Abstract
The IL-33/ST2 axis is reported to be controversially associated with coronary artery disease (CAD). A systematic review of the association between the IL-33/ST2 axis and CAD revealed that IL-33/ST2 plays a protective role in CAD and serum sST2 and IL-33 levels are increased in patients with cardiovascular disease. Therefore, the association of IL-33/ST2 single nucleotide polymorphisms (SNPs) with CAD prevalence, prognosis, and risk factors was assessed by performing a meta-analysis. Through a literature search of relevant articles in various databases using the relevant keywords, seven studies were included in the analysis. The meta-analysis showed that the IL-33/ST2 axis was associated with increased CAD risk [pooled odds ratio (OR) = 1.17, 95% confidence interval (CI): 1.13–1.20]. Gene subgroup analysis showed a close association of IL1RL1 (OR = 1.25, 95% CI: 1.20–1.30; I2 = 85.9%; p = 0.000) and IL1RAcP (OR = 1.42, 95% CI: 1.26–1.60; I2 = 27.1%; p = 0.203) with increased CAD risk. However, the association for the IL-33 gene was not statistically significant. SNPs rs7044343 (T), rs10435816 (G), rs11792633 (C) in IL-33 gene were associated with a protective effect in CAD. However, rs7025417 (T) in IL-33, rs11685424 (G) in IL1RL1, rs950880 (A) in sST2, and rs4624606 (A) in IL1RAcP were related to increased CAD risk. Overall, polymorphisms in IL-33/ST2 axis components were associated with increased CAD risk. These results may help identify key features of IL-33/ST2 immunobiology in CAD along with potential treatment strategies to lower disease burden.
Keywords: IL-33/ST2, gene, SNPs, CAD, immunobiology
Introduction
Interleukin-33 (IL-33), a member of the IL-1 cytokine superfamily, is an important regulator of pathological inflammation, immune homeostasis, fibrosis, and repair processes (1). The IL-33 receptors ST2L and sST2 are constitutively expressed in cells of the cardiovascular system, especially endothelial cells (2). Coronary artery disease (CAD) is caused by complex chronic inflammatory processes facilitated by the innate and acquired immune systems. Many cytokines are involved in CAD development (3). The IL-33/ST2 axis is involved in diverse areas of cardiovascular disease. IL-33 plays a role in cardiovascular disease either at the genetic level through regulation of transcription or as a classically active IL-33 functions that acts as an “alarmin” or cytokine. IL-33, upon interaction with ST2L, has been reported to prevent myocardial apoptosis, and alleviate myocardial fibrosis and myocardial hypertrophy (4). Thus, it inhibits the progression of atherosclerosis. However, clinically, serum IL-33 levels are elevated in patients with cardiac failure and stent restenosis after myocardial infarction (5). Further, sST2 is a biomarker of disease severity and prognosis for most cardiovascular diseases. Many studies have suggested that sST2 attenuates the cellular and beneficial actions of IL-33 in the cardiovascular system (6). However, there are no actual studies to prove that sST2 is harmful in CAD. Therefore, the theoretically beneficial effect of the IL-33/ST2 axis on the cardiovascular system is not reflected in practice.
Only a few recent studies on genetic linkage have attempted to explain the paradoxical relation between IL-33/ST2 and CAD (7, 8). Therefore, this article describes the known immunobiological functions of the IL-33/ST2 pathway in CAD occurrence and development and also presents a meta-analysis of the IL-33/ST2 gene polymorphisms associated with an increased risk of CAD.
Systematic review
IL-33/ST2: Biological structure and function
The human IL-33 gene is present on chromosome 9p24.1. IL-33 is known to bind chromatin through the H2A-H2B histone complex (9), which alters the interaction between nucleosomes to regulate the degree of chromosome compression, and thus alters the transcription of target genes (Figure 1). The IL-33 protein possesses an N-terminal nuclear domain, a C-terminal IL-1-like cytokine domain, and a homeodomain-like helix-turn-helix (HTH) motif at the divergence part in the middle of its sequence (10). The chromatin-binding motif (CBM, aa 40–58) at its N-terminal binds to chromatin through protein-protein interactions and co-determines the nuclear targeting of IL-33 through a predicted bipartite nuclear localization sequence (NLS, aa 65–78) (9) (Figure 2). The precursor of IL-33 (proIL-33) normally resides in the nucleus. When cells are stimulated by mechanical stress, inflammatory cytokines, or necrosis, extracellular proteases process proIL-33 such that it can act in an autocrine/paracrine manner as an “alarmin” on neighboring cells or on various ST2 receptor-expressing immune cells (11). This complex biological structure and function of IL-33 is an important reason for the multiple roles of IL-33 in CAD. For example, SNPs of IL-33 in different cellular localization fragments have different or even opposite roles in the pathogenesis of CAD.
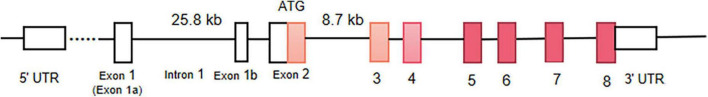
FIGURE 1.
The human IL-33 gene. The molecular weight of human IL-33 is about 18 kDa, and its gene span is > 42 kb. The main promoter of human IL-33 mRNA is upstream of the untranslated exon. The human IL-33-encoding gene contains eight exons. Exon 1b may replace the promoter. There are CAD related SNPs in promoter and intron 1 regions. Previous studies have reported the absence of splicing variants on exons 3/4/5/8, which may lead to the production of spliced IL-33 proteins.
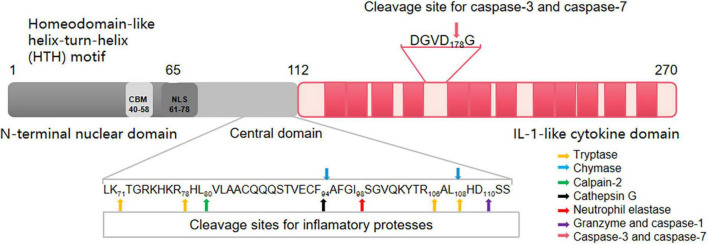
FIGURE 2.
The human IL-33 protein. Human IL-33 consists of 270 amino acids (aa). The protein includes the N-terminal nuclear localization signal, C-terminal IL-1-like cytokine domain, and intermediate divergence. The homeodomain-like helix-turn-helix (HTH) motif is constituted of aa 1–65 in the N-terminal region. Aa 40–58 in the N-terminal region of IL-33 form the chromatin-binding motif (CBM). CBM mediates IL-33 to bind to chromatin in a protein-protein interaction. The aa at positions 65–78 form the nuclear localization signal (NLS). Notably, CBM and NLS are critical for the nuclear localization of IL-33. Human mature IL-33 residues aa 112–270 (mouse mIL-33 residues aa 109–266). Many cleavage sites for inflammatory proteases (including chymase, tryptase, neutrophil elastase, cathepsin G, and elastase process) are found in the central domain of IL-33. These inflammatory proteases cleave full-length IL-33 in the central domain and produce cytokines with a more bioactive mature. Amino acids at position 111 of IL-33 is inactivated by caspase-1. Granzyme B processes full-length IL-33 into 18-kDa form which corresponds to IL-33111–270. The IL-1-like cytokine domain at aa 112–270 is the C-terminal region that is structurally homologous to other cytokines of the IL-1 family (IL-1α, IL-1β, and IL-18). After being digested by caspase-3 and caspase-7 at aa 178, IL-33 is often released as a mature cytokine that subsequently binds to its receptor, ST2. Different parts of the IL-33 sequence control different aspects of its biology, including subcellular localization, exocrine secretion, and functional maturation. Based on these sequence signals, IL-33 plays a dual role as a cytokine that activates downstream signals when released from necrotic cells and as a nuclear factor that contains transcriptional regulation in cells.
The human IL1RL1 gene is present on chromosome 2q12 (12). IL1RL1 (ST2) has four transcript isoforms, of which the most important are ST2L (IL1RL1-b) and sST2 (IL1RL1-a). The IL1RL1 gene encodes both ST2L and sST2 through alternative promoter activation. Two promoters at the proximal and distal parts of the ST2 gene regulate ST2 gene transcription. There are two SNPs in the distal parts are related to the susceptibility of CAD. IL-33 can self-regulate both ST2L and sST2 mRNA transcription (13). The link between the gene structure of IL-33 and its receptor IL1RL1 and CAD determines the important role of IL-33/ST2 pathway in CAD disease.
IL-33/ST2 and coronary artery disease
IL-33, a member of the IL-1 family of cytokines, is the ligand of the orphan receptor for ST2 (IL1RL1) (1). ST2 undergoes differential splicing to form the following subtypes: a transmembrane ligand (ST2L), a soluble component (sST2), and ST2V (13). ST2L is a transmembrane receptor; sST2 is a soluble receptor existing in blood circulation; and ST2V is a new variant of IL-33. ST2L and sST2 in the circulatory system are majorly found in the endothelial cells of the aorta and coronary arteries as well as immune cells such as T cells (2). The detailed biological structure and function of IL-33 have been described previously. IL-33 combined with chromatin is mainly stored in endothelial and epithelial cells nuclear. Then, it is released extracellular as a cytokine (14). IL-33 and ST2L bind to inflammatory cell membranes, which trigger intracellular signaling cascades that are enhanced by histones. A heterodimeric receptor complex is formed by IL-33 and ST2L with IL-1R accessory protein (IL-1RAcP) (15). This heterodimeric receptor complex utilizes the Toll/IL-1 receptor domain of IL-1RAcP for recruiting IRAK1, IRAK4, TRAF6 and Myd88, and subsequently activates multiple signaling pathways such as IKK/NF-KB, MAPK/AP-1, and PI3K/mTORC1, thus promoting gene expression, protein and lipid synthesis, and cellular metabolism (16) (Figure 3). Further, extracellular IL-33 is reported to indirectly inhibit the MAPK/p38/NF-κB pathway activation in the heart following MI (17). IL-33 reduces proinflammatory responses by affecting the activation of these cytokines, thus becoming a potential therapeutic target for cardiac remodeling after MI.
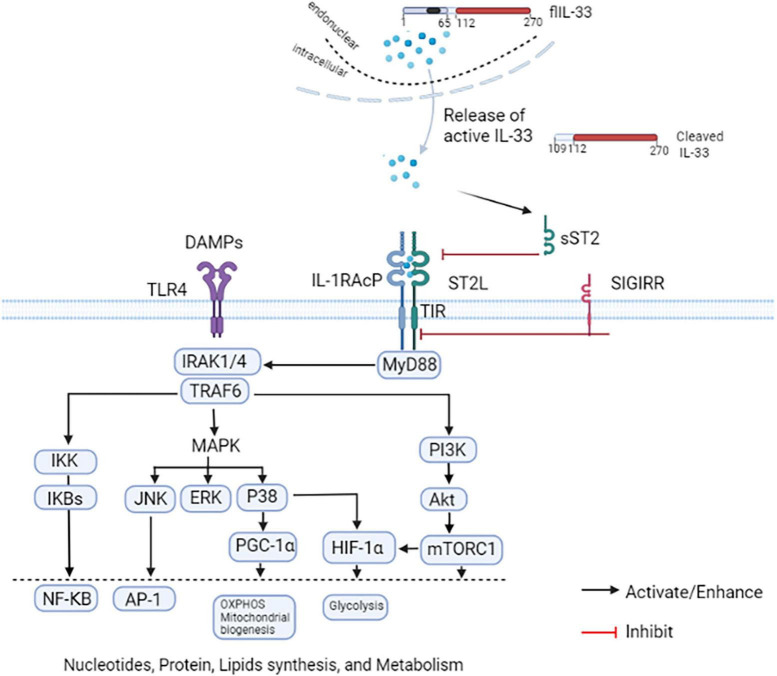
FIGURE 3.
IL-33/ST2 axis. IL-33 is normally localized in the cell nucleus. The full-length (fl) IL-33 protein is released in the endoplasmic reticulum-Golgi independent but ATP-dependent pathway during cell damage or necrosis. The flIL-33 is then cleaved by inflammatory proteases into a form with increased activity. A heterodimeric receptor complex is formed by IL-33 and ST2L with IL-1R accessory protein (IL-1RAcP) The complex activates the downstream MyD88 pathway, and then activates NF-κB, AP-1, PGC-1α, and HIF-1α to promote gene, protein, and lipid synthesis as well as cellular metabolism. A single IL-1-associated receptor SIGIRR negatively regulates the IL-33/ST2 axis. NF-κB and AP-1 are involved in the pathogenesis of various diseases by promoting inflammatory responses and inhibiting apoptosis. Activation of P38 can promote PGC-1α, OXPHOS, and mitochondrial fission and fusion; it can also lead to the activation of HIF-1α and enhancement of glycolysis. mTORC1 can also enhance HIF-1α expression and glycolysis enhancement in monocytes/macrophages.
A major part of atherosclerosis is artery wall chronic inflammation (18). IL-33 binding to ST2L inhibits Th1 cytokine production (reduces IFN-γ levels) and significantly increases Th2 cytokine production (IL-4, IL-5, IL-6, IL-8, and IL-13) (19). Thus, IL-33/ST2L induces polarization of the anterior atherosclerotic immune response from the Th1 to the Th2 type and inhibits atherosclerosis progression (20). Simultaneously, IL-33/ST2L induces the amplification of type 2 innate lymphoid cells (ILC2s) and promotes their egress from the immune organ (21). ILC2s also activate the Th2 response (22) and release cytokines. The activated downstream cytokine IL-5 may stimulate the proliferation of B1 cells (23) and production of atheroprotective natural IgM antibodies, which inhibit oxidized low-density lipoprotein production by acting against the phosphorylcholine head group of the oxidized phospholipids within low-density lipoproteins (24). Th2 cytokines, especially IL-13, polarize macrophages into the M2 phenotype (25). This mechanism limits the formation of foamy cells and lipid accumulation within plaques. Together with IL-10, IL-33 elevates the ATP-binding cassette transporter A1 expression (26), promotes the excretion of human cholesterol (27), and inhibits the formation of macrophage foam cells (28). Regulatory T cells (Tregs) support metabolic function and adipocyte differentiation (29, 30). IL-33 can selectively amplify ST2+ Tregs. ST2+ Tregs promote IL-13 and TGF-β release to inhibit inflammation and CD4+ T cell proliferation (31, 32). Enhanced IL-10 production in macrophages promotes macrophage polarization to the M2 anti-inflammatory phenotype, which suppresses foam cell production (25) (Figure 4).
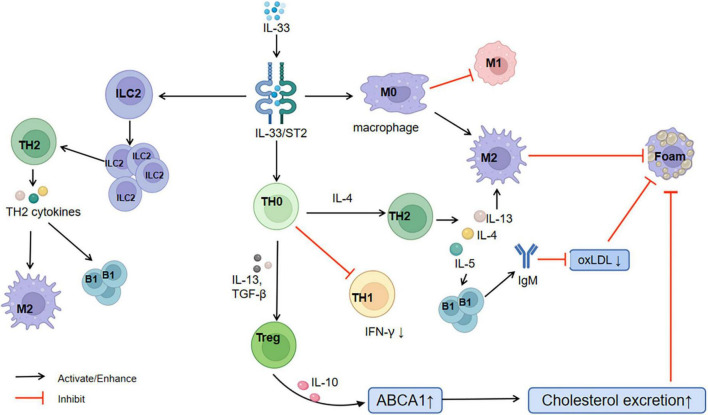
FIGURE 4.
Relationship between IL-33/ST2 and CAD. CAD, coronary artery disease; M1, M1 phenotype of macrophages; M2, M2 phenotype of macrophages; TH1, T-helper 1; TH2, T-helper 2; ILC2, type 2 innate lymphoid cells; B1: B1 cells; Treg, regulatory T cells; Foam, foam cells; oxLDL, oxidized low-density lipoprotein; ABCA1, ATP-binding cassette transporter A1.
However, sST2 binds IL-33 reducing the amount of IL-33 that can bind to ST2L and altering the anti-atherosclerosis protective activity of IL-33 (33). During cellular stress, IL-33, ST2L, and sST2 are upregulated, and sST2 can be considered a regulatory mechanism for avoiding excessive activation of IL-33. Altara et al. (34) previously summarized the following reasons underlying increased sST2 levels during enhanced IL-33/ST2L responses: AP1 and NF-KB signaling can activate sST2-related promoters; and increased ST2L expression by increased endogenous IL-1β levels because the counter-regulated PI3K/AKT/mTOR signaling is inhibited by ST2L to decrease the levels of IL-1β. IL-33 promotes the activation of adhesion molecules (such as VCAM-1, ICAM-1, and E-selectin) and induces the expression of chemokines (such as CXCL1 and MCP-1) in human endothelial cells and activates inflammation, which can possibly enhance atherosclerotic lesion development in the vascular wall (18, 35). Clinically, serum IL-33 levels are elevated in patients with cardiac failure and stent restenosis after myocardial infarction (5, 36). However, the mechanisms of these paradoxical roles of IL-33/ST2 remain unclear.
Relationship between single nucleotide polymorphisms in IL-33/ST2 and coronary artery disease: A meta-analysis
Considering the paradoxical role of the IL-33/ST2 axis on the cardiovascular system, we sought to explain the relationship between IL-33/ST2 and CAD based on the genetic linkage of the IL-33/ST2 axis. We thus performed a meta-analysis of all the studies related to CAD and the SNPs of IL-33/ST2 to identify the key features of the IL-33/ST2 immunobiology that have significance in CAD and the potential treatment strategies to lower disease burden. We searched for relevant articles published before April 1, 2022, without any restrictions on language. The following keywords and MeSH terms were used to search PubMed, Web of Science, Cochrane Library, and Clinicaltrials.gov databases: IL-33/ST2, Single Nucleotide Polymorphisms, Coronary Artery Diseases, nuclear protein, localization, metabolic, immunity, and Diseases.
Study characteristics
Figure 5 shows the literature-screening process. We first screened 44 studies, and after removing duplicates, selected the collected studies according to the following inclusion standards: (1) the study should be a case-control or clinical cohort study focusing on the link between IL-33/ST2 gene polymorphism and susceptibility to cardiovascular disease; (2) the study must provide adequate genotypic frequency information; (3) all cases must meet the diagnostic standard for cardiovascular disease; (4) the healthy controls should have their genotype frequencies in the Hardy-Weinberg equilibrium; (5) the studies should have a Newcastle-Ottawa scale score ≥ 5. Studies that did not meet these standards were excluded. Thus, seven case-control studies met our inclusion criteria for qualitative data analysis, and contained a total of 10686 cases and 10775 healthy subjects (7, 8, 37–41). Two researchers simultaneously screened the studies back-to-back and obtained consistent results. Detailed information on the finally included studies is shown in Table 1.
FIGURE 5.
Flowchart of search strategy. CAD, coronary artery disease; SNP, single nucleotide polymorphism.
TABLE 1.
Characteristics of the studies included.
| References | Country | Ethnicity | Sample size |
Genotyping method | SNP type (gene) |
HWE test (p-value) |
|
| Case | Control | ||||||
| Tu et al. (37) | China | Asian | 4,521 | 4,809 | Rotor-Gene 6000 High-Resolution Melt (HRM) system | rs7025417(IL-33) rs11685424(IL1RL1) |
>0.001 |
| Wu et al. (8) | China | Asian | 1,146 | 1,146 | Sequenom MassARRAY and TaqMan assays | rs4624606(IL-1RAcP) | > 0.001 |
| Yang et al. (38) | China | Asian | 490 | 929 | Sequenom MassARRAY and TaqMan assays | rs4624606(IL-1RAcP) | >0.05 |
| Lin et al. (39) | China | Asian | 532 | 601 | TaqMan assays | rs950880(IL1RL1) rs13001325(IL1RL1) |
>0.05 |
| Angeles-Martínez et al. (40) | Mexico | Caucasian | 1,095 | 1,118 | TaqMan assays | rs7044343(IL-33) | >0.001 |
| Nie et al. (41) | China | Asian | 1,736 | 1,093 | DNA sequencing analysis | rs7025417(IL-33) | >0.001 |
| Li et al. (7) | China | Asian | 1,166 | 1,079 | Multiple Ligase Detection Reaction platform | rs7025417(IL-33) rs7044343(IL-33) rs10435816(IL-33) rs11792633(IL-33) |
>0.05 |
SNP, single nucleotide polymorphism; HWE, Hardy-Weinberg equilibrium.
Meta-analysis
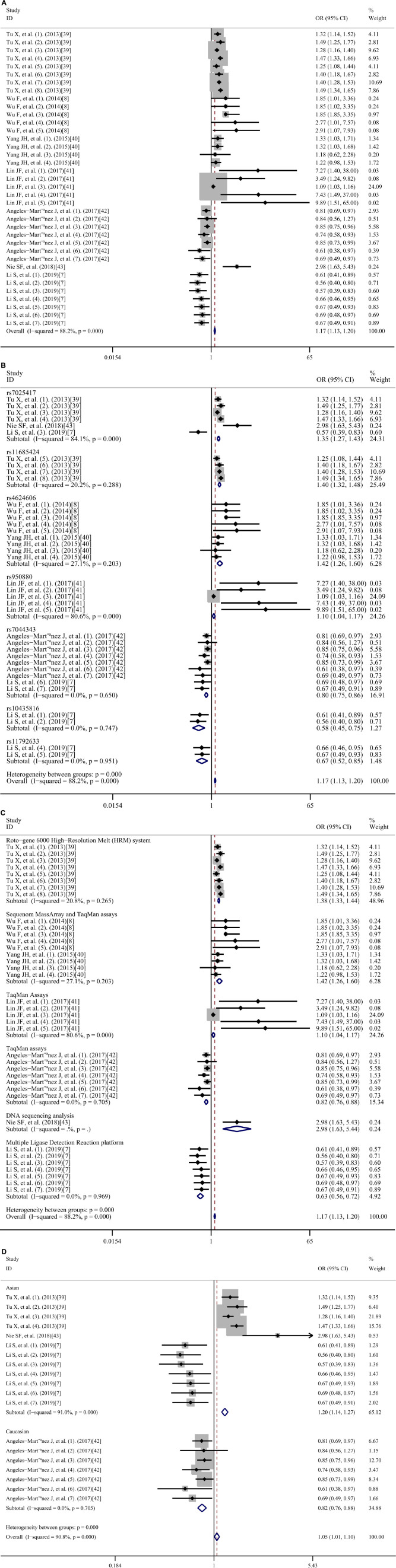
Figure 6 shows the multivariate-adjusted odds ratio (OR) for IL-33/ST2. The meta-analysis indicated that the IL-33/ST2 axis was significantly associated with increased CAD risk. The pooled OR was 1.17 (95% confidence interval (CI): 1.13–1.20), and high heterogeneity was observed (I2 = 88.2%; p < 0.001).
FIGURE 6.
Meta-analysis of the relationship between IL-33/ST2 and coronary artery diseases risk OR, odds ratio; CI, confidence interval.
Subgroup analysis
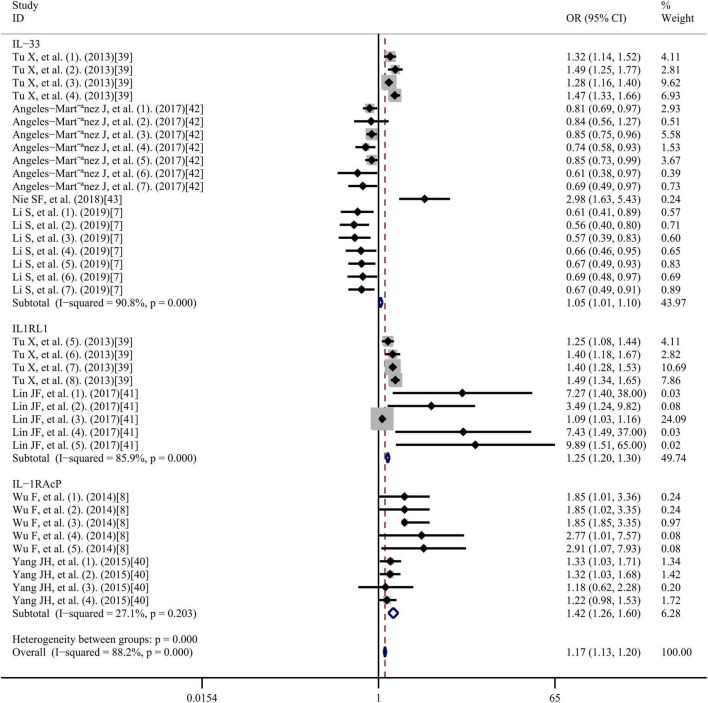
The impact of potential factors on the individual risk of developing CAD was investigated using subgroup analysis (Figure 7). Gene subgroup analysis suggested that IL1RL1 (OR = 1.25, 95% CI: 1.20–1.30; I2 = 85.9%; p = 0.000) and IL1RAcP (OR = 1.42, 95%CI: 1.26-1.60; I2 = 27.1%; p = 0.203) may be closely associated with increased CAD risk (Figure 7A). However, the association of the IL-33 gene was not statistically significant, possibly because of polymorphisms. Analysis results of the SNP subgroups (Figure 7B) showed that rs7044343 (T) (OR = 0.80; 95% CI: 0.75–0.86; I2 = 0.0%; p = 0.650), rs10435816 (G) (OR = 0.58, 95% CI: 0.45–0.75; I2 = 0.0%; p = 0.747), and rs11792633 (C) (OR = 0.67, 95% CI: 0.52-0.85; I2 = 0.0%; p = 0.951) polymorphisms in the IL-33 gene may be closely associated with a protective effect against CAD. However, rs7025417 (T) (OR = 1.35, 95% CI; 1.27–1.43; I2 = 84.1%; p = 0.000) in IL-33, rs11685424 (G) (OR = 1.40, 95% CI; 1.32–1.48; I2 = 20.2%; p = 0.288) in IL1RL1, rs950880 (A) (OR = 1.10, 95% CI; 1.04–1.17; I2 = 80.6%; p = 0.000) in sST2, and rs4624606 (A) (OR = 1.42, 95% CI: 1.26–1.60; I2 = 27.1%; p = 0.203) in IL1RAcP may be closely associated with increased CAD risk. Subgroup analysis of the genotyping methods (Figure 7C) showed that the use of TaqMan assays and the Multiple Ligase Detection Reaction platform were negatively associated with increased CAD risk, whereas the others were positively associated with increased CAD risk. We also performed a subgroup analysis of ethnicity for IL-33 (7, 37, 40, 41) (Figure 7D), and found that Caucasian ethnicity may be closely associated with a protective effect against CAD, whereas Asian ethnicity was associated with increased CAD risk. The ethnicity of the study subjects for both IL1RL1 and IL1RAcP was Asian. All the OR values obtained were adjusted for risk factors associated with CAD, including age, smoking, drinking, sex, triglyceride levels, body mass index, diabetes mellitus, hypertension, and a family history of CAD.
FIGURE 7.
Subgroup analyses based on (A) genes, (B) single nucleotide polymorphisms, (C) genotyping methods, and (D) ethnicity. OR, odds ratio; CI, confidence interval.
Meta-regression
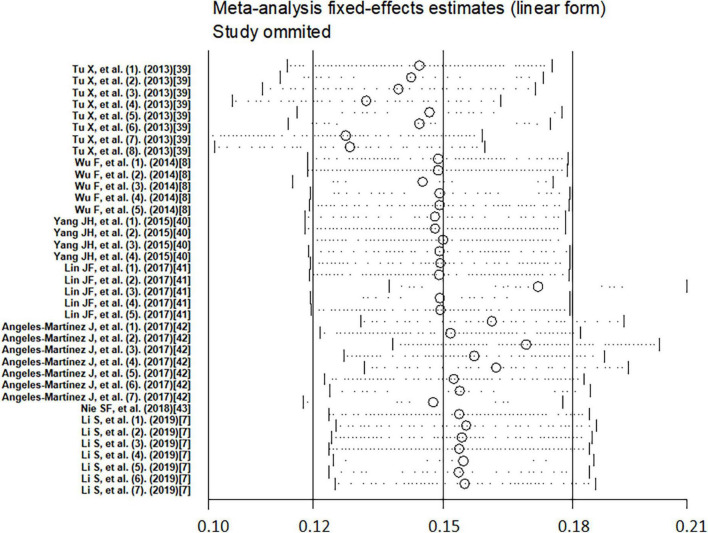
To investigate the source of heterogeneity, we performed meta-regression using genes, SNPs, genotyping methods, alleles, and ethnicity as covariates (Table 2). The results showed that genes, SNPs, alleles, and ethnicity are the main sources of possible heterogeneity in the association of IL-33/ST2 genetic polymorphisms with CAD susceptibility. The other covariates had no significant effect on inter-study heterogeneity. The sensitivity analysis results revealed that no study significantly affected the overall pooled OR (Figure 8). The funnel plot was used for assessing publication bias (Figure 9), Begg test, and Egger test; however, no publication bias was found (Begg test p = 0.349; Egger test p = 0.213).
TABLE 2.
Meta-regression based on genes, SNPs, genotyping method, and alleles as covariates of coronary artery disease.
| Covariate | p |
| Gene | 0.001 |
| SNPs | 0.001 |
| Genotyping method | 0.207 |
| Alleles | 0.048 |
| Ethnicity | 0.028 |
SNP, single nucleotide polymorphism.
FIGURE 8.
Sensitivity analysis of the association between IL-33/ST2 and coronary artery disease.
FIGURE 9.
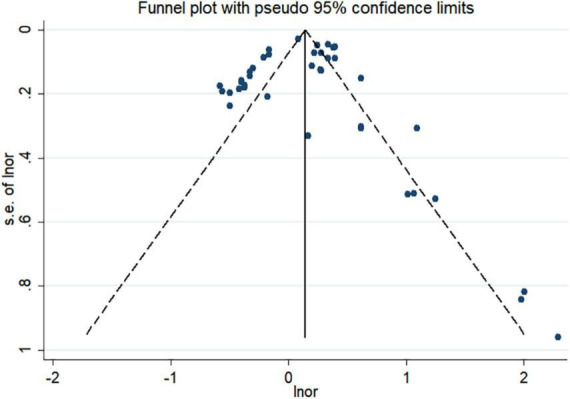
Funnel plot of the association between IL-33/ST2 and coronary artery disease. s.e, standard error; lnor, logarithm of the odds ratio.
Discussion
The beneficial and harmful effects of the IL-33/ST2 axis in patients with CAD are debatable (34, 42–44). Although several studies have reported the role of the IL-33/ST2 axis in CAD from a genetic perspective, the results remain controversial. In this study, we performed a meta-analysis to quantify this aspect based on previously published studies. Our meta-analysis results indicated that the IL-33/ST2 axis was significantly associated with increased CAD risk, consistent with the findings targeting IL1RL1 and IL1RaCP.
Among the results of the subgroup analysis, several factors deserve consideration. First, the results were explained by the differences in the target genes of the IL-33/ST2 axis among the included study participants. The gene subgroup analysis results showed that IL1RL1 and IL1RAcP might be closely associated with increased CAD risk. However, the results for the IL-33 gene were not statistically significant, possibly because of the genetic polymorphisms in IL-33; another possible explanation is that the expression levels of the IL-33 gene may be affected by genetic variations in it, thereby altering its function. To date, no other genes in the IL-33/ST2 axis have been reported to be associated with CAD. The SNP subgroup analysis showed that the rs7044343 (T), rs10435816 (G), and rs11792633 (C) in IL-33 may be closely related to CAD protection. A previous genetic study evaluated the potential influence of the IL-33 rs3939286 polymorphism in the development of carotid intima media wall thickness (a surrogate marker of cardiovascular disease) in Caucasian patients with rheumatoid arthritis (RA) (a chronic inflammatory disease associated with atherosclerosis) (45). With respect to this, those patients with RA carrying the IL-33 rs3939286 TT genotype exhibited lower carotid intima media wall values than those homozygous for the CC genotype. Also, patients with RA carrying the IL-33 rs3939286 mutant T allele showed significantly lower carotid intima media wall thickness values than those carrying the wild allele C in this study. Interestingly, this potential effect conferred by IL33 rs3939286 was independent of confounder factors (45). In contrast, rs7025417 (T) in IL-33, rs11685424 (G) in IL1RL1, rs950880 (A) in sST2, and rs4624606 (A) in IL1RAcP may be closely related with increased CAD risk. This dual role of IL-33 is closely related to the complex biological structure and function of IL-33. The mechanism of IL-33 in cardiovascular disease acts on the one hand at the genetic level through regulation of transcription, and also through a classically active IL-33 as an “alarmin” or cytokine. Different IL-33 segments have different cellular localization and different functions. As a nuclear factor plays a transcriptional regulatory role, as a cytokine processed by inflammatory proteases to a 10-fold expanded activity. The different IL-33 SNPs in our meta-analysis results have different or even opposite roles in the pathogenesis of CAD, consistent with the dual effects of IL-33 in the clinic.
Subgroup analysis of the genotyping methods showed that the use of TaqMan assays and Multiple Ligase Detection Reaction platform were negatively associated with increased CAD risk, whereas the others method was positively. However, no association was found between genotyping methods and gene polymorphisms. Age was a major risk factor for CVD, but all the ages of the included studies were clustered between 55 and 75, which were at high risk of cardiovascular disease, and the subgroup analysis of age was not too significant.
As heterogeneity is evident, we performed a subgroup analysis of the ethnicity for IL-33. Our results suggested that IL-33 gene polymorphism is associated with increased CAD risk in Asians and strongly associated with CAD protection in Caucasians. The ethnicity of the subjects included in the studies on IL1RL1 and IL1RAcP was Asian and could not be classified. However, based on the ethnic typing of IL-33, ethnic differences could possibly be a source of inter-study heterogeneity regarding the role of IL-33 gene polymorphisms in the pathogenesis of CAD. Natural selection and random genetic drift may be factors affecting individual genetic susceptibility.
Furthermore, related studies have identified obesity, diabetes, hypertension, serum sST2 levels, and GDF-15 levels as significant factors affecting the increased risk of CAD (39).
Heterogeneity is significant for meta-analysis and directly influences the interpretation of its results. Therefore, exploring the potential sources of heterogeneity is important for this study. Our meta-regression results showed that genes, SNPs, alleles, and ethnicity may be the major sources of heterogeneity in the association of IL-33/ST2 genetic polymorphisms with susceptibility to CAD. As raw data from the included studies were not included, the influence of other individual CAD risk factors such as sex could not be assessed with respect to the potential role of IL-33/ST2 gene polymorphisms in CAD development. Moreover, information on allele genotype frequencies was insufficient to be included in the study; thus, allele genotype frequencies may also be a cause of heterogeneity. Although this study has many limitations, this study is among the first meta-analysis to focus on the association between IL-33/ST2 and CAD risk from the perspective of gene polymorphisms. The sensitivity analysis results reveal that no single study significantly affected the overall pooled OR. Further, no publication bias was found based on the funnel plot, Begg test, and Egger test.
Conclusion
Our meta-analysis indicated that the IL-33/ST2 axis was significantly associated with increased CAD risk. This is consistent with the findings targeting both IL1RL1 and IL1RaCP. The SNPs rs7044343 (T), rs10435816 (G), and rs11792633 (C) in the IL-33 gene of individuals with Asian ethnicity may be closely related to the protective effect of CAD. Further, rs7025417 (T) in the IL-33 gene, rs11685424 (G) in IL1RL1, rs950880 (A) in sST2, and rs4624606 (A) in IL1RAcP in individuals of Asian ethnicity, may be closely associated with increased CAD risk. Thus, IL-33/ST2 gene polymorphisms may function as potentially useful biomarkers for the early diagnosis of CAD. However, owing to the abovementioned limitations, further studies with large sample sizes are required for obtaining a more representative statistical analysis. Overall, these results may help identify the key features of the IL-33/ST2 immunobiology in CAD and facilitate the development of potential treatment strategies to lower disease burden.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RL: conceiving the research, data analysis, and manuscript writing. LL: data analysis. CW: screening of studies. DL: manuscript review. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Shoumeng Yan for his help in the use of statistical software.
Funding
This work was supported by the Education Department of Jilin Province (grant no. JJKH20211039KJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF, Jr, et al. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity. (2021) 54:151–63.e6. 10.1016/j.immuni.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M, Duan L, Cai Y, Hao B, Chen J, Li H, et al. Prognostic value of soluble suppression of tumorigenesis-2 (sST2) for cardiovascular events in coronary artery disease patients with and without diabetes mellitus. Cardiov Diabetol. (2021) 20:49. 10.1186/s12933-021-01244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, MacFadyen JG, Glynn RJ, Bradwin G, Hasan AA, Rifai N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the cardiovascular inflammation reduction trial. Eur Heart J. (2020) 41:2952–61. 10.1093/eurheartj/ehaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circul Heart Fail. (2009) 2:684–91. 10.1161/CIRCHEARTFAILURE.109.873240 [DOI] [PubMed] [Google Scholar]
- 5.Demyanets S, Tentzeris I, Jarai R, Katsaros KM, Farhan S, Wonnerth A, et al. An increase of interleukin-33 serum levels after coronary stent implantation is associated with coronary in-stent restenosis. Cytokine. (2014) 67:65–70. 10.1016/j.cyto.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg VJ, Vroegindewey MM, Umans VA, van der Harst P, Asselbergs FW, Akkerhuis KM, et al. Persistently elevated levels of sST2 after acute coronary syndrome are associated with recurrent cardiac events. Biomarkers. (2022) 27:264–9. 10.1080/1354750X.2022.2032350 [DOI] [PubMed] [Google Scholar]
- 7.Li S, Wang Z, Liu X, Li Y, Shi C, Wu J, et al. Association of common variants in the IL-33/ST2 axis with ischemic stroke. Curr Neurovas Res. (2019) 16:494–501. 10.2174/1567202616666191029112334 [DOI] [PubMed] [Google Scholar]
- 8.Wu F, He M, Wen Q, Zhang W, Yang J, Zhang X, et al. Associations between variants in IL-33/ST2 signaling pathway genes and coronary heart disease risk. Int J Mol Sci. (2014) 15:23227–39. 10.3390/ijms151223227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. (2008) 9:1006–12. 10.1038/embor.2008.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. (2007) 104:282–7. 10.1073/pnas.0606854104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman HA. Nuclear IL-33 as a growth and survival agent within basal cells. J Clin Invest. (2021) 131:e153203. 10.1172/JCI153203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba Y, Maeda K, Yashiro T, Inage E, Kasakura K, Niyonsaba F, et al. GATA2 is a critical transactivator for the human IL1RL1/ST2 promoter in mast cells/basophils: opposing roles for GATA2 and GATA1 in human IL1RL1/ST2 gene expression. J Biol Chem. (2012) 287:32689–96. 10.1074/jbc.M112.374876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmieder A, Multhoff G, Radons J. Interleukin-33 acts as a pro-inflammatory cytokine and modulates its receptor gene expression in highly metastatic human pancreatic carcinoma cells. Cytokine. (2012) 60:514–21. 10.1016/j.cyto.2012.06.286 [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Ameri AH, Dempsey KE, Conrad DN, Kem M, Mino-Kenudson M, et al. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. (2021) 40:e106151. 10.15252/embj.2020106151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Sun L, He Y, Yuan X, Niu J, Su J, et al. Deficiency in IL-33/ST2 axis reshapes mitochondrial metabolism in lipopolysaccharide-stimulated macrophages. Front Immunol. (2019) 10:127. 10.3389/fimmu.2019.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. (2016) 16:676–89. 10.1038/nri.2016.95 [DOI] [PubMed] [Google Scholar]
- 17.Yin H, Li P, Hu F, Wang Y, Chai X, Zhang Y. IL-33 attenuates cardiac remodeling following myocardial infarction via inhibition of the p38 MAPK and NF-κB pathways. Mol Med Rep. (2014) 9:1834–8. 10.3892/mmr.2014.2051 [DOI] [PubMed] [Google Scholar]
- 18.Jia M, Li Q, Guo J, Shi W, Zhu L, Huang Y, et al. Deletion of BACH1 attenuates atherosclerosis by reducing endothelial inflammation. Circul Res. (2022) 130:1038–55. 10.1161/CIRCRESAHA.121.319540 [DOI] [PubMed] [Google Scholar]
- 19.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. (2008) 205:339–46. 10.1084/jem.20071868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munjal A, Khandia R. Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Adv Protein Chem Struct Biol. (2020) 120:85–122. 10.1016/bs.apcsb.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, et al. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. (2020) 75:1606–17. 10.1111/all.14196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stier MT, Zhang J, Goleniewska K, Cephus JY, Rusznak M, Wu L, et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J Exp Med. (2018) 215:263–81. 10.1084/jem.20170449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newland SA, Mohanta S, Clément M, Taleb S, Walker JA, Nus M, et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat Commun. (2017) 8:15781. 10.1038/ncomms15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay JT, Haro MA, Daly CA, Yammani RD, Pang B, Swords WE, et al. PD-L2 regulates B-1 cell antibody production against phosphorylcholine through an IL-5-dependent mechanism. J Immunol. (2017) 199:2020–9. 10.4049/jimmunol.1700555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley ML, Williams JO, Chan YH, Laubertová L, Gallagher H, Moss JWE, et al. The interleukin-33-mediated inhibition of expression of two key genes implicated in atherosclerosis in human macrophages requires MAP kinase, phosphoinositide 3-kinase and nuclear factor-κB signaling pathways. Sci Rep. (2019) 9:11317. 10.1038/s41598-019-47620-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HF, Wu MX, Lin YQ, Xie SL, Huang TC, Liu PM, et al. IL-33 promotes IL-10 production in macrophages: a role for IL-33 in macrophage foam cell formation. Exp Mol Med. (2017) 49:e388. 10.1038/emm.2017.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, et al. IL-33 reduces macrophage foam cell formation. J Immunol. (2010) 185:1222–9. 10.4049/jimmunol.1000520 [DOI] [PubMed] [Google Scholar]
- 28.Stankovic M, Ljujic B, Babic S, Maravic-Stojkovic V, Mitrovic S, Arsenijevic N, et al. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine. (2019) 120:242–50. 10.1016/j.cyto.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 29.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. (2015) 16:276–85. 10.1038/ni.3085 [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circul Res. (2020) 127:335–53. 10.1161/CIRCRESAHA.119.316461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lima MHF, Hiroki CH, de Fátima Borges V, Cebinelli GCM, Santos J, Rosa MH, et al. Sepsis-induced immunosuppression is marked by an expansion of a highly suppressive repertoire of FOXP3+ T-regulatory cells expressing TIGIT. J Infect Dis. (2022) 225:531–41. 10.1093/infdis/jiab405 [DOI] [PubMed] [Google Scholar]
- 32.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, et al. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. (2012) 32:605–12. 10.1161/ATVBAHA.111.242800 [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Duffen JL, Nocka KH, Kasaian MT. IL-13 controls IL-33 activity through modulation of ST2. J Immunol. (2021) 207:3070–80. 10.4049/jimmunol.2100655 [DOI] [PubMed] [Google Scholar]
- 34.Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiov Res. (2018) 114:1578–94. 10.1093/cvr/cvy166 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed A, Hollan I, Curran SA, Kitson SM, Riggio MP, Mikkelsen K, et al. Brief report: proatherogenic cytokine microenvironment in the aortic adventitia of patients with rheumatoid arthritis. Arthrit Rheumatol. (2016) 68:1361–6. 10.1002/art.39574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai V, Agrawal DK. Immunomodulation of IL-33 and IL-37 with vitamin D in the neointima of coronary artery: a comparative study between balloon angioplasty and stent in hyperlipidemic microswine. Int J Mol Sci. (2021) 22:8824. 10.3390/ijms22168824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu X, Nie S, Liao Y, Zhang H, Fan Q, Xu C, et al. The IL-33-ST2L pathway is associated with coronary artery disease in a Chinese Han population. Am J Hum Genet. (2013) 93:652–60. 10.1016/j.ajhg.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JH, Wu FQ, Wen Q, Zhang WC, Wang YE, Xiong X, et al. Association of IL33/ST2 signal pathway gene polymorphisms with myocardial infarction in a Chinese Han population. J Huazhong Univ Sci Technolog Med Sci. (2015) 35:16–20. 10.1007/s11596-015-1382-9 [DOI] [PubMed] [Google Scholar]
- 39.Lin JF, Wu S, Juang JJ, Chiang FT, Hsu LA, Teng MS, et al. IL1RL1 single nucleotide polymorphism predicts sST2 level and mortality in coronary and peripheral artery disease. Atherosclerosis. (2017) 257:71–7. 10.1016/j.atherosclerosis.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 40.Angeles-Martínez J, Posadas-Sánchez R, Llorente L, Alvarez-León E, Ramírez-Bello J, Villarreal-Molina T, et al. The rs7044343 polymorphism of the Interleukin 33 gene is associated with decreased risk of developing premature coronary artery disease and central obesity, and could be involved in regulating the production of IL-33. PLoS One. (2017) 12:e0168828. 10.1371/journal.pone.0168828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie SF, Zha LF, Fan Q, Liao YH, Zhang HS, Chen QW, et al. Genetic regulation of the thymic stromal lymphopoietin (TSLP)/TSLP teceptor (TSLPR) gene expression and influence of epistatic interactions between IL-33 and the TSLP/TSLPR axis on risk of coronary artery disease. Front Immunol. (2018) 9:1775. 10.3389/fimmu.2018.01775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes MF, Appelbaum S, Havulinna AS, Jagodzinski A, Zeller T, Kee F, et al. ST2 may not be a useful predictor for incident cardiovascular events, heart failure and mortality. Heart. (2014) 100:1715–21. 10.1136/heartjnl-2014-305968 [DOI] [PubMed] [Google Scholar]
- 43.Ghali R, Habeichi NJ, Kaplan A, Tannous C, Abidi E, Bekdash A, et al. IL-33 induces type-2-cytokine phenotype but exacerbates cardiac remodeling post-myocardial infarction with eosinophil recruitment, worsened systolic dysfunction, and ventricular wall rupture. Clin Sci. (2020) 134:1191–218. 10.1042/CS20200402 [DOI] [PubMed] [Google Scholar]
- 44.Zhao YN, Li H, Zhao C, Liu GH. ST2 silencing aggravates ventricular remodeling and chronic heart failure in rats by mediating the IL-33/ST2 axis. J Tissue Eng Regen Med. (2020) 14:1201–12. 10.1002/term.3091 [DOI] [PubMed] [Google Scholar]
- 45.López-Mejías R, Genre F, Remuzgo-Martínez S, Robustillo-Villarino M, García-Bermúdez M, et al. Protective role of the interleukin 33 rs3939286 gene polymorphism in the development of subclinical atherosclerosis in rheumatoid arthritis patients. PLoS One. (2015) 10:e0143153. 10.1371/journal.pone.0143153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.