Abstract
Female individuals are more likely to be diagnosed with PTSD following trauma exposure than males, potentially due, in part, to underlying neurobiological factors. Several brain regions underlying fear learning and expression have previously been associated with PTSD, with the hippocampus, amygdala, dorsal anterior cingulate cortex (dACC), and rostral ACC (rACC) showing altered volume and function in those with PTSD. However, few studies have examined how sex impacts the predictive value of subcortical volumes and cortical thickness in longitudinal PTSD studies. As part of an emergency department study completed at the Grady Trauma Project in Atlanta, GA, N = 93 (40 Female) participants were enrolled within 24 h following a traumatic event. Multi-echo T1-weighted MRI images were collected one-month post-trauma exposure. Bilateral amygdala and hippocampal volumes and rACC and dACC cortical thickness were segmented. To assess the longitudinal course of PTSD, the PTSD Symptom Scale (PSS) was collected 6 months post-trauma. We investigated whether regional volume/thickness interacted with sex to predict later PTSD symptom severity, controlling for PSS score at time of scan, age, race, and trauma type, as well as intracranial volume (ICV) for subcortical volumes. There was a significant interaction between sex and rACC for 6-month PSS, such that right rACC thickness was positively correlated with 6-month PSS scores in females, but not in males. In examining PTSD symptom subtypes and depression symptoms, greater rACC thickness in females predicted greater avoidance symptoms, while smaller rACC thickness in males predicted greater depression symptoms. Amygdala and hippocampus volume and dACC thickness showed no main effect or interaction with sex. The current findings provide evidence for sex-based differences in how brain volume predicts future PTSD severity and symptoms and supports the rACC as being a vital region regarding PTSD. Gender differences should be assessed in future longitudinal PTSD MRI studies for more accurate identification of future PTSD risk following trauma.
Subject terms: Risk factors, Stress and resilience
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder with known differences in occurrence between male and female individuals. Females are twice as likely as males to develop PTSD after experiencing a traumatic event [1–4], and this sex difference is not explained by differences in the type of trauma experienced nor amount of trauma exposure [2, 5, 6]. It is possible there are biological risk factors that influence the chance of developing PTSD in female individuals following trauma. Thus, it is necessary to identify potential risk factors, including neurobiological and symptom variance in females vs. males who develop PTSD post-trauma.
Neuroimaging studies suggest three main areas involved in threat neurocircuitry play an important role in PTSD - the amygdala, hippocampus, and medial prefrontal or anterior cingulate cortical areas. The amygdala is associated with emotion and fear response in both animal models and humans [7]. The hippocampus is vital for contextual memory and the contextualization of fear response, providing inhibitory signaling to the amygdala [8, 9]. Finally, several prefrontal areas play important roles in fear regulation. One is the rostral anterior cingulate cortex (rACC), which is considered to have overlap with the ventromedial prefrontal cortex (vmPFC) in functional neuroimaging studies. The rACC is important for top-down regulation of the amygdala, and is thought to be involved in emotion regulation [10–14]. A second important prefrontal area is the dorsal anterior cingulate cortex (dACC), which plays a role in conflict monitoring and salience of environmental threat cues [12, 15]. The amygdala, hippocampus, rACC, and dACC play main roles in threat response and/or salience of threat, and show functional dysregulation or changes in volume in those with PTSD.
Previous functional magnetic resonance imaging (fMRI) studies of PTSD have identified associations with amygdala hyperactivity and hippocampal and anterior cingulate/prefrontal cortex hypoactivity [13, 14, 16–18]. Regarding structural MRI data, case-control studies demonstrate an association between PTSD and smaller hippocampal volumes [19, 20]. Longitudinal studies, in which MRI scans were collected early post-trauma and participants were assessed for later PTSD symptoms, suggest correlations between the development of PTSD and reduced hippocampal volume [19, 21–25] and reduced rACC and dACC volume [26, 27]. There are mixed findings regarding amygdala structure, with either no correlations being seen longitudinally [26], or associations with reduced amygdala volume in case-control studies [20, 28], or differences being seen between nuclei in a case-control study [29].
However, few studies have examined the influence of sex on the relationship between PTSD and brain volumes, and early predictors of risk in females vs. males remain unknown. Animal studies, which can help inform our hypotheses regarding humans, suggests sex plays a role in the relationship between stress and dendritic spine loss, with stress increasing hippocampal dendritic spine density in males and reducing density in females [30], while having an opposite pattern in the medial prefrontal cortex (decreased in males, increased in females) [31]. Differences are shown to be influenced partially by estradiol, which increases dendritic growth between the mPFC and amygdala in females in response to stress [32, 33]. In humans, cross-sectional and developmental studies inform our current understanding, though their results are equivocal. One study saw smaller amygdala volumes in female participants with PTSD compared to male participants with PTSD [28], but another found early life trauma exposure was associated with decreased PFC, amygdala, and hippocampal gray matter in males but increased amygdala volume in females [34, 35]. Other studies do not find any strong evidence for sex differences in brain structure in those with PTSD [19, 36]. One potential reason for these discrepancies is the influence of trauma type, with females more likely to experience sexual assault and males more likely to experience non-sexual assault, accidents, or combat [1]. Differences in trauma type can influence hippocampal volume findings [37], and therefore sex differences in trauma type exposure may play a role in study results. Overall, there is little understanding regarding how sex differences impact the predictive value of brain volume for prospective PTSD across time points following trauma, with most studies focusing on both male and female participants combined with no differentiation. As there is an active attempt to understand how biological sex differences impact PTSD symptoms, it is important to determine whether differences in brain structure predict PTSD differently in females vs. males, or may predict a distinct symptom presentation in females vs. males.
In the current study, we investigated sex differences in the predictive value of brain structure for longitudinal PTSD symptoms in individuals recruited following a traumatic event in the emergency department (ED). Given previous findings implicating threat-related neurocircuitry in PTSD risk [7, 8, 10–13, 38], and the current literature regarding sex differences in brain structural alterations associated with PTSD longitudinally in adults [28, 34, 35], we hypothesized that reduced hippocampus and amygdala volumes, and rACC and dACC thickness would correlate with higher PTSD symptomology, and that we would see sex differences across these correlations. We predicted decreased dACC, rACC, and hippocampal thickness/volume in male participants, and decreased amygdala volume in female participants, based on limited prior neuroimaging findings [28, 34, 35]. Secondly, we hypothesized that sex-dependent associations might predict differences in risk for specific types of PTSD symptoms (re-experiencing, avoidance, numbing, hyperarousal) and depressive symptoms.
Materials and methods
Participants
Participants were recruited from a larger Emergency Department (ED) study consisting of 504 participants [39–41]. Of the 504 participants, 419 were approached regarding MRI participation. 93 participants were eligible (did not present contraindications including ferrous metal, pregnancy, pacemakers, etc.) and agreed to undergo MRI scans which were used for the current study. All subjects were ED patients at Grady Memorial Hospital in Atlanta, GA, who had experienced a traumatic event within the past 24 h of arrival to the ED. All participants were English-speaking, 18–65 years of age, experienced a criterion-A-trauma as defined by the DSM-IV-TR, and provided contact information for follow-up visits. Exclusion criteria included previous hospitalization for mental health reasons, current suicidal ideation, attempted suicide in the past 3 months, current intoxication, or altered mental status during the ED visit. 53 participants self-identified as male and 40 as female. Trauma types included 54 motor vehicle collisions, 14 pedestrian versus automobile accidents, 5 sexual assaults, 4 motorcycle crashes, 4 bike or bike versus automobile accidents, 4 industrial or home accidents, 3 non-sexual assaults, 3 gunshot wounds, 1 stabbing, and 1 animal attack. Participants provided written informed consent for all parts of the study, and study procedures were approved by the Institutional Review Boards of Emory University and Grady Memorial Hospital.
Emergency department (ED) assessment and follow-up assessments
Demographic information and trauma index information was gathered using the Standardized Trauma Interview (STI), a 41-item clinician-administered interview gathering information on relevant aspects of the trauma at baseline as well as demographic information [42]. Following the ED visit, PTSD symptoms were assessed at 1 month, 3 months, 6 months, and 12 months. PTSD symptom severity in response to the index trauma was measured utilizing the PTSD Symptom Scale (PSS) [43], a 17-item scale measuring PTSD symptom severity according to DSM-IV-TR criteria [44]. For this paper, PSS measures at 1 month (baseline) and 6 months were used, because the 6-month timepoint is critical in the recovery process when individuals who will recover spontaneously begin to clearly diverge in symptom severity from those who will maintain chronic PTSD symptoms at high levels [38, 45–47]. Table 1 displays demographics and mean scores split by sex. PSS subcategories including intrusion, avoidance, numbing, and hyperarousal were differentiated. The Beck Depression Inventory (BDI) was collected across timepoints as a measure of depression severity. The Childhood Trauma Questionnaire (CTQ) and a self-report count of prior traumas were also collected.
Table 1.
Clinical and demographic features of the sample.
| Female | Male | Effect of sex | |
|---|---|---|---|
| N = 40 | N = 53 | ||
| Age, mean (SD) | 34 (12.46) | 37 (12.88) | t(85.53) = 1.17, p = 0.25 |
| Race (%) | χ2(3) = 3.07, p = 0.28 | ||
| Hispanic | 1 (2.50%) | 4 (7.40%) | |
| Non-Hispanic White | 4 (10.00%) | 10 (18.52%) | |
| Non-Hispanic Black | 34 (85.00%) | 37 (68.52%) | |
| Non-Hispanic Other | 1 (2.50%) | 2 (3.70%) | |
| Education level (%) | χ2(6) = 5.24, p = 0.51 | ||
| Doctoral degree | 0 (0.00%) | 1 (1.89%) | |
| Master’s degree | 1 (2.50%) | 2 (3.77%) | |
| Some graduate school | 0 (0.00%) | 1 (1.89%) | |
| Bachelor’s degree | 2 (5.00%) | 8 (15.09%) | |
| Associate’s/some college | 22 (55.00%) | 21 (39.62%) | |
| High school degree | 10 (25.00%) | 15 (28.30%) | |
| Some high school | 5 (12.50%) | 5 (9.43%) | |
| Trauma type (%) | χ2(9) = 20.73, p = 0.01* | ||
| Non-sexual assault | 0 (0.00%) | 3 (5.66%) | |
| Motor vehicle collision | 27 (67.50%) | 27 (50.94%) | |
| Motorcycle crash (MCC) | 0 (0.00%) | 4 (7.55%) | |
| Ped v. auto | 5 (12.50%) | 9 (16.98%) | |
| Gunshot wound | 0 (0.00%) | 3 (5.66%) | |
| Stabbing | 1 (2.50%) | 0 (0.00%) | |
| Industrial/home accident | 0 (0.00%) | 4 (7.54%) | |
| Animal bite/attack | 0 (0.00%) | 1 (1.89%) | |
| Bike accident/bike v. auto | 2 (5.00%) | 2 (3.77%) | |
| Sexual assault | 5 (12.50%) | 0 (0.00%) | |
| 1-month PSS, mean (SD) | 19.32 (11.09) | 13.98 (11.31) | t(84.98) = 2.28, p = 0.03* |
| 6-month PSS, mean (SD) | 11.79 (9.29) | 8.30 (9.36) | t(60.00) = 1.13, p = 0.26 |
| Change in PSS score, mean (SD) | −8.69 (9.42) | −5.5 (6.56) | t(45.14) = 1.59, p = 0.12 |
| 1-month beck depression inventory (BDI), mean (SD) | 16.19 (10.89) | 12.57 (10.05) | t(73.89) = 1.59, p = 0.12 |
| 6-month beck depression inventory (BDI), mean (SD) | 14.22 (11.41) | 7.82 (7.95) | t(41.57) = 2.56, p = 0.01* |
| Childhood trauma questionnaire (CTQ), mean (SD) | 42.91 (18.00) | 36.34 (15.94) | t(63.54) = 1.68, p = 0.10 |
| Number of prior traumatic events, mean (SD) | 2.29 (1.75) | 2.92 (2.01) | t(83.14) = 1.55, p = 0.12 |
PTSD Symptom Scale (PSS) Range: 0–40, Childhood Trauma Questionnaire (CTQ) Range: 5–125. Beck Depression Inventory (BDI) Range: 0–63.
*p < 0.05.
Structural brain imaging data acquisition
MRI sessions were completed within three weeks of the 1-month follow-up assessment. This period was targeted to account for potential injury recovery time. Brain imaging data were acquired on three Siemens 3.0-Tesla Magnetom Trio TIM MRI scanners (Siemens, Malvern, PA) using a 12-channel head coil. N = 12 participants were scanned on the first scanner, N = 26 on the second, and N = 55 on the third. Changes in scanner site were necessitated due to an upgrade from Trio to Prisma. For the first two MRI scanners, structural images were acquired using a gradient-echo, T1- weighted pulse sequence (TR = 2300 ms, TE = 2.78 ms; 1.2 mm × 1.3 mm × 1.3 mm voxel size). For the third MRI scanner, structural images were acquired using multi-echo T1-weighted image (176 slices, TR = 2530 ms, TE1 = 1.74 ms, TE2 = 3.6 ms, TE3 = 5.46 ms, TE4 = 7.32 ms, voxel size 1 × 1 × 1 mm3). Images were analyzed using an automated multistep segmentation process (Freesurfer version 5.3) [48, 49]. Automated segmentation was used to compute total intracranial volume, bilateral hippocampal volume, and amygdala volume using the included FreeSurfer subcortical atlas [48]. Bilateral dACC and rACC thickness were calculated using the Desikan-Killiany Atlas with FreeSurfer [50]. Total intracranial volume (ICV) was also measured. Segmentation quality checks were performed using the ENIGMA 2 (subcortical volume) and ENIGMA 3 (cortical thickness and surface area) protocols (http://enigma.ini.usc.edu/protocols/imaging-protocols/), designed to standardize quality control procedures and facilitate study replication [51] Table 2 displays mean regional brain volume and thickness split by sex.
Table 2.
Regional brain volume and thickness by sex.
| Regional volume/thickness (μL for hippocampus and amygdala, mm for dACC and rACC | Female M(SD) | Male M(SD) |
|---|---|---|
| Total intracranial volume | 1,308,609.00 (140,029.10) | 1,557,524.00 (173,987.30) |
| Right amygdala | 1,496.76 (206.76) | 1,665.86 (246.55) |
| Left amygdala | 1,463.81 (196.16) | 1,668.59 (219.88) |
| Right hippocampus | 3,791.43 (404.58) | 4,163.26 (581.15) |
| Left hippocampus | 3,742.74 (405.42) | 4,069.49 (482.03) |
| Right dACC | 2.55 (0.29) | 2.45 (0.22) |
| Left dACC | 2.71 (0.34) | 2.57 (0.26) |
| Right rACC | 2.79 (0.24) | 2.69 (0.23) |
| Left rACC | 2.91 (0.26) | 2.76 (0.22) |
dACC dorsal anterior cingulate cortex, rACC rostral anterior cingulate cortex.
We focused on cortical thickness based on an increasing number of studies that use it as a measure of cortical integrity [52–55], in addition to cortical thinning being a common finding in PTSD studies [56–58]. However, surface area for cortical areas was also recorded and included in supplemental analyses.
Statistical analysis overview
Data were analyzed and visualized using R v.4.0.3. The dplyr package [59] was used for data organization and transformation. T-tests were utilized to analyze sex differences in age, PSS scores, change in PSS score between time points (1-month PSS subtracted from 6-month PSS), BDI, CTQ, and number of traumatic events, and Chi-squared tests were performed to determine if race, level of education, and trauma type were significantly different between male and female participants (Table 1). Z-scores were calculated and values were winsorized if they fell outside 3 standard deviations from the mean to control for outliers. To test the hypothesis that threat neurocircuitry may show differential associations with PTSD symptoms in depending on sex, regression models were implemented with sex, region of interest (ROI), and sex*ROI effects on PTSD symptoms. Symptoms concurrent with the MRI scan (at 1-month post-trauma) and future symptoms at 6-months post-trauma were investigated in a separate regression analysis. Significant sex*ROI interaction effects were then split by sex and analyzed for correlations between ROI volume /thickness and PSS total. Spearman’s rho correlations were used when data had a non-normal distribution, according to the Shapiro-Wilks test, but otherwise Pearson correlations were utilized. Variables with non-normal distributions included left dorsal ACC, right amygdala, right rostral ACC, 1-month PSS scores, 6 month PSS scores, and 6 month BDI. Correlation matrices were created using the Hmisc package [60]. The statistical threshold correcting for multiple comparisons was set at p < 0.00625 (bonferroni-corrected p < 0.05), across models for the 8 ROIs.
Statistical models of subcortical volumes included ICV as a covariate to account for significant correlations between ICV and hippocampal and amygdala volumes (p < 0.001). There was no significant correlation between ICV and cortical thickness (p > 0.05), and it was not included for cortical thickness models. Participant age, race, and education level did not differ by sex (p > 0.05; Table 1), but we included age and self-reported racial identity as covariates because of their established associations with PTSD risk [61–63]. Trauma type did significantly differ by sex (p = 0.004). Covariates therefore included total ICV (for subcortical volumes), scanner site, trauma type, age and race. An additional regression including 1-month PSS as a covariate was conducted to account for PSS at time of scan. Exploratory analyses of regions outside this set of ROIs were conducted using the same statistical models with Bonferroni correction. To further examine the significant relationships between brain volume and PTSD symptom severity, PSS avoidance, numbing, hyperarousal, and intrusive subscales based on the DSM-IV were analyzed in regression models to examine the influence of specific PTSD symptoms. Avoidance and numbing scores were separated according to a prior confirmatory factor analysis [64]. BDI was analyzed to examine the relationship between depression symptoms and brain volume. Additionally, two separate models including CTQ and number of prior traumas were performed to analyze the influence of prior trauma on results. The ggplot2 package [65] was used to visualize data.
Results
Demographic and trauma-related characteristics of the final sample are reported in Table 1. Female participants had significantly higher PTSD symptom severity than male participants 1 month post-trauma (t(85) = 2.28, p = 0.03). There was no significant difference in PSS scores between females and males at 6 months (t(60) = 1.13, p = 0.26). There was no significant sex difference in change in PSS scores between timepoints (t(45) = 1.59, p = 0.12).
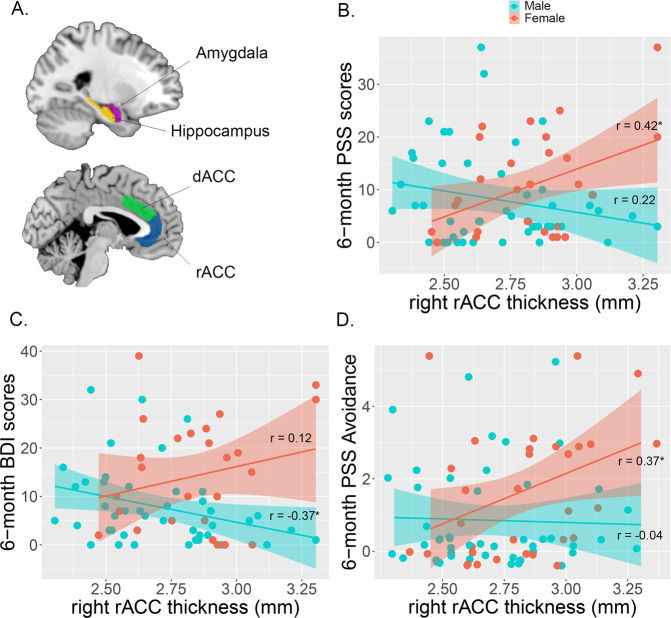
There was a significant sex interaction effect with right rACC thickness in predicting 6-month PTSD symptom severity (Fig. 1, Supplementary Table S1; ß = 33.96, ΔR2 = 0.16, t = 3.41, p = 0.001; Model: F(7,67) = 2.61, p = 0.01). This interaction effect persisted when 1-month PSS was included in the model (Supplementary Table S2, ß = 18.98, ΔR2 = 0.05, t = 2.59, p = 0.01; Model: F(8,66) = 12.88, p < 0.001). Follow-up analyses showed a significant positive correlation between right rACC and 6-month PTSD symptoms in female participants (r(27) = 0.42, p = 0.02), but not male participants (r(44) = −0.22, p = 0.15). There was no main effect or interaction for the left or right amygdala, hippocampus, or dACC. Furthermore, exploratory analyses of regions outside our ROIs showed no significant interactions with sex at a corrected significance threshold, for either regional thickness (Supplementary Table S3), or surface area (Supplementary Table S4). These findings suggest that greater early post-trauma rACC thickness is related to PTSD risk in female participants only.
Fig. 1. Regions of interest and sex-by-region interaction effects predicting subsequent PTSD symptoms.
Amygdala, hippocampus, rACC, and dACC regions segmented (A). There was a significant sex interaction effect (B) for right rostral ACC thickness and 6-month PSS total scores when accounting for scanner site, trauma type, age, and race (t = 3.41, p = 0.001). Female participants showed a significant positive correlation between right rACC thickness and 6-month PSS total scores (r(27) = 0.42, p = 0.02), while male participants did not show a significant correlation (r(44) = −0.22, p = 0.15). To further understand the relationship between right rACC thickness and 6-month PSS, PSS subcategories and depression symptoms were analyzed. Male participants showed a significant negative correlation (C) between right rACC and 6-month depression symptoms (r(42) = −0.37, p = 0.01). Female participants showed a significant positive correlation (D) between right rACC and 6-month avoidance symptoms (r(27) = 0.37, p = 0.04). *p < 0.05.
To evaluate whether this sex-dependent effect may be driven by specific PTSD symptom subcategories or depression symptoms, we conducted regression models investigating the sex*right rACC effect on 6-month intrusive symptoms, avoidance, symptoms, numbing symptoms, hyperarousal symptoms, and depression symptoms from the BDI (Fig. 1C, D). Regression models showed that right rACC thickness interacted with sex to predict 6-month avoidance symptoms (ß = 3.61, ΔR2 = 0.12, t = 2.13, p = 0.04), numbing symptoms (ß = 8.15, ΔR2 = 0.11, t = 2.69, p = 0.01), hyperarousal symptoms (ß = 12.27, ΔR2 = 0.13, t = 2.67, p = 0.01), and intrusive symptoms (ß = 6.99, ΔR2 = 0.08, t = 2.39, p = 0.02). Follow-up analyses showed that right rACC thickness was positively correlated with avoidance symptoms in females (r(27) = 0.37, p = 0.04), but not males (r(44) = −0.04, p = 0.79). There were no significant correlations for numbing, hyperarousal, or intrusive symptoms for either sex (ps > 0.05). For 6-month depression symptoms, there was a sex*right rACC interaction effect (ß = 34.85, ΔR2 = 0.24, t = 3.19, p = 0.002). Here, interestingly, right rACC thickness correlated negatively with 6-month BDI in male participants (r(42) = −0.37, p = 0.01), but not female participants (r(25) = 0.12, p = 0.56). There were no sex differences between avoidance symptoms, numbing symptoms, hyperarousal symptoms, or intrusive symptoms at 6 months (p > 0.05). There was a significant difference for depression symptoms, with female participants having significantly higher depression symptoms (t(41.57) = 2.56, p = 0.01).
To see if prior trauma had an impact on results, we further analyzed two self-report measures, the CTQ and a self-report number of prior traumatic events, as covariates. Female and male participants did not significantly differ in their CTQ scores nor their amount of prior reported trauma (p > 0.05; Table 1). When CTQ was included in our regression model as a covariate, the interaction between sex and right rACC was attenuated, but still significant (CTQ: ß = 0.20, ΔR2 = 0.08, t = 2.41, p = 0.003; sex*right rACC: ß = 12.00, ΔR2 = 0.08, t = 2.41, p = 0.02). A second model including number of prior traumatic events as a covariate showed a strong remaining sex * rACC effect (traumatic events: ß = 1.28, ΔR2 = 0.15, t = 2.39, p = 0.01; sex*right rACC: ß = 30.57, ΔR2 = 0.15, t = 2.93, p = 0.005). This suggests that the observed sex-dependent rACC effect predicts PTSD symptoms above and beyond lifetime or childhood trauma history.
Discussion
The results of the current study provide evidence for sex-related differences in how early-post-trauma brain cortical thickness predicts longitudinal PTSD severity. In female participants, greater right rACC thickness 1 month post-trauma predicted PTSD severity at 6 months post-trauma, as well as avoidance symptoms, while smaller rACC thickness in male participants predicted greater subsequent depression symptom severity. Amygdala volume, hippocampal volume, dACC, and left rACC thickness 1-month post-trauma did not predict 6-month PTSD severity. These findings support the rACC as a vital neural region regarding mood and PTSD. This study fills a gap in the literature by investigating the influence of sex on how brain structure predicts PTSD longitudinally, which has not been examined previously.
For females, these results differ from prior rACC findings with respect to PTSD. Previous longitudinal studies showed reduced rACC thickness and surface area predict later probable PTSD diagnosis 3 months [26] and 6 months [26, 27]. However, within these studies, individuals were either only exposed to motor vehicle accidents [27] or also experienced mild traumatic brain injury during a traumatic event [26]. These studies also did not differentiate by sex nor examine PTSD symptom subtypes, making it difficult to draw direct comparisons.
Interestingly, a similar phenotype has been identified in neuroimaging studies of the dissociative subtype of PTSD. Those studies suggest that greater PFC activation relates to emotional overmodulation and dissociation symptoms. There is evidence for a dissociative subtype of PTSD, with findings suggesting that, within this subtype, overmodulation of prefrontal areas over the amygdala leads to diminished fear response. This is in contrast to traditional PTSD models, in which under-modulation of prefrontal areas over the amygdala leads to hyperactive fear response [66, 67]. Our findings may be consistent with this differentiation, with larger right rACC correlating with avoidance symptoms. While we did not collect information about dissociative symptoms in the current study, avoidance and numbing symptoms are known to reflect hypoarousal, and to share some overlap with dissociation symptoms [68]. Notably, much of the neuroimaging work on trauma-related dissociation or the dissociative subtype of PTSD has been conducted in females [69–71]. It may be the case that the neural features associated with PTSD in females have been undercharacterized to date, and that a more male-typical pattern drives the models of PTSD neurocircuitry in the existing literature. There are no structural or fMRI studies that have focused on PTSD subtypes and differences across sex longitudinally. However, one study looking specifically at the influence of interpersonal violence in women with PTSD compared to non-trauma controls found PTSD subtypes did predict differences in cortical thickness and gray matter volume throughout the brain [72], consistent with our findings.
For males, smaller right rACC thickness correlated with greater depression symptoms. This supports prior findings, which suggest that this region is thinner in those with depression, and thickens in those who show clinical improvement following transcranial magnetic stimulation treatment [73–75]. Interestingly, one prior study found the rACC to be thinner only in boys with depressive symptoms, but not girls [74]. The results of the current study build upon these findings and suggest the rACC plays a role in vulnerability to post-trauma depression in males following trauma, but not in females.
These contrasting findings point to the importance of considering sex differences in assessing biological risk factors for PTSD. As mentioned, differences in PTSD symptom presentation between females and males may influence these results, with a dissociative/overmodulatory phenotype correlating with rACC thickness in female participants in this study, and with depression symptoms correlating with lower rACC thickness in male participants in this study. It would be of interest to examine populations of male and female individuals post-trauma who develop emotional overmodulation vs. undermodulation symptoms as well as depression symptoms, to examine more closely the relationship between sex, brain volume, and PTSD subtypes. It is worth noting that, when 1-month PSS symptoms were included in models as a baseline collected at the time of the scan, this removed significant sex interaction effects when correcting for multiple comparisons. 1-month PSS scores were correlated with 6-month PSS scores (r = 0.74, p < 0.001), with this high collinearity likely obscuring the association with rACC. Other studies also suggest predisposing factors pre-index trauma, such as childhood trauma, may influence rACC volume and PTSD symptomology [76–81]. However, when childhood trauma and number of prior traumas were added to regression models as covariates, the interaction between sex and right rACC was still significant, meaning sex-dependent rACC predicts PTSD symptoms regardless of earlier trauma. Hormonal differences between males and females may also explain differences in rACC thickness. Estradiol is known to mediate dendritic growth in the mPFC and its projections to the amygdala in response to stress in female rodents [32, 33]. Regarding humans, hormone fluctuations have been shown to play a role in fear learning in females, and may also play a role in PTSD severity [82–84] and sex differences through influence on the rACC and the vmPFC [33, 85]. However, this is outside the scope of the current study. Differences in trauma type experienced between male and female individuals may also influence rACC thickness results [1]. Trauma type is shown to impact hippocampal volume [37], although there is no current evidence for how trauma type influences rACC thickness.
Weaknesses of the current study include sample size and generalizability. Only 93 of the 504 participants recruited for this study had MRI scans collected, when it is becoming clearer that larger sample sizes are needed for observational studies [86]. While findings suggest that rACC may be causally involved in PTSD development, these are observational findings done in a small sample size. Due to the nature of trauma research, in which participants are usually only able to be recruited for longitudinal studies after a traumatic event has occurred, it is difficult to assess the exact influence of traumatic events on the brain from pre-trauma to post-trauma, thus making it more difficult to determine a causal influence of the rACC in PTSD neuroimaging studies. Participants were also only recruited from the ED at Grady Hospital in Atlanta, GA, meaning that findings may be specific to this population. Biological sex was indicated by participants via survey, and there was no data collected regarding gender identity. Thus, this paper is limited to the analysis of sex. Additionally, only 6-month follow-up data were used for this analysis. While this is a strong indicator of which participants will go on to develop chronic symptoms [87], we did not evaluate the influence of chronic vs. acute PTSD symptoms post-trauma within this analysis. Individuals with chronic PTSD may wait years before pursuing treatment, and we did not collect PSS scores at timepoints past one year. We also utilized the PSS as a self-report measure to evaluate PTSD symptom severity rather than relying on a clinical diagnostic interview. Further longitudinal research is necessary to understand brain volume differences and what they might mean for individuals with PTSD long-term.
In summary, findings support incorporating both PTSD symptom subtypes and sex differences into models of predicting PTSD from rACC thickness. In female participants, greater right rACC thickness predicted later overall PTSD and avoidance symptoms, suggesting a relation with emotional overmodulation. While in male participants, smaller right rACC thickness predicted later depression severity, in line with prior findings. In utilizing our understanding of how brain volume differentially predicts symptoms across sex, we may continue to improve upon efforts to find neurobiological predictors of PTSD following traumatic events. This may lead to more accurate identification of individuals at risk of PTSD immediately following trauma, refining patient-specific targets for preventative care.
Supplementary information
Author contributions
Design and conceptualization of the study: ARR, SS, VM, BOR, TJ, KJR, JSS. Data collection and recruitment: VM, RH, SJHVR, JSS. Data processing and statistical analyses: ARR, SS, VM, JSS. Initial drafting of the paper: ARR, SS, JSS. All authors revised the paper critically for important intellectual context and agree to be accountable for all aspects of the work, and ensure the accuracy and integrity of the findings.
Funding
This research was supported by the National Institute of Mental Health (R01 MH094757 [to KJR], R21 MH106902 [to TJ], R01 MH117009 [to JSS], F31 MH126623 [to ARR]), and a NARSAD award from the Brain & Behavior Research Foundation to BOR.
Competing interests
KJR has received consulting fees or sponsored research support from Alkermes, BrainsWay, and Genomind, and he serves on scientific advisory boards for Janssen, Takeda, and Verily. KJR and JSS are on the NPP Editorial Board. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01452-9.
References
- 1.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Trauma. 2008;S:37–85. [DOI] [PubMed]
- 2.Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 3.Dell’Osso L, Carmassi C, Massimetti G, Stratta P, Riccardi I, Capanna C, et al. Age, gender and epicenter proximity effects on post-traumatic stress symptoms in L’Aquila 2009 earthquake survivors. J Affect Disord. 2013;146:174–80. doi: 10.1016/j.jad.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Kline A, Ciccone DS, Weiner M, Interian A, St Hill L, Falca-Dodson M, et al. Gender differences in the risk and protective factors associated with PTSD: a prospective study of National Guard troops deployed to Iraq. Psychiatry. 2013;76:256–72. doi: 10.1521/psyc.2013.76.3.256. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29:813–21. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–43. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 13.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 14.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–57. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 16.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 17.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81:1023–9. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller Hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244–53. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–78. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress. 2011;14:227–32. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- 22.Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, et al. Relationship of Hippocampal volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:968–75. doi: 10.1016/j.bpsc.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Zion Z, Artzi M, Niry D, Keynan NJ, Zeevi Y, Admon R, et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:311–9. doi: 10.1016/j.bpsc.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quidé Y, Andersson F, Dufour-Rainfray D, Descriaud C, Brizard B, Gissot V, et al. Smaller hippocampal volume following sexual assault in women is associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2018;138:312–24. doi: 10.1111/acps.12920. [DOI] [PubMed] [Google Scholar]
- 25.Koch SBJ, van Ast VA, Kaldewaij R, Hashemi MM, Zhang W, Klumpers F, et al. Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms. Neuropsychopharmacology. 2021;46:1283–92. doi: 10.1038/s41386-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein MB, Yuh E, Jain S, Okonkwo DO, Mac Donald CL, Levin H, et al. Smaller Regional Brain Volumes Predict Posttraumatic Stress Disorder at 3 Months After Mild Traumatic Brain Injury. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:352–9. doi: 10.1016/j.bpsc.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu H, Sun Y, Su S, Wang Y, Qiu Y, Yang X, et al. Cortical surface area reduction in identification of subjects at high risk for post-traumatic stress disorder: A pilot study. Aust N Z J Psychiatry. 2018;52:1084–91. doi: 10.1177/0004867417750757. [DOI] [PubMed] [Google Scholar]
- 28.Starcevic A, Postic S, Radojicic Z, Starcevic B, Milovanovic S, Ilankovic A, et al. Volumetric analysis of amygdala, hippocampus, and prefrontal cortex in therapy-naive PTSD participants. Biomed Res Int. 2014;2014:968495. doi: 10.1155/2014/968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morey RA, Clarke EK, Haswell CC, Phillips RD, Clausen AN, Mufford MS, et al. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:281–90. doi: 10.1016/j.bpsc.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–13. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, et al. Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Dev Psychopathol. 2015;27:1555–76. doi: 10.1017/S0954579415000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson GM, Camins JS, Wisse L, Wu J, Duda JT, Cook PA, et al. Childhood socioeconomic status and childhood maltreatment: Distinct associations with brain structure. PLoS One. 2017;12:e0175690. doi: 10.1371/journal.pone.0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woon F, Hedges DW. Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: a meta-analysis. Hippocampus. 2011;21:243–52. doi: 10.1002/hipo.20746. [DOI] [PubMed] [Google Scholar]
- 37.Hinojosa CA. Does Hippocampal volume in patients with posttraumatic stress disorder vary by trauma type? Harv Rev Psychiatry. 2022;30:118–34. doi: 10.1097/HRP.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 38.Stevens JS, Harnett NG, Lebois LAM. Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. Am J Psychiatry. 2021;79:1037–49. [DOI] [PMC free article] [PubMed]
- 39.Hinrichs R, Michopoulos V, Winters S, Rothbaum AO, Rothbaum BO, Ressler KJ, et al. Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress Anxiety. 2017;34:502–7. doi: 10.1002/da.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, et al. Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry. 2020;177:58–65. doi: 10.1176/appi.ajp.2019.19010039. [DOI] [PubMed] [Google Scholar]
- 41.Lalonde CS, Mekawi Y, Ethun KF, Beurel E, Gould F, Dhabhar FS, et al. Sex differences in peritraumatic inflammatory cytokines and steroid hormones contribute to prospective risk for nonremitting posttraumatic stress disorder. Chronic Stress. 2021;5:24705470211032208. doi: 10.1177/24705470211032208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foa EB, Rothbaum BO. Treating the trauma of rape: cognitive-behavioral therapy for PTSD. New York, NY: Guilford Press; 2001.
- 43.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
- 45.Galatzer-Levy IR, Karstoft K-I, Statnikov A, Shalev AY. Quantitative forecasting of PTSD from early trauma responses: a Machine Learning application. J Psychiatr Res. 2014;59:68–76. doi: 10.1016/j.jpsychires.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, et al. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol Psychiatry. 2018;84:106–15. doi: 10.1016/j.biopsych.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb EK, Huggins AA, Belleau EL, Taubitz LE, Hanson JL, deRoon-Cassini TA, et al. Acute posttrauma resting-state functional connectivity of periaqueductal gray prospectively predicts posttraumatic stress disorder symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:891–900. doi: 10.1016/j.bpsc.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 49.Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84.. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Van Erp TG, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biological psychiatry. 2018;84:644–54. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickerson BC, Wolk DA. Alzheimer’s Disease Neuroimaging Initiative. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addiction Biol. 2013;18:379–91. doi: 10.1111/j.1369-1600.2011.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almeida Montes LG, Prado Alcántara H, Martínez García RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: age, sex, and clinical correlations. J Atten Disord. 2013;17:641–54. doi: 10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- 55.Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Bäckman L, Li S-C, et al. Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp. 2012;33:1607–20. doi: 10.1002/hbm.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27:515–25. doi: 10.1016/j.euroneuro.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadeh N, Spielberg JM, Logue MW, Wolf EJ, Smith AK, Lusk J, et al. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Mol Psychiatry. 2016;21:357–63. doi: 10.1038/mp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601–11. doi: 10.1016/j.nicl.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham H, François R, Henry L, Müller K dplyr: a grammar of data manipulation. 2022. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr.
- 60.Harrell Jr FE. Hmisc—Harrell miscellaneous—R package version 4.7-1: The Comprehensive R Archive Network web page. 2021. https://CRAN.R-project.org/package=Hmisc.
- 61.Kobayashi I, Sledjeski EM, Delahanty DL. Gender and age interact to predict the development of posttraumatic stress disorder symptoms following a motor vehicle accident. Psychol Trauma. 2019;11:328–36. doi: 10.1037/tra0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koenen KC, Stellman JM, Stellman SD, Sommer JF., Jr Risk factors for course of posttraumatic stress disorder among Vietnam veterans: a 14-year follow-up of American Legionnaires. J Consult Clin Psychol. 2003;71:980–6. doi: 10.1037/0022-006X.71.6.980. [DOI] [PubMed] [Google Scholar]
- 63.Sibrava NJ, Bjornsson AS, Pérez Benítez ACI, Moitra E, Weisberg RB, Keller MB. Posttraumatic stress disorder in African American and Latinx adults: Clinical course and the role of racial and ethnic discrimination. Am Psychol. 2019;74:101–16. doi: 10.1037/amp0000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King DW, Leskin GA, King LA, Weathers FW. Confirmatory factor analysis of the clinician-administered PTSD Scale: evidence for the dimensionality of posttraumatic stress disorder. Psychol Assess. 1998;10:90–96. [Google Scholar]
- 65.Wickham H. Data analysis. In: Wickham H, editor. ggplot2: elegant graphics for data analysis. Cham: Springer International Publishing; 2016;189–201.
- 66.Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Prim. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 67.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion Modulation in PTSD: clinical and Neurobiological Evidence for a Dissociative Subtype. AJP. 2010;167:640–7. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1071:110–24. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- 69.Lebois LAM, Li M, Baker JT, Wolff JD, Wang D, Lambros AM, et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am J Psychiatry. 2021;178:165–73. doi: 10.1176/appi.ajp.2020.19060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seligowski AV, Lebois LAM, Hill SB, Kahhale I, Wolff JD, Jovanovic T, et al. Autonomic responses to fear conditioning among women with PTSD and dissociation. Depress Anxiety. 2019;36:625–34. doi: 10.1002/da.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–8. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- 72.Crombie KM, Ross MC, Letkiewicz AM, Sartin-Tarm A, Cisler JM. Differential relationships of PTSD symptom clusters with cortical thickness and grey matter volumes among women with PTSD. Sci Rep. 2021;11:1825. doi: 10.1038/s41598-020-80776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmaal L, for the ENIGMA-Major Depressive Disorder Working Group. Hibar DP, Sämann PG, Hall GB, Baune BT, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boes AD, McCormick LM, Coryell WH, Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol Psychiatry. 2008;63:391–7. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boes AD, Uitermarkt BD, Albazron FM, Lan MJ, Liston C, Pascual-Leone A, et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul. 2018;11:575–81. doi: 10.1016/j.brs.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23:185–222. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxbe D, Khoddam H, Piero LD, Stoycos SA, Gimbel SI, Margolin G, et al. Community violence exposure in early adolescence: Longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev Sci. 2018;21:e12686. doi: 10.1111/desc.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whittle S, Simmons JG, Hendriksma S, Vijayakumar N, Byrne ML, Dennison M, et al. Childhood maltreatment, psychopathology, and the development of hippocampal subregions during adolescence. Brain Behav. 2017;7:e00607. doi: 10.1002/brb3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–36. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 80.Samplin E, Ikuta T, Malhotra AK, Szeszko PR, DeRosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res. 2013;47:1174–9. doi: 10.1016/j.jpsychires.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rinne-Albers MA, Pannekoek JN, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse. Eur Neuropsychopharmacol. 2017;27:1163–71. doi: 10.1016/j.euroneuro.2017.08.432. [DOI] [PubMed] [Google Scholar]
- 82.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ravi M, Stevens JS, Michopoulos V. Neuroendocrine pathways underlying risk and resilience to PTSD in women. Front Neuroendocrinol. 2019;55:100790. doi: 10.1016/j.yfrne.2019.100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–7. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galatzer-Levy IR, Nickerson A, Litz BT, Marmar CR. Patterns of lifetime PTSD comorbidity: a latent class analysis. Depress Anxiety. 2013;30:489–96. doi: 10.1002/da.22048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.