Abstract
Background
Inappropriate antibiotic prescribing is common in primary care (PC), particularly for respiratory tract diagnoses (RTDs). However, the optimal approach for improving prescribing remains unknown.
Methods
We conducted a stepped-wedge study in PC practices within a health system to assess the impact of a provider-targeted intervention on antibiotic prescribing for RTDs. RTDs were grouped into tiers based on appropriateness of antibiotic prescribing: tier 1 (almost always indicated), tier 2 (may be indicated), and tier 3 (rarely indicated). Providers received education on appropriate RTD prescribing followed by monthly peer comparison feedback on antibiotic prescribing for (1) all tiers and (2) tier 3 RTDs. A χ 2 test was used to compare the proportion of visits with antibiotic prescriptions before and during the intervention. Mixed-effects multivariable logistic regression analysis was performed to assess the association between the intervention and antibiotic prescribing.
Results
Across 30 PC practices and 185 755 total visits, overall antibiotic prescribing was reduced with the intervention, from 35.2% to 23.0% of visits (P < .001). In multivariable analysis, the intervention was associated with a reduced odds of antibiotic prescription for tiers 2 (odds ratio [OR] 0.57; 95% confidence interval [CI] .52–.62) and 3 (OR 0.57; 95% CI .53–.61) but not for tier 1 (OR 0.98; 95% CI .83–1.16).
Conclusions
A provider-focused intervention reduced overall antibiotic prescribing for RTDs without affecting prescribing for infections that likely require antibiotics. Future research should examine the sustainability of such interventions, potential unintended adverse effects on patient health or satisfaction, and provider perceptions and acceptability.
Keywords: antibiotic stewardship, antimicrobial stewardship, respiratory tract infections, primary care, antibiotic prescribing
A provider-targeted education- and peer comparison feedback-based intervention focused on antibiotic prescribing for respiratory tract infections was implemented in primary care practices. Across 30 practices, antibiotic prescribing was reduced from 35.2% to 23.0% of visits (P < .001).
Inappropriate antibiotic prescribing is common in primary care (PC) practices [1, 2]. In particular, antibiotic prescribing for respiratory tract diagnoses (RTDs), including respiratory tract infections (RTIs) and other noninfectious respiratory syndromes, is common and frequently unwarranted [2, 3]. Inappropriate prescription of antibiotics, especially broad-spectrum antibiotics, can have adverse effects, including medication side effects, Clostridioides difficile infection, and multidrug-resistant organism colonization or infection [4–6]. Additionally, over half of antibiotic expenditures occur in the outpatient setting, often for RTIs, and antibiotic stewardship programs are uncommon in ambulatory practices [2, 7, 8].
Antibiotic stewardship interventions for RTIs have previously demonstrated reductions in antibiotic prescribing in PC with variable levels of effectiveness, including those utilizing combinations of provider education, communications training, and feedback [9–17]. However, these studies have primarily focused on prescribing for a limited group of RTI diagnoses and have not examined the impact of interventions in a broader group of RTDs, including noninfectious respiratory diseases and non-specific respiratory symptoms. Conversely, the potential for an adverse impact on RTIs for which an antibiotic is typically indicated has also not been previously assessed. Here we present the results of a study conducted to assess the impact of a provider focused education- and feedback-based intervention on antibiotic prescribing for RTDs in PC. In this study, the largest in the United States to examine adult RTIs at the visit level, we also demonstrate the use of 2 easily generated, novel metrics, previously shown to be associated with inappropriate antibiotic prescribing, in our provider peer comparison feedback [18].
METHODS
Study Design and Setting
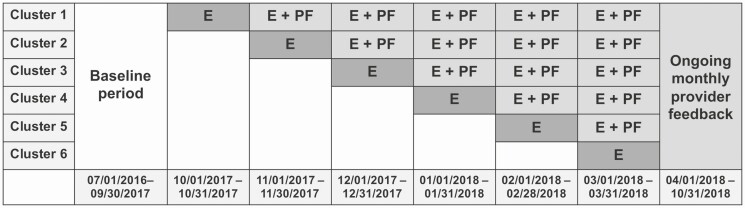
A stepped-wedge cluster randomized study was performed to assess the impact of an education- and feedback-based intervention to improve prescribing for RTDs. This study was conducted in 31 PC practices in the University of Pennsylvania Healthcare System (UPHS) located throughout the greater Philadelphia region, encompassing a variety of practice types (teaching/non-teaching, family/internal medicine) and locations (urban/non-urban). PC clinics were randomly assigned to one of 6 clusters. The study intervention was implemented in each cluster at monthly intervals, with one cluster per interval (Figure 1). Following a baseline data collection period from 1 July 2016 to 30 September 2017, the intervention was implemented in the first cluster starting 1 October 2017. The last cluster completed crossover into the intervention by 31 March 2018, and the intervention continued through 31 October 2018. This study was approved by the Institutional Review Board at the University of Pennsylvania.
Figure 1.
Stepped-wedge interventional study design. Abbreviations: E, educational session; PF, provider feedback.
Intervention
The intervention consisted of 2 components: (1) an initial one-time educational session on appropriate prescribing for RTIs and patient communication strategies, and (2) monthly electronic feedback to providers on their performance regarding antibiotic prescribing for RTDs. The educational sessions focused on evidence-based recommendations for common RTIs, as well as methods for effective communication with patients, particularly when not prescribing an antibiotic; further details are provided in the Supplementary materials. Monthly feedback reports were then sent via email and automatically generated using two previously developed metrics [18]. To generate these metrics for each provider, all RTD International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes were grouped into 3 tiers, adapted from previous ICD-9-CM codes classifications: tier 1, diagnoses for which an antibiotic is almost always indicated (eg, bacterial pneumonia); tier 2, diagnoses for which an antibiotic may be indicated (eg, sinusitis); and tier 3, diagnoses for which an antibiotic is rarely indicated (eg, acute bronchitis) (Supplementary Table 1) [2]. In the reports, providers received individual and peer comparison feedback on the percentage of their visits with an antibiotic prescription for all RTD visits (metric 1) as well as for a subset of visits with only tier 3 diagnoses (metric 2), detailed in the Supplementary materials (Supplementary Figure 1). These metrics were selected based on a prior study demonstrating that they were the administrative data metrics most strongly associated with inappropriate antibiotic prescribing and because they are easily extractable from the electronic health record (EHR) for a large population [18]. Additionally, using both metrics enabled detection of shifting of diagnostic codes between tiers hypothesized to occur in response to feedback.
Outcome Assessment and Definitions
Visits for patients at least 18 years of age that occurred between 1 July 2016 and 31 October 2018 and included 1 or more ICD-10-CM codes for an RTD were included (Supplementary Table 1). Only visits conducted by an attending physician or advanced practice provider (APP) were included; medical resident and fellow trainee visits were excluded, as they did not participate in the intervention. Providers without at least one patient visit both before and during the intervention were excluded from the analysis. Visits from the month during which the educational session occurred at each practice were excluded, given that the exact date varied across practices within each cluster.
The primary outcome was presence of an antibiotic prescription at each in-person visit. The analysis was performed at the visit level, allowing patients to contribute more than 1 visit during the study time period. Only oral antibacterial agents that would plausibly be prescribed for an RTI were considered (eg, nitrofurantoin excluded). As in the provider feedback reports to group visits into tiers, we adapted a prior classification of ICD-9-CM codes for ICD-10-CM codes (Supplementary Table 1) [2]. For those with codes falling into multiple tiers, the lowest number tier was utilized. ICD-10-CM codes were also used to classify diagnoses into disease groups: sinusitis, bronchitis, pharyngitis, pneumonia, otitis media, pertussis, and other [2]. One visit could be classified into more than 1 disease group, and some disease groups could overlap tiers (eg, bacterial vs viral pneumonia).
Data Collection
Demographic and antibiotic prescription data were obtained from the database that captures all data from the EHR, including all antibiotic prescriptions. ICD-10-CM codes from the index encounter and encounters 6 months prior to the index visit were collected and used to calculate the Charlson comorbidity index (CCI) score for each patient [19]. Data from the pre-intervention period were retrospectively collected. Provider- and practice-specific data were also collected.
Statistical Analysis
At the visit level, patient-specific variables were compared in the pre- and post-intervention periods using χ 2 or Wilcoxon rank-sum testing as appropriate. For the primary analysis, a multivariable mixed effects logistic regression model was performed at the visit level, with the intervention as the primary binary exposure of interest. A mixed effects model with nested clustering on patient and provider was used to account for the inclusion of multiple visits over time for some patients and all providers (see Supplementary materials for additional details). A similar secondary analysis was performed excluding patients with chronic obstructive pulmonary disease (COPD) (emphysema or chronic bronchitis by ICD-10-CM code), given the potential for this diagnosis to affect prescribing. The proportion of antibiotic prescribing pre- and post-intervention was compared overall and for specific diseases using a χ2 test. For all calculations, a 2-tailed P value of .05 was considered statistically significant. All calculations were performed using STATA v14.2 (Stata Corp, College Station, Texas USA).
RESULTS
The intervention was implemented in 31 practices, although one practice was excluded from the analysis due to dissolution of the practice. Thirty PC practices were included in the final analysis, with 183 unique providers, of which 167 (91.3%) participated in the educational session. The 16 nonparticipating providers were distributed across 9 practices and 4 clusters. A total of 185 755 unique office visits were included, with 127 324 (68.5%) from the pre-intervention period and 58 431 (31.5%) from the intervention period. These visits represented 113 620 unique patients, with a median number of visits per patient of 1 (interquartile range [IQR] 1–2). Patient and visit characteristics are provided in Table 1, and provider and practice characteristics are described in Table 2. Small increases in amoxicillin-clavulanate, amoxicillin, and doxycycline were seen in the intervention period, with a decrease in azithromycin (Table 3).
Table 1.
Patient Demographics, Tiers, and Diseases Seen in Primary Care Practices During the Study Time Period, as Assessed by Unique Visit
| Pre-intervention (n = 127 324) | Intervention (n = 58 431) | P Value | |
|---|---|---|---|
| Gender (%) | |||
| Male | 44 194 (34.7) | 20 547 (35.2) | .056 |
| Female | 83 130 (65.3) | 37 884 (64.8) | |
| Median age (IQR) | 54 (39–66) | 57 (41–68) | <.001 |
| Race (%) | |||
| White | 87 710 (68.9) | 39 638 (67.8) | <.001 |
| Black or African American | 28 443 (22.3) | 13 338 (22.8) | |
| Asian | 3308 (2.6) | 1482 (2.5) | |
| Native Hawaiian or other Pacific Islander | 242 (0.2) | 111 (0.2) | |
| American Indian or Native Alaskan | 70 (0.1) | 39 (0.1) | |
| Other | 3543 (2.8) | 1614 (2.8) | |
| Unknown | 4008 (3.2) | 2209 (3.8) | |
| Median Charlson comorbidity index (IQR) | 0 (0 – 1) | 0 (0 – 1) | <.001 |
| Lowest visit diagnosis tier (%) | |||
| 1 | 2875 (2.3) | 1526 (2.6) | <.001 |
| 2 | 33 700 (26.5) | 12 385 (21.2) | |
| 3 | 90 749 (71.3) | 44 520 (76.2) | |
| Visit disease groupsa (%) | |||
| Bronchitis (acute/unspecified) | 9088 (7.1) | 3092 (5.3) | <.001 |
| Sinusitis | 23 381 (18.4) | 7500 (12.8) | <.001 |
| Pharyngitis | 8903 (7.0) | 3849 (6.6) | .001 |
| Pneumonia | 2730 (2.1) | 1432 (2.5) | <.001 |
| Otitis media | 6690 (5.3) | 3172 (5.4) | .12 |
| Pertussis | 22 (0.02) | 20 (0.03) | .024 |
| Other | 91 772 (72.1) | 45 256 (77.5) | <.001 |
Abbreviation: IQR, interquartile range.
aOne visit could be classified into more than 1 disease group.
Table 2.
Provider and Practice Characteristics, as Assessed by Unique Visit
| All Visits (n = 185 755) | |
|---|---|
| Provider gender (%) | |
| Male | 72 752 (39.2) |
| Female | 113 003 (60.8) |
| Provider specialty (%) | |
| Internal medicine | 88 268 (47.5) |
| Family medicine | 97 487 (52.5) |
| Teaching practice (%) | |
| Teaching | 25 577 (13.8) |
| Nonteaching | 160 178 (86.2) |
| Urban practice (%) | |
| Urban | 68 317 (36.8) |
| Nonurban | 117 438 (63.2) |
| Provider type (%) | |
| MD/DO | 132 619 (71.4) |
| NP/PA | 53 136 (28.6) |
| Median years since certification (IQR) | 13 (6–25) |
Abbreviations: DO, doctor of osteopathic medicine; IQR, interquartile range; MD, doctor of medicine; NP, nurse practitioner; PA, physician assistant.
Table 3.
Most Commonly Prescribed Antibiotics During the Pre-intervention and Intervention Periods
| Antibiotic | Pre-intervention (n = 46 110)a | Intervention (n = 13 976)a |
|---|---|---|
| Azithromycin (%) | 17 062 (37.0) | 4445 (31.8) |
| Amoxicillin-clavulanate (%) | 12 024 (26.1) | 4163 (29.8) |
| Amoxicillin (%) | 4112 (8.9) | 1342 (9.6) |
| Doxycycline (%) | 3541 (7.7) | 1346 (9.6) |
| Levofloxacin (%) | 3420 (7.4) | 1023 (7.3) |
| Other (%) | 5951 (12.9) | 1657 (11.9) |
an = total number of antibiotics prescribed.
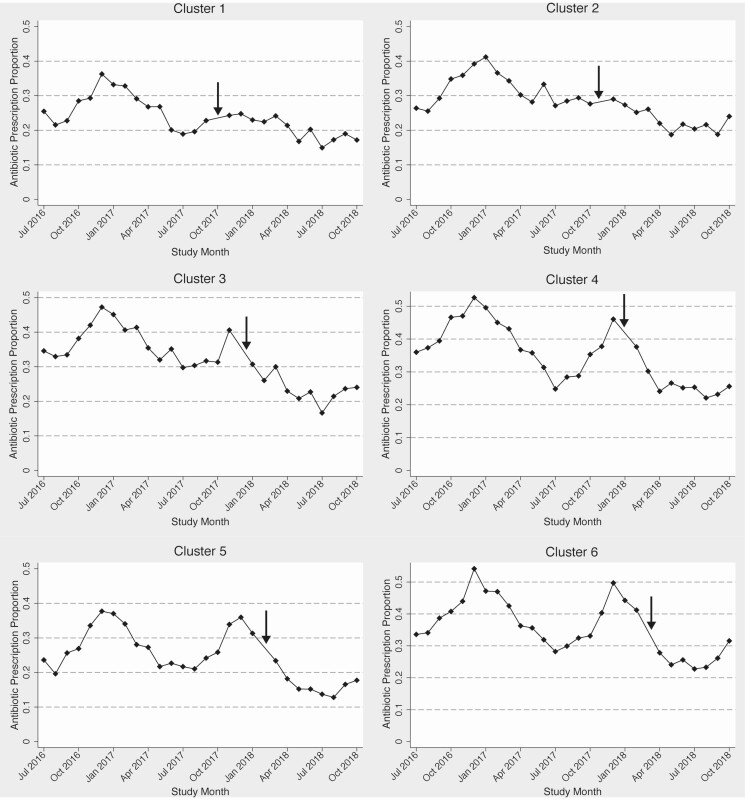
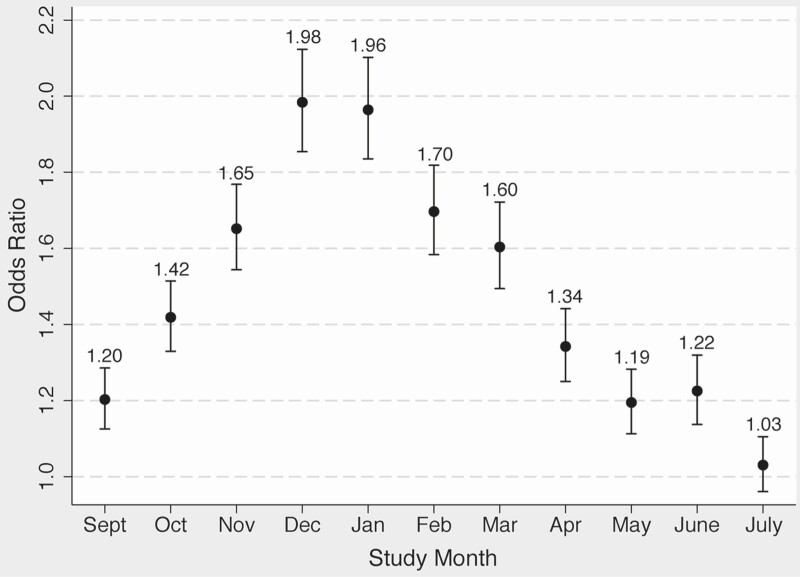
The proportion of visits with an antibiotic prescription decreased from the pre-intervention period to the intervention period, from 35.2% to 23.0% (P < .001), displayed in Figure 2 monthly by cluster and in Supplementary Table 2 by individual practice. In the unadjusted analysis, the intervention was associated with a decreased odds of antibiotic prescription, odds ratio (OR) 0.47 (95% confidence interval [CI] .45–.48) (Table 4). In the multivariable analysis, there was a significant interaction between the intervention and diagnosis tier. Rather than construct separate models for each tier, one model including a tier-intervention interaction term was created, given the sufficient sample size and desire to compare antibiotic prescribing across tiers. Due to inclusion of this interaction term in the final model, the ORs for the intervention are presented separately for each tier, and the ORs for the tiers are presented by pre-intervention and intervention periods. For tiers 2 and 3, the intervention remained significantly associated with decreased odds of antibiotic prescription, although in tier 1, the association was not significant (Table 5). The seasonal variation in antibiotic prescribing is shown in Figure 3. A secondary analysis excluding patients with a concomitant diagnosis of COPD revealed similar results (data not shown). Decreases in proportional antibiotic prescribing was seen for several disease groups (bronchitis, sinusitis, pharyngitis, pneumonia, and other categories), as well as for all tiers, most notably tiers 2 and 3 (Table 6).
Figure 2.
Monthly proportion of visits with antibiotic prescription by cluster. Arrow denotes month of intervention for each cluster.
Table 4.
Univariate Analysis to Assess for Characteristics Associated With Antibiotic Prescribing
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Intervention | 0.47 (.45–.48) | <.001 |
| Tier (reference = 1) | ||
| 2 | 3.40 (3.14–3.69) | <.001 |
| 3 | 0.20 (.18–.21) | <.001 |
| Nonurban practice location (reference = urban) | 4.49 (3.45–5.84) | <.001 |
| Family medicine practice (reference = internal medicine) | 3.04 (2.24–4.12) | <.001 |
| Provider NP/PA degree (reference = MD/DO)a | 2.39 (1.63–3.50) | <.001 |
| Nonteaching practice (reference = teaching) | 4.64 (3.47–6.19) | <.001 |
| Years since board certification (reference = 0–6) | ||
| 7–13 | 0.57 (.53–.62) | <.001 |
| 14–25 | 0.34 (.30–.39) | <.001 |
| ≥26 | 0.28 (.23–.35) | <.001 |
| Provider female gender (reference = male) | 1.48 (1.05–2.09) | .026 |
| Patient age (reference = 18–40) | ||
| 41–55 | 0.95 (.91–.98) | .003 |
| 56–67 | 0.82 (.79–.85) | <.001 |
| ≥68 | 0.58 (.55–.60) | <.001 |
| Patient female gender (reference = male) | 1.15 (1.12–1.19) | <.001 |
| Patient race (reference = white) | ||
| Black or African American | 0.68 (.65–.71) | <.001 |
| Asian | 0.73 (.67–.80) | <.001 |
| Native Hawaiian or other Pacific Islander | 1.07 (.79–1.44) | .679 |
| American Indian or Native Alaskan | 1.20 (.70–2.07) | .505 |
| Other | 0.94 (.87–1.02) | .165 |
| Unknown | 0.81 (.75–.87) | <.001 |
| Charlson comorbidity index (reference = 0) | 0.50 (.49–.51) | <.001 |
| Month (reference = August) | ||
| September | 1.20 (1.13–1.28) | <.001 |
| October | 1.33 (1.25–1.41) | <.001 |
| November | 2.06 (1.93–2.19) | <.001 |
| December | 2.56 (2.40–2.72) | <.001 |
| January | 2.02 (1.91–2.15) | <.001 |
| February | 1.68 (1.58–1.79) | <.001 |
| March | 1.54 (1.45–1.64) | <.001 |
| April | 1.14 (1.07–1.21) | <.001 |
| May | 0.99 (.93–1.05) | .684 |
| June | 1.04 (.97–1.11) | .285 |
| July | 1.03 (.96–1.10) | .390 |
| Year (reference = 2016) | ||
| 2017 | 0.86 (.83–.88) | <.001 |
| 2018 | 0.46 (.45–.48) | <.001 |
Abbreviations: CI, confidence interval; DO, doctor of osteopathic medicine; MD, doctor of medicine; NP, nurse practitioner; PA, physician assistant.
Table 5.
Multivariable Analysis to Assess for Characteristics Associated With Antibiotic Prescribing
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| McKelvey and Zavoina Pseudo-R2 = 0.504 | ||
| Interventiona | ||
| Tier 1 | 0.98 (0.83–1.16) | .812 |
| Tier 2 | 0.57 (.52–.62) | <.001 |
| Tier 3 | 0.57 (.53–.61) | <.001 |
| Tier without interventiona (reference = tier 1) | ||
| 2 | 3.75 (3.40–4.14) | <.001 |
| 3 | 0.23 (.21–.25) | <.001 |
| Tier with interventiona (reference = tier 1) | ||
| 2 | 2.16 (1.89–2.47) | <.001 |
| 3 | 0.13 (.12–.15) | <.001 |
| Non-urban practice location (reference = urban) | 1.49 (1.12–1.97) | .006 |
| Family medicine practice (reference = internal medicine) | 1.81 (1.46–2.24) | <.001 |
| Provider NP/PA degree (reference = MD/DO) | 1.31 (1.02–1.68) | .034 |
| Non-teaching practice (reference = teaching) | 2.16 (1.61–2.90) | <.001 |
| Years since board certification (reference = 0–6) | ||
| 7–13 | 0.94 (.86–1.02) | .146 |
| 14–25 | 0.88 (.77–1.01) | .074 |
| ≥26 | 0.99 (.81–1.20) | .886 |
| Patient age (reference = 18–40) | ||
| 41–55 | 1.17 (1.12–1.22) | <.001 |
| 56–67 | 1.20 (1.15–1.25) | <.001 |
| ≥68 | 1.02 (.98–1.07) | .399 |
| Patient female gender (reference = male) | 1.04 (1.01–1.07) | .008 |
| Patient race (reference = Caucasian) | ||
| Black or African American | 0.82 (.78–.86) | <.001 |
| Asian | 0.81 (.74–.89) | <.001 |
| Native Hawaiian or other Pacific Islander | 1.06 (.77–1.45) | .731 |
| American Indian or Native Alaskan | 1.56 (.90–2.72) | .115 |
| Other | 0.96 (.88–1.04) | .298 |
| Unknown | 0.84 (.78–.91) | <.001 |
| Charlson comorbidity index (reference = 0) | 0.83 (.81–.86) | <.001 |
| Month (reference = August) | ||
| September | 1.20 (1.13–1.29) | <.001 |
| October | 1.42 (1.33–1.51) | <.001 |
| November | 1.65 (1.54–1.77) | <.001 |
| December | 1.98 (1.85–2.12) | <.001 |
| January | 1.96 (1.84–2.10) | <.001 |
| February | 1.70 (1.58–1.82) | <.001 |
| March | 1.60 (1.49–1.72) | <.001 |
| April | 1.34 (1.25–1.44) | <.001 |
| May | 1.19 (1.11–1.28) | <.001 |
| June | 1.22 (1.14–1.32) | <.001 |
| July | 1.03 (.96–1.10) | .400 |
| Year (reference = 2016) | ||
| 2017 | 0.86 (.82–.90) | <.001 |
| 2018 | 0.78 (.72–.85) | <.001 |
Abbreviations: CI, confidence interval; DO, doctor of osteopathic medicine; MD, doctor of medicine; NP, nurse practitioner; PA, physician assistant.
aVariables included in interaction term in final multivariable model.
Figure 3.
Variation of odds ratios of antibiotic prescription by study month for entire study period. Reference = August.
Table 6.
Changes in Antibiotic Prescription by Disease Groups and Tiers
| Disease Group/Tier (Total Number Visits With Diagnosis/Tier) | Pre-intervention Antibiotic Prescription (%) | Intervention Antibiotic Prescription (%) | P Value |
|---|---|---|---|
| Acute/unspecified bronchitis | |||
| All (12 180) | 6839 (75.3) | 1532 (49.6) | <.001 |
| Excluding COPD (11 609) | 6538 (75.3) | 1433 (48.9) | <.001 |
| Sinusitis (30 881) | 20 410 (87.3) | 5689 (75.9) | <.001 |
| Pharyngitis (12 752) | 4637 (52.1) | 1346 (35.0) | <.001 |
| Pneumonia (4162) | 1248 (45.7) | 597 (41.7) | .013 |
| Otitis media (9862) | 3365 (50.3) | 1414 (44.6) | <.001 |
| Pertussis (42) | 14 (63.6) | 14 (70.0) | .662 |
| Other diagnoses (137 028) | 19 440 (21.2) | 6234 (13.8) | <.001 |
| Tier | |||
| 1 (4401) | 1331 (46.3) | 648 (42.5) | .015 |
| 2 (46 085) | 26 045 (77.3) | 7798 (63.0) | <.001 |
| 3 (135 269) | 17 410 (19.2) | 5011 (11.3) | <.001 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
DISCUSSION
This stepped-wedge interventional study demonstrated a significant reduction in antibiotic prescribing for RTDs across a variety of PC practice types and patient populations using provider-focused education and peer comparison feedback. Importantly, an effect was seen only in tiers 2 and 3, those diagnoses for which an antibiotic is frequently not indicated, and no effect was seen for diagnoses for which an antibiotic should typically be prescribed. This differential impact suggests that the study intervention was able to reduce inappropriate antibiotic prescribing without adversely impacting appropriate prescribing. However, only modest effects were seen in some specific conditions, such as sinusitis, suggesting there remains room for improvement.
This study is one of the largest in the United States to demonstrate a significant reduction in antibiotic prescribing for RTDs. Prior studies have demonstrated improvements in antibiotic prescribing in PC using various intervention methods, including education, communications training and peer comparison feedback [11–14, 16, 17, 20–24]. However, in comparison to other randomized trials that employed regular provider-level audit and feedback or peer comparison, our study is unique in its use of two validated metrics, shown to be associated with appropriate prescribing, in peer comparison feedback [11, 13, 17, 18]. These metrics are easily extracted from the EHR and could therefore be widely implemented. Furthermore, unlike prior studies, we examined the impact of our intervention on each diagnosis tier and included respiratory symptoms and other non-infectious respiratory tract diagnoses that were excluded from other studies [11–17, 20–23]. By including this larger group of RTDs, we captured a broader patient population for which antibiotics are prescribed, often inappropriately. Additionally, we distinguished between diagnosis tiers, thus gaining a critical understanding of the differential impact of an antibiotic stewardship intervention on a broad array of diagnoses, including diagnoses that warrant an antibiotic prescription and those for which it is not needed. Finally, by accounting for seasonal variation, as well as patient-, provider-, and practice-level variables, all of which may impact antibiotic prescribing, we can gain a better understanding of how to implement focused interventions.
We considered that providers might change visit diagnoses in response to feedback to increase their number of visits in lower number tiers, but this did not appear to occur (Table 1). Small changes in frequency of specific diagnoses were seen, most notably a decrease in sinusitis visits; this may have been due to seasonal variation in specific diagnoses, given that the pre-intervention period contained a higher proportion of winter months. We found that antibiotic prescribing in tier 1 was unexpectedly low (45.0% overall) in comparison to tier 2 (73.4% overall). The majority of tier 1 visits were for bacterial pneumonia; one possible explanation for low antibiotic prescribing is the practice of carrying forward some diagnoses on follow-up visits for the same problem. Supporting this hypothesis, we found that upon manual review of 20 randomly selected visits for bacterial pneumonia, 17 visits were found to be for follow-up of a recent prior diagnosis of pneumonia, with no new antibiotic prescription.
Consistent with prior studies, there was marked seasonal variation in antibiotic prescribing [25, 26]. This variation suggests that patients are more likely to receive an antibiotic for similar diagnoses in the winter months compared to the summer months, perhaps due to providers feeling overwhelmed due to higher volume of RTD visits or perceiving patients to have a higher expectation for an antibiotic during the winter months. It is possible that much of the increase in winter months is inappropriate because clusters that received the intervention either before or during the winter months (clusters 1–3, Figure 2) either did not have a winter spike in proportion of visits in which an antibiotic was prescribed or had that spike curtailed. However, more investigation into the drivers and appropriateness of increased antibiotic prescribing in winter months is needed. We also noted an overall decrease in prescribing during each subsequent year of the intervention, independent of the intervention effect. There were no other specific healthcare system-wide interventions during this period that would account for this secular trend, although national campaigns to improve ambulatory antibiotic prescribing may have played a role [27].
Prescribing was highly heterogeneous between practices, and there were several patient, provider, and practice characteristics found to be associated with antibiotic prescribing. Non-white patients were less likely to receive an antibiotic, a racial disparity that is consistent with prior literature [28, 29]. Patients with a higher CCI also had lower odds of antibiotic receipt, compared to those with a CCI of zero. Prior literature has shown an association between presence of chronic comorbidities and increased antibiotic prescribing, making our finding unexpected [30, 31]. There may be unmeasured factors that impacted this finding in our study, although we did adjust for provider factors that might be potential confounders with CCI, such as provider training or specialty [18]. Potential explanations might be care-seeking for less severe symptoms or overall increased frequency of contact with the healthcare system for more chronically ill patients, providing close follow-up opportunities when withholding antibiotic prescriptions. We also identified multiple provider- and practice-level factors associated with increased antibiotic prescribing, some of which had been previously reported [31–33]. Several of these factors, such as provider training type, practice specialty and practice teaching status may help guide more targeted future antibiotic stewardship approaches. However, there are likely other factors that we did not account for that are important, such as practice size and structure, patient volume and socioeconomic composition, or more intangible factors such as practice prescribing culture and provider perceptions of patient satisfaction. These other factors are likely important in gaining a more nuanced understanding of how prescribing decisions are made [31, 33–35].
Limitations
We acknowledge that we did not directly assess appropriateness of antibiotic prescribing, given the infeasibility of chart review in a large population. Although the metrics used in provider feedback were previously shown to be associated with appropriateness of prescribing, they are likely imperfect in capturing decision-making surrounding antibiotic prescribing [18]. Additionally, in examining antibiotic prescribing as a binary outcome, we did not directly assess changes in appropriateness of prescribing. However, with the reductions seen in prescribing in tiers 2 and 3, where antibiotics are sometimes or rarely indicated, it is likely that at least some of the reduction in prescribing was due to a decrease in inappropriate prescribing. We also additionally noted reductions in prescribing for specific diagnoses where antibiotics are frequently inappropriately prescribed, including acute bronchitis and sinusitis. Furthermore, although we did not examine other potential improvements in antibiotic prescribing, such as antibiotic selection and duration, we did note a decrease in azithromycin prescribing (Table 3). Suda et al demonstrated increases in macrolide prescribing in winter months, much of which is likely inappropriate, suggesting that the azithromycin reduction seen in our study may have been due to reductions in inappropriate prescribing [25]. Additionally, patient outcomes, including antibiotic-related adverse events, antibiotic resistance, or potential unintended consequences of not receiving an antibiotic were not assessed in this study, also due to limitations in ability to perform manual chart review in this large study. Given the association of inappropriate antibiotic prescribing with adverse events, we expect that a reduction in prescribing may improve outcomes, although data directly examining the impact of antibiotic stewardship interventions in primary care, including possible unintended negative consequences, are limited [4–6, 10, 36].
We are also unable to assess the differential impact of the individual components of the intervention and also cannot exclude the possibility that provider knowledge of being observed also played a role in changing behavior during the intervention, separately from peer comparison (Hawthorne effect). Additionally, use of ICD-10-CM codes may lead to disease/tier misclassification, given that providers may enter inaccurate codes or carry forward codes from prior visits regardless of active medical problems; however, the frequency of this practice was unlikely to have changed substantially during the intervention. Finally, we were unable to confirm that antibiotic prescriptions were associated with RTDs (rather than another diagnosis at the same visit) or if prescribers entered delayed prescriptions, for use only pending worsening symptoms.
CONCLUSION
This study demonstrated a significant reduction in antibiotic prescribing using provider-focused education and peer comparison feedback only in diagnosis tiers where antibiotics are only sometimes or rarely indicated. In the future, it will be important to assess the sustainability of this intervention, particularly following removal of the monthly provider feedback, given that it is well known that the impact of many behavioral interventions wane over time [37, 38]. In addition, building on prior literature focused on the drivers of prescribing behavior will be important, in order to better target future antibiotic stewardship initiatives, particularly focusing on both providers who did not change practice following the intervention and those for whom there was a large effect [39, 40]. Finally, assessment of patient outcomes and unintended negative consequences from this intervention is important, including examination of patient satisfaction ratings as well as antibiotic prescriptions for RTIs in the time period following each visit.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial Support. This work was supported by a Centers for Disease Control and Prevention (CDC) Cooperative Agreement Funding Opportunity Announcement (FOA) CK16-004, Epicenters for the Prevention of Healthcare Associated Infections.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Lauren Dutcher, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Kathleen Degnan, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Afia B Adu-Gyamfi, Medical College of Wisconsin, Milwaukee, Wisconsin, USA.
Ebbing Lautenbach, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Leigh Cressman, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Michael Z David, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA; Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Valerie Cluzet, Division of Infectious Diseases, Health Quest, Poughkeepsie, New York, USA.
Julia E Szymczak, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
David A Pegues, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Warren Bilker, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Pam Tolomeo, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Keith W Hamilton, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
References
- 1. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 3. Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open 2018; 1:e180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 5. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016; 316:2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340:c2096. [DOI] [PubMed] [Google Scholar]
- 7. Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin Infect Dis 2017; 66:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eudy JL, Pallotta AM, Neuner EA, et al. Antimicrobial stewardship practice in the ambulatory setting from a national cohort. Open Forum Infect Dis 2020; 7:ofaa513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implementation Science 2018; 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tonkin-Crine SK, Tan PS, van Hecke O, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev 2017; 9:CD012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016; 387:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care: a randomized clinical trial. JAMA Intern Med 2017; 177:176–83. [DOI] [PubMed] [Google Scholar]
- 14. McNulty C, Hawking M, Lecky D, et al. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: pragmatic randomized controlled trial of the TARGET antibiotics workshop. J Antimicrob Chemother 2018; 73:1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 2012; 344:d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med 2013; 173:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013; 309:2345–52. [DOI] [PubMed] [Google Scholar]
- 18. Degnan KO, Cluzet V, David MZ, et al. Development and validation of antibiotic stewardship metrics for outpatient respiratory tract diagnoses and association of provider characteristics with inappropriate prescribing. Infect Control Hosp Epidemiol 2021.. doi:10.1017/ice.2021.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 20. Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ 2012; 184:E726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buehrle DJ, Shively NR, Wagener MM, Clancy CJ, Decker BK. Sustained reductions in overall and unnecessary antibiotic prescribing at primary care clinics in a veterans affairs healthcare system following a multifaceted stewardship intervention. Clin Infect Dis 2020; 71:e316–22. [DOI] [PubMed] [Google Scholar]
- 22. Gjelstad S, Høye S, Straand J, Brekke M, Dalen I, Lindbæk M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ 2013; 347:f4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linder JA, Schnipper JL, Tsurikova R, et al. Documentation-based clinical decision support to improve antibiotic prescribing for acute respiratory infections in primary care: a cluster randomised controlled trial. Inform Prim Care 2009; 17:231–40. [DOI] [PubMed] [Google Scholar]
- 24. Kronman MP, Gerber JS, Grundmeier RW, et al. Reducing antibiotic prescribing in primary care for respiratory illness. Pediatrics 2020; 146:e20200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 2014; 58:2763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient antibiotic prescription trends in the United States: a national cohort study. Infect Control Hosp Epidemiol 2018; 39:584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 28. Olesen SW, Grad YH. Racial/ethnic disparities in antimicrobial drug use, United States, 2014–2015. Emerg Infect Dis 2018; 24:2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics 2013; 131:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shallcross L, Beckley N, Rait G, Hayward A, Petersen I. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017; 72:1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKay R, Mah A, Law MR, McGrail K, Patrick DM. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother 2016; 60:4106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003; 289:719–25. [DOI] [PubMed] [Google Scholar]
- 33. Fleming-Dutra KE, Bartoces M, Roberts RM, Hicks LA. Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis 2018; 5:ofx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni ML. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infect Control Hosp Epidemiol 2015; 36:153–9. [DOI] [PubMed] [Google Scholar]
- 35. Dempsey PP, Businger AC, Whaley LE, Gagne JJ, Linder JA. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract 2014; 15:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drekonja DM, Filice GA, Greer N, et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36:142–52. [DOI] [PubMed] [Google Scholar]
- 37. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014; 312:2569–70. [DOI] [PubMed] [Google Scholar]
- 38. Linder JA, Meeker D, Fox CR, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA 2017; 318:1391–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeffs L, McIsaac W, Zahradnik M, et al. Barriers and facilitators to the uptake of an antimicrobial stewardship program in primary care: a qualitative study. PLoS One 2020; 15:e0223822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szymczak JE, Feemster KA, Zaoutis TE, Gerber JS. Pediatrician perceptions of an outpatient antimicrobial stewardship intervention. Infect Control Hosp Epidemiol 2014; 35Suppl 3:S69–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.