Abstract
Infection of BALB/c mice with Listeria monocytogenes stimulates an antilisterial immune response evident by the appearance of H2-Kd-restricted CD8+ cytotoxic T lymphocytes (CTLs) specific for the nanomer peptides amino acids (aa) 91 to 99 of listeriolysin O (LLO 91–99) and aa 217 to 225 of the p60 molecule (p60 217–225). We have introduced point mutations at anchor residues within LLO 91–99 (92F) or p60 217–225 (218F), and BALB/c mice infected with L. monocytogenes strains containing these point mutations do not develop CTLs specific for LLO 91–99 or p60 217–225, respectively. We have used these strains to test whether primary CTL responses against L. monocytogenes-derived determinants can be stimulated within an environment of existing antilisterial immunity. We found that the development of a primary L. monocytogenes-specific CTL response is not altered by existing immunity to L. monocytogenes. For example, primary immunization with the p60 218F strain of L. monocytogenes followed by a secondary immunization with wild-type L. monocytogenes results in stimulation of p60 217–225-specific CTLs at primary response levels and LLO 91–99-specific effectors at levels consistent with a memory CTL response. Similarly, primary immunization with the 92F strain of L. monocytogenes followed by a secondary immunization with wild-type L. monocytogenes results in stimulation of LLO 91–99-specific CTLs at primary response levels and p60 217–225-specific effectors at levels consistent with a memory CTL response. These results provide additional support for the use of L. monocytogenes as a recombinant vaccine vector and show that antivector immunity does not inhibit the development of a primary CTL response when the epitope is delivered by L. monocytogenes as the vaccine strain.
The induction of a protective immune response against the intracytoplasmic pathogen Listeria monocytogenes occurs following subclinical infection with a viable listeriolysin O (LLO)-producing strain. Nonviable L. monocytogenes preparations as well as non-LLO-secreting strains are avirulent and do not trigger protective antilisterial immunity (1, 2, 13, 27). It has been established that protective antilisterial immunity can be adoptively transferred with T cells of the CD8+ subset (4, 16). As a facultative intracellular pathogen, L. monocytogenes can infect and then replicate within professional major histocompatibility complex (MHC) class II-positive phagocytes (macrophages) and MHC class II-negative nonprofessional phagocytic cells, such as fibroblasts (36). Thus, a required involvement of the MHC class I-restricted CD8+ T-cell subset is consistent with clearance of a pathogen which can replicate within MHC class II-negative cells, because MHC class II-negative cells are not typically armed with the necessary mechanisms to kill intracellular bacteria. This is supported by a recent report showing that Kb-restricted Listeria-specific CD8+ T cells adoptively transfer antilisterial protection in transgenic mice in which Kb was only expressed on hepatocytes (17).
As greater understanding of disease processes and protective immunity is developed, novel approaches for the design of effective vaccine delivery systems are being tested, including recombinant vaccinia virus and adenovirus vectors (14, 29, 41). L. monocytogenes has also been proposed as a vaccine carrier, a concept founded on the observation that L. monocytogenes is a pathogen that replicates within the intracytoplasmic environment, thus facilitating delivery of antigen to the endogenous antigen-processing–presentation pathway. An initial report found that β-galactosidase-specific CTLs were stimulated in mice following immunization with an L. monocytogenes strain that secreted this molecule (38). Subsequent studies showed that immunization of mice with recombinant L. monocytogenes strains that secrete foreign gene products, including the p55 molecule of human immunodeficiency virus (15), tumor antigens (29, 34), lymphocytic choriomeningitis virus (LCMV) nucleoprotein (NP)-derived epitope (39), or an influenza virus NP-derived epitope (21), stimulate the desired peptide-specific CD8+ CTL population. The CD8+ CTLs are protective in the tumor model (34), and mice immunized with the LCMV recombinant strain are protected against a lethal challenge with LCMV (39). Finally, rabbits immunized with an L. monocytogenes strain that secretes a papillomavirus-derived antigen are protected against papillomavirus infection (22).
Collectively, these data argue strongly for the continued assessment of the efficacy of recombinant L. monocytogenes as a vaccine strain. However, an issue that is not resolved for the general use of any vaccine vector is what the constraints to the stimulation of a specific primary response are when existing immunity to the vector is present. This is an important consideration, given the unknown status of immunity to any vector within the general population. We have addressed this consideration with repeated immunization utilizing strains of L. monocytogenes that do or do not specifically stimulate peptide-specific CTL responses. We found that L. monocytogenes-specific primary CTL responses can be stimulated in an environment of established antilisterial immunity. These results are consistent with the use of L. monocytogenes as a candidate vaccine strain.
MATERIALS AND METHODS
Bacteria.
L. monocytogenes 10403 serotype 1 was originally obtained from the American Type Culture Collection (Rockville, Md.). Virulence was maintained by repeated passage in BALB/c mice. The development of the 92F strain has been described previously (9).
Development of the p60 218F strain.
Overlap extension PCR (20) was used to introduce mutations in the sequence encoding the p60 217–225 (amino acids [aa] 217 to 225) epitope. Codon 218 of p60 was changed from TAC to TTC, resulting in substitution of phenylalanine for tyrosine. In addition, an AatII site was created that resulted in substitution of valine for isoleucine at position 225 (ATT to GTC). These mutations were introduced into the L. monocytogenes genome by allelic exchange (39). To identify the desired mutant, the p60 gene was amplified from genomic DNA of individual isolates by PCR and screened for the presence of the introduced AatII site. The point mutations were further confirmed by direct sequencing of the PCR products.
Mice and Immunization.
Six-week-old female BALB/c mice were purchased from Bantin-Kingman (Freemont, Calif.). Mice were provided unrestricted access to food and water. Eight-week-old mice were immunized with approximately 0.1 50% lethal dose (LD50) of viable L. monocytogenes in 0.2 ml of phosphate-buffered saline (PBS) injected via the tail vein. The same immunization protocol was used for all primary and secondary immunizations.
Cell lines and reagents.
The J774 cell line was maintained by culture in Dulbecco’s modified Eagle’s medium (DMEM) (antibiotic free) supplemented with nonessential amino acids (Gibco, Grand Island, N.Y.) and supplemented with 5% fetal calf serum (FCS) (Tissue Culture Biologicals, Tulare, Calif.). The RMAS-Kd cell line (obtained from Mike Bevan, University of Washington, Seattle, Wash.) was maintained in RPMI 1640 (Gibco) supplemented with 10% FCS. The LLO 91–99 (GYKDGNEYI) and p60 217–225 (KYGVSVQDI) peptides were synthesized with an Applied Biosystems Synergy apparatus by using standard Fmoc chemistry at the Portland Veterans Administration Medical Center. The p60 449–457 (IYVGNGQMI) peptide was synthesized by SynPep Corp. (Dublin, Calif.).
Cell culture.
Spleen cells from mice immunized 6 days previously with L. monocytogenes (either as a primary or secondary injection) were stimulated in culture with 1.0 μg of concanavalin A (ConA) (Sigma, St. Louis, Mo.) per ml in RPMI 1640 containing 100 U of penicillin (Sigma) per ml 100 μg of streptomycin (Sigma) per ml, 5% FCS, and 5 × 10−5 M 2-mercaptoethanol 2-ME (Sigma). A total of 108 cells in 50 ml were cultured in a 75-cm2 flask for 72 h at 37°C in humidified air with 7.5% CO2. Following culture, the recovered cells were used in assays of CTL activity.
CFU reduction assay.
J774 target cells were deposited at 1 × 105 to 2 × 105 cells/well in 24-well tissue culture plates in 1.0 ml of antibiotic-free DMEM supplemented with nonessential amino acids and 5% FCS 18 h before the assay (2, 6). The target cell monolayers were infected with L. monocytogenes (obtained from a log-phase culture) at a multiplicity of infection (MOI) of 2 to 5. After 60 min, the monolayers were washed twice with sterile PBS (37°C) and covered with 0.5 ml of DMEM containing 5% FCS and 40 μg of gentamicin sulfate per ml. Effector cells, as obtained following culture stimulation were added (typically effector/target [E/T] ratio of 20:1) in 0.5 ml of DMEM with 5% FCS 3 to 4 h after initiation of the infection. The assays were terminated 4 to 5 h later, and the number of intracellular bacteria remaining in each well was determined. Specifically, the medium was aspirated and replaced with 1 ml of distilled water. Five minutes later, dilutions were plated onto brain heart infusion (BHI) agar plates, which were incubated for 24 h at 37°C, and the number of CFU was determined. Data are presented as percent CFU reduction = [1 − (CFU in target monolayers incubated with effector cells)/(CFU in target monolayers incubated without effector cells)] × 100.
51Cr release assay.
RMAS-Kd target cells were labeled with 51Cr (5 × 106 cells, 250 μCi of 51Cr) for 60 min and then washed twice. The 51Cr-labeled target cells were pulsed with a 10−9 M concentration of the LLO 91–99, p60 217–225, or p60 449–457 peptide for 60 min. The peptide-pulsed target cells were added in 100-μl volumes to 96-well round-bottom microtiter plates at 104 cells/well. Effector cells in a 100-μl volume were added at an E/T ratio of 50:1. Following a 4-h incubation at 37°C, 150 μl of supernatant was collected, and the percent lysis was calculated as 100 × [(experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm)]. The data presented are the mean of triplicate wells. Spontaneous release was less than 10% for all experiments.
CTL frequency analysis.
51Cr-labeled RMAS-Kd target cells were pulsed with either 10−9 M LLO 91–99, p60 217–225, or p60 449–457 for 60 min and then plated in a 100-μl volume to round-bottom microtiter plates at 5 × 103 targets/well. Serial dilutions of the antilisterial effectors were added in 100-μl volumes, in replicates of 10 to 20 for each peptide, typically beginning with a 50:1 E/T ratio. The assay was terminated 4 to 6 h later, the 96-well plates were centrifuged, and 0.15 ml of supernatant was removed from each well for analysis. Wells were scored positive if release exceeded 3 standard deviations above the spontaneous release. The fraction of negative wells was plotted against the number of input cells, and the CTL frequency was calculated as the number of cells in the total population that corresponds to the 37% negative value (40).
RESULTS
L. monocytogenes strains used for the present studies.
We have shown previously that LLO 91–99-specific CTLs are absent in mice immunized with a strain of L. monocytogenes in which the tyrosine (single-letter-code Y) at amino acid position 92 within LLO has been substituted for by a phenylalanine (single-letter-code F) (9). In these studies, we were unable to detect any stimulation of LLO 91–99-specific CTLs in mice immunized with the 92F mutant. In addition, we were unable to detect the 91-92F-99 peptide as an MHC class I-associated target for LLO 91–99-specific CTLs with J774 cells infected with the 92F strain. Based upon these results with the 92F mutant and the ability to manipulate the CTL response following immunization with L. monocytogenes, we developed a strain of L. monocytogenes in which the tyrosine at position 2 within the p60 217–225 epitope has been changed to phenylalanine, thus eliminating the anchor residue at position 2 that is required for Kd binding. In addition, the isoleucine at amino acid 225 has been changed to valine. We refer to this mutant as the p60 218F strain. Table 1 shows the amino acid sequences of the LLO 91–99 and p60 217–225 determinants for the 92F and p60 218F strains used for the studies presented in this report.
TABLE 1.
Amino acid sequences of LLO 91–99 or p60 217–225 for L. monocytogenes mutants
| L. monocytogenes straina | Amino acid sequenceb
|
|
|---|---|---|
| LLO 91–99 | p60 217–225 | |
| Wild type | GYKDGNEYI | KYGVSVQDI |
| p60 218F | GYKDGNEYI | KFGVSVQDV |
| 92F | GFKDGNEYI | KYGVSVQDI |
The L. monocytogenes strains utilized for study include the wild type; the 92F mutant, which contains a Y-to-F substitution at aa 92 of LLO; or p60 218F, which contains a Y-to-F substitution at aa 218 of p60 and an I-to-V substitution at aa 225. (Substitutions are underlined.)
The amino acid sequence is given in the single-letter code for each of the strains listed. The amino acid sequence was determined by DNA sequencing as described in Materials and Methods.
Peptide-specific CTLs are absent from mice immunized with the p60 218F or 92F mutants.
In order to verify that the mutants as listed in Table 1 had specific defects for stimulation of peptide-specific CTLs, BALB/c mice were immunized with viable wild-type L. monocytogenes or the 92F or p60 218F mutants. Six days later, immune spleen cells were stimulated in vitro to obtain effector CTLs that were then assessed for peptide-specific CTL responses. The data presented in Fig. 1 show that LLO 91–99 and p60 217–225-specific CTLs are stimulated following immunization with wild-type L. monocytogenes. Immunization with the p60 218F mutant results in the stimulation of LLO 91–99-specific but not p60 217–225-specific CTLs. As previously reported (9) and again shown in Fig. 1, p60 217–225-specific but not LLO 91–99-specific CTLs are stimulated in mice immunized with the 92F mutant.
FIG. 1.
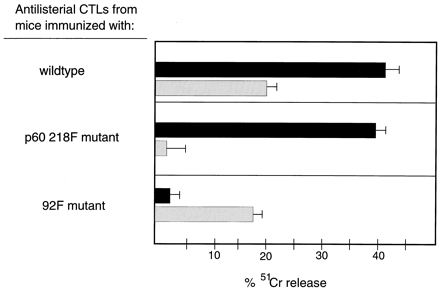
p60 217–225-specific CTLs are absent from mice immunized with the p60 218F mutant. BALB/c mice were immunized either with wild-type L. monocytogenes, the 92F mutant, or the p60 218F mutant. Six days later, spleen cells were stimulated in culture for 72 h, and the recovered cells were utilized as effector CTLs. 51CR-labeled RMAS-Kd cells were pulsed with a 10−9 M concentration of either the LLO 91–99 (solid bars) or p60 217–225 (stippled bars) peptide for 60 min and then added at 104 cells/well. Effector CTLs were added at an E/T ratio of 50:1. The assay was terminated 4 h later, and the percent 51Cr was determined. The data are representative of four experiments.
The CTL activity of these populations was also tested against targets cells infected with wild-type L. monocytogenes. The data presented in Fig. 2 show that J774 target cells infected with wild-type L. monocytogenes are lysed by effector CTLs from mice previously immunized with the 92F or p60 218F mutants. Thus, although LLO 91–99- or p60 217–225-specific CTLs are absent in mice immunized with the 92F or p60 218F mutants, respectively, the capacity to lyse L. monocytogenes-infected targets does not appear to be altered with these immune effector populations.
FIG. 2.
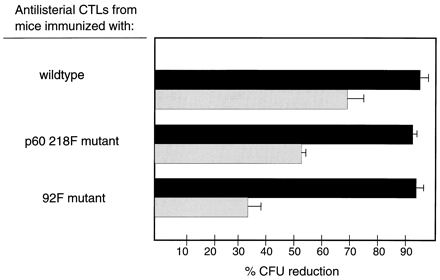
Effector CTLs from mice immunized with the p60 218F mutant lyse L. monocytogenes-infected target cells. BALB/c mice were immunized either with wild-type L. monocytogenes, the 92F mutant or the p60 218F mutant. Six days later, spleen cells were stimulated in culture for 72 h, and the recovered cells were utilized as effector CTLs. J774 target cells were infected with wild-type L. monocytogenes at an MOI of 2:1 to 5:1 and washed 60 min later, and 40 μg of gentamicin per ml was added. Three hours later, effector CTLs at an E/T ratio of 20:1 (solid bars) or 5:1 (stippled bars) were added. The assay was terminated 4 h later, and the percent CFU reduction was determined. The data are representative of four experiments. The mean number of CFU per well for L. monocytogenes-infected J774 cells in the absence of antilisterial CTLs for this experiment was 9.2 × 106.
Induction of active immunity.
The results presented in Fig. 1 show that LLO 91–99- and p60 217–225-specific CTL effectors are absent in mice immunized with the mutants in which amino acid substitutions for tyrosine have been placed at the second amino acid position for LLO 91–99 (Y to F) or p60 217–225 (Y to F). In order to determine whether the absence of LLO 91–99 or p60 217–225 CTL responses influences antilisterial protection, BALB/c mice were immunized with the 92F or p60 218F mutant, and the levels of antilisterial protection were assessed 3 weeks later. The levels of protection were compared to the level of protection in mice previously immunized with wild-type L. monocytogenes. Figure 3A shows that mice immunized previously with wild-type L. monocytogenes, the 92F mutant, or the p60 218F mutant and then challenged with 10 LD50 of wild-type L. monocytogenes show a log10 protection value in the range of 3.5 to 4. We found the levels of antilisterial protection following wild-type challenge in the group immunized previously with the p60 218F mutant to be equivalent to control levels. Similarly, and as previously reported (9), the levels of antilisterial protection following wild-type challenge in the group immunized previously with the 92F mutant were also found to be equivalent to control levels. Additional experiments were conducted in which mice were immunized with wild-type L. monocytogenes, the 92F mutant, or the p60 218F mutant and then challenged 3 weeks later with the p60 218F strain. Figure 3B shows log10 protection values for the experimental groups to be in the range of 3.5 to 4 as well.
FIG. 3.
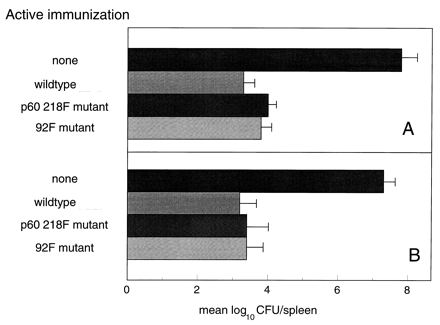
Active immunization with the p60 218F mutant protects against challenge with wild-type L. monocytogenes. BALB/c mice were immunized with approximately 0.1 LD50 of the indicated strain of L. monocytogenes. Three weeks later, the animals were challenged with wild-type L. monocytogenes (A) or the p60 218F mutant (B). Forty-eight hours later, the numbers of splenic CFU were determined. The data are presented as the mean log10 CFU per spleen for each group. The data are representative of two experiments.
The absence of p60 217–225-specific CTLs does not alter intermolecular or intramolecular CTL responses.
Results from our previous study suggest that the presence or absence of LLO 91–99-specific CTLs does not alter the magnitude of other L. monocytogenes-derived peptide-specific CTL responses (7). In order to evaluate this further, experiments were conducted with populations of effector CTLs from mice immunized with the p60 218F mutant. As shown in Table 2, the absence of p60 217–225-specific CTLs does not alter the levels of LLO 91–99-specific CTLs. In addition, the absence of p60 217–225-specific CTLs does not alter or enhance the levels of p60 449–457-specific CTLs compared to the levels measured in mice immunized with wild-type L. monocytogenes. Furthermore, the data show that p60 449–457-specific CTLs occur less frequently than CTLs to the p60 217–225 determinant. Table 2 also includes data showing results with effector CTLs from mice previously immunized with the 92F mutant. Consistent with our previous report (7), the absence of LLO 91–99-specific CTLs does not alter the levels of p60 217–225-specific CTLs. In addition, the absence of LLO 91–99-specific CTLs does not alter the level of p60 449–457-specific CTLs. Culture-activated spleen cells from normal mice or mice previously immunized with a heat-killed preparation of L. monocytogenes do not contain LLO 91–99-, p60 217–225-, or p60 449–457-specific CTLs (data not shown).
TABLE 2.
Immunization with the p60 218F mutant does not stimulate p60 217–225-specific CTLs
| Immunizationa | Effector CTL frequencyb
|
||
|---|---|---|---|
| LLO 91–99 | p60 217–225 | p60 449–457 | |
| Wild type | 1/58,500 | 1/84,500 | 1/181,000 |
| p60 218F mutant | 1/52,900 | NDc | <1/200,000 |
| 92F mutant | ND | 1/93,480 | <1/200,000 |
BALB/c mice were immunized with the indicated strains of L. monocytogenes. Six days later, spleen cells were stimulated in culture, and the recovered effectors were assessed for frequencies of peptide-specific CTLs.
51Cr-labeled RMAS-Kd cells were pulsed with a 10−9 M concentration of the indicated peptide for 60 min and then added at 5 × 103 cells/well. Serial dilutions of effector cells were added, and 4 to 6 h later, supernatant was collected for assay of 51Cr release. CTL frequency was determined as previously described (7). The data are representative of three experiments.
ND, not detected.
CTL development in an environment of existing antivector immunity.
Mice injected with the p60 218F mutant do not possess CTLs to the p60 217–225 determinant, and the levels of LLO 91–99-specific CTLs are similar to the levels observed in wild-type-immunized donors (Table 2). Similarly, mice injected with the 92F mutant do not possess CTLs to the LLO 91–99 determinant, and the levels of p60 217–225-specific CTLs are similar to the levels observed in wild-type-immunized donors (7). These observations allow experiments to test whether L. monocytogenes-specific CTLs are stimulated in an environment of existing antilisterial immunity. BALB/c mice were immunized with the p60 218F mutant or 92F mutant, and 2 months later, they were injected with wild-type L. monocytogenes. (At the time the secondary L. monocytogenes injection is given, the immune animals are resistant to a 1,000-LD50 challenge of wild-type L. monocytogenes [data not shown].) Six days later, spleen cells were stimulated to generate effector CTLs, and the frequency of effector CTLs was determined (7). Control groups include animals given a single immunization with wild-type L. monocytogenes (to establish primary CTL response levels), as well as groups of animals immunized twice with wild-type L. monocytogenes (to establish memory CTL response levels). (In order to verify the presence or absence of peptide-specific CTLs following the initial immunization, effector CTLs were obtained from a subgroup of animals 6 days following the initial injection. We found that BALB/c mice initially injected with the 92F mutant did not possess LLO 91–99-specific CTLs and that animals initially injected with the p60 218F mutant did not possess p60 217–225 CTLs. Mice initially injected with wild-type L. monocytogenes possessed LLO 91–99- and p60 217–225-specific CTLs. For these three groups, positive CTL responses were consistent with primary levels [data not shown].) The data presented in Table 3 for experiment A show that the numbers of LLO 91–99, p60 217–225, and p60 449–457-specific CTLs from the animals given a single injection of wild-type L. monocytogenes are at levels consistent with a primary CTL response. Animals that received a primary injection and a secondary injection of wild-type L. monocytogenes possess enhanced numbers of CTLs specific for LLO 91–99, p60 217–225, and p60 449–457, a finding consistent with the presence of memory CTLs. When effector CTLs from animals that received an initial immunization with the p60 218F mutant and then were given a secondary injection with wild-type L. monocytogenes are assessed, the frequencies of effector cells specific for LLO 91–99 and p60 449–457 are consistent with the presence of a memory CTL response. However, the numbers of CTLs specific for p60 217–225 are equivalent to a level measured as a primary CTL response. For animals that received an initial immunization with the 92F mutant and then were given a secondary injection with wild-type L. monocytogenes, the frequencies of effector cells specific for p60 217–225 are consistent with the presence of a memory CTL response (Table 3 [experiment B]). However, the frequency of CTLs specific for LLO 91–99 is equivalent to that detected in a primary CTL response. The results presented in Table 3, experiment C, show that, in some studies, although injection of wild-type L. monocytogenes into animals previously immunized with the 92F strain results in stimulation of LLO 91–99-specific CTLs, the effector frequency is not always equivalent to levels considered to be a primary response. However, it is apparent from this experiment that the CTL response to the p60 217–225 determinant is consistent with a memory response.
TABLE 3.
Existing antilisterial immunity does not alter the development of primary CTLs to L. monocytogenes-derived determinants
| Expt | Immunizationa
|
Effector CTL frequencyb
|
|||
|---|---|---|---|---|---|
| Primary | Secondary | LLO 91–99 | p60 217–225 | p60 449–457 | |
| A | |||||
| Wild type | 1/21,840 | 1/90,800 | 1/226,000 | ||
| Wild type | Wild type | 1/2,350 | 1/5,600 | 1/19,000 | |
| p60 218F | Wild type | 1/3,300 | 1/81,310 | 1/23,070 | |
| B | |||||
| Wild type | 1/31,500 | 1/99,050 | NDc | ||
| 92F | Wild type | 1/32,900 | 1/62,460 | ND | |
| C | |||||
| Wild type | 1/19,375 | 1/68,444 | ND | ||
| 92F | Wild type | 1/57,930 | 1/25,010 | ND | |
BALB/c mice were immunized with the indicated strains, and 2 months later, they were injected with wild-type L. monocytogenes. Control animals were immunized with the wild-type strain at this time. Six days later, spleen cells were stimulated in culture, and the recovered cells were assessed for peptide-specific effector CTLs.
51Cr-labeled RMAS-Kd cells were pulsed with a 10−9 M concentration of the indicated peptide for 60 min, and then they were added at 5 × 103 cells/well. Serial dilutions of effector cells were added, and 4 to 6 h later, supernatant was collected for assay of 51Cr release. CTL frequency was determined as previously described (7). The data are representative of three experiments.
ND, not determined.
DISCUSSION
The effectiveness of vaccines for the general population in which a virus or bacteria is used as a vector to deliver heterologous antigen may depend on the immune response to the vector itself. It is not difficult to envision that for any given vector that is utilized, individuals within the population may have existing immunity to the vector. Thus, a situation may be encountered in which vaccine efficacy requires that primary responses develop even though existing immunity to the vector may result in a more rapid elimination of the vaccine carrier, thus compromising the effort. For example, with adenovirus as a carrier, existing antiviral immunity significantly alters the development of a primary response (37). Existing immunity to adenovirus also limits its effectiveness in models of gene therapy (23). Concerns over the use of vaccinia virus as a carrier for recombinant molecules have also been reported (30). One study showed that stimulation of a primary antibody response when using vaccinia virus to deliver the recombinant antigen was suppressed for at least 9 months because of anti-vaccinia virus immunity that was stimulated from a previous exposure to the vaccinia virus (24). Whether these observations are due to unknown virus-associated influences on antigen processing and presentation remains to be determined (19).
L. monocytogenes has been proposed and tested as a vaccine delivery system. Because of knowledge about the pathogenesis of this bacterium (12, 35), as well as an understanding of the protective cell-mediated immune response that develops following infection (5), this model system can be utilized to additionally test the concept of priming of CTL responses when immunity to the vector exists. Injection of BALB/c with an immunizing dose of L. monocytogenes (0.1 LD50) results in relatively uncontrolled bacterial replication in the spleen for the first 72 h, which then begins to decline (6, 25). Six days following injection, splenic CFU are barely detectable. This finding is in marked contrast to the clearance of L. monocytogenes from the spleens of immune animals. Rather than uncontrolled growth, a rapid decline in splenic CFU is observed. By 48 h following challenge, splenic CFU are typically below detection limits (data not shown). Thus, when considering utilization of L. monocytogenes or any other vector as a vaccine carrier, a key question of concern asks if there is sufficient time for stimulation of a primary response to the recombinant antigen of interest. This is relevant given the narrow window that the vector would be expected to persist in an environment of existing antivector immunity. For the use of L. monocytogenes as a vaccine carrier, this concern is underscored from the results of a previous study showing that development of antilisterial immunity is significantly reduced or does not develop when immunized animals are treated with antibiotics to halt the infection 1 to 2 days after the immunization (31).
The data presented in this report suggest that existing antilisterial immunity does not inhibit subsequent development of a primary CTL response when the epitope of interest is delivered within L. monocytogenes. Thus, primary immunization with the p60 218F L. monocytogenes mutant that does not stimulate the development of p60 217–225-specific CTLs followed by secondary immunization with the wild-type strain results in development of p60 217–225-specific CTLs (Table 3). Similarly, primary immunization with an L. monocytogenes mutant that does not stimulate the development of LLO 91–99-specific CTLs followed by secondary immunization with the wild-type strain results in development of LLO 91–99-specific CTLs. Even though existing antilisterial immunity results in rapid clearance of the wild-type strain, priming can still occur to an L. monocytogenes-specific determinant. In addition, determinants that would be expected to elicit a memory response following the secondary injection with L. monocytogenes are at an increased frequency of peptide-specific CTLs. These data suggest that primary and secondary CTL responses develop independently.
The studies that have defined the LLO 91–99 and p60 217–225 epitopes as targets of antilisterial CTLs utilized cell lines or clones continuously stimulated in the presence of L. monocytogenes-infected target cells or crude preparations of L. monocytogenes-derived peptides (32, 33). The studies presented in this report showing peptide-specific CTL responses were done by utilizing effector cell populations obtained after primary culture stimulation of L. monocytogenes immune cells with the polyclonal T-cell mitogen ConA. Studies with ConA-stimulated Listeria-immune spleen cells have shown that peptide-specific MHC class I-restricted CTLs reside solely within the CD8+ T-cell subset, with no peptide-specific MHC class I-restricted CTL activity measured in non-CD8+ cells (8, 10). Furthermore, we found that the peptide concentration (10−9 M) for sensitization of target cells for cytolysis by the polyclonally stimulated effector population is similar to that observed for peptide-specific T-cell lines and clones (16, 18). This would suggest that the CTL effector cells used in our studies possess similar properties in terms of recognition of peptide-pulsed cells and subsequent lytic functions, as observed with CTLs which have been selected for epitope-specific responsiveness. Antilisterial effectors obtained following culture stimulation have also been shown to possess enhanced in vivo activity, as measured by the levels and the duration of protection to L. monocytogenes challenge (3). In addition, experiments with ConA-stimulated Listeria-immune cells showed the importance of the immune CD8+ T-cell subset for the in vivo expression of antilisterial immunity (1, 4). Subsequent studies with Listeria-specific CD8+ T-cell lines, as well as peptide-specific CD8+ T-cell lines and clones, are consistent with this earlier report (16, 18).
Because this polyclonally stimulated CTL population has not been selected in vitro for a specific MHC class I-presented target peptide, the nature of the response is reflective of the array of responses that occur in vivo. This is supported by published data showing that the relative ratio of LLO 91–99- compared to p60 217–225 peptide-specific CTLs is not altered following the ConA culture stimulation step compared to the ratio of effector CTLs assessed directly ex vivo (7). This observation was recently confirmed, and the authors further suggested that some aspects of in vitro peptide restimulation may cause disparate expansion of CTLs specific for different epitopes (11).
The development of L. monocytogenes mutants that do not stimulate LLO 91–99- or p60 217–225-specific CTLs has allowed for studies assessing the development of responses in the presence or absence of CTLs to intermolecular or intramolecular determinants. We originally demonstrated the feasibility of this approach following immunization with an L. monocytogenes mutant in which the tyrosine anchor residue within LLO 91–99 was changed to phenylalanine. We now extend these findings with effector CTLs from mice immunized with an L. monocytogenes mutant in which the tyrosine anchor residue within p60 217–225 has been changed. The numbers of intermolecular (LLO 91–99) or intramolecular (p60 447–457) specific CTLs that develop are not influenced, even though effector CTLs to the p60 217–225 determinant are absent (Table 2).
L. monocytogenes, which as a pathogen is a strong stimulator of Th1 cytokines and cellular immune responses (26, 28), has been utilized as a candidate vaccine delivery system. Recombinant strains have been tested for the successful stimulation of protective antitumor and antiviral immune responses (21, 34, 39). However, a major concern for the rational design of effective subunit recombinant vaccines is whether existing immunity to the carrier or vector influences the development of a primary response to the determinant(s) of interest. This is an issue for the general application of this approach to the clinical setting. The results from experiments presented in this report show that L. monocytogenes-specific primary CTL responses can develop within an environment of existing antilisterial immunity. These data suggest that existing immunity to L. monocytogenes may not compromise its utility as a vaccine carrier, a concern that is underscored by results obtained when vaccinia virus or adenovirus is utilized as the carrier (23, 24, 30, 37). Thus, these data are consistent with the continued evaluation of the efficacy of L. monocytogenes as a vaccine delivery vector. We are currently investigating whether CTLs with specificity for heterologous (non-L. monocytogenes-derived) determinants when delivered by recombinant L. monocytogenes can also be stimulated in an environment of existing antilisterial immunity.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1 AI40698 to H.G.A.B., RO1 AI23455 to D.J.H., and AI38955 and ACS IM-791 to J.F.M.; NIH training grant T32 CA09120 to H.S.; and a grant from the Department of Veterans Affairs to D.J.H.
REFERENCES
- 1.Baldridge J R, Barry R A, Hinrichs D J. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990;58:654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry R A, Bouwer H G A, Portnoy D A, Hinrichs D J. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992;60:1625–1632. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry R A, Hinrichs D J. Enhanced adoptive transfer of immunity to Listeria monocytogenes after in vitro culture of murine spleen cells with concanavalin A. Infect Immun. 1982;35:560–565. doi: 10.1128/iai.35.2.560-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 5.Bouwer H G A, Barry R A, Hinrichs D J. Acquired immunity to an intracellular pathogen: immunologic recognition of L. monocytogenes-infected cells. Immunol Rev. 1997;158:137–146. doi: 10.1111/j.1600-065x.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouwer H G A, Gibbins B L, Jones S, Hinrichs D J. Antilisterial immunity includes specificity to listeriolysin O (LLO) and non-LLO-derived determinants. Infect Immun. 1994;62:1039–1045. doi: 10.1128/iai.62.3.1039-1045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouwer H G A, Hinrichs D J. Cytotoxic-T-lymphocyte responses to epitopes of listeriolysin O and p60 following infection with Listeria monocytogenes. Infect Immun. 1996;64:2515–2522. doi: 10.1128/iai.64.7.2515-2522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouwer H G A, Lindahl K F, Baldridge J R, Wagner C R, Barry R A, Hinrichs D J. An H2-T MHC class Ib molecule presents Listeria monocytogenes-derived antigen to immune CD8+ cytotoxic T cells. J Immunol. 1994;152:5352–5360. [PubMed] [Google Scholar]
- 9.Bouwer H G A, Moors M, Hinrichs D J. Elimination of the listeriolysin O-directed immune response by conservative alteration of the immunodominant listeriolysin O amino acid 91 to 99 epitope. Infect Immun. 1996;64:3728–3735. doi: 10.1128/iai.64.9.3728-3735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouwer H G A, Nelson C S, Gibbins B L, Portnoy D A, Hinrichs D J. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175:1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch D H, Pamer E G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 12.Cossart P, Mengaud J. Listeria monocytogenes. A model system for the molecular study of intracellular parasitism. Mol Biol Med. 1989;6:463–474. [PubMed] [Google Scholar]
- 13.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ertl H C, Xiang Z. Novel vaccine approaches. J Immunol. 1996;156:3579–3582. [PubMed] [Google Scholar]
- 15.Frankel F R, Hedge S, Lieberman J, Paterson Y. Induction of cell mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J Immunol. 1998;155:4775–4782. [PubMed] [Google Scholar]
- 16.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harty J T, Bevan M J. CD8 T-cell recognition of macrophages and hepatocytes results in immunity to Listeria monocytogenes. Infect Immun. 1996;64:3632–3640. doi: 10.1128/iai.64.9.3632-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harty J T, Pamer E G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J Immunol. 1995;154:4642–4650. [PubMed] [Google Scholar]
- 19.Hayder H, Mullbacher A. Molecular basis of immune evasion strategies by adenovirus. Immunol Cell Biol. 1996;74:504–512. doi: 10.1038/icb.1996.83. [DOI] [PubMed] [Google Scholar]
- 20.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomidis G, Paterson Y, Kos F J, Portnoy D A. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med. 1994;180:2209–2218. doi: 10.1084/jem.180.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen E R, Shen H, Wettstein F O, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle and a probe for studying cell-mediated immunity. Immunol Rev. 1997;158:147–157. doi: 10.1111/j.1600-065x.1997.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 23.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundig T M, Kalberer C P, Hengartner H, Zinkernagel R M. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine. 1993;11:1154–1158. doi: 10.1016/0264-410x(93)90079-d. [DOI] [PubMed] [Google Scholar]
- 25.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 26.Mielke M E, Peters C, Hahn H. Cytokines in the induction and expression of T-cell mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev. 1998;158:79–93. doi: 10.1111/j.1600-065x.1997.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller M A, Skeen M J, Ziegler H K. Nonviable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. J Immunol. 1995;155:4817–4828. [PubMed] [Google Scholar]
- 28.Mocci S, Dalrymple S A, Nishinakamura R, Murray R. The cytokine stew and innate resistance to L. monocytogenes. Immunol Rev. 1998;158:107–114. doi: 10.1111/j.1600-065x.1997.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 29.Mosier D E, Gulizia R J, MacIsaac P D, Corey L, Greenberg P D. Resistance to human immunodeficiency virus infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc Natl Acad Sci USA. 1993;90:2443–2447. doi: 10.1073/pnas.90.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata K, Garcia-Sastre A, Tsuji M, Rodrigues M, Rodriguez D, Rodriguez J R, Nussenzweig R S, Palese P, Esteban M, Zavala F. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 31.North R J, Berche P, Newborg M F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981;127:342–346. [PubMed] [Google Scholar]
- 32.Pamer E G. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J Immunol. 1994;152:686–694. [PubMed] [Google Scholar]
- 33.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 35.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaria A, St George J A, Gregory R J, Noelle R J, Wadsworth S C, Smith A E, Kaplan J M. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- 38.Schafer R, Portnoy D A, Brassell S A, Paterson Y. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J Immunol. 1992;149:53–59. [PubMed] [Google Scholar]
- 39.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 41.Xiang Z Q, Yang Y, Wilson J M, Ertl H C. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]


