Abstract
Antibody-based therapeutics are now standard in the treatment of neuroinflammatory diseases, and the spectrum of neurological diseases targeted by those approaches continues to grow. The efficacy of antibody-based drug platforms is largely determined by the specificity-conferring antigen-binding fragment (Fab) and the crystallizable fragment (Fc) driving antibody function. The latter provides specific instructions to the immune system by interacting with cellular Fc receptors and complement components. Extensive engineering efforts have enabled tuning of Fc functions to modulate effector functions and to prolong or reduce antibody serum half-lives. Technologies that improve bioavailability of antibody-based treatment platforms within the CNS parenchyma are being developed and could invigorate drug discovery for a number of brain diseases for which current therapeutic options are limited. These powerful approaches are currently being tested in clinical trials or have been successfully translated into the clinic. Here, we review recent developments in the design and implementation of antibody-based treatment modalities in neurological diseases.
Keywords: antibody, therapy, neurology, IgG, Fc
Ruck et al. describe recent developments in next-generation antibody-based therapies for brain diseases, including progress in Fc engineering, and efforts to improve the bioavailability of antibody-based platforms in CNS parenchyma. They highlight the potential benefits as well as limitations of such therapies in neurology.
Introduction
Therapeutic antibodies can be separated into two broad categories. The first category comprises intravenous immunoglobulins (IVIg), a preparation of polyclonal serum IgG pooled from thousands of blood donors; recombinantly produced monoclonal antibodies (mAbs) represent a second category.1 While IVIg products have been used to treat neurological disease conditions such as epilepsy or neuromuscular diseases since the 1980s,2 it was not before 2004 that mAbs received regulatory approval for a neurological indication. Natalizumab, marketed as Tysabri®, was the first mAb to be approved in the USA and Europe for the treatment of multiple sclerosis.3 The distinction between both categories started to blur with the technological development of recombinant replacement products for IVIg, based on progress in our understanding of IVIg's mechanisms of action and the subsequent use of these technologies to additionally improve mAbs. So far, mAbs and IVIg are used for treating a wide and growing spectrum of neurological diseases (Table 1), and neurological disease conditions are among the most frequent non-cancer indications for testing the safety and efficacy of new Ab-based treatment platforms.4,5 All of the compounds discussed in our article are designed based on the structure and function of Abs. Since there is no specific term to encompass all the newly developed Ab-based drugs, we chose the term ‘Ab-based therapeutics’ for simplicity reasons to cover all Ab- and recombinant Ig-domain-based molecules. Here, we illustrate the biology of Ab-based therapeutics and highlight new technologies that could reinvigorate drug discovery for a number of brain diseases for which current therapeutic options are limited.
Table 1.
Antibody-based treatments in neurological diseases
| Indications | Antibody/FDA approval | Molecular target | IgG subclass; Fc variant; Fc function | Key references |
|---|---|---|---|---|
| Alzheimer’s disease | Aducanumab/2021 | Aggregated amyloid-β | Human IgG1; n/a; binds aggregated amyloid-β forms | Sabbagh et al.112 |
| CIDP/on-label GBS/off-label MMN/on-label MG/off-label LEMS/off-label Myositis/off-label |
Intravenous Immunoglobulins/off-label | Immune modulation | All subclasses; n/a; pleiotropic effects | Chen et al.2 Lünemann et al.115 |
| Glioblastoma | Bevacizumab/2017 (not approved by EMA) | VEGF | Humanized IgG1k; n/a; binds VEGF | Wick et al.116 Friedman et al.117 |
| Migraine | Erenumab/2018 | CGRP receptor | Human IgG2; n/a; competes for binding to CGRP receptor | Dodick et al.118 Goadsby et al.119 Reuter et al.120 |
| Migraine | Fremanezumab/2018 | CGRP | Humanized IgG2; n/a; binds CGRP | Ferrari et al.121 Silberstein et al.122 Dodick et al.123 |
| Migraine/cluster headache | Galcanezumab 2018 and 2019 | CGRP | Humanized IgG4; S228P, F234A, L235A; binds CGRP | Detke et al.124 Skljarevski et al.125 Stauffer et al.126 Goadsby et al.127 Dodick et al.128 |
| Migraine | Eptinezumab/2020 (not yet approved by EMA) | CGRP | Humanized IgG1; N297A; binds CGRP | Lipton et al.129 Silberstein et al.130,131 |
| NMOSD MG |
Eculizumab/2019 and 2017 | Complement factor 5 (C5) | Humanized IgG2/4; IgG2 until T260, then IgG4; binds and inhibits cleavage of C5 | Pittock et al.132 Howard et al.133 Muppidi et al.134 |
| NMOSD | Inebilizumab/2020 | CD19 | Humanized IgG1k; afucosylated; ADCC | Cree et al.135 |
| NMOSD | Satralizumab/2020 | IL-6 receptor | Humanized IgG2; SMART-Ig®; binds membrane-bound and soluble IL-6 receptors | Traboulsee et al.27 Yamamura et al.29 |
| RRMS | Natalizumab/2004 | Integrin α4β1 | Humanized IgG4k; n/a; binds α4β1 | Yednock et al.136 Rudick et al.137 Polman et al.138 |
| RRMS | Alemtuzumab/2013 | CD52 | Humanized IgG1κ mAb; n/a; ADCC > CDC | Ruck et al.139 Cohen et al.140 Coles et al.141 |
| RRMSPPMS | Ocrelizumab/2017 and 2017 | CD20 | Humanized IgG1; n/a; ADCC | Hauser et al.9 Bittner et al.142 Montalban et al.143 |
| RRMS/off-label NMOSD/off-label MG/off-label Myositis/off-label |
Rituximab/off-label | CD20 | Chimeric IgG1k; n/a; ADCC + CDC | Yamout et al.144 De Flon et al.145 Hauser et al.146 Cabre et al.147 Nikoo et al.148 Nowak et al.149 Díaz-Manera et al.150 Stieglbauer et al.151 Oddis et al.152 |
CDC = complement-dependent cytotoxicity; CGRP = calcitonin-gene related peptide; CIDP = chronic inflammatory demyelinating polyneuropathy; EMA = European Medicines Agency; FDA = Food and Drug Administration; GBS = Guillain–Barré syndrome; IL = interleukin; LEMS = Lambert–Eaton myasthenic syndrome; MMF = multifocal motor neuropathy; MG = myasthenia gravis; PPMS = primary progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis. A fucosylation of the Fc glycan increases the FcγRIIIA binding affinity and enhances ADCC.59 The S228P Fc mutation increases stability by abolishing formation of half antibody molecules. F234A/L235A/N297A mutations and IgG2/4 fusion lead to reduced FcγR and C1q (complement) binding.95,153 SMART-Ig® increases FcRn binding at pH 6.0 and increases half-life.
Harnessing IgG-Fc biology to improve therapeutic antibodies
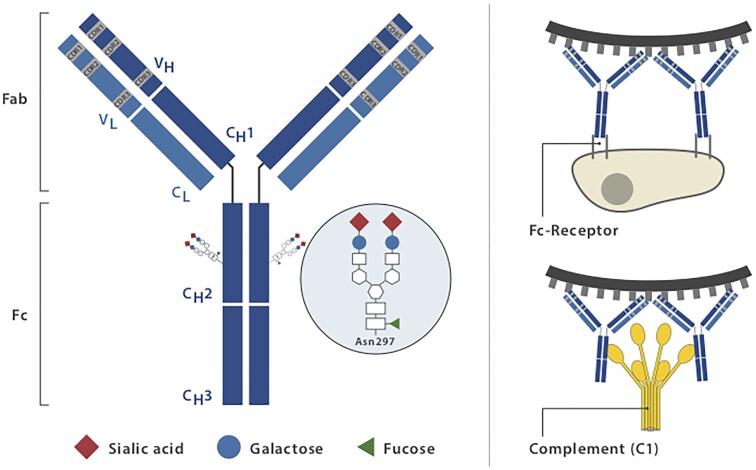
The design and clinical implementation of therapeutic antibodies are principally based on the biological functions of IgG molecules that confer protection against infectious diseases. Immunoglobulins evolved to specifically recognize target structures (antigens) mediated by the fragment antigen-binding (Fab) domains, while the fragment crystallizable (Fc) domain contains the binding sites for immune effector molecules such as the C1q component of the complement system and through binding to Fc receptors. (Fig. 1).6 In the context of infectious diseases, the Fab domain may directly prevent infection by neutralizing pathogens.7 The Fc domain triggers immune effector functions by interacting with Fc receptors, c-type lectins or the complement system to ensure that antibody-opsonized material can additionally be visualized and appropriately eliminated by the immune system.8 The same principles apply to monoclonal and polyclonal therapeutic antibodies. Their simplest mode of action is to bind to target molecules and thereby interfere with their activity and interaction with binding partners. However, even those mAbs specifically produced to block soluble or membrane-bound target molecules elicit Fc-mediated effector functions as long as they contain a functional Fc domain.8 Other mAbs, for example, CD20-targeting antibodies, are specifically designed to recruit immune effectors through their Fc domain after binding to their target epitopes.9 Cell-depleting therapeutic IgG antibodies such as those targeting B lineage cells lyse target cells through at least three mechanisms: antibody-dependent cell-mediated cytotoxicity (ADCC) triggered by signalling through activating Fc receptors (FcγRs) expressed by cytotoxic innate immune effector cells, including natural killer cells or myeloid cells; complement-dependent cytotoxicity through binding of C1q, which initiates activation of the classical complement pathway and antibody-dependent cellular phagocytosis mediated by phagocytes recognizing opsonized target cells.10 In vitro assays provided evidence that all of the described Fc-mediated effector mechanisms may contribute to the depleting efficacy of a single mAb.11 To what extent these different effector mechanisms contribute to cell-depleting or, in general, therapeutic Ab activity in vivo is less well understood and might depend on the disease condition treated and on the organ environment in which the antibody mediates its activity. It has become clear across many preclinical animal model systems that cytotoxic antibody binding to cellular FcγRs is critical for their therapeutic activity in vivo.12,13 New developments in Fc-engineering technologies are now being used to specifically address and improve particular effector functions and to create entirely new Ab-based therapies. Improving access to the CNS by mAb modifications is another important target for Ab- and Ig-domain-based therapies (see section ‘Evolving strategies to overcome the blood–brain barrier’).
Figure 1.
Structure and effector functions of immunoglobulin G (IgG). IgG is composed of two heavy and two light chains linked by disulphide bonds. The antigen-binding fragment (Fab) consists of two moieties with identical structures, which define the antigen-specificity through their complementarity-determining regions CDR), highlighted in grey. The crystallizable fragment (Fc) mediates antibody effector functions through binding to Fc receptors and interaction with the C1q component of the complement system. A highly conserved IgG-Fc N-glycan (Fc glycan) is attached to each of the asparagine 297 (N297) residues in the CH2-domains of the two Fc fragments. The Fc glycan has an essential role on Ab structure and function. Its common core-structure consists of an N-acetylglucosamine (GlcNAc) attached to the asparagine, to which a second GlcNAc and three mannoses are attached. This core can be further extended by a bisecting GlcNAc attached to the core mannose (not shown) as well as by galactose (blue circle), sialic acid (red diamond) and fucose (green triangle) residues.
IgG-Fc engineering generates a growing repertoire of antibody-based therapeutics
The costs of producing entire multimeric therapeutic antibodies and the supply shortages for IVIg generated an urgent need for alternatives. The most promising developments are: first, recombinant antibody preparations that degrade IgG; second, multimeric IgG-Fc preparations that block binding to activating Fcγ receptors and finally, IVIg preparations with enhanced levels of anti-inflammatory sialic acid-rich IgG glycovariants (sIVIg). Similar Fc-engineering technologies have been applied to modify therapeutic mAbs. These developments will be outlined next.
Targeting degradation
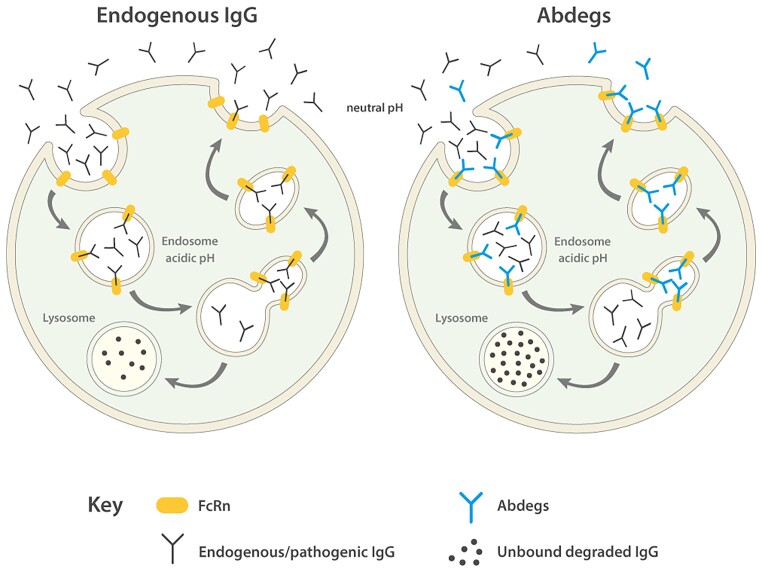
The neonatal Fc receptor (FcRn) is a major histocompatibility class I-related receptor responsible for the transfer of humoral immunity from the mother to the newborn. Throughout life, FcRn contributes to effective humoral immunity by recycling IgG and extending its half-life in the circulation (Fig. 2). The receptor, mainly expressed by endothelial and myeloid cells, binds tightly to the Fc portion of IgG at acidic pH (pH 6.0) but not at physiological pH (pH 7.4). The cells internalize serum IgG, which binds to FcRn in an acidic endosomal compartment. FcRn then recycles IgG back to the cell surface where it releases IgG at physiological pH, thus extending its serum half-life, which ranges between 3 and 4 weeks, the longest of any plasma protein.14 The rate of FcRn-mediated IgG recycling has been estimated to be 40% greater than the rate of IgG production, indicating that recycling of IgG, and not its production, is the dominant process for maintaining the IgG plasma concentration in humans.15 Serum proteins that do not bind to a recycling receptor are destined for lysosomal degradation.14 FcRn-mediated recycling can be blocked therapeutically by Abdegs (Abs that enhance IgG degradation). Abdegs are engineered to have Fc regions that bind to FcRn with an unusually high affinity at both near neutral and acidic pH, thereby out-competing endogenous antibody binding to FcRn and forcing the rapid catabolism of pathogenic antibodies.16 One example of an Abdeg is efgartigimod, a recombinant IgG1 Fc portion mutated at five residues to increase FcRn affinity. A single administration of efgartigimod in humans reduced total IgGs by about 50%, while repeated administration at a saturating dose of 10 mg/kg further lowered IgG levels by ∼75%.17 Enhancing the degradation of endogenous IgG might also contribute to the anti-inflammatory efficacy of high doses of IVIg, which oversaturate FcRn.18 Alternative strategies to interfere with IgG-FcRn interactions are mAbs or antibody variable fragments that block the FcRn binding to IgG, for example, rozanolixizumab or nipocalimab, humanized high-affinity anti-human FcRn monoclonal antibodies or small molecules inhibiting FcRn function.19,20 To date, the results from studies in non-human primates and clinical trials for several FcRn-based inhibitors indicate that they induce significant and sustained decreases in endogenous IgG levels in healthy volunteers while being safe and well-tolerated, and also have beneficial clinical efficacy in patients with myasthenia gravis, as outlined next (NCT03457649, NCT03971422, NCT03052751).17,19
Figure 2.
Harnessing FcRn function through Abdegs. IgG enters cells by fluid-phase pinocytosis in small tubulovesicular transport carriers that fuse with larger, FcRn-positive early acidic endosomes in which binding to FcRn can occur. Bound IgG molecules are recycled and released by exocytosis, involving the fusion of recycling compartments with the plasma membrane. By contrast, IgG that does not bind to FcRn in sorting endosomes enters lysosomes and is degraded. Abdegs bind to FcRn with an increased affinity at both near neutral and acidic pH and compete with endogenous IgGs for FcRn binding in acidic endosomes. Consequently, more endogenous IgG molecules are driven into lysosomes and are degraded.
Seldegs (selective degradation of antigen-specific antibodies) have been designed to selectively deplete antibodies of a particular antigen (Ag)-specificity while avoiding global reduction in IgG levels. Seldegs consist of an Ag molecule combined with an Fc domain with increased affinity to FcRn at both near neutral and acidic pH. Consequently, circulating Abs that bind to Ag-Fc fusion proteins are delivered to lysosomes for enhanced degradation. Due to their Ag-specificity, seldegs can be applied at lower doses as compared to the other FcRn-targeting approaches and are, therefore, less prone to lower total IgG levels.21 While seldegs have been shown to capture Ag-specific antibodies, such as myelin oligodendrocyte glycoprotein (MOG)-targeting antibodies, and direct them into degradative lysosomal compartments in vitro,21 their therapeutic efficacy in vivo has yet to be shown.
In principle, those Fc domain modifications promoting FcRn interaction can also be harnessed to increase serum persistence of Ab-based therapeutics resulting in reduced dose and administration frequencies.22 A strategy that might be of special interest in the treatment of chronic diseases, where—despite their long half-lives—mAb must be administered repetitively. However, Fc domain modifications might also interfere with biological function and clinical efficacy of the mAb. Of note, Fc variants [e.g. YD (M252Y/T256D) DQ (T256D/T307Q) and DW (T256D/T307W)] that improve serum half-life while retaining effector functions in vitro by enhanced FcRn binding have already been identified,23,24 whereas their efficacy in vivo awaits evaluation in preclinical disease models.
Lysosomal degradation can also be used to enhance Ab-mediated clearance of antigens. So-called acid-switched antibodies are designed to bind their target antigen with a higher affinity at near-neutral pH than at acidic pH. Ag–Ab complexes enter acidic sorting endosomes in which Ag dissociates from Abs and enters the lysosomal pathway for degradation while the FcRn-bound antibody is recycled.16 Most acid-switched antibodies have been generated to support degradation antigens that exist in soluble forms, such as the complement factor C5 or interleukin-6 (IL-6), but are also feasible for IL-6R, which can be membrane-bound or soluble.25,26 One example is satralizumab, an anti-IL-6 receptor monoclonal antibody optimized for FcRn binding and recently approved for the treatment of aquaporin 4 water channel autoantibody (AQP4-IgG) seropositive neuromyelitis optica spectrum disorder (NMOSD).27,28 In the acidic environment of the late endosomal compartment, satralizumab bound to FcRn dissociates from the IL-6 receptor, is transported back to the plasma membrane and released from FcRn, ready to bind another IL-6 receptor.25 The two phase 3 trials SakuraSky (satralizumab as add-on therapy to immunosuppressants) and SakuraStar (satralizumab monotherapy) demonstrated significant reduction of relapse rates in NMOSD for satralizumab treatment compared to placebo. In contrast to other trials, also seronegative NMOSD patients were included, however, SakuraSky did not detect significant reduction of relapse rate in this subgroup.27,29,30
Targeting FcγR signalling
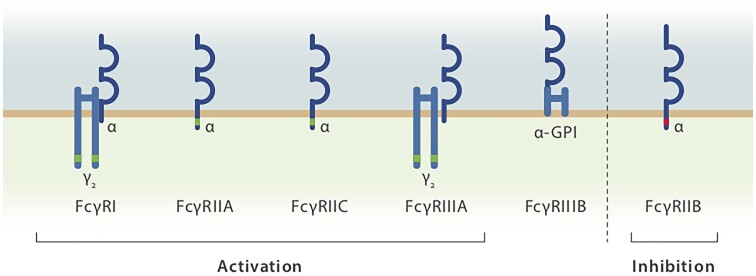
The family of FcγRs consists of several activating members (FcγRIA, IIA, IIC, IIIA and IIIB in humans) and one inhibitory member (FcγRIIB) (Fig. 3). Both activating and the inhibitory receptors are co-expressed on many innate immune effector cells residing in lymphoid organs such as macrophages.31–33 Thus, Abs and immune complexes (IC) trigger both activating and inhibitory signalling pathways. FcγRIIB is the only FcγR expressed on B cells, in which it transduces an inhibitory signal on colligation with the B cell receptor; among circulating blood cells, FcγRIIB expression levels are highest on B cells and basophils.31,32,34 The observation that the IgG-Fc fragment is the predominant mediator of the anti-inflammatory activity of IVIg spurred interest in FγR-dependent anti-inflammatory signalling.35,36 Notably, aged IVIg preparations were found to be more potent in suppressing autoantibody activity in an immune thrombocytopenia (ITP) animal model due to an increase in IgG dimers within the IVIg preparation.37 Furthermore, the critical role of activating FcγRs in mediating autoantibody activity in humans was demonstrated by a clinical trial using FcγR-specific blocking antibodies to ameliorate autoimmune pathology in ITP patients.38 On the basis of the aforementioned findings, several groups started to develop recombinant IgG multimers. These synthetic small immune complex (IC-)like molecules bind to cellular FcγRs without triggering cell activation and competitively block the binding of autoantibodies to these receptors.39 IgG multimers show therapeutic efficacy in experimental animal models of autoimmune neuritis and myasthenia gravis as well as in preclinical models of ITP, inflammatory arthritis and skin blistering diseases.40–44 Recombinant human IgG-Fc multimers have been developed, and preclinical data support the potential of Fc multimers as a synthetic alternative to IVIg.45 The mechanisms by which Fc multimers confer their anti-inflammatory activity might well go beyond simply shielding activating FcγR from auto-Abs or pathogenic ICs. Immunomodulatory effects similar to those reported for IVIg have been described in preclinical models, including FcRn blockade, stimulation and upregulation of the inhibitory FcγRIIB, and expansion of regulatory T cells.44
Figure 3.
The human FcγR family. FcγRIA, FcγRIIA/IIC and FcγRIIIA initiate activating signalling pathways via immune tyrosine-based activation motifs (ITAMs, highlighted in green), whereas FcγRIIB is an inhibitory FcγR carrying an immune tyrosine-based activation motif (ITIM, highlighted in red). FcγRIII lacks a signalling domain. The strength of the signal mediated through both activating and inhibitory FcγRs, which are often co-expressed on inflammatory immune cells, sets the threshold for the initiation of FcγR-dependent effector responses.
Alternative therapeutic strategies to modulate FcγR signalling that are currently being tested in the context of autoimmunity include mAbs directed against activating FcγRs (FcγRI, FcγRIIIA) or the inhibitory FcγRIIB with anti-inflammatory agonistic function. For example, the mAb SM201 recognizes an epitope outside the IgG-binding site of FcγRIIB and mediates IC dependent inhibition of B cells in vitro, whereas the antagonistic FcγRI mAb 197 induced clinical improvement and specific down-modulation of FcγRI expression on monocytes in an ITP patient 38,46–48 In contrast, to support anti-tumour immunity, FcγRIIB antagonistic mAb are being developed. For example, a fully human FcγRIIB antagonistic antibody was shown to overcome resistance to and to functionally augment anti-tumour activity of rituximab in chronic lymphocytic leukaemia.49
Tyrosine-kinase inhibitors harness effector functions similar to Abs even though they cannot be defined as Ab-based therapeutics. Tyrosine-kinase inhibitors target signalling molecules downstream of Ab-induced crosslinking of activating FcγRs, such as the common FcRγ-chain, the spleen tyrosine kinase (Syk) and the Bruton’s tyrosine kinase (BTK). These play a critical role in activating innate immune effector cells and initiating the release of pro-inflammatory cytokines, chemokines and other pro-inflammatory events.50,51 Therefore, small inhibitory molecules directed to TK have been identified as a potential pathway to block autoimmune inflammation. Spleen tyrosine-kinase inhibitors, for example, fostamatinib, have shown potent activity in blocking ITP in mice and humans (NCT00706342) and have been approved by the FDA for chronic ITP.52 Along the same lines, BTK inhibitors such as rilzabrutinib have shown promising results in investigational studies in patients with ITP and a phase 3 trial is ongoing (NCT04562766).53 Other BTK inhibitors showed promising results in patients with relapse-onset multiple sclerosis,54 resulting in three ongoing phase 3 studies (NCT04338022, NCT04338061 and NCT04586023).
Targeting Fc glycosylation
Glycosylation of IgG-Fc domains contributes to both the stability and biological activity of antibody molecules and is essential for effector functions.55 The N-linked glycosylation at asparagine 297 in the Fc domain of IgG1 is composed of a heptameric core sugar structure with variable amounts of branching and terminal sugar residues such as galactose, sialic acid (SA), N-acetylglucosamine and fucose 56 (Fig. 1). Fc glycosylation changes have been exploited in the monoclonal therapeutics field. Removal of fucose from the core biantennary structure of the IgG1 glycan enhances FcγRIIIA binding and ADCC.57,58 These observations led to the development of so-called glycoengineered, i.e. afucosylated, therapeutic cell-depleting mAbs such as the CD20-targeting antibodies obinutuzumab or ublituximab.59 Afucosylation has become a clinically approved strategy to improve the efficacy of anti-cancer antibodies through enhanced ADCC.60,61 Whether enhanced ADCC translates into increased clinical efficacy in neurological diseases as compared to fucosylated CD20-targeting mAbs remains to be shown. Ublituximab is currently being tested in phase 3 clinical trials in patients with relapse-onset multiple sclerosis and has also been investigated in a pilot safety study in patients with aquaporin-4 IgG+ NMOSD (NCT02276963).62 A potential benefit is that its increased biological efficacy may allow lower doses and shorter infusion times versus other anti-CD20 mAbs.63
The addition of terminal sialic acid to the Fc glycan is thought to improve Fc domain binding to non-classical Fc receptors such as lectins and to confer anti-inflammatory activities to IgG molecules.64,65 In animal models of autoantibody-mediated tissue inflammation, IVIg preparations and isolated Fc fragments enriched for terminal sialic acid residues have a >10-fold higher anti-inflammatory activity than non-enriched preparations, while the removal of sialic acid residues results in reduced immunoprotective activity.66–68 Despite strong evidence from studies in various model system for an important role of sialylation in the in vivo therapeutic activity of IVIg,69 desialylation of IgG has shown no clinical effect in some animal models of autoimmunity, for example, experimental autoimmune encephalomyelitis (model for multiple sclerosis).70,71 Recently, highly tetrasialylated IVIg, in which both sugar domains contained the maximal level of two sialic acid residues, were tested for clinical efficacy in comparison to IVIg in a small ITP patient cohort and showed superior clinical efficacy (NCT03866577).72 To date, no studies have been performed using tetrasialylated IVIg in IVIg-responsive neurological disease.
Evolving strategies to overcome the blood–brain barrier
Currently, neurological diseases susceptible to Ab-based therapies such as multiple sclerosis or neuromuscular disorders are driven, at least in part, by immune factors accessible outside of the CNS, whereas diseases largely confined to the CNS parenchyma, such as neurodegenerative disease conditions, are less accessible to Ab-based therapies.73
Among the largest obstacles to effective CNS delivery is the blood–brain barrier, formed by tight junctions between brain endothelial and epithelial cells that limit the transfer of therapeutic molecules between the blood and the interstitial fluid of the CNS.73 Large molecules such as Abs can only traverse the blood–brain barrier by receptor-mediated transport through endothelial cells. The transferrin receptor (TfR) and the insulin receptor (IR) expressed by endothelial cells are natural brain portals, and mAbs specific for either one of these receptors could improve the ability of Abs to penetrate the CNS parenchyma.74–76 Indeed, bi-specific Ab platforms targeting the TfR or IR on the one hand and CNS disease-associated targets such as BACE1 (β-site amyloid precursor protein-cleaving enzyme 1), an enzyme that cleaves the amyloid precursor protein to generate the pathogenic form of amyloid-β in Alzheimer disease, on the other hand, have been developed with promising results in preclinical models.76–78 The drawbacks of this strategy are that both the TfR and IR are not specific for brain endothelial cells and have essential physiological functions that have raised important safety concerns.79
Alternative attempts to use receptor-mediated transcytosis to increase brain uptake of therapeutic Abs with binding sites distant from the natural ligands of TfR or IR are currently being developed and could reinvigorate drug discovery for a number of brain diseases for which current therapeutic options are limited.80,81
Engineering antibody therapeutics to improve treatment safety
mAb side-effects are the result from the interaction with the target protein and/or its function, off-target effects due to antibody polyspecificity or reactions to the foreign proteins by the host immune system.82
A prominent example for a target-mediated side-effect of mAb is the cytokine release syndrome, which is often found for cell-depleting mAb such as rituximab and alemtuzumab.83 The release of cytokines including interferon gamma (IFNγ), tumour necrosis factor alpha (TNFα) and interleukin-6 (IL-6) leads to systemic inflammation and corresponding symptoms such as fever, chills and malaise, and might proceed to multi-organ failure.83 Another prominent target-mediated side-effect in neurology is progressive multifocal leukoencephalopathy (PML) in the context of natalizumab. Natalizumab (Tysabri®) is a recombinant, humanized mAb to integrin α-4 and inhibits the interaction with VCAM-1 (vascular cell adhesion molecule-1) on endothelial cells of the blood–brain barrier. Thereby, natalizumab prevents the transmigration of activated lymphocytes and monocytes into the CNS. The reduced immunosurveillance of the CNS is assumed to be the cause for opportunistic JC-virus infections that cause PML.84
Polyspecificity of mAbs describes the binding of multiple epitopes on different antigens by one antibody. The identified main mechanism of polyspecificity are rigid adaptation, conformational flexibility and differential ligand positioning.82 For rituximab, a nonimmune off-target effect has been reported in recurrent focal segmental glomerulosclerosis after renal transplantation, where it was shown to bind SMPDL-3b (sphingomyelinase-like phosphodiesterase 3b protein) improving podocyte survival.85 However, whether off-target effects are also associated with clinical relevant adverse events is understudied and has not been investigated in detail so far.
As a reaction to foreign proteins are generated antidrug-antibodies (ADAs) by T cell dependent and independent mechanism. ADAs can bind to the therapeutic antibody, diminish its effect and lead to the formation of ICs. ICs can induce type III hypersensitivities such as serum sickness with fever and lymphadenopathy occurring 6–21 days after drug administration.86 Particularly antibodies of rodent origin cause intense ADA responses, however also humanized and fully human mAb can induce ADA responses. For example, around 30% of patients with multiple sclerosis treated with the chimeric rituximab developed ADAs,87 whereas humanized natalizumab induced ADAs in around 6%.88 However, the humanized alemtuzumab induced ADAs in 85% of treated multiple sclerosis patients.89 Therefore, sequence homology is not the only determinant of ADA generation. Additional factors might include the biopharmaceutical parameters, patient’s background and specific treatment factors (administration route and duration).82 Further posttranslational modifications such as glycosylation occurring after protein synthesis or while manufacturing and storage can influence immunogenicity and ADA generation of mAbs.90
Also, specific Fc modifications might influence adverse event risk. For example, afucosylated mAbs might increase infusion-related reactions due to enhanced affinities to FcγRIII.61
Different engineering strategies have been developed to counteract those undesired mAb effects. Chimerization and humanization are used to reduce immunogenicity of mAb.82 Further, unwanted immunogenic reactions through FcγR and C1q binding can be ameliorated by Fc isotype selection or Fc modification. IgG2 and IgG4 are used when strong effector (ADCC or complement-dependent cytotoxicity, respectively) functions are undesired and ADCC is suppressed by specific Fc mutations (e.g. L234A/L235A, L234A/L235A/P329G).91,92 Moreover, different computational models have been developed to detect immunogenic T cell epitopes, which might be used to engineer mAbs with less ADA induction.93 ADA induction through posttranslational modifications can be avoided by optimization of production and storage processes as well as by stabilizing sequence mutations.82,94
Next-generation antibody therapeutics in neurology: evidence from clinical trials
Currently approved mAbs in neurology are already very diverse in terms of IgG subclasses, Fc variants and Fc functions (Table 1). The next generation of mAb will implement sophisticated Fc engineering to provide superior characteristics compared to earlier mAb. Those characteristics might comprise optimized serum half-lives, enhanced or reduced effector functions such as target cell depletion, or Fc functions in selected targets. Many of those new concepts are currently subject to clinical investigation in several neurological disorders, and are outlined in the following as well as in Table 2.
Table 2.
Engineered next-generation antibody therapeutics in neurology: clinical trials
| Next-gen mechanism | Exemplary mAb or Ab biologics | Target | Neurological indications | Clinical trialsa |
|---|---|---|---|---|
| Optimized half-life | Ravulizumab | Complement factor 5 | MG | Phase 3, NCT03920293 |
| NMOSD | Phase 3, NCT04201262 | |||
| Satralizumab | IL-6 receptor | NMOSD | Phase 3, NCT02073279, NCT02028884 | |
| Enhanced Fc effector function/IgG stability | Ublituximab | CD20 | RRMS | Phase 3, NCT03277248 Phase 2, NCT02738775 |
| NMOSD | Phase 1, NCT02276963 | |||
| Inebilizumab | CD19 | NMOSD | Phase 2/3, NCT02200770 | |
| Rozanolixizumab | FcRn | MG, | Phase 3, NCT03971422 Phase 2, NCT03052751 |
|
| CIDP | Phase 2, NCT03861481 | |||
| Reduced Fc effector function | Aquaporumab | AQP4 | NMOSD | Preclinical studies |
| Crenezumab | Monomeric + aggregated amyloid-β | Alzheimer’s disease | Phase 2, NCT 01343966 Phase 3, NCT02670083, NCT03114657 |
|
| Eculizumab | Complement factor 5 | MG, | Phase 3, NCT01997229 | |
| NMOSD | Phase 3, NCT01892345 | |||
| Eptinezumab | CGRP | Migraine | Phase 3 NCT02559895, NCT02974153 | |
| Galcanezumab | CGRP | Migraine/cluster Headache | Phase 3, NCT02614261, NCT02614183, NCT02614196, NCT02397473 | |
| Superselective targets | Aducanumab | Aggregated amyloid-β | Alzheimer’s disease | Phase 3, NCT02484547, NCT02477800 |
CIDP = chronic inflammatory demyelinating polyneuropathy; MG = myasthenia gravis; RRMS = relapsing-remitting multiple sclerosis.
ClinicalTrials.gov.
Increased serum half-live reduces application frequency and thereby reduces the therapeutic burden imposed on patients. An example of optimizing half-live by Fc engineering is ravulizumab (Ultomiris®). Ravulizumab is a humanized monoclonal antibody directed to complement component C5 and was engineered from eculizumab permitting longer dosing intervals (8 weeks compared to 2 weeks for eculizumab). A targeted substitution of four amino acids in the complementary binding and neonatal Fc regions in the eculizumab backbone results in enhanced endosomal dissociation of the ravulizumab–C5 complex, lysosomal degradation of C5 and recycling of ravulizumab to the extracellular space.30 Moreover, the fusion of IgG2 and 4 molecules (IgG2 until T260, then IgG4) reduces binding of FcγR and C1q.95 Ravulizumab has already been approved for paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome.96 For neurological indications, ravulizumab is currently investigated in phase 3 trials in NMOSD (NCT04201262), anti-acetylcholine receptor positive myasthenia gravis (NCT03920293) patients and a trial in amyotrophic lateral sclerosis is active, but not yet recruiting (NCT04248465). For the myasthenia gravis trial, first results were announced in July 2021 in an interim analysis.97 The trial met the primary end point reduction of the Myasthenia Gravis-Activities of Daily Living Profile (MG-ADL) (ravulizumab: −3.1, placebo: −1.4, treatment difference: −1.6, P < 0.001). In addition, the proportion of patients experiencing an improvement of Quantitative Myasthenia Gravis total score of at least five points was higher in the ravulizumab group (30.0 versus 11.3%). The benefits were detected as early as of Week 1 and throughout the study period of 52 weeks. The trial showed no new safety signals; headache, diarrhoea and nausea were the most common adverse events. No cases of meningococcal infection have been observed so far.97
With ublituximab (TG-1101) another mAb was designed to improve applicability compared to previous mAb generations by enhancing Fc receptor functions. Ublituximab is a glycoengineered anti-CD20 IgG1 mAb. The Fab domain of ublituximab targets a unique CD20 epitope, and its Fc region with low fucose content enhances affinity for all variants of FcγRIIIA receptors and thereby ADCC (100× facilitated ADCC compared to rituximab, the prototypic CD20-targeting Ab, in vitro).98 The results of the two twin phase 3 trials (NCT03277248 and NCT03277261) of ublituximab in relapse-onset multiple sclerosis (RRMS) and active secondary progressive multiple sclerosis have been recently presented at the yearly European Academy of Neurology congress.99 Ublituximab was infused on the first day over 4 h followed by 1-h infusions at Day 15 and then every 6 weeks. Teriflunomide was used as an active comparator. In comparison to teriflunomide, ublituximab reduced the mean annual relapse rate in both trials (0.188 versus 0.076 relapses per year in ULTIMATE I, and 0.178 versus 0.091 relapses per year in ULTIMATE II) and no evidence of disease activity rates were significantly higher (44.6 versus 15% in ULTIMATE I, and 43 versus 11.4% in ULTIMATE II), whereas confirmed disability progression was similar for ublituximab and teriflunomide at 12 weeks and at 24 weeks. The most common adverse events associated with ublituximab comprised infusion-related reactions, nasopharyngitis and headaches.99 In a previous phase 2 trial, ublituximab was tested in RRMS patients against placebo (NCT02738775). Ublituximab depleted >99% of B cells: a depletion that was maintained over 48 weeks.63 The higher efficacy for ADCC allows for substantially shorter infusion times as compared to non-defucosylated glycovariants of CD20-targeting antibodies. However, afucosylated mAbs might increase infusion-related reactions due to enhanced affinities for FcγRIII, which might interfere with the desired engineering effect.61 Consistent with this, 43% of patients with multiple sclerosis experienced an infusion-related reactions treated with ublituximab, whereas several cohort studies report significantly lower infusion-related reactions rates, e.g. with 16.7 or 25.7%.99–101
In addition, stabilization of the antibody molecule might also improve durability and efficacy of monoclonal antibodies. An example is the S228P mutation in the hinge region of rozanolixizumab, a human IgG4 anti-FcRn mAb.95 In accordance with increased blocking function of IgG recycling, a phase 2 study (NCT03052751) in myasthenia gravis demonstrated a 68% decrease in IgG and acetylcholine receptor (AChR)-Ab levels as well as a dose-dependent improvement in the myasthenia gravis clinical disease activity.102 A consecutive phase 3 trial is ongoing (NCT03971422). The success of FcRn modulation led to an expansion to further neurological indications. Consequently, rozanolixizumab (NCT03861481) is currently tested in phase 2 studies for chronic inflammatory demyelinating polyneuropathy.
To prevent side-effects, Fc engineering can also be used to reduce unfavourable Fc functions and one example is aquaporumab, a non-pathogenic human IgG1 mAb against AQP4. Antibodies to AQP4 water channels play a fundamental role in the pathogenic processes in NMOSD.103 It was generated from clonally expanded plasmablasts from the CSF of NMOSD patients and the Fc domain was mutated (L234A/L235A) to neutralize effector functions such as complement activation.104 Animal and mechanistic studies with human materials showed that aquaporumab blocks autoantibody binding to aquaporin-4 and prevents complement and cellular cytotoxicity. Those data are promising and support further clinical development.104,105 Similar strategies of Fc engineering have been used in amyloid-β targeted therapy in Alzheimer’s disease. In Alzheimer’s disease, protein misfolding and increased production and deposition of neurotoxic amyloid-β leads to progressive neuroaxonal degeneration.106 Several mAbs were designed to interrupt this self-perpetuating pathology reducing amyloid-β deposition. To reduce vascular side-effects such as vasogenic oedema and micro-haemorrhages, IgG4 (crenezumab) instead of IgG1 or Fc mutations (AAB-003, three mutations in CH3; GSK933776, L235A/G237A) have been used to reduce FcγR and C1q binding by mAb.95 However, in a phase 2 trial (NCT01343966) crenezumab failed to reach the predefined primary end points of improved cognition, as the anti-amyloid-β mAb bapineuzumab (IgG1) and solanezumab (IgG1) did in corresponding phase 3 studies (NCT00575055, NCT00574132; NCT00905372, NCT00904683).107,108 All three antibodies bind monomers and aggregated forms of amyloid-β.109 Thus suboptimal efficacy might, at least in part, be related to the saturation of antibodies by soluble amyloid-β monomers, which thus cannot engage the deposited Aβ. Aducanumab (BIIB037), a fully human anti-amyloid-β (N terminus of amyloid-β3-6) IgG1 mAb, is able to circumvent this problem by selective binding of aggregated amyloid-β forms (both the insoluble fibrils and the soluble oligomers). Moreover, IgG1 related FcγR binding induces effective antibody-dependent cellular phagocytosis.109,110 Correspondingly, in a phase 3 clinical trial (NCT02484547), high-dose aducanumab met the primary end point (change in Clinical Dementia Rating Sum of Boxes) at Week 78 (23% reduction of decline versus placebo, P = 0.01). Consistent with this, these patients also showed a reduction of clinical decline in the Mini-Mental State Examination (15% versus placebo, P = 0.06), the AD Assessment Scale-Cognitive Subscale 13 Items (27% versus placebo, P = 0.01) and the AD Cooperative Study-Activities of Daily Living Inventory Mild Cognitive Impairment Version (40% versus placebo, P = 0.001). Amyloid plaques were reduced with low- and high-dose aducanumab compared to placebo at 26 and 78 weeks (P < 0.001).111 However, in interpreting those data it has to be considered that the second phase 3 trial (NCT02477800) did not show any clinical benefits, whereas amyloid plaque burden was reduced in a dose-dependent fashion.112 Those contradictory findings might be related to several factors such as different durations of exposure to high-dose aducanumab, variation in the performance of placebo groups or prove missing efficacy.112 Nevertheless, in June 2021 the FDA granted accelerated approval of aducanumab for Alzheimer’s disease.113 Further antibody-engineering approaches are currently used to improve immunotherapy in Alzheimer’s disease (e.g. to cross the blood–brain barrier) and are reviewed elsewhere in detail.114 Overall, Fc engineering is instrumental in improving efficacy, safety and applicability of mAb in the treatment of several neurological disorders, which is supported by first clinical trials.
Future of antibody-based therapies
Improved concepts of Ab biology, the increasing research and development investments and the adoption of collaborative research strategies by pharmaceutical companies, increasing prevalence rates for chronic diseases, and the growing clinical experience based on both clinical trials as well as community use of approved drugs will foster the development of Ab-based therapies in the future. As of 2020, >80 Ab-based therapeutics have been approved in the USA or EU, and a growing number of Abs are in regulatory review.111 The global market for the use of recombinant mAbs alone is currently valued at US $140 billion and is estimated to grow to US $370 billion by the end of 2027.
Many neurological diseases are among the most recently identified new and approved indications for Ab-based therapies. However, despite the significant progress, there remain central outstanding questions or problems that need to be addressed in the future:
Despite the rapid development in broadening and improving therapeutic applications of antibodies in neurological diseases, gaps in our armamentarium, including strategies that deliver Ab biologics into the CNS, remain to be addressed. Further, it is not known whether strategies that allow Ab-based platforms to cross the blood–brain barrier, such as receptor-mediated transport through transferrin and insulin receptors, are safe and efficient in humans.
It is not clear whether improving pharmacodynamics and bioavailability of Ab-based treatment platforms within the CNS parenchyma, combined with efficient target validation processes, reinvigorate drug discovery for neurological diseases currently not amenable to immunotherapy.
It remains to be explained whether technologies and processes that decrease production and processing costs of Ab-based treatments together with validated biomarker development programs bring more efficient and affordable Ab-based treatments to the clinic.
Conclusions
Substantial progress has been made over the past decades and has led to improved engineering technologies, safety and efficacy of the first generation of therapeutic Abs in neurology. These developments, along with a greater understanding of the immunomodulatory properties of Abs, have paved the way for the next generation of new and improved Ab-based treatment platforms. Fc-engineering technologies are now being used to specifically address and improve particular effector functions and safety issues to create entirely new Ab-based therapies for immune-mediated neurological diseases. Effector functions of therapeutic Abs can further be improved by regulating FcγR binding and signalling. It remains to be demonstrated, however, that enhanced effector functions indeed translate into higher clinical efficacy. Improving access to the CNS is another important target for Ab- and Ig-domain-based therapies. With dedicated attention to basic, translational and clinical research, we shall soon build even better, more effective and safe Ab-based treatment platforms able to target CNS diseases currently not amenable to immunotherapy.
Abbreviations
- ADAs
antidrug-antibodies
- Abdegs
antibodies that enhance IgG degradation
- ADCC
antibody-dependent cell-mediated cytotoxicity
- Fc
crystallizable fragment
- IR
insulin receptor
- ITP
immune thrombocytopenia
- FcγR
Fc receptor
- FcRn
neonatal Fc receptor
- IVIg
intravenous immunoglobulins
- (m)Abs
(monoclonal) antibodies
- NMOSD
neuromyelitis optica spectrum disorder
Contributor Information
Tobias Ruck, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, 48149 Münster, Germany; Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, 40225 Düsseldorf, Germany.
Falk Nimmerjahn, Department of Biology, Division of Genetics, University of Erlangen-Nuremberg, 91058 Erlangen, Germany.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, 48149 Münster, Germany.
Jan D Lünemann, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, 48149 Münster, Germany.
Funding
This work has been supported by grants to T.R. from Else-Kröner Fresenius Foundation (2018_A03), German Research Foundation (RU 2169/2-1) and Bundesministerium für Bildung und Forschung (BMBF) (01EC1901A). F.N. was supported by the Deutsche Forschungsgemeinschaft (DFG) project grants DFG-TRR130-P13 and D-A-CH NI 711/9-1 to F.N. F.N. was further supported by National Institute of Allergy and Infectious Diseases (NIAID) grant U01 AI-148119-01. H.W.’s research is funded by the Bundesministerium für Bildung und Forschung (BMBF), German Research Foundation, Else-Kröner Fresenius Foundation, Hertie Foundation, Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen, Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Münster and Sanofi-Genzyme. Research in J.D.L.’s laboratory is supported by the Swiss National Science Foundation (31003A_169664) and the German Research Foundation (SFB-CRC128; LU 900/3-1).
Competing interests
T.R. has received honoraria and consultation fee support from Celgene/BMS, Biogen, Roche, Sanofi-Aventis, Alexion, Novartis and Teva and personal support from Merck Serono. F.N. reports no conflicts of interest. H.W. receives honoraria for acting as a member of Scientific Advisory Boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd, Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, TEVA and WebMD Global. H.W. is acting as a paid consultant for Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis and the Swiss Multiple Sclerosis Society. J.D.L. received speaker fees, research support, travel support and/or served on advisory boards by Abbvie, Alexion, Biogen, Merck, Novartis, Roche and Sanofi.
References
- 1. Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: Rationale and mechanisms of action. Nat Clin Pract Rheumatol. 2007;3(5):262–272. [DOI] [PubMed] [Google Scholar]
- 2. Chen Y, Wang C, Xu F, Ming F, Zhang H. Efficacy and tolerability of intravenous immunoglobulin and subcutaneous immunoglobulin in neurologic diseases. Clin Ther. 2019;41(10):2112–2136. [DOI] [PubMed] [Google Scholar]
- 3. Brandstadter R, Sand IK. The use of natalizumab for multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banker DD. Monoclonal antibodies. A review. Indian J Med Sci. 2001;55(12):651.– . [PubMed] [Google Scholar]
- 5. Gklinos P, Papadopoulou M, Stanulovic V, Mitsikostas DD, Papadopoulos D. Monoclonal antibodies as neurological therapeutics. Pharmaceuticals. 2021;14(2):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: From structure to effector functions. Front Immunol. 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821): 353–363. [DOI] [PubMed] [Google Scholar]
- 8. Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. [DOI] [PubMed] [Google Scholar]
- 9. Bittner S, Ruck T, Wiendl H, Grauer OM, Meuth SG. Targeting B cells in relapsing-remitting multiple sclerosis: From pathophysiology to optimal clinical management. Ther Adv Neurol Disord. 2017;10(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang TH, Jung ST. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp Mol Med. 2019;51(11):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pincetic A, Bournazos S, Dilillo DJ, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15(8):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biburger M, Lux A, Nimmerjahn F. How immunoglobulin G antibodies kill target cells: Revisiting an old paradigm. Adv Immunol. 2014;124:67–94. [DOI] [PubMed] [Google Scholar]
- 14. Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725. [DOI] [PubMed] [Google Scholar]
- 15. Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J Allergy Clin Immunol. 2020;146(3):467–478. [DOI] [PubMed] [Google Scholar]
- 16. Ward ES, Ober RJ. Targeting FcRn to generate antibody-based therapeutics. Trends Pharmacol Sci. 2018;39(10):892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128(10):4372–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li N, Zhao M, Hilario-Vargas J, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115(12):3440–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med. 2017;9(414):eaan1208. [DOI] [PubMed] [Google Scholar]
- 20. Shock A, Humphreys D, Nimmerjahn F. Dissecting the mechanism of action of intravenous immunoglobulin in human autoimmune disease: Lessons from therapeutic modalities targeting Fcγ receptors. J Allergy Clin Immunol. 2020;146(3):492–500. [DOI] [PubMed] [Google Scholar]
- 21. Devanaboyina SC, Khare P, Challa DK, Ober RJ, Ward ES. Engineered clearing agents for the selective depletion of antigen-specific antibodies. Nat Commun. 2017;8:15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghetie V, Popov S, Borvak J, et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15(7):637–640. [DOI] [PubMed] [Google Scholar]
- 23. Robbie GJ, Criste R, Dall’Acqua WF, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57(12):6147–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mackness BC, Jaworski JA, Boudanova E, et al. Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. MAbs. 2019;11(7):1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Igawa T, Ishii S, Tachibana T, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28(11):1203–1207. [DOI] [PubMed] [Google Scholar]
- 26. Fukuzawa T, Sampei Z, Haraya K, et al. Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Sci Rep. 2017;7(1):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Traboulsee A, Greenberg B, Bennett JL, et al. Efficacy and safety of satralizumab monotherapy for relapse prevention in neuromyelitis optica spectrum disorder (NMOSD): Results from SAkuraStar, a double-blind placebo-controlled phase 3 clinical study. J Neurol Sci. 2019;405:171. [Google Scholar]
- 28. Yamamura T, Kleiter I, Fujihara K, et al. Efficacy of satralizumab in subgroups of patients in SAkuraSky: A phase III double-blind, placebo-controlled, add-on study in patients with neuromyelitis optica spectrum disorder (NMOSD). J Neurol Sci. 2019;405:11–12. [Google Scholar]
- 29. Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114–2124. [DOI] [PubMed] [Google Scholar]
- 30. Duchow A, Chien C, Paul F, Bellmann-Strobl J. Emerging drugs for the treatment of neuromyelitis optica. Expert Opin Emerg Drugs. 2020;25(3):285–297. [DOI] [PubMed] [Google Scholar]
- 31. Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. [DOI] [PubMed] [Google Scholar]
- 32. Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. [DOI] [PubMed] [Google Scholar]
- 33. Kerntke C, Nimmerjahn F, Biburger M. There is (scientific) strength in numbers: A comprehensive quantitation of fc gamma receptor numbers on human and murine peripheral blood leukocytes. Front Immunol. 2020;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anania JC, Chenoweth AM, Wines BD, MarkHogarth P. The human FcγRII (CD32) family of leukocyte FCR in health and disease. Front Immunol. 2019;10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291(5503):484–486. [DOI] [PubMed] [Google Scholar]
- 36. Debré M, Bonnet MC, Fridman WH, et al. Infusion of Fcγ fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342(8877):945–949. [DOI] [PubMed] [Google Scholar]
- 37. Teeling JL, Jansen-Hendriks T, Kuijpers TW, et al. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: Studies in experimental immune thrombocytopenia. Blood. 2001;98(4):1095–1099. [DOI] [PubMed] [Google Scholar]
- 38. Clarkson SB, Bussel JB, Kimberly RP, Valinsky JE, Nachman RL, Unkeless JC. Treatment of refractory immune thrombocytopenic purpura with an anti-Fcγ-receptor antibody. N Engl J Med. 1986;314(19):1236–1239. [DOI] [PubMed] [Google Scholar]
- 39. Fitzpatrick EA, Wang J, Strome SE. Engineering of Fc multimers as a protein therapy for autoimmune disease. Front Immunol. 2020;11:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qureshi OS, Rowley TF, Junker F, et al. Multivalent Fcγ-receptor engagement by a hexameric Fc-fusion protein triggers Fcγ-receptor internalisation and modulation of Fcγ-receptor functions. Sci Rep. 2017;7(1):17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis BJB, Ville J, Blacquiere M, et al. Using the K/BxN mouse model of endogenous, chronic, rheumatoid arthritis for the evaluation of potential immunoglobulin-based therapeutic agents, including IVIg and Fc-μTP-L309C, a recombinant IgG1 Fc hexamer. BMC Immunol. 2019;20(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ortiz DF, Lansing JC, Rutitzky L, et al. Elucidating the interplay between IgG-Fc valency and FcγR activation for the design of immune complex inhibitors. Sci Transl Med. 2016;8(365):365ra158. [DOI] [PubMed] [Google Scholar]
- 43. Niknami M, Wang MX, Nguyen T, Pollard JD. Beneficial effect of a multimerized immunoglobulin Fc in an animal model of inflammatory neuropathy (experimental autoimmune neuritis). J Peripher Nerv Syst. 2013;18(2):141–152. [DOI] [PubMed] [Google Scholar]
- 44. Thiruppathi M, Sheng JR, Li L, Prabhakar BS, Meriggioli MN. Recombinant IgG2a Fc (M045) multimers effectively suppress experimental autoimmune myasthenia gravis. J Autoimmun. 2014;52:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Owens J, Olsen HS, et al. A recombinant human IgG1 Fc multimer designed to mimic the active fraction of IVIG in autoimmunity. JCI Insight. 2019;4(2):e121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rieth N, Carle A, Müller MA, et al. Characterization of SM201, an anti-hFcγRIIB antibody not interfering with ligand binding that mediates immune complex dependent inhibition of B cells. Immunol Lett. 2014;160(2):145–150. [DOI] [PubMed] [Google Scholar]
- 47. Ericson SG, Coleman KD, Wardwell K, et al. Monoclonal antibody 197 (anti-FcγRI) infusion in a patient with immune thrombocytopenia purpura (ITP) results in down-modulation of FcγRI on circulating monocytes. Br J Haematol. 1996;92(3):718–724. [DOI] [PubMed] [Google Scholar]
- 48. Flaherty MM, Maclachlan TK, Troutt M, et al. Nonclinical evaluation of GMA161-an antihuman CD16 (FcγRIII) monoclonal antibody for treatment of autoimmune disorders in CD16 transgenic mice. Toxicol Sci. 2012;125(1):299–309. [DOI] [PubMed] [Google Scholar]
- 49. Roghanian A, Teige I, Mårtensson L, et al. Antagonistic Human FcγRIIB (CD32B) Antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell. 2015;27(4):473–488. [DOI] [PubMed] [Google Scholar]
- 50. Pottier C, Fresnais M, Gilon M, Jérusalem G, Longuespée R, Sounni NE. Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers. 2020;12(3):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. García-Merino A. Bruton’s tyrosine kinase inhibitors: A new generation of promising agents for multiple sclerosis therapy. Cells. 2021;10(10):2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: An open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154–3160. [DOI] [PubMed] [Google Scholar]
- 53. Miltiadous O, Hou M, Bussel JB. Identifying and treating refractory ITP: Difficulty in diagnosis and role of combination treatment. Blood. 2020;135(7):472–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Montalban X, Arnold DL, Weber MS, et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med. 2019;380(25):2406–2417. [DOI] [PubMed] [Google Scholar]
- 55. Yang C, Gao X, Gong R. Engineering of Fc fragments with optimized physicochemical properties implying improvement of clinical potentials for Fc-based therapeutics. Front Immunol. 2018;8:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rudd PM, Leatherbarrow RJ, Rademacher TW, Dwek RA. Diversification of the IgG molecule by oligosaccharides. Mol Immunol. 1991;28(12):1369–1378. [DOI] [PubMed] [Google Scholar]
- 57. Ferrara C, Grau S, Jag¨er C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108(31):12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci USA. 2017;114(13):3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van der Horst HJ, Nijhof IS, Mutis T, Chamuleau MED. Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies. Cancers. 2020;12(10):3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 61. Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mealy MA, Levy M. A pilot safety study of ublituximab, a monoclonal antibody against CD20, in acute relapses of neuromyelitis optica spectrum disorder. Medicine. 2019;98(25):e15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fox E, Lovett-Racke AE, Gormley M, et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler J. 2020;27(3):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci USA. 2013;110(24):9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354): 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ortiz RS, Mariotti KC, Schwab NV, et al. Fingerprinting of sildenafil citrate and tadalafil tablets in pharmaceutical formulations via X-ray fluorescence (XRF) spectrometry. J Pharm Biomed Anal. 2012;58:7–11. [DOI] [PubMed] [Google Scholar]
- 67. Bozza S, Käsermann F, Kaveri SV, Romani L, Bayry J. Intravenous immunoglobulin protects from experimental allergic bronchopulmonary aspergillosis via a sialylation-dependent mechanism. Eur J Immunol. 2019;49(1):195–198. [DOI] [PubMed] [Google Scholar]
- 68. Washburn N, Schwabb I, Ortiz D, et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci USA. 2015;112(11):E1297–E1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwab I, Nimmerjahn F. Role of sialylation in the anti-inflammatory activity of intravenous immunoglobulin – F(ab′)2 versus Fc sialylation. Clin Exp Immunol. 2014;178(Suppl 1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campbell IK, Miescher S, Branch DR, et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc Portion and independent of sialylation or basophils. J Immunol. 2014;192(11):5031–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Othy S, Topçu S, Saha C, et al. Sialylation may be dispensable for reciprocal modulation of helper T cells by intravenous immunoglobulin. Eur J Immunol. 2014;44(7):2059–2063. [DOI] [PubMed] [Google Scholar]
- 72. Arroyo S, Tiessen RG, Denney WS, et al. Hyper-sialylated IgG M254, an innovative therapeutic candidate, evaluated in healthy volunteers and in patients with immune thrombocytopenia purpura: Safety, tolerability, pharmacokinetics, and pharmacodynamics. Blood. 2019;134(Supplement1):1090–1090. [Google Scholar]
- 73. Freskgård PO, Urich E. Antibody therapies in CNS diseases. Neuropharmacology. 2017;120:38–55. [DOI] [PubMed] [Google Scholar]
- 74. Pardridge WM. Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015;12(2):207–222. [DOI] [PubMed] [Google Scholar]
- 75. Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6(261):61ra154. [DOI] [PubMed] [Google Scholar]
- 76. Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81(1):49–60. [DOI] [PubMed] [Google Scholar]
- 77. Atwal JK, Chen Y, Chiu C, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med. 2011;3(84):84ra43. [DOI] [PubMed] [Google Scholar]
- 78. Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3(84):84ra44. [DOI] [PubMed] [Google Scholar]
- 79. Couch JA, Yu YJ, Zhang Y, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med. 2013;5(183):183ra57. [DOI] [PubMed] [Google Scholar]
- 80. Ullman JC, Arguello A, Getz JA, et al. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci Transl Med. 2020;12(545):eaay1163. [DOI] [PubMed] [Google Scholar]
- 81. Kariolis MS, Wells RC, Getz JA, et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. 2020;12(545):eaay1359. [DOI] [PubMed] [Google Scholar]
- 82. Ulitzka M, Carrara S, Grzeschik J, Kornmann H, Hock B, Kolmar H. Engineering therapeutic antibodies for patient safety: Tackling the immunogenicity problem. Protein Eng Des Sel. 2020;33:gzaa025. [DOI] [PubMed] [Google Scholar]
- 83. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schwab N, Schneider-Hohendorf T, Melzer N, Cutter G, Wiendl H. Natalizumab-associated PML: Challenges with incidence, resulting risk, and risk stratification. Neurology. 2017;88(12):1197–1205. [DOI] [PubMed] [Google Scholar]
- 85. Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dunn N, Juto A, Ryner M, et al. Rituximab in multiple sclerosis: Frequency and clinical relevance of anti-drug antibodies. Mult Scler J. 2018;24(9):1224–1233. [DOI] [PubMed] [Google Scholar]
- 88. Link J, Ramanujam R, Auer M, et al. Clinical practice of analysis of anti-drug antibodies against interferon beta and natalizumab in multiple sclerosis patients in Europe: A descriptive study of test results. PLoS ONE. 2017;12(2):e0170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baker D, Ali L, Saxena G, et al. The irony of humanization: Alemtuzumab, the first, but one of the most immunogenic, humanized monoclonal antibodies. Front Immunol. 2020;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu H, Ponniah G, Zhang HM, et al. In vitro and in vivo modifications of recombinant and human IgG antibodies. MAbs. 2014;6(5):1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu D, Alegre ML, Varga SS, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200(1):16–26. [DOI] [PubMed] [Google Scholar]
- 92. Lo M, Kim HS, Tong RK, et al. Effector-attenuating substitutions that maintain antibody stability and reduce toxicity in mice. J Biol Chem. 2017;292(9):3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuroda D, Tsumoto K. Engineering stability, viscosity, and immunogenicity of antibodies by computational design. J Pharm Sci. 2020;109(5):1631–1651. [DOI] [PubMed] [Google Scholar]
- 94. Chen X, Zeng F, Huang T, Cheng L, Liu H, Gong R. Optimization on Fc for improvement of stability and aggregation resistance. Curr Pharm Biotechnol. 2016;17(15):1353–1359. [DOI] [PubMed] [Google Scholar]
- 95. Dumet C, Pottier J, Gouilleux-Gruart V, Watier H. Insights into the IgG heavy chain engineering patent landscape as applied to IgG4 antibody development. MAbs. 2019;11(8):1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Syed YY. Ravulizumab: A review in atypical haemolytic uraemic syndrome. Drugs. 2021;81(5):587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alexion . Alexion Announces Positive Topline Results from Phase 3 Study of ULTOMIRIS® (ravulizumab-cwvz) in Adults with Generalized Myasthenia Gravis (gMG). 15 July 2021. https://ir.alexion.com/news-releases/news-release-details/alexion-announces-positive-topline-results-phase-3-study
- 98. Babiker HM, Glode AE, Cooke LS, Mahadevan D. Ublituximab for the treatment of CD20 positive B-cell malignancies. Expert Opin Investig Drugs. 2018;27(4):407–412. [DOI] [PubMed] [Google Scholar]
- 99. Tg Therapeutics Press Release . 14 October 2021. https://www.tgtherapeutics.com/wp-content/uploads/2021/06/ULTIMATE-I-II-EAN2021-Steinman-FNAL-PDF-6.18.21.pdf
- 100. Torgauten HM, Myhr KM, Wergeland S, Bø L, Aarseth JH, Torkildsen Ø. Safety and efficacy of rituximab as first- and second line treatment in multiple sclerosis – A cohort study. Mult Scler J - Exp Transl Clin. 2021;7(1):2055217320973049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brown BA, Torabi M. Incidence of infusion-associated reactions with rituximab for treating multiple sclerosis: A retrospective analysis of patients treated at a US centre. Drug Saf. 2011;34(2):117–123. [DOI] [PubMed] [Google Scholar]
- 102. Bril V, Benatar M, Andersen H, et al. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: A phase 2 randomized control trial. Neurology. 2021;96(6):e853–e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rosenthal JF, Hoffman BM, Tyor WR. CNS inflammatory demyelinating disorders: MS, NMOSD and MOG antibody associated disease. J Investig Med. 2020;68(2):321–330. [DOI] [PubMed] [Google Scholar]
- 104. Duan T, Tradtrantip L, Phuan PW, Bennett JL, Verkman AS. Affinity-matured ‘aquaporumab’ anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology. 2020;162:107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tradtrantip L, Zhang H, Saadoun S, et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71(3):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. [DOI] [PubMed] [Google Scholar]
- 107. Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–321. [DOI] [PubMed] [Google Scholar]
- 108. Soejitno A, Tjan A, Purwata TE. Alzheimer’s disease: Lessons learned from amyloidocentric clinical trials. CNS Drugs. 2015;29(6):487–502. [DOI] [PubMed] [Google Scholar]
- 109. van Dyck CH. Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: Pitfalls and promise. Biol Psychiatry. 2018;83(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. [DOI] [PubMed] [Google Scholar]
- 111. Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12(1):1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sabbagh MN, Cummings J. Open peer commentary to “Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen December 2019”. Alzheimer’s Dement. 2021;17(4):702–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Food and Drug Administration. FDA announcement; FDA grants accelerated approval for Alzheimer’s drug . 2021. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- 114. Sumner IL, Edwards RA, Asuni AA, Teeling JL. Antibody engineering for optimized immunotherapy in Alzheimer’s disease. Front Neurosci. 2018;12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lünemann JD, Quast I, Dalakas MC. Efficacy of intravenous immunoglobulin in neurological diseases. Neurotherapeutics. 2016;13(1):34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 117. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 118. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. [DOI] [PubMed] [Google Scholar]
- 119. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132. [DOI] [PubMed] [Google Scholar]
- 120. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287. [DOI] [PubMed] [Google Scholar]
- 121. Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–1040. [DOI] [PubMed] [Google Scholar]
- 122. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. [DOI] [PubMed] [Google Scholar]
- 123. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of Fremanezumab compared with placebo for prevention of episodic migraine a randomized clinical trial. JAMA - J Am Med Assoc. 2018;319(19):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):E2211–E2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454. [DOI] [PubMed] [Google Scholar]
- 126. Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goadsby PJ, Dodick DW, Leone M, et al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019;381(2):132–141. [DOI] [PubMed] [Google Scholar]
- 128. Dodick DW, Goadsby PJ, Lucas C, et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: Results from 3-month double-blind treatment. Cephalalgia. 2020;40(9):935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lipton RB, Saper J, Ashina M, et al. A phase 3 study to evaluate eptinezumab for the preventive treatment of chronic migraine: results of the promise-2 (prevention of migraine via intravenous eptinezumab safety and efficacy-2) trial. Headache. 2018;58:80. [Google Scholar]
- 130. Silberstein SD, Kudrow D, Saper J, et al. Eptinezumab results for the prevention of episodic migraine over one year in the PROMISE-1 (prevention of migraine via intravenous eptinezumab safety and efficacy-1) trial. Headache. 2018;58(8):1298. [Google Scholar]
- 131. Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: Efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J Headache Pain. 2020;21(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4–positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–625. [DOI] [PubMed] [Google Scholar]
- 133. Howard JF, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–986. [DOI] [PubMed] [Google Scholar]
- 134. Muppidi S, Utsugisawa K, Benatar M, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–1363. [DOI] [PubMed] [Google Scholar]
- 136. Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental allergic encephalomyelitis by antibodies against alpha-4-beta-1 integrin. Nature. 1992;356:63–66. [DOI] [PubMed] [Google Scholar]
- 137. Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–923. [DOI] [PubMed] [Google Scholar]
- 138. Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. [DOI] [PubMed] [Google Scholar]
- 139. Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in multiple sclerosis: Mechanism of action and beyond. Int J Mol Sci. 2015;16(7):16414–16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828. [DOI] [PubMed] [Google Scholar]
- 141. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. [DOI] [PubMed] [Google Scholar]
- 142. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. [DOI] [PubMed] [Google Scholar]
- 143. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab in primary progressive and relapsing multiple sclerosis. N Engl J Med. 2017;376(17):209–220. [DOI] [PubMed] [Google Scholar]
- 144. Yamout BI, El-Ayoubi NK, Nicolas J, Kouzi YE, Khoury SJ, Zeineddine MM. Safety and efficacy of rituximab in multiple sclerosis: A retrospective observational study. J Immunol Res. 2018;2018:9084759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. De Flon P, Gunnarsson M, Laurell K, et al. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology. 2016;87(2):141–147. [DOI] [PubMed] [Google Scholar]
- 146. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. [DOI] [PubMed] [Google Scholar]
- 147. Cabre P, Mejdoubi M, Jeannin S, et al. Treatment of neuromyelitis optica with rituximab: A 2-year prospective multicenter study. J Neurol. 2018;265(4):917–925. [DOI] [PubMed] [Google Scholar]
- 148. Nikoo Z, Badihian S, Shaygannejad V, Asgari N, Ashtari F. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: A randomized clinical trial. J Neurol. 2017;264(9):2003–2009. [DOI] [PubMed] [Google Scholar]
- 149. Nowak RJ, O’Connor KC, Coffey C, Yankey JW, Uribe L, Goldstein JM, et al. 15th International Congress on Neuromuscular Diseases, July 6–10, 2018 Vienna, Austria. J Neuromuscul Dis. 2018;5(s1):S1–S408. [Google Scholar]
- 150. Díaz-Manera J, Martínez-Hernández E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78(3):189–193. [DOI] [PubMed] [Google Scholar]
- 151. Stieglbauer K, Pichler R, Topakian R. 10-year-outcomes after rituximab for myasthenia gravis: Efficacy, safety, costs of in-hospital care, and impact on childbearing potential. J Neurol Sci. 2017;375:241–244. [DOI] [PubMed] [Google Scholar]
- 152. Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]