Abstract
Background
Zinc supplementation was hypothesized to have therapeutic potential against prostate cancer, but its influence on prostate cancer incidence especially at high doses is controversial.
Methods
A total of 47,240 men from the Health Professionals Follow-up Study were followed from 1986 to 2016. Men reported their zinc supplement use at baseline and biennially thereafter. Clinical features of prostate cancer included stage, grade, lethal and aggressive (T4 or N1 or M1 or Gleason 8–10) outcome. Multivariable Cox proportional hazards models were used to evaluate the association between zinc supplement use and incidence of prostate cancer.
Results
During a median follow-up of 28.3 years, we documented 6,980 incident prostate cancer cases including 1,053 lethal and 1,143 aggressive. Zinc supplement use was not associated with overall, localized, low- and intermediate-grade prostate cancer. However, compared to never-users, men who used supplement zinc more than 75 mg/day were at higher risk for lethal (HR: 1.76, 95% CI: 1.16–2.66, Ptrend = 0.001) and aggressive prostate cancer (HR: 1.80, 95% CI: 1.19–2.73, Ptrend = 0.006). Similarly, men who took supplemental zinc for 15 or more years had a higher risk for lethal (HR: 1.91, 95% CI: 1.28–2.85, Ptrend <0.001) and aggressive prostate cancer (HR: 1.55, 95% CI: 1.03–2.33, Ptrend = 0.004).
Conclusion
Zinc supplementation of more than 75 mg per day or over 15 years may substantially increase risk of lethal and aggressive prostate cancer. Caution is warranted regarding excessive usage of zinc supplements among adult men.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10654-022-00922-0.
Keywords: Zinc, Dietary supplement, Prostate cancer, Cohort study
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer death among men in the United States (US). Normal prostate tissue contains one of the highest concentrations of zinc in the body, and mechanistic evidence indicates it is important for prostate health [1]. The role of zinc in prostate cancer pathogenesis has not been elucidated, but evidence suggests zinc can suppress angiogenic and metastatic potential of malignant prostate cells [2].
The primary dietary sources of zinc are red meat, seafood, poultry, grains, and legumes. In the US, the recommended dietary allowance (RDA) of zinc for men aged 19 or above is 11 mg/day [3]. In Europe, the Population Reference Intakes (PRIs) of zinc by European Food Safety Authority (EFSA) range from 7.5 to 12.7 mg/day for women and from 9.4 to 16.3 mg/day for men, based on phytate intakes observed in European population [4]. Most Americans consume the recommended amounts of zinc [5] however, 20–25% adults aged 60 years or older have inadequate zinc intakes [6]. Supplemental zinc use is common among US adults, with prevalence ranging 30–39% from 1999 to 2014 [7, 8]. While typical doses of individual zinc supplements are 30 to 50 mg/tablet, some supplements have even higher dose, exceeding the RDA ten-fold. In fact, the upper intake limits (UL) for zinc is 40 mg/day in US and 25 mg/day by EFSA [3, 4]. The no observed adverse effect level (NOAEL), which was the highest exposure level at which no harmful effects were seen in the organ system studied, was set to be 50 mg/day in both US and Europe [4, 9]. Anecdotally, use of dietary supplements including zinc is increasing due to the COVID-19 pandemic in US and many parts of the world [10–12], although current guidelines did not authorize the use of any dietary supplements for the prevention or management of COVID-19 [13].
Epidemiologic studies examining zinc and prostate cancer have had mixed results [14–22], and few examined supplemental zinc specifically [23–27]. A 2016 meta-analysis suggested a 10% increase in risk of prostate cancer for total zinc intake, but not for supplemental zinc intake, which showed strong evidence for heterogeneity among studies [28]. Previously in the Health Professionals Follow-up Study (HPFS) with follow-up to 2000, we found that men who used supplemental zinc ≥ 100 mg/day or for ≥ 10 years had an increased risk of advanced prostate cancer [24]. With an additional 16 years of follow-up, we aimed to further evaluate zinc supplement use in relation to clinically relevant prostate cancer. In a population expected to be zinc sufficient, we hypothesized that high-dose, long-term zinc supplement use may increase risk of aggressive prostate cancer.
Methods
Study population
The HPFS is an ongoing prospective cohort study of 51,529 US male health professionals who enrolled in 1986 at age of 40 to 75. Participants were mailed questionnaires at baseline and biennially to collect updated information on demographic, lifestyle factors, medical history, medication, and disease outcome. Dietary information was collected using a validated food frequency questionnaire (FFQ) of approximately 130 food items at baseline and every 4 years thereafter [29, 30]. Follow up rates exceeded 90% for each questionnaire cycle. The study protocol was approved by the institutional review boards of the Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Assessment of zinc supplement and dietary zinc
Participants provided detailed information on use and dosage of selected supplements every two years since 1986 [31]. At baseline, participants were asked to identify themselves as “never-user”, “past only” or “current regular user” for zinc supplement. Current users further provided duration of use (0–1, 2–4, 5–9 or ≥ 10 years) and amount per day (pre-defined four levels: < 25, 25–74, 75–100, ≥ 101 mg/d). Current use status (yes, no) and dosage was updated at each subsequent biennial questionnaire. Never-users were defined as participants who had never reported supplement zinc use up to each questionnaire cycle. Men who reported current supplement zinc use but missing dosage (< 2.0% for each questionnaire cycle) were assigned the most common dosage level (25–74 mg/d). At baseline, we assumed men who answered supplement section questionnaire but did not respond (yes/no) to zinc supplement question (7.2%) as never-users. The duration of zinc supplement use was calculated based on reported duration at baseline and updated with subsequent response to current use status. Men who reported supplement zinc current use but missing duration at baseline (1.5%) were assigned the most common duration category (2–4 years).
Dietary zinc intake was assessed repeatedly by FFQs in which participants were asked how often, on average, they consumed each food of a standard portion size during the previous year. Daily intake of zinc was calculated by multiplying the reported frequency of consumption of each item by its zinc content, summing across from all foods, and adjusting for total caloric intake using the nutrient residual methods [32]. The validity of zinc intake by these health professionals was confirmed with validation studies using dietary records (Pearson correlation is 0.71) [29].
Ascertainment of prostate cancer cases
Diagnoses of prostate cancer were initially self-reported on biennial questionnaires by participants and confirmed by review of medical records and pathology reports. Clinical and pathologic stage, Gleason score, and prostate cancer progression (i.e., distant metastases and biochemical recurrence) were collected by medical records and questionnaires sent to prostate cancer survivors and their attending physicians. Deaths were ascertained through reports by family members, autopsy reports, and searches of National Death Index. Underlying cause of death were determined by study physicians based on all available data (including medical records) and who were blinded to any exposure information. Mortality ascertainment in the cohort is more than 98% [33].
For overall prostate cancer endpoint, stage T1a cases were excluded since they are incidentally diagnosed and prone to detection bias. We classified clinical subtypes of prostate cancer based on stage (localized, advanced), tumor grade (low-grade, intermediate-grade, high-grade), lethal (distant metastases or prostate cancer specific death) and aggressive (T4 or N1 or M1 or Gleason 8–10) [34].
Population for analysis
At baseline, we excluded participants who had a history of cancer (other than nonmelanoma skin cancer), who did not complete supplements questionnaire section, and those who left more than 70 items blank on FFQ or reported implausible energy intake (< 800 or > 4200 kcal/day). The remaining 47,240 men were included in current analysis.
Statistical analyses
Cox proportional hazards models were applied to estimate the hazard ratios (HR’s) and 95% confidence intervals (CI’s) for the association between zinc supplement use and risk of prostate cancer. Person-time was calculated from return of the baseline questionnaire until date of prostate cancer diagnosis, death, or end of follow-up (January 2017), whichever came first. Age in months and calendar year at start of follow-up for each 2-year questionnaire cycle were used as stratification variables in the model.
We evaluated zinc supplement by dosage (never-user, past-user, 1–24, 25–74, ≥ 75 mg/day) and duration (never-use, past use of any duration, 1–4, 5–9, 10–14, ≥ 15 years). Multivariable model included race, family history of prostate cancer, prostate-specific antigen (PSA) testing in previous cycle, PSA testing in > 50% of previous cycles, history of diabetes, aspirin use, body mass index (BMI) at age 21, current BMI, height, smoking, vigorous physical activity, total calories, red meat, tomato-based foods, fish, total zinc intake without supplement and history of prostatitis or prostatic infection. To control for potential confounding from other supplements, we additionally adjusted for multivitamin, selenium, vitamin A, vitamin E and number of overall supplement use. Additional adjustment for history of negative biopsy did not affect the main estimates and was excluded from multivariate models. The proportional hazards assumption was verified by adding interactions between the exposure and the time scale. Linear trends across categories were evaluated using median of each category as a continuous variable.
As our previous report followed up to 2000 [24], we further examined the association starting from 2000 to 2016 to assess if association persisted over independent follow-up time. To address residual confounding from PSA screening, we examined the association separately during pre-PSA (1986–1994) and post-PSA era (1994–2016). To evaluate potential latency and minimize possible reverse causation, we assessed zinc supplement use reported at different latencies (i.e. 2–4, 4–6, 6–8, 8–10, and 10–12 years) before prostate cancer diagnosis. For example, in 8–10 years latency analysis, we examined the association of zinc supplement use in 1990 with risk of prostate cancer between 1998 and 2000. We conducted stratified analysis by age of onset (< 65 vs. ≥65 years), dietary zinc intake (< 11 vs. ≥11 mg/d), multivitamin use (non-users vs. users) and performed sensitivity analysis by restricting to men with complete baseline information on use, dosage, and duration of zinc supplements.
We further examined the association between cumulative dosage and duration of zinc supplement use with risk of prostate cancer. The cumulative dosage was calculated by assigning median value for each dosage group and took average dosage since baseline through the follow-up period. The cumulative duration is similar to ‘pack-year’ cigarette smoking, regardless their current use status of supplement zinc. For example, if someone had taken zinc supplement for 6 years but currently stopped, he was classified into 5-9-year category. We also calculated the E-value to explore contribution by unmeasured confounding [35, 36]. Finally, we examined total zinc intake in relation to risk of prostate cancer.
All analyses were performed using SAS version 9.4 (SAS Institute, Inc; Cary, NC) and results with a two-sided p-value < 0.05 were considered statistically significant.
Results
During a median follow-up of 28.3 years, we documented 6,980 incident prostate cancer cases including 1,053 lethal and 1,143 aggressive cases. Age-standardized characteristics by dosage of supplemental zinc use in 1986 and 2000 were presented in Table 1. At baseline, zinc supplement users of ≥ 75 mg/day had more vigorous physical activity and were more likely to use other supplements. Similar patterns were observed in 2000.
Table 1.
Age-standardized characteristics of participants in the Health Professionals Follow-up Study by dosage of supplemental zinc use in 1986 and 2000
| Characteristic | Level of supplement zinc dosage, mg/day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1986 | 2000 | ||||||||||
| Past use | Never use | 1–24 mg/d | 25–74 mg/d | ≥ 75 mg/d | Past use | Never use | 1–24 mg/d | 25–74 mg/d | ≥ 75 mg/d | ||
| (n = 3768) | (n = 37,699) | (n = 1984) | (n = 3105) | (n = 684) | (n = 6808) | (n = 25,335) | (n = 2239) | (n = 3613) | (n = 714) | ||
| Age in years, mean (SD) | 53.5 (9.5) | 54.3 (9.8) | 56.4 (9.6) | 56.6 (9.5) | 56 (9.1) | 66.8 (9.2) | 66.1 (9.2) | 67.4 (9.1) | 68.1 (9.0) | 67.8 (8.9) | |
| White race, % | 95.4 | 95.7 | 95.8 | 96.4 | 96.0 | 96.2 | 95.6 | 95.9 | 95.7 | 97.2 | |
| Family history of prostate cancer, % | 12.8 | 11.9 | 11.1 | 11.6 | 13.1 | 12.3 | 11.6 | 11.8 | 12.6 | 12.1 | |
| History of prostatitis or prostatic infection in 1992, % | 16.9 | 12.1 | 14.4 | 15.7 | 14.4 | 16.1 | 11.6 | 15.2 | 15.7 | 16.8 | |
| PSA test in 1994, % | 39.2 | 36.7 | 38.3 | 39.4 | 36.3 | 42.2 | 35.4 | 42.3 | 41.7 | 38.1 | |
| PSA test in 2000, % | 69.6 | 68.8 | 69.5 | 69.9 | 63.8 | 77.2 | 72.2 | 76.5 | 77.4 | 75.2 | |
| Ever had negative biopsy in 1994, % | 10.7 | 9.5 | 10.3 | 10.1 | 10.0 | 11.1 | 8.9 | 10.0 | 11.1 | 9.7 | |
| History of diabetes, % | 3.5 | 3.2 | 2.6 | 2.9 | 4.4 | 8.1 | 8.6 | 8.1 | 8.1 | 9.6 | |
| Current aspirin use, % | 30.6 | 28.7 | 34.1 | 33.6 | 34.7 | 40.6 | 38.1 | 40.6 | 39.4 | 39.0 | |
| BMI at age 21 in kg/m2, mean (SD) | 23.0 (2.9) | 23.0 (3.0) | 22.8 (2.6) | 23.1 (2.8) | 23.2 (3.1) | 23.0 (2.7) | 23.1 (2.9) | 23.0 (2.7) | 23.3 (3.1) | 23.3 (2.8) | |
| Current BMI in in kg/m2, mean (SD) | 25.5 (3.3) | 25.6 (3.3) | 25.1 (3.0) | 25.2 (3.2) | 25.4 (3.5) | 26.1 (3.7) | 26.3 (3.7) | 25.9 (3.4) | 26.1 (3.6) | 26.5 (3.9) | |
| Height in inches, mean (SD) | 70.2 (2.8) | 70.1 (2.9) | 70.2 (2.7) | 70.2 (2.7) | 70.4 (2.9) | 70.3 (2.7) | 70.1 (2.8) | 70.1 (2.8) | 70.3 (2.8) | 70.4 (2.5) | |
| Current smoker, % | 8.6 | 9.9 | 7.9 | 8.0 | 10.2 | 4.9 | 5.8 | 3.8 | 4.0 | 5.8 | |
| Vigorous activity in MET-h/week, mean (SD)a | 6.4 (18.1) | 6.1 (19.5) | 7.0 (19.4) | 7.1 (26.6) | 8.4 (24.8) | 10.9 (15.0) | 9.4 (14.6) | 10.7 (13.9) | 11.2 (14.7) | 11.4 (15.7) | |
| Total calories in kcal/d, mean (SD) | 1979 (622) | 1985 (610) | 2010 (609) | 1970 (616) | 1999 (654) | 1964 (530) | 1979 (545) | 1993 (536) | 1971 (535) | 1987 (569) | |
| Total dietary zinc intake in mg/d, mean (SD)b | 13 (4) | 13 (5) | 13 (3) | 13 (4) | 13 (4) | 13 (3) | 13 (3) | 13 (3) | 13 (3) | 13 (2) | |
| Red meat in servings/week, mean (SD) | 6.3 (4.9) | 7.0 (5.1) | 5.9 (4.9) | 5.5 (4.6) | 5.4 (4.7) | 5.8 (4.0) | 6.6 (4.3) | 5.8 (4.1) | 5.5 (4.1) | 5.8 (4.2) | |
| Tomato-based foods in servings/week, mean (SD) | 4.8 (3.6) | 4.7 (3.6) | 4.8 (3.5) | 4.9 (3.7) | 5.0 (4.0) | 5.0 (2.9) | 4.9 (2.9) | 5.0 (2.9) | 5.1 (3.2) | 5.3 (3.0) | |
| Fish in servings/week, mean (SD) | 2.5 (2.2) | 2.3 (2.1) | 2.7 (2.3) | 2.9 (2.5) | 3.1 (2.5) | 2.4 (1.8) | 2.2 (1.7) | 2.4 (1.8) | 2.5 (1.9) | 2.5 (1.9) | |
| Current multivitamin use, % | 32.9 | 27.2 | 64.5 | 60.6 | 65.9 | 74.5 | 54.1 | 82.1 | 79.6 | 76.3 | |
| Current selenium supplement use, % | 2.4 | 0.9 | 36.2 | 31.2 | 49.0 | 13.5 | 4.9 | 55.3 | 49.2 | 56.1 | |
| Current vitamin A supplement use, % | 5.9 | 1.6 | 45.7 | 36.7 | 53.2 | 6.5 | 2.5 | 40.6 | 30.0 | 35.9 | |
| Current vitamin E supplement use, % | 15.6 | 9.0 | 70.1 | 62.1 | 76.4 | 60.0 | 42.5 | 89.9 | 86.4 | 91.7 | |
a MET = Metabolic Equivalents of Task per week
b Without supplements. Nutrients were adjusted for total energy intake
We did not find statistically significant associations for zinc supplement and risk of overall, localized, low- or intermediate-grade prostate cancer. However, men who took zinc supplements of more than 75 mg/day were at increased risk for lethal (HR [95% CI], 1.76 [1.16–2.66], Ptrend = 0.001) and aggressive (1.80 [1.19–2.73], Ptrend = 0.006) prostate cancer compared to never-users (Table 2). In terms of duration, men who took supplement zinc for 15 years or more had increased risk for lethal (1.91 [1.28–2.85], Ptrend <0.001) and aggressive prostate cancer (1.55 [1.03–2.33], Ptrend = 0.004) compared to never-users (Table 3).
Table 2.
Multivariable hazard ratios (and 95% confidence intervals, CI) of prostate cancer in relation to supplemental zinc dose (simple update) in the Health Professionals Follow-up Study, 1986–2016
| Risk of prostate cancer subtypes a | Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Never use | Past use | 1–24 mg/d | 25–74 mg/d | ≥ 75 mg/d | P for trendd | |
| Overall prostate cancer, n | 4365 | 1269 | 366 | 580 | 121 | |
| Model 1b | 1.0 | 1.03 (0.96, 1.10) | 1.04 (0.94, 1.16) | 1.04 (0.95, 1.14) | 1.18 (0.98, 1.42) | 0.08 |
| Model 2c | 1.0 | 1.00 (0.92, 1.07) | 1.03 (0.91, 1.16) | 1.03 (0.92, 1.14) | 1.16 (0.96, 1.41) | 0.16 |
| Localized prostate cancer, n | 3204 | 926 | 264 | 423 | 83 | |
| Model 1b | 1.0 | 0.98 (0.91, 1.06) | 1.00 (0.88, 1.13) | 1.02 (0.92, 1.14) | 1.10 (0.88, 1.37) | 0.39 |
| Model 2c | 1.0 | 0.95 (0.87, 1.04) | 0.99 (0.85, 1.14) | 1.01 (0.89, 1.14) | 1.09 (0.86, 1.37) | 0.39 |
| Advanced prostate cancer, n | 358 | 105 | 33 | 52 | 18 | |
| Model 1b | 1.0 | 1.37 (1.09, 1.71) | 1.34 (0.93, 1.92) | 1.34 (0.99, 1.81) | 2.36 (1.45, 3.84) | < 0.001 |
| Model 2c | 1.0 | 1.34 (1.03, 1.75) | 1.37 (0.90, 2.09) | 1.38 (0.96, 1.98) | 2.43 (1.42, 4.16) | 0.009 |
| Low-grade, n | 1732 | 488 | 136 | 203 | 54 | |
| Model 1b | 1.0 | 0.99 (0.89, 1.10) | 0.95 (0.79, 1.13) | 0.91 (0.79, 1.06) | 1.32 (1.00, 1.73) | 0.85 |
| Model 2c | 1.0 | 0.96 (0.85, 1.08) | 0.94 (0.77, 1.15) | 0.90 (0.75, 1.06) | 1.30 (0.97, 1.74) | 0.65 |
| Intermediate-grade, n | 1352 | 432 | 125 | 203 | 26 | |
| Model 1b | 1.0 | 1.07 (0.96, 1.20) | 1.15 (0.96, 1.39) | 1.22 (1.05, 1.42) | 0.86 (0.58, 1.28) | 0.13 |
| Model 2c | 1.0 | 1.02 (0.89, 1.16) | 1.13 (0.91, 1.40) | 1.19 (0.99, 1.42) | 0.84 (0.56, 1.27) | 0.56 |
| High-grade, n | 606 | 165 | 49 | 78 | 23 | |
| Model 1b | 1.0 | 0.95 (0.80, 1.14) | 1.00 (0.74, 1.34) | 1.00 (0.79, 1.28) | 1.55 (1.01, 2.36) | 0.21 |
| Model 2c | 1.0 | 1.01 (0.82, 1.24) | 1.13 (0.81, 1.59) | 1.15 (0.86, 1.53) | 1.80 (1.14, 2.84) | 0.01 |
| Lethal prostate cancer, n | 672 | 175 | 59 | 118 | 29 | |
| Model 1b | 1.0 | 1.14 (0.96, 1.36) | 1.17 (0.89, 1.54) | 1.42 (1.16, 1.74) | 1.84 (1.26, 2.70) | < 0.001 |
| Model 2c | 1.0 | 1.06 (0.86, 1.30) | 1.12 (0.82, 1.53) | 1.38 (1.07, 1.76) | 1.76 (1.16, 2.66) | 0.001 |
| Aggressive prostate cancer, n | 745 | 204 | 66 | 100 | 28 | |
| Model 1b | 1.0 | 1.04 (0.89, 1.22) | 1.14 (0.88, 1.47) | 1.08 (0.87, 1.34) | 1.58 (1.08, 2.32) | 0.05 |
| Model 2c | 1.0 | 1.09 (0.90, 1.31) | 1.28 (0.95, 1.72) | 1.22 (0.94, 1.57) | 1.80 (1.19, 2.73) | 0.006 |
a Overall cases: T1a excluded; Localized cases: stage T1 or T2 and N0, M0; Advanced cases: stage T3b, T4, N1, or M1; Low -grade cases: Gleason score 2–6; Intermediate-grade cases: Gleason score 7; High-grade cases: Gleason score 8–10; Lethal cases: distant metastases or prostate cancer specific death; Aggressive cases: stage T4 or N1 or M1 or Gleason 8–10
b Model 1: adjusted for current age, time period, race (white, African American, Asian American, other), family history of prostate cancer (yes, no), lagged PSA testing history (yes, no, lagged by one period to avoid counting diagnostic PSA tests), lagged PSA testing in > 50% of possible time periods (yes, no, lagged by one period to avoid counting diagnostic PSA tests), history of diabetes (yes, no), current aspirin use (yes, no), body mass index at age 21 (< 20, 20-22.5, 22.5–25, ≥ 25 kg/m2), current body mass index (< 21, 21–25, 25–30, ≥ 30 kg/m2), height (< 68, 68–70, 70–72, ≥ 72 inches), smoking (never, former/quit > 10 years ago, former/quit ≤ 10 years ago, current), vigorous physical activity (quintiles of metabolic equivalent off task (MET)-h/week), total calories (quintiles of kcal/day), red meat (quintiles of servings/week), tomato-based foods (quintiles of servings/week), fish (quintiles of servings/week), total zinc intake without supplement (quintiles of mg/day) and history of prostatitis or prostatic infection (yes, no)
c Model 2: additionally adjusted for multivitamin use (past, never, current), selenium use (past, never, current), vitamin A use (past, never, current), vitamin E use (past, never, current) and number of overall supplement use (0, 1, 2, 3, ≥ 4 per day)
d Using median value of each category (0, 12.5, 50, 90). Past use as missing
Table 3.
Multivariable hazard ratios (and 95% confidence intervals) of prostate cancer in relation to duration of supplemental zinc use in the Health Professionals Follow-up Study, 1986–2016
| Risk of prostate cancer subtypes a | Hazard Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Never use | Past use of any duration | 1–4 years | 5–9 years | 10–14 years | ≥ 15 years | P for trendd | |
| Overall prostate cancer, n | 4365 | 1269 | 490 | 253 | 161 | 163 | |
| Model 1b | 1.0 | 1.03 (0.96, 1.10) | 1.03 (0.94, 1.14) | 1.09 (0.95, 1.23) | 1.05 (0.90, 1.24) | 1.08 (0.92, 1.27) | 0.15 |
| Model 2c | 1.0 | 1.00 (0.92, 1.08) | 1.03 (0.92, 1.14) | 1.07 (0.93, 1.23) | 1.04 (0.87, 1.24) | 1.04 (0.87, 1.24) | 0.36 |
| Localized prostate cancer, n | 3204 | 926 | 372 | 180 | 107 | 111 | |
| Model 1b | 1.0 | 0.98 (0.91, 1.06) | 1.06 (0.95, 1.18) | 1.04 (0.90, 1.22) | 0.95 (0.78, 1.15) | 0.95 (0.78, 1.15) | 0.66 |
| Model 2c | 1.0 | 0.94 (0.86, 1.03) | 1.04 (0.92, 1.18) | 1.02 (0.86, 1.21) | 0.93 (0.75, 1.15) | 0.91 (0.73, 1.12) | 0.48 |
| Advanced prostate cancer, n | 358 | 105 | 45 | 23 | 18 | 17 | |
| Model 1b | 1.0 | 1.37 (1.09, 1.72) | 1.34 (0.97, 1.85) | 1.36 (0.89, 2.09) | 1.58 (0.97, 2.57) | 1.83 (1.10, 3.03) | 0.001 |
| Model 2c | 1.0 | 1.35 (1.04, 1.76) | 1.40 (0.97, 2.01) | 1.43 (0.88, 2.31) | 1.64 (0.94, 2.85) | 1.93 (1.08, 3.44) | 0.04 |
| Low-grade, n | 1732 | 488 | 189 | 95 | 54 | 55 | |
| Model 1b | 1.0 | 0.99 (0.89, 1.10) | 0.98 (0.84, 1.14) | 1.03 (0.83, 1.26) | 0.89 (0.67, 1.17) | 0.91 (0.70, 1.20) | 0.36 |
| Model 2c | 1.0 | 0.95 (0.84, 1.08) | 0.96 (0.81, 1.14) | 1.01 (0.80, 1.27) | 0.88 (0.65, 1.18) | 0.88 (0.66, 1.19) | 0.45 |
| Intermediate-grade, n | 1352 | 432 | 168 | 80 | 50 | 56 | |
| Model 1b | 1.0 | 1.07 (0.96, 1.20) | 1.18 (1.00, 1.38) | 1.14 (0.90, 1.43) | 1.11 (0.83, 1.47) | 1.20 (0.91, 1.57) | 0.08 |
| Model 2c | 1.0 | 1.02 (0.89, 1.16) | 1.16 (0.97, 1.40) | 1.11 (0.86, 1.43) | 1.08 (0.79, 1.47) | 1.13 (0.84, 1.53) | 0.52 |
| High-grade, n | 606 | 165 | 62 | 38 | 22 | 28 | |
| Model 1b | 1.0 | 0.95 (0.80, 1.14) | 0.94 (0.72, 1.23) | 1.22 (0.87, 1.70) | 1.00 (0.65, 1.54) | 1.24 (0.84, 1.83) | 0.27 |
| Model 2c | 1.0 | 1.02 (0.83, 1.25) | 1.07 (0.79, 1.44) | 1.44 (0.99, 2.09) | 1.20 (0.74, 1.93) | 1.50 (0.96, 2.34) | 0.01 |
| Lethal prostate cancer, n | 672 | 175 | 87 | 36 | 46 | 37 | |
| Model 1b | 1.0 | 1.15 (0.96, 1.36) | 1.28 (1.02, 1.61) | 1.00 (0.71, 1.41) | 1.82 (1.34, 2.47) | 1.92 (1.36, 2.72) | < 0.001 |
| Model 2c | 1.0 | 1.08 (0.88, 1.32) | 1.26 (0.97, 1.64) | 1.00 (0.69, 1.45) | 1.81 (1.26, 2.59) | 1.91 (1.28, 2.85) | < 0.001 |
| Aggressive prostate cancer, n | 745 | 204 | 83 | 45 | 33 | 33 | |
| Model 1b | 1.0 | 1.04 (0.89, 1.22) | 1.06 (0.84, 1.34) | 1.19 (0.88, 1.61) | 1.24 (0.87, 1.77) | 1.29 (0.90, 1.85) | 0.05 |
| Model 2c | 1.0 | 1.10 (0.91, 1.32) | 1.18 (0.91, 1.54) | 1.37 (0.97, 1.93) | 1.46 (0.98, 2.17) | 1.55 (1.03, 2.33) | 0.004 |
a Overall cases: T1a excluded; Localized cases: stage T1 or T2 and N0, M0; Advanced cases: stage T3b, T4, N1, or M1; Low -grade cases: Gleason score 2–6; Intermediate-grade cases: Gleason score 7; High-grade cases: Gleason score 8–10; Lethal cases: distant metastases or prostate cancer specific death; Aggressive cases: stage T4 or N1 or M1 or Gleason 8–10
b Model 1: adjusted for current age, time period, race(white, African American, Asian American, other), family history of prostate cancer (yes, no), lagged PSA testing history (yes, no, lagged by one period to avoid counting diagnostic PSA tests), lagged PSA testing in > 50% of possible time periods (yes, no, lagged by one period to avoid counting diagnostic PSA tests), history of diabetes (yes, no), current aspirin use (yes, no), body mass index at age 21 (< 20, 20-22.5, 22.5–25, ≥ 25 kg/m2), current body mass index (< 21, 21–25, 25–30, ≥ 30 kg/m2), height (< 68, 68–70, 70–72, ≥ 72 inches), smoking (never, former/quit > 10 years ago, former/quit ≤ 10 years ago, current), vigorous physical activity (quintiles of metabolic equivalent off task (MET)-h/week), total calories (quintiles of kcal/day), red meat (quintiles of servings/week), tomato-based foods (quintiles of servings/week), fish (quintiles of servings/week), total zinc intake without supplement (quintiles of mg/day) and history of prostatitis or prostatic infection (yes, no)
c Model 2: additionally adjusted for multivitamin use (past, never, current), selenium use (past, never, current), vitamin A use (past, never, current), vitamin E use (past, never, current) and number of overall supplement use (0, 1, 2, 3, ≥ 4 per day)
d Using median of each category. (0, 2.5, 7, 12, 17). Past use of any duration as missing
When stratified by dietary zinc intake (< 11 vs. ≥11 mg/day), supplemental zinc of ≥ 101 mg/day or ≥ 15 years were associated with increased risk of lethal and aggressive prostate cancer in both groups, while significant trend tests were seen in high dietary zinc group but not the other (eTable 1). When examining associations for total zinc intake, compared to men with total zinc intake of 12–15 mg/day, those with zinc intake < 9 mg/day had suggestively higher risk of lethal prostate cancer (1.18 [0.90–1.55]), and men with excessive zinc intake were also at higher risk of lethal prostate cancer (65–75 mg/day: 1.25 [0.81, 1.94], > 75 mg/day: 1.56 [1.14–2.12], Fig. 1).
Fig. 1.
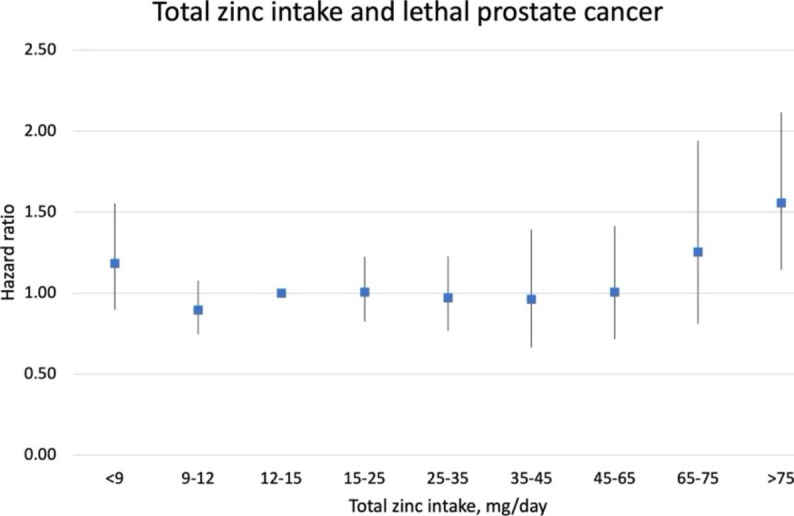
Multivariable hazard ratios (and 95% confidence intervals) of total zinc intake in relation to lethal prostate cancer in the Health Professionals Follow-up Study, 1986–2016. Multivariable model was adjusted for the same set of covariates as in model2 in Table 3 except for total zinc intake without supplement
We observed similar positive associations for follow-up from 2000 to 2016 as in earlier follow-up (eTable 2). The association pattern was qualitatively similar in pre-PSA and post-PSA era (eTable 3). The trend tests remained statistically significant, with slight attenuation of the point estimates. In latency analyses, the associations between high dose or long duration of zinc supplement use and lethal and aggressive prostate cancer remained after applying a 4–10 year lag (eTable 4). We observed similar patterns of increased risk of lethal prostate cancer with excessive use of zinc supplement in groups with different age of onset (eTable 5) and in both multivitamin non-user and user group (eTable 6). When examining cumulative version of the exposure, men with high cumulative dose or long cumulative duration of supplemental zinc use had increased risk of lethal and aggressive prostate cancer (eTables 7 and 8) compared to never-users. Restriction to men with complete supplement zinc information at baseline did not change the result (data not shown). The E-value for the observed association between ≥ 75 mg/day of supplement zinc and lethal prostate cancer was 2.92 (eFigure 1), suggesting an unmeasured confounder that was associated with both the exposure and the outcome by a relative risk of 2.92-fold each, above and beyond the measured confounders, could explain away the estimate, but weaker confounding could not.
Discussion
In this large prospective cohort study with thirty years of follow-up, we found that excessively high-dose and long-duration supplemental zinc intake was associated with increased risk of lethal and aggressive prostate cancer. Separate analyses of follow-up from 2000 to 2016 confirmed our initial report [24]. The association remained in both pre-PSA and post-PSA era and persisted when various latency analyses were performed. Given the strong association between excessive supplemental zinc use and increased risk of clinically important prostate cancer, caution is warranted regarding usage of excessive zinc supplement among adult males. Our data suggests supplemental zinc no more than 25 mg/day may be relatively safe, while the risk of aggressive prostate cancer may increase with higher dosage, especially at extreme levels of more than 75 mg/day. Our conclusions align with the ULs of zinc in both US (40 mg/day) and Europe (25 mg/day) [3, 4], and provides additional evidence of long-term effects of excessive zinc on chronic disease to the current NOAEL guideline of 50 mg/day for zinc in both US and Europe, which were usually based on expected toxicity and short-term controlled metabolic studies [4, 9].
High intracellular zinc concentrations within healthy prostate tissues are important in the production of prostatic fluid [37]. In prostate cancer, decreased intracellular zinc levels are consistently observed in tumor compared to normal tissue [38]. One well-established reason is the downregulation of the hZIP1 transporter, which normally functions to uptake zinc from circulation and causes accumulation in the prostate gland [39]. Based on this observation, researchers have been interested in restoration of high zinc to cancerous prostate tissue and potential zinc treatment to slow or inhibit prostate cancer growth and invasion [40]. However, zinc may enhance activity of telomerase which has been associated with prostate tumor proliferation [41] and excess zinc can reverse potential inhibitory effect of bisphosphonate, which was often used to treat osteolytic metastases [42]. Zinc also has insulin-mimetic properties that can activate insulin-like growth factor-I receptor and epidermal growth factor receptor signaling pathways, both of which play a role in prostate cancer development [43].
Currently, few studies examined supplemental zinc and risk of prostate cancer (eTable 9). Most of these studies were not able to examine the association between high-dose (e.g., ≥ 75 mg/d) supplemental zinc and risk prostate cancer, either with no dosage information [23, 26] or unable to separate true high-dose group [25, 27]. Similar to our observations, a hospital-based case-control study found men who used supplemental zinc of ≥ 10 years had an increased risk of overall prostate cancer (Odds ratio (OR): 1.9, 95% CI: 1.0-3.6) [26], while the Prostate Cancer Prevention Trial reported a null association, possibly because of narrow dosage range (highest category > 22 mg/day) [27]. Another case-control study from 1990s reported supplement zinc use frequency of ≥ 7 times/week over 2 years before diagnosis was associated with lower risk of prostate cancer (OR: 0.55, 95% CI: 0.30-1.00) [23]. However, information on dosage or duration was not collected, and the controls may have been a biased sample of men who were more interested in health and more likely to use dietary supplements than the overall population. Finally, one cohort study reported 10-year average supplemental zinc intake of > 15 mg/day was associated with decreased risk of advanced prostate cancer (HR: 0.34, 95% CI: 0.14–1.09, Ptrend = 0.04) [25]. Caution is warranted in interpretation of this result because of short follow-up time (5 years), PSA testing information was only available at baseline, and the multivariable model did not adjust for important risk factors for advanced prostate cancer including BMI and physical activity.
Although zinc is essential for prostate cells, it might only be beneficial for prostate health within a certain range. In populations with insufficient zinc intake, low zinc might increase prostate cancer risk or mortality, while in populations with sufficient zinc intake from food, additional excessive supplemental zinc may do harm. One Swedish cohort study found men with adequate dietary zinc intake (15.6–20.1 mg/day) had lower risk of prostate cancer-specific mortality compared to men with lower dietary zinc intake (9-12.8 mg/day) [14]. Our analysis on total zinc intake showed, compared to men with adequate zinc intake (12–15 mg/day), having inadequate zinc intake (< 9 mg/day) or excessive zinc intake (> 65 mg/day) was suggested to have higher risk of lethal prostate cancer (Fig. 1). Chronic high intake of zinc can cause abnormalities of genitourinary functions [44]. Laboratory evidence also suggested zinc at optimal levels is preventive whereas at both deficient and higher levels it may enhance tumor growth [45–47]. An in vitro study showed that high Zn2+ has suppressive effects on prostate cancer cell growth but continuous exposure to an environment of high Zn2+ can lead to overexpression of cancer promoting genes such as FBL and CD164 [45]. In current stratified analysis, we saw increased risk of lethal prostate cancer in both high and low dietary zinc group for excessive zinc supplementation, though trend tests were only significant in high dietary zinc group but not the other. This may because high dietary zinc group is similar to the entire population as most of the men (80%) in our population were zinc sufficient, while low zinc group has limited sample size. Furthermore, the variation of dietary zinc intake between two groups is small (medians for low and high dietary zinc groups were 10 and 13 mg/day respectively). With a much higher supplemental dosage of 75 mg/day, it is not likely to have different effects on these two groups.
Zinc supplements might not be an efficient way to increase zinc concentration in prostate cancer cells. Besides potential contaminants of supplement preparations (notably cadmium and lead), the bioavailability also varies across different zinc supplement compounds (sulfate, gluconate, and less frequently citrate [48]). Significantly elevated Zn concentration in prostate was found in rats supplemented with zinc gluconate or zinc citrate, while zinc sulfate was found to have no impact [49]. Most of the zinc absorbed from intestine was stored in bones and muscles, and only around 10% of zinc in human body is readily exchangeable [50]. Even when zinc supplements can increase serum zinc level, they may not necessarily increase intraprostatic zinc to the desirable levels due to the downregulation of zinc transporters in prostate cancer cells. Direct intra-tumoral injection of zinc was shown to halt prostate cancer cell growth in xenograft mice [51] but the practicality of using intra-tumoral administration in humans remains in question.
Our results may not be generalizable to all populations as most of our study participants are white and health professionals who are mostly dietary zinc sufficient. However, the biological mechanisms are likely to be similar in other zinc sufficient populations, and our main findings should be broadly applicable. One exception might be certain ethnic groups who may have impaired ability to incorporate zinc into the prostate. Evidence suggests African American men may have down-regulated zinc transporter, hZIP1, due to selection from long-term zinc-rich ancestral West African soils [52]. Further investigation to examine supplemental zinc and prostate cancer in this special population is warranted.
Because zinc has long been associated with prostate health, the observed associations may reflect the effect of self-medication of longstanding prostate symptoms with excessive amount of supplemental zinc (“reverse causation”). However, accounting for detailed PSA screening history, ever had a negative biopsy and history of prostatic infection and applying up to 10 years latency did not alter the results. In addition, zinc supplement use was not higher in men with prostatitis (Table 1). There is potential measurement error in self-reported supplement use. However, given the prospective study design, any mismeasurement in the exposures would tend to be non-differential, generally attenuating associations. Moreover, the E-value indicated that unmeasured confounders need to be very strong to fully explain the observed association away. Finally, zinc supplement dosage collected from questionnaires were pre-defined and we were unable to separate dosage into smaller intervals especially for the low-dosage level. Study strengths include prospective and repeated assessment of supplement use and dosage every two years, long follow-up, large number of events, and comprehensive information on lifestyle factors and PSA screening.
In conclusion, high-dose, long-duration zinc supplement use may increase risk of lethal and aggressive prostate cancer. Caution is warranted regarding excessive zinc supplementation among men.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yiwen Zhang. The first draft of the manuscript was written by Yiwen Zhang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The Health Professionals Follow-up study is supported by grant from the National Institutes of Health (U01 CA167552).
Statements and declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li D, Stovall DB, Wang W, Sui G. Advances of Zinc Signaling Studies in Prostate Cancer. Int J Mol Sci. 2020;21(2). [DOI] [PMC free article] [PubMed]
- 2.To PK, Do MH, Cho JH, Jung C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci. 2020;21(8). [DOI] [PMC free article] [PubMed]
- 3.National Institutes of Health Office of Dietary Supplements. Zinc, Fact Sheet for Health Professionals 2022 [Available from: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/.
- 4.EFSA Panel on Dietetic Products N. Allergies Scientific opinion on dietary reference values for zinc. EFSA J. 2014;12(10):3844. doi: 10.2903/j.efsa.2014.3844. [DOI] [Google Scholar]
- 5.Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011;141(10):1847–54. [DOI] [PMC free article] [PubMed]
- 6.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132(11):3422–7. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 7.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316(14):1464–74. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li X, Gathirua-Mwangi W, Song Y. Prevalence and trends in dietary supplement use among US adults with diabetes: the National Health and Nutrition Examination Surveys, 1999–2014. BMJ Open Diabetes Res Care. 2020;8(1). [DOI] [PMC free article] [PubMed]
- 9.Roney N. Toxicological profile for zinc. Agency for Toxic Substances and Disease Registry; 2005. [PubMed]
- 10.Grebow J. Dietary supplement sales skyrocket during coronavirus pandemic 2020 [Available from: https://mdanderson.libanswers.com/faq/26219.
- 11.Aysin E, Urhan M. Dramatic increase in dietary supplement use during Covid-19. Curr Developments Nutr. 2021;5(Supplement_2):207. doi: 10.1093/cdn/nzab029_008. [DOI] [Google Scholar]
- 12.Radwan H, Hasan H, Jaafar Z, Abbas N, Rashed Saif E, Al Kitbi M, et al. Diets and dietary supplements used during the COVID-19 pandemic in the United Arab Emirates: A cross-sectional survey. Saudi Pharm J. 2022;30(4):421–32. doi: 10.1016/j.jsps.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The US. Food and Drug Administration. Fraudulent Coronavirus Disease 2019 (COVID-19) Products 2022 [Available from: https://www.fda.gov/consumers/health-fraud-scams/fraudulent-coronavirus-disease-2019-covid-19-products.
- 14.Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93(3):586–93. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52(4):1052–6. doi: 10.1016/j.eururo.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 16.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol. 1988;127(5):999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- 17.Andersson SO, Wolk A, Bergstrom R, Giovannucci E, Lindgren C, Baron J, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68(6):716–22. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76(5):678–87. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M. Case-control study of diet and prostate cancer in China. Cancer Causes Control. 1998;9(6):545–52. doi: 10.1023/A:1008840105531. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Wilkens LR, Morris JS, Henderson BE, Kolonel LN. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. Prostate. 2013;73(3):261–6. doi: 10.1002/pros.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate. 2002;52(4):288–96. doi: 10.1002/pros.10115. [DOI] [PubMed] [Google Scholar]
- 22.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2(2):85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 23.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(10):887–92. [PubMed] [Google Scholar]
- 24.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95(13):1004–7. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer. 2009;61(2):206–15. doi: 10.1080/01635580802419749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer Causes Control. 2009;20(5):691–8. doi: 10.1007/s10552-008-9282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172(5):566–77. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad A, Mahmoud AM, Al-Alem U, Dabbous F, Ali MM, Batai K, et al. Zinc Intake and Risk of Prostate Cancer: Case-Control Study and Meta-Analysis. Plos One. 2016;11(11). [DOI] [PMC free article] [PubMed]
- 29.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-26; discussion 27–36. [DOI] [PubMed]
- 30.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Giovannucci E, Rosner B, Willett WC, Cho E. Longitudinal and secular trends in dietary supplement use: nurses’ health study and health professionals follow-up study, 1986–2006. J Acad Nutr Dietetics. 2014;114(3):436–43. doi: 10.1016/j.jand.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett W. Nutritional epidemiology. Oxford university press; 2012.
- 33.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz LM, Agalliu I, Albanes D, Barry KH, Berndt SI, Cai Q, et al. Recommended Definitions of Aggressive Prostate Cancer for Etiologic Epidemiologic Research. J Natl Cancer Inst. 2021;113(6):727–34. doi: 10.1093/jnci/djaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 36.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web Site and R Package for Computing E-values. Epidemiology. 2018;29(5):e45-e7. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7(2):111–7. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vartsky D, Shilstein S, Bercovich A, Huszar M, Breskin A, Chechik R, et al. Prostatic zinc and prostate specific antigen: an experimental evaluation of their combined diagnostic value. J Urol. 2003;170(6 Pt 1):2258–62. doi: 10.1097/01.ju.0000095795.86327.b8. [DOI] [PubMed] [Google Scholar]
- 39.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463(2):211–7. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med. 2020;17(3):612–25. doi: 10.20892/j.issn.2095-3941.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemoto K, Kondo Y, Himeno S, Suzuki Y, Hara S, Akimoto M, et al. Modulation of telomerase activity by zinc in human prostatic and renal cancer cells. Biochem Pharmacol. 2000;59(4):401–5. doi: 10.1016/S0006-2952(99)00334-2. [DOI] [PubMed] [Google Scholar]
- 42.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60(11):2949–54. [PubMed] [Google Scholar]
- 43.Pooi-Fong W, Sazaly A. Zinc Supplementation and Prostate Cancer. Prostate Cancer: From Bench to Bedside. 2011:423.
- 44.Johnson AR, Munoz A, Gottlieb JL, Jarrard DF. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007;177(2):639–43. doi: 10.1016/j.juro.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 45.Wong PF, Abubakar S. Comparative transcriptional study of the effects of high intracellular zinc on prostate carcinoma cells. Oncol Rep. 2010;23(6):1501–16. doi: 10.3892/or_00000789. [DOI] [PubMed] [Google Scholar]
- 46.Prasad AS, Mukhtar H, Beck FW, Adhami VM, Siddiqui IA, Din M, et al. Dietary zinc and prostate cancer in the TRAMP mouse model. J Med Food. 2010;13(1):70–6. doi: 10.1089/jmf.2009.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko YH, Woo YJ, Kim JW, Choi H, Kang SH, Lee JG, et al. High-dose dietary zinc promotes prostate intraepithelial neoplasia in a murine tumor induction model. Asian J Androl. 2010;12(2):164–70. doi: 10.1038/aja.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haase H, Overbeck S, Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol. 2008;43(5):394–408. doi: 10.1016/j.exger.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Sapota A, Darago A, Skrzypinska-Gawrysiak M, Nasiadek M, Klimczak M, Kilanowicz A. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: a comparative study. Biometals. 2014;27(3):495–505. doi: 10.1007/s10534-014-9724-9. [DOI] [PubMed] [Google Scholar]
- 50.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292(5526):2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 51.Shah MR, Kriedt CL, Lents NH, Hoyer MK, Jamaluddin N, Klein C, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. 2009;28:84. doi: 10.1186/1756-9966-28-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, et al. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl Immunohistochem Mol Morphol. 2003;11(3):253–60. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


