Abstract
Background
Bronchial Dieulafoy's disease (BDD) is a rare vascular anomaly that was first described in 1995. The main symptom is recurrent hemoptysis. It can be diagnosed through angiography, bronchoscopy, and sometimes histology and endobronchial ultrasound scan (EBUS). Treatment includes embolization and surgery.
Case presentation
A 77-year-old male with dyspnea and CT scan revealing an interstitial pattern underwent bronchoscopy for bronchoalveolar lavage (BAL). During bronchoscopy, a protruding white non-pulsatile lesion was biopsied. The biopsy triggered a massive hemorrhage, which required an embolization procedure. Bronchial Dieulafoy's disease was diagnosed. There was no need for surgery in this case. The interstitial pattern was diagnosed as idiopathic pulmonary fibrosis.
Conclusions
This report describes a novel case of BDD leading to bronchial hemorrhage. Considering the endoscopic differential diagnosis, including rather frequent carcinoid tumor and broncholithiasis, we highlight the need for extreme caution when considering endoscopic biopsy of protruding white lesions. Indeed, biopsy – or even contact – with a BDD lesion is frequently associated with massive hemorrhage. According to our review, BDD is the most hemorrhage-prone lesion when biopsied, associated with significant bleeding in 90% of cases and 30% mortality, compared with significant bleeding in only 2.6% of carcinoid tumors and 3.1% of broncholithiasis cases.
This case of BDD is also original since associated with idiopathic pulmonary fibrosis. It is to our knowledge the first time that such an association has been reported.
Keywords: Bronchial Dieulafoy's disease, Carcinoid tumor, Idiopathic pulmonary fibrosis, Hemoptysis, Bronchial hemorrhage
1. Background
More than a century ago, Dieulafoy's ulcer was described as superficial large-caliber arteriole in the upper part of the stomach, exposed to gastric erosion leading to massive bleeding [1]. Since then, it has been described as occurring all along the digestive tract [2].
The first description of Dieulafoy's lesion in the bronchus was in 1995 through two case reports of hemoptysis and biopsied lesions with the same histologic pattern: “large artery showing focal thinning of the media extended between the bronchial cartilages into the submucosa” [3]. It was thus similar to Dieulafoy's original description and suggested a congenital nature rather than progression of a peptic ulceration. Since then, bronchial Dieulafoy's disease (BDD) has been very rarely described. A comprehensive literature search in 2019 reported only 74 cases of BDD, mainly in the Asian population [4].
The main symptom is recurrent or massive hemoptysis even though other symptoms such as cough and chronic bronchitis have also been reported [4]. Diagnosis, which was initially obtained with histology, is now conducted mainly through bronchoscopy, angiography and sometimes endobronchial ultrasound scan or histology [5,6]. Treatment can include medication and sometimes local coagulation but generally requires embolization or surgery [5,7].
2. Case presentation
Our patient is a 77-year-old male who had professional exposure to asbestosis and hydrocarbon, and a 20-pack-year history of smoking that ceased when he was 35 years old. He reported no regular alcohol consumption. His past medical history revealed atrial fibrillation and gallbladder removal. Daily medications included oral anticoagulation, beta blockers, digoxin and proton pump inhibitors. This case occurred more than 6 months before the COVID-19 epidemic began in France.
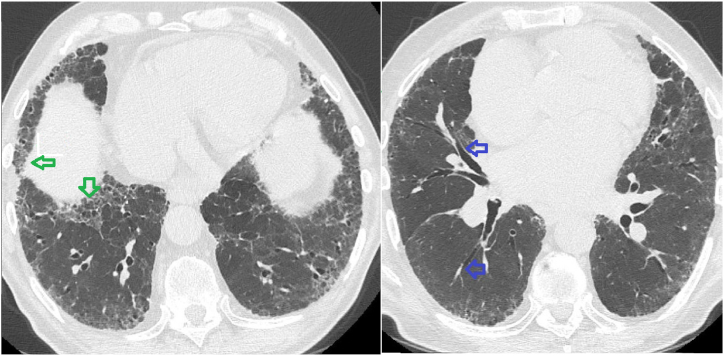
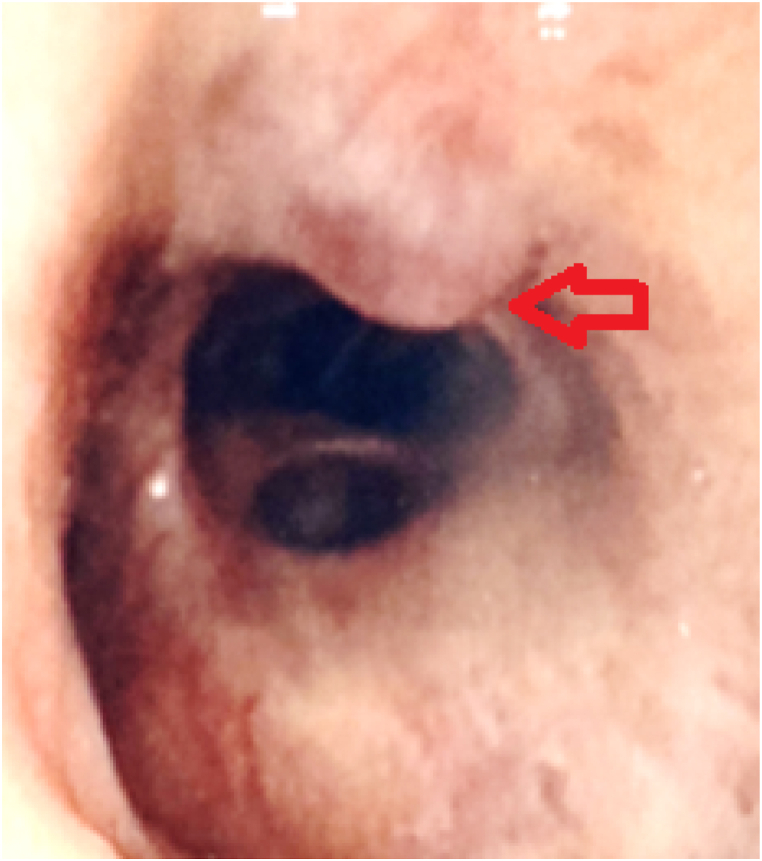
The initial clinical presentation included a three-month history of increasing dyspnea associated with occasional dry cough, but no general symptoms such as weight loss or asthenia. On physical examination, SpO2 was 92% without oxygen, and pulmonary auscultation found fine bibasilar crackles. There were no extra-respiratory complaints or clinical abnormalities, particularly no clubbing, digestive disorders or cutaneous anomalies. The results of the pulmonary function test (PFT) showed restrictive pulmonary syndrome, with a total lung capacity of 4.78L, i.e. 74% of the theoretical value, associated with low diffusing capacity of the lungs for carbon monoxide (KCO) at 50% of the theoretical value. There was no associated obstructive syndrome, and forced expiratory volume in the first second (FEV1) was normal. Non-contrast chest CT [Fig. 1] showed signs of peripheral honeycombing and reticulations associated with mild bronchiectasis as well as basal micro calcifications and a couple of mediastinal adenopathies with a maximal diameter of 14 mm. The laboratory returned normal results for the complete blood count, blood electrolytes, markers of renal and hepatic function, C-reactive protein, and autoantibodies usually associated with interstitial diseases. After due interruption of oral anticoagulation, a bronchoscopy was performed with bronchoalveolar lavage (BAL). A protruding white non-pulsatile lesion at the entrance of the right intermediate bronchus [Fig. 2] was observed and subsequently biopsied, which led to massive hemorrhage.
Fig. 1.
CT Interstitial pattern ( honeycombing
honeycombing  mild bronchiectasis).
mild bronchiectasis).
Fig. 2.
Bronchoscopy view ( protruding white lesion).
protruding white lesion).
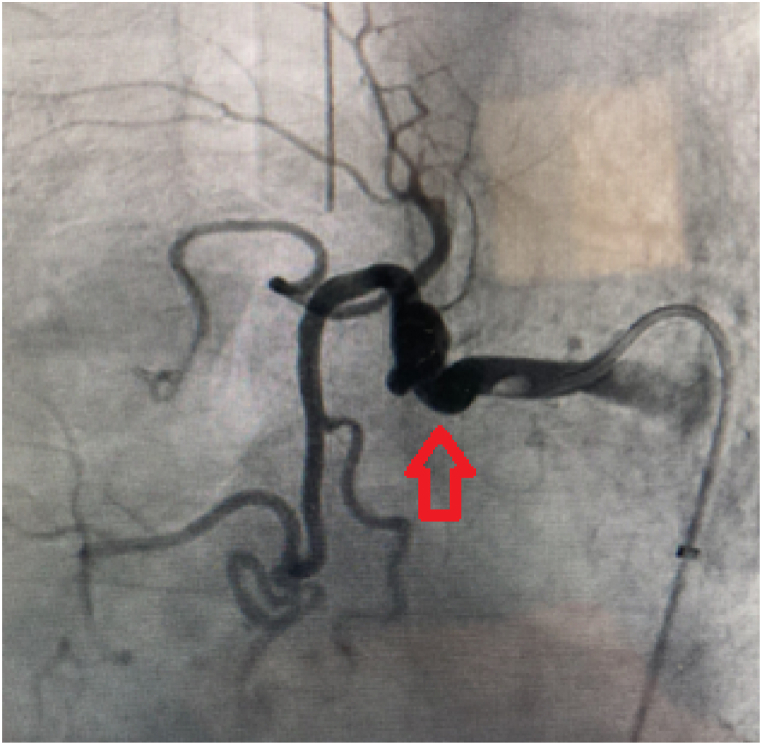
The patient was rapidly admitted to intensive care where medical treatment with glypressin, tranexamic acid and etamsylate successfully controlled the bleeding. Chest CT angiography showed tortuous ectasis of the right bronchial artery [Fig. 3]. The results of the BAL performed prior to bleeding showed mainly macrophages (95%) associated with neutrophils (4%), eosinophils (1%) and signs of alveolar hemorrhage with a Golde score of 320. Biopsies showed unspecific inflammatory lung tissue. The patient was discharged from intensive care after anticoagulation, preventive and then curative, was reintroduced. Massive hemoptysis reoccurred at day 19 and led to hemorrhagic shock. The patient was intubated and successfully treated for shock, and right bronchial artery embolization was performed with 500μ microparticles. Anticoagulation was contraindicated and a left atrial appendage occlusion procedure was performed. The bronchoscopy and angiography led to the diagnosis of bronchial Dieulafoy's disease. To date, surgery has not been required, and there were no new episodes of hemorrhage in the 7 months after the embolization.
Fig. 3.
Angiography of bronchial vessels ( right bronchial artery ectasis).
right bronchial artery ectasis).
At the same time, the patient was diagnosed with idiopathic pulmonary fibrosis (IPF) based on the clinical findings, CT Scan, PFT, and BAL results. The diagnosis of asbestosis was also ruled out. Antifibrotic therapy will soon be introduced. Moreover, one month after discharge following the hemorrhagic episodes, our patient suffered from severe hypoxic dyspnea, which was compatible with exacerbation of IPF and successfully treated as such.
3. Discussion
3.1. Endoscopic differential diagnosis
A review of differential diagnoses on a vascular perspective is conducted in Ref. [4]. It includes among others bronchial arteriovenous malformation, bronchial artery aneurysm, lobular capillary hemangioma or even early stage cancer. Our case suggests differential diagnoses considering the findings of the bronchoscopy.
In our opinion, the presence of a protruding white non-pulsatile lesion should lead the clinician to consider the following diagnoses.
-
-First, bronchial carcinoid tumor, which is far more common than BDD, since it is estimated to represent around 2% of lung tumors [8].
-
oBronchoscopy findings typically report round lesions that are often white or raspberry colored [9].
-
oRisk of bleeding was assessed in a review of 587 biopsies, which showed 15 cases of hemorrhage, i.e. 2.5%, among which 4 cases required hemostatic surgery and 1 case resulted in death [8,10]. The authors suggest that biopsy with local epinephrine is a rather safe procedure if it is performed in a hospital with a thoracic surgery department. This was also confirmed in the European Neuroendocrine Tumor Society recommendations [11].
-
o
-
-Secondly, broncholithiasis should be considered. Similar to BDD, it occurs more frequently in the right lung and often stems from tuberculosis or histoplasmosis.
-
oBronchoscopic aspect depends on the localization, which can be endobronchial, transbronchial or peribronchial [12]. The peribronchial form is very similar to BDD and bronchial carcinoid tumor.
-
oRisk of bleeding was assessed among 256 cases, which showed 7 minor bleed and 1 major bleed requiring surgery i.e. 3.1 of cases [12].
-
o
In comparison with bronchial carcinoid tumor or broncholithiasis, 17/19 patients with BDD who underwent biopsy had significant bleeding, including 6 deaths due to massive hemorrhage [5]. We can add our case to these results, which makes 18/20 significant bleeding events and 6 deaths. Thus, biopsy led to 30% mortality in cases of BDD.
BDD is thus by far the most hemorrhagic lesion when biopsied. Major bleeding occurred in 90% of cases and death in 30%, compared with major bleeding in 2.6% of carcinoid tumor cases and 3.1% of broncholithiasis cases. This difference and the difference in mortality were significant according to Pearson's Chi2 Test [Table 1].
Table 1.
Risk of significant bleeding and death for BDD, Broncholithiasis and Carcinoid tumor.
| Bronchial Dieulafoy Disease | Broncholithiasis | Bronchial carcinoid tumor | ||
|---|---|---|---|---|
| Cases biopsied | 20 | 256 | 587 | |
| Significant bleeding | 18 (90,0%) | 8 (3.1%) | 15 (2.6%) | Pearson Chi 2 p < 0.001 |
| Deaths | 6 (30,0%) | 0 | 1 (0.2%) | Pearson Chi 2 p < 0.001 |
These results highlight the importance of proceeding with extreme caution before biopsying a protruding white carcinoid-like lesion in the bronchus.
3.2. Unexpected association with IPF
Our literature review found no cases of BDD associated with IPF. Most cases report either normal lung parenchyma on CT, or a pattern linked to bronchial hemorrhage, including ground glass opacities [4]. Out of the 73 cases of BDD, 21 patients had respiratory disorders, including tuberculosis, bronchiectasis, asthma and COPD [5]. None of the case reports mentioned IPF.
Epidemiology and risk factors in BDD are as follows: the male to female ratio is approximately 2:1; 48% had a history of smoking, 52% were reported in Asia, and average age at diagnosis was 47.2 years [5]. Concerning IPF, the sex ratio is similar, smoke exposure is also frequent, and age at diagnosis is frequently around 60–70 years [13]. The similarities in epidemiology and exposure may explain the association between BDD and IPF in our case report.
However, given that the genetic factors for IPF and BDD remain poorly known, we consider that this association needs to be reported. All the more as BBD is highly underdiagnosed and because this case may support decision-making when lesions are observed fortuitously during bronchoscopy in patients suffering from IPF.
4. Conclusion
This report of a novel case of bronchial Dieulafoy's disease is original in that it was associated with interstitial disease diagnosed as IPF. It is, to our knowledge, the first time that this association has been reported. It may or may not be purely random since genetic factors for IPF and BDD are still mostly unknown.
BDD is generally diagnosed in patients with recurrent or massive hemoptysis, and biopsy is associated with a risk of life-threatening bleeding and even death.
Based on endoscopic differential diagnosis, including carcinoid tumor and broncholithiasis, we suggest extreme caution when endoscopic findings include a protruding white lesion since biopsy or even contact with such lesions is frequently associated with massive hemorrhage.
Data sharing
Data and material are available upon request at University of Burgundy, CHU de Dijon, Dijon, France.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent
Informed consent for publication of clinical details and clinical images was obtained from the patient.
Declaration of competing interest
All authors declare no competing interests.
List of abbreviations
- BAL
Bronchoalveolar Lavage
- BDD
Bronchial Dieulafoy Disease
- COPD
Chronic Obstructive Pulmonary Disease
- CT
Computed Tomography
- IPF
Idiopathic Pulmonary Fibrosis
- PFT
Pulmonary Function Test
References
- 1.Karamanou M., Fiska A., Demetriou T., Androutsos G. Georges-Paul Dieulafoy (1839-1911) and the first description of « exulceratio simplex. Ann. Gastroenterol. Q Publ. Hell Soc. Gastroenterol. 2011;24:188–191. 8 janv. [PMC free article] [PubMed] [Google Scholar]
- 2.Fockens P., Tytgat G.N. Dieulafoy's disease. Gastrointest. Endosc. Clin. N Am. 1996;6(4):739–752. oct. [PubMed] [Google Scholar]
- 3.Sweerts M., Nicholson A.G., Goldstraw P., Corrin B. Dieulafoy's disease of the bronchus. Thorax. 1995;50(6):697–698. doi: 10.1136/thx.50.6.697. juin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing X., Liu J., Xu S., Deng Y., Yang J. Research advances in Dieulafoy's disease of the bronchus (Review) Exp. Ther. Med. 2022;23(1):100. doi: 10.3892/etm.2021.11023. janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian X., Du Q., Wei N., Wang M., Wang H., Tang Y. Bronchial Dieulafoy's disease: a retrospective analysis of 73 cases. BMC Pulm. Med. 2019;19(1):104. doi: 10.1186/s12890-019-0863-1. déc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurioli C., Casoni G.L., Gurioli C., Tomassetti S., Romagnoli M., Ravaglia C., et al. Endobronchial ultrasound in Dieulafoy's disease of the bronchus: an additional application of EBUS. Monaldi Arch. Chest Dis. Arch. Monaldi Mal Torace. 2010;73(4):166–168. doi: 10.4081/monaldi.2010.287. déc. [DOI] [PubMed] [Google Scholar]
- 7.Dalar L., Sökücü S.N., Özdemir C., Büyükkale S., Altın S. Endobronchial argon plasma coagulation for treatment of Dieulafoy disease. Respir. Care. 2015;60(1):e11–e13. doi: 10.4187/respcare.03307. janv. [DOI] [PubMed] [Google Scholar]
- 8.Dusmet M., McKneally M.F. Bronchial and thymic carcinoid tumors: a review. Digestion. 1994;55(Suppl 3):70–76. doi: 10.1159/000201205. [DOI] [PubMed] [Google Scholar]
- 9.Papaporfyriou A., et al. Bronchoscopic diagnosis and treatment of endobronchial carcinoid: case report and review of the literature. Eur. Respir. Rev. 2021;30(159):6–30. doi: 10.1183/16000617.0115-2020. https://err.ersjournals.com/content/30/159/200115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limited and radical resection for tracheal and bronchopulmonary carcinoid tumour. Report on 227 cases. Eur. J. Cardio. Thorac. Surg. 1 oct 1990;4(10):527–532. doi: 10.1016/1010-7940(90)90140-u. [DOI] [PubMed] [Google Scholar]
- 11.Caplin M.E., Baudin E., Ferolla P., Filosso P., Garcia-Yuste M., Lim E., et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015;26(8):1604–1620. doi: 10.1093/annonc/mdv041. août. [DOI] [PubMed] [Google Scholar]
- 12.Alshabani K., Ghosh S., Arrossi A.V., Mehta A.C. Broncholithiasis: a review. Chest. 2019;156(3):445–455. doi: 10.1016/j.chest.2019.05.012. sept. [DOI] [PubMed] [Google Scholar]
- 13.Maher T.M., Bendstrup E., Dron L., Langley J., Smith G., Khalid J.M., et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021;22(1):197. doi: 10.1186/s12931-021-01791-z. 7 juill. [DOI] [PMC free article] [PubMed] [Google Scholar]