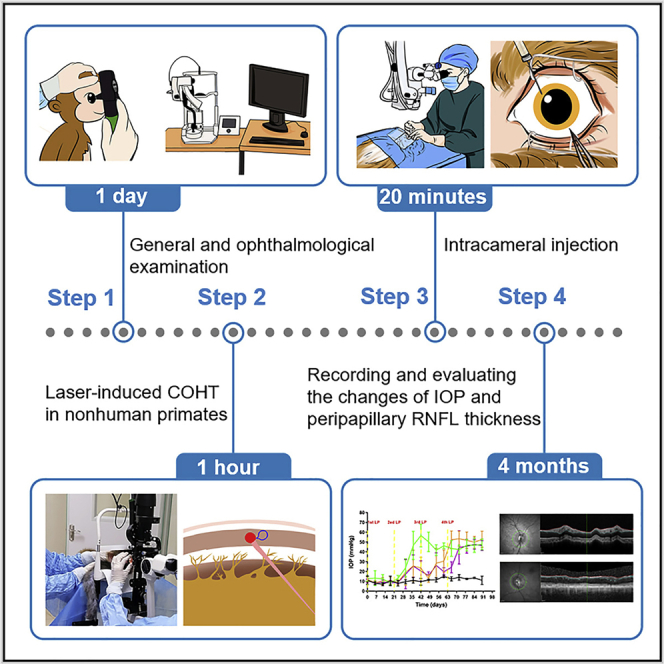
Summary
Laser-induced hypertension in nonhuman primates is used to mimic human glaucoma, the leading cause of irreversible blindness. In this protocol, we detail steps for laser-induced ocular hypertension in nonhuman primates by laser photocoagulation of the trabecular meshwork and subsequent intracameral injection. We further describe recording and evaluation of intraocular pressure changes and peripapillary retinal nerve fiber layer thickness. This protocol can assist researchers improve the success rate and repeatability of the procedure and reduce the number of nonhuman primates needed.
For complete details on the use and execution of this protocol, please refer to Sun et al. (2022).
Subject areas: Health sciences, Model organisms, Neuroscience
Graphical abstract
Highlights
-
•
Detailed description for general and ophthalmological examination before experiment
-
•
Protocol to generate laser-induced chronic ocular hypertension in rhesus monkeys
-
•
Laser photocoagulation of the trabecular meshwork followed by intracameral injection
-
•
Approach is highly reproducible and offers a tool to test potential treatment strategies
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Laser-induced hypertension in nonhuman primates is used to mimic human glaucoma, the leading cause of irreversible blindness. In this protocol, we detail steps for laser-induced ocular hypertension in nonhuman primates by laser photocoagulation of the trabecular meshwork and subsequent intracameral injection. We further describe recording and evaluation of intraocular pressure changes and peripapillary retinal nerve fiber layer thickness. This protocol can assist researchers improve the success rate and repeatability of the procedure and reduce the number of nonhuman primates needed.
Before you begin
Glaucoma is one of the most common causes of irreversible blindness worldwide. Nonhuman primates are homologous to humans, with consistent anatomical structures and physiological functions with human eyes (Stewart et al., 2011; Rasmussen and Kaufman, 2005). To better study the effectiveness and safety of potential anti-glaucoma medicines (intraocular pressure reduction and/or optic nerve protection) for the treatment of human glaucoma in the future, it is necessary to evaluate such agents in nonhuman primate ocular hypertension models with high repeatability and stability (long-lasting high intraocular pressure). The laser-induced chronic ocular hypertension (COHT) model in nonhuman primates is similar to human glaucoma, involving increased intraocular pressure (IOP) and subsequently damaged retinal nerve fiber layer. Furthermore, it has the advantages of being noninvasive, producing only a mild inflammatory response, keeping the refractive stroma clear, and maintaining continuous high IOP (several weeks to several months) (Pelzel et al., 2006; Quigley and Hohman, 1983; Tu et al., 2019a). Due to the high cost and shortage of nonhuman primates, the successful construction of this model is particularly important to minimize the number of nonhuman primates needed. Although some studies involve the research and application of the laser-induced COHT model (Bastia et al., 2021; Fuwa et al., 2018; Quigley and Hohman, 1983; Takahashi et al., 2021; Tu et al., 2019a, Tu et al., 2019b), a detailed protocol describing the complete procedure is still lacking. Here, we provide a step-by-step guide based on literature reports and our own experience (Quigley and Hohman, 1983; Sun et al., 2022; Tu et al., 2019a, Tu et al., 2019b), to improve the success rate and repeatability between laboratories. Moreover, intracameral injection is a common therapeutic technique to introduce therapuetics for ocular diseases in clinical and scientific research, especially in the research and development of anti-glaucoma drugs, which is rarely described in detail. This protocol presented below describes the specific steps of laser-induced COHT and intracameral injection in rhesus monkeys (Macaca mulatta).
Institutional permissions
All experiments involving animals were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research. The experimental procedures were approved by the Ethics Committee of Guangdong Laboratory Animals Monitoring Institute (IACUC2020141).
Part 0: Preoperative examination
Timing: 1 day (for step 1)
This part introduces the general evaluation and ophthalmological examination of rhesus monkeys before laser photocoagulation, as well as relevant baseline data collection.
-
1.General evaluation.
-
a.Identify animals from distributors that do not have significant health problems (epilepsy, trauma, deformity, epilation, parasitic infection, or ocular damage).
-
b.Check the ID number and record the sex, weight, and age of the rhesus monkeys.
-
c.Observe general health and activity evaluation of the rhesus monkeys, including locomotor activity, reaction capacity, nutritional status, eating status.
-
d.Check whether the following test results meet the required standards: fecal bacteria examination (shigella, salmonella, etc), blood B virus detection, and tuberculin reactivity.
-
a.
-
2.Ophthalmological examination.
-
a.IOP measurements.
-
i.Measure baseline IOP using a rebound tonometer (Tono Vet; iCare, Finland) according to the manufacturer’s instructions.
-
ii.Perform deep general anesthetized in Rhesus monkeys with an intramuscular injection of ketamine hydrochloride (5 mg/kg) plus medetomidine (0.05 mg/kg).Note: Measure IOP as soon as possible after anesthesia takes effect (within 5 min). The criteria of anesthesia onset are pain response disappeared and muscular relaxation.
-
iii.Perform the measurement in an upright, sitting position to avoid the influence of posture on IOP.
-
iv.Measure IOP within a specific time period (10–12 a.m.) to reduce the impact of circadian rhythm regulation (Aptel et al., 2016).
-
v.Obtain six consecutive IOP readings by the same researcher using the same tonometer, and calculate the mean value from three repeated measurements (for a total of 18 separate measurements).Note: Only consecutive readings with little deviation (<3 mmHg) are considered as valid measurements.
-
i.
-
b.Perform slit-lamp biomicroscopy examination (eyelids, conjunctiva, cornea, sclera, anterior chamber, iris, lens, anterior vitreous body, etc) and direct ophthalmoscopy (optic nerve head, macula, retina, retinal vessels, etc) after pupil dilation with Compound Tropicamide Eye Drops (Zhuobian®; Sinqi, China) to exclude common eye diseases.
-
c.Optical coherence tomography (OCT).
-
i.Perform a spectral-domain optical coherence tomography (SD-OCT; Heidelberg Engineering, Heidelberg, Germany) examination in the dark according to the manufacturer’s instructions.
-
ii.Detect and record the central corneal thickness (CCT) and the anterior chamber angle, especially Schlemm’s canal (SC).
-
iii.Detect and analyze the optic nerve head and macular retina, including the retinal nerve fiber layer (RNFL) thickness of the optic nerve head and the retina, the ganglion cell layer (GCL) and ganglion cell inner plexiform layer (GCIPL) thickness of the macula.Note: Dilate pupil will help shorten the fundus examination time and facilitate the acquisition of high-quality images. In addition, use 0.1% Sodium Hyaluronate Eye Drops (Aili®; Santen Pharmaceutical Co., Ltd, Japan) to keep the cornea hydrated. Besides, peripapillary RNFL thickness is automatically acquired and recorded by the software, while the macular retinal thickness and CCT are measured manually by two researchers independently.
 CRITICAL: The IOP values measured by different tonometers are usually consistent when the IOP is in the normal range, but there are certain deviations when the IOP is too high or too low (Gloe et al., 2019; McAllister et al., 2018; Tu et al., 2019b). Besides, the measured IOP can increase when animals are alert or struggling (anesthesia has not fully taken effect). On the contrary, if animals are anesthetized for too long (including general and/or topical anesthesia), the measured IOP will be lower (Kelly and Farrell, 2018; Sarchahi and Eskandari, 2019). Therefore, to ensure an accurate IOP measurement, each measurement should be completed as soon as possible after anesthesia takes effect. It is important to keep the cornea hydrated in both eyes during anesthesia by using artificial tears locally. Furthermore, it is recommended to use Levofloxacin Hydrochloride Eye Gel (Jieqi ®; EBE Pharmaceutical Co., Ltd, China) locally after examination to prevent infection.
CRITICAL: The IOP values measured by different tonometers are usually consistent when the IOP is in the normal range, but there are certain deviations when the IOP is too high or too low (Gloe et al., 2019; McAllister et al., 2018; Tu et al., 2019b). Besides, the measured IOP can increase when animals are alert or struggling (anesthesia has not fully taken effect). On the contrary, if animals are anesthetized for too long (including general and/or topical anesthesia), the measured IOP will be lower (Kelly and Farrell, 2018; Sarchahi and Eskandari, 2019). Therefore, to ensure an accurate IOP measurement, each measurement should be completed as soon as possible after anesthesia takes effect. It is important to keep the cornea hydrated in both eyes during anesthesia by using artificial tears locally. Furthermore, it is recommended to use Levofloxacin Hydrochloride Eye Gel (Jieqi ®; EBE Pharmaceutical Co., Ltd, China) locally after examination to prevent infection.
-
i.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Rhesus monkey (Macaca mulatta, 3–4 years old, male) | Guangdong Laboratory Animals Monitoring Institute | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Ketamine hydrochloride | Controlled Substance – contact your animal facility veterinarian | N/A |
| Medetomidine | Controlled Substance – contact your animal facility veterinarian | N/A |
| Compound Tropicamide Eye Drops | Sinqi | N/A |
| 0.1% Sodium Hyaluronate Eye Drops | Santen Pharmaceutical Co., Ltd | N/A |
| Levofloxacin Hydrochloride Eye Gel | EBE Pharmaceutical Co., Ltd | N/A |
| Pilocarpine Nitrate Eye Drops | Bausch & Lomb | N/A |
| Oxybuprocaine Hydrochloride Eye Drops | Santen Pharmaceutical Co., Ltd | N/A |
| Tobramycin and Dexamethasone Eye Ointment | Alcon | N/A |
| Tobramycin Eye Drops | Alcon | N/A |
| Tobramycin Eye Ointment | Alcon | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.0 | GraphPad Software | https://www.graphpad.com/ |
| Other | ||
| Rebound tonometer | iCare | Tono Vet |
| Slit-lamp biomicroscope | Suzhou Kangjie Medical Co., Ltd | KJ5DII |
| SD-OCT | Heidelberg | Heidelberg Engineering |
| TX532 laser photocoagulation instrument | IRIDEX | Oculight TX |
| Syringe | Hamilton | Cat#80565 |
| Insulin Syringe | Bidi medical devices (Shanghai) Co., Ltd | Cat#1012907 |
| Eye speculum | Suzhou Mingren Medical Instrument Co., Ltd | MR-0103 |
| Ophthalmic microforceps | Suzhou Xiehe Medical Instrument Co., Ltd | MR-F101A-1 |
Materials and equipment
Anesthetic: Ketamine and medetomidine
| Reagent | Final concentration | Amount |
|---|---|---|
| Ketamine hydrochloride | 5 mg/kg | 2 mL (100 mg) |
| Medetomidine | 0.05 mg/kg | 1 mL (1 mg) |
| Total | 3 mL |
Storage: The anesthetic has a shelf life of one month at room temperature from when it is mixed.
CRITICAL: These are controlled substances which should be got from your animal facility veterinarian.
Step-by-step method details
Part 1: Laser-induced COHT in nonhuman primates
Timing: 30–60 min (for step 1)
This section presents the detailed procedures to create a COHT model in rhesus monkeys by laser photocoagulation of the trabecular meshwork.
-
1.Anesthesia and eye treatment for pupil contraction.
-
a.After 6–8 h of fasting, perform deep general anesthesia via an intramuscular anesthesia of ketamine hydrochloride (5 mg/kg) plus medetomidine (0.05 mg/kg).Note: This procedure is performed by a veterinarian.
-
b.Administer pilocarpine nitrate eye drops (Zhenrui ®; Bausch & Lomb, China) locally for pupil contraction.Note: The lacrimal sac area should be pressed after the local application of the pilocarpine nitrate eye drops, and the drug should not be used too many times (using 1–2 drops of mydriasis medicine 10–15 min before photocoagulation is recommended), so as to prevent excessive secretion of saliva, which might affect respiration. After 10–15 min, the pupil shrinks to 1–2 mm.
-
c.Use Oxybuprocaine Hydrochloride Eye Drops (Santen Pharmaceutical Co., Ltd, Japan) locally for topical anesthesia.
-
a.
-
2.
Positioning fixation.
Position the rhesus monkey subsequently as shown in Figure 1A.
Note: A researcher is required to help fix the rhesus monkey head position to facilitate the examination and operation.
-
3.Laser photocoagulation.
-
a.After placing the gonioscope, photocoagulate the whole middle trabecular meshwork of the rhesus monkeys in a dark room by a TX532 laser photocoagulation instrument (Oculight TX; IRIDEX).
-
b.The laser parameters are as follows: 50 μm spot size, 0.1–0.5 s duration, and 800–1,000 mW laser power.
-
c.Locate the trabecular meshwork accurately (Figures 1B and 1C).Note: The structure of the rhesus monkeys' anterior chamber angle is similar to that in humans. To ensure an optimal tissue response, it is essential to accurately focus the aiming beam on the trabecular meshwork (a well-defined dot).
-
d.Adjust laser power and exposure time according to the spot’s reaction.Note: The optimal signs of a successful trabecular meshwork reaction after the photocoagulation include whitening, slight shrinkage, depigmentation at the points, and particularly the generation of a bubble (Figure 1D).
-
e.Treatment in the 360° range.Note: It usually requires 150–250 continuous and non-overlapping light spots on the trabecular meshwork. Histopathological analysis showed that there was still some remaining trabecular meshwork and Schlemm's canal with light structural damage, which might still have a partial function, although laser photocoagulation of the middle trabecular meshwork was performed in the entire circumference (Sun et al., 2022).
-
f.Avoid photocoagulation in blood vessels.Note: If bleeding occurs, press the gonioscope at the corresponding direction to the bleeding for a short time (just a few seconds) to stop bleeding.
-
g.The focus avoids damage to the ciliary body band.Note: It may lead to partial cyclodialysis, which makes it difficult to increase IOP.
-
a.
-
4.
Postoperative medication.
Figure 1.
Methods of laser-induced COHT model
(A) An operation picture of laser photocoagulation in a rhesus monkey.
(B) Anterior chamber angle structure of rhesus monkey and positioning of trabecular meshwork under gonioscopy using a smartphone camera. Scale bar 1 mm.
(C) A diagram of anterior chamber angle structure in rhesus monkeys.
(D) The laser spot location and a bubble generation.
Topical application of Tobramycin and Dexamethasone Eye Ointment (Tobramycin®; Alcon, USA) and Compound Tropicamide Eye Drops (Zhuobian®; Sinqi, China) for 3–7 days after photocoagulation can alleviate non-infectious inflammation.
Note: Significant fibrous exudation in the anterior chamber usually occurs on the first postoperative day.
-
5.
Monitor IOP changes.
Measure IOP in a specific time window (10–12 a.m.) on the first day after photocoagulation and every 3–7 days.
Note: IOP is likely to be lower than normal on the first postoperative day.
-
6.Repeat photocoagulation if necessary.
-
a.If the IOP is not consistently increased (>21 mmHg), repeated photocoagulation is required at intervals of three weeks.Note: The baseline IOP of rhesus monkeys is similar to that of humans (Lin et al., 2021; Pasquale et al., 2021; Quigley and Hohman, 1983; Tu et al., 2019a, Tu et al., 2019b).
-
b.It is generally necessary to perform 2–4 laser photocoagulation sessions.Note: It is usually difficult to obtain continuous high IOP (>21 mmHg at least for 1 month) after one-time laser photocoagulation according to previous literature and based on our experience (Quigley and Hohman, 1983; Sun et al., 2022; Tu et al., 2019a). High IOP usually occurs 1–2 weeks after the second photocoagulation session, and some eyes may gradually return to the normal IOP range after 1–2 weeks, and require further laser photocoagulation, while others may maintain high IOP for several weeks to several months. In addition, locating the trabecular meshwork is more accurate and simpler when laser photocoagulation is repeated, especially in the inferior of globe, where there are more pigments attached to the trabecular meshwork (pigment particles from the previous photocoagulation accumulated here).
 CRITICAL: Use Levofloxacin Hydrochloride Eye Gel locally in both eyes to keep the ocular surface hydrated and prevent infection during anesthesia. It is recommended to use covers and bedding made of disposable sterile surgical towels for the operated monkey to keep rhesus monkeys warm throughout the process.
CRITICAL: Use Levofloxacin Hydrochloride Eye Gel locally in both eyes to keep the ocular surface hydrated and prevent infection during anesthesia. It is recommended to use covers and bedding made of disposable sterile surgical towels for the operated monkey to keep rhesus monkeys warm throughout the process.
-
a.
Part 2: Intracameral injection
Timing: 10–20 min (for step 7)
After successfully established COHT model in rhesus monkeys, this section provides detailed steps for intracameral injection of anti-glaucoma drugs.
-
7.
Slit-lamp biomicroscopy examination before intracameral injection.
Verify that rhesus monkeys are free from infectious inflammation such as conjunctivitis, keratitis, and dacryocystitis.
-
8.
Prepare drugs to be validated according to experimental requirements.
IκBα-siRNA plus Lipofectamine® RNAiMAX Reagent were used in our study.
-
9.
Anesthesia.
After 6–8 h of fasting, perform intramuscular anesthesia by a veterinarian, and then use Oxybuprocaine Hydrochloride Eye Drops for topical anesthesia.
-
10.
Positioning fixation.
Place the rhesus monkey in a supine position on the operating table, and position the sterile sheet (Figure 2A).
-
11.Preoperative measures to prevent ocular infection.
-
a.Administer Tobramycin Eye Drops (Tobrex®, Alcon, USA) topically (Figure 2B).
-
b.Use an eye speculum (Suzhou Mingren, China) to open the upper and lower eyelids of the rhesus monkey after disinfecting the periorbital skin;
-
c.Add povidone iodine to the conjunctival sac to completely cover the conjunctiva and cornea for 1 min (Figure 2C), and rinse the conjunctival sac with a large amount of normal saline.
-
a.
-
12.Intracameral injection.
-
a.Fix the eyeball with ophthalmic microforceps (Xiehe Medical Instruments, China) under the ophthalmic surgical microscope (Carl Zeiss, Germany).
-
b.Use an insulin syringe and insert the needle approximately 1 mm along the corneal tangent direction on the contralateral upper temporal or nasal side and then parallel to the iris to create a self-sealing opening.Note: Keep away from limbal vessels during puncture to avoid bleeding. Besides, keep the bevel oriented upward (toward the corneal endothelium) into the anterior chamber to avoid damage to the cornea, iris, and lens.
-
c.Insert 1 mm inside the limbal of the cornea to release about 40 μL of aqueous humor by siphoning through an empty needle.Note: The speed of anterior chamber paracentesis should be slow, to avoid the sudden shallowing of the anterior chamber and scratching the iris and lens with the tip of the needle.
-
d.Deliver the drug to the anterior chamber using a Hamilton glass syringe (50 μL volume) with a 30G sharp needle (Hamilton, Reno, NV, USA) or insulin syringe through the same puncture port (Figure 2D).Note: The inclined plane of the needle should be inserted completely into the anterior chamber, not too deep, keeping the tip of the needle over the iris and not over the pupil. The intracameral injection speed should not be too fast, avoiding the anterior chamber deepens suddenly, so as to lest the anterior chamber fluctuation, and the lens-iris diaphragm moves back sharply.
-
a.
-
13.
Withdrawing the needle.
Figure 2.
Procedure of intracameral injection
(A) Intracameral injection operation.
(B) Using antibiotic eye drops to prevent infection.
(C) Local disinfection.
(D) Needle tip position in intracameral injection. Scale bar 1 mm.
(E) Using antibiotic eye ointment after operation.
Gently withdraw the needle and massage the puncture port with ophthalmic microforceps to prevent or minimize the efflux of aqueous humor containing drugs.
-
14.Postoperative medication.
-
a.Administer Tobramycin Eye Ointment (Tobrex®, Alcon, USA) topically to prevent ocular infection and, finally, remove the eyelid opener (Figure 2E).
-
b.Apply Tobramycin Eye Ointment locally for 7 days after intracameral injection.
-
a.
Expected outcomes
After the first laser photocoagulation of the trabecular meshwork in rhesus monkeys, the IOP was found to be within the normal range (<21 mmHg, 14 eyes). Among them, 4 eyes had obvious increased IOP (>30 mmHg) after the second laser photocoagulation, 6 eyes showed elevated IOP after the third laser photocoagulation, and the last 4 eyes finally showed high IOP after the fourth laser photocoagulation (Figure 3A). Besides, SD-OCT was used to scan the thickness of the peripapillary RNFL in the two groups. It was found that the peripapillary RNFL thickness at baseline in the two groups was similar and within the normal range. However, there was obvious thinning in the peripapillary RNFL thickness after 2 months of elevated IOP in the COHT group (Figure 3C). Furthermore, hematoxylin and eosin (H&E) staining of paraffin sections showed that trabecular meshwork cells and SC of the COHT group were damaged to different degrees, which limited the outflow of aqueous humor and led to elevated IOP in rhesus monkeys (Figure 3B). Significantly, the residual trabecular meshwork cells provide the site of action for the subsequent IOP lowering drugs.
Figure 3.
Experimental images from COHT model in rhesus monkeys
(A) Time course of IOP changes in 14 eyes after 2–4 times laser photocoagulation compared to 2 normal eyes.
(B) Representative H&E staining images in trabecular meshwork from normal and COHT eyes. Scale bar 100 μm.
(C) Representative SD-OCT pictures showed the progression of peripapillary RNFL in a normal eye and a COHT eye. Scale bar 200 μm.
Limitations
The successful construction of this model (obtaining stable high IOP) often requires repeated laser photocoagulation (generally 1–4 times). Furthermore, high IOP may fluctuate from 30 to 60 mmHg, and the duration of high IOP may vary from several weeks to several months.
Troubleshooting
Problem 1
The IOP of some rhesus monkeys might not rise after multiple (>4) laser photocoagulations (steps 3–6 of Part 1).
Potential solution
The success rate of this model does not reach 100%, which may be related to individual differences. We believe that the success rate can be greatly improved by performing accurate full-circle laser photocoagulation of the middle trabecular meshwork and making more effective spots (generation of a bubble instead of just local graying in the trabecular meshwork).
Problem 2
The photocoagulation of nasal and temporal trabecular meshwork is difficult for inexperienced operators (step 3 of Part 1).
Potential solution
The rhesus monkey’s body position can then be adjusted (e.g., lateral lying position) as needed. For example, when the nasal or temporal trabecular meshwork needs to be photocoagulated, the rhesus monkey can be placed in a lateral position so that it can be imaged in the superior of the gonioscope, which is more convenient for observation and photocoagulation.
Problem 3
In a monkey model of COHT, the measurements obtained by different types of tonometers might be inconsistent, higher IOPs associated with larger measurement errors (step 2.a of Part 0, and step 5 of Part 1).
Potential solution
In addition to the influence of anesthesia, body position and circadian rhythm regulation on IOP, there may be systemic errors and deviations in IOP measurements among different tonometers ( Tu et al., 2019b). The same approved tonometer should be used throughout the experiment to reduce the influence of errors on the experimental results.
Problem 4
Repeated use of Hamilton glass syringes might increase the risk of infection (step 12 of Part 2).
Potential solution
Monkey eyes have a relatively large anterior chamber space (Lin et al., 2021), which is similar to the structure of human eyes. Hamilton glass syringes should be sterilized with ethylene oxide after intracameral injection. For drugs with an injection volume greater than 25 μL (insulin syringe min scale), disposable insulin syringes can be used for intracameral injection.
Problem 5
There is a leakage at the puncture port (step 13 of Part 2).
Potential solution
It is suggested that the needle remain in the anterior chamber for a few seconds after injection and that it be withdrawn slowly. The operator should then massage the puncture port with ophthalmic microforceps to prevent or minimize leakage.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yuqing Lan (lanyq@mail.sysu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81570845) and the Sun Yat-sen Clinical Research Cultivation Project (No. SYS-C-201705). We thank Guangdong Laboratory Animals Monitoring Institute and Zhongshan Ophthalmic Center for their support of this study. We also thank the research group staff for their technical assistance.
Author contributions
D.S. wrote the paper. Y.L., D.S., Z.Z., J.L., and B.W. conceived and design the project. D.S., B.W., J.L., Z.Z., and Z.Y. performed the experiments and analyzed the data. All authors have read and approved the paper.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Difang Sun, Email: 15269612636@163.com.
Zongyi Zhan, Email: zzy19902009@163.com.
Jun Li, Email: doctor_li@126.com.
Yuqing Lan, Email: lanyq@mail.sysu.edu.cn.
Data and code availability
This study did not generate datasets or code.
References
- Aptel F., Weinreb R.N., Chiquet C., Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog. Retin. Eye Res. 2016;55:108–148. doi: 10.1016/j.preteyeres.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Bastia E., Toris C.B., Brambilla S., Galli C., Almirante N., Bergamini M.V.W., Masini E., Sgambellone S., Unser A.M., Ahmed F., et al. NCX 667, a novel nitric oxide donor, lowers intraocular pressure in rabbits, dogs, and non-human primates and enhances TGFβ2-induced outflow in HTM/HSC constructs. Invest. Ophthalmol. Vis. Sci. 2021;62:17. doi: 10.1167/iovs.62.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa M., Toris C.B., Fan S., Taniguchi T., Ichikawa M., Odani-Kawabata N., Iwamura R., Yoneda K., Matsugi T., Shams N.K., Zhang J.Z. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J. Ocul. Pharmacol. Ther. 2018;34:531–537. doi: 10.1089/jop.2017.0146. [DOI] [PubMed] [Google Scholar]
- Gloe S., Rothering A., Kiland J.A., McLellan G.J. Validation of the Icare(®) TONOVET plus rebound tonometer in normal rabbit eyes. Exp. Eye Res. 2019;185:107698. doi: 10.1016/j.exer.2019.107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D.J., Farrell S.M. Physiology and role of intraocular pressure in contemporary anesthesia. Anesth. Analg. 2018;126:1551–1562. doi: 10.1213/ANE.0000000000002544. [DOI] [PubMed] [Google Scholar]
- Lin K.H., Tran T., Kim S., Park S., Stout J.T., Chen R., Rogers J., Yiu G., Thomasy S., Moshiri A. Advanced retinal imaging and ocular parameters of the rhesus macaque eye. Transl. Vis. Sci. Technol. 2021;10:7. doi: 10.1167/tvst.10.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F., Harwerth R., Patel N. Assessing the true intraocular pressure in the non-human primate. Optom. Vis. Sci. 2018;95:113–119. doi: 10.1097/OPX.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale L.R., Gong L., Wiggs J.L., Pan L., Yang Z., Wu M., Yang Z., Chen D.F., Zeng W. Development of primary open angle glaucoma-like features in a rhesus macaque colony from southern China. Transl. Vis. Sci. Technol. 2021;10:20. doi: 10.1167/tvst.10.9.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel H.R., Schlamp C.L., Poulsen G.L., Ver Hoeve J.A., Nork T.M., Nickells R.W. Decrease of cone opsin mRNA in experimental ocular hypertension. Mol. Vis. 2006;12:1272–1282. [PubMed] [Google Scholar]
- Quigley H.A., Hohman R.M. Laser energy levels for trabecular meshwork damage in the primate eye. Invest. Ophthalmol. Vis. Sci. 1983;24:1305–1307. [PubMed] [Google Scholar]
- Rasmussen C.A., Kaufman P.L. Primate glaucoma models. J. Glaucoma. 2005;14:311–314. doi: 10.1097/01.ijg.0000169409.01635.bc. [DOI] [PubMed] [Google Scholar]
- Sarchahi A.A., Eskandari M. Effect of four local anesthetics (tetracaine, proparacaine, lidocaine, and bupivacaine) on intraocular pressure in dogs. Int. Ophthalmol. 2019;39:1467–1474. doi: 10.1007/s10792-018-0969-0. [DOI] [PubMed] [Google Scholar]
- Stewart W.C., Magrath G.N., Demos C.M., Nelson L.A., Stewart J.A. Predictive value of the efficacy of glaucoma medications in animal models: preclinical to regulatory studies. Br. J. Ophthalmol. 2011;95:1355–1360. doi: 10.1136/bjo.2010.188508. [DOI] [PubMed] [Google Scholar]
- Sun D., Zhan Z., Zeng R., Liu X., Wang B., Yang F., Huang S., Li Y., Yang Z., Su Y., Lan Y. Long-term and potent IOP-lowering effect of IκBα-siRNA in a nonhuman primate model of chronic ocular hypertension. iScience. 2022;25:104149. doi: 10.1016/j.isci.2022.104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Matsunaga N., Natsume T., Kitazawa C., Itani Y., Hama A., Hayashi I., Shimazawa M., Hara H., Takamatsu H. A longitudinal comparison in cynomolgus macaques of the effect of brimonidine on optic nerve neuropathy using diffusion tensor imaging magnetic resonance imaging and spectral domain optical coherence tomography. Heliyon. 2021;7:e06701. doi: 10.1016/j.heliyon.2021.e06701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., Li K., Ding X., Hu D., Li K., Ge J. Relationship between intraocular pressure and retinal nerve fibre thickness loss in a monkey model of chronic ocular hypertension. Eye. 2019;33:1833–1841. doi: 10.1038/s41433-019-0484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., Li K., Ding X., Zuo C., Hu D., Ge J. TonoVet versus Tonopen in a high intraocular pressure monkey model. Mol. Vis. 2019;25:391–399. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets or code.





