Abstract
Apathy and disinhibition are common and highly distressing neuropsychiatric symptoms associated with negative outcomes in persons with dementia. This paper is a critical review of functional and structural neuroimaging studies of these symptoms transdiagnostically in dementia of the Alzheimer type, which is characterized by prominent amnesia early in the disease course, and behavioural variant frontotemporal dementia, characterized by early social-comportmental deficits. We describe the prevalence and clinical correlates of these symptoms and describe methodological issues, including difficulties with symptom definition and different measurement instruments. We highlight the heterogeneity of findings, noting however, a striking similarity of the set of brain regions implicated across clinical diagnoses and symptoms. These regions involve several key nodes of the salience network, and we describe the functions and anatomical connectivity of these brain areas, as well as present a new theoretical account of disinhibition in dementia. Future avenues for research are discussed, including the importance of transdiagnostic studies, measuring subdomains of apathy and disinhibition, and examining different units of analysis for deepening our understanding of the networks and mechanisms underlying these extremely distressing symptoms.
Keywords: neuropsychiatric symptoms, neural networks, behavioural variant frontotemporal dementia, dementia of the Alzheimer’s type, salience network
Neuropsychiatric symptoms of dementia, including apathy and disinhibition, are distressing and difficult to treat. Jenkins et al. review functional and structural neuroimaging studies of these symptoms across behavioural variant frontotemporal dementia and dementia of the Alzheimer type.
Introduction
Worldwide in 2015 the number of individuals living with dementia was estimated at 47.47 million, and this is projected to increase to 135.46 million by 2050.1 Two frequent clinical dementia syndromes, defined by the domain of the earliest appearing symptoms, are dementia of the Alzheimer type (DAT) and behavioural variant frontotemporal dementia (bvFTD). Individuals with these syndromes show high rates of neuropsychiatric symptoms (NPS) such as disinhibition, apathy, delusions, aggression, agitation and depression, with one US community-based study of dementia reporting a 5-year prevalence rate of 97%.2 Apathy and disinhibition are amongst the most distressing NPS in dementia,3 and are predictive of increased institutionalization, disease progression and mortality. Among individuals with dementia living in long-term institutionalized care, prevalence of apathy and disinhibition increase over time.4 Unfortunately, these symptoms are notoriously difficult to treat, owing in part to the complex relationships between clinical syndromes, neuropathological causes and neuroanatomical predilection of disease. For example, both DAT and bvFTD clinical syndromes are associated with apathy, and apathy is also present in mild cognitive impairment (MCI) and mild behavioural impairment5 as well as other clinical dementia syndromes including Parkinson’s disease, Huntington’s disease and vascular dementia.6 An individual can even exhibit apathy and disinhibition concurrently,7 particularly in later stages.8 Understanding the affected neural networks is needed to inform treatments, and to identify and improve therapeutic targets for non-invasive neurostimulation treatments such as repetitive transcranial magnetic stimulation (rTMS), for which some initial small pilot studies have found encouraging results.9,10 The present review is a critical summary of functional and grey matter structural neuroimaging studies of apathy and/or disinhibition, focused specifically on the clinical syndromes of bvFTD and DAT. We consider the prevalence, measurement, clinical correlates, neuroimaging findings, prominent and emerging theoretical positions, and future directions for research, emphasizing the utility of studies with a Research Domain Criteria (RDoC) focus11 and a network neuroscience approach.
Background and rationale for our transdiagnostic approach
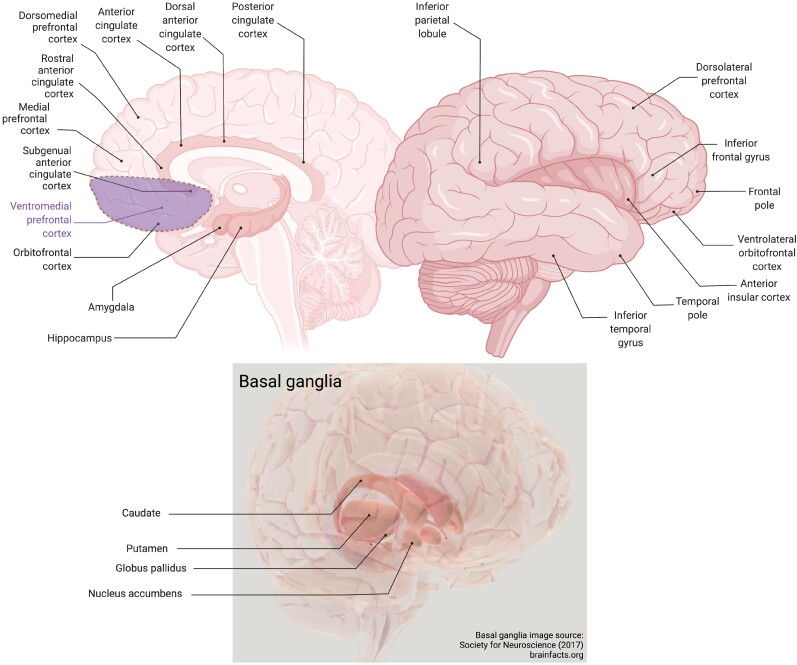
Neuropathological diseases that cause dementia share fundamental processes associated with progressive neuronal dysfunction and death, including oxidative stress, programmed cell death, neuroinflammation and proteotoxic stress. However, they are defined by specific pathological processes. The family of diseases under the neuropathologic rubric of frontotemporal lobar degeneration (FTLD) include microtubule-associated protein tau (MAPT), TAR DNA binding protein 43 (TDP-43), and fused-in-sarcoma (FUS) immunoreactive inclusions. Alzheimer’s disease is defined neuropathologically by the presence and density of amyloid-β accumulation and tau neurofibrillary tangles. Generally, cells that accumulate neuropathology-associated proteins are the cells that succumb to cytotoxic events such as synaptic toxicity, neuroinflammation or cell death-related signalling.12 However, NPS tend to reflect the topology of the grey matter pathology, rather than the specific neuropathologies.13,14 Evidence indicates a prion-like spread of misfolded disease protein conformers across synapses in common neurodegenerative disorders.15 This body of evidence is consistent with findings from human neuroimaging research that brain regions that are targeted by distinct neurodegenerative diseases correspond to functional brain networks in healthy individuals.16 For example, individuals with DAT show a primary deficit in episodic memory and have prominent atrophy in the medial temporal lobe, precuneus, posterior cingulate cortex (PCC) and lateral temporo-parietal regions,16 which form the default mode network (DMN).17 These are areas that underlie episodic memory, visuospatial skills and motivational systems that first decline in DAT. Frontotemporal dementia (FTD) refers to the class of clinical syndromes most often associated with FTLD neuropathology (but not always). Subtypes feature distinct patterns of atrophy, e.g. individuals with semantic dementia have loss of word meaning and bilateral anterior temporal atrophy,18 while individuals with bvFTD exhibit disturbances in behaviour and atrophy in the frontoinsular, anterior cingulate, striatum and frontopolar cortex (Fig. 1), which form part of the salience network (SN).16 The SN (Fig. 2 and Box 1) is involved in signalling personal salience, whether emotional, cognitive or homeostatic, that requires changes in sympathetic tone, including reward processing, conflict monitoring and interoceptive-autonomic processing.29,30
Figure 1.
Brain regions involved in apathy and disinhibition in dementia. Figure created with BioRender.com.
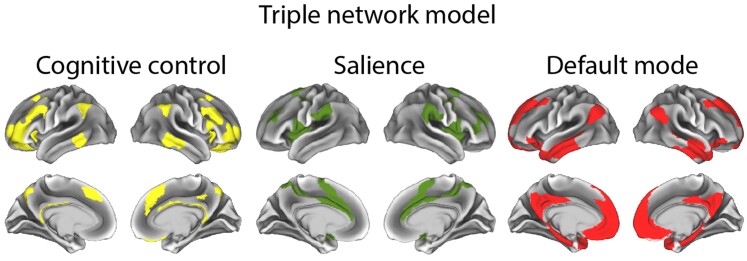
Figure 2.
One visual conceptualization of the triple network model of large-scale intrinsic connectivity networks.
Box 1. Large scale intrinsic connectivity networks.
Complex behaviours rely on a distributed network of regions, and researchers have identified several large-scale intrinsic connectivity networks. For example, a predominant triple network model19 defines a cognitive control network (CCN, also known as the central executive, executive control or fronto-parietal control network), salience network (SN, also known as the ventral attention network or the salience and emotion network) and default mode network (DMN). One depiction of areas comprising these networks based on Ji et al.20 is shown in Fig. 2, although there are several variations within the literature.
Key regions of the SN are the amygdala, orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), subgenual ACC (sgACC), nucleus accumbens (NAc), and the anterior insula. Key regions of the CCN are the dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), and dorsal ACC. Key regions of the DMN are the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), precuneus, hippocampus and inferior parietal lobule (IPL). See Fig. 1 for locations of these regions.
Variations in the literature are due to the fact that these models are over-simplifications based on averages across many participants, often using a winner takes all approach, whereas in reality, brain regions are involved in multiple functions, and individuals differ in their resting state connectivity patterns.21 So, for example, the VMPFC is often considered part of the DMN, and involved in self-referential processing. However, it is also sometimes described as part of the SN and important for the perception of self-relevant salient stimuli,22 and others emphasize the importance of some of its subregions in salience and reward processing, or affiliation.23 Another widely employed functional network parcellation includes the OFC and sgACC in a ‘limbic’ network,24 and these regions are likely to correspond to the SN, along with the amygdala and anterior insula. Others consider the basolateral amygdala to belong to a semantic appraisal network (SAN), along with the anterior temporal lobes, NAc, sgACC and caudate head.25–27 The SAN is argued to highly overlap with the SN and to support personalized hedonic evaluations of emotional and social experiences and concepts.26
Relating the triple network model to RDoC constructs
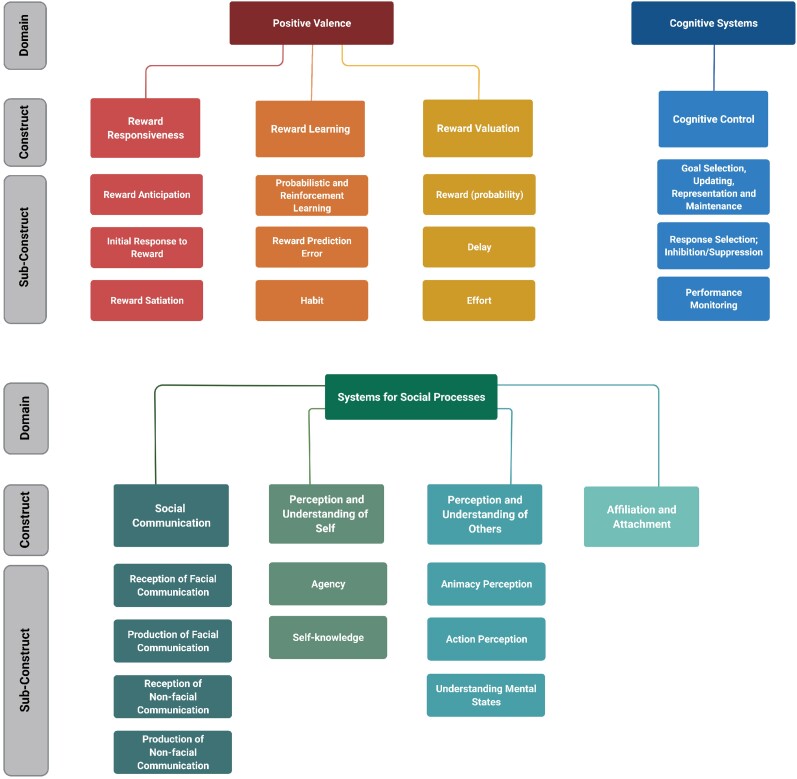
The DMNs closest RDoC construct is likely the declarative memory construct within the cognitive systems domain, as it contains the critical constructs for episodic and semantic memory.28 Also relevant for the DMN is the RDoC domain of systems for social processes (Fig. 3). The CCN is most closely related to the cognitive systems RDoC domain, with the construct of cognitive control and its subdomains. Other constructs in the cognitive systems domain (not shown in Fig. 3) are attention; perception; language; and working memory. The SN is related to several RDoC domains, including positive valence (Fig. 3), systems for social processes, and negative valence, particularly the acute threat (Fear) construct. Other constructs contained within the negative valence domain are potential threat (anxiety), sustained threat, loss and frustrative non-reward.28
The majority of neuroimaging studies up until now have relied on clinical diagnosis of a dementia syndrome, and in this review we indicate the few studies that have incorporated biomarker, genetic mutations or neuropathological confirmation of diagnosis. Combinations of pathologies are commonly observed at autopsy,31 and some researchers argue that neurodegenerative diseases exist upon a single continuum, citing evidence for a clinical, pathologic and genetic spectrum of diseases that contains both FTLD and Alzheimer’s disease.32 Ossenkoppele et al.33 found that 52% of individuals with a behavioural (i.e. ‘frontal’) variant of DAT met the clinical diagnostic criteria for possible bvFTD. Such absence of one-to-one correspondence between NPS and disease pathology, manifesting as clinic-anatomical convergence and phenotypic diversity, obscure our understanding of relationships between brain and behaviour, impeding treatment efforts. These factors make NPS suited to a dimensional analysis such as that of the RDoC. RDoC is a framework for pathophysiology research launched by the National Institutes for Mental Health (NIMH) to address some of the limitations of a categorical approach to studying mental disorders, such as poor predictions of treatment response and a failure to capture fundamental mechanisms.11 It conceives of mental disorders as disorders of brain circuitry, and symptoms are posited to share risk factors, and potentially underlying disease processes. As such it encourages the study of symptoms across multiple disorders, transdiagnostically. The present review also takes an RDoC approach (Fig. 3 and Boxes 2 and 3), the utility of which for studying NPS such as apathy in neurodegenerative diseases has recently been highlighted.53
Figure 3.
RDoC domains, constructs and subconstructs relevant to apathy and disinhibition. Figure created with BioRender.com.
Box 2. RDoC domains and apathy.
RDoC is a research framework to provide information about the basic cognitive and biological processes that lead to mental illness. As such, RDoC is concerned with uncovering behavioural, biological and physiological components and mechanisms via which risk and protective factors exist.28 RDoC is implicated as a matrix of elements. Six major domains of human functioning include negative valence, positive valence, cognitive systems, systems for social processes, arousal/regulatory systems and sensorimotor systems. Domains contain constructs, or behavioural elements, comprising aspects of the overall range of functions within that domain. Constructs can be measured using varying levels or units of analysis, including genes, molecules, cells, circuits, physiology, behaviour and self-report.
The RDoC domain of positive valence is highly relevant to studying apathy.34 Figure 3 shows the constructs and subconstructs within this domain. Examples of paradigms used to measure constructs within the positive valence domain include the monetary incentive delay task for the reward responsiveness construct, probabilistic reward task for the reward learning construct, and the delay discounting task for the reward valuation construct.28 So far, only a few neuroimaging studies of individuals with bvFTD or DAT have examined RDoC positive valence constructs. Of relevance to this review, Manuel et al.35 gave the delay discounting task to individuals with bvFTD and DAT. However, prior to each choice on the delay discounting task, individuals were shown positive, negative or neutral pictures and asked to vividly imagine witnessing the event. They found that individuals with bvFTD did not have emotion-related modulation of the rate of delay discounting. On the other hand, individuals with DAT were more impulsive when primed by negative pictures, and this impulsivity was associated with reduced bilateral amygdala integrity in individuals with DAT. These researchers concluded that the amygdala is a key locus of interaction of emotion and decision-making.
Another RDoC Domain relevant to apathy (perhaps cognitive apathy), given the brain regions it incorporates (including DLPFC, dorsomedial PFC, and dorsal ACC), is the cognitive systems domain. In addition to cognitive control shown in Fig. 3, the cognitive systems domain contains the constructs of attention, perception, declarative memory, language and working memory. Paradigms to assess the cognitive control construct include task-switching, flanker, Simon, Stroop colour-word, Go/No-go and stop signal reaction time tasks.28 One neuroimaging study that gave such a task to individuals with dementia is Li et al.36 These authors found that individuals with DAT showed decreased and individuals with MCI increased functional MRI activity in several regions implicated in the above studies of apathy, including dorsal ACC, middle and inferior frontal gyri, IPL and bilateral insula.
The studies in this review are focused on identifying neuroimaging associations of apathy, disinhibition, and in some cases their separable effects using disjunction analyses. However, we want to highlight an RDoC-inspired study by Lansdall et al.37 who were interested in studying apathy and disinhibition together across individuals with FTD subtypes, and PSP and CBS syndromes. These researchers derived dimensions of apathy and impulsivity from a principal components analysis of items from caregiver-ratings, self-reported questionnaires and objective tasks. They found that a component that reflected caregiver ratings of ‘challenging behaviours’ including apathy and disinhibition was associated with extensive decreased grey matter in the temporal pole, frontal pole, OFC, and mPFC. The authors maintain that apathy and impulsivity are not distinct ends of the same spectrum, but that both involve disrupted motivation and reward processing circuitry. They advocate that a dimensional approach such as theirs enables improved characterization of neural systems responsible for behaviour across disorders, which can allow for identification of symptomatic therapies for one illness that might help patients and caregivers that are affected by another.
Box 3. RDoC domains and disinhibition.
Disinhibition, manifesting as impulsivity may be related to impaired prediction errors, value representations, reversal learning, or temporal discounting.38 In terms of RDoC, these fall within the cognitive control construct (Fig. 3). Cognitive control is impaired in bvFTD, e.g. using the flanker task (conflict monitoring), participants with bvFTD who were early in their disease course and had otherwise normal neuropsychological test scores were impaired on this task of cognitive control.39 One study found that neuropsychological tests of inhibitory control (e.g. the Hayling Test, Stroop, verbal fluency and other tests) were not able to distinguish between 27 individuals with bvFTD and 25 individuals with DAT.40 However, the caregiver-rated Barratt Impulsiveness Scale (BIS) did reveal that individuals with bvFTD were rated as more disinhibited than those with DAT.40 This finding highlights the difficulty of assessing disinhibited behaviour within a controlled laboratory setting and the importance of measuring behaviours using multiple methods, in particular caregiver report for bvFTD.
Few studies have attempted to examine neural correlates of cognitive control in bvFTD or DAT, other than the Krueger et al.41 study mentioned above. These researchers found a double dissociation, whereby NPI-measured disinhibition was associated with OFC cortical thickness, and executive functioning measures of inhibition (e.g. Stroop, Trail-making test) were associated with cortical thickness in the DLPFC.
Much more research investigating the mechanisms underlying disinhibition has focused on inappropriate social behaviour, which may result from impairments in theory of mind, empathy or facial emotion processing.38 These relate to the RDoC domain of systems for social processes (Fig. 3). Several neuroimaging studies in individuals with dementia, including in bvFTD, have examined perception of facial42 and bodily expressions.43 The Reading the Mind in the Eyes test (RMET) of social cognition44 requires inferring mental states based only on pictures of eyes, and individuals with MCI have been reported to have reduced activation in the posterior temporal sulcus and temporal pole during this test, compared to healthy controls.45 Others have highlighted the importance of context in making social judgements. For example, Kumfor et al.46 found that individuals with bvFTD were more impaired at judging emotions that were incongruent with the bodily context they were presented in, than individuals with semantic dementia and healthy controls. Furthermore, the influence of abnormal context was associated with reduced grey matter in the right amydala/parahippocampal gyrus and left precentral gyrus. The Awareness of Social Inferences Test (TASIT)47 is an even more ecologically valid measure as it presents dynamic (i.e. video) vignettes of everyday social interactions. It’s been used in studies of bvFTD and DAT where it was shown that the emotion evaluation subtest was associated with grey matter reductions in regions important for emotion processing, including the amygdala and insula, whereas the social inference subtest, requiring judgements of sincerity or sarcasm was associated with grey matter loss in theory of mind regions, including the precuneus and temporal pole.48 Using resting state functional MRI, Sedoño et al.49 found that performance on social-executive tasks, including the TASIT and the RMET, as well a measure of executive functioning, was associated with significantly decreased network centrality (a measure of the importance of a node for efficient communication and integration across a network) in a fronto-temporo-insular network resembling the SN bvFTD.
Bickart et al.23 examined subcomponents of social functioning in individuals with FTD clinical subtypes using the Social Impairment Rating Scale. They looked at grey matter atrophy of three partially dissociable amygdala networks that they had defined previously in healthy adults. Atrophy across all three networks was associated with greater social impairment overall. Atrophy in a mesolimbic, reward-related network that included the VMPFC, rostral ACC, sgACC and dorsal temporal pole was associated with most severe socio-emotional detachment. Atrophy in an interoceptive, pain-related network that included the insula, putamen and dorsal ACC was associated with lack of social apprehension. Atrophy in a perceptual network which included the lateral OFC and ventral temporal pole was associated with severe lack of awareness or understanding of others’ behaviour. Amygdala atrophy also predicted greater impairment in three SIRS domains, although this variance was not unique to the amygdala.
Two studies by Toller and colleagues50 also examined SN functional connectivity in transdiagnostic samples including individuals with bvFTD, DAT, svPPA, non-fluent PPA and cognitively normal individuals. Toller et al.50 found that across diagnoses, higher functional connectivity in the SN predicted higher informant-rated socioemotional sensitivity (on the Revised Self-Monitoring Scale). Individuals with bvFTD also show reduced interpersonal warmth, and in a subsequent study Toller et al.51 also found that higher resting state functional MRI connectivity of the SN predicted increased interpersonal warmth, even after controlling for grey matter volume in this network. Pasquini et al.52 related in vivo structural MRI and quantitative histopathological data to questionnaire-based empathy measures in 16 individuals with bvFTD or amyotrophic lateral sclerosis (ALS). They found that TDP-43 pathobiology within right frontoinsular Von Economo neurons and fork cells was associated with SN atrophy, including in insular and medial frontal regions. Furthermore, they found that degeneration of grey matter within these regions mediated loss of empathy. This study is important because it provides evidence for a link between cellular, network and behavioural features in bvFTD. This integration of units of analysis, from moleucles to circuits to behaviour, is particularly RDoC. Future studies such as this that combine neuropathology data with in vivo neuroimaging and behavioural findings will be invaluable for our mechanistic understanding of symptoms, and our ability to predict and monitor them in vivo.
Apathy
Apathy definition and measurement
Previously defined as a disorder of motivation, an international consensus group has recently defined apathy in brain disorders as a quantitative reduction of goal-directed activity compared to a person’s previous level of functioning.54 Symptoms must be present for most of the time over a period of at least 4 weeks, and affect at least two of three dimensions of behaviour/cognition; emotion; or social interaction, and result in identifiable functional impairments. Symptoms of apathy such as anhedonia and reduced volition can overlap with those of depression; however, the two have been found to be distinct.55 Due to the multidimensional definition of apathy, some researchers focus on measuring its subcomponents such as emotion, cognition and behaviour (Box 4). Some argue that the clinical profile of apathy differs between bvFTD and DAT. For example, Kumfor et al.75 found that individuals with DAT had higher cognitive than affective and behavioural apathy whereas those with bvFTD had higher affective and cognitive than behavioural apathy. The same group found that in the early stage of disease, emotional apathy was more common in bvFTD, and later, executive apathy was more common in DAT.76
Box 4. Instruments for measurement of apathy and disinhibition.
Some of the more commonly used assessments of apathy and disinhibition available clinically and for research are described below. The NPI is the most widely used in clinical research studies. The Behaviour, Comportment and Personality Box56 that is supplemental to the Clinical Dementia Rating (CDR) summarizes a range of symptoms using a single rating. Some measures examine multiple dimensions of behaviours using an aggregation of items and can be useful for identifying subtypes. Self-rated measures are problematic due to impaired insight and self-appraisal, particularly prevalent in bvFTD.57 Close confidants such as caregivers are therefore considered the best source for clinical data in bvFTD58; however, clinical interviews are susceptible to informant and clinician biases. To overcome these issues, task-based assessments have been developed to objectively measure apathetic and disinhibited behaviour, and we present one example of each. However, complex human behaviour is difficult to study in the laboratory, independent of a person’s usual situational and interpersonal contexts. See Henry et al.58 for a review of assessments of social-behavioural abnormalities, including measures of social perception, empathy and theory of mind. See Chapman and Spitznagel59 for a review of measures of sexual disinhibition in dementia. See Radakovic, Harley, Abrahams and Starr60 for a review of apathy scales in neurodegenerative conditions.
Questionnaire and interview-based measures
Neuropsychiatric Inventory Questionnaire (NPI-Q)61
This caregiver-based behavioural rating system for dementia evaluates 12 global behaviours over the previous month on their presence and severity (1 = mild to 3 = severe). One item per behaviour measures delusions, hallucinations, agitation/aggression, depression, anxiety, elation/euphoria, apathy, disinhibition, irritability/lability, aberrant motor behaviour, sleep disturbances and appetite/eating disturbances. Frequency of each behaviour is also recorded (1 = least frequent to 4 = most frequent) in the full NPI version,62 allowing calculation of a severity index by multiplying frequency by severity scores. A Caregiver Distress scale indicates the impact of the symptoms on the caregivers. The disinhibition item includes prompts like acting impulsively, talking familiarly to strangers, and saying things that are hurtful to others. The apathy question is focused on motivational aspects and not initiation or planning.
Frontal Systems Behaviour Scale (FrSBe)63
This is a 46-item questionnaire designed to assess behaviour before and after damage or illness. It is completed by the patient (self-assessment) and/or caregiver (informant-rated). Questions are rated on 5-point Likert scales. Three theoretically derived subscales include Apathy (subscale A), e.g. ‘sits around doing nothing’, Disinhibition (subscale D), e.g. ‘does risky things’ and Executive dysfunction (subscale E). Disinhibition items have been further categorized into ‘person-based’ and ‘generalized impulsivity’ subscales.64
Behaviour, Comportment and Personality box of CDR56
Along with a language supplement, this scale was designed to measure key FTD characteristics that are not captured by the standard CDR. It is scored following a semi-structured interview with the caregiver and patient. Scoring is similar to CDR (0 = socially appropriate; 0.5 = questionable changes in comportment, empathy, appropriateness of actions; 1 = mild but definite changes in behaviour, comportment, empathy, appropriateness of actions; 2 = moderate behavioural changes affecting interpersonal relationships and interactions in a significant manner; and 3 = severe behavioural changes, making interpersonal interactions all unidirectional).
Social Impairment Rating Scale (SIRS)23
This instrument is rated by a clinician after a structured interview with a caregiver that takes around an hour and a half. It grades types and severity of social behavioural symptoms in six domains: e.g. lack of attention to social cues, socioemotional detachment, lack of adherence to social norms.
Frontal Behavioural Inventory (FBI)65
This 24-item screening instrument is administered via structured caregiver interview. Items measuring apathy, disinhibition, loss of insight, inappropriateness, indifference, impulsivity, personal neglect, etc. can be classified as negative symptoms (apathy) or positive symptoms (disinhibition).
Frontal Assessment Battery (FAB)66
This short bedside cognitive evaluation is designed to assess functions primarily attributed to large-scale frontal neuroanatomical networks. Its six subtests include inhibitory control, mental flexibility, conceptualization, motor programming, sensitivity to interference and environmental autonomy.
Cambridge Behavioural Inventory-Revised67
This 45-item informant-based questionnaire assesses behaviours across 10 domains. It includes behavioural subsections for disinhibition (e.g. socially embarrassing behaviour, inappropriate comments) and apathy (e.g. lacking motivation or enthusiasm, appearing indifferent).
Dimensional Apathy Scale68
This measure is appropriate for use in individuals with neurodegenerative disease with motor symptoms, e.g. Parkinson’s disease. It comprises 24 items forming 3 subscales: Executive, Emotional and Initiation. There is a self-report and an informant-rated version. Responses are made on a 4-point Likert-type scale based on the frequency of occurrence in the previous month.
Apathy Evaluation Scale69
This comprises 18 items assessing emotional, behavioural and cognitive apathy based on the frequency of occurrence in the previous month. It has clinician, informant and self-rated versions. The total score ranges from 18–72, and there are cognitive, behavioural and insight subcomponent scores.
Lille Apathy Rating Scale (LARS)70
This clinician-rated scale is completed using responses from the patient and the caregiver. It contains 33 items assessing symptoms over the previous four weeks. Items form nine subscales, and can be reduced to four dimensions: intellectual curiosity, emotion, action initiation and self-awareness.
The Apathy Inventory (AI)71
This is a rapid rating scale for global assessment of apathy with caregiver and self-report versions. It addresses changes in behaviour that have occurred since the beginning of the disease. It includes separate items assessing emotional blunting, lack of initiative and lack of interest. Based on the NPI model, the questionnaire first contains Yes/No questions to determine if a behaviour is present or absent. If present, ratings are also made for the frequency (1–4) and severity (1–3) of the behaviours, as well as how distressing they are to the patient.
The Starkstein Apathy Scale (SAS)72
This is a 14-item questionnaire. Scores range from 0 to 42, with scores ≥14 considered to indicate clinically relevant apathy.
Task measures
Philadelphia Apathy Computerized Test (PACT)73
Developed as a behavioural measure of apathy, this computerized test measures three aspects of goal directed behaviour: initiation, planning and motivation. It involves responding to shapes on a computer, measures reaction time, and includes response feedback and monetary incentive.
Hayling Sentence Completion Task74
This verbal test of inhibitory function assesses impulsive and stimulus-bound behaviour. It takes 5 min to complete and consists of two sets of 15 sentences that are missing the last word. An examiner reads a sentence aloud and the participant completes it with an appropriate word (control condition), or inappropriate word, which requires suppression of an appropriate response.
Apathy prevalence in DAT and bvFTD
Apathy is the most frequent NPS in dementia, with one large population-based study reporting a prevalence of 36%.77 In bvFTD, apathy prevalence has been reported as high as 90%.78 A retrospective study of 887 neuropathologically confirmed cases from the National Alzheimer Coordinating Center (NACC) with Neuropsychiatric Inventory (NPI) data found that apathy alone occurred in 33% of Alzheimer’s disease cases, compared to 25% of those with FTLD neuropathology.79 In Alzheimer’s disease, apathy is present from early on in the disease stage,80,81 and frequently presents as an early symptom in amnestic MCI.82 Apathy is often, paradoxically, associated with disinhibition. For example, Le Ber et al.83 reported that of 61 individuals with bvFTD, at onset of the disease 57% had a mixed subtype, 25% were inert (apathetic) and 18% disinhibited. After 4 years, behaviours had progressed to a mixed or inert profile, with all of the disinhibited individuals developing at least one sign of inertia and 55% of individuals with inertia predominant at baseline developing at least one sign of disinhibition. Similarly, Malpetti et al.84 reported that of 82 individuals with bvFTD, the proportion with first-presenting symptoms of disinhibition only was 14%, whereas isolated apathy occurred in 34–40%, and a combination of both apathy and disinhibition in 43–57%. The abovementioned study of NACC data by Borges et al.79 also found that the combination of apathy and disinhibition was more common in those with FTLD neuropathology (43%) than those with Alzheimer’s disease neuropathology (14%). The format of this review is to first discuss studies focused only on apathy, then discuss studies focused on disinhibition only, followed by studies that consider both apathy and disinhibition. Please refer to Supplementary Table 1 for the methodological details, including sample, measurement instrument(s), neuroimaging modality, and covariates of these studies.
Clinical correlates of apathy in DAT and bvFTD
Apathy in dementia is associated with heightened caregiver burden and distress, greater functional disability,85,86 and worsens over time.87,88 In FTD, apathy is associated with functional disability,89,90 lack of interest in previous hobbies and social isolation,91 dementia severity92,93 and risk of mortality.94 Studies in bvFTD have found that caregiver-rated apathy correlates with deficits in executive functioning, theory of mind and caregiver-rated empathy95; however, not all studies report a correlation with executive dysfunction.96 Apathy in DAT has also been associated with dementia severity,93 faster cognitive and functional decline,97,98 and an increased likelihood of progressing from MCI to DAT,99 e.g. one study reported an almost 7-fold likelihood of progressing to DAT in those with apathy than those without.100 Apathy in DAT is associated with increased risk for mortality and its incidence is predicted by antipsychotic use.101 Correlations between apathy and tau and p-tau in CSF have been reported in mild DAT.102
Neuroimaging studies of apathy
Categorical neuroimaging studies of apathy
In dementia, neuroimaging studies comparing individuals with apathy to those without (or sometimes a cognitively normal group) have been conducted in several modalities. These categorical studies have frequently implicated the orbitofrontal cortex (OFC) in apathy, including showing reduced cortical thickness,103 and reduced regional cerebral blood flow (rCBF) or metabolism in DAT,104–106 and bvFTD.107 (Figure 1 depicts the OFC and other regions most commonly described in this review.) The anterior cingulate cortex (ACC) is also frequently reported in studies of apathy. For example, in DAT lower rCBF105 and metabolism104 have been reported in those with apathy, as well as in a mixed sample of individuals with DAT, MCI and organic personality disturbances, regardless of diagnosis.108 Grey matter reductions in the ACC between those with apathy and without have been reported in DAT,103 and in a mixed sample of DAT and progressive supranuclear palsy (PSP).109 This latter study additionally found decreased volume in the left insula in those with apathy, regardless of diagnosis. One study examined resting state functional connectivity in DAT and found that the left insula was hypoconnected to the superior parietal lobule but the right dorsolateral prefrontal cortex (DLPFC) was hyperconnected to the superior parietal lobule in those with apathy compared to those without.110 DAT studies have also found inferior frontal gyrus (IFG) and lateral OFC atrophy in individuals with apathy compared to those without.103 Hypometabolism in DAT in the bilateral thalamus104 and in MCI in the PCC111 have also been reported in individuals with apathy. For the temporal lobes, Lanctôt et al.105 found that individuals with DAT and apathy had greater rCBF in the bilateral hippocampi, left superior temporal gyrus and right middle temporal gyrus than those without apathy. Few other categorical studies reported positive associations with apathy, although Stanton et al.109 did find some positive associations between reduced initiative and emotional blunting subtypes of apathy, and grey matter volume in regions including the cerebellum and occipital cortex.
Studies of DAT or MCI that find associations between apathy and posterior regions such as the temporal or occipital lobes tend to predominantly include individuals early in the course of disease, or include large proportions of cognitively unimpaired individuals, who are more likely to have mild severity of apathy. It’s possible that these studies were detecting cortical localization of Alzheimer’s disease pathology, which is associated with apathy and may even precede a clinical diagnosis of DAT, rather than or in addition to an association with apathy. This highlights the importance of co-varying disease severity when investigating neuroimaging correlates in dementia. Supplementary Table 1 lists the covariates controlled for in the studies of apathy and disinhibition we review. The Clinical Dementia Rating (CDR) scale is the preferred measure of dementia severity (over other instruments, e.g. the Mini-Mental State Examination, which is a screening instrument for dementia), although this is not optimal for bvFTD, which is why the Behaviour, Comportment and Personality Box was developed (Box 4).
Dimensional neuroimaging studies of apathy
Categorical studies are useful, and sometimes dichotomizing data is the only option when it is restricted in range. However, RDoC considers behaviours to lie on a dimension or spectrum of functioning from normal to abnormal, and therefore studies that have taken such a continuous approach, e.g. relating to symptom severity rather than presence, are considered in this section. Some studies report both categorical and dimensional analyses.3,107,112–115 As with categorical studies, dimensional studies using a variety of apathy instruments and neuroimaging measures, including PET, single-photon emission computed tomography (SPECT) and grey matter volume report a correlation with general apathy severity in the OFC. These include studies in DAT,113 and early-onset DAT.116 In some studies, the correlations with OFC changes encompassed, or were accompanied by, changes to the broader ventromedial prefrontal cortex (VMPFC) and adjacent ACC, including in individuals with bvFTD only,96 DAT,117 and transdiagnostic samples including bvFTD, non-fluent variant PPA (nfvPPA) and semantic dementia,3 and bvFTD, DAT, semantic dementia and nfvPPA.118 However, in some studies, the ACC was affected in the absence of the OFC or VMPFC,112 particularly those affecting the more dorsal, mid-cingulate,119 which in one instance also involved the dorsomedial PFC.120 One study found an aberrant pattern whereby ACC volume correlated positively with apathy in MCI,121 which the authors suggested could be a compensatory or inflammatory response that precedes atrophy. Similarly, using a variety of instruments, apathy severity has been associated with decreased insular cortical thickness in bvFTD,114 MCI,122 as well as grey matter volume in bvFTD,95 low frequency fluctuations during resting state in bvFTD and semantic dementia123 and hypometabolism in bvFTD and DAT.112 Several of the studies already cited additionally found associations between apathy severity and the DLPFC,116 with some additionally reporting associations in the IFG region as well, either bilaterally3,119 or in the left112 or right114 hemisphere. Others reported associations in ventrolateral PFC but not DLPFC.95,117 Kazui et al.124 measured grey matter volume in 98 MCI individuals classified as Alzheimer’s disease positive or negative based on SPECT scanning. They found that across individuals, apathy was associated with hypoperfusion in the right DLPFC and left superior frontal gyrus, and reduced grey matter in the right caudate. A mixed FTD study found apathy was associated with atrophy of the right caudate.3 Caudate and putamen atrophy has been associated with apathy in DAT.119 In bvFTD right caudate and ventral striatum atrophy has been associated with apathy also.95 In MCI, self and informant-rated apathy were also associated with reduced volumes in subcortical structures including the nucleus accumbens, putamen, thalamus and hippocampus.122 This study also found that amygdala volume was positively associated with apathy. Several studies of grey matter cortical thickness114,121,122 or volume3,95 and one SPECT study113 also found negative associations with apathy in the temporal lobes, primarily in the middle or inferior portions. Parietal atrophy, including superior114 and inferior95,114 cortices has been associated with apathy, as has reduced grey matter volume and cortical thickness in the PCC in both DAT117 but also FTD subtypes.114 There are a lack of longitudinal studies of apathy in bvFTD125; however, a longitudinal study of individuals with MCI and DAT examined associations of apathy with FDG-PET in five regions of interest.126 They found that cross-sectionally, apathy severity was associated with PCC hypometabolism, whereas longitudinally, baseline supramarginal hypometabolism was associated with rate of increase of apathy over time, when age, sex, diagnosis, apolipoprotein E (APOE) genotype, premorbid intelligence, cognition and antidepressant use were co-varied.
Again, we want to emphasize the importance of considering disease stage when interpreting neuroimaging findings. Findings of increased volume or activity tend to occur in samples of MCI,121,122 and could reflect attempted compensatory processes. Findings in regions such as the PCC and supramarginal gyrus that atrophy early in DAT127 could reflect disease-related changes, rather than the symptoms which are associated with increased likelihood of progression to dementia. This argument would explain the findings of one study of MCI which did not co-vary disease stage, and reported that increased apathy was associated with greater cortical amyloid burden but not hypometabolism in the ACC or OFC.128 Another important feature to consider is the measurement instrument used, as research has found that the type of apathy changes over time. For example, Wei et al.76 showed that in the early stage of the disease (<5 years since onset), emotional apathy was greater in bvFTD than DAT, whereas in the later stages of disease (>5 years since onset), executive apathy was greater in DAT than bvFTD.
Large-scale intrinsic-connectivity network correlates of apathy
A handful of studies of individuals with MCI or DAT have related resting state functional connectivity to apathy severity. Balthazar et al.129 combined NPI-apathy with NPI-appetite and eating disturbances, and did not find a significant relationship between severity of this construct and connectivity of the SN or DMN (Fig. 2 shows an example of these networks); however, this was a small sample of 20 individuals with DAT. Two studies of MCI with sample sizes more than double that of Balthazar et al.129 have reported significant associations with apathy. Munro et al.130 examined seed-to-seed resting state functional MRI connectivity in four networks, the dorsal attention network, ventral attention network (also known as the SN, see Box 1), the DMN and the fronto-parietal control network (also known as the cognitive control network, CCN). They found that an affective factor (comprising NPI apathy, anxiety, depression, appetite disturbances and sleep disturbances) was associated with reduced within-network fronto-parietal control connectivity, with follow-up analysis indicating this effect was driven by apathy severity. Joo et al.131 examined seed-to-whole brain resting state functional MRI connectivity in MCI with ‘clinically significant’ apathy, that is individuals scoring 4 or more on the Apathy Inventory. They also examined connectivity of major intrinsic connectivity networks, the DMN, SN and CCN. They found that within the DMN (PCC seed), functional connectivity to the ACC was negatively correlated with apathy, whereas within the CCN (bilateral DLPFC seeds), connectivity to the DLPFC, IFG and supramarginal gyrus were positively associated with apathy.
Multidimensional theoretical models of apathy
Several theoretical models have conceptualized apathy as a multidimensional construct,132–134 and this is reflected in the current diagnostic criteria for apathy in brain disorders, stated earlier.54 In their influential theoretical model of the neurobiology of apathy subcomponents, Levy and DuBois133 argued that apathy occurs when there is damage to the systems that generate and control voluntary actions. They assert that goal-directed behaviour involves several steps, from processing determinants that influence the intention to act, to elaboration of action plans, initiation, execution and feedback of the behavioural response. Dysfunction can arise from any of these steps and result in apathy. Levy and Dubois distinguish three subtypes of apathy, each of which emerge following disruption to distinct but closely interconnected regions of the PFC and basal ganglia. Emotional-affective apathy occurs following damage to the orbitomedial region and related limbic basal ganglia subregions (e.g. ventral striatum). As these regions provide the contextual, relative value of a stimulus, individuals with decreased sensitivity to reward may demonstrate a decreased number of voluntary actions. The cognitive subtype of apathy follows damage to the DLPFC and related associative territory subregions of the basal ganglia (i.e. dorsal caudate nucleus). This damage is posited to result in reduced goal directed behaviours due to impaired cognition needed to elaborate action plans, particularly impairments in executive functions, including planning, working memory, rule-finding and set-shifting, which are necessary for elaborating, maintaining and manipulating goals and sub-goals. The auto-activation form of apathy is argued to be the most severe form of apathy, and to result from bilateral lesions to the associative and limbic territories of the globus pallidus. Apathy following lesions here results from a failure to self-generate initiation or activation of thoughts or actions, although responses to external stimuli are relatively intact. This syndrome is also posited to occur after extensive bilateral lesions of the medial prefrontal cortex (mPFC) in the anterior cerebral artery territory, i.e. akinetic mutism, where individuals don’t spontaneously speak or move. More recently in their review of apathy in FTD, Johnson and Kumfor125 integrate and refine these multidimensional theories of apathy and refer to them as affective, behavioural and cognitive subtypes, the so-called ABC model of apathy.75 In their model, the affective subtype corresponds to the emotional-affective subtype of Levy and Dubois,133 and includes symptoms of indifference, emotional blunting, and loss of empathy. The behavioural subtype corresponds to the auto-activation subtype of Levy and Dubois and is characterized by a loss of self-initiated behaviour and conversation, social withdrawal, requires external prompting to perform physical activity and with poor persistence. The cognitive subtype, like Levy and Dubois, includes difficulty planning and implementing and maintaining actions to achieve a goal, as well as reduced curiosity and lack of interest in routine or novel activities.
Empirical neuroimaging studies of multidimensional apathy
As described in Box 4, measures have been developed that have separate scales assess subtypes of apathy. Some neuroimaging studies have identified associations using these subscales. For example, using the apathy inventory, Benoit et al.135 measured SPECT in individuals with DAT. They found that lack of initiative was associated with reduced perfusion in the right ACC, lack of interest with hypoperfusion in the right OFC and emotional blunting with hypoperfusion in the left superior DLPFC. Subsequently, Massimo et al.73 examined individuals with bvFTD using the Philadelphia Apathy Computerized Test (PACT) to relate the initiation, planning and motivational components of apathy to grey matter atrophy. They found that poor initiation was related to atrophy in the ACC, planning impairment to atrophy in DLPFC, and poor motivation to atrophy in OFC. They interpreted their findings as supporting the three-process theory described above. They also examined grey matter atrophy as it related to NPI apathy, and found that the results were most similar to the motivational component of the PACT, i.e. in the OFC.
Two transdiagnostic studies involving individuals with bvFTD and DAT related voxel-based morphometry to multiple dimensions of apathy, including affective or emotional, cognitive or executive, and behavioural or initiative related apathy.75,76 Affective apathy was associated with atrophy of ventral PFC regions, including the bilateral OFC, subcallosal cingulate and insula, and the left temporal pole,75 as well as the bilateral cerebellum, left amygdala, IFG and posterior regions including the right postcentral and supramarginal gyri.76 Behavioural apathy was associated with atrophy of the basal ganglia [caudate and nucleus accumbens (NAc)]75 as well as the frontal pole,75,76 right mPFC, OFC, paracingulate and ACC.76 Cognitive apathy was associated with atrophy in the left OFC and temporal regions75,76 as well as mPFC regions including the subcallosal region and ACC, as well as the superior frontal gyrus, PCC75 and bilateral frontal pole.76 Both studies reported that across all apathy subtypes, there was an association with atrophy of fronto-insular regions, including the left or right insula encroaching upon the operculum cortex, left basal ganglia regions, the left or bilateral frontal poles,75,76 as well as the left IFG.76
Summary of neuroimaging findings associated with apathy across bvFTD and DAT
Across the functional and structural neuroimaging studies reviewed above, several key regions were consistently implicated in apathy, most frequently the ACC and OFC, and particularly among categorical studies that compared groups with apathy to those without. Dimensional studies such as those that correlate with severity are more in line with the RDoC conceptualization of behaviour as existing on a spectrum. These studies supported the findings implicating OFC and ACC in apathy, and additionally identified associations in regions such as the insula and DLPFC, with a few studies also reporting findings in the inferior frontal gyrus, and portions of the temporal and parietal lobes, PCC and basal ganglia. Studies that have examined resting state connectivity of intrinsic functional brain networks in DAT and MCI perhaps surprisingly have not found associations with salience network connectivity; however, they offer some preliminary support for the involvement of the cognitive control network in apathy. Studies that measured apathy as a multidimensional construct provided evidence that apathy subtypes can occur following damage to distinct regions, although there are also regions of commonalities. There was a pattern to these studies that suggested the OFC is important for emotional apathy, as this region was associated with affective symptoms of apathy as well as a lack of interest and reduced motivation. Similarly, findings converged to identify the ACC as important for behavioural apathy, as initiation impairments were associated with this region in three of the four multidimensional studies. Other than two studies identifying associations between cognitive apathy and the temporal lobes, there were few other converging findings. More studies that investigate the multiple dimensions of apathy are needed in the future, as different dimensions of apathy may be responsive to different treatment strategies.76
Disinhibition
Disinhibition definition and measurement
Early disinhibition is one of the six possible behaviours that must be present for a clinical diagnosis of bvFTD.136 However, outside of this bvFTD-specific definition, there is no standardized definition of disinhibition in dementia generally, and disinhibition is thus somewhat of a heterogeneous construct. A majority of measures focus on behaviours that are socially inappropriate, such as hypersexual comments, inappropriate approaching of strangers, excessive jocularity, and impulsive decision-making.38 Some measures make a distinction between social disinhibition (e.g. violating social tact and interpersonal boundaries) and behavioural disinhibition (e.g. stealing, utilization behaviour), and suggest that they may have distinct neural correlates.64 Other researchers make a distinction between emotional and behavioural disinhibition, arguing that behavioural disinhibition includes socially inappropriate behaviours whereas emotional disinhibition refers to excessive emotional output, resulting in excessive maladaptive arousal and emotional distress.137 Still others make a distinction between cognitive inhibition (e.g. the ability to resist internal or external interference, inhibit cognitive content, or suppress inappropriate responses) and behavioural disinhibition, which they argue encompasses the control of emotional behaviours in a social context.138 Indeed, a major function of emotions is to communicate our internal states to others, and another is to drive our goal-directed behaviour, therefore we have chosen to discuss studies that have used a range of measures of disinhibition. Many definitions consider impulsivity to be a subcomponent of disinhibition, and the NPI disinhibition question prompts ‘Does the patient seem to act impulsively, for example, talking to strangers as if he/she knows them, or saying things that may hurt people’s feelings?’139 Disinhibited behaviour often fails to conform to lawful boundaries and can lead to arrest in individuals with FTD,140 or psychiatric misdiagnoses, as it resembles the disinhibited behaviour seen in primary mania.141 Disinhibition in dementia has been referred to as ‘acquired sociopathy’,142 a term first used to describe similar behaviour in patients with lesions to the VMPFC that encompass both the OFC and ventral regions of the mPFC, including the rostral and subgenual ACC.143,144
Disinhibition prevalence in DAT and bvFTD
Problems defining disinhibition make it difficult to estimate its prevalence in dementia. It is clear though, that compared with other types of dementia (e.g. vascular or dementia with Lewy bodies), disinhibition is more common in bvFTD and less common in DAT.88 Disinhibition is also more severe in bvFTD,145 and more frequent in those with an onset prior to 65 years of age.136 A meta-analysis of 48 studies that used the NPI found that disinhibition was present in 17% of individuals with DAT,146 and a large community sample of 435 individuals with DAT who had dementia for at least 3 years found that NPI-measured disinhibition was present in 30% in the course of their dementia and 23% in the past month.147 In bvFTD, the prevalence is much higher, e.g. Chow et al.78 found that 69% of 48 individuals with bvFTD had disinhibition. A large neuropathologically confirmed sample of 137 bvFTD found that early disinhibition was the second most common feature (76%), with only apathy (84%) more common.136 It’s important to consider disease stage when estimating prevalence of disinhibition. In a large transdiagnostic community sample of 779 individuals with dementia, Brodaty et al.88 found that across diagnoses, the percentage of individuals with NPI-measured disinhibition increased over 3 years. This result may reflect increased disinhibition in DAT over time though, as two-thirds of the sample were DAT, with only 5% with FTD. Indeed, research indicates that in DAT, disinhibition has a median onset time of 30 months.147 In contrast, early disinhibition is a diagnostic criterion for bvFTD.136
Clinical correlates of disinhibition in DAT and bvFTD
Disinhibition is related to worse CDR sum of boxes score (CDR-SB) in DAT.38 However, in bvFTD O’Connor et al.148 found that those with a primarily disinhibited phenotype with mild apathy did not differ from those with a primarily apathetic or combined subtypes on functional impairment, cognition or time to residential care. Another study of individuals with bvFTD even found that individuals who were less disinhibited had faster rates of functional decline as measured by the CDR-SB, and worse performance on executive tests.92 The reasons for this are not clear; however, the authors suggested that a subtype of the disease that progresses more slowly may be responsible for this finding, noting, e.g. that disinhibition was the predominant behavioural symptom in individuals with MAPT mutations.149 Regarding the course of disinhibition over time in bvFTD, there have been some conflicting results. Reus et al.87 found that FBI-measured disinhibition worsened over 3 years but was stable in other neurodegenerative disorders presenting with these NPS. O’Connor et al.90 investigated individuals with bvFTD with a similar disease duration; however, they measured disinhibition using the Cambridge Behavioural Inventory-Revised. They found that disinhibition decreased in bvFTD over a follow-up period of 2–4 years. These discrepant results may be explained by the different symptom profiles of the individuals in these two studies. Reus et al.87 specifically sought to recruit individuals with late-onset ‘frontal lobe syndrome’, only including individuals with a score of ≥ 11 on the Frontal Behavioural Inventory (FBI) or ≥ 10 on the Stereotypy Rating Inventory. O’Connor et al.’s90 bvFTD sample were likely less disinhibited, as they did not differ significantly from a semantic variant primary progressive aphasia (svPPA) group on disinhibition severity, but they were significantly more apathetic. A recent longitudinal study found disinhibition increased up to the intermediate disease stage in bvFTD, and then declined in the severe stage,150 whereas apathy increased until the intermediate stages of the disease then remained somewhat stable. Overall, the evidence suggests that with disease progression, disinhibition decreases in bvFTD and increases in DAT. In DAT, disinhibition is also associated with younger age, worse memory and executive functioning scores.38 Individuals with disinhibition may have impaired ability to delay gratification, often have an inability to plan for the future, and may show perseverative behaviour. Compared to those with an apathetic subtype, bvFTD with a disinhibited subtype are more likely to attempt to eat non-food items,151 and show a lack of concern for others, emotional lability, relentlessness, impulsiveness, perseveration and utilization behaviour. Both subtypes show executive deficits.8
Neuroimaging studies of disinhibition
Categorical neuroimaging studies of disinhibition
Categorical studies have also examined individuals with dementia with and without disinhibition. Mendez et al.142 used expert raters to identify acquired sociopathy in individuals with bvFTD. Using PET and SPECT, they found that individuals with acquired sociopathy had more right anterior temporal involvement that those considered not to have acquired sociopathy. In the Alzheimer’s Disease Neuroimaging Initiative (ADNI) sample, Finger et al.38 compared 177 individuals with DAT and disinhibition on the NPI-Q (defined using the disinhibition and euphoria scales, not the disinhibition scale alone) to 581 individuals with DAT without disinhibition. Disinhibition was associated with reduced right frontal pole cortical thickness, even after co-varying for age, sex, CDR-SB, total intracranial volume, and APOE. However, the disinhibited group had worse CDR-SB scores than the non-disinhibited group, so it’s possible that co-varying for this obscured some findings. When they compared the disinhibition group to a subset of the no disinhibition group that was matched on CDR-SB, no brain regions predicted disinhibition.
Dimensional neuroimaging studies of disinhibition
Dimensional studies of disinhibition in dementia have identified associations with severity of disinhibition using a variety of neuroimaging modalities and reported changes to similar brain regions as those described in studies of apathy. Disinhibition has been measured used a variety of methods, e.g. one study of bvFTD found frequency of antisocial behaviours (e.g. stealing, physical assault) was associated with reduced rCBF in the OFC extending to the VMPFC, ACC, IFG, right caudate and left insula.152 NPI disinhibition has been associated with reduced OFC metabolism in FDG-PET in bvFTD.107 A study of individuals with FTD subtypes found NPI disinhibition was associated with reduced grey matter volume in the bilateral OFC, insula and IFG, as well as the right middle temporal gyrus.3 Transdiagnostic studies have also found NPI disinhibition associated with reduced OFC grey matter volume or cortical thickness, including in samples that combined individuals with bvFTD and DAT64,153 with semantic dementia, nfvPPA, PSP, corticobasal degeneration (CBD), and MCI.41 The Haylings test of inhibitory function is a test of cognitive (verbal) inhibition, and has also been associated with reduced grey matter or cortical thickness in the OFC, including in bvFTD and DAT,153,154 for whom the atrophy associated with disinhibition extended to the subgenual (sg)ACC, as well as in studies of bvFTD and PSP,155 and across Parkinson’s disease and bvFTD.156 This latter study gave participants an additional test of cognitive control, the go/no-go test, and found that no-go accuracy was associated with NAc atrophy. Flanagan et al.154 also gave participants the Rey Auditory Verbal Learning Test and found that false positive identifications on this test were associated with reduced grey matter in the OFC, thalamus, IFG, temporal pole and hippocampus. These regions were also associated with worse Haylings test performance, in addition to the PCC, the precuneus in DAT and the amygdala in bvFTD. Few dimensional studies of disinhibition found an association in the DLPFC. Serra et al.157 found in individuals with DAT and MCI, NPI disinhibition was associated with decreased grey matter in the DLPFC and bilateral dorsal cingulate. In a study of individuals with various clinical FTD syndromes (including bvFTD) and corticobasal syndrome (CBS), those with selective impairment on a social concept task (but not other semantic impairments) had higher rates of disinhibition on the Neurobehaviour Rating Scale and more pronounced hypometabolism in the right lateral OFC and dorsal mPFC, and the right superior anterior temporal lobe and left temporal pole.158 The anterior temporal lobes have also been associated with NPI and Haylings disinhibition.153
Multidimensional neuroimaging studies of disinhibition
It’s been argued that single-test measures are unlikely to be adequate to assess disinhibition in bvFTD,159 and although some studies have measured the NPI and a cognitive measure of inhibition such as the Haylings Test, few neuroimaging studies have sought to measure multiple dimensions of disinhibition. One neuroimaging study that did attempt to is by Paholpak et al.64 These authors used the Frontal Systems Behaviour Scale (FrSBe) to separate ‘person-based’ disinhibition (associated with violating personal boundaries) and ‘generalized impulsivity’. They found that person-based disinhibition was more prevalent in bvFTD than Alzheimer’s disease, whereas generalized impulsivity was comparable. They found the left anterior temporal lobe grey matter volume (along with right OFC) was decreased in individuals with generalized impulsivity, and the left anterior temporal sulcus was associated with person-based disinhibition. These findings indicate the need to identify and measure multiple dimensions of disinhibition, as has been done with apathy.
Summary of neuroimaging studies of disinhibition
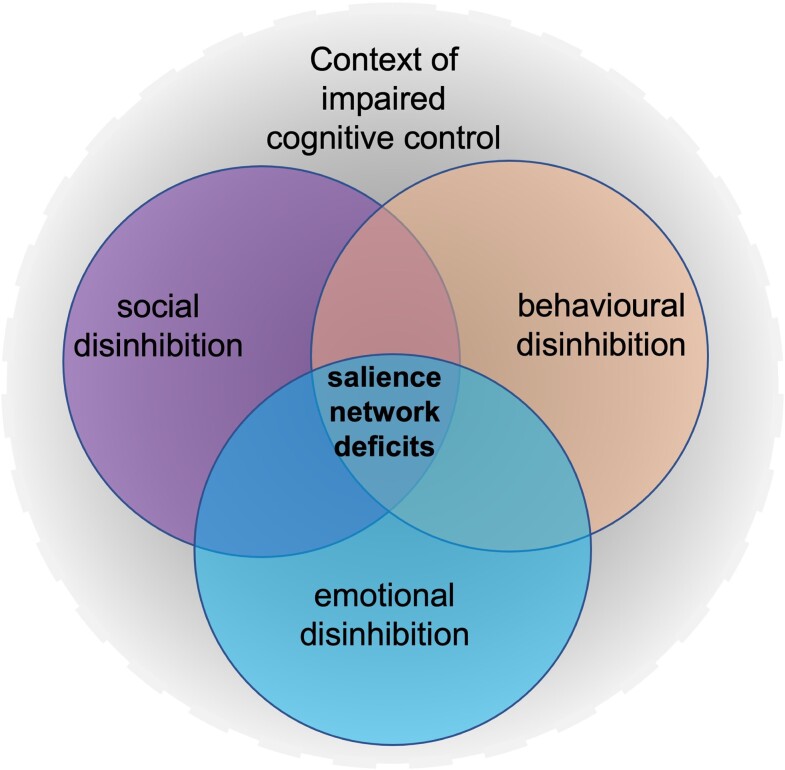
In summary, the findings from the neuroimaging studies of disinhibition in dementia are strikingly similar to the findings from studies of apathy. In particular, the OFC, ACC and VMPFC are affected by both. One region of divergence is the temporal lobes, with the inferior portions possibly more affected in apathy whereas the anterior temporal lobes/poles are more involved in disinhibition. The results of these studies have informed a new theoretical model of disinhibition described below and presented in Fig. 4.
Figure 4.
A new theoretical model of disinhibition in dementia.
Within-individual studies of disinhibition and apathy
Given the comorbidity and neuroanatomical overlap, some neuroimaging studies of bvFTD have measured both apathy and disinhibition in the same individuals, and categorized participants according to their initial presenting or predominant symptom profiles. For example, Le Ber et al.83 measured SPECT in bvFTD compared to healthy controls. They found that compared to healthy controls, those with an apathetic profile had anterior bilateral frontal, cingular and insular hypoperfusion particularly within in the frontomesial and ACC and the DLPFC. Compared to healthy controls, those with a disinhibited profile had predominant VMPFC and temporal hypoperfusion. Morbelli et al.160 classified individuals into those with a predominant negative symptom profile (including apathy and depression) and those with a predominant positive symptom profile (including disinhibition, agitation, euphoria and irritability). They found that in those with positive symptoms, PET hypometabolism occurred in the bilateral medial and basal frontal cortex, including inferior frontal gyrus, left caudate, and right insula and superior temporal gyrus. Grey matter reduction was also found in the left ACC and inferior frontal gyrus and the right insula and claustrum. In individuals with negative symptoms, atrophy and hypometabolism involved the inferior parietal lobule and lateral frontal cortex, including left superior and inferior frontal gyri. Liu et al.161 classified individuals with FTD into frontal and temporal variants based on grey matter volume loss in healthy controls and individuals with DAT. They found that individuals with the frontal variant had more apathy than the temporal variant, and across FTD groups, disinhibition was associated with decreased volume of the right VMPFC, amygdala and anterior temporal cortex.
Some categorical studies have taken the approach of using neuroimaging to contrast individuals with different behavioural profiles. For example, using PET, Franceschi et al.162 found that compared to apathetic individuals, those who were disinhibited had reduced metabolism in the posterior OFC, NAc, ACC and inferior temporal cortex bilaterally, and the right hippocampus and amygdala. Apathetic individuals had hypometabolism in the DLPFC, insula and mPFC, bilaterally, compared to those with disinhibition. More recently, O’Connor et al.148 subclassified individuals with bvFTD into four groups based on the severity of apathy and disinhibition from the Cambridge Behaviour Inventory-Revised. They found that compared to a subgroup of individuals with severe apathy only, those with severe apathy and disinhibition had thinning in the left temporal pole and right middle and inferior temporal gyri.
Other neuroimaging studies measured apathy and disinhibition dimensionally within the same individuals living with dementia. In addition to looking at the correlates of either symptom alone, these studies have further been able to control for apathy when examining the neural correlates of disinhibition and vice versa using statistical techniques such as multiple regression. One of the earlier studies to attempt this was a transdiagnostic study by Rosen et al.,163 who examined the NPI in 148 individuals with dementia and a variety of clinical syndromes. They found that apathy was independently associated with grey matter loss in the ventromedial superior frontal gyrus of the VMPFC/mPFC, and disinhibition with grey matter loss in the sgACC, but only in the bvFTD+ semantic dementia group. Subsequently, several others have also examined the unique relationships between apathy and disinhibition on grey matter loss in either bvFTD, other FTD clinical subtypes, or transdiagnostic samples. Some replicated the finding that apathy was associated with VMPFC grey matter loss when disinhibition was covaried,164,165 and others reported grey matter loss in mPFC regions associated with disinhibition when apathy was co-varied.115 However, a consistent finding across these studies was that apathy was associated with reduced grey matter in the DLPFC and disinhibition was associated with reduced grey matter in the temporal lobes, in particular the amygdala.8,115,164,165 This pattern was also reported in studies that used PET166 and SPECT.167 In their study of resting state functional MRI connectivity, Farb et al.168 found that hyperconnectivity of an executive control network (that included the rostral ACC and anterior insula) to the mPFC was associated with apathy. They also found associations with apathy and connectivity of the mPFC within the salience network and the right angular gyrus within the DMN, and an association between left insula connectivity within the SN and disinhibition. However, this study had a very small sample of only eight individuals with bvFTD and eight with semantic dementia. Nevertheless, findings from this study are consistent with those that measured grey matter, PET and SPECT, that suggest that apathy is associated with changes to regions within the executive control network, such as the DLPFC, whereas disinhibition is associated with changes to regions in the SN, including the amygdala.
Theoretical models of disinhibition
Several neuroanatomical models for disinhibition and social-behavioural control have been proposed. Some focus on motor response inhibition, whereas others emphasize the importance of social context, whereas others still are more generalized to describe different dimensions of non-adaptive behaviour.169 Recently, Guimet et al.25 proposed a model of disinhibition within a controlled semantic cognition theory170,171 framework, arguing that behavioural disinhibition may be fundamentally related to language and object knowledge. This framework posits that semantic cognition comprises two interconnected representation and process control systems. The representational system is concerned with acquisition and storage of conceptual knowledge, with hubs in the bilateral anterior temporal lobes. These hubs integrate transmodal information coming from modality-specific ‘spokes’ such as face processing and valence. The process control system, proposed to be located bilaterally in the ventrolateral PFC and temporoparietal cortex, directs conceptual knowledge to produce operations, guiding efficient and rapid retrieval of relevant information to enable decisions and actions. Guimet et al.25 argue that an impairment in the representational system could lead to loss of knowledge needed to recognize, understand and evaluate social rules, thereby preventing inappropriate behaviour, whereas process control dysfunction may compromise executive mechanisms needed to prevent impulsive or disorganized behaviour.
We present an alternative, more generalized model of disinhibition in dementia that is less semantic-focused. We argue that disinhibition comprises separable but overlapping dimensions of behavioural disinhibition, social disinhibition, and emotional disinhibition, all of which result from damage to regions within the SN, and all of which occur in the context of impaired cognitive control (Fig. 4 ), thus cognitive disinhibition is not a separate dimension. We agree with others who have suggested that damage to the SN impacts disinhibition via dysconnectivities with other major canonical networks,172 including the DMN173 and the CCN, as disinhibition occurs within the context of impaired cognitive control. These connections will influence precisely how disinhibition manifests, e.g. more social, more behaviourally or more emotionally. The phenomenology and severity of disinhibition also depends on the region or combination of regions involved, as different nodes within the SN serve different purposes. The most severe disinhibition is observed when there is either damage to both the VMPFC and the amygdala or a disconnection of the VMPFC and amygdala.
Social disinhibition is certainly an important domain within this model, however we distinguish a behavioural domain, as not all behaviours occur in a social context. For example, impulsive or careless actions, such as over-spending, or environmental dependency syndrome, occur when individuals have an excessive dependence on environmental cues.174 Other types of behavioural disinhibition could include stealing or reckless driving that do not occur within a social context; however, it could be argued that these behaviours represent a violation of social norms. Although not really covered in our above review, a third aspect of disinhibition that we propose is emotional disinhibition, which may manifest as emotional lability, irritability or aggression, owing to a decreased inhibition of emotion. Some reviews of the literature highlight the importance of regions of the salience network, including the amgydala, ACC and insula for aggression and agitation.175,176 Indeed, it has been suggested that agitation in dementia may be related to misinterpretation of threats and affective regulation.177
A new theoretical model of disinhibition in dementia
The amygdala is important for stimulus-reinforcement learning178 and signalling the emotional significance of stimuli, or its salience.179 For example, the amygdala will respond more to food when an individual is hungry than when satiated.180 Impaired salience signalling, rather than social cognitive deficits, is argued to underlie the disinhibited social behaviour shown by rhesus monkeys with selective amygdala lesions.181 The amygdala has bidirectional connections with other regions of the salience network, including the insula182 and while it has connections with all regions of the prefrontal cortex, those with the ACC and OFC are particularly dense.183 These VMPFC regions serve to inhibit the amygdala. For example, Motzkin et al.184 showed that individuals with bilateral VMPFC lesions had increased amygdala activity to emotional stimuli compared to controls.
Subregions within the VMPFC, including the ACC/mPFC and the OFC, are richly interconnected185 and play overlapping but separate roles. The mPFC is important for cognitive aspects of emotion processing, such as self-awareness,186 which is necessary to understand the mental states of others.187 Thus lesions to this region would be expected to result in disinhibited social behaviour due to impaired Theory of Mind and ability to self-monitor. This region is also important for the perception of self-relevant salient stimuli,22 consistent with findings of apathy following damage to this region, as individuals may not appreciate the importance of certain environmental stimuli to themselves and therefore fail to act on them. The OFC is crucial for signalling the reward value of stimuli,188 which is also important for appropriate social behaviour, as well as perception of emotional stimuli189 and subjective emotional response.190 Therefore lesions to this region may result in behaviour that is either inappropriate for the social context or an emotional response that is lacking the intensity that an individual with intact emotional perception and subjective feeling would display. Lesions here also result in perseverative behaviour that may appear disinhibited, as individuals are unable to update stimulus-reinforcement contingencies due to this OFC damage. Both the ACC and the OFC are connected to the insula and the amygdala, although the inhibitory projections to the amygdala are more robust from the ACC than from the OFC. The dual inhibition theory of disinhibited behaviour following lesions that encompass both the ACC and OFC argues that the dense yet independent interconnections of the ACC and OFC with areas that are important for emotion (e.g. the amygdala, which they both inhibit) allow for compensation when one of these areas is damaged in isolation.191–193
Other brain regions within the salience network are involved in disinhibition; however, the most profound disturbances are likely to be seen in individuals with the combined damage to or disconnection of the above regions. The insula is also important for signalling salience,194 and modulates awareness of internal body states and observations of another’s body state.195 This role in interoception may constitute part of the subjective feeling state.196 The anterior insular cortex (along with the ACC) contains large, spindle-shaped bipolar neurons known as Von Economo neurons which may be a potential substrate for the rapid and intuitive assessment of complex situations such as human social networks.197 Also part of the salience network, the ventral striatum and its major component the NAc partially mediates the motivational effects of emotionally significant stimuli. The NAc is heavily innervated by dopaminergic neurons and plays a crucial part in reward,198 and dysfunction in these neurons may play a role in the disinhibited and apathetic behaviour observed in individuals with Parkinson’s disease,199 another neurodegenerative disease that can result in dementia. Finally, although not part of the SN, the anterior temporal cortices are important for semantic knowledge, and in particular, social semantic knowledge,200 although close interconnections with the OFC and amygdala are also likely to play a role in social disinhibition following damage to the anterior portions of the temporal lobes.201
Summary, conclusions and future directions
Apathy and disinhibition can occur in the clinical syndrome of DAT, particularly those with a ‘frontal’ presentation, but they occur more frequently and severely in the clinical syndrome of bvFTD. In DAT, apathy is common from early in the disease course, whereas disinhibition emerges later. In contrast, in bvFTD, early disinhibition is a key diagnostic criterion. Apathy and disinhibition often occur in the same individual, and some studies attempt to characterize individuals with bvFTD according to which symptom predominates or presents first. Studies that treat apathy and disinhibition as multidimensional constructs and include measures that examine the nuances of these symptoms do show evidence for neuroanatomical correlates that are reflective of the different functions of these distinct but related brain regions, many of which lie within the salience network.
The regions implicated in apathy and disinhibition in neuroimaging studies of both DAT and bvFTD are important for emotion and signalling the salience of stimuli to the individual. They form part of a network, and damage to one or a subset of nodes within the network can show similar symptoms. These regions work together and play complementary roles in high-level integrative functions involving emotion and salient internal states for the control of goal-directed behaviour and cognition. Of note, one overarching theory of bvFTD conceptualizes the broad array of clinical symptoms as reflecting a common underlying deficiency in goal-directed behaviour,202 and our transdiagnostic model aligns well with this framework.
Future studies that use a network approach will be valuable for testing the theoretical model of disinhibition proposed in this review. Previously, Reyes et al.203 investigated whole-brain, global network associations with apathy and disinhibition; however, future studies should identify relationships between apathy and disinhibition and subnetworks such as the SN or specific hubs. This would potentially allow identification of nodes that might be targeted for interventions, such as via rTMS. Interactions of the SN with other networks in the context of these symptoms will be important to explore, particularly in transdiagnostic studies. Connectome-based methods such as structural covariance networks, which have been related to cognitive symptoms in bvFTD and DAT204 could be informative for studying apathy and disinhibition. Atrophy network mapping is another method that can identify networks underlying NPS in dementia.205
Future studies that take a transdiagnostic approach by studying symptoms across different dementia syndromes that target different neural networks can deepen our understanding of the brain regions responsible for behavioural impairments regardless of underlying neuropathology. Doing so may inform symptomatic treatments of these distressing behaviours. Studies such as Pasquini et al.52 will help to elucidate relationships between symptoms, in vivo brain functioning, and post-mortem pathology. Currently there are a lack of neuropathologic findings that are relatable to NPS. A handful of post-mortem studies in Alzheimer’s disease have found correlations between apathy and neurofibrillary tangle counts in the ACC,206,207 neuronal loss in the hippocampus and nucleus basalis of Meynert, and higher tangle counts in medial frontal and parietal regions and the parahippocampal gyrus.208,209 However, there are a lack of similar studies focused on disinhibition, although a couple of small post-mortem studies have found that the frontal lobes of Alzheimer’s disease cases with so-called ‘frontal’ symptoms have higher rates of neurofibrillary tangle pathology210,211 and Aβ plaques211 than those with the more typical amnestic form of Alzheimer’s disease. A study of FTLD found that individuals who were tau-positive had significantly more initial behavioural problems and personality changes than those who were tau-negative,212 although groups did not differ on disinhibition specifically, and the tau-positive finding was not confirmed in a slightly larger sample using the same NACC sample pool.213 However, both studies were able to show that individuals who were clinically diagnosed with bvFTD but found to have Alzheimer’s disease pathology were significantly more likely to have delusions, hallucinations and agitation than those with FTLD pathology at autopsy.212,213 While these studies are informative, what is needed is to be able to distinguish between frontal-type DAT presentations and bvFTD in vivo. To date, a couple of molecular PET imaging studies using tau-PET and/or amyloid-PET have in fact been able to achieve this differentiation214,215; however, such studies are rare. The field would benefit immensely from research that relates neuropathology to more readily available neuroimaging measures such as T1-weighted or resting-state fMRI. This knowledge would improve our understanding of relationships between NPS and associated brain networks, to guide future treatments. Whole-brain neuropathology data will be informative for future research, as currently, neuropathological studies are restricted to a few a priori regions of interest given the laborious requirements involved. Future studies should also investigate the role of white matter damage, as this is also a risk factor for dementia and is related to vascular risk factors,216 which are also relevant to the neurobiology of NPS,217 including both apathy and disinhibition in bvFTD.164,218 Ultimately, the goal of these investigations of structural and functional networks is to provide new therapeutic targets for clinical trials. In addition to informing treatment, studies that provide a better understanding of biological mechanisms can also aid in development of reliable biomarkers that allow for early intervention, and predicting disease progression and response to treatment.
Supplementary Material
Abbreviations
- ACC
anterior cingulate cortex
- bvFTD
behavioural variant frontotemporal dementia
- CCN
cognitive control network
- CDR
Clinical Dementia Rating scale
- DAT
clinical dementia of the Alzheimer type
- DLPFC
dorsolateral prefrontal cortex
- DMN
default mode network
- FTD
frontotemporal dementia
- IFG
inferior frontal gyrus
- MCI
mild cognitive impairment
- mPFC
medial prefrontal cortex
- NPI
Neuropsychiatric Inventory
- NPS
neuropsychiatric symptoms
- OFC
orbitofrontal cortex
- PCC
posterior cingulate cortex
- PFC
prefrontal cortex
- RDoC
Research Domain Criteria
- SPECT
single-photon emission computed tomography
- SN
salience network
- VMPFC
ventromedial prefrontal cortex
Contributor Information
Lisanne M Jenkins, Department of Psychiatry and Behavioral Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA.
Lei Wang, Department of Psychiatry and Behavioral Health, Wexner Medical Center, Ohio State University, Columbus, OH 43210, USA.
Howie Rosen, Weill Institute for Neurosciences, School of Medicine, University of California, San Francisco, CA, USA 94158.
Sandra Weintraub, Department of Psychiatry and Behavioral Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; Mesulam Center for Cognitive Neurology and Alzheimer’s Disease, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA 60611.
Funding
Research reported in this publication was supported, in part, by the National Center for Advancing Translational Sciences, Grant Number KL2 TR001424 (L.M.J.), and by National Institute on Aging grants P30 AG013854 (S.W.), and R01 AG055121 (L.W., H.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Alzheimer’s Disease International. Policy brief for heads of government: the global impact of dementia 2013–2050. Accessed 17 May 2022. https://www.alzint.org/u/2020/08/GlobalImpactDementia2013.pdf
- 2. Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massimo L, Powers C, Moore P, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wetzels R, Zuidema S, Jansen I, Verhey F, Koopmans R. Course of neuropsychiatric symptoms in residents with dementia in long-term care institutions: a systematic review. Int Psychogeriatr. 2010;22(7):1040–1053. [DOI] [PubMed] [Google Scholar]
- 5. Sherman C, Liu CS, Herrmann N, Lanctôt KL. Prevalence, neurobiology, and treatments for apathy in prodromal dementia. Int Psychogeriatr. 2018;30(2):177–184. [DOI] [PubMed] [Google Scholar]
- 6. Cipriani G, Lucetti C, Danti S, Nuti A. Apathy and dementia. Nosology, assessment and management. J Nerv Ment Dis. 2014;202(10):718–724. [DOI] [PubMed] [Google Scholar]
- 7. Chow TW, Binns MA, Cummings JL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66(7):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71(10):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padala PR, Padala KP, Lensing SY, et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018;261:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Commu C, Treur J, Dols A, Pijnenburg YAL. An adaptive network model for a possible therapy for the effects of a certain type of dementia on social functioning. Biol Inspired Cogn Archit. 2018;26:145–158. [Google Scholar]
- 11. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Hardy J, Duff KE. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21(10):1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seeley WW. Mapping neurodegenerative disease onset and progression. Cold Spring Harb Perspect Biol. 2017;9:a023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain. 2009;132(Pt 11):2906–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31(9):3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. [DOI] [PubMed] [Google Scholar]
- 20. Ji JL, Spronk M, Kulkarni K, Repovs G, Anticevic A, Cole MW. Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage. 2019;185:35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon EM, Laumann TO, Gilmore AW, et al. Precision functional mapping of individual human brains. Neuron. 2017;95(4):791–807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. [DOI] [PubMed] [Google Scholar]
- 23. Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, Dickerson BC. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J Neurol Neurosurg Psychiatry. 2014;85(4):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guimet N, Miller BL, Allegri RF, Rankin KP. What do we mean by behavioral disinhibition in frontotemporal dementia? Front Neurol. 2021;12:707799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rankin KP. Brain networks supporting social cognition in dementia. Curr Behav Neurosci Rep. 2020;7:203–211. [Google Scholar]
- 27. Ranasinghe KG, Rankin KP, Pressman PS, et al. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol. 2016;73(9):1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute of Mental Health. Research Domain Criteria Initiative. Accessed 26 December 2021. https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc
- 29. Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J comp Neurol. 2005;493(1):154–166. [DOI] [PubMed] [Google Scholar]
- 30. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996–2005. [DOI] [PubMed] [Google Scholar]
- 32. van der Zee J, Sleegers K, Van Broeckhoven C. The Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology. 2008;71(15):1191–1197. [DOI] [PubMed] [Google Scholar]
- 33. Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: Clinical, neuroimaging and pathological features. Brain. 2015;138(Pt 9):2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thant T, Yager J. Updating apathy: Using research domain criteria to inform clinical assessment and diagnosis of disorders of motivation. J Nerv Ment Dis. 2019;207(9):707–714. [DOI] [PubMed] [Google Scholar]
- 35. Manuel AL, Roquet D, Landin-Romero R, et al. Interactions between decision-making and emotion in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Soc Cogn Affect Neurosci. 2020;15(6):681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li CM, Zheng J, Wang J, Gui L, Li CA. An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer’s disease. Curr Alzheimer Res. 2009;6(6):525–530. [DOI] [PubMed] [Google Scholar]
- 37. Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain. 2017;140:1792–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finger E, Zhang J, Dickerson B, Bureau Y, Masellis M; Alzheimer’s Disease Neuroimaging Initiative . Disinhibition in Alzheimer’s disease is associated with reduced right frontal pole cortical thickness. J Alzheimers Dis. 2017;60(3):1161–1170. [DOI] [PubMed] [Google Scholar]
- 39. Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73(5):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mariano LI, O’Callaghan C, Guimaraes HC, et al. Disinhibition in frontotemporal dementia and Alzheimer’s disease: A neuropsychological and behavioural investigation. J Int Neuropsychol Soc. 2020;26(2):163–171. [DOI] [PubMed] [Google Scholar]
- 41. Krueger CE, Laluz V, Rosen HJ, Neuhaus JM, Miller BL, Kramer JH. Double dissociation in the anatomy of socioemotional disinhibition and executive functioning in dementia. Neuropsychology. 2011;25(2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Virani K, Jesso S, Kertesz A, Mitchell D, Finger E. Functional neural correlates of emotional expression processing deficits in behavioural variant frontotemporal dementia. J Psychiatry Neurosci. 2013;38(3):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van den Stock J, De Winter FL, de Gelder B, et al. Impaired recognition of body expressions in the behavioral variant of frontotemporal dementia. Neuropsychologia. 2015;75:496–504. [DOI] [PubMed] [Google Scholar]
- 44. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘reading the mind in the eyes’ test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- 45. Baglio F, Castelli I, Alberoni M, et al. Theory of mind in amnestic mild cognitive impairment: an FMRI study. J Alzheimers Dis. 2012;29(1):25–37. [DOI] [PubMed] [Google Scholar]
- 46. Kumfor F, Ibañez A, Hutchings R, Hazelton JL, Hodges JR, Piguet O. Beyond the face: how context modulates emotion processing in frontotemporal dementia subtypes. Brain. 2018;141(4):1172–1185. [DOI] [PubMed] [Google Scholar]
- 47. McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K. Reliability and validity of the awareness of social inference test (TASIT): a clinical test of social perception. Disabil Rehabil. 2006;28(24):1529–1542. [DOI] [PubMed] [Google Scholar]
- 48. Kumfor F, Honan C, McDonald S, Hazelton JL, Hodges JR, Piguet O. Assessing the ‘social brain’ in dementia: Applying TASIT-S. Cortex. 2017;93:166–177. [DOI] [PubMed] [Google Scholar]
- 49. Sedoño L, Couto B, Garcia-Cordero I, et al. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc. 2016;22(2):250–262. [DOI] [PubMed] [Google Scholar]
- 50. Toller G, Brown J, Sollberger M, et al. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex. 2018;103:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toller G, Yang WFZ, Brown JA, et al. Divergent patterns of loss of interpersonal warmth in frontotemporal dementia syndromes are predicted by altered intrinsic network connectivity. Neuroimage Clin. 2019;22:101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pasquini L, Nana AL, Toller G, et al. Salience network atrophy links neuron type-specific pathobiology to loss of empathy in frontotemporal dementia. Cereb Cortex. 2020;30(10):5387–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Husain M, Roiser JP. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat Rev Neurosci. 2018;19(8):470–484. [DOI] [PubMed] [Google Scholar]
- 54. Robert PH, Lanctôt KL, Agüera-Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur psychiatry. 2018;54:71–76. [DOI] [PubMed] [Google Scholar]
- 55. Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry. 2005;76(8):1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(Pt 11):2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Massimo L, Libon DJ, Chandrasekaran K, et al. Self-appraisal in behavioural variant frontotemporal degeneration. J Neurol Neurosurg psychiatry. 2013;84(2):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Henry JD, von Hippel W, Molenberghs P, Lee T, Sachdev PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. 2016;12(1):28–39. [DOI] [PubMed] [Google Scholar]
- 59. Chapman KR, Spitznagel MB. Measurement of sexual disinhibition in dementia: A systematic review. Int J Geriatr Psychiatry. 2019;34(12):1747–1757. [DOI] [PubMed] [Google Scholar]
- 60. Radakovic R, Harley C, Abrahams S, Starr JM. A systematic review of the validity and reliability of apathy scales in neurodegenerative conditions. Int Psychogeriatr. 2015;27(6):903–923. [DOI] [PubMed] [Google Scholar]
- 61. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. [DOI] [PubMed] [Google Scholar]
- 62. Cummings JL. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–S16. [DOI] [PubMed] [Google Scholar]
- 63. Grace J, Malloy P. Frontal systems behavior scale (FrSBe): professional manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 64. Paholpak P, Carr AR, Barsuglia JP, et al. Person-based versus generalized impulsivity disinhibition in frontotemporal dementia and Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29(6):344–351. [DOI] [PubMed] [Google Scholar]
- 65. Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24(1):29–36. [DOI] [PubMed] [Google Scholar]
- 66. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A frontal assessment battery at bedside. Neurology. 2000;55(11):1621–1626. [DOI] [PubMed] [Google Scholar]
- 67. Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge behavioural inventory revised. Demen Neuropsychol. 2008;2(2):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Radakovic R, Abrahams S. Developing a new apathy measurement scale: Dimensional Apathy Scale. Psychiatry Res. 2014;219(3):658–663. [DOI] [PubMed] [Google Scholar]
- 69. Clarke DE, Reekum R, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: An examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19(1):57–64. [DOI] [PubMed] [Google Scholar]
- 70. Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L. The lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(5):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Robert PH, Clairet S, Benoit M, et al. The apathy inventory: Assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17(12):1099–1105. [DOI] [PubMed] [Google Scholar]
- 72. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–139. [DOI] [PubMed] [Google Scholar]
- 73. Massimo L, Powers JP, Evans LK, et al. Apathy in frontotemporal degeneration: Neuroanatomical evidence of impaired goal-directed behavior. Front Hum Neurosci. 2015;9:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burgess P, Shallice T. Hayling and brixton tests. Thames Valley Test Company; 1997. [Google Scholar]
- 75. Kumfor F, Zhen A, Hodges JR, Piguet O, Irish M. Apathy in Alzheimer’s disease and frontotemporal dementia: Distinct clinical profiles and neural correlates. Cortex. 2018;103:350–359. [DOI] [PubMed] [Google Scholar]
- 76. Wei G, Irish M, Hodges JR, Piguet O, Kumfor F. Disease-specific profiles of apathy in Alzheimer’s disease and behavioural-variant frontotemporal dementia differ across the disease course. J Neurol. 2020;267(4):1086–1096. [DOI] [PubMed] [Google Scholar]
- 77. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment—results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. [DOI] [PubMed] [Google Scholar]
- 78. Chow TW, Miller BL, Boone K, Mishkin F, Cummings JL. Frontotemporal dementia classification and neuropsychiatry. Neurologist. 2002;8(4):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Borges LG, Rademaker AW, Bigio EH, Mesulam MM, Weintraub S. Apathy and disinhibition related to neuropathology in amnestic versus behavioral dementias. Am J Alzheimers Dis Other Demen. 2019;34(5):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. [DOI] [PubMed] [Google Scholar]
- 81. Robert PH, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. [DOI] [PubMed] [Google Scholar]
- 82. Donovan NJ, Wadsworth LP, Lorius N, et al. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22(11):1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Le Ber I, Guedj E, Gabelle A, et al. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129(Pt 11):3051–3065. [DOI] [PubMed] [Google Scholar]
- 84. Malpetti M, Carli G, Sala A, et al. Variant-specific vulnerability in metabolic connectivity and resting-state networks in behavioural variant of frontotemporal dementia. Cortex. 2019;120:483–497. [DOI] [PubMed] [Google Scholar]
- 85. Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s disease. J Am Geriatr Soc. 2001;49(12):1700–1707. [DOI] [PubMed] [Google Scholar]
- 86. Onyike CU, Sheppard JM, Tschanz JT, et al. Epidemiology of apathy in older adults: The cache county study. Am J Geriatr Psychiatry. 2007;15(5):365–375. [DOI] [PubMed] [Google Scholar]
- 87. Reus LM, Vijverberg EGB, Tijms BM, et al. Disease trajectories in behavioural variant frontotemporal dementia, primary psychiatric and other neurodegenerative disorders presenting with behavioural change. J Psychiatr Res. 2018;104:183–191. [DOI] [PubMed] [Google Scholar]
- 88. Brodaty H, Connors MH, Xu J, Woodward M, Ames D. The course of neuropsychiatric symptoms in dementia: A 3-year longitudinal study. J Am Med Dir Assoc. 2015;16(5):380–387. [DOI] [PubMed] [Google Scholar]
- 89. Yassuda MS, da Silva TBL, O’Connor CM, et al. Apathy and functional disability in behavioral variant frontotemporal dementia. Neurol Clin Pract. 2018;8(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O’Connor CM, Clemson L, Hornberger M, et al. Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurol Clin Pract. 2016;6(5):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol. 2011;10(2):162–172. [DOI] [PubMed] [Google Scholar]
- 92. Josephs KA Jr, Whitwell JL, Weigand SD, et al. Predicting functional decline in behavioural variant frontotemporal dementia. Brain. 2011;134(Pt 2):432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Akyol MA, Kucukguclu O, Yener G. Investigation of factors affecting apathy in three major types of dementia. Noro Psikiyatr Ars. 2020;57(2):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lansdall CJ, Coyle-Gilchrist ITS, Vázquez Rodríguez P, et al. Prognostic importance of apathy in syndromes associated with frontotemporal lobar degeneration. Neurology. 2019;92(14):e1547–e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behav Neurol. 2012;25(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gonçalves SAB, Caramelli P, Mariano LI, et al. Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. 2020;1737:146799. [DOI] [PubMed] [Google Scholar]
- 97. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lechowski L, Benoit M, Chassagne P, et al. Persistent apathy in Alzheimer’s disease as an independent factor of rapid functional decline: The REAL longitudinal cohort study. Int J Geriatr Psychiatry. 2009;24(4):341–346. [DOI] [PubMed] [Google Scholar]
- 99. Robert PH, Berr C, Volteau M, et al. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer’s disease: A one-year follow-up study. Clin Neurol Neurosurg. 2006;108(8):733–736. [DOI] [PubMed] [Google Scholar]
- 100. Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: The role of depression and apathy. J Alzheimers Dis. 2010;20(1):175–183. [DOI] [PubMed] [Google Scholar]
- 101. Vilalta-Franch J, Calvó-Perxas L, Garre-Olmo J, Turró-Garriga O, López-Pousa S. Apathy syndrome in Alzheimer’s disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. J Alzheimers Dis. 2013;33(2):535–543. [DOI] [PubMed] [Google Scholar]
- 102. Skogseth R, Mulugeta E, Jones E, et al. Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):559–563. [DOI] [PubMed] [Google Scholar]
- 103. Tunnard C, Whitehead D, Hurt C, et al. Apathy and cortical atrophy in Alzheimer’s disease. Int J Geriatr Psychiatry. 2011;26(7):741–748. [DOI] [PubMed] [Google Scholar]
- 104. Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64(7):1015–1020. [DOI] [PubMed] [Google Scholar]
- 105. Lanctôt KL, Moosa S, Herrmann N, et al. A SPECT study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(1):65–72. [DOI] [PubMed] [Google Scholar]
- 106. Holthoff VA, Beuthien-Baumann B, Kalbe E, et al. Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biol Psychiatry. 2005;57(4):412–421. [DOI] [PubMed] [Google Scholar]
- 107. Peters F, Perani D, Herholz K, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21(5-6):373–379. [DOI] [PubMed] [Google Scholar]
- 108. Migneco O, Benoit M, Koulibaly PM, et al. Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: A study in Alzheimer’s disease and nondemented patients. Neuroimage. 2001;13(5):896–902. [DOI] [PubMed] [Google Scholar]
- 109. Stanton BR, Leigh PN, Howard RJ, Barker GJ, Brown RG. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J Neurol. 2013;260(10):2481–2490. [DOI] [PubMed] [Google Scholar]
- 110. Jones SA, De Marco M, Manca R, et al. Altered frontal and insular functional connectivity as pivotal mechanisms for apathy in Alzheimer’s disease. Cortex. 2019;119:100–110. [DOI] [PubMed] [Google Scholar]
- 111. Delrieu J, Desmidt T, Camus V, et al. Apathy as a feature of prodromal Alzheimer’s disease: An FDG-PET ADNI study. Int J Geriatr Psychiatry. 2015;30(5):470–477. [DOI] [PubMed] [Google Scholar]
- 112. Fernández-Matarrubia M, Matías-Guiu JA, Cabrera-Martín MN, et al. Different apathy clinical profile and neural correlates in behavioral variant frontotemporal dementia and Alzheimer’s disease. Int J Geriatr Psychiatry. 2018;33(1):141–150. [DOI] [PubMed] [Google Scholar]
- 113. Robert PH, Darcourt G, Koulibaly MP, et al. Lack of initiative and interest in Alzheimer’s disease: A single photon emission computed tomography study. Eur J Neurol. 2006;13(7):729–735. [DOI] [PubMed] [Google Scholar]
- 114. Basavaraju R, Feng X, France J, Huey ED, Provenzano FA. Depression is associated with preserved cortical thickness relative to apathy in frontotemporal dementia. J Geriatr Psychiatry Neurol. 2022;35(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Santamaria-Garcia H, Reyes P, Garcia A, et al. First symptoms and neurocognitive correlates of behavioral variant frontotemporal dementia. J Alzheimers Dis. 2016;54(3):957–970. [DOI] [PubMed] [Google Scholar]
- 116. Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum Brain Mapp. 2016;37(12):4234–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huey ED, Lee S, Cheran G, Grafman J, Devanand DP; Alzheimer’s Disease Neuroimaging Initiative . Brain regions involved in arousal and reward processing are associated with apathy in Alzheimer’s disease and frontotemporal dementia. J Alzheimers Dis. 2017;55(2):551–558. [DOI] [PubMed] [Google Scholar]
- 118. Shaw SR, El-Omar H, Roquet D, et al. Uncovering the prevalence and neural substrates of anhedonia in frontotemporal dementia. Brain. 2021;144(5):1551–1564. [DOI] [PubMed] [Google Scholar]
- 119. Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455–2463. [DOI] [PubMed] [Google Scholar]
- 120. Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(2):91–97. [DOI] [PubMed] [Google Scholar]
- 121. Guercio BJ, Donovan NJ, Ward A, et al. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. 2015;27(1):e22–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Johansson M, Stomrud E, Lindberg O, et al. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol Aging. 2020;85:74–82. [DOI] [PubMed] [Google Scholar]
- 123. Day GS, Farb NA, Tang-Wai DF, et al. Salience network resting-state activity: Prediction of frontotemporal dementia progression. JAMA Neurol. 2013;70(10):1249–1253. [DOI] [PubMed] [Google Scholar]
- 124. Kazui H, Takahashi R, Yamamoto Y, et al. Neural basis of apathy in patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2017;55(4):1403–1416. [DOI] [PubMed] [Google Scholar]
- 125. Johnson E, Kumfor F. Overcoming apathy in frontotemporal dementia: challenges and future directions. Curr Opin Behav Sci. 2018;22:82–89. [Google Scholar]
- 126. Gatchel JR, Donovan NJ, Locascio JJ, et al. Regional 18F-fluorodeoxyglucose hypometabolism is associated with higher apathy scores over time in early Alzheimer disease. Am J Geriatr Psychiatry. 2017;25(7):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McDonald CR, McEvoy LK, Gharapetian L, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73(6):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Marshall GA, Donovan NJ, Lorius N, et al. Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 2013;25(4):302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Balthazar MLF, Pereira FRS, Lopes TM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. 2014;35(4):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Munro CE, Donovan NJ, Guercio BJ, et al. Neuropsychiatric symptoms and functional connectivity in mild cognitive impairment. J Alzheimers Dis. 2015;46(3):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Joo SH, Lee CU, Lim HK. Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr Dis Treat. 2017; 13:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–254. [DOI] [PubMed] [Google Scholar]
- 133. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16(7):916–928. [DOI] [PubMed] [Google Scholar]
- 134. Stuss DT, van Reekum R, Murphy KJ. Differentiation of states and causes of apathy. In: Borod J, ed. The neuropsychology of emotion. Oxford University Press; 2000:340–363. [Google Scholar]
- 135. Benoit M, Clairet S, Koulibaly PM, Darcourt J, Robert PH. Brain perfusion correlates of the apathy inventory dimensions of Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19(9):864–869. [DOI] [PubMed] [Google Scholar]
- 136. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huey ED. A critical review of behavioral and emotional disinhibition. J Nerv Ment Dis. 2020;208(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Migliaccio R, Tanguy D, Bouzigues A, et al. Cognitive and behavioural inhibition deficits in neurodegenerative dementias. Cortex. 2020;131:265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 140. Miller BL, Darby A, Benson DF, Cummings JL, Miller MH. Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. Br J Psychiatry. 1997;170:150–155. [DOI] [PubMed] [Google Scholar]
- 141. Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mendez MF, Chen AK, Shapira JS, Miller BL. Acquired sociopathy and frontotemporal dementia. Dement Geriatr Cogn Disord. 2005;20(2-3):99–104. [DOI] [PubMed] [Google Scholar]
- 143. Tranel D. ‘Acquired sociopathy’: The development of sociopathic behavior following focal brain damage. Prog Exp Pers Psychopathol Res. 1994;17:285–311. [PubMed] [Google Scholar]
- 144. Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18(3):355–381. [DOI] [PubMed] [Google Scholar]
- 145. Blair M, Kertesz A, Davis-Faroque N, et al. Behavioural measures in frontotemporal lobar dementia and other dementias: The utility of the frontal behavioural inventory and the neuropsychiatric inventory in a national cohort study. Dement Geriatr Cogn Disord. 2007;23(6):406–415. [DOI] [PubMed] [Google Scholar]
- 146. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. [DOI] [PubMed] [Google Scholar]
- 147. Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2005;13(6):460–468. [DOI] [PubMed] [Google Scholar]
- 148. O’Connor CM, Landin-Romero R, Clemson L, et al. Behavioral-variant frontotemporal dementia: Distinct phenotypes with unique functional profiles. Neurology. 2017;89(6):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: Comparison with patients with MAPT and no known mutations. Brain. 2008;131(Pt 3):721–731. [DOI] [PubMed] [Google Scholar]
- 150. Cosseddu M, Benussi A, Gazzina S, et al. Progression of behavioural disturbances in frontotemporal dementia: a longitudinal observational study. Eur J Neurol. 2020;27(2):265–272. [DOI] [PubMed] [Google Scholar]
- 151. Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nakano S, Asada T, Yamashita F, et al. Relationship between antisocial behavior and regional cerebral blood flow in frontotemporal dementia. Neuroimage. 2006;32(1):301–306. [DOI] [PubMed] [Google Scholar]
- 153. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134:2502–2512. [DOI] [PubMed] [Google Scholar]
- 154. Flanagan EC, Wong S, Dutt A, et al. False recognition in behavioral variant frontotemporal dementia and Alzheimer’s disease: Disinhibition or amnesia? Front Aging Neurosci. 2016;8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Santillo AF, Lundblad K, Nilsson M, et al. Grey and white matter clinico-anatomical correlates of disinhibition in neurodegenerative disease. PLoS One. 2016;11(10):e0164122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. O’Callaghan C, Naismith SL, Hodges JR, Lewis SJ, Hornberger M. Fronto-striatal atrophy correlates of inhibitory dysfunction in Parkinson’s disease versus behavioural variant frontotemporal dementia. Cortex. 2013;49(7):1833–1843. [DOI] [PubMed] [Google Scholar]
- 157. Serra L, Perri R, Cercignani M, et al. Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration? J Alzheimers Dis. 2010;21(2):627–639. [DOI] [PubMed] [Google Scholar]
- 158. Zahn R, Moll J, Iyengar V, et al. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132(Pt 3):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ahmed RM, Hodges JR, Piguet O. Behavioural variant frontotemporal dementia: Recent advances in the diagnosis and understanding of the disorder. Adv Exp Med Biol. 2021;1281:1–15. [DOI] [PubMed] [Google Scholar]
- 160. Morbelli S, Ferrara M, Fiz F, et al. Mapping brain morphological and functional conversion patterns in predementia late-onset bvFTD. Eur J Nucl Med Mol Imaging. 2016;43(7):1337–1347. [DOI] [PubMed] [Google Scholar]
- 161. Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Franceschi M, Anchisi D, Pelati O, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57(2):216–225. [DOI] [PubMed] [Google Scholar]
- 163. Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sheelakumari R, Bineesh C, Varghese T, Kesavadas C, Verghese J, Mathuranath PS. Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal dementia. Brain Imaging Behav. 2020;14(5):2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Huey ED, Lee S, Brickman AM, et al. Neuropsychiatric effects of neurodegeneration of the medial versus lateral ventral prefrontal cortex in humans. Cortex. 2015;73:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Schroeter ML, Vogt B, Frisch S, et al. Dissociating behavioral disorders in early dementia: An FDG-PET study. Psychiatry Res. 2011;194(3):235–244. [DOI] [PubMed] [Google Scholar]
- 167. Borroni B, Grassi M, Premi E, et al. Neuroanatomical correlates of behavioural phenotypes in behavioural variant of frontotemporal dementia. Behav Brain Res. 2012;235(2):124–129. [DOI] [PubMed] [Google Scholar]
- 168. Farb NAS, Grady CL, Strother S, et al. Abnormal network connectivity in frontotemporal dementia: Evidence for prefrontal isolation. Cortex. 2013;49(7):1856–1873. [DOI] [PubMed] [Google Scholar]
- 169. Ibáñez A. Brain oscillations, inhibition and social inappropriateness in frontotemporal degeneration. Brain. 2018;141(10):e73. [DOI] [PubMed] [Google Scholar]
- 170. Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. [DOI] [PubMed] [Google Scholar]
- 171. Binney RJ, Ramsey R. Social semantics: The role of conceptual knowledge and cognitive control in a neurobiological model of the social brain. Neurosci Biobehav Rev. 2020;112:28–38. [DOI] [PubMed] [Google Scholar]
- 172. Kumfor F, Dermody N, Irish M. Considering the impact of large-scale network interactions on cognitive control. J Neurosci. 2015;35(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chiong W, Wilson SM, D’Esposito M, et al. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136(Pt 6):1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lhermitte F. Human autonomy and the frontal lobes. Part II: Patient behavior in complex and social situations: the ‘environmental dependency syndrome’. Ann Neurol. 1986;19(4):335–343. [DOI] [PubMed] [Google Scholar]
- 175. Gotovac K, Nikolac Perković M, Pivac N, Borovečki F. Biomarkers of aggression in dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2016;69:125–130. [DOI] [PubMed] [Google Scholar]
- 176. Ruthirakuhan M, Lanctôt KL, Di Scipio M, Ahmed M, Herrmann N. Biomarkers of agitation and aggression in Alzheimer’s disease: A systematic review. Alzheimers Dement. 2018;14(10):1344–1376. [DOI] [PubMed] [Google Scholar]
- 177. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Aspects Med. 2015;43-44:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gaffan D, Harrison S. Amygdalectomy and disconnection in visual learning for auditory secondary reinforcement by monkeys. J Neurosci. 1987;7(8):2285–2292. [PMC free article] [PubMed] [Google Scholar]
- 179. Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–733. [DOI] [PubMed] [Google Scholar]
- 180. LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. [DOI] [PubMed] [Google Scholar]
- 181. Amaral DG, Bauman MD, Capitanio JP, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41(4):517–522. [DOI] [PubMed] [Google Scholar]
- 182. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22(3):229–244. [DOI] [PubMed] [Google Scholar]
- 183. Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (macaca fascicularis). J Comp Neurol. 1984;230(4):465–496. [DOI] [PubMed] [Google Scholar]
- 184. Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77(3):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. [DOI] [PubMed] [Google Scholar]
- 186. Stuss DT. Disturbance of self-awareness after frontal system damage. In: Prigatano GP, Schacter DL, eds. Awareness of deficit after brain injury: clinical and theoretical issues. Oxford University Press; 1991:63–83. [Google Scholar]
- 187. Frith CD, Frith U. Interacting minds: A biological basis. Science. 1999;286(5445):1692–1695. [DOI] [PubMed] [Google Scholar]
- 188. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. [DOI] [PubMed] [Google Scholar]
- 189. Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry. 2004;161(7):1247–1255. [DOI] [PubMed] [Google Scholar]
- 190. Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. [DOI] [PubMed] [Google Scholar]
- 191. Jenkins LM, Andrewes DG, Nicholas CL, et al. Emotional reactivity following surgery to the prefrontal cortex. J Neuropsychol. 2018;12(1):120–141. [DOI] [PubMed] [Google Scholar]
- 192. Jenkins LM, Andrewes DG, Nicholas CL, et al. Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Res Neuroimaging. 2014;224(3):192–203. [DOI] [PubMed] [Google Scholar]
- 193. Andrewes DG, Jenkins LM. The role of the amygdala and the ventromedial prefrontal cortex in emotion regulation: Implications for post-traumatic stress disorder. Neuropsychol Rev. 2019;29(2):220–243. [DOI] [PubMed] [Google Scholar]
- 194. Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ibañez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct. 2010;214(5-6):397–410. [DOI] [PubMed] [Google Scholar]
- 196. Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33(4):653–663. [DOI] [PubMed] [Google Scholar]
- 197. Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: A possible role for von Economo neurons. Trends Cogn sci. 2005;9(8):367–373. [DOI] [PubMed] [Google Scholar]
- 198. Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. [DOI] [PubMed] [Google Scholar]
- 199. Sinha N, Manohar S, Husain M. Impulsivity and apathy in Parkinson’s disease. J Neuropsychol. 2013;7(2):255–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104(15):6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Wong S, Balleine BW, Kumfor F. A new framework for conceptualizing symptoms in frontotemporal dementia: from animal models to the clinic. Brain. 2018;141(8):2245–2254. [DOI] [PubMed] [Google Scholar]
- 203. Reyes P, Ortega-Merchan MP, Rueda A, et al. Functional connectivity changes in behavioral, semantic, and nonfluent variants of frontotemporal dementia. Behav Neurol. 2018:2018:9684129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Hafkemeijer A, Moller C, Dopper EGP, et al. Differences in structural covariance brain networks between behavioral variant frontotemporal dementia and Alzheimer’s disease. Hum Brain Mapp. 2016;37(3):978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tetreault AM, Phan T, Orlando D, et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2020;143:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(3):144–147. [DOI] [PubMed] [Google Scholar]
- 207. Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49(3):355–361. [PubMed] [Google Scholar]
- 208. Förstl H, Burns A, Levy R, Cairns N, Luthert P, Lantos P. Neuropathological correlates of behavioural disturbance in confirmed Alzheimer’s disease. Br J Psychiatry. 1993;163:364–368. [DOI] [PubMed] [Google Scholar]
- 209. Burns A, Förstl H. The institute of psychiatry Alzheimer’s disease cohort: Part 2: Clinicopathological observations. Int J Geriatr Psychiatry. 1996;11:321–327. [Google Scholar]
- 210. Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–1239. [DOI] [PubMed] [Google Scholar]
- 211. Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):63–70. [DOI] [PubMed] [Google Scholar]
- 212. Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013;80(6):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Léger GC, Banks SJ. Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2014;37(1-2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Li CH, Fan SP, Chen TF, Chiu MJ, Yen RF, Lin CH. Frontal variant of Alzheimer’s disease with asymmetric presentation mimicking frontotemporal dementia: Case report and literature review. Brain Behav. 2020;10(3):e01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Paquin V, Therriault J, Pascoal TA, Rosa-Neto P, Gauthier S. Frontal variant of Alzheimer disease differentiated from frontotemporal dementia using in vivo amyloid and tau imaging. Cogn Behav Neurol. 2020;33(4):288–293. [DOI] [PubMed] [Google Scholar]
- 216. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Agüera-Ortiz L, Hernandez-Tamames JA, Martinez-Martin P, et al. Structural correlates of apathy in Alzheimer’s disease: A multimodal MRI study. Int J Geriatr Psychiatry. 2017;32(8):922–930. [DOI] [PubMed] [Google Scholar]
- 218. Powers JP, Massimo L, McMillan CT, et al. White matter disease contributes to apathy and disinhibition in behavioral variant frontotemporal dementia. Cogn Behav Neurol. 2014;27(4):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.