ABSTRACT
Background
The positive association of choline for cognition has been reported in both animal and human studies, yet the associations of choline with the risks of incident dementia or Alzheimer's disease (AD) in humans is unclear.
Objectives
Our objective was to test the hypothesis that lower or higher dietary choline intake is associated with increased or decreased, respectively, risks of incident dementia and AD.
Methods
Data from the Framingham Heart Study Offspring Cohort exam 5 to exam 9 were used. Participants were free of dementia and stroke, with a valid self-reported 126-item Harvard FFQ at exam 5. The intakes of total choline, its contributing compounds, and betaine were estimated based on a published nutrient database. The intakes were updated at each exam to represent the cumulative average intake across the 5 exams. The associations between dietary choline intakes and incident dementia and AD were examined in mixed-effect Cox proportional hazard models, adjusting for covariates.
Results
A total of 3224 participants (53.8% female; mean ± SD age, 54.5 ± 9.7 y) were followed up for a mean ± SD of 16.1 ± 5.1 y (1991–2011). There were 247 incident dementia cases, of which 177 were AD. Dietary choline intake showed nonlinear relationships with incident dementia and AD. After adjusting for covariates, low choline intake (defined as ≤ 219 and ≤ 215 mg/d for dementia and AD, respectively) was significantly associated with incident dementia and incident AD.
Conclusions
Low choline intake was associated with increased risks of incident dementia and AD.
Keywords: nutrition, diet, choline, Alzheimer's disease, dementia, Framingham Heart Study
Introduction
Dementia, including Alzheimer's disease (AD) and other causes, is among the biggest global public health and social care challenges (1). Unfortunately, there have been no major medical treatment breakthroughs that effectively stop dementia progression. The world's largest survey revealed that almost 80% of the general public are concerned about developing dementia at some point, and 1 in 4 people think that there is nothing that can be done to prevent dementia (2). Yet, recent studies suggest that dietary factors can influence disease risk and prevention (3). As an essential nutrient in humans, choline is a dietary nutrient closely related to brain development (4, 5). It is a methyl group donor to homocysteine as an alternative way to synthesize methionine (4), a precursor for the synthesis of phospholipids that are essential for the integrity of cell membranes and intracellular signaling (4, 5), and a precursor for the synthesis of acetylcholine, a neurotransmitter of cholinergic neural networks associated with memory (4–6). Animal experiments indicated the protective effects of dietary choline supplementation on reducing the brain β amyloid load and microglia activation and improving cognitive performance (7–9).
However, choline's effects on the risk of incident dementia, or specifically on AD in humans, is unclear. A previous analysis by Poly et al. (10) in the Framingham Heart Study (FHS) found that total choline intake was positively correlated with the performance of verbal and visual memory tasks and inversely correlated with white-matter hyperintensity volume. The positive association of dietary total choline intake for cognition was also confirmed in another population-based Finnish sample of 2497 male participants by Ylilauri et al. (11). They also reported that a lower dementia risk was associated with higher phosphatidylcholine (PtdCho; a choline-contributing compound) intake. Nonetheless, no significant association was found between incident dementia and total choline intake, nor between incident AD and total choline or PtdCho intake, in their study.
There is a dearth of literature that investigates the effects of the amount of dietary choline intake on human cognition and the risks of incident dementia or AD, and the limited available findings are inconsistent. Thus, the present paper aimed to investigate the associations of dietary choline intakes with the risks of incident dementia or AD in FHS participants.
Methods
Study population
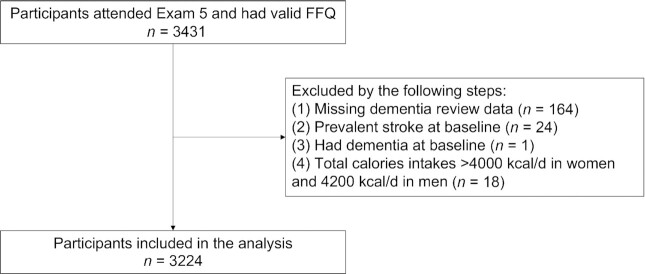
The FHS, an ongoing, community-based, prospective cohort study, was established in 1948. Details about the FHS can be found in previous publications (12–14). Participants in the FHS Offspring Cohort who attended their fifth health exam (1991–1995) were invited to participate in the dietary survey using the well-validated 126-item Harvard FFQ (n = 3431) (15, 16). Participants were excluded (Figure 1) if they had missing dementia review data (n = 164) or had dementia or stroke at baseline (n = 25). Also excluded were participants who did not have valid FFQs (≥12 items blank) or who reported energy intakes from the FFQs of <2.51 MJ/d (600 kcal/d) or >16.74 MJ/d (4000 kcal/d) for females and >17.57 MJ/d (4200 kcal/d) for males (n = 18) (10). After all exclusions, a total of 3224 participants were included as the baseline sample for the present analysis and were followed for incident dementia and AD.
FIGURE 1.
Flowchart of the study.
Measurement of dietary choline
Dietary intake was assessed with the semiquantitative Harvard FFQ, covering 126 food items and vitamin supplements to assess habitual dietary intakes (16). Participants were asked to report how often they had eaten each particular food from a standard list of foods for the prior 12-month period. The questionnaire was mailed to participants, who were asked to complete the form and bring it to their on-site interviews from exam 5 to exam 9. Participants who had <12 blank items were included in analyses and considered nonconsumers of the blank items. The choline and betaine composition of individual foods was evaluated using values published by Zeisel et al. (17) and from the USDA's choline database (18). The validity of choline intake as measured by FFQs was supported by the work of Cho et al. (19). Total choline intake was calculated as the sum of intakes from the choline-contributing compounds: PtdCho, free choline (FrCho), glycerophosphocholine (GpCho), phosphocholine (PCho), and sphingomyelin (Sphingo). Supplements were included as part of the definition of total choline intake. The overall diet quality was assessed by the Dietary Guideline Adherence Index (DGAI) (20).
Case ascertainment of dementia and AD
The detailed cognitive evaluation methods and case ascertainment procedures have been published elsewhere (21, 22). Every 4–6 years, the Offspring Cohort were given a neuropsychological test battery that assessed multiple cognitive domains. Neuropsychological results, along with all available data, which included a neurological examination, a family interview, FHS cycle exam records, and/or hospital or nursing home medical records, were reviewed by a panel including at least 1 neurologist and 1 neuropsychologist to ascertain the cognitive status and, if demented, to assign the possible onset date. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria had to be met for a diagnosis of dementia (23) and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria had to be met for a diagnosis of AD (24), but the presence of vascular dementia did not disqualify a concomitant diagnosis of AD if indicated. The review panel experts were blinded to the participants’ diet questionnaires during the case review process.
Ethics
The Boston University Medical Campus and Boston Medical Center Institutional Review Board approved the study procedures and protocols. Written informed consent was obtained from all participants. The Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for observational studies were followed (26).
Statistical analysis
Our primary and secondary outcomes were dementia and AD, respectively. The exposure of interest was the cumulative average choline intake, which was calculated by updating the choline intake at each exam prior to censoring or until reaching the updating-stop rule for demented participants. If a participant was missing intake data at ≥1 of the follow-up exams, existing intake data were used to calculate the cumulative average of choline intake. The updating-stop rule for demented participants is described here. 1) If the difference between the exam date and dementia diagnosis date was ≥1825 days, we stopped the update at the exam prior to the dementia diagnosis. 2) If the difference was <1825 days, then we stopped the update at 2 exams prior to the exam at which dementia was diagnosed. 3) If the participants died without a diagnosis of dementia, we stopped the update at the exam prior to their death. 4) For participants who survived until the end of the study, we stopped the update at the last exam (exam 9). The same cumulative average approach was used to update choline-contributing compounds, choline without supplements, betaine, and the covariates at each exam, except baseline age, sex, education, and apoE ε4 allele.
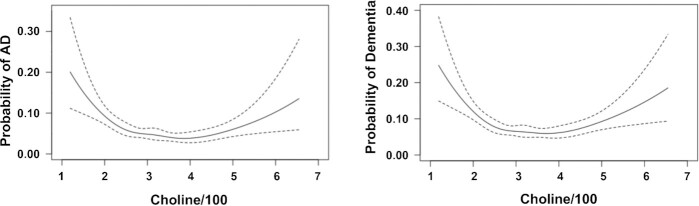
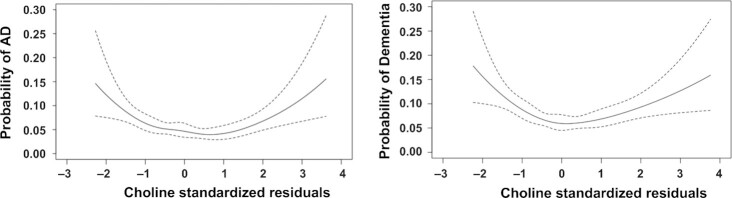
To visualize whether there is a nonlinear association between dementia and choline intake, we produced figures using the R package “Hmisc” (Harrell, FE, Jr, https://cran.r-project.org/web/packages/Hmisc/index.html). With no covariate adjustment, we observed nonlinear relationships between the total choline intake (per 100 mg/d increase) and the probabilities of incident AD and dementia (Figure 2). We further examined the nonlinear relationships after adjustments for age, sex, and education (Figure 3; Supplemental Figures 1–4). To realize this, we generated residuals for choline intake by regressing choline intake on age, sex, and education. The residuals were standardized to a mean of 0 and SD of 1 and used as the x-axis in the plot. The probability of incident AD or dementia was presented on the y-axis. A similar approach was conducted to generate residuals for betaine, the average of choline plus betaine, choline without supplements, and each of the 5 choline-contributing compounds.
FIGURE 2.
Nonlinear associations between total choline intakes and incident AD and dementia. Crude nonlinear associations between total choline intakes and incident AD (177 AD cases/3224) and dementia (247 demented cases/3224). The total choline intake (per 100 mg/d increase) was used as the x-axis and the probability of incident AD or dementia was used as the y-axis. AD, Alzheimer's disease.
FIGURE 3.
Nonlinear associations between AD and dementia and standardized residuals of choline. Nonlinear associations between AD (177 AD cases/3224) and dementia (247 demented cases/3224) and standardized residuals of choline. Residuals of the exposures of interest were regressed on age, sex, and education. The residuals were standardized to a mean of 0 and SD of 1 and were used as the x-axis in the plot. The probability of incident AD or dementia was used as the y-axis. AD, Alzheimer's disease.
Given the nonlinear relationship shown in Figure 2, we determined the choline intake cutoffs to be the points where incident dementia and AD probabilities were 8%. Based on the cutoffs, we defined choline intake as low (≤ 219 and ≤ 215 mg/d), medium (between 220 and 516 mg/d and between 216 and 552 mg/d), and high (≥ 517 and ≥ 553 mg/d) for dementia and AD, respectively.
Then, we built mixed-effect Cox proportional hazards regression models to estimate the associations of low and high intakes (compared to medium intake) with dementia and AD. The follow-up time was calculated as the interval from baseline to the time of dementia onset, time of death free of dementia, or time of the latest exam visit in which dementia was known to not exist. Three models were constructed in our analyses:
Model 1 was adjusted for age, sex, education, and family structure.
Model 2 was adjusted for the model 1 covariates plus BMI; apoE ε 4; methionine, vitamin B6, vitamin B12 and folate intake; total energy intake; DGAI score; and Framingham Stroke Risk Profile score, a marker of estimated stroke risk over the subsequent 10-year period.
Model 3 was adjusted for the model 2 covariates plus alcohol intake, current smoking, and Physical Activity Index (PAI) score (25).
We further examined the nonlinear relationship by adjusting the squared term of the total choline intake (per 100 mg/d increase) in the models.
Secondary analyses were performed in the mixed-effect Cox models with adjustment of the squared term of the exposures of interest and covariates. The secondary analyses explored the associations between dementia and AD and 1) choline without supplements and choline without PtdCho, because dietary PtdCho is the largest dietary contributor of choline but may not be as bioavailable as other dietary choline-containing compounds (20); 2) choline-contributing compounds, all expressed as per 100 mg/d increases except for PCho and Sphingo; 3) choline plus betaine and betaine only, considering the effects of betaine in the choline metabolic pathway; and 4) stratification by sex, physical activity (PAI score; ≥35 and < 35), BMI (≥27 and <27 kg/m2), DGAI score (≥61 and <61), and alcoholic drinks (≥5/wk and <5/wk). The significance level was set to 0.05, and Bonferroni correction was used for multiple comparisons when appropriate. All analyses were performed using R 3.5.3 software (https://www.R-project.org/).
Results
Baseline characteristics are shown in Table 1. The mean ± SD age was 54.5 ± 9.7 years, and 53.8% of participants were females. In general, participants were highly educated (64.2% had received a college-level education or higher). The mean ± SD total choline intake was 324.5 ± 88.5 mg/d. The average amounts of choline-contributing compounds decreased in an order of PtdCho, FrCho, GpCho, PCho, and Sphingo. After a mean ± SD follow-up time of 16.1 ± 5.1 years, 247 participants were diagnosed as having incident dementia, of which 177 cases were AD.
TABLE 1.
Characteristics of the study sample at baseline1
| Characteristics | Value |
|---|---|
| N | 3224 |
| Age, y | 54.5 ± 9.7 |
| Female, n (%) | 1734 (53.8%) |
| Education,2n (%) | |
| At most high school degree | 1110 (35.8%) |
| Some college or college graduate | 1987 (64.2%) |
| ApoE ε 4 positiveness,3n (%) | 643 (20.8%) |
| Total choline intake, mg/d | 324.5 ± 88.5 |
| Choline constituents, mg/d | |
| Phosphatidylcholine | 159.6 ± 52.4 |
| Free choline | 77.6 ± 22.5 |
| Glycerophosphocholine | 54.4 ± 20.9 |
| Phosphocholine | 14.2 ± 4.8 |
| Sphingomyelin | 18.6 ± 6.4 |
| Betaine, mg/d | 189.6 ± 82.7 |
| Methionine, g/d | 1.8 ± 0.6 |
| Vitamin B6, mg/d | 7.5 ± 15.0 |
| Vitamin B12, μg/d | 12.5 ± 15.0 |
| Folate intake, μg/d | 699.8 ± 335.3 |
| BMI, kg/m2 | 28.0 ± 5.0 |
| DGAI score | 60.4 ± 10.5 |
| Total energy, kcal/d | 1866.8 ± 492.4 |
| FSRP score,4 % | 5.5 ± 4.5 |
| Alcohol intake, g/d | 10.8 ± 14.0 |
| Current smoking, n (%) | 739 (23%) |
| Physical Activity Index score | 35.4 ± 4.8 |
Values are shown as mean ± SD or n (%). DGAI, Dietary Guidelines Adherence Index; FSRP, Framingham Stroke Risk Profile.
Education data were missing for 127 participants.
The apoE genotype was missing for 77 participants.
The FSRP score provides the estimated stroke risk over the subsequent 10-y period.
As illustrated in Figure 2, choline intake showed nonlinear relationships with incident dementia and AD. The lowest probabilities for incident dementia and AD were for choline intake of 371 mg/d and 385 mg/d, respectively. Compared to medium intake of choline, low intake was significantly associated with increased risks of dementia and AD in all 3 models (Table 2). High intake was not significantly associated with incident dementia or AD.
TABLE 2.
Associations of total choline intakes (low or high levels compared to a medium level) with incident dementia and AD1
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Total choline intake2 and dementia | ||||||
| Medium intake | Reference | — | — | — | — | — |
| Low intake | 0.81 ± 0.23 | <0.001 | 0.75 ± 0.32 | 0.02 | 0.84 ± 0.32 | 0.009 |
| High intake | 0.44 ± 0.47 | 0.35 | −0.07 ± 0.64 | 0.92 | −0.20 ± 0.65 | 0.76 |
| Total choline intake2 and AD | ||||||
| Medium intake | Reference | — | — | — | — | — |
| Low intake | 0.81 ± 0.23 | <0.001 | 0.68 ± 0.33 | 0.04 | 0.76 ± 0.33 | 0.02 |
| High intake | 0.85 ± 0.55 | 0.13 | 0.50 ± 0.74 | 0.50 | 0.30 ± 0.75 | 0.70 |
The analysis was based on mixed-effect Cox proportional hazards regression models. Model 1 was adjusted for age, sex, education, and family structure. Model 2 was adjusted for the model 1 covariates plus BMI, apoE ε 4, methionine, vitamin B6, vitamin B12, folate intake, total energy intake, Dietary Guidelines Adherence Index score, and Framingham Stroke Risk Profile score. Model 3 was adjusted for the model 2 covariates plus alcohol intake, current smoking, and Physical Activity Index score. AD, Alzheimer's disease.
β and SE based on total choline intake expressed as per 100 mg/d increases. Medium intake was choline between 220 and 516 mg/d in models for dementia and between 216 and 552 mg/d in models for AD. Low intake was choline ≤219 mg/d in models for dementia and ≤215 mg/d in models for AD. High intake was choline ≥517 mg/d in models for dementia and ≥553 mg/d in models for AD.
In Table 3, in the models adjusting for the squared term of choline and covariates, choline intake (per 100 mg/d increase) was significantly associated with dementia (model 1) and AD (model 1 and model 3).
TABLE 3.
Associations of total choline intake (per 100 mg/d increase) with incident dementia and AD1
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Total choline and dementia | ||||||
| Choline (squared) | 0.09 ± 0.03 | 0.007 | 0.08 ± 0.05 | 0.11 | 0.09 ± 0.05 | 0.09 |
| Choline | −0.77 ± 0.27 | 0.005 | −0.64 ± 0.49 | 0.19 | −0.78 ± 0.50 | 0.12 |
| Total choline and AD | ||||||
| Choline (squared) | 0.17 ± 0.05 | <0.001 | 0.13 ± 0.07 | 0.04 | 0.15 ± 0.07 | 0.03 |
| Choline | −1.31 ± 0.37 | <0.001 | −1.09 ± 0.59 | 0.06 | −1.30 ± 0.59 | 0.03 |
The analysis was based on mixed-effect Cox proportional hazards regression models. The exposures of interest were expressed as per 100 mg/d increases. The choline-squared term was added into the model for nonlinear analysis. Model 1 was adjusted for age, sex, education, squared term of interest, and family structure. Model 2 was adjusted for the model 1 covariates plus BMI, apoE ε 4, methionine, vitamin B6, vitamin B12, folate intake, total energy intake, Dietary Guidelines Adherence Index score, and Framingham Stroke Risk Profile score. Model 3 was adjusted for the model 2 covariates plus alcohol intake, current smoking, and Physical Activity Index score. AD, Alzheimer's disease.
In the secondary analyses, nonlinear relationships were also observed between incident dementia and AD and choline without supplements, choline without PtdCho, choline-contributing compounds, the average of choline plus betaine, and betaine only (Supplemental Figures 1–4).
As shown in Supplemental Table 1, choline intake without supplements was consistently and significantly associated with dementia (model 1) and AD (model 1 and model 3). Choline intake (without PtdCho) was significantly associated with dementia and AD in all 3 models.
For choline-contributing compounds, GpCho, PCho, PtdCho, and Sphingo were all significantly associated with dementia and AD in model 1, while only PCho still held significance in the fully adjusted model 3 (Supplemental Table 2).
As shown in Supplemental Table 3, betaine was significantly associated with dementia (model 1 and 2) but not with incident AD. The average of choline plus betaine did not show any significant association with incident dementia or AD.
In the stratification analyses, in model 1, choline intake was significantly associated with dementia in women and in those with a DGAI score < 61, but the results did not hold in the fully adjusted model 3 (Supplemental Table 4). Similarly, choline intake was significantly associated with AD in women, those with a PAI score < 35, those with a DGAI score < 61, and those consuming < 5 drinks per week, but the association remained statistically significant only in those with a PAI score < 35 in the fully adjusted model 3 (Supplemental Table 5).
Discussion
The main finding of our study is that dietary choline intake was nonlinearly related to incident dementia and AD. Low intake of dietary choline was associated with increased risks of dementia and AD, compared to medium intake. High intake was associated with increased risks but did not reach statistical significance. The results held when dietary supplements or the contributing compound (PtdCho) was taken out from total choline intake.
A previous study conducted in Finnish people reported that total choline intake was not associated with dementia or AD, but PtdCho was inversely associated with dementia (11). Differences in methodology might partly explain the inconsistent results. The probable reason is that the nonlinear relationship between choline and incident dementia may veil the results. In addition, the Finnish study was conducted in males, used 4-day food records, only had a subset of 482 out of 2497 male participants who had received cognitive tests, and retrieved dementia and AD diagnoses from health registers. By contrast, the present analysis included both sexes, used a 12-month FFQ, and had diagnoses confirmed by consensus in a dementia review.
We found that low intake of dietary choline was associated with increased risks of dementia and AD. The results are consistent with previous findings that dietary choline intake showed positive associations with concurrent cognition in the FHS (10) and the Finnish study (11). We found that high intake of choline was associated with increased risks of incident dementia and AD, but the results did not reach statistical significance. A possible reason is that the choline intake amount was not high enough to show the harm effect, because only a few people were in the high-choline group in our sample. Above these cutoffs we set for high intake (517 mg/d in models for dementia and 553 mg/d in models for AD), there were 9 demented cases out of 82 participants in the high-intake group and 5 AD cases out of 45 participants in the high-intake group. Besides, the average choline intake in the Finnish cohort was 431±88 mg/d and the corresponding amount in our sample was 324.5 ± 88.5 mg/d, which was below the Adequate Intake for choline (5).
A further argument for a nonlinear relationship in which high choline may not protect against dementia risk is its role as one of the primary sources of a microbial-derived metabolite, trimethylamine N-oxide (TMAO) (27, 28). TMAO has been reported to be associated with increased risks of mild cognitive impairment, AD dementia, more severe AD pathology (as measured by cerebrospinal fluid biomarkers), and cardiovascular disease (29–31). However, the role of choline-derived TMAO remains uncertain given that TMAO production could also be affected by the gut microbe and liver function.
Dietary choline intake comes from a variety of different food sources, including red meat, poultry, dairy, fish, cruciferous vegetables, legumes, nuts, and seeds (19). Choline is an essential nutrient for humans, and it may be that the combined effects of multinutrient approaches or healthy dietary patterns with balanced dietary choline, such as the Mediterranean diet or the MIND diet (a hybrid Mediterranean–Dietary Approaches to Stop Hypertension diet), may be more important to reduce dementia or AD risks (32, 33). To rule out confounding by consumption of healthy dietary patterns, we used the DGAI score as a covariate to account for the variety of different food sources of choline and betaine.
The strengths of this study include the high response rate on the FFQ among exam 5 participants (90.0%), cumulative average intake assessment to reflect long-term effects, long follow-up time for the occurrence of dementia or AD, and systematic ascertainment of dementia and AD diagnoses, which capitalizes on the longitudinal data. There are several limitations of this study that need to be considered. First, FHS participants are primarily non-Hispanic White, limiting the generalization of these results to other populations. Second, the FFQ data are subject to possible misclassification of intakes, likely weakening any true association. To help account for possible misclassification, we have adjusted our analyses for total energy intakes. Recall may be affected by cognitive impairment during the preclinical stage of dementia and AD, so we set updating-stop rules for updates to dietary data, based on the times between the dietary collection dates and dementia diagnoses. Because of our concern that choline intake may be associated with the overall diet quality, we also adjusted for the DGAI score as a marker of a healthy diet pattern. While most of the foods that are good sources of choline or betaine are included in the calculation of the DGAI score (e.g., eggs, beef, chicken, fish, dairy, cruciferous vegetables, legumes, nuts, seeds, cereals, and other grain products), it would have been difficult to exclude those foods from the calculation of this index given the wide distributions of choline and betaine in the diet. Lastly, the effects of high choline intake on dementia and AD need to be investigated in populations with high choline dietary patterns.
In conclusion, low dietary choline intake was associated with increased risks of incident dementia and AD. Further investigations regarding the effects of high choline intake on dementia and AD risks are warranted.
Supplementary Material
Acknowledgements
We thank the Framingham Heart Study (FHS) participants for their decades of dedication and the FHS staff members for their hard work in collecting and preparing the data.
The authors’ responsibilities were as follows – PFJ, RA, JY, JKB: designed the research; PFJ, RA, JY, XL: conducted the research; XL, CL, JM: analyzed the data; JY: wrote the paper; SAD, SHA: did the dementia review; AFAA: provided data; PFJ, JY: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by the Framingham Heart Study's National Heart, Lung, and Blood Institute contract (N01-HC-25195; HHSN268201500001I), NIH grants from the National Institute on Aging (AG008122, AG016495, AG033040, AG054156, AG049810, AG062109 and AG057768), Pfizer, and the USDA Agricultural Research Service (agreement no. #58-1950-9-004). Support for JY was also provided by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016I2M1004).
Author disclosures: JY was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016I2M1004). RA is a scientific advisor to Signant Health. All other authors report no conflicts of interest.
Supplemental Figures 1–4 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
JY and XL contributed equally to this work.
Abbreviations used: AD, Alzheimer's disease; DGAI, Dietary Guidelines Adherence Index; FHS, Framingham Heart Study; FrCho, free choline; GpCho, glycerophosphocholine; PAI, Physical Activity Index; PCho, phosphocholine; PtdCho, phosphatidylcholine; Sphingo, sphingomyelin; TMAO, trimethylamine N-oxide.
Contributor Information
Jing Yuan, Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China; Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA, USA.
Xue Liu, Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
Chunyu Liu, Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
Alvin F A Ang, Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA, USA; Slone Epidemiology Center, Boston University School of Medicine, Boston, MA, USA.
Joseph Massaro, Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA, USA.
Sherral A Devine, Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA, USA.
Sanford H Auerbach, Framingham Heart Study, Boston University School of Medicine, Boston, MA, USA; Department of Neurology, Boston University School of Medicine, Boston, MA, USA.
Jan Krzysztof Blusztajn, Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, MA, USA.
Rhoda Au, Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA, USA; Framingham Heart Study, Boston University School of Medicine, Boston, MA, USA; Slone Epidemiology Center, Boston University School of Medicine, Boston, MA, USA; Department of Neurology, Boston University School of Medicine, Boston, MA, USA; Department of Epidemiology, Boston University School of Public Health, Boston, MA, USA.
Paul F Jacques, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA; Gerald J. and Dorothy R. Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request, pending application to and approval by the Framingham Heart Study.
References
- 1. Alzheimer's Disease International . World Alzheimer Report 2015: The global impact of dementia. [Internet]. London, UK: Alzheimer's Disease International; 2015; [cited 1 June 2020]. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf [Google Scholar]
- 2. Alzheimer's Disease International . World Alzheimer Report 2019: Attitudes to dementia. [Internet]. London, UK: Alzheimer's Disease International; 2019; [cited 1 June 2020]. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf [Google Scholar]
- 3. Marchand NE, Jensen MK. The role of dietary and lifestyle factors in maintaining cognitive health. Am J Lifestyle Med. 2018;12(4):268–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14(1):269–96. [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine . Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: The National Academies Press;1998. [PubMed] [Google Scholar]
- 6. Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–96. [DOI] [PubMed] [Google Scholar]
- 7. Velazquez R, Ferreira E, Winslow W, Dave N, Piras IS, Naymik Met al. . Maternal choline supplementation ameliorates Alzheimer's disease pathology by reducing brain homocysteine levels across multiple generations. Mol Psychiatry. 2020;25:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow Wet al. . Lifelong choline supplementation ameliorates Alzheimer's disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019;18(6):e13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poly C, Massaro JM, Seshadri S, Wolf PA, Cho E, Krall Eet al. . The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am J Clin Nutr. 2011;94(6):1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ylilauri MPT, Voutilainen S, Lonnroos E, Virtanen HEK, Tuomainen TP, Salonen JTet al. . Associations of dietary choline intake with risk of incident dementia and with cognitive performance: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2019;110(6):1416–23. [DOI] [PubMed] [Google Scholar]
- 12. Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet. 2014;383(9921):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsao CW, Vasan RS. Cohort profile: The Framingham Heart Study (FHS): Overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44(6):1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolf PA. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Arch Neurol. 2012;69(5):567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi Jet al. . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 16. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 17. Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133(5):1302–7. [DOI] [PubMed] [Google Scholar]
- 18. Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM. USDA database for the choline content of common foods, Release 2. Beltsville, MD: US Department of Agriculture;2008. [Google Scholar]
- 19. Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GAet al. . Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83(4):905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauder KA, Proctor DN, Chow M, Troy LM, Wang N, Vita JAet al. . Endothelial function, arterial stiffness and adherence to the 2010 Dietary Guidelines for Americans: A cross-sectional analysis. Br J Nutr. 2015;113(11):1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Au R, Piers RJ, Lancashire L. Back to the future: Alzheimer's disease heterogeneity revisited. Alzheimers Dement (Amst). 2015;1(3):368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White Ret al. . Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–504. [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and statistical manual of mental disorders IV (DSM-IV). Washington, DC: American Psychiatric Association;1994. [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. [DOI] [PubMed] [Google Scholar]
- 25. Kannel WB, Belanger A, D'Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;11(4):820–25. [DOI] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JPet al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci. 2012;109(52):21307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37(1):157–81. [DOI] [PubMed] [Google Scholar]
- 29. Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CMet al. . The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Del Rio D, Zimetti F, Caffarra P, Tassotti M, Bernini F, Brighenti Fet al. . The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients. 2017;9(10):1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guasch-Ferré M, Hu FB, Ruiz-Canela M, Bulló M, Toledo E, Wang DDet al. . Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J Am Heart Assoc. 2017;6(11):e006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv Nutr. 2015;6(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request, pending application to and approval by the Framingham Heart Study.