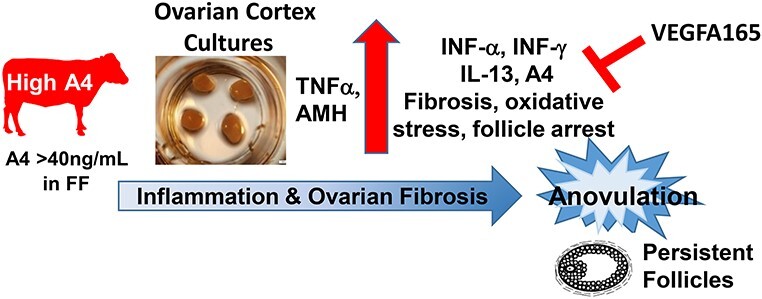
Abstract
A population of cows with excess androstenedione (A4; High A4) in follicular fluid, with follicular arrest, granulosa cell dysfunction, and a 17% reduction in calving rate was previously identified. We hypothesized that excess A4 in the ovarian microenvironment caused the follicular arrest in High A4 cows and that vascular endothelial growth factor A would rescue the High A4 phenotype. In trial 1, prior to culture, High A4 ovarian cortex (n = 9) had greater numbers of early stage follicles (primordial) and fewer later-stage follicles compared to controls (n = 11). Culture for 7 days did not relieve this follicular arrest; instead, High A4 ovarian cortex had increased indicators of inflammation, anti-Mullerian hormone, and A4 secretion compared to controls. In trial 2, we tested if vascular endothelial growth factor A isoforms could rescue the High A4 phenotype. High A4 (n = 5) and control (n = 5) ovarian cortex was cultured with (1) PBS, (2) VEGFA165 (50 ng/mL), (3) VEGFA165B (50 ng/mL), or (4) VEGFA165 + VEGFA165B (50 ng/mL each) for 7 days. Follicular progression increased with VEGFA165 in High A4 cows with greater early primary, primary, and secondary follicles than controls. Similar to trial 1, High A4 ovarian cortex secreted greater concentrations of A4 and other steroids and had greater indicators of inflammation compared to controls. However, VEGFA165 rescued steroidogenesis, oxidative stress, and fibrosis. The VEGFA165 and VEGFA165b both reduced IL-13, INFα, and INFβ secretion in High A4 cows to control levels. Thus, VEGFA165 may be a potential therapeutic to restore the ovarian steroidogenic microenvironment and may promote folliculogenesis.
Keywords: VEGFA165, oxidative stress, fibrosis, A4, in vitro culture, ovarian cortex
VEGFA165 angiogenic isoform can rescue High A4 steroid excess, inflammation, and follicular arrest and may be a potential therapeutic for anovulatory disorders.
Graphical Abstract
Ovarian cortex cultures from High A4 cows secrete increased A4 and other steroid hormones, which may contribute to increased anti-Mullerian hormone, pro-inflammatory cytokines, fibrosis, oxidative stress, follicular arrest, persistent follicles, and anovulation. The VEGFA165 treatment rescued steroidogenesis, oxidative stress, and fibrosis. The VEGFA165 and VEGFA165b both reduced IL-13, INFα, and INFα secretion in High A4 cows to control levels. Thus, VEGFA165 may be a potential therapeutic to restore the ovarian steroidogenic microenvironment and may promote folliculogenesis, resulting in ovulation.
Introduction
Anovulation is a major cause of infertility, and it is the leading reproductive disorder in many species, including women [1] and cattle [2]. Thus, to increase female fertility, it is important to understand the factors that influence ovulation, follicular development, and atresia, which can contribute to anovulation. Ovulatory disorders due to endocrine hormone excess or reduction account for 25% of infertility experienced by couples [3]. Specifically, androgen excess can cause dysregulation of the hypothalamus–pituitary–gonadal axis and can alter gonadotropin secretion to promote greater LH to FSH ratios as seen in some women diagnosed with polycystic ovary syndrome (PCOS) [4, 5].
The PCOS is a common anovulatory androgen excess reproductive disorder affecting 5–7% of women of reproductive age [6, 7]. Women are diagnosed with PCOS if they meet two of the three criteria: (1) hyperandrogenism, (2) polycystic ovaries, and/or (3) anovulation [8]. In addition to these criteria, PCOS women also have arrested follicle growth and are predisposed to metabolic disorders such as type II diabetes [9]. To understand the etiology of PCOS, several non-human models have been created. These include androgenized rats, which had fewer estrous cycles and lower numbers of mature and ovulated follicles [10]. Sheep treated with testosterone, early to mid-gestation, displayed ovarian, hormonal, and metabolic characteristics of PCOS [11]. Studies with mice [12], cows [13], and primates [14] suggest that androgens promote the growth of pre-antral follicles and, in moderation, are critical to follicle development.
Our laboratory has previously identified a naturally occurring androgen excess model [High A4] population of cows within our research herd [15]. Previous reports from our laboratory characterized the similarities between these High A4 cows and women with PCOS [15] with increased mRNA abundance of LHCGR, CYP11A1, CYP17A1, and GATA6 in the theca cells, which were findings similar to the expression patterns reported in PCOS women [16]. These High A4 cows have intrafollicular A4 excess, follicular arrest, and are often anovulatory with reduced fertility [15]. Furthermore, granulosa cell gene expression in High A4 cows compared to controls had reductions in cell cycle regulation genes, indicating reduced proliferation and arrest of the cell cycle [17]. Increased expression of CYP17A1 was also present in High A4 granulosa cells, presumably, due to reduced FOS mRNA [18], which has been shown to allow for expression of CYP17A1 in granulosa cells previously. Culture of primary granulosa cells, in our lab, with increasing concentrations of A4 also recapitulated these characteristics of High A4 cows with reduced proliferation and increased secretion of anti-Mullerian hormone (AMH) [17]. Thus, High A4 cows are a naturally occurring androgen excess domestic livestock model that has similar characteristics to PCOS. [15]. Furthermore, the cow is an excellent model for women; since, both have similar sized ovaries, follicular waves resulting in a single ovulation, similar length luteal phase, endocrine profiles, and gestation length [19]. Thus, our naturally occurring High A4 cow model is an excellent model to unravel the mechanisms contributing to anovulation and female infertility.
Previously, we demonstrated that treatment with vascular endothelial growth factor A (VEGFA) angiogenic isoform (VEGFA165) in bovine cortex cultures induces more growing follicles [20, 21], while VEGFA antiangiogenic isoforms (VEGFA165b) cause follicular arrest [20]. The signal transduction pathways that are activated through each of these isoforms alone, VEGFA165 or VEGFA165b, or in combination have also been identified [20]. The KDR receptor can be activated through VEGFA165 angiogenic isoform to stimulate cell proliferation through MAPK and PKC as well as cell migration through ROCK and ACT1 and cell proliferation and cell survival through PI3K. However, VEGFA165b inhibits these pathways, and VEGFA165b in combination with VEGFA165 results in neither inhibition nor expression of these pathways, suggesting VEGFA165b can inhibit VEGFA165’s angiogenic actions [20].
Other experiments within our laboratory utilizing rat ovarian organ cultures treated with an angiogenic isoform of VEGFA (VEGFA165) resulted in fewer early stage follicles (primordial follicles) and more follicles that developed to later stages, suggesting that VEGFA165 stimulated follicular progression [22]. However, when they treated the ovaries with VEGFAxxxB antibody, which binds to antiangiogenic VEGFA isoforms, there was greater follicle progression compared to controls [22]. Similarly, treatment of bovine ovarian cortex with VEGFA165 [21] increased primordial follicle activation and formation of secondary follicles (3- to 4-fold) over 10 days in culture. Therefore, the presence of exogenous VEGFA165 in the culture medium might have increased theca and granulosa cell proliferation, resulting in the development of larger follicles and follicular progression.
Therefore, the objective of the current study was to determine potential mechanisms of follicular arrest in High A4 cows and how excess A4 production by the ovarian microenvironment may be affecting follicular development. Furthermore, we sought to determine how VEGFA isoforms may affect follicular development and progression. We hypothesized that ovarian steroidogenic microenvironment is a critical mediator of follicle arrest and follicular fate in cows.
Materials and methods
All procedures were approved by the Animal Care and Use Committee at the University of Nebraska-Lincoln. The University of Nebraska-Lincoln is AAA-LAC-certified.
Animals
Non-lactating, composite beef cows [25% MARC III (1/4 Angus, 1/4 Hereford, 1/4 Pinzgauer, and 1/4 Red Poll) and 75% Red Angus] from the beef physiology herd located at the University of Nebraska Eastern Nebraska Research and Extension Center was used in this study.
Classifications of cows into High A4 and control groups
Thirty cows were randomly selected from the physiology herd, and estrous cycles were synchronized by two injections of PGF2α (PGF2α, 25 mg/mL; i.m.; Lutalyse, Zoetis Animal Health, Parsippany, NJ) 14 days apart (Figure 1A) to stimulate progesterone removal with corpus luteum (CL) lysis. At 6–56 h after the second PGF2α injection, the dominant follicles were aspirated and the cows were classified according to the androstenedione (A4) concentration in the follicular fluid into control (A4 < 20 ng/mL) and High A4 (A4 > 40 ng/mL) [15]. In the current manuscript, we have conducted two trials; the first investigates the different steroid and paracrine factors that are secreted by the control and High A4 cows in in vitro bovine cortex cultures. Because we were examining the steroid ovarian microenvironment, we wanted to ensure a natural estrous cycle without addition of steroids to determine differences that may occur in our High A4 and control cows. In the second trial, we were treating both control and High A4 with VEGFA angiogenic (VEGFA165) and antiangiogenic (VEGFA165b) isoforms alone and in combination to determine if any of these VEGFA isoforms would rescue factors contributing to follicle arrest in High A4 bovine cortex cultures. Because the steroidogenesis in the dominant follicle may be critical to the starting material in each bovine ovary, we decided to use a progestin to synchronize the dominant follicle to ensure similar steroidogenic profiles. As seen in the data, there were no differences whether we used the synchronization regime in trials 1 or 2 since High A4 cows had similar inflammatory and steroidogenic characteristics in each trial.
Figure 1.
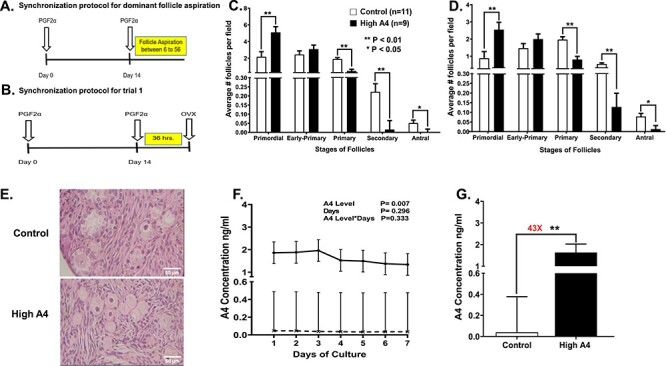
Demonstrates the synchronization protocol (A) to obtain follicular fluid through dominant follicle aspiration to classify the cows into High A4 or control groups for all trials; (B) synchronization of estrous cycles prior to ovariectomy to obtain ovarian cortex pieces to culture for trial 1; (C) the average number of follicles per fields at different follicle stages in control and High A4 cows before culture; (D) the average number of follicles for each stage and per field after 7 days of ovarian cortex culture; (E) representative H and E staining for control and High A4 cows, demonstrating follicle stages present in each cow classification; (F) the concentrations of A4 daily during the 7 day ovarian cortex culture for each cow classification; and (G) the total amount of A4 that was collected for all 7 days during the ovarian cortex culture in control and High A4 cows. Control (n = 11); High A4 (n = 9); difference is shown between bars with *P < 0.05; **P < 0.01; NS = not significant.
Trial 1
After classification, 20 cows (n = 9 High A4, n = 11 control) were synchronized by lysing the CL with two injections of PGF2α (PG; 25 mg/mL; i.m.; Lutalyse, Zoetis Animal Health, Parsippany, NJ) 14 days apart (Figure 1B). Ovariectomy was performed 36–42 h after the second PGF2α injection (Figure 1B) and the ovaries were removed to collect ovarian cortex for tissue culture.
Trial 2
To determine if VEGFA isoforms would rescue the High A4 phenotype, cows were synchronized with a progestin [controlled internal drug release device (CIDR)] since this synchronization method allows for better synchronization of dominant follicle to similar stages and allows for ovaries to be exposed to similar steroids from the dominant follicle. The estrous cycles of 10 cows (n = 5 High A4, n = 5 control) were synchronized with the Co-Synch + CIDR timed artificial insemination (AI) protocol, except ovariectomy was performed after synchronization rather than timed AI [17] (Figure 2A). Briefly, cows received a single injection (100 μg/cow; i.m.) of gonadotropin-releasing hormone (Cystorelin, Merial Limited, Duluth, GA) on treatment day 0 to induce ovulation, and thus, initiate a new follicular wave. Also, on day 0, an intravaginal insert (CIDR; Zoetis, Florham Park, NJ) containing 1.38 g of P4 was inserted. Ovariectomy (n = 10) was performed approximately 36–42 h after CIDR removal, which coincided with the administration of PGF2α to eliminate a functional CL (Figure 2A). Similar results were observed regardless of the synchronization method that was used in these trials.
Figure 2.
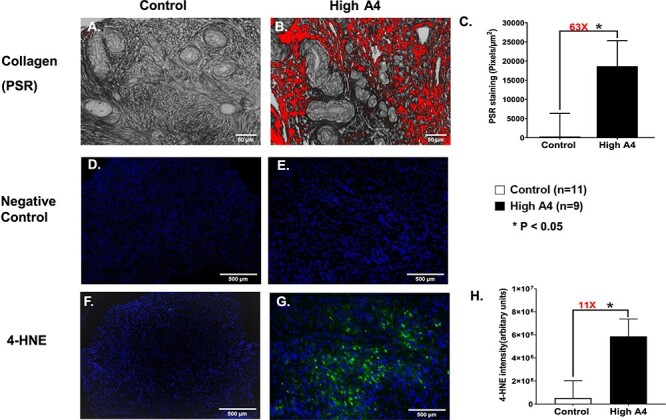
Collagen staining (PSR) for (A) control and (B) High A4 ovarian cortex cultures. (C) Graph comparing the average area of PSR-positive staining per ovarian cortex field (pixels/μm2) between High A4 and control cows; control (n = 11), High A4 (n = 9). (D–H) Immunofluorescent staining for oxidative stress (4-HNE) negative controls for (D) control and (E) High A4 cow (4-HNE) and (F) controls. (G) High A4 cow ovarian cortex and (H) graph quantitating the integrated intensity of 4-HNE-positive staining per ovarian cortex field (arbitrary units) between High A4 and control cows. *P < 0.05.
Ovarian tissue collection
In both trials, ovaries were removed using a right flank laparotomy using aseptic technique [15, 23]. Local anesthesia was induced using an inverted L-block with 2% lidocaine. Each ovary was measured, weighed, and a count of surface antral follicles was performed.
Culture of ovarian cortical pieces
Ovarian cortical pieces were collected from each ovary from each cow to determine the differences in folliculogenesis based on cow A4 classification within follicular fluid [15, 20, 24].
Trial 1
An in vitro cortex culture was conducted with no treatment (PBS) as published [24]. Portions of the ovarian cortex were cut into eight ovarian cortex pieces of 0.5–1 mm3 per ovary. Four ovarian cortical pieces per ovary were fixed immediately in Bouin’s solution overnight at 4°C as day 0 (uncultured). The other four cortical pieces were placed on uncoated culture well inserts (four pieces/well; one well/treatment/ovary/cow; Millicell-CM, 0.4 μm pore size; Millipore Corporation, MA) in the wells of 24-well Costar culture plates (Corning, Inc., Corning, NY) with 350 μL serum-free Waymouth’s medium MB 752/1 (Sigma-Aldrich, St. Louis, MO) and supplemented with 75 μg/mL penicillin, 50 μg/mL streptomycin sulfate (Sigma), and ITS [1000 mg insulin, 550 mg transferrin, 6.7 ng selenious acid (Corning; Manassas, VA)], 1.25 mg BSA (Sigma), and 50 ng/mL PBS as stated previously [20, 24].
Trial 2
The cortical cultures were treated with different VEGFA isoforms to determine effects on follicular development. The bovine ovarian cortex culture method is published in more detail in Sutton et al. [24]. The ovarian cortex pieces were cut into 20 ovarian cortex pieces with 0.5–1 mm3 for each piece. Four ovarian cortical pieces were fixed immediately in Bouin’s overnight at 4°C as day 0 (uncultured). We used the same protocol in trial 1 for trial 2 for cortex culture as mentioned above, except that, instead of just PBS, each well was treated with one of the following treatment: (1) PBS, (2) VEGFA165 (50 ng/mL), (3) VEGFA165B (50 ng/mL), or (4) VEGFA165 + VEGFA165B (50 ng/mL each) per ovary/cow with 10 replicates per treatment one for each ovary on each cow.
In both trials, cortical pieces were cultured at 37°C in a humidified incubator with 5% CO2, and 70% of 350 μL of culture medium was replaced (240–250 μL) with fresh medium every day. After 7 days of culture in both trials, the four ovarian cortical pieces were fixed immediately in Bouin’s overnight at 4°C as day 7 (cultured) [25]. After that, day 0 and day 7 cortical pieces were embedded in paraffin. Serial sections of 6 μm were cut into 10 slides using a microtome, and five serial sections were mounted on each gelatin-coated slide. Sections from slides 1, 5, and 10 were selected from each ovary per each cow, which were stained with hematoxylin and eosin.
We took three images per slide for a total of nine images per ovary, and follicles were staged and counted by three individuals and were averaged to determine the number of follicles at each stage classified in one of five groups: (0) primordial follicle—an oocyte surround by a single layer of squamous pregranulosa cells; (1) transitional follicle or early primary—an oocyte surrounded by mostly squamous pregranulosa cells with some cuboidal granulosa cells; (2) primary follicle—an oocyte surround by 1–1.5 layers of cuboidal granulosa cells; (3) secondary follicle—an oocyte surrounded by two or more cuboidal granulosa cells; and (4) antral follicle with an oocyte surrounded by two or more layers of granulosa cells that contains a distinct antrum [15, 25, 26]. From our imaging and counting, we counted approximately 30% of follicles in each ovarian cortex per ovary as published previously [20, 24].
Androstenedione hormone assay
To evaluate the level of androstenedione (A4) based on cow A4 classification, we measured daily the concentration of A4 in culture media in trial 1 and in trial 2 using Immuchem™ Double Antibody, Androstenedione 125I RIA Kit (MP Diagnostics). Briefly, culture media was diluted 1:5 by using 100 μL of culture media and 400 μL of steroid diluent. Following this step, the samples were assayed by direct competitive binding determination. Samples values were corrected for dilution factor [15]. Intra- and inter-assay CVs for A4 in trial 1 were 6.18% and 4.48%, respectively, and the intra- and inter-assay CVs for A4 in trial 2 were 7.37% and 4.59%, respectively.
In trial 2, in order to analyze the concentration of multiple steroid hormones from a single sample, a pool from first 5 days of culture media that were treated with PBS, VEGFA165, VEGFA165B, and VEGFA165 plus VEGFA165B were analyzed by Biocrates Life Sciences AG (Innsbruck, Austria) using the SterolDQ HPLC-MS/MS kit as previously reported [15, 24, 27].
Picro-Sirius Red staining
Picro-Sirius Red (PSR) is a stain used for histological visualization of collagen I and III fibers (as an indicator of fibrosis) [24]. The PSR staining was conducted in both trials as published previously [24, 28]. Briefly, tissue sections were deparaffinized in xylene and then rehydrated in a series of graded ethanol baths (100%, 70%, and 30%). Tissue sections were immersed for 1 h at room temperature in a PSR staining solution prepared by dissolving Sirius Red in picric acid (Direct Red 80, Sigma). Tissues were washed 3 × 5 min in 0.5% glacial acetic acid in water. Excess acidified water was carefully wicked away from the tissue sections, and the tissue was rapidly dehydrated in 100% ethanol (a total of three, 1-min incubations each). The tissues were cleared in xylene for 5 min and were mounted with Permount mounting media (SP15-100 Toluene Solution; Fisher Scientific). To quantify the area of ovarian tissue that was positive for PSR staining, we followed the protocol as described in [25, 28] using cows of the same age. Two images were taken from different field using a 40X objective. ImageJ was used to quantify the area of positive PSR staining above a threshold that was set based on the staining in the Control cows. This threshold was constant for all images analyzed. This procedure from our laboratory is published [25].
Immunofluorescence staining
For immunofluorescence (IF) for 4-hydroxynonenal (4-HNE; oxidative stress marker), we used representative ovarian cortex pieces in both trials fixed in Bouin’s solution, embedded in paraffin, and sectioned (6 μm) into gelatin-coated slides with five serial sections on each slide, four cortex pieces per serial section. The IF was performed as previously described [20, 29]. The 4-HNE antibody (catalog no. ab46545; abcam) was a rabbit polyclonal primary antibody. We used donkey anti-rabbit secondary antibody (Alexa Fluor 488; catalog no. ab150061; abcam). The primary antibody was diluted 1:100 and the secondary antibody was diluted 1:500 in 10% normal donkey serum. For a negative control, one or two sections were processed without primary antibody. ImageJ, as published previously [24], was used to quantify the area of positive 4-HNE staining.
Quantibody cytokine array
Because of reports of inflammatory cytokines being involved in fibrosis [25], ovarian cortex culture media from the first 5 days of culture in control and High A4 cows were pooled from trial 1 and then the samples were analyzed with commercially available antibody arrays (Table 3; Quantibody Cytokine Q3 and Q1 Arrays, RayBiotech, GA, USA) [24]. These arrays measured 20 different cytokines and chemokines in the pro- or anti-inflammatory pathway: angiopoietin 1 (ANG1), CD40 ligand, chemokine (C-C motif) ligand 4 (CCL4, also known as macrophage inflammatory protein-1β (MIP-1β)], chemokine (C-C motif) ligand 5 (CCL5, also known as RANTES—regulated on activation, normal T cell expressed and secreted), chemokine (C-X-C motif) ligand 9 (CXCL9, also known as MIG—macrophage interferon gamma), C-X-C motif chemokine ligand 10 (CXCL10, also known as interferon-inducible cytokine, IP-10), decorin, interferon beta 1 (IFNβ), interferon gamma (IFNγ), interleukin-18 (IL18), leukemia inhibitory factory (LIF), interleukin 13 (IL13), interleukin 21 (IL21), interleukin 36 receptor antagonist (IL36RA, also known as IL1F5—interleukin 1 family member 5), tumor necrosis factor alpha (TNFα), interferon alpha A, interleukin 10 (IL-10), interleukin 17 alpha (also known as cytotoxic T-lymphocyte-associated protein 8), which have been validated for bovine samples [30, 31] Cortex culture samples were assayed without any dilution. Array slides were shipped back to the company for data extraction and retrieval. Data were received from Raybiotech and were analyzed with their analysis program. For trial 2, the same procedure was followed, except the samples were treated with PBS (control), VEGFA165, VEGFA165b, or a combination as stated previously. For trial 2, the cytokines and chemokines that were significantly reduced due to VEGFA isoforms are graphically represented in Figure 7B–D.
Table 3.
Concentrations of cytokines and chemokines in the cortex culture media in control and High A4 in trial 1
| Group | ||||
| Control | High A4 | SEM | P-value | |
| n | 11 | 9 | ||
| Cytokine/chemokine, pg/mL | ||||
| ANG-1 | 56.03 | 64.76 | 26.96 | 0.83 |
| CD40 ligand | 3624.31 | 1103.59 | 261.58 | 0.19 |
| CCL4/MIP-1β | 371.74 | 616.61 | 138.60 | 0.25 |
| CCL5/RANTES | 2198.06 | 2800.50 | 1058.11 | 0.70 |
| CXCL9 | 0.16 | 90.43 | 45.45 | 0.19 |
| CXCL10 | 1121.92 | 2179.34 | 378.69 | 0.07† |
| Decorin | 2104.51 | 2274.92 | 383.00 | 0.77 |
| INF-α | 104.81 | 227.51 | 30.58 | 0.01** |
| INF-β1 | 544.81 | 710.25 | 167.25 | 0.51 |
| INF-γ | 7.40 | 15.69 | 3.21 | 0.10† |
| IL-1α | 30.56 | 55.49 | 10.26 | 0.12 |
| IL-1β | 18.93 | 17.60 | 9.15 | 0.92 |
| IL-10 | 13 399 | 10 854 | 5700.58 | 0.76 |
| IL-13 | 5.34 | 26.13 | 5.18 | 0.01** |
| IL-17A | 54.19 | 36.50 | 27.19 | 0.66 |
| IL-18 | 464.97 | 713.38 | 334.88 | 0.62 |
| IL-21 | 31.05 | 38.41 | 14.83 | 0.74 |
| IL36RA/IL-1F5 | 1.08 | 2.72 | 0.73 | 0.15 |
| TNFα | 22.78 | 95.66 | 14.88 | 0.001*** |
| LIF | 28 110 | 54 877 | 15 232 | 0.25 |
* P-value <0.05.
** P-value <0.01.
*** P-value < 0.001.
† P-value <0.10.
MIG = monokine induced by gamma interferon, IP-10 = interleukin protein 10, IFN-α = interferon alpha, IL-1α = interleukin 1 alpha, IL-1β = interleukin 1 beta, IL-17A = Interleukin-17A.
Figure 7.
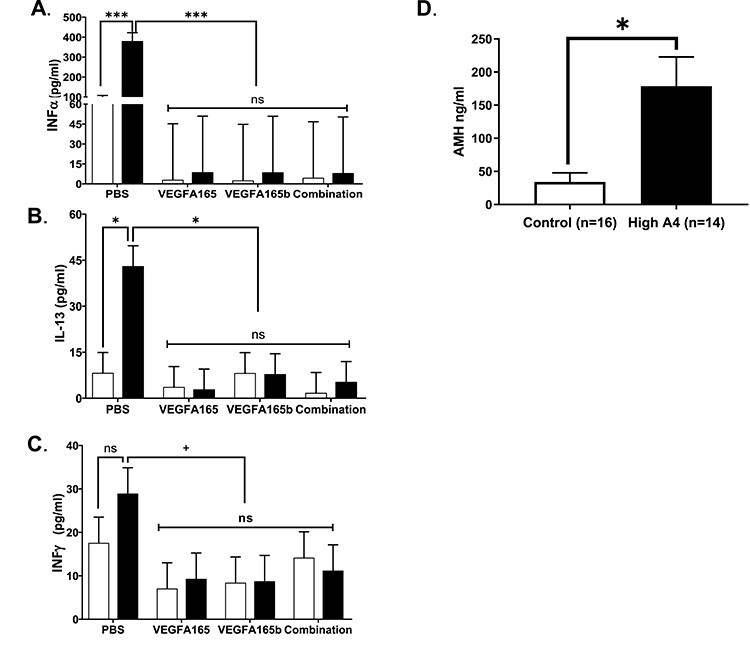
Effects of VEGFA isoforms on cytokines and chemokines in ovarian cortex culture media. Graphs representing PBS, VEGFA165, VEGFA165b, and combination treatments for (A) INFα, (B) IL-13, and (C) INFγ. (D) Concentrations of AMH in ovarian cortex cultures in trials 1 and 2. Control, n = 16; High A4, n = 14. Statistical differences are denoted on the graphs where P < 0.05 are different and P > 0.05 but <0.1 tend to be different.
AMH ELISA
Media from bovine cortex cultures were measured for AMH from samples combined from trials 1 and 2 by utilizing a commercially available bovine AMH ELISA kit (Ansh Labs, Webster, TX, Ca# AL-114) validated in cattle [32–34] and used by us previously [17]. For the analysis, culture media was not diluted and the manufacture’s protocol was followed. The intra-assay CV was 1.96%.
Statistical analyses
In both trials, the average length, width, and surface antral follicle count (AFC) were compared using the MIXED procedure of SAS with group as the fixed effect. Animals were considered the experimental unit in both trials. All data are presented as means ± SEM and a P-value < 0.05 was used as threshold for significance and a P-value of 0.05–0.10 was used as a tendency for all analyses.
Trial 1
The histological evaluations of primordial, primary, secondary, and antral follicles were analyzed using the MIXED procedure of SAS with A4 classification groups (High or control) as a fixed effect. Culture media collection data were analyzed using the MIXED procedure of SAS with days of culture, in vivo treatments, and the interactions as fixed effects in a repeated measures model. Cortex culture media cytokines and chemokines collection data were analyzed using the MIXED procedure of SAS with in vivo treatments as fixed effects. Correlation was calculated using CORR procedure of SAS between the amounts of A4 produced in the culture media and the area that stained positively for fibrosis.
Trial 2
The A4 classification groups (High A4 or control) and in vitro culture treatments (PBS, VEGFA165, VEGFA165B, and VEGFA165 plus VEGFA165B) were used as fixed effects. The fold changes were calculated for each treatment after culture by dividing the number of follicles after in vitro culture over number of follicles before culture within stage. Follicle numbers to which comparisons were made were set as a minimum value of one to avoid dividing by zero, and then we analyzed the change in primordial, primary, secondary, and antral follicles after culture using the PROC GLIMMIX procedure of SAS. The interaction between in vivo and in vitro treatments was significant for some of follicles stages, and the interaction between in vivo and in vitro treatments will be discussed. Culture media collection data were analyzed using the MIXED procedure of SAS with days of culture, in vitro treatments, in vivo treatments, and the interactions as fixed effects in a repeated measures model. Cortex culture media cytokines and AMH collection data were analyzed using the MIXED procedure of SAS with in vivo treatments as fixed effects.
Results
Trial 1
Phenotype measurements
There were no differences in ovarian area, ovarian weight, and surface AFC based on A4 classification as demonstrated in Table 1.
Table 1.
Ovarian area, weight, and surface AFC in control and High A4 cows at ovariectomy in trial 1
| Control1 N = 11 | High A42 N = 9 | P-value | |
| Ovarian area, mm2 | 589.75 ± 73.59 | 706.27 ± 81.36 | 0.30 |
| Ovarian weight, g | 7.60 ± 0.88 | 9.08 ± 0.97 | 0.28 |
| Surface AFC | 33.91 ± 3.55 | 29.22 ± 3.92 | 0.39 |
1Dominant follicle follicular fluid A4 concentration of <20 ng/mL.
2Dominant follicle follicular fluid A4 concentration of >40 ng/mL.
Follicle stage in ovarian cortex cultures
The number of follicles classified primordial, early primary, primary, secondary, and antral in control ovaries were compared to High A4 ovaries. Before culture, ovarian cortex pieces from High A4 ovaries had more (P = 0.005) primordial follicles (2.42-fold; stage 0) compared to the cortex pieces from the control ovaries (Figure 1C). There was no difference (P = 0.336) in the number of early primary follicles (stage 1) (Figure 1C) between ovarian cortex of control and High A4 cows. However, ovarian cortex from control cows had more follicles classified as primary (4-fold; P = 0.001), secondary (4-fold; P = 0.001), and antral (5-fold; P = 0.048) (stages 2–4, Figure 1C) compared to ovarian cortex of High A4 cows. Conversely, the cortex pieces from High A4 ovaries had more (P = 0.009) primordial follicles (2.9-fold; stage 0) compared to the cortex pieces from control ovaries (Figure 1D) after 7 days of culture. After culture, there was no difference (P = 0.199) in the number of early primary (stage 1, Figure 1D) between the ovarian cortex of control and High A4 cows. Additionally, ovarian cortex from the control had more follicles classified as primary (P = 0.002), secondary (P = 0.003), and antral (P = 0.014) (stages 2–4, Figure 1D) compared to the ovarian cortex from High A4 cows after culture.
Steroids’ concentrations in the culture media
There was no significant difference (P = 0.333, Figure 1E) for the interaction between our groups (High A4 vs. control cows) and days of culture (D1–D7). However, the ovarian cortex from High A4 cows secreted greater (43X) amounts of A4 (P = 0.007; Figure 1E and F) compared to control cows.
Markers of inflammation
Fibrosis
There was increased positive staining for a collagen marker (PSR, an indicator of fibrosis) in High A4 compared to control ovarian cortex pieces (Figure 2A and B). Ovarian cortex from the High A4 cows had nearly 63-fold the amount of PSR-positive collagen staining in the ovarian cortex and stroma relative to the control cows (P = 0.05; Figure 2C). Additionally, there was a positive correlation (P = 0.02; R = 0.51) between the amount of A4 produced by the ovarian cortex and the area that stained for fibrosis in the High A4 cows.
Oxidative stress
There was increased positive staining for the oxidative stress marker, 4-HNE, in the ovarian cortex and ovarian stroma of High A4 cows compared with the control cows (Figure 2D–G). Ovarian cortex from the High A4 cows had nearly 11-fold the amount of integrated intensity of 4-HNE staining in the ovarian cortex and stroma relative to the control cows (P = 0.03; Figure 2H).
Cytokines and chemokines
Twenty different cytokines and/or chemokines that were either pro-inflammatory or anti-inflammatory or considered both were analyzed in cortex culture media in trial 1 (Table 3). High A4 ovarian cortex culture media had increased concentrations of INFα (P < 0.01), IL13 (P < 0.01), and TNFαα (P < 0.001) with a tendency for increased CXCL10/IP10 (P < 0.07) and INF-γ (P < 0.1) compared to controls.
Trial 2
Phenotype measurements
There were no differences in ovarian area (P = 0.93), ovarian weight (P = 0.71), and surface AFC (P = 0.55) based on A4 classification (Table 2).
Table 2.
Ovarian area, weight, and surface AFC in control and High A4 cows at ovariectomy in trial 2
| Control1 N = 5 | High A42 N = 5 | P-value | |
| Ovarian area, mm2 | 768.44 ± 70.05 | 777.87 ± 70.05 | 0.93 |
| Ovarian weight, g | 9.92 ± 0.88 | 10.40 ± 0.88 | 0.71 |
| Surface AFC | 31.20 ± 3.64 | 28.00 ± 3.64 | 0.55 |
1Dominant follicle follicular fluid A4 concentration of <20 ng/mL.
2Dominant follicle follicular fluid A4 concentration of >40 ng/mL.
Follicle stage in ovarian cortex cultures
There were greater numbers of follicles classified as primary, secondary, and antral in the control ovarian cortex compared to High A4 before culture (Figure 3B). While, cortex pieces from High A4 ovaries contained greater numbers of follicles classified as primordial follicles (P = 0.004; Figure 3B) prior to culture with fewer numbers of follicles classified as primary (P = 0.01; Figure 3B), secondary (P = 0.0001; Figure 3B), and antral (P = 0.008; Figure 3B) compared to control ovarian cortex before culture. There was no difference (P = 0.48) in the number of early primary (stage 1) (Figure 3B) between the ovarian cortex of control and High A4 cows before culture.
Figure 3.
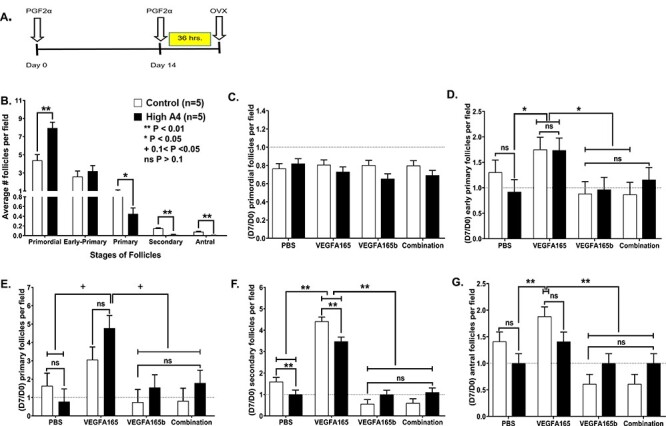
Synchronization of estrous cycles of cows in trial 2 (A) and graphs of follicular counts at each follicular stage for High A4 and control cows (control, n = 5; High A4, n = 5) (B) before culture all stages; and (C–G) after culture in trial 2 treated with different VEGFA isoforms; (C) primordial; (D) early primary; (E) primary; (F) secondary; and (G) antral follicles. Differences are denoted by **P < 0.01; *P < 0.05; ns = not significant.
None of the in vitro treatments during culture altered the number of primordial follicles between ovarian cortex of High A4 and Control cows (Figure 3C). The ovarian cortex treated with VEGFA165 in High A4 and control cows had the greatest increase in numbers of early primary follicles (P = 0.05; Figure 3D), with reductions in the number of early primary follicles treated with VEGFA165b or combination compared to VEGFA165.
Numbers of primary follicles (Figure 3E) were not changed due to treatment in either control or High A4 ovarian cortex cultures.. However, numbers of secondary follicles were greater in controls compared to High A4 ovarian cortex (PBS). The VEGFA165 treatment increased secondary follicles in both control and High A4 classifications compared to PBS treatment; but, secondary follicles numbers were still greater in controls compared to High A4 ovarian cortex (Figure 3F). There also were reductions in numbers of secondary follicles in VEGFA165b and combination treatments when compared to VEGFA165-treated ovarian cortex (Figure 3F). Similarly, antral follicle numbers were greater in VEGFA165-treated ovarian cortex when compared to PBS or VEGFA165b or combination treatment regardless of cow classification (Figure 3G). Thus, VEGFA165 treatment can stimulate increased follicles at the early primary, secondary, and antral follicles stage and can increase these follicle stages in High A4 cows so they are not different from controls. Furthermore, the VEGFA165b and combination treatment indicate that VEGFA165b can inhibit the actions of VEGFA165 angiogenic isoforms and can reduce the number of follicles to these later stages of development.
Steroids’ concentrations in the culture media
There was a significant difference (P = 0.001; Figure 4A) between our experimental groups (High A4 vs. control), in vitro culture treatment (PBS, VEGFA165, VEGFA165B, and VEGFA165 plus VEGFA165B), and days of culture (D1–D7). Ovarian cortex from High A4 cows that were treated with PBS, VEGFA165B, and VEGFA165 plus VEGFA165B secreted the greatest amounts of A4 (Figure 4A and B) in the culture media compared with control cows. By contrast, treatment with VEGFA165 significantly reduced (4.2 times; P = 0.001) the amount of A4 secreted by the ovarian cortex of High A4 cows treated with PBS, and this was also reduced from VEGFA165b or combination isoform treatment (Figure 4A and B).
Figure 4.
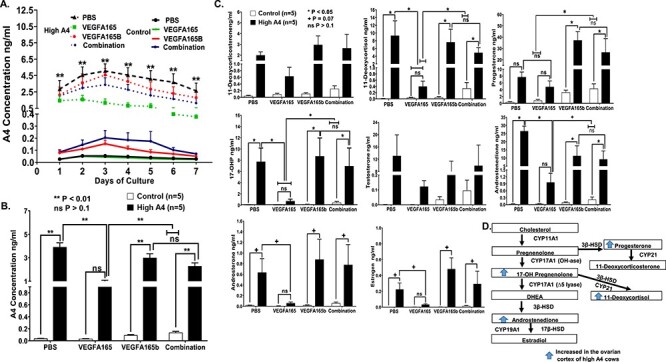
Concentrations of steroids secreted into ovarian cortex media in trial 2 for High A4 and control cows with different in vitro treatment (A) over each day of the culture period and (B) average of 7 days in culture. (C) HPLC-MS analysis for different steroid hormones and metabolites secreted from PBS, VEGFA165, VEGFA165B, and combination-treated cortex culture media for control and High A4 cows. (D) Schematic of increased steroid in ovarian cortex of High A4 cows. Statistical differences are denoted by *P < 0.05; +P < 0.07; n for control = 5; n for High A4 = 5; ns = not significant.
The concentrations of A4 and other steroids and steroid metabolites in the culture media, including 11-deoxycortisol, 17-OHP, androstenedione, were greater, while E2 and androsterone tended to be greater in High A4 cows compared to controls (PBS; Figure 4C and D). The VEGFA165 treatment in all of the previously mentioned steroid hormones reduced concentrations of steroids to the levels of the control cows (PBS). However, VEGFA165b and the combination treatment did not reduce steroid production. In fact, both VEGFA165b and the combination treatment increased progesterone production in ovarian cortex cultures from High A4 cows compared to controls. Thus, the VEGFA165 treatment rescued much of the steroid excess that was present in the ovarian cortex cultures of High A4 cows, while VEGFA165b and the combination of VEGFA165 plus VEGFA165b stimulated increased progesterone production (Figure 4C).
Fibrosis
There was increased positive staining for a collagen marker (PSR, an indicator of fibrosis) in High A4 ovarian cortex compared to control ovarian cortex pieces before culture (D0; P = 0.001;Figure 5A and B). Treatment with VEGFA165 dramatically (P = 0.001) reduced the staining of collagen (Figure 7A and B) compared with VEGFA165B and combination of VEGFA165 plus VEGFA165B after 7 days of culture.
Figure 5.
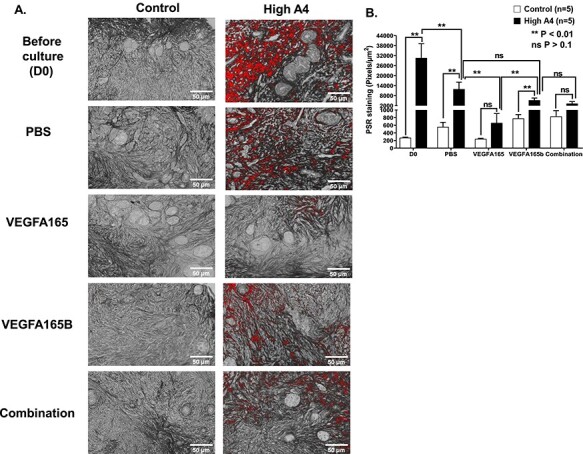
(A) Collagen staining (PSR) for High A4 and control ovarian cortexes before culture and after culture with different VEGFA isoforms in trial 2; (B) graph comparing the average area of PSR-positive staining per ovarian cortex field (pixels/μm2) between High and control cows before culture and after culture with different VEGFA isoforms. Statistical differences are denoted with **P < 0.01 or ns P > 0.10. n for control = 5; n for High A4 = 5.
Figure 6.
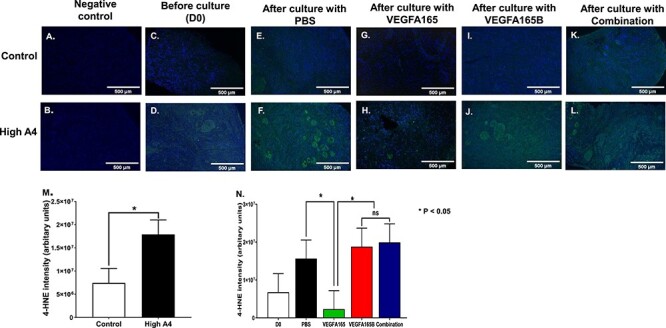
(A–L) Immunofluorescent staining for oxidative stress (4-HNE) negative controls for (A) control and (B) High A4 cows; before culture (C) controls and (D) High A4 cows; after culture with PBS (E) control and (F) High A4 cow; after culture with VEGFA165 (G) control and (H) High A4 cow; after culture with VEGFA165B (I) control and (J) High A4 cow; after culture with combination (K) control and (L) High A4 cow ovarian cortex. (M) Graph comparing the integrated intensity of 4-HNE-positive staining per ovarian cortex field (arbitrary units) between High and control cows; (N) graph quantitating the integrated intensity of 4-HNE-positive staining per ovarian cortex field (arbitrary units) between different in vitro culture treatments. Statistical differences are denoted by *P < 0.05. n for control = 5; n for High A4 = 5.
Oxidative stress
There was increased positive staining for the oxidative stress marker (4-HNE) in the ovarian cortex of High A4 compared with the control cows (Figure 8A–M). The interaction between in vivo and in vitro treatments (level of A4 and VEGFA isoforms) was not statistically significant. When in vitro treatments had an effect, the main effect was discussed. Ovarian cortex from the High A4 cows had an increased integrated intensity of 4-HNE staining in the ovarian cortex relative to the control cows (P = 0.02; Figure 8M). Positive staining for 4-HNE was present in oocytes, granulosa, and stroma cells. Treatment with VEGFA165 dramatically (P = 0.05) reduced the staining of the oxidative stress (Figure 8N) compared with PBS, VEGFA165B, and VEGFA165 plus VEGFA165B treatments. While VEGFA165B did not reduce the staining of the oxidative stress in ovarian cortex from the High A4 cows compared to control cows. Moreover, VEGFA165B had a higher staining of the oxidative stress compared to VEGFA165 within the ovarian cortex of High A4 cows.
Cytokines and chemokines
Twenty different cytokines and/or chemokines that were either pro-inflammatory or anti-inflammatory (or considered both) were analyzed in cortex culture media in trial 2, and the effects of VEGFA isoforms were evaluated to determine how they impacted the cytokines and chemokines that were increased in trial 1 (Table 3). The same cytokines were elevated as in Table 3; however, only three were affected by all VEGFA isoform treatments such that INFα (P < 0.01) and IL-13 (P = 0.03) were reduced by all VEGFA isoform treatments compared to PBS and INFγ tended (P < 0.06) to be reduced. Thus, all isoforms of VEGFA can reduce these three cytokines/chemokines to concentrations found in the control ovarian cortex culture media.
Concentration of AMH in ovarian cortex culture media from trials 1 and 2
Ovarian cortex media from both trials 1 and 2 were pooled from D1–D5 for individual cows and were used to analyze AMH production in media from ovarian cortex culture between control and High A4 cows. The concentration of AMH was greater in High A4 ovarian cortex media (P < 0.03) compared to controls, indicating that increased concentrations of AMH may also contribute to follicular arrest in the High A4 cow ovarian cortex.
Discussion
The results of the present study confirm our hypothesis that the ovarian steroidogenic environment influences the follicular development in the cow. The ovarian cortex in the High A4 cows displayed follicular arrest and increased markers of oxidative stress and fibrosis within increased secretion of AMH and pro-inflammatory cyokines. Similarly, women with PCOS are characterized to have excess ovarian androgen production, abnormal follicle growth, increased pro-inflammatory cytokines and AMH, anovulation, and infertility [35]. As we observed in both trials before culture (D0), High A4 cows had more primordial follicles compared with our control cows. This increase in the number of primordial follicles indicates that follicle activation is being inhibited and does not necessarily suggest increased ovarian reserve. According to recent research, collagen controls the stiffness of the ovary [36], and an in vitro study found that physically stiffer conditions allow for primordial follicle survival and growth in nonhuman primates [37]. As a result, the increased fibrotic and collagen-rich cortex in the ovaries of High A4 cows may be retaining primordial follicles and not allowing for the development of later stage follicles. These High A4 cows also have sporadic ovulation and often are anovulatory [38], indicating that this stiffer ovarian structure and steroid excess microenvironment may inhibit the development and ovulation of antral follicles necessary for normal reproductive function.
The High A4 cows had fewer primary, secondary, and antral follicles compared with the control cows, supporting our previous report of reducing High A4 compared with control cows [15]. Also other experiments [39] have demonstrated that hyper-androgenism or abnormal androgen levels seriously suppress normal follicular development and increase follicular atresia and degeneration in human and rhesus monkey females. Likewise, excess androgen is associated with ovarian dysfunction in cattle [40] and with an increase in cell cycle arrest, autophagy, and apoptosis in granulosa cells [17]. Chen et al. [10] also reported that androgenized rats had fewer estrous cycles and lower numbers of mature and ovulated follicles. These studies indicate that excess A4 disrupts follicle growth and prevents follicle progression to primary, secondary, and antral follicle stages.
Androgen production by the ovarian cortex in the High A4 cows may be the factor causing altered ovarian morphology and leading to oxidative stress. Oxidative stress results when there is an imbalance between pro-oxidant molecules and antioxidant defense systems, which results in a build-up of reactive oxygen species (ROS); which is a common characteristic of inflammatory processes [41–43]. Furthermore, the oxidation of unsaturated lipids by ROS can cause excessive levels of lipid peroxidation followed by the generation of degradation products [42]. The resulting aldehyde compounds are more toxic, such as malondialdehyde and 4-hydroxy-2-nonenal (4-HNE) [42]. Excessive ROS production during follicle development and oocyte maturation may also cause negative effects on the growth and development of follicles and on oocyte competence [43]. Oxidative stress is also a major contributor to the pathogenesis and development of PCOS [32, 43]. Previous studies found that the excess androgen in circulation is associated with increased oxidative stress in women with or without PCOS [33]. The ROS is also released in physiologic processes, such as ovulation and regression of the CL [41, 42]. Thus, the increased A4 secreted from the High A4 cow ovarian cortex may cause increased ROS accumulation in the High A4 follicle, resulting in aberrant folliculogenesis.
The bovine ovarian cortex is highly steroidogenic, producing many steroids and steroid metabolites. In the current study, we demonstrated that, in addition to increased concentrations of A4, we also observed elevated 11-deoxycorticosterone, 11-deoxycortisol, 17-OHP, androsterone, A4, and E2 compared to control cows. To our knowledge, this is the first study to report steroid and steroid metabolites secreted from the bovine ovarian cortex. These data support previous results reported by Summers et al. [15] who found that 11-dehydroxy-corticosterone and E2 were elevated in follicular fluid of High A4 compared to control cows.
In addition to evidence of oxidative stress, we also found a positive correlation between the amount of A4 produced by the ovarian cortex and the area that stained for fibrosis in the High A4 cows. Fibrosis occurs when the homeostasis between synthesis and degradation of extracellular matrix components is altered [28], and as fibrosis continues to develop, tissue architecture and function are compromised. Women diagnosed with PCOS have an increase in the thickness of ovarian stroma due to increased collagen deposition and development of fibrous tissue [34] with an increase in protease activity [44]. Protease activity disrupts the normal tissue remodeling process, availability of different growth factors, and the communication through cellular gap junctions, contributing to the abnormal ovarian phenotype associated with PCOS [44]. Follicle arrest observed in women with PCOS is also associated with increased collagen deposition and fibrosis along with increased cytokine production leading to inflammation in these women. Elevated circulating concentrations of TNFα contribute to inflammation in PCOS patients and also may influence the alterations in structure occurring at the level of the ovary [35].
In general, when ovarian organ cultures or pieces of ovarian tissue are placed into culture, there is a spontaneous development of follicles to later stages of development, with the majority of follicles progressing past secondary follicles. Thus, it was unexpected in both trials with no treatment (PBS) that the ovarian cortex from High A4 cows did not display increased follicle progression after 7 days of culture. Given increased evidence of fibrosis and follicular arrest, the High A4 cortex may be secreting factors from the extracellular matrix which lead to fibrosis. High A4 ovarian cortex media had increased cytokines, IL-13, INFα, and TNFα, and a tendency for increased CXCL10/IP-10 and INFγ. Interleukin-13 has been shown to collaborate with TNFα to cause fibrosis by inducing secretion of transforming growth factor beta (TGFβ) TGFβ−1, a known fibrosis inducing growth factor [25].Thus, increased A4 concentrations may be increasing IL-13 along with other pro-inflammatory cytokines to impair follicle growth and development.
Another ovarian cortex secreted factor AMH, a TGFβ family member, may also contribute to follicular arrest and fibrosis. We demonstrated previously that treatment of granulosa primary cultures with increasing A4 concentrations increases AMH [17]. We also observed greater concentrations of AMH secreted in our High A4 bovine cortex media, indicating that excess A4 may increase AMH, which can contribute to reduced granulosa cell proliferation [17] and follicular arrest. Therefore, additional studies are necessary to determine which factor is the originator of this phenotype culminating in or initiating follicle arrest.
Previous research from our laboratory has demonstrated that angiogenic VEGFA isoforms and VEGFA signal transduction inhibitors cause follicle progression in both the bovine [20] and rat [29] model systems. However, we have not previously used a model displaying follicular arrest to test the VEGFA angiogenic isoform treatment. As expected, angiogenic isoform, VEGFA165, increased follicular progression to later stages of development by increasing the number of early primary, primary, secondary, and antral follicles in both High A4 and control cows. These results agree with Yang and Fortune [21] who, in 2007, found that VEGFA increased the number of secondary follicles in bovine cortical pieces by 3- to 4-fold over 10 days in culture, suggesting a role for VEGFA in preantral follicle growth in cattle. Moreover, the addition of VEGFA165 to the culture medium improved the development of goat preantral follicles cultured in vitro, allowing the production of mature oocytes [45]. In vivo, Danforth et al. [46] found a 75–100% increase in the number of primary and secondary follicles after the injection of VEGFA (within 24 h) into rat ovaries, suggesting a role in regulating growth of early follicles.
Interestingly, in the current study, VEGFA165 not only advanced follicle development, but it also reduced A4 and other steroid hormones being secreted into the culture media from High A4 ovarian cortex. Recently, Asadi et al. [47] found an increase in the concentrations of 17β-estradiol after 6 days of treatment with VEGFA165 in human ovarian cortex cultures. However, we found that VEGFA165 dramatically reduced steroids and metabolites in culture media to the levels of these steroids in the control cow ovarian cortex. We are not sure of the specific mechanisms of this steroid reduction. Yamashita et al. [48] reported that treatment with VEGFA165 modulated the expression of bovine placenta steroidogenesis in vitro by increasing or decreasing P4 and estrone sulfate (E1) concentrations as well as aromatase P450-positive cell density, depending on gestational age of the fetus [49]. Our samples were analyzed on a 16 steroid and metabolite panel (see Materials and methods). It is possible that VEGFA165 increases aromatase activity to convert steroid precursors to E2. The steroid and metabolite panel measured E1, and we did not observe changes in our High A4 bovine cortex culture media.
In addition to the reduction of steroids, VEGFA165 also diminished fibrosis and oxidative stress in High A4 ovarian cortex to the level of controls, indicating that this growth factor may rescue the High A4 phenotype. This finding agrees with Wang et al. [50] who found that the administration of VEGF165 triggers angiogenesis and inhibits both apoptosis and fibrosis. Asadi et al. [47] also demonstrated that, in ovarian cortex cultures treated with VEGFA165, there was an increase in secondary follicles with a reduction in atretic, suggesting that VEGA165 promotes the proliferation and viability of granulosa cells to allow for follicle progression. These previous reports would support our previous research, where we found that cultured bovine cortex with VEGFA165 stimulated the expression of genes related to cell cycle, cell proliferation, and growth [20]. In the current study, bovine cortex treated with the anti-angiogenic isoform VEGFA165B or the combination of VEGFA165 plus VEGFA165B did not reduce steroids, fibrosis, or oxidative stress in High A4 cows compared to controls. Furthermore, VEGFA165B appears to antagonize the actions of angiogenic isoform VEGFA165 neutralizing its actions when given in combination.
Interestingly, after VEGFA isoform treatment, three of the cytokines that were elevated, IL-13, INFα, and INFγ, were reduced to control levels, suggesting that the VEGFA isoforms are relieving inflammation and inhibiting cytokine production. This may be due to VEGFA’s ability to make tissues more permeable as well as its ability to reduce fibrosis and oxidative stress as demonstrated by our studies.
Havelock et al. [51] demonstrated the presence of all enzymes necessary for androgen production in the postmenopausal ovarian stroma in women; however, there have been no studies to demonstrate this in bovine stroma. The excess A4 secreted into the media from ovarian cortex cultures of High A4 cows may be due to the stroma cell production of A4 or enhanced production of A4 by other cells within the High A4 cow ovary or excess expression by these cells. We have demonstrated [20] there is increased gene expression in CYP17A1, a steroid enzyme that produces androgens, in granulosa cells from High A4 cows. So it seems highly plausible that other cell populations may have expression of CYP17A1, producing androgen in greater quantities than control cows. Also excess A4 may be due to steroidogenesis being arrested due to lack of activation of aromatase and reduced conversion to estrogens.
Conclusion
As indicated in the graphical abstract, the ovarian cortex from High A4 cows secretes greater concentrations of A4, which may associated with increased fibrosis, oxidative stress, AMH secretion, and increased secretion of pro-inflammatory cytokines and chemokines, resulting in arrested follicle development. Stroma or other ovarian cells within the ovarian cortex could be a primary source or contributing sources of A4 in the High A4 cow population. Angiogenic VEGFA165 isoforms can reduce A4, other steroid hormones, rescue follicle development, oxidative stress, and fibrosis and selective cytokine production. Thus, VEGFA165 may be used as a potential therapeutic to restore the ovarian microenvironment and to enhance follicular maturation. In conclusion, our High A4 bovine cortical cultures provide an excellent in vitro model to understand and unravel the mechanisms involved in follicular arrest and ovarian steroid microenvironment effects on follicle fate.
Conflict of interest
The authors have no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Mohamed A Abedal-Majed, Department of Animal Production, School of Agriculture, University of Jordan, Amman-Jordan, Jordan.
Shelby A Springman, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Courtney M Sutton, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Alexandria P Snider, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Brooke E Bell, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Scott G Kurz, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Jeff Bergman, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Adam F Summers, Department of Animal and Range Sciences, New Mexico State University, Las Cruces, NM, USA.
Renee M McFee, School of Veterinary and Biomedical Sciences, Veterinary Medicine and Biomedical Sciences Hall (VBS), University of Nebraska-Lincoln, Lincoln, NE, USA.
John S Davis, Olson Center for Women’s Health, University of Nebraska Medical Center, Omaha, NE, USA; VA Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Jennifer R Wood, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA.
Andrea S Cupp, Department of Animal Science, Animal Science Building, University of Nebraska-Lincoln, Lincoln, NE, USA; VA Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
References
- 1. Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health 2011; 3:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hackbart KS, Cunha PM, Meyer RK, Wiltbank MC. Effect of glucocorticoid-induced insulin resistance on follicle development and ovulation. Biol Reprod 2013; 88:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA 2003; 290:1767–1770. [DOI] [PubMed] [Google Scholar]
- 4. Beshay VE, Carr BR. Hypothalamic-pituitary-ovarian axis and control of the menstrual cycle. 2013; 31–42.
- 5. Wiltbank MC, Gumen A, Sartori R. Physiological classification of anovulatory conditions in cattle. Theriogenology 2002; 57:21–52. [DOI] [PubMed] [Google Scholar]
- 6. Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, Tesone M, Abramovich D. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015; 156:1453–1463. [DOI] [PubMed] [Google Scholar]
- 7. Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 2007; 8:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician 2016; 94:106–113. [PubMed] [Google Scholar]
- 9. Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol 2002; 174:1–5. [DOI] [PubMed] [Google Scholar]
- 10. Chen MJ, Chou CH, Chen SU, Yang WS, Yang YS, Ho HN. The effect of androgens on ovarian follicle maturation: dihydrotestosterone suppress FSH-stimulated granulosa cell proliferation by upregulating PPARgamma-dependent PTEN expression. Sci Rep 2015; 5:18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity—implication for polycystic ovary syndrome. Endocrinology 2009; 150:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 1998; 113:27–33. [DOI] [PubMed] [Google Scholar]
- 13. Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod 2006; 75:924–932. [DOI] [PubMed] [Google Scholar]
- 14. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 1998; 101:2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Summers AF, Pohlmeier WE, Sargent KM, Cole BD, Vinton RJ, Kurz SG, McFee RM, Cushman RA, Cupp AS, Wood JR. Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess. PLoS One 2014; 9:e110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss JF 3rd. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol 2004; 63:51–60. [DOI] [PubMed] [Google Scholar]
- 17. McFee RM, Romereim SM, Snider AP, Summers AF, Pohlmeier WE, Kurz SG, Cushman RA, Davis JS, Wood JR, Cupp AS. A high-androgen microenvironment inhibits granulosa cell proliferation and alters cell identity. Mol Cell Endocrinol 2021; 531:111288. [DOI] [PubMed] [Google Scholar]
- 18. Jones MR, Chazenbalk G, Xu N, Chua AK, Eigler T, Mengesha E, Chen Y-H, Lee J-M, Pall M, Li X, Chen Y-DI, Taylor KD et al. Steroidogenic regulatory factor FOS is underexpressed in polycystic ovary syndrome (PCOS) adipose tissue and genetically associated with PCOS susceptibility. J Clin Endocrinol Metab 2012; 97:E1750–E1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abedal-Majed MA, Cupp AS. Livestock animals to study infertility in women. Anim Front 2019; 9:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abedal-Majed MA, Kurz SG, Springman SA, McNeel AK, Freetly HC, Largen V, Magamage M, Sargent KM, Wood JR, Cushman RA, Cupp AS. Vascular endothelial growth factor A isoforms modulate follicle development in peripubertal heifers independent of diet through diverse signal transduction pathways. Biol Reprod 2020; 102:680–692. [DOI] [PubMed] [Google Scholar]
- 21. Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev 2007; 74:1095–1104. [DOI] [PubMed] [Google Scholar]
- 22. McFee RM, Rozell TG, Cupp AS. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res 2012; 349:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Youngquist RS, Garverick HA, Keisler DH. Use of umbilical cord clamps for ovariectomy in cows. J Am Vet Med Assoc 1995; 207:474–475. [PubMed] [Google Scholar]
- 24. Sutton CM, Springman SA, Abedal-Majed MA, Cupp AS. Bovine ovarian cortex tissue culture. J Vis Exp 2021. [DOI] [PubMed] [Google Scholar]
- 25. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13a2 receptor is involved in induction of TGF-b1 production and fibrosis. Nat Med 2006; 12:99–106. 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 26. Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil 1997; 109:165–171. [DOI] [PubMed] [Google Scholar]
- 27. Koal T, Schmiederer D, Pham-Tuan H, Röhring C, Rauh M. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol 2012; 129:129–138. [DOI] [PubMed] [Google Scholar]
- 28. Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, Duncan FE. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016; 152:245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McFee RM, Artac RA, McFee RM, Clopton DT, Smith RA, Rozell TG, Cupp AS. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod 2009; 81:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poole RK, Brown AR, Poore MH, Pickworth CL, Poole DH. Effects of endophyte-infected tall fescue seed and protein supplementation on stocker steers: II. Adaptive and innate immune function. J Anim Sci 2019; 97:4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu W, Fang S, Wang Y, Chi X, Ma X, Zhang T, Hu S. Receptor and signaling pathway involved in bovine lymphocyte activation by Atractylodis macrocephalae polysaccharides. Carbohydr Polym 2020; 234:115906. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91:336–340. [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez F, Nair KS, Daniels JK, Basal E, Schimke JM, Blair HE. Hyperandrogenism sensitizes leukocytes to hyperglycemia to promote oxidative stress in lean reproductive-age women. J Clin Endocrinol Metab 2012; 97:2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi N, Harada M, Hirota Y, Nose E, Azhary JM, Koike H, Kunitomi C, Yoshino O, Izumi G, Hirata T, Koga K, Wada-Hiraike O et al. Activation of endoplasmic reticulum stress in granulosa cells from patients with polycystic ovary syndrome contributes to ovarian fibrosis. Sci Rep 2017; 7:10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lobo RA. What are the key features of importance in polycystic ovary syndrome? Fertil Steril 2003; 80:259–261. [DOI] [PubMed] [Google Scholar]
- 36. Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, Rowley JE, Villanueva CE, Hornick JE, Shekhawat GS, Wei JJ, Pavone ME et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020; 19:e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod 2012; 27:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spuri-Gomes R, Tenley SC, Abedal-Majed MA, Kurz SG, Bergman J, Wood JR, Cupp AS. Cows with excess androgen are anovulatory and have a differing patterns of progesterone secretion. Nebraska Beef Cattle Reports 2016; 22–24. [Google Scholar]
- 39. Pan JX, Zhang JY, Ke ZH, Wang FF, Barry JA, Hardiman PJ, Qu F. Androgens as double-edged swords: induction and suppression of follicular development. Hormones (Athens) 2015; 14:190–200. [DOI] [PubMed] [Google Scholar]
- 40. Mossa F, Jimenez-Krassel F, Folger JK, Ireland JL, Smith GW, Lonergan P, Evans AC, Ireland JJ. Evidence that high variation in antral follicle count during follicular waves is linked to alterations in ovarian androgen production in cattle. Reproduction 2010; 140:713–720. [DOI] [PubMed] [Google Scholar]
- 41. Tamate K, Sengoku K, Ishikawa M. The role of superoxide dismutase in the human ovary and fallopian tube. J Obstet Gynaecol (Tokyo 1995) 1995; 21:401–409. [DOI] [PubMed] [Google Scholar]
- 42. Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol 2005; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yilmaz N, Inal HA, Gorkem U, Sargin Oruc A, Yilmaz S, Turkkani A. Follicular fluid total antioxidant capacity levels in PCOS. J Obstet Gynaecol 2016; 36:654–657. [DOI] [PubMed] [Google Scholar]
- 44. Puttabyatappa M, Irwin A, Martin JD, Mesquitta M, Veiga-Lopez A, Padmanabhan V. Developmental programming: gestational exposure to excess testosterone alters expression of ovarian matrix metalloproteases and their target proteins. Reprod Sci 2017; 1933719117697127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Araujo VR, Silva GM, Duarte AB, Magalhaes DM, Almeida AP, Goncalves RF, Bruno JB, Silva TF, Campello CC, Rodrigues AP, Figueiredo JR. Vascular endothelial growth factor-A(165) (VEGF-A(165)) stimulates the in vitro development and oocyte competence of goat preantral follicles. Cell Tissue Res 2011; 346:273–281. [DOI] [PubMed] [Google Scholar]
- 46. Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod 2003; 68:1736–1741. [DOI] [PubMed] [Google Scholar]
- 47. Asadi E, Najafi A, Moeini A, Pirjani R, Hassanzadeh G, Mikaeili S, Salehi E, Adutwum E, Soleimani M, Khosravi F, Barati M, Abolhassani F. Ovarian tissue culture in the presence of VEGF and fetuin stimulates follicle growth and steroidogenesis. J Endocrinol 2017; 232:205–219. [DOI] [PubMed] [Google Scholar]
- 48. Yamashita H, Kamada D, Shirasuna K, Matsui M, Shimizu T, Kida K, Berisha B, Schams D, Miyamoto A. Effect of local neutralization of basic fibroblast growth factor or vascular endothelial growth factor by a specific antibody on the development of the corpus luteum in the cow. Mol Reprod Dev 2008; 75:1449–1456. [DOI] [PubMed] [Google Scholar]
- 49. Sousa LM, Campos DB, Fonseca VU, Viau P, Kfoury JR Jr, Oliveira CA, Binelli M, Buratini J Jr, Papa PC. Vascular endothelial growth factor A (VEGFA) modulates bovine placenta steroidogenesis in vitro. Placenta 2012; 33:788–794. [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Ying YF, Ouyang YL, Wang JF, Xu J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J Assist Reprod Genet 2013; 30:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Havelock JC, Rainey WE, Bradshaw KD, Carr BR. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Hum Reprod 2006; 21:309–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


