduced insulin transcription (Figure 7), ER stress may cause the reduced total content of insulin in pancreatic islets in LP rats (Figure 6). In addition, although the fold changes (7.72 in control rats; 6.67 in LP rats) of secreted insulin in response to low and high concentration of glucose seem similar between control and LP groups (Figure 4), the different fold changes in the amount of secreted insulin in response to the three chemicals, more obviously the nonresponse of pancreatic islets in LP rats to L-Arg (Figure 5), support that the decreased secretion in LP rat is mechanistic.
This study aimed to investigate whether insulin secretion is reduced in pancreatic islets of pregnant rats fed the LP diet and to exploring whether the classical GSIS pathway is interrupted in pancreatic islets of LP rats and thus, reducing insulin secretion. Our data are supportive for proving our hypothesis; however, cautions should be taken when interpreting them. First, we explored primarily KATP activity in this study, but calcium channel, calcium influx, glucose transport, and metabolism in the KATP-dependent GSIS pathway were not included, so it was with other recently proposed mechanisms such as KATP and calcium channel-independent GSIS [10]. All these players or events in GSIS warrant to be screened in future studies in order to find potential interventions. Second, we could not report the physiological blood glucose levels in pregnant rats in fed status because many factor could affect blood glucose measurement [47]. Instead of seeking correlation between blood glucose and insulin levels, glucose clamp techniques, the golden standard for measuring insulin secretion and resistance, will be applied in future studies. Third, we did not address whether the reduced insulin content in beta cells of LP rats affect insulin secretion. The decreased insulin content in LP islets may be due to the reduced expression of Ins (Figure 6), and unaltered expression of Gcg in LP islets may indicate that the effects of LP diet are specific for beta cells (Figure 7). Fourth, our data do not reveal which factors in the LP diet fed animals will lead to the reduced insulin secretion, because the increased sucrose content in LP diet, increased non-essential amino acids in the plasma, altered diet intake, and body composition in dams may play a role in insulin resistance and glucose intolerance.
It is noteworthy that fetal growth is restricted in pregnant rats fed LP diet [19,20], despite more severe glucose intolerance. In contrast, gestational diabetes in humans often results in fetal macrosomia. This discrepancy may be due to species differences. Adipose tissue development and associated fat accumulation occur postnatally in rodents but prenatally in humans; therefore, many nutritional manipulations during pregnancy failed to generate fetal macrosomia in rodents [48]. More importantly, insulin acts as a growth factor for placental and fetal growth by stimulating the mTOR signaling pathway [19,20]; therefore, reduced insulin levels may be responsible for impaired mTOR signaling (downstream effector of insulin activity) and macronutrient (glucose and amino acids) transport in placentas in pregnant rats fed LP diet [30,49,50]. In addition, fetal growth restriction is also associated with glucose intolerance in the adult offspring of LP dams [51–54]; thus, the reduced maternal insulin levels may play an important role in the programming of diabetes in offspring.
In summary, decreased GSIS of pancreatic islets in pregnant rats fed LP diet is responsible for the reduced plasma insulin levels and consequently enhanced glucose intolerance, while reduced insulin gene expression in LP rats is also involved in impaired insulin secretion. The disrupted KATP channels and/or perhaps events following plasma membrane depolarization in GSIS contribute to the reduced GSIS in pregnant rats fed the low protein diet and are schematically shown in Figure 8. Based on these findings, we propose that the reduced insulin availability in pregnant rats fed LP diet may be one of maternal mechanisms for restricted placental and fetal growth and associated fetal developmental programming, and this hypothesis will be tested in future studies.
Figure 8.
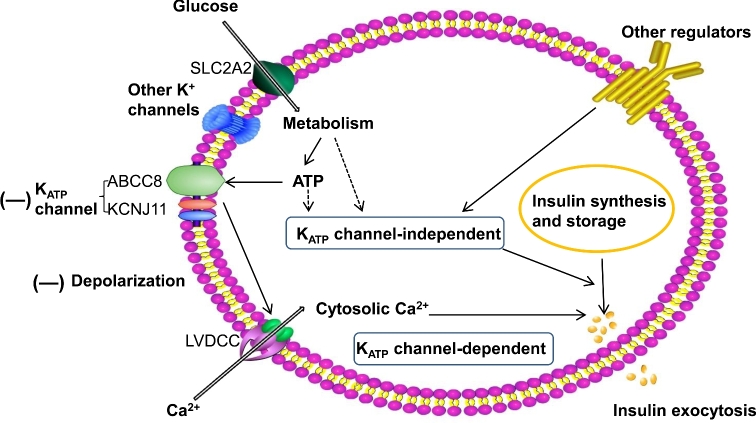
Schematic model of reduced GSIS pancreatic beta cells in pregnant rats fed the low protein diet. GSIS via the classical KATP channel-dependent pathway has been measured in pancreatic islets from CT and LP rats in late pregnancy. In both CT and LP rats, high extracellular K+ causes plasma membrane depolarization and glibenclamide specifically blocks the activities of KATP channel via ABCC8, but insulin secretion was lower in LP rats. Extracellular L-arginine increases insulin secretion via stimulating plasma membrane depolarization in CT rats, but not LP rats. Therefore, the reduced insulin secretion in LP rats could be attributed to disrupted KATP channel activities and perhaps other downstream events in GSIS following plasma membrane depolarization (indicated by bold minus signs). Other KATP channel-independent GSIS pathways were not investigated in this animal model. CT: normal protein diet; LP: low protein diet; ABCC8: sulfonylurea receptor 1 (Alias: SUR1); ATP, adenosine triphosphate; KCNJ11: K+ channel 6.2 subunit (Alias: Kir6.2); LVDCC, L-type voltage-dependent calcium channel; SLC2A1: glucose transporter (Alias: GLUT1); (—): reduced activities or procedures (modified from Reference [10]).
Acknowledgments
The authors thank Dr Daniel Simoes and Jong Kyung Lee in Division of Diabetes, Endocrinology and Metabolism, Department of Medicine, Baylor College of Medicine for their technic supports and also Sandra Dale for editorial work on this manuscript and administrative support.
Contributor Information
Haijun Gao, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, Texas, USA; Texas Children's Hospital, Houston, Texas, USA.
Eric Ho, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, Texas, USA; Texas Children's Hospital, Houston, Texas, USA.
Meena Balakrishnan, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, Texas, USA; Texas Children's Hospital, Houston, Texas, USA.
Vijay Yechoor, Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Chandra Yallampalli, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, Texas, USA; Texas Children's Hospital, Houston, Texas, USA.
References
- 1. Leturque A, Ferre P, Satabin P, Kervran A, Girard J. In vivo insulin resistance during pregnancy in the rat. Diabetologia 1980; 19:521–528. [DOI] [PubMed] [Google Scholar]
- 2. Munoz C, Lopez-Luna P, Herrera E. Glucose and insulin tolerance tests in the rat on different days of gestation. Biol Neonate 1995; 68:282–291. [DOI] [PubMed] [Google Scholar]
- 3. Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992; 130:1459–1466. [DOI] [PubMed] [Google Scholar]
- 4. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 1997; 29:301–307. [DOI] [PubMed] [Google Scholar]
- 5. Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000; 71:1256S–1261S. [DOI] [PubMed] [Google Scholar]
- 6. Di CG, Miccoli R, Volpe L, Lencioni C, Del PS. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res Rev 2003; 19:259–270. [DOI] [PubMed] [Google Scholar]
- 7. Knopp RH, Ruder HJ, Herrera E, Freinkel N. Carbohydrate metabolism in pregnancy. VII. Insulin tolerance during late pregnancy in the fed and fasted rat. Acta Endocrinol (Copenh) 1970; 65:352–360. [PubMed] [Google Scholar]
- 8. Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, Koizumi M, Sone H, Nakata M, Kakei M, Dezaki K. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes Obes Metab 2014; 16(Suppl 1):111–117. [DOI] [PubMed] [Google Scholar]
- 9. Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine 2000; 13:251–262. [DOI] [PubMed] [Google Scholar]
- 10. Komatsu M, Takei M, Ishii H, Sato Y. Glucose-stimulated insulin secretion: a newer perspective. J Diabetes Investig 2013; 4:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henquin JC, Nenquin M, Ravier MA, Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes Metab 2009; 11(Suppl 4):168–179. [DOI] [PubMed] [Google Scholar]
- 12. Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, Ma K, Yechoor VK. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets 2011; 3:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iglesias-Barreira V, Ahn MT, Reusens B, Dahri S, Hoet JJ, Remacle C. Pre- and postnatal low protein diet affect pancreatic islet blood flow and insulin release in adult rats. Endocrinology 1996; 137:3797–3801. [DOI] [PubMed] [Google Scholar]
- 14. de-Mello MA, Cury L. Effects of protein-calorie malnutrition on endocrine pancreatic function in young pregnant rats. Braz J Med Biol Res 1989; 22:791–794. [PubMed] [Google Scholar]
- 15. de Mello MA, Luciano E, Carneiro EM, Latorraca MQ, Machado de Oliveira CA, Boschero AC. Glucose homeostasis in pregnant rats submitted to dietary protein restriction. Res Commun Mol Pathol Pharmacol 2003; 113–114:229–246. [PubMed] [Google Scholar]
- 16. Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond) 2008; 114:381–392. [DOI] [PubMed] [Google Scholar]
- 17. Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 2005; 566:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, Yang K. Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab 2005; 288:E663–E673. [DOI] [PubMed] [Google Scholar]
- 19. Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 2009; 37:295–298. [DOI] [PubMed] [Google Scholar]
- 20. Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 2014; 28:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao H, Tanchico DT, Yallampalli U, Balakrishnan MP, Yallampalli C. Appetite regulation is independent of the changes in ghrelin levels in pregnant rats fed low-protein diet. Physiol Rep 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967; 16:35–39. [DOI] [PubMed] [Google Scholar]
- 23. Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 2004; 53:3142–3151. [DOI] [PubMed] [Google Scholar]
- 24. Alves ES, Haidar AA, Quadros CD, Carvalho DS, Morgan D, Rocha MS, Curi R, Carpinelli AR, Hirata AE. Angiotensin II-induced JNK activation is mediated by NAD(P)H oxidase in isolated rat pancreatic islets. Regul Pept 2012; 175:1–6. [DOI] [PubMed] [Google Scholar]
- 25. Gao H, Sisley S, Yallampalli C. Blunted hypothalamic ghrelin signaling reduces diet intake in rats fed a low-protein diet in late pregnancy. Physiol Rep 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu 2010; 39:133–137, 142. [PubMed] [Google Scholar]
- 27. Kautzky-Willer A, Handisurya A. Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Invest 2009; 39:631–648. [DOI] [PubMed] [Google Scholar]
- 28. Gao H, Yallampalli U, Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol Reprod 2012; 86:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao H, Sathishkumar KR, Yallampalli U, Balakrishnan M, Li X, Wu G, Yallampalli C. Maternal protein restriction regulates IGF2 system in placental labyrinth. Front Biosci (Elite Ed) 2012; 4:1434–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 2006; 576:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr 1999; 81:243–250. [PubMed] [Google Scholar]
- 32. Oron-Herman M, Kamari Y, Grossman E, Yeger G, Peleg E, Shabtay Z, Shamiss A, Sharabi Y. Metabolic syndrome: comparison of the two commonly used animal models. Am J Hypertens 2008; 21:1018–1022. [DOI] [PubMed] [Google Scholar]
- 33. Vasanji Z, Cantor EJ, Juric D, Moyen M, Netticadan T. Alterations in cardiac contractile performance and sarcoplasmic reticulum function in sucrose-fed rats is associated with insulin resistance. Am J Physiol Cell Physiol 2006; 291:C772–C780. [DOI] [PubMed] [Google Scholar]
- 34. Souza DF, Ignacio-Souza LM, Reis SR, Reis MA, Stoppiglia LF, Carneiro EM, Boschero AC, Arantes VC, Latorraca MQ. A low-protein diet during pregnancy alters glucose metabolism and insulin secretion. Cell Biochem Funct 2012; 30:114–121. [DOI] [PubMed] [Google Scholar]
- 35. Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 1995; 270:1166–1170. [DOI] [PubMed] [Google Scholar]
- 36. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002; 51(Suppl 3):S368–S376. [DOI] [PubMed] [Google Scholar]
- 37. Thams P, Capito K. L-arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur J Endocrinol 1999; 140:87–93. [DOI] [PubMed] [Google Scholar]
- 38. Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology 2012; 153:4608–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leiss V, Flockerzie K, Novakovic A, Rath M, Schonsiegel A, Birnbaumer L, Schurmann A, Harteneck C, Nurnberg B. Insulin secretion stimulated by L-arginine and its metabolite L-ornithine depends on Galpha(i2). Am J Physiol Endocrinol Metab 2014; 307:E800–E812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 2007; 56:2319–2327. [DOI] [PubMed] [Google Scholar]
- 41. Roe MW, Worley JF III, Mittal AA, Kuznetsov A, DasGupta S, Mertz RJ, Witherspoon SM III, Blair N, Lancaster ME, McIntyre MS, Shehee WR, Dukes IDet al. Expression and function of pancreatic beta-cell delayed rectifier K+ channels. Role in stimulus-secretion coupling. J Biol Chem 1996; 271:32241–32246. [DOI] [PubMed] [Google Scholar]
- 42. MacDonald PE, Ha XF, Wang J, Smukler SR, Sun AM, Gaisano HY, Salapatek AM, Backx PH, Wheeler MB. Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion. Mol Endocrinol 2001; 15:1423–1435. [DOI] [PubMed] [Google Scholar]
- 43. Chu KY, Cheng Q, Chen C, Au LS, Seto SW, Tuo Y, Motin L, Kwan YW, Leung PS. Angiotensin II exerts glucose-dependent effects on Kv currents in mouse pancreatic beta-cells via angiotensin II type 2 receptors. Am J Physiol Cell Physiol 2010; 298:C313–C323. [DOI] [PubMed] [Google Scholar]
- 44. Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003; 144:916–921. [DOI] [PubMed] [Google Scholar]
- 45. Umeda M, Hiramoto M, Watanabe A, Tsunoda N, Imai T. Arginine-induced insulin secretion in endoplasmic reticulum. Biochem Biophys Res Commun 2015; 466:717–722. [DOI] [PubMed] [Google Scholar]
- 46. Mullooly N, Vernon W, Smith DM, Newsholme P. Elevated levels of branched-chain amino acids have little effect on pancreatic islet cells, but L-arginine impairs function through activation of the endoplasmic reticulum stress response. Exp Physiol 2014; 99:538–551. [DOI] [PubMed] [Google Scholar]
- 47. Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010; 3:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol 2016; 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011; 152:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhasin KK, van NA, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 2009; 58:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peixoto-Silva N, Frantz ED, Mandarim-de-Lacerda CA, Pinheiro-Mulder A. Maternal protein restriction in mice causes adverse metabolic and hypothalamic effects in the F1 and F2 generations. Br J Nutr 2011; 106:1364–1373. [DOI] [PubMed] [Google Scholar]
- 52. Blesson CS, Schutt AK, Balakrishnan MP, Pautler RG, Pedersen SE, Sarkar P, Gonzales D, Zhu G, Marini JC, Chacko SK, Yallampalli U, Yallampalli C. Novel lean type 2 diabetic rat model using gestational low-protein programming. Am J Obstet Gynecol 2016; 214: 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cox AR, Gottheil SK, Arany EJ, Hill DJ. The effects of low protein during gestation on mouse pancreatic development and beta cell regeneration. Pediatr Res 2010; 68:16–22. [DOI] [PubMed] [Google Scholar]
- 54. Heywood WE, Mian N, Milla PJ, Lindley KJ. Programming of defective rat pancreatic beta-cell function in offspring from mothers fed a low-protein diet during gestation and the suckling periods. Clin Sci (Lond) 2004; 107:37–45. [DOI] [PubMed] [Google Scholar]