Abstract
Anthelmintic resistance is reported in equine nematodes with increasing frequency in recent years, and no new anthelmintic classes have been introduced during the past 40 years. This manuscript reviews published literature describing anthelmintic resistance in cyathostomins, Parascaris spp., and Oxyuris equi with special emphasis on larvicidal efficacy against encysted cyathostomin larvae and strongylid egg reappearance periods (ERP). Resistance to benzimidazoles and pyrimidines is highly prevalent in cyathostomin populations around the world, and macrocyclic lactone resistance has been documented in cyathostomins in recent years as well. Two recent studies have documented resistance to the larvicidal regimen of fenbendazole, whereas the larvicidal efficacy of moxidectin is variable, but with no evidence of a reduction from historic levels. In the 1990s, ERP estimates were 8–10 and 12–16 weeks for ivermectin and moxidectin, respectively, while several studies published after year 2000 found ERPs to be 5 weeks for both compounds. This is a clear change in anthelmintic performance, but it remains unclear if this is due to development of anthelmintic resistance or selection for other biological traits leading to a quicker resumption of strongylid egg shedding following anthelmintic treatment. Macrocyclic lactone resistance is common in Parascaris spp. around the world, but recent reports suggests that resistance to the two other classes should be monitored as well. Finally, O. equi has been reported resistant to ivermectin and moxidectin in countries representing four continents. In conclusion, multi-drug resistance is becoming the norm in managed cyathostomin populations around the world, and a similar pattern may be emerging in Parascaris spp. More work is required to understand the mechanisms behind the shortened ERPs, and researchers and veterinarians around the world are encouraged to routinely monitor anthelmintic efficacy against equine nematodes.
Keywords: Strongylid, Ascarid, Cyathostomin, Parascaris, Oxyuris, Egg reappearance period, Larvicidal
Graphical abstract
Highlights
-
•
Cyathostomins are widely resistant to pyrimidines and benzimidazoles.
-
•
Cyathostomins have been reported resistant to macrocyclic lactones in recent years.
-
•
The interpretation of shortened egg reappearance periods is not clear.
-
•
Parascaris spp. are widely resistant to macrocyclic lactones.
-
•
Oxyuris equi has been documented resistant to macrocyclic lactones.
1. Introduction
Horses around the world are constantly exposed to infection with multiple nematode species. Since the introduction of modern anthelmintic classes in the 1960s, parasite control efforts have been based on routine and frequent administration of anthelmintics to entire populations of horses in a primarily prophylactic manner (Drudge and Lyons, 1966). This approach, later termed the interval-dose program, has been widely adopted in equine establishments during the past several decades (O'Meara and Mulcahy, 2002; Robert et al., 2015; Becher et al., 2018). However, a heavy reliance on anthelmintic products comes with a risk of development of drug resistance to the compounds used, and this was evident already in the 1960s, where benzimidazole resistance was reported just a few years after the introduction of this class (Drudge et al., 1965).
In most parts of the world, only three anthelmintic drug classes are available for treatment of nematode parasites in horses. These include the benzimidazoles, the pyrimidines, and the macrocyclic lactones. The newest anthelmintic class to be introduced for equine usage was the macrocyclic lactones with ivermectin being launched in the early 1980s (Egerton et al., 1981). Thus, no new anthelmintic classes with new modes of action have been introduced for equine usage during the past 40 years, and no such new products are expected in a foreseeable future. This emphasizes the need to monitor efficacy of existing anthelmintic classes in nematodes of veterinary importance and work towards identifying the most sustainable parasite control strategies.
Currently available data suggest that once developed within a parasite population, anthelmintic resistance persists for many years and multiple parasite generations. In one study, benzimidazole resistance was documented in a cyathostomin population, which was then left untreated for a period of 22 years. When benzimidazole efficacy was subsequently evaluated in this population, resistance was still evident to this drug class (Lyons et al., 2007). In another study conducted by the same group, ponies harboring a population of benzimidazole-resistant cyathostomins were treated bimonthly with pyrantel pamoate for a period of eight years. Efficacy evaluations conducted after the eight-year period demonstrated that the benzimidazole resistance status did not change, but that resistance had now developed to pyrantel as well (Lyons et al., 2001). Thus, these data suggest that acquiring benzimidazole resistance is not associated with any apparent fitness deficits in cyathostomin populations. However, no information is currently available regarding these aspects for resistance to other drug classes and in other parasite categories. Nonetheless, once anthelmintic resistance has established in cyathostomins to a given anthelmintic class, there is no reason to expect a reversion to susceptibility.
Diagnosing anthelmintic resistance in equine nematodes has long been challenging due to a lack of guidelines. A World Association for the Advancement of Veterinary Parasitology (WAAVP) sanctioned guideline paper was published in 1992 (Coles et al., 1992), but it was primarily focused on diagnosing anthelmintic resistance in sheep nematodes and included very limited guidance for equine parasites. In recent years, a WAAVP subcommittee has been working on a new set of guidelines for FECRT studies in large animals, and publication of these can be expected in a relatively near future. Given that these guidelines are undergoing revision at the time of writing this review, it is not appropriate to describe them in detail herein. However, a few principles are worth highlighting. The new guidelines will include treatment efficacy thresholds based on historic data from when each anthelmintic compound was first introduced. These thresholds will be specified for each anthelmintic class and each parasite category. Furthermore, the guidelines will introduce a modern approach for statistical analysis of FECRT data and will provide suggested group sizes required to reach sufficient statistical power. One factor affecting statistical power, and, hence, group size considerations is the number of parasite eggs counted in the group pre-treatment (Dobson et al., 2012). The statistical unit of interest is not the estimated fecal egg count expressed in eggs per gram of feces (EPG), but rather the number of eggs counted under the microscope prior to applying the multiplication factor. An egg counting technique with a low multiplication factor will count more eggs under the microscope than one with a higher multiplication factor, as long as both techniques perform with similar accuracy. The value of this ‘eggs counted’ principle has been demonstrated by Levecke et al. (2018), and a recent equine FECRT study adopted these new concepts (Nielsen et al., 2022a). Furthermore, in equine parasite control it is important to clearly delineate between reduced efficacy determined at two weeks post treatment and strongylid egg reappearance, which can only be demonstrated in subsequent weeks and only if full efficacy is first demonstrated at two weeks post treatment. Thus, it is important to emphasize that anthelmintic resistance can only be determined when efficacy is evaluated at two weeks post treatment. In cases, where strongylid egg counts are evaluated beyond the initial two-week interval, the study becomes an evaluation of egg reappearance, which has different implications, as will be discussed in this review. Once the new WAAVP guidelines get published, a more uniform use of study designs can be expected in equine anthelmintic efficacy studies.
The status of anthelmintic resistance in equine nematodes has been reviewed multiple times over the past couple of decades (Kaplan, 2002, 2004; von Samson-Himmelstjerna, 2012; Matthews, 2014; Peregrine et al., 2014; Silva et al., 2019). However, several new developments reported in the past decade prompts a new review. These include several reports of anthelmintic resistance in Oxyuris equi, cyathostomin resistance to the larvicidal regimen of fenbendazole, documentation of resistance to the macrocyclic lactone class in cyathostomins, and a substantial body of literature documenting shortened strongylid egg reappearance periods (ERPs) following administration of ivermectin and moxidectin.
The aims with this literature study were to 1) review published reports of anthelmintic resistance in cyathostomins since the year 2000, 2) critically analyze all published data describing strongylid ERPs following ivermectin and moxidectin administration, 3) summarize recent work evaluating larvicidal efficacy in cyathostomin populations, 4) review all studies reporting anthelmintic resistance in Parascaris spp., and 5) review studies reporting anthelmintic resistance in O. equi.
2. Cyathostomins
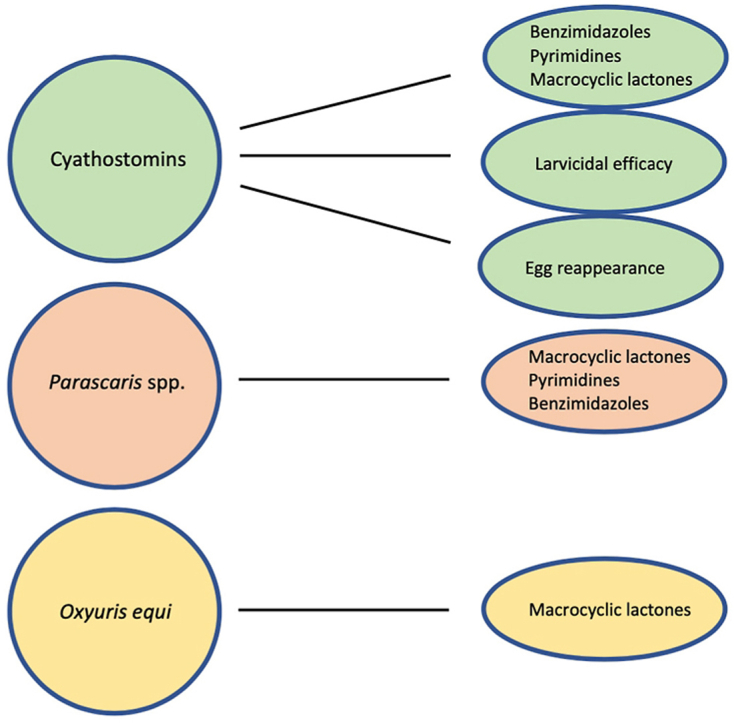
Given the numerous previous reviews of the status of anthelmintic resistance in equine strongylids, this review is focused on reports published since year 2000. A total of 71 equine strongylid anthelmintic resistance studies had been published in this millennium, and these are presented in Table 1. Of these, 30 studies were published during 2000–2009, while 29 were published during 2010–2019, and 12 were published between 2020 and 2022 at the time of writing this manuscript. The studies were conducted in 31 countries and six continents. Benzimidazole efficacy was evaluated in in 58 studies, and resistance was reported in all of these. In comparison, pyrimidine was evaluated in 37 studies, with resistance documented in 34 of these (92%). Finally, macrocyclic lactones were evaluated in 57 studies and evidence of resistance to this class was reported in 13 of these (23%).
Table 1.
Studies reporting anthelmintic resistance in equine strongylids since 2000. For each study, anthelmintic classes evaluated are indicated with an X. Studies reporting anthelmintic resistance to a given class are marked with AR. Thus, X means that resistance was tested, but not found, and X-AR means that resistance was tested and found.
Based on the above, benzimidazole resistance is widespread in equine strongylids around the world, as it was documented in every study evaluating this class published since 2000. Remarkably, resistance to this drug class has been documented in strongylids harbored by working equids with histories of limited anthelmintic use (Kumar et al., 2016; Seyoum et al., 2017; Salas-Romero et al., 2018) as well as in feral equids (Kuzmina et al., 2020), indicating that benzimidazole resistant strongylids should be expected in most locations regardless of anthelmintic treatment intensity. Pyrantel resistance was first documented in equine cyathostomins in 1996 (Chapman et al., 1996), and Table 1 documents that resistance to the pyrimidine class has become common over the past couple of decades with over 90% of all studies documenting resistance. In addition to the data presented in Table 1, two US studies evaluating observed efficacy of anthelmintics administered by horse owners further suggested a very high occurrence of pyrantel resistant strongylids (Nielsen et al., 2018a; Cain et al., 2019). Because of this development, it is not uncommon to find cyathostomin populations that are resistant to both the benzimidazole and the pyrimidine classes, which was documented in 19 of 22 studies evaluating both classes (Table 1). In contrast, resistance to macrocyclic lactones has been slow to develop in cyathostomins. This is remarkable, given that this drug class is the most widely used in equine operations (Stratford et al., 2014b; Nielsen et al., 2018b), and that macrocyclic lactone resistance is very common in ruminant trichostrongylids (Kaplan, 2004; Sutherland and Leathwick, 2011).
A study published by Molento and colleagues has long been regarded the first report of macrocyclic lactone resistance in equine strongylids (Molento et al., 2008). However, this paper did not describe the timing of the post treatment samples relative to the day of treatment, and the first author has subsequently relayed that the data reported were based on egg counts determined from samples collected four weeks post treatment (M.B. Molento, personal communication). Thus, this study did not document anthelmintic resistance, but rather four-week ERPs following administration of ivermectin, moxidectin, and abamectin (Molento et al., 2008). As will be discussed herein, the interpretation of shortened ERPs is not clear, and they cannot be solely regarded as evidence of emerging anthelmintic resistance. However, several reports of apparent macrocyclic lactone resistance in equine strongylids were published in subsequent years. The first reports were all based on ivermectin fecal egg count reductions (FECRs) in single groups of relatively small size with no follow-up testing done to confirm the suspicion of resistance (Traversa et al., 2009; Milillo et al., 2009; Näreaho et al., 2011; Canever et al., 2013; Relf et al., 2014), and other causes of the reduced efficacy could, therefore, not be ruled out. But stronger evidence of macrocyclic lactone resistant cyathostomins has been published in the past decade. It is remarkable that Table 1 contains six Brazilian studies reporting macrocyclic lactone resistance, which is almost half of all studies reporting resistance to this anthelmintic class. Several of these studies documented clearly reduced macrocyclic lactone efficacy in several groups and to several members of this class (Toscan et al., 2012; Flores et al., 2020; Martins et al., 2021; Lignon et al., 2021), and one of them was a terminal study with intestinal worm counts confirming resistance to ivermectin in two cohorts (Felippelli et al., 2015), providing strong evidence for these findings. In 2020, we documented the first case of macrocyclic lactone resistance in equine strongylids in the US (Nielsen et al., 2020). In this study, it was remarkable that of the over 110 yearlings in the study, only the 59 imported from Ireland as weanlings displayed reduced ivermectin efficacy, while the US-born counterparts treated with the same products at the same time points displayed full efficacy on the FECRTs. This clearly suggested that the resistant cyathostomins were imported from Ireland and introduced to the US operation. The study consistently documented reduced ivermectin efficacy in three different groups over several treatments, and also documented resistance to moxidectin among the imported yearlings (Nielsen et al., 2020). A subsequent study documented ivermectin resistance in a group of US born yearlings raised on a different farm in the same area (Nielsen et al., 2022a), demonstrating that macrocyclic lactone resistance is established in the domestic US equine population as well. Furthermore, moxidectin resistance was recently demonstrated in groups of weanlings and yearlings in Australia (Abbas et al., 2021). Taken together, these recent data suggest that a long-awaited breakthrough of macrocyclic lactone resistance in equine strongylids has finally happened, and that many more reports can be expected in coming years.
2.1. Larvicidal efficacy
The concept of larvicidal efficacy deserves particular attention in the context of anthelmintic resistance. For cyathostomins, this term is used for anthelmintics with efficacy against encysted larval stages. The parasitic phase of the cyathostomin life cycle contains three distinct larval stages; the early third stage (EL3), the late third stage (LL3), and the mucosal fourth stage (ML4). Collectively, the LL3 and ML4 stages are referred to as developing larvae, whereas the EL3 can undergo arrested development for up to several years and are sometimes referred to as hypobiotic larvae (Gibson, 1953). Traditionally, larvicidal efficacy is determined separately for EL3s and LL3/ML4s (developing larvae), as different efficacy levels can be observed between these. Two anthelmintic compounds are currently registered with efficacy against encysted cyathostomin larvae: moxidectin (single dose) and a five-day regimen of fenbendazole. The fenbendazole regimen was originally reported to have high efficacy (>90%) against all larval stages (DiPietro et al., 1997a; Duncan et al., 1998), whereas moxidectin displayed lower and more variable efficacy levels with the percent reduction of EL3s ranging between 0 and 37% and the efficacy against LL3/ML4s in the 50–89% range (Xiao et al., 1994; Monahan et al., 1995, 1996). It should be mentioned that due to these data, moxidectin products do not have a label claim for efficacy against EL3s in North America, whereas the fenbendazole regimen has label claims for all encysted larval stages. However, given the widespread occurrence of benzimidazole resistance documented at the adult cyathostomin stage through FECRTs and the multiple reports of shortened strongylid ERPs following moxidectin treatment summarized in the next section, it is legitimate to question whether the larvicidal efficacies of these two compounds are still intact today.
In recent years, we have conducted two studies investigating the larvicidal efficacy of the five-day fenbendazole regimen and three studies examining the same property of moxidectin. These studies were all terminal controlled efficacy studies based on mucosal digestion and enumeration of the aforementioned encysted larval stages. In all these studies, the horses were kept on pasture for the duration of the trial to mimic a realistic field scenario, where horses are subjected to continued exposure to infective larvae following anthelmintic treatment. The two studies evaluating fenbendazole were conducted at two different sites with different resident parasite populations, but results were remarkably similar. In both cases, FECRTs indicated a lack of fenbendazole efficacy, which was confirmed by adult worm count data clearly documenting anthelmintic resistance at the adult stages (Reinemeyer et al., 2015; Bellaw et al., 2018). The larvicidal efficacy against EL3s and LL3/ML4s were around 30–40% and 70%, respectively (Reinemeyer et al., 2015; Bellaw et al., 2018), which was a substantial reduction from historic levels, and, hence, confirmed resistance of all parasitic larval stages to fenbendazole.
In all three studies evaluating moxidectin, FECRTs indicated 100% efficacy and adult worm count data documented efficacy levels above 99.9% (Reinemeyer et al., 2015; Bellaw et al., 2018). In two of the studies, ERP was found to be five weeks following moxidectin administration (Bellaw et al., 2018; Nielsen et al., in press). The first two studies evaluating moxidectin efficacy found larvicidal efficacy estimates at or above the higher end of the historic ranges with the efficacy against EL3s in the 60–70% range and the reduction of LL3/ML4s at 75 and 85% (Reinemeyer et al., 2015; Bellaw et al., 2018). However, the most recent moxidectin study returned substantially lower efficacy estimates with 18% against EL3s and 60% against LL3/ML4s (Nielsen et al., In press). Given the largely variable historic larvicidal efficacy estimates for moxidectin, it is not possible to determine if these three recent studies suggest a change from historic levels. It can be argued that keeping horses on pasture for the duration of these studies might have falsely lowered the efficacy against EL3s due to newly ingested L3s establishing during the post-treatment intervals. However, this is not supported by available evidence, as in three historic studies, where horses were confined post treatment, EL3 efficacy estimates ranged from 0% to 37% (Xiao et al., 1994; Monahan et al., 1995, 1996), which is either lower or similar to our three recent studies, where horses were kept on pasture. Furthermore, it can also be argued that confinement of horses during the interval between anthelmintic treatment and necropsy could falsely increase efficacy estimates against encysted stages, as removal of luminal burdens may trigger hypobiotic EL3s to resume development and develop into LL3s and ML4s. While this does not appear to have been the case in the historic studies cited herein (Xiao et al., 1994; Monahan et al., 1995, 1996), a study design involving stall or gravel/dirt paddock confinement for eight weeks post treatment returned larvicidal efficacy estimates >90% for moxidectin against EL3s (Bairden et al., 2001, 2006), and it seems plausible that a proportion of these larvae could have progressed to later stages during the eight weeks of confinement.
In our two most recent studies, the efficacy levels were evaluated at two different time intervals; the standard two weeks post treatment, and again at five weeks post treatment. In both of these studies, the larvicidal efficacy estimates for moxidectin declined between the two time points (Bellaw et al., 2018; Nielsen et al., in press), but the trend was most pronounced in the most recent study, where encysted larval counts were similar to those of the untreated control group at five weeks post treatment (Nielsen et al., in press). Taken together, these studies demonstrate that the larvicidal efficacy of moxidectin is partial and that the effect may be short-lived in horses kept on pasture. Although a reduction of encysted larval stages was observed for both compounds in each study, the differences between the treated groups and untreated control groups were not statistically significant, and the clinical benefit from larvicidal therapy with either compound is highly questionable. However, more studies are needed from different parts of the world and under different climatic and seasonal conditions to further investigate the value of larvicidal efficacy against cyathostomins.
2.2. Strongylid egg reappearance periods
Strongylid ERPs were initially introduced to identify appropriate treatment intervals for various anthelmintic products (Borgsteede et al., 1993; Jacobs et al., 1995; Boersema et al., 1996). This gained particular attention when moxidectin was first introduced in the 1990s, and several studies demonstrated substantially longer ERPs for this new compound in comparison with ivermectin (Demeulenaere et al., 1997; Mercier et al., 2001). Several explanations were proposed for these observations: a) The labelled dose of moxidectin is twice that of ivermectin (400 vs. 200 μg/kg) and this was suggested to partly explain the different ERPs (Taylor and Kenny, 1995; Demeulenaere et al., 1997), b) Moxidectin has activity against encysted cyathostomin larvae, which ivermectin does not, and this has been proposed to lead to longer ERPs (Jacobs et al., 1995; Demeulenaere et al., 1997), and c) Moxidectin's pharmacokinetic profiles were substantially different from ivermectin's with much longer plasma half-lives, which led to speculations of possible persistent efficacy, which, again, could explain the longer ERPs (Pérez et al., 1999; Mercier et al., 2001; Gokbulut et al., 2001).
In the late 1990s, Sangster (1999) proposed a different use of ERP monitoring. He postulated that ivermectin and moxidectin resistance in cyathostomins would be preceded by a shortening of ERPs. His rationale was that species with shorter prepatent periods would be under stronger selection pressure for developing resistance and that cyathostomin species composition would shift towards these species as resistance developed. This, in turn, would manifest as a reduced ERP. These predictions led scientists to start tracking strongylid ERPs across the world. However, ERP was never clearly defined, and a consensus of methodology was never reached. This led to a multitude of different definitions and methods used over the years, which has complicated comparisons across time and between regions. Most ERP studies have been based on determining weekly or biweekly fecal egg counts in cohorts of horses for variable periods of time following anthelmintic treatment, but the criteria for defining the week of egg reappearance have been vastly different. Some have simply defined the week of the first positive egg count as the week of egg reappearance (Lyons et al., 2008a, 2011a), while others declared egg reappearance when 50% of horses in the group exceeded a predetermined egg count threshold (Jacobs et al., 1995; Demeulenaere et al., 1997). Some tracked the group mean egg count relative to a predetermined threshold (Boersema et al., 1996), while others made use of a FECR calculation and defined an efficacy threshold for the group FECR estimate to fall below to define the week of ERP (von Samson-Himmelstjerna et al., 2007). To address this lack of consensus, we recently defined equine strongylid ERP in a guideline paper sanctioned by the WAAVP (Nielsen et al., 2022b). Here, the FECR calculation is used, and the week of ERP is when the upper 95% confidence (or credible) limit falls below a threshold defined as the FECR determined at two weeks post treatment minus 10% (Nielsen et al., 2022b). Using this definition, ERP can only be determined in cases where no evidence of reduced anthelmintic efficacy is found at two weeks post treatment. We strongly encourage everyone to use this definition in ERP studies going forward to allow for an easier comparison between studies and across regions and time.
To objectively assess the development of ivermectin and moxidectin ERPs across time, all publications reporting strongylid fecal egg counts during the weeks and months following treatment with either of these active ingredients were reviewed. The WAAVP definition described above for determining ERP was applied, where possible. However, given that none of the publications provided access to the original raw data and very few of the studies included a calculation of confidence or credible intervals for the mean FECR estimates, a slight modification to the WAAVP principle was necessary. Instead of using the upper confidence (credible) limit, ERP determination was based on the mean FECR falling below the threshold, which was determined as the FECR calculated at two weeks post treatment minus 10%. In the large majority of cases, the FECR at two weeks post treatment was 100%, which meant that the ERP threshold was 90%. The data were acquired using three possible methods; 1) FECRs already calculated by the study authors, 2) FECRs calculated from fecal egg count data presented in data tables, or 3) FECRs calculated from fecal egg count data estimated from graphs included in the publications. A few ERP publications did not include a presentation of fecal egg count or FECR data in any form (Jacobs et al., 1995; Little et al., 2003; Daniels and Proudman, 2016b; Zak et al., 2017), and were, thus, not included in this exercise. In total, 36 studies provided data describing ivermectin ERP (Table 2) and 23 studies documented moxidectin ERP (Table 3). Table 2 summarizes strongylid ERP reports for ivermectin from 1989 to 2022 and includes data from 14 countries and four continents. While estimates ranged between 8 and 10 weeks during the 1990s, they became more variable during the 2000s and 2010s with several studies reporting four and five weeks. Similarly, Table 3 presents ERP data for moxidectin spanning from 1995 to 2022, and studies were conducted in ten countries and four continents. The moxidectin data demonstrate that estimates have declined from an initial 12–16 weeks to five weeks in three of the most recently published studies. Taken together, these tables provide clear evidence that ERPs have reduced dramatically for both actives over the past decades. It is also remarkable that while moxidectin originally had substantially longer ERPs in comparison with ivermectin, the two compounds now appear to be performing with very similar ERPs.
Table 2.
Estimates of strongylid Egg Reappearance Periods (ERP) following ivermectin treatment in horses over the course of four decades. The table includes all publications that either calculated the percent Fecal Egg Count Reduction (FECR) during weeks post ivermectin treatment or presented Fecal Egg Count data allowing this calculation to me made. The definition of ERP was the week post treatment, where the mean FECR fell below 90%.
FECR calculated from data presented.
FECR calculated by study authors
Table 3.
Estimates of strongylid Egg Reappearance Periods (ERP) following moxidectin treatment in horses over the course of four decades. The table includes all publications that either calculated the percent Fecal Egg Count Reduction (FECR) during weeks post ivermectin treatment or presented Fecal Egg Count data allowing this calculation to me made. The definition of ERP was the week post treatment, where the mean FECR fell below 90%.
| Study | Country | Estimate (weeks) |
|---|---|---|
| Taylor and Kenny (1995)a | UK | 14 |
| Boersema et al. (1998)a | Netherlands | 15 |
| Demeulenaere et al. (1997)a | Belgium | >12 |
| DiPietro et al., 1997ba | USA | >12 |
| Rolfe et al. (1998)b | Australia | 13 |
| Mercier et al. (2001)b | Australia and Brazil | 7 |
| Martin-Downum et al. (2001)a | USA | >16 |
| Cirak et al. (2005)a | Turkey | >25 |
| Molento et al. (2008) | Brazil | 4 |
| Rossano et al. (2010)b | USA | 5 |
| Lyons et al. (2011a)a | USA | 5, 6, and 8 |
| Lyons et al. (2011b)a | USA | 5 and 6 |
| Francisco et al. (2012)a | Spain | 5 and 8 |
| Relf et al. (2014)b | UK | 8 and 9 |
| Geurden et al. (2014)b | Belgium | 6, 10, 12, and >12 |
| Geurden et al. (2014)b | Italy | 6, 12, and >12 |
| Geurden et al. (2014)b | Netherlands | 6, 8, and >12 |
| Mason et al. (2014)b | USA | 6 |
| Sanna et al. (2016)b | Italy | >21 |
| Tzelos et al. (2017)b | UK | 6 and 10 |
| Shea Porr et al. (2017)a | USA | 10 |
| Bellaw et al. (2018)a | USA | 5 |
| Abbas et al. (2021)b | Australia | 5 |
| Nielsen et al. (2022c)b | USA | 5 |
FECR calculated from data presented.
FECR calculated by study authors
Based on the data presented above, Sangster's suggestion of monitoring strongylid ERP is strongly supported. However, the interpretation of shortened ERPs remains obscure. In 2009, a study associated a shortened ERP following ivermectin administration with apparent resistance at the luminal L4 stage (Lyons et al., 2009), and a subsequent study provided further evidence supporting this claim (Lyons and Tolliver, 2013). While it was conceivable that luminal L4s surviving anthelmintic treatment could quickly complete the last molt to become egg producing adults, which could explain a shortened ERP, studies evaluating cyathostomin populations exhibiting shortened ERPs following moxidectin treatment provided a less clear picture. The two ivermectin studies referenced above found the efficacy against luminal L4s to be 55.3% and 9.5%, respectively (Lyons et al., 2009; Lyons and Tolliver, 2013). However, two studies investigating moxidectin efficacy in cyathostomin populations with ERPs of five weeks, found efficacy estimates against luminal L4s to be substantially higher; 93.8% and 98.3% (Lyons et al., 2010; Bellaw et al., 2018). Thus, the hypothesis that shortened ERPs were due to anthelmintic resistance at the luminal L4 stage was not strongly supported by the moxidectin data. These observations leave several questions. Why are moxidectin and ivermectin ERPs so similar, when the efficacy estimates against luminal L4s are so different? Why do the larvicidal properties and the substantially different pharmacokinetic profiles of moxidectin not appear to influence ERP? The fundamental question is still whether shortened ERP can be considered a sign of emerging anthelmintic resistance or whether it reflects selection for other biological traits.
We recently conducted a study to compare ivermectin and moxidectin efficacy over a five-week interval and evaluate the efficacy against adults, luminal L4s, and encysted larval stages (Nielsen et al., 2022c). Egg reappearance was documented at five weeks post treatment in both treated groups. While the adulticidal efficacy was >99.5% in both groups at two weeks post treatment, the efficacies against the luminal L4s were 69.7% and 84.3% for ivermectin and moxidectin, respectively. Thus, at a first glance, this appeared to support the hypothesis that shortened ERPs can be explained by resistance at the luminal L4 stage. However, luminal L4s accounted for less than 3% of total luminal worm burdens in the untreated control group and mean luminal L4 counts in the two treated groups at two weeks post treatment were in the range of a few hundred, which is very low. In comparison, at five weeks post treatment coinciding with ERP, adult worm counts were in the range of several thousand with the luminal L4 counts remaining in the hundreds. Thus, the number of luminal L4s at two weeks post treatment did not correspond with the number of adult worms recovered in the treated groups three weeks later, and the hypothesis that shortened ERPs is due to luminal L4s surviving treatment and completing the life cycle sooner after treatment could, therefore, not be supported. In fact, it is not given that the luminal L4s encountered post ivermectin and moxidectin treatment were even present in the intestinal lumen at the day of treatment, as it is plausible that they could have emerged from the mucosal walls as a response to removal of luminal burdens. If the latter is the case, recovering luminal L4s post treatment would not be indicative of anthelmintic resistance. A larvicidal efficacy within historic ranges was demonstrated for moxidectin (Nielsen et al., 2022c), but as mentioned in the previous section, only a proportion of larvae were eliminated, and encysted larval counts were similar to those of the two other groups at five weeks post treatment. Thus, the larvicidal efficacy of moxidectin did not appear to be high enough to substantially suppress the dynamics of recruitment of new adults from the pool of encysted ML4s. But given that larvicidal efficacy did not differ from historic levels suggests that the reduced ERP could not be linked to signs of emerging anthelmintic resistance. The question remains whether ERP shortening could be a result of selection for parasite species or strains with a quicker emergence of L4s from the mucosal walls. The same study evaluated cyathostomin species composition at the time of egg reappearance (five weeks) and found that a selection of the globally most abundant species contributed to the egg production with no apparent major shifts from the untreated control group (Nielsen et al., 2022c). However, it was noteworthy that one species, Cylicocyclus nassatus, represented over half the worms recovered in the moxidectin treated group, whereas it only accounted for about 25% of the adult worm burden in the ivermectin treated group, which could suggest some differential species-specific activity between the two compounds. However, the most abundant species encountered in this study were all with the most abundant species reported worldwide over a 40-year period (Bellaw and Nielsen, 2020), so there is no clear evidence suggesting that shortened ERPs could be due to selection for species with shorter prepatent periods as suggested by Sangster (1999). However, it remains possible that a selection could be happening on an isolate or strain basis rather than on a species level. However, cyathostomin isolates or strains have not been genetically characterized on a population level, and it remains unclear how a selection might occur within and between species. Clearly, more work is needed to characterize cyathostomin populations genetically in the context of shortened ERPs.
In the context of the above discussion, it should be kept in mind that for cyathostomins, prepatent periods are not defined and fixed attributes, due to the arrested development at the EL3 stage, which can last a couple of years or more (Gibson, 1953). In addition to this, the available evidence suggests that cyathostomin prepatent periods get longer as horses age. In a series of experimental inoculations of a group of ponies, Smith (1976a) first kept the ponies in a low transmission environment for three years and administered several thiabendazole treatments to eliminate naturally acquired cyathostomin infection. When the ponies were 4–5 years old, he experimentally inoculated them with a mixed-species cyathostomin isolate and documented that strongylid egg shedding began 12–15 weeks later (Smith, 1976b). He then repeated the exercise of keeping the ponies in a low transmission environment for another three years and administered several thiabendazole treatments over the course of this period to clear out the infection (Smith, 1978). Following this, he, again, experimentally inoculated the ponies, now 9–10 years old and this time documented egg shedding 17–18 weeks post inoculation (Smith, 1978). It should be acknowledged that the cyathostomin species composition of the inocula was unknown in these experiments, and the two inocula may not have been identical. However, taken together, these data do suggest that prepatent periods may lengthen with age and parasite exposure. Furthermore, experimental data have demonstrated that preconditioning infective larvae to autumn temperatures between 0 and 10 °C leads to a significantly higher proportion of EL3s compared to ponies inoculated with larvae preconditioned at higher temperatures (Scháňková et al., 2014). This suggests that infective larvae exposed to the cooler temperatures are more prone to undergoing arrested development at the EL3 stage, which would lead to longer prepatent periods. Thus, prepatent periods appear to be affected by both environmental and host-dependent factors, and the suggestion that prepatent period is a primary factor affecting development rates of anthelmintic resistance in cyathostomins, and that anthelmintic treatment would select for species with shorter prepatent periods, is likely too simplistic. Thus, while Sangster was correct that monitoring ERPs would be valuable, his reasoning behind this suggestion has not been supported, and more work is needed to fully understand and interpret the shortened ERPs observed around the world.
In summary, ivermectin and moxidectin strongylid ERPs have shortened substantially over the past decades. Moxidectin originally displayed ERPs about 4–6 weeks longer than ivermectin, but recent estimates have been very similar for the two compounds. While our recent investigations do not support the suggestion that shortened ERPs are signs of emerging anthelmintic resistance, it is important to emphasize that this development is a substantial change of drug performance with obvious implications for strongyle control. Analogous observations have been made in studies evaluating ivermectin efficacy against Onchocerca volvulus in human populations. Here, the anthelmintic has high anti-microfilarial efficacy regardless of treatment history, but in populations subjected to mass administration of ivermectin in the past, microfilariae return much quicker following treatment (Churcher et al., 2009; Pion et al., 2013). While this is a clear change in anthelmintic performance of ivermectin, it does not appear to represent anthelmintic resistance, but rather a selection of other biological traits allowing the adult worms to more quickly resume production of microfilariae following treatment. Clearly, more work is clearly needed to fully elucidate the mechanisms behind shortened strongylid ERPs following macrocyclic lactone administration and to monitor the progression of this development in different parts of the world. With the guidance recently provided by the WAAVP (Nielsen et al., 2022b), a better consensus regarding study design and methodology should be expected in the future.
3. Parascaris spp
Compared to the situation in cyathostomins, anthelmintic resistance in equine ascarids is a fairly new development with the first peer-reviewed report occurring in 2002 (Boersema et al., 2002). However, anecdotal reports of apparent ivermectin treatment failure existed well before, but these were dismissed as likely errors such as false positive fecal samples due to coprophagy and the time required to kill adult ascarids and eliminate all eggs from the intestinal tract (Boraski, 1987). However, no data were presented to support or dismiss these claims. It should also be noted that while one early study suggested complete elimination of all larval ascarid stages (DiPietro et al., 1988) another study demonstrated 100% ivermectin efficacy against intestinal stages of the parasite, but only 76.9% efficacy against migrating stages (DiPietro et al., 1987). These data suggest a variable efficacy against migrating stages and may have been an early sign of developing resistance.
All resistance reports in equine ascarids are summarized in Table 4. The table includes 32 studies from 20 different countries and five continents. Of these, 29 evaluated macrocyclic lactone efficacy and all reported evidence of resistance to this class. Four of 16 studies (25%) reported pyrimidine resistance, and three of 13 studies (23%) reported benzimidazole resistance. Thus, it can be concluded that resistance to macrocyclic lactones is widespread around the world and is highly likely to be documented whenever a study is conducted. In contrast, resistance to the two other classes was reported in about a quarter of studies conducted. It should be noted, however, that the sporadic reports for the benzimidazole and pyrimidine classes may to some extent reflect a limited number of studies conducted evaluating these classes. Thus, it is possible that resistance to these two anthelmintic classes may be more common in equine ascarids than suggested in the scientific literature, and this is supported by recent reports of pyrantel and benzimidazole resistance in Nordic countries (Martin et al., 2018, 2021b; Hautala et al., 2019). It should also be noted that in addition to the studies included in the table, Lyons and colleagues published two studies evaluating the activity of several different anthelmintics against equine ascarids (Lyons et al., 2008b, 2011c). However, these studies did not make use of FECR measurements, but rather counted the number of foals testing positive for ascarid eggs pre and post treatment. Given this unconventional study design, these studies were not included in Table 4. However, both studies suggested reduced efficacy of pyrantel pamoate, and one of them indicated a lack of ivermectin efficacy as well (Lyons et al., 2008b). Overall, reports of multi-drug resistance in equine ascarids are rare. Of 21 studies evaluating more than one anthelmintic class, only three (14%) documented resistance to two or more classes. Researchers and clinicians around the world should be encouraged to monitor the efficacy of all three anthelmintic classes against Parascaris spp. With resistance being so widely documented to the macrocyclic lactone class, there is more value in monitoring the efficacy of the two other classes.
Table 4.
Reports of anthelmintic resistance in equine ascarids. For each study, anthelmintic classes evaluated are indicated with an X. Studies reporting anthelmintic resistance to a given class are marked with AR. Thus, X means that resistance was tested, but not found, and X-AR means that resistance was tested and found.
Some consideration should be given to the design of ascarid FECRT studies, as the biology is substantially different from the cyathostomins. Ascarid egg shedding is highly age dependent with foals and weanlings usually only shedding eggs for a short period of time before an age dependent immunity eliminates the parasites (Fabiani et al., 2016). Typically, foals shed ascarid eggs between three and six months of age, with egg counts peaking at four-five months of age (Fabiani et al., 2016; Nielsen et al., 2021). This challenges the study design of ascarid FECRT studies, because age can be a considerable confounder affecting efficacy estimates. To account for this bias, a recent study demonstrated the feasibility of using age-matched untreated control groups under field settings (Morris et al., 2019). To increase the number of foals meeting the eligibility criteria of being ascarid egg count positive, study subjects were enrolled in a rolling manner over the course of several months (Morris et al., 2019). This was meaningful since foals are typically born over the course of several months starting in late winter and extending well into the spring and sometimes early summer. Consequently, only a subset of foals will be at the optimum ascarid shedding age at any single time point, but by sampling foals repeatedly over the course of several months, more foals will reach the optimum age for ascarid shedding and can be enrolled. This lengthens the duration of the study and requires several more farm visits, but the increased number of eligible study subjects may be worth the effort. These principles will be outlined in the upcoming WAAVP guidelines for FECRT studies.
A few comments should be made about the species designation. Historically, the equine ascarid was referred to as P. equorum, which was the case in the majority of studies summarized herein. However, for a period of several decades, the veterinary parasitology community appears to have been unaware of the existence of the other equine ascarid species, P. univalens (Nielsen et al., 2014), and scientists assumed populations to be P. equorum without attempting to confirm the species identity. The only established technique for identifying equine ascarid specimens to species is karyotyping, which requires fresh viable worm specimens or viable ascarid eggs at the single cell stage, whereas no morphological or molecular techniques exist for this purpose. Consequently, identifying equine ascarid eggs to species can be challenging in a field setting. Nonetheless, two recent studies demonstrated the feasibility of including karyotyping in anthelmintic resistance surveys and determined the studied populations to be 100% P. univalens in both studies (Martin et al., 2018, 2021a). Given this, it is appropriate to refer to the equine ascarid as Parascaris spp., unless the species has been verified by karyotyping or other techniques, if they become available in the future.
4. Oxyuris equi
Ivermectin and moxidectin were originally reported to have near 100% efficacy levels against O. equi adults and L4s (Lyons et al., 1980, 1992; Yazwinski et al., 1982). However, in recent years, several reports have suggested reduced efficacy of both compounds against this parasite (Table 5). In 2009, Lyons and colleagues reported <50% efficacy of ivermectin against O. equi L4s in a series of critical tests in Kentucky, USA (Lyons et al., 2009). In a second US study, eight horses harboring patent O. equi infections were first treated with ivermectin and then with pyrantel pamoate 14 days later. All feces passed by each horse were collected for a period of 72 h and washed and sieved for recovery of parasite specimens (Reinemeyer, 2012). Several adult O. equi were recovered suggesting that they had survived the ivermectin treatment but were subsequently eliminated by the pyrantel pamoate treatment (Reinemeyer, 2012). Similar observations were made in New Zealand, where naturally infected horses were first administered ivermectin or abamectin and subsequently treated with oxfendazole, which resulted in expulsion of several adult O. equi (Rock et al., 2013). In Europe, studies conducted in Germany (Wolf et al., 2014), Czech Republic (Scháňková et al., 2013), and France (Sallé et al., 2016) have all documented resistance to macrocyclic lactones in O. equi. The German study documented a lack of efficacy of ivermectin as well as moxidectin (Wolf et al., 2014), and all three European studies included recovery of adult O. equi from the treated horses, adding strength to the evidence. Finally, a large terminal study conducted in Brazil documented a lack of efficacy of ivermectin, abamectin, and moxidectin (Felippelli et al., 2015), demonstrating resistance to the entire macrocyclic lactone class. Remarkably, no further studies reporting anthelmintic efficacy against O. equi have been published since 2016. Given the findings summarized herein, researchers should be encouraged to provide further information about anthelmintic efficacy against this parasite around the world.
Table 5.
Reports of anthelmintic resistance in equine pinworms (Oxyuris equi). For each study, anthelmintic classes evaluated are indicated with an X. Studies reporting anthelmintic resistance to a given class are marked with AR. Thus, X means that resistance was tested, but not found, and X-AR means that resistance was tested and found.
| Country | Reference | Benzimidazoles | Pyrimidines | Macrocyclic lactones |
|---|---|---|---|---|
| Brazil | Felippelli et al. (2015) | X-AR | ||
| Czech Republic | Scháňková et al. (2013) | X-AR | ||
| France | Sallé et al. (2016) | X | X | X-AR |
| Germany | Wolf et al. (2014) | X | X-AR | |
| New Zealand | Rock et al. (2013) | X | X-AR | |
| USA | Reinemeyer (2012) | X | X-AR |
Although the number of studies reporting evidence of anthelmintic resistance in O. equi is small, the representation of four continents strongly suggests that macrocyclic lactone resistant isolates of this parasite may be very common around the world. Anecdotally, veterinarians and horse owners frequently report an apparent lack of efficacy of benzimidazoles and pyrimidines as well. However, no such evidence has been reported in the peer-reviewed literature at this time (Table 5), but this should be monitored and documented if found.
Evaluating anthelmintic efficacy against O. equi can be challenging, as a standardized FECRT has yet to be defined. However, the studies summarized herein provided good examples of how good quality data can be generated. While a terminal study can provide the ultimate documentation of anthelmintic resistance as demonstrated by the study conducted by Fellipelli and colleagues (2015), several of the other studies made use of follow-up treatments with an effective anthelmintic and collected expulsed parasites in the feces (Reinemeyer, 2012; Rock et al., 2013; Scháňková et al., 2013; Wolf et al., 2014; Sallé et al., 2016). However, this latter approach can become challenging if multi-drug resistance is suspected. Relying on O. equi egg counts alone as determined by the scotch tape test should be done with caution but can provide meaningful indications of anthelmintic activity if performed daily and thoroughly washing the perianal area after each sampling (Sallé et al., 2016).
5. Concluding remarks
In conclusion, anthelmintic resistance in equine nematodes has progressed considerably over the past decades. Macrocyclic lactone resistant cyathostomins have now been clearly documented in Brazil and USA and is very likely to exist elsewhere as well. Resistance to the larvicidal regimen of fenbendazole has been demonstrated in all parasitic stages of the cyathostomin life cycle, including the encysted larval stages. A growing body of literature has demonstrated widespread occurrence of macrocyclic lactone resistance in Parascaris spp. around the world, and resistance to macrocyclic lactones has been documented in O. equi as well. This paper included the first comprehensive review of published data describing strongylid ERPs following administration of ivermectin and moxidectin and addressed the historic discrepancies in ERP definition and use of methodology by applying the same criteria to all available data. These data clearly documented a trend of shortened ERPs over the course of the past three decades. While this represents a substantial change in the anthelmintic performance of ivermectin and moxidectin, it is not yet clear whether this reflects anthelmintic resistance or a selection for parasites possessing other biological traits allowing them to reach the egg producing stage sooner after anthelmintic treatment. This review has emphasized the need for continued monitoring of anthelmintic efficacy in equine operations around the world and has identified several knowledge gaps in need of being addressed in future research studies.
Declaration of competing interest
The author declares no conflict of interest.
References
- Abbas G., Ghafar G., Hurley J., Bauquier J., Beasley A., Wilkes E.J.A., Jacobson C., El-Hage C., Cudmore L., Carrigan P., Tennent-Brown B., Gauci C.G., Nielsen M.K., Hughes K.J., Beveridge I., Jabbar A. Cyathostomin resistance to moxidectin and combinations of anthelmintics in Australian horses. Parasites Vectors. 2021;14:597. doi: 10.1186/s13071-021-05103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A.D., Mukbel R.M., Alyousif M.S., AlShehri Z.S., Alanazi I.O., Al-Mohammed H.I. A field study on the anthelmintic resistance of Parascaris spp. in Arab foals in the Riyadh region, Saudi Arabia. Vet. Q. 2017;37:200–205. doi: 10.1080/01652176.2017.1334981. [DOI] [PubMed] [Google Scholar]
- Arbittier E. Comparative effects of three anthelmintics in the control of equine intestinal parasites. Bios. 1996;67:140–148. [Google Scholar]
- Armstrong S.K., Woodgate R.G., Gough S., Heller J., Sangster N.C., Hughes K.J. The efficacy of ivermectin, pyrantel and fenbendazole against Parascaris equorum infection in foals on farms in Australia. Vet. Parasitol. 2014;205:575–580. doi: 10.1016/j.vetpar.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Bairden K., Brown S.R., McGoldrick J., Parker L.D., Talty P.J. Efficacy of moxidectin 2 percent gel against naturally acquired strongyle infections in horses, with particular reference to larval cyathostomins. Vet. Rec. 2001;148:138–141. doi: 10.1136/vr.148.5.138. [DOI] [PubMed] [Google Scholar]
- Bairden K., Davies H.S., Gibson N.R., Hood A.J.O., Parker L.D. Efficacy of moxidectin 2 per cent oral gel against cyathostomins, particularly third-stage inhibited larvae, in horses. Vet. Rec. 2006;158:766–768. doi: 10.1136/vr.158.22.766. [DOI] [PubMed] [Google Scholar]
- Baranova M.V., Panova O.A., Polukhina D.N., Panova D.S. Reduction of the nematode egg reappearance period in horses after anthelmintic therapy. Vet. World. 2022;15:1530–1534. doi: 10.14202/vetworld.2022.1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley A., Coleman G., Kotze A.C. Suspected ivermectin resistance in a south-east Queensland Parascaris equorum population. Aust. Vet. J. 2015;93:305–307. doi: 10.1111/avj.12352. [DOI] [PubMed] [Google Scholar]
- Becher A.M., van Doorn D.C., Pfister K., Kaplan R.M., Reist M., Nielsen M.K. Equine parasite control and the role of national legislation - a multinational questionnaire survey. Vet. Parasitol. 2018;259:6–12. doi: 10.1016/j.vetpar.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Bellaw J.L., Krebs K., Reinemeyer C.R., Norris J.K., Scare J.A., Pagano S., Nielsen M.K. Anthelmintic therapy of equine cyathostomin nematodes – larvicidal efficacy, egg reappearance period, and drug resistance. Int. J. Parasitol. 2018;48:97–105. doi: 10.1016/j.ijpara.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Bellaw J.L., Nielsen M.K. Meta-analysis of cyathostomin species-specific prevalence and relative abundance in domestic horses from 1975-2020: emphasis on geographic region and specimen collection method. Parasites Vectors. 2020;13:509. doi: 10.1186/s13071-020-04396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R.M., Scott I., Gee E.K., Rogers C.W., Pomroy W.E., Mayhew I.G. Sub-optimal efficacy of ivermectin against Parascaris equorum in foals on three Thoroughbred stud farms in the Manawatu region of New Zealand. N. Z. Vet. J. 2014;62:91–95. doi: 10.1080/00480169.2013.843146. [DOI] [PubMed] [Google Scholar]
- Boraski E.A. Efficacy of ivermectin against Parascaris equorum. J. Am. Vet. Med. Assoc. 1987;191:278. [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., Maas J., van der Aar W.M. Comparison of the reappearance of strongyle eggs in foals, yearlings, and adult horses after treatment with ivermectin or pyrantel. Vet. Q. 1996;18:7–9. doi: 10.1080/01652176.1996.9694602. [DOI] [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., van der Aar W.M. The reappearance of strongyle eggs in the faeces of horses after treatment with moxidectin. Vet. Q. 1998;20:15–17. doi: 10.1080/01652176.1998.9694828. [DOI] [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., Nas J.W.M. Apparent resistance of Parascaris equorum to macrocyclic lactones. Vet. Rec. 2002;150:279–281. doi: 10.1136/vr.150.9.279. [DOI] [PubMed] [Google Scholar]
- Bodeček S., Svetlikova J., Hargitaiova K., Kecerova Z., Mrackova M. Monitoring the avermectin and pyrantel resistance status of nematode parasites of horses in the Czech Republic. Vet. Med. (Praha) 2018;63:299–305. [Google Scholar]
- Borgsteede F.H.M., Boersema J.H., Gaasenbeek C.P.H., van der Burg W.P.J. The reappearance of eggs in faeces of horses after treatment with ivermectin. Vet. Q. 1993;15:24–26. doi: 10.1080/01652176.1993.9694363. [DOI] [PubMed] [Google Scholar]
- Boulkaboul A., Bouakkaz A., Kerboeuf D. Detection of resistance to benzimidazoles in horse strongyles, in Algeria. Rev. Med. Vet. (Toulouse) 2006;157:59–64. [Google Scholar]
- Brazik E.L., Luquire J.T., Little D. Pyrantel pamoate resistance in horses receiving daily administration of pyrantel tartrate. J. Am. Vet. Med. Assoc. 2006;228:101–103. doi: 10.2460/javma.228.1.101. [DOI] [PubMed] [Google Scholar]
- Butler S.J., Greenbank H., Parish R., Nielsen M.K., Stoughton W.B. Prevalence of anthelmintic resistant cyathostomins in prince edward island, Canada. Vet. Parasitol. Reg. Stud. Rep. 2021;26 doi: 10.1016/j.vprsr.2021.100629. [DOI] [PubMed] [Google Scholar]
- Buzatu M.C., Mitrea I.L., Miron L., Ionita M. Efficacy of two anthelmintic products on strongyles in horses from stud farms in Romania. Agric. Agric. Sci. Procedia. 2015;6:293–298. [Google Scholar]
- Cain J.L., Foulk D., Jedrzejewski E., Stofanak H., Nielsen M.K. The importance of anthelmintic efficacy monitoring: results of an outreach effort. Parasitol. Res. 2019;118:2877–2883. doi: 10.1007/s00436-019-06423-6. [DOI] [PubMed] [Google Scholar]
- Canever R.J., Braga P.R.C., Boeckh A., Grycajuck M., Bier D., Molento M.B. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet. Parasitol. 2013;194:35–39. doi: 10.1016/j.vetpar.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Čerňanská D., Paoletti B., Král’ová-Hromadová I., Iorio R., Čudeková P., Milillo P., Traversa D. Application of a Reverse Line Blot hybridisation assay for the species specific identification of cyathostomins (Nematoda, Strongylida) from benzimidazole-treated horses in the Slovak Republic. Vet. Parasitol. 2009;160:171–174. doi: 10.1016/j.vetpar.2008.10.078. [DOI] [PubMed] [Google Scholar]
- Cernea M., Cristina R.T., Ştefănuţ L.C., Carvalho L.M.M.d., Marian A., Taulescu M.A., Cozma V. Screening for anthelmintic resistance in equid strongyles (Nematoda) in Romania. Folia Parasitol. 2015;63 doi: 10.14411/fp.2015.023. [DOI] [PubMed] [Google Scholar]
- Chapman M.R., French D.D., Monahan C.M., Klei T.R. Identification and characterization of a pyrantel pamoate resistant cyathostome population. Vet. Parasitol. 1996;66:205–212. doi: 10.1016/s0304-4017(96)01014-x. [DOI] [PubMed] [Google Scholar]
- Churcher T.S., Pion S.D., Osei-Atweneboana M.Y., Prichard R.K., Awadzi K., Boussinesq M., Collins R.C., Whitworth J.A., Basanez M.G. Identifying sub-optimal responses to ivermectin in the treatment of river blindness. Proc. Natl. Acad. Sci. USA. 2009;106:16716–16721. doi: 10.1073/pnas.0906176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirak V.Y., Gülegen E., Bauer C. Benzimidazole resistance in cyathostomin populations on horse farms in western Anatolia, Turkey. Parasitol. Res. 2004;93:392–395. doi: 10.1007/s00436-004-1143-3. [DOI] [PubMed] [Google Scholar]
- Cirak V.Y., Gülegen E., Bauer C. The prevalence of strongyle infections and persistent efficacy of pyrantel embonate, ivermectin and moxidectin in Turkish horses. Turk. J. Vet. Anim. Sci. 2005;29:175–181. [Google Scholar]
- Cirak V.Y., Kar S., Girisgin O. A survey on anthelmintic resistance in strongyles to ivermectin and pyrantel and macrocyclic lactone-resistance in Parascaris equorum. Turk. Parazitoloji Derg. 2010;34:35–39. [PubMed] [Google Scholar]
- Coles G., Bauer C., Borgsteede F., Geerts S., Klei T., Taylor M., Waller P. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Comer K.C., Hillyer M.H., Coles G.C. Anthelmintic use and resistance on thoroughbred training yards in the UK. Vet. Rec. 2006;158:596–598. doi: 10.1136/vr.158.17.596. [DOI] [PubMed] [Google Scholar]
- Cooper L.G., Caffe G., Cerutti J., Nielsen M.K., Anziani O.S. Reduced efficacy of ivermectin and moxidectin against Parascaris spp. in foals from Argentina. Vet. Parasitol. Reg. Stud. Reports. 2020;20 doi: 10.1016/j.vprsr.2020.100388. [DOI] [PubMed] [Google Scholar]
- Craig T.M., Diamond P.L., Ferwerda N.S., Thompson J.A. Evidence of ivermectin resistance by Parascaris equorum on a Texas horse farm. J. Equine Vet. Sci. 2007;27:67–71. [Google Scholar]
- Daniels S.P., Proudman C.J. Ovicidal efficacy of fenbendazole after treatment of horses naturally infected with cyathostomins. Vet. Parasitol. 2016;227:151–156. doi: 10.1016/j.vetpar.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Daniels S.P., Proudman C.J. Shortened egg reappearance after ivermectin or moxidectin use in horses in the UK. Vet. J. 2016;218:36–39. doi: 10.1016/j.tvjl.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Dauparaitė E., Kupčinskas T., Samson-Himmelstjerna G.v., Petkevičius S. Anthelmintic resistance of horse strongyle nematodes to ivermectin and pyrantel in Lithuania. Acta Vet. Scand. 2021;63:5. doi: 10.1186/s13028-021-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.A., Schwalbach L.M.J. A study to evaluate the field efficacy of ivermectin, fenbendazole and pyrantel pamoate, with preliminary observations on the efficacy of doramectin, as anthelmintics in horses. Tydskr. S.-Afr. Vet. Ver. 2000;71:144–147. doi: 10.4102/jsava.v71i3.703. [DOI] [PubMed] [Google Scholar]
- Demeulenaere D., Vercruysse J., Dorny P., Claerebout E. Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses. Vet. Rec. 1997;15:383–386. doi: 10.1136/vr.141.15.383. [DOI] [PubMed] [Google Scholar]
- DiPietro J.A., Lock T.F., Todd K.S., Reuter V.E. Evaluation of ivermectin paste in the treatment of ponies for Parascaris equorum infections. J. Am. Vet. Med. Assoc. 1987;190:1181–1183. [PubMed] [Google Scholar]
- DiPietro J.A., Lock T.F., Todd K.S., Sanecki R.K. Evaluation of ivermectin for larvicidal effect in experimentally induced Parascaris equorum infections. Am. J. Vet. Res. 1988;49:1983–1985. [PubMed] [Google Scholar]
- DiPietro J.A., Klei T.R., Reinemeyer C.R. Efficacy of fenbendazole against encysted small strongyle larvae. Proceeding American Association of Equine Practitioners Convention. 1997;43:343–344. [Google Scholar]
- DiPietro J.A., Hutchens D.E., Lock T.F., Walker K., Paul A.J., Shipley C., Rulli D. Clinical trial of moxidectin oral gel in horses. Vet. Parasitol. 1997;72:167–177. doi: 10.1016/s0304-4017(97)01108-4. [DOI] [PubMed] [Google Scholar]
- Dobson R.J., Hosking B.C., Jacobson C.L., Cotter J.L., Besier R.B., Stein P.A., Reid S.A. Preserving new anthelmintics: a simple method for estimating faecal egg count reduction test (FECRT) confidence limits when efficacy and/or nematode aggregation is high. Vet. Parasitol. 2012;186:79–93. doi: 10.1016/j.vetpar.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Dorny P., Meijer I., Smets K., Vercruysse J. A survey of anthelmintic resistance on Belgian horse farms. Vlaams Diergeneeskd Tijdschr. 2000;69:334–337. [Google Scholar]
- Drudge J.H., Lyons E.T., Szanto J. World Association for the Advancement of Veterinary Parasitology. Academic Press Inc; New York: 1965. Pathogenesis of migrating stages of helminths, with special reference to Strongylus vulgaris; pp. 199–214. [Google Scholar]
- Drudge J.H., Lyons E.T. Control of internal parasites of horses. J. Am. Vet. Med. Assoc. 1966;148:378–383. [PubMed] [Google Scholar]
- Duncan J.L., Bairden K., Abbott E.M. Elimination of mucosal cyathostome larvae by five daily treatments with fenbendazole. Vet. Rec. 1998;142:268–271. doi: 10.1136/vr.142.11.268. [DOI] [PubMed] [Google Scholar]
- Egerton J.R., Brokken E.S., Suhayda D., Eary C.H., Wooden J.W., Kilgore R.L. The antiparasitic activity of ivermectin in horses. Vet. Parasitol. 1981;8:83–88. [Google Scholar]
- Fabiani J.V., Lyons E.T., Nielsen M.K. Dynamics of Parascaris and Strongylus spp. parasites in untreated juvenile horses. Vet. Parasitol. 2016;230:62–66. doi: 10.1016/j.vetpar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Felippelli G., Cruz B.C., Gomes L.V.C., Lopes W.D.Z., Teixeira W.F.P., Buzzulini W.G.M.C., Murilo Bichuette M.A., Campos G.P., Soares V.E., Bergamasco P.L.F., Oliveira G.P.d., Costa A.J.d. Susceptibility of helminth species from horses against different chemical compounds in Brazil. Vet. Parasitol. 2015;212:232–238. doi: 10.1016/j.vetpar.2015.07.041. [DOI] [PubMed] [Google Scholar]
- Fischer J.K., Hinney B., Denwood M.J., Traversa D., Samson-Himmelstjerna G.v., Clausen P.-H. Efficacy of selected anthelmintic drugs against cyathostomins in horses in the federal state of Brandenburg, Germany. Parasitol. Res. 2015;114:4441–4450. doi: 10.1007/s00436-015-4685-7. [DOI] [PubMed] [Google Scholar]
- Flores A.G., Osmari V., Ramos F., Marques C.B., Ramos D.J., Botton A.S., Vogel F.S.F., Sangioni L.A. Multiple resistance in equine cyathostomins: a case study from military establishments in Rio Grande do Sul, Brazil. Braz. J. Vet. Parasitol. 2020;29 doi: 10.1590/S1984-29612020086. [DOI] [PubMed] [Google Scholar]
- Francisco R., Paz-Silva A., Francisco I., Cortiñas F.J., Miguélez S., Suárez J., Cazapal-Monteiro C.F., Suárez J.L., Arias M.S., Sánchez-Andrade R. Preliminary analysis of the results of selective therapy against strongyles in pasturing horses. J. Equine Vet. Sci. 2012;32:274–280. [Google Scholar]
- Garcia A., Brady H.A., Nichols W.T., Prien S. Equine cyathostomin resistance to fenbendazole in Texas horse facilities. J. Equine Vet. Sci. 2013;33:223–228. [Google Scholar]
- Geurden T., Betsch J.M., Maillard K., Vanimisetti B., D'Espois M., Besognet B. Determination of anthelmintic efficacy against equine cyathostomins and Parascaris equorum in France. Equine Vet. Educ. 2013;25:304–307. [Google Scholar]
- Geurden T., van Doorn D., Claerebout E., Kooyman F., Keersmaecker S.D., Vercruysse J., Besognet B., Vanimisetti B., di Regalbono A.F., Beraldo P., Cesare A.D., Traversa D. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet. Parasitol. 2014;204:291–296. doi: 10.1016/j.vetpar.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Gibson T.E. The effect of repeated anthelmintic treatment with phenothiazine on fecal egg counts of housed horses, with some observations on the life cycle of Trichonema spp. in the horse. J. Helminthol. 1953;27:29–40. [Google Scholar]
- Gokbulut C., Nolan A.M., McKellar Q.A. Plasma pharmacokinetics and faecal excretion of ivermectin, doramectin and moxidectin following oral administration in horses. Equine Vet. J. 2001;33:494–498. doi: 10.2746/042516401776254835. [DOI] [PubMed] [Google Scholar]
- Hautala K., Näreaho A., Kauppinen O., Nielsen M.K., Sukura A., Rajala-Schultz P.J. Risk factors for equine intestinal parasite infections and reduced efficacy of pyrantel embonate against Parascaris sp. Vet. Parasitol. 2019;273:52–59. doi: 10.1016/j.vetpar.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Hearn F.P.D., Peregrine A.S. Identification of foals infected with Parascaris equorum apparently resistant to ivermectin. J. Am. Vet. Med. Assoc. 2003;223:482–485. doi: 10.2460/javma.2003.223.482. [DOI] [PubMed] [Google Scholar]
- Jacobs D.E., Hutchinson M.J., Parker L., Gibbons L.M. Equine Cyathostome infection: suppression of faecal egg output with moxidectin. Vet. Rec. 1995;137:545. doi: 10.1136/vr.137.21.545. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Anthelmintic resistance in nematodes of horses. Vet. Res. 2002;33:491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Klei T.R., Lyons E.T., Lester G., Courtney C.H., French D.D., Tolliver S.C., Vidyashankar A.N., Zhao Y. Prevalence of anthelmintic resistant cyathostomes on horse farms. J. Am. Vet. Med. Assoc. 2004;225:903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- Kivipelto J., Asquith R.L. Duration of fecal egg count reduction for antiparasitic compounds in the young horse. Equine Pract. 1994;16:10–14. [Google Scholar]
- Königová A., Várady M., Čorba J. Comparison of in vitro methods and faecal egg count reduction test for the detection of benzimidazole resistance in small strongyles of horses. Vet. Res. Commun. 2003;27:281–288. doi: 10.1023/a:1024079907895. [DOI] [PubMed] [Google Scholar]
- Kumar S., Garg R., Kumar S., Banerjee P.S., Ram H., Prasad A. Benzimidazole resistance in equine cyathostomins in India. Vet. Parasitol. 2016;218:93–97. doi: 10.1016/j.vetpar.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Kuzmina T.A., Kharchenko V.O. Anthelmintic resistance in cyathostomins of brood horses in Ukraine and influence of anthelmintic treatments on strongylid community structure. Vet. Parasitol. 2008;154:277–288. doi: 10.1016/j.vetpar.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Kuzmina T.A., Zvegintsova N.S., Yasynetska N.I., Kharchenko V.A. Anthelmintic resistance in strongylids (nematoda: strongylidae) parasitizing wild and domestic equids in the askania nova biosphere reserve, Ukraine. Ann. Parasitol. 2020;66:49–60. [PubMed] [Google Scholar]
- Kyvsgaard N.C., Lindbom J., Andreasen L.L., Luna-Olivares L.A., Nielsen M.K., Monrad J. Prevalence and anthelmintic control of strongyles in working horses in Nicaragua. Vet. Parasitol. 2011;181:248–254. doi: 10.1016/j.vetpar.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Langrová I., Borovsky M., Jankovská I., Navratíl J., Slavik V. The benzimidazole resistance of cyathostomes on five horse farms in the Czech Republic. Helminthologia. 2002;39:211–216. [Google Scholar]
- Larsen M.L., Ritz C., Petersen S.L., Nielsen M.K. Determination of ivermectin efficacy and egg reappearance period on horse farms using selective therapy. Vet. J. 2011;188:44–47. doi: 10.1016/j.tvjl.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Lassen B., Peltola S.-M. Anthelmintic resistance of intestinal nematodes to ivermectin and pyrantel in Estonian horses. J. Helminthol. 2015;89:760–763. doi: 10.1017/S0022149X14000510. [DOI] [PubMed] [Google Scholar]
- Laugier C., Sevin C., Ménard S., Maillard K. Prevalence of Parascaris equorum infection in foals on French stud farms and first report of ivermectin-resistant P. equorum populations in France. Vet. Parasitol. 2012;188:185–189. doi: 10.1016/j.vetpar.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Lester H.E., Spanton J., Stratford C.H., Bartley D.J., Morgan E.R., Hodgkinson J.E., Coumbe K., Mair T., Swan B., Lemon G., Cookson R., Matthews J.B. Anthelmintic efficacy against cyathostomins in horses in Southern England. Vet. Parasitol. 2013;197:189–196. doi: 10.1016/j.vetpar.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Levecke B., Kaplan R.M., Thamsborg S.M., Torgerson P.R., Vercruysse J., Dobson R.J. How to improve the standardization and the diagnostic performance of the fecal egg count reduction test? Vet. Parasitol. 2018;15:71–78. doi: 10.1016/j.vetpar.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Lignon J.S., Gonçalves N.F., Cunha L.L., Antunes T.A., Leão M.S., Camassola J.L.T., Pellegrin T.G., Ripoll P.K., Pappen F.G., Pinto D.M. Anthelmintic resistance in creole horses in the south of rio grande do sul, Brazil. Arq. Bras. Med. Vet. Zootec. 2021;73:598–604. [Google Scholar]
- Lind E.O., Kuzmina T., Uggla A., Waller P.J., Hoglund J. A field study on the effect of some anthelmintics on cyathostomins of horses in Sweden. Vet. Res. Commun. 2007;31:53–65. doi: 10.1007/s11259-006-3402-5. [DOI] [PubMed] [Google Scholar]
- Lind E.O., Christensson D. Anthelmintic efficacy on Parascaris equorum in foals on Swedish studs. Acta Vet. Scand. 2009;51:45. doi: 10.1186/1751-0147-51-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren K., Ljungvall Ö., Nilsson O., Ljungström B.-L., Lindahl C., Höglund J. Parascaris equorum in foals and in their environment on a Swedish stud farm, with notes on treatment failure of ivermectin. Vet. Parasitol. 2008;151:337–343. doi: 10.1016/j.vetpar.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Little D., Flowers J.R., Hammerberg B.H., Gardner S.Y. Management of drug-resistant cyathostominosis on a breeding farm in central North Carolina. Equine Vet. J. 2003;35:246–251. doi: 10.2746/042516403776148264. [DOI] [PubMed] [Google Scholar]
- Lumsden G.G., Quan-Taylor R., Smith S.M., Washbrooke I.M. Field efficacy of ivermectin, fenbendazole and pyrantel embonate paste anthelmintics in horses. Vet. Rec. 1989;125:497–499. doi: 10.1136/vr.125.20.497. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Drudge J.H., Tolliver S.C. Antiparasitic activity of ivermectin in critical tests in equids. Am. J. Vet. Res. 1980;41:2069–2070. [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Drudge J.H., Granstrom D.E., Collins S.S., Stamper S. Critical and controlled tests of activity of moxidectin (CL 301,432) against natural infections of internal parasites of equids. Vet. Parasitol. 1992;41:255–284. doi: 10.1016/0304-4017(92)90086-o. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Drudge J.H., Collins S.S., Swerczek T.W. Continuance of studies on Population S benzimidazole-resistant small strongyles in a Shetland pony herd in Kentucky: effect of pyrantel pamoate (1992–1999) Vet. Parasitol. 2001;94:247–256. doi: 10.1016/s0304-4017(00)00382-4. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Study (1991 to 2001) of drug-resistant population B small strongyles in critical tests in horses in Kentucky at the termination of a 40-year investigation. Parasitol. Res. 2007;101:680–701. doi: 10.1007/s00436-007-0535-6. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Ionita M., Lewellen A., Collins S.S. Field studies indicating reduced activity of ivermectin on small strongyles in horses on a farm in Central Kentucky. Parasitol. Res. 2008;103:209–215. doi: 10.1007/s00436-008-0959-7. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Ionita M., Collins S.S. Evaluation of parasiticidal activity of fenbendazole, ivermectin, oxibendazole, and pyrantel pamoate in horse foals with emphasis on ascarids (Parascaris equorum) in field studies on five farms in Central Kentucky in 2007. Parasitol. Res. 2008;103:287–291. doi: 10.1007/s00436-008-0966-8. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Probable reason why small strongyle EPG counts are returning “early” after ivermectin treatment of horses on a farm in Central Kentucky. Parasitol. Res. 2009;104:569–574. doi: 10.1007/s00436-008-1231-x. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Kuzmina T.A., Collins S.S. Critical tests evaluating efficacy of moxidectin against small strongyles in horses from a herd for which reduced activity had been found in field tests in Central Kentucky. Parasitol. Res. 2010;107:1495–1498. doi: 10.1007/s00436-010-2025-5. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Reduced activity of moxidectin and ivermectin on small strongyles in young horses on a farm (BC) in Central Kentucky in two field tests with notes on variable counts of eggs per gram of feces (EPGs) Parasitol. Res. 2011;108:1315–1319. doi: 10.1007/s00436-010-2225-z. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S., Ionita M., Kuzmina T.A., Rossano M. Field tests demonstrating reduced activity of ivermectin and moxidectin against small strongyles in horses on 14 farms in Central Kentucky in 2007–2009. Parasitol. Res. 2011;108:355–360. doi: 10.1007/s00436-010-2068-7. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Kuzmina T.A., Collins S.S. Further evaluation in field tests of the activity of three anthelmintics (fenbendazole, oxibendazole, and pyrantel pamoate) against the ascarid Parascaris equorum in horse foals on eight farms in Central Kentucky (2009–2010) Parasitol. Res. 2011;109:1193–1197. doi: 10.1007/s00436-011-2379-3. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C. Further indication of lowered activity of ivermectin on immature small strongyles in the intestinal lumen of horses on a farm in Central Kentucky. Parasitol. Res. 2013;112:889–891. doi: 10.1007/s00436-012-3098-0. [DOI] [PubMed] [Google Scholar]
- Martin F., Höglund J., Bergström T.F., Lindsjö O.K., Tyden E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet. Parasitol. 2018;264:69–73. doi: 10.1016/j.vetpar.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Martin F., Svansson V., Eydal M., Oddsdóttir C., Ernback M., Persson I., Tydén E. First report of resistance to ivermectin in Parascaris univalens in Iceland. J. Parasitol. 2021;107:16–22. doi: 10.1645/20-91. [DOI] [PubMed] [Google Scholar]
- Martin F., Halvarsson P., Delhomme N., Höglund J., Tydén E. Exploring the β-tubulin gene family in a benzimidazole-resistant Parascaris univalens population. Int. J. Parasitol. Drugs Drug Resist. 2021;17:84–91. doi: 10.1016/j.ijpddr.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Downum K., Yazwinski T., Tucker C., Fincher M., Ralph J., Hamilton J. Cyathostome fecal egg count trends in horses treated with moxidectin, ivermectin or fenbendazole. Vet. Parasitol. 2001;101:75–79. doi: 10.1016/s0304-4017(01)00495-2. [DOI] [PubMed] [Google Scholar]
- Martins N.S., Pinto D.M., Cunha L.L.d., Lignon J.S., Santos T.C.d., Evaristo T.A., Pappen F.G., Leandro Quintana Nizoli L.Q. Assessment of the efficacy of commercial anthelmintics in horses naturally infected with gastrointestinal nematodes. Med. Vet. UFRPE. 2021;15:28–32. [Google Scholar]
- Mason M.E., Voris N.D., Ortis H.A., Geeding A.A., Kaplan R.M. Comparison of a single dose of moxidectin and a five-day course of fenbendazole to reduce and suppress cyathostomin fecal egg counts in a herd of embryo transfer–recipient mares. J. Am. Vet. Med. Assoc. 2014;245:944–951. doi: 10.2460/javma.245.8.944. [DOI] [PubMed] [Google Scholar]
- Matthews J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014;4:310–315. doi: 10.1016/j.ijpddr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaki A.M., Mohammed F.F., Idris S.B. Anthelmintic resistance and associated management practices in local horses in Sokoto metropolis, Nigeria. Maced. Vet. Rev. 2018;41:55–64. [Google Scholar]
- McFarlane D., Hale G.H., Johnson E.M., Maxwell L.K. Fecal egg counts after anthelmintic administration to aged horses and horses with pituitary pars intermedia dysfunction. J. Am. Vet. Med. Assoc. 2010;236:330–334. doi: 10.2460/javma.236.3.330. [DOI] [PubMed] [Google Scholar]
- Meier A., Hertzberg H. Equine strongyles. II. Occurrence of anthelmintic resistance in Switzerland. Schweiz. Arch. Tierheilkd. 2005;147:389–396. doi: 10.1024/0036-7281.147.9.389. [DOI] [PubMed] [Google Scholar]
- Mercier P., Chick B., Alves-Branco F., White C.R. Comparative efficacy, persistent effect, and treatment intervals of anthelmintic pastes in naturally infected horses. Vet. Parasitol. 2001;99:29–39. doi: 10.1016/s0304-4017(01)00453-8. [DOI] [PubMed] [Google Scholar]
- Milillo P., Boeckh A., Cobb R., Otranto D., Lia R.P., Perrucci S., di Regalbono A.F., Beraldo P., von Samson-Himmelstjerna G., Demeler J., Bartolini R., Traversa D. Faecal cyathostomin egg count distribution and efficacy of anthelmintics against cyathostomins in Italy: a matter of geography? Parasites Vectors. 2009;2(Suppl. 2):S4. doi: 10.1186/1756-3305-2-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molena R.A., Peachey L.E., Cesare A.D., Traversa D., Cantacessi C. Cyathostomine egg reappearance period following ivermectin treatment in a cohort of UK Thoroughbreds. Parasites Vectors. 2018;11:61. doi: 10.1186/s13071-018-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molento M.B., Antunes J., Bentes R.N., Coles G.C. Anthelmintic resistant nematodes in Brazilian horses. Vet. Rec. 2008;162:384–385. doi: 10.1136/vr.162.12.384. [DOI] [PubMed] [Google Scholar]
- Molento M.B., Anese J.D., Pontarolo D.V., Brandão Y.d.O. Strong ivermectin resistance in cyathostomins of horses: a grim picture for sustainable parasite control. 2022. Available at: SSRN: [DOI] [PMC free article] [PubMed]
- Monahan C.M., Chapman M.R., French D.D., Taylor H.W., Klei T.R. Dose titration of moxidectin oral gel against gastrointestinal parasites of ponies. Vet. Parasitol. 1995;59:241–248. doi: 10.1016/0304-4017(94)00762-2. [DOI] [PubMed] [Google Scholar]
- Monahan C.M., Chapman M.R., Taylor H.W., French D.D., Klei T.R. Comparison of moxidectin oral gel and ivermectin oral paste against a spectrum of internal parasites of ponies with special attention to encysted cyathostome larvae. Vet. Parasitol. 1996;63:225–235. doi: 10.1016/0304-4017(95)00910-8. [DOI] [PubMed] [Google Scholar]
- Morris L.H., Colgan S., Leathwick D.M., Nielsen M.K. Anthelmintic efficacy of single active and combination products against commonly occurring parasites in foals. Vet. Parasitol. 2019;268:46–52. doi: 10.1016/j.vetpar.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Nápravníková J., Várady M., Vadlejch J. Total failure of fenbendazole to control strongylid infections in Czech horse operations. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.833204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näreaho A., Vainio K., Oksanen A. Impaired efficacy of ivermectin against Parascaris equorum, and both ivermectin and pyrantel against strongyle infections in trotter foals in Finland. Vet. Parasitol. 2011;182:372–377. doi: 10.1016/j.vetpar.2011.05.045. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Steuer A.E., Anderson H.P., Gavriliuc S., Carpenter A.B., Redman E.M., Gilleard J.S., Reinemeyer C.R., Poissant J. Shortened egg reappearance periods of equine cyathostomins following ivermectin or moxidectin treatment: morphologic and molecular investigation of efficacy and species composition. Int. J. Parasitol. 2022 doi: 10.1016/j.ijpara.2022.09.003. (in press) [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Vidyashankar A.N., Hanlon B.M., Diao G., Petersen S.L., Kaplan R.M. Hierarchical model for evaluating pyrantel efficacy against strongyle parasites in horses. Vet. Parasitol. 2013;197:614–622. doi: 10.1016/j.vetpar.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Wang J., Davis R., Bellaw J.L., Lyons E.T., Lear T.L., Goday C. Parascaris univalens – a victim of large-scale misidentification? Parasitol. Res. 2014;113:4485–4490. doi: 10.1007/s00436-014-4135-y. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Branan M.A., Wiedenheft A.M., Digianantonio R., Scare J.A., Bellaw J.L., Garber L.P., Kopral C.A., Phillippi-Taylor A.M., Traub-Dargatz J.L. Anthelmintic efficacy against equine strongyles in the United States. Vet. Parasitol. 2018;259:53–60. doi: 10.1016/j.vetpar.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Branan M.A., Wiedenheft A.M., Digianantonio R., Garber L.P., Kopral C.A., Phillippi-Taylor A.M., Traub-Dargatz J.L. Parasite control strategies used by equine owners in the United States: a national survey. Vet. Parasitol. 2018;250:45–51. doi: 10.1016/j.vetpar.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Banahan M., Kaplan R.M. Importation of macrocyclic lactone resistant cyathostomins on a US Thoroughbred farm. Int. J. Parasitol. Drugs Drug Resist. 2020;14:99–104. doi: 10.1016/j.ijpddr.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.K., Gee E.K., Hansen A., Waghorn T., Bell J., Leathwick D.M. Monitoring equine ascarid and cyathostomin parasites: evaluating health parameters under different treatment regimens. Equine Vet. J. 2021;53:902–910. doi: 10.1111/evj.13374. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Littman B.A., Orzech S.W., Ripley N.E. Equine strongylids: ivermectin efficacy and fecal egg shedding patterns. Parasitol. Res. 2022;121:1691–1697. doi: 10.1007/s00436-022-07509-4. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., von Samson-Himmelstjerna G., Kuzmina T.A., van Doorn D.C.K., Meana A., Rehbein S., Elliott T., Reinemeyer C.R. World Association for the Advancement of Veterinary Parasitology (WAAVP): third edition of guideline for evaluating the efficacy of equine anthelmintics. Vet. Parasitol. 2022;303 doi: 10.1016/j.vetpar.2022.109676. [DOI] [PubMed] [Google Scholar]
- O'Meara B., Mulcahy G. A survey of helminth control practices in equine establishments in Ireland. Vet. Parasitol. 2002;109:101–110. doi: 10.1016/s0304-4017(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Papadopoulos E., Hamhougias K., Himonas C., Dorchies P. Strongyle anthelmintic resistance in horses and cattle from Greece. Rev. Med. Vet. 2000;151:1139–1142. [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Piché C.A., Kennedy M.J., Herbers H.A., Newcomb K.M. Comparison of ivermectin, oxibendazole and pyrantel pamoate in suppressing fecal egg output in horses. Can. Vet. J. 2001;32:104–107. [PMC free article] [PubMed] [Google Scholar]
- Pion S.D., Nana-Djeunga H.C., Kamgno J., Tendongfor N., Wanji S., Njiokou F., Prichard R.K., Boussinesq M. Dynamics of Onchocerca volvulus microfilarial densities after ivermectin treatment in an ivermectin-naive and a multiply treated population from Cameroon. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez R., Cabezas I., García M., Rubilar L., Sutra J.F., Galtier P., Alvinerie M. Comparison of the pharmacokinetics of moxidectin (Equest) and ivermectin (Eqvalan) in horses. J. Vet. Pharmacol. Therapeut. 1999;22:174–180. doi: 10.1046/j.1365-2885.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Pook J.F., Power M.L., Sangster N.C., Hodgson J.L., Hodgson D.R. Evaluation of tests for anthelmintic resistance in cyathostomes. Vet. Parasitol. 2002;106:331–343. doi: 10.1016/s0304-4017(02)00093-6. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R., Prado J.C., Nichols E.C., Marchiondo A.A. Efficacy of pyrantel pamoate against a macrocyclic lactone-resistant isolate of Parascaris equorum in horses. Vet. Parasitol. 2010;171:111–115. doi: 10.1016/j.vetpar.2010.02.041. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R., Prado J.C., Nielsen M.K. Comparison of the larvicidal efficacies of moxidectin or a five-day regimen of fenbendazole in horses harbouring cyathostomin populations resistant to the adulticidal dosage of fenbendazole. Vet. Parasitol. 2015;214:100–107. doi: 10.1016/j.vetpar.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R. Anthelmintic resistance among non‐strongylid parasites of horses. Vet. Parasitol. 2012;185:9–15. doi: 10.1016/j.vetpar.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Relf V.E., Lester H.E., Morgan E.R., Hodgkinson J.E., Matthews J.B. Anthelmintic efficacy on UK Thoroughbred stud farms. Int. J. Parasitol. 2014;44:507–514. doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Repeta D.L., Birnbaum N., Courtney C.H. Anthelmintic resistance on pleasure horse farms in North Central Florida. Equine Pract. 1993;15:8–12. [Google Scholar]
- Robert M., Hu W., Nielsen M.K., Stowe C.J. Attitudes towards implementation of surveillance-based parasite control on Kentucky Thoroughbred farms – current strategies, awareness, and willingness-to-pay. Equine Vet. J. 2015;47:694–700. doi: 10.1111/evj.12344. [DOI] [PubMed] [Google Scholar]
- Rock C., Pomroy W., Gee E., Scott I. Macrocyclic lactone resistant Oxyuris equi in New Zealand. Proceedings of 24th International Conference of the WAAVP. 2013;25–29 August:520. [Google Scholar]
- Rolfe P.F., Dawson K.L., Holm-Martin M. Efficacy of moxidectin and other anthelmintics against small strongyles in horses. Aust. Vet. J. 1998;76:332–334. doi: 10.1111/j.1751-0813.1998.tb12361.x. [DOI] [PubMed] [Google Scholar]
- Rosanowski S.M., Bolwell C.F., Scott I., Sells P.D., Rogers C.W. The efficacy of Ivermectin against strongyles in yearlings on Thoroughbred breeding farms in New Zealand. Vet. Parasitol. Reg. Stud. Rep. 2017;8:70–74. doi: 10.1016/j.vprsr.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Rossano M.G., Smith A.R., Lyons E.T. Shortened strongyle-type egg reappearance periods in naturally infected horses treated with moxidectin and failure of a larvicidal dose of fenbendazole to reduce fecal egg counts. Vet. Parasitol. 2010;173:349–352. doi: 10.1016/j.vetpar.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Saeed K., Qadir Z., Khan S.A., Ashraf K., Nazir S. Evaluation of some broad spectrum antiparasitic drugs against natural strongyle infections in horses. J. Anim. Pl. Sci. 2008;18:64–66. [Google Scholar]
- Saes I.d.L., Vera J.H.S., Fachiolli D.F., Yamada P.H., Dellaqua J.V.T., Saes R.d.L., Amarante A.F.T., Soutello R.V.G. Time required by different anthelmintics to reach expected efficacy levels in horses infected by strongyles. Vet. Parasitol. 2016;229:90–92. doi: 10.1016/j.vetpar.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Salas-Romero J., Gómez-Cabrera K.A., Salas J.E., Vázquez R., Arenal A., Nielsen M.K. First report of anthelmintic resistance of equine cyathostomins in Cuba. Vet. Parasitol. Reg. Stud. Rep. 2018;13:220–223. doi: 10.1016/j.vprsr.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Sallé G., Cortet J., Koch C., Gascogne T., Reigner F., Cabaret J. Ivermectin failure in the control of Oxyuris equi in a herd of ponies in France. Vet. Parasitol. 2016;229:73–75. doi: 10.1016/j.vetpar.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Sallé G., Cortet J., Bois I., Dubès C., Guyot-Sionest Q., Larrieu C., Landrin V., Majorel G., Wittreck S., Woringer E., Couroucé A., Guillot J., Jacquiet P., Guégnard F., Blanchard A., Leblond A. Risk factor analysis of equine strongyle resistance to anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2017;7:407–415. doi: 10.1016/j.ijpddr.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster N.C. Pharmacology of anthelmintic resistance in cyathostomes: will it occur with the avermectin/milbemycins? Vet. Parasitol. 1999;85:189–204. doi: 10.1016/s0304-4017(99)00099-0. [DOI] [PubMed] [Google Scholar]
- Sanna G., Pipia A.P., Tamponi C., Manca R., Varcasia A., Traversa D., Scala A. Anthelmintics efficacy against intestinal strongyles in horses of Sardinia, Italy. Parasite Epidemiol. Control. 2016;1:15–19. doi: 10.1016/j.parepi.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scháňková Š., Maršálek M., Wagnerová P., Lukešová D., Starostová L., Jankovská I., Čadková Z., Kudrnáčová M., Brožová A., Truněčková J., Langrová I. Treatment failure of ivermectin for Oxyuris equi in naturally infected ponies in Czech Republic. Helminthologia. 2013;50:232–234. [Google Scholar]
- Scháňková Š., Maršálek M., Wagnerová P., Langrová I., Starostová L., Stupka R., Navrátil J., Brožová A., Truněčková J., Kudrnáčová M., Jankovská I., Vadlejch J., Čadková Z., Křivská D. Arrested development of experimental Cyathostominae infections in ponies in Czech Republic. Vet. Parasitol. 2014;2016:328–332. doi: 10.1016/j.vetpar.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Schougaard H., Nielsen M.K. Apparent ivermectin resistance of Parascaris equorum in Danish foals. Vet. Rec. 2007;160:439–440. doi: 10.1136/vr.160.13.439. [DOI] [PubMed] [Google Scholar]
- Seyoum Z., Zewdu A., Dagnachew S., Bogale B. Anthelmintic resistance of strongyle nematodes to ivermectin and fenbendazole on cart horses in gondar, northwest Ethiopia. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/5163968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea Porr C.A., Hedinger V.F., Hamm L.R., Ernst M.M., Papajeski B.M., Santiago M.L., Davis A.J. Effects of ivermectin and moxidectin on fecal egg count and egg reappearance rate in horses. J. Equine Vet. Sci. 2017;57:51–55. [Google Scholar]
- Silva P.A., Cernea M., Carvalho L.M.d. Anthelmintic resistance in equine nematodes – a review on the current situation, with emphasis in europe. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. 2019;76:133–142. [Google Scholar]
- Slocombe J.O.D., Coté J.F., de Gannes R.V.G. The persistence of benzimidazole-resistant cyathostomes on horse farms in Ontario over 10 years and the effectiveness of ivermectin and moxidectin against these resistant strains. Can. Vet. J. 2008;49:56–60. [PMC free article] [PubMed] [Google Scholar]
- Slocombe J.O.D., de Gannes R.V.G., Lake M.C. Macrocyclic lactone resistant Parascaris equorum on stud farms in Canada and effectiveness of fenbendazole and pyrantel pamoate. Vet. Parasitol. 2007;145:371–376. doi: 10.1016/j.vetpar.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Slocombe J.O.D., de Gannes R.V.G. Cyathostomes in horses in Canada resistant to pyrantel salts and effectively removed by moxidectin. Vet. Parasitol. 2006;140:181–184. doi: 10.1016/j.vetpar.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Smith H.J. Strongyle infections in ponies. I. Response to intermittent thiabendazole treatments. Can. J. Comp. Med. 1976;40:327–333. [PMC free article] [PubMed] [Google Scholar]
- Smith H.J. Strongyle infections in ponies. II. Reinfection of treated animals. Can. J. Comp. Med. 1976;40:334–340. [PMC free article] [PubMed] [Google Scholar]
- Smith H.J. Experimental Trichonema infections in mature ponies. Vet. Parasitol. 1978;4:265–273. [Google Scholar]
- Smith M.A., Nolan T.J., Rieger R., Aceto H., Levine D.G., Nolen-Walston R., Smith B.I. Efficacy of major anthelmintics for reduction of fecal shedding of strongyle-type eggs in horses in the Mid-Atlantic region of the United States. Vet. Parasitol. 2015;214:139–143. doi: 10.1016/j.vetpar.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Stoneham S., Coles G. Ivermectin resistance in Parascaris equorum. Vet. Rec. 2006;158:572. doi: 10.1136/vr.158.16.572-b. [DOI] [PubMed] [Google Scholar]
- Stratford C.H., Lester H.E., Pickles K.J., McGorum B.C., Matthews J.B. An investigation of anthelmintic efficacy against strongyles on equine yards in Scotland. Equine Vet. J. 2014;46:17–24. doi: 10.1111/evj.12079. [DOI] [PubMed] [Google Scholar]
- Stratford C.H., Lester H.E., Morgan E.R., Pickles K.J., Relf V., McGorum B.C., Matthews J.B. A questionnaire study of equine gastrointestinal parasite control in Scotland. Equine Vet. J. 2014;46:25–31. doi: 10.1111/evj.12101. [DOI] [PubMed] [Google Scholar]
- Studzińska M.B., Sallé G., Roczeń-Karczmarz M., Szczepaniak K., Demkowska-Kutrzepa M., Tomczuk K. A survey of ivermectin resistance in Parascaris species infected foals in south-eastern Poland. Acta Vet. Scand. 2020;62:28. doi: 10.1186/s13028-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarigo-Martinie J.L., Wyatt A.R., Kaplan R.M. Prevalence and clinical implications of anthelmintic resistance in cyathostomes of horses. J. Am. Vet. Med. Assoc. 2001;218:1957–1960. doi: 10.2460/javma.2001.218.1957. [DOI] [PubMed] [Google Scholar]
- Taylor S.M., Kenny J. Comparison of moxidectin with ivermectin and pyrantel pamoate for reduction of faecal egg counts in horses. Vet. Rec. 1995;137:516–518. doi: 10.1136/vr.137.20.516. [DOI] [PubMed] [Google Scholar]
- Toscan G., Cezar A.S., Pereira R.C.F., Silva G.B., Sangioni L.A., Oliveira L.S.S., Vogel F.S.F. Comparative performance of macrocyclic lactones against large strongyles in horses. Parasitol. Int. 2012;61:550–553. doi: 10.1016/j.parint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Traversa D., Klei T.R., Iorio R., Paoletti B., Lia R.P., Otranto D., Sparagano O.A., Giangaspero A. Occurrence of anthelmintic resistant equine cyathostome populations in central and southern Italy. Prev. Vet. Med. 2007;82:314–320. doi: 10.1016/j.prevetmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Traversa D., von Samson-Himmelstjerna G., Demeler J., Milillo P., Schurmann S., Barnes H., Otranto D., Perrucci S., di Regalbono A.F., Beraldo P., Boeckh A., Cobb R. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasites Vectors. 2009;2(Suppl. 2):S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Sconza S., Seghetti M., di Regalbono A.F., Milillo P., Marcer F., Beraldo P. Efficacy of moxidectin against fenbendazole-resistant cyathostomins. Ippologia. 2011;22:19–23. [Google Scholar]
- Traversa D., Castagna G., von Samson-Himmelstjerna G., Meloni S., Bartolini R., Geurden T., Pearce M.C., Woringer E., Besognet B., Milillo P., D'Espois M. Efficacy of major anthelmintics against horse cyathostomins in France. Vet. Parasitol. 2012;188:294–300. doi: 10.1016/j.vetpar.2012.03.048. [DOI] [PubMed] [Google Scholar]
- Tzelos T., Barbeito J.S.G., Nielsen M.K., Morgan E.R., Hodgkinson J.E., Matthews J.B. Strongyle egg reappearance period after moxidectin treatment and its relationship with management factors in UK equine populations. Vet. Parasitol. 2017;237:70–76. doi: 10.1016/j.vetpar.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Várady M., Königová A., Corba J. Benzimidazole resistance in equine cyathostomes in Slovakia. Vet. Parasitol. 2000;94:67–74. doi: 10.1016/s0304-4017(00)00366-6. [DOI] [PubMed] [Google Scholar]
- Vera J.H.S., Fachiolli D.F., Ramires L.M., Saes I.d.L., Yamada P.H., Gonçalves J.A., Oliveira K.d., Amarante A.F.T.d., Ricardo Velludo Gomes de Soutello R.V.G.d. Efficacy of ivermectin, moxidectin and fenbendazole in equine in Brazil. Vet. Parasitol. Reg. Stud. Rep. 2020;20 doi: 10.1016/j.vprsr.2020.100374. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Moretta I., Moretti A., Fioretti D.P., Genchi C. Field effectiveness of pyrantel and failure of Parascaris equorum egg count reduction following ivermectin treatment in Italian horse farms. Vet. Parasitol. 2009;161:138–141. doi: 10.1016/j.vetpar.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Fioretti D.P., Genchi C. Are macrocyclic lactones useful drugs for the treatment of Parascaris equorum infections in foals? Vet. Parasitol. 2010;172:164–167. doi: 10.1016/j.vetpar.2010.04.019. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G., von Witzendorff C., Sievers G., Schnieder T. Comparative use of faecal egg count reduction test, egg hatch assay and beta-tubulin codon 200 genotyping in small strongyles (cyathostominae) before and after benzimidazole treatment. Vet. Parasitol. 2002;108:227–235. doi: 10.1016/s0304-4017(02)00197-8. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G., Fritzen B., Demeler J., Schurmann S., Rohn K., Schnieder T., Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet. Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G. Anthelmintic resistance in equine parasites – detection, potential clinical relevance and implications for control. Vet. Parasitol. 2012;185:2–8. doi: 10.1016/j.vetpar.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Vyšniauskas A., Kaziūnaitė V., Kharchenko V.A., Pereckienė A., Tolliver S.C., Lyons E.T. Determining anthelmintic resistance of cyathostomes using anthelmintics from two drug classes. Med. Weter. 2006;62:883–886. [Google Scholar]
- Wilkes E.J.A., McConaghy F.F., Thompson R.L., Dawson K., Sangster N.C., Hughes K.J. Efficacy of a morantel–abamectin combination for the treatment of resistant ascarids in foals. Aust. Vet. J. 2017;95:85–88. doi: 10.1111/avj.12559. [DOI] [PubMed] [Google Scholar]
- Wirtherle N., Schnieder T., von Samson-Himmelstjerna G. Prevalence of benzimidazole resistance on horse farms in Germany. Vet. Rec. 2004;154:39–41. doi: 10.1136/vr.154.2.39. [DOI] [PubMed] [Google Scholar]
- Wolf D., Hermosilla C., Taubert A. Oxyuris equi: lack of efficacy in treatment with macrocyclic lactones. Vet. Parasitol. 2014;201:163–168. doi: 10.1016/j.vetpar.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Xiao L.H., Herd R.P., Majewski G.A. Comparative efficacy of moxidectin and ivermectin against hypobiotic and encysted cyathostomes and other equine parasites. Vet. Parasitol. 1994;53:83–90. doi: 10.1016/0304-4017(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Yazwinski T.A., Hamm D., Williams M., Greenway T., Tilley W. Effectiveness of ivermectin in the treatment of equine Parascaris equorum and Oxyuris equi infections. Am. J. Vet. Res. 1982;43:1095. [PubMed] [Google Scholar]
- Zak A., Siwinska N., Slowikowska M., Borowicz H., Kubiak K., Hildebrand J., Popiolek M., Niedzwiedz A. Searching for ivermectin resistance in a Strongylidae population of horses stabled in Poland. BMC Vet. Res. 2017;13:210. doi: 10.1186/s12917-017-1133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet S., Battisti E., Labate F., Oberto F., Ferroglio E. Reduced efficacy of fenbendazole and pyrantel pamoate treatments against intestinal nematodes of stud and performance horses. Vet. Sci. 2021;8:42. doi: 10.3390/vetsci8030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouiten H., Berrag B., Oukessou M., Sadak A., Cabaret J. Poor efficacy of the most commonly used anthelmintics in sport horse nematodes in Morocco in relation to resistance. Parasite. 2005;12:347–351. doi: 10.1051/parasite/2005124347. [DOI] [PubMed] [Google Scholar]


