Abstract
Background
Leukotriene receptor antagonists (LTRAs) are widely used for asthma and allergic rhinitis (AR), but concerns about the risk of neuropsychiatric events (NPEs) have been raised since the first Drug Safety Communication by the US Food and Drug Administration in 2008. This study evaluates the association between LTRA use and NPEs in children, adolescents and young adults with asthma or AR.
Methods
A self-controlled case series study was conducted using the Korean National Health Insurance Service claims database from two 3-year observation periods (observation period 1 (Obs1): 2005–2007; observation period 2 (Obs2): 2016–2018). Asthma or AR patients aged 3–30 years who were prescribed LTRAs and diagnosed with NPEs were included. The incidence rate ratios (IRRs) for the exposed period and risk periods (1–3, 4–7, 8–14, 15–30, 31–90 and >90 days from initiation of LTRA) compared with unexposed periods were calculated using conditional Poisson regression. Subgroup analysis according to age group, type of NPEs and indication of LTRA was performed.
Results
Among 17 001 included patients, the risk of NPEs increased in Obs2 (IRR 1.11, 95% CI 1.00–1.22), but did not increase in Obs1. Risk was increased during risk periods 4–7 days (IRR 2.36, 95% CI 1.99–2.76) and 8–14 days (IRR 1.78, 95% CI 1.46–2.15) after initiation of LTRA, particularly in adolescents (IRR 1.28, 95% CI 1.05–1.55) and young adults (IRR 1.14, 95% CI 1.02–1.28), while risk was decreased in children (3–11 years). Risk was not increased for any single type of NPE. AR patients were at increased risk (IRR 1.19, 95% CI 1.01–1.39), but not those with asthma.
Conclusions
Overall, risk of NPEs with LTRA use differed between risk periods and subgroups. Physicians should prescribe LTRAs according to indications and inform patients about possible NPEs.
Short abstract
Risk of neuropsychiatric events associated with leukotriene receptor antagonist use differs between risk periods and age groups. Risk is increased 4–14 days after initiation of the medication in adolescents and young adults, but not in children. https://bit.ly/3kcKFvE
Introduction
Leukotriene receptor antagonists (LTRAs), including montelukast and pranlukast, are prescribed as control medications, in combination with inhaled corticosteroids (ICSs), in moderate-to-severe asthma or as an alternative to ICSs in mild persistent asthma [1]. LTRAs are approved preventive agents for exercise-induced asthma and also control allergic rhinitis (AR). In 2008, LTRAs were widely prescribed due to ease of use and minimal systemic side-effects. However, post-marketing reports by the drug manufacturer and pharmacovigilance studies suggested a possible association between LTRAs and adverse neuropsychiatric events (NPEs), specifically agitation, aggression, anxiousness, nightmares and hallucinations, depressive mood, insomnia, irritability, restlessness, and suicidality [2, 3].
Prior studies have investigated the relationship between LTRA use and adverse NPEs. Through pooled analysis of adverse events from randomised controlled trials, the US Food and Drug Administration (FDA) suggested that behaviour-related adverse events and suicidality did not increase with montelukast treatment [4, 5]; observational studies reported contradicting results [6–10]. Nonetheless, in 2020, the US FDA required a boxed warning (also referred to as a “black box warning”) and revised the AR indication, thereby reserving montelukast for patients with inadequate response or intolerance to alternative therapies [11]. The association between LTRAs and adverse NPEs remains controversial with scarce evidence on whether and when LTRA use triggers adverse events.
This study aimed to explore whether there are increased risk periods of NPEs following LTRA exposure. Using a self-controlled case series (SCCS) design, wherein each case acts as its control, we compared the incidence of NPEs between the periods during which patients were exposed and unexposed to LTRAs.
Materials and methods
Database
We used data from the Korean National Health Insurance Service (NHIS), a mandatory healthcare system covering 99.4% of the 51 million people in South Korea. The database contains patient-specific information on demographics, healthcare utilisation, diagnoses coded as per the International Statistical Classification of Diseases and Related Health Problems, 10th Edition (ICD-10) and medications prescribed. The NHIS database has been used in previous studies [12, 13].
We obtained reimbursement records of patients aged 3–30 years with at least one diagnosis of asthma or AR (ICD-10 codes J45, J46 and J30), one LTRA prescription (montelukast or pranlukast) and one neuropsychiatric diagnosis (ICD-10 codes in supplementary table S1) between 1 January 2004 and 31 December 2018. The study protocol was approved by the Review Committee of the NHIS (REQ0000037612) and the Institutional Review Board of Seoul National University Hospital (E-2003-175-1112). The need for informed consent was waived because the NHIS database was anonymised.
SCCS study design
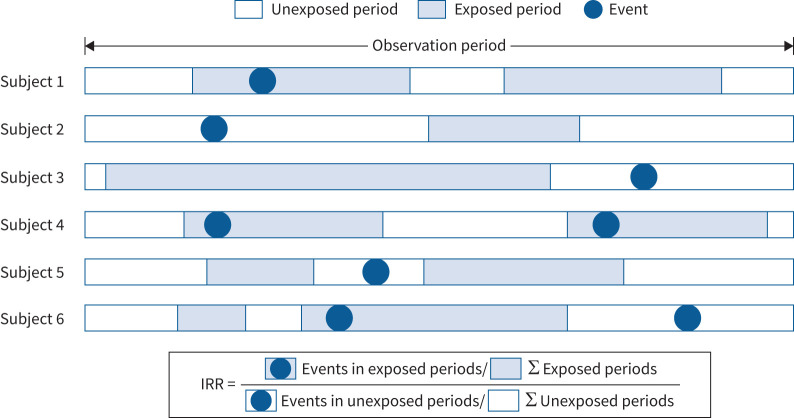
An SCCS study design was used to investigate the association between LTRA use and NPEs [12]. This design calculates the incidence rate ratio (IRR) of an acute event in relation to a brief time-dependent exposure by comparing the incidence rate of the event during the exposure and unexposed periods. Since each case acts as its own control, the known and unknown time-invariant confounders specific to individual patients, such as sex, socioeconomic status, genetics and underlying state of health, are controlled. We adjusted for age in the present study. Figure 1 depicts a graphical representation of the study design and timeline for the hypothetical patients.
FIGURE 1.
Design of a self-controlled case series (SCCS) study of hypothetical subjects with leukotriene receptor antagonist (LTRA) use and neuropsychiatric events (NPEs). The SCCS calculates the incidence rate ratio (IRR) of an event in relation to a transient exposure by comparing the incidence rate of the event during the exposed and unexposed periods. Σ: Sum of.
Participants
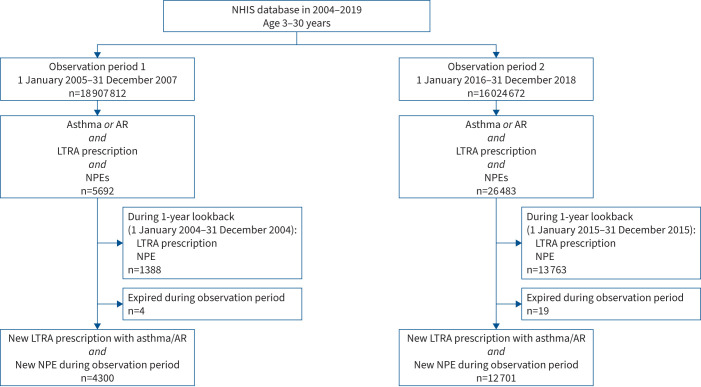
We included two patient cohorts from two study periods, before (observation period 1 (Obs1): 1 January 2005–31 December 2007) and after (observation period 2 (Obs2): 1 January 2016–31 December 2018) the first Drug Safety Communication from the US FDA [2]. Children, adolescents and young adults aged 3–30 years at the beginning of each study period with a record of neuropsychiatric diagnoses, LTRA use and asthma or AR diagnosis during the study period were included. To ensure that patients were not recently prescribed LTRAs or recently diagnosed with neuropsychiatric diseases at the start of the observation periods, patients who were prescribed LTRAs or had a neuropsychiatric diagnosis within 1 year preceding each observation period (1 year preceding Obs1: 1 January 2004–31 December 2004; 1 year preceding Obs2: 1 January 2015–31 December 2015) were excluded. Patients who died during the study period were excluded because the SCCS design assumes that the occurrence of an event of interest must not censor or affect the observation period [14, 15]. Patients were included in both observation periods on meeting the inclusion and exclusion criteria for both periods. Figure 2 presents a flowchart of case definition and selection.
FIGURE 2.
Flow diagram of study participants included in this study. Patients from two 3-year observation periods before and after the first Drug Safety Communication by the US Food and Drug Administration in 2008 were chosen. NHIS: National Health Insurance Service; AR: allergic rhinitis; LTRA: leukotriene receptor antagonist; NPE: neuropsychiatric event.
Assessment of exposure
Montelukast and pranlukast were the LTRAs investigated. Zafirlukast was excluded because it was indicated only for patients aged ≥12 years and was withdrawn from the market in 2019 in South Korea. All the LTRA prescriptions were identified for each patient, and all formulations (tablets, chewable tablets, capsules, granules and syrup) and doses were included in the analysis, encompassing combinations with other antihistamines. The prescription date was defined as exposure day 0, with the next day being day 1. The exposed period was defined as day 1 to the end of the prescription. Day 0 was not included in the exposed period because when an event of interest occurred on the day of the prescription, it was impossible to determine which occurred first. A grace window of 2 days between prescriptions was allowed for late repeat prescriptions; if two exposed periods were within 2 days of each other, they were considered as one continuous exposed period. The exposed period was divided into different exposed risk periods of days 1–3, 4–7, 8–14, 15–30, 31–90 and >90 days to the end of the exposed period. Unexposed periods comprised all other time intervals of the observation period that were not included in the exposed period. Indications for LTRA included asthma, if there was an asthma diagnosis regardless of AR diagnosis, and AR only, if there was an AR diagnosis without asthma diagnosis, during the observation period. One of the main assumptions of an SCCS study is that events, in this case NPEs, do not influence subsequent exposures, in this case LTRA prescription. To investigate this assumption, a histogram of the time interval between the first NPE of each patient and LTRA prescription dates was plotted (the methods are explained in greater detail in the supplementary material).
Outcome
We defined NPEs as newly diagnosed neuropsychiatric diagnoses (ICD-10 codes in supplementary table S1) during the observation period. The NPEs were categorised into psychotic, mood, anxiety, sleep-related, cognitive, movement and personality disorders (supplementary table S1). Patients were considered newly diagnosed if they did not have a neuropsychiatric diagnosis within 1 year before the observation period. For patients with multiple diagnoses within the observation periods, we included diagnoses at least 6 months apart to avoid potential bias of the latter event being influenced by the former.
Statistical analysis
The association between LTRA treatment and NPEs was determined by comparing the incidence of NPEs during the exposed and unexposed periods. Adjusted IRRs and their 95% confidence intervals during the exposed periods in relation to the unexposed periods were calculated using conditional Poisson regression, adjusted for age in 1-year bands. IRRs were estimated for each and the total (Obs1 and Obs2 combined) observation periods.
We performed subgroup analyses according to age groups (children (3–11 years), adolescents (12–18 years) and young adults (19–30 years)), sex, type of NPEs, indication for LTRA (asthma or AR only) and type of LTRA (montelukast or pranlukast). Sensitivity analyses were performed, allowing for washout periods (1–3, 4–7 and 8–20 days) to account for the possibility of missing doses and exposure to longer periods than the prescribed duration. Additional sensitivity analyses were performed for severe asthma, defined as at least one visit to the emergency room or hospital admission with an asthma diagnosis. In addition, a sensitivity analysis allowing for 3 months between NPEs was performed. To further characterise the NPEs related to LTRA use, we determined the frequency of each type of NPE during the exposure and total periods according to age groups.
Categorical variables are presented as number (percentage) and continuous variables are presented as mean with standard deviation. A two-tailed p-value <0.05 was considered statistically significant. Data manipulation from the NHIS database was performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA) and statistical analysis was performed using R version 4.0.3 (www.R-project.org).
Results
Out of 18 907 812 and 16 024 672 insurance holders aged 3–30 years at the beginning of Obs1 and Obs2, respectively, 32 175 patients had an LTRA prescription, asthma or AR diagnosis and an NPE during the observation periods. We excluded 15 151 patients with an LTRA prescription or an NPE within 1 year prior to the observation period. Additionally, four and 19 patients were excluded due to censored follow-up periods by death. Finally, 17 001 patients were included in the analysis, of which 4300 and 12 701 were from Obs1 and Obs2, respectively (figure 2). At the start of the observation period, the mean±sd age was 21.18±7.33 years and females constituted 57.77% of the study population. While 10 517 (61.86%) patients had an asthma diagnosis with or without AR, 6484 (38.14%) had only AR diagnoses. While 12 989 (76.40%) patients were prescribed montelukast alone, 2232 (13.13%) were prescribed pranlukast alone and 1780 (10.47%) were prescribed both medications. The mean±sd duration of LTRA exposure was 31.28±54.37 days per participant and the mean±sd length of each prescription was 13.57±35.37 days. The overall follow-up period was 51 002.87 person-years and the overall incidence of NPEs was 21 599 per 51 002.87 person-years (table 1). The histogram of time intervals from LTRA prescription to first NPE showed that the number of prescriptions before and after the first NPE was not significantly different (supplementary figure S1).
TABLE 1.
Baseline characteristics
| Total period | Observation period 1 | Observation period 2 | |
| Patients | 17 001 | 4300 | 12 701 |
| Total events | 21 599 | 5424 | 16 175 |
| Age (years) | 21.18±7.33 | 19.44±8.89 | 21.77±6.62 |
| Age group | |||
| Children (3–11 years) | 2258 (13.28) | 1073 (24.95) | 1185 (9.33) |
| Adolescents (12–18 years) | 2784 (16.38) | 585 (13.60) | 2199 (17.31) |
| Young adults (19–30 years) | 11 959 (70.34) | 2642 (61.44) | 9317 (73.36) |
| Sex | |||
| Male | 7179 (42.23) | 1613 (37.51) | 5566 (43.82) |
| Female | 9822 (57.77) | 2687 (62.49) | 7135 (56.18) |
| Neuropsychiatric events | |||
| Psychotic disorder | 132 (0.78) | 37 (0.86) | 95 (0.75) |
| Mood disorder | 2834 (16.67) | 741 (17.23) | 2093 (16.48) |
| Anxiety disorder | 9095 (53.5) | 2141 (49.79) | 6954 (54.75) |
| Personality disorder | 100 (0.59) | 36 (0.84) | 64 (0.5) |
| Sleep-related disorder | 6068 (35.69) | 1237 (28.77) | 4831 (38.04) |
| Cognitive | 993 (5.84) | 605 (14.07) | 388 (3.05) |
| Movement | 370 (2.18) | 54 (1.26) | 316 (2.49) |
| Indication of LTRA | |||
| Asthma (±allergic rhinitis) | 10 517 (61.86) | 3586 (83.4) | 6931 (54.57) |
| Allergic rhinitis only | 6484 (38.14) | 714 (16.60) | 5770 (45.43) |
| ER visit due to asthma | 164 (0.96) | 108 (2.51) | 56 (0.44) |
| Admission due to asthma | 521 (3.06) | 313 (7.28) | 208 (1.64) |
| Exposure period length (days) | 31.28±54.37 | 31.55±57.05 | 31.19±53.44 |
| Type of LTRA | |||
| Montelukast only | 12 989 (76.40) | 2976 (69.21) | 10 013 (78.84) |
| Pranlukast only | 2232 (13.13) | 1024 (23.81) | 1208 (9.51) |
| Both | 1780 (10.47) | 300 (6.98) | 1480 (11.65) |
Data are presented as n, mean±sd or n (%). LTRA: leukotriene receptor antagonist; ER: emergency room.
We found no increase in the risk of NPEs during the total observation period (IRR 1.05, 95% CI 0.96–1.15) and Obs1 (IRR 0.88, 95% CI 0.73–1.06). However, a slight increase in risk was seen during Obs2 (IRR 1.11, 95% CI 1.00–1.22). In the total observation period and Obs2, IRR was highest 4–7 days (total: IRR 2.10, 95% CI 1.80–2.43; Obs2: IRR 2.36, 95% CI 1.99–2.76) and 8–14 days (total: IRR 1.60, 95% CI 1.34–1.89; Obs2: IRR 1.78, 95% CI 1.46–2.15) after the prescription, decreased risk of NPEs was observed 1–3, 15–30 and 31–90 days after the prescription, and no increase with longer prescriptions (>90 days). In Obs1, the risk was decreased 1–3 days after the prescription, and tended to increase at 4–7 and 8–14 days; however, it was not statistically significant (table 2).
TABLE 2.
Risk of neuropsychiatric events in relation to leukotriene receptor antagonist (LTRA) use in patients with asthma or allergic rhinitis
| Total period | Observation period 1 | Observation period 2 | ||||||||||
| Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | |
| Unexposed | 21 028 | 49 546.89 | 1 | 5294 | 12 519.69 | 1 | 15 734 | 37 027.2 | 1 | |||
| Exposed | 571 | 1455.98 | 1.05 (0.96–1.15) | 0.266 | 130 | 371.49 | 0.88 (0.73–1.06) | 0.185 | 441 | 1084.5 | 1.11 (1.00–1.22) | 0.0468 |
| Time since initiation of LTRA (days) | ||||||||||||
| 1–3 | 115 | 444.70 | 0.68 (0.57–0.82) | <0.001 | 28 | 107.86 | 0.65 (0.44–0.93) | 0.025 | 87 | 336.85 | 0.69 (0.56–0.85) | <0.001 |
| 4–7 | 180 | 228.38 | 2.10 (1.80–2.43) | <0.001 | 31 | 57.92 | 1.35 (0.93–1.9) | 0.099 | 149 | 170.46 | 2.36 (1.99–2.76) | <0.001 |
| 8–14 | 133 | 221.99 | 1.60 (1.34–1.89) | <0.001 | 27 | 61.66 | 1.11 (0.74–1.59) | 0.603 | 106 | 160.33 | 1.78 (1.46–2.15) | <0.001 |
| 15–30 | 66 | 244.77 | 0.71 (0.55–0.90) | 0.007 | 20 | 68.13 | 0.74 (0.46–1.12) | 0.183 | 46 | 176.64 | 0.70 (0.51–0.92) | 0.015 |
| 31–90 | 51 | 219.15 | 0.61 (0.46–0.80) | <0.001 | 16 | 53.61 | 0.74 (0.43–1.18) | 0.243 | 35 | 165.54 | 0.57 (0.40–0.78) | <0.001 |
| >90 | 26 | 96.99 | 0.73 (0.47–1.07) | 0.130 | 8 | 22.30 | 0.95 (0.42–1.89) | 0.903 | 18 | 74.69 | 0.66 (0.39–0.82) | 0.094 |
IRR: incidence rate ratio.
Furthermore, a decreased risk of NPEs in children aged 3–11 years (IRR 0.66, 95% CI 0.53–0.80) and an increased risk in adolescents aged 12–18 years (IRR 1.28, 95% CI 1.05–1.55) and young adults aged 19–30 years (IRR 1.14, 95% CI 1.02–1.28) was noted (table 3). Similar temporal trends in IRR were seen in Obs2; however, IRR did not increase in adolescents and young adults during Obs1 (supplementary table S2).
TABLE 3.
Risk of neuropsychiatric events in relation to leukotriene receptor antagonist (LTRA) use in patients with asthma or allergic rhinitis according to age group
| Children (3–11 years) | Adolescents (12–18 years) | Young adults (19–30 years) | ||||||||||
| Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | |
| Unexposed | 2467 | 6393.79 | 1 | 3345 | 8101.2 | 1 | 15 216 | 35 051.9 | 1 | |||
| Exposed | 105 | 378.82 | 0.66 (0.53–0.80) | <0.001 | 118 | 251.11 | 1.28 (1.05–1.55) | 0.014 | 348 | 826.05 | 1.14 (1.02–1.28) | 0.019 |
| Time since initiation of LTRA (days) | ||||||||||||
| 1–3 | 15 | 107.73 | 0.34 (0.20–0.55) | <0.0001 | 23 | 71.96 | 0.84 (0.54–1.23) | 0.399 | 77 | 265.02 | 0.78 (0.62–0.97) | 0.029 |
| 4–7 | 24 | 58.84 | 0.99 (0.64–1.45) | 0.970 | 32 | 37.69 | 2.27 (1.56–3.17) | <0.001 | 124 | 131.86 | 2.52 (2.10–3.00) | <0.001 |
| 8–14 | 23 | 62.37 | 0.89 (0.57–1.31) | 0.570 | 29 | 36.83 | 2.13 (1.43–3.03) | <0.001 | 81 | 122.80 | 1.76 (1.40–2.19) | <0.001 |
| 15–30 | 19 | 68.37 | 0.65 (0.40–0.99) | 0.064 | 15 | 41.68 | 0.97 (0.55–1.57) | 0.912 | 32 | 134.72 | 0.63 (0.44–0.88) | 0.010 |
| 31–90 | 16 | 60.57 | 0.61 (0.35–0.97) | 0.054 | 14 | 40.94 | 0.94 (0.52–1.55) | 0.823 | 21 | 117.63 | 0.47 (0.29–0.71) | <0.001 |
| >90 | 8 | 20.94 | 0.90 (0.40–1.78) | 0.784 | 5 | 22.01 | 0.62 (0.21–1.43) | 0.315 | 13 | 54.03 | 0.66 (0.35–1.13) | 0.158 |
IRR: incidence rate ratio.
No increased risk was identified for each type of NPE in all the risk periods combined. However, a higher risk was observed in mood, anxiety, sleep-related and movement disorders 4–7 days after LTRA prescription. An increased risk of psychotic, mood, anxiety and personality disorders was also seen 8–14 days after LTRA prescription. The risk for cognitive disorders decreased in the overall exposed period and did not increase during any specific risk period (tables 4 and 5). Comparable trends were observed during Obs2; however, the results were not statistically significant during Obs1 (supplementary table S3).
TABLE 4.
Risk of neuropsychiatric events (NPEs) in relation to leukotriene receptor antagonist (LTRA) use in patients with asthma or allergic rhinitis according to type of NPE: psychotic, mood, anxiety and sleep-related disorders
| Psychotic disorder | Mood disorder | Anxiety disorder | Sleep-related disorder | |||||||||||||
| Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | |
| Unexposed | 466 | 968.7 | 1 | 5298 | 11 120.18 | 1 | 13 108 | 29 123.82 | 1 | 8973 | 19 983.28 | 1 | ||||
| Exposed | 18 | 42.33 | 1.19 (0.69–1.91) | 0.506 | 172 | 375.86 | 1.16 (0.98–1.36) | 0.072 | 307 | 786.61 | 1.01 (0.89–1.13) | 0.911 | 219 | 603.5 | 0.92 (0.80–1.06) | 0.259 |
| Time since initiation of LTRA (days) | ||||||||||||||||
| 1–3 | 2 | 9.09 | 0.56 (0.14–2.25) | 0.413 | 30 | 96.68 | 0.75 (0.51–1.06) | 0.121 | 53 | 248.15 | 0.54 (0.41–0.70) | <0.001 | 62 | 179.41 | 0.87 (0.67–1.11) | 0.289 |
| 4–7 | 2 | 5.74 | 0.91 (0.23–3.70) | 0.901 | 43 | 53.57 | 1.96 (1.42–2.62) | <0.001 | 107 | 123.67 | 2.20 (1.81–2.66) | <0.001 | 62 | 95.91 | 1.64 (1.26–2.09) | <0.001 |
| 8–14 | 6 | 6.44 | 2.56 (1.12–5.84) | 0.025 | 39 | 56.03 | 2.16 (1.6–2.84) | <0.001 | 68 | 115.42 | 1.51 (1.17–1.90) | <0.001 | 44 | 94.48 | 1.18 (0.86–1.57) | 0.281 |
| 15–30 | 2 | 7.98 | 0.69 (0.17–2.81) | 0.607 | 26 | 67.12 | 0.97 (0.64–1.41) | 0.882 | 40 | 127.52 | 0.80 (0.57–1.08) | 0.159 | 20 | 103.15 | 0.49 (0.3–0.74) | 0.001 |
| 31–90 | 5 | 8.54 | 1.78 (0.70–4.48) | 0.224 | 18 | 67.95 | 0.68 (0.41–1.05) | 0.106 | 25 | 114.21 | 0.56 (0.37–0.82) | 0.004 | 21 | 93.66 | 0.56 (0.35–0.84) | 0.009 |
| >90 | 1 | 4.54 | 0.78 (0.10–6.03) | 0.808 | 6 | 34.51 | 0.47 (0.18–1.00) | 0.079 | 14 | 57.63 | 0.67 (0.37–1.13) | 0.164 | 10 | 36.89 | 0.64 (0.31–1.17) | 0.181 |
IRR: incidence rate ratio.
TABLE 5.
Risk of neuropsychiatric events (NPEs) in relation to leukotriene receptor antagonist (LTRA) use in patients with asthma or allergic rhinitis according to type of NPE: cognitive, movement and personality disorders
| Cognitive disorder | Movement disorder | Personality disorder | ||||||||||
| Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | |
| Unexposed | 1366 | 3205.13 | 1 | 798 | 1656.32 | 1 | 251 | 501.22 | 1 | |||
| Exposed | 55 | 171.90 | 0.75 (0.56–0.98) | 0.045 | 30 | 65.86 | 1.18 (0.78–1.71) | 0.410 | 9 | 20.74 | 1.05 (0.48–2.03) | 0.883 |
| Time since initiation of LTRA (days) | ||||||||||||
| 1–3 | 12 | 47.55 | 0.6 (0.32–1.01) | 0.079 | 4 | 16.96 | 0.58 (0.18–1.35) | 0.274 | 2 | 5.24 | 0.86 (0.21–3.49) | 0.830 |
| 4–7 | 12 | 26.37 | 1.08 (0.57–1.82) | 0.801 | 9 | 8.85 | 2.51 (1.19–4.60) | 0.007 | 1 | 3.13 | 0.76 (0.10–5.45) | 0.782 |
| 8–14 | 9 | 29.10 | 0.73 (0.35–1.32) | 0.344 | 6 | 8.71 | 1.7 (0.67–3.50) | 0.202 | 4 | 3.16 | 2.99 (1.08–8.22) | 0.0344 |
| 15–30 | 7 | 33.44 | 0.48 (0.21–0.94) | 0.057 | 5 | 10.94 | 1.14 (0.41–2.51) | 0.768 | 1 | 3.36 | 0.71 (0.10–5.16) | 0.735 |
| 31–90 | 12 | 27.18 | 1.02 (0.54–1.75) | 0.950 | 3 | 12.44 | 0.66 (0.16–1.76) | 0.478 | 1 | 3.60 | 0.65 (0.09–4.82) | 0.673 |
| >90 | 3 | 8.27 | 0.89 (0.21–2.46) | 0.841 | 3 | 7.96 | 1.16 (0.27–3.48) | 0.810 | 0 | 2.24 | NA | NA |
IRR: incidence rate ratio.
The risk of NPEs was found to be increased in AR (IRR 1.19, 95% CI 1.01–1.39) but not in asthma (IRR 1.00, 95% CI 0.90–1.10) patients during the overall exposure. In both asthma and AR patients, the risk decreased 1–3 days after LTRA prescription, increased 4–7 and 8–14 days after the prescription, and further decreased 31–90 days after the prescription (supplementary table S4).
The risk of NPEs increased on using pranlukast (IRR 1.81, 95% CI 1.36–2.36) but not montelukast (IRR 1.07, 95% CI 0.96–1.18). Both groups showed an increased risk during 4–7 and 8–14 days (supplementary table S5). When categorised by sex, the overall risks of NPE were not increased for both male and female patients; however, a secular trend of increased IRR during 4–7 and 8–14 days was shown (supplementary table S6).
Contrary to the primary analysis, sensitivity analysis with washout periods showed increased risk estimates for the exposure period during the entire observation period (IRR 1.17, 95% CI 1.06–1.27). The risk of NPEs was higher in the washout periods than in the unexposed periods, excluding washout periods. The risk did not increase during Obs1 in the sensitivity analysis, although it increased during the washout period (table 6). When the analysis was limited to severe asthma, the risk of NPEs did not increase for any risk period (supplementary table S7). When 3 months were allowed between repeated events, the overall risk of NPEs was decreased during the exposed period but increased during exposure risk periods of 4–7 and 8–14 days (supplementary table S8). When divided into age groups, the risk was decreased for children and neither increased nor decreased for adolescents and adults, but the temporal trend of increased risk during 4–7 and 8–14 days was preserved (supplementary table S9).
TABLE 6.
Sensitivity analysis with washout periods of risk of neuropsychiatric events in relation to leukotriene receptor antagonist (LTRA) use in patients with asthma or allergic rhinitis
| Total period | Observation period 1 | Observation period 2 | ||||||||||
| Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | Events (n) | Person-years | IRR (95% CI) | p-value | |
| Primary analysis | ||||||||||||
| Unexposed | 21 028 | 49 546.89 | 1 | 5294 | 12 519.69 | 1 | 15 734 | 37 027.20 | 1 | |||
| Exposed | 571 | 1455.98 | 1.05 (0.96–1.15) | 0.266 | 130 | 371.49 | 0.88 (0.73–1.06) | 0.185 | 441 | 1084.50 | 1.11 (1.00–1.22) | 0.047 |
| Secondary analysis | ||||||||||||
| Unexposed | 19 827 | 47 394.77 | 1 | 5004 | 11 971.52 | 1 | 14 823 | 35 423.26 | 1 | |||
| Exposed | 571 | 1455.98 | 1.17 (1.06–1.27) | <0.001 | 130 | 371.49 | 0.98 (0.81–1.17) | 0.797 | 441 | 1084.50 | 1.23 (1.11–1.36) | <0.001 |
| Time since initiation of LTRA (days) | ||||||||||||
| 1–3 | 115 | 444.70 | 0.73 (0.61–0.88) | <0.001 | 28 | 107.86 | 0.69 (0.46–0.98) | 0.052 | 87 | 336.85 | 0.74 (0.60–0.91) | 0.006 |
| 4–7 | 180 | 228.38 | 2.25 (1.93–2.60) | <0.001 | 31 | 57.92 | 1.43 (0.98–2.01) | 0.050 | 149 | 170.46 | 2.53 (2.14–2.97) | <0.001 |
| 8–14 | 133 | 221.99 | 1.71 (1.43–2.03) | <0.001 | 27 | 61.66 | 1.17 (0.78–1.69) | 0.419 | 106 | 160.33 | 1.91 (1.57–2.31) | <0.001 |
| 15–30 | 66 | 244.77 | 0.76 (0.59–0.97) | 0.016 | 20 | 68.13 | 0.78 (0.48–1.19) | 0.280 | 46 | 176.64 | 0.75 (0.55–0.99) | 0.050 |
| 31–90 | 51 | 219.15 | 0.66 (0.49–0.86) | <0.001 | 16 | 53.61 | 0.78 (0.45–1.25) | 0.344 | 35 | 165.54 | 0.60 (0.42–0.83) | 0.004 |
| >90 | 26 | 96.99 | 0.78 (0.51–1.15) | 0.241 | 8 | 22.30 | 1.01 (0.45–2.00) | 0.970 | 18 | 74.69 | 0.71 (0.42–0.83) | 0.168 |
| Washout period (days) | ||||||||||||
| 1–3 | 198 | 363.44 | 1.54 (1.33–1.77) | <0.001 | 32 | 91.47 | 0.93 (0.64–1.29) | 0.665 | 166 | 271.97 | 1.75 (1.50–2.04) | <0.001 |
| 4–7 | 331 | 460.87 | 2.03 (1.81–2.26) | <0.001 | 84 | 117.15 | 1.90 (1.51–2.35) | <0.001 | 247 | 343.71 | 2.06 (1.81–2.34) | <0.001 |
| 8–20 | 672 | 1327.81 | 1.42 (1.31–1.54) | <0.001 | 174 | 339.55 | 1.35 (1.15–1.57) | <0.001 | 498 | 988.26 | 1.44 (1.31–1.58) | <0.001 |
IRR: incidence rate ratio.
In children, the three most common types of NPEs were cognitive, anxiety and sleep-related disorders; however, anxiety, sleep-related and mood disorders were the three most common types of NPEs in adolescents and young adults (table 7).
TABLE 7.
Proportion of neuropsychiatric events in each age group
| Children (3–11 years) | Adolescents (12–18 years) | Young adults (19–30 years) | |||||||
| Total | Exposed | Total | Exposed | Total | Exposed | ||||
| Total events | 2572 | 105 | 3463 | 118 | 15 564 | 118 | |||
| Type of event | Cognitive disorder | 1060 (41.21) | 42 (40.00) | Anxiety disorder | 2381 (68.76) | 74 (62.71) | Anxiety disorder | 10 177 (65.39) | 74 (62.71) |
| Anxiety disorder | 857 (33.32) | 28 (26.67) | Sleep-related disorder | 993 (28.67) | 35 (29.66) | Sleep-related disorder | 7461 (47.94) | 35 (29.66) | |
| Sleep-related disorder | 738 (28.69) | 29 (27.62) | Mood disorder | 882 (25.47) | 36 (30.51) | Mood disorder | 4341 (27.89) | 36 (30.51) | |
| Mood disorder | 247 (9.60) | 16 (15.24) | Cognitive disorder | 230 (6.64) | 11 (9.32) | Movement disorder | 546 (3.51) | 11 (9.32) | |
| Movement disorder | 82 (3.19) | 5 (4.76) | Movement disorder | 200 (5.78) | 9 (7.63) | Psychotic disorder | 357 (2.29) | 9 (7.63) | |
| Personality disorder | 47 (1.83) | 4 (3.81) | Psychotic disorder | 93 (2.69) | 7 (5.93) | Personality disorder | 172 (1.11) | 7 (5.93) | |
| Psychotic disorder | 34 (1.32) | 2 (1.90) | Personality disorder | 41 (1.18) | 3 (2.54) | Cognitive disorder | 131 (0.84) | 3 (2.54) | |
Data are presented as n or n (%).
Discussion
The overall risk of NPEs did not increase with LTRA use in asthma or AR patients aged 3–30 years. However, an increased risk was found 4–7 and 8–14 days after initiation of LTRAs. Results for different age groups were contrasting: while an increased risk of NPEs was seen in adolescents (12–18 years) and young adults (19–30 years), no increase was seen in children (3–11 years). In young adults, the risk of NPEs increased during 4–14 days but decreased during 15–90 days post-prescription. Therefore, the use of LTRAs for asthma and AR is associated with a transient increase in the risk of NPEs 4–14 days after the prescription, which gets attenuated possibly with improved control of asthma or AR.
Our findings on the temporal changes in the risk of NPEs are similar to previous clinical studies and experimental mouse studies on montelukast. Pharmacovigilance studies from Spain on nightmares in children using montelukast and those from the World Health Organization VigiBase and Swedish Adverse Drug Reaction database (SWEDIS) on psychiatric events reported that most NPEs occurred within 1 or 2 weeks of the prescription [3, 16, 17]. Other case–control studies also reported similar findings [7]. Moreover, in asthma patients, the therapeutic effects of montelukast are seen 1 day after starting the medication, continuously improving over 12 weeks [18]. Interestingly, in mouse models of asthma, montelukast treatment did not lead to depression-like behaviour, while mice that received saline showed symptoms of depression [19]. Therefore, the possible risk of LTRA-associated NPEs may transiently increase during the first 2 weeks and later decrease as asthma/AR symptoms are controlled.
On using LTRAs, the risk of NPEs decreased in children and increased in adolescents and young adults. A possible explanation is the different types of common NPEs in the age groups and their different pathophysiologies. Cognitive disorder is the most common (41.21%) NPE in children impacting memory, attention and behaviour. Attention deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder in South Korean children with a prevalence of 3.5–5.5% [20]. ADHD in children was found to be associated with asthma and its severity [21, 22]. The decreased risk of NPEs in children could be attributed to the decreased risk of cognitive disorders with control of asthma symptoms. Moreover, there is increasing evidence that montelukast improves cognitive function in multiple cerebral pathologies [23–26].
The most common NPEs in adolescents and young adults were anxiety, sleep and mood disorders. The prevalence of NPEs in our cohort is consistent with the common neuropsychiatric diagnoses, besides substance use, in the general South Korean population [27, 28]. Although the association between LTRA use and NPEs is unclear, the purinergic cysteinyl leukotriene receptor P2Y12 possibly plays a role in the pathophysiology [29]. Montelukast inhibits the expression and biological effects of the P2Y12 receptor [30, 31], increasing anxiety and decreasing synapse plasticity associated with mood disorders [32–34]. Montelukast crosses the blood–brain barrier by active transport and accumulates in the cerebrospinal fluid of humans and mice [23].
In young adults and patients with anxiety and sleep-related disorders, there was a decreased risk of NPEs 1–3 months after initiating LTRAs. Symptoms of asthma and AR, including the pro-inflammatory effects, contribute to anxiety and sleep disturbance [35, 36]. However, the associated psychiatric disorders improve with continuous asthma medication [37, 38].
During exposure to LTRAs, the risk of NPEs increased in AR but not asthma patients. This should not be interpreted as LTRAs posing a higher risk of NPEs in AR than in asthma. The IRR of this study represents the relative risk of NPEs during the exposed periods compared with the unexposed periods within the selected patients. Therefore, the incidence between the two groups should not be directly compared using the IRR. If we hypothesise that the baseline risk of NPEs is higher in asthma than in AR patients, when the protective effects of LTRAs due to symptom control and possible neuropsychiatric hazardous effects are integrated, the overall relative effect compared with baseline could be higher in AR patients. The same rationale can be applied to the sensitivity analysis of severe asthma, where none of the risk periods showed increased risk compared with the baseline.
While the secular trends of IRR were similar in Obs1 and Obs2, the increase in IRR during 4–7 and 8–4 days was not statistically significant in Obs1. The different outcomes could be attributed to two factors. First, the use of LTRAs increased after 2012, when the patent on montelukast expired and generic medications entered the market; thus, the use of LTRAs was more common in Obs2. Additionally, the raising of drug safety concerns by the US FDA during 2008–2009 may have increased awareness of the neuropsychiatric symptoms, leading to increased NPE diagnoses. However, there was no evidence in our data supporting a change in LTRA prescription patterns based on prior NPEs.
To the best of our knowledge, this is the first SCCS study examining the risk of NPEs associated with LTRA use. This design allows for controlling fixed known and unknown confounders, such as genetic and socioeconomic factors. Controlling for the genetic background is crucial as neuropsychiatric diseases are usually hereditary. Using this design, we were able to identify the association between LTRA use and a subsequent NPE. Moreover, we also performed a detailed study of the temporal relationship of LTRA use and NPEs with IRRs from sequential risk periods during the exposure period [39]. While recent studies have found no relationship between LTRA use and NPEs [9, 40], our analysis using the SCCS study design revealed important aspects of the relationship.
Our study has some limitations. First, the NPEs were defined using ICD-10 codes; therefore, only those requiring medical attention with subsequent diagnoses were included, and while we have set a 6-month refractory period to not include follow-up visits within a single episode, it is possible that some separate events of a shorter interval may not be detected. Second, although the SCCS is a well-designed method to assess the relevant risk of an event during the exposure period compared with the unexposed period, it does not provide the total incidence of the event. Third, due to data availability restrictions, we could not adjust for time-dependant confounders, such as concomitant medication or disease severity. Moreover, the diagnosis of allergic diseases and NPE according to ICD-10 codes is less accurate compared with clinician examination or questionnaires. Finally, the prescription data in the NHIS is based on prescriptions that were filled out; however, we did not have information on whether the patient actually took the medication on the prescribed days or at a different time period. Nonetheless, by using the NHIS claims database, the study population was large, including the entire national population, which enabled detailed subgroup analysis based on clinical needs.
In conclusion, the overall risk of NPEs did not increase on using LTRAs; it increased within a 4–14-day window of initiation. Moreover, the risk differed according to age, underlying allergic disease and type of NPE. Physicians should, therefore, be aware of the transient risk of NPEs when prescribing LTRAs, educate patients regarding potential risks and schedule short-term follow-ups.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02467-2021.Supplement (620.1KB, pdf)
Shareable PDF
Footnotes
Conflict of interest: J.S. Park has nothing to disclose. Y.J. Cho has nothing to disclose. J-Y. Yun reports lecture fees from the Korean Society for Human Brain Mapping, outside the submitted work. H.J. Lee has nothing to disclose. J. Yu has nothing to disclose. H-J. Yang reports lecture fees from the Korean Academy of Pediatric Allergy and Respiratory Disease (KAPARD), Korea Disease Control and Prevention Agency, and Korea National Enterprise for Clinical Trials, outside the submitted work. D.I. Suh reports research grants from KAPARD for the submitted work and lecture fees from KAPARD, Organon Korea and Sama Pharm, outside the submitted work.
Support statement: This study was funded by the Korean Academy of Pediatric Allergy and Respiratory Disease research grant for the Korean childhood Asthma REsearch (KARE) team. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
- 2.US Food and Drug Administration . Updated information on leukotriene inhibitors: montelukast (marketed as Singulair), zafirlukast (marketed as Accolate), and zileuton (marketed as Zyflo and Zyflo CR). 2009. https://wayback.archive-it.org/7993/*/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htm Date last accessed: 29 April 2022.
- 3.Wallerstedt SM, Brunlof G, Sundstrom A, et al. Montelukast and psychiatric disorders in children. Pharmacoepidemiol Drug Saf 2009; 18: 858–864. doi: 10.1002/pds.1794 [DOI] [PubMed] [Google Scholar]
- 4.Philip G, Hustad C, Noonan G, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol 2009; 124: 691–696. doi: 10.1016/j.jaci.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 5.Philip G, Hustad CM, Malice MP, et al. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol 2009; 124: 699–706. doi: 10.1016/j.jaci.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 6.Schumock GT, Stayner LT, Valuck RJ, et al. Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: a nested case-control study. J Allergy Clin Immunol 2012; 130: 368–375. doi: 10.1016/j.jaci.2012.04.035 [DOI] [PubMed] [Google Scholar]
- 7.Benard B, Bastien V, Vinet B, et al. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J 2017; 50: 2000148. doi: 10.1183/13993003.00148-2017 [DOI] [PubMed] [Google Scholar]
- 8.Glockler-Lauf SD, Finkelstein Y, Zhu J, et al. Montelukast and neuropsychiatric events in children with asthma: a nested case-control study. J Pediatr 2019; 209: 176–182. doi: 10.1016/j.jpeds.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Sansing-Foster V, Haug N, Mosholder A, et al. Risk of psychiatric adverse events among montelukast users. J Allergy Clin Immunol Pract 2021; 9: 385–193. doi: 10.1016/j.jaip.2020.07.052 [DOI] [PubMed] [Google Scholar]
- 10.Ali MM, O'Brien CE, Cleves MA, et al. Exploring the possible association between montelukast and neuropsychiatric events among children with asthma: a matched nested case-control study. Pharmacoepidemiol Drug Saf 2015; 24: 435–445. doi: 10.1002/pds.3758 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. 2020. www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug Date last accessed: 29 April 2022.
- 12.Shin JY, Roughead EE, Park BJ, et al. Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ 2016; 353: i2550. doi: 10.1136/bmj.i2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JH, Kim BS, Kwak N, et al. Impact of body mass index on development of nontuberculous mycobacterial pulmonary disease. Eur Respir J 2021; 57: 2000454. doi: 10.1183/13993003.00454-2020 [DOI] [PubMed] [Google Scholar]
- 14.Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 2009; 18: 7–26. doi: 10.1177/0962280208092342 [DOI] [PubMed] [Google Scholar]
- 15.Ramsay EN, Pratt NL, Ryan P, et al. Proton pump inhibitors and the risk of pneumonia: a comparison of cohort and self-controlled case series designs. BMC Med Res Methodol 2013; 13: 82. doi: 10.1186/1471-2288-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldea Perona A, Garcia-Saiz M, Sanz Alvarez E. Psychiatric disorders and montelukast in children: a disproportionality analysis of the VigiBase. Drug Saf 2016; 39: 69–78. doi: 10.1007/s40264-015-0360-2 [DOI] [PubMed] [Google Scholar]
- 17.Cereza G, Garcia Dolade N, Laporte JR. Nightmares induced by montelukast in children and adults. Eur Respir J 2012; 40: 1574–1575. doi: 10.1183/09031936.00092812 [DOI] [PubMed] [Google Scholar]
- 18.Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001; 108: E48. doi: 10.1542/peds.108.3.e48 [DOI] [PubMed] [Google Scholar]
- 19.Tel BC, Telli G, Onder S, et al. Investigation of the relationship between chronic montelukast treatment, asthma and depression-like behavior in mice. Exp Ther Med 2021; 21: 27. doi: 10.3892/etm.2020.9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Kim BN, Cho SC, et al. Prevalence, correlates, and comorbidities of DSM-IV psychiatric disorders in children in Seoul, Korea. Asia Pac J Public Health 2015; 27: NP1942-51. doi: 10.1177/1010539513475656 [DOI] [PubMed] [Google Scholar]
- 21.Holmberg K, Lundholm C, Anckarsater H, et al. Impact of asthma medication and familial factors on the association between childhood asthma and attention-deficit/hyperactivity disorder: a combined twin- and register-based study: Epidemiology of Allergic Disease. Clin Exp Allergy 2015; 45: 964–973. doi: 10.1111/cea.12529 [DOI] [PubMed] [Google Scholar]
- 22.Chen MH, Su TP, Chen YS, et al. Asthma and attention-deficit/hyperactivity disorder: a nationwide population-based prospective cohort study. J Child Psychol Psychiatry 2013; 54: 1208–1214. doi: 10.1111/jcpp.12087 [DOI] [PubMed] [Google Scholar]
- 23.Marschallinger J, Schaffner I, Klein B, et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat Commun 2015; 6: 8466. doi: 10.1038/ncomms9466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Sun X, Shi Y, et al. Cysteinyl leukotriene receptor type 1 antagonist montelukast protects against injury of blood–brain barrier. Inflammopharmacology 2019; 27: 933–940. doi: 10.1007/s10787-019-00611-7 [DOI] [PubMed] [Google Scholar]
- 25.Gelosa P, Colazzo F, Tremoli E, et al. Cysteinyl leukotrienes as potential pharmacological targets for cerebral diseases. Mediators Inflamm 2017; 2017: 3454212. doi: 10.1155/2017/3454212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwimmbeck F, Staffen W, Hohn C, et al. Cognitive effects of montelukast: a pharmaco-EEG study. Brain Sci 2021; 11: 547. doi: 10.3390/brainsci11050547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwangbo R, Chang H, Hong M, et al. The diagnostic distribution of psychiatric disorders among the population under 19 years old: based on the national insurance data. J Korean Acad Child Adolesc Psychiatry 2016; 27: 139–145. doi: 10.5765/jkacap.2016.27.2.139 [DOI] [Google Scholar]
- 28.Lee YM. National Mental Health Statistics 2019. Seoul, National Center for Mental Health, 2020. [Google Scholar]
- 29.Singh RK, Gupta S, Dastidar S, et al. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology 2010; 85: 336–349. doi: 10.1159/000312669 [DOI] [PubMed] [Google Scholar]
- 30.Mamedova L, Capra V, Accomazzo MR, et al. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol 2005; 71: 115–125. doi: 10.1016/j.bcp.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinh HKT, Suh DH, Nguyen TVT, et al. Characterization of cysteinyl leukotriene-related receptors and their interactions in a mouse model of asthma. Prostaglandins Leukot Essent Fatty Acids 2019; 141: 17–23. doi: 10.1016/j.plefa.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Zheng F, Zhou Q, Cao Y, et al. P2Y12 deficiency in mouse impairs noradrenergic system in brain, and alters anxiety-like neurobehavior and memory. Genes Brain Behav 2019; 18: e12458. doi: 10.1111/gbb.12458 [DOI] [PubMed] [Google Scholar]
- 33.Sipe GO, Lowery RL, Tremblay ME, et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun 2016; 7: 10905. doi: 10.1038/ncomms10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavalali ET, Monteggia LM. Targeting homeostatic synaptic plasticity for treatment of mood disorders. Neuron 2020; 106: 715–726. doi: 10.1016/j.neuron.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzeng NS, Chang HA, Chung CH, et al. Increased risk of psychiatric disorders in allergic diseases: a nationwide, population-based, cohort study. Front Psychiatry 2018; 9: 133. doi: 10.3389/fpsyt.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavanagh J, Jackson DJ, Kent BD. Sleep and asthma. Curr Opin Pulm Med 2018; 24: 569–573. doi: 10.1097/MCP.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 37.Sastre J, Crespo A, Fernandez-Sanchez A, et al. Anxiety, depression, and asthma control: changes after standardized treatment. J Allergy Clin Immunol Pract 2018; 6: 1953–1959. doi: 10.1016/j.jaip.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 38.Prasad B, Nyenhuis SM, Imayama I, et al. Asthma and obstructive sleep apnea overlap: what has the evidence taught us? Am J Respir Crit Care Med 2020; 201: 1345–1357. doi: 10.1164/rccm.201810-1838TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maclure M. ‘Why me?’ versus ‘why now?’ – differences between operational hypotheses in case-control versus case-crossover studies. Pharmacoepidemiol Drug Saf 2007; 16: 850–853. doi: 10.1002/pds.1438 [DOI] [PubMed] [Google Scholar]
- 40.Shim JS, Kim MH, Kim MH, et al. Risk of neuropsychiatric diseases according to the use of a leukotriene receptor antagonist in middle-aged and older adults with asthma: a nationwide population-based study using health claims data in Korea. J Allergy Clin Immunol Pract 2021; 9: 4290–4297. doi: 10.1016/j.jaip.2021.06.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02467-2021.Supplement (620.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02467-2021.Shareable (483.6KB, pdf)