Abstract
Background
Comprehensive studies investigated the role of T-cells in asthma which led to personalised treatment options targeting severe eosinophilic asthma. However, little is known about the contribution of B-cells to this chronic inflammatory disease. In this study we investigated the contribution of various B-cell populations to specific clinical features in asthma.
Methods
In the All Age Asthma Cohort (ALLIANCE), a subgroup of 154 adult asthma patients and 28 healthy controls were included for B-cell characterisation by flow cytometry. Questionnaires, lung function measurements, blood differential counts and allergy testing of participants were analysed together with comprehensive data on B-cells using association studies and multivariate linear models.
Results
Patients with severe asthma showed decreased immature B-cell populations while memory B-cells were significantly increased compared with both mild–moderate asthma patients and healthy controls. Furthermore, increased frequencies of IgA+ memory B-cells were associated with impaired lung function and specifically with parameters indicative for augmented resistance in the peripheral airways. Accordingly, asthma patients with small airway dysfunction (SAD) defined by impulse oscillometry showed increased frequencies of IgA+ memory B-cells, particularly in patients with mild–moderate asthma. Additionally, IgA+ memory B-cells significantly correlated with clinical features of SAD such as exacerbations.
Conclusions
With this study we demonstrate for the first time a significant association of increased IgA+ memory B-cells with asthma and SAD, pointing towards future options for B-cell-directed strategies in preventing and treating asthma.
Short abstract
Circulating B-cells are altered in asthma patients. In particular, IgA+ memory B-cells are significantly increased in patients with impaired lung function, especially of the small airways, suggesting a contribution to inflammation in the peripheral lung. https://bit.ly/3r0xBNA
Introduction
Asthma is one of the most prevalent chronic respiratory diseases characterised by airway inflammation, airway hyperreactivity and impaired lung function with obstruction of the central and peripheral airways [1, 2]. In the past decades, a better understanding of distinct phenotypes and endotypes of this heterogeneous disease supported the development of personalised therapeutic approaches, mainly directed against type 2 cytokines in severe eosinophilic asthma [3]. In contrast, knowledge of the impact of Bcells on asthma is still very limited and mostly acknowledges their role in allergic asthma as IgE producers [4]. More recently, research revealed immunomodulatory functions of regulatory B-cells on allergic airway inflammation [5] and allergen tolerance [6]. Additionally, we could show that B-cells control airway hyperreactivity and airway remodelling in a murine asthma model [7], pointing towards a possible role of B-cells for future diagnostic and preventive strategies in asthma.
The peripheral B-cell compartment consists of various populations ranging from immature so-called transitional B-cells to mature naïve B-cells. Activation of naïve B-cells leads to highly specialised antigen-experienced CD27+ memory B-cells or plasma cells producing IgM, IgA, IgG or IgE [8, 9]. Additionally, less antigen-specific and therefore polyreactive IgA is produced by CD27− memory B-cells which play a role in mucosal host–microbiome homeostasis [10]. Memory B-cells recirculate in blood, secondary lymphoid tissues [11] and mucosal organs such as the lung [12], and their re-activation results in a strengthened immunoglobulin response [11, 13].
In particular, IgA and IgA+ B-cells are crucial for pulmonary mucosal immune defence [14] and also show immunomodulatory properties [15]. Histology studies in chronic obstructive pulmonary disease (COPD) connected IgA+ B-cells and locally impaired secretion of IgA to inflammation of the small airways [16, 17]. This is of particular interest as inflammation and obstruction of the peripheral airways (bronchioles <2 mm) is also an important clinical feature of asthma called small airway dysfunction (SAD) [18, 19]. SAD occurs in patients with mild–moderate and severe asthma, and significantly affects exacerbation rates, quality of life and daily physical activity [20, 21]. While lung function and imaging correlates of SAD have been frequently investigated in recent years [18, 21], little is known about the inflammatory processes contributing to SAD due to the relative inaccessibility of the small airways for cellular specimen collection.
Based on our previous findings in experimental asthma mouse models [5, 7], we hypothesised that B-cells influence asthma pathogenesis in humans and are linked to specific clinical characteristics in asthma patients. We therefore analysed immature, mature and memory B-cells in peripheral blood of asthma patients and healthy controls of the All Age Asthma Cohort (ALLIANCE). We used supervised and unsupervised statistical methods to search for associations between specific B-cell populations and essential clinical asthma features such as disease severity, markers of airway inflammation and lung function. Overall, we aimed to delineate the influence of B-cells on inflammatory processes driving asthma pathogenesis or specific traits to address the existing knowledge gap about B-cells and asthma, and to explore the potential of B-cells for disease phenotyping and diagnostics to improve personalised asthma care.
Materials and methods
Subjects and sample collection
B-cell analysis was done in probands with available blood specimens comprising 154 adult patients and 28 healthy controls of the ALLIANCE cohort, a longitudinal multicentre clinical cohort of the German Center for Lung Research (DZL) recruiting children with pre-school wheeze and asthma as well as adult asthma patients [22]. All local medical ethics committees of the participating centres approved the study protocol and all participants gave their written informed consent. Adults were recruited at the DZL sites of the Airway Research Center North (ARCN). The study was registered at ClinicalTrials.gov (adult arm: NCT02419274). Study design, inclusion and exclusion criteria, detailed data, and biomaterial collected at yearly study visits have been reported elsewhere [22]. Adult patients with asthma diagnosed according to international [23] and national guidelines [24] were eligible for inclusion as well as healthy controls without a previous asthma diagnosis and respective clinical symptoms. Further information concerning study design, methods and definition of clinical variables is available in the supplementary material.
B-cell characterisation
Isolated peripheral blood mononuclear cells were used for phenotypic characterisation of B-cell subpopulations. Cells were blocked, stained and analysed via flow cytometry. Further details are specified in the supplementary material.
Statistical analysis
The analysis was done using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) with the R packages stats (version 4.0.4), q-value (version 2.20.0) and ggpubr (version 0.4.0).
For patient characterisation, the median (interquartile range) or percentage was calculated for continuous or categorical variables, respectively. The Wilcoxon test or Chi-squared test of independence was used to calculate p-values.
The association between pairs of B-cell populations and clinical variables was analysed using the Kruskal–Wallis test for categorical and Spearman's correlation for continuous clinical variables. To adjust for multiple testing, the Benjamini–Hochberg procedure was used. For continuous variables, linear regression lines were generated and for categorical variables the p-values (using the Wilcoxon test) between the categories were calculated. The same method was also used to examine the association between pairs of clinical variables.
Multivariate linear regression was used to assess the relationship between B-cell populations (percentage of CD19+ B-cells) and all clinical variables as used in the association analysis while accounting for additional confounders such as age and oral corticosteroid (OCS) intake. To determine the significance of the clinical variable term, a model comparison approach was taken. A null model consisting of age and OCS (but without the clinical variable of interest) was compared with the full model consisting of the clinical variable, age and OCS using ANOVA. The resulting ANOVA derived p-values were subsequently corrected for multiple testing using Storey q-values [25].
To define SAD, the upper limit of normal and percentage predicted values of impulse oscillometry (IOS) parameters were determined according to the 95th centile of a German cohort of healthy adults [26].
To analyse the relationship between SAD and IgA+ B-cells and clinical variables, a multivariate linear model was built. Features for the model were chosen from age, gender, exhaled nitric oxide fraction (FENO), sputum and blood eosinophils, sum of allergen-specific IgE, smoking (pack-years), body mass index (BMI), and OCS intake using a stepwise model selection by Akaike Information Criteria. Further information regarding the clinical variables is available in the supplementary material.
Results
Study population
The study population included 154 patients with asthma and 28 healthy subjects from the ALLIANCE cohort. Mean age was comparable between patients and controls. 40% of patients suffered from severe asthma according to the European Respiratory Society/American Thoracic Society guidelines [27]. More details are presented in tables 1 and 2.
TABLE 1.
Clinical characteristics of patients with asthma and healthy controls
| Asthma patients (n=154) | Healthy controls (n=28) | p-value | |
| Age (years) | 53.1 (45.0–64.9) | 56.2 (36.1–68.7) | 0.97 |
| BMI (kg·m–2) | 27.2 (24.4–30.7) | 24.9 (22.4–27.1) | 0.012 |
| Female | 86 (56) | 12 (43) | 0.288 |
| Current or ex-smoker ≥10 pack-years | 40 (26) | 4 (14) | 0.276 |
| Atopy, blood and sputum differential counts | |||
| Atopy | 88 (59) | 9 (33) | 0.024 |
| Blood eosinophil granulocytes (×103 µL−1) | 0.29 (0.14–0.49) | 0.12 (0.07–0.17) | <0.001 |
| Blood neutrophil granulocytes (×103 µL−1) | 4.32 (3.37–5.88) | 3.20 (2.53–3.59) | <0.001 |
| Sputum eosinophil granulocytes (%) | 1.8 (0.5–6.7) | 0.1 (0.0–0.5) | <0.001 |
| Sputum neutrophil granulocytes (%) | 56.0 (32.0–73.1) | 53.4 (21.4–72.8) | 0.490 |
| Blood eosinophils ≥300 µL−1 | 75 (49) | 2 (7) | <0.001 |
| Sputum eosinophils ≥2% | 65 (49) | 0 (0) | <0.001 |
| Lung function | |||
| FEV1 (z-score) | −1.53 (−2.40– −0.49) | −0.03 (−0.49–0.46) | <0.001 |
| FEV1 (% pred) | 78.55 (65.18–92.8) | 99.62 (92.26–107.68) | <0.001 |
| FEV1/FVC (z-score) | −1.73 (−2.69– −0.81) | −0.65 (−0.95– −0.12) | <0.001 |
| FEV1/FVC (% pred) | 84.95 (74.09–92.99) | 94.52 (90.95–98.94) | <0.001 |
| FEF25–75% (z-score) | −1.69 (−2.78– −0.80) | −0.43 (−0.77–0.06) | <0.001 |
| FEF25–75% (% pred) | 51.55 (30.23–75.27) | 86.05 (73.52–101.97) | <0.001 |
| FENO (ppb) | 26.0 (16.0–44.0) | 17.0 (13.0–19.8) | <0.001 |
| R5−R20 (kPa·L–1·s–1) | 0.11 (0.06–0.19) | 0.03 (0.01–0.06) | <0.001 |
| R5−R20 (% pred) | 186 (107–331) | 93 (30–125) | <0.001 |
| AX (kPa·L–1·s–1) | 0.67 (0.31–1.61) | 0.17 (0.10–0.27) | <0.001 |
| AX (% pred) | 244 (116–498) | 60 (25–107) | <0.001 |
| FRES (Hz) | 17.07 (12.68–21.29) | 9.44 (8.45–13.08) | <0.001 |
| FRES (% pred) | 134 (109–166) | 98 (80–124) | <0.001 |
Data are presented as median (interquartile range) or n (%), unless otherwise stated. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75%: forced expiratory flow at 25–75% of FVC; FENO: exhaled nitric oxide fraction; R5–R20: resistance at 5 Hz−resistance at 20 Hz; AX: reactance area; FRES: resonance frequency.
TABLE 2.
Clinical characteristics of asthma patients (n=154)
| Disease duration (years) | 19 (8–32) |
| Adult-onset asthma | 102 (67) |
| Patients with ≥1 severe exacerbations | 82 (53) |
| Asthma severity | |
| Mild–moderate | 92 (60) |
| Severe | 62 (40) |
| Asthma Control Test score | 20 (14–23) |
| GINA control status | |
| Controlled | 48 (31) |
| Partly controlled | 46 (30) |
| Uncontrolled | 60 (39) |
| Medication | |
| Mean ICS dose# (µg·day–1) | 450±480 |
| LTRA | 25 (16) |
| LABA | 129 (84) |
| LAMA | 37 (24) |
| OCS | 36 (23) |
| Omalizumab | 5 (3) |
| Mepolizumab | 2 (1) |
Data are presented as median (interquartile range), n (%) or mean±sd, unless otherwise stated. GINA: Global Initiative for Asthma; ICS: inhaled corticosteroids; LTRA: leukotriene antagonists; LABA: long-acting β2-agonists; LAMA: long-acting muscarinic antagonists; OCS: oral corticosteroids. #: fluticasone-equivalent.
Patients with severe asthma have altered frequencies of B-cell populations
We investigated peripheral B-cells of patients and healthy volunteers by flow cytometry (supplementary figure S1).
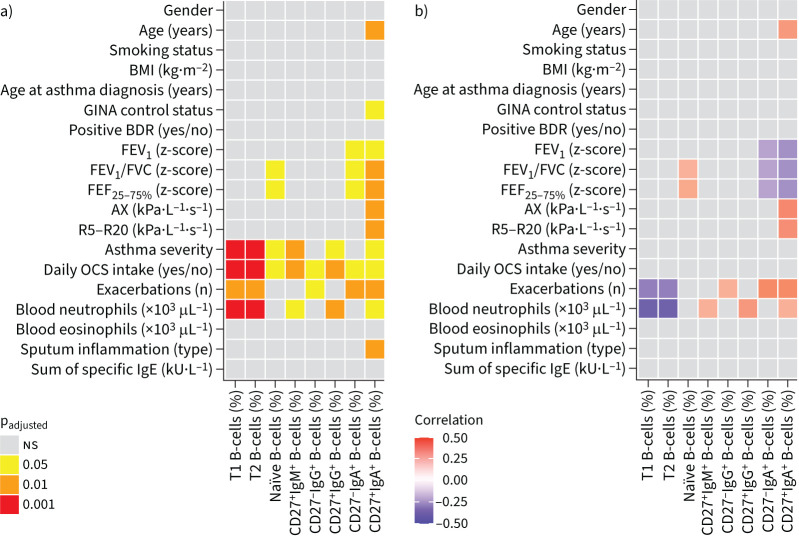
Percentages of different B-cell subpopulations were significantly associated with important clinical characteristics such as asthma severity, exacerbation frequency, blood neutrophils, sputum eosinophilia and lung function parameters (figure 1 and supplementary table S3).
FIGURE 1.
Pairwise a) comparisons and b) correlations between B-cell populations and clinical parameters of asthma patients and healthy controls. Colour code shows a) significant p-values and b) estimate for significant correlations analysed by Kruskal–Wallis or Spearman's correlation, respectively, with adjustment for multiple testing (padjusted). B-cell subsets are presented as percentage of total CD19+ B-cells. BMI: body mass index; GINA: Global Initiative for Asthma; BDR: bronchodilator response; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75%: forced expiratory flow at 25–75% of FVC; AX: reactance area; R5–R20: resistance at 5 Hz−resistance at 20 Hz; OCS: oral corticosteroids; T1: transitional 1; T2: transitional 2. ns: nonsignificant.
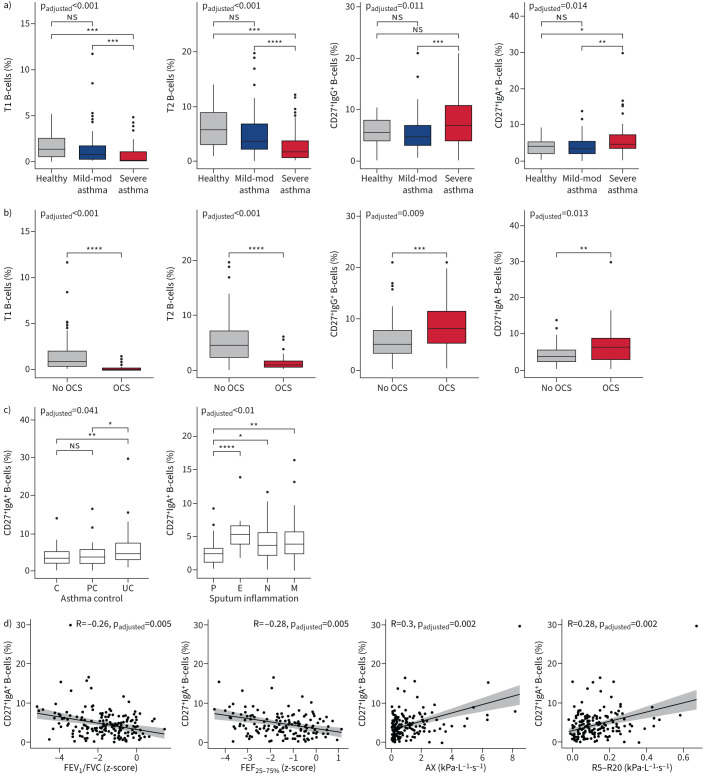
Patients with severe asthma showed a significant reduction of the immature early transitional 1 (T1) and late transitional 2 (T2) B-cell populations compared with patients with mild–moderate asthma and healthy subjects. Similarly, percentages of mature naïve B-cells were diminished in patients with severe compared with mild–moderate asthma, but comparable to the percentage of healthy volunteers (figure 2a, supplementary figure S2a and supplementary table S3). Conversely, proportions of unswitched CD27+IgM+ as well as class-switched CD27+IgG+ and CD27+IgA+ memory B-cells were strongly increased in severe compared with mild–moderate asthma. In addition, CD27+IgM+ and CD27+IgA+ but not CD27+IgG+ memory B-cells were increased in severe asthma patients compared with healthy controls (figure 2a and supplementary figure S2a).
FIGURE 2.
Associations between B-cell subsets and clinical parameters of asthma patients and healthy controls. Associations with a) asthma severity, b) oral corticosteroid (OCS) intake, c) asthma control and sputum inflammation, and d) forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC), forced expiratory flow at 25–75% of FVC (FEF25–75%), reactance area (AX) and resistance at 5 Hz−resistance at 20 Hz (R5−R20) are shown for asthma patients and healthy controls. Overall adjusted p-values after multiple test corrections and p-values from categorical group comparisons are shown as well as R and adjusted p-values from Spearman correlations. Further significant associations are shown in supplementary figure S2. T1: transitional 1; mod: moderate; T2: transitional 2; OCS: oral corticosteroids; C: controlled; PC: partly controlled; UC: uncontrolled; P: paucigranulocytic; E: eosinophilic; N: neutrophilic; M: mixed granulocytic. ns: nonsignificant; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
Patients with regular OCS intake showed similar findings as patients with severe asthma (figure 2b and supplementary figure S2b). An increased frequency of CD27+IgA+ memory B-cells occurred in uncontrolled disease according to the 2019 Global Initiative for Asthma definition [23] and was also associated with sputum eosinophilia, but not with blood eosinophilia or atopy (figure 2c and supplementary table S3).
Impaired lung function is associated with increased CD27+IgA+ memory B-cell frequency
Several lung function parameters indicative for airway obstruction were moderately associated with distinct B-cell patterns. Increased frequencies of IgA+ memory B-cells were associated with central airway obstruction measured by forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity (FVC) and small airway obstruction measured by forced expiratory flow at 25–75% of FVC (FEF25–75%) as well as IOS parameters reactance area (AX) and resistance at 5 Hz−resistance at 20 Hz (R5−R20) (figure 2d, supplementary figure S2c and supplementary table S3).
Association of IgA+ memory B-cells and airway obstruction is independent from OCS treatment
As already presented, regular treatment with OCS showed a significant association with all investigated B-cell populations (figure 1 and supplementary table S3). We chose a linear model to investigate if any of the associations seen in figure 1 remained significant independently of OCS intake and age (table 3).
TABLE 3.
Linear model
| B-cell variable | Clinical variable | Independent variables per model | q-value of clinical variable | |||
| Term | Estimate | se | p-value | |||
| CD27+IgA+ memory B-cells | AX | AX | 0.886 | 0.167 | <0.001 | <0.001 |
| Age | 0.034 | 0.016 | 0.039 | |||
| Regular OCS | 2.001 | 0.624 | 0.002 | |||
| (Intercept) | 1.358 | 0.869 | ||||
| CD27+IgA+ memory B-cells | R5−R20 | R5−R20 | 9.117 | 2.240 | <0.001 | 0.002 |
| Age | 0.031 | 0.017 | 0.066 | |||
| Regular OCS | 2.156 | 0.627 | 0.001 | |||
| (Intercept) | 1.307 | 0.896 | ||||
| CD27+IgA+ memory B-cells | FEF25–75% (z-score) | FEF25–75% (z-score) | −0.683 | 0.225 | 0.003 | 0.026 |
| Age | 0.046 | 0.019 | 0.020 | |||
| Regular OCS | 2.154 | 0.713 | 0.003 | |||
| (Intercept) | 0.764 | 1.097 | ||||
| CD27+IgA+ memory B-cells | FEV1 (z-score) | FEV1 (z-score) | −0.541 | 0.177 | 0.003 | 0.026 |
| Age | 0.049 | 0.017 | 0.005 | |||
| Regular OCS | 2.137 | 0.663 | 0.002 | |||
| (Intercept) | 0.902 | 0.940 | ||||
| CD27+IgA+ memory B-cells | FEV1/FVC (z-score) | FEV1/FVC (z-score) | −0.597 | 0.192 | 0.002 | 0.026 |
| Age | 0.044 | 0.017 | 0.010 | |||
| Regular OCS | 2.279 | 0.649 | 0.001 | |||
| (Intercept) | 0.815 | 0.944 | ||||
| CD27−IgA+ memory B-cells | Number of severe exacerbations | Number of severe exacerbations | 0.223 | 0.068 | 0.001 | 0.026 |
| Age | 0.0003 | 0.011 | 0.975 | |||
| Regular OCS | 0.613 | 0.475 | 0.199 | |||
| (Intercept) | 2.097 | 0.591 | ||||
| CD27−IgA+ memory B-cells | AX | AX | 0.339 | 0.111 | 0.003 | 0.026 |
| Age | −0.003 | 0.011 | 0.803 | |||
| Regular OCS | 0.997 | 0.415 | 0.017 | |||
| (Intercept) | 2.173 | 0.578 | ||||
| CD27−IgA+ memory B-cells | R5−R20 | R5−R20 | 4.224 | 1.448 | 0.004 | 0.032 |
| Age | −0.004 | 0.011 | 0.718 | |||
| Regular OCS | 1.152 | 0.406 | 0.005 | |||
| (Intercept) | 2.084 | 0.579 | ||||
Linear model describing B-cell subpopulations as a function of clinical characteristics with oral corticosteroids (OCS) and age as confounders. Coefficient estimate, standard error and p-value are given for each term in the model. p-values for the clinical variables were corrected for multiple tests (q-value) and all significant results are shown (q<0.05). AX: reactance area; R5–R20: resistance at 5 Hz−resistance at 20 Hz; FEF25–75%: forced expiratory flow at 25–75% of FVC; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
The linear model confirmed the association of increased percentages of CD27+IgA+ memory B-cells with SAD which was independent from OCS intake and age. The OCS-independent association was strongest between CD27+IgA+ B-cells and the IOS parameters AX (p=3.3×10−7) and R5−R20 (p=7.2×10−5), both indicating small airway obstruction (table 3). Comparing the association between R5−R20 and CD27+IgA+ B-cells in the linear model between patients with and without regular OCS intake showed no significant difference in the slope describing the association (p=0.148); however, percentages of IgA+ memory B-cells were overall higher in patients with OCS (supplementary figure S3).
Additional associations were found for FEF25–75%, FEV1 and FEV1/FVC (table 3). Furthermore, percentages of CD27−IgA+ B-cell frequencies were also associated with AX and R5−R20, and additionally with frequency of severe exacerbations. All other associations seen between B-cell populations were not independent from effects of OCS intake (supplementary table S4).
IgA+ memory B-cells are increased in asthma patients with SAD
As shown by the linear model, lung function parameters indicative of peripheral airway obstruction showed a significant association with CD27+ and CD27−IgA+ memory B-cells. The strongest association was seen for both IOS parameters AX and R5−R20, which measure airway distensibility and small airway obstruction, respectively. We were therefore interested to further investigate if these cells were also increased in patients with SAD. The IOS parameter R5−R20 has been shown to appropriately reflect resistance of the small airways [18], detect SAD in asthma patients [21] and corresponds well to important clinical outcomes of SAD in asthma patients [18, 21]. We consecutively used R5−R20 values above the upper limit of normal (95th centile) according to published reference equations [26] to define SAD in our cohort and to analyse its association with proportions of IgA+ B-cells in more detail.
SAD was present in 42% (63 out of 152) of all asthma patients in our cohort. Of these, 43% (27 out of 63) had mild–moderate asthma and 57% (36 out of 63) had severe asthma. Further clinical characteristics of all asthma patients with and without SAD are specified in supplementary table S5.
Percentages of CD27+IgA+ memory B-cells were increased in patients with SAD (figure 3a), while CD27−IgA+ memory B-cells did not show any differences (supplementary figure S4a). Furthermore, we observed differences in CD27+IgA+ memory B-cells depending on disease severity (figure 3b). IgA+ memory B-cells were lowest in patients with mild–moderate asthma who did not have SAD and were significantly higher in mild–moderate asthma patients with SAD. Patients with severe asthma had overall increased percentages of IgA+ B-cells without a difference between patients with and without SAD.
FIGURE 3.
IgA+ memory B-cells and small airway dysfunction (SAD). a) CD27+IgA+ memory B-cells in patients with or without SAD. b) CD27+IgA+ B-cells in patients with or without SAD in mild–moderate (mild-mod) asthma and severe asthma. c) CD27+IgA+ B-cells in patients with or without central airway obstruction (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)<lower limit of normal (LLN; z-score −1.64)) and with or without SAD. **: p<0.01; ***: p<0.001.
SAD and peripheral airway obstruction can occur independently from central airway obstruction. IgA+ memory B-cells were only increased in patients with both central airway obstruction measured by FEV1/FVC and small airway obstruction measured by IOS (figure 3c). In patients with normal FEV1/FVC and SAD, the increase in the percentage of IgA+ memory B-cells did not reach statistical significance compared with patients without central or peripheral airway obstruction (p=0.0662).
To investigate if IgA+ memory B-cells were associated with SAD in the context of other known risk factors, including age, gender, BMI, smoking, markers of type 2 inflammation such as blood and sputum eosinophils, specific IgE, and FENO [20], we used a multivariate regression model. In patients with mild–moderate asthma, IgA+ memory B-cells were associated with SAD in addition to other known risk factors such as sputum eosinophils and gender (table 4). Adding severe asthma patients to the model did not show an association between SAD and IgA+ memory B-cells (supplementary table S6).
TABLE 4.
Regression model for small airway dysfunction (SAD) defined by resistance at 5 Hz−resistance at 20 Hz (R5−R20) in mild–moderate asthma patients
| Estimate | se | p-value | 95% CI lower bound | 95% CI upper bound | |
| CD27+IgA+ memory B-cell | 0.29 | 0.109 | 0.008 | 1.087 | 1.683 |
| Sputum eosinophils | 0.095 | 0.04 | 0.017 | 1.022 | 1.197 |
| Blood eosinophils | −4.805 | 2.015 | 0.017 | 0.0001 | 0.294 |
| Gender (female) | −1.392 | 0.599 | 0.02 | 0.071 | 0.769 |
| Sum of sIgE | 0.004 | 0.002 | 0.13 | 0.998 | 1.008 |
Result of stepwise multivariate regression model (n=80). The dependent variable is SAD defined by the 95th centile of R5−R20. A stepwise-forward regression was used to find the best model using Akaike Information Criteria. The table shows the variables with best model fit (CD27+IgA+ memory B-cells (%), sputum eosinophils (%), blood eosinophils (×103 μL−1), gender and sum of 36 allergen-specific IgE (sIgE; kU·L−1)). Variables not selected by best model fit are not shown (regular OCS intake (yes/no), exhaled nitric oxide fraction (ppb), body mass index (kg·m−2), age (years) and smoking (pack-years)).
IgA+ memory B-cells and clinical features of SAD
Patients with SAD present more often with uncontrolled asthma [28], frequent exacerbations [21] and impaired quality of life [18]. Percentages of CD27+IgA+ and CD27−IgA+ B-cells correlated with the number of exacerbations similarly as sputum eosinophils (figure 4 and supplementary table S7). Equally, CD27+IgA+ B-cells were correlated with impaired asthma control and reduced quality of life as assessed by the Asthma Control Questionnaire (ACQ-7) and Asthma Quality of Life Questionnaire, respectively (supplementary figure S5, and supplementary tables S8 and S9).
FIGURE 4.
Correlations of number of exacerbations with clinical and B-cell parameters. Dark red defines the highest positive correlation between the parameters and dark blue shows the lowest negative correlation between the variables. Adjusted p-values after multiple test corrections are shown next to the bars. AX: reactance area; ICS: inhaled corticosteroids; R5–R20: resistance at 5 Hz−resistance at 20 Hz; BMI: body mass index; FENO: exhaled nitric oxide fraction; OCS: oral corticosteroids; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75%: forced expiratory flow at 25–75% of FVC. ns: nonsignificant; *: p<0.05; **: p<0.01; ***: p<0.001.
Since IgA+ B-cells play an important role in mucosal immune defence, we analysed IgA+ memory B-cells in SAD patients with frequent (two or more per year) respiratory tract infections (RTIs). In the ALLIANCE cohort, patients with and without SAD did not differ regarding the occurrence of frequent RTIs (17 out of 63 patients with SAD reported frequent RTIs compared with 17 out of 89 without SAD and with frequent RTIs; p=0.341). Equally, in patients with SAD, frequencies of IgA+ memory B-cells did not differ depending on the presence or absence of frequent RTIs (supplementary figure S6).
Discussion
In this study we demonstrated significant alterations of immature and mature B-cell populations in asthma. Importantly, we described for the first time an unappreciated connection of IgA+ memory B-cells with SAD. IgA+ memory B-cells were associated with peripheral airway obstruction measured by R5−R20 irrespective of disease severity, and correlated with an increased number of severe exacerbations and worse asthma control.
OCS intake is known to be a major confounder of systemic immune responses, an effect that was evident for most B-cell populations examined in our study. Importantly, the link between increased systemic IgA+ memory B-cell frequencies and lung function parameters indicative for peripheral airway obstruction such as R5−R20, AX and FEF25–75% [18, 21] was independent of the influence of OCS intake and age. R5−R20 has been shown to reflect increased narrowing of the small airways [18, 21] and important clinical outcomes [18, 29]. We therefore used published reference equations for R5−R20 to define SAD and demonstrated an increase of IgA+ B-cells in patients with SAD. This link was particularly evident in patients with mild–moderate asthma, indicating a role for IgA+ B-cells in SAD irrespective of disease severity. This is important as SAD is not only found in severe asthma patients but also in mild–moderate disease [20, 21].
Little is known so far about inflammation or remodelling processes in the peripheral airways in asthma mostly due to their difficult accessibility. Histology data originate mostly from patients with fatal asthma attacks [30], limiting translation to asthma patients in general. There is some evidence for a role of type 2 inflammation in SAD from in vitro experiments [31] and histology of transbronchial biopsies revealed eosinophilic inflammation, particularly in the parenchyma of patients with nocturnal asthma symptoms [32], which are symptoms that are connected to SAD [28]. Clinical markers of type 2 inflammation, e.g. blood and sputum eosinophils, have also been linked to the presence of SAD [20] and type 2-targeting biologicals have been shown to ameliorate peripheral airway resistance measured by R5−R20 [33]. However, overall knowledge about inflammation connected to SAD is still very limited, particularly also regarding the impact of B-cells on SAD in asthma patients.
While our study is the first to link IgA+ memory B-cells with SAD in asthma, our finding is supported by several studies linking IgA+ B -cells to small airway inflammation in COPD. Histology studies of lungs from patients with COPD show increased IgA+ B-cell frequencies in lymphoid follicles, particularly in the distal lung parenchyma and close to small airways, which correlate with disease severity [16]. Furthermore, in COPD there is a strong link between localised mucosal deficiency of secretory IgA (sIgA) and increased inflammation and airway remodelling most likely driven by impaired local pathogen defence [17, 34]. Additionally, reduced capacity for transcytosis of IgA over the epithelial barrier has been shown in both small airways of COPD patients [34] and airway epithelial cells in asthma [35], and sIgA in bronchoalveolar lavage fluid inversely correlates with asthma symptoms [36].
However, it remains unsolved if the increased presence of IgA+ B-cells in the lung periphery of COPD patients with small airway disease reflects an exacerbated response against pathogens, potentially due to intraluminal sIgA deficiency, or if they could drive inflammation and remodelling, e.g. by producing antibodies against self-antigens [16].
Here, we showed for the first time that SAD in asthma patients is associated with increased frequencies of circulatory IgA+ memory B-cells. This is in concordance with previous observations showing that systemic and pulmonary memory B-cell pools are connected, as memory B-cells in the lung depend on both local induction [37] as well as replenishment from extrapulmonary organs [38, 39]. Furthermore, we carefully investigated and excluded other reasons for increased blood IgA+ memory B-cells in the context of asthma, e.g. frequent RTIs, smoking [40] and atopy [41].
Based on our analysis and previously published histological evidence [30], IgA+ memory B-cells could serve as a biomarker for inflammation of the small airways, a compartment of the lung that is difficult to reach for diagnostic evaluation especially in asthma patients in whom lung histology is usually not available. However, due to the observational character of the ALLIANCE cohort, we cannot answer the question whether increased IgA+ memory B-cells are a cause or co-phenomenon of SAD. Future studies need to confirm this link and assess its use as a biomarker for SAD development and progression.
In addition to our results regarding IgA+ memory B-cells, we demonstrate substantial changes of other B-cell populations in asthma. Naïve mature B-cells as well as T1 and T2 B-cells were reduced in patients with severe asthma compared with mild–moderate asthma patients, while IgG+ and IgM+ memory B-cells were increased in severe asthma. However, these findings did not remain significant after correction for regular OCS intake, a treatment which affected 58% of the severe asthma patients in our cohort demonstrating the importance of considering steroid effects in the analysis. Further differentiation between asthma-specific or steroid effects or a combination of both was therefore not possible for these B-cell populations, a problem that has been described by other authors as well, particularly with regard to early B-cell differentiation [42, 43]. Equally, the association seen between several B-cell populations and blood neutrophils in our dataset did not remain significant after correction for OCS, most likely also due to effects of OCS on neutrophil frequencies [44]. Noteworthy, we were able to uncover a significant and OCS-independent association between IgA+ memory B-cells and SAD. Still, we cannot completely exclude an additional effect of OCS on IgA+ memory B-cell frequencies in patients with severe asthma. This could also explain why the multivariate model which compared IgA+ memory B-cells to other known risk factors for SAD only revealed a significant association when focusing on patients with mild–moderate asthma who are not exposed to OCS or high inhaled doses of corticosteroids.
A particular strength of our study is the stringent statistical design. Treatment effects are an inevitable problem in asthma research since most patients are already under treatment at the time of inclusion into an observational study. Therefore, appropriate statistical measures need to be applied to control for OCS effects, which confirmed in our study a new and until now undescribed role of IgA+ memory B-cells in asthma patients with SAD.
A weakness of our study is that we did not investigate B-cells in lung tissue or in the airways. Lung histology as used in COPD studies is rarely available for patients with asthma. Future studies should explore and correlate lung and blood IgA+ memory B-cells using bronchoalveolar lavage fluid or sputum and ideally lung tissue in combination with additional support from experimental murine models [7]. Additionally, more data are needed regarding the predictive use of IgA+ memory B-cells for SAD development.
In conclusion, we showed that B-cell populations are altered in asthma compared with controls, and differ between mild–moderate and severe asthma, and we described disease-specific changes in the B-cell repertoire that are independent from systemic corticosteroid effects.
Our results reveal a new and until now undescribed association of IgA+ memory B-cells in asthma patients with SAD, an important clinical feature of asthma with significant impact on symptom burden and quality of life. Most importantly, our data highlight for the first time a role for IgA+ B-cells in asthma and particularly in SAD even in milder disease stages. Future studies are needed to elucidate the specific effects of IgA+ B-cells on the development of SAD, and to investigate the use of IgA+ memory B-cells as a biomarker for early diagnosis of SAD in asthma and prevention of lung function decline.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary materials and methods. ERJ-02130-2021.Supplement (106.4KB, pdf)
Supplementary figure S1. Flow cytometry gating strategy of peripheral blood B cell subpopulations. After dead cell exclusion, total B cells were gated as CD19+. CD27− B cells were further subdivided into transitional 1 (T1) B cells, transitional 2 (T2) B cells and naïve B cells via CD24 and CD38. CD19+ memory B cell subpopulations were gated as IgM+, IgG+ and IgA+ cells, and subdivided into CD27+IgM+ cells, as well as CD27+ and CD27− IgG+ and IgA+ cells, respectively. ERJ-02130-2021.Figure_S1 (47.6KB, jpg)
Supplementary figure S2. Associations between B cell subsets and clinical parameters. Association with asthma severity (a), and OCS intake (b), FEV1 and FEV1/FVC, FEF25–75 (c) and age (d) are shown. Overall adjusted p-values after multiple test corrections and p-values from categorical group comparisons are shown as well as R and adjusted p-values from Spearman correlations. H: healthy; mild-mod A: mild-moderate asthma; sev A: severe asthma; OCS: oral corticosteroids; w/o OCS: without OCS; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of FVC; AX: reactance area [kPa·L−1·s−1]; R5−R20: resistance at 5 Hz−resistance at 20 Hz; ns: not significant. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. ERJ-02130-2021.Figure_S2 (199.4KB, jpg)
Supplementary figure S3. OCS independent association between R5−R20 and CD27+IgA+ B cells. CD27+IgA+ B cell frequencies were associated with R5−R20 regardless of OCS treatment (adjusted p-value <0.002). The linear associations for all patients is given as a solid line and the dotted lines represent the linear associations for OCS (---) and non-OCS (-·-) groups. The difference in slope between the two groups is not significant (p=0.148). Age is illustrated by colour. Triangle represents OCS intake, circles no OCS intake. OCS: oral corticosteroids. ERJ-02130-2021.Figure_S3 (50.6KB, jpg)
Supplementary figure S4. CD27−IgA+ memory B cells and small airway dysfunction. CD27−IgA+ B cells in patients with and without SAD (a), in patients with mild-moderate or severe asthma (b). SAD: small airway dysfunction; sev A: severe asthma; mild-mod A: mild-moderate asthma. ns: not significant. *: p<0.05. ERJ-02130-2021.Figure_S4 (31.7KB, jpg)
Supplementary figure S5. Correlations of Asthma Control Questionnaire 7 and Asthma Quality of Life Questionnaire with clinical and B cell parameters. Dark red defines the highest positive correlation between the parameters and dark blue shows the lowest negative correlation between the variables. Adjusted p-values are depicted at the right bar side. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of FVC; OCS: oral corticosteroids; FeNO: fractional exhaled nitric oxide; BMI: body mass index; ICS: inhaled corticosteroids; AX: reactance area (kPa·L−1·s−1); R5−R20: resistance at 5 Hz−resistance at 20 Hz (kPa·L−1·s−1); ns: not significant. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. ERJ-02130-2021.Figure_S5 (194.9KB, jpg)
Supplementary figure S6.IgA+ memory B cells of asthmatic patients with or without SAD in combination with frequent respiratory infections. SAD: small airways dysfunction; fr: frequent; RTIs: respiratory tract infections. *: p<0.05; **: p<0.01. ERJ-02130-2021.Figure_S6 (23.7KB, jpg)
Supplementary table S3. Pairwise comparisons between B cell populations and clinical variables in asthma patients and healthy controls. ERJ-02130-2021.Table_S3 (294.7KB, pdf)
Supplementary table S4. Linear Model. ERJ-02130-2021.Table_S4 (611.8KB, pdf)
Supplementary table S5. Clinical characteristics of patients with versus without SAD. ERJ-02130-2021.Table_S5 (90.5KB, pdf)
Supplementary table S6. Regression model for SAD defined by R5−R20. ERJ-02130-2021.Table_S6 (38.8KB, pdf)
Supplementary table S7. Correlations between exacerbation frequency and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S7 (77.4KB, pdf)
Supplementary table S8. Correlations between Asthma Control Questionnaire (ACQ-7) and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S8 (78KB, pdf)
Supplementary table S9. Correlations between Asthma Quality Of Life Questionnaire (AQLQ) and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S9 (78KB, pdf)
Shareable PDF
Acknowledgements
We thank the patients who participate in the ALLIANCE cohort for their invaluable contribution to our research. We thank Susann Prange and Corinna Derwort (LungenClinic Grosshansdorf, Grosshansdorf, Germany) and Jana Bergmann, Anika Dreier, Beate Junk, Michaela Bartsch and Christin Albrecht (Hannover Medical School, Hannover, Germany) for their excellent technical support, and Julia Kontsendorn (Hannover Medical School) for critical data documentation and quality control.
Footnotes
Members of the ALLIANCE Study Group as part of the German Center for Lung Research (DZL) Study Group: Oliver Fuchs (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany; and Dept of Pediatric Respiratory Medicine, Inselspital, University Children's Hospital of Bern, University of Bern, Bern, Switzerland), Barbara Roesler (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Nils Welchering (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Naschla Kohistani-Greif (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Johanna Kurz (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany; and Dept of Pediatric Respiratory Medicine, Inselspital, University Children's Hospital of Bern, University of Bern, Bern, Switzerland), Katja Landgraf-Rauf (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Kristina Laubhahn (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Nicole Maison (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany; and Institut für Asthma- und Allergieprävention (IAP), Helmholtz Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), Munich, Germany), Claudia Liebl (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Bianca Schaub (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Markus Ege (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Sabina Illi (Institut für Asthma- und Allergieprävention (IAP), Helmholtz Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), Munich, Germany), Alexander Hose (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Esther Zeitlmann (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Mira Berbig (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Carola Marzi (Institut für Asthma- und Allergieprävention (IAP), Helmholtz Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), Munich, Germany), Christina Schauberger (Dept of Pediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, and Comprehensive Pneumology Center-Munich (CPC-M), German Center for Lung Research (DZL), Germany), Ulrich Zissler (Center of Allergy and Environment (ZAUM), Technical University of Munich and Helmholtz Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), German Center for Lung Research (DZL), Munich, Germany), Carsten Schmidt-Weber (Center of Allergy and Environment (ZAUM), Technical University of Munich and Helmholtz Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), German Center for Lung Research (DZL), Munich, Germany), Isabell Ricklefs (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Gesa Diekmann (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Lena Liboschik (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Gesche Voigt (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Laila Sultansei (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Markus Weckmann (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Gyde Nissen (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Anne-Marie Kirsten (Pulmonary Research Institute at LungenClinic Grosshansdorf, Grosshansdorf, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Benjamin Waschki (LungenClinic Grosshansdorf GmbH, Grosshansdorf, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Christian Herzmann (Research Center Borstel – Medical Clinic, Borstel, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Heike Biller (LungenClinic Grosshansdorf GmbH, Grosshansdorf, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Karoline I. Gaede (Research Center Borstel – Medical Clinic, Borstel, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Xenia Bovermann (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Alena Steinmetz (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Berrit Liselotte Husstedt (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Catharina Nitsche (University Children's Hospital, Lübeck, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Vera Veith (LungenClinic Grosshansdorf GmbH, Grosshansdorf, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Marlen Szewczyk (LungenClinic Grosshansdorf GmbH, Grosshansdorf, and Airway Research Center North (ARCN), German Center for Lung Research (DZL), Germany), Folke Brinkmann (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany; and Dept of Pediatric Pneumology, University Children's Hospital, Ruhr-University Bochum, Bochum, Germany), Aydin Malik (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Nicolaus Schwerk (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Christian Dopfer (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Mareike Price (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Adan Chari Jirmo (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Bin Liu (Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Mifflin-Rae Calveron (Hannover Medical School, Hannover, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Germany), Stefanie Weber (University Children's Hospital Marburg, University of Marburg, and University of Giessen Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Germany), Svenja Foth (University Children's Hospital Marburg, University of Marburg, and University of Giessen Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Germany), Chrysanthi Skevaki (Institute of Laboratory Medicine and Pathobiochemistry, Molecular Diagnostics, University of Marburg, and University of Giessen, Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Germany), Harald Renz (Institute of Laboratory Medicine and Pathobiochemistry, Molecular Diagnostics, University of Marburg, and University of Giessen, Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Germany), Meike Meyer (University of Cologne, Faculty of Medicine and University Hospital Cologne, Dept of Pediatrics, Cologne, Germany), Tom Schildberg (University of Cologne, Faculty of Medicine and University Hospital Cologne, Dept of Pediatrics, Cologne, Germany), Ernst Rietschel (University of Cologne, Faculty of Medicine and University Hospital Cologne, Dept of Pediatrics, Cologne, Germany), Silke van Koningsbruggen-Rietschel (University of Cologne, Faculty of Medicine and University Hospital Cologne, Dept of Pediatrics, Cologne, Germany) and Miguel Alcazar (University of Cologne, Faculty of Medicine and University Hospital Cologne, Translational Experimental Pediatrics – Experimental Pulmonology, Dept of Pediatric and Adolescent Medicine, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Center for Molecular Medicine Cologne (CMMC), Germany; Excellence Cluster on Stress Responses in Aging-associated Diseases (CECAD), University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; and Institute for Lung Health, University of Giessen and Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Giessen, Germany).
Conflict of interest: C. Happle reports grants from Novartis and Pari, outside the submitted work. M.V. Kopp reports grants from Allergopharma GmbH and Vertex GmbH; honoraria for lectures from Allergopharma GmbH, Sanofi GmbH, Infectopharm GmbH, Vertex GmbH and Leti GmbH; advisory board membership at Allergopharma GmbH and Sanofi GmbH; outside the submitted work. E. von Mutius reports royalties from Elsevier GmbH, Georg Thieme Verlag, Springer-Verlag GmbH and Elsevier Ltd; consulting fees from the Chinese University of Hong Kong, European Commission, HiPP GmbH & Co KG and AstraZeneca; lecture honoraria from Massachusetts Medical Society, Springer-Verlag GmbH, Elsevier Ltd, Boehringer Ingelheim International GmbH, European Respiratory Society, Universiteit Utrecht – Faculteit Diergeneeskunde, Universität Salzburg, Springer Medizin Verlag GmbH, Japanese Society of Pediatric Allergy and Clinical Immunology (JSPACI), Klinikum Rechts der Isar, University of Colorado, Paul-Martini-Stiftung and Imperial College London; travel support from Verein zur Förderung der Pneumologie am Krankenhaus Großhansdorf eV, Pneumologie Développement, Mondial Congress & Events GmbH & Co. KG, American Academy of Allergy, Asthma & Immunology, Imperial College London, Margaux Orange, Volkswagen Stiftung, Boehringer Ingelheim International GmbH, European Respiratory Society, Universiteit Utrecht – Faculteit Diergeneeskunde, Österreichische Gesellschaft für Allergologie und Immunologie, Massachusetts Medical Society, OM Pharma SA, Hanson Wade Ltd, iKOMM GmbH, DSI Dansk Borneastma Center, American Thoracic Society, HiPP GmbH & Co. KG and Universiteit Utrecht – Faculteit Bètawetenschappen; outside the submitted work. In addition, E. von Mutius has patent LU101064 (Barn dust extract for the prevention and treatment of diseases) pending, royalties paid to ProtectImmun for patent EP2361632 (Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders, granted on 19 March 2014), and patents EP1411977 (Composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases, granted on 18 April 2007), EP1637147 (Stable dust extract for allergy protection, granted on 10 December 2008) and EP1964570 (Pharmaceutical compound to protect against allergies and inflammatory diseases, granted on 21 November 2012) licensed to ProtectImmun. In addition, E. von Mutius is a member of the EXPANSE (funded by European Commission) Scientific Advisory Board, member of the BEAMS External Scientific Advisory Board (ESAB), member of the Editorial Board of the Journal of Allergy and Clinical Immunology: In Practice, member of the Scientific Advisory Board of the Children's Respiratory and Environmental Workgroup (CREW), member of the International Scientific and Societal Advisory Board (ISSAB) of Utrecht Life Sciences (ULS), University of Utrecht, member of the External Review Panel of the Faculty of Veterinary Science, University of Utrecht, member of the Selection Committee for the Gottfried Wilhelm Leibniz Programme (DFG), member of the International Advisory Board of the Asthma UK Centre for Applied Research (AUKCAR), member of the International Advisory Board of The Lancet Respiratory Medicine, and member of the Scientific Advisory Board of the CHILD (Canadian Healthy Infant Longitudinal Development) study, McMaster University, Hamilton, Canada. T. Bahmer reports grants from Network University Medicine (NUM): National Pandemic Cohort Network (NAPKON); consulting fees and lecture honoraria from AstraZeneca, Novartis, GlaxoSmithKline, Roche and Chiesi; travel support from Chiesi and AstraZeneca; outside the submitted work. K.F. Rabe reports lecture honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Novartis, Sanofi Regeneron, GlaxoSmithKline, Berlin-Chemie and Roche; advisory board membership at AstraZeneca and Sanofi Regeneron; leadership roles with the German Center for Lung Research (DZL), German Chest Society (DGP) and American Thoracic Society; outside the submitted work. A. Meyer-Bahlburg reports lecture honoraria from Pfizer; travel support from CSL Behring; advisory board membership with Pfizer; outside the submitted work. G. Hansen reports consulting fees from Sanofi GmbH; lecture honoraria from MedUpdate and AbbVie; outside the submitted work. All other authors have nothing to disclose.
Support statement: This research was supported by the German Center for Lung Research (DZL; via BMBF (Federal Ministry of Education and Research)) and Cluster of Excellence RESIST (EXC 2155, DFG (German Research Foundation)). Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
the ALLIANCE Study Group as part of the German Center for Lung Research (DZL):
Oliver Fuchs, Barbara Roesler, Nils Welchering, Naschla Kohistani-Greif, Johanna Kurz, Katja Landgraf-Rauf, Kristina Laubhahn, Nicole Maison, Claudia Liebl, Bianca Schaub, Markus Ege, Sabina Illi, Alexander Hose, Esther Zeitlmann, Mira Berbig, Carola Marzi, Christina Schauberger, Ulrich Zissler, Carsten Schmidt-Weber, Isabell Ricklefs, Gesa Diekmann, Lena Liboschik, Gesche Voigt, Laila Sultansei, Markus Weckmann, Gyde Nissen, Anne-Marie Kirsten, Benjamin Waschki, Christian Herzmann, Heike Biller, Karoline I. Gaede, Xenia Bovermann, Alena Steinmetz, Berrit Liselotte Husstedt, Catharina Nitsche, Vera Veith, Marlen Szewczyk, Folke Brinkmann, Aydin Malik, Nicolaus Schwerk, Christian Dopfer, Mareike Price, Adan Chari Jirmo, Bin Liu, Mifflin-Rae Calveron, Stefanie Weber, Svenja Foth, Chrysanthi Skevaki, Harald Renz, Meike Meyer, Tom Schildberg, Ernst Rietschel, Silke van Koningsbruggen-Rietschel, and Miguel Alcazar
References
- 1.Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020; 8: 585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 3.McGregor MC, Krings JG, Nair P, et al. Role of biologics in asthma. Am J Respir Crit Care Med 2019; 199: 433–445. doi: 10.1164/rccm.201810-1944CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akdis CA, Arkwright PD, Bruggen MC, et al. Type 2 immunity in the skin and lungs. Allergy 2020; 75: 1582–1605. doi: 10.1111/all.14318 [DOI] [PubMed] [Google Scholar]
- 5.Happle C, Jirmo AC, Meyer-Bahlburg A, et al. B cells control maternofetal priming of allergy and tolerance in a murine model of allergic airway inflammation. J Allergy Clin Immunol 2018; 141: 685–696. doi: 10.1016/j.jaci.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 6.Ma S, Satitsuksanoa P, Jansen K, et al. B regulatory cells in allergy. Immunol Rev 2021; 299: 10–30. doi: 10.1111/imr.12937 [DOI] [PubMed] [Google Scholar]
- 7.Habener A, Happle C, Grychtol R, et al. Regulatory B cells control airway hyperreactivity and lung remodeling in a murine asthma model. J Allergy Clin Immunol 2021; 147: 2281–2294. doi: 10.1016/j.jaci.2020.09.041 [DOI] [PubMed] [Google Scholar]
- 8.Bemark M, Holmqvist J, Abrahamsson J, et al. Translational mini-review series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation – revelation of B cell developmental pathways and lineage phenotypes. Clin Exp Immunol 2012; 167: 15–25. doi: 10.1111/j.1365-2249.2011.04469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol 2013; 131: 959–971. doi: 10.1016/j.jaci.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 10.Berkowska MA, Schickel JN, Grosserichter-Wagener C, et al. Circulating human CD27−IgA+ memory B cells recognize bacteria with polyreactive Igs. J Immunol 2015; 195: 1417–1426. doi: 10.4049/jimmunol.1402708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisel F, Shlomchik M. Memory B cells of mice and humans. Annu Rev Immunol 2017; 35: 255–284. doi: 10.1146/annurev-immunol-041015-055531 [DOI] [PubMed] [Google Scholar]
- 12.Koutsakos M, Wheatley AK, Loh L, et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med 2018; 10: eaan8405. doi: 10.1126/scitranslmed.aan8405 [DOI] [PubMed] [Google Scholar]
- 13.Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15: 149–159. doi: 10.1038/nri3802 [DOI] [PubMed] [Google Scholar]
- 14.Onodera T, Takahashi Y, Yokoi Y, et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci USA 2012; 109: 2485–2490. doi: 10.1073/pnas.1115369109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha C, Das M, Patil V, et al. Monomeric immunoglobulin A from plasma inhibits human Th17 responses in vitro independent of FcalphaRI and DC-SIGN. Front Immunol 2017; 8: 275. doi: 10.3389/fimmu.2017.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladjemi MZ, Martin C, Lecocq M, et al. Increased IgA expression in lung lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2019; 199: 592–602. doi: 10.1164/rccm.201802-0352OC [DOI] [PubMed] [Google Scholar]
- 17.Polosukhin VV, Richmond BW, Du RH, et al. Secretory IgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med 2017; 195: 1010–1021. doi: 10.1164/rccm.201604-0759OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foy BH, Soares M, Bordas R, et al. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med 2019; 200: 982–991. doi: 10.1164/rccm.201812-2322OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall H, Kenworthy JC, Horn FC, et al. Peripheral and proximal lung ventilation in asthma: short-term variation and response to bronchodilator inhalation. J Allergy Clin Immunol 2021; 147: 2154–2161. doi: 10.1016/j.jaci.2020.11.035 [DOI] [PubMed] [Google Scholar]
- 20.Abdo M, Trinkmann F, Kirsten AM, et al. Small airway dysfunction links asthma severity with physical activity and symptom control. J Allergy Clin Immunol Pract 2021; 9: 3359–3368. doi: 10.1016/j.jaip.2021.04.035 [DOI] [PubMed] [Google Scholar]
- 21.Postma DS, Brightling C, Baldi S, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med 2019; 7: 402–416. doi: 10.1016/S2213-2600(19)30049-9 [DOI] [PubMed] [Google Scholar]
- 22.Fuchs O, Bahmer T, Weckmann M, et al. The All Age Asthma cohort (ALLIANCE) – from early beginnings to chronic disease: a longitudinal cohort study. BMC Pulm Med 2018; 18: 140. doi: 10.1186/s12890-018-0705-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2019. Available from: http://ginasthma.org/
- 24.Bundesärztekammer, Kassenärztliche Bundesvereinigung, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften . Nationale VersorgungsLeitlinie Asthma Langfassung; 2009, 2. Auflage, Version 1.3. [National asthma health care guideline, long version; 2009, 2nd edition, version 1.3.] 2011. www.leitlinien.de/themen/asthma/archiv/pdf/asthma-2-aufl-lang-1-3.pdf Date last accessed: 14 April 2022.
- 25.Storey J. A direct approach to false discovery rates. J R Stat Soc Series B Stat Method 2002; 64: 479–498. doi: 10.1111/1467-9868.00346 [DOI] [Google Scholar]
- 26.Schulz H, Flexeder C, Behr J, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One 2013; 8: e63366. doi: 10.1371/journal.pone.0063366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 28.van der Wiel E, Postma DS, van der Molen T, et al. Effects of small airway dysfunction on the clinical expression of asthma: a focus on asthma symptoms and bronchial hyper-responsiveness. Allergy 2014; 69: 1681–1688. doi: 10.1111/all.12510 [DOI] [PubMed] [Google Scholar]
- 29.Bahmer T, Waschki B, Schatz F, et al. Physical activity, airway resistance and small airway dysfunction in severe asthma. Eur Respir J 2017; 49: 1601827. doi: 10.1183/13993003.01827-2016 [DOI] [PubMed] [Google Scholar]
- 30.Mauad T, Silva LF, Santos MA, et al. Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med 2004; 170: 857–862. doi: 10.1164/rccm.200403-305OC [DOI] [PubMed] [Google Scholar]
- 31.Manson ML, Safholm J, James A, et al. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol 2020; 145: 808–817. doi: 10.1016/j.jaci.2019.10.037 [DOI] [PubMed] [Google Scholar]
- 32.Kraft M, Martin RJ, Wilson S, et al. Lymphocyte and eosinophil influx into alveolar tissue in nocturnal asthma. Am J Respir Crit Care Med 1999; 159: 228–234. doi: 10.1164/ajrccm.159.1.9804033 [DOI] [PubMed] [Google Scholar]
- 33.Abdo M, Watz H, Veith V, et al. Small airway dysfunction as predictor and marker for clinical response to biological therapy in severe eosinophilic asthma: a longitudinal observational study. Respir Res 2020; 21: 278. doi: 10.1186/s12931-020-01543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polosukhin VV, Cates JM, Lawson WE, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184: 317–327. doi: 10.1164/rccm.201010-1629OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladjemi MZ, Gras D, Dupasquier S, et al. Bronchial epithelial IgA secretion is impaired in asthma. Role of IL-4/IL-13. Am J Respir Crit Care Med 2018; 197: 1396–1409. doi: 10.1164/rccm.201703-0561OC [DOI] [PubMed] [Google Scholar]
- 36.Balzar S, Strand M, Nakano T, et al. Subtle immunodeficiency in severe asthma: IgA and IgG2 correlate with lung function and symptoms. Int Arch Allergy Immunol 2006; 140: 96–102. doi: 10.1159/000092252 [DOI] [PubMed] [Google Scholar]
- 37.Allie SR, Bradley JE, Mudunuru U, et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 2019; 20: 97–108. doi: 10.1038/s41590-018-0260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathew NR, Jayanthan JK, Smirnov IV, et al. Single-cell BCR and transcriptome analysis after influenza infection reveals spatiotemporal dynamics of antigen-specific B cells. Cell Rep 2021; 35: 109286. doi: 10.1016/j.celrep.2021.109286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng W, Zhang B, Schwartz GW, et al. An atlas of B-cell clonal distribution in the human body. Nat Biotechnol 2017; 35: 879–884. doi: 10.1038/nbt.3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandsma CA, Kerstjens HA, van Geffen WH, et al. Differential switching to IgG and IgA in active smoking COPD patients and healthy controls. Eur Respir J 2012; 40: 313–321. doi: 10.1183/09031936.00011211 [DOI] [PubMed] [Google Scholar]
- 41.Looman KIM, van Meel ER, Grosserichter-Wagener C, et al. Associations of Th2, Th17, Treg cells, and IgA+ memory B cells with atopic disease in children: the Generation R Study. Allergy 2020; 75: 178–187. doi: 10.1111/all.14010 [DOI] [PubMed] [Google Scholar]
- 42.Bigler J, Boedigheimer M, Schofield JPR, et al. A severe asthma disease signature from gene expression profiling of peripheral blood from U-BIOPRED cohorts. Am J Respir Crit Care Med 2017; 195: 1311–1320. doi: 10.1164/rccm.201604-0866OC [DOI] [PubMed] [Google Scholar]
- 43.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am J Transplant 2016; 16: 3443–3457. doi: 10.1111/ajt.13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleishaker DL, Mukherjee A, Whaley FS, et al. Safety and pharmacodynamic dose response of short-term prednisone in healthy adult subjects: a dose ranging, randomized, placebo-controlled, crossover study. BMC Musculoskelet Disord 2016; 17: 293. doi: 10.1186/s12891-016-1135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary materials and methods. ERJ-02130-2021.Supplement (106.4KB, pdf)
Supplementary figure S1. Flow cytometry gating strategy of peripheral blood B cell subpopulations. After dead cell exclusion, total B cells were gated as CD19+. CD27− B cells were further subdivided into transitional 1 (T1) B cells, transitional 2 (T2) B cells and naïve B cells via CD24 and CD38. CD19+ memory B cell subpopulations were gated as IgM+, IgG+ and IgA+ cells, and subdivided into CD27+IgM+ cells, as well as CD27+ and CD27− IgG+ and IgA+ cells, respectively. ERJ-02130-2021.Figure_S1 (47.6KB, jpg)
Supplementary figure S2. Associations between B cell subsets and clinical parameters. Association with asthma severity (a), and OCS intake (b), FEV1 and FEV1/FVC, FEF25–75 (c) and age (d) are shown. Overall adjusted p-values after multiple test corrections and p-values from categorical group comparisons are shown as well as R and adjusted p-values from Spearman correlations. H: healthy; mild-mod A: mild-moderate asthma; sev A: severe asthma; OCS: oral corticosteroids; w/o OCS: without OCS; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of FVC; AX: reactance area [kPa·L−1·s−1]; R5−R20: resistance at 5 Hz−resistance at 20 Hz; ns: not significant. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. ERJ-02130-2021.Figure_S2 (199.4KB, jpg)
Supplementary figure S3. OCS independent association between R5−R20 and CD27+IgA+ B cells. CD27+IgA+ B cell frequencies were associated with R5−R20 regardless of OCS treatment (adjusted p-value <0.002). The linear associations for all patients is given as a solid line and the dotted lines represent the linear associations for OCS (---) and non-OCS (-·-) groups. The difference in slope between the two groups is not significant (p=0.148). Age is illustrated by colour. Triangle represents OCS intake, circles no OCS intake. OCS: oral corticosteroids. ERJ-02130-2021.Figure_S3 (50.6KB, jpg)
Supplementary figure S4. CD27−IgA+ memory B cells and small airway dysfunction. CD27−IgA+ B cells in patients with and without SAD (a), in patients with mild-moderate or severe asthma (b). SAD: small airway dysfunction; sev A: severe asthma; mild-mod A: mild-moderate asthma. ns: not significant. *: p<0.05. ERJ-02130-2021.Figure_S4 (31.7KB, jpg)
Supplementary figure S5. Correlations of Asthma Control Questionnaire 7 and Asthma Quality of Life Questionnaire with clinical and B cell parameters. Dark red defines the highest positive correlation between the parameters and dark blue shows the lowest negative correlation between the variables. Adjusted p-values are depicted at the right bar side. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of FVC; OCS: oral corticosteroids; FeNO: fractional exhaled nitric oxide; BMI: body mass index; ICS: inhaled corticosteroids; AX: reactance area (kPa·L−1·s−1); R5−R20: resistance at 5 Hz−resistance at 20 Hz (kPa·L−1·s−1); ns: not significant. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. ERJ-02130-2021.Figure_S5 (194.9KB, jpg)
Supplementary figure S6.IgA+ memory B cells of asthmatic patients with or without SAD in combination with frequent respiratory infections. SAD: small airways dysfunction; fr: frequent; RTIs: respiratory tract infections. *: p<0.05; **: p<0.01. ERJ-02130-2021.Figure_S6 (23.7KB, jpg)
Supplementary table S3. Pairwise comparisons between B cell populations and clinical variables in asthma patients and healthy controls. ERJ-02130-2021.Table_S3 (294.7KB, pdf)
Supplementary table S4. Linear Model. ERJ-02130-2021.Table_S4 (611.8KB, pdf)
Supplementary table S5. Clinical characteristics of patients with versus without SAD. ERJ-02130-2021.Table_S5 (90.5KB, pdf)
Supplementary table S6. Regression model for SAD defined by R5−R20. ERJ-02130-2021.Table_S6 (38.8KB, pdf)
Supplementary table S7. Correlations between exacerbation frequency and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S7 (77.4KB, pdf)
Supplementary table S8. Correlations between Asthma Control Questionnaire (ACQ-7) and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S8 (78KB, pdf)
Supplementary table S9. Correlations between Asthma Quality Of Life Questionnaire (AQLQ) and clinical parameters and IgA+ memory B cells. ERJ-02130-2021.Table_S9 (78KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02130-2021.Shareable (487.2KB, pdf)