ABSTRACT
Background
Production of SCFAs from food is a complex and dynamic saccharolytic fermentation process mediated by both human and gut microbial factors. Knowledge of SCFA production and of the relation between SCFA profiles and dietary patterns is lacking.
Objectives
Temporal changes in SCFA concentrations in response to 2 contrasting diets were investigated using a novel GC-MS method.
Methods
Samples were obtained from a randomized, controlled, crossover trial designed to characterize the metabolic response to 4 diets. Participants (n = 19) undertook these diets during an inpatient stay (of 72 h). Serum samples were collected 2 h after breakfast (AB), after lunch (AL), and after dinner (AD) on day 3, and a fasting sample (FA) was obtained on day 4. The 24-h urine samples were collected on day 3. In this substudy, samples from the 2 extreme diets representing a diet with high adherence to WHO healthy eating recommendations and a typical Western diet were analyzed using a bespoke GC-MS method developed to detect and quantify 10 SCFAs and precursors in serum and urine samples.
Results
Considerable interindividual variation in serum SCFA concentrations was observed across all time points, and temporal fluctuations were observed for both diets. Although the sample collection timing exerted a greater magnitude of effect on circulating SCFA concentrations, the unhealthy diet was associated with a lower concentration of acetic acid (FA: coefficient: –17.0; SE: 5.8; P-trend = 0.00615), 2-methylbutyric acid (AL: coefficient: –0.1; SE: 0.028; P-trend = 4.13 × 10–4 and AD: coefficient: –0.1; SE: 0.028; P-trend = 2.28 × 10–3), and 2-hydroxybutyric acid (FA: coefficient: –15.8; SE: 5.11; P-trend: 4.09 × 10–3). In contrast, lactic acid was significantly higher in the unhealthy diet (AL: coefficient: 750.2; SE: 315.2; P-trend = 0.024 and AD: coefficient: 1219.3; SE: 322.6; P-trend: 8.28 × 10–4).
Conclusions
The GC-MS method allowed robust mapping of diurnal patterns in SCFA concentrations, which were affected by diet, and highlighted the importance of standardizing the timing of SCFA measurements in dietary studies. This trial was registered on the NIHR UK clinical trial gateway and with ISRCTN as ISRCTN43087333.
Keywords: short-chain fatty acids, branched-chain fatty acid, diet, fiber, metabolite, GC-MS
Introduction
In recent years, the relevance of gut microbial metabolites in nutrition research has gained increasing recognition (1, 2). Multiple classes of metabolites deriving from bacterial conversion of dietary chemicals, including SCFAs, provide an energy source and act as signaling molecules that affect physiological and pathological processes, including immunity, adipocyte physiology, and neuronal signaling. SCFAs are the end product of fermentation of nondigestible carbohydrates, undigested proteins, and amino acids (3, 4). Typically, most research only reports the 3 SCFAs that are produced by the microbiota in the largest quantities: acetic, propionic, and butyric acids. However, new studies are demonstrating other molecules that are interconnected with these 3 SCFAs, such as lactic acid, are also mechanistically important in driving physiological and pathological processes (5). There is therefore a need to explore a wider panel of SCFAs and related molecules. SCFAs have been shown to have a therapeutic value in the prevention of obesity and insulin resistance in both animals and humans (6–10), with several mechanisms such as immunomodulation of intestinal macrophages and reduction of cholesterol synthesis being proposed (11).
SCFAs and branched short-chain fatty acids (BSCFAs) have been associated with both protective and causal roles with respect to metabolic diseases (12–15). For example, microbial capacity for butyric acid production has been linked to lower blood glucose concentrations (16). With regard to metabolic disorders, 2-hydroxybutyric and lactic acid are two 2-hydroxycarboxylic acids of interest, with 2-hydroxybutyric acid being proposed as an early predictor of insulin resistance and impaired glucose tolerance (17, 18). Based on previous published studies on SCFAs and related metabolites, a panel of 10 SCFAs, BSCFAs, and 2-hydroxycarboxylic acids was chosen as the most pertinent panel of analytes to quantify in terms of understanding dietary impact on SCFA-related metabolism.
The potential of SCFAs to act as anti-inflammatory and anticarcinogenic mediators opens a window of opportunity for dietary modulation to achieve preventative or therapeutic impact for various diseases via delivery of SCFAs. However, the complex interactions between food, diet, microbiota, health, and metabolic phenotype are, as yet, poorly understood. Diet is the main source of fermentable substrates, as it directly influences gut microbiota fermentation rates and metabolite production. To date, there are no detailed clinical studies that explore how SCFAs can be modulated exclusively by mixed diets under highly controlled food intake conditions, and knowledge of the effect of dietary SCFA sources on human metabolism, without the inclusion of any specific dietary supplements of SCFAs or their precursors, is inadequate and poorly documented.
To explore the interplay between SCFAs and diet, we developed an analytical method for quantifying SCFA-related metabolites, including acetic acid, propionic acid, butyric acid, methyl-branched SCFAs, and hydroxylated carboxylic acids, and applied it to serum and urine samples collected in a controlled, randomized, crossover trial monitoring response to a WHO-compliant (healthy) (19) and a standard typical Western (unhealthy) diet. Our study was a substudy from an inpatient clinical trial previously published (20). Specifically, we monitored the profile of circulating SCFA-related metabolites throughout the day in response to these 2 contrasting diets to better understand the systemic exposure to dietary SCFAs.
Methods
Study design and participants
In this randomized, controlled, crossover trial to assess the impact of healthiness of diet on metabolism, 19 participants (aged 21–65 y; 9 female, 10 male) were recruited from the healthy volunteer database of the UK National Institute for Health Research/Wellcome Trust Imperial Clinical Research Facility (Supplemental Figure 1 and Supplemental Table 1). BMI (in kg/m2) of participants was distributed between 21.1 and 33.3 (Table 1).
TABLE 1.
Baseline sociodemographic and clinical characteristics of participants1
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 10 (53) |
| Female | 9 (47) |
| Age, y | 55.8 (12.6; 29–65) |
| Ethnic origin | |
| White | 18 (95) |
| Asian | 1 (5) |
| Anthropometric and biochemical parameters | |
| Weight, kg | 74.5 (12.5; 52.8–107.9) |
| BMI, kg/m2 | 25.6 (3.2; 21.1–33.3) |
| Glucose,2 mmol/L | 4.8 (0.4; 4.1–5.4) |
| HbA1c,2 % | 5.5% (0.1, 5.1–5.8) |
| HbA1c,2 mmol/mol | 36.4 (0.9; 32–40) |
| Triglycerides,3 mmol/L | 0.9 (0.3; 0.5–1.4) |
| Cholesterol,3 mmol/L | |
| Total | 5.1 (0.7; 3.9–6.1) |
| LDL | 3.1 (0.7; 1.7–4.2) |
| HDL | 1.6 (0.4; 0.9–2.6) |
| Liver function tests,3 IU/L | |
| Alanine transaminase | 21.2 (7.4; 12.3–40.0) |
| Aspartate transaminase | 19.5 (3.2; 15.0–24.3) |
Data are in n (%) or mean (SD; range). IU, international units.
From plasma samples.
From serum samples.
Inclusion and exclusion criteria are listed in the “Supplemental Methods.” This study was approved by the London–Brent Research Ethics Committee and carried out in accordance with the Declaration of Helsinki (13/LO/0078). This study (ISRCTN number: ISRCTN43087333) is a substudy from a previously published study (20). All participants provided written informed consent. The number of individuals enrolled in the study was selected based on the literature reports of observed differences in circulating SCFA concentrations between 2 or more contrasting diets, in which the number of participants ranged between 10 and 18 (21–23).
In our previous work, 4 dietary interventions were designed accordingly to WHO healthy eating recommendations (19, 20). These interventions were designed with stepwise variance in concordance with the WHO healthy eating guidelines, ranging from 100% to 25% concordance. In this substudy, we investigated the temporal changes in SCFA concentrations in the 2 extreme diets designated “healthy” and “unhealthy” as subgroups because these diets differ in their dietary profile, including macronutrient composition. Importantly, the 2 contrasting diets demonstrate differences in fiber and protein amounts, both of which are associated with SCFA production (3, 25–28). The “healthy” diet that was fully compliant with the WHO healthy eating guidelines (19) was rich in fruit, vegetables, and fiber and low in saturated fats, sugars, and salt, whereas the “unhealthy” diet represented a contrasting diet that was low in fruit and vegetables and 3 times less the amount of fiber (for differences in dietary composition and diet information, see Table 2 and Supplemental Tables 2–3). The 2 diets were provided in random order, and for each diet period, participants attended a clinical research facility on different occasions for 72 h. Dietary interventions were delivered during 3 d in an inpatient setting, served at the same time each day. To avoid any carryover of metabolic effects from the previous dietary intervention, a minimum washout period of 5 d was required following completion of the first diet and the subsequent dietary intervention.
TABLE 2.
Macronutrients content and characteristics of the 2 contrasting dietary interventions1
| Characteristic | Healthy diet | Unhealthy diet |
|---|---|---|
| Energy, kcal | 2260 | 2490 |
| Energy density, kcal/g | 1.2 | 1.9 |
| Proportion of protein, % | 24 | 13 |
| Proportion of carbohydrate, % | 51 | 44 |
| Total sugar, g | 14 | 25 |
| Proportion of fat, % | 23 | 42 |
| Saturated fatty acids, g | 5 | 20 |
| Monounsaturated fatty acids, g | 8 | 12 |
| Polyunsaturated fats, g | 8 | 2 |
| Total trans fatty acids, g | 0.5 | 1 |
| Fiber, g | 45.9 | 13.6 |
| Sodium, mg | 2367 | 3066 |
| Fruit and vegetables, g | 600 | 100 |
| Dash score | 37 | 11 |
1Macronutrients and micronutrients were calculated using a nutrient software (Dietplan v.6; Forestfield Software Ltd). DASH, Dietary Approaches to Stop Hypertension (24).
Sample collection
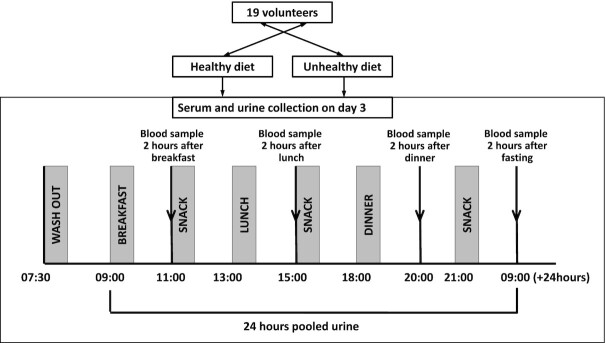
Timed blood and 24-h urine samples were collected during the third day of each diet to allow equilibration to the diet prior to measuring the dietary response. Blood samples were collected 2 h after breakfast (AB), after lunch (AL), and after dinner (AD). Fasting (FA) samples were collected on the morning of day 4. Serum was obtained from whole blood within 15 min of collection and stored at –80°C (Figure 1 and Supplemental Table 1). Urine samples were stored at –80°C and were centrifuged prior to analysis. Samples were not exposed to freeze-thaw cycles prior to SCFA analysis.
FIGURE 1.
Flowchart of sample collection for serum and urine. Samples were obtained after consumption of a healthy and an unhealthy diet. Short-chain fatty acids from both biofluids were analyzed by GC-MS.
SCFA analysis in serum and urine by GC-MS
A total of 5 SCFAs, 3 methyl-branched SCFAs (branched-chain fatty acids), and 2 hydroxylated carboxylic acids were analyzed by GC-MS using a method adapted from Moreau et al. (29), which was validated as described in the Supplemental Methods.
A volume of 100 µL of sample was suspended in 500 µL methyl tert-butyl ether with 100 ppm methyl stearate as an internal standard (IS) to correct injection variability across the various samples batches and to compensate for analytical drift or minor system instabilities. Subsequently, 0.01% of hydrochloric acid was added to each sample for acidification and precipitation of proteins. A short homogenization step was included by vortexing each tube for 3 s individually, followed by a further 20 min using a plate vortex, after which samples were centrifuged for 5 min at 4°C at 10,000 rcf. Immediately, 90 µL supernatant was transferred to a silanized vial, and 60 µL derivatizer N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide with 1% tert-butyldimethylchloro-silane was added. The sample was incubated for 45 min at 60°C. Prior to MS analysis, 70 µL of sample was taken into a regular vial with a deactivated insert. The GC system consisted of an Agilent 7890 B spectrometer (Agilent Technologies), equipped with an automatic liquid sampler model 7693 (Agilent Technologies). The GC was fitted with an HP-5 MS UI inert capillary column (30-m × 0.25-mm × 0.25-μm film thickness; Agilent J&W Scientific), and helium was used as the carrier gas at 1.5 mL/min. Each injection was made in split mode with an injection of 1 µL and injector temperature of 250°C. A glass liner ultra-inert, splitless, single-taper and glass wool (5190–2293; Agilent Technologies) was used to avoid possible column contamination with nonvolatile elements. The oven temperature program was optimized with an initial temperature of 40°C and final temperature of 300°C. The total runtime of the method was 9 min. The GC system was connected to an Agilent 7010 MS/MS triple quadrupole mass spectrometric detector with an electron ionization source, operating at 70 eV. The source temperature was maintained at 230°C, whereas the temperature of both quadrupoles was set at 150°C. The helium quench gas flow was set at 2.25 mL/min and the nitrogen collision gas flow at 1.5 mL/min. A solvent delay of 2 min was implemented. Analyses were performed in positive multiple reaction monitoring mode, with collision energy optimized for each selected transition during the method development (Supplemental Figure 2 and Supplemental Table 4). Data acquisition was achieved using Mass Hunter workstation software for triple quadrupole (Agilent Technologies). SCFA concentrations obtained from 24-h urine samples were normalized to the total volume collected to partially account for differences in urinary osmolality.
This assay was validated in terms of linearity (lower limit of quantification and upper limit of quantification), precision, and accuracy on inter- and intraday analysis and achieved good recovery of the metabolites in serum and urine samples (Supplemental Tables 5–9).
Acetic acid (C2), propionic acid (C3), lactic acid (C3), butyric acid (C4), isobutyric acid (i-C4), 2-hydroxybutyric acid (C4), valeric acid (C5), isovaleric acid (i-C5), 2-methylbutyric acid (C5), caproic acid (C5), and methyl stearate (IS) were purchased from Sigma-Aldrich for use as chemical standards.
Statistical analysis
This study is a substudy from a previously published clinical trial (20) and was a pioneer pilot trial on metabolic profiling under controlled feeding conditions. To provide a sample size calculation number, data from the previous work of Heinzmann et al. (30) were considered a baseline using urinary proline betaine excretion to quantify the intake of oranges, because this metabolite is derived almost exclusively from citrus fruit and not extensively metabolized prior to excretion. Each orange consumed typically increased proline betaine concentrations by 50 µmol/L; therefore, a sample size of 12 was deemed sufficient to detect a difference of 50 μmol/L, considering a SD of 40 μmol/L, power of 0.95, and α of 0.05. Based on this example, and taking into account the considerable volunteer inpatient time required (12 d in total across the 4 original dietary interventions), a high dropout rate was expected. Therefore, accounting for an estimated 33% dropout, permission to recruit 30 participants was sought, aiming to enroll a cohort of 20 participants. We assumed a similar behavior for SCFA excretion, and the power calculation was supported by data from other published studies based on 10–18 participants in whom changes in circulating SCFAs were detected following diets with different fiber composition (21–23). Similarly, other dietary biomarker studies (30, 31) found individual differences in biomarker intake after controlled feeding with a sample size of 20 participants or fewer. A total of 19 participants completed the full protocol and were included in the statistical analysis (Supplemental Table 1 and Supplemental Figure 1).
Statistical analyses, with the exception of ANOVA and Skillings–Mack, were performed using MATLAB R2014a (Version 8.3). Normal distribution was tested by using the Shapiro–Wilk method. None of the SCFA concentrations in either serum or urine were found to exhibit a normal distribution, and results were expressed as median values with IQR. AUCs for SCFAs concentrations were calculated by the trapezoidal rule. For each SCFA, differences in concentrations between time points throughout the day were analyzed with the Skillings–Mack test and subsequent Wilcoxon signed-rank tests corrected by Hommel's method. Pairwise differences in SCFA concentrations and differences in the AUCs of SCFA concentrations between the 2 diets were compared for each time point using the Wilcoxon signed-rank test and adjusted using false discovery rate (32). Differences with adjusted P value (Padj) <0.05 were considered statistically significant.
For metabolites with a concentration that was affected by diet at a particular time point, the interaction between diet and time and the association between diet and metabolite concentration were assessed by 2-factor ANOVA using R statistical tools. The assumptions of normal distribution and homogeneity of variance were tested for the entire SCFA metabolite panel using the Shapiro–Wilk test and Levene test, respectively, after the major outliers were removed. In no case was more than 5% of the total data set removed. In cases where the diet-by-time interaction was not significant, the result from the additive model was reported (Table 3). Associations of diet with SCFA concentrations were evaluated by regression. Graphs were produced using GraphPad Prism 8 version 8.4.2 (GraphPad Software) or inkscape 1.0.2, The inkscape team and the ggplot2, Tidyverse library for R 4.0.2.
TABLE 3.
Diet-by-time interactions based on a 2-factor ANOVA1
| Direction compared with healthy | P-value | Residual number | Number of outliers | |||
|---|---|---|---|---|---|---|
| SCFA | Diet | Time | Diet by time | |||
| Acetic acid | + | 4.71 × 10–2 | 6.86 × 10–33 | 3.87 × 10–1 | 134 | 6 |
| Isobutyric acid | + | 3.90 × 10–1 | 5.61 × 10–18 | 3.17 × 10–1 | 133 | 6 |
| Butyric acid | NS | 7.36 × 10–1 | 4.18 × 10–59 | NS | 133 | |
| 2-Methyl butyric acid | + | 7.32 × 10–7 | 5.42 × 10–25 | 5.53 × 10–2 | 133 | 6 |
| Isovaleric acid | + | 5.89 × 10–2 | 3.58 × 10–18 | 1.98 × 10–1 | 133 | 6 |
| Valeric acid | NS | 9.93 × 10–1 | 2.69 × 10–82 | NS | 133 | |
| Caproic acid | NS | 9.90 × 10–1 | 5.09 × 10–54 | NS | 139 | |
| Lactic acid | – | 4.05 × 10–11 | 1.13 × 10–31 | 1.21 × 10–2 | 128 | 8 |
| 2-Hydroxy butyric acid | + | 3.12 × 10–4 | 9.64 × 10–2 | 7.02 × 10–1 | 133 | 6 |
| Propionic acid | NS | 6.05 × 10–1 | 2.58 × 10–23 | NS | 133 | |
NS, not significant.
Results
SCFA concentrations vary accordingly to biofluid type, diet, and time of sampling
Diet-by-time interactions
For serum SCFA concentrations, where metabolites were measured at 4 time points throughout the day, the diet-by-time interactions were calculated (Table 3). Acetic acid, isobutyric acid, 2-methylbutyric acid, isovaeric acid, lactic acid, and 2-hydroxybutyric acid showed mostly weak diet-by-time interactions, of which only lactic acid retained significance after removing outliers to obtain normality of distribution and homogeneity of variance. The number of outliers based on the residual plots is provided in Table 3 and consisted mainly of samples from participants ID6 and ID15.
Comparison of serum concentrations of SCFAs between the 2 contrasting diets at different time points over a 24-h period
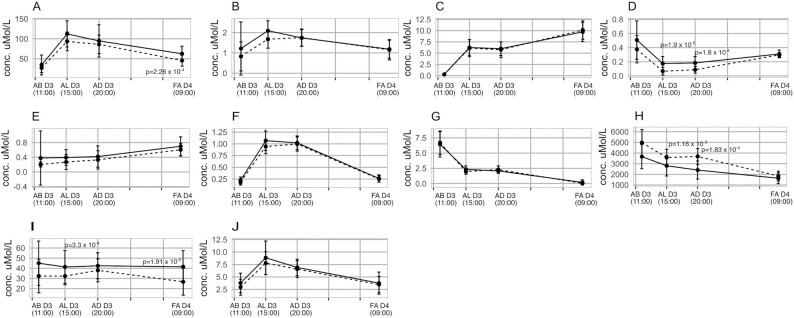
Serum SCFA concentrations were compared across the 4 timed collection periods (AB, AL, and AD on day 3 and an FA sample on day 4 following commencement of the diet). For the SCFAs at time points where a significant concentration difference between the 2 diets was found based on the Wilcoxon rank-sum test (see P values provided in Figure 2), the association between the diet and the SCFA concentration was established via a 2-factor ANOVA. In the AL samples, 2-methylbutyric acid concentrations (coefficient: –0.1; SE: 0.028; P-trend = 4.13 × 10–4) were present in higher concentrations in the healthier diet (Figure 2D, depicted in solid black lines), whereas lactic acid concentrations (coefficient: 750.2; SE: 315.2; P-trend = 0.024) were higher in the unhealthy diet (Figure 2H, depicted by dashed black lines). In the AD samples, 2-methylbutyric acid concentrations (coefficient: –0.1; SE: 0.028; P-trend = 2.28 × 10–3) remained higher in the healthy diet, with lower lactic acid (coefficient: 1219.3; SE: 322.6; P-trend = 8.28 × 10–4) concentrations (Figure 2D and H). The FA serum samples obtained on day 4, after consumption of the healthier diet, contained higher concentrations of acetic acid (coefficient: –17.0; SE: 5.8; P-trend = 0.00615) and 2-hydroxybutyric acid (coefficient: –15.8; SE: 5.11; P-trend = 4.09 × 10–3) (Figure 2A and I). The AUC for each of the SCFAs over the summed 24-h period in response to the 2 contrasting diets is provided in Supplemental Table 10. The results showed that significantly higher concentrations of serum acetic, 2-methylbutyric, and 2-hydroxybutyric acid were present in the total 24-h response to the healthy diet, whereas lactic acid area was higher in the unhealthy diet.
FIGURE 2.
Comparison of serum short-chain fatty acid concentrations through the day for the same time points for the healthy and unhealthy diets. Values are shown as median and IQR; n = 19. The healthy and unhealthy diets are represented by solid and dashed lines, respectively. Differences between the same time points for different diets were determined by Wilcoxon signed-rank test and false discovery rate post hoc correction. Padj < 0.05 indicates statistical significance. (A) Acetic acid, (B) isobutyric acid, (C) butyric acid, (D) 2-methylbutyric acid, (E) isovaleric acid, (F) valeric acid, (G) caproic acid, (H) lactic acid, (I) 2-hydroxybutyric, and (J) propionic acid. AB D3, after breakfast on day 3; AD D3, after dinner on day 3; AL D3, after lunch on day 3; FA D4, fasting on day 4.
Variation of serum SCFAs throughout the day within and across diets
Differences across diets were observed when SCFA concentration patterns were stratified by collection time points (Supplemental Table 11). Most of the SCFAs were significantly different between the AB and AL samples, as well as between the AB and AD samples (Padj < 0.05) with the exception of 2-hydroxybutyric acid and isovaleric acid for both diets and isobutyric acid in the healthy diet.
Significant differences were found between AL and AD for acetic acid (Padj < 0.05), as well as caproic acid (Padj < 0.05) in the healthy diet and 2-hydroxybutyric acid (Padj < 0.05) in the unhealthy diet. For most SCFAs, a significant difference between AD and FA (Padj < 0.05) was evident, except for isobutyric and isovaleric acids in both diets and 2-hydroxybutyric acid in the healthy diet. In the case of AL compared with FA, 2-hydroxybutyric was the only SCFA that was not significantly different at these time points for either diet. In the comparison of samples collected in AB compared with FA, acetic acid (Padj < 0.05), butyric acid (Padj < 0.05), and isovaleric acid (Padj < 0.05) were significantly higher in the FA samples in both diets. In contrast, caproic acid (Padj < 0.05) and lactic acid (Padj < 0.05) were found in higher concentrations in the samples collected AB for both diets. 2-Methylbutyric acid was significantly higher AB compared with post-FA in the healthy diet (Padj < 0.05) but not in the unhealthy diet, whereas valeric acid was significantly lower AB compared with post-FA in the unhealthy diet (Padj < 0.05) but not in the healthy diet. See Supplemental Figures 3–4.
Variability in the response between participants in serum SCFA concentrations
Interindividual variability in the serum profile
As described, the mean serum SCFA concentrations showed different transient variations throughout the day (Figure 3). The variability in serum SCFA concentrations stratified by diet across the 4 time points is shown in the box-and-whiskers plots in Supplemental Figure 5 along with the median concentration values. However, when taken at the individual level, some participants showed a different temporal response in SCFA profiles compared with the median response for a given diet. Examples are provided for acetic, 2-methylburytic, and propionic acids (Figure 3), in which it can be seen, for example, that participant ID3 generally demonstrated a different response to the other participants. For this participant, we observed a dampened response to the unhealthy diet in terms of circulating SCFA concentrations compared with other participants, whereas the response to the healthy diet was heightened in comparison to the other participants, particularly in the samples obtained after lunch.
FIGURE 3.
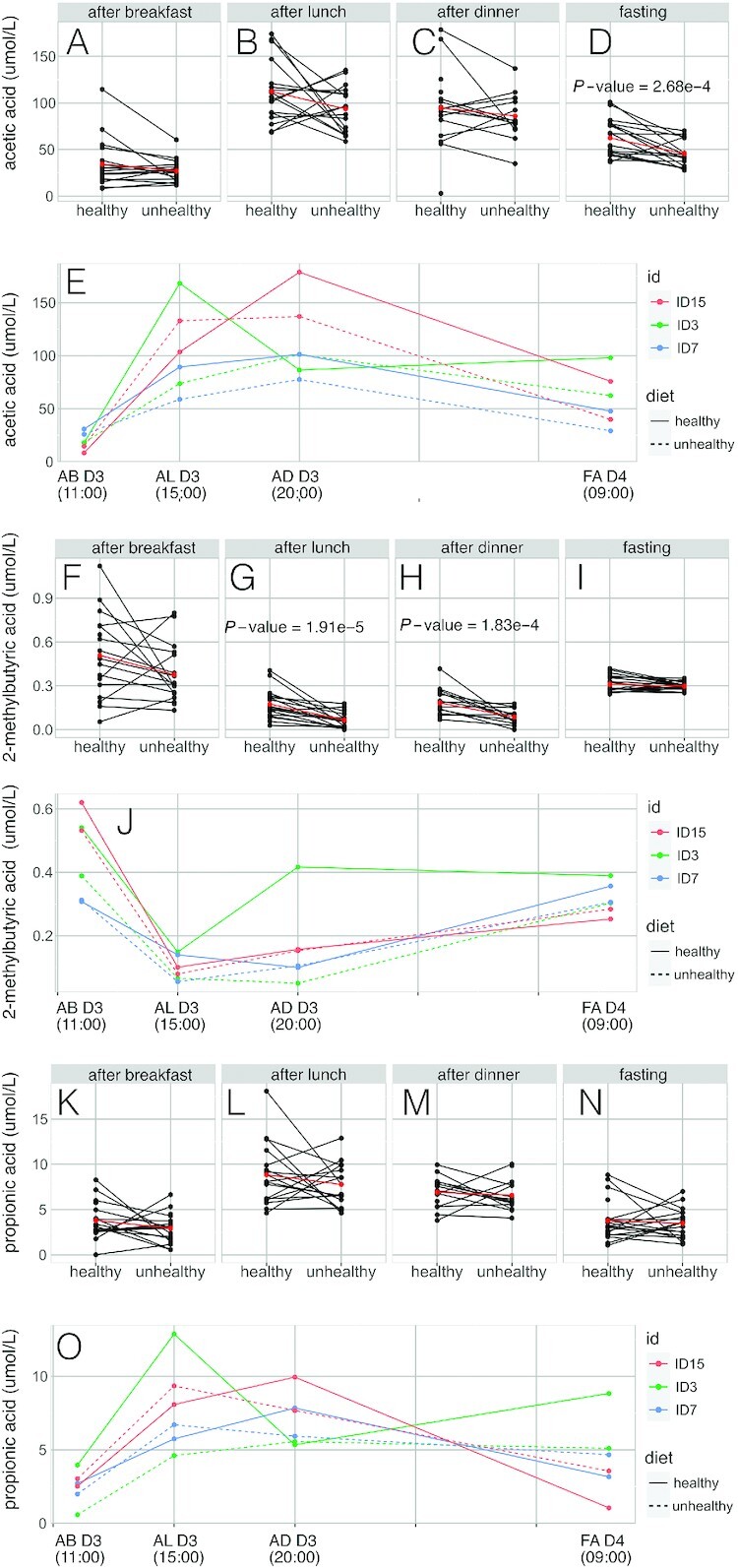
Serum variation response in selected short-chain fatty acids between participants. (A) Interindividual variation in serum acetic acid concentrations after breakfast on day 3. (B) Interindividual variation in serum acetic acid concentrations after lunch on day 3. (C) Interindividual variation in serum acetic acid concentrations after dinner on day 3. (D) Interindividual variation in serum acetic acid concentrations fasting on day 4. (E) Interindividual variation in serum acetic acid concentrations in selected participants (showing 2 “typical” dietary responders and 1 “atypical” dietary responder). (F) Interindividual variation in serum propionic acid concentrations after breakfast on day 3. (G) Interindividual variation in serum propionic acid concentrations after lunch on day 3. (H) Interindividual variation in serum propionic acid concentrations after dinner on day 3. (I) Interindividual variation in serum propionic acid concentrations after dinner on day 3. (J) Interindividual variation in serum propionic acid concentrations in selected participants. (K) Interindividual variation in serum 2-methylbutyric acid concentrations after breakfast on day 3. (L) Interindividual variation in serum 2-methylbutyric acid concentrations after lunch on day 3. (M) Interindividual variation in serum 2-methylbutyric acid concentrations after lunch on day 3. (N) Interindividual variation in serum 2-methylbutyric acid concentrations after dinner on day 3. (O) Interindividual variation in serum 2-methylbutyric acid concentrations in selected participants. AB D3, after breakfast on day 3; AD D3, after dinner on day 3; AL D3, after lunch on day 3; FA D4, fasting on day 4; ID, participant number. In interindividual graphics variation, blue dots represent results of 19 participants and red lines indicate median. Padj < 0.05 indicates statistical significance.
SCFA concentrations in urine
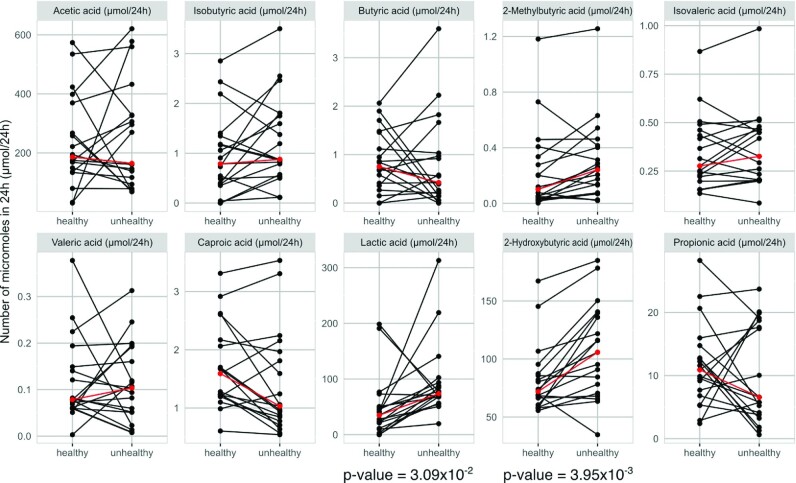
The 24-h urine concentrations for the SCFA panel are provided in Supplemental Table 9. In general, the concentrations of SCFA-related metabolites measured in urine were lower than those in serum, and there was an inverse relationship between serum and urine concentrations for most SCFAs. In most cases, with the notable exception of lactic acid, in which lactic acid was directly associated with the unhealthy diet for both urine and serum, the trend was that for serum, SCFA concentrations were higher for the healthy diet, whereas in urine, the higher concentrations of most SCFAs were observed with the unhealthy diet (Supplemental Figure 6). This trend in diet-related concentration differences was significant for isobutyric (Padj < 0.05), 2-methylbutyric (Padj < 0.05), isovaleric (Padj < 0.05), lactic, and 2-hydroxybutyric (Padj < 0.05) acids (Figure 4B, D, E, H, and I). Urinary acetic acid was also slightly higher in the healthy diet as determined from both NMR and GC data, however this observation was not statistically significant and there was extreme interperson variance with most participants excreting lower concentrations following the unhealthy diet with The correlation matrix for the comparison between the 24-h urine and fasting serum SCFA concentrations (selected for proximity in time of collection) is provided in Supplemental Figure 7 and shows the lack of correlation between urine and plasma concentrations in response to diet for any given SCFA-related metabolite, with the exception of isovaleric acid. However, urinary 2-methylbutyric acid showed inverse associations with several serum SCFAs, including acetic acid, valeric acid, isovaleric acid, caproic acid, propionic acid, and isobutyric acid.
FIGURE 4.
Urine concentrations of short-chain fatty acids (24-h collection) for the healthy and unhealthy diets. Differences between 24-h urine for different diets were determined by Wilcoxon signed-rank test and false discovery rate post hoc correction. Padj < 0.05 indicates statistical significance. Blue dots with lines represent results of 19 participants. Red dots and lines represent median. (A) Acetic acid, (B) propionic acid, (C) isobutyric acid, (D) butyric acid, (E) 2-methylbutyric acid, (F) isovaleric acid, (G) valeric acid, (H) caproic acid, (I) lactic acid, and (J) 2-hydroxybutyric acid.
Discussion
A growing body of research highlights the association of SCFAs with health outcomes, including colon cancer and cardiometabolic diseases. However, within this body of literature, the results are conflicting as to whether SCFAs behave in a beneficial or adverse capacity (33–36). Most published work only reports dietary impact on acetic, propionic, and butyric acids, and the relation between SCFA concentrations in different biofluids and tissues has not been well studied. Here we developed a GC-MS–based method for capturing plasma and urine concentrations of an extended panel of SCFAs and illustrate the difference in SCFA profiles in response to 2 contrasting diets. In addition, more granularity in the stability of circulating SCFA concentrations has been provided by measuring the SCFA profiles throughout the day in relation to timed meals, and thus we were able to take the dynamic postprandial profile into account. Despite the increase of literature on SCFAs, little is known about the temporal changes in SCFA concentrations following dietary change (e.g., from unhealthy to healthy diets or throughout the day). Thus, by characterizing serum SCFA profiles over time in response to a healthy and an unhealthy diet, we were better able to understand their variation between meals and how meals with different amounts of fermentable substrates can influence their concentrations over different periods of time.
Diet-related differences in serum SCFA concentrations
Based on the concentrations of serum SCFA concentrations throughout the day, strong diet-dependent differences were observed in 4 of the 10 measured SCFA-related metabolite panel in serum samples, including acetic acid, 2-methylbutyric acid, lactic acid, and 2-hydroxybutyric acid (Supplemental Table 10). Differences in the AUCs for acetic acid were mainly driven by significant differences between the diets in the fasting samples (54.28 μmol/L in the healthy diet and 42.8 μmol/L in the unhealthy diet), although the healthy diet was associated with higher serum concentrations of acetic acid regardless of time (Figure 2). Fasting serum acetic acid concentrations were significantly higher in the healthy diet compared with the unhealthy diet. In the fed state, acetic acid is likely to be mainly derived from the colonic bacteria, whereas in starvation periods, the liver probably regulates its production, along with ketones bodies, to ensure delivery of suitable oxidizable substrates to tissues such as the brain and myocardium (37). The role of acetic acid on metabolic health is still controversial and widely discussed (38). Previous studies have reported that consumption of fiber in the form of 20 g of pectin/d resulted in increased FA serum acetic acid concentrations (31), whereas consumption of barley kernels that included high amylose or high β-glucans resulted in increased serum concentrations of butyric acid the following morning (23). This evidence, together with other published studies, suggests that the type of dietary substrate plays a role in the SCFA production and metabolism, influencing the production of specific or total SCFAs (39, 40).
The AL and AD serum concentrations of 2-methylbutyric acid were significantly higher in the healthy diet that contained double the concentration of protein (mostly animal based) compared with the unhealthy diet. This metabolite belongs to the group of BSCFAs, together with isobutyric acid and isovaleric acid. Their production indicates protein breakdown in gut lumen by proteolytic fermentation by microbiota (4). BSCFAs are derived from branched amino acids, and 2-methylbutyric acid derives directly from leucine: high serum concentrations have been associated with metabolic syndrome (13–15). In addition, consumption of a high-protein diet together with a low-fiber diet has been associated with the production of 2-methylbutyric acid (41), which could reflect the balance between proteolytic fermentation and saccharolytic fermentation in the colon.
Serum concentrations of 2-hydroxybutyric acid were higher in the healthy diet in samples obtained AL and FA, although there was a high degree of interindividual variation in the serum profiles. The biological significance of this observation is unclear. 2-Hydroxybutyric acid is produced in the liver from 2-ketobutyric acid, which derives from threonine catabolism or methionine metabolism via cystathionine, and is metabolized to propionyl-CoA and carbon dioxide (42). Although high urinary concentrations of 2-hydroxybutyric acid can be indicative of an inborn metabolic error or impaired intestinal absorption of methionine (43, 44), the concentration differences observed in the current study are not consistent with an overt genetic disorder and more likely reflect complex interindividual differences in gene–environment interactions. This metabolite is associated with high oxidative stress levels, which have been shown to contribute to insulin resistance and other comorbidities (17, 45).
In contrast to the other metabolites, lactic acid demonstrated the opposite trend, with higher concentrations encountered after consumption of the unhealthy diet (AUC of 67,665.71 μmol/L in the healthy diet compared with 88,849.06 μmol/L in the unhealthy diet). Lactic acid was also present in higher concentrations in the postprandial samples taken from AL and AD samples following consumption of the unhealthy diet, consistent with literature reports in which “typical” Western diets have been associated with increased blood lactic acid concentrations (46, 47). Similarly, fasting serum lactic acid concentrations have been associated with type 2 diabetes (48). Typical Western diets, such as the one consumed in the current study, are characterized by high levels of sugar and subsequent glycemia (49). Although glucose is generally oxidized to carbon dioxide via the tricarboxylic cycle under aerobic conditions, under anaerobic conditions, lactic acid can be produced via glycolysis (50). In addition to its contrasting dietary response compared with the other metabolites, lactic acid was the only metabolite that showed significant diet-by-time interactions. Diurnal differences in postprandial lactic acid and urea concentrations in pigs suggested decreased efficiency of carbohydrate metabolism in the morning and increased efficiency in postprandial protein metabolism (51). Changes in the systemic concentrations of lactic acid have been shown to be at least partially dependent on the circadian modulation of the microbiome (52). Because the GC-MS method does not distinguish between L-lactic acid, which is endogenous, and D-lactic acid, which is predominantly of microbial origin, it is not possible to ascertain the role of microbial metabolism in the current study but warrants further investigation.
Serum SCFA concentrations fluctuate throughout the day
We demonstrated the concentrations of serum SCFAs were more dependent on time of day and the relation to timing of meals rather than diet. Nevertheless, diet-dependent differences in circulating SCFA profiles were evident for several SCFAs. However, diet-related differences were not observed for any of the metabolites in samples obtained AB. Nutritional studies focused on assessing diet-dependent SCFA concentrations generally measure SCFAs in postprandial conditions immediately after a short-term food challenge or intervention or alternatively measure a paired before-and-after response following a longer time intervention (10, 30). The observations in this study would urge caution in selection of sampling time in relation to a dietary intervention. Another challenge in understanding the behavior of SCFAs is that food in daily life is consumed in mixtures, and unsurprisingly, systemic concentrations of SCFAs derived from a “typical” diet appear to be different compared with SCFAs that are derived from single dietary components or SCFA supplementation (10, 31, 53, 54).
In the current study, clear time-related differences were found in the serum profiles of multiple SCFAs in response to contrasting diets that varied in nutritional profile with differences in fiber and protein content (Table 2 and Supplemental Tables 2 and 3). However, although the fiber contents of the breakfast used in the healthy and unhealthy diets were markedly different (14.9 g and 3.6 g, respectively), no significant difference between the 2 diets was found for any of the SCFAs in the samples collected AB, highlighting the importance of the time of sampling. Fermentation processes are initiated in the colon 6–8 h after food intake (55, 56), and dietary fibers are fermented to different degrees: cellulose and lignins are less fermentable compared with pectin, oligosaccharides, resistant starch, inulin, and β-glucans (57, 58). Therefore, consistent with previous research evaluating SCFA concentrations after dietary fiber supplement or SCFA intake as part of the first meal of the day (30, 54, 58), it is unsurprising that we did not observe dietary differences in SCFAs within 2 h of the first meal of the day because serum SCFA concentrations are likely to have equilibrated after an overnight fast.
The fermentation of nondigestible carbohydrates (NDCs) in the colon takes several hours and is dependent on the chemical physical properties of the NDCs and the composition and/or activity of the host microbiota (57, 59). Thus, the impact of diet on SCFA production can be prolonged, and regular consumption of a high-fiber diet results in continuous stimulation of the colonic microbiota to produce and absorb SCFAs (59). For example, high-fiber diets have been shown to result in higher FA serum acetic acid concentrations compared with a diet poor in fiber (30, 60). The dietary patterns examined in this study incorporated a healthy diet based on WHO recommendations (19), in which diverse types of NDCs were consumed (57), compared with a typical Western diet with high fat and sugar content with low fiber. None of the SCFAs measured peaked in concentration in the AD samples, supporting the fact that the impact of a meal on systemic SCFA concentrations lasts 6 h or more. These responses were similar to those found in previous studies in which SCFA concentrations were quantified after food intake (21, 30). Most of the SCFAs remained stable in the period AL compared with AD, suggesting that postprandial concentrations of SCFAs derived from these meals would not be evident in venous blood after 2 h postconsumption of a food or meal (30).
The difference in the systemic profiles of butyric acid, which manifested peak concentrations in the overnight FA sample compared with acetic and propionic acids, which peaked in the AL sample, was striking. This difference in profiles is consistent with the expectation that the 3 main SCFAs behave somewhat independently, as reflected by the difference in metabolic origin of acetic, propionic, and butyric acids and in line with the fact that these SCFAs have different metabolic functions mediated by different receptors (61–63).
It was notable that although the 2 contrasting diets resulted in differences in the serum concentrations of certain SCFAs, the diets did not influence the overall diurnal profile of the SCFAs such that if highest concentrations of a SCFA were observed in the AB sample in the healthy diet, then peak concentrations also occurred in the AB sample for the unhealthy diet. As expected, the AUCs of SCFA concentrations compared between diets throughout the day showed significant changes in the same metabolites identified as being significantly different at specific time points.
Interindividual variability in serum SCFA profiles
Interindividual differences in the temporal variation of serum SCFA concentrations were evident, consistent with previous studies (31, 32, 53). Suggested origins of this interindividual variability include differences in both genetics (64) and environment/behavior (e.g., food mastication, digestion processes, and colonic transit time) (55, 65–68). Perhaps more important, the gut microbiota, which is unique to each individual and the main source of SCFA synthesis, contributes to metabolic processing of dietary compounds and affects the regulation of physiologic responses at the systemic level (2, 24).
The study has several limitations, and although it clearly shows that the circulating SCFA profile depends on time of day and diet patterns, it must be noted that there is a carryover effect with typical colonic transit times around 6 h (55, 56), and thus serum samples taken 2 h after each meal would also reflect the influence of the previous meal on SCFA production. SCFA concentrations in blood reflect the balance between production and utilization rates, with SCFAs being substrates for fatty acid, glucose, and amino acid metabolism. Our study design does not allow us to determine fluctuations, interconversions, and directionality of these metabolic changes that influence circulating SCFA concentrations but rather captures the gross effect of diet on circulating SCFA concentrations. It is also possible that different diets introduce variation in transit time: for example, glutinous barley has been shown to have a shorter colonic transit time than white rice (69), and a long colonic transit time is associated with high colonic protein catabolism (70). Similarly, although the gut microbiome is the major source of SCFA production, fecal samples were not taken in the current study, and therefore, direct associations between SCFA concentrations and microbial taxonomy or metagenomic data could not be made. Nevertheless, characterization of changes in SCFA concentrations throughout the day after the consumption of 2 extreme diets showed clear variation in profile in relation to time, diet, and individual.
Impact of diet on urine SCFA concentrations
The 24-h urinary excretion of SCFAs also showed a high degree of interindividual variability within diet, even though all participants followed the same dietary intervention under rigorously controlled conditions. Higher concentrations of lactic acid, 2-hydroxybutyric acid, and BSCFAs were found in 24-h urine samples from participants who consumed an unhealthy diet. For all SCFAs, the variability in response to the unhealthy diet was greater than that observed for the healthy diet. As the urinary SCFAs were calculated for the 24-h sample, unlike the full serum profiles, and because urine provides a time-averaged as opposed to a snapshot measure of metabolite concentrations, meaningful comparison between serum and urinary SCFA concentrations was difficult.
Conclusions
This study developed a robust method for measuring a panel of SCFAs and related metabolites and applied it to study their circulating concentrations in relation to 2 contrasting diets under randomized, controlled, and crossover conditions. Although we demonstrated a consistent diet-induced shift resulting in increased circulating SCFA concentrations with the “healthier” diet, in all metabolites except lactic acid, temporal variation in serum SCFA profiles was stronger than dietary influence over the controlled 3-d diet and underscores the need to consider the time of measurement of SCFAs in relation to dietary behavior.
We chose to explore the physiologic SCFA concentrations in a relatively small cohort of healthy participants exposed to 2 contrasting diets rather than to elucidate the detailed relation between specific nutrients or foods and SCFAs because foods are rarely consumed in isolation. Although several texts have outlined the impact of specific foods on SCFA concentrations and provide valuable mechanistic information (23, 54, 58), we extended this research by exploring the impact of 2 contrasting, whole diets on an extended range of SCFAs and related metabolites. This provides a more realistic picture of the relation between diet and SCFAs, because humans are exposed to complex dietary patterns in which foods and nutrients can demonstrate synergistic effects. Despite the rigorously controlled diet and environment, interindividual variations in both absolute concentrations and serum profiles of SCFAs were detected, indicative of differences in gut microbial presence or functionality.
The method used in this study allowed reliable quantification of SCFAs in serum and urine that can be easily applied in different research studies to understand SCFA metabolism. The significant temporal differences in SCFA profiles emphasize the need for standardization of study protocols with respect to timing of sample collection. Further studies are required to explore how the consumption of diverse dietary patterns for a longer period can modulate SCFA production, providing a better understanding of how diets affect SCFA profiles and how their manipulation may be used to deliver personalized nutrition to aid prevention and management of chronic diseases.
Supplementary Material
Acknowledgements
The authors’ contributions were as follows—IG-P, EH, and GF: conceptualized the study; IG-P, JKN, JMP, GF, and EH: supervised the study; JB, SF, IG-P, EH, and GF: wrote the manuscript; JB, SF, and IG-P: conducted the spectroscopic analysis; JMP, JW, JB, and SF: statistically analyzed the data; IG-P and GF: ran the clinical trial; IG-P, ESC, EH, and GF: designed the clinical trial; EH: assumes responsibility for the completeness and accuracy of the data and analyses; GF: assumes responsibility for adherence to the study protocol; and all authors: read and approved the final manuscript and approved the final submitted version. GF is lead for the Imperial Nestlé Collaboration and reports personal fees from Unilever, both outside the submitted work. GF, EH and IGP are directors of Melico Ltd also outside the scope of the submitted work.
Notes
Supported by the UK National Institute for Health Research (NIHR) and Medical Research Council (MRC). The views expressed are those of the authors and not necessarily those of the UK National Health Service (NHS), the NIHR, or the UK Department of Health. IG-P is supported by a NIHR Career Development Research Fellowship (NIHR-CDF-2017-10-032). JB is supported by the Chilean National Agency for Research and Development (ANID). JMP is supported by a Rutherford Fund Fellowship at Health Data Research (HDR) UK (MR/S004033/1). GF is supported by an NIHR Senior Investigator award. EH and GF are supported by an MRC grant entitled “Metabolomics for Monitoring Dietary Exposure” (MR/J010308/1). EH received support from the Western Australian Government via provision of the Premier's Science Fellowship and from the Australian Research Council Laureate Fellowship. This study was supported by the NIHR/Wellcome Trust Imperial Clinical Research Facility, and infrastructure support was provided by the NIHR Imperial Biomedical Research Centre (BRC) in line with the Gut Health research theme. The Section of Nutrition Research is funded by grants from the MRC, UK Biotechnology and Biological Sciences Research Council, NIHR, and an Integrative Mammalian Biology (IMB) Capacity Building Award.
JB and SF contributed equally to this work.
IG-P and EH contributed equally to this work.
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–7, Supplemental Tables 1–11, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AB, after breakfast; AD, after dinner; AL, after lunch; BSCFA, branched-chain fatty acid; FA, fasting; IS, internal standard; NDC, nondigestible carbohydrate.
Contributor Information
Jerusa Brignardello, Section for Nutrition Research, Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom.
Sofia Fountana, Department of Surgery & Cancer, Imperial College London, London, United Kingdom.
Joram Matthias Posma, Section of Bioinformatics, Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom.
Edward S Chambers, Section for Nutrition Research, Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom.
Jeremy K Nicholson, Australian National Phenome Centre, Health Futures Institute, Murdoch University, Harry Perkins Institute of Medical Research, Perth, Western Australia, Australia; Institute of Global Health Innovation, Imperial College London, London, United Kingdom.
Julien Wist, Chemistry Department, Universidad del Valle, Cali, Colombia; Centre for Computational and Systems Medicine, Health Futures Institute, Murdoch University, Harry Perkins Institute of Medical Research, Perth, Western Australia, Australia.
Gary Frost, Section for Nutrition Research, Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom.
Isabel Garcia-Perez, Section for Nutrition Research, Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom.
Elaine Holmes, Section for Nutrition Research, Division of Digestive Diseases, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom; Australian National Phenome Centre, Health Futures Institute, Murdoch University, Harry Perkins Institute of Medical Research, Perth, Western Australia, Australia; Centre for Computational and Systems Medicine, Health Futures Institute, Murdoch University, Harry Perkins Institute of Medical Research, Perth, Western Australia, Australia.
Data availability
Data described in the manuscript will be made available upon request.
References
- 1. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele Iet al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–89. [DOI] [PubMed] [Google Scholar]
- 3. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60. [DOI] [PubMed] [Google Scholar]
- 4. Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Yet al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68(1):95–107. [DOI] [PubMed] [Google Scholar]
- 5. Abrantes HD, Briquet M, Schmuziger C, Restivo G, Puyal J, Rosenberg Net al. The lactate receptor HCAR1 modulates neuronal network activity through the activation of G(alpha) and G(beta gamma) subunits. J Neurosci. 2019;39(23):4422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody Let al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci Rep. 2016;6:37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24(1):151–7. [DOI] [PubMed] [Google Scholar]
- 9. Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWMet al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEet al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–73. [DOI] [PubMed] [Google Scholar]
- 12. Gao F, Lv YW, Long J, Chen JM, He JM, Ruan XZet al. Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Front Pharmacol. 2019;10:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heimann E, Nyman M, Palbrink AK, Lindkvis-Petersson K, Degermann E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte. 2016; 5(4):359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barengolts E, Green SJ, Chlipala GE, Eisenberg Y, Privadarshini M, Dugas LR. Predictors of obesity among gut microbiota biomarkers in African American men with and without diabetes. Microorgaisms. 2019;7(9):1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanna S, Vosa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting Met al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gall WE, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra Set al. Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. Alpha-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care. 2016;39(6):988–95. [DOI] [PubMed] [Google Scholar]
- 19. WHO . Global strategy on diet, physical activity and health. Geneva (Switzerland): World Health Organization; 2004. [Google Scholar]
- 20. Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard Het al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5(3):P184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolever TM, Josse RG, Leiter LA, Chiasson JL. Time of day and glucose tolerance status affect serum short-chain fatty acid concentrations in humans. Metabolism. 1997;46(7):805–11. [DOI] [PubMed] [Google Scholar]
- 22. Gill PA, van Zelm MC, Ffrench RA, Muir JG, Gibson PR. Successful elevation of circulating acetate and propionate by dietary modulation does not alter T-regulatory cell or cytokine profiles in healthy humans: a pilot study. Eur J Nutr. 2020;59(6):2651–61. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson AC, Ostman EM, Knudsen KE, Holst JJ, Bjorck IM. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr. 2010;140(11):1932–6. [DOI] [PubMed] [Google Scholar]
- 24. Byrne CS, Preston T, Brignardello J, Garcia-Perez I, Holmes E, Frost GSet al. The effect of L-rhamnose on intestinal transit time, short chain fatty acids and appetite regulation: a pilot human study using combined. J Breath Res. 2018;12(4):046006. [DOI] [PubMed] [Google Scholar]
- 25. Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Yet al. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host.”. Pharmacol Res. 2013;69(1):114–26. [DOI] [PubMed] [Google Scholar]
- 26. Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020;111(3):545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed Ket al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han S, Gao H, Song R, Zhang W, Li Y, Zhang J. Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short Chain Fatty Acids. J Agric Food Chem. 2021;69(51):15624–15635. [DOI] [PubMed] [Google Scholar]
- 29. Moreau NM, Goupry SM, Antignac JP, Monteau FJ, Le Bizec BJ, Champ MMet al. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J Chromatogr B. 2003;784(2):395–403. [DOI] [PubMed] [Google Scholar]
- 30. Heinzmann SS, Brown IJ, Chan Q, Bictash M, Dumas ME, Kochhar Set al. Metabolic profiling strategy for discovery of nutritional biomarkers: proline betaine as a marker of citrus consumption. Am J Clin Nutr. 2010;92(2):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest. 1985;75(5):1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters SG, Pomare EW, Fisher CA. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33(9):1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 34. Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 36. de la Cuesta-Zuluaga J, Mueller NT, Alvarez-Quintero R, Velasquez-Mejia EP, Sierra JA, Corrales-Agudelo Vet al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weng J, Wu H, Xu Z, Xi H, Chen C, Chen Det al. The role of propionic acid at diagnosis predicts mortality in patients with septic shock. J Crit Care. 2018;43:95–101. [DOI] [PubMed] [Google Scholar]
- 38. Scheppach W, Pomare EW, Elia M, Cummings JH. The contribution of the large intestine to blood acetate in man. Clin Sci. 1991;80(2):177–82. [DOI] [PubMed] [Google Scholar]
- 39. Canfora EE, Blaak EE. Acetate: a diet-derived key metabolite in energy metabolism: good or bad in context of obesity and glucose homeostasis?. Curr Opin Clin Nutr Metab Care. 2017;20(6):477–83. [DOI] [PubMed] [Google Scholar]
- 40. Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter Vet al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. 2015;39(9):1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton Ket al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landaas S. The formation of 2-hydroxybutyric acid in experimental animals. Clin Chim Acta. 1975;58(1):23–32. [DOI] [PubMed] [Google Scholar]
- 44. Smith AJ, Strang LB. An inborn error of metabolism with the urinary excretion of alpha-hydroxy-butyric acid and phenylpyruvic acid. Arch Dis Child. 1958;33(168):109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hooft C, Timmermans J, Snoeck J, Antener I, Oyaert W, Van den Hende C. Methionine malabsorption syndrome. Ann Paediatr. 1965;205(1):73–104. [PubMed] [Google Scholar]
- 46. Varvel SA, Pottala JV, Thiselton DL, Caffrey R, Dall T, Sasinowski Met al. Serum alpha-hydroxybutyrate (alpha-HB) predicts elevated 1 h glucose levels and early-phase beta-cell dysfunction during OGTT. BMJ Open Diabetes Res Care. 2014;2(1):e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams RS, Kozan P, Samocha-Bonet D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie. 2016;124:171–7. [DOI] [PubMed] [Google Scholar]
- 48. Frayn KN, Coppack SW, Humphreys SM, Clark ML, Evans RD. Periprandial regulation of lipid metabolism in insulin-treated diabetes mellitus. Metabolism. 1993;42(4):504–10. [DOI] [PubMed] [Google Scholar]
- 49. Juraschek SP, Shantha GP, Chu AY, Miller ER, Guallar E, Hoogeveen RCet al. Lactate and risk of incident diabetes in a case-cohort of the Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2013;8(1):e55113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martinez KB, Leone V, Chang EB. Western diets, gut dysbiosis, and metabolic diseases: are they linked?. Gut Microbes. 2017;8(2):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu Wet al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koopmans SJ, van der Meulen J, Dekker R, Corbijn H, Mroz Z. Diurnal rhythms in plasma cortisol, insulin, glucose, lactate and urea in pigs fed identical meals at 12-hourly intervals. Physiol Behav. 2005;84(3):497–503. [DOI] [PubMed] [Google Scholar]
- 53. Montagner A, Korecka A, Polizzi A, Lippi Y, Blum Y, Canlet Cet al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci Rep. 2016;6:20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28(6):657–61. [DOI] [PubMed] [Google Scholar]
- 55. Rahat-Rozenbloom S, Fernandes J, Cheng J, Wolever TMS. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur J Clin Nutr. 2017;71(8):953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cummings JH, Englyst HN. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45(5):1243–55. [DOI] [PubMed] [Google Scholar]
- 58. Stephen AM, Champ MM, Cloran SJ, Fleith M, van Lieshout L, Mejborn Het al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30(2):149–90. [DOI] [PubMed] [Google Scholar]
- 59. Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JAet al. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 2015;7(11):8916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jenkins DJ, Thorne MJ, Camelon K, Jenkins A, Rao AV, Taylor RHet al. Effect of processing on digestibility and the blood glucose response: a study of lentils. Am J Clin Nutr. 1982;36(6):1093–101. [DOI] [PubMed] [Google Scholar]
- 61. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels Det al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. [DOI] [PubMed] [Google Scholar]
- 63. Le Poul E, Loison C, Struyf S, Springael J-Y, Lannoy V, Decobecq M-Eet al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9. [DOI] [PubMed] [Google Scholar]
- 64. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou T, Wang M, Ma H, Li X, Heianza Y, Qi L. Dietary fiber, genetic variations of gut microbiota-derived short-chain fatty acids, and bone health in UK biobank. J Clin Endocrinol Metab. 2021;106(1):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Snow P, O'Dea K. Factors affecting the rate of hydrolysis of starch in food. Am J Clin Nutr. 1981;34(12):2721–7. [DOI] [PubMed] [Google Scholar]
- 67. Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8(3):117–29. [DOI] [PubMed] [Google Scholar]
- 68. Suzuki H, Fukushima M, Okamoto S, Takahashi O, Shimbo T, Kurose Tet al. Effects of thorough mastication on postprandial plasma glucose concentrations in nonobese Japanese subjects. Metabolism. 2005;54(12):1593–9. [DOI] [PubMed] [Google Scholar]
- 69. Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6(8):1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim JY, Son BK, Lee SS. Effects of adlay, buckwheat, and barley on transit time and the antioxidative system in obesity induced rats. Nutr Res Pract. 2012;6(3):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request.